Abstract
Alzheimer’s disease (AD) is characterized by three major histopathological hallmarks: β-amyloid plaques, neurofibrillary tangles and gliosis. While neglected for decades, neuroinflammatory processes coordinated by microglia are now accepted as etiologic events in AD evolution. Microglial cells are found in close vicinity to amyloid plaques and display various activation phenotypes determined by expression of a wide range of cytokines, chemokines, and innate immune cell surface receptors. During the development of AD pathology, microglia fail to restrict amyloid plaques and may contribute to neurotoxicity and cognitive deficit. Nevertheless, under specific conditions, microglia can participate in cerebral amyloid clearance. This review focuses on the complex relationship between microglia and Aβ pathology, and highlights both deleterious and beneficial roles of microglial activation in the context of AD. A deeper understanding of microglial biology will hopefully pave the way for next-generation AD therapeutic approaches aimed at harnessing these enigmatic innate immune cells of the central nervous system.
Keywords: Alzheimer disease, Amyloid-β peptide, Chemokine, Cytokine, Gliosis, Inflammation, Innate Immunity, Microglia, Neuroinflammation, Phagocytosis
Introduction
Alzheimer’s disease (AD), the most common form of dementia, currently affects more than five million Americans and newly diagnosed cases are expected to reach 15 million by the year 2050. Pathological features include large-scale damage to the entorhinal cortex, hippocampus and basal forebrain, leading to memory impairment, temporal and spatial disorientation and altered cognitive function. The disease is earmarked by presence of two histopathological lesions: extracellular amyloid deposits [chiefly composed of amyloid-β (Aβ) peptides] and intracellular neurofibrillary tangles [NFTs, made up primarily of abnormally folded tau protein]. Over the last hundred years, intense focus has been directed toward understanding AD pathoetiology and potential treatment approaches aimed at controlling these two hallmark pathologies.
In 1906, Alois Alzheimer described post-mortem analysis of Auguste Deter, a 51-year-old female patient that suffered from dementia. In this first report, he identified a third pathological feature of the disease that would later bear his name. What he termed “gliose ”, we now know as gliosis, or inflammation of the brain’s glial support cells [1]. At the light microscopic level, gliosis in the AD brain is characterized by presence of reactive microglia and astrocytes that typically surround “senile” β-amyloid plaques [2]. Interestingly, microglial cells found in close proximity to amyloid plaques express the human leukocyte antigen-DR surface immune marker for mononuclear phagocyte activation as well as pro-inflammatory cytokines including interleukin(IL)-1β and IL-6 [3, 4]. While once regarded as epiphenomenon, more recent clinico-pathological studies show a strong association between microglial abundance and disease severity, and suggest that microglial activation is an early event in AD pathogenesis [5–8]. These results suggest that reactive microglia play both active and essential roles in the pathophysiology of AD [3, 9, 10]. In this review, we examine the broader implications of gliosis and innate immunity in the pathobiology of AD. A complex relationship emerges whereby the interactions between microglia and Aβ are characterized by discrete phases of attraction, interaction and activation, leading to context-dependent detrimental or beneficial outcomes in AD.
Attraction of microglia to amyloid plaques
As resident innate immune cells of the central nervous system (CNS), microglia play an indispensible role in surveying the brain milieu and form a first line of defense against invading pathogens. To carry out these important functions, microglia utilize: phagocytosis, cytokine production, activation of the protein complement cascade, and production of oxyradicals [11]. But before microglia can mount a response to a stimulus, they must first be attracted to the tissue site. The attraction process is often mediated by the interaction of chemokines and cytokines with their receptors, both of which are expressed by microglial cells. In the context of AD, several lines of evidence support the notion that β-amyloid plaque-associated factors, including misfolded Aβ peptides themselves, act as microglial attractants.
Indeed, the numerous chemokine receptors expressed by microglia and corresponding chemokines produced by Aβ-stimulated cells indicate that these macromolecules play an important role in microglia accumulation in the AD brain. More precisely, chemokine receptors such as CCR2, CCR3, CCR5 and CX3CR1 are all present on microglia in vitro as well as in AD patient brains, and CXCR2 and CXCR3 are expressed in close vicinity of neuritic plaques [12, 13]. That microglial cells readily express these chemokine receptors implies that they are primed to respond to cognate chemokine ligands. In this regard, in vitro experiments reveal that Aβ-stimulated microglia can induce mRNA and secretion of several chemokines, including CCL4 and CXCL2, CCL3, CXCL8 and CCL5 [14], suggesting an autocrine mode of chemokine signaling. Furthermore, several cell types appear to be a source of CCL2 in response to Aβ stimulation: astrocytes, neurons, oligodendrocytes and microglial cells themselves [14–16]. In fact, the central role of CCR2-CCL2 interaction in attraction of microglia towards Aβ deposits has been demonstrated in mouse models. For example, 3xTg-AD mice carrying amyloid precursor protein “Swedish” (APPsw), presenilin 1 (PS1) M146V and Tau P301L mutant human transgenes [17] have increased CCL2 levels in entorhinal cortex correlating with recruitment of microglia/macrophages to this brain region [18]. Furthermore, aged mice bearing human mutant APP and PS1 transgenes (designated PSAPP mice) develop β-amyloid plaques accompanied by activated microglial cells expressing CCL2 [19]. These data are clinically strengthened by post-mortem analysis of AD patient brains, indicating that CCL2 is expressed in microglia present in mature senile plaques [20]. Additionally, El Khoury and collaborators established that CCR2 deficiency leads to reduced accumulation of microglia in brains of aged transgenic APPsw mice (line Tg2576) [21, 22]. Moreover, intracerebral injections of Aβ into wild-type mice induces accumulation of microglia at the injection site that is completely abolished in CCR2−/− mice. These data confirm that CCR2 is required in vivo for the recruitment of microglia to sites of Aβ deposition. Another chemokine receptor, CX3CR1 (also known as fractalkine receptor) is exclusively expressed on microglia whereas its ligand, CX3CL1, is highly expressed on neurons [23, 24]. The CX3CL1/CX3CR1 pathway is implicated in neuronal-microglial cross-talk, and may play a role in microglia attraction to amyloid plaques. In support, CX3CR1 deficiency reduces numbers of total and activated microglia surrounding amyloid plaques in two different mouse models of cerebral amyloidosis: PSAPP and R1–40 [25–27]. Furthermore, CX3CR1 deletion rescues microglial-dependent neuronal loss observed in 3xTg-AD mice [28].
Cytokines have also been suggested to be involved in chemoattraction of microglia to amyloid lesions. Interestingly, elevated Aβ1–40 and Aβ1–42 levels in aging Tg2576 and PSAPP transgenic mice are associated increased pro-inflammatory cytokines including tumor necrosis factor-alpha (TNF-α), IL-6, IL-1α and granulocyte macrophage-colony stimulating factor (GM-CSF) [29]. These results suggest that pathological accumulation of Aβ is a key factor leading to neuroinflammatory responses in AD [29]. In addition, in vitro experiments demonstrate that exposure of cultured microglia to pre-aggregated Aβ1–42 increases production of proinflammatory cytokines (i.e., pro-IL-1β, IL-6, TNF-α), macrophage inflammatory peptide (MIP-1α) and macrophage colony-stimulating factor (M-CSF) [30]. These molecules are all classically associated with monocyte chemoattraction, and others have implicated IL-6, IL-34 and M-CSF as microglial mitogens [31–33]. Furthermore, M-CSF levels in the plasma and CNS of AD patients are significantly increased compared to age-matched healthy controls or patients with mild cognitive impairment (MCI) [34]. Thus, there is little doubt that Aβ accumulation and perhaps neuronal injury endorse signals that trigger microglial recruitment and proliferation. This strong attraction of microglia towards Aβ prompts the question of how microglia immunologically respond once recruited to these lesions.
Activation of microglia in response to Aβ
Once microglia have moved toward Aβ deposits, they express various cell surface receptors allowing them to recognize and interact with misfolded Aβ peptides. In this context, the scavenger receptors (SRs) have garnered attention, as they can bind diverse ligands and affect the activation level, inflammatory status and phagocytic function of microglia [35]. Class A and B scavenger receptors were first described to bind Aβ in fibrillar or oligomeric conformations [36–39]. Several members of this family seem to be implicated in microglial interaction with Aβ. A prototypical example is CD36, which is expressed by microglia in vitro and in AD brain and endorses secretion of reactive oxygen species (ROS) by microglia in response to Aβ [14, 40]. Furthermore, in microglial cells isolated from CD36 null mice, fibrillar Aβ-induced secretion of cytokines, chemokines and ROS is reduced. Additionally, intracerebral injection of fibrillar Aβ results in significantly lower recruitment of microglia in brains of CD36−/− mice compared to wild-type mice [14]. Finally, Bamberger and collaborators described a receptor complex including CD36, the integrin-associated protein CD37 and the α6β1 integrin, which interacts with Aβ fibrils and activates microglial secretion of ROS [41]. Another key SR, the receptor for advanced glycation end products (RAGE), has also been identified on CD68+ microglial cells close to senile plaques in AD patient brains and in cultured rat and mouse microglia [42]. In vitro, RAGE is expressed on the surface of microglial cells and is able to bind Aβ [42]. Furthermore, Alarcon and colleagues reported that SRs expressed on neonatal rat microglia endorse binding of both fibrillar and non-fibrillar Aβ and another SR, MARCO, specifically mediates fibrillar Aβ binding [43]. Furthermore, APP23 transgenics have elevated levels of the SR SCARA-1 on microglia around Aβ plaques. Thus, SCARA-1 also seems to be implicated in microglial recognition of Aβ. Finally, other reports suggest that the low density lipoprotein receptor-related protein, expressed on microglial cells, may function as an Aβ clearance receptor [44, 45].
Another family of innate immune receptors, the Toll-like receptors (TLRs), function as sensors for pathogen-associated molecular patterns (PAMPs) and can also recognize pathogenic endogenous proteins. TLR engagement initiates intracellular signaling pathways resulting in pro-inflammatory cytokine and chemokine secretion as well as nitric oxide (NO) release [46]. Remarkably, TLRs and associated receptors (e.g., CD14) are highly expressed by microglia in close proximity to plaques in AD patient brains and in mouse models of the disease [47–51]. Aβ infusion into hippocampi of wild-type mice increases TLR2 expression, supporting these observations made in human AD [52]. Several reports suggest that TLRs are involved in recognition of amyloid peptides by microglia. For example, in a mouse model of AD, TLR4 deficiency modulates activation of microglia [53]. Others have reported that the hydrophobic β-sheet conformation is recognized and binds to the TLR-associated receptor CD14, initiating microglial secretion of NO, IL-6 and other neurotoxic factors [48–50]. This hypothesis is consistent with observations that TLR2 or TLR4 inhibition reduces Aβ-mediated microglial production of NO, TNF-α, IL-6 and IL-1β [49, 54, 55], and that CD14 deficiency in PSAPP mice inhibits microgliosis and lowers CD45 immunoreactivity [56]. At some level, these observations in mouse models have been corroborated by genetic studies showing that TLR4 or CD14 polymorphisms that attenuate inflammatory responses decrease AD risk [57, 58].
Activation of immune cells typically requires co-stimulatory signals in addition to the primary stimulus. CD40 is an immunoregulatory cell surface glycoprotein which, upon stimulation with its cognate ligand, CD40 ligand (CD40L), promotes activation of microglia and production of inflammatory proteins [59]. Interestingly, AD patient brains exhibit elevated abundance of microglial-associated CD40 concomitant with astrocyte-derived CD40L in and around amyloid plaques [60, 61]. Furthermore, Aβ stimulated microglia co-challenged with CD40L or agonistic CD40 antibody produce copious amounts of TNF-α that injure primary cortical neurons in vitro [62, 63]. In cultured microglial cells, CD40-CD40L interaction stimulates release of pro-inflammatory cytokines, inhibits Aβ phagocytosis, and promotes loading of Aβ peptides onto immunostimulatory major histocompatibility class II molecules [64]. Broadly then, concomitant stimulation with Aβ plus CD40L shifts microglial activation from a beneficial phagocytic phenotype to a potentially deleterious pro-inflammatory state endorsing antigen-presenting cell function. Furthermore, microglial cells derived from CD40L–deficient PSAPP AD model mice have reduced expression of TNF-α compared to control PSAPP littermates, and exhibit attenuated cell surface markers of activation [65]. Interestingly, CD40L–induced pro-inflammatory immune responses can be blocked by stimulation of the microglial transmembrane protein tyrosine phosphatase, CD45 [66, 67]. Complementarily, CD45 deficient PSAPP mice present a pro-inflammatory microglial phenotype accompanied by elevated levels of TNF-α and IL-1β, indicating that CD45 is a negative regulator of microglial activation [68]. Remarkably, CD45 is expressed by microglia in the frontal cortex and hippocampus of normal aging individuals and is markedly increased in close vicinity of β-amyloid plaques in AD patient brains [69], possibly representing a compensatory response in an attempt to reduce Aβ-mediated microgliosis.
Just recently, the list of potential Aβ innate immune receptors has grown. Of particular interest is the concept that misfolded Aβ peptides may in fact mimic activators of the prototypical NOD-like receptor 3 (NLRP3) pathway. NLRP3 is a cytoplasmic receptor that associates with an adapter protein, apoptosis-associated speck-like protein, and caspase-1 to form a multiprotein complex known as the “Inflammasome” [70]. Interestingly, a recent report showed that Inflammasome activation is initiated after Aβ phagocytosis by microglia. The interaction between Aβ and the NLRP3 Inflammasome leads to release of IL-1β, the cardinal downstream Inflammasome effector cytokine [71]. We await further confirmation of these exciting results linking Aβ-induced microglial activation to the Inflammasome cascade.
Heterogeneity of microglial activation states
In the healthy adult CNS, microglial cells display a classic “ramified” or resting morphology characterized by a small soma with fine cellular processes. While often associated with a quiescent state, contrary to what the name implies, this ramified morphology is actually an active state in which microglia dynamically scan the brain milieu by extending and retracting many cytoplasmic extensions [72, 73]. This phenotype shifts to a reactive state in case of disturbance in homeostasis, injury or recognition of danger signals- when rapid changes in morphology, gene expression and function occur. The purpose of this shift toward activation is to allow microglia to divide, acquire mobility, and mount innate immune responses. Once activated, microglia present with a characteristic enlarged cytoplasm and shortened processes, morphologically referred to as “amoeboid” [74]. The activation states of microglia have been discretely classified into: classical activation (M1), alternative activation (M2), and acquired deactivation. However, a complementary concept is that a continuum of functional phases exist between two extremes, designated M1 and M2 [75]. Microglial activation is both characterized and modulated by cytokines, cell surface antigen interactions, and the inflammatory milieu. Classical activation is earmarked by elevated proinflammatory cytokines including TNF-α, IL-1, IL-6, IL-12 and IL-18, cell surface receptors, NO and prostaglandins accompanied by poor phagocytic capacity [76]. The M2 state is characterized by secretion of the anti-inflammatory cytokines IL-4, IL-10, IL-13 and TGF-β, and elevated phagocytic capacity without supraphysiologic production of toxic NO [77–79]. In neurodegenerative diseases, heterogeneity of microglial activation states is likely to impact the development of the disorder [80]. In the brain of AD patients or mouse models, microglia surrounding plaques exhibit both M1 and M2 activation markers, indicating that a shift from one state to the other could occur during the progression of the disease or that one or several intermediate activation states are involved [81, 82]. This perspective is particularly interesting in view of the complex relationship between microglia and amyloid deposits, detailed below.
Inappropriate activation of microglia: reinforcement of amyloid load and neurotoxicity
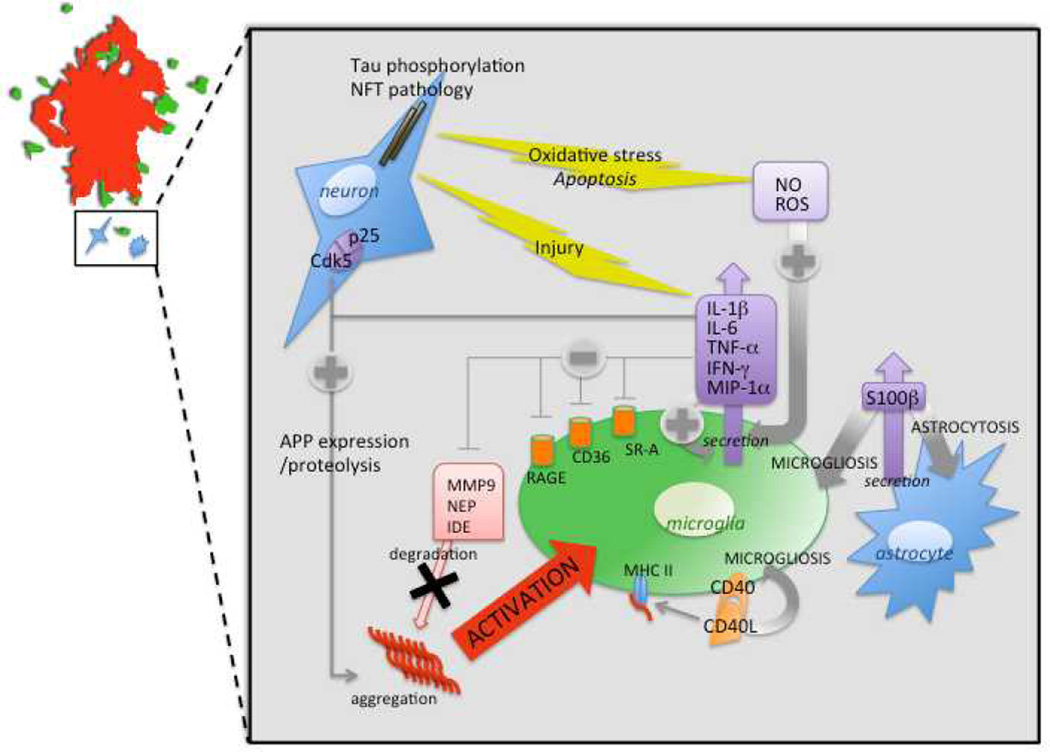
In health, microglia are immunocompetent cells that survey the brain milieu and, if needed, become acutely activated in order to repair tissue damage. However, in the context of AD, microglia fail in this primary function, as 1) Aβ deposits are not cleared despite abundant microgliosis surrounding amyloid plaques, 2) it is not evident that microglia are capable of degrading Aβ, and 3) chronic microglial activation is damaging to neurons, via production of numerous cytokines and acute-phase reactants. This inappropriate activation of microglia in the AD brain is represented in Figure 1.
Figure 1. Inappropriate activation of microglia in Alzheimer’s disease.
A schematic representation is shown of a mature amyloid plaque (red) surrounded by activated microglia (green) and a neuron and astrocyte (shaded blue; top left corner). The inset summarizes the effects of Aβ-induced activation of microglia on cytokine/chemokine secretion, expression of cell surface immune receptors, oxidative stress, and the resultant deleterious vicious cycle reinforcing Aβ deposition and neurotoxicity. Abbreviations used: APP, amyloid precursor protein; Cdk5, cyclin-dependent kinase 5; IDE, insulin degrading enzyme; IFN-γ, interferon-gamma; IL, interleukin; MHC II, major histocompatibility complex, class II; MIP-1α, macrophage inflammatory protein, type 1alpha; MMP9, matrix metalloprotease, type 9; NEP, neprilysin; NFT, neurofibrillary tangle; NO, nitric oxide; RAGE, receptor for advanced glycation endproducts; ROS, reactive oxygen species; SR-A, scavenger receptor, type A, TNF-α, tumor necrosis factor-alpha.
Several studies have cast doubt on the effectiveness of microglia in amyloid clearance. Poor microglial Aβ clearance aptitude could be at least partially due to age-related structural deterioration and cellular senescence of microglia [83]. Another explanation involves the particular phenotype that microglia adopt during the course of the disease, which fails to avail these cells of the appropriate molecular tools for amyloid clearance [82]. This line of reasoning is supported by Hickman and collaborators, who reported that aged PSAPP mice have reduced expression of Aβ-binding receptors including scavenger receptor A (SR-A), CD36, the receptor for advanced glycation endproducts (RAGE) and Aβ degrading enzymes such as insulin degrading enzyme (IDE), neprilysin (NEP), and matrix metalloprotease 9 (MMP9) [84]. Ex vivo experiments indicate that microglia from aged mice secrete constitutively high amounts of the pro-inflammatory cytokines IL-6 and TNF-α, and this is associated with reduced capacity to internalize Aβ peptide compared to younger mouse microglia [85]. This evidence supports the above-mentioned concept that microglia committed to a strong inflammatory response are less efficient at phagocytosing Aβ [78]. All together, these data suggest that the aging process biases microglial activation toward an M1-like state that fails to restrict AD pathology. Furthermore, even if microglia are able to take up Aβ during the early stage of the disease, there is a paucity of evidence to suggest that these cells effectively degrade the peptide. In this regard, it is interesting to note that, in vitro, the majority of Aβ internalized by mouse microglia is still not degraded after 72 hours [86]. Furthermore, Aβ extracted from human AD patients and phagocytosed by primary cultured canine microglia can be detected in phagosomes for up to 19 days [87], and others have shown that non-degraded Aβ is released by microglia [88]. While opsonisation of Aβ with Immunoglobulin G or complement improves Aβ phagocytic receptor binding, this approach fails to improve phagolysosomal degradation of the peptide [89].
This inability of microglia to efficiently phagocytose and degrade Aβ during the evolution of AD has been termed “frustrated phagocytosis.” Part and parcel of this response is persistent release of pro-inflammatory molecules, ROS, cytokines and chemokines [90]. Importantly, this maladaptive phenotype not only negatively impacts Aβ clearance, but can also be neurotoxic. For example, in microglial/neuronal co-culture or in organotypic slice cultures, the ratio of dead to live neurons is increased by microglial Aβ stimulation [91, 92]. In these experiments, presence of microglia in the culture is obligatory for the deleterious effects on neurons. These experimental results are not without validation from human AD cases. In fact, high levels of the cardinal proinflammatory cytokine IL-1β are detected in microglial cells surrounding Aβ plaques in AD patient brains and cerebrospinal fluid (CSF) [93, 94]. In vitro, IL-1β is released by activated microglia after stimulation with Aβ [95], and in turn induces expression of the astrocyte pro-inflammatory cytokine-like molecule, S100β, which is implicated in neuritic plaque formation. Forced expression of S100β accelerates AD-like pathology in Tg2567 mice by increasing amyloidogenic APP cleavage, astrocytosis, microgliosis and pro-inflammatory cytokine abundance [96]. IL-1β can, at least under certain circumstances, favor Aβ deposition by modulating APP expression and proteolysis [97]. This phenomenon sets off a vicious loop leading to chronic cytokine-mediated neuronal injury and subsequent microglial activation.
There are multiple lines of evidence suggesting that the pro-inflammatory milieu present in the AD brain and in transgenic mouse models of the disease is damaging. For instance, risk for conversion from mild cognitive impairment to AD is high in subjects presenting with elevated CSF abundance of the pro-inflammatory cytokine TNF-α and decreased anti-inflammatory TGF-β levels [98]. Accordingly, TgCRND8 AD model mice have enhanced expression of TNF-α, NO and the pro-apoptotic protein Bax, while the anti-apoptotic protein Bcl-2 is decreased [99]. Elevated expression of additional cytokines/chemokines and innate immune receptors favor an M1-like activation state in the context of AD. For example, in neuron-microglia co-cultures, the synergistic action of Aβ with either interferon-gamma (IFN-γ) or CD40L triggers TNF-α secretion and production of ROS that is neurotoxic [62, 100]. Furthermore, CD40L deficiency in Tg2576 mice results in decreased microgliosis and tau hyperphosphorylation– a key index of neuronal stress [62]. As pharmacologic validation of this genetic approach, intracerebroventricular delivery of CD40L depleting antibody to PSAPP AD model mice leads to striking reduction of β-amyloid pathology associated with decreased amyloidogenic processing of APP and brain-to-blood Aβ efflux. Taken together then, these data indicate that the CD40-CD40L interaction favors a pathogenic form of microglial activation in the context of AD [62]. In addition, the innate immune receptor, TLR4, is responsible for elevated levels of TNF-α and MIP-1α in AD model mice [53]. As the disease progresses in association with persistent production of pro-inflammatory cytokines such as TNF-α and IL-1β, unresolved neuroinflammation leads to chronicity. Deregulation of the stress responsive cyclin-dependent kinase 5 (Cdk5) is also involved in the development of AD-like pathology, including glial activation and MIP-1α, TNF-α, TGF-β and IL-1β secretion [101]. This form of neuroinflammation is deleterious, as the Cdk5/p25 complex induces hyperphosphorylation of tau, NFT formation and aberrant APP processing [102–107].
Finally, it deserves mentioning that Aβ-induced M1-type activation of microglia via SRs, TLRs or M-CSF leads to oxidative stress by release of NO and other ROS. Oxidative stress is well-known to provoke various types of damage including: apoptosis [108, 109], tau hyperphosphorylation and NFT formation, and increased β- and γ-secretase expression and activity associated with amyloidogenic APP metabolism [110, 111]. Furthermore, via stimulation of microglia and astrocytes, oxidative stress induces release of pro-inflammatory cytokines including IL-1, TNF-α and IL-6 that enhance Aβ generation [112–115]. Thus, oxidative stress is also a key perpetrator of the chronic, vicious cycle triggered by Aβ and reactive microglia.
Appropriate activation of microglia: resolution of cerebral amyloidosis
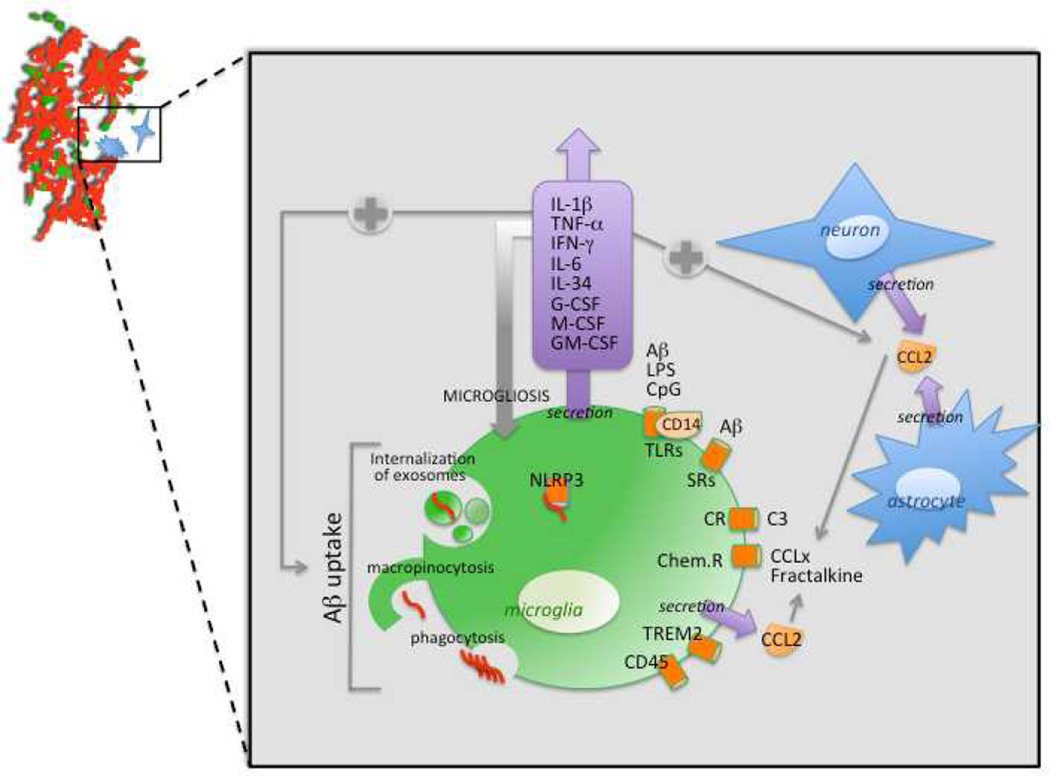
The primary goal of inflammation is to resolve injury or defend against foreign invaders while minimizing damage to surrounding tissues. In the case of AD, this would theoretically consist of clearing the brain of damaging forms of Aβ peptides. Several studies suggest that appropriate activation of microglia can be achieved and lead to resolution of cerebral amyloidosis in AD mouse models [68, 116–119]. The importance of microglia for restricting and remodeling Aβ plaques is underscored by transplantation of exogenous microglia into rat brains. Once transplanted, these cells migrate into the parenchyma and clear intracerebrally injected Aβ peptides [120]. Bone marrow-derived microglia also have an important role in plaque clearance via Aβ phagocytosis, when appropriately activated [121]. For instance, under defined conditions, microglia can internalize Aβ [78, 119, 122, 123] via macropinocytosis for soluble Aβ peptides, phagocytosis for insoluble aggregated species [124] and by phosphatidylserine-dependent internalization of exosomes [125]. Evidence supporting beneficial activation of microglia in the context of AD is summarized in Figure 2 and detailed below.
Figure 2. Beneficial activation of microglia in the context of Alzheimer’s disease.
A schematic representation of an amyloid plaque (red) being phagocytosed by appropriately-activated microglia (green) is shown in the top left corner. A neuron and an astrocyte are represented in blue. The inset summarizes the principle receptors and cytokines/chemokines that play roles in mediating Aβ clearance by microglia. Abbreviations used: CCL, chemokine ligand; Chem.R, chemokine receptor; CpG, unmethylated DNA containing CpG motifs; CRs, protein complement receptors; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte macrophage colony-stimulating factor; IFN-γ, interferon-gamma; IL, interleukin; LPS, lipopolysaccharide; M-CSF, macrophage colony stimulating factor; NLRP3, NOD-like receptor, type 3; NO, nitric oxide; ROS, reactive oxygen species; SRs, scavenger receptors; TLRs, Toll-like receptors; TNF-α, tumor necrosis factor-alpha; TREM, triggering receptor expressed on myeloid cells; SRs, scavenger receptors.
It is now appreciated that targeting specific receptors involved in microglial recognition of Aβ can lead to beneficial phagocytic activation. A good example of this is genetic deletion of the chemokine receptor, CCR2, in Tg2576 mice. These crossed mice manifest reduced migration of microglia towards amyloid plaques and higher cerebral Aβ levels associated with increased mortality [21]. Another study in PSAPP mice confirmed that lack of CCR2 aggravates amyloid pathology and behavioral deficit. In this model, expression is enhanced of the typical anti-inflammatory molecules TGF-β1 ligand and TGF-β receptors [126]. These results suggest that CCR2-mediated activation of microglia promotes brain Aβ clearance and protects mice from neurotoxicity, a concept further supported by a study designed to overexpress IL-1β in PSAPP mouse brains. Interesting, these animals have dramatic elevation of CCL2, increased microglial accumulation and activation, and reduced AD-like pathology [127]. Furthermore, the scavenger receptor SCARA-1 is also implicated in this mechanism, as its deficiency in mouse-derived microglia drastically reduces Aβ uptake [9, 39, 43, 128]. TLR-mediated activation of microglia also seems to impact Aβ clearance. For instance, in mouse models of AD, TLR2 deficiency or expression of a TLR4 mutant increases Aβ deposition and worsens cognitive impairment [52, 129, 130]. Similarly, intrahippocampal injections of the TLR4 ligand, lipopolysaccharide, promote Aβ clearance in PSAPP mice [131]. In primary neuron-microglia co-cultures, activation of microglia via interaction between TLR9 and its ligand, unmethylated CpG DNA, mediates oligomeric Aβ clearance by microglial cells and attenuates Aβ1–42 oligomer-induced neurotoxicity. These observations were accompanied by high levels of the antioxidant enzyme, heme oxygenase-1, and absence of potentially neurotoxic NO and glutamate. Those authors further confirmed these effects in vivo, by demonstrating that intracerebroventricular injection of CpG DNA ameliorates impaired associative learning and cognition induced by oligomeric Aβ in Tg2576 mice [132]. When taken together, these reports indicate that selective targeting of innate immune receptors can favor microglial Aβ uptake and support a protective form of activation.
Cytokines are also powerful modulators of neuroinflammation and can impact microglial Aβ clearance. Two good examples are M-CSF and granulocyte-colony stimulating factor (G-CSF), which are reported to promote Aβ phagocytic clearance by microglia and to rescue learning and memory deficits in mouse models [133, 134]. Additionally, unilateral hippocampal injection of M-CSF, G-CSF or GM-CSF in transgenic AD model mice reduces amyloid load when compared to the (control) contralateral hemisphere. Furthermore, chronic GM-CSF subcutaneous injection reduces Aβ burden, ameliorates cognitive deficit, and increases hippocampal synaptic area [135]. GM-CSF binding to its receptor promotes proliferation of human fetal and adult microglia in primary cultures and acts as a neurotrophic factor without enhancing Aβ-induced microglial secretion of potentially damaging pro-inflammatory cytokines [136]. These experimental results are even more valid when considering that GM-CSF receptor expression is reduced in the hippocampus of AD patients compared to age-matched controls [137]. However, when Manczak and colleagues administered GM-CSF neutralizing antibody to a mouse model of AD, they observed suppression of microglial activation and decreased Aβ1–42 levels [138]. Interestingly, stimulation of pro-inflammatory signaling via treatment of microglial cells with IFN-γ and IL-1β results in increased Aβ1–42 uptake concomitant with enhanced IL-6 secretion [139], and pharmacological induction of IL-6 and TNF-α secretion by microglia favors Aβ peptide uptake [140]. Similarly, overexpression of IL-6 in TgCRND8 or Tg2567 model mice induces massive gliosis accompanied by increased expression of the phagocytosis marker, CD68, resulting in microglial clearance of amyloid aggregates [141]. Furthermore, in primary neuron and microglia co-cultures, an M-CSF-related cytokine modulator of inflammation, IL-34, promotes microglial proliferation and clearance of soluble oligomeric Aβ. Moreover, intracerebroventricular injection of IL-34 in PSAPP mice reduces oligomeric Aβ levels and improves learning and memory [33].
While often regarded as pro-inflammatory molecules, NO and components of the protein complement cascade can also play beneficial roles in the context of AD pathobiology. NO is inducibly generated in the brain by the inducible nitric oxide synthase (iNOS) enzyme, encoded by the NOS2 gene. Strikingly, NOS2 deficiency diminishes NO levels and exacerbates cerebral amyloidosis, tauopathy and neuronal loss in AD model mice [142, 143]. Those authors suggested that AD-like pathology was directly related to NO abundance, where high levels produced by microglia were neuroprotective whereas NO deficiency favored development of AD-like pathology [144]. Regarding the protein complement pathway, components of this cascade have been found to colocalize with amyloid plaques and tangles and may also participate in beneficial effects of microglia in AD [145]. Thus, inhibition of complement C3 activation or genetic ablation of C3 in a transgenic mouse model of AD activates microglia, augments Aβ deposition and promotes neuronal degeneration [146, 147]. Additionally, in vitro experiments showed that synthetic Aβ peptides enhance C3 production by cultured microglial cells [148]. Finally, despite the controversy surrounding degradation of fibrillar Aβ by microglia, it has been demonstrated that activation of microglial cells by M-CSF or other inflammatory stimuli increases their ability to degrade internalized fibrillar Aβ in vitro. In this regard, it is interesting that quiescent microglial cells have unusually high lysosomal pH (which does not support optimal Aβ degradation), while activated cells have acidification of these compartments, endorsing efficient degradation of Aβ [149].
Harnessing beneficial microglial responses to Aβ: a therapeutic perspective
In past decades, one of the key therapeutic strategies for AD has been aimed at reducing cerebral Aβ load. In one embodiment, preventive therapy would target β- and γ-secretases, preventing amyloidogenic APP metabolism and thereby reducing Aβ generation. However, because the secretases: 1) target a variety of substrates and 2) have negative side-effects and associated adverse events, the scientific community has asked for consideration of alternative therapeutic approaches. One such approach has been to target the other side of the equation: Aβ clearance. This alternate approach may be even more important when considering a recent report strongly suggesting that failure in Aβ clearance machinery rather than overproduction of the peptides may be the etiologic culprit in human AD [150].
In a seminal report, Schenk and colleagues reported that so-called “active” vaccination with Aβ1–42 peptide plus QuilA adjuvant strikingly ameliorated cerebral Aβ load in PDAPP AD model mice, and Bard and coworkers followed-up by showing that “passive” transfer of Aβ antibodies produced a similar result [151, 152]. These exciting results led to an early developmental clinical trial, AN-1792 (Elan Pharmaceuticals/Wyeth) which revealed Aβ antibody-dependent microglial clearance of amyloid plaques in post-mortem analyses of a limited AD patient cohort [153]. Unfortunately though, this phase IIa trial was prematurely halted after 4 months of treatment, when ∼6 % of the patients developed a severe form of brain inflammation known as aseptic meningoencephalis [154]. This adverse event was believed to have been caused by T helper type 1 (Th1) cells that infiltrated the brain and mounted an autoaggressive response to cerebral Aβ. Furthermore, even though patients that received AN-1792 produced Aβ-specific antibodies and had reduced amyloid plaques, they did not demonstrate durable improvement in cognitive function. It is interesting to note that immunization with Aβ1–42 plus Freund’s adjuvant in either Tg2576 or wild-type mice results in decreased Th1 cytokines IL-2 and IFN-γ and elevated Th2 cytokines IL-4 and IL-10 concomitant with attenuation of Aβ burden [155]. These data suggest that the choice of adjuvant is a critical determinant of the character of the Aβ-specific immune response. Other studies have shown similar Th2 responses to active Aβ vaccines in transgenic AD mice associated with robust reduction of Aβ deposits [156–158], while less efficient clearance of Aβ is typically observed in immunization approaches that favor a Th1 response [159]. Although imperfect, the AN-1792 trial has nonetheless paved the way for additional immunotherapeutic studies aiming at preventing plaque formation by the use of Aβ-specific antibodies.
Broadly stated, one such approach has been to convert microglial activation from harmful M1-like neurotoxic to helpful M2-like pro-phagocytic phenotype– and not simply to suppress inflammatory processes all together. Numerous in vitro and in vivo studies, detailed below, provide evidence that microglial phagocytosis of amyloid peptide can, in fact, be successfully achieved.
One such approach has been to selectively target receptor complexes that bind Aβ and induce inflammatory responses. Peroxisome proliferator-activated receptor gamma (PPARγ) is involved in the regulation of apolipoprotein E and acts as a molecular chaperone for Aβ. Oral treatment of PSAPP mice with a synthetic PPARγ agonist causes phenotypic polarization of microglial cells from pro- to anti-inflammatory, enhances phagocytosis of soluble and insoluble Aβ peptides, and ameliorates memory deficits [160, 161]. Another example is inhibition of RAGE receptor in transgenic AD model mice, which reduces β-secretase activity and Aβ production, suppresses microglial activation and inflammatory responses, and ameliorates cognitive performance [162]. Blockade of TLR2 also reduces inflammation and increases Aβ uptake by shifting microglia from M1 to M2 activation states [163]. Furthermore, inhibition of CD36-dependent microglial activation results in beneficial blockade of ROS production [164].
A different strategy that has shown promise is modulation of cytokines. TGF-βs are key cytokine regulators of inflammation that also play cardinal roles in immune homeostasis and tissue repair [165]. In brains of AD mouse models, several studies indicate that overexpression of TGF-β1 promotes microgliosis and accelerates Aβ deposition [166, 167], while others have show resolution of microglial activation [168] and clearance of Aβ deposits via phagocytosis in this scenario [119, 169, 170]. Another report demonstrated that neuronal reduction of TGF-β signaling in a mouse model of AD induces Aβ accumulation, dendritic loss and age-dependent neurodegeneration, suggesting that increasing neuronal TGF-β signaling may be beneficial in AD by being neurotrophic [171]. More recently, Tichauer and collaborators evaluated the participation of the TGF-β-Smad3 pathway on expression of SRs and activation of wild-type rat-derived microglia in culture. They reported that TGF-β1 increased SR-A but decreased SR-B1 expression, and had no effect on SR-MARCO or CD36. In addition, microglial activation by TGF-β1 leads to increased clearance of Aβ and reduced neurotoxicity [172]. Nevertheless, in AD patient brains, TGF-β1 is increased while Aβ clearance is reduced. Interestingly, TGF-β1, nuclear Smad7 and β-catenin are strikingly augmented in cortical brain regions of TgCRND8 AD model mice. Furthermore, interaction between Smad7 and β-catenin, as revealed by coimmunoprecipitation, is enhanced in TgCRND8 mice. In mouse primary cortical neuronal cultures, exposure to Aβ1–42 peptide induces TGF-β1 and elevates nuclear β-catenin and Smad7. Moreover, addition of TGF-β1 to mouse cortical neuron cultures increases apoptosis via a Smad7 and β-catenin-dependent pathway. These data suggest that TGF-β1 amplifies Aβ1–42-mediated neurodegeneration via a Smad7/β-catenin-dependent mechanism [173].
Our group developed a transgenic mouse model with blockade of TGF-β receptor and downstream Smad2/3 signaling on peripheral macrophages (designated CD11c–DNR mice) [174, 175]. When these mice were crossed with Tg2576 mice, doubly transgenic animals had betterment of behavioral impairment [176]. Importantly, these animals had striking reduction of Aβ deposits in brain parenchyma, accompanied by increased infiltration of peripheral macrophages in and around β-amyloid plaques. In vitro, CD11c–DNR-derived peripheral macrophages exhibit blockade of the classical TGF-β-activated Smad2/3 pathway accompanied by hyperactivation of the alternative Smad1/5/8 signaling cascade and marked increase in Aβ phagocytosis [176]. It is noteworthy that the peripheral macrophages implicated in this beneficial Aβ phagocytosis effect have increased levels of IL-10, characteristic of an anti-inflammatory M2-like phenotype. Taken together, these results indicate that blockade of TGF-β-Smad2/3 signaling in peripheral macrophages represents a new therapeutic target for AD [177, 178].
Concluding remarks
An impressive array of studies, reviewed here, clearly implicate the innate immune response in the pathobiology of AD. In toto, these reports reveal a highly complex relationship between microglia and Aβ that can either be deleterious or beneficial. If left unchecked, the chronic neuroinflammatory process can perpetrate bystander neuronal injury; but if controlled, reactive microgliosis can be harnessed to clear the brain of damaging Aβ species. In mouse models of AD, targeted activation of microglia induces plaque clearance associated with improvement in learning and memory. Nevertheless, extensive microglial recruitment to plaques in human AD is accompanied by very little if any phagocytosis of Aβ, allowing plaques to accumulate over many years– or even decades. A central message that emerges is that the specific microglial activation profile is of utmost importance in determining whether the response is ultimately damaging or helpful. A key challenge in coming years will be to translate these basic science discoveries into potential AD therapeutics favoring pro-phagocytic activation of microglia and/or blocking the chronic, unresolved pro-inflammatory form of reactive microgliosis that wreaks havoc on the AD brain.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health Grants 5R00AG029726-04 and 1R01NS076794-01 from the NIA and the NINDS (to T. T), an Alzheimer’s Association Zenith Fellows Award ZEN-10-174633 (to T.T.), and an American Federation of Aging Research/Ellison Medical Foundation Julie Martin Mid-Career Award in Aging Research M11472 (to T. T.). T. T. is the inaugural holder of the Ben Winters Endowed Chair in Regenerative Medicine. We would like to thank Tara Weitz, Joshua J. Breunig and David Gate (Cedars-Sinai Medical Center) for helpful discussion of this work.
List of abbreviations
- Aβ
amyloid-β peptide
- AD
Alzheimer’s Disease
- APP
Amyloid Precursor Protein
- CDn
Cluster of Differentiation
- CCL
Chemokine C-C motif Ligand
- CCR
Chemokine C-C motif Receptor
- CNS
Central Nervous System
- CSF
Cerebrospinal Fluid
- CXnCLn
Chemokine C-X-C motif Ligand
- CXnCRn
Chemokine C-X-C motif Receptor
- G-CSF
Granulocyte Colony-Stimulating Factor
- GM-CSF
Granulocyte-Macrophage Colony-Stimulating Factor
- HLA-DR
Human Leukocyte Antigen
- IFN
Interferon
- IL
Interleukin
- MCI
Mild Cognitive Impairment
- M-CSF
Macrophage Colony-Stimulating Factor
- MHC II
Major Histocompatibility Complex II
- MIP-1α
Macrophage Inflammatory Peptide 1-alpha
- NFT
Neurofibrillary Tangle
- NO
Nitric Oxide
- PPAR-γ
Peroxisome Proliferator-Activated Receptor-gamma
- PS
presenilin
- RAGE
Receptor for Advanced Glycation End product
- ROS
Reactive Oxygen Species
- SR
Scavenger Receptor
- TGF-β
Transforming Growth Factor-β
- Th2
T helper 2
- TLRs
Toll Like Receptors
- TNF-α
Tumor Necrosis Factor-alpha
References
- 1.Blümcke I. History of Neuroscience: Alois Alzheimer's First Report on Cortical neurodegeneration. IBRO History of Neuroscience. 2003 [Google Scholar]
- 2.Perlmutter LS, Barron E, Chui HC. Morphologic association between microglia and senile plaque amyloid in Alzheimer's disease. Neurosci Lett. 1990;119(1):32–36. doi: 10.1016/0304-3940(90)90748-x. [DOI] [PubMed] [Google Scholar]
- 3.McGeer PL, Itagaki S, Tago H, McGeer EG. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci Lett. 1987;79(1–2):195–200. doi: 10.1016/0304-3940(87)90696-3. [DOI] [PubMed] [Google Scholar]
- 4.Dickson DW, Farlo J, Davies P, Crystal H, Fuld P, Yen SH. Alzheimer's disease. A double-labeling immunohistochemical study of senile plaques. Am J Pathol. 1988;132(1):86–101. [PMC free article] [PubMed] [Google Scholar]
- 5.Arends YM, Duyckaerts C, Rozemuller JM, Eikelenboom P, Hauw JJ. Microglia, amyloid and dementia in alzheimer disease. A correlative study. Neurobiol Aging. 2000;21(1):39–47. doi: 10.1016/s0197-4580(00)00094-4. [DOI] [PubMed] [Google Scholar]
- 6.Cagnin A, Kassiou M, Meikle SR, Banati RB. In vivo evidence for microglial activation in neurodegenerative dementia. Acta Neurol Scand Suppl. 2006;185:107–114. doi: 10.1111/j.1600-0404.2006.00694.x. [DOI] [PubMed] [Google Scholar]
- 7.Okello A, Edison P, Archer HA, Turkheimer FE, Kennedy J, Bullock R, Walker Z, Kennedy A, Fox N, Rossor M, Brooks DJ. Microglial activation and amyloid deposition in mild cognitive impairment: a PET study. Neurology. 2009;72(1):56–62. doi: 10.1212/01.wnl.0000338622.27876.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vehmas AK, Kawas CH, Stewart WF, Troncoso JC. Immune reactive cells in senile plaques and cognitive decline in Alzheimer's disease. Neurobiol Aging. 2003;24(2):321–331. doi: 10.1016/s0197-4580(02)00090-8. [DOI] [PubMed] [Google Scholar]
- 9.Bornemann KD, Wiederhold KH, Pauli C, Ermini F, Stalder M, Schnell L, Sommer B, Jucker M, Staufenbiel M. Abeta-induced inflammatory processes in microglia cells of APP23 transgenic mice. Am J Pathol. 2001;158(1):63–73. doi: 10.1016/s0002-9440(10)63945-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolmont T, Haiss F, Eicke D, Radde R, Mathis CA, Klunk WE, Kohsaka S, Jucker M, Calhoun ME. Dynamics of the microglial/amyloid interaction indicate a role in plaque maintenance. J Neurosci. 2008;28(16):4283–4292. doi: 10.1523/JNEUROSCI.4814-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graeber MB, Streit WJ. Microglia: biology and pathology. Acta Neuropathol. 2010;119(1):89–105. doi: 10.1007/s00401-009-0622-0. [DOI] [PubMed] [Google Scholar]
- 12.Boddeke EW, Meigel I, Frentzel S, Gourmala NG, Harrison JK, Buttini M, Spleiss O, Gebicke-Harter P. Cultured rat microglia express functional beta-chemokine receptors. J Neuroimmunol. 1999;98(2):176–184. doi: 10.1016/s0165-5728(99)00096-x. [DOI] [PubMed] [Google Scholar]
- 13.Van Coillie E, Van Damme J, Opdenakker G. The MCP/eotaxin subfamily of CC chemokines. Cytokine Growth Factor Rev. 1999;10(1):61–86. doi: 10.1016/s1359-6101(99)00005-2. [DOI] [PubMed] [Google Scholar]
- 14.El Khoury JB, Moore KJ, Means TK, Leung J, Terada K, Toft M, Freeman MW, Luster AD. CD36 mediates the innate host response to beta-amyloid. J Exp Med. 2003;197(12):1657–1666. doi: 10.1084/jem.20021546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prat E, Baron P, Meda L, Scarpini E, Galimberti D, Ardolino G, Catania A, Scarlato G. The human astrocytoma cell line U373MG produces monocyte chemotactic protein (MCP)-1 upon stimulation with beta-amyloid protein. Neurosci Lett. 2000;283(3):177–180. doi: 10.1016/s0304-3940(00)00966-6. [DOI] [PubMed] [Google Scholar]
- 16.Johnstone M, Gearing AJ, Miller KM. A central role for astrocytes in the inflammatory response to beta-amyloid; chemokines, cytokines and reactive oxygen species are produced. J Neuroimmunol. 1999;93(1–2):182–193. doi: 10.1016/s0165-5728(98)00226-4. [DOI] [PubMed] [Google Scholar]
- 17.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39(3):409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 18.Janelsins MC, Mastrangelo MA, Oddo S, LaFerla FM, Federoff HJ, Bowers WJ. Early correlation of microglial activation with enhanced tumor necrosis factor-alpha and monocyte chemoattractant protein-1 expression specifically within the entorhinal cortex of triple transgenic Alzheimer's disease mice. J Neuroinflammation. 2005;2:23. doi: 10.1186/1742-2094-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, Copeland NG, Lee MK, Younkin LH, Wagner SL, Younkin SG, Borchelt DR. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet. 2004;13(2):159–170. doi: 10.1093/hmg/ddh019. [DOI] [PubMed] [Google Scholar]
- 20.Ishizuka K, Kimura T, Igata-yi R, Katsuragi S, Takamatsu J, Miyakawa T. Identification of monocyte chemoattractant protein-1 in senile plaques and reactive microglia of Alzheimer's disease. Psychiatry Clin Neurosci. 1997;51(3):135–138. doi: 10.1111/j.1440-1819.1997.tb02375.x. [DOI] [PubMed] [Google Scholar]
- 21.El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13(4):432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- 22.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274(5284):99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 23.Nishiyori A, Minami M, Ohtani Y, Takami S, Yamamoto J, Kawaguchi N, Kume T, Akaike A, Satoh M. Localization of fractalkine and CX3CR1 mRNAs in rat brain: does fractalkine play a role in signaling from neuron to microglia? FEBS Lett. 1998;429(2):167–172. doi: 10.1016/s0014-5793(98)00583-3. [DOI] [PubMed] [Google Scholar]
- 24.Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, Botti P, Bacon KB, Feng L. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci U S A. 1998;95(18):10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehman EJ, Kulnane LS, Lamb BT. Alterations in beta-amyloid production and deposition in brain regions of two transgenic models. Neurobiol Aging. 2003;24(5):645–653. doi: 10.1016/s0197-4580(02)00153-7. [DOI] [PubMed] [Google Scholar]
- 26.Lee S, Varvel NH, Konerth ME, Xu G, Cardona AE, Ransohoff RM, Lamb BT. CX3CR1 deficiency alters microglial activation and reduces beta-amyloid deposition in two Alzheimer's disease mouse models. Am J Pathol. 2010;177(5):2549–2562. doi: 10.2353/ajpath.2010.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurt MA, Davies DC, Kidd M, Duff K, Rolph SC, Jennings KH, Howlett DR. Neurodegenerative changes associated with beta-amyloid deposition in the brains of mice carrying mutant amyloid precursor protein and mutant presenilin-1 transgenes. Exp Neurol. 2001;171(1):59–71. doi: 10.1006/exnr.2001.7717. [DOI] [PubMed] [Google Scholar]
- 28.Fuhrmann M, Bittner T, Jung CK, Burgold S, Page RM, Mitteregger G, Haass C, LaFerla FM, Kretzschmar H, Herms J. Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer's disease. Nat Neurosci. 2010;13(4):411–413. doi: 10.1038/nn.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel NS, Paris D, Mathura V, Quadros AN, Crawford FC, Mullan MJ. Inflammatory cytokine levels correlate with amyloid load in transgenic mouse models of Alzheimer's disease. J Neuroinflammation. 2005;2(1):9. doi: 10.1186/1742-2094-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lue LF, Rydel R, Brigham EF, Yang LB, Hampel H, Murphy GM, Jr, Brachova L, Yan SD, Walker DG, Shen Y, Rogers J. Inflammatory repertoire of Alzheimer's disease and nondemented elderly microglia in vitro. Glia. 2001;35(1):72–79. doi: 10.1002/glia.1072. [DOI] [PubMed] [Google Scholar]
- 31.Raivich G, Moreno-Flores MT, Moller JC, Kreutzberg GW. Inhibition of posttraumatic microglial proliferation in a genetic model of macrophage colony-stimulating factor deficiency in the mouse. Eur J Neurosci. 1994;6(10):1615–1618. doi: 10.1111/j.1460-9568.1994.tb00552.x. [DOI] [PubMed] [Google Scholar]
- 32.Streit WJ, Hurley SD, McGraw TS, Semple-Rowland SL. Comparative evaluation of cytokine profiles and reactive gliosis supports a critical role for interleukin-6 in neuron-glia signaling during regeneration. J Neurosci Res. 2000;61(1):10–20. doi: 10.1002/1097-4547(20000701)61:1<10::AID-JNR2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 33.Mizuno T, Doi Y, Mizoguchi H, Jin S, Noda M, Sonobe Y, Takeuchi H, Suzumura A. Interleukin-34 selectively enhances the neuroprotective effects of microglia to attenuate oligomeric amyloid-beta neurotoxicity. Am J Pathol. 2011;179(4):2016–2027. doi: 10.1016/j.ajpath.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laske C, Stransky E, Hoffmann N, Maetzler W, Straten G, Eschweiler GW, Leyhe T. Macrophage colony-stimulating factor (M-CSF) in plasma and CSF of patients with mild cognitive impairment and Alzheimer's disease. Curr Alzheimer Res. 2010;7(5):409–414. doi: 10.2174/156720510791383813. [DOI] [PubMed] [Google Scholar]
- 35.Krieger M, Herz J. Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptors and LDL receptor-related protein (LRP) Annu Rev Biochem. 1994;63:601–637. doi: 10.1146/annurev.bi.63.070194.003125. [DOI] [PubMed] [Google Scholar]
- 36.El Khoury J, Hickman SE, Thomas CA, Cao L, Silverstein SC, Loike JD. Scavenger receptor-mediated adhesion of microglia to beta-amyloid fibrils. Nature. 1996;382(6593):716–719. doi: 10.1038/382716a0. [DOI] [PubMed] [Google Scholar]
- 37.Paresce DM, Ghosh RN, Maxfield FR. Microglial cells internalize aggregates of the Alzheimer's disease amyloid beta-protein via a scavenger receptor. Neuron. 1996;17(3):553–565. doi: 10.1016/s0896-6273(00)80187-7. [DOI] [PubMed] [Google Scholar]
- 38.Husemann J, Loike JD, Anankov R, Febbraio M, Silverstein SC. Scavenger receptors in neurobiology and neuropathology: their role on microglia and other cells of the nervous system. Glia. 2002;40(2):195–205. doi: 10.1002/glia.10148. [DOI] [PubMed] [Google Scholar]
- 39.Chung H, Brazil MI, Irizarry MC, Hyman BT, Maxfield FR. Uptake of fibrillar beta-amyloid by microglia isolated from MSR-A (type I and type II) knockout mice. Neuroreport. 2001;12(6):1151–1154. doi: 10.1097/00001756-200105080-00020. [DOI] [PubMed] [Google Scholar]
- 40.Coraci IS, Husemann J, Berman JW, Hulette C, Dufour JH, Campanella GK, Luster AD, Silverstein SC, El-Khoury JB. CD36, a class B scavenger receptor, is expressed on microglia in Alzheimer's disease brains and can mediate production of reactive oxygen species in response to beta-amyloid fibrils. Am J Pathol. 2002;160(1):101–112. doi: 10.1016/s0002-9440(10)64354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bamberger ME, Harris ME, McDonald DR, Husemann J, Landreth GE. A cell surface receptor complex for fibrillar beta-amyloid mediates microglial activation. J Neurosci. 2003;23(7):2665–2674. doi: 10.1523/JNEUROSCI.23-07-02665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt AM. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382(6593):685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 43.Alarcon R, Fuenzalida C, Santibanez M, von Bernhardi R. Expression of scavenger receptors in glial cells. Comparing the adhesion of astrocytes and microglia from neonatal rats to surface-bound beta-amyloid. J Biol Chem. 2005;280(34):30406–30415. doi: 10.1074/jbc.M414686200. [DOI] [PubMed] [Google Scholar]
- 44.Marzolo MP, von Bernhardi R, Bu G, Inestrosa NC. Expression of alpha(2)-macroglobulin receptor/low density lipoprotein receptor-related protein (LRP) in rat microglial cells. J Neurosci Res. 2000;60(3):401–411. doi: 10.1002/(SICI)1097-4547(20000501)60:3<401::AID-JNR15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 45.Narita M, Holtzman DM, Schwartz AL, Bu G. Alpha2-macroglobulin complexes with and mediates the endocytosis of beta-amyloid peptide via cell surface low-density lipoprotein receptor-related protein. J Neurochem. 1997;69(5):1904–1911. doi: 10.1046/j.1471-4159.1997.69051904.x. [DOI] [PubMed] [Google Scholar]
- 46.Han J, Ulevitch RJ. Limiting inflammatory responses during activation of innate immunity. Nat Immunol. 2005;6(12):1198–1205. doi: 10.1038/ni1274. [DOI] [PubMed] [Google Scholar]
- 47.Letiembre M, Liu Y, Walter S, Hao W, Pfander T, Wrede A, Schulz-Schaeffer W, Fassbender K. Screening of innate immune receptors in neurodegenerative diseases: a similar pattern. Neurobiol Aging. 2009;30(5):759–768. doi: 10.1016/j.neurobiolaging.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y, Walter S, Stagi M, Cherny D, Letiembre M, Schulz-Schaeffer W, Heine H, Penke B, Neumann H, Fassbender K. LPS receptor (CD14): a receptor for phagocytosis of Alzheimer's amyloid peptide. Brain. 2005;128(Pt 8):1778–1789. doi: 10.1093/brain/awh531. [DOI] [PubMed] [Google Scholar]
- 49.Walter S, Letiembre M, Liu Y, Heine H, Penke B, Hao W, Bode B, Manietta N, Walter J, Schulz-Schuffer W, Fassbender K. Role of the toll-like receptor 4 in neuroinflammation in Alzheimer's disease. Cell Physiol Biochem. 2007;20(6):947–956. doi: 10.1159/000110455. [DOI] [PubMed] [Google Scholar]
- 50.Fassbender K, Walter S, Kuhl S, Landmann R, Ishii K, Bertsch T, Stalder AK, Muehlhauser F, Liu Y, Ulmer AJ, Rivest S, Lentschat A, Gulbins E, Jucker M, Staufenbiel M, Brechtel K, Walter J, Multhaup G, Penke B, Adachi Y, Hartmann T, Beyreuther K. The LPS receptor (CD14) links innate immunity with Alzheimer's disease. FASEB J. 2004;18(1):203–205. doi: 10.1096/fj.03-0364fje. [DOI] [PubMed] [Google Scholar]
- 51.Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002;61(11):1013–1021. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- 52.Richard KL, Filali M, Prefontaine P, Rivest S. Toll-like receptor 2 acts as a natural innate immune receptor to clear amyloid beta 1–42 and delay the cognitive decline in a mouse model of Alzheimer's disease. J Neurosci. 2008;28(22):5784–5793. doi: 10.1523/JNEUROSCI.1146-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jin JJ, Kim HD, Maxwell JA, Li L, Fukuchi K. Toll-like receptor 4-dependent upregulation of cytokines in a transgenic mouse model of Alzheimer's disease. J Neuroinflammation. 2008;5:23. doi: 10.1186/1742-2094-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Udan ML, Ajit D, Crouse NR, Nichols MR. Toll-like receptors 2 and 4 mediate Abeta(1–42) activation of the innate immune response in a human monocytic cell line. J Neurochem. 2008;104(2):524–533. doi: 10.1111/j.1471-4159.2007.05001.x. [DOI] [PubMed] [Google Scholar]
- 55.Jana M, Palencia CA, Pahan K. Fibrillar amyloid-beta peptides activate microglia via TLR2: implications for Alzheimer's disease. J Immunol. 2008;181(10):7254–7262. doi: 10.4049/jimmunol.181.10.7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reed-Geaghan EG, Reed QW, Cramer PE, Landreth GE. Deletion of CD14 attenuates Alzheimer's disease pathology by influencing the brain's inflammatory milieu. J Neurosci. 2010;30(46):15369–15373. doi: 10.1523/JNEUROSCI.2637-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Minoretti P, Gazzaruso C, Vito CD, Emanuele E, Bianchi M, Coen E, Reino M, Geroldi D. Effect of the functional toll-like receptor 4 Asp299Gly polymorphism on susceptibility to late-onset Alzheimer's disease. Neurosci Lett. 2006;391(3):147–149. doi: 10.1016/j.neulet.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 58.Rodriguez-Rodriguez E, Sanchez-Juan P, Mateo I, Infante J, Sanchez-Quintana C, Garcia-Gorostiaga I, Berciano J, Combarros O. Interaction between CD14 and LXRbeta genes modulates Alzheimer's disease risk. J Neurol Sci. 2008;264(1–2):97–99. doi: 10.1016/j.jns.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 59.McGeer PL, McGeer EG. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Brain Res Rev. 1995;21(2):195–218. doi: 10.1016/0165-0173(95)00011-9. [DOI] [PubMed] [Google Scholar]
- 60.Togo T, Akiyama H, Kondo H, Ikeda K, Kato M, Iseki E, Kosaka K. Expression of CD40 in the brain of Alzheimer's disease and other neurological diseases. Brain Res. 2000;885(1):117–121. doi: 10.1016/s0006-8993(00)02984-x. [DOI] [PubMed] [Google Scholar]
- 61.Calingasan NY, Erdely HA, Altar CA. Identification of CD40 ligand in Alzheimer's disease and in animal models of Alzheimer's disease and brain injury. Neurobiol Aging. 2002;23(1):31–39. doi: 10.1016/s0197-4580(01)00246-9. [DOI] [PubMed] [Google Scholar]
- 62.Tan J, Town T, Paris D, Mori T, Suo Z, Crawford F, Mattson MP, Flavell RA, Mullan M. Microglial activation resulting from CD40-CD40L interaction after beta-amyloid stimulation. Science. 1999;286(5448):2352–2355. doi: 10.1126/science.286.5448.2352. [DOI] [PubMed] [Google Scholar]
- 63.Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10(12):1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- 64.Townsend KP, Town T, Mori T, Lue LF, Shytle D, Sanberg PR, Morgan D, Fernandez F, Flavell RA, Tan J. CD40 signaling regulates innate and adaptive activation of microglia in response to amyloid beta-peptide. Eur J Immunol. 2005;35(3):901–910. doi: 10.1002/eji.200425585. [DOI] [PubMed] [Google Scholar]
- 65.Tan J, Town T, Crawford F, Mori T, DelleDonne A, Crescentini R, Obregon D, Flavell RA, Mullan MJ. Role of CD40 ligand in amyloidosis in transgenic Alzheimer's mice. Nat Neurosci. 2002;5(12):1288–1293. doi: 10.1038/nn968. [DOI] [PubMed] [Google Scholar]
- 66.Tan J, Town T, Mori T, Wu Y, Saxe M, Crawford F, Mullan M. CD45 opposes beta-amyloid peptide-induced microglial activation via inhibition of p44/42 mitogen-activated protein kinase. J Neurosci. 2000;20(20):7587–7594. doi: 10.1523/JNEUROSCI.20-20-07587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tan J, Town T, Mullan M. CD45 inhibits CD40L–induced microglial activation via negative regulation of the Src/p44/42 MAPK pathway. J Biol Chem. 2000;275(47):37224–37231. doi: 10.1074/jbc.M002006200. [DOI] [PubMed] [Google Scholar]
- 68.Zhu Y, Hou H, Rezai-Zadeh K, Giunta B, Ruscin A, Gemma C, Jin J, Dragicevic N, Bradshaw P, Rasool S, Glabe CG, Ehrhart J, Bickford P, Mori T, Obregon D, Town T, Tan J. CD45 deficiency drives amyloid-beta peptide oligomers and neuronal loss in Alzheimer's disease mice. J Neurosci. 2011;31(4):1355–1365. doi: 10.1523/JNEUROSCI.3268-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Masliah E, Mallory M, Hansen L, Alford M, Albright T, Terry R, Shapiro P, Sundsmo M, Saitoh T. Immunoreactivity of CD45, a protein phosphotyrosine phosphatase, in Alzheimer's disease. Acta Neuropathol. 1991;83(1):12–20. doi: 10.1007/BF00294425. [DOI] [PubMed] [Google Scholar]
- 70.Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, Rentsendorj A, Vargas M, Guerrero C, Wang Y, Fitzgerald KA, Underhill DM, Town T, Arditi M. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36(3):401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9(8):857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8(6):752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 73.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 74.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91(2):461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 75.Town T, Nikolic V, Tan J. The microglial “activation” continuum: from innate to adaptive responses. J Neuroinflammation. 2005;2:24. doi: 10.1186/1742-2094-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 77.Goerdt S, Orfanos CE. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity. 1999;10(2):137–142. doi: 10.1016/s1074-7613(00)80014-x. [DOI] [PubMed] [Google Scholar]
- 78.Koenigsknecht-Talboo J, Landreth GE. Microglial phagocytosis induced by fibrillar beta-amyloid and IgGs are differentially regulated by proinflammatory cytokines. J Neurosci. 2005;25(36):8240–8249. doi: 10.1523/JNEUROSCI.1808-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zelcer N, Khanlou N, Clare R, Jiang Q, Reed-Geaghan EG, Landreth GE, Vinters HV, Tontonoz P. Attenuation of neuroinflammation and Alzheimer's disease pathology by liver x receptors. Proc Natl Acad Sci U S A. 2007;104(25):10601–10606. doi: 10.1073/pnas.0701096104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Colton CA, Wilcock DM. Assessing activation states in microglia. CNS Neurol Disord Drug Targets. 2010;9(2):174–191. doi: 10.2174/187152710791012053. [DOI] [PubMed] [Google Scholar]
- 81.Colton CA, Mott RT, Sharpe H, Xu Q, Van Nostrand WE, Vitek MP. Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD. J Neuroinflammation. 2006;3:27. doi: 10.1186/1742-2094-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jimenez S, Baglietto-Vargas D, Caballero C, Moreno-Gonzalez I, Torres M, Sanchez-Varo R, Ruano D, Vizuete M, Gutierrez A, Vitorica J. Inflammatory response in the hippocampus of PS1M146L/APP751SL mouse model of Alzheimer's disease: age-dependent switch in the microglial phenotype from alternative to classic. J Neurosci. 2008;28(45):11650–11661. doi: 10.1523/JNEUROSCI.3024-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Flanary BE, Streit WJ. Progressive telomere shortening occurs in cultured rat microglia, but not astrocytes. Glia. 2004;45(1):75–88. doi: 10.1002/glia.10301. [DOI] [PubMed] [Google Scholar]
- 84.Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer's disease mice. J Neurosci. 2008;28(33):8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Njie EG, Boelen E, Stassen FR, Steinbusch HW, Borchelt DR, Streit WJ. Ex vivo cultures of microglia from young and aged rodent brain reveal age-related changes in microglial function. Neurobiol Aging. 2012;33(1):195. doi: 10.1016/j.neurobiolaging.2010.05.008. e1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Paresce DM, Chung H, Maxfield FR. Slow degradation of aggregates of the Alzheimer's disease amyloid beta-protein by microglial cells. J Biol Chem. 1997;272(46):29390–29397. doi: 10.1074/jbc.272.46.29390. [DOI] [PubMed] [Google Scholar]
- 87.Frackowiak J, Wisniewski HM, Wegiel J, Merz GS, Iqbal K, Wang KC. Ultrastructure of the microglia that phagocytose amyloid and the microglia that produce beta-amyloid fibrils. Acta Neuropathol. 1992;84(3):225–233. doi: 10.1007/BF00227813. [DOI] [PubMed] [Google Scholar]
- 88.Chung H, Brazil MI, Soe TT, Maxfield FR. Uptake, degradation, and release of fibrillar and soluble forms of Alzheimer's amyloid beta-peptide by microglial cells. J Biol Chem. 1999;274(45):32301–32308. doi: 10.1074/jbc.274.45.32301. [DOI] [PubMed] [Google Scholar]
- 89.Brazil MI, Chung H, Maxfield FR. Effects of incorporation of immunoglobulin G and complement component C1q on uptake and degradation of Alzheimer's disease amyloid fibrils by microglia. J Biol Chem. 2000;275(22):16941–16947. doi: 10.1074/jbc.M000937200. [DOI] [PubMed] [Google Scholar]
- 90.Heiple JM, Wright SD, Allen NS, Silverstein SC. Macrophages form circular zones of very close apposition to IgG-coated surfaces. Cell Motil Cytoskeleton. 1990;15(4):260–270. doi: 10.1002/cm.970150408. [DOI] [PubMed] [Google Scholar]
- 91.Giulian D, Haverkamp LJ, Yu JH, Karshin W, Tom D, Li J, Kirkpatrick J, Kuo LM, Roher AE. Specific domains of beta-amyloid from Alzheimer plaque elicit neuron killing in human microglia. J Neurosci. 1996;16(19):6021–6037. doi: 10.1523/JNEUROSCI.16-19-06021.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.London JA, Biegel D, Pachter JS. Neurocytopathic effects of beta-amyloid-stimulated monocytes: a potential mechanism for central nervous system damage in Alzheimer disease. Proc Natl Acad Sci U S A. 1996;93(9):4147–4152. doi: 10.1073/pnas.93.9.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL, 3rd, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A. 1989;86(19):7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Blum-Degen D, Muller T, Kuhn W, Gerlach M, Przuntek H, Riederer P. Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer's and de novo Parkinson's disease patients. Neurosci Lett. 1995;202(1–2):17–20. doi: 10.1016/0304-3940(95)12192-7. [DOI] [PubMed] [Google Scholar]
- 95.Akama KT, Van Eldik LJ. Beta-amyloid stimulation of inducible nitric-oxide synthase in astrocytes is interleukin-1beta- and tumor necrosis factor-alpha (TNFalpha)-dependent, and involves a TNFalpha receptor-associated factor- and NFkappaB-inducing kinase-dependent signaling mechanism. J Biol Chem. 2000;275(11):7918–7924. doi: 10.1074/jbc.275.11.7918. [DOI] [PubMed] [Google Scholar]
- 96.Mori T, Koyama N, Arendash GW, Horikoshi-Sakuraba Y, Tan J, Town T. Overexpression of human S100B exacerbates cerebral amyloidosis and gliosis in the Tg2576 mouse model of Alzheimer's disease. Glia. 2010;58(3):300–314. doi: 10.1002/glia.20924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mrak RE, Sheng JG, Griffin WS. Glial cytokines in Alzheimer's disease: review and pathogenic implications. Hum Pathol. 1995;26(8):816–823. doi: 10.1016/0046-8177(95)90001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tarkowski E, Andreasen N, Tarkowski A, Blennow K. Intrathecal inflammation precedes development of Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2003;74(9):1200–1205. doi: 10.1136/jnnp.74.9.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Luccarini I, Grossi C, Traini C, Fiorentini A, Ed Dami T, Casamenti F. Abeta plaque-associated glial reaction as a determinant of apoptotic neuronal death and cortical gliogenesis: a study in APP mutant mice. Neurosci Lett. 2012;506(1):94–99. doi: 10.1016/j.neulet.2011.10.056. [DOI] [PubMed] [Google Scholar]
- 100.Meda L, Cassatella MA, Szendrei GI, Otvos L, Jr, Baron P, Villalba M, Ferrari D, Rossi F. Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature. 1995;374(6523):647–650. doi: 10.1038/374647a0. [DOI] [PubMed] [Google Scholar]
- 101.Sundaram JR, Chan ES, Poore CP, Pareek TK, Cheong WF, Shui G, Tang N, Low CM, Wenk MR, Kesavapany S. Cdk5/p25-induced cytosolic PLA2-mediated lysophosphatidylcholine production regulates neuroinflammation and triggers neurodegeneration. J Neurosci. 2012;32(3):1020–1034. doi: 10.1523/JNEUROSCI.5177-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Otth C, Concha II, Arendt T, Stieler J, Schliebs R, Gonzalez-Billault C, Maccioni RB. AbetaPP induces cdk5-dependent tau hyperphosphorylation in transgenic mice Tg2576. J Alzheimers Dis. 2002;4(5):417–430. doi: 10.3233/jad-2002-4508. [DOI] [PubMed] [Google Scholar]
- 103.Town T, Zolton J, Shaffner R, Schnell B, Crescentini R, Wu Y, Zeng J, DelleDonne A, Obregon D, Tan J, Mullan M. p35/Cdk5 pathway mediates soluble amyloid-beta peptide-induced tau phosphorylation in vitro. J Neurosci Res. 2002;69(3):362–372. doi: 10.1002/jnr.10299. [DOI] [PubMed] [Google Scholar]
- 104.Cruz JC, Tseng HC, Goldman JA, Shih H, Tsai LH. Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron. 2003;40(3):471–483. doi: 10.1016/s0896-6273(03)00627-5. [DOI] [PubMed] [Google Scholar]
- 105.Noble W, Olm V, Takata K, Casey E, Mary O, Meyerson J, Gaynor K, LaFrancois J, Wang L, Kondo T, Davies P, Burns M, Veeranna, Nixon R, Dickson D, Matsuoka Y, Ahlijanian M, Lau LF, Duff K. Cdk5 is a key factor in tau aggregation and tangle formation in vivo. Neuron. 2003;38(4):555–565. doi: 10.1016/s0896-6273(03)00259-9. [DOI] [PubMed] [Google Scholar]
- 106.Lopes JP, Oliveira CR, Agostinho P. Role of cyclin-dependent kinase 5 in the neurodegenerative process triggered by amyloid-Beta and prion peptides: implications for Alzheimer's disease and prion-related encephalopathies. Cell Mol Neurobiol. 2007;27(7):943–957. doi: 10.1007/s10571-007-9224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Saito T, Konno T, Hosokawa T, Asada A, Ishiguro K, Hisanaga S. p25/cyclin-dependent kinase 5 promotes the progression of cell death in nucleus of endoplasmic reticulum-stressed neurons. J Neurochem. 2007;102(1):133–140. doi: 10.1111/j.1471-4159.2007.04540.x. [DOI] [PubMed] [Google Scholar]
- 108.Velez-Pardo C, Ospina GG, Jimenez del Rio M. A beta;[25–35] peptide and iron promote apoptosis in lymphocytes by an oxidative stress mechanism: involvement of H2O2, caspase-3, NF-kappaB, p53 and c-Jun. Neurotoxicology. 2002;23(3):351–365. doi: 10.1016/s0161-813x(02)00081-5. [DOI] [PubMed] [Google Scholar]
- 109.Tamagno E, Parola M, Guglielmotto M, Santoro G, Bardini P, Marra L, Tabaton M, Danni O. Multiple signaling events in amyloid beta-induced, oxidative stress-dependent neuronal apoptosis. Free Radic Biol Med. 2003;35(1):45–58. doi: 10.1016/s0891-5849(03)00244-2. [DOI] [PubMed] [Google Scholar]
- 110.Tamagno E, Guglielmotto M, Bardini P, Santoro G, Davit A, Di Simone D, Danni O, Tabaton M. Dehydroepiandrosterone reduces expression and activity of BACE in NT2 neurons exposed to oxidative stress. Neurobiol Dis. 2003;14(2):291–301. doi: 10.1016/s0969-9961(03)00131-1. [DOI] [PubMed] [Google Scholar]
- 111.Chan A, Shea TB. Folate deprivation increases presenilin expression, gamma-secretase activity, and Abeta levels in murine brain: potentiation by ApoE deficiency and alleviation by dietary S-adenosyl methionine. J Neurochem. 2007;102(3):753–760. doi: 10.1111/j.1471-4159.2007.04589.x. [DOI] [PubMed] [Google Scholar]
- 112.Agostinho P, Cunha RA, Oliveira C. Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer's disease. Curr Pharm Des. 2010;16(25):2766–2778. doi: 10.2174/138161210793176572. [DOI] [PubMed] [Google Scholar]
- 113.Ayasolla K, Khan M, Singh AK, Singh I. Inflammatory mediator and beta-amyloid (25–35)-induced ceramide generation and iNOS expression are inhibited by vitamin E. Free Radic Biol Med. 2004;37(3):325–338. doi: 10.1016/j.freeradbiomed.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 114.Rogers JT, Bush AI, Cho HH, Smith DH, Thomson AM, Friedlich AL, Lahiri DK, Leedman PJ, Huang X, Cahill CM. Iron and the translation of the amyloid precursor protein (APP) and ferritin mRNAs: riboregulation against neural oxidative damage in Alzheimer's disease. Biochem Soc Trans. 2008;36(Pt 6):1282–1287. doi: 10.1042/BST0361282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rogers JT, Lahiri DK. Metal and inflammatory targets for Alzheimer's disease. Curr Drug Targets. 2004;5(6):535–551. doi: 10.2174/1389450043345272. [DOI] [PubMed] [Google Scholar]
- 116.Grathwohl SA, Kalin RE, Bolmont T, Prokop S, Winkelmann G, Kaeser SA, Odenthal J, Radde R, Eldh T, Gandy S, Aguzzi A, Staufenbiel M, Mathews PM, Wolburg H, Heppner FL, Jucker M. Formation and maintenance of Alzheimer's disease beta-amyloid plaques in the absence of microglia. Nat Neurosci. 2009;12(11):1361–1363. doi: 10.1038/nn.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Herber DL, Roth LM, Wilson D, Wilson N, Mason JE, Morgan D, Gordon MN. Time-dependent reduction in Abeta levels after intracranial LPS administration in APP transgenic mice. Exp Neurol. 2004;190(1):245–253. doi: 10.1016/j.expneurol.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 118.Wilcock DM, Munireddy SK, Rosenthal A, Ugen KE, Gordon MN, Morgan D. Microglial activation facilitates Abeta plaque removal following intracranial anti-Abeta antibody administration. Neurobiol Dis. 2004;15(1):11–20. doi: 10.1016/j.nbd.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 119.Wyss-Coray T, Lin C, Yan F, Yu GQ, Rohde M, McConlogue L, Masliah E, Mucke L. TGF-beta1 promotes microglial amyloid-beta clearance and reduces plaque burden in transgenic mice. Nat Med. 2001;7(5):612–618. doi: 10.1038/87945. [DOI] [PubMed] [Google Scholar]
- 120.Takata K, Kitamura Y, Yanagisawa D, Morikawa S, Morita M, Inubushi T, Tsuchiya D, Chishiro S, Saeki M, Taniguchi T, Shimohama S, Tooyama I. Microglial transplantation increases amyloid-beta clearance in Alzheimer model rats. FEBS Lett. 2007;581(3):475–478. doi: 10.1016/j.febslet.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 121.Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer's disease. Neuron. 2006;49(4):489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 122.Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6(8):916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 123.Jantzen PT, Connor KE, DiCarlo G, Wenk GL, Wallace JL, Rojiani AM, Coppola D, Morgan D, Gordon MN. Microglial activation and beta -amyloid deposit reduction caused by a nitric oxide-releasing nonsteroidal anti-inflammatory drug in amyloid precursor protein plus presenilin-1 transgenic mice. J Neurosci. 2002;22(6):2246–2254. doi: 10.1523/JNEUROSCI.22-06-02246.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mandrekar S, Jiang Q, Lee CY, Koenigsknecht-Talboo J, Holtzman DM, Landreth GE. Microglia mediate the clearance of soluble Abeta through fluid phase macropinocytosis. J Neurosci. 2009;29(13):4252–4262. doi: 10.1523/JNEUROSCI.5572-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yuyama K, Sun H, Mitsutake S, Igarashi Y. Sphingolipid-modulated Exosome Secretion Promotes Clearance of Amyloid-beta by Microglia. J Biol Chem. 2012;287(14):10977–10989. doi: 10.1074/jbc.M111.324616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Naert G, Rivest S. CC chemokine receptor 2 deficiency aggravates cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer's disease. J Neurosci. 2011;31(16):6208–6220. doi: 10.1523/JNEUROSCI.0299-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shaftel SS, Kyrkanides S, Olschowka JA, Miller JN, Johnson RE, O'Banion MK. Sustained hippocampal IL-1 beta overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. J Clin Invest. 2007;117(6):1595–1604. doi: 10.1172/JCI31450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brandenburg LO, Konrad M, Wruck CJ, Koch T, Lucius R, Pufe T. Functional and physical interactions between formyl-peptide-receptors and scavenger receptor MARCO and their involvement in amyloid beta 1-42-induced signal transduction in glial cells. J Neurochem. 2010;113(3):749–760. doi: 10.1111/j.1471-4159.2010.06637.x. [DOI] [PubMed] [Google Scholar]
- 129.Tahara K, Kim HD, Jin JJ, Maxwell JA, Li L, Fukuchi K. Role of toll-like receptor signalling in Abeta uptake and clearance. Brain. 2006;129(Pt 11):3006–3019. doi: 10.1093/brain/awl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Song M, Jin J, Lim JE, Kou J, Pattanayak A, Rehman JA, Kim HD, Tahara K, Lalonde R, Fukuchi K. TLR4 mutation reduces microglial activation, increases Abeta deposits and exacerbates cognitive deficits in a mouse model of Alzheimer's disease. J Neuroinflammation. 2011;8:92. doi: 10.1186/1742-2094-8-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.DiCarlo G, Wilcock D, Henderson D, Gordon M, Morgan D. Intrahippocampal LPS injections reduce Abeta load in APP+PS1 transgenic mice. Neurobiol Aging. 2001;22(6):1007–1012. doi: 10.1016/s0197-4580(01)00292-5. [DOI] [PubMed] [Google Scholar]
- 132.Doi Y, Mizuno T, Maki Y, Jin S, Mizoguchi H, Ikeyama M, Doi M, Michikawa M, Takeuchi H, Suzumura A. Microglia activated with the toll-like receptor 9 ligand CpG attenuate oligomeric amyloid {beta} neurotoxicity in in vitro and in vivo models of Alzheimer's disease. Am J Pathol. 2009;175(5):2121–2132. doi: 10.2353/ajpath.2009.090418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sanchez-Ramos J, Song S, Sava V, Catlow B, Lin X, Mori T, Cao C, Arendash GW. Granulocyte colony stimulating factor decreases brain amyloid burden and reverses cognitive impairment in Alzheimer's mice. Neuroscience. 2009;163(1):55–72. doi: 10.1016/j.neuroscience.2009.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Boissonneault V, Filali M, Lessard M, Relton J, Wong G, Rivest S. Powerful beneficial effects of macrophage colony-stimulating factor on beta-amyloid deposition and cognitive impairment in Alzheimer's disease. Brain. 2009;132(Pt 4):1078–1092. doi: 10.1093/brain/awn331. [DOI] [PubMed] [Google Scholar]
- 135.Boyd TD, Bennett SP, Mori T, Governatori N, Runfeldt M, Norden M, Padmanabhan J, Neame P, Wefes I, Sanchez-Ramos J, Arendash GW, Potter H. GM-CSF upregulated in rheumatoid arthritis reverses cognitive impairment and amyloidosis in Alzheimer mice. J Alzheimers Dis. 2010;21(2):507–518. doi: 10.3233/JAD-2010-091471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee SC, Liu W, Brosnan CF, Dickson DW. GM-CSF promotes proliferation of human fetal and adult microglia in primary cultures. Glia. 1994;12(4):309–318. doi: 10.1002/glia.440120407. [DOI] [PubMed] [Google Scholar]
- 137.Ridwan S, Bauer H, Frauenknecht K, von Pein H, Sommer CJ. Distribution of granulocyte-monocyte colony-stimulating factor and its receptor alpha-subunit in the adult human brain with specific reference to Alzheimer's disease. J Neural Transm. 2012 doi: 10.1007/s00702-012-0794-y. [DOI] [PubMed] [Google Scholar]
- 138.Manczak M, Mao P, Nakamura K, Bebbington C, Park B, Reddy PH. Neutralization of granulocyte macrophage colony-stimulating factor decreases amyloid beta 1–42 and suppresses microglial activity in a transgenic mouse model of Alzheimer's disease. Hum Mol Genet. 2009;18(20):3876–3893. doi: 10.1093/hmg/ddp331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hjorth E, Frenkel D, Weiner H, Schultzberg M. Effects of immunomodulatory substances on phagocytosis of abeta(1–42) by human microglia. Int J Alzheimers Dis. 2010;2010 doi: 10.4061/2010/798424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kakimura J, Kitamura Y, Taniguchi T, Shimohama S, Gebicke-Haerter PJ. Bip/GRP78-induced production of cytokines and uptake of amyloid-beta(1–42) peptide in microglia. Biochem Biophys Res Commun. 2001;281(1):6–10. doi: 10.1006/bbrc.2001.4299. [DOI] [PubMed] [Google Scholar]
- 141.Chakrabarty P, Jansen-West K, Beccard A, Ceballos-Diaz C, Levites Y, Verbeeck C, Zubair AC, Dickson D, Golde TE, Das P. Massive gliosis induced by interleukin-6 suppresses Abeta deposition in vivo: evidence against inflammation as a driving force for amyloid deposition. FASEB J. 2010;24(2):548–559. doi: 10.1096/fj.09-141754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wilcock DM, Lewis MR, Van Nostrand WE, Davis J, Previti ML, Gharkholonarehe N, Vitek MP, Colton CA. Progression of amyloid pathology to Alzheimer's disease pathology in an amyloid precursor protein transgenic mouse model by removal of nitric oxide synthase 2. J Neurosci. 2008;28(7):1537–1545. doi: 10.1523/JNEUROSCI.5066-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wilcock DM, Gharkholonarehe N, Van Nostrand WE, Davis J, Vitek MP, Colton CA. Amyloid reduction by amyloid-beta vaccination also reduces mouse tau pathology and protects from neuron loss in two mouse models of Alzheimer's disease. J Neurosci. 2009;29(25):7957–7965. doi: 10.1523/JNEUROSCI.1339-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Colton CA, Wilcock DM, Wink DA, Davis J, Van Nostrand WE, Vitek MP. The effects of NOS2 gene deletion on mice expressing mutated human AbetaPP. J Alzheimers Dis. 2008;15(4):571–587. doi: 10.3233/jad-2008-15405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Webster S, Lue LF, Brachova L, Tenner AJ, McGeer PL, Terai K, Walker DG, Bradt B, Cooper NR, Rogers J. Molecular and cellular characterization of the membrane attack complex, C5b-9, in Alzheimer's disease. Neurobiol Aging. 1997;18(4):415–421. doi: 10.1016/s0197-4580(97)00042-0. [DOI] [PubMed] [Google Scholar]
- 146.Wyss-Coray T, Yan F, Lin AH, Lambris JD, Alexander JJ, Quigg RJ, Masliah E. Prominent neurodegeneration and increased plaque formation in complement-inhibited Alzheimer's mice. Proc Natl Acad Sci U S A. 2002;99(16):10837–10842. doi: 10.1073/pnas.162350199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Maier M, Peng Y, Jiang L, Seabrook TJ, Carroll MC, Lemere CA. Complement C3 deficiency leads to accelerated amyloid beta plaque deposition and neurodegeneration and modulation of the microglia/macrophage phenotype in amyloid precursor protein transgenic mice. J Neurosci. 2008;28(25):6333–6341. doi: 10.1523/JNEUROSCI.0829-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Haga S, Ikeda K, Sato M, Ishii T. Synthetic Alzheimer amyloid beta/A4 peptides enhance production of complement C3 component by cultured microglial cells. Brain Res. 1993;601(1–2):88–94. doi: 10.1016/0006-8993(93)91698-r. [DOI] [PubMed] [Google Scholar]
- 149.Majumdar A, Capetillo-Zarate E, Cruz D, Gouras GK, Maxfield FR. Degradation of Alzheimer's amyloid fibrils by microglia requires delivery of ClC-7 to lysosomes. Mol Biol Cell. 2011;22(10):1664–1676. doi: 10.1091/mbc.E10-09-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ. Decreased clearance of CNS beta-amyloid in Alzheimer's disease. Science. 2010;330(6012):1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, Chishti MA, Horne P, Heslin D, French J, Mount HT, Nixon RA, Mercken M, Bergeron C, Fraser PE, St George-Hyslop P, Westaway D. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature. 2000;408(6815):979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- 152.Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Wilcock D, Connor K, Hatcher J, Hope C, Gordon M, Arendash GW. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature. 2000;408(6815):982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 153.Boche D, Denham N, Holmes C, Nicoll JA. Neuropathology after active Abeta42 immunotherapy: implications for Alzheimer's disease pathogenesis. Acta Neuropathol. 2010;120(3):369–384. doi: 10.1007/s00401-010-0719-5. [DOI] [PubMed] [Google Scholar]
- 154.Check E. Nerve inflammation halts trial for Alzheimer's drug. Nature. 2002;415(6871):462. doi: 10.1038/415462a. [DOI] [PubMed] [Google Scholar]
- 155.Town T, Vendrame M, Patel A, Poetter D, DelleDonne A, Mori T, Smeed R, Crawford F, Klein T, Tan J, Mullan M. Reduced Th1 and enhanced Th2 immunity after immunization with Alzheimer's beta-amyloid(1–42) J Neuroimmunol. 2002;132(1–2):49–59. doi: 10.1016/s0165-5728(02)00307-7. [DOI] [PubMed] [Google Scholar]
- 156.Lemere CA, Maron R, Selkoe DJ, Weiner HL. Nasal vaccination with beta-amyloid peptide for the treatment of Alzheimer's disease. DNA Cell Biol. 2001;20(11):705–711. doi: 10.1089/10445490152717569. [DOI] [PubMed] [Google Scholar]
- 157.Lemere CA, Maron R, Spooner ET, Grenfell TJ, Mori C, Desai R, Hancock WW, Weiner HL, Selkoe DJ. Nasal A beta treatment induces anti-A beta antibody production and decreases cerebral amyloid burden in PD-APP mice. Ann N Y Acad Sci. 2000;920:328–331. doi: 10.1111/j.1749-6632.2000.tb06943.x. [DOI] [PubMed] [Google Scholar]
- 158.Nikolic WV, Bai Y, Obregon D, Hou H, Mori T, Zeng J, Ehrhart J, Shytle RD, Giunta B, Morgan D, Town T, Tan J. Transcutaneous beta-amyloid immunization reduces cerebral beta-amyloid deposits without T cell infiltration and microhemorrhage. Proc Natl Acad Sci U S A. 2007;104(7):2507–2512. doi: 10.1073/pnas.0609377104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Das P, Murphy MP, Younkin LH, Younkin SG, Golde TE. Reduced effectiveness of Abeta1-42 immunization in APP transgenic mice with significant amyloid deposition. Neurobiol Aging. 2001;22(5):721–727. doi: 10.1016/s0197-4580(01)00245-7. [DOI] [PubMed] [Google Scholar]
- 160.Mandrekar-Colucci S, Karlo JC, Landreth GE. Mechanisms Underlying the Rapid Peroxisome Proliferator-Activated Receptor-gamma-Mediated Amyloid Clearance and Reversal of Cognitive Deficits in a Murine Model of Alzheimer's Disease. J Neurosci. 2012;32(30):10117–10128. doi: 10.1523/JNEUROSCI.5268-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shie FS, Nivison M, Hsu PC, Montine TJ. Modulation of microglial innate immunity in Alzheimer's disease by activation of peroxisome proliferator-activated receptor gamma. Curr Med Chem. 2009;16(6):643–651. doi: 10.2174/092986709787458399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Deane R, Singh I, Sagare AP, Bell RD, Ross NT, LaRue B, Love R, Perry S, Paquette N, Deane RJ, Thiyagarajan M, Zarcone T, Fritz G, Friedman AE, Miller BL, Zlokovic BV. A multimodal RAGE-specific inhibitor reduces amyloid beta-mediated brain disorder in a mouse model of Alzheimer disease. J Clin Invest. 2012;122(4):1377–1392. doi: 10.1172/JCI58642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu S, Liu Y, Hao W, Wolf L, Kiliaan AJ, Penke B, Rube CE, Walter J, Heneka MT, Hartmann T, Menger MD, Fassbender K. TLR2 is a primary receptor for Alzheimer's amyloid beta peptide to trigger neuroinflammatory activation. J Immunol. 2012;188(3):1098–1107. doi: 10.4049/jimmunol.1101121. [DOI] [PubMed] [Google Scholar]
- 164.Wilkinson K, Boyd JD, Glicksman M, Moore KJ, El Khoury J. A high content drug screen identifies ursolic acid as an inhibitor of amyloid beta protein interactions with its receptor CD36. J Biol Chem. 2011;286(40):34914–34922. doi: 10.1074/jbc.M111.232116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 166.Wyss-Coray T, Lin C, Sanan DA, Mucke L, Masliah E. Chronic overproduction of transforming growth factor-beta1 by astrocytes promotes Alzheimer's disease-like microvascular degeneration in transgenic mice. Am J Pathol. 2000;156(1):139–150. doi: 10.1016/s0002-9440(10)64713-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wyss-Coray T, Masliah E, Mallory M, McConlogue L, Johnson-Wood K, Lin C, Mucke L. Amyloidogenic role of cytokine TGF-beta1 in transgenic mice and in Alzheimer's disease. Nature. 1997;389(6651):603–606. doi: 10.1038/39321. [DOI] [PubMed] [Google Scholar]
- 168.Brionne TC, Tesseur I, Masliah E, Wyss-Coray T. Loss of TGF-beta 1 leads to increased neuronal cell death and microgliosis in mouse brain. Neuron. 2003;40(6):1133–1145. doi: 10.1016/s0896-6273(03)00766-9. [DOI] [PubMed] [Google Scholar]
- 169.Magnus T, Chan A, Linker RA, Toyka KV, Gold R. Astrocytes are less efficient in the removal of apoptotic lymphocytes than microglia cells: implications for the role of glial cells in the inflamed central nervous system. J Neuropathol Exp Neurol. 2002;61(9):760–766. doi: 10.1093/jnen/61.9.760. [DOI] [PubMed] [Google Scholar]
- 170.Frautschy SA, Cole GM, Baird A. Phagocytosis and deposition of vascular beta-amyloid in rat brains injected with Alzheimer beta-amyloid. Am J Pathol. 1992;140(6):1389–1399. [PMC free article] [PubMed] [Google Scholar]
- 171.Tesseur I, Zou K, Esposito L, Bard F, Berber E, Can JV, Lin AH, Crews L, Tremblay P, Mathews P, Mucke L, Masliah E, Wyss-Coray T. Deficiency in neuronal TGF-beta signaling promotes neurodegeneration and Alzheimer's pathology. J Clin Invest. 2006;116(11):3060–3069. doi: 10.1172/JCI27341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tichauer JE, von Bernhardi R. Transforming growth factor-beta stimulates beta amyloid uptake by microglia through Smad3-dependent mechanisms. J Neurosci Res. 2012 doi: 10.1002/jnr.23082. [DOI] [PubMed] [Google Scholar]
- 173.Salins P, He Y, Olson K, Glazner G, Kashour T, Amara F. TGF-beta1 is increased in a transgenic mouse model of familial Alzheimer's disease and causes neuronal apoptosis. Neurosci Lett. 2008;430(1):81–86. doi: 10.1016/j.neulet.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 174.Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nat Immunol. 2005;6(6):600–607. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- 175.Laouar Y, Town T, Jeng D, Tran E, Wan Y, Kuchroo VK, Flavell RA. TGF-beta signaling in dendritic cells is a prerequisite for the control of autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2008;105(31):10865–10870. doi: 10.1073/pnas.0805058105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Town T, Laouar Y, Pittenger C, Mori T, Szekely CA, Tan J, Duman RS, Flavell RA. Blocking TGF-beta-Smad2/3 innate immune signaling mitigates Alzheimer-like pathology. Nat Med. 2008;14(6):681–687. doi: 10.1038/nm1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gate D, Rezai-Zadeh K, Jodry D, Rentsendorj A, Town T. Macrophages in Alzheimer's disease: the blood-borne identity. J Neural Transm. 2010;117(8):961–970. doi: 10.1007/s00702-010-0422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rezai-Zadeh K, Gate D, Town T. CNS infiltration of peripheral immune cells: D-Day for neurodegenerative disease? J Neuroimmune. Pharmacol. 2009;4(4):462–475. doi: 10.1007/s11481-009-9166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]