Summary
Background
Treatment with bisphosphonates decreases bone loss and can increase disease-free survival in patients with breast cancer. The aim of our study was to assess the effect of zoledronic acid on clearance of disseminated tumour cells (DTCs) from the bone marrow in women undergoing neoadjuvant chemotherapy for breast cancer.
Methods
Patients were recruited for this open-label, phase 2 randomised trial between March 17, 2003, and May 19, 2006, at a single centre. Eligible patients had clinical stage II–III (≥T2 and/or ≥N1) newly diagnosed breast cancer, Eastern Cooperative Oncology Group performance status of 0 or 1, and normal cardiac, renal, and liver function. 120 women were randomly assigned, using allocation concealment, to receive 4 mg zoledronic acid intravenously every 3 weeks (n=60), or no zoledronic acid (n=60), for 1 year concomitant with four cycles of neoadjuvant epirubicin (75 mg/m²) plus docetaxel (75 mg/m²) and two cycles of adjuvant epirubicin plus docetaxel. The primary endpoint was the number of patients with detectable DTCs at 3 months. Final analysis was done 1 year after the last patient was enrolled. Analyses were done for all patients with available data at 3 months. This study is registered with ClinicalTrials.gov, number NCT00242203.
Findings
Of the 120 patients initially enrolled, one withdrew after signing consent and one patient’s baseline bone marrow was not available. Both of these patients were in the control group. At 3 months, 109 bone-marrow samples were available for analysis. In the zoledronic acid group, bone marrow was not collected from one patient because of disease progression, one patient was taken off study because of severe diarrhoea, and two patients had not consented at the time of surgery. In the control group, bone marrow was not collected from two patients because of disease progression, one patient withdrew consent, and three patients were not consented at the time of surgery. At baseline, DTCs were detected in 26 of 60 patients in the zoledronic acid group and 28 of 58 patients in the control group. At 3 months, 17 of 56 patients receiving zoledronic acid versus 25 of 53 patients who did not receive zoledronic acid had detectable DTCs (p=0·054). The most common grade 3–4 toxicities were infection (five of 60 patients in the zoledronic acid group and six of 59 in the control group) and thrombosis (five of 60 in the zoledronic acid and two of 59 in the control group). There was one documented case of osteonecrosis in the zoledronic acid group.
Interpretation
Zoledronic acid administered with chemotherapy resulted in a decreased proportion of patients with DTCs detected in the bone marrow at the time of surgery. Our study supports the hypothesis that the antimetastatic effects of zoledronic acid may be through effects on DTCs.
Funding
Novartis Pharmaceuticals and Pfi zer Inc.
Introduction
Early tumour-cell dissemination from the primary site is the first step in the development of distant metastases. Disseminated tumour cells (DTCs) have been found in the bone marrow of patients with breast cancer and are an independent prognostic indicator of increased risk of distant disease development and death.1 Moreover, patients with detectable DTCs after cytotoxic chemotherapy have a high risk of recurrence.2
The bone microenvironment seems to be a sanctuary site for DTCs, allowing the cells to adapt and disseminate to other organs.3 Preclinical models suggest that tumour growth in bone is enhanced when osteoclast activity is increased, possibly due to the release of bone-derived growth factors.4 Breast-cancer therapies can increase osteoclastic-mediated bone resorption through development of osteoporosis.5
Several clinical trials have examined the use of osteoclast-inhibiting bisphosphonates for the prevention of bone loss during adjuvant therapy for breast cancer. Bisphosphonates are analogues of pyrophosphates that bind to hydroxyapatite crystals in bone and inhibit bone resorption. Zoledronic acid, a third-generation bisphosphonate, inhibits osteoclastic resorptive activity partly through inhibition of farnesyl-diphosphate synthase and protein prenylation.6 Zoledronic acid prevents bone loss in preclinical models,7 in women with postmenopausal osteoporosis,8 in patients with breast cancer with bone metastases,9 in postmenopausal patients with breast cancer receiving adjuvant aromatase inhibitors,10 and in premenopausal patients with breast cancer who develop ovarian failure or in whom ovarian suppression has been induced.11 In preclinical models, bisphosphonates directly inhibit tumour growth and angiogenesis.12 Two recent clinical trials, ABCSG13 and Z/Zo-FAST,14 have shown a disease-free survival benefit with zoledronic acid in women receiving adjuvant endocrine therapy.
We postulated that zoledronic acid could improve the effect of neoadjuvant chemotherapy in women with breast cancer, by increasing the clearance of DTCs from bone marrow. We did a randomised phase 2 study to investigate the effects of zoledronic acid on DTCs, bone-turnover markers, and bone-mineral density, in women undergoing neoadjuvant chemotherapy for stage II–III breast cancer.
Methods
Patients
120 women were enrolled between March 17, 2003, and May 19, 2006, at Siteman Cancer Center, Washington University, St Louis, MO, USA. Patients with clinical stage II–III (≥T2 and/or ≥N1) newly diagnosed breast cancer, Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and normal cardiac, renal, and liver function were eligible. Exclusion criteria included evidence of distant metastasis by CT scan of the chest, abdomen, pelvis, or bone scan. Other exclusion criteria were prior malignancies, serious functional disorders of the heart, liver, or kidneys, pregnancy, or women below 18 years of age. Menopause was defined as 1 year with no menstrual activity, previous bilateral oophorectomy, or age older than 56 years. The Internal Review Board of Washington University approved the study and patients gave written consent. The Siteman Cancer Center’s quality assurance and safety monitoring committee oversaw patient safety.
Randomisation and masking
Randomisation was done using a formal probability model and implemented with an SAS proc plan generated by the statistician. Assignments were balanced within blocks of random size. Allocations were placed in sequentially numbered, opaque envelopes that were kept in a locked file cabinet and accessed only by the study’s patient coordinator. After enrolment, the group assignment was revealed to the study coordinator and the patient. The interpreting pathologists were masked to the study group.
Procedures
Women were randomly assigned to receive 4 mg intravenous zoledronic acid every 3 weeks for 1 year (total 17 doses) commencing with the first dose of chemotherapy, or no zoledronic acid (chemotherapy alone). The zoledronic acid dosing schedule for bone metastasis was used.15 All women received four cycles of intravenous neoadjuvant epirubicin (75 mg/m²) plus docetaxel (75 mg/m²) every 3 weeks, with granulocyte-stimulating factor support and oral dexamethasone premedication (20 mg), followed by surgery and two cycles of adjuvant epirubicin plus docetaxel administered every 3 weeks. Adjuvant radiation, endocrine, and trastuzumab therapies were administered when indicated. Patients were encouraged to take 1000 mg of calcium with 800 IU vitamin D daily. Adverse events were assessed at each follow-up. Patients were removed from the study for safety reasons, progression during chemotherapy, or recurrent disease development. The primary endpoint was the number of patients with detectable DTCs in bone marrow, measured at baseline, 3 months (after four cycles of chemotherapy). Secondary endpoints were changes in bone-turnover markers (N-telopeptide [NTx], osteocalcin, serum bone-specific alkaline phosphatase [BSAP]), measured at baseline, 3 months, and 12 months, and in bone-mineral density, measured at baseline and 12 months.
Bone marrow was collected from each anterior iliac crest and processed as described by Fehm and colleagues.16 Briefly, 2 million cells from each specimen were stained using anti-pan-cytokeratin (CK) antibodies AE1AE3 (Chemicon International, Temecula, California, USA) at a dilution of 1:500. Slides were reviewed by two pathologists who were masked to treatment assignment. DTCs were defined as CK-positive, morphologically consistent cells. Patients with at least one CK-positive cell in the bone marrow from either iliac crest, as read by either pathologist, were scored as positive for micrometastases. The proportion of discordant slide interpretations between pathologists was 13·8% (kappa 0·63; 95% CI 0·55–0·70). In cases of disagreement, all discordant slides were reviewed by both pathologists and a consensus was reached.
Immunostaining for oestrogen receptor, progesterone receptor, and human epidermal growth-factor receptor 2 (HER2) was done at Washington University, using ER-Sp1, PR-1E2, and HER2-4B5 antibodies (Ventana Inc, Tucson, AZ, USA). Oestrogen receptor and progesterone receptor were considered positive if there was any detectable staining by immuno histochemistry. HER2 was considered positive if HercepTest (Dako Inc, St. Louis, Missouri, USA) was 3+ or Fluorescent in situ hybridisation (FISH) score was higher than 2·0. FISH analysis was done for all specimens scored as 2+ on HercepTest.
Bone-mineral density at the lumbar spine, proximal femur, and distal radius was measured using dual x-ray absorptiometry on a single unit densitometer (Hologic Inc, Bedford, MA, USA). Bone-mineral density was expressed as g per cm² or as T-scores. In-vivo coefficients of variation for bone-mineral density were 0·02. Bone-turnover markers (urinary NTx, serum osteocalcin, and BSAP) were assayed by use of immunoassays at Mayo Medical Laboratories (Rochester, MN, USA). NTx was expressed as a ratio to urinary creatinine.
Pretreatment tumour size was defined as the largest tumour dimension documented by mammogram, breast ultrasound, or MRI. Pretreatment lymph-node status was defined as any abnormal lymph nodes on CT or ultrasound imaging, or clinical exam, or by the presence of metastatic disease from fine needle aspiration or sentinel lymph-node biopsy. Pathological complete response (pCR) was defined as no residual invasive tumour in the breast specimen according to the National Surgical Adjuvant Breast and Bowel Project (NSABP) definition. Disease-free survival was defined as the interval between date of diagnosis and date of first clinical evidence of distant disease.
Statistical analysis
We estimated that 60–80% of the patients at our centre with locally advanced breast cancer would have DTCs at baseline, and that neoadjuvant chemotherapy would decrease this by about 20% at 3 months (similar to the percentage of pCR expected in this population). The additional benefit attributable to zoledronic acid was unknown; however, we estimated this effect to be roughly equivalent to doubling the pCR rate. The trial was designed to have at least 80% power at a 0·05 significance level to detect a 20–26% difference in DTCs at baseline versus 3 months, in patients with and without zoledronic acid therapy, even if no DTCs were detected at baseline. Treatment with zoledronic acid was expected to show increasing bone-mineral density at 12 months and decreasing concentrations of bone-turnover markers at 3 months and 12 months compared with the non-zoledronic acid group, as in previous studies of women with low bone-mineral density.9
Patients’ baseline characteristics were analysed with descriptive statistics. Categorical data, including DTC analysis, were described using frequencies and percentages and tested using χ² and one-sided Fisher’s exact tests. DTC analysis included all patients with data at each timepoint. All patients were treated according to the group into which they were randomly assigned.
Continuous data were described using means and standard deviations for normally distributed variables, and otherwise by median and percentiles. Patients with analysable bone-turnover markers were defined as those with a baseline and either a 3-month or 12-month measurement. Patients were defined as analysable for bone-mineral density if they had two determinations. Paired t tests were done for change from baseline in bone-turnover markers and bone-mineral density. Comparison across treatment groups was done using an unpaired t-test. Multiple linear regression analysis was done to control for smoking, body-mass index (BMI), and endocrine therapy in the analysis of bone-mineral density. Mixed models (random intercept and slopes) were used, with each of the bone-turnover markers as dependent variables, to model the treatment effect over time while adjusting for possible confounders. For analysis of bone-turnover markers, time was modelled as linear and the independent variables were BMI, use of adjuvant endocrine therapy, and smoking habits. All tests for bone-turnover markers and bone-mineral density were two-sided and an alpha of less than 0·05 was considered significant. SAS version 9.1 was used for statistical analysis. This study is registered with ClinicalTrials.gov, NCT00242203.
Role of the funding source
The study sponsors had no role in the trial design, implementation, analysis and interpretation of the data, and did not have access to the raw data. The corresponding author had unrestricted access to the study data and was responsible for the accuracy and completeness of the analyses. The corresponding author had final responsibility for the decision to submit for publication.
Results
Between March 17, 2003, and May 19, 2006, 120 patients with locally advanced breast cancer were randomly assigned to receive zoledronic acid or no zoledronic acid, concurrent with neoadjuvant chemotherapy. Women in the two groups had similar baseline characteristics (table 1). About half the patients in each group were premenopausal and 56% of all patients were oestrogen-receptor positive. Mean pretreatment tumour size was similar in both groups. After chemotherapy, three of the postmenopausal women with hormone-receptor positive breast cancer received tamoxifen, 24 received an aromatase inhibitor, and three received no endocrine therapy. Of the premenopausal women with oestrogen-receptor positive tumours, 11 received an aromatase inhibitor, 24 received tamoxifen, and two received no endocrine therapy.
Table 1.
Patient and tumour characteristics at baseline
| ZA (n=60) | Control (n=59) | |
|---|---|---|
| Median age in years (range) | 49 (29–67) | 47 (31–68) |
|
| ||
| Ethnic origin, n | ||
| White | 39 | 45 |
| African American | 20 | 11 |
| Other | 1 | 3 |
|
| ||
| Menopausal status, n | ||
| Premenopausal | 31 | 33 |
| Postmenopausal | 29 | 26 |
|
| ||
| Pathology, n | ||
| Ductal carcinoma | 47 | 49 |
| Lobular carcinoma | 7 | 7 |
| Other | 6 | 3 |
|
| ||
| Mean tumour size in cm (SD) | 3·81 (2·03) | 3·56 (2·41) |
|
| ||
| Lymph-node positive, n | 38 | 33 |
|
| ||
| Grade, n | ||
| I | 7 | 2 |
| II | 20 | 28 |
| III | 33 | 29 |
|
| ||
| Oestrogen-receptor positive, n | 32 | 35 |
|
| ||
| Progesterone-receptor positive, n | 24 | 31 |
|
| ||
| HER2 positive, n | 13 | 10 |
|
| ||
| Positive for DTCs at baseline, n* | 26 | 28 |
|
| ||
| T-score, median (range)† | ||
| Wrist | 0·7 (−2·8 to 3·0) | 0·2 (−2·2 to 3·1) |
| Spine | 0·1 (−3·1 to 4·5) | −0·02 (−2·8 to 3·3) |
| Hip | 0·15 (−2·6 to 3·2) | 0·05 (−2·3 to 3·4) |
|
| ||
| BMD in g/cm², mean (SD)† | ||
| Wrist | 0·5769 (0·063) | 0·5834 (0·067) |
| Spine | 1·030 (0·201) | 0·9943 (0·156) |
| Hip | 0·9512 (0·186) | 0·9049 (0·146) |
|
| ||
| BTM, mean (SD) | ||
| NTx in nmol/mol | 33·44 (19·02) | 32·82 (21·53) |
| BSAP in μg/L | 8·62 (4·16) | 9·22 (4·25) |
| Osteocalcin in ng/mL | 10·78 (5·62) | 10·08 (4·89) |
|
| ||
| BMI in kg/m², mean (SD) | 31·5 (7·2) | 28·6 (8·3) |
|
| ||
| Smokers, n | 31 | 28 |
ZA=zoledronic acid. HER2=human epidermal growth-factor receptor 2. DTCs=disseminated tumour cells. BMD=bone-mineral density. BTM=bone turnover markers. NTx=N-telopeptide. BSAP=bone-specific alkaline phosphatase. BMI=body-mass index. *For DTC analysis, total n=118: 58 in the control group and 60 in the ZA group. †For dual-energy x-ray absorptiometry scans, total n=110 (54 in the control group and 56 in the ZA group).
Of the 120 patients initially enrolled, one withdrew after signing consent and one did not have bone marrow available at baseline. At 3 months, bone-marrow specimens were not collected from ten patients. In the zoledronic acid group, bone marrow was not collected from one patient because of disease progression, one patient was taken off study with severe diarrhoea, and two patients had not consented at the time of surgery. In the control group, bone marrow was not collected from two patients because of disease progression, one patient withdrew consent, and three patients had not consented at the time of surgery. Before the end of the 12 months, three patients experienced disease progression during neoadjuvant chemotherapy, 14 developed metastatic disease, and six withdrew (figure 1). The distribution of off-study patients was similar in both groups. At baseline, 118 patients were assessable for DTCs, 119 for bone-turnover markers, and 110 for bone-mineral density. At 3 months, 109 patients were assessable for DTCs and bone-turnover markers, and at 1 year of follow-up, 79 patients were assessable for DTCs and 89 for bone-mineral density and bone-turnover markers.
Figure 1. Trial profile.
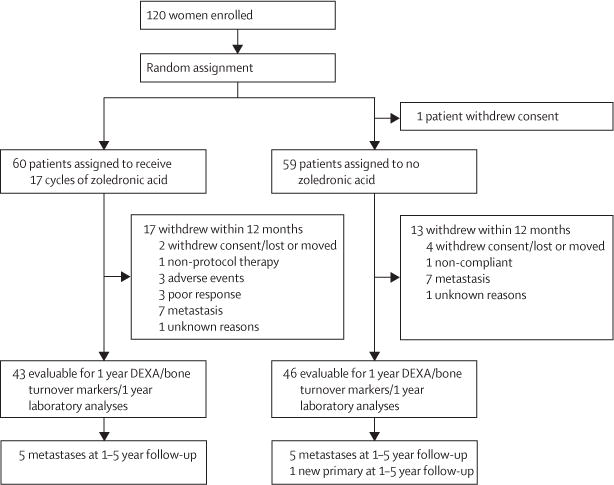
DEXA=dual energy x-ray absorptiometry.
26 of 60 patients in the zoledronic acid group and 28 of 58 in the control group had detectable DTCs at diagnosis (table 1). After 3 months, after four cycles of neoadjuvant chemotherapy, 17 of 56 patients treated with zoledronic acid had detectable DTCs, compared with 25 of 53 in the control group (p=0·054; figure 2A). We also analysed the primary endpoint using all randomised patients—attributing a negative outcome to patients with missing data points. The outcome of this analysis was similar (p=0·058). Of the patients that had no detectable DTCs at baseline, 27 of 31 in the zoledronic acid group remained negative for DTCs at 3 months, compared with 15 of 25 in the control group (p=0·030; figure 2B). There was no significant difference in detectable DTCs at 3 months in those patients who were positive at baseline (table 2). At 1 year, bone marrow was not collected from 40 patients because of relapse (n=17), withdrawal from the study (n=6), or the procedure was not performed (n=17). Of the 79 patients with evaluable DTCs at 1 year, there was no significant difference in the proportion of DTC-positive patients in the zoledronic acid and control groups (16 of 40 patients vs 13 of 39).
Figure 2. Proportion of patients with DTC-positive bone marrow.
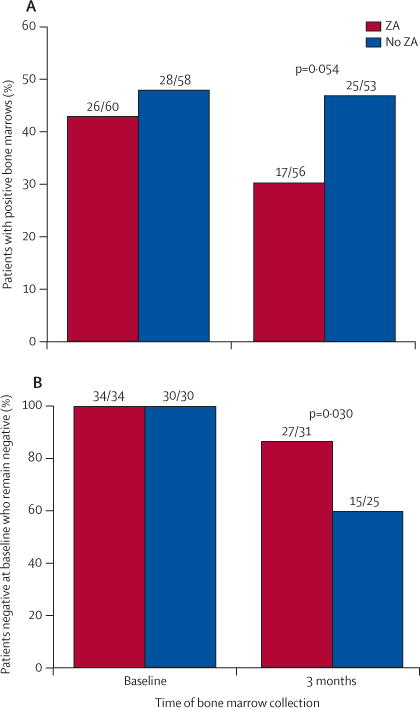
(A) Proportion of patients in each group with detectable DTCs in their bone marrow at baseline and 3 months. (B) Proportion of patients in each group with no DTCs detectable in their bone marrow at baseline who remained negative at 3 months. DTCs=disseminated tumour cells. ZA=zoledronic acid.
Table 2.
Presence of DTCs in the bone marrow, according to treatment group
| Baseline status | 3-month status | |
|---|---|---|
| Control | ||
|
| ||
| Positive | 28/58 | ·· |
| Positive | ·· | 15/28 |
| Negative | ·· | 13/28 |
| Negative | 30/58 | ·· |
| Positive | ·· | 10/25 |
| Negative | ·· | 15/25 |
|
| ||
| Zoledronic acid | ||
|
| ||
| Positive | 26/60 | ·· |
| Positive | ·· | 13/25 |
| Negative | ·· | 12/25 |
| Negative | 34/60 | ·· |
| Positive | ·· | 4/31 |
| Negative | ·· | 27/31 |
DTCs=disseminated tumour cells. ZA=zoledronic acid
Baseline values for bone-turnover markers were similar in the zoledronic acid and control groups (table 1). Women in the control group had significant increases in NTx and osteocalcin at 3 months, and in all three bone-turnover markers at 12 months, compared with baseline (p<0·05 for all comparisons; webappendix p 1). Changes in the concentrations of all bone-turnover markers after 3 months of neoadjuvant chemotherapy, and at 12 months, were significantly different between the control group and zoledronic acid group, regardless of menopausal status (p<0·05; webappendix p 3).
Women in the control group experienced significant losses in bone density at both the hip (−3·53%) and spine (−4·42%) at 12 months (p=0·0054) compared with baseline (webappendix p 2). Women randomly assigned to zoledronic acid treatment had a significant gain in bone density at the hip at 12 months compared with baseline (p=0·0275). Comparisons between the zoledronic acid and control groups showed significant differences in bone-mineral density changes from baseline at the hip (5·79 vs −3·53) and wrist (1·17 vs −1·41; p<0·05; webappendix p 4). In regression analysis, smoking, BMI, and endocrine therapy did not affect the change in bone-mineral density (data not shown).
Pathological tumour response to chemotherapy is shown in figure 3. Overall, 22 of 118 patients had a pCR: 13 of 60 in the zoledronic acid group and nine of 58 in the control group (p=0·63). In analysis by tumour subtype, few pCRs were observed in the oestrogen-receptor-positive subgroup, regardless of study treatment (four patients in each group). Six of 21 patients with oestrogen-receptor/HER2-negative tumours who were treated with zoledronic acid had a pCR, versus 2 of 19 for the control group, although this was not statistically significant (table 3).
Figure 3. Percent pathological complete response by treatment group and tumour subtype.
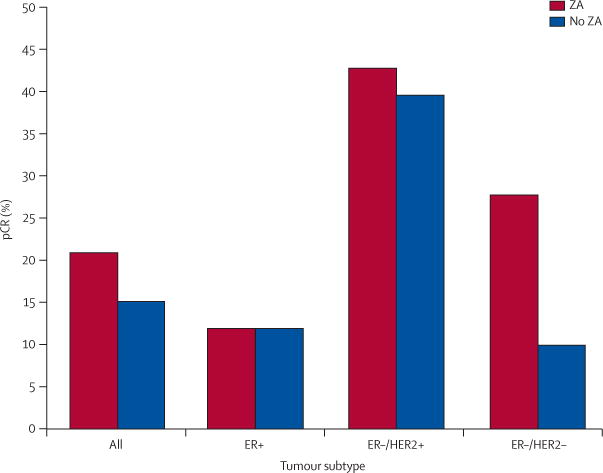
pCR=pathological complete response. ER=oestrogen receptor. HER2=human epidermal growth-factor receptor 2.
Table 3.
Pathological tumour response to chemotherapy by treatment group and tumour subtype
| Residual tumour size in ZA group, n
|
Residual tumour size in control group, n
|
|||||
|---|---|---|---|---|---|---|
| pCR (no tumour) | <1 cm | >1 cm | pCR (no tumour)* | <1 cm | >1 cm | |
| ER+/HER2− | 2 | 3 | 21 | 3 | 6 | 20 |
|
| ||||||
| ER+/HER2+ | 2 | 1 | 3 | 1 | 2 | 2 |
|
| ||||||
| ER−/HER2+ | 3 | 1 | 3 | 2 | 2 | 1 |
|
| ||||||
| ER−/HER2− | 6 | 3 | 12 | 2 | 2 | 14 |
ZA=zoledronic acid. pCR=pathological complete response. ER=oestrogen receptor. HER2=human epidermal growth-factor receptor 2. *Biomarker status was not available for one patient.
There was no difference in disease-free survival between the two treatment groups at 12 and 24 months. 12-month and 24-month recurrence-free survival for the control group was 89·3% (95% CI 77·7–95·0) and 85·7% (75·3–92·6), respectively. 12-month and 24-month overall survival for the zoledronic-acid group was 86·2% (74·3–92·8) and 81·0% (68·4–89·0), respectively.
Safety analysis consisted of 119 women. National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 were followed. Adverse events were similar in the two treatment groups. Serious adverse events (grade 3 and 4) are shown in table 4. There were no fractures reported in either group. One case of osteonecrosis of the jaw (ONJ) occurred. This patient had chronic sinusitis at the start of chemotherapy and zoledronic acid, and developed ONJ of the maxillary sinus after 11 infusions of zoledronic acid. She underwent partial excision of the right maxilla with evidence of nonviable bone with bacterial colonies. The ONJ resolved with treatment and the patient wears a partial hard-palate prosthesis.
Table 4.
Number of grade 3–4 serious adverse events
| ZA (N=60) | Control (N=59) | |
|---|---|---|
| Infection | 5 | 6 |
| Thrombosis/DVT | 5 | 2 |
| Neutropenic fever | 3 | 2 |
| Pneumothorax | 1 | 3 |
| Arrhythmia | 1 | 2 |
| Diarrhoea | 2 | 0 |
| Abdominal pain | 1 | 1 |
| Bone pain | 1 | 1 |
| Pleural effusion | 1 | 0 |
| ONJ | 1 | 0 |
| Bronchospasm | 1 | 0 |
ZA=zoledronic acid. DVT=deep-vein thrombosis. ONJ=osteonecrosis of the jaw.
Discussion
We postulated that the decrease of osteoclast activity with bisphosphonates in patients with breast cancer undergoing chemotherapy would alter the bone microenvironment and result in a decrease in the development of micrometastatic disease. We noted that around 46% of patients with clinical stage II–III breast cancer had DTCs at the time of diagnosis. Chemotherapy alone had little effect on the proportion of patients with DTCs at 3 months. By contrast, treatment with zoledronic acid resulted in fewer patients with detectable DTCs at 3 months than at diagnosis. Patients who had no detectable DTCs in their bone marrow at the time of diagnosis were more likely to remain negative for DTCs at 3 months if they received zoledronic acid. The number of patients with DTCs detectable at 12 months did not differ significantly between treatment groups, which may be related to the reduced number of evaluable patients or the variation of adjuvant treatment after 3 months. Our study was randomised, had a uniform chemotherapy regimen, with patients recruited at a single centre, and included a variety of patient subgroups, which allows our primary endpoint of DTC results at 3 months to be informative. Two smaller, non-randomised pilot studies reported that zoledronic acid clears DTCs from bone marrow.17,18
Identification of a single DTC in bone marrow is an independent indicator of poor survival,2 and the detection of CK-positive cells after adjuvant chemotherapy is significantly correlated with decreased overall survival.3 We did not observe a decrease in the proportion of patients with DTCs in their bone marrow after cytotoxic chemotherapy with taxanes and anthracyclines, which is consistent with previous findings3 and supports the hypothesis that the bone marrow serves as a sanctuary for DTCs, or that DTCs that survive in the bone marrow are chemotherapy resistant, possibly by acquiring stem-cell-like properties.4
The decrease in the proportion of women with detectable DTCs in the bone marrow after 3 months of treatment with zoledronic acid and chemotherapy observed in this study was not statistically significant. A possible explanation is that a threshold effect exists, whereby detection of a particular level of DTCs at baseline indicates a tumour burden that is too large for chemotherapy with or without zoledronic acid to have an effect. Moreover, some negative bone marrow may have DTCs present that are below the threshold of detection by the current methodology, but with time these DTCs may proliferate or become detectable because of changes in the bone-marrow composition with chemotherapy. Furthermore, a lower number of patients with detectable DTCs at baseline than expected, and a lower number of patients with analysable bone marrow at 12 months. Our results do not exclude the possibility that treatment with zoledronic acid may reduce established DTCs in subsets of women with localised breast cancer.
The increased proportion of patients in the control versus zoledronic acid group who were initially negative for detectable DTCs and became positive after chemotherapy may indicate that chemotherapy mobilises tumour cells to or from the bone marrow. Previous studies suggest that chemotherapy with growth-factor support mobilises tumour cells from the bone marrow and other metastatic sites by poorly understood mechanisms.19 Thus, the presence of DTCs in the bone marrow could represent a balance between chemotherapy-dependent mobilisation and therapy-induced elimination.20
Zoledronic acid administered with chemotherapy led to a significant proportion of patients who had no detectable DTCs at baseline to remain negative. We postulate that chemotherapy leads to increased bone turnover and the release of growth factors, providing a favourable environment for DTCs, and that this effect is abrogated by treatment with bisphosphonates. However, our study was not designed to compare the proportion of patients who remained DTC-free with treatment, and these findings should be confirmed in larger clinical trials.
Interpretation of the appearance or disappearance of several cells as either success or failure of applied therapy should be done cautiously. False-negative bone marrow results due to sampling cannot be excluded. To minimise this, we assessed aspirates from each iliac crest, a procedure that allows the detection of about 90% of patients with positive bone marrows.21
Recent results from the neoadjuvant subgroup analysis of the AZURE trial22 showed a significant increase in pCRs in patients treated with zoledronic acid. In our study, pCR was not significantly different between treatment groups. There may be differences in the patient populations or chemotherapy administered, or the increase in pCR may be an artifact of the dataset and, as such, is in need of confirmation.
Data from our study and others suggest that zoledronic acid has antimetastatic properties within the bone marrow and systemically.13,14 Two of three randomised clinical trials of another bisphosphonate, clodronate, used as adjuvant therapy in patients with non-metastatic breast cancer have reported a survival benefit,23–25 and two recent trials have shown a survival benefit in premenopausal and postmenopausal women treated with zoledronic acid and adjuvant aromatase inhibitors.13,14 Zoledronic acid may work through several mechanisms, including alteration of osteoclastic resorption with local release of bone-derived growth factors, resulting in a bone marrow micro environment less favourable for tumour-cell growth; inhibition of neoangiogenesis;26 induction of tumour-cell apoptosis;27 synergy with cytotoxic chemotherapy;28 and immunomodulatory effects.29 In preclinical models, zoledronic acid synergises with chemotherapy, with the greatest effect noted when zoledronic acid was administered sequentially after chemotherapy rather than concomitantly.30 In our study, zoledronic acid was administered on the day of chemotherapy. Future studies are needed to address the optimum timing of zoledronic acid administration.
Our data show that premenopausal and postmenopausal women with breast cancer had an increase in serum markers of bone activity after 3 months of neoadjuvant chemotherapy, with concomitant bone loss indicated by increased bone-turnover markers and decreases in bone-mineral density. Roughly 10% of women with normal bone-mineral density at baseline became osteopenic at the hip, spine, or wrist during the 12-month treatment period. Increased bone-turnover markers persisted in the control group at 12 months, irrespective of menopausal status or administration of endocrine or trastuzumab therapy. The addition of zoledronic acid to the treatment regimen significantly improved bone-mineral density at the hip and wrist and reversed the effect on bone-turnover markers. 8 of 18 women (44%) with mild osteopenia at baseline had normal bone-mineral density at 1 year after receiving concurrent chemotherapy and zoledronic acid, and significant decreases in bone-turnover markers at both 3 months and 12 months after initiation of treatment.
In postmenopausal breast-cancer patients, bone loss is primarily attributed to endocrine therapy with aromatase inhibitors.31 However, postmenopausal women under going adjuvant chemotherapy have lower bone-mineral density at 1 year than those who do not receive chemotherapy, suggesting an effect of chemotherapy treatment itself on bone-mineral density.32 We found a 35% change from baseline in osteocalcin at the time of completion of four cycles of treatment in postmenopausal women, which suggests a direct effect on bone turnover by the chemotherapy regimen. Bone loss at the lumbar spine was not as high as reported in other studies,11 possibly because of the negative hormone-receptor status of 55% of the postmenopausal women, who did not receive an aromatase inhibitor.
Our results show that administration of zoledronic acid at the start of chemotherapy prevented bone-density loss at 1 year of follow-up, consistent with other studies examining bisphosphonates in breast cancer.33–35 The increase in bone-turnover markers after four cycles of chemotherapy suggests that treatment effects on the bone are fairly rapid and that some patients may benefit from zoledronic acid administration at the start of chemotherapy. This possibility deserves further investigation, in view of reports suggesting that zoledronic acid might improve overall survival, compared with placebo, in patients with high bone-turnover rates because of bone metastases.36 However, the clinical implications of increasing bone-mineral density in patients with a normal baseline bone-mineral density are unclear.37 Questions remain regarding bisphosphonate treatment for patients with breast cancer—including dose, time of administration, and target population. The optimum dose of zoledronic acid will likely depend on the reason for administration. Several large randomised trials are in progress (NASBP B-34 and South West Oncology Group 0307) to clarify the role of zoledronic acid in the adjuvant setting.
Zoledronic acid was well tolerated with no treatment discontinuations. ONJ was observed in one of the 60 patients receiving zoledronic acid. Although this is within the reported incidence of ONJ (0·6–9%) in patients receiving intravenous bisphospho nates,38 it might be related to dosing and patient factors.
In summary, we report that fewer women had detectable DTCs after neoadjuvant chemotherapy with concurrent zoledronic acid than with chemotherapy alone, and that zoledronic acid appeared to decrease the rate at which DTC-negative breast-cancer patients developed subsequent micrometastatic disease at 3 months. Further, zoledronic acid was effective in preventing treatment-related loss in bone-mineral density, irrespective of menopausal status or tumour biomarkers, adding to the growing literature on the possible benefit of zoledronic acid in the treatment of women with breast cancer.11,12,39,40
Supplementary Material
Acknowledgments
We thank our patients, study coordinators, and the doctors and nurses involved in their care. We thank Susan Fox for administrative support and Michael Tomasson and Priya Gopalan for critical review of this manuscript.
Footnotes
Contributors
RA, MN, KW, and JM were involved in the conception and design of the study. RA, KW, MW, MN, VH, JD, AA, ME, PW, TE, CM, PMF, IZ, MT, WG, and TP were involved in the provision of study material and patients. LY and JZ interpreted the pathology slides. RA, MN, K W, MC-MG, KT, WS, KD, and SK did the data analysis and interpretation. KT was in charge of the statistical design of the study. RA, KW, MN, MC-MG, and KT wrote the manuscript. RA, KW, and KT approved the final version.
Conflicts of interest
RA and KW have received honoria from Novartis. MN has received honoria from Novartis, Sanofi-Aventis, and Pfizer. ME is a consultant for Novartis and has received honoria and research funds from Novartis. KD has received honoria from Novartis. WS received funds for portions of the statistical analyses. All other authors declared no conflicts of interest.
For the full protocol for this study see www.siteman.wustl.edu/ZoledronatebreastcancerDTCstudy.aspx
References
- 1.Braun S, Vogl FD, Naume B, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005;353:793–802. doi: 10.1056/NEJMoa050434. [DOI] [PubMed] [Google Scholar]
- 2.Braun S, Kentenich C, Janni W, et al. Lack of effect of adjuvant chemotherapy on the elimination of single dormant tumour cells in bone marrow of high-risk breast cancer patients. J Clin Oncol. 2000;18:80–86. doi: 10.1200/JCO.2000.18.1.80. [DOI] [PubMed] [Google Scholar]
- 3.Pantel K, Alix-Panabieres C, Riethdorf S. Cancer micrometastases. Nat Rev Clin Oncol. 2009;6:339–51. doi: 10.1038/nrclinonc.2009.44. [DOI] [PubMed] [Google Scholar]
- 4.Käkönen S-M, Mundy G. Mechanisms of osteolytic bone metastases in breast carcinoma. Cancer. 2003;97:834–39. doi: 10.1002/cncr.11132. [DOI] [PubMed] [Google Scholar]
- 5.Hirbe A, Morgan EA, Uluckan O, Weilbaecher K. Skeletal complications of breast cancer therapies. Clin Cancer Res. 2006;12:6309–14. doi: 10.1158/1078-0432.CCR-06-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimmel DB. Mechanism of Action, Pharmacokinetic and pharmacodynamic profile, and clinical applications of nitrogen-containing bisphosphonates. J Dent Res. 2007;86:1022–33. doi: 10.1177/154405910708601102. [DOI] [PubMed] [Google Scholar]
- 7.Saad F. Zoledronic acid: past, present and future roles in cancer treatment. Future Oncol. 2005;1:149–59. doi: 10.1517/14796694.1.2.149. [DOI] [PubMed] [Google Scholar]
- 8.Reid IR, Brown JP, Burckhardt P, et al. Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N Engl J Med. 2002;346:653–61. doi: 10.1056/NEJMoa011807. [DOI] [PubMed] [Google Scholar]
- 9.Nagy Z. Zoledronic acid (ZOMETA): a significant improvement in the bone metastases. Pathol Oncol Res. 2005;11:186–87. doi: 10.1007/BF02893400. [DOI] [PubMed] [Google Scholar]
- 10.Brufsky A, Harker WG, Beck JT, et al. Zoledronic acid inhibits adjuvant letrozole-induced bone loss in postmenopausal women with early breast cancer. J Clin Oncol. 2007;25:829–36. doi: 10.1200/JCO.2005.05.3744. [DOI] [PubMed] [Google Scholar]
- 11.Gnant M, Mlineritsch B, Luschin-Ebengreuth G, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 5-year follow-up of the ABCSG-12 bone-mineral density substudy. Lancet Oncol. 2008;9:840–49. doi: 10.1016/S1470-2045(08)70204-3. [DOI] [PubMed] [Google Scholar]
- 12.Guise TA. Antitumour effects of bisphosphonates: promising preclinical evidence. Cancer Treatment Reviews. 2008;34(suppl 1):19. doi: 10.1016/j.ctrv.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Gnant M, Mlineritsch B, Schippinger W, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679–91. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- 14.Coleman R, Bundred N, De Boer R, Llombarto A, Campbell ID, Neven P. Impact of zoledronic acid in post-menopausal women with early breast cancer receiving adjuvant letrozole: Z-FAST, ZO-FAST, and E-ZO-FAST. Cancer Research. 2009;70(suppl):4082. [Google Scholar]
- 15.Kohno N, Aogi K, Minami H, et al. Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trial. J Clin Oncol. 2005;23:3314–21. doi: 10.1200/JCO.2005.05.116. [DOI] [PubMed] [Google Scholar]
- 16.Fehm T, Braun S, Muller V, et al. A concept for the standardized detection of disseminated tumour cells in bone marrow from patients with primary breast cancer and its clinical implementation. Cancer. 2006;107:885–92. doi: 10.1002/cncr.22076. [DOI] [PubMed] [Google Scholar]
- 17.Rack B, Schindlbeck C, Strobl B, Sommer H, Friese K, Janni W. Efficacy of zoledronate in treating persisting isolated tumour cells in bone marrow in patients with breast cancer. Dtsch Med Wochenschr. 2008;133:285–89. doi: 10.1055/s-2008-1046707. [DOI] [PubMed] [Google Scholar]
- 18.Lin A, Park J, Melisko M, et al. Zoledronic acid as adjuvant therapy for women with early stage breast cancer and occult tumour cells in bone marrow. Cancer Res. 2007;68 abstr 510. [Google Scholar]
- 19.Brugger W, Bross KJ, Glatt M, Weber F, Mertelsmann R, Kanz L. Mobilization of tumour cells and hematopoietic progenitor cells into peripheral blood of patients with solid tumours. Blood. 1994;83:636–40. [PubMed] [Google Scholar]
- 20.Shpall EJ, Jones RB. Release of tumour cells from bone marrow. Blood. 1994;83:623–25. [PubMed] [Google Scholar]
- 21.Pantel K, Schlimok G, Angstwurm M, et al. Methodological analysis of immunocytochemical screening for disseminated epithelial tumour cells in bone marrow. J Hematother. 1994;3:165–73. doi: 10.1089/scd.1.1994.3.165. [DOI] [PubMed] [Google Scholar]
- 22.Winter MC, Thorpe HC, Burkinshaw R, Beevers SJ, Coleman R. The addition of zoledronic acid to neoadjuvant chemotherapy may influence pathological response exploratory evidence for direct anti-tumour activity in breast cancer. Cancer Res. 2009;69 doi: 10.1038/sj.bjc.6605604. abstr 5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saarto T, Vehmanen L, Virkkunen P, Blomqvist C. Ten-year follow-up of a randomized controlled trial of adjuvant clodronate treatment in node-positive breast cancer patients. Acta Oncol. 2004;43:650–56. doi: 10.1080/02841860410032885. [DOI] [PubMed] [Google Scholar]
- 24.Powles T, Paterson A, McCloskey E, et al. Reduction in bone relapse and improved survival with oral clodronate for adjuvant treatment of operable breast cancer. Breast Cancer Res. 2006;8:13. doi: 10.1186/bcr1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diel IJ, Solomayer EF, Costa SD, et al. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N Engl J Med. 1998;339:357–63. doi: 10.1056/NEJM199808063390601. [DOI] [PubMed] [Google Scholar]
- 26.Santini D, Vincenzi B, Dicuonzo G, et al. Zoledronic acid induces significant and long-lasting modifications of circulating angiogenic factors in cancer patients. Clin Cancer Res. 2003;9:2893–97. [PubMed] [Google Scholar]
- 27.Jagdev SP, Coleman RE, Shipman CM, Rostami HA, Croucher PI. The bisphosphonate, zoledronic acid, induces apoptosis of breast cancer cells: evidence for synergy with paclitaxel. Br J Cancer. 2001;84:1126–34. doi: 10.1054/bjoc.2001.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neville-Webbe HL, Evans CA, Coleman RE, Holen I. Mechanisms of the synergistic interaction between the bisphosphonate zoledronic acid and the chemotherapy agent paclitaxel in breast cancer cells in vitro. Tumour Biol. 2006;27:92–103. doi: 10.1159/000092489. [DOI] [PubMed] [Google Scholar]
- 29.Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96:384–92. [PubMed] [Google Scholar]
- 30.Ottewell PD, Monkkonen H, Jones M, Lefley DV, Coleman RE, Holen I. Antitumour effects of doxorubicin followed by zoledronic acid in a mouse model of breast cancer. J Natl Cancer Inst. 2008;100:1167–78. doi: 10.1093/jnci/djn240. [DOI] [PubMed] [Google Scholar]
- 31.Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol. 2005;23:619–29. doi: 10.1200/JCO.2005.09.121. [DOI] [PubMed] [Google Scholar]
- 32.Greep NC, Giuliano AE, Hansen NM, Taketani T, Wang H-J, Singer FR. The effects of adjuvant chemotherapy on bone density in postmenopausal women with early breast cancer. Am J Med. 2003;114:653. doi: 10.1016/s0002-9343(03)00127-x. [DOI] [PubMed] [Google Scholar]
- 33.Hershman DL, McMahon DJ, Crew KD, et al. Zoledronic acid prevents bone loss in premenopausal women undergoing adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol. 2008;26:4739–45. doi: 10.1200/JCO.2008.16.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuleihan Gel H, Salamoun M, Mourad YA, et al. Pamidronate in the prevention of chemotherapy-induced bone loss in premenopausal women with breast cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2005;90:3209–14. doi: 10.1210/jc.2004-1444. [DOI] [PubMed] [Google Scholar]
- 35.Greenspan SL, Brufsky A, Lembersky BC, et al. Risedronate prevents bone loss in breast cancer survivors: a 2-year, randomized, double-blind, placebo-controlled clinical trial. J Clin Oncol. 2008;26:2644–52. doi: 10.1200/JCO.2007.15.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lipton A, Cook R, Saad F, et al. Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumours and elevated bone resorption receiving zoledronic acid. Cancer. 2008;113:193–201. doi: 10.1002/cncr.23529. [DOI] [PubMed] [Google Scholar]
- 37.Brufsky A, Bundred N, Coleman R, et al. Integrated analysis of zoledronic acid for prevention of aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole. Oncologist. 2008;13:503–14. doi: 10.1634/theoncologist.2007-0206. [DOI] [PubMed] [Google Scholar]
- 38.Hoff AO, Toth BB, Altundag K, et al. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res. 2008;23:826–36. doi: 10.1359/JBMR.080205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gnant MFX, Mlineritsch B, Luschin-Ebengreuth G, et al. Zoledronic acid prevents cancer treatment-induced bone loss in premenopausal women receiving adjuvant endocrine therapy for hormone-responsive breast cancer: a report from the Austrian Breast and Colorectal Cancer Study Group. J Clin Oncol. 2007;25:820–28. doi: 10.1200/JCO.2005.02.7102. [DOI] [PubMed] [Google Scholar]
- 40.Bundred N, Campbell I, Davidson N, et al. Effective inhibition of aromatase inhibitor-associated bone loss by zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole. Cancer. 2008;112:1001–10. doi: 10.1002/cncr.23259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


