Abstract
Human cytomegalovirus (CMV) reactivation and infection can lead to poor outcomes after allogeneic stem cell transplantation. We hypothesized that anti-CD3 activated T cells (ATCs) armed with chemically heteroconjugated anti-CD3 × polyclonal anti-CMV bispecific antibody (CMVBi) will target and eliminate CMV-infected cells. Arming doses of CMVBi as low as 0.01 ng/106 ATCs was able to mediate specific cytotoxicity (SC) directed at CMV-infected target cells significant above unarmed ATCs at mutiplicities of infection (MOI) between 0.01 and 1. At effector-to-target ratios (E:T) of 25:1, 12.5:1, 6.25:1, and 3.125:1, armed ATCs significantly enhanced killing of CMV-infected targets compared with unarmed ATCs. At an MOI of 1.0, the mean % SC directed at CMV-infected targets cells for CMVBi-armed ATCs at E:T of 3.12, 6.25, and 12.5 were 79%, 81%, and 82%, respectively; whereas the mean % SC for unarmed ATCs at the same E:T were all <20%. ATCs, Cytogam®, or CMVBi alone did not lyse uninfected or CMV-infected targets. Co-cultures of CMVBi-armed ATCs with CMV-infected targets induced cytokine and chemokine release from armed ATCs. This nonmajor histocompatibility complex restricted strategy for targeting CMV could be used to prevent or treat CMV infections after allogeneic stem cell transplantation or organ transplantation.
Keywords: CMV, Activated T cells, Bispecific antibodies, Peripheral blood mononuclear cells, Immunotherapy, Cytogam®
INTRODUCTION
Despite the use of appropriate antiviral agents, cytomegalovirus (CMV) reactivation or infection limits the success of allogenenic stem cell transplantation (alloSCT) or organ transplantation. Reactivation of CMV augments acute graft-versus-host disease (GVHD) and is associated with organ rejection. Treatment of GVHD with immunosuppressive agents enhances the vicious cycle of further CMV reactivation. Most of the well-established strategies are not completely effective in preventing or treating CMV infections after transplantation, and vaccine strategies against CMV infection have failed in immunocompromised hosts and after alloSCT [1,2].
Post-alloSCT infusions of cloned donor CMV-specific cytotoxic T lymphocytes (CTLs) have been successful and encouraging [3,3–9] as have been Epstein-Barr virus-specific CTLs for the treatment of posttransplantation lymphoproliferative disease [10–13]. But the logistics, cost, and labor intensity involved in generating histocompatible virus-specific CTLs remain challenging.
Numerous approaches have been tested or are in development for amplification of CMV-specific protective CTLs, nearly all of which depend on histocompatibility. The requirement for histocompatibility eliminates the possibility for using CTLs from universal donors and leaves the daunting task of generating either donor/recipient-specific CTLs or generating sufficient numbers of amplified CTL populations to provide coverage for a wide range of recipient HLA profiles. Another difference is that HLA-matched CTL approaches are based on the relatively restricted array of CMV T cell epitopes in a given individual.
This study addresses the aforementioned challenge and presents a novel approch to the clinical problem through the use of a combination of existing immunologic reagents. If successful, it would be a relatively simple and high-impact approach to the treatment of life-threatening CMV infections post-alloSCT. The strategy takes advantage of the non-major histocompatibility complex-restricted, perforin/granzyme-mediated cytotoxic properties of anti-CD3 activated T cells (ATCs) by redirecting their cytotoxicity to CMV-infected targets by arming ATCs with chemically heteroconjugated anti-CD3 × anti-CMV bispecific antibodies (CMVBi). We used polyclonal Cytogam®, which has the diverse array of HLA independent anti-CMV antibody epitopes. This method was adapted from the use of bispecific antibodies (BiAbs) to retarget ATCs to tumors. Our earlier studies showed that ATCs armed with anti-CD3 × anti-Her2/neu [14], anti-CD3 × anti-CD20 [15], or anti-CD3 × anti-EGFR [16] BiAbs have high levels of specific cytotoxicity directed at cancers of the breast [14], prostate [17,18], colon, lung, head [16], and ovaries [19]. The BiAb retargeting approach enables essentially every ATC in a population to specifically target and kill cells bearing the antigen(s) of interest. The activating end of the BiAb is a monoclonal antibody against CD3, which binds to and activates CTLs; the arming end of the BiAb is an antibody that can bind specifically to a tumor-associated antigen or antigen(s) from bacterial or viral pathogens. In this work, we take advantage of the diverse array of HLA-independent anti-CMV antibody epitopes available in the Cytogam® IgG pool. CMVBi-armed ATCs could be produced in 2 weeks from donors of bone marrow transplantation recipients to prevent or treat CMV disease using a similar approach.
Obviously, GVHD is a life-threatening concern whenever donor-derived T cells are used in adoptive transfer approaches. Bulk or polyclonally anti-CD3/anti-CD28 coactivated donor T cells have been used in combination with donor lymphocyte infusions in patients without exacerbation of GVHD in patients who had relapsed with hematologic malignancy [20], and others have shown polarized CD4+ type 2 cells did not cause life-threatening GVHD [21]. We present data in this study that suggests that activated T cells may actually suppress allogeneic immune responses. This study asked the following questions: (1) Can polyclonal human immune globulin enriched in antibodies against CMV (Cytogam®) be used to produce chemically heteroconjugated anti-CD3 × anti-CMV bispecific antibodies that specifically target and kill fibroblasts infected with CMV? (2) What is the optimal arming dose of CMVBi for inducing specific cytotoxicity and optimal time to harvest ATCs for optimal CMV-specific cytotoxicity? (3) Will CMVBi-armed ATCs secrete Th1 cytokines and chemokines upon engagement with CMV-infected target cells? (4) Will CMVBi-armed ATCs cause or augment GVHD in the alloSCT setting? The results of this preclinical study show that healthy related or unrelated donor anti-CD3 activated T cells can be armed with very low doses of CMVBi to specifically kill CMV-infected cells. CMVBi-armed ATCs secrete cytokines and chemokines upon engaging infected targets and exhibit low levels of alloreactivity. Our results support the feasibility of clinical trials of CMVBi-armed ATCs to prevent or treat CMV in hematopoietic stem cell or solid-organ transplantations.
MATERIALS AND METHODS
Blood and Perpheral Blood Mononuclear Cell Separation
Perpheral Blood Mononuclear Cells (PBMCs) were isolated from heparinized whole blood of normal healthy donors by Ficoll-hypaque density gradient centrifugation and resuspended in RPMI-1640 (Lonza, Inc., Allendale, NJ) supplemented with 10% FCS (Lonza), L-glutamine (Lonza), and penicillin-streptomycin (Lonza). The Wayne State University institutional review board approved research protocols for blood collection. All blood donors signed consent forms.
Cells and Virus
A human lung fibroblast cell line (MRC-5) was maintained in RPMI-1640 culture medium supplemented with 10% fetal bovine serum, 2 mM L-glutamine, and antibiotics. These cells were infected with a green fluorescent protein-expressing version of CMV strain (AD169) at various multiplicities of infection (MOI) ranging from 0.01 to 1.
Expansion and Generation of ATC
PBMCs were expanded using 20 ng/mL of OKT3 and 100 IU of IL-2 for 14 days at a concentration of 1 × 106 PBMC/mL in RPMI-1640 supplemented with 10% fetal bovine serum. Cells were maintained at 1 × 106 cells/mL, and 100 IU/mL IL-2 was added every 2 to 3 days throughout the initial culture period. ATCs were either used fresh or cryopreserved for later use.
Production of Anti-OKT3 × Anti-CMVBi Antibodies
BiAb were produced by chemical heteroconjugation of OKT3 (a murine IgG2a anti-CD3 monoclonal antibody; Ortho Biotech, Horsham, PA) and Cytogam® (CSL Behring, King of Prussia, PA), as described previously using Trauts reagent to crosslink OKT3 and Sulfo-SMCC to cross-link Cytogam [15]. Cytogam® is a pooled CMV-specific human IgG already approved for routine clinical use by the FDA. In the initial experiments, ATCs were armed using an arbitrarily selected concentration of BiAb (50 ng/106 ATCs) for 30 minutes with either CMVBi or irrelevant anti-CD3 × anti-CD33 bispecific antibody (CD33Bi) to verify that CMVBi-armed ATCs would react specifically with CMV-infected fibroblasts. Following arming, armed ATCs were washed thrice to eliminate any unbound BiAb before use in these experiments.
Immunofluorescence Microscopy
CMV-infected human lung fibroblasts fixed on slides (MBL-BION, Des Plaines, IL) were permeabilized in phosphate-buffered saline (PBS) containing 0.2% Triton X-100 and 10% normal goat serum for 15 min followed by incubation for 1 hour in blocking buffer (10% normal goat serum and 5% glycine in PBS). Permeabilized and blocked CMV-infected cells were incubated for 1 hour at room temperature with primary antibodies (CMVBi, anti-CMV immediate early protein 2 [anti-IE-2], Cytogam®, anti-CD3 × anti-CD20 [CD20Bi]) diluted in blocking buffer. Cells were then washed 3 times with PBS and allowed to react for 1 hour with similarly prepared corresponding secondary antibody (goat antihuman IgG-PE was used to detect Cytogam® or Cytogam® arm of the CMVBi, goat antimouse IgG-FITC was used to detect anti-IE2). At the end of the incubation, cells were washed and mounted using Vectashield with 40′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA). Images were captured with a Leica TCS-SP digital confocal microscope.
Cytotoxicity Assay
Cytotoxic activity of ATCs or CMVBi-armed ATCs to CMV-infected fibroblasts (targets) was evaluated to assess the specific and nonspecific target killing by chromium (51Cr) release assay in 96-well flat-bottomed microtiter plates as previously described [14]. Briefly, ATCs or ATCs armed with CMVBi were plated in triplicate onto 51CR-loaded target cells (4 × 104 cells/well) at E:T ratio of 25:1, 12.5:1, 6.25:1, and 3.12:1.51Cr release was measured after 18 hours, and percent cytotoxicity was calculated using the following formula: (experimental cpm − spontaneous cpm)/(maximum cpm − spontaneous cpm) × 100%.
Mixed Lymphocyte Cultures
PBMC from the responder and unarmed ATCs or armed ATCs derived from the responder were stimulated with irradiated autologous PBMC or the irradiated allogeneic PBMC. All cocultures contained 100,000 responders and 100,000 irradiated stimulators. Cells were irradiated with 2,500 rads using a Cesium− source. Proliferative responses were measured by CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison, WI). The assay determines the number of viable cells in cultures based on quantitation of the ATPs present, which signal the presence of metabolically active cells. The number of viable cells serves as a surrogate marker for proliferation in the mixed lymphocyte cultures. In order to compare experiments, the various cocultures were normalized to the allogeneic control culture: PBMC responder (Pr) × allogeneic PBMC irradiated stimulator (P*s) = 100%.
Cytokine Profiling of Cocultures
Cytokines were quantitated in culture supernatants collected from CMV-infected or uninfected MRC-5 cells with ATCs or CMVBi-armed ATCs cocultures using a 25-plex human cytokine Luminex Array (Invitrogen, Carlsbad, CA) on a Bio-Plex system (Bio-Rad Lab, Hercules, CA). The limit of detection for these assays is <10 pg/mL based on detectable signal of >two fold above background (Bio-Rad). Cytokine concentrations were automatically calculated by the BioPlex Manager Software (Bio-Rad).
Statistical Methods
Descriptive statistics (means, ranges, and SD) were used to analyze most data sets. Comparison of paired data between groups was performed using nonparametric Wilcoxon rank-sum test. All P values are two sided, with P < .05 considered statistically significant. All of the analyses were performed using SigmaStat Version 3.5 (2006 Systat Software, Chicago, IL).
RESULTS
Characterization of the ATCs
ATCs that were grown for 14 days expanded a mean of 10 ± five fold (n=10) with mean proportions of CD3+ = 95%,CD4+ = 20%±10,CD8+ = 60%±10, CD45RA/CD45RO+ = 70 ± 10, CD25+/CD4+/CD127− = 2.5 ± 2, CD25+/CD8+ = 2 ± 1.5.
Production of CMVBi
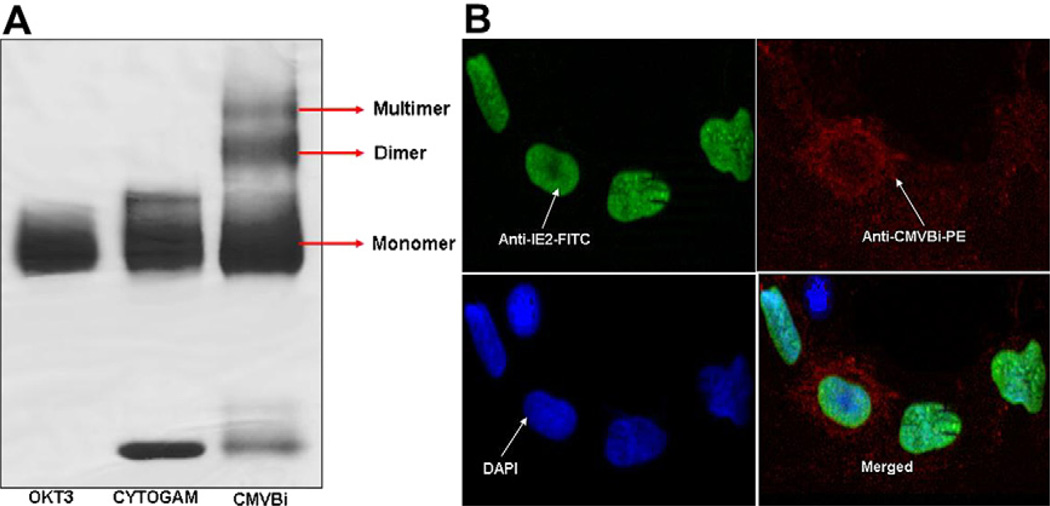
CMVBi was produced by chemical heteroconjugating OKT3 and Cytogam® as previously described [14], and the proportion of monomers, dimers, and multimers was characterized in a nonreducing PAGE gel (Figure 1A). CMVBi (lane 3) shows distinct bands of monomeric unconjugated OKT3 and Cytogam®, plus dimers and multimers of CMVBi. The product contains approximately 30% dimer, 52% unconjugated monomers, and 18% multimers. These results are comparable to the proportions seen in the conjugation of other bispecific antibodies.
Figure 1.
(A) Production of CMVBi.OKT3 was cross-linked with Traut’s reagent, and Cytogam® was cross-linked with SMCC before mixing followed by overnight heterconjugation. The reactants were resolved by nonreducing PAGE gel and stained with Coomassie blue. All lanes were loaded with 8 µg of protein. Lane 1: OKT3; lane 2: Cytogam; lane 3: CMVBi. (B) Staining of CMV-infected cells.The series of panels show dual staining for infected fibroblasts by staining for IE-2 (FITC, green) expressed in the nucleus, cytoplasm, and cell surface viral proteins by staining with CMVBi (PE, red). Nuclei of infected and noninfected cells were counterstained with blue DAPI (blue, lower left panel). The rightlower panel shows merged images for anti-IE2 and anti-CMVBi staining at 20 × magnification.
CMVBi Binds to Cell Surface, Cytoplasmic, and Nuclear Viral Antigens
We determined whether CMVBi retained the ability to bind CMV viral antigens on infected target cells (Figure 1B). CMV-infected cells stained with anti-CMV IE-2 (FITC-labeled secondary antibody) and CMVBi (PE-labeled secondary antibody). The cells were then counterstained with the nuclear stain DAPI for all infected and noninfected cells. Fluorescent microscopy revealed strong anti-IE2 nuclear staining in most cells, demonstrating CMV infection. Staining with CMVBi or Cytogam showed surface and cytoplasmic staining of CMV-infected cells with little background staining in uninfected cells by either CMVBi or Cytogam®. Low-level CMVBi or Cytogam® staining in some IE2-positive cells suggests that the antibodies in CMVBi and Cytogam® target genes expressed late in the CMV replication cycle.
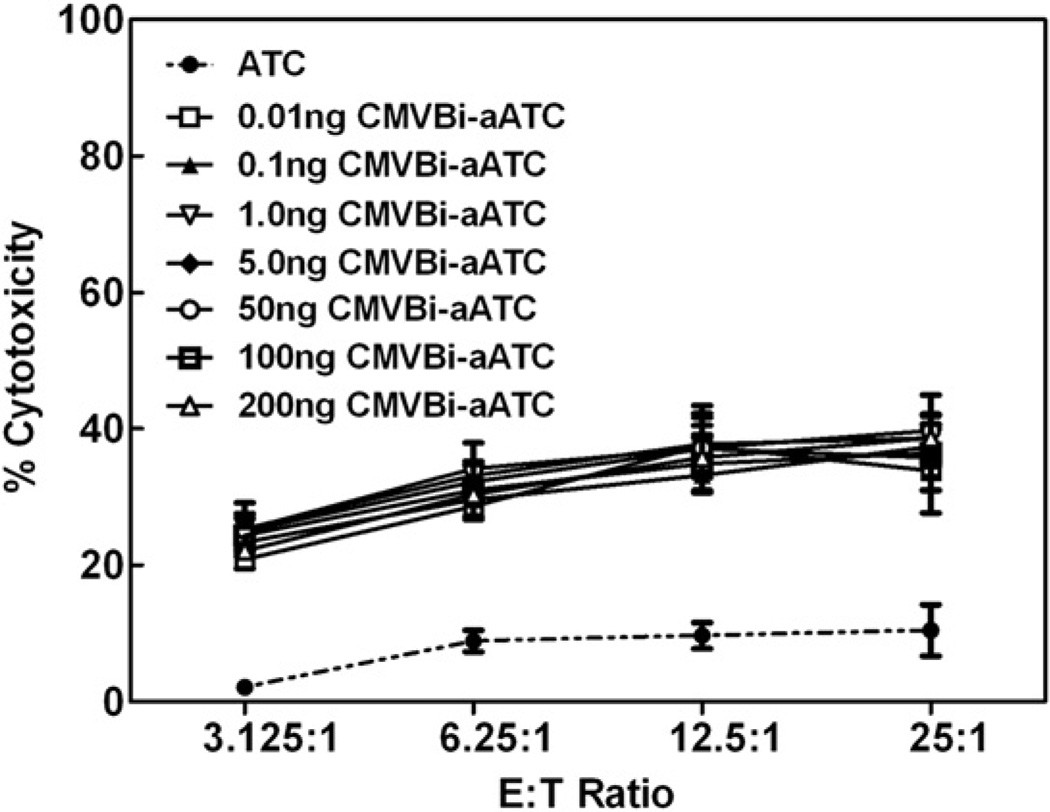
CMVBi Arming Dose Titration
To determine the optimal arming dose, ATCs were armed with increasing doses of CMVBi ranging from 0.01 ng to 200 ng of CMVBi/106 ATCs. The dose titration curve showed arming doses as low as 0.01 ng of CMVBi/106 ATCs could mediate ~33% specific cytotoxicity, while maximum cytotoxicity (39%) at the same E:T of 12.5:1 was observed at 50 ng of CMVBi/106 ATCs against CMV-infected MRC-5 at an MOI of 0.1 (Figure 2). Based on the titration dose response curve shown in Figure 2 (representative of 3 separate individuals), a dose of 50 ng/106 ATCs was chosen for all subsequent experiments. Fresh and cryopreserved ATCs exhibited comparable specific cytotoxicity when armed before or after cyro-preservation. Different E:T ratios, ranging from 3.125:1 to 25:1, were tested to delineate the specific cytotoxicity curve as a function of the E:T ratio. Because approximately 40% of the heterconjugate was active in binding with a 20% arming efficiency (based on the gel scans in Figure 1A), ATCs armed with 0.01 ng of CMVBi per 106 ATCs would bear ~2 × 106 IgG molecules on the surface of each cell. Furthermore, assuming 10 or 1% of the IgG were CMV specific, 200 or 20 × 103 CMV-specific IgG molecules would theoretically be bound to the surface of a single T cell. As a negative control, we tested ATCs armed with anti-CD3 × anti-CD33 BiAb for cytotoxicity directed at CMV-infected or noninfected MRC-5. The level of cytotoxicity is seen at levels equivalent to unarmed ATCs alone (data not shown).
Figure 2.
Cytotoxicity directed at CMV-infected MRC-5 fibroblasts as a function of the CMVBi arming dose. Cytotoxicity mediated by ATCs armed with increasing doses of CMVBi directed at MRC-5 human lung fibroblasts infected with CMV strain AD169. ATCs were armed with 0.01, 0.1, 1, 5, 50, 100, and 200 ng per 106 ATCs and tested for specific cytotoxicity against CMV-infected cells with an MOI of 0.1 after 96 hours of infection. Unarmed ATCs from the same normal donor are shown. The data represent the mean cytotoxicity ± standard error of the mean (SEM) of triplicate determinations.
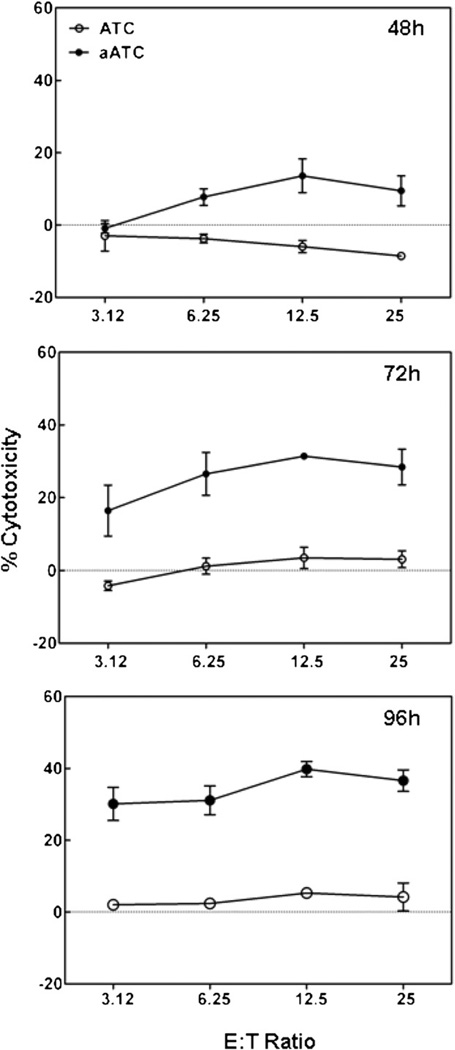
Kinetics of CMV Infection for Optimal Cytotoxicity
The development of cytotoxicity was measured at 48, 72, and 96 hours after CMV infection at a MOI of 0.1. Cytotoxicity mediated by CMVBi-armed ATCs as a function of CMV infection (Figure 3). Peak cytotoxicity mediated by CMVBi-armed ATCs from 3 donors occurred at 96 hours after CMV infection in Figure 3 (bottom panel). This suggests that viral late genes are important targets for the cytotoxic reaction and provide further evidence of the specificity of the reaction. Unarmed ATCs did not lyse CMV-infected fibroblasts.
Figure 3.
Cytotoxicity as a function of time of CMV infection. CMVBi-armed ATCs and unarmed ATCs from one donor were tested against MRC-5 targets at E:T from 3.12 to 25:1 at 48, 72, and 96 hours after infection at an MOI of 0.1. The data are presented as mean ± SEM of triplicate determinations.
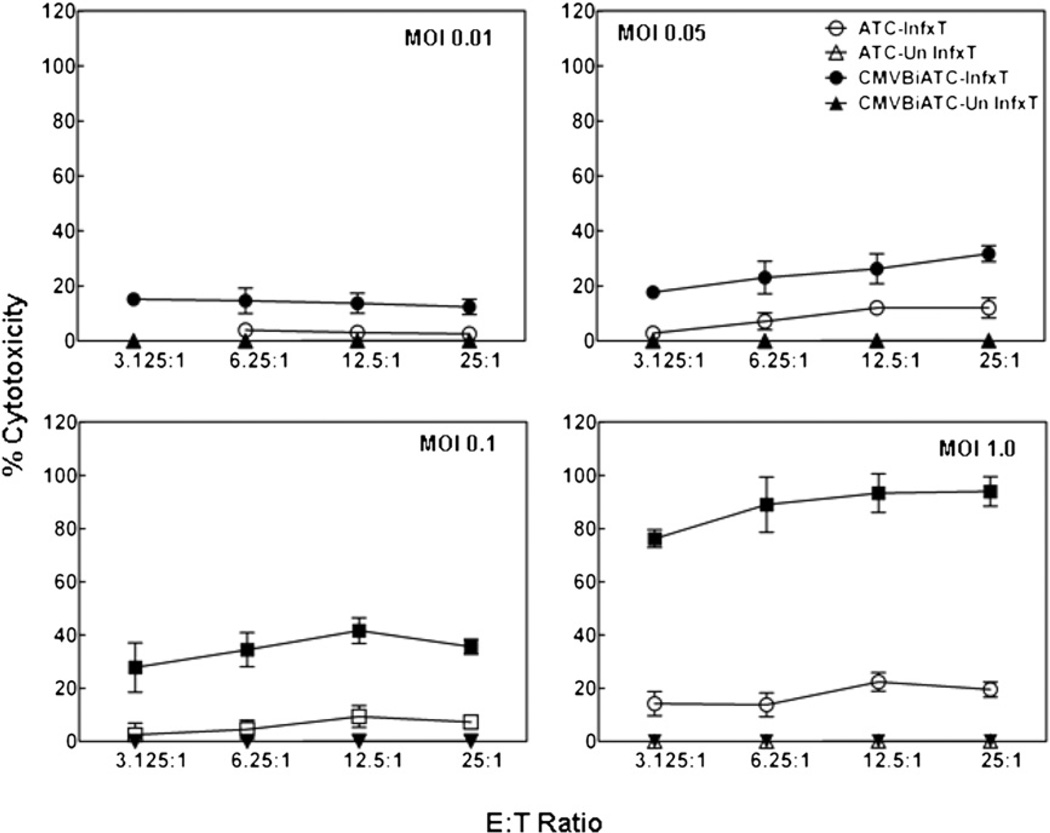
Cytotoxicity of CMV-Infected Targets Increases with Increase in MOI
MRC-5 cells were infected with the AD169 strain of CMV at MOI ranging from 0.01 to 1.0 to determine the interaction between MOI and the E:T ratio for cytotoxicity mediated by the CMVBi-armed ATCs. Uninfected MRC-5 and MRC-5 infected with AD169 strain of CMV at MOI ranging from 0.01 to 1 were plated in 96-well plates at a concentration of 40,000 cells/well for 96 hours postinfection. Normal donor ATCs unarmed and armed with CMVBi at 50 ng/106 ATCs were added to 96-well plates to MRC-5 targets that were labeled with 51Cr for 4 hours, at E:T ranging from 3.125 to 25:1. As shown in Figure 4, as the MOI increased, so did the percentage of specific cytotoxicity mediated by armed ATCs, whereas cytotoxicity mediated by unarmed ATCs did not increase. This experiment shows that killing of CMV-infected fibroblasts also increases as a function of the increased number of CMV-infected targets in the pool. Neither unarmed nor CMVBi-armed ATCs were able to kill uninfected fibroblasts at any of the MOIs. It is important to note that most published studies of CTL killing of CMV-infected cells used a much higher MOI of 5 [7]. These results suggest that CMVBi-armed ATCs are remarkably effective at specifically targeting the scattered infected cells in cultures at low MOI. Based on the data in Figure 3, we used CMV targets that were infected for 96 hours at an MOI of 0.1 for subsequent experiments unless otherwise specified.
Figure 4.
CMVBi-armed ATCs targeting of CMV-infected and uninfected fibroblast as a function MOI. MRC-5 human lung fibroblasts were plated after infected for 96 hours (I) at the MOIs of 0.01, 0.05, 0.1 and 1.0 or left uninfected as controls (UnI) as indicated in each panel. ATC-I (ATCs + infected targets), armed ATC-I (CMVBi-armed ATCs + infected targets); ATC-UnI (ATCs + unInfected targets); and armed ATC-UnI (CMVBi-armed ATCs + uninfected targets).
Cytotoxicity Mediated by CMVBi-armed ATCs from High to Low E:T
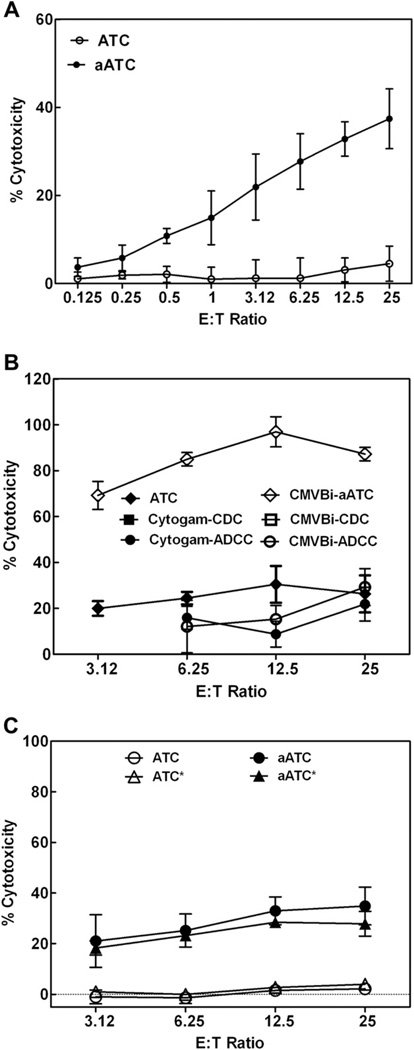
In this set of experiments, we asked whether CMVBi-armed ATCs can mediate the cytotoxicity at low E:T. CMVBi-armed ATCc and ATCc from 3 normal donors were tested at an MOI of 0.1 at 96 hours of CMV exposure at a range of E:T from 25:1 down to 0.125:1 (1:8) (Figure 5A). The mean cytotoxicity exhibited by armed ATCs was consistently higher than ATCs at all E:T ratios. More intriguingly, E:T as low as 1:1 showed 8% to 20% killing of specific targets. These data clearly show that CMVBi-armed ATCs are highly effective cytotoxic effector cells that are capable of lysing multiple CMV-infected targets at a low E:T ratio.
Figure 5.
(A) Specific cytotoxicity directed at CMV-infected fibroblasts at various E:T ratios. CMVBi-armed ATCs (aATC) and unarmed ATCs (ATC) from 4 donors were tested for cytotoxicity-directed MRC-5 targets at an MOI of 0.1 at E:T from 25:1 to 0.125:1 in 51Cr release cytotoxicity. The data are presented as the mean ± SEM of 4 donors. (B) Comparison of cytotoxicity seen in ADCC, CDC, and CMVBi with ATCs and armed ATCs. PBMC or ATCs from 2 normal subjects were used for this experiment. Antibody-dependent cellular cytotoxicity was tested using CMVBi at 50 ng/mL (CMVBi-ADCC) and Cytogam® 10 µg/mL (Cytogam®-ADCC) in the presence of fresh PBMC at the indicated E:T, ATCs armed with 50 ng/106 ATCs (CMVBi-armed ATCs) or unarmed ATCs (ATC) were tested for cytotoxicity at the indicated E:T Complement-dependent cytotoxicity (CDC) was tested using fresh human complement (1:10 dilutions of fresh human serum) either in the presence of CMVBi at 50 ng/mL or Cytogam® 10 µg/mL on MRC-5 targets. The experiments were conducted at an MOI of 1. (C) The effects of irradiation on cytotoxicity mediated by CMVBi-armed ATCs. Unarmed ATCs (ATC), CMVBi-armed ATCs (aATC) from 2 donors were tested against irradiated ATCs (ATC*) and irradiated CMVBi-armed ATCs (aATC* for specific cytotoxicity directed at CMV-infected fibroblasts) (0.1 MOI after 96 hours of infection) at E:T from 3.12 to 25:1. The data are presented as mean ± SEM of triplicate determinations.
CMVBi-Armed ATCs Cytotoxicity Is Not Because of Antibody-Dependent Cell-Mediated Cytotoxicity or Complement-Mediated Cytotoxicity
In order to determine if Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC) or Complement-Mediated Cytotoxicity (CDC) of Cytogam® or CMVBi would mediate comparable cytotoxicty to CMVBi-armed ATCs. CMV-infected MRC-5 cells were cocultured with Cytogam® or CMVBi alone in the presence of PBMC or human complement (Figure 5B). Fresh PBMC or fresh human complement were added to test ADCC or CDC, respectively, against CMV-infected targets. Cytogam® and CMVBi alone in the presence of 10% human serum (source of complement) did not kill CMV-infected targets (<1% cytotoxicity), whereas CMVBi or Cytogram® in the presence of PBMC exhibited 0% to 24% and 1.2 to 35% ADCC, respectively. These data were compared with armed and unarmed ATCs from 2 normal subjects at E:T from 25 to 3.125:1 (Figure 5B). CMVBi-armed ATCs exhibited high levels of specific cytotoxicity ranging from 70% to 100% at E:T from 25:1 to 3.125:1 at an MOI of 1.0.
Radioresistant Cytotoxicity Mediated by CMVBi-Armed ATCs
Because CMVBi-armed ATCs may respond to alloantigens, CMVBi-armed ATCs were tested to determine whether they would exhibit CMV-specific cytotoxicity after 2,500 rad of irradiation. Specific cytotoxicity mediated by irradiated (*) CMVBi-armed ATCs was comparable to the cytotoxicity directed at CMV-infected fibroblasts by unirradiated CMVBi-armed ATCs from 2 separate individuals. Irradiated and unirradiated unarmed ATCs did not exhibit cytotoxicity (Figure 5C).
Interferon-gamma, Macrophase Inflammatory Protein 1-alpha, and MIP-1β Secreted by CMVBi-Armed ATCs
Because in vitro targeting of breast cancer with anti-CD3 × anti-Her2/neu BiAb or lymphoma with anti-CD3 × anti-CD20 BiAb triggers a Th1 pattern of chemokine secretion from ATCs [14,15], we tested whether armed ATCs would secrete cytokines upon engaging CMV-infected targets. CMVBi-armed ATCs from 2 normal individuals were co-cultured with CMV-infected or uninfected targets for 24 hours at a 10:1 E:T and an MOI of 0.1. Cytokine concentration was adjusted to pg/106 armed or unarmed ATCs. Culture supernatants from cocultures of armed ATCs with CMV-infected fibroblasts showed an average of 6-fold more interferon-gamma (IFN-γ), 15-fold more macrophase inflammatory protein 1-alpha (MIP-1α), and 31-fold more MIP-1β than cocultures of ATCs and infected fibroblasts. The mean absolute increments in pg/mL/106 cells were 128, 1,167, and 3,031 pg/mL for IFN-γ, MIP-1α, and MIP-1β, respectively (Table 1). Uninfected fibroblasts cocultured with ATCs had mean background levels of 26 pg/mL for IFN-γ, 293 pg/mL for MIP-1α, and 440 pg/mL for MIP-1β (data not shown).
Table 1.
IFN-γ, MIP-1α, and MIP-1β Secretion (pg/mL/24 hours/106)
| Culture Conditions | IFN-γ | MIP1α | MIP1β |
|---|---|---|---|
| Infected targets + ATC1 | 14 | 133 | 84 |
| Infected targets + ATC2 | 38 | 31 | 116 |
| Mean infected targets + ATC | 26 | 82 | 100 |
| Infected targets + CMVBi-armed ATC1 | 202 | 2250 | 5335 |
| Infected targets + CMVBi-armed ATC2 | 105 | 248 | 926 |
| Mean infected targeted + armed ATC | 154 | 1249 | 3131 |
| Net mean increment in secretion | 128 | 1167 | 3031 |
ATC1 and ATC2 indicate 2 normal subjects; infected targets, CMV-infected fibroblasts, MOI 0.1 at 96 hours after infection; net mean increment, amount produced by CMVBi-armed ATCs above the amount produced by ATC.
Single determinations were performed for each data point. The bioplex system has been consistently reproducible on standards with ± 10 pg.
Depressed Mixed Lymphocyte Culture Responses
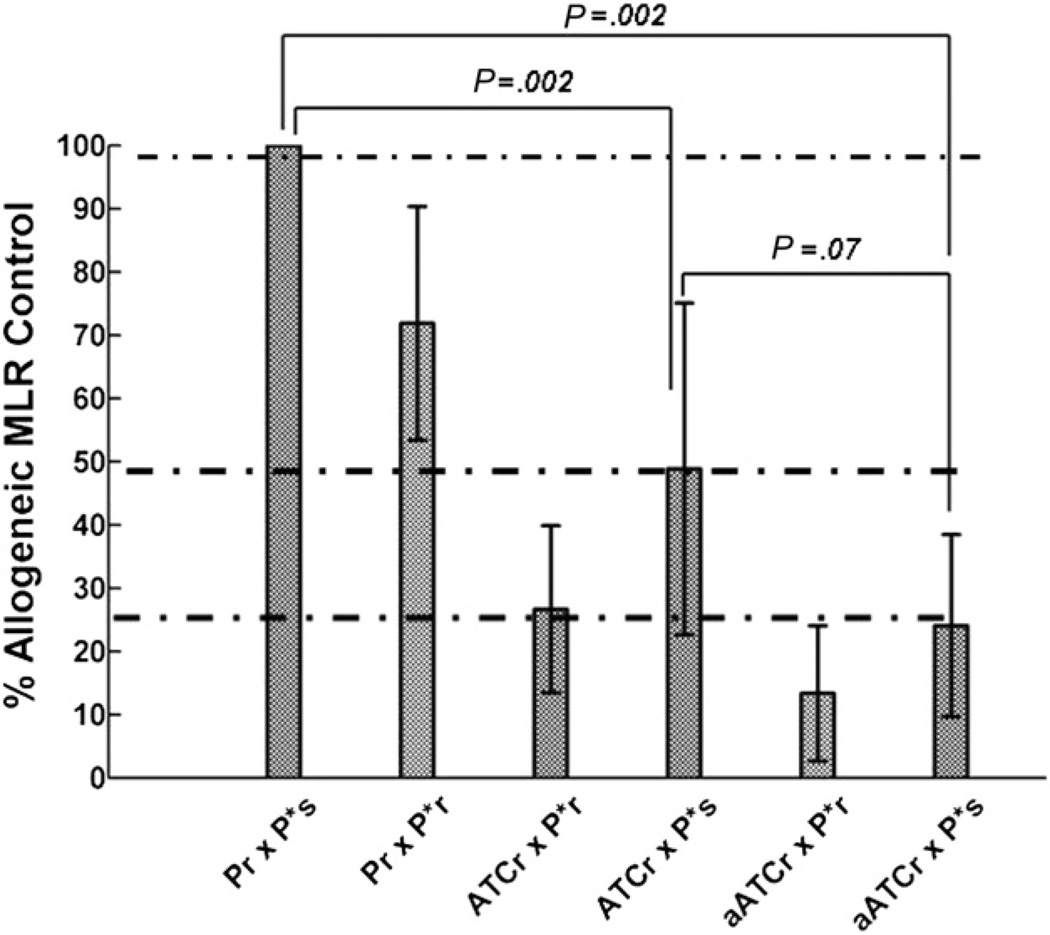
Mixed lymphocyte culture responses (MLRs) of CMVBi-armed ATCs were tested to determine whether random or non–HLA-matched normal donor samples could respond to host alloantigens in patients undergoing organ or alloSCT. Both ATCs and CMVBi-armed ATCs showed depressed allogeneic responses in MLR in 6 unrelated responder and stimulation combinations tested (Figure 6). The allogeneic response to alloantigens by unarmed ATCs was (26.7) 73% less than the allogeneic MLR control (P = .002, n = 6, Mann-Whitney rank-sum test) and the allogeneic response to alloantigens by armed ATCs was (24.1) 75.9% less than the allogeneic MLR control (P = .002, n = 6). There was no difference in the allogeneic responses between unarmed and armed ATCs (P = .07).
Figure 6.
Marked depressed allogeneic responses from ATCs and armed ATCs. Six experiments involving cocultures of unrelated allogeneic responder PBMC (Pr) and irradiated PBMC stimulator cells (P*s) in the allogeneic MLR control. Pr and autologous irradiated PBMC (P*r) from the same individual cocultured in an autologous MLR; ATCs from the responder (ATCr) cocultured with autologous P*r in an autologous MLR (control); ATCr cocultured with P*s in allogeneic MLR to assess allo-reactivity ofATC; responder armed ATCs (aATCr) cocultured with autologous P*r in an autologous MLR (control); and aATCr cocultured with P*s in allogeneic MLR to assess allo-reactivity of aATC.
DISCUSSION
Adoptive immunotherapy using antigen-specific CTL to treat patients with CMV viral infection needs to be safe, effective, easily reproduced, and transportable. This new approach for manufacturing CMV-specific cytotoxic T lymphocytes incorporates these elements for rapid translation to the clinic. This study shows that polyclonal anti-CMV IgG can be used to produce CMVBi for arming ATCs to specifically target and lyse CMV-infected fibroblasts. CMVBi specifically bound to T cells and CMV antigens and cell membranes of CMV-infected fibroblasts at an optimal dose of 50 ng/106 TC. Binding of the armed ATCs to CMV-infected targets triggered secretion of IFN-γ, MIP1-α, and MIP1-β. Irradiation of armed ATCs to eliminate allogeneic proliferative responses did not impair cytotoxicity, and armed ATCs did not respond to allo-antigens.
Since the initial approach to expand antigen-specific T cells using anti-CD3 and CMV antigen [3,6,22], a number of different strategies have been developed for producting CMV-specific CTL [23–39]. Despite these extensive preclinical experiments, only a few approaches have reached clinical trials [3,40] because of applicability. Our platform of customizable BiAb production from pooled human sera and the proven feasibility of clinical-scale manufacturing of T cells allows us to target the broadly relevant epitopes expressed on the CMV-infected cells.
This study shows that a polyclonal CMVBi can be produced, and very low doses (0.01 ng/106 ATC) can be used to arm ex vivo expanded ATCs that can then specifically target and kill CMV-infected fibroblasts at low E:T and at low and high MOI without nonspecific cytotoxicity. Such a high level of specific cytotoxicity might have been unexpected from a polyclonal bispecific antibody construct because only a fraction of the total Ig in Cytogam® (already enriched for anti-CMV activity in the manufacturing) may be CMV specific. Assuming that only 5% to 10% of poly-clonal Cytogam® isCMV specific, our CMVBi may be at least as potent on a per/ng basis than a BiAb derived from a CMV monoclonal antibody. Unfortunately, there are no clinical grade anti-CMV mAbs that could be used for this approach. In our prior studies using anti-CD3 × anti-Her2 BiAb, we found that the dimer and multimer fractions from a size fractionation column mediated high levels of comparable cytotoxicity directed at breast cancer cell lines, while the monomer fraction lacked specific cytotoxicity. Because the unfractionated material turned out to be so potent, we did not affinity purify the CMV-specific antibodies from Cytogam®.
It is not clear how many CMV antigens or which CMV antigens need to be expressed on the surface of the CMV-infected fibroblasts or other cells lines (eg, endothelial cells) to achieve the threshold for binding with the armed ATCs to trigger target lysis mediated by granzyme B. If CMV targeting is analogous to that seen in Her2Bi ATCs targeting of cancer cells lines (low Her2 expressing MCF-7 breast cancer or PC-3 prostate cancer), then only low-level expression of viral antigens may be required to achieve the targeting and cytotoxicity threshold. Additional studies are underway to assess the ability of CMVBi-armed ATCs to recognize clinical strains of CMV and to kill other types of CMV-infected cells (eg, endothelial cells).
Our studies show that Her2Bi-armed ATCs are capable of high levels of cytotoxicity directed at MCF-7 (breast cancer line) that express nearly undetectable levels of Her2 receptors (less than 5–10 × 10 Her2) [14]. ATCs armed with Her2Bi can target Her2-positive cells, and Her2Bi-armed ATCs can kill multiple target cells, divide and secrete IFN-γ, tumor necrosis factor-alpha, granulocyte macrophage colony-stimulating factor, and chemokines after engaging Her2/neu tumor targets in vitro [41]. Cytokines secreted by armed ATCs (eg, IL-2, granulocyte macrophage colony-stimulating factor, and chemokines such as RANTES, and MIP-1α) at the tumor site may provide an in vivo milieu conducive to induction of an endogenous immune response to tumor antigens. Furthermore, the armed ATCs are able to mediate repeated cycles of cytotoxicity and cytokine/chemokine secretion upon repeated T cell engagement with new tumor cells [41]. Therefore, infusions of autologous CMVBi armed ATCs may not only act as a antiviral agent to kill CMV-infected targets but can also divide, proliferate, secrete cytokines/chemokines, and kill multiple CMV-infected targets.
Arming ATCs with CMVBi may circumvent some of the potential major limitations of infusing CMVBi alone, which could lead to cytokine storm mediated by binding of the BiAb to Fc-R bearing natural killer cells, monocytes, and mast cells [42]. Cytokine storm with dose-limiting toxicities has been observed in nearly all infusions of BiAb alone and keep BiAbs from clinical development. The in vitro cytotoxicity mediated by armed ATCs is clearly superior to CMVBi in complement-mediated cytotoxicity or ADCC. Use of polyclonal CMV-specific IgG (Cytogam®) as a component of CMVBi may enable ATC targeting of multiple CMV antigens that would not be recognized by single mAb. The broad repertoire of naturally occurring IgG anti-CMV antibodies in the Cytogam® pool represents the range of naturally occurring human immune responses against CMV. Thus, CMVBi may enable armed ATCs to target CMV antigens that have not been recognized or for which humanized mAb are not available. Polyclonal targeting may result in marked improvement in the clinical management of CMV infections after alloSCT or organ transplantation. This strategy may also be applicable to treatment of Epstein-Barr virus, herpes simplex virus, and BK virus infections.
The downside of using a polyclonal antiserum from normal donors that include women who have been pregnant is the potential of exacerbating GVHD because of antibodies directed at paternal antigens that are present in any preparation of human polyclonal immunoglobulin preparation (eg, intravenous immunoglobulin or Cytogam®) is a theoretical concern. In clinical trials of intravenous immunoglobulin to prevent infectious complications after alloSCTs or Cytogam® in combination with ganciclovir for the treatment of CMV, increased incidence or severity of GVHD were not observed.
The repertoire of anti-CMV antibodies in Cytogam® preparation will vary, leading to batch-to-batch variation of CMVBi produced for clinical trials. In order to adjust for the specific CMV toxicity exhibited by the armed ATC product, each lot of CMVBi will be titered for specific cytotoxicity directed at specific and nonspecific targets. A major barrier to application of CTL therapies to treatment of CMV infections is that donor-derived CTL may cause life-threathening GVHD. Therefore, strategies to deplete alloreactive cells or control the allogeneic response need to be incorporated into approaches that employ nonautologous products. Irradiated ATCs can mediate specific CMV cytotoxicity but lack of responses to alloantigens, which makes it possible to produce ATCs from healthy HLA matched or mismatched donors to produce ATCs. One approach would involve infusing unrelated matched or partially matched-irradiated armed ATCs to treat CMV infections. This would be a universal “soldiers” armed anti-CMV T cell product that could be produced, cryopreserved in multiple aliquots for multiple infusions, and thawed as needed. These “presuicide” or irradiated universal soldiers would not allow proliferation of the CMVBi armed ATCs to potentiate GVHD.
The second approach would be to use unirradiated ATCs that we have shown to consistently not respond to alloantigens. Because the MLR studies involved randomly selected donor:stimulator pairs, they may have significant HLA disparities. Both armed and unarmed ATCs exhibited mean responses <20% of the allostimulated control cultures indicating a lack of responsivness. We anticipate that the alloreactivity by ATCs from HLA-identical sibling donors should be no more than the low levels seen in our in vitro studies using HLA-disparate cells. A murine study using anti-CD3 activated donor T cells had a reduced ability to cause lethal murine GVHD but retained their ability to facilitate alloengraftment [43]. A possible explanation for the “lack of response” in our systems is that nonspecific polyclonal activation with anti-CD3 in the absence of nominal antigens expanded clones nonspecifically and, therefore, by 14 days of culture, the precursor frequency of allo reactive clones that can develop an alloresponse have been overgrown or suppressed by regulatory cells generated in the ATC culture.
In summary, our strategy of using cryopreserved HLA-identical donor T cells or unrelated donor T cells armed with CMVBi to target CMV-infected cells in a nonmajor histocompatibility complex-restricted manner offers the possibility of a straightforward and inexpensive method for producing “polyclonal” CTL directed at multiple CMV antigens from related or unrelated donor T cells. The strategy eliminates the 2 weeks or more required for generation of infusible histocompatible CMV-specific T cell clones. If armed ATCs do not augment the rate of GVHD in phase I trials, phase II trials could be conducted to determine whether multiple infusions of CMVBi armed could be used as a prophylaxis or to treatment when there is a rise in CMV viral load. The cost of production is estimated at $5,000 per 80 billion CMVBi-armed ATCs, including cost of labor, antibodies, media, plastic ware, bioreactor bags, and quality-control release testing. Based on our experience, armed ATC infusions per se are not likely to have dose-limiting toxicities. Our data strongly support evaluation of CMVBi-armed ATCs in phase I/II clinical trials to treat patients CMV infections in allogeneic SCT or organ transplantation recipients.
ACKNOWLEDGMENTS
Financial disclosure: These studies were supported in part by start-up funds from the Karmanos Cancer Institute (L.G.L.), funding from the Department Oncology, Wayne State University (M.R.), funding from DHHS NCI R01 092344 and NCI R01 140314 (L.G.L), by Wayne State University start-up funds (P.P), and start-up funds from the Department of Oncology. The authors thank Sri Vidya Kondadusula for graphic contributions and proof-reading, and Christina Woodard for her contributions to the development of the virologic approaches. The authors thank Dr. Voravit Ratanatharathorn for his careful review and constructive comments.
Footnotes
AUTHORSHIP STATEMENT
L.G.L. designed research, analyzed, interpreted data, and wrote the article. M.R. designed, performed, and interpreted experiments. AT. designed, performed experiments, analyzed data, and assisted in writing. S.M. performed experiments. A.D. provided clinical input for the design and assisted in writing. J.U. provided clinical input for design and assisted in writing. P.E.P. assisted with design, analysis, and writing.
AUTHOR CONFLICT OF INTEREST STATEMENT
L.G.L. is a founder of Transtarget, Inc. The other authors have no conflicts of interest.
REFERENCES
- 1.Einsele H, Kapp M, Grigoleit GU. CMV-specific T cell therapy. Blood Cells Mol Dis. 2008;40:71–75. doi: 10.1016/j.bcmd.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Hebart H, Einsele H. Clinical aspects of CMV infection after stem cell transplantation. Hum Immunol. 2004;65:432–436. doi: 10.1016/j.humimm.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Walter EA, Greenberg PD, Gilbert MJ, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 4.Feuchtinger T, Opherk K, Bethge WA, et al. Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood. 2010;116:4360–4367. doi: 10.1182/blood-2010-01-262089. [DOI] [PubMed] [Google Scholar]
- 5.Lucas KG, Sun Q, Burton RL, et al. A phase I-II trial to examine the toxicity of CMV- and EBV-specific cytotoxic T lymphocytes when used for prophylaxis against EBV and CMV disease in recipients of CD34-selected/T cell-depleted stem cell transplants. Hum Gene Ther. 2000;11:1453–1463. doi: 10.1089/10430340050057521. [DOI] [PubMed] [Google Scholar]
- 6.Riddell SR, Greenberg PD. The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand human antigen-specific T cells. J Immunol Methods. 1990;128:189–201. doi: 10.1016/0022-1759(90)90210-m. [DOI] [PubMed] [Google Scholar]
- 7.Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha ME, Greenberg PD. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257:238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 8.Reusser P, Riddell SR, Meyers JD, Greenberg PD. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood. 1991;78:1373–1380. [PubMed] [Google Scholar]
- 9.Riddell SR, Greenberg PD. Cellular adoptive immunotherapy after bone marrow transplantation. Cancer Treat Res. 1995;76:337–369. doi: 10.1007/978-1-4615-2013-9_16. [DOI] [PubMed] [Google Scholar]
- 10.Heslop HE, Brenner MK, Rooney C, et al. Administration of neomycin-resistance-gene-marked EBV-specific cytotoxic T lymphocytes to recipients of mismatched-related or phenotypically similar unrelated donor marrow grafts. Hum Gene Ther. 1994;5:381–397. doi: 10.1089/hum.1994.5.3-381. [DOI] [PubMed] [Google Scholar]
- 11.Rooney CM, Smith CA, Ng CY, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–1555. [PubMed] [Google Scholar]
- 12.Savoldo B, Cubbage ML, Durett AG, et al. Generation of EBV-specific CD4+ cytotoxic T cells from virus naive individuals. J Immunol. 2002;168:909–918. doi: 10.4049/jimmunol.168.2.909. [DOI] [PubMed] [Google Scholar]
- 13.Smith CA, Ng CYC, Heslop HE, et al. Production of genetically modified Epstein-Barr virus-specific cytotoxic T cells for adoptive transfer to patients at high risk of EBV-associated lympho-proliferative disease. J Hematother. 1995;4:73–79. doi: 10.1089/scd.1.1995.4.73. [DOI] [PubMed] [Google Scholar]
- 14.Sen M, Wankowski DM, Garlie NK, et al. Use of anti-CD3 × anti-HER2/neu bispecific antibody for redirecting cytotoxicity of activated T cells toward HER2/neu tumors. J Hematother Stem Cell Res. 2001;10:247–260. doi: 10.1089/15258160151134944. [DOI] [PubMed] [Google Scholar]
- 15.Gall JM, Davol PA, Grabert RC, Deaver M, Lum LG. T cells armed with anti-CD3 × anti-CD20 bispecific antibody enhance killing of CD20+ malignant B cells and bypass complement-mediated rituximab resistance in vitro. Exp Hematol. 2005;33:452–459. doi: 10.1016/j.exphem.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Reusch U, Sundaram M, Davol PA, et al. Anti-CD3 × anti-epidermal growth factor receptor (EGFR) bispecific antibody redirects T-cell cytolytic activity to EGFR-positive cancers in vitro and in an animal model. Clin Cancer Res. 2006;12:183–190. doi: 10.1158/1078-0432.CCR-05-1855. [DOI] [PubMed] [Google Scholar]
- 17.Lum HE, Miller M, Davol PA, Grabert RC, Davis JB, Lum LG. Preclinical studies comparing different bispecific antibodies for redirecting T cell cytotoxicity to extracellular antigens on prostate carcinomas. Anticancer Res. 2005;25:43–52. [PubMed] [Google Scholar]
- 18.Davol PA, Smith JA, Kouttab N, Elfenbein GJ, Lum LG. Anti-CD3 × Anti-HER2 bispecific antibody effectively redirects armed T cells to inhibit tumor development and growth in hormone-refractory prostate cancer-bearing SCID-Beige mice. Clin Prostate Cancer. 2004;3:112–121. doi: 10.3816/cgc.2004.n.021. [DOI] [PubMed] [Google Scholar]
- 19.Chan JK, Hamilton CA, Cheung MK, et al. Enhanced killing of primary ovarian cancer by retargeting autologous cytokine-induced killer cells with bispecific antibodies: a preclinical study. Clin Cancer Res. 2006;12:1859–1867. doi: 10.1158/1078-0432.CCR-05-2019. [DOI] [PubMed] [Google Scholar]
- 20.Porter DL, Levine BL, Bunin N, et al. A phase 1 trial of donor lymphocyte infusions expanded and activated ex vivo via CD3/ CD28 costimulation. Blood. 2006;107:1325–1331. doi: 10.1182/blood-2005-08-3373. [DOI] [PubMed] [Google Scholar]
- 21.Fowler DH, Odom J, Steinberg SM, et al. Phase I clinical trial of costimulated, IL-4 polarized donor CD4+ T cells as augmentation of allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2006;12:1150–1160. doi: 10.1016/j.bbmt.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg PD, Reusser P, Goodrich JM, Riddell SR. Development of a treatment regimen for human cytomegalovirus (CMV) infection in bone marrow transplantation recipients by adoptive transfer of donor-derived CMV-specific T cell clones expanded in vitro. Ann N Y Acad Sci. 1991;636:184–195. doi: 10.1111/j.1749-6632.1991.tb33450.x. [DOI] [PubMed] [Google Scholar]
- 23.Bao L, Sun Q, Lucas KG. Rapid generation of CMV pp65-specific T cells for immunotherapy. J Immunother. 2007;30:557–561. doi: 10.1097/CJI.0b013e31803b945b. [DOI] [PubMed] [Google Scholar]
- 24.Hamel Y, Blake N, Gabrielsson S, et al. Adenovirally transduced dendritic cells induce bispecific cytotoxic T lymphocyte responses against adenovirus and cytomegalovirus pp65 or against adenovirus and Epstein-Barr virus EBNA3C protein: a novel approach for immunotherapy. Hum Gene Ther. 2002;13:855–866. doi: 10.1089/10430340252899028. [DOI] [PubMed] [Google Scholar]
- 25.Foster AE, Gottlieb DJ, Marangolo M, et al. Rapid, large-scale generation of highly pure cytomegalovirus-specific cytotoxic T cells for adoptive immunotherapy. J Hematother Stem Cell Res. 2003;12:93–105. doi: 10.1089/152581603321210172. [DOI] [PubMed] [Google Scholar]
- 26.Foster AE, Forrester K, Gottlieb DJ, Barton GW, Romagnoli JA, Bradstock KF. Large-scale expansion of cytomegalovirus-specific cytotoxic T cells in suspension culture. Biotechnol Bioeng. 2004;85:138–146. doi: 10.1002/bit.10801. [DOI] [PubMed] [Google Scholar]
- 27.Micklethwaite K, Hansen A, Foster A, et al. Ex vivo expansion and prophylactic infusion of CMV-pp65 peptide-specific cytotoxic T-lymphocytes following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:707–714. doi: 10.1016/j.bbmt.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Oelke M, Maus MV, Didiano D, June CH, Mackensen A, Schneck JP. Ex vivo induction and expansion of antigen-specific cytotoxic T cells by HLA-Ig-coated artificial antigen-presenting cells. Nat Med. 2003;9:619–624. doi: 10.1038/nm869. [DOI] [PubMed] [Google Scholar]
- 29.Papanicolaou GA, Latouche JB, Tan C, et al. Rapid expansion of cytomegalovirus-specific cytotoxic T lymphocytes by artificial antigen-presenting cells expressing a single HLA allele. Blood. 2003;102:2498–24505. doi: 10.1182/blood-2003-02-0345. [DOI] [PubMed] [Google Scholar]
- 30.Sili U, Huls MH, Davis AR, et al. Large-scale expansion of dendritic cell-primed polyclonal human cytotoxic T-lymphocyte lines using lymphoblastoid cell lines for adoptive immunotherapy. J Immunother. 2003;26:241–256. doi: 10.1097/00002371-200305000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Peggs K, Verfuerth S, Mackinnon S. Induction of cytomegalovirus (CMV)-specific T-cell responses using dendritic cells pulsed with CMV antigen: a novel culture system free of live CMV virions. Blood. 2001;97:994–1000. doi: 10.1182/blood.v97.4.994. [DOI] [PubMed] [Google Scholar]
- 32.Mackinnon S, Thomson K, Verfuerth S, Peggs K, Lowdell M. Adoptive cellular therapy for cytomegalovirus infection following allogeneic stem cell transplantation using virus-specific T cells. Blood Cells Mol Dis. 2008;40:63–67. doi: 10.1016/j.bcmd.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Rauser G, Einsele H, Sinzger C, et al. Rapid generation of combined CMV-specific CD4+ and CD8+ T-cell lines for adoptive transfer into recipients of allogeneic stem cell transplants. Blood. 2004;103:3565–3572. doi: 10.1182/blood-2003-09-3056. [DOI] [PubMed] [Google Scholar]
- 34.Trivedi D, Williams RY, O’Reilly RJ, Koehne G. Generation of CMV-specific T lymphocytes using protein-spanning pools of pp65-derived overlapping pentadecapeptides for adoptive immunotherapy. Blood. 2005;105:2793–2801. doi: 10.1182/blood-2003-05-1433. [DOI] [PubMed] [Google Scholar]
- 35.Dasari V, Smith C, Zhong J, Scott G, Rawlinson W, Khanna R. Recombinant glycoprotein B vaccine formulation with Toll-like receptor 9 agonist and immune-stimulating complex induces specific immunity against multiple strains of cytomegalovirus. J Gen Virol. 2011;92:1021–1031. doi: 10.1099/vir.0.029413-0. [DOI] [PubMed] [Google Scholar]
- 36.La RC, Wang Z, Lacey SF, et al. In vitro expansion of polyclonal T-cell subsets for adoptive immunotherapy by recombinant modified vaccinia Ankara. Exp Hematol. 2006;34:497–507. doi: 10.1016/j.exphem.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 37.Cobbold M, Khan N, Pourgheysari B, et al. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J Exp Med. 2005;202:379–386. doi: 10.1084/jem.20040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schub A, Schuster IG, Hammerschmidt W, Moosmann A. CMV-specific TCR-transgenic T cells for immunotherapy. J Immunol. 2009;183:6819–6830. doi: 10.4049/jimmunol.0902233. [DOI] [PubMed] [Google Scholar]
- 39.Full F, Lehner M, Thonn V, et al. T cells engineered with a cytomegalovirus-specific chimeric immunoreceptor. J Virol. 2010;84:4083–4088. doi: 10.1128/JVI.02117-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaz-Santiago J, Lule J, Rohrlich P, et al. Ex vivo stimulation and expansion of both CD4(+) and CD8(+) T cells from peripheral blood mononuclear cells of human cytomegalovirus-seropositive blood donors by using a soluble recombinant chimeric protein, IE1-pp65. J Virol. 2001;75:7840–7847. doi: 10.1128/JVI.75.17.7840-7847.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grabert RC, Cousens LP, Smith JA, et al. Human T cells armed with Her2/neu bispecific antibodies divide, are cytotoxic, and secrete cytokines with repeated stimulation. Clin Cancer Res. 2006;12:569–576. doi: 10.1158/1078-0432.CCR-05-2005. [DOI] [PubMed] [Google Scholar]
- 42.Thakur A, Lum LG. Cancer therapy with bispecific antibodies: clinical experience. Curr Opin Mol Ther. 2010;12:340–349. [PMC free article] [PubMed] [Google Scholar]
- 43.Drobyski WR, Majewski D, Ozker K, Hanson G. Ex vivo anti-CD3 antibody-activated donor T cells have a reduced ability to cause lethal murine graft-versus-host disease but retain their ability to facilitate alloengraftment. J Immunol. 1998;161:2610–2619. [PubMed] [Google Scholar]