Abstract
The theory of cancer stem cells (CSCs) has provided evidence on fundamental clinical implications because of the involvement of CSCs in cell migration, invasion, metastasis, and treatment resistance, which leads to the poor clinical outcome of cancer patients. Therefore, targeting CSCs will provide a novel therapeutic strategy for the treatment and/or prevention of tumors. However, the regulation of CSCs and its signaling pathways during tumorigenesis are not well understood. MicroRNAs (miRNAs) have been proved to act as key regulators of the post-transcriptional regulation of genes, which involve in a wide array of biological processes including tumorigenesis. The altered expressions of miRNAs are associated with poor clinical outcome of patients diagnosed with a variety of tumors. Therefore, emerging evidence strongly suggest that miRMAs play critical roles in tumor development and progression. Emerging evidence also suggest that miRNAs participate in the regulation of tumor cell growth, migration, invasion, angiogenesis, drug resistance, and metastasis. Moreover, miRNAs such as let-7, miR-21, miR-22, miR-34, miR-101, miR-146a, and miR-200 have been found to be associated with CSC phenotype and function mediated through targeting oncogenic signaling pathways. In this article, we will discuss the role of miRNAs in the regulation of CSC phenotype and function during tumor development and progression. We will also discuss the potential role of naturally occurring agents (nutraceuticals) as potent anti-tumor agents that are believed to function by targeting CSC-related miRNAs.
Keywords: CSCs, miRNAs, natural agents
1. INTRODUCTION
Within a decade, cancer stem cells (CSCs) have attracted tremendous attentions due to its potential role in tumor aggressive phenotypes such as treatment resistance, metastasis, and tumor recurrence or relapse. Similar to normal stem cells, the CSCs have common properties such as long lifespan, angiogenic induction, apoptosis resistance, the capacity of self-renewal and differentiation, orchestrated by very small sub-population of cells consistent with the expression of stem cell genes [1]. These findings suggest a possible role of CSCs in tumor development and that CSCs might be originating from normal adult stem cells, such as mesenchymal stem cells, due to the alterations of intrinsic and extrinsic microenvironments, leading to the development of tumors [2].
It has been recognized that the CSCs only constitute a very small percentage (0.05–1%) of sub-populations of tumor cells within a tumor mass or within the tumor microenvironment, and posses the ability to self-renew leading to heterogeneous tumor cell populations with a complex group of differentiated tumor cells [1, 3–6]. The theory of CSCs has basic clinical implications especially because the CSCs have been found in the majority of malignant tumor tissues, and the CSCs have been widely considered to be resistant to chemo-radiation therapy relative to its differentiated progenies [3–5, 7]. This theory affords a good explanation for the clinical observation that tumor regression alone may not be associated with patient survival [7] because of tumor recurrence due to the existence and sustenance of CSCs after therapy. It has been suggested that the CSCs are known to play critical roles in treatment resistance, tumor metastasis and recurrence after conventional therapy, which is mediated through deregulation of multiple mechanisms and networks, as reviewed by us recently [6, 8, 9]. However, the molecular mechanism(s) of CSCs and its regulation in tumor aggressiveness is not fully understood. Here, we will discuss the role of microRNAs (miRNAs) in the regulation of CSC phenotype and function. We will also discuss the potential role of naturally occurring agents (nutraceuticals) such as polyphenolic compounds and vitamin D in targeting CSC phenotype and function with respect to tumor development and progression.
2. CSCs AND TUMOR AGGRESSIVENESS
Over the past decade, a large number of clinical and experimental studies have demonstrated that CSCs are exclusively involved in the chemotherapy resistance and metastasis, leading to poor clinical outcome of patients with malignancies such as pancreatic tumor, prostate tumor, liver tumor, breast tumor, and brain tumors [6, 10–12]. Moreover, the CSCs also contribute to resistance to radio-therapy through preferential activation of the DNA damage response, and an increase in DNA repair capacity [13]. It has been found that the subpopulation of glioma cells expressing CD133, a marker for brain CSCs, is enriched after radiation in gliomas [13, 14]. The CD133+ glioma cells survived after ionizing radiation showed remarkably increased proportions relative to large numbers of CD133− tumor cells. These findings suggest that the CD133+ tumor cells represent glioma CSCs that confers glioma radio-resistance. One animal study has revealed that CSCs in mouse mammary tumors may contribute to cisplatin resistance [15]. Similarly, CSCs in human colorectal cancers are also found to be responsible for resistance to chemotherapeutic drugs [16, 17]. Moreover, human breast cancer cells containing CSC-like cells have displayed high metastatic capacity, and showed distinct molecular signature of CSCs [18], which suggest that the CSCs of breast cancer are involved in the regulation of breast cancer metastasis.
It has also been shown that the CSCs are implicated in drug resistance and that the drug resistant cells from a variety of tumors such as pancreatic, colon, breast, and brain tumors are more tumorigenic and metastatic in vitro and in vivo. The data show that human pancreatic cancer tissues contain CD133+ CSCs that are exclusively tumorigenic and highly resistant to standard chemotherapy [6, 19]. Elimination of the CSC populations suppresses the metastatic phenotype of pancreatic tumors without altering their tumorigenic potential. These findings suggest that CSCs contribute to drug resistance and tumor metastasis. Other experimental studies have confirmed that CSCs in tumor tissues are strongly associated with drug resistance and metastatic phenotype. Patients with CSC cell surface marker positive tumor cells were found to correlate with histological grade and poor clinical outcome [20]. These findings suggest that CSCs or CSC-like cells (CSLCs) play important roles in tumor aggressiveness.
3. THE ROLE OF miRNAs IN THE REGULATION OF CSC CHARACTERISTICS
MicroRNAs (miRNAs) are a large family of small non-protein-coding RNAs with around 18–24 nucleotides in length, which function as potent post-transcriptional regulators of genes by binding to their specific binding sites in the 3’ untranslated region (3’-UTR) of their target mRNAs, leading to either degradation of mRNA or inhibition of protein translation [21, 22]. Currently, more than two thousands of miRNAs have been identified in human genome. The miRNAs are broadly recognized as regulators of the expression of, at least, one-third human mRNAs, and consequently play critical roles in a wide array of biological processes, such as cell differentiation, proliferation, cell death, metabolism and energy homeostasis [23, 24]. A large number of evidence has suggested that miRNAs might have an important role in tumorigenesis. Increased numbers of clinical studies have provided the clear supportive evidence showing that the altered expressions of miRNAs are associated with clinical prognosis of tumor, resistance to chemo-radiation therapy and tumor recurrence. More importantly, miRNAs have been considered to be the regulators of CSC phenotype and function mediated through the regulation of multiple signaling pathways, and thus miRNAs appear to play important roles in tumor development and progression. In the following sections, we will provide example of some well-characterized miRNAs that are associated with CSCs and tumor aggressiveness.
3.1. Let-7
A great number of experimental studies have suggested that let-7 family plays a key regulatory role in tumor development and progression by targeting multiple signaling pathways. The expression of let-7 has been shown to be negatively related to clinical outcome. The expression of let-7 family members, for instance, let-7a, b, c, has been identified as negative regulators of epithelial-to-mesenchymal transition (EMT) and the function of CSCs, which is in part mediated through the regulation of phosphatase and tensin homolog deleted on chromosome 10 (PTEN, a known tumor suppressor which inactivates the PI3K/Akt/mTOR signaling pathway), and the regulation of CSC gene signature marker Lin28B in pancreatic and prostate cancer cells [25–29]. The acquisition of EMT is strongly associated with treatment resistance, metastasis, and tumor recurrence in the clinical setting, has been considered as a process that is reminiscent of CSCs or CSLCs. Moreover, the expression of let-7 family has been found to be remarkably low in CSCs or CSLCs, compared to its parental cells associated with in vitro and in vivo differentiated progenies [30, 31]. In breast CSCs, forced over-expression of let-7a inhibited cell proliferation, mammosphere formation, tumor formation, and metastasis in mouse xenograft tumor, and also led to a reduction in the proportion of undifferentiated cells in vitro [31]. Increased expression of let-7 resulted in decreased levels in the expression of Lin28, and interestingly Lin28 has been identified to inhibit the expression of let-7 [25, 29, 32, 33]. It has also been shown that let-7 targets proto-oncogene N-Myc (neuroblastoma derived V-myc myelocytomatosis viral related oncogene) and inhibits the proliferative and clonogenic growth of neuroblastoma cells [34]. Recently, we found that let-7 family inhibited the expression of enhancer of zeste homolog 2 (EZH2), a major epigenetic component of polycomb repressive complex 2 (PRC2) that functions in the embryonic and adult stem cells to inhibit the expression of developmental genes that are preferentially activated during differentiation [35]. Emerging evidence suggest that EZH2 is a potential regulator for maintaining the phenotype and function of CSCs. Therefore, let-7 may have an important function in the regulation of CSCs characteristics by targeting multiple cell signaling pathways. However, its exact molecular mechanism requires further investigation.
3.2. miR-21
The miR-21 has been widely considered as an oncogenic miRNA which mediates its function by targeting multiple signaling pathways. Clinical studies showed increased expression of miR-21 in a wide range of tumors such as pancreatic cancer, prostate cancer, breast cancer and brain cancers, and it is tightly associated with poor clinical outcome of cancer patients [36, 37]. It has been reported that high expression of miR-21 results in decreased expression of PTEN in cancers [38, 39]. It has also documented that the miR-21 exerts anti-apoptotic, proliferative, invasive and angiogenic properties in cancer cells [37, 40–42]. Moreover, the data have shown that the expression of miR-21 is increased in CSCs, compared to non-CSC cancer cells [30, 43]. It has also been known that the expressions of miR-21 and HIF-1α, a potential positive mediator of CSC phenotype and function, which is consistent with the regulation of CSC signature genes, are increased in breast CSLCs [44]. Increased expression of miR-21 has also been reported in cancer cells such as breast cancer cells under hypoxic conditions [45, 46]. Forced over-expression of miR-21 in breast cancer cells led to an increase in the expression of HIF-1α and VEGF along with tumor angiogenesis. Moreover, it has been shown that over-expression of miR-21 could promote the survival of bone marrow mesenchymal stem cells under hypoxic condition. Down-regulation of miR-21 increased apoptosis of mesenchymal stem cells [47]. Our unpublished data shows that functional loss of miR-21 by its inhibitor leads to decreased formation of pancreatospheres of human pancreatic cancer cells. These findings strongly suggest that miR-21 plays a key role in the regulation of CSC characteristics mediated by regulation of multiple signaling pathways.
3.3. miR-22
Increasing evidence suggests that miR-22 appears to play an important role in tumorigenesis in a cell-type specific manner. It has been shown that the expression of miR-22 is increased in human senescent fibroblasts and epithelial cells, but decreased in a variety of cancer cells such as colon cancer, liver cancer, ovarian cancer, and breast cancer cells [48–53]. The decreased levels of miR-22 have been reported to be associated with poor clinical outcome of liver cancer patients [51]. Increased numbers of experimental studies have demonstrated that over-expression of miR-22 reduces cell growth, invasion, and metastasis in several cancer cells by targeting PTEN, p21, and p53 [48–51], which suggest that miR-22 may act as a tumor suppressor. However, one recent study showed that UV lights increased miR-22 expression and decreased PTEN expression, suggesting that the expression of PTEN might be inversely correlated with miR-22 induction. UV-induced PTEN repression is attenuated by the functional loss of miR-22 via a miR-22 inhibitor [54]. Moreover, forced over-expression of miR-22 significantly inactivates caspase signaling cascade, which leads to increased cell survival upon UV radiation. The exact role of miR-22 in the regulation of CSC characteristic is not fully understood, suggesting more investigations are required to elucidate the role of miR-22 in tumor aggressiveness.
3.4. miR-26a
A number of clinical and experimental studies have provided a clear evidence to support the potential role of miR-26a as a tumor suppressor molecule. Several reports have indicated that miR-26a modulates the cancer epigenome by repressing the polycomb group protein, EZH2, a potential regulator of CSC characteristic [55–60]. EZH2 has been identified to be associated with tumor angiogenesis, the self-renewal capacity and the gene expression of CSCs, and tumor metastasis and invasion [56, 61, 62]. Altered expression of miR-26a have been found in a wide variety of cancers such as breast cancer, prostate cancer, gastric cancer, pancreatic cancer, and nasopharyngeal carcinoma [63, 64]. Re-expression of miR-26a in cancer cells causes the down-regulation of EZH2, resulting in the inhibition of tumor metastasis and invasion [55, 58, 60]. Therefore, targeting miR-26a provides a novel therapeutic approach for cancer treatment. We have reported that re-expression of miR-26a results in the down-regulation in the expression of EZH2, Oct4, Notch-1, and EpCAM in pancreatic cancer cells [65], which strongly suggest that miR-26a appears to have an important role in the regulation of CSC characteristic modulated by targeting CSC signature genes.
3.5. miR-34a
The miR-34a is under-expressed in various tumors such as prostate cancer, gastric cancer, oral squamous cell carcinoma, colon cancer and pancreatic cancer. We and other groups have demonstrated that the methylation of miR-34a in its promoter region results in the loss of miR-34a expression in various cancer cells [66, 67]. Lower levels of miR-34a have been reportedly associated with poor clinical outcome of cancer patients [66, 67]. Emerging evidence clearly suggest that miR-34a function as a potent suppressor of tumorigenesis by inhibition of cell survival, proliferation, invasion, and metastasis mediated in part through the activation of p53 and inactivation of Cyclin D1, E2F1/2, and CDK6 in cancer [68–72]. It has also been found that miR-34a can inhibit LPS-induced inflammatory response in murine macrophages [73]. In addition, several recent reports have demonstrated that miR-34a decreases the expression of CSC genes such as CD44, CD133, and Notch-1, which is consistent with the down-regulation of the CSC self-renewal capacity in various cancer cells [22, 67, 74, 75]. It has also been demonstrated that re-expression of miR-34a,b,c could reverse the EMT phenotype by down-regulation of EMT mesenchymal markers ZEB1, Snail, and Slug [76]. Moreover, miR-34a and Snail are known to form a double-negative feedback loop to regulate EMT phenotype and function [76]. Our unpublished data show that re-expression of miR-34a decreased the formation of pancreatospheres, consistent with the inhibition in the expression of CSC cell surface proteins CD44 and EpCAM in CSC-like sphere-forming cells of pancreatic cancer cells. These data clearly suggest that miR-34a plays a key role as a potential tumor suppressor in the down-regulation of CSC phenotype and function.
3.6. miR-101
The miR-101 has been reported to act as a tumor suppressor by targeting multiple signaling pathways. The low expression of miR-101 has been found in a variety of cancers such as lung, prostate, pancreatic, and liver tumors. The clinical data shows that the low levels of miR-101 are associated with poor clinical outcome of patients diagnosed with different types of cancers [63, 64]. A number of in vitro and in vivo studies have provided some clear evidence showing that miR-101 plays a protective role in tumor aggressiveness mediated through the inhibition of CSC characteristics via repression of EZH2, an epigenetic regulator of cell survival, proliferation, and CSC phenotype and function, in various cancer cells [39, 77]. It is known that EZH2 has an important role in the development and maintenance of CSC and EMT characteristics [78]. Functional loss of miR-101 expression by its inhibitor results in the activation of EZH2 signaling network, consistent with increased capacity of CSC self-renewal, leading to the tumor aggressive phenotype. We have recently demonstrated that re-expression of miR-101 by transfection of its miRNA precursor results in the inhibition of EZH2 expression consistent with the suppression of the CSC self-renewal capacity in human pancreatic cancer cells and its CSC-like sphere cells [39], which is consistent with the results reported by others [79–82]. These findings clearly suggest that miR-101 may have a pivotal role in the regulation of CSC phenotype and function. However, the precise role of miR-101 in CSC characteristics during tumor development and progression requires further in-depth investigation.
3.7. miR-146a
Low levels of expression of miR-146a have been found to be highly associated with poor prognosis of various cancers such as prostate cancer and pancreatic cancer [64, 83]. A large number of experimental studies have suggested that miR-146a may function as a potent tumor suppressor molecule via regulation of multiple signaling pathways in a variety of tumors such as pancreatic cancer and prostate cancer. The evidence shows that miR-146a impairs NF-ϰB activity and suppresses the expression of NF-ϰB target genes such as IL-1β, IL-6, IL-8, and TNF-α mediated by the regulation in the expression of IL-1 receptor associated kinase 1 (IRAK1) and TNF receptor associated factor 6 (TRAF6) [84]. The activation of NF-ϰB signaling has been reported to be involved in the enrichment of CSC characteristic by the regulation of CSC genes such as Nanog, Sox2, and Lin28 [85]. Recently, we demonstrated that the expression of miR-146a is lost in pancreatic cancer cells while re-expression of miR-146a causes decreased capacity of tumor cell invasion, consistent with the inactivation of EGFR and NF-ϰB, leading to the down-regulation of NF-ϰB targets [83]. However, one study showed that oral squamous cell carcinoma tissue samples have high levels of miR-146a and increased expression of miR-146a enhanced the oncogenicity of oral squamous cell carcinoma cells [86]. These results suggest that the role of miR-146a in development and progression of tumors appears to be cell lineage specific, suggesting that further investigations are required to elucidate the role of miR-146 in the regulation of CSC characteristics.
3.8. miR-200
Numerous clinical and experimental studies have demonstrated that miR-200 family members play very important roles in the development and progression of tumors by targeting multiple cell signaling pathways. It has been shown that the expression of miR-200 family is decreased in a wide variety of tumors such as prostate, pancreatic, colon, gastric, and breast cancers. The altered expressions of miR-200 family are highly associated with poor clinical outcome of cancer patients [29, 40, 87]. We have reported that drug-resistant human cancer cells have decreased expression of miR-200a,b,c, and displayed more mesenchymal phenotype such as EMT characteristics. Re-expressions of miR-200a,b,c by transfection technique in drug-resistant pancreatic cancer cells or PDGF-D-induced EMT prostate cancer cells decreased the expression of ZEB1, ZEB2, Slug, and increased the expression of E-cadherin, an epithelial marker, which was consistent with findings reported by other investigators [28, 29, 66]. It has also been shown that miR-200 decreases the expression of Bmil-1, Suz12, and Notch-1, known regulators of CSC and EMT phenotypes and functions in various cancer cells, which is consistent with the inhibition of CSC formation [88–90]. More importantly, the down-regulation of miR-200 family members such as miR-200a, b, and c has been observed in CSC-like (CD44+/CD24−) cells of breast cancer [91]. These data clearly suggest that miR-200 family play a key role in the regulation of CSCs via regulating multiple signaling pathways.
3.9. Other CSC-Associated miRNAs
Other miRNAs that have been reported to be associated with CSCs came from several experimental studies. For instance, miR-16, miR-107, and miR-128 are expressed at a much lower levels in CSCs or CSLCs of tumors, compared to non-CSC tumor cells or normal cells of human tissues [1, 22, 91, 92]. However, molecular targets of CSC signature genes or mediators by these miRNAs are not reported yet. It has also been reported that forced over-expression of miR-30 and miR-181 decreased the capacity of CSC self-renewal, whereas knock-down of these miRNAs by its inhibitors increased the CSC self-renewal capacity of cancer cells [1, 22, 91, 92]. These limited studies suggest that further investigations are required to elucidate the role of these miRNAs in the regulation of CSC characteristics.
4. THE ROLE OF NATURALLY OCCURRING AGENTS (NUTRACEUTICALS) IN THE REGULATION OF CSC-RELATED miRNAs
Over a decade, the naturally occurring agents and their structurally-derived compounds have attracted remarkable attentions because of their potential role in the prevention and/or treatment of human malignancies. Considering the non-toxic characteristics of these natural agents, targeting CSC-related miRNAs by these agents could provide a novel, safe, and effective approach for controlling tumor aggressiveness. In the following paragraphs, we are discussing the potential role of several common natural agents collectively referred as nutraceuticals in the regulation of CSC-related miRNAs, leading to the attenuation of CSC characteristics, and eventually contributing to the suppression of tumor aggressiveness.
4.1. Curcumin
Curcumin, a diferuloylmethane, is a bioactive polyphenolic compound, which originates from the plant Curcuma longa (Linn) grown in tropical Southeast Asia [93–95]. Curcumin is the main component of the spice tumeric, which is responsible for the yellow pigmentation of the curry. This bioactive dietary component has received considerable attention due to its remarkable anti-inflammatory, anti-oxidative, immuno-modulating, anti-atherogenic, and anti-carcinogenic activities, and it is widely used as a therapeutic agent in Asia [96]. It has been reported that curcumin inhibits the growth of a variety of tumor cells. A large body of experimental studies has shown that curcumin induces cell apoptosis, suppress cell proliferation, cell migration and invasion, and tumor growth in vitro and in vivo, consistent with its deregulation of multiple cellular signaling pathways such as NF-ϰB, Wnt, Notch-1, and hedgehog [97–102]. The role of curcumin in the regulation of CSC-related miRNAs has been not fully investigated. One study showed that curcumin appears to decrease the expression of miR-21 in colorectal cancer cells, along with its inhibition of tumor growth in vitro and in vivo [103]. Curcumin also reportedly increased miR-22 in pancreatic cancer cells [104]. Recently, we have developed a novel synthetic compound of curcumin, 3,4-difluoro-benzo-curcumin, referred to as Difluorinated-Curcumin (CDF), which shows greater bioavailability in multiple tissues such as pancreas and prostate compared to its parental compound curcumin [98, 105]. It has been demonstrated that CDF inhibits cell growth, NF-ϰB DNA-binding activity, COX-2, Akt, and the production of VEGF and PGE2 in pancreatic cancer cells [38, 39]. Moreover, CDF has shown to inhibit the tumor growth of human pancreatic cancer in mouse xenograft models, consistent with inhibition of NF-ϰB, VEGF, EZH2, COX2, and CSC characteristics [39]. More importantly, CDF appears to increase the expression of let-7, miR-26a, miR-101 and miR-200, and decrease miR-21, which is consistent with the inhibition of pancreatosphere formation and the expression of CSC signature proteins in CSC-like sphere cells of pancreatic cancer and prostate cancer [38, 39, 106–108]. Our unpublished data also shows that CDF could increase the expression of miR-34a in pancreatic cancer cells and tissues of mouse xenografts. These data clearly suggest the role of CDF as a potent anti-tumor agent, which in part could be due to deregulation of CSC-related miRNAs.
4.2. Soy Isoflavone
Isoflavones belong to the flavanoid group of compounds, the largest class of polyphenolic compounds and are primarily exists in the Leguminosae family of plants such as soybean, lentil bean, and chickpea. However, soybean is the most common food that contains great amounts of isoflavones. Genistein, daidzein, and glycitein are the three major components of isoflavones found in soybeans and soy protein-rich products such tofu, soy milk, and soy sauce. Genistein, also referred as phytoestrogen, and it is the most studied of these bioactive compounds. A number of epidemiological and clinical studies have indicated that isoflavone-rich soy products could have protective effects against certain cancers such as prostate cancer [109–112] in addition to other cancers.
A large number of in vitro and in vivo studies from our group and others have shown that isoflavones, particularly genistein and daidzein, exhibit anti-tumor effects by targeting multiple signaling pathways such as NF-ϰB, Wnt, Notch-1, and Akt/mTOR in many types of cancers [99–101, 113– 116]. Recently, we have demonstrated that genistein treatment increases the expression of let-7b, c, d, e, miR-26a, miR-101, miR-146a, and miR-200, and decreases the expression of miR-21, CSC cell surface markers CD44 and EpCAM, and the formation of pancreatospheres, all of which are in direct agreement with its anti-tumor activity against human pancreatic cancer cells [28, 88, 117]. These data suggest that genistein has an anti-tumor activity, which is in part mediated by deregulation of CSC-related miRNAs that is important during the development and progression of tumors.
4.3. Tea Polyphenols
Tea is the secondly most consumed beverage in the world, following to water. It has been widely accepted that consumption of green tea, one of the most popular types of tea, has been highly associated with better human health including the prevention of cancers and heart disease. The epidemiological studies have shown that high dietary intake of green tea among Asian men significantly decreases the incidence of certain cancers such as prostate cancer, which suggests that green tea could be useful as a chemopreventive agent against the development and progression of cancers [118]. For instance, one epidemiological report from Japan's Public Health Center-based Prospective Study revealed that the high consumption of green tea is clearly associated with reduced risk of advanced prostate cancer [119]. Green tea and its bioactive constituents have been robustly investigated both in vitro and in vivo. One sub-group of polyphenols, referred as catechins, is the most abundant of the bioactive compounds in green tea. These catechins include epicatechin (EC), epicatechin-3-gallate, epigallocatechin, and epigallocatechin-3-gallate (EGCG). However, EGCG, accounting for more than 50% of active compounds in green tea, which has been shown to be the most potent agent contributing to the suppression of tumorigenesis and the inhibition of oxidative stress among these catechins [120]. Increased numbers of experimental studies have demonstrated that EGCG has an anti-tumor activity in vitro and in vivo, potentially related to its down-regulation of NF-ϰB, hedgehog, and Wnt pathways in a variety of cancers, such as lung, oral, colon, prostate, colon, pancreatic, gastric, ovarian, and breast cancer [121– 128]. The regulatory effects of EGCG on CSC-related miRNAs have been reported by several in vitro and in vivo studies. It has been noted that EGCG treatment inhibits the expression of androgen-induced miR-21 in prostate cancer cells [129]. Other reports also indicate that EGCG increases the expression of let-7 and miR-34a in human hepatocellular carcinoma cells and neuroblastoma cells [130, 131]. However, the decreased expression of miR-200a is observed in EGCG-treated HepG2 cells by microarray analysis [131]. These findings suggest that bioactive compounds from green tea appear to involve in the regulation of CSC characteristics. However, our knowledge is still limited, and thus further in-depth investigations are required to elucidate the role of bioactive compounds of green tea in inhibiting tumor aggressiveness, which could indeed be in part due to deregulation of CSC-related miRNAs.
4.4. Resveratrol
Resveratrol (trans-3,5,4'-trihydroxystilbene) is another type of dietary polyphenolic compounds found in several plants such as red grapes, berries and peanuts [132], but it is most abundant in the skin of grapes [96]. Resveratrol is also consumed in the form of grape-derived red wine. It has been recognized that resveratrol has antioxidant and antiinflammatory properties mediated through various molecular and biochemical pathways. Increased evidence from in vitro and in vivo experimental studies have indicated that resveratrol could suppress many types of cancers by regulation of cell proliferation, apoptosis, angiogenesis and tumor metastasis mediated through deregulation of multiple cellular signaling pathways such as Akt, Wnt, hedgehog, NF-ϰB [132– 138]. There is only limited report showing that resveratrol treatment could decrease the level of miR-21 expression in lung and colon cancer cells as documented by microarray analysis [139, 140]. However, one study reports that resveratrol decreases the expression of miR-146a in cancer cells [141]. These findings suggest that the anti-tumor activity of resveratrol appears to be in part mediated through the regulation in the expression of CSC-related miRNAs; however, more studies are required to elucidate the role of resveratrol in the suppression of CSC characteristics that may be mediated through the regulation of miRNAs during tumor development and progression.
4.5. Indole-3-Carbinol and 3,3 ′-Diindolylmethane
3,3′-diindolylmethane (DIM) is one of the dimeric products of indole-3-carbinol (I3C) which is generated from naturally occurring glucosinolates found in a wide variety of plants including members of the family “Cruciferae” such as broccoli, brussel sprouts, cauliflowers and cabbages. Under the acidic environment of the stomach, I3 C is chemically unstable and undergoes extensive and rapid reactions of self-condensation to generate several derivative products. A major condensation product of I3 C in the stomach is DIM, which appears to be the active compound. A number of epidemiological studies have suggested that human exposure to indoles through consumption of cruciferous vegetables could decrease the risk of a variety of cancers such as breast, cervical, and endometrial cancer [142]. It has been documented that DIM reduces oxidative stress and stimulate the expression of anti-oxidant response element-driven genes, suggesting the anti-oxidant function of indole compounds [143, 144]. A large number of in vitro and in vivo experimental studies have shown that DIM inhibits tumorigenesis and cancer cell growth, and induces apoptosis in cancer cells. These findings suggest that DIM could function as a potent anti-tumor agent for the prevention and/or treatment of tumors, potentially associated with its inhibition of multiple cellular signaling pathways such as NF-ϰB, Akt, and Wnt as documented by recent studies from our laboratory and others [142–147]. The role of I3C and its bioactive compounds in the regulation of CSC-related miRNAs has been not widely investigated. One experimental study showed that I3C treatment decreases the expression of miR-21 and miR-146b in vinyl carbamate-induced mouse lung tumor, along with the inhibition of tumor growth [148]. Our recent experimental studies have shown that DIM treatment increases the expression of let-7b, c, d, e and miR-146a, and decreases the expression of miR-21 and miR-22b, along with the inhibition of cell growth and invasion of human pancreatic cancer cells [28]. However, one report shows increased expression of miR-21 by DIM [149], which is very controversial and thus requires more in-depth investigations. Overall, the regulatory role of DIM appears to be important in prostate cancer as recently documented by our reports [35, 67], and thus we believe that DIM mediates its anti-tumor activity by targeting CSC-related miRNAs although further investigations are warranted.
4.6. Vitamin D
Vitamin D has long been considered as an essential nutrient for human health. There are two major types of vitamin D, namely vitamin D2 (ergocalciferol) and D3 (cholecalciferol) in the diets. The active form of vitamin D in the body is 1α, 25-dihydroxyvitamin D (1, 25-(OH)2D), which can be derived from either vitamin D2 or vitamin D3. Vitamin D2 is commonly present in fungi and mushrooms irradiated with UV lights, whereas vitamin D3 is obtained from animal sources such as fish oils and liver. Vitamin D also can be synthesized from 7 dehydrocholesterol substrate in the skin of the human body by long exposure to sun lights. The evidence from epidemiologic and experimental studies have indicated that higher intakes of vitamin D from food and/or supplements leads to higher blood levels of vitamin D, which are highly associated with reduced risks of certain cancers [150, 151]. It has been reported that reduced levels of active vitamin D in the body resulted in a higher incidence and mortality of prostate cancer [150, 151]. These findings clearly suggest that vitamin D could be useful as a chemopreventive agent for cancers. A large number of in vitro and in vivo studies have indicated that vitamin D inhibits cell growth, invasion, and angiogenesis in tumors through the regulation of multiple signaling pathways, such as Wnt, VEGF, and NF-ϰB [152, 153]. Two recent experimental studies have shown that vitamin D treatment up-regulates the expression of miR-22, and down-regulates the expression of miR-146a in cancer cells as assessed by real time PCR assay [154, 155]. One human subject study showed that 12 months of supplementation with high dose of vitamin D (40,000 IU/week) in normal subjects led to increased levels of plasma miR-let-7f and miR-26a, and reduced levels of plasma miR-22 [156]. However, the precise role of vitamin D on the CSC-related miRNAs is not yet clear. Therefore, further investigations are required to elucidate the role of vitamin D in the regulation of CSC characteristics and miRNAs in the development and progression of human malignancies.
5. CONCLUSION
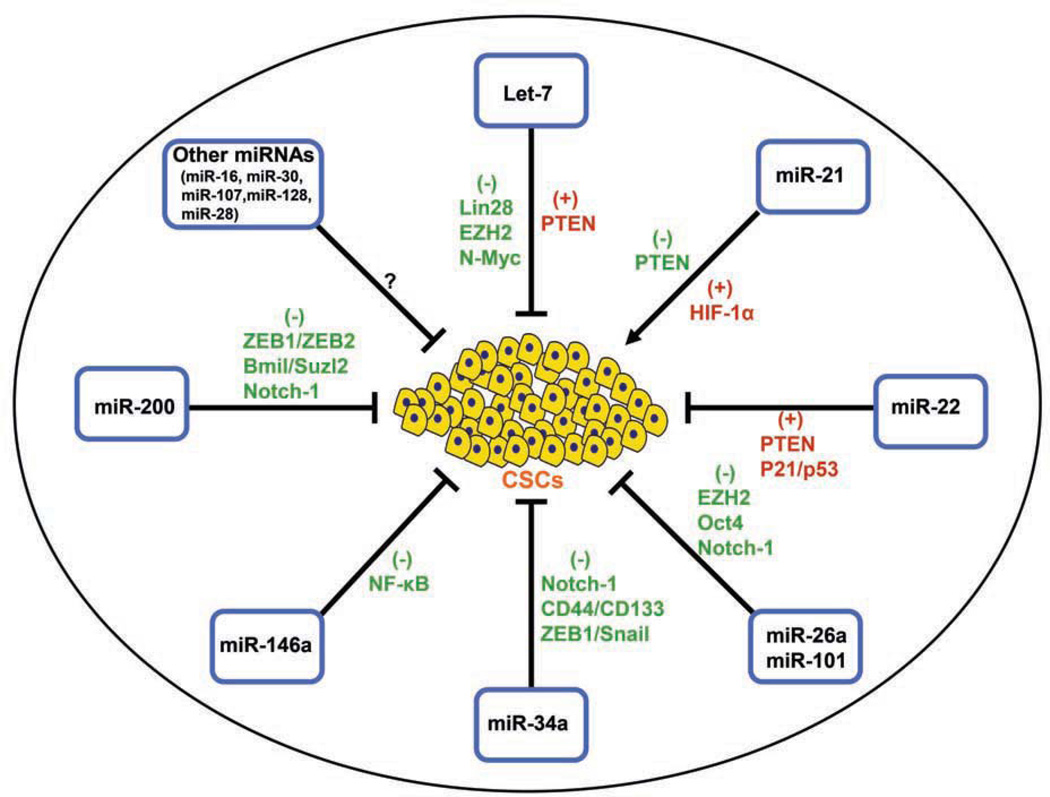
A large body of clinical and experimental studies have provided some credible evidence in support of the role of a very small sub-populations of CSCs in tumors or within the tumor microenvironment that are strongly associated with tumor aggressive phenotypes. The tumor aggressiveness is associated with increased resistance to cell apoptosis, increased growth rate, proliferation, migration, invasion, and metastasis, leading to treatment resistance and tumor recurrence or relapse, which eventually contributes to reduced overall disease-free survival rate and increased mortality of cancer patients. Extensive efforts have been focused on investigating CSC characteristics in the fields of cancer research over the past decade; however, the regulation of CSCs and its signal pathways during the development and progression of tumors are not well understood. A large number of studies have suggested that miRNAs plays pivotal roles in the post-transcriptional regulation of genes, and miRNAs are involved in a wide array of biological processes including tumorigenesis. The altered expressions of miRNAs are associated with poor clinical outcome of the patients diagnosed with a variety of cancers. Emerging evidence suggest that miRNAs have important roles in the regulation of tumor cell growth, migration, invasion, angiogenesis, drug resistance, and metastasis. Moreover, miRNAs such as let-7, miR-21, miR-22, miR-34, miR-101, miR-146a, and miR-200 have been found to be involved in the regulation of CSC characteristics mediated by targeting oncogenic signaling pathways Fig. (1). Therefore, targeting CSC signature genes along with relevant miRNAs will provide a novel and more effective therapeutic approach for the eradication of CSCs within the tumor microenvironment, which will lead to the inhibition of tumor aggressiveness. To that end, naturally occurring agents such as dietary polyphenolic and flavonoid compounds and vitamin D have been shown to have the inhibitory effects on cell growth, migration, invasion, angiogenesis, and metastasis in variety of tumors which is in part to deregulation of multiple signaling pathways and CSC-related miRNAs. Therefore targeting CSCs-related miRNAs would likely lead to the inhibition of tumor growth in vivo, suggesting the potential role of naturally occurring agents as anti-tumor agents for the prevention and/or treatment of human malignancies.
Fig. (1).
The potential roles of selected miRNAs in the regulation of CSC characteristics.
(→ : indicating an activation; : indicating an inhibition).
ACKNOWLEDGEMENTS
We thank Puschelberg and Guido foundations for their generous financial contribution. We also thank Ms. Ahmedi Bee Fnu, Mr. Anthony Badie Oraha, and Mr. Evan Bao for their technical assistance.
GRANT SUPPORT
National Cancer Institute, NIH grants, R01CA131151, R01CA132794 and R01CA154321 awarded to F.H. Sarkar, and a DOD Exploration-Hypothesis Development Award PC101482 awarded to B Bao.
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Yu C, Yao Z, Jiang Y, Keller ET. Prostate cancer stem cell biology. Minerva Urol Nefrol. 2012;64(1):19–33. [PMC free article] [PubMed] [Google Scholar]
- 2.Bao B, Ahmad A, Li Y, et al. Targeting CSCs within the tumor microenvironment for cancer therapy: a potential role of mesenchymal stem cells. Expert Opin Ther Targets. 2012;16(10):1041–1054. doi: 10.1517/14728222.2012.714774. [DOI] [PubMed] [Google Scholar]
- 3.Hermann PC, Bhaskar S, Cioffi M, Heeschen C. Cancer stem cells in solid tumors. Semin. Cancer Biol. 2010;20:77–84. doi: 10.1016/j.semcancer.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Ischenko I, Seeliger H, Kleespies A, et al. Pancreatic cancer stem cells: new understanding of tumorigenesis, clinical implications. Langenbecks Arch Surg. 2010;395(1):1–10. doi: 10.1007/s00423-009-0502-z. [DOI] [PubMed] [Google Scholar]
- 5.Lee CJ, Dosch J, Simeone DM. Pancreatic cancer stem cells. J Clin Oncol. 2008;26:2806–2812. doi: 10.1200/JCO.2008.16.6702. [DOI] [PubMed] [Google Scholar]
- 6.Sarkar FH, Li Y, Wang Z, Kong D. Pancreatic cancer stem cells and EMT in drug resistance and metastasis. Minerva Chir. 2009;64:489–500. [PMC free article] [PubMed] [Google Scholar]
- 7.Creighton CJ, Chang JC, Rosen JM. Epithelial-mesenchymal transition (EMT) in tumor-initiating cells and its clinical implications in breast cancer. J Mammary Gland Biol Neoplasia. 2010;15:253–260. doi: 10.1007/s10911-010-9173-1. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z, Li Y, Ahmad A, Azmi AS, et al. Targeting miRNAs involved in cancer stem cell and EMT regulation: An emerging concept in overcoming drug resistance. Drug Resist Updat. 2010;13:109–118. doi: 10.1016/j.drup.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, Li Y, Ahmad A, et al. Pancreatic cancer: understanding and overcoming chemoresistance. Nat. Rev. Gastroenterol Hepatol. 2011;8:27–33. doi: 10.1038/nrgastro.2010.188. [DOI] [PubMed] [Google Scholar]
- 10.Bauerschmitz GJ, Ranki T, Kangasniemi L, et al. Tissue-specific promoters active in CD44+CD24−/low breast cancer cells. Cancer Res. 2008;68:5533–5539. doi: 10.1158/0008-5472.CAN-07-5288. [DOI] [PubMed] [Google Scholar]
- 11.Folkins C, Man S, Xu P, Shaked Y, Hicklin DJ, Kerbel RS. Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res. 2007;67:3560–3564. doi: 10.1158/0008-5472.CAN-06-4238. [DOI] [PubMed] [Google Scholar]
- 12.Matsui W, Wang Q, Barber JP, et al. Clonogenic multiple myeloma progenitors, stem cell properties, and drug resistance. Cancer Res. 2008;68:190–197. doi: 10.1158/0008-5472.CAN-07-3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 14.Bao S, Wu Q, Sathornsumetee S, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 15.Shafee N, Smith CR, Wei S, et al. Cancer stem cells contribute to cisplatin resistance in Brca1/p53-mediated mouse mammary tumors. Cancer Res. 2008;68:3243–3250. doi: 10.1158/0008-5472.CAN-07-5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dylla SJ, Beviglia L, Park IK, et al. Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PLoS One. 2008;3:e2428. doi: 10.1371/journal.pone.0002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Todaro M, Alea MP, Di Stefano AB, et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of inter-leukin-4. Cell Stem Cell. 2007;1:389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Charafe-Jauffret E, Ginestier C, Iovino F, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69:1302–1313. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hermann PC, Huber SL, Herrler T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Hong SP, Wen J, Bang S, Park S, Song SY. CD44-positive cells are responsible for gemcitabine resistance in pancreatic cancer cells. Int J Cancer. 2009;125:2323–2331. doi: 10.1002/ijc.24573. [DOI] [PubMed] [Google Scholar]
- 21.Garzon R, Pichiorri F, Palumbo T, et al. MicroRNA gene expression during retinoic acid-induced differentiation of human acute promyelocytic leukemia. Oncogene. 2007;26:4148–4157. doi: 10.1038/sj.onc.1210186. [DOI] [PubMed] [Google Scholar]
- 22.Liu C, Tang DG. MicroRNA regulation of cancer stem cells. Cancer Res. 2011;71:5950–5954. doi: 10.1158/0008-5472.CAN-11-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeSano JT, Xu L. MicroRNA regulation of cancer stem cells and therapeutic implications. AAPS J. 2009;11:682–692. doi: 10.1208/s12248-009-9147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perera RJ, Ray A. MicroRNAs in the search for understanding human diseases. BioDrugs. 2007;21:97–104. doi: 10.2165/00063030-200721020-00004. [DOI] [PubMed] [Google Scholar]
- 25.Kong D, Banerjee S, Ahmad A, et al. Epithelial to mesenchymal transition is mechanistically linked with stem cell signatures in prostate cancer cells. PLoS One. 2010;5:e12445. doi: 10.1371/journal.pone.0012445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang CJ, Hsu CC, Chang CH, et al. Let-7d functions as novel regulator of epithelial-mesenchymal transition and chemoresistant property in oral cancer. Oncol Rep. 2011;26:1003–1010. doi: 10.3892/or.2011.1360. [DOI] [PubMed] [Google Scholar]
- 27.McCarty MF. Metformin may antagonize Lin28 and/or Lin28B activity, thereby boosting let-7 levels and antagonizing cancer progression. Med Hypotheses. 2012;78:262–269. doi: 10.1016/j.mehy.2011.10.041. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, VandenBoom TG, Kong D, et al. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009;69:6704–6712. doi: 10.1158/0008-5472.CAN-09-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peter ME. Let-7 and miR-200 microRNAs: guardians against pluripotency and cancer progression. Cell Cycle. 2009;8:843–852. doi: 10.4161/cc.8.6.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Golestaneh AF, Atashi A, Langroudi L, Shafiee A, Ghaemi N, Soleimani M. miRNAs expressed differently in cancer stem cells and cancer cells of human gastric cancer cell line MKN-45. Cell Biochem Funct. 2012;30(5):411–418. doi: 10.1002/cbf.2815. [DOI] [PubMed] [Google Scholar]
- 31.Yu F, Yao H, Zhu P, et al. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 32.Gunaratne PH. Embryonic stem cell microRNAs: defining factors in induced pluripotent (iPS) and cancer (CSC) stem cells? Curr Stem Cell Res Ther. 2009;4:168–177. doi: 10.2174/157488809789057400. [DOI] [PubMed] [Google Scholar]
- 33.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buechner J, Tomte E, Haug BH, et al. Tumour-suppressor microRNAs let-7 and mir-101 target the proto-oncogene MYCN and inhibit cell proliferation in MYCN-amplified neuroblastoma. Br J Cancer. 2011;105:296–303. doi: 10.1038/bjc.2011.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kong D, Heath E, Chen W, et al. Loss of Let-7 Up-Regulates EZH2 in Prostate Cancer Consistent with the Acquisition of Cancer Stem Cell Signatures That Are Attenuated by BR-DIM. PLoS One. 2012;7:e33729. doi: 10.1371/journal.pone.0033729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dillhoff M, Liu J, Frankel W, Croce C, Bloomston M. MicroRNA-21 is overexpressed in pancreatic cancer and a potential predictor of survival. J Gastrointest Surg. 2008;12:2171–2176. doi: 10.1007/s11605-008-0584-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moriyama T, Ohuchida K, Mizumoto K, et al. MicroRNA-21 modulates biological functions of pancreatic cancer cells including their proliferation, invasion, and chemoresistance. Mol Cancer Ther. 2009;8:1067–1074. doi: 10.1158/1535-7163.MCT-08-0592. [DOI] [PubMed] [Google Scholar]
- 38.Ali S, Ahmad A, Banerjee S, et al. Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res. 2010;70:3606–3617. doi: 10.1158/0008-5472.CAN-09-4598. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Bao B, Ali S, Banerjee S, et al. Curcumin Analogue CDF Inhibits Pancreatic Tumor Growth by Switching on Suppressor microRNAs and Attenuating EZH2 Expression. Cancer Res. 2012;72:335–345. doi: 10.1158/0008-5472.CAN-11-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olson P, Lu J, Zhang H, et al. MicroRNA dynamics in the stages of tumorigenesis correlate with hallmark capabilities of cancer. Genes Dev. 2009;23:2152–2165. doi: 10.1101/gad.1820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Z, Sun H, Dai H, et al. MicroRNA miR-210 modulates cellular response to hypoxia through the MYC antagonist MNT. Cell Cycle. 2009;8:2756–2768. doi: 10.4161/cc.8.17.9387. [DOI] [PubMed] [Google Scholar]
- 43.Han M, Wang Y, Liu M, et al. MiR-21 regulates epithelial-mesenchymal transition phenotype and hypoxia-inducible factor-1alpha expression in third-sphere forming breast cancer stem cell-like cells. Cancer Sci. 2012;103(6):1058–1064. doi: 10.1111/j.1349-7006.2012.02281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bao B, Azmi AS, Ali S, et al. The biological kinship of hypoxia with CSC and EMT and their relationship with deregulated expression of miRNAs and tumor aggressiveness. Biochim Biophys Acta. 2012;1826(12):272–296. doi: 10.1016/j.bbcan.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kulshreshtha R, Ferracin M, Negrini M, Calin GA, Davuluri RV, Ivan M. Regulation of microRNA expression: the hypoxic component. Cell Cycle. 2007;6:1426–1431. [PubMed] [Google Scholar]
- 46.Kulshreshtha R, Ferracin M, Wojcik SE, et al. A microRNA signature of hypoxia. Mol. Cell Biol. 2007;27:1859–1867. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nie Y, Han BM, Liu XB, et al. Identification of MicroRNAs involved in hypoxia- and serum deprivation-induced apoptosis in mesenchymal stem cells. Int J Biol Sci. 2011;7:762–768. doi: 10.7150/ijbs.7.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li J, Zhang Y, Zhao J, Kong F, Chen Y. Overexpression of miR-22 reverses paclitaxel-induced chemoresistance through activation of PTEN signaling in p53-mutated colon cancer cells. Mol Cell Biochem. 2011;357:31–38. doi: 10.1007/s11010-011-0872-8. [DOI] [PubMed] [Google Scholar]
- 49.Tsuchiya N, Izumiya M, Ogata-Kawata H, et al. Tumor suppressor miR-22 determines p53-dependent cellular fate through post-transcriptional regulation of p21. Cancer Res. 2011;71:4628–4639. doi: 10.1158/0008-5472.CAN-10-2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li J, Liang S, Yu H, Zhang J, Ma D, Lu X. An inhibitory effect of miR-22 on cell migration and invasion in ovarian cancer. Gynecol Oncol. 2010;119:543–548. doi: 10.1016/j.ygyno.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 51.Zhang J, Yang Y, Yang T, et al. microRNA-22, downregulated in hepatocellular carcinoma and correlated with prognosis, suppresses cell proliferation and tumourigenicity. Br J Cancer. 2010;103:1215–1220. doi: 10.1038/sj.bjc.6605895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pandey DP, Picard D. miR-22 inhibits estrogen signaling by directly targeting the estrogen receptor alpha mRNA. Mol Cell Biol. 2009;29:3783–3790. doi: 10.1128/MCB.01875-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiong J, Yu D, Wei N, et al. An estrogen receptor alpha suppressor, microRNA-22, is downregulated in estrogen receptor alpha-positive human breast cancer cell lines and clinical samples. FEBS J. 2010;277:1684–1694. doi: 10.1111/j.1742-4658.2010.07594.x. [DOI] [PubMed] [Google Scholar]
- 54.Tan G, Shi Y, Wu ZH. MicroRNA-22 promotes cell survival upon UV radiation by repressing PTEN Biochem. Biophys Res Commun. 2012;417:546–551. doi: 10.1016/j.bbrc.2011.11.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Banerjee R, Mani RS, Russo N, et al. The tumor suppressor gene rap1GAP is silenced by miR-101-mediated EZH2 overexpression in invasive squamous cell carcinoma. Oncogene. 2011;30(42):4339–4349. doi: 10.1038/onc.2011.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bracken AP, Pasini D, Capra M, Prosperini E, Colli E, Helin K. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J. 2003;22:5323–5335. doi: 10.1093/emboj/cdg542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Y, Xie D, Yin LW, et al. RNAi targeting EZH2 inhibits tumor growth and liver metastasis of pancreatic cancer in vivo. Cancer Lett. 2010;297:109–116. doi: 10.1016/j.canlet.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 58.Friedman JM, Liang G, Liu CC. The putative tumor suppressor microRNA-101 modulates the cancer epigenome by repressing the polycomb group protein EZH2. Cancer Res. 2009;69:2623–2629. doi: 10.1158/0008-5472.CAN-08-3114. [DOI] [PubMed] [Google Scholar]
- 59.Fujii S, Ito K, Ito Y, Ochiai A. Enhancer of zeste homologue 2 (EZH2) down-regulates RUNX3 by increasing histone H3 methylation. J Biol Chem. 2008;283:17324–17332. doi: 10.1074/jbc.M800224200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu J, He ML, Wang L, et al. MiR-26a Inhibits Cell Growth and Tumorigenesis of Nasopharyngeal Carcinoma through Repression of EZH2. Cancer Res. 2011;71:225–233. doi: 10.1158/0008-5472.CAN-10-1850. [DOI] [PubMed] [Google Scholar]
- 61.Chase A, Cross NC. Aberrations of EZH2 in Cancer Clin Cancer Res. 2011;17:2613–2618. doi: 10.1158/1078-0432.CCR-10-2156. [DOI] [PubMed] [Google Scholar]
- 62.Suva ML, Riggi N, Janiszewska M, et al. EZH2 is essential for glioblastoma cancer stem cell maintenance. Cancer Res. 2009;69:9211–9218. doi: 10.1158/0008-5472.CAN-09-1622. [DOI] [PubMed] [Google Scholar]
- 63.Lu L, Tang D, Wang L, et al. Gambogic acid inhibits TNF-alpha-induced invasion of human prostate cancer PC3 cells in vitro through PI3K/Akt and NF-kappaB signaling pathways. Acta Pharmacol Sin. 2012;33:531–541. doi: 10.1038/aps.2011.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pang Y, Young CY, Yuan H. MicroRNAs and prostate cancer. Acta Biochim. Biophys. Sin.(Shanghai) 2010;42:363–369. doi: 10.1093/abbs/gmq038. [DOI] [PubMed] [Google Scholar]
- 65.Bao B, Wang Z, Ali S, et al. Metformin Inhibits Cell Proliferation, Migration and Invasion by Attenuating CSC Function Mediated by Deregulating miRNAs in Pancreatic Cancer Cells. Cancer Prev Res (Phila) 2012;5(3):355–364. doi: 10.1158/1940-6207.CAPR-11-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kent OA, Mullendore M, Wentzel EA, et al. A resource for analysis of microRNA expression and function in pancreatic ductal adenocarcinoma cells. Cancer Biol Ther. 2009;8:2013–2024. doi: 10.4161/cbt.8.21.9685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kong D, Heath E, Chen W, et al. Epigenetic silencing of miR-34a in human prostate cancer cells and tumor tissue specimens can be reversed by BR-DIM treatment. Am J Transl Res. 2012;4:14–23. [PMC free article] [PubMed] [Google Scholar]
- 68.Aranha MM, Santos DM, Sola S, Steer CJ, Rodrigues CM. miR-34a regulates mouse neural stem cell differentiation. PLoS One. 2011;6:e21396. doi: 10.1371/journal.pone.0021396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guo Y, Li S, Qu J, et al. MiR-34a inhibits lymphatic metastasis potential of mouse hepatoma cells. Mol. Cell Biochem. 2011;354:275–282. doi: 10.1007/s11010-011-0827-0. [DOI] [PubMed] [Google Scholar]
- 70.Lodygin D, Tarasov V, Epanchintsev A, et al. Inactivation of miR-34a by aberrant CpG methylation in multiple types of cancer. Cell Cycle. 2008;7:2591–2600. doi: 10.4161/cc.7.16.6533. [DOI] [PubMed] [Google Scholar]
- 71.Sun F, Fu H, Liu Q, et al. Downregulation of CCND1 and CDK6 by miR-34a induces cell cycle arrest. FEBS Lett. 2008;582:1564–1568. doi: 10.1016/j.febslet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 72.Wang X, Meyers C, Guo M, Zheng ZM. Upregulation of p18Ink4c expression by oncogenic HPV E6 via p53-miR-34a pathway. Int J Cancer. 2011;129:1362–1372. doi: 10.1002/ijc.25800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jiang P, Liu R, Zheng Y, et al. MiR-34a inhibits lipopolysaccharide-induced inflammatory response through targeting Notch1 in murine macrophages. Exp Cell Res. 2012;318:1175–1184. doi: 10.1016/j.yexcr.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 74.Chang SJ, Weng SL, Hsieh JY, Wang TY, Chang MD, Wang HW. MicroRNA-34a modulates genes involved in cellular motility and oxidative phosphorylation in neural precursors derived from human umbilical cord mesenchymal stem cells. BMC Med Genomics. 2011;4:65. doi: 10.1186/1755-8794-4-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nalls D, Tang SN, Rodova M, Srivastava RK, Shankar S. Targeting epigenetic regulation of miR-34a for treatment of pancreatic cancer by inhibition of pancreatic cancer stem cells. PLoS One. 2011;6:e24099. doi: 10.1371/journal.pone.0024099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Siemens H, Jackstadt R, Hunten S, et al. miR-34 and SNAIL form a double-negative feedback loop to regulate epithelial-mesenchymal transitions. Cell Cycle. 2011;10:4256–4271. doi: 10.4161/cc.10.24.18552. [DOI] [PubMed] [Google Scholar]
- 77.Sparmann A, van LM. Polycomb silencers control cell fate, development and cancer. Nat Rev Cancer. 2006;6:846–856. doi: 10.1038/nrc1991. [DOI] [PubMed] [Google Scholar]
- 78.Chang CJ, Hung MC. The role of EZH2 in tumour progression. Br J Cancer. 2012;106:243–247. doi: 10.1038/bjc.2011.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alajez NM, Shi W, Hui AB, et al. Enhancer of Zeste homolog 2 (EZH2) is overexpressed in recurrent nasopharyngeal carcinoma and is regulated by miR-26a, miR-101, and miR-98. Cell Death Dis. 2010;1:e85. doi: 10.1038/cddis.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leung-Kuen AS, Chak-Lui WC, Man-Fong LJ, et al. Enhancer of zeste homolog 2 (EZH2) epigenetically silences multiple tumor suppressor miRNAs to promote liver cancer metastasis. Hepatology. 2012;56(2):622–631. doi: 10.1002/hep.25679. [DOI] [PubMed] [Google Scholar]
- 81.Smits M, Nilsson J, Mir SE, et al. miR-101 is down-regulated in glioblastoma resulting in EZH2-induced proliferation, migration, and angiogenesis. Oncotarget. 2010;1:710–720. doi: 10.18632/oncotarget.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang JG, Guo JF, Liu DL, Liu Q, Wang JJ. MicroRNA-101 exerts tumor-suppressive functions in non-small cell lung cancer through directly targeting enhancer of zeste homolog 2. J Thorac Oncol. 2011;6:671–678. doi: 10.1097/JTO.0b013e318208eb35. [DOI] [PubMed] [Google Scholar]
- 83.Li Y, VandenBoom TG, Wang Z, et al. miR-146a suppresses invasion of pancreatic cancer cells. Cancer Res. 2010;70:1486–1495. doi: 10.1158/0008-5472.CAN-09-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bhaumik D, Scott GK, Schokrpur S, Patil CK, Campisi J, Benz CC. Expression of microRNA-146 suppresses NF-kappaB activity with reduction of metastatic potential in breast cancer cells. Oncogene. 2008;27:5643–5647. doi: 10.1038/onc.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu M, Sakamaki T, Casimiro MC, et al. The canonical NF-kappaB pathway governs mammary tumorigenesis in transgenic mice and tumor stem cell expansion. Cancer Res. 2010;70:10464–10473. doi: 10.1158/0008-5472.CAN-10-0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hung PS, Chang KW, Kao SY, Chu TH, Liu CJ, Lin SC. Association between the rs2910164 polymorphism in pre-mir-146a and oral carcinoma progression. Oral Oncol. 2012;48:404–408. doi: 10.1016/j.oraloncology.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 87.Wendlandt EB, Graff JW, Gioannini TL, McCaffrey AP, Wilson ME. The role of MicroRNAs miR-200b and miR-200c in TLR4 signaling and NF-kappaB activation. Innate Immun. 2012 Apr 20; doi: 10.1177/1753425912443903. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bao B, Wang Z, Ali S, et al. Notch-1 induces epithelial-mesenchymal transition consistent with cancer stem cell phenotype in pancreatic cancer cells. Cancer Lett. 2011;307:26–36. doi: 10.1016/j.canlet.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 89.Iliopoulos D, Lindahl-Allen M, Polytarchou C, Hirsch HA, Tsichlis PN, Struhl K. Loss of miR-200 inhibition of Suz12 leads to polycomb-mediated repression required for the formation and maintenance of cancer stem cells. Mol Cell. 2010;39:761–772. doi: 10.1016/j.molcel.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leal JA, Lleonart ME. MicroRNAs and cancer stem cells: Therapeutic approaches and future perspectives. Cancer Lett. 2012 Apr 30; doi: 10.1016/j.canlet.2012.04.020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 91.Shimono Y, Zabala M, Cho RW, et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138:592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Navarro A, Monzo M. MicroRNAs in human embryonic and cancer stem cells. Yonsei Med J. 2010;51:622–632. doi: 10.3349/ymj.2010.51.5.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abas F, Lajis NH, Shaari K, Israf DA, Stanslas J, Yusuf UK, Raof SM. A labdane diterpene glucoside from the rhizomes of Curcuma mangga. J Nat Prod. 2005;68:1090–1093. doi: 10.1021/np0500171. [DOI] [PubMed] [Google Scholar]
- 94.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol. Life Sci. 2008;65:1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Narayan S. Curcumin, a multi-functional chemopreventive agent, blocks growth of colon cancer cells by targeting beta-catenin-mediated transactivation and cell-cell adhesion pathways. J Mol Histol. 2004;35:301–307. doi: 10.1023/b:hijo.0000032361.98815.bb. [DOI] [PubMed] [Google Scholar]
- 96.Hardy TM, Tollefsbol TO. Epigenetic diet: impact on the epigenome and cancer. Epigenomics. 2011;3:503–518. doi: 10.2217/epi.11.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mukherjee PK, Maity N, Nema NK, Sarkar BK. Bioactive compounds from natural resources against skin aging. Phytomedicine. 2011;19:64–73. doi: 10.1016/j.phymed.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 98.Padhye S, Chavan D, Pandey S, Deshpande J, Swamy KV, Sarkar FH. Perspectives on chemopreventive and therapeutic potential of curcumin analogs in medicinal chemistry. Mini Rev Med Chem. 2010;10:372–387. doi: 10.2174/138955710791330891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sarkar FH, Li Y. Harnessing the fruits of nature for the development of multi-targeted cancer therapeutics. Cancer Treat Rev. 2009;35:597–607. doi: 10.1016/j.ctrv.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sarkar FH, Li Y, Wang Z, Padhye S. Lesson learned from nature for the development of novel anti-cancer agents: implication of isoflavone, curcumin, and their synthetic analogs. Curr Pharm Des. 2010;16:1801–1812. doi: 10.2174/138161210791208956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang Z, Desmoulin S, Banerjee S, et al. Synergistic effects of multiple natural products in pancreatic cancer cells. Life Sci. 2008;83:293–300. doi: 10.1016/j.lfs.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang Z, Li Y, Ahmad A, Banerjee S, et al. Down-regulation of Notch-1 is associated with Akt and FoxM1 in inducing cell growth inhibition and apoptosis in prostate cancer cells. J Cell Biochem. 2011;112:78–88. doi: 10.1002/jcb.22770. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 103.Mudduluru G, George-William JN, Muppala S, et al. Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Biosci Rep. 2011;31:185–197. doi: 10.1042/BSR20100065. [DOI] [PubMed] [Google Scholar]
- 104.Sun M, Estrov Z, Ji Y, Coombes KR, Harris DH, Kurzrock R. Curcumin (diferuloylmethane) alters the expression profiles of microRNAs in human pancreatic cancer cells. Mol. Cancer Ther. 2008;7:464–473. doi: 10.1158/1535-7163.MCT-07-2272. [DOI] [PubMed] [Google Scholar]
- 105.Padhye S, Banerjee S, Chavan D, et al. Fluorocurcumins as cyclooxygenase-2 inhibitor: molecular docking, pharmacokinetics and tissue distribution in mice. Pharm Res. 2009;26:2438–2445. doi: 10.1007/s11095-009-9955-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ali S, Ahmad A, Aboukameel A, et al. Increased Ras GTPase activity is regulated by miRNAs that can be attenuated by CDF treatment in pancreatic cancer cells. Cancer Lett. 2012;319(2):173–181. doi: 10.1016/j.canlet.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 107.Bao B, Ali S, Kong D, et al. Anti-tumor activity of a novel compound-CDF is mediated by regulating miR-21, miR-200, and PTEN in pancreatic cancer. PLoS One. 2011;6:e17850. doi: 10.1371/journal.pone.0017850. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 108.Bao B, Ahmad A, Kong D, et al. Hypoxia induced aggressiveness of prostate cancer cells is linked with deregulated expression of VEGF, IL-6 and miRNAs that are attenuated by CDF. PLoS One. 2012;7(8):e43726. doi: 10.1371/journal.pone.0043726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Adlercreutz H, Honjo H, Higashi A, et al. Urinary excretion of lignans, isoflavonoid phytoestrogens in Japanese men and women consuming a traditional Japanese diet. Am J. Clin Nutr. 1991;54:1093–1100. doi: 10.1093/ajcn/54.6.1093. [DOI] [PubMed] [Google Scholar]
- 110.Adlercreutz H, Markkanen H, Watanabe S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet. 1993;342:1209–1210. doi: 10.1016/0140-6736(93)92188-y. [DOI] [PubMed] [Google Scholar]
- 111.Hebert JR, Hurley TG, Olendzki BC, Teas J, Ma Y, Hampl JS. Nutritional and socioeconomic factors in relation to prostate cancer mortality: a cross-national study. J Natl Cancer Inst. 1998;90:1637–1647. doi: 10.1093/jnci/90.21.1637. [DOI] [PubMed] [Google Scholar]
- 112.Jacobsen BK, Knutsen SF, Fraser GE. Does high soy milk intake reduce prostate cancer incidence? The Adventist Health Study (United States) Cancer Causes Control. 1998;9:553–557. doi: 10.1023/a:1008819500080. [DOI] [PubMed] [Google Scholar]
- 113.Kuang HB, Miao CL, Guo WX, Peng S, Cao YJ, Duan EK. Dick-kopf-1 enhances migration of HEK293 cell by beta-catenin/E-cadherin degradation. Front Biosci. 2009;14:2212–2220. doi: 10.2741/3373. [DOI] [PubMed] [Google Scholar]
- 114.Su Y, Simmen FA, Xiao R, Simmen RC. Expression profiling of rat mammary epithelial cells reveals candidate signaling pathways in dietary protection from mammary tumors. Physiol Genomics. 2007;30:8–16. doi: 10.1152/physiolgenomics.00023.2007. [DOI] [PubMed] [Google Scholar]
- 115.Su Y, Simmen RC. Soy isoflavone genistein upregulates epithelial adhesion molecule E-cadherin expression and attenuates beta-catenin signaling in mammary epithelial cells. Carcinogenesis. 2009;30:331–339. doi: 10.1093/carcin/bgn279. [DOI] [PubMed] [Google Scholar]
- 116.Wagner J, Lehmann L. Estrogens modulate the gene expression of Wnt-7a in cultured endometrial adenocarcinoma cells. Mol. Nutr. Food Res. 2006;50:368–372. doi: 10.1002/mnfr.200500215. [DOI] [PubMed] [Google Scholar]
- 117.Bao B, Wang Z, Ali S, et al. Over-expression of FoxM1 leads to epithelial-mesenchymal transition and cancer stem cell phenotype in pancreatic cancer cells. J Cell Biochem. 2011;112:2296–2306. doi: 10.1002/jcb.23150. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 118.Jian L, Lee AH, Binns CW. Tea and lycopene protect against prostate cancer. Asia Pac J Clin Nutr. 2007;16(Suppl 1):453–457. [PubMed] [Google Scholar]
- 119.Kurahashi N, Sasazuki S, Iwasaki M, Inoue M, Tsugane S. Green tea consumption and prostate cancer risk in Japanese men: a prospective study. Am J Epidemiol. 2008;167:71–77. doi: 10.1093/aje/kwm249. [DOI] [PubMed] [Google Scholar]
- 120.Khan N, Adhami VM, Mukhtar H. Review: green tea polyphenols in chemoprevention of prostate cancer: preclinical and clinical studies. Nutr Cancer. 2009;61:836–841. doi: 10.1080/01635580903285056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dashwood WM, Orner GA, Dashwood RH. Inhibition of beta-catenin/Tcf activity by white tea, green tea, and epigallocatechin-3-gallate (EGCG): minor contribution of H(2)O(2) at physiologically relevant EGCG concentrations. Biochem Biophys Res Commun. 2002;296:584–588. doi: 10.1016/s0006-291x(02)00914-2. [DOI] [PubMed] [Google Scholar]
- 122.Gao Z, Xu Z, Hung MS, Lin YC, et al. Promoter demethylation of WIF-1 by epigallocatechin-3-gallate in lung cancer cells. Anticancer Res. 2009;29:2025–2030. [PubMed] [Google Scholar]
- 123.Pahlke G, Ngiewih Y, Kern M, Jakobs S, Marko D, Eisenbrand G. Impact of quercetin and EGCG on key elements of the Wnt pathway in human colon carcinoma cells. J Agric Food Chem. 2006;54:7075–7082. doi: 10.1021/jf0612530. [DOI] [PubMed] [Google Scholar]
- 124.Mount JG, Muzylak M, Allen S, Althnaian T, McGonnell IM, Price JS. Evidence that the canonical Wnt signalling pathway regulates deer antler regeneration. Dev Dyn. 2006;235:1390–1399. doi: 10.1002/dvdy.20742. [DOI] [PubMed] [Google Scholar]
- 125.Bose M, Hao X, Ju J, Husain A, Park S, Lambert JD, Yang CS. Inhibition of tumorigenesis in ApcMin/+ mice by a combination of (−)-epigallocatechin-3-gallate and fish oil. J Agric Food Chem. 2007;55:7695–7700. doi: 10.1021/jf071004r. [DOI] [PubMed] [Google Scholar]
- 126.Liu L, Lai CQ, Nie L, et al. The modulation of endothelial cell gene expression by green tea polyphenol-EGCG. Mol Nutr Food Res. 2008;52:1182–1192. doi: 10.1002/mnfr.200700499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim J, Zhang X, Rieger-Christ KM, et al. Suppression of Wnt signaling by the green tea compound (−)-epigallocatechin 3-gallate(EGCG) in invasive breast cancer cells. Requirement of the transcriptional repressor HBP1. J Biol Chem. 2006;281:10865–10875. doi: 10.1074/jbc.M513378200. [DOI] [PubMed] [Google Scholar]
- 128.Tang GQ, Yan TQ, Guo W, et al. (−)-Epigallocatechin-3-gallate induces apoptosis and suppresses proliferation by inhibiting the human Indian Hedgehog pathway in human chondrosarcoma cells. J Cancer Res Clin Oncol. 2010;136:1179–1185. doi: 10.1007/s00432-010-0765-3. [DOI] [PubMed] [Google Scholar]
- 129.Siddiqui IA, Asim M, Hafeez BB, Adhami VM, Tarapore RS, Mukhtar H. Green tea polyphenol EGCG blunts androgen receptor function in prostate cancer. FASEB J. 2011;25:1198–1207. doi: 10.1096/fj.10-167924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chakrabarti M, Khandkar M, Banik NL, Ray SK. Alterations in expression of specific microRNAs by combination of 4-HPR and EGCG inhibited growth of human malignant neuroblastoma cells. Brain Res. 2012;1454:1–13. doi: 10.1016/j.brainres.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tsang WP, Kwok TT. Epigallocatechin gallate up-regulation of miR-16 and induction of apoptosis in human cancer cells. J Nutr Biochem. 2010;21:140–146. doi: 10.1016/j.jnutbio.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 132.Shakibaei M, Harikumar KB, Aggarwal BB. Resveratrol addiction: to die or not to die. Mol. Nutr. Food Res. 2009;53:115–128. doi: 10.1002/mnfr.200800148. [DOI] [PubMed] [Google Scholar]
- 133.Vanamala J, Reddivari L, Radhakrishnan S, Tarver C. Resveratrol suppresses IGF-1 induced human colon cancer cell proliferation and elevates apoptosis via suppression of IGF-1R/Wnt and activation of p53 signaling pathways. BMC. Cancer. 2010;10:238. doi: 10.1186/1471-2407-10-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hope C, Planutis K, Planutiene M, et al. Low concentrations of resveratrol inhibit Wnt signal throughput in colon-derived cells: implications for colon cancer prevention. Mol Nutr Food Res. 2008;52(Suppl 1):S52–S61. doi: 10.1002/mnfr.200700448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Roccaro AM, Leleu X, Sacco A, et al. Resveratrol exerts antiproliferative activity and induces apoptosis in Waldenstrom's macroglobulinemia. Clin. Cancer Res. 2008;14:1849–1858. doi: 10.1158/1078-0432.CCR-07-1750. [DOI] [PubMed] [Google Scholar]
- 136.Cho SW, Her SJ, Sun HJ, et al. Differential effects of secreted frizzled-related proteins (sFRPs) on osteoblastic differentiation of mouse mesenchymal cells and apoptosis of osteoblasts. Biochem Biophys Res Commun. 2008;367:399–405. doi: 10.1016/j.bbrc.2007.12.128. [DOI] [PubMed] [Google Scholar]
- 137.Cho SW, Yang JY, Sun HJ, et al. Wnt inhibitory factor (WIF)-1 inhibits osteoblastic differentiation in mouse embryonic mesenchymal cells. Bone. 2009;44:1069–1077. doi: 10.1016/j.bone.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 138.Zhou H, Shang L, Li X, et al. Resveratrol augments the canonical Wnt signaling pathway in promoting osteoblastic differentiation of multipotent mesenchymal cells. Exp Cell Res. 2009;315:2953–2962. doi: 10.1016/j.yexcr.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 139.Lancon A, Kaminski J, Tili E, Michaille JJ, Latruffe N. Control of MicroRNA Expression as a New Way for Resveratrol To Deliver Its Beneficial Effects. J Agric Food Chem. 2012;60(36):8783–8789. doi: 10.1021/jf301479v. [DOI] [PubMed] [Google Scholar]
- 140.Tili E, Michaille JJ. Resveratrol, MicroRNAs, Inflammation, and Cancer. J Nucleic Acids. 2011;2011:102431. doi: 10.4061/2011/102431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lukiw WJ, Zhao Y, Cui JG. An NF-kappaB-sensitive micro RNA-146a-mediated inflammatory circuit in Alzheimer disease and in stressed human brain cells. J Biol Chem. 2008;283:31315–31322. doi: 10.1074/jbc.M805371200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007;55:224–236. doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nho CW, Jeffery E. Crambene, a bioactive nitrile derived from glucosinolate hydrolysis, acts via the antioxidant response element to upregulate quinone reductase alone or synergistically with in-dole-3-carbinol. Toxicol Appl Pharmacol. 2004;198:40–48. doi: 10.1016/j.taap.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 144.Benabadji SH, Wen R, Zheng JB, Dong XC, Yuan SG. Anticarcinogenic and antioxidant activity of diindolylmethane derivatives. Acta Pharmacol Sin. 2004;25:666–671. [PubMed] [Google Scholar]
- 145.Fares F, Azzam N, Appel B, Fares B, Stein A. The potential efficacy of 3,3'-diindolylmethane in prevention of prostate cancer development. Eur J Cancer Prev. 2010;19:199–203. doi: 10.1097/CEJ.0b013e328333fbce. [DOI] [PubMed] [Google Scholar]
- 146.Li Y, Wang Z, Kong D, et al. Regulation of FOXO3a/beta-catenin/GSK-3beta signaling by 3,3'-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in prostate cancer cells. J Biol Chem. 2007;282:21542–21550. doi: 10.1074/jbc.M701978200. [DOI] [PubMed] [Google Scholar]
- 147.Li Y, Zhang T, Korkaya H, et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res. 2010;16:2580–2590. doi: 10.1158/1078-0432.CCR-09-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Melkamu T, Zhang X, Tan J, Zeng Y, Kassie F. Alteration of microRNA expression in vinyl carbamate-induced mouse lung tumors and modulation by the chemopreventive agent indole-3-carbinol. Carcinogenesis. 2010;31:252–258. doi: 10.1093/carcin/bgp208. [DOI] [PubMed] [Google Scholar]
- 149.Jin Y, Zou X, Feng X. 3,3'-Diindolylmethane negatively regulates Cdc25A and induces a G2/M arrest by modulation of microRNA 21 in human breast cancer cells. Anticancer Drugs. 2010;21:814–822. doi: 10.1097/CAD.0b013e32833e53ea. [DOI] [PubMed] [Google Scholar]
- 150.Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, Holick MF. The role of vitamin D in cancer prevention. Am. J. Public Health. 2006;96:252–261. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ahn J, Albanes D, Peters U, et al. Dairy products, calcium intake, and risk of prostate cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol. Biomarkers Prev. 2007;16:2623–2630. doi: 10.1158/1055-9965.EPI-07-0601. [DOI] [PubMed] [Google Scholar]
- 152.Ben-Shoshan M, Amir S, Dang DT, Dang LH, Weisman Y, Mab-jeesh NJ. 1alpha,25-dihydroxyvitamin D3 (Calcitriol) inhibits hypoxia-inducible factor-1/vascular endothelial growth factor pathway in human cancer cells. Mol Cancer Ther. 2007;6:1433–1439. doi: 10.1158/1535-7163.MCT-06-0677. [DOI] [PubMed] [Google Scholar]
- 153.Pike JW, Meyer MB, Martowicz ML, et al. Emerging regulatory paradigms for control of gene expression by 1,25-dihydroxyvitamin D3. J Steroid Biochem. Mol Biol. 2010;121:130–135. doi: 10.1016/j.jsbmb.2010.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang WL, Chatterjee N, Chittur SV, Welsh J, Tenniswood MP. Effects of 1alpha,25 dihydroxyvitamin D3 and testosterone on miRNA and mRNA expression in LNCaP cells. Mol Cancer. 2011;10:58. doi: 10.1186/1476-4598-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lutherborrow M, Bryant A, Jayaswal V, et al. Expression profiling of cytogenetically normal acute myeloid leukemia identifies microRNAs that target genes involved in monocytic differentiation. Am J Hematol. 2011;86:2–11. doi: 10.1002/ajh.21864. [DOI] [PubMed] [Google Scholar]
- 156.Jorde R, Svartberg J, Joakimsen RM, Coucheron DH. Plasma profile of microRNA after supplementation with high doses of vitamin D3 for 12 months. BMC Res Notes. 2012;5:245. doi: 10.1186/1756-0500-5-245. [DOI] [PMC free article] [PubMed] [Google Scholar]