Abstract
In the present study the antihistaminic activity of tricyclic benzothieno 1,2,3-triazine derivatives namely CP-3 (3-(phenyl)-5,6,7,8-tetrahydro,3H-benzo[4,5] thieno [2,3-d][1,2,3] triazin-4-one), CP-5 (3-(3-methyl phenyl)-5,6,7,8-tetrahydro,3H-benzo[4,5] thieno [2,3-d][1,2,3] triazin-4-one) and CP-8 (3-(4-chloro phenyl)-5,6,7,8-tetrahydro,3H-benzo[4,5] thieno [2,3-d][1,2,3] triazin-4-one) were evaluated using in vitro (isolated guinea pig ileum) and in vivo (bronchodilator activity in guinea pigs) models and the sedative potential of the test compounds were evaluated using actophotometer in mice. In in vitro antihistaminic study, the CP-3, CP-5, CP-8 and chlorpheniramine maleate (CPM) have shown a rightward shift in concentration response curve (CRC) of histamine with a change in EC50 values of histamine in all the four tissue preparations. The slope obtained in the schild plot indicated that CP-5, CP-8 and CPM were competitive in nature for H1-receptors. However, CP-3 has shown non-competitive antagonism. In in vivo antihistaminic study, the CP-3, CP-5, CP-8 and CPM have shown mean increase in exposition time against histamine challenge compared to control group (p < 0.001). All the test drugs (10 mg/kg) and CPM (2 mg/kg) have offered a significant (p < 0.001) protection against preconvulsive dyspnoea (PCD) compared to control. In conclusion, all the test drugs have shown very good antihistaminic activity and the test drugs have very little sedative action compared to CPM.
Keywords: Antihistaminic activity; Histamine induced bronchospasm; Tricyclic benzothieno1,2,3-triazines
1. Introduction
Allergy is one of the common diseases that affects mankind with diverse manifestations, allergy can refer to several kinds of immune reactions including type-I hypersensitivity reactions and activation of mast cells (Ring et al., 2001). The mast cell is a key effector cell in allergic inflammation and the release of histamine from activated mast cells and basophils contributes significantly to the symptoms of allergic rhinitis, conjunctivitis, urticaria and other allergic reactions including allergic asthma anaphylaxis, autoimmune hemolytic anemia, erythroblastosis fetalis, pernicious anemia, immune complex glomerulonephritis, rheumatoid arthritis, serum sickness, contact dermatitis and temporal arteritis (Larson et al., 2007). Currently antihistamines, glucocorticoids and mast cell stabilizers are widely prescribed for the management of these allergic reactions, but these drugs exhibit a high side effect profile which includes sedation, diminished alertness and concentration, light headedness, motor in coordination, fatigue and tendency to fall asleep (Klaassen., 2001).
The substituted triazines are reported for many uses like anti-inflammatory activity (Daidone et al., 1998), analgesic activity (Daidone et al., 1998), purine antagonism activity (Biesele, 1952), anti cancer and trypanocidal activities (Gatta et al., 1989), antineoplastic activity (Dattolo et al., 1989), inhibition of nitric oxide and eicosanoid biosynthesis (Quintela et al., 1999), 5-HT3 receptor antagonists with gastric motility enhancement activity (David, 1990), antianaphylatic activity (Siegfried et al., 1990), anti platelet aggregation activity (Antonio et al., 1992), anti thrombotic and elastase inhibitor activity (Thomas et al., 1995), antiallergic activity (Youssefyeh et al., 1984), xanthine oxidase inhibitory activity (Shaw and Woolley, 1952), antiviral/antitumor activity (Manfredini et al., 1992), fungicidal activity (Anthony et al., 1993), antimicrobial activity and antineoplastic activity (Monge et al., 1991).
The 1,2,3-triazines are the class of heterocyclic compounds, unlike other triazines there is very minimal research that has been carried out on this class of compounds and based on the structural similarities of these compounds with the other triazines like 1,2,4-triazines and 1,3,5-triazine, it is thought that 1,2,3-triazine derivatives may also found to possess prostaglandin inhibition property, analgesic, anti-inflammatory and antihistaminic properties. Based on the results of preliminary antihistaminic pilot study, the present study was under taken to investigate the synthesized tricyclic benzothieno 1,2,3-triazines for their anti-histaminic activity.
2. Materials and methods
2.1. Drugs, chemicals and instruments
Histamine hydrochloride (Otto Kemi, Mumbai) and Chlorpheniramine maleate (Alfa Labs, Pigdambari). The solvents and chemicals used for the synthesis of thienotriazines and for the preparation of physiological salt solutions were of analytical grade procured from local firms.
Analytical TLC was performed on Silica plates-GF254 (Merck) with visualization by UV or iodine vapors. Melting points were determined in open capillaries on a Thermonic Melting point apparatus and are uncorrected. The IR spectra (KBr, cm−1) were run on Perkin Elmer FTIR Spectrophotometer. 1H NMR and 13C NMR (CDCl3/DMSO-d6) spectra were recorded using Bruker AMX-400 with TMS as internal standard, MS spectra were recorded on (AMD-604) and elemental analyses were performed on Carlo Erba 1108 elemental analyzer and were within ±0.4% of theoretical values.
2.2. Test compounds
2.2.1. Synthesis of thienotriazines
The starting compounds 2-amino-3-(N-substituted carboxamido)-4,5-tetramethylene thiophenes, CP-3a, CP-5a and CP-8a were synthesized in three steps by the adaptation of well known and versatile Gewald reaction (Gewald et al., 1966). Later, the compounds CP-3a, CP-5a and CP-8a were diazotized to yield a series of 3-substituted amino-5,6-tetramethylene thieno[2,3-d] [1,2,3]-triazin-4(3H)-ones, namely CP-3, CP-5 and CP-8, respectively. In this reaction, the starting compounds 2-amino-3-(N-substitutes carboxamido)-4,5-tetramethylene thiophenes (CP-3a, CP-5a and CP-8a) react with NaNO2 in the presence of HCl to give respective triazine-4-ones.
2.2.2. General procedure for the syntheses of 2-amino-3-(N-substituted carboxamido/carboxanilido)-4,5-tetramethylene thiophenes (CP-3a, CP-5a, CP-8a)
A mixture of appropriate active methylenic ketone (0.04 mol), substituted cyano acetamide/acetanilide (0.04 mol), ammonium acetate (2 g) and glacial acetic acid (2 ml) in cyclohexane (80 ml) was refluxed for 10 h in a Dean stark apparatus with an arrangement for water separation. The reaction mixture was cooled, diluted with cyclohexane and washed successively with water, 10% aqueous sodium carbonate solution, and dried over anhydrous sodium sulfate. The solvent was removed under vacuum. The crude alpha (N-substituted carboxamido/carboxanilido) acetonitrile derivative thus obtained was employed directly for further reaction.
To a mixture of the above crude intermediate, sulfur (0.04 mol) in ethanol (40 ml) and diethylamine (4.0 ml) was added drop wise with stirring. The mixture was stirred for 1 h at 45–50 °C, chilled overnight and the solid obtained was filtered, washed with ethanol to yield yellow crystalline solids. Recrystallized from suitable solvents. Yield 45–50%.
2.2.2.1. General method for the syntheses of 4,5-tetramethylene thieno [2,3-d] [1,2,3]-triazin-4(3H)-ones (CP-3, CP-5, CP-8)
A mixture of the corresponding 2-amino-3-N-(substituted carboxamido/carboxanilido)-4,5-substituted thiophenes (CP-3a, CP-5a, CP-8a) (0.01 mol) in 30 ml of glacial acetic acid was warmed until the starting material dissolved. The mixture was cooled to room temperature, 20 ml of concentrated HCl was added and the reaction mixture was cooled to a temperature below 5 °C. To this mixture an ice cold solution of NaNO2 (0.03 mol) in water (25 ml) was added drop wise with constant stirring. Temperature was maintained below 5 °C. The product separated as bright yellow solid, which was filtered, dried and washed with methanol to obtain pure triazines (CP-3, CP-5, CP-8). The synthetic route employed for the synthesis of the title compounds is outlined in Fig. 1. The physical properties and elemental analysis of CP-3, CP-5 and CP-8 are given in Table 1.
Figure 1.
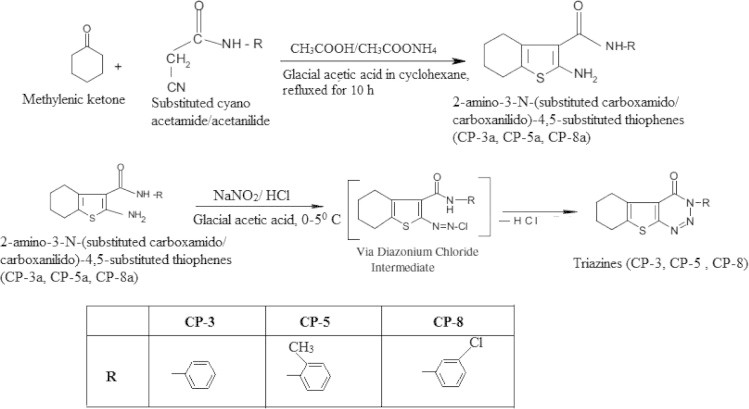
General experimental scheme for the synthesis of thieno 1,2,3 triazine-4-ones.
Table 1.
Physical properties and elemental analysis of CP-3, CP-5 and CP-8.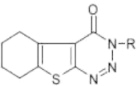
| Compound | Mol. formula | Mol. wt | R | M.P (°C) | % Yield | Rf value (solvent CHC13:MeOH) (70:30) | Elemental analysis |
|
|---|---|---|---|---|---|---|---|---|
| Theoretical | Actual | |||||||
| CP-3 | Ci5H13N3OS | 283 | 112–114 | 47 | 0.71 | C = 63.58 H = 4.62 N = 14.84 S = 11.32 |
C = 6i.60 H = 4.63 N = 14.82 S = 11.33 |
|
| CP-5 | C16H15N3OS | 297 |  |
138–140 | 64 | 0.80 | C = 64.62 H = 5.0S N = 14.14 S = 10.77 |
C = 64.59 H = 5.07 N = 14.12 S = 10.78 |
| CP-8 | C15H12N3OSCl | 317.5 |  |
110–112 | 62 | 0.72 | C = 56.69 H = 3.81 N = 13.22 S = 10.07 |
C = 56.60 H = 3.S2 N = 13.21 S = 10.09 |
2.2.3. Spectral data
CP-3a: Melting Point: 112–114 °C, yield: 47%, MS (%): 283 (M+ 100.0%), 284.08(17.7%), 285.07(4.4%), 285.08(1.8%), 284.07(1.8%).
IR max cm−1: Ar-CH 3035.23; -Ali-CH 2982.54; Arom C C 1498.63; –CO– 1692; (C–N) −759.23.
1H NMR (CDCl3): 7.25–7.5 (m, 5H, Ar-H), 2.0–3.0 (m, 8H, tetramethylenic protons), 1.5 (m, 4H, methylenic protons).
13C NMR (DMSO-d6): 164.5, 138.5, 136.5, 135.6, 128.5, 125.5, 125.7, 122.3, 115.5, 25.5, 23.4, 20.5.
CP-5a: Melting point: 138–140 °C, yield: 65%, MS (%): 297.80 (M+) (100.0%), 298.69(17.3%), 299.88(4.5%), 299.10(1.4%), 298.29(1.1%).
IR max cm−1: Ar-CH 3013.34; -Ali-CH 2963.25; Arom C C1507.26; –CO– 1684; (C–N) −742.25.
1H NMR (CDCl3): 7.3–7.4 (d, 2H, Ar-H), 7.1 (s, 1H, Arom), 6.7 (s, 1H, Arom), 2.4 (s, 3H, methyl protons), 1.7 (m, 4H, methylenic protons), 2.2 (m, 4H, methylenic protons).
13C NMR (DMSO-d6): 164.7, 138.5, 136.7, 135.6, 134.9, 127.4, 126.4, 125.7, 123.4, 121.3, 115.3, 25.6, 23.4, 20.4, 15.7.
CP-8a: Melting point: 110–112 °C, yield: 62%, MS (%): 317 (M+) (100.0%), 319.04(32.5%), 318.04(18.6%), 320.04(6.4%), 319.03(4.4%), 321.04(1.4%), 319.05(1.3%).
IR max cm−1: Ar-CH 3098.35; -Ali-CH 2968.57; Arom C C 1508.24; –CO– 1688; (C–N) −7734.2.
1H NMR (CDCl3): 7.8 (d, 2H, Arom), 7.2 (d, 2H, Arom), 2.8 (m, 4H, methylenic protons), 1.6 (m, 8H, methylenic protons).
13C NMR (DMSO-d6): 164.5, 138.5, 136.5, 135.6, 134.5, 129.5, 125.5, 125.7, 122.3, 118.5, 115.5, 25.5, 23.4, 20.5.
2.3. Experimental animals
Guinea pigs of either sex (200–300 g) and female swiss albino mice (18–22 g) were purchased from Bioneeds, Nelamangala, Tumkur, India. They were housed in a separate room in animal facility of PES College of Pharmacy. Mice were maintained in polypropylene cages, while guinea pigs were maintained in stainless steel cages at a temperature of 25 ± 1 °C and relative humidity of 45–55% in a clean environment under 12 h light–dark cycle. The animals had free access to food pellets (Pranav Agro Industry, Bangalore, India) and purified water ad libitum.
All the experimental protocols were approved by Institutional Animal Ethics Committee (IAEC) of PES College of Pharmacy (No. PESCP/IAEC/03/2005-06) and were conducted according to the guidelines of CPCSEA, India.
3. Experimental protocol
3.1. Acute toxicity studies
The acute intraperitoneal toxicity for the test compounds was determined in female, nulliparous and non pregnant swiss albino mice weighing 18–22 g. After administration of different doses of test compounds, the mortality with each dose was noted at 48 h (acute) and 14 days (chronic) as per OECD guideline no. 425. LD50 was calculated using AOT425 stat program (http://www.oecd.org/dataoecd/17/51/1948378.pdf).
3.2. Antihistaminic activity
3.2.1. In vitro antihistaminic activity by using isolated guinea pig ileum
The guinea pig was sacrificed after 24 h of fasting, the ileocaecal junction was exposed by giving the midline abdominal incision and the ileum was isolated by discarding the terminal 10 cm length nearer to ileocaecal junction, the lumen of the isolated ileum was thoroughly cleaned by using a warm tyrode solution with the help of a 50 ml volumetric pipette. A small piece of the ileum with 2–4 cm length was cut; at the two opposite ends a thread is passed through the lumen with the help of a fine suturing needle and tied securely without occluding the lumen. One end of the segment is secured tightly to the tissue holder and transferred to the organ bath already filled with Tyrode solution of following composition: 136.9 mM NaCl, 11.9 mM NaHCO3, 2.68 mM KCl, 1.05 mM MgSO4, 1.8 mM CaCl2, 0.37 mM NaH2PO4 and 5.6 mM glucose. The solution was kept at 37 ± 1 °C and it was continuously aerated with 95% O2 and 5% CO2. The pH of the solution was maintained at 7.4, The tissue holder is fixed in position with clamps and the long thread from the tissue is tied to the force transducer (T 305) connected to student physiograph (Bio-Devices, Ambala, India) against a constant load of 1 g. The tissue was allowed to stabilize for a period of 30 min, after which concentration response curves (CRC) to histamine (contact time 30 s) were constructed by a cumulative addition of log increment dose of histamine in the absence or presence of antagonists. Antagonists were incubated with ileum for 30 min before the addition of cumulative concentrations of histamine (Shames et al., 1999).
Mean CRCs to contraction rate were analyzed by the equation (given below) using non-linear regression of GraphPad Prism trial version software (GraphPad Software Inc., San Diego, USA).
where X is logarithm of molar concentration of the relaxant, Y is the response produced by the agonist and P is the slope of the CRCs. EC50 is the concentration (M) of the relaxant that produces 50% of its maximum response and pEC50 is the negative logarithm of EC50.
Agonist concentration ratios(r) were determined from the EC50 on the CRCs with or without antagonists. The plot of log [agonist concentration (dose) ratio (r) − 1] vs. log [antagonist] was analyzed by linear regression.
Antagonism was considered to be competitive if the slope (m) of regression line was not significantly different from unity. In some cases a mean pA2 value was obtained from individuals estimated using the equation.
In the cases where the slope or regression line significantly differed from unity, the value obtained from the above equation was pKB rather than pA2. A statistically significant difference between two means was analyzed using one-way analysis of variance followed by the Tukey test, where comparison was made to the same control group. P < 0.05 was considered significant.
3.2.2. In vivo antihistaminic activity by histamine induced bronchospasm in guinea pigs
Guinea pigs were treated with vehicle/test compounds/Chlorpheniramine maleate (2 mg/kg) intraperitoneally. After 30 min of the vehicle/test drug/CPM treatment, the bronchospasm was induced by exposing the animals to 2% histamine acid phosphate under constant pressure and rate (1 kg/cm2) in a histamine chamber (24 × 14 × 24 cm, INCO, Ambala, India) made up of perplex glass, the preconvulsive time (PCT) was determined from the time of exposure to the onset of dyspnoea leading to the appearance of convulsions which is known as preconvulsive dyspnoea (PCD).
As soon as the PCD were noted, the animals were removed from the chamber and placed in fresh air. The animals which withstand exposure to histamine aerosol for 15 min were considered to be completely protected (Gopumadhavan et al.,2005).
3.2.3. CNS depressant activity of CPM and test drug by actophotometer
Each animal was placed individually on Actophotometer (INCO, Ambala, India) and basal locomotor activity score of all the animals were noted. Mice were injected with test/CPM/vehicle and after 30 min each animal was tested for locomotor activity score for 10 min and the percentage decrease in motor activity was calculated (Kulkarni, 2005).
3.2.4. Statistical analysis
The results of various studies were expressed as mean ± S.E.M. Data analysis was done by one way analysis of variance (ANOVA) followed by Tukey test. Probability values of 0.05 (p < 0.05) or less were considered statistically significant.
4. Results
4.1. Acute toxicity studies
Intraperitoneal administration of CP-3, CP-5 and CP-8 at 55 and 175 mg/kg dose levels did not show any toxic signs during short term and long term observation period. However, at 550 mg/kg, i.p. dose of CP-3, CP-5 and CP-8 produced 100% mortality during the short-term observation period. LD50 value of all the test drugs was found to be 310 mg/kg.
4.2. Antihistaminic activity
4.2.1. In vitro studies
CP-3, CP-5, CP-8 and CPM shifted the CRCs of histamine toward the right with a change in EC50 values of histamine. The schild plot yielded a line with a slope close to unity for CP-5, CP-8 and CPM in guinea pig ileum preparation, indicating that the antagonists were competitive in nature for H1-receptors. However, the slope of the schild plot was significantly different from unity for the test drug CP-3, indicating non competitive antagonism, the rightward shift of histamine CRCs and schild plots for histamine in the presence of CP-3, CP-5, CP-8 and CPM are shown in the Figure 2, Figure 3, respectively, the EC50 and pA2 values are shown in Table 2.
Figure 2.
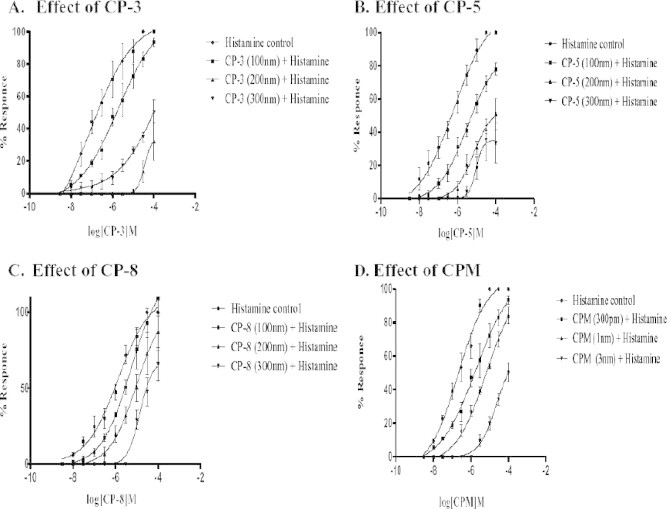
Effect of CP-3, CP-5, CP-8 and CPM on histamine induced contractions on guinea pig ileum. Results are expressed as percentage decrease in contractions induced by histamine.
Figure 3.
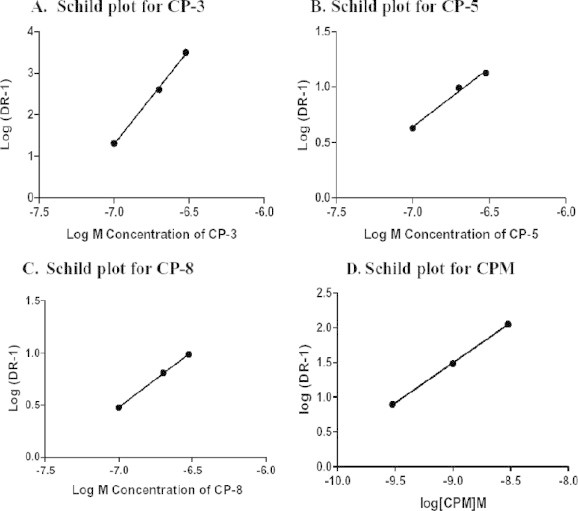
Schild plot for CP-3, CP-5, CP-8 and CPM.
Table 2.
Effect of CPM, CP-3, CP-5 and CP-8 on contractions induced by histamine in isolated guinea pig ileum.
| Treatment | EC50 (μM) | pEC50 | pA2/pKB value |
|---|---|---|---|
| Histamine alone | 4.65 (4.07–5.03) | 6.449 ± 0.237 | – |
| CP-3 (100 nm) | 1.88 (5.21–6.81) | 5.724 ± 0.275 | 10.211 ± 0.42 |
| CP-3 (200 nm) | 3.53 (1.40–8.90) | 4.451 ± 0.197 | |
| CP-3 (300 nm) | 0.0002 (5.97–126.3) | 3.561 ± 2.79 | |
| CP-5 (100 nm) | 3.36 (1.27–8.90) | 5.472 ± 0.208 | 8.65 ± 0.01 |
| CP-5 (200 nm) | 6.94 (2.66–1.81) | 5.158 ± 0.205 | |
| CP-5 (300 nm) | 9.20 (4.93–1.71) | 5.036 ± 0.133 | |
| CP-8 (100 nm) | 5.33 (1.69–1.68) | 5.272 ± 0.246 | 8.50 ± 0.009 |
| CP-8 (200 nm) | 9.95 (1.91–5.15) | 5.002 ± 0.352 | |
| CP-8 (300 nm) | 1.42 (8.46–2.40) | 4.845 ± 0.111 | |
| CPM (300 pm) | 1.88 (5.21–6.81) | 5.724 ± 0.275 | 10.491 ± 0.038 |
| CPM (1 nm) | 6.64 (2.48–1.77) | 5.177 ± 0.210 | |
| CPM (3 nm) | 2.41 (8.54–6.80) | 4.617 ± 0.222 |
The values in the parenthesis indicate EC50 range at 95% confidence interval. The values of pEC50 and pA2/pKB are expressed as mean ± S.E.M.
4.2.2. In vivo studies
All the test drugs (CP-3, CP-5 and CP-8) and chlorpheniramine maleate have shown the mean increase in exposition time against histamine challenge compared to control group (p < 0.001). All the test drugs (10 mg/kg) and standard antihistaminic chlorpheniramine maleate (2 mg/kg) have offered significant (p < 0.001) protection against PCD compared to control group, However, significant antihistaminic effect was not observed in lower dose (5 mg/kg) of test compounds. The results are shown in the Table 3.
Table 3.
Effect of CP-3, CP-5 and CP-8 and CPM on histamine induce bronchospasm in guinea pigs.
| Sl. no. | Groups | Onset of PCD (in sec) | % Protection against mortality |
|---|---|---|---|
| I | Control | 195 ± 12.4 | 0 |
| II | CPM (2 mg/kg) | 800 ± 25.69∗∗∗ | 100 |
| III | CP-3 (5 mg/kg) | 225 ± 12.84 | 100 |
| IV | CP-3 10 mg/kg) | 860 ± 24.08∗∗∗ | 100 |
| V | CP-5 (5 mg/kg) | 230 ± 16.73 | 83.34 |
| VI | CP-5 (10 mg/kg) | 720 ± 26.83∗∗∗ | 100 |
| VII | CP-8(5 mg/kg) | 240 ± 17.32 | 66.65 |
| VIII | CP-8 (10 mg/kg) | 690 ± 37.14∗∗∗ | 100 |
Values are expressed as mean ± S.E.M from six guinea pigs. ∗∗∗P < 0.001; compared with control group using one way ANOVA followed by Tukey test.
4.2.3. CNS depressant activity of CPM and test drug by actophotometer
The standard drug chlorpheniramine maleate has shown a significant decrease in the locomotor activity at 10 mg/kg dose and none of the test drugs (CP-3, CP-5 and CP-8) have shown a significant decrease in the locomotor activity at 50 mg/kg dose compared to CPM treated group, indicating the very less CNS depression property of test drugs. The results are shown in Table 4.
Table 4.
Effect of CP-3, CP-5, CP-8 and CPM on spontaneous locomotor activity in mice.
| Groups | Control | CPM treated group (Std) (10 mg/kg) | CP-3 (50 mg/kg) | CP-5 (50 mg/kg) | CP-8 (50 mg/kg) |
|---|---|---|---|---|---|
| Spontaneous locomotor activity | 679.16 ± 21.4 | 102.66 ± 2.69∗∗∗ | 513.83 ± 6.11∗∗∗ | 484.16 ± 7.12∗∗∗ | 474.66 ± 4.77∗∗∗ |
| % Inhibition in locomotor activity | – | 84.88344 | 24.34 | 28.71 | 30.11 |
Values are expressed as mean ± S.E.M from six mice. ∗∗∗P < 0.001; compared with control group using one way ANOVA followed by Tukey test.
5. Discussion
The results of the present investigation indicate that, CP series of compounds exerts specific antihistaminic action. However, in all these preparations histamine manifests its effect by acting upon H1-receptors (Patnaik et al., 1979). The purpose behind selecting CPM as a standard for both the models was to find out whether the action of CP series of compounds is dependent on H1-receptors or not. The compounds were able to antagonize the action of histamine on H1-receptors in both the models.
Histamine has a key role in many physiological processes including inflammation and the drugs that target H1-receptors have been successful for the treatment of allergy. H1-receptors are expressed on multiple cell types including endothelial cells and smooth muscle cells, where they mediate vasodilation and bronchoconstriction. Antagonists of H1-receptors, such as diphenhydramine and loratadine have been used for many years in the treatment of allergic inflammatory responses (Thurmond et al., 2008).In the assessment of H1-receptor antihistaminic activity in vitro, the parallel rightward shift in agonist concentration response curves in the presence of increasing concentrations of antagonist is observed with competitive antagonists. The occurred inhibition with competitive antagonists can be overcome by increasing the concentration of agonist. Finally a maximal effect can be achieved by using sufficient agonist (Thurmond et al., 2008).
The results of this study indicate a similar rightward shift in dose response curves of histamine in the presence of test drugs (CP-3, CP-5 and CP-8) and CPM. By increasing the dose of test drug, the EC50 has increased. The slopes of drugs CP-5, CP-8 and CPM are near to unity indicating that the antagonism of these drugs is competitive in nature whereas the CP-3 had shown noncompetitive type of antagonism. All the drugs had shown good antagonistic activity as indicated by their pA2 values. Currently antihistamines (H1-receptor blockers) are not used as front-line drugs for the treatment asthma; the provocative similarity between the physiological effects of histamine and the pathological symptoms of asthma has resulted in an enduring investigation into the possible utility of antihistamines in various models of bronchospasm (Thurmond et al., 2008). As in the case of above study, the increase in the onset of PCD (in sec) against histamine challenge was significantly (p < 0.001) increased with the increasing doses of test drugs.
The H1-receptor occupancy in brain is an indicator of central side effects of antihistamines (Gupta et al., 2007). The antagonism of H1-receptors in the brain can lead to side effects like sedation and impairment of cognitive functions as experienced with first generation antihistaminics in the treatment of allergic disorders (Simons et al., 1996). So we have evaluated the test drug for sedating potential by using spontaneous locomotor activity (Actophotometer) in mice. Based on LD50 value and therapeutic dose level in in vivo study, the dose range was selected as five times that of in vivo therapeutic dose i.e. 10 mg/kg in case of CPM and 50 mg/kg in case of test drugs. CPM has significantly decreased the spontaneous locomotor activity in mice compared to control but all the three test drugs have a very little effect on spontaneous locomotor activity compared to standard drug CPM.
The overall study has suggested that all the three test drugs (CP-3, CP-5 and CP-8) possess very good antihistaminic activity, the CP-5 and CP-8 were found to possess competitive antagonism and CP-3 was found to possess non-competitive antagonism at H1-receptor site and having very low sedative potential compared to standard drug CPM.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors are thankful to Prof. Dr. S. Mohan, Principal and management members of P.E.S. College of Pharmacy for providing all necessary facilities to carry out the research work.
References
- Antonio M., Ignacio A., Maria F., Angel A.J., Esteban S., Jose I.J. New indole and triazino [5,4-b] indol-4-one derivatives: synthesis and studies as inotropics and inhibitors of blood platelet aggregation. Chem. Abst. 1992;117:171387v. doi: 10.1002/ardp.19923250712. [DOI] [PubMed] [Google Scholar]
- Anthony W.P., Martin C.J., Richard G.C., Thomas S.I. Preparation of me 3-methoxy 2-[2-(4-phenoxy-1,2,3-triazin-6-yloxy) phenyl] acrylates as agrochemical fungicides. Chem. Abst. 1993;118:147584g. [Google Scholar]
- Biesele J.J. Purine antagonism and differential toxicity of some imidazo-1,2,3-triazines in mouse-tumor tissue cultures. Cancer. 1952;5:787–791. doi: 10.1002/1097-0142(195207)5:4<787::aid-cncr2820050419>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Daidone G., Maggio B., Plescia S., Raffa D., Schillaci D., Migliara O. Synthesis and pharmacological evaluation of 1-methyl-5-[substituted-4(3H)-oxo-1,2,3-benzotriazin-3-yl]-1H-pyrazole-4-acetic acid derivatives. IlFarmaco. 1998;53:350–356. doi: 10.1016/s0014-827x(98)00034-2. [DOI] [PubMed] [Google Scholar]
- Dattolo G., Cirrincione G., Almerico A.M., Aiello E. Polycondensed nitrogen heterocycles pyrrolo [3,4-d]-1,2,3-triazines: a new ring system as potential antineoplastic agent. J. Heterocycl. Chem. 1989;26:1747–1749. [Google Scholar]
- David K.F. Preparation of 3-(azabicycloalkyl)-3,4-dihydro-4-oxobenzotriazines and quinazolines as 5-HT3 receptor antagonists. Chem. Abst. 1990;112:7515m. [Google Scholar]
- Gatta F., Luciani M., Palazzo G. Pyrazolo [3,4-d] pyrimidines related to lonidamine. J. Heterocycl. Chem. 1989;26:613–618. [Google Scholar]
- Gewald K., Schinke E., Bohcher H. 2-Aminothiophene aus methylenaktiven nitrilen, carbonylverbindungen and schwefel. Chem. Ber. 1966;99:94–100. [Google Scholar]
- Gopumadhavan S., Rafiq M., Venkataranganna M.V., Mitra S.K. Antihistaminic and anaphylactic activity of HK-07, a herbal formulation. Indian J. Pharmacol. 2005;37:300–303. [Google Scholar]
- Gupta A., Gillard M., Christophe B., Chatelain P., Massingham R., Hammarlund-Udenaes M. Peripheral and central H1 histamine receptor occupancy by levocetirizine a non-sedating Antihistamine; a time course study in the guinea pig. Br. J. Pharmcol. 2007;151:1129–1136. doi: 10.1038/sj.bjp.0707318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen C.D. In: The pharmacological Basis of Therapeutics. tenth ed. Gilman A.G., Hardman J.G., Limbird L.E., editors. McGraw-Hill Publication Corp.; New York: 2001. Priniciples of toxicology in the treatment of poisoning; pp. 71–72. [Google Scholar]
- Kulkarni S.K. third ed. Vallabh Prakashan; New Delhi: 2005. Handbook of Experimental Pharmacology. pp 117–119. [Google Scholar]
- Larson A., Fumagalli F., DiGennaro A., Anderson M., Lundbers J., Edenitus C. A new class of nitric oxide–releasing derivtives of cetrizine. Br. J. Pharmacol. 2007;151:35–44. doi: 10.1038/sj.bjp.0707214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredini S., Bazzanini R., Baraldi P.G., Mario Guarneri M., Simoni D., Marongiu M.E. Pyrazole-related nucleosides. Synthesis and antiviral/antitumour activity of some substituted pyrazole and pyrazolo [4,3-d]-1,2,3-triazine-4-one nucleosides. J. Med. Chem. 1992;35:917–924. doi: 10.1021/jm00083a017. [DOI] [PubMed] [Google Scholar]
- Monge A., Aldana I., Arraras J.A., Alvarez E.F. Synthesis of 3-amino-5H-1,2,3-triazino [5,4-b]-indol-4-one. New compounds with blood platelet antiaggregation activity. J. Heterocycl. Chem. 1991;28:557–560. [Google Scholar]
- Patnaik G.K., Sabir M., Dhawan B.N. Antihistaminic activity of 4′-Fluoro-(1-Piperidyl) Propiophenone – a Centrally acting skeletal muscle relaxant. Indian J. Pharmacol. 1979;11:107–111. [PubMed] [Google Scholar]
- Quintela J.M., Peinador C., Gonzalez L.M., Riguera R., Rioja I., Terencio M.C. Synthesis and pharmacological evaluation of some 8-cyanopyrido [3/, 2/:4, 5] thieno [3,2-d] triazine derivatives as inhibitors of nitric oxide and eicosanoid biosynthesis. J. Med. Chem. 1999;42:4720–4724. doi: 10.1021/jm991085l. [DOI] [PubMed] [Google Scholar]
- Ring J., Kramer U., Shafer T., Beherendt H. Why are allergies increasing. Curr. Options Immunol. 2001;13:701–708. doi: 10.1016/s0952-7915(01)00282-5. [DOI] [PubMed] [Google Scholar]
- Shames F., Ahmadiani A., Khosrokhavar R. Antihistaminic and Anticholinergic activity of Barberry fruit (Berberis vulgaris) in the guinea pig ileum. J. Ethnopharmacol. 1999;64:161–166. doi: 10.1016/s0378-8741(98)00122-6. [DOI] [PubMed] [Google Scholar]
- Shaw E., Woolley D.W. Imidazo-1,2,3-triazines as substrates and inhibitors for xanthine oxidase. J. Biol. Chem. 1952;194:641–654. [PubMed] [Google Scholar]
- Siegfried L., Guenther W., Thomas S., Helmut V., Renate G. Preparation of 8,9,10,11–tetrahydro–1,2,3–triazino[4/5/:4, 5]-thieno[2,3-c]isoquinolines as antianaphylactics. Chem. Abst. 1990;113:40736c. [Google Scholar]
- Simons F.E.R., Fraser T.G., Reggin J.D., Roberts J.R., Simons K.J. Adverse central nervous system effects of older antihistamines in children. Pediatr. Allergy Immunol. 1996;7:22–27. doi: 10.1111/j.1399-3038.1996.tb00101.x. [DOI] [PubMed] [Google Scholar]
- Thomas P., Peter Z., Wofram R., Rainer K. Preparation of heterocyclic carbamates as antithrombotics and elastase inhibitors. Chem. Abst. 1995;122:290895x. [Google Scholar]
- Thurmond R.L., Gelfand R.W., Dunford P.J. The role of histamine H1 and H4 receptors in allergic inflammation the search for new antihistamines. Nat. Rev. Drug Discov. 2008;7:41–52. doi: 10.1038/nrd2465. [DOI] [PubMed] [Google Scholar]
- Youssefyeh R.D., Brown R.E., Wilson J., Shah U., Jones H., Love B. Pyrido[3/, 2/:4, 5] thieno [3,2-d]-N-triazines: a new series of orally active antiallergic agents. J. Med. Chem. 1984;27:1639–1643. doi: 10.1021/jm00378a019. [DOI] [PubMed] [Google Scholar]


