Abstract
The Eph family of receptor tyrosine kinases regulates numerous biological processes. To examine the biochemical and developmental contributions of specific structural motifs within Eph receptors, wild-type or mutant forms of the EphA4 receptor were ectopically expressed in developing Xenopus embryos. Wild-type EphA4 and a mutant lacking both the SAM domain and PDZ binding motif were constitutively tyrosine phosphorylated in vivo and catalytically active in vitro. EphA4 induced loss of cell adhesion, ventro-lateral protrusions, and severely expanded posterior structures in Xenopus embryos. Moreover, mutation of a conserved SAM domain tyrosine to phenylalanine (Y928F) enhanced the ability of EphA4 to induce these phenotypes, suggesting that the SAM domain may negatively regulate some aspects of EphA4 activity in Xenopus. Analysis of double mutants revealed that the Y928F EphA4 phenotypes were dependent on kinase activity; juxtamembrane sites of tyrosine phosphorylation and SH2 domain-binding were required for cell dissociation, but not for posterior protrusions. The induction of protrusions and expansion of posterior structures is similar to phenotypic effects observed in Xenopus embryos expressing activated FGFR1. Furthermore, the budding ectopic protrusions induced by EphA4 express FGF-8, FGFR1, and FGFR4a. In addition, antisense morpholino oligonucleotide-mediated loss of FGF-8 expression in vivo substantially reduced the phenotypic effects in EphA4Y928F expressing embryos, suggesting a connection between Eph and FGF signaling.
INTRODUCTION
During embryonic development, Eph receptor tyrosine kinases (RTKs) and their activating ligands, termed ephrins, control numerous developmental processes such as neural crest cell migration (Smith et al., 1997), axon pathfinding (Henkemeyer et al., 1996), hindbrain segmentation (Xu et al., 1995), angiogenesis (Wang et al., 1998), and somitogenesis (Durbin et al., 1998). Interaction of Eph receptors with ephrins can activate bidirectional signaling pathways (Holland et al., 1996; Davy et al., 1999), frequently resulting in repulsive cell-cell signaling (Gale and Yancopoulos, 1997). Eph receptors are characterized by an extracellular region, containing an N-terminal ephrin-binding domain, a cysteine-rich region, and two fibronectin type III repeats, and a cytoplasmic sequence comprising a juxtamembrane regulatory element, a tyrosine kinase domain, a SAM (sterile alpha motif) domain, and a C-terminal PDZ (post synaptic density protein, disk large, zona occludens) binding motif.
On ephrin binding, Eph receptors autophosphorylate on tyrosine residues that both regulate kinase activity and serve as binding sites for cytoplasmic target proteins (reviewed by Kalo and Pasquale, 1999b). Several in vitro and in vivo tyrosine phosphorylation sites have been identified that reside within the juxtamembrane region, the kinase domain, and the SAM domain (Kalo and Pasquale, 1999a). Two conserved tyrosine residues in the juxtamembrane region repress the intrinsic kinase activity of EphA4 and EphB2 in their unphosphorylated state (Binns et al., 2000; Zisch et al., 2000; Wybenga-Groot et al., 2001). Autophosphorylation of these juxtamembrane tyrosines concomitantly stimulates kinase activity and creates binding sites for SH2-containing targets such as Abl, Arg, Fyn, Nck1, Ras GAP, Src, SLAP, and SHEP1 (Pandey et al., 1995; Ellis et al., 1996; Holland et al., 1997; Zisch et al., 1998; Dodelet et al., 1999; Yu et al., 2001). Autophosphorylation within the kinase domain activation segment potentiates Eph kinase activity. Furthermore, a conserved tyrosine residue within the SAM domain of EphB1 has been suggested to recruit signaling molecules such as Grb10 and the low-molecular-weight protein tyrosine phosphatase (Stein et al., 1996, 1998). The SAM domains of Eph receptors can self-associate to form dimers or oligomers (Smalla et al., 1999; Stapleton et al., 1999; Thanos et al., 1999), but their functional role has remained obscure (Gu and Park, 2001). Indeed, despite being highly conserved, the SAM domain appears entirely dispensable in the context of EphA4 signaling in the mouse (Kullander et al., 2001).
During Xenopus development, expression of EphA4 (Pagliaccio/Sek-1) begins early in gastrulation and continues through to the tadpole stage. EphA4 expression is observed in the forebrain, olfactory placode, hindbrain (r3 and r5), otic placode, third visceral arch, and pronephros (Winning and Sargent, 1994; Xu et al., 1995). Ectopic expression of dominant negative EphA4 in Xenopus induces abnormal boundary formation in the hindbrain (Xu et al., 1995) and inappropriate migration of branchial neural crest cells (Smith et al., 1997). Stimulation of a chimeric EGFR/EphA4 receptor results in a loss of cell adhesion and disrupts polarity during early Xenopus development (Winning et al., 1996, 2001).
To pursue the functional contributions of specific Eph domains and motifs to signaling in an in vivo system, we examined the effect of overexpressing wild-type (WT) and mutant forms of EphA4 during Xenopus development. Here we show that EphA4 receptors harboring a deletion of the SAM domain, or mutation of tyrosine 928 contained within this domain, continue to display tyrosine kinase activity and induce ectopic protruding structures during Xenopus embryogenesis, which is dependent on FGF signaling. Moreover, we have exploited this system to explore the in vivo functions of Eph-mediated phosphorylation and protein-protein interactions.
MATERIALS AND METHODS
Molecular Cloning and Mutagenesis
Mouse EphA4 cDNA was obtained from Regeneron (Tarrytown, NY). XbaI sites were added to the 5′ and 3′ end of the EphA4 cDNA using PCR and subcloned into the XbaI site of the Xenopus expression vector, pCS2+. Desired mutations were introduced using a QuikChange site-directed mutagenesis strategy (Stratagene, La Jolla, CA) and were confirmed by DNA sequence analysis. Deletion of the globular domain (amino acid 33–202) and addition of the N-terminal Flag tag (DYKDDDDK) were conducted by PCR. Full-length Xenopus FGF-8 cDNA was isolated by PCR using a Xenopus stage 30 head cDNA library (a gift from R. Harland), and then sequenced. An HA-tag (YPYDVPDYA) was introduced at the C-termini of both FGF-8a and FGF-8b by PCR and cloned into BamHI and EcoRI sites of pCS2+.
Preparation of Xenopus Embryos, Synthetic RNA, Lineage Trace, and Animal Cap Explants
Embryos were obtained by artificial insemination after induction of females with 500 IU of human chorionic gonadotropin (Sigma, St. Louis, MO). Capped mRNA was made using the SP6 mMessage mMachine kit (Ambion, Austin, TX). pCS2+ plasmids containing EphA4 constructs, β-galactosidase, FGFR1K562E (an activated FGFR1, a gift from R. Friesel) and HA-tagged FGF-8a and FGF-8b were linearized with NotI. Control, FGF-8-AS (5′-CCA GGA TGG AGG TGA TGT AGT TCA T) and FGF-8-AS-4PM (5′-CCA GGA TGG AGG TCA TCT ACT TGA T) morpholino oligonucleotides were obtained from Gene Tools, LLC (Corvallis, OR). Embryos were injected into both blastomeres at the two-cell stage with indicated amounts of RNAs or antisense morpholino oligonucleotides. Lineage tracing was performed by β-galactosidase staining as previously described (Sive et al., 2000). Briefly, Xenopus embryos injected with 200 pg of β-gal mRNA at the two-cell stage were cultured until stage 30 and then rinsed with 0.7× phosphate-buffered saline (PBS), and fixed for 1 h on ice in freshly prepared 0.7× PBS containing 2% formaldehyde, 0.2% glutaraldehyde, 0.02% Triton X-100, and 0.01% sodium deoxycholate. Embryos were rinsed twice with 0.7× PBS and stained at 30°C in 0.7× PBS containing 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, 1 mg/ml X-gal, and 1 mM MgCl2. Embryos were fixed in MEMPFA for 1 h and photographed with a Nikon CCD camera (Garden City, NY). Animal cap explants were made using forceps or the Gastromaster (Xenotek Engineering, Belleville, IL) at stage 8. The explants were cultured as described previously (Maeda et al., 2001).
Whole Mount In Situ Hybridization
Plasmids containing cDNAs were obtained and linearized, and digoxygenin-labeled riboprobes were synthesized using MegaScript kit (Ambion) as specified: Xenopus Ap-2 (T. Sargent, HindIII, T7 polymerase), Nrp-1 (I. Dawid, BamHI, T3 polymerase), Xpo (T. Sargent, PstI, T7 polymerase), Xhox-3 (J. Howard, DdeI, T7 polymerase), Xslug (M. Sargent, BglII, SP6 polymerase), ADAM13 (D. DeSimone, XhoI, T3 polymerase), FGF-8 (J. Slack, XbaI, T3 polymerase), FGFR1 (R. Friesel, NcoI, T7 polymerase), and Xenopus EphA4 (A. Brandli, BamHI, T7 polymerase). A ClaI/XhoI fragment from pSP64Xwnt3a (R. Moon) was subcloned into pBluscript SK+ and linearized with ClaI, and the riboprobe was synthesized with T7 polymerase. A KpnI/SalI fragment from pSP64TXFGFR4a (I. Hongo and H. Okamoto) was subcloned into pBluscript SK+ and linearized with KpnI, and T3 polymerase was used to synthesize the riboprobe. Whole mount in situ hybridization was essentially done as described by Harland with modification (Maeda et al., 2001). For detection, BM Purple (Roche, Indianapolis, IN) was used as a chromogenic substrate. The reaction was stopped and the embryos were refixed in MEMPFA (0.1 M MOPS [pH 7.4], 2 mM EGTA, 1 mM MgSO4, and 4% paraformaldehyde) for 1 h. When pigmented embryos were used, embryos were bleached with a solution containing 1% H2O2, 5% formamide, and 0.5× SSC after staining (Mayor et al., 1995).
RT-PCR Assay
Total RNA from animal cap explants and staged embryos was extracted using Trizol (Life Technologies, Rockville, MD). cDNA was synthesized from 1 μg total RNA using SuperScriptII (Life Technologies). PCR was performed in 50 μl containing 1 μl of cDNA, 1× Taq buffer, 1.5 mM MgCl2, 0.2 mM each of dNTPs, 350 ng of each primer, and 1 U of AmpliTaq DNA polymerase (Perkin-Elmer Cetus, Norwalk, CT). Primers and cycle number used for FGF-8 were as follows: forward, 5′-CAC CTC CAT CCT GGG CTA TCT G and reverse, 5′-GAT TAA CTT GGC GTG TGG GTC for 27 cycles at 64°C annealing temperature. Primers and PCR condition for eFGF were as follows: forward, 5′-CGG GTT TCA TAT CCA GGT TTT AC and reverse, 5′-GCG TTA TAG TTG TTG GGC AGA AG for 29 cycles at 62°C. The primer sequences and conditions for NCAM, Otx-2, Krox-20, HoxB9, muscle actin, and EF-1α have been previously published (Hemmati-Brivanlou and Melton, 1994). Xtwist primer sequences were posted at the Xenbase web page: www.xenbase.org/genomics/genomics.html.
Immunoprecipitation and Western Blot Analysis
Embryos were prepared with ice-cold lysis buffer (20 mM Tris [pH 8.0], 150 mM NaCl, 1% Nonidet P-40 containing 1:50 diluted Protease Inhibitor Cocktail Set III, Calbiochem, La Jolla, CA), as previously described (Chong et al., 2000), followed by extraction with Freon (Sigma) at a 1:1 (vol/vol) ratio. IPs were conducted on 10 embryo equivalents with antibody raised against phosphotyrosine (4G10, UBI) or EphA4 (a rabbit polyclonal antibody raised against peptide: VLEDDPEAAYTTRG) for 4 h or overnight and protein-A/G agarose (Santa Cruz Biotechnology, Santa Cruz, CA) for an additional 1 h. After three washes with lysis buffer, IPs were separated by SDS-PAGE. The proteins were transferred to a membrane (Immobilon-P, Millipore, Bedford, MA), blocked with 5% nonfat dry milk in TBS containing 0.1% Tween-20 (TBST), and incubated with anti-Sek1/EphA4 (Transduction Laboratories, Lexington, KY). For antiphosphotyrosine blotting, 5% BSA in TBST was used to block. Proteins were visualized using an appropriate secondary antibody coupled to HRP (UBI, Lake Placid, NY), followed by application of enhanced chemiluminescence reagents (Amersham, Piscataway, NJ).
In Vitro Kinase Assays
EphA4 IPs were washed three times with HNTG Buffer (20 mM HEPES [pH 7.5], 150 mM NaCl, 10% glycerol, 0.1% Triton X-100, 100 μM sodium vanadate) and twice more with kinase buffer (25 mM HEPES [pH 7.5], 2.5 mM MgCl2, 4 mM MnCl2, 100 μM sodium vanadate). Ten percent of each sample was used for Western blot analysis to confirm equal levels of expression, and the remaining sample was incubated in kinase buffer containing 5 μg of acid denatured Enolase (Sigma) and 5 μCi of [γ-32P]ATP at room temperature for 30 min. The reaction was stopped by adding SDS/PAGE sample buffer and heating to 100°C. The reaction products were resolved using SDS polyacrylamide (10%) gels and subsequently stained, destained, and dried before autoradiography.
RESULTS
The SAM Domain Does Not Contribute to EphA4 Activity In Vitro, But Displays Negative Regulation In Vivo
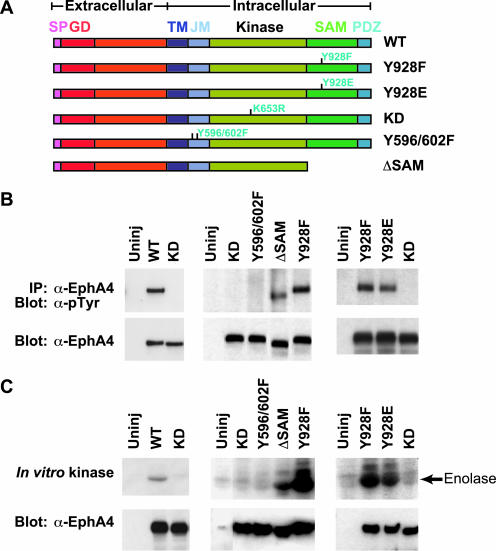
To explore the functional effect of specific Eph motifs in vivo, we expressed WT and mutant forms of mouse EphA4 in Xenopus embryos. In particular, we examined the effects of mutating the SAM domain, the kinase domain, and tyrosine autophosphorylation sites in the juxtamembrane region and the SAM domain (Figure 1A). mRNAs (2.5 ng/embryo) encoding WT or mutant receptors were injected into the animal pole of two-cell stage embryos in the absence of exogenous ligand. Embryos were harvested at stage 9 to examine the phosphorylation state of the receptor. At this stage of development, the WT receptor displayed in vitro kinase activity and was significantly tyrosine phosphorylated in vivo (Figure 1, B and C). Kinase dead forms of the receptor with mutations in the kinase domain or juxtamembrane tyrosines were inactive in vitro and were not significantly tyrosine phosphorylated in vivo (Figure 1, B and C). In contrast, the ΔSAM EphA4 mutant lacking both the SAM domain and PDZ binding motif was tyrosine phosphorylated in Xenopus embryos and showed significant in vitro kinase activity (Figure 1, B and C). These results suggest that neither the SAM domain nor the PDZ domain is essential for EphA4 kinase activity in Xenopus embryos.
Figure 1.
EphA4 mutants, their tyrosine phosphorylation state, and their kinase activity. Xenopus embryos were left uninjected or injected with RNA encoding wild type (WT) or various mutant EphA4 receptors. (A) Schematic representations of the kinase dead (KD, K653R), juxtamembrane tyrosine (Y596/602F), SAM domain deletion (ΔSAM), Y928F, and Y928E mutants are depicted. Embryos were harvested at stage 9, whole embryo lysates were immunoprecipitated with anti-EphA4 antibody and analyzed by Western blot analysis with antiphosphotyrosine antibodies (B) or in vitro kinase assays (C) were performed. SP, signal peptide; GD, globular ligand binding domain; TM, transmembrane domain; JM, juxtamembrane region; SAM, sterile alpha motif; PDZ, postsynaptic density protein, discs large, zona occludens binding motif.
To further examine the role of the SAM domain in activating EphA4, individual residues within the SAM domain were mutated. Mutation of a conserved SAM domain tyrosine residue, a potential site for phosphorylation and binding to cytoplasmic proteins, to either Phe or Glu resulted in robust EphA4 tyrosine phosphorylation in vivo and kinase activity in vitro (Figure 1, B and C). Sequence alignments show that this tyrosine residue is present in all Eph SAM domains, with the exception of EphA3 paralogs. These data suggest that the SAM domain may normally exert an inhibitory effect on EphA4 activity, at least in the context of the developing Xenopus embryo.
EphA4 and SAM Domain Mutants Induce Ectopic Posterior Structures
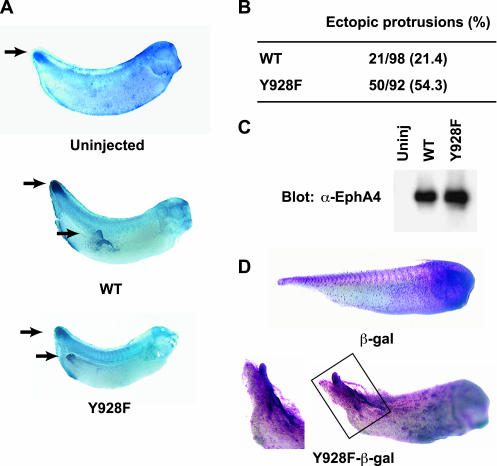
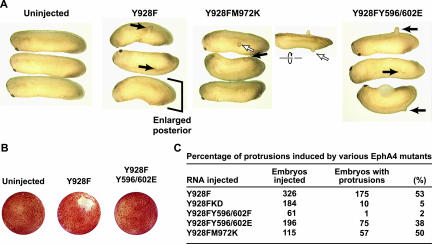
WT and Y928F EphA4 expressing embryos developed ectopic protrusions that evaginate from the posterior region beginning at the neurula stage (Figure 2A). In situ hybridization reveals that these ectopic protrusions express the posterior marker, Xpo (Figure 2A). Furthermore, the Y928F mutant induced these posterior protrusions at a frequency 2.5 times that of WT, despite being expressed at equal levels (Figure 2, B and C). These protruding structures were formed from a subset of the tissue expressing Y928F as evidenced by lineage tracing via coexpression of β-galactosidase (Figure 2D). Thus, the Y928F mutant was used in subsequent experiments. Embryos injected with RNA encoding the wild-type EphA4, Y928F EphA4, or ΔSAM EphA4 mutants (hereafter referred to as WT, Y928F, and ΔSAM, respectively) displayed a dramatic loss of cell adhesion at the blastula stage, whereas embryos expressing KD mutants were similar to uninjected controls (Figure 3A and unpublished data). This result is consistent with a previous report that an EGFR/EphA4 chimera stimulated with TGF-α leads to cell dissociation in Xenopus embryos (Winning et al., 1996). As development proceeded to the tadpole stage, tail-like structures evolved from these protrusions (Figure 3A) and whole mount in situ hybridizations of 1-day embryos showed expression of the posterior markers Xpo, Xwnt3a, and Xhox-3 (Ruiz i Altaba and Melton, 1989; Sato and Sargent, 1991; Wolda et al., 1993; Beck and Slack, 1998, 1999; Figures 2A and 3B). These results confirm that Y928F-induced protrusions display posterior molecular characteristics.
Figure 2.
Comparison of EphA4 WT and EphA4 Y928F mutant expressing embryos. Embryos were left uninjected or injected with RNA encoding WT or Y928F mRNA. (A) Phenotypic effect and whole mount in situ hybridization with Xpo probe (posterior marker) of embryos expressing WT or Y928F mutant protein. Arrows indicate normal or ectopic posterior expression of the Xpo marker. (B) Summary of the frequency and percentage of Xenopus embryos displaying ectopic posterior structures when injected with either WT or Y928F EphA4 (number of embryos displaying protrusions/number of embryos injected). (C) WT or mutant EphA4 receptor expression was examined by Western blot analysis of stage 9 embryonic lysates. (D) Embryos stained for β-galactosidase activity after injection with mutant EphA4 receptor RNA plus β-galactosidase RNA or β-galactosidase RNA alone as a lineage tracer. Insert is enlargement of boxed area.
Figure 3.
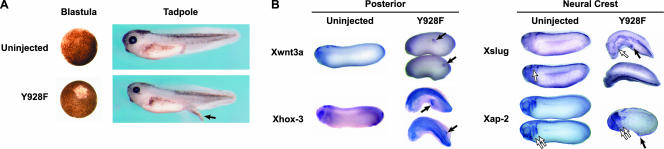
Phenotypes and whole mount in situ hybridization of posterior and neural crest markers in Y928F injected embryos. (A) Cell dissociation at the blastula stage and ectopic protrusion formation at the stage 37/38 tadpole are observed in Y928F expressing embryos and not in uninjected controls. The arrow indicates a representative posterior protrusion. (B) Albino embryos were left uninjected or injected with Y928F mRNA (1.25 ng mRNA was injected into one blastomere at the 2-cell stage). Embryos at stage 26/28 were fixed with MEMPFA. Whole mount in situ hybridizations were performed with posterior markers (Xwnt3a and Xhox-3) and neural crest markers (Xslug and Xap-2). Filled arrowheads indicate staining of the posterior protrusions. Double-open arrowheads indicate third and fourth branchial arches; a single-open arrowhead denotes cephalic slug expression.
A truncated EphA4 receptor has previously been shown to induce inappropriate migration of branchial neural crest cells (Smith et al., 1997). We examined the expression of neural and neural crest markers in Y928F embryos and found normal neural crest patterning was disrupted as evidenced by the neural crest markers, Xslug and Xap-2 (Figure 3B, open arrows). These two markers (Winning et al., 1991; Mayor et al., 1995) were robustly expressed in the protrusions of Y928F expressing embryos (Figure 3B, filled arrows). Taken together, these results suggest that Y928F can induce ectopic protrusions that display both posterior and neural crest molecular characteristics. Although such markers were induced in embryos in vivo, Y928F failed to directly induce posterior neural tissue in naïve ectodermal explants and failed to promote a more caudal fate in neuralized explants in vitro, suggesting that the activated EphA4 receptor does not alter cell fate in explants (unpublished data).
Other EphA4 Motifs and Domains Are Required for the Phenotype Induced by Y928F
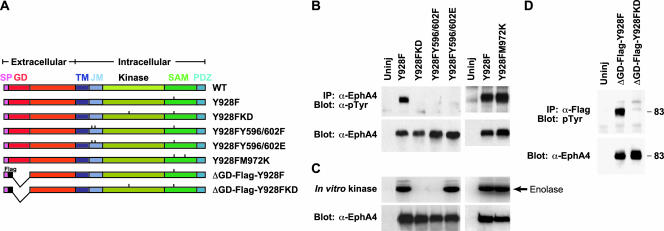
We used the Y928F mutant to explore motifs and domains required in a positive manner for Eph receptor signaling by engineering additional mutations into the Y928F receptor (Figure 4A). mRNAs (2.5 ng/embryo) encoding these double mutants were injected into the animal pole of two-cell stage embryos. EphA4 kinase activity and the consequent binding of SH2 domain proteins to the EphA4 juxtamembrane autophosphorylation sites could potentially play a role in inducing the phenotypes observed in Y928F expressing embryos. Mutating the two regulatory juxtamembrane tyrosines to phenylalanine (Y928FY596/602F) resulted in a kinase dead receptor (Figure 4, B and C) unable to induce loss of cell adhesion or ectopic posterior protrusions (Figure 5C), as anticipated from previous work showing that substitution of the juxtamembrane tyrosines with phenylalanine locks Eph receptors in an autoinhibited state (Wybenga-Grout et al., 2001).
Figure 4.
Tyrosine phosphorylation state and kinase activity for various EphA4 Y928F double mutants. (A) Xenopus embryos were left uninjected or injected with RNA encoding the various Y928F double mutants depicted. (B) Embryos were harvested at stage 9 and the tyrosine phosphorylation state of the various EphA4 receptors was examined by IP-Western blot analysis. (C) In vitro kinase assays were performed on EphA4 immunoprecipitates to determine kinase activity of the various Y928F double mutants. (D) A Flag tag was introduced next to the signal peptide in ephrin binding domain mutants to create ΔGD-Flag-Y928F and ΔGD-Flag-Y928FKD mutants (noted in A). Various EphA4 mutants were immunoprecipitated with anti-Flag antibody and probed with antiphosphotyrosine antibody. Anti-EphA4 blots show equivalent levels of receptor expression. Abbreviations as in Figure 1.
Figure 5.
Morphological analysis and percentage of ectopic protrusions produced by the various Y928F double mutants. (A) Xenopus embryos were left uninjected or injected with RNA encoding the indicated Y928F double mutants. Photographs were taken at stage 26/28. Arrows indicate ectopic protrusions. Inset area represents dorsal view, and open arrowhead marks reference protrusion. (B) Embryos injected with Y928F RNA or Y928FY596/602E RNA were cultured and photographed at stage 9. (C) The frequency of the posterior protrusion phenotype was scored. Note: Both the Y928FM972K (Y928F SAM dimerization mutant) and Y928FY596/602E (Y928F juxtamembrane-SH2 binding mutant) induce budding protrusions (A and C), but Y928FY596/602E mutant does not induce cell dissociation (B).
Similarly, a kinase dead Y928FK653R double mutant was not tyrosine phosphorylated or kinase active and did not induce loss of cell adhesion or ectopic protrusions (Figures 4, B and C, and 5C). To obtain an EphA4 mutant that retains kinase activity but is defective for binding to SH2 proteins, the juxtamembrane tyrosines were changed to glutamate. This substitution is anticipated to simulate juxtamembrane phosphorylation by maintaining a negative charge, thereby overcoming the inhibitory effects of the unphosphorylated juxtamembrane region. However, glutamate residues do not support binding to SH2 domains, and such a mutant should be compromised in its ability to recruit cytoplasmic targets to the juxtamembrane region. The Y928FY596/602E mutant was active as a kinase (Figure 4C) and induced ectopic protrusions (Figure 5, A and C), indicating that phosphotyrosine-dependent binding of SH2 proteins to the juxtamembrane region is not necessary for induction of ectopic protrusions by the Y928F mutant. This mutant was not robustly tyrosine phosphorylated (Figure 4B), likely reflecting the loss of three major tyrosine phosphorylation sites. In contrast, the loss of cell adhesion observed in Y928F embryos during early embryogenesis was not observed in embryos expressing the Y928FY596/602E mutant receptor (Figure 5B). These data suggest that phosphorylation of the juxtamembrane tyrosines and potential recruitment of SH2 domain targets to these sites is necessary for Y928F-induced cell dissociation, and that de-adhesion is separable from formation of ectopic posterior structures.
To determine if Y928F-induced tyrosine phosphorylation is dependent on ligand binding, N-terminally Flag-tagged mutants lacking the globular ligand-binding domain were constructed (Figure 4A). The ΔGD-Flag-Y928F mutant receptor displayed a high level of tyrosine phosphorylation in vivo (Figure 4D), demonstrating that signaling by microinjected EphA4 is ephrin independent. This may be due to activation of the Y928F receptor by overexpression or to compensating dimerization motifs in the extracellular domain of the receptor, whose activity may be enhanced in the absence of a functional SAM domain. Previous in vitro studies indicate that purified Y928F EphA4 SAM domain protein behaves like a dimer in solution (DS; unpublished data). To determine if SAM domain–mediated dimerization is required for activity of the Y928F mutant, an Y928FM972K double mutant was constructed (Figure 4A). Kinase activity and protrusion-induction are not affected by a mutation within the SAM dimerization interface (Y928FM972K; Stapleton et al., 1999), suggesting that SAM dimerization is not essential for Y928F-induced activities (Figures 4, B and C, and 5, A and C).
Activated FGFR1 Induces Ectopic Posterior Protrusions with the Same Molecular Characteristics as Y928F
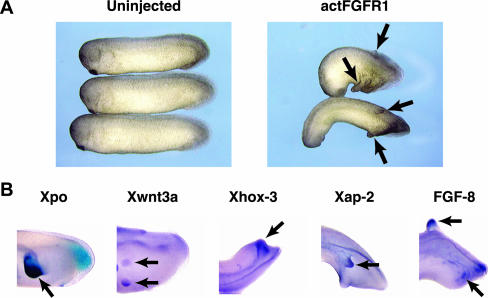
The phenotype of Xenopus embryos expressing the Y928F EphA4 receptor is similar to that of embryos expressing activated FGF receptor 1 (actFGFR1; Weinstein et al., 1998; Figure 6A). In situ hybridizations were performed on Xenopus embryos expressing actFGFR1 to determine whether the molecular characteristics of the actFGFR1-induced posterior protrusions are the same as those induced by Y928F. The posterior markers Xpo, Xwnt3a, Xhox-3, the neural crest marker Xap-2, and FGF-8 (Figure 6B) were expressed in both the Y928F EphA4-induced protrusions and the ectopic protrusions of actFGFR1-expressing embryos. This suggested a connection between Eph and FGF signaling pathways.
Figure 6.
Activated FGFR1 induces ectopic posterior protrusions in whole embryos. (A) Morphology of embryos left uninjected or injected with 50 pg of an activated FGFR1 (actFGFR1). (B) Whole mount in situ hybridization analysis of ectopic protrusions produced by actFGFR1. Stage 26/28 embryos injected with actFGFR1 were fixed and probed with posterior markers (Xpo, Xwnt3a, and Xhox-3), neural crest marker (Xap-2), and FGF-8. Arrows indicate staining of ectopic protrusions by the indicated probes.
EphA4 Induces Expression of FGFs and Their Receptors
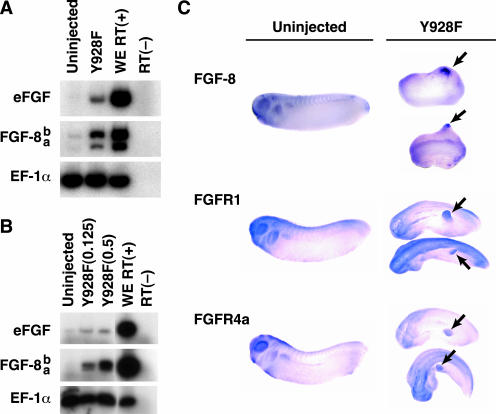
To pursue the relationship between Eph and FGF receptor signaling, the expression of FGF was examined in explants expressing the Y928F EphA4 mutant. Y928F EphA4 mRNA (1.5 ng/embryo) was injected into the animal pole of two-cell stage embryos. Ectodermal animal caps were excised at stage 8 and cultured until stage 26. RT-PCR analysis revealed that eFGF and particularly FGF-8 were induced by Y928F EphA4 (Figure 7A). To determine whether the induction eFGF and FGF-8 was an early event in response to Y928F EphA4 expression, explants were prepared from gastrula stage embryos injected with increasing doses of Y928F EphA4 RNA (0.125–0.5 ng). RT-PCR analysis demonstrated that eFGF was weakly induced, but a dose-dependent induction was not observed (Figure 7B). In contrast, FGF-8 was robustly induced in a dose-dependent manner, suggesting that FGF-8 induction is an early event and represents a tightly linked response to EphA4 activity in explant culture (Figure 7B).
Figure 7.
EphA4 Y928F induces expression of eFGF, FGF-8, FGFR1, and FGFR4a. (A and B) Embryos were left uninjected or injected with Y928F mRNA (1 ng/embryo for A; 0.125 ng or 0.5 ng for B) into both blastomeres at the 2-cell stage. Animal caps were excised at stage 8 and cultured until stage 26 (A) or stage 10.5/11 (B). Expression of eFGF and FGF-8 were analyzed by RT-PCR. EF-1α was used to normalize cDNAs as a control. PCR reactions were performed in the absence of reverse transcriptase [RT(-)] as a negative control. A reaction performed using stage 26 (A) or stage 11 (B) whole embryo [WE RT (+)] RNA served as a positive control. (C) Analysis of ectopic protrusions by whole mount in situ hybridization of stage 26/28 embryos. Embryos left uninjected or injected with Y928F mRNA (2.5 ng/embryo) were fixed and probed with DIG-labeled FGF-8 (top three embryos), FGFR1 (middle three embryos), or FGFR4a (bottom three embryos). Arrowheads indicate staining of ectopic protrusions by the indicated probes.
Furthermore, whole mount in situ hybridizations of 1-day embryos revealed that FGF-8 was prominently expressed in the budding posterior structures of Y928F EphA4 embryos (Figure 7C), whereas eFGF was only weakly expressed (unpublished data). Because FGF ligand is present in the ectopic protrusions, the presence of possible cognate receptors was examined. Whole mount in situ hybridization indicated that Y928F embryos expressed both FGFR1 (Figure 7C) and FGFR4a (Figure 7C) in the budding posterior structures. These results raise the possibility of a link between EphA4 and FGF signaling.
FGF-8 Is Involved in Activated EphA4-induced Posterior Protrusions
eFGF has been shown to induce mesoderm tissues during early Xenopus development (Kimelman and Kirschner, 1987; Slack et al., 1987), but muscle actin (a late mesoderm marker) is not expressed in ectodermal explants or ectopic tails expressing Y928F (unpublished data). FGF-8, however, has been shown to induce neural differentiation through FGFR4a and inhibit mesoderm induction during Xenopus development (Hardcastle et al., 2000). FGF-8 has the ability to induce posterior expansion and anterior reduction when overexpressed (Beck and Slack, 1998; Christen and Slack, 1999). These data make FGF-8 a promising candidate for mediating EphA4 induced ectopic protrusion formation.
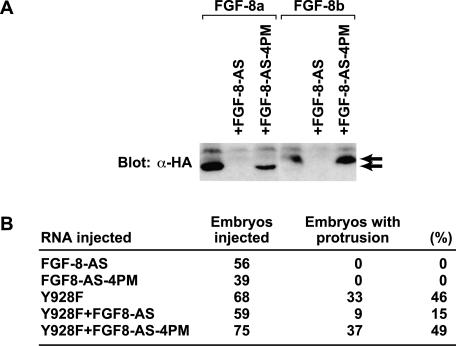
To test whether FGF-8 may participate in Y928F EphA4 signaling in the embryo, an FGF-8 loss-of-function approach was used. FGF-8a and FGF-8b cDNAs were isolated from a Xenopus stage 30 head library. The FGF-8a cDNA sequence is identical to the previously reported Xenopus FGF-8 sequence (Christen and Slack, 1999). FGF-8b, however, has an additional 11 amino acids containing a glycosylation site in the N-terminal region (GenBank Accession number: AF484745). Antisense FGF-8 morpholino oligonucleotides (FGF-8-AS) and a control morpholino oligonucleotide containing four point mutations within the FGF-8-AS sequence (FGF-8-AS-4PM) were designed. The specificity of FGF-8-AS and FGF-8-AS-4PM were confirmed in vitro. Western blot analysis of in vitro–translated HA-tagged FGF-8 in the presence or absence of FGF-8-AS or FGF-8-AS-4PM demonstrates specific inhibition of FGF-8 translation by FGF-8-AS and not FGF-8-AS-4PM (Figure 8A). High concentrations of FGF-8-AS block gastrulation movements (unpublished data), a phenotype similar to that observed with the disruption of FGF-8 in mice (Sun et al., 1999). To avoid this, the amount of FGF-8-AS was titered to a level that would not have a dramatic effect on development (14 ng/embryo), and this was used for future experiments (unpublished data). Coinjection of FGF-8-AS with Y928F EphA4 (2.5 ng/embryo) resulted in a substantial reduction in the incidence of ectopic posterior protrusion formation (Figure 8B). In contrast, injection of FGF-8-AS-4PM did not affect formation of these structures induced by Y928F (Figure 8B). These results are consistent with a causative role for FGF-8 in the phenotypic effects induced by Y928F in the embryo.
Figure 8.
Inhibition of FGF-8 expression using antisense morpholino oligonucleotides represses Y928F-induced ectopic protrusion formation in vivo. (A) FGF-8-AS blocks translation of FGF-8 expression in vitro. HA-tagged FGF-8a or FGF-8b mRNAs (1 ng) were in vitro–translated in the presence or absence of FGF-8-AS (14 ng) or a control antisense reagent with 4 point mutations [FGF-8-AS-4PM] (14 ng) as indicated. Western blot analysis of the reaction products with anti-HA antibody was used to examine inhibition of FGF-8a and FGF-8b translation in the presence or absence of the indicated antisense reagent. The top and bottom arrows indicate the bands corresponding to FGF-8b and FGF-8a, respectively. (B) Antisense FGF-8 (FGF-8-AS) and (FGF-8-AS-4PM) were injected into the indicated number of Xenopus embryos with or without Y928F and scored for ectopic protrusions at stage 26.
DISCUSSION
Eph RTKs are subject to complex regulation, involving interaction with cell-associated ephrins and subsequent receptor oligomerization, followed by receptor autophosphorylation at multiple regulatory tyrosines. Activated Eph receptors can potentially bind cytoplasmic targets through several motifs, including the juxtamembrane region, SAM domain, and C-terminal PDZ-binding sequence. We tested the contribution of specific tyrosines within the EphA4 juxtamembrane region and SAM domain using a Xenopus microinjection system to assess the biological activity of these highly conserved sites in Eph receptor signaling.
SAM domains have been proposed to mediate homotypic or heterotypic protein-protein interactions. Indeed, the SAM domains of EphA4 and EphB2 are able to form homotypic oligomers (Smalla et al., 1999; Stapleton et al., 1999; Thanos et al., 1999). Here, we have found that the SAM domain of ectopically expressed EphA4 is not required for either activation of the receptor's tyrosine kinase domain or signaling that can elicit morphogenetic changes during Xenopus development (Figure 1, B and C). Deletion of both the SAM and PDZ domains did not affect tyrosine phosphorylation and activation of EphA4 (Figure 1, B and C). Similarly, substituting Phe for Tyr at position 928 within the SAM domain maintained EphA4 kinase activity and constitutive EphA4 tyrosine phosphorylation in vivo (Figure 1, B and C). However, this mutation resulted in an observable increase in ectopic induction of posterior protrusions when compared with WT EphA4 (Figure 2B), suggesting that tyrosine 928 may act, in part, to suppress EphA4 in vivo activity. We have no evidence to support an inhibitory effect of the Y928 site on EphA4 kinase activity. Alternatively, this site may bind a negative regulator that in turn suppresses kinase activity toward a specific substrate. Y929 in EphB1 corresponds to Y928 in EphA4 and has been identified as a potential binding site for LMW-PTP and/or Grb10 (Stein et al., 1996, 1998). It is possible that the Y928F mutation may preclude binding of LMW-PTP or Grb10 and thus partially enhance EphA4 activity. Although we have no evidence indicating that LMW-PTP binds to EphA4 in Xenopus (our unpublished results and Park et al., 2002), our data are consistent with the hypothesis that Y928 is a protein-protein interaction site for a negative regulator, which might be recruited upon phosphorylation of Y928 and serve to down-regulate the receptor.
Most existing Eph mutations in invertebrates and mammals have led to a decrease or loss of receptor activity. Here we consider the phenotypic effects of EphA4 overexpression. Overexpression of either the WT or the Y928F EphA4 mutant causes a striking phenotype in which animal blastomeres lose cell adhesion in early Xenopus development (Figure 3A), but revert to apparently normal adherence at late gastrulation. As development proceeds, tail-like protrusions and severely expanded posterior structures are observed (Figure 3). In situ hybridization analysis established the posterior character of these protrusions (Figures 2 and 3B). Furthermore, Xap-2 staining reveals that Y928F disrupts patterning of the third and fourth branchial arches (Figure 3B). Although the second arch appears normal, the third and fourth branchial arches fuse and expand toward the posterior and ventral regions (Figure 3B). This is consistent with previous observations demonstrating that expression of a truncated EphA4 receptor disrupts the migration of cranial neural crest cells during Xenopus development (Xu et al., 1995; Smith et al., 1997). It is possible that neural crest cells expressing activated EphA4 migrate abnormally, perhaps contributing to the protruding cell mass that forms the ectopic posterior structures. Supporting this concept, Y928F-induced ectopic protrusions express neural crest markers such as Xslug, Xap-2 (Figure 3B), and ADAM-13 (unpublished data; Alfandari et al., 1997). The idea that neural crest migration rather than induction accounts for marker expression in the ectopic protrusions is supported by the observation that ectodermal explants expressing Y928F fail to induce neural or neural crest markers (our unpublished results). Alternatively, EphA4 may disrupt normal embryo patterning through its early effects on cell adhesion, resulting in an abnormal positioning of cells expressing neural and neural crest marker genes. This is unlikely because Y928FY596/602E induces ectopic protrusions, but not cell dissociation (Figure 5, A–C).
We have used the Y928F EphA4 mutant to test the functions of receptor kinase activity and autophosphorylation in the regulation of Xenopus cell adhesion and posterior structure formation. A mutation in the kinase domain that abrogates catalytic activity caused a loss of EphA4 Y928F biological activity. Similarly, conversion of juxtamembrane tyrosines 596/602 to phenylalanines, which locks EphA4 in an autoinhibited state through repressive interactions of the juxtamembrane region with the kinase domain, also blocked Y928F-induced phenotypes. Once phosphorylated, these same tyrosines, which lie in YXXP motifs, can serve as docking sites for SH2-containing proteins (reviewed in Kalo and Pasquale, 1999b; Boyd and Lackmann, 2001). Substitution of the juxtamembrane tyrosines with glutamates, in the context of the Y928F EphA4 mutant, maintains constitutive kinase activity (because the introduction of negatively charged residues interferes with the inhibitory interaction between the juxtamembrane region and the kinase domain), but should block phosphotyrosine-dependent binding to SH2 proteins (Figure 4). The Y596/602E substitutions block EphA4 Y928F-induced disruption of cell-cell adhesion, but not ectopic protrusion formation (Figure 5). These data argue that interaction of the phosphorylated juxtamembrane tyrosines is important for signaling that leads to the loss of cell adhesion, but is not essential for ectopic posterior protrusion formation. Furthermore, these data indicate that these ectopic structures are not simply derived from disrupted patterning due to cell dissociation.
FGF signaling plays multiple roles during Xenopus development including mesoderm induction, posteriorization of neural tissue, and limb regeneration. Activated FGF receptor 1 (actFGFR1), like several FGF pathway elements (Pownall et al., 1996; Tada et al., 1997; Weinstein et al., 1998; Brewster et al., 2000; Hama et al., 2001), induces ectopic protrusions in addition to anterior reduction and posterior expansion (Figure 6A). Ectopic structures induced by actFGFR1 have molecular characteristics similar to those induced by activated EphA4 (Figure 6B), suggesting a possible link between Eph and FGF signaling. An active EphA4 receptor induces eFGF and FGF-8 (both FGF-8a and FGF-8b) in early stage ectodermal explants (Figure 7B), and robust expression of FGF-8 as well as FGFR1 and R4a is found in developing ectopic posterior structures (Figure 7C). Although it is formally possible that eFGF plays a role in ectopic protrusion formation, we have no direct evidence that this is the case. eFGF is a strong mesoderm inducer (Kimelman and Kirschner, 1987; Slack et al., 1987), but mesoderm tissue and markers were not evident in the ectopic structures or ectodermal explants expressing Y928F EphA4. Furthermore, the eFGF induction by Y928F EphA4 did not appear to be dose dependent, implying that the induction may not be direct. Supporting these data, eFGF antisense (AS) morpholino oligonucleotides have no observable effect on the Y928F EphA4-induced phenotypes (E-K.P. and I.O.D., unpublished results). Alternatively, expression of eFGF may be too weak to induce mesoderm tissue, but may still have an influence on the induction of ectopic protrusions.
In contrast, experiments using FGF-8 AS morpholino oligonucleotides result in a substantial reduction in the incidence of ectopic posterior structure formation (Figure 8B). We have also found that coexpression of WT FGFR1 and Y928F increases the incidence of these ectopic structures in embryos (E-K.P. and I.O.D., unpublished results), supporting a role for FGF in the formation of these posterior structures. Xenopus FGF-8 (XFGF-8) has been reported to induce ectopic neural tissue in Xenopus embryos (Hardcastle et al., 2000), but does not increase proliferation of BaF3 cells expressing FGFR1c, suggesting FGF-8 may not bind to FGFR1 (MacArthur et al., 1995). However, XFGF-8-induced ectopic neuralization is strongly inhibited by a dominant negative FGFR4a, and only weakly by a dominant negative FGFR1, suggesting that both receptors may be involved in this FGF-8 mediated event. Moreover, weak induction of mesoderm by XFGF-8 is blocked by dominant negative FGFR1, supporting involvement or utilization of FGFR1 by the FGF-8 ligand (Christen and Slack, 1999; Hardcastle et al., 2000).
Previous studies in Xenopus used only the XFGF-8a isoform (Christen and Slack, 1999; Hardcastle et al., 2000); however, an XFGF-8b isoform was also isolated, which is the predominant isoform induced by the active EphA4 (Figure 7). Unlike FGF-8a, the FGF-8b isoform is able to induce significant activation of MAPK when expressed during early Xenopus embryogenesis (E-K.P. and I.O.D., unpublished results), consistent with the possible use of FGFR1 or other FGF receptors that are expressed at this time (Robbie et al., 1995). It is possible that activated EphA4 can induce another family of FGFs that activate FGFR1 directly or indirectly. Functional redundancy of the FGF family has been proposed in many studies including the knock-out of specific FGF isoforms (reviewed in Powers et al., 2000). Regardless of whether one or more FGF receptors are involved, FGF signaling is clearly activated and, at least in part, necessary for the induction of ectopic protrusions by an active EphA4 receptor.
Acknowledgments
We thank Tom Sargent, Igor Dawid, Richard Harland, Jonathan Slack, Aurora Lombardo, Michael Sargent, Douglas DeSimone, Robert Friesel, Andre Brandli, Randy Moon, Ikuko Hongo, and Harumasa Okamoto for reagents. This work was supported by grants from the Canadian Institutes for Health Research (CIHR) and the National Cancer Institute of Canada to T.P. T.P. is a distinguished scientist of the CIHR. Predoctoral support for N.W. was provided by OGS and NSERC studentships. Postdoctoral support for R.M. was provided by a fellowship from the Japanese Society for the Promotion of Science.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–09–0674. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03–09–0674.
References
- Alfandari, D., Wolfsberg, T.G., White, J.M., and DeSimone, D.W. (1997). ADAM 13, a novel ADAM expressed in somitic mesoderm and neural crest cells during Xenopus laevis development. Dev. Biol. 182, 314-230. [DOI] [PubMed] [Google Scholar]
- Beck, C.W., and Slack, J.M. (1998). Analysis of the developing Xenopus tail bud reveals separate phases of gene expression during determination and outgrowth. Mech. Dev. 72, 41-52. [DOI] [PubMed] [Google Scholar]
- Beck, C.W., and Slack, J.M. (1999). A developmental pathway controlling outgrowth of the Xenopus tail bud. Development 126, 1611-1620. [DOI] [PubMed] [Google Scholar]
- Binns, K.L., Taylor, P.P. Sicheri, F. Pawson, T., and Holland, S.J. (2000). Phosphorylation of tyrosine residues in the kinase domain and juxtamembrane region regulates the biological and catalytic activities of Eph receptors. Mol. Cell. Biol. 20, 4791-4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd, A.W., and Lackmann, M. (2001). Signals from Eph and ephrin proteins: A developmental tool kit. Sci. STKE 2001, RE20. [DOI] [PubMed] [Google Scholar]
- Brewster, R., Mullor, J. L., and Ruiz i Altaba, A. (2000). Gli2 functions in FGF signaling during antero-posterior patterning. Development 127, 4395-4405. [DOI] [PubMed] [Google Scholar]
- Chong, L. D., Park, E-K. Latimer, E., Friesel, R., and Daar. I. O. (2000). Fibroblast growth factor receptor-mediated rescue of x-ephrin B1-induced cell dissociation in Xenopus embryos. Mol. Cell. Biol. 20, 724-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen, B., and Slack, J.M. (1999). Spatial response to fibroblast growth factor signalling in Xenopus embryos. Development 126, 119-125. [DOI] [PubMed] [Google Scholar]
- Davy, A., Gale, N.W., Murray, E.W., Klinghoffer, R.A., Soriano, P., Feuerstein, C., and Robbins, S.M. (1999). Compartmentalized signaling by GPI-anchored ephrin-A5 requires the Fyn tyrosine kinase to regulate cellular adhesion. Genes Dev. 13, 3125-3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodelet, V.C., Pazzagli, C., Zisch, A.H., Hauser, C.A., and Pasquale, E.B. (1999). A novel signaling intermediate, SHEP1, directly couples Eph receptors to R-Ras and Rap1A. J. Biol. Chem. 274, 31941-31946. [DOI] [PubMed] [Google Scholar]
- Durbin, L., Brennan, C., Shiomi, K., Cooke, J., Barrios, A., Shanmugalingam, S., Guthrie, S., Lindberg, R., and Holder, N. (1998). Eph signaling is required for segmentation and differentiation of the somites. Genes Dev. 12, 3096-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis, C., Kasmi, F., Ganju, P., Walls, E., Panayotou, G., and Reith, A.D. (1996). A juxtamembrane autophosphorylation site in the Eph family receptor tyrosine kinase, Sek, mediates high affinity interaction with p59fyn. Oncogene 12, 1727-1736. [PubMed] [Google Scholar]
- Gale, N.W., and Yancopoulos, G.D. (1997). Ephrins and their receptors: a repulsive topic?. Cell Tissue Res. 290, 227-241. [DOI] [PubMed] [Google Scholar]
- Gu, C., and Park, S. (2001). The EphA8 receptor regulates integrin activity through p110gamma phosphatidylinositol-3 kinase in a tyrosine kinase activity-independent manner. Mol. Cell. Biol. 21, 4579-4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama, J., Xu, H., Goldfarb, M., and Weinstein, D.C. (2001). SNT-1/FRS2alpha physically interacts with Laloo and mediates mesoderm induction by fibroblast growth factor. Mech. Dev. 109, 195-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardcastle, Z., Chalmers, A.D., and Papalopulu, N. (2000). FGF-8 stimulates neuronal differentiation through FGFR-4a and interferes with mesoderm induction in Xenopus embryos. Curr. Biol. 10, 1511-1514. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou, A., and Melton, D.A. (1994). Inhibition of activin receptor signaling promotes neuralization in Xenopus. Cell 77, 273-281. [DOI] [PubMed] [Google Scholar]
- Henkemeyer, M., Orioli, D., Henderson, J.T., Saxton, T.M., Roder, J., Pawson, T., and Klein, R. (1996). Nuk controls pathfinding of commissural axons in the mammalian central nervous system. Cell 86, 35-46. [DOI] [PubMed] [Google Scholar]
- Holland, S.J., Gale, N.W., Gish, G.D., Roth, R.A., Songyang, Z., Cantley, L.C., Henkemeyer, M., Yancopoulos, G.D., and Pawson, T. (1997). Juxtamembrane tyrosine residues couple the Eph family receptor EphB2/Nuk to specific SH2 domain proteins in neuronal cells. EMBO J. 16, 3877-3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland, S.J., Gale, N.W., Mbamalu, G., Yancopoulos, G.D., Henkemeyer, M., and Pawson, T. (1996). Bidirectional signalling through the EPH-family receptor Nuk and its transmembrane ligands. Nature 383, 722-725. [DOI] [PubMed] [Google Scholar]
- Kalo, M.S., and Pasquale, E.B. (1999a). Multiple in vivo tyrosine phosphorylation sites in EphB receptors. Biochemistry 38, 14396-14408. [DOI] [PubMed] [Google Scholar]
- Kalo, M.S., and Pasquale, E.B. (1999b). Signal transfer by Eph receptors. Cell Tissue Res. 28, 91-99. [PubMed] [Google Scholar]
- Kimelman, D., and Kirschner, M. (1987). Synergistic induction of mesoderm by FGF and TGF-beta and the identification of an mRNA coding for FGF in the early Xenopus embryo. Cell 51, 869-877. [DOI] [PubMed] [Google Scholar]
- Kullander, K., Mather, N.K., Diella, F., Dottori, M., Boyd, A.W., and Klein, R. (2001). Kinase-dependent and kinase-independent functions of EphA4 receptors in major axon tract formation in vivo. Neuron 29, 73-84. [DOI] [PubMed] [Google Scholar]
- MacArthur, C.A., Lawshe, A., Shankar, D.B., Heikinheimo, M., and Shackleford, M. (1995). FGF-8 isoforms differ in NIH3T3 cell transforming potential. Cell Growth Differ. 6, 817-825. [PubMed] [Google Scholar]
- Maeda, R., Mood, K., Jones, T.J., Aruga, J., Buchberg, A.M., and Daar, I.O. (2001). Xmeis1, a protooncogene involved in specifying neural crest cell fate in Xenopus embryos. Oncogene 20, 1329-1342. [DOI] [PubMed] [Google Scholar]
- Mayor, R., Morgan, R., and Sargent, M.G. (1995). Induction of the prospective neural crest of Xenopus. Development 121, 767-777. [DOI] [PubMed] [Google Scholar]
- Pandey, A., Duan, H., and Dixit, V.M. (1995). Characterization of a novel Src-like adapter protein that associates with the Eck receptor tyrosine kinase. J. Biol. Chem. 270, 19201-19204. [DOI] [PubMed] [Google Scholar]
- Park, E.K., Warner, N., Mood, K., Pawson, T., and Daar, I.O. (2002). Low-molecular-weight protein tyrosine phosphatase is a positive component of the fibroblast growth factor receptor signaling pathway. Mol. Cell. Biol. 22, 3404-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers, C.J., McLeskey, S.W., and Wellstein, A. (2000). Fibroblast growth factors, their receptors and signaling. Endocr. Relat. Cancer 7, 165-197. [DOI] [PubMed] [Google Scholar]
- Pownall, M.E., Tucker, A.S., Slack, J.M., and Isaacs, H.V. (1996). eFGF, Xcad3 and Hox genes form a molecular pathway that establishes the anteroposterior axis in Xenopus. Development 122, 3881-3892. [DOI] [PubMed] [Google Scholar]
- Robbie, E.P., Peterson, M., Amaya, E., and Musci, T.J. (1995). Temporal regulation of the Xenopus FGF receptor in development: a translation inhibitory element in the 3′ untranslated region. Development 121, 1775-1785. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba, A., and Melton, D.A. (1989). Bimodal and graded expression of the Xenopus homeobox gene Xhox3 during embryonic development. Development 106, 173-183. [DOI] [PubMed] [Google Scholar]
- Sato, S.M., and Sargent, T.D. (1991). Localized and inducible expression of Xenopus-posterior (Xpo), a novel gene active in early frog embryos, encoding a protein with a `CCHC' finger domain. Development 112, 747-753. [DOI] [PubMed] [Google Scholar]
- Sive, H.L., Grainger, R.M., and Harland, R.M. (2000). Early Development of Xenopus laevis: A Laboratory Manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press.
- Slack, J.M., Darlington, B.G., Heath, J.K., and Godsave, S.F. (1987). Mesoderm induction in early Xenopus embryos by heparin-binding growth factors. Nature 326, 197-200. [DOI] [PubMed] [Google Scholar]
- Smalla, M., Schmieder, P., Kelly, M., Ter Laak, A., Krause, G., Ball, L., Wahl, M., Bork, P., and Oschkinat, H. (1999). Solution structure of the receptor tyrosine kinase EphB2 SAM domain and identification of two distinct homotypic interaction sites. Protein Sci. 8, 1954-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, A., Robinson, V., Patel, K., and Wilkinson, D.G. (1997). The EphA4 and EphB1 receptor tyrosine kinases and ephrin-B2 ligand regulate targeted migration of branchial neural crest cells. Curr. Biol. 7, 561-570. [DOI] [PubMed] [Google Scholar]
- Stapleton, D., Balan, I., Pawson, T., and Sicheri, F. (1999). The crystal structure of an Eph receptor SAM domain reveals a mechanism for modular dimerization. Nat. Struct. Biol. 6, 44-49. [DOI] [PubMed] [Google Scholar]
- Stein, E., Cerretti, D.P., and Daniel, T.O. (1996). Ligand activation of ELK receptor tyrosine kinase promotes its association with Grb10 and Grb2 in vascular endothelial cells. J. Biol. Chem. 271, 23588-23593. [DOI] [PubMed] [Google Scholar]
- Stein, E., Lane, A.A., Cerretti, D.P., Schoecklmann, H.O., Schroff, A.D., Van Etten, R.L., and Daniel, T.O. (1998). Eph receptors discriminate specific ligand oligomers to determine alternative signaling complexes, attachment, and assembly responses. Genes Dev. 12, 667-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, X., Meyers, E.N., Lewandoski, M., and Martin, G.R. (1999). Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse embryo. Genes Dev. 13, 1834-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada, M., O'Reilly, M.A., and Smith, J.C. (1997). Analysis of competence and of Brachyury autoinduction by use of hormone-inducible Xbra. Development 124, 2225-2234. [DOI] [PubMed] [Google Scholar]
- Thanos, C.D., Goodwill, K.E., and Bowie, J.U. (1999). Oligomeric structure of the human EphB2 receptor SAM domain. Science 283, 833-836. [DOI] [PubMed] [Google Scholar]
- Wang, H.U., Chen, Z.F., and Anderson, D.J. (1998). Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 93, 741-753. [DOI] [PubMed] [Google Scholar]
- Weinstein, D.C., Marden, J., Carnevali, F., and Hemmati-Brivanlou, A. (1998). FGF-mediated mesoderm induction involves the Src-family kinase Laloo. Nature 394, 904-908. [DOI] [PubMed] [Google Scholar]
- Winning, R.S., and Sargent, T.D. (1994). Pagliaccio, a member of the Eph family of receptor tyrosine kinase genes, has localized expression in a subset of neural crest and neural tissues in Xenopus laevis embryos. Mech. Dev. 46, 219-229. [DOI] [PubMed] [Google Scholar]
- Winning, R.S., Scales, J.B., and Sargent, T.D. (1996). Disruption of cell adhesion in Xenopus embryos by Pagliaccio, an Eph-class receptor tyrosine kinase. Dev. Biol. 179, 309-319. [DOI] [PubMed] [Google Scholar]
- Winning, R.S., Shea, L.J., Marcus, S.J., and Sargent, T.D. (1991). Developmental regulation of transcription factor AP-2 during Xenopus laevis embryogenesis. Nucleic Acids Res. 19, 3709-3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winning, R.S., Wyman, T.L., and Walker, G.K. (2001). EphA4 activity causes cell shape change and a loss of cell polarity in Xenopus laevis embryos. Differentiation 68, 126-132. [DOI] [PubMed] [Google Scholar]
- Wolda, S.L., Moody, C.J., and Moon, R.T. (1993). Overlapping expression of Xwnt-3A and Xwnt-1 in neural tissue of Xenopus laevis embryos. Dev. Biol. 155, 46-57. [DOI] [PubMed] [Google Scholar]
- Wybenga-Groot, L.E., Baskin, B., Ong, S.H., Tong, J., Pawson, T., and Sicheri, F. (2001). Structural basis for autoinhibition of the Ephb2 receptor tyrosine kinase by the unphosphorylated juxtamembrane region. Cell 106, 745-757. [DOI] [PubMed] [Google Scholar]
- Xu, Q., Alldus, G., Holder, N., and Wilkinson, D.G. (1995). Expression of truncated Sek-1 receptor tyrosine kinase disrupts the segmental restriction of gene expression in the Xenopus and zebrafish hindbrain. Development 121, 4005-4016. [DOI] [PubMed] [Google Scholar]
- Yu, H.H., Zisch, A.H. Dodelet, V.C., and Pasquale, E.B. (2001). Multiple signaling interactions of Abl and Arg kinases with the EphB2 receptor. Oncogene 20, 3995-4006 [DOI] [PubMed] [Google Scholar]
- Zisch, A.H., Kalo, M.S., Chong, L.D., and Pasquale, E.B. (1998). Complex formation between EphB2 and Src requires phosphorylation of tyrosine 611 in the EphB2 juxtamembrane region. Oncogene 16, 2657-2670. [DOI] [PubMed] [Google Scholar]
- Zisch, A.H., Pazzagli, C., Freeman, A.L., Schneller, M., Hadman, M., Smith, J.W., Ruoslahti, E., and Pasquale, E.B. (2000). Replacing two conserved tyrosines of the EphB2 receptor with glutamic acid prevents binding of SH2 domains without abrogating kinase activity and biological responses. Oncogene 19, 177-187. [DOI] [PubMed] [Google Scholar]