Abstract
The mmd1 mutation causes temperature-sensitive growth and defects in mitochondrial morphology and distribution in the fission yeast Schizosaccharomyces pombe. In mutant cells, mitochondria aggregate at the two cell ends, with increased aggregation at elevated temperatures. Microtubules, which mediate mitochondrial positioning in fission yeast, seem normal in mmd1 cells at permissive temperature and after several hours at the nonpermissive temperature but display aberrant organization after prolonged periods at 37°C. Additionally, cells harboring both mmd1 and ban5-4, a temperature-sensitive allele of α2-tubulin, display synthetic defects in growth and mitochondrial distribution. The mmd1 mutation maps to an open reading frame encoding a novel 35.7-kDa protein. The Mmd1p sequence features repeating EZ-HEAT motifs and displays high conservation with uncharacterized homologues found in a variety of organisms. Saccharomyces cerevisiae cells depleted for their MMD1 homologue show increased sensitivity to the antimicrotubule drug benomyl, and the S. cerevisiae gene complemented the S. pombe mutation. Mmd1p was localized to the cytosol. Mmd1p is the first identified component required for the alignment of mitochondria along microtubules in fission yeast.
INTRODUCTION
Cell proliferation and cellular function require the appropriate distribution of mitochondria and other organelles. This organelle positioning is coordinated with key events of the cell division cycle and largely depends on activities of the cytoskeleton (Yaffe, 1999; Rogers and Gelfand, 2000). In particular, microtubules play a major role in determining organellar distribution in many cell types. Microtubule-based motor proteins, kinesin and cytoplasmic dynein, function as key mediators of organelle movement (Vale, 2003), but many details of the underlying mechanisms and regulation of organelle positioning have yet to be described.
Microtubules mediate mitochondrial distribution in the fission yeast Schizosaccharomyces pombe. In contrast to the situation in the budding yeast S. cerevisiae in which microtubules play no apparent role in determining mitochondrial position or behavior (Huffaker et al., 1988; Jacobs et al., 1988), mitochondria in fission yeast coalign with microtubules during interphase (Kanbe et al., 1989; Yaffe et al., 1996), and mutations or inhibitors that disrupt microtubules cause aberrant mitochondrial distribution (Yaffe et al., 1996). However, depletion of dynein or kinesin homologues has no effect on mitochondrial positioning or morphology (Yamamoto et al., 1999; Brazer et al., 2000; Weir and Yaffe, unpublished data), and the mechanisms by which microtubules mediate mitochondrial distribution in S. pombe are largely obscure.
To identify components that coordinate microtubule function with mitochondrial dynamics, we have screened a collection of mutant S. pombe strains for cells displaying aberrant mitochondrial distribution or morphology. Analysis of one of these mutants has led to the identification of a novel protein required for the alignment of mitochondria along microtubules.
MATERIALS AND METHODS
Strains and Genetic Techniques
Yeast strains used in this study are listed in Table 1. All S. pombe strains are derived from wild-type strain FY254 or FY261. S. cerevisiae strains used are derived from MYY290 or MYY297. Media, growth conditions, and genetic methods for S. pombe strains were essentially as described previously (Moreno et al., 1991). Media preparation and genetic methods for S. cerevisiae followed standard procedures (Rose et al., 1990). Plasmid DNA was prepared from Escherichia coli strain DH5α.
Table 1.
Yeast strains
| Strain | Genotype | Source or Reference |
|---|---|---|
| FY254 | h-, ura4-D18, leu1-32, ade6-M210, can1-1 | S. Forsburg |
| FY261 | h+, ura4-D18, leu1-32, ade6-M216, can1-1 | S. Forsburg |
| h-, ura4-D18, leu1-32, his3-D1, ade6-M216, | ||
| 645 | nmt1+:atb1GFP::LEU2 | R. McIntosh |
| MYP2 | h+, ban5-4, leu1-32 | Yaffe et al. (1996) |
| ARC 4402 | h-, ura4-D18, ade6-M210, tea1GFP::kanr | P. Nurse |
| MYP102 | h+, mmd1-1, ura4-D18, leu1-32, ade6-M210, can1-1 | This study |
| MYP103 | h-, mmd1-1, ura4-D18, leu1-32, ade6-M216, can1-1 | This study |
| MYP104 | h+, mmd1-1, ura4-D18, leu1-32, ade6-M216, can1-1 h-, Δmmd1::ura4+, ura4-D18, | This study |
| leu1-32, ade6-M216, | ||
| MYP105 | can1-1, nmt1+:COXIV-GFP:leu1+ h+, mmd1-1, ura4-D18, leu1-32, ade6-216, can1-1, | This study |
| MYP106 | nmt1+:COXIV-GFP:leu1+ h-, gly1-1, ura4-D18, leu1-32, ade6-M216, can1-1, | This study |
| MYP107 | nmt1+:COXIV-GFP:leu1+ | This study |
| h+, mmd1-1, gly1-1, ura4-D18, leu1-32, ade6-M210, | ||
| MYP108 | can1-1, nmt1+:COXIV-GFP:leu1+ | This study |
| MYP109 | h+, ade6-M210, ura4-D18, leu1-32, mmd1-1, ban5-4 h-, ura4-D18, leu1-32, ade6-M210, | This study |
| can1-1, | ||
| MYP110 |
mmd1HA |
This study |
| MYP112 |
mmd1GFP |
This study |
| MYP113 | pUR19scMMD1 h-, ura4-D18, leu1-32, ade6-M210, can1-1, | This study |
| MYP114 | pUR19-mmd1+ h-, ura4-D18, leu1-32, ade6-M210, can1-1, | This study |
| MYP115 | nmt1+:COXIV-GFP |
This study |
| MYP219 | h-, ura4-D18, leu1-32, ade6-M210, can1-1, pUR19 h+, ura4-D18, leu1-32, ade6-M210, | This study |
| can1-1, mmd1-1, | ||
| MYP220 | pUR19 | This study |
| MYP223 | h+, mmd1-1, ura4-D18, ade6-M216, tea1GFP | This study |
| MYY290 | MATa, ura3, leu2, his3 | Smith and Yaffe (1991) |
| MYY297 | MATa/α ura3, leu2, his3 | Smith and Yaffe (1991) |
| MYY2043 | MATa, Δmmd1::HIS3, ura3, leu2, his3 | This study |
Isolation of mmd Mutants
Wild-type S. pombe cells were mutagenized with ethylmethane sulfonate (EMS; Sigma-Aldrich, St. Louis, MO) as described previously (Moreno et al., 1991). Mutant cells were cultured at 25°C on YES medium, and temperature-sensitive colonies were identified by screening colonies for growth at 37°C by replica-plating. Temperature-sensitive strains were further screened by fluorescence microscopy to identify cells displaying abnormal mitochondrial morphology and/or distribution. Candidate mmd mutants were backcrossed three times to the wild-type parental strain. Meiotic progeny from the final cross showed 2:2 cosegregation of the temperature-sensitive defect with the mitochondrial morphology and distribution defects.
Microscopic Analysis
Mitochondria in S. pombe strains were visualized microscopically with the vital dye 2-(4-dimethylaminostyryl)-1-methylpyridinium iodide (DASPMI; Sigma-Aldrich) as described previously (Yaffe et al., 1996). Alternatively, strains containing a stably integrated green fluorescent protein (GFP) fused in frame to the mitochondrial targeting sequence of S. cerevisiae cytochrome oxidase subunit IV were used for some studies. Indirect immunofluorescence microscopy using methanol or formaldehyde fixation was performed essentially as described previously (Hagan and Hyams, 1988). Microtubules were visualized with the monoclonal TAT-1 antibody (Woods et al., 1989) (provided by Keith Gull, University of Oxford, Oxford, United Kingdom), and mitochondria were visualized with an affinity-purified rabbit antibody directed against the β-subunit of the mitochondrial F1-ATPase protein (Jensen and Yaffe, 1988).
Cloning and Sequence Analysis of mmd1+
The mmd1+ gene was cloned by complementation of the mmd1-1 temperature-sensitive phenotype. Cells with the mmd1-1 mutation were transformed with an S. pombe genomic DNA library in the vector pUR19 (Barbet et al., 1992) (obtained from S. Forsburg, Salk Institute, San Diego, CA). Ura+ transformants were selected at 25°C and screened for growth at 37°C by replica plating. One clone was obtained that allowed mmd1-1 cells to grow at the nonpermissive temperature. The S. pombe DNA insert in the complementing plasmid was partially sequenced with primers to the pUR19 vector, pUR19-U1 (5′ AACGCCAGGGTTTTCCCAGTCACGA 3′) and pUR19-L1 (5′ GCTATGACCATGATTACGCCAAG 3′). Internal primers complementary to sequences of the plasmid insert were also used for sequencing.
After its identification by complementation (as described above), a copy of mmd1+ was synthesized by polymerase chain reaction (PCR) by using primers mdmx-f2 (5′ CAATATGGCGGGGTGAGTAAANGAC) and mdmx-r2 (5′ CAAGAGTCAGGAGAAGTGGGAGAAC) and Pfu polymerase. The product was cloned into the pCR2.1 Topo vector (Invitrogen, Carlsbad, CA). mmd1+ sequences were isolated from this plasmid on a 2.2-kb HindIII fragment and cloned into the HindIII site of plasmid pUR19 to yield plasmid pUR19mmd1+.
mmd1+ Gene Replacement
A null allele of the mmd1+ gene was created by integrative transformation of S. pombe cells with a replacement cassette composed of the ura4+ gene flanked by sequences 5′ and 3′, respectively, to the mmd1+ open reading frame (ORF). The 5′-flanking sequence was synthesized by PCR, by using primers KO1mmd1–5′ (5′ GTCGACATATTAGCGACGTTGC) and 3′-mmd1KO1a (5′ATCGATCTGACAAGAAATTTCTC). The 3′-flanking sequence was synthesized by PCR by using primers KO2mmd1–5′ (5′ GGATCCGTATGGTTGGGTTCGCTA) and mdmx-f2 (5′ CAATATGGCGGGGTGAGTAAANGAC). Both 5′- and 3′-flanking sequences were cloned into the pCR2.1 Topo vector, yielding plasmids pCR2.1mmd1KO1 and pCR2.1mmd1KO2, respectively. The S. pombe ura4+ gene was isolated on a BamHI-EcoRI fragment from plasmid pTZura4 (obtained from S. Forsburg, Salk Institute, La Jolla, CA) and cloned into the BamHI and EcoRI sites of plasmid pBluescript to yield pB-Sura4. The 3′-flanking sequences were subcloned from plasmid pCR2.1mmd1KO2 into the BamHI and SpeI sites of pBSura4 to yield pBSura4mmd1KO2. The 5′-flanking sequences were subcloned from plasmid pCR2.1mmd1KO1 into the SalI and EcoRI sites of pBSura4mmd1KO2. The disruption cassette was excised from the resulting plasmid by digestion with SalI and SpeI and transformed into strain FY254 as described previously (Keeney and Boeke, 1994).
Mmd1p Tagging
The mmd1+ gene was engineered to create versions encoding Mmd1p tagged at its carboxy terminus with GFP or three copies of the hemagglutinin epitope (3HA), respectively. Mmd1+ was synthesized by PCR by using primers 5′mmd1 × hoI (5′ GGCTCGAGAGTTAAACATGTCTTC) and mmd1–3′NotI (5′ ACGAAGCGGCCGCATGCACTAAC) and cloned into the pCR2.1 Topo vector. The resulting plasmid was digested with XhoI and NotI, and the fragment containing mmd1+ was cloned into the XhoI and NotI sites of plasmids pSGP572a and pSGP72 (obtained from S. Forsburg). In the resulting plasmids, pSGP572mmd1+ and pSGP72mmd1+, mmd1+ is under control of the inducible nmt promoter, and the gene is fused in frame with sequences encoding GFP or 3HA, respectively.
The GFP- and HA-tagged versions of mmd1+ were integrated into the chromosomal DNA at the wild-type mmd1+ locus. Plasmids pSGP572mmd1+ and pSGP72mmd1+ were each digested with XhoI and SmaI, and Mmd1pGFP and Mmd1pHA were then ligated into the SalI and filled BamHI sites of vector pTZura4. The resulting plasmids, pTZura4mmd1GFP and pTZura4mmd1HA, were linearized with NruI and HincII, respectively, and transformed into strain FY254. The resulting strains have one copy of tagged mmd1+ under the control of the endogenous mmd1 promoter and one wild-type copy of mmd1+ with no promoter.
Cloning and Mutagenesis of S. cerevisiae MMD1
S. cerevisiae MMD1 (YJR070c) was synthesized by PCR by using primers YJR070c-F (5′ GCAAAGACGAACGTCAGATA 3′) and YJR070c-R (5′ TCAAAATCGACTGCACTTCC 3′) and genomic DNA as a template and cloned into pCR2.1 Topo vector to generate pCR2.1scMMD1. This plasmid was digested with HindIII and NsiI, and the fragment encoding MMD1 was cloned into the HindIII and PstI sites of plasmid pUR19 (Barbet et al., 1992) to yield plasmid pUR19scMMD1.
An S. cerevisiae strain deleted for MMD1 was created by PCR-mediated gene disruption as described previously (Baudin et al., 1993). A disruption cassette was generated using primers YJR070c-U1 (5′ ATGTCTACTAACTTTGAAAAACATTTCCAAGAAAACGTCGGATGTACTGAGAGTGCACC 3′) and YJR070c-L1 (5′ CTAATTAGCAGTTGGAGCATATTCTAGTTCGTTGCTGTTTGTGCGGTATTTCACACCGC 3′) to amplify the HIS3 gene from plasmid pRS303 (Sikorski and Hieter, 1989). The disruption cassette was transformed into diploid strain MYY297, and the replacement of one copy of YJR070c by HIS3 was confirmed by PCR analysis. The resulting strain was sporulated, and a haploid segregant disrupted for YJR070c, strain MYY2043, was isolated.
Mmd1p Antibody Preparation
Antibodies against Mmd1p were raised against a β-galactosidase-Mmd1p fusion protein. A 1.1-kb PvuII-XhoI fragment encoding the C-terminal 217 amino acids of Mmd1p was isolated from plasmid pCR2.1mmd1+ and ligated into the SalI and filled BamHI sites of plasmid pTRB0 (Burglin and DeRobertis, 1987) to create plasmid pTRB0mmd1+. The fusion protein was expressed in E. coli strain 71-18 by induction with isopropylthio-β-d-galactoside, purified by SDS-PAGE and electroelution, and used to immunize rabbits (Harlow and Lane, 1988).
Subcellular Fractionation and Analysis
Cells were grown in Edinburgh minimal medium (EMM) complete medium overnight at 30°C. Cells were collected, converted to spheroplasts, homogenized, and subcellular fractions were isolated as described previously for S. cerevisiae (Daum et al., 1982) with the following modifications. Cells were converted to spheroplasts by treatment with 4 mg Zymolase-20T (ICN Biomedicals, Costa Mesa, CA) and 4 mg of lysing enzymes (Sigma-Aldrich) per gram of cells, homogenate was centrifuged at 3000 × g to yield the low-speed pellet fraction, and the high-speed pellet (HSP) fraction was isolated by centrifugation at 55,000 × g for 15 min. The mitochondrial fraction was washed an additional two times, the intermediate-speed pellet fraction was washed an additional four times, and the HSP fraction was washed two additional times.
Respiration Analysis
Whole-cell respiration rates were measured using a Clark-type polarographic O2 electrode. Cells were grown to log phase at 23°C in YES medium and diluted to 2 × 106 cells/ml into fresh media for 3 h. One aliquot of each sample was used for measurement of oxygen consumption at 23°C, whereas a second aliquot was analyzed to determine cell number per milliliter. The rate of oxygen consumption was normalized for 1 × 106 cells/ml. Only cultures with cell concentrations between 1 × 106 and 4 × 106 cells/ml were use for rate calculations.
RESULTS
Isolation and Analysis of mmd1
S. pombe mutants displaying aberrant mitochondrial distribution and morphology were isolated by screening a collection of temperature-sensitive strains by fluorescence microscopy. Of 106 strains analyzed, three mutants, designated mmd1, mmd2, and mmd3 (for defective in mitochondrial morphology and distribution), were identified. Genetic analysis confirmed that single, nuclear mutations cause defects in both growth at high temperature and mitochondrial distribution and morphology in each of these strains and that each of the mutations defined unique genes. The mmd2-1 and mmd3-1 mutants will be described elsewhere.
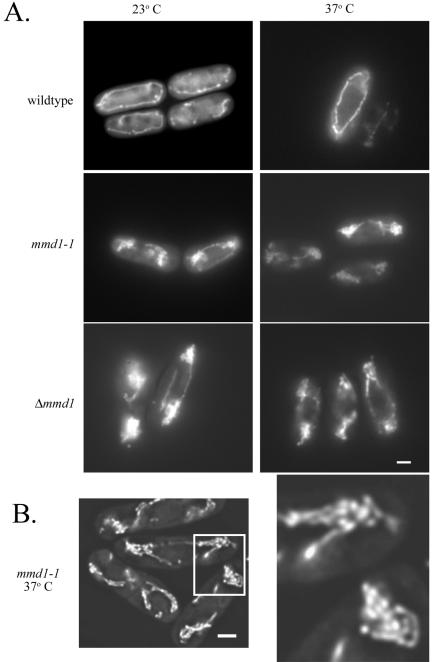
The mitochondria in the mmd1-1 mutant aggregate slightly at 23°C and severely after a 4-h incubation at the nonpermissive temperature of 37°C (Figure 1A). Mitochondria in mmd1-1 cells generally form two aggregations, with one at each pole of the cell. These clusters of mitochondria seem to be a collapsed mass of tubules containing some shorter mitochondrial fragments as well, rather than a single, enlarged mitochondrion (Figure 1B). 4,6-Diamidino-2-phenylindole staining in mmd1-1 cells revealed normal nuclear segregation in the mutant cells at both permissive and nonpermissive temperatures (our unpublished data).
Figure 1.
Mutant mmd1 cells display abnormal mitochondrial morphology and distribution. Wild-type (MYP115), mmd1-1 (MYP106), and Δmmd1 (MYP105) were grown in YES medium at 23°C and incubated for 4 h at 23°C or 37°C. (A) Mitochondrially targeted GFP was visualized in wild-type, mmd1-1 and Δmmd1 cells by using fluorescence microscopy. (B) Mitochondrially-targeted GFP was visualized in mmd1-1 cells incubated for 4 h at 37°C with fluorescence microscopy on an Olympus IX-70 inverted microscope. Images were taken and deconvolution was performed on 64 optical sections with a step of 0.2 μm by using Delta Vision 2.10 software (Applied Precision, Issaquah, WA). Bar, 2 μm.
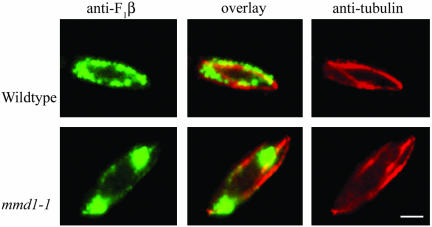
Microtubules play a central role in mediating mitochondrial distribution in S. pombe (Yaffe et al., 1996). To examine whether mitochondrial aggregation in mmd1-1 cells was accompanied by alterations in the microtubule network, microtubules were visualized by indirect immunofluorescence microscopy. Mutant mmd1-1 cells displayed intact and apparently normal microtubules at the permissive temperature and after incubation for up to 7 h at the nonpermissive temperatures (Figure 2). Additionally, mitochondria in mmd1-1 cells seemed to retain at least partial association with microtubules, despite their aggregated distribution (Figure 2). This association included the presence of one or two mitochondrial tubules associated with microtubules in ∼50% of the cells.
Figure 2.
Mitochondria remain associated with microtubules in mmd1 cells. Wild-type (FY254) and mmd1-1 (MYP102) cells were grown in YES medium, incubated at 37°C for 4 h, fixed with methanol, and processed for indirect immunofluorescence microscopy. Microtubules were visualized with mouse monoclonal TAT-1 antibody followed by rhodamine-conjugated donkey anti-mouse IgG. Mitochondria were detected with affinity-purified, anti-F1β antibody and fluorescein-conjugated goat anti-rabbit IgG. Pseudocolor was added to the digitized images. Bar, 2 μm.
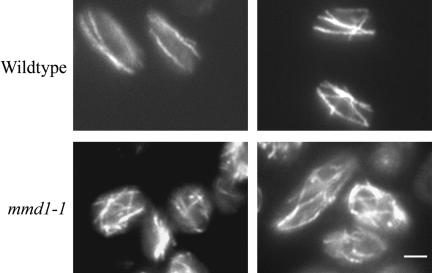
After extended incubation (e.g., 20 h) at the nonpermissive temperature, microtubules in mmd1-1 cells became misoriented (Figure 3). Instead of the normal two to four microtubules aligned parallel to the long cellular axis, 87% (n = 100) of mutant cells contained multiple microtubules arrayed in disorganized patterns. Although 32% (n = 100) of wild-type cells incubated for 20 h at 37°C also displayed some microtubule disorganization, the extent of misorientation was less in wild-type than in mutant cells (Figure 3). However, mutant mmd1 strains did not show altered sensitivity to the antimicrotubule drug thiabendazole, and after short incubations at 37°C, they were not defective in microtubule-dependent processes such as nuclear segregation and localization of Tea1-GFP to cellular ends (our unpublished data).
Figure 3.
The mmd1 mutation leads to severe misorientation of microtubules after extended times at the nonpermissive temperature. Wild-type (MYP115) and mmd1-1 (MYP106) cells were grown at 23°C in YES media and then incubated at 37°C for 20 h. During this incubation cultures were back-diluted into prewarmed media after 5 h. Wild-type and mmd1-1 cultures reached a final OD600 of 0.6 and 0.1, respectively. Cells were fixed in methanol and processed for immunofluorescence with anti-tubulin antibody as described in Figure 2. Two representative images of wild-type and mmd1-1 cells are shown. Bar, 2 μm.
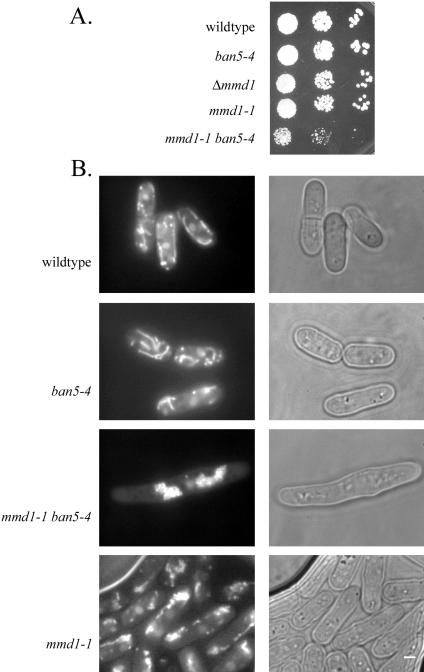
A previous study revealed that the temperature-sensitive, ban5-4 mutation in α2-tubulin causes mitochondrial aggregation and aberrant microtubules at the nonpermissive temperature (Yaffe et al., 1996). Cells harboring both ban5-4 and mmd1-1 displayed synthetic phenotypes more severe than those caused by either of the single mutations alone (Figure 4). At the permissive-temperature, double-mutant cells displayed severely aggregated mitochondria and possessed aberrantly short or even absent microtubules. These doublemutant cells were elongated and grew more slowly at 23°C, with a doubling time of 9 h compared with doubling times of 5–6 h for wild-type, ban5-4, and mmd1-1 cells.
Figure 4.
Synthetic genetic interactions between mmd1 and a mutation in an α-tubulin gene. (A) Wild type (FY254), mmd1-1 (MYP102), ban5-4 (MYP2), and mmd1-1 ban5-4 (MYP109) were grown in liquid YES medium and diluted to 106 cells/ml. Tenfold serial dilutions (10-μl aliquots) were spotted on solid YES medium and incubated at 25°C for 5 d. (B) Cells were grown in YES medium at 23°C, stained with DASPMI, and visualized with fluorescence microscopy. Bar, 2 μm.
mmd1+ Encodes a Novel, Conserved Protein
To determine the molecular basis of the mutant phenotypes caused by mmd1-1, the wild-type mmd1+ gene was cloned by complementation. One plasmid from an S. pombe genomic DNA library was found to rescue the growth of mmd1-1 cells at 37°C. This plasmid also partially rescued the mitochondrial distribution defects. DNA sequencing and comparison to sequences in the S. pombe genome database revealed that the plasmid genomic insert corresponded to sequences from chromosome 1 adjacent to (and including a portion of) the dynein heavy chain gene, dhc1+. When the ORF immediately adjacent to dhc1+, SPAC30C2.02, was disrupted in the complementing plasmid, complementation of mutant phenotypes was lost. A plasmid containing only this ORF and flanking sequences, generated by PCR-based cloning, complemented the temperature-sensitivity of mmd1-1 cells. To demonstrate that SPAC30C2.02 corresponded to the mmd1+ gene (rather than to a suppressor of the mutation) mmd1-1 was mapped genetically to less than one cM from dhc1+. These linkage and complementation results indicate that SPAC30C2.02 is mmd1+.
The mmd1+ gene encodes a 35.7-kDa, acidic protein. The protein sequence features six copies of EZ-HEAT motifs (Schultz et al., 2000) and is predicted to be composed primarily of alpha-helical structure, with the first half of the protein very similar to the second half.
Comparison of Mmd1p with sequences in GenBank database revealed a single, homologous protein of unknown function in every currently sequenced organism (Table 2 and Figure 5) (Altschul et al., 1990). Arabadopsis thaliana is an exception, because it also has a second, less conserved homologue. Mmd1p shows high sequence identity with its homologues: 51% with S. cerevisiae gene YJR070c, 44% with the C. elegans homologue, and 45% with the human homologue.
Table 2.
Mmd1p homologs
| Organism | % Identity | % Similarity |
|---|---|---|
| S. cerevisiae | 51 | 69 |
| Lentinula edodes | 47 | 63 |
| Drosophila melanogaster | 46 | 64 |
| Mus musculus | 46 | 60 |
| Anopheles gambiae str. PEST | 45 | 60 |
| Homo sapiens | 45 | 59 |
| Dictyostelium discoideum | 44 | 63 |
| C. elegans | 44 | 60 |
| A. thaliana (F9D24.90) | 41 | 59 |
| Leishmania major | 41 | 57 |
| Encephalitozoon cuniculi | 38 | 55 |
| Babesia bovis | 34 | 54 |
| A. thaliana (T12C14.230) | 33 | 59 |
| Synechococcus sp. PCC 7002 (CpcE) | 29 | 48 |
Figure 5.
Alignment of S. pombe Mmd1p and its homologues in diverse organisms. Amino acid sequences were aligned using the ClustalX program (Thompson et al., 1997) and the alignment saved in GCG/MSF format. The alignment file was processed with the GeneDoc program (http://www.psc.edu/biomed/genedoc). Shading levels represent 100% (darkest), >80%, >60%, and <60% conservation of amino acid residues among homologues for a given position. Bars over the Mmd1p sequence of S. pombe show the position of the EZ-HEAT motifs, as identified by the SMART database (Schultz et al., 2000). The residue mutated in the mmd1-1 strain is indicated by the Λ.
Cellular Requirements for Mmd1p in Fission and Budding Yeast
Cloning and analysis of the mmd1-1 gene from mutant cells revealed that the temperature-sensitive phenotypes are caused by the point mutation, G195A. This lesion leads to a replacement by a lysine residue of a conserved glutamic acid (Glu66), which is within an E-Z HEAT motif. To examine the cellular requirement for mmd1+, a null mutation was created by gene replacement (see MATERIALS AND METHODS). Cells deleted for mmd1+ were viable, but temperature sensitive for growth. The deletion strain, displayed a phenotype similar to the original mmd1-1 mutant with aggregated mitochondria at each cellular pole at both 23 and 37°C.
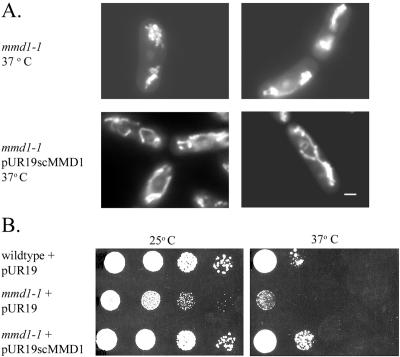
To determine whether the function of Mmd1p remains conserved between budding and fission yeast, the S. cerevisiae MMD1 gene (YJR070c) was deleted by integrative transformation of wild-type cells. The deletion strain displayed normal growth on both fermentable and nonfermentable carbon sources at a variety of temperatures and showed wild-type mitochondrial distribution and morphology. These results indicate that S. cerevisiae MMD1 plays no apparent role in maintaining the morphology, distribution, or function of mitochondria in budding yeast. S. cerevisiae cells deleted for MMD1 did display increased sensitivity to the antimicrotubule drug benomyl (our unpublished data), suggesting a role of this protein in mediating microtubule stability. However, expression of S. cerevisiae MMD1 in S. pombe mmd1-1 mutant cells complemented the mutant phenotypes (Figure 6), indicating a conservation of basic molecular function between the homologues in budding and fission yeast.
Figure 6.
S. cerevisiae MMD1 gene complements the mitochondrial and temperature sensitive defects of mmd1-1 cells. (A) S. pombe mmd1-1 cells (MYP102) were grown in EMM medium, and mmd1-1 cells transformed with a multicopy plasmid (pURscMMD1) encoding S. cerevisiae MMD1 (MYP113) were cultured in EMM medium without uracil. Cells were grown at 23°C, incubated for 4 h at 37°C, stained with DASPMI, and analyzed by fluorescence microscopy. Bar, 2 μm. (B) Wild type and mmd1-1 transformed with the empty plasmid pUR19 (MYP219 and MYP220) as well as mmd1-1 transformed with pUR19scMMD1 were grown in liquid EMM medium without uracil and diluted to 107 cells/ml. Tenfold serial dilutions (10-μl aliquots) were spotted on solid EMM-uracil medium and incubated at 25 and 37°C for 4 d.
Mitochondrial Function in mmd1–1 Cells
Mutant mmd1 cells grew poorly on glycerol, suggesting a defect in mitochondrial energy metabolism. The lack of growth on this carbon source could indicate a defect in respiration, but mitochondria in mmd1 cells readily stained with DASPMI, a vital dye whose uptake depends on mitochondrial membrane potential (Bereiter-Hahn, 1976). Additionally, import of mitochondrially targeted GFP, a process dependent on membrane potential, seemed normal in mutant cells. To further evaluate the effect of mmd1 mutations on mitochondrial membrane potential, cells were analyzed by fluorescence-activated cell sorting after treatment with the potential-sensitive dye JC-1. Wild-type, mmd1-1, and Δmmd1 cells displayed the same fluorescence-activated cell sorting profiles (our unpublished data), indicating no effect of the mutations on mitochondrial membrane potential. To determine whether mmd1 affects cellular respiration, respiratory rates were measured using a polarographic Clark-type O2 electrode. Whole-cell respiratory rates of mmd1 cells were 44 ± 13% (n = 11) of the wild-type rate, indicating a modest respiratory deficiency.
To further investigate how the inability to grow on glycerol relates to the defects in mitochondrial morphology apparent in mmd1 cells, suppressors of the glycerol growth defect were isolated. Six strains were identified that were able to grow on glycerol but also possessed aggregated mitochondria (like the original mmd1-1 mutant). Genetic analysis of one of these strains revealed that recovery of growth on glycerol was due to a single suppressing mutation (designated gly1-1), which mapped to an unidentified gene distinct from mmd1. Although cells with both mmd1 and gly1-1 grew at normal rates on glycerol, they remained temperature sensitive for growth on all carbon sources and possessed mitochondria with aberrant morphology and distribution (our unpublished data). In addition, the presence of the gly1 suppressor in mmd1 mutant cells restored respiratory rates to 73 ± 13% of wild-type levels. These results indicate that the abnormalities of mitochondrial distribution and morphology caused by mmd1-1 are not the direct result of an inability to grow on glycerol.
Mmd1p Is a Cytosolic Protein
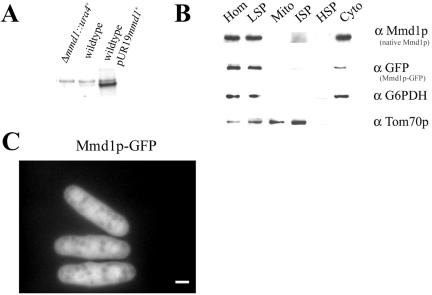
To determine the cellular location of Mmd1p function, the protein was localized by subcellular fractionation and by fluorescence microscopy. To facilitate this analysis, antiserum was raised against a lacZ-fusion protein containing the C-terminal 2/3 of Mmd1p. This serum detected a polypeptide of ∼36 kDa on immunoblots of proteins from total cellular extracts (Figure 7A). This species was absent when extracts were prepared from mmd1-null mutant cells and greatly increased in extracts from cells harboring a multicopy plasmid encoding mmd1+ (Figure 7A). Immunoblotting of subcellular fractions isolated from wild-type S. pombe cells revealed enrichment of Mmd1p in the cytosolic fraction. To confirm this localization, cells expressing a version of Mmd1p tagged with GFP (Mmd1p-GFP) were generated and analyzed. In separate experiments, we found that this fusion protein could fully replace the wild-type protein in complementing all mutant phenotypes (our unpublished data). Immunoblotting of fractions from cells expressing the tagged protein also showed enrichment of Mmd1p-GFP, as well as native Mmd1p, in the cytosolic fraction (Figure 7B).
Figure 7.
Mmd1p is a cytosolic protein. (A) Cellular homogenates were prepared from Δmmd1 (MYP105) and wild-type (FY254) cells and from wild-type cells harboring a multicopy plasmid (pUR19mmd1+) encoding mmd1+ (MYP114). Proteins were analyzed by SDS-PAGE on an 8–16% gradient gel and immunoblotted with antibodies against Mmd1p. Each lane contained 25 μg of total protein. (B) Subcellular fractions were prepared from cells harboring a single copy of the gene encoding Mmd1p-GFP (MYP112). Proteins were separated by SDS-PAGE and analyzed by immunoblotting. Fractions were probed with anti-Mmd1p, anti-GFP, anti-Glucose-6-phosphate dehydrogenase (G6PDH), a cytosolic marker, and anti-Tom70p, a mitochondrial marker. Subcellular fractions are: HOM, total cell homogenate; LSP, low-speed pellet; Mito, mitochondrial fraction; ISP, intermediate-speed pellet; HSP, high-speed pellet; and Cyto, cytosolic fraction. Each lane contained 10 μg of total protein. (C) Microscopic localization of Mmd1p. Cells containing a single copy of Mmd1p-GFP (strain MYP112) were grown on YES medium at 30°C and analyzed by fluorescence microscopy. Bar, 2 μm.
To further characterize Mmd1p localization, live cells expressing the GFP-tagged Mmd1p construct were visualized using fluorescence microscopy. The fluorescence pattern in these cells confirmed a cytosolic localization (Figure 7C). Indirect immunofluorescence microscopic analysis by using cells expressing an HA-tagged version of Mmd1p revealed a similar subcellular distribution (our unpublished data). These results demonstrate that Mmd1p is a component of the cytosol.
DISCUSSION
We have described a novel protein, Mmd1p, required for normal mitochondrial morphology and distribution in S. pombe. The role of this protein was revealed through the analysis of mmd1 mutant cells that display mitochondria aggregated toward the two cellular poles rather than extended as elongated tubules along the long cellular axis. This wild-type mitochondrial distribution depends on microtubules (Yaffe et al., 1996), and although mitochondria in the mutant cells seem to remain associated with these cytoskeletal structures, the mutant phenotype reflects a partially defective alignment and incomplete extension of mitochondrial tubules along microtubules. The aberrant mitochondrial distribution in mmd1 cells suggests a function of Mmd1p in facilitating the extended, lateral interactions of mitochondria and microtubules.
Mmd1p is highly conserved through evolution and shares high sequence identity with a homologue in all currently sequenced organisms. Only the S. pombe protein has been linked (current study) to a specific cellular process, but complementation of mmd1 defects with the homologous gene from S. cerevisiae, indicates at least partial conservation of function between homologues from two disparate species. The cellular role of the homologue (scMmd1p) in budding yeast is distinct from that of the fission yeast protein, because S. cerevisiae cells depleted of scMmd1p display normal mitochondrial morphology and distribution. This dis-similarity may reflect a fundamental divergence between the essential role of microtubules in mediating mitochondrial distribution in S. pombe and a complete lack of involvement of microtubules in facilitating mitochondrial behavior in S. cerevisiae (Huffaker et al., 1988; Jacobs et al., 1988). Furthermore, this difference is consistent with a function of Mmd1p in mediating the alignment of mitochondria with microtubules in fission yeast.
A connection between Mmd1p and microtubule function is further supported by the synthetic phenotypes apparent in cells harboring both mmd1 and the ban5-4 mutation in α2-tubulin. This synthetic interaction could indicate either a direct association (and cooperative function) of Mmd1p and tubulin or function of Mmd1p and tubulin in distinct but parallel pathways that both contribute to normal mitochondrial distribution. Although localization data do not support a stable binding of Mmd1p to tubulin-containing structures, the protein may interact transiently to modify microtubule function. The disorganized microtubules in S. pombe mmd1 mutant cells after long incubation at 37°C and the increased benomyl sensitivity of S. cerevisiae cells deleted for MMD1 further support a role for Mmd1p in modulating the structure or activity of microtubules.
The molecular activity of Mmd1p is unknown, but this protein and its homologues contain a repeating sequence motif, the EZ-HEAT or HEAT-PBS motif, that may provide clues to its biochemical function. This conserved motif was originally described in cyanobacterial lyase subunits responsible for covalently attaching chromophores to phycobilisomes, light-harvesting complexes in cyanobacteria and red algae (Fairchild et al., 1992). The motif is related to the more generalized HEAT motif, which is thought to mediate protein–protein interactions, and serve a molecular scaffolding role (Andrade and Bork, 1995; Andrade et al., 2001). The functional importance of this motif in Mmd1p was demonstrated by characterization of the mmd1 mutation; this lesion alters a conserved amino acid in the second EZ-HEAT motif and eliminates the protein's function in mediating mitochondrial distribution.
The structural features of Mmd1p suggest a model for its function in which the protein acts as a molecular scaffold that facilitates assembly of a complex needed for the lateral attachment of mitochondria with microtubules. Such a role might involve only a transient association of Mmd1p with microtubules and/or mitochondria and would be consistent with the protein's cytosolic distribution. Another possibility is that Mmd1p binds to and modifies another protein, which then can influence mitochondrial morphology and distribution. The modified protein might normally act as a linker between mitochondria and microtubules, or it could alter features of the mitochondrial membranes to support an extended, tubular morphology. The identification of proteins that interact with Mmd1p should provide new insights into the molecular role of this novel component.
Acknowledgments
We thank Susan Forsburg for the gift of plasmids, yeast strains, and valuable advice; DeWight Williams and Dick McIntosh for the gift of strain 645; Keith Gull for the gift of the TAT-1 anti-tubulin antibody; and the Aroian laboratory for use of a microscope and assistance with deconvolution microscopy. This work was supported by National Institutes of Health grant GM-44614 (to M.P.Y.).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–06–0371. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03–06–0371.
References
- Altschul, S.F., Gish, W., Miller, W., Myers, E.W., and Lipman, D.J. (1990). Basic local alignment search tool. J. Mol. Biol. 215, 403-410. [DOI] [PubMed] [Google Scholar]
- Andrade, M.A., and Bork, P. (1995). HEAT repeats in the Huntington's disease protein. Nat. Genet. 11, 115-116. [DOI] [PubMed] [Google Scholar]
- Andrade, M.A., Petosa, C., O'Donoghue, S.I., Muller, C.W., and Bork, P. (2001). Comparison of ARM and HEAT protein repeats. J. Mol. Biol. 309, 1-18. [DOI] [PubMed] [Google Scholar]
- Barbet, N., Muriel, W.J., and Carr, A.M. (1992). Versatile shuttle vectors and genomic libraries for use with Schizosaccharomyces pombe. Gene 114, 59-66. [DOI] [PubMed] [Google Scholar]
- Baudin, A., Ozier-Kalogeropoulos, O., Denouel, A., Lacroute, F., and Cullin, C. (1993). A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. Nucleic Acids Res. 21, 3329-3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereiter-Hahn, J. (1976). Dimethylaminostyrylmethylpyridiniumiodine (DASPMI) as a fluorescent probe for mitochondria in situ. Biochim. Biophys. Acta 423, 1-14. [DOI] [PubMed] [Google Scholar]
- Brazer, S.C., Williams, H.P., Chappell, T.G., and Cande, W.Z. (2000). A fission yeast kinesin affects Golgi membrane recycling. Yeast 16, 149-166. [DOI] [PubMed] [Google Scholar]
- Burglin, T.R., and DeRobertis, E.M. (1987). The nuclear migration signal of Xenopus laevis nucleoplasmin. EMBO J. 6, 2617-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum, G., Bohni, P.C., and Schatz, G. (1982). Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J. Biol. Chem. 257, 13028-13033. [PubMed] [Google Scholar]
- Fairchild, C.D., Zhao, J., Zhou, J., Colson, S.E., Bryant, D.A., and Glazer, A.N. (1992). Phycocyanin alpha-subunit phycocyanobilin lyase. Proc. Natl. Acad. Sci. USA 89, 7017-7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan, I.M., and Hyams, J.S. (1988). Use of cell division cycle mutants to investigate the control of microtubule distribution in the fission yeast Schizosaccharomyces pombe. J. Cell Sci. 89, 343-357. [DOI] [PubMed] [Google Scholar]
- Harlow, E., and Lane, D. (1988). Antibodies. A Laboratory Manual, Cold Spring Harbor: NY: Cold Spring Harbor Laboratory Press.
- Huffaker, T.C., Thomas, J.H., and Botstein, D. (1988). Diverse effects of β-tubulin mutations on microtubule formation and function. J. Cell Biol. 106, 1997-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs, C.W., Adams, A.E.M., Szaniszlo, P.J., and Pringle, J.R. (1988). Functions of microtubules in the Saccharomyces cerevisiae cell cycle. J. Cell Biol. 107, 1409-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, R.E., and Yaffe, M.P. (1988). Import of proteins into yeast mitochondria: the nuclear MAS2 gene encodes a component of the processing protease that is homologous to the MAS1-encoded subunit. EMBO J. 7, 3863-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanbe, T., Kobayashi, I., and Tanaka, K. (1989). Dynamics of cytoplasmic organelles in the cell cycle of the fission yeast Schizosaccharomyces pombe: three-dimensional reconstruction from serial sections. J. Cell Sci. 94, 647-656. [DOI] [PubMed] [Google Scholar]
- Keeney, J.B., and Boeke, J.D. (1994). Efficient targeted integration at leu1-32 and ura4-294 in Schizosaccharomyces pombe. Genetics 136, 849-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno, S., Klar, A., and Nurse, P. (1991). Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194, 795-823. [DOI] [PubMed] [Google Scholar]
- Rogers, S.L., and Gelfand, V.I. (2000). Membrane trafficking, organelle transport, and the cytoskeleton. Curr. Opin. Cell Biol. 12, 57-62. [DOI] [PubMed] [Google Scholar]
- Rose, M.D., Winston, F., and Hieter, P. (1990). Methods in Yeast Genetics: A Laboratory Course Manual, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Schultz, J., Copley, R.R., Doerks, T., Ponting, C.P., and Bork, P. (2000). SMART: a web-based tool for the study of genetically mobile domains. Nucleic Acids Res. 28, 231-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski, R.S., and Hieter, P. (1989). A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, B.J., and Yaffe, M.P. (1991). A mutation in the yeast heat-shock factor gene causes temperature-sensitive defects in both mitochondrial protein import and the cell cycle. Mol. Cell. Biol. 11, 2647-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, J.D., Gibson, T.J., Plewniak, F., Jeanmougin, F., and Higgins, D.G. (1997). The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25, 4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale, R.D. (2003). The molecular motor toolbox for intracellular transport. Cell 112, 467-480. [DOI] [PubMed] [Google Scholar]
- Woods, A., Sherwin, T., McRae, T.H., Baines, A.J., and Gull, K. (1989). Definition of individual components within the cytoskeleton of Trypanosoma brucei by a library of monoclonal antibodies. J. Cell Sci. 93, 491-500. [DOI] [PubMed] [Google Scholar]
- Yaffe, M.P. (1999). The machinery of mitochondrial inheritance and behavior. Science 283, 1493-1497. [DOI] [PubMed] [Google Scholar]
- Yaffe, M.P., Harata, D., Verde, F., Eddison, M., Toda, T., and Nurse, P. (1996). Microtubules mediate mitochondrial distribution in fission yeast. Proc. Natl. Acad. Sci. USA 93, 11664-11668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, A., West, R.R., McIntosh, J.R., and Hiraoka, Y. (1999). A cytoplasmic dynein heavy chain is required for oscillatory nuclear movement of meiotic prophase and efficient meiotic recombination in fission yeast. J. Cell Biol. 145, 1233-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]