Abstract
The checkpoint kinase Chk1 undergoes ATR-mediated phosphorylation and activation in response to unreplicated DNA, but the precise mechanism of Chk1 activation is not known. In this study, we have analyzed the domain structure of Xenopus Chk1 and explored the mechanism of its activation by ATR-mediated phosphorylation. We show that the C-terminal region of Xenopus Chk1 contains an autoinhibitory region (AIR), which largely overlaps with a bipartite, unusually long (∼85-amino acid) nuclear localization signal. When coexpressed in oocytes or embryos, the AIR can interact with and inhibit the kinase domain of Chk1, but not full-length Chk1, suggesting an autoinhibitory intramolecular interaction in the Chk1 molecule. If linked with the preceding ATR phosphorylation domain that has either phospho-mimic mutation or genuine phosphorylation, however, the AIR can no longer interact with or inhibit the kinase domain, suggesting a conformational change of the AIR by ATR-mediated phosphorylation. Even in full-length Chk1, such phospho-mimic mutation can interfere with the autoinhibitory intramolecular interaction, but only if this interaction is somewhat weakened by an additional mutation in the AIR. These results provide significant insights into the mechanism of Chk1 activation at the DNA replication checkpoint.
INTRODUCTION
In eukaryotic cells, genotoxic stress that damages the DNA or inhibits DNA synthesis causes activation of checkpoints (Hartwell and Weinert, 1989), which lead to diverse cellular responses such as cell cycle arrest, DNA repair, and cell death (Zhou and Elledge, 2000). The cell cycle checkpoints elicit signaling pathways that ultimately inhibit cyclin-dependent kinases, thereby delaying or arresting the cell cycle at specific stages (Nurse, 1997; Walworth, 2000). In vertebrates, upstream elements of the cell cycle checkpoint pathways include the kinase ATM and its relative ATR (Abraham, 2001). ATM and ATR phosphorylate and activate the effector kinases Cds1 (also called Chk2) and Chk1, respectively, which in turn directly target regulators of the cell cycle (Rhind and Russell, 2000; Shiloh, 2001). Although the ATM-Cds1 pathway is activated primarily by ionizing radiation (IR)-induced DNA damage and is nonessential for cell viability (Barlow et al., 1996; Matsuoka et al., 1998), the ATR-Chk1 pathway is induced primarily by unreplicated DNA and is essential at least for early embryogenesis (Liu et al., 2000; Shimuta et al., 2002). Recent studies also show that ATM (but not ATR) activates Chk1 in response to IR-induced DNA damage (Gatei et al., 2003; Sørensen et al., 2003).
Although the activation mechanism of Cds1 has recently been elucidated in some detail (Xu et al., 2002), that of Chk1 remains largely unknown. Chk1 is a nuclear protein like Cds1 but is structurally distinct from Cds1 (Rhind and Russell, 2000; Bartek and Lukas, 2003). In all eukaryotes so far examined, Chk1 protein has a well-conserved primary structure, containing an N-terminal kinase domain, a putative flexible linker region, an SQ/TQ domain, and a C-terminal domain with ill-defined function (Sanchez et al., 1997; Chen et al., 2000). A crystal structure of the kinase domain of recombinant human Chk1 protein revealed an open kinase conformation, implying that the kinase domain itself is catalytically active without any modification (Chen et al., 2000). The SQ/TQ domain has several conserved Ser-Gln (SQ) or Thr-Gln (TQ) motifs, in which the serine or threonine residues are preferred phosphorylation sites by ATR in vitro (Kim et al., 1999; Abraham, 2001). Indeed, phosphorylation of the SQ/TQ motifs occurs principally in an ATR-dependent manner and is essential for the activation of Chk1 at the DNA damage/replication checkpoint (Guo et al., 2000; Liu et al., 2000; Lopez-Girona et al., 2001; Zhao and Piwnica-Worms, 2001). However, several other proteins such as Claspin and Brca1 are required for the activation of Chk1 by ATR, although exactly how they function for Chk1 activation is not known (Kumagai and Dunphy, 2000, 2003; Yarden et al., 2002).
Under normal conditions, the C-terminal domain of Chk1 seems to function to negatively regulate Chk1 kinase activity. Thus, in vitro, full-length human Chk1 has >20-fold less kinase activity than the kinase domain itself, indicating that at least part of the C-terminal half (∼220 amino acids) of Chk1 negatively impacts Chk1 kinase activity (Chen et al., 2000). Indeed, in Xenopus oocytes, truncations of the C-terminal 15–60 amino acids progressively increase the Chk1 kinase activity up to a 25-fold activity compared with full-length Chk1; the sequence surrounding the C-terminal amino acid 15 is also required for nuclear localization of Xenopus Chk1 (Oe et al., 2001). These results raise the possibility that at least part of the C-terminal region of Chk1 may have an autoinhibitory sequence (as well as a nuclear localization signal, NLS) that might interact with and inhibit the kinase domain. Consistent with this possibility, a recent study shows that the C-terminal half of rat Chk1 can physically interact with the kinase domain (Shann and Hsu, 2001). So far, however, whether this interaction occurs intermolecularly or intramolecularly in full-length Chk1 molecules or whether the interaction can inhibit the kinase domain of Chk1 or not is not known. It is also unclear whether phosphorylation of the SQ/TQ motifs by ATR can affect the potential interaction in Chk1 molecules. In addition, whether SQ/TQ phosphorylation by ATR is sufficient for the activation of Chk1 or not remains an elusive question, because phospho-mimic mutation of the SQ/TQ motifs alone cannot activate human or fission yeast Chk1 proteins (Capasso et al., 2002; Gatei et al., 2003).
In this study, we have analyzed the C-terminal domain structure of Xenopus Chk1 in more detail and examined the effects of mutation or phosphorylation of the SQ/TQ motifs on the domain–domain interaction and kinase activity of Chk1. We show that the C-terminal region after the SQ/TQ domain contains an autoinhibitory region (AIR), which largely overlaps with a bipartite, unusually long NLS. When coexpressed in oocytes or embryos, the AIR can bind to and inhibit the kinase domain of Chk1, but not full-length Chk1. If linked with the preceding SQ/TQ domain that has either phospho-mimic DQ/EQ mutations or ATR-phosphorylated SQ/TQ motifs, however, the AIR can no longer interact with or inhibit the kinase domain. Even in full-length Chk1 molecules, such phospho-mimic mutations can interfere with the autoinhibitory intramolecular interaction, but only if this interaction is somewhat weakened by an additional mutation in the AIR. These results provide significant insights into the mechanism of Chk1 activation at the DNA damage/replication checkpoint.
MATERIALS AND METHODS
Preparation, Microinjection, and Treatment of Oocytes and Embryos
Xenopus oocytes and embryos were prepared, cultured, and microinjected as described previously (Furuno et al., 1994; Shimuta et al., 2002). To induce oocyte maturation, stage VI oocytes were treated with progesterone (5 μg/ml). Early blastula embryos (at stage 7.5) were treated with aphidicolin (100 μg/ml) or caffeine (10 mM) as described previously (Shimuta et al., 2002).
Subcellular Fractionation of Oocytes
Oocytes were manually dissected into the nuclear and cytoplasmic fractions in mineral oil to prevent any leakage of nuclear contents (Lund and Paine, 1990). In some cases, fractionation was performed by using oocytes fixed with 10% trichloroacetic acid (Oe et al., 2001).
Embryo Extracts for Binding Assay
Two groups of 40 embryos expressing either GST-KD or Myc-C3 constructs were homogenized separately in 80 μl of an extraction buffer (EB) (80 mM β-glycerophosphate, 20 mM EGTA, 15 mM MgCl2, 0.3% Triton X-100, 10 μM pepstatin A, 20 μM leupeptin, 2 mM phenylmethylsulfonyl fluoride) in the presence of 5 μM tautomycin and 5 μM okadaic acid. After brief centrifugation, supernatants from the two groups of embryos were mixed, incubated for 45 min at 4°C, and subjected to glutathione S-transferase (GST)-pulldown assays (see below) in the presence of tautomycin and okadaic acid.
Plasmid Constructs and In Vitro Transcription
All the Xenopus Chk1 cDNA constructs were subcloned into the pT7G(UKII+) vector (Oe et al., 2001); the encoded protein was tagged N-terminally with either GST or a c-Myc epitope, depending on the constructs. Point mutations in Chk1 (374KR→AA, 404KK→AA, 418RR→AA, 451KR→AA, 456KIK→AAA, and 314TQ/344SQ/356SQ/365SQ→4AQ or 4DQ or 4EQ) were introduced by site-directed mutagenesis (Okamoto et al., 2002). (The amino acid numbering of Xenopus Chk1 was according to Kumagai et al., 1998.) To construct the internal deletion mutants [Δ381–403, Δ404–426, Δ427–450, Δ381–450, Δ386–445, ΔSQ/TQ (or Δ314–367), and ΔNLS (or Δ367–474)], pT7G(UKII+)-Myc-Chk1 fragments other than the regions to be deleted were amplified by polymerase chain reaction (PCR) by using EcoRI site-containing primers. The resulting PCR products were digested with EcoRI and self-ligated; as a consequence, two extra amino acids, Glu-Phe, were introduced at the ligation (or deletion) site. To construct Δ384–443/GST, the internal region (384–443) of Chk1 was replaced by a GST fragment (1–60) by using appropriate primers. The N-terminally GST-tagged Chk1 constructs [GST-C1(415–474), GST-C2(360–474), GST-C3(283–474), and GST-KD(1–277)] were made by a PCR-based method by using appropriate primers. Point mutations or deletions of all cDNA constructs were confirmed by DNA sequencing. In vitro transcription of the cDNAs was performed as described previously (Okamoto et al., 2002).
GST-Pulldown Assay
Oocytes or embryos expressing GST-fusion proteins were homogenized in the above-described EB buffer (10 μl/oocyte or embryo) and centrifuged briefly to obtain supernatants. (In the case of the embryos, the EB buffer was supplemented with 3 μM tautomycin.) For GST-pulldown assays, glutathione-Sepharose beads (Amersham Biosciences, Piscataway, NJ) were added to the supernatants, which were then incubated for 30 min at 4°C under constant agitation. The beads were washed four times with the EB buffer and boiled in an SDS-sample buffer for immunoblotting.
Immunoblotting
Routinely, proteins equivalent to one oocyte or embryo were subjected to immunoblot analysis by using anti-Myc antibody (Santa Cruz Biotechnology, Santa Cruz, CA), anti-GST antibody (Santa Cruz Biotechnology), or anti-phospho-Ser344 antibody (or anti-human Chk1 phospho-Ser345 antibody; Cell Signaling Technology, Beverly, MA), essentially as described previously (Shimuta et al., 2002). (The anti-human Chk1 phospho-Ser345 antibody can recognize well the phosphorylated Ser344 of Xenopus Chk1; Shimuta et al., 2002.) Detections were performed by using the ECL Plus system (Amersham Biosciences) or SuperSignal (PIERCE).
RESULTS
Identification of an Unusually Long Bipartite NLS at the C Terminus
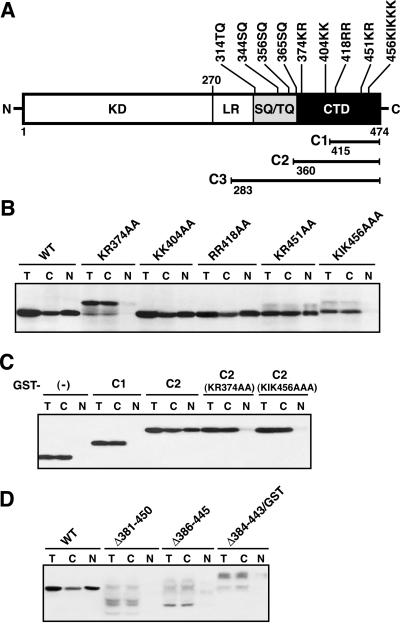
Chk1 proteins generally contain an N-terminal kinase domain (∼270-amino acid residues), a central flexible linker region (∼50 residues), an SQ/TQ domain (∼60 residues), and a C-terminal domain with ill-defined function (∼100 residues) (Sanchez et al., 1997; Chen et al., 2000; see also Figure 1A). At least part of the C-terminal domain (here called the CTD; Figure 1A) of Xenopus Chk1 is required for its nuclear localization in oocytes (Oe et al., 2001). First, we intended to identify a NLS in the CTD of Xenopus Chk1. The Xenopus Chk1 CTD has five small domains rich in basic Arg (R) or Lys (K) residues (Kumagai et al., 1998; Nakajo et al., 1999), one or more of which could constitute a classical monopartite or bipartite NLS (Mattaj and Englmeier, 1998) (Figure 1A). We constructed five Chk1 mutants each with alanine substitutions in one of the five basic domains, expressed them in Xenopus oocytes (by injection of their mRNA), and analyzed their subcellular localization by manual dissection of the oocytes followed by Western blotting. Three mutants (KK404AA, RR418AA, and KR451AA) as well as wild-type Chk1 localized to both the nucleus and the cytoplasm (Figure 1B), essentially as shown previously for endogenous as well as ectopic wild-type Chk1 (Oe et al., 2001). In contrast, the other two mutants, KR374AA and KIK456AAA, localized nearly exclusively to the cytoplasm (Figure 1B), indicating that two basic domains, 347KR and 456KIK(KK), were essential for nuclear localization of Chk1. We then asked whether a C-terminal C2 region containing the two basic domains (Figure 1A) could confer nuclear localization on GST. GST-C2 fusion protein localized nearly normally to the nucleus (as well as the cytoplasm) of the oocyte, whereas either GST alone or GST-C1 fusion protein (with a shorter C-terminal region lacking the 374KR residues; Figure 1A) localized exclusively to the cytoplasm (Figure 1C). Moreover, and importantly, GST-C2 mutants in which either the 374KR or 456KIK residues were replaced by alanine residues failed to localize to the nucleus (Figure 1C). Thus, the C2 region containing the two basic domains apart was sufficient for nuclear localization. These results suggest that Chk1 has a multipartite, probably bipartite, NLS in the CTD.
Figure 1.
Identification of a bipartite NLS in Xenopus Chk1. (A) Domain organization of Xenopus Chk1 protein, showing the approximate domain boundaries of the KD, flexible linker region (LR), SQ/TQ domain (SQ/TQ), and CTD. Positions of the four SQ/TQ motifs, five basic domains, and three C-terminal fragments used in this study (C1–C3) are indicated. (B–D) Subcellular localization of various Chk1 constructs. Oocytes injected with 1.5 ng of mRNA encoding the indicated Chk1 constructs (tagged with a Myc-epitope or GST) were cultured for 12 h and fractionated into the nuclear and cytoplasmic fractions. Proteins extracted from the total (T), nuclear (N), or cytoplasmic (C) fractions (each equivalent to one oocyte) were analyzed by immunoblotting by using either anti-Myc or anti-GST antibodies. In (C), (-) denotes GST itself.
Typically, a bipartite NLS consists of two basic domains separated by as short as 10 residues (Dingwall and Laskey, 1991). However, the bipartite NLS of Chk1 had two basic domains (374KR and 456KIKKK) separated by as long as 80 residues. To determine whether the unusually long internal sequence of the Chk1 NLS was required for an NLS function, we deleted parts of the internal sequence so that the two basic domains would be separated by 10 or 20 residues. Interestingly, both of the deletion mutants, Δ381–450 and Δ386–445, localized nearly exclusively to the cytoplasm (Figure 1D). Moreover, another mutant in which the long internal sequence was replaced by a GST-derived sequence of the same length (Δ384–443/GST) was mostly cytoplasmic (Figure 1D). Thus, the long internal sequence was not simply a “spacer” of the Chk1 NLS but was essential for an NLS function. These results strongly suggest that the two basic domains and the unusually long internal sequence, as a whole, function as an NLS of Chk1 protein.
Identification of an Autoinhibitory Region: Colocalization with the NLS
Previous studies indicated that the kinase domain of Chk1 has an open kinase conformation, at least part of the C-terminal region probably acting as an autoinhibitory region (Chen et al., 2000; Oe et al., 2001). Thus, when expressed in Xenopus oocytes, a series of C-terminal truncation mutants (lacking C-terminal 15–60 amino acids) of Xenopus Chk1 showed progressively increased kinase activities, as determined by their ability to inhibit oocyte maturation (or germinal vesicle breakdown, GVBD), by in vitro kinase assays, and by their electrophoretic mobility upshifts (due to auto-phosphorylation) (Oe et al., 2001). Interestingly, all the Chk1 mutants that failed to localize to the nucleus (KR374AA, KIK456AAA, Δ381–450, Δ386–445, and Δ384–443/GST) showed electrophoretic mobility upshifts (although to different degrees) (Figure 1, B and D; also Figure 2A), suggesting that these NLS-disrupted mutants were catalytically activated. Indeed, when assayed by their GVBD-inhibiting activity (which should correlate well with their kinase activity in vitro; Oe et al., 2001), the NLS-disrupted mutants all showed a much greater activity than wild-type Chk1 (Figure 2A). (Kinase-dead versions of these mutants did not show any mobility upshift or biological activity.) At first glance, these results seemed to indicate that the cytoplasmic localization of Chk1 activates Chk1 kinase activity (including autophosphorylation activity). Significantly, however, even the KR451AA mutant, which localized essentially normally to the nucleus (Figure 1B), showed an increased biological activity and a mobility upshift (Figures 1B and 2A). Moreover, a T377A mutant, which is constitutively active and exhibits a mobility upshift (Wang and Dunphy, 2000), showed essentially normal nuclear localization (Figure 2A). Thus, it was not the cytoplasmic localization itself but the specific mutations in the NLS that caused activation of Chk1. These results imply that the NLS contains at least part of the proposed autoinhibitory region.
Figure 2.
Identification of an AIR in Xenopus Chk1. (A–C) Typical sets of results on the biological activity of various Chk1 constructs. After injection of the same amount of mRNAs (1.5 ng) into oocytes, different Chk1 protein constructs were produced at somewhat different levels, probably due to their different stability (Figure 1, B and D). Therefore, here, (30) oocytes were injected with various amounts of mRNAs (1–3 ng, depending on the mRNA constructs) so that the various Chk1 protein constructs would be produced at comparable levels after 12 h of the mRNA injection. The oocytes were then treated with progesterone to induce maturation and, 7 h later, scored for the percentage GVBD. The activity of Chk1, represented by the percentage GVBD inhibition, correlates well with its kinase activity in vitro (Oe et al., 2001). The presence (+) or absence (-) of the size-shift or the functional NLS in each Chk1 construct is shown at the bottom of each histogram; most of the data in A are taken from Figure 1. For A–C, at least four experiments were performed and, for each, a typical set of results is shown.
To determine whether the autoinhibitory region (hereafter called the AIR) was restricted to the NLS, we deleted either the NLS region (ΔNLS) or the immediately preceding SQ/TQ domain (ΔSQ/TQ) from the C-terminal region of Chk1. Although the ΔNLS mutant showed prominent mobility shifts and a very strong biological activity as expected, the ΔSQ/TQ mutant showed only a weak activity and no appreciable mobility shift, similar to wild-type Chk1 (Figure 2B). Thus, the autoinhibitory region, or AIR, apparently was restricted to the NLS. We then determined whether most part of the long internal sequence of the NLS was required for the AIR function. As shown in Figure 2C, all of the three mutants in which either of the three consecutive 20-amino acid internal sequences was deleted from the NLS had a strong biological activity as well as mobility upshifts, whether large or small. Thus, in all respects, the AIR totally overlapped with the 85-amino acid long NLS, indicating that the AIR colocalizes with the NLS in the CTD.
Binding and Inhibition of the Kinase Domain by the AIR
Given the above-mentioned points as well as previous results (Chen et al., 2000; Oe et al., 2001), the AIR might directly interact with and inhibit the kinase domain of Chk1. Indeed, a recent study shows that the C-terminal half of rat Chk1 can directly interact with the kinase domain, although neither its precise region for the interaction nor the effect of this interaction on the kinase activity is known (Shann and Hsu, 2001). We also tested the possible interaction between the kinase domain (KD) and the C-terminal half region (C3; Figure 1A) of Xenopus Chk1. For this, we coexpressed the KD (tagged with a Myc-epitope) and the C3 region (fused to GST) in oocytes and subjected the oocyte extracts to GST-pulldown assays. GST-C3, but not GST itself, was able to bind efficiently to the KD (Figure 3A, bottom), confirming the interaction between the kinase domain and the C-terminal half of Chk1. We then coexpressed GST-C3 and a C-terminal 60-amino acid-deleted Chk1 mutant (Δ60-Chk1), which is constitutively active due to the partial deletion of the AIR (Oe et al., 2001). GST-C3 could also bind to Δ60-Chk1 very efficiently (Figure 3A, bottom), indicating that the kinase domain of Δ60-Chk1, like the isolated kinase domain, is freely accessible to the coexpressed, intact C-terminal half. Importantly, even a (GST-fused) C2 region, which consisted mostly of the AIR (Figure 1A), could bind to Δ60-Chk1 (albeit somewhat less efficiently than the C3 region), whereas its KR374AA mutant, which lacked a functional NLS/AIR (Figures 1C and 2A), could not (bottom). Thus, these results indicate that the AIR, if intact, can interact with the kinase domain. In contrast to these, neither the C3 region nor the C2 region (our unpublished data) was able to bind to full-length wild-type Chk1 (Figure 3A, bottom), suggesting a preformed interaction between the kinase domain and the AIR in full-length Chk1 molecules. To determine whether this preformed interaction occurred intramolecularly or intermolecularly, we coexpressed Myc-tagged full-length Chk1 and GST-tagged full-length Chk1 in oocytes. GST-pulldown assays revealed no detectable interaction between the two Chk1 molecules under the conditions in which the interaction between GST-C3 and (Myc-tagged) Δ60-Chk1 was readily observed (Figure 3B). Thus, it seems very likely that in full-length Chk1 molecules, the AIR interacts with the kinase domain in an intramolecular manner.
Figure 3.
Binding and inhibition of the kinase domain of Chk1 by the AIR. (A and B) Interaction between the kinase domain of Chk1 and the AIR in the oocyte. Twelve oocytes were coinjected with 1 ng of mRNA encoding the indicated Myc-Chk1 constructs and 12 ng of mRNA encoding the indicated GST-Chk1 constructs and were cultured for 12 h. (For unstable Myc-Δ60-Chk1, 3 ng of mRNA was injected.) Two oocytes were then subjected to direct immunoblotting (IB) with anti-GST or anti-Myc antibodies, and the remaining 10 oocytes were subjected to GST-pulldown assays (PD) followed by immunoblotting with anti-Myc antibody. (C) Inhibition of the biological activity of the kinase domain by the AIR. Thirty oocytes were coinjected with mRNAs as in A and B, cultured for 12 h, treated with progesterone, and, a further 8 h later, scored for the percentage of GVBD inhibition. A typical set of results from four independent experiments is shown.
When expressed alone or coexpressed with control GST, Δ60-Chk1 showed prominent mobility upshifts (Figure 3A, middle), due to its very strong autophosphorylation activity (Oe et al., 2001). Importantly, however, when coexpressed with GST-C3 or GST-C2 but not with GST-C2(KR374AA), Δ60-Chk1 showed substantially reduced mobility shifts (middle), suggesting that the binding of the AIR suppressed the kinase activity of Δ60-Chk1. Indeed, coexpression of GST-C3 or GST-C2, but not of GST alone or GST-C2(KR374AA), strongly reduced the biological activity of Δ60-Chk1 (Figure 3C). The KD alone, although catalytically very active similar to Δ60-Chk1 (Chen et al., 2000; Oe et al., 2001), did not show any detectable mobility shift (Figure 3A, middle), most probably due to its lacking autophosphorylation sites (Chen et al., 2000). Nevertheless, coexpression of GST-C2 (our unpublished data) or GST-C3 strongly reduced the activity of the KD (Figure 3C). Thus, binding of the AIR most probably suppressed the kinase activities of Δ60-Chk1 and the KD. In contrast to these, coexpression of GST-C3 had no appreciable effect on the intrinsic (low) activity of full-length wild-type Chk1 (Figure 3C). These results, together with the above-mentioned results, strongly suggest that under normal conditions, the kinase activity of Chk1 is maintained low by the intramolecular interaction between the kinase domain and the AIR.
Inhibition of the KD-AIR Interaction by Phospho-Mimic Mutation of the SQ/TQ Motifs
At the DNA damage/replication checkpoint, ATR-mediated phosphorylation on the SQ/TQ motifs is essential for the activation of Chk1 (Guo et al., 2000; Lopez-Girona et al., 2001; Zhao and Piwnica-Worms, 2001). This, together with the above-mentioned results, raises the possibility that SQ/TQ phosphorylation may somehow inhibit the interaction between the kinase domain and the AIR in Chk1, thereby activating Chk1 kinase activity. To test this possibility, first we mutated together Thr314, Ser344, Ser356, and Ser365 (within the four SQ/TQ motifs in the C3 region; Figure 1A) to either nonphosphorylatable Ala or phospho-mimic Asp or Glu residues, thus producing GST-C3(4AQ) or GST-C3(4DQ or 4EQ). (All the four Ser/Thr residues in Xenopus Chk1 can be phosphorylated by ATR both in vivo and in vitro; Guo et al., 2000.) When coexpressed in oocytes, although GST-C3(4AQ) could bind to Δ60-Chk1 and reduce its mobility upshifts as efficiently as wild-type GST-C3(WT), neither GST-C3(4DQ) nor GST-C3(4EQ) could do so (Figure 4A). In fact, both GST-C3(4DQ) and GST-C3(4EQ) bound to Δ60-Chk1 significantly less efficiently even than GST-C2 (which contained only the AIR) (Figure 4A). (This particular result could imply that phospho-mimic mutation of the nearby SQ/TQ motifs can cause a conformational change of the AIR, thereby inhibiting the kinase domain–AIR interaction.) Consistent with their very low ability to bind to Δ60-Chk1, GST-C3(4DQ) or GST-C3(4EQ) could not inhibit the activity of Δ60-Chk1 as efficiently as GST-C3(WT), GST-C3(4AQ), or even GST-C2 (Figure 4B).
Figure 4.
Inhibition of the kinase domain–AIR interaction by phospho-mimic mutation of the SQ/TQ motifs. (A and C) Interaction of Δ60-Chk1 and SQ/TQ-mutated C3 constructs. Twelve oocytes were coinjected with 3 ng of mRNA encoding Myc-Δ60-Chk1 and 12 ng of mRNA encoding the indicated GST constructs, cultured for 12 h, and analyzed as described in A and B of Figure 3. (B and D) Inhibition of the biological activity of Δ60-Chk1 by SQ/TQ-mutated C3 constructs. Thirty oocytes were coinjected with mRNAs as described above, cultured for 12 h, treated progesterone, and, a further 7 h later, scored for the percentage of GVBD inhibition. For both B and D, a typical set of results from four independent experiments is shown.
Phosphorylation of Ser345, a residue in one of the conserved SQ motifs, is essential for the activation of human and fission yeast Chk1 proteins (Lopez-Girona et al., 2001; Zhao and Piwnica-Worms, 2001). Ser344 of Xenopus Chk1 corresponds to the Ser345 residue of human and yeast Chk1 proteins. Therefore, we also tested whether a single phospho-mimic Glu mutation of Ser344 (1EQ) could affect the properties of GST-C3. When coexpressed in oocytes, GST-C3(1EQ) bound to Δ60-Chk1 and reduced its mobility upshifts even less efficiently than GST-C3(WT) but not GST-C3(4EQ) (Figure 4C). Consistent with this, GST-C3(1EQ) inhibited Δ60-Chk1 activity significantly less efficiently than GST-C3(WT) but not GST-C3(4EQ) (Figure 4D). Thus, even the single phospho-mimic mutation of the essential SQ motif was able to affect the activities of GST-C3, although somewhat less strongly than the 4EQ mutation. Together, these results would support the idea that phosphorylation of the SQ/TQ motifs inhibits the AIR from interacting with the kinase domain probably by causing its conformational change.
Inhibition of the KD-AIR Interaction by ATR-Mediated Phosphorylation of the SQ/TQ Motifs
We then asked, by using embryo extracts, whether ATR-mediated phosphorylation on the SQ/TQ motifs could indeed inhibit the interaction between the kinase domain of Chk1 and the AIR. To this end, we first determined whether a Myc-tagged C3 fragment could be efficiently phosphorylated by ATR in embryos treated with aphidicolin, a potent inducer of the DNA replication checkpoint (Dasso and Newport, 1990). We expressed Myc-C3(WT) or Myc-C3(4AQ) in just fertilized embryos and then treated the embryos (at the early blastula stage) with aphidicolin. In these embryos, a significant fraction (∼70%) of Myc-C3(WT), but not of Myc-C3(4AQ), showed a prominent upward size-shift after aphidicolin treatment; it was strongly recognized by anti-phospho-Ser344 antibody and was not detected in embryos treated with caffeine, a potent inhibitor of ATR (Sarkaria et al., 1999) (Figure 5A). Thus, Myc-C3(WT) was efficiently phosphorylated on the SQ/TQ motifs in an ATR-dependent manner in aphidicolin-treated embryos.
Figure 5.
Inhibition of the kinase domain-AIR interaction by ATR-mediated phosphorylation of the SQ/TQ motifs. (A) ATR-mediated SQ/TQ phosphorylation of the C3 constructs in aphidicolin-treated embryos. Fertilized embryos were injected with 2 ng of mRNA encoding either Myc-C3(WT) or Myc-C3(4AQ), incubated until the early blastula stage (stage 7.5), treated with aphidicolin (APH) in the presence or absence of caffeine (CAF) for 1 h, and then subjected to immunoblot analysis with either anti-Myc or anti-phospho-Ser344 antibodies. (B) Inhibition of the kinase domain-AIR interaction by SQ/TQ phosphorylation in embryo extracts. Forty fertilized embryos were injected with either 6 ng of mRNA encoding (kinase-dead) GST-KD or GST alone or with 6 ng of mRNA encoding Myc-C3 constructs, cultured until the early blastula stage, and then treated with aphidicolin for 1 h. Extracts prepared from the two groups of embryos were mixed and incubated for 45 min; a portion (equivalent to one embryo) was then subjected directly to immunoblot analyses (Input) and the remaining portion to GST-pulldown assays followed by immunoblotting (GST-pulldown). (C) Abrogation of the preformed kinase domain–AIR interaction by SQ/TQ phosphorylation. Twenty fertilized embryos were coinjected with the above-described mRNAs, cultured, and treated with aphidicolin as described in B; the embryos before (-) and after (+) aphidicolin treatment were then subjected to either direct immunoblotting (Expression) or GST-pulldown assays followed by immunoblotting (GST-pulldown) as described above.
To answer the above-mentioned question, we next expressed Myc-C3(WT) and a GST-fused kinase domain (GST-KD) separately in two groups of embryos, treated the embryos with aphidicolin, prepared extracts from the two groups of embryos, and then mixed and incubated them. (In these experiments, we used a kinase-inactive D148A version of the KD to allow the embryos to develop normally until aphidicolin treatment.) GST-pulldown assays showed that the above-described SQ/TQ-phosphorylated form of Myc-C3(WT) failed to bind to GST-KD, whereas its unphosphorylated form as well as Myc-C3(4AQ) was able to bind to GST-KD but not GST itself (Figure 5B, middle and bottom). In these experiments, the SQ/TQ-phosphorylated form of Myc-C3(WT) was not appreciably dephosphorylated during incubation and pulldown assays of the mixed extracts (our unpublished data; but see Figure 5B, input). Thus, these results strongly indicate that SQ/TQ phosphorylation by ATR can indeed inhibit the interaction between the kinase domain and the AIR.
We also tested whether a preformed interaction between the kinase domain and the AIR could be abrogated by ATR-mediated SQ/TQ phosphorylation. For this, we coexpressed Myc-C3 and (kinase-inactive) GST-KD in fertilized embryos and then treated the embryos (at the early blastula stage) with aphidicolin. Although the amount of Myc-C3(4AQ) that bound to GST-KD did not appreciably change before and after aphidicolin treatment, that of Myc-C3(WT) did decrease to about one-third after aphidicolin treatment (Figure 5C, middle, GST-pulldown). Apparently, this decrease was due to the conversion, after aphidicolin treatment, of two-thirds of the total Myc-C3(WT) to the SQ/TQ-phosphorylated form that was unable to bind to GST-KD (Figure 5C, expression). Thus, most likely, SQ/TQ phosphorylation was able to abrogate the preformed interaction between Myc-C3 and GST-KD. Together, these results strongly suggest that, at the DNA replication checkpoint, ATR-mediated phosphorylation on the SQ/TQ motifs inhibits the interaction between the kinase domain and the AIR in Chk1.
A Possible Requirement for a Weak Intramolecular Interaction in Phosphorylation-Dependent Activation of Full-Length Chk1
Given the above-mentioned results (Figures 4 and 5) and if phosphorylation of the SQ/TQ motifs were the sole and direct mechanism of Chk1 activation, then mutation of the SQ/TQ motifs to 4DQ or 4EQ alone could strongly activate the full-length Chk1 molecule, causing a large increase in its mobility shift (due to autophosphorylation) and in its biological activity. When expressed in oocytes, however, only a very small fraction of full-length Chk1 with 4DQ or 4EQ (but not 4AQ) showed a slight mobility shift (see asterisk in the figure), compared with the large mobility shifts of the AIR-disrupted full-length Chk1 mutant (KR374AA; Figures 1B and 2A) (Figure 6A). [In this case, even the major unshifted fraction of Chk1(4DQ) or Chk1(4EQ) migrated slightly more slowly than wild-type Chk1 and Chk1(4AQ), presumably due to their increased negative charge.] Indeed, Chk1(4DQ) or Chk1(4EQ), unlike Chk1(KR374AA), did not appreciably show a greater biological activity than wild-type Chk1 (Figure 6B). Moreover, these mutants could not appreciably bind the coexpressed AIR (our unpublished observation). Thus, these results indicate that in full-length Chk1 molecules, phospho-mimic mutation of the SQ/TQ motifs alone cannot abrogate the intramolecular interaction or cause a strong enzymatic activation, although it does seem to confer a very small autophosphorylation activity (Figure 6A).
Figure 6.
Activation of a full-length Chk1 mutant (KR451AA) by phospho-mimic mutation of the SQ/TQ motifs. (A and C) Mobility shifts of full-length Chk1 molecules by phospho-mimic mutation of the SQ/TQ motifs. Oocytes were injected with 1 ng of mRNA encoding the indicated full-length Chk1 mutants (tagged with a Myc-epitope), cultured for 12 h, and subjected to immunoblot analysis. For approximately twofold less stable mutants (KR374AA, KR451AA, and KR451AA/4DQ), 2 ng of mRNA was injected. In A, a very small fraction of 4DQ or 4EQ showed a small mobility upshift (marked with asterisk). (B and D) Thirty oocytes injected with mRNAs as described above were treated with progesterone and scored for the percentage GVBD inhibition as described in Figure 2. For both B and D, a typical set of results from three independent experiments is shown. (E) Interaction of full-length Chk1 mutants with GST-C3. Twelve oocytes were coinjected with 10 ng of mRNA encoding GST-C3 and 2 ng of mRNA encoding the indicated full-length Myc-Chk1 mutants, cultured for 12 h, and analyzed as described in Figure 3A.
These results show an apparent discrepancy with those obtained with GST-C3(4DQ or 4EQ) or SQ/TQ-phosphorylated GST-C3, either of which was unable to interact with the kinase domain, probably due to the conformational change of the AIR (Figures 4 and 5). This discrepancy could be reconciled, however, if we assume that phospho-mimic mutation or phosphorylation of the SQ/TQ motifs can affect the conformation of the full-length Chk1's AIR only when this AIR is not tightly bound to the kinase domain, somewhat like the (free) AIR of GST-C3. To test this possibility, we introduced a 4DQ mutation into the previously described full-length Chk1 mutant, KR451AA, because this mutant seemed to have a weak intramolecular interaction judging from its relatively small mobility shift, weak biological activity, and poor binding by GST-C3, compared with other AIR-disrupted mutants (Figures 1B, 2A, and 6E). Intriguingly, the 4DQ-introduced double-mutant KR451AA/4DQ showed much larger mobility shifts and a significantly stronger biological activity than the parental mutant KR451AA as well as Chk1(4DQ) (Figure 6, C and D). Moreover, this double-mutant, unlike the parental mutant, was able to bind the coexpressed GST-C3 very efficiently, comparable with two other AIR-disrupted mutants, Δ60-Chk1 and KR374AA (Figure 6E). Thus, phospho-mimic mutation of the SQ/TQ motifs was able to activate strongly the full-length Chk1 molecule if this molecule had a weak intramolecular interaction. These results may suggest that activation of full-length Chk1, or probably the conformational change of the AIR, requires both SQ/TQ phosphorylation and a somewhat weakened intramolecular interaction between the kinase domain and the AIR.
DISCUSSION
In this study, we have analyzed the C-terminal domain structure of Xenopus Chk1 and explored the mechanism of its activation by ATR-mediated phosphorylation. Our results, employing various Chk1 mutants, provide significant insights into the mechanism of Chk1 activation at the DNA damage/replication checkpoint.
Colocalization of the NLS and the AIR
Typically, a bipartite NLS consists of two small basic domains separated by as short as nine to 12 residues (Dingwall and Laskey, 1991). Surprisingly, however, Xenopus Chk1 contained at the C terminus a bipartite NLS consisting of two basic domains separated by as long as 80 residues (residues 376–455); the unusually long internal sequence was essential for the NLS function (Figure 1). Chk1 proteins from all other known species such as fission yeast (Walworth et al., 1993), Drosophila (Fogarty et al., 1997), and human (Sanchez et al., 1997) also contain two or three basic domains approximately at the corresponding sites. Thus, Chk1 proteins may generally contain an unusually long bipartite NLS at the C terminus. Two basic domains of a typical bipartite NLS, separated by as short as 10 residues, seem to bind to two unique binding sites of importin α, enabling nuclear localization of the NLS-containing protein (Conti et al., 1998; Mattaj and Englmeier, 1998). The very long internal sequence of the Chk1 NLS might therefore be structured so that the two basic domains could become sterically close to each other to bind to the two unique sites of importin α.
Previous studies suggested that although the N-terminal kinase domain of Chk1 has an open kinase conformation, at least part of the C-terminal region contains an autoinhibitory region (Chen et al., 2000; Oe et al., 2001). Interestingly, deletion of the NLS (but not the SQ/TQ domain) from the C-terminal half of Xenopus Chk1 caused prominent mobility upshifts (due to autophosphorylation) and a marked activation of the kinase in oocytes (Figure 2B). More specifically, both mutations of the two basic domains and deletions of the internal sequence of the NLS caused a prominent activation of the kinase (Figure 2A). This activation was not due to the cytoplasmic localization of the mutants but was due to their specific mutations or deletions themselves in the NLS (Figure 2A; see also Oe et al., 2001). Thus, the AIR totally overlapped with the long bipartite NLS, its integrity being largely dependent on the structure of the NLS. Currently, the biological significance of colocalization of the NLS and the AIR is not known. However, in the cytoplasm the binding of importin α to the NLS might help stabilizing the conformation of the AIR (to prevent a spontaneous activation of Chk1), whereas in the nucleus the release of importin α from the NLS might allow a conformational change of the AIR by ATR (see below). In any case, as long as the NLS/AIR is bound to the kinase domain of Chk1 (see below), the two basic domains of the NLS might be sterically exposed to bind importin α.
Binding and Inhibition of the Kinase Domain by the AIR
Reportedly, the C-terminal half of rat Chk1 can directly interact with the kinase domain, although the functional significance of this interaction is not known (Shann and Hsu, 2001). When coexpressed in oocytes, not only the C-terminal half but also the AIR of Xenopus Chk1 was able to bind to the kinase domain and even to the constitutively active Chk1 mutant (Δ60-Chk1) in which the AIR was partially deleted; the AIR mutant (KR374AA) in which the N-terminal basic domain (which constituted the bipartite NLS) was mutated to alanine bound to Δ60-Chk1 very poorly (Figure 3A). In contrast to these, the AIR could not bind to full-length Chk1 (Figure 3A), and two full-length Chk1 molecules (with different tags) failed to interact with each other (Figure 3B). Thus, the AIR, if intact, was able to interact with the kinase domain, and, in full-length Chk1 molecules, this interaction most probably occurred intramolecularly.
Interestingly, coexpression of the AIR but not the mutated AIR (that could not bind to the kinase domain) significantly reduced the electrophoretic mobility shift (or autophosphorylation) and biological activity of Δ60-Chk1 in oocytes (Figure 3A). Similarly, coexpression of the AIR (but not the mutated AIR) strongly inhibited the activity of the kinase domain, which itself did not show a mobility shift most probably due to its lacking autophosphorylation sites (Figure 3, A and C; Chen et al., 2000). Thus, the binding of the AIR most certainly suppressed the intrinsic kinase activity of the kinase domain. Together, these results strongly suggest that, under normal conditions, the kinase activity of Chk1 is maintained low by the intramolecular interaction between the kinase domain and the AIR.
Inhibition of the Kinase Domain–AIR Interaction by Phosphorylation of the SQ/TQ Motifs
At the DNA damage/replication checkpoint, the kinase activity of Chk1 is increased at least severalfold via the ATR-mediated phosphorylation on the SQ/TQ motifs (Kumagai and Dunphy, 2000; Zhao and Piwnica-Worms, 2001). Thus, given our results, phosphorylation of Chk1 on the SQ/TQ motifs could somehow inhibit the interaction between the kinase domain and the AIR. Consistent with this possibility, phospho-mimic mutation (to 4DQ or 4EQ), but not nonphosphorylatable mutation (to 4AQ), of the SQ/TQ motifs strongly reduced the abilities of the isolated C-terminal C3 region (containing both the SQ/TQ domain and the AIR) to bind to, to decrease the mobility shift of, and to inhibit the biological activity of, Δ60-Chk1 in oocytes (Figure 4, A and B). Even a single Glu mutation of Ser344, a residue of the essential SQ motif (Lopez-Girona et al., 2001; Zhao and Piwnica-Worms, 2001), had similar effects on the activities of the C3 region, albeit to somewhat smaller degrees than the 4EQ mutation (Figure 4, C and D). Importantly, the C2 region, which contained only the AIR, was able to bind to and inhibit Δ60-Chk1 significantly more strongly even than the C3(4DQ) or C3(4EQ) constructs (Figure 4, A and B). Thus, these results would support the idea that the nearby SQ/TQ motifs, if phosphorylated, can inhibit the AIR from interacting with the kinase domain probably by causing its conformational change.
Consistent with the results obtained with phospho-mimic mutation of the SQ/TQ motifs, the isolated C3 region, if phosphorylated on the SQ/TQ motifs by ATR, could not interact with the kinase domain in embryo extracts (Figure 5, A and B). Moreover, and importantly, the preformed interaction between the kinase domain and the C3 region was disrupted by ATR-mediated phosphorylation of the SQ/TQ motifs in aphidicolin-treated embryos (Figure 5C). Together, these results strongly suggest that, at the DNA replication checkpoint, ATR-mediated phosphorylation on the SQ/TQ motifs interferes with the interaction between the kinase domain and the AIR in Chk1, thereby activating Chk1 kinase activity.
A Model for the Activation of Full-Length Chk1 by ATR
Unexpectedly, but as reported recently for both fission yeast and human Chk1 proteins (Capasso et al., 2002; Gatei et al., 2003), phospho-mimic mutation of the SQ/TQ motifs alone could not greatly alter the properties of full-length Xenopus Chk1. When expressed in oocytes, although both Chk1(4DQ) and Chk1(4EQ) showed a slight mobility shift in a very small fraction (Figure 6A), neither of them appreciably showed a greater biological activity than wild-type Chk1 (Figure 6B) or could bind the coexpressed AIR (our unpublished observation). Thus, unlike the case with the isolated C3 region, phospho-mimic mutation of the SQ/TQ motifs apparently could not cause a (large) conformational change of the AIR in full-length Chk1. Formally, this could imply that 4DQ or 4EQ cannot mimic phosphorylated SQ/TQ motifs in the case of full-length Chk1 molecules, as suggested (Capasso et al., 2002). However, phospho-mimic mutation or phosphorylation of the SQ/TQ motifs might be able to affect the conformation of the full-length Chk1's AIR if this AIR is not tightly bound to the kinase domain, somewhat like the (free) AIR of the isolated C3 fragment. Consistent with this possibility, introduction of a 4DQ mutation could markedly increase the mobility shift, biological activity, and binding by the coexpressed AIR, of the full-length Chk1 mutant (KR451AA) that had a somewhat weakened intramolecular interaction (Figure 6, C–E). Thus, it seems likely that SQ/TQ phosphorylation can, in fact, activate full-length Chk1 if this molecule has a weak intramolecular interaction. At the DNA replication checkpoint, the adaptor-like protein Claspin binds to Chk1 and is absolutely required for the activation of Chk1 by ATR (Kumagai and Dunphy, 2002, 2003). The tumor suppressor protein Brca1 also becomes associated with Chk1 and is required for the activation of Chk1 at the DNA damage checkpoint (Yarden et al., 2002). At the DNA damage/replication checkpoint, therefore, some additional factor such as Claspin or Brca1 might act to weaken or loosen the intramolecular interaction of Chk1, thereby enabling the conformational change of the AIR by ATR-mediated phosphorylation.
Together, our results would allow us to propose a model for the activation of Chk1 at the DNA replication checkpoint (Figure 7). Under normal conditions, Chk1 kinase activity is maintained low by the tight intramolecular interaction between the kinase domain and the AIR. At the DNA replication checkpoint, however, some factor (e.g., Claspin) might first act to weaken or loosen the tight intramolecular interaction of Chk1. ATR may then phosphorylate Chk1 on the SQ/TQ motifs and cause a large conformational change of the AIR, thereby abolishing the intramolecular interaction and, hence, markedly increasing Chk1 kinase activity. In addition to these, binding of 14-3-3 proteins or autophosphorylation might also contribute, at some step, to Chk1 activation (Walworth and Bernards, 1996; Chen et al., 1999; Jiang et al., 2003). Further work will elucidate a more precise mechanism of Chk1 activation at the DNA damage/replication checkpoint.
Figure 7.
Model for Chk1 activation at the DNA replication checkpoint. Under normal conditions, Chk1 activity is maintained low by the tight intramolecular interaction between the KD and the AIR. At the DNA replication checkpoint, ATR-mediated phosphorylation on the SQ/TQ motifs, together with a somewhat weakened intramolecular interaction, may activate Chk1 by causing a conformational change of the AIR. A tertiary factor (X) might act to weaken or loosen the tight intramolecular interaction of Chk1 in either ATR-dependent or ATR-independent manner. S*Q/T*Q represents ATR-phosphorylated SQ/TQ motifs.
Acknowledgments
We thank Drs. N. Nakajo, K. Uto, and K. Okamoto for valuable discussions and K. Gotoh for typing the manuscript. This work was supported by the scientific grants from the Ministry of Education, Science and Culture of Japan and the Core Research for Evolution Science and Technology Research Project of Japan Science and Technology Agency to N.S.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–12–0874. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-12-0874.
Abbreviations used: AIR, autoinhibitory region; CTD, C-terminal domain; GST, glutathione S-transferase; GVBD, germinal vesicle breakdown; KD, kinase domain; NLS, nuclear localization signal.
References
- Abraham, R.T. (2001). Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15, 2177-2196. [DOI] [PubMed] [Google Scholar]
- Barlow, C., et al. (1996). Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell 86, 159-171. [DOI] [PubMed] [Google Scholar]
- Bartek, J., and Lukas, J. (2003). Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 3, 421-429. [DOI] [PubMed] [Google Scholar]
- Capasso, H., Palermo, C., Wan, S., Rao, H., John, U.P., O'Connell, M.J., and Walworth, N.C. (2002). Phosphorylation activates Chk1 and is required for checkpoint-mediated cell cycle arrest. J. Cell Sci. 115, 4555-4564. [DOI] [PubMed] [Google Scholar]
- Chen, L., Liu, T.H., and Walworth, N.C. (1999). Association of Chk1 with 14-3-3 proteins is stimulated by DNA damage. Genes Dev. 13, 675-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, P., et al. (2000). The 1.7Å crystal structure of human cell cycle checkpoint kinase Chk 1: implications for Chk1 regulation. Cell 100, 681-692. [DOI] [PubMed] [Google Scholar]
- Conti, E., Uy, M., Leighton, L., Blobel, G., and Kuriyan, J. (1998). Crystallographic analysis of the recognition of a nuclear localization signal by the nuclear import factor karyopherin α. Cell 94, 193-204. [DOI] [PubMed] [Google Scholar]
- Dasso, M., and Newport, J.W. (1990). Completion of DNA replication is monitored by a feedback system that controls the initiation of mitosis in vitro: studies in Xenopus. Cell 61, 811-823. [DOI] [PubMed] [Google Scholar]
- Dingwall, C., and Laskey, R.A. (1991). Nuclear targeting sequences—a consensus? Trends Biochem. Sci. 16, 478-481. [DOI] [PubMed] [Google Scholar]
- Fogarty, P., Campbell, S.D., Abu-Shumays, R., Phalle, B.S., Yu, K.R., Uy, G.L., Goldberg, M.L., and Sullivan, W. (1997). The Drosophila grapes gene is related to checkpoint gene chk1/rad27 and is required for late syncytial division fidelity. Curr. Biol. 7, 418-426. [DOI] [PubMed] [Google Scholar]
- Furuno, N., Nishizawa, M., Okazaki, K., Tanaka, H., Iwashita, J., Nakajo, N., Ogawa, Y., and Sagata, N. (1994). Suppression of DNA replication via Mos function during meiotic divisions in Xenopus oocytes. EMBO J. 13, 2399-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatei, M., et al. (2003). Ataxia-telangiectasia-mutated (ATM) and NBS1-dependent phosphorylation of Chk1 on Ser-317 in response to ionizing radiation. J. Biol. Chem. 278, 14806-14811. [DOI] [PubMed] [Google Scholar]
- Guo, Z., Kumagai, A., Wang, S.X., and Dunphy, W.G. (2000). Requirement for Atr in phosphorylation of Chk1 and cell cycle regulation in response to DNA replication blocks and UV-damaged DNA in Xenopus egg extracts. Genes Dev. 14, 2745-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell, L.H., and Weinert, T.A. (1989). Checkpoints: controls that ensure the order of cell cycle events. Science 246, 629-634. [DOI] [PubMed] [Google Scholar]
- Jiang, K., Pereira, E., Maxfield, M., Russell, B., Goudelock, D.M., and Sanchez, Y. (2003). Regulation of Chk1 includes chromatin association and 14–3-3 binding following phosphorylation on Ser-345. J. Biol. Chem. 278, 25207-25217. [DOI] [PubMed] [Google Scholar]
- Kim, S., Lim, D., Canman, C.E., and Kastan, M.B. (1999). Substrate specificities and identification of putative substrates of ATM kinase family members. J. Biol. Chem. 274, 37538-37543. [DOI] [PubMed] [Google Scholar]
- Kumagai, A., Guo, Z., Emami, K.H., Wang, S.X., and Dunphy, W.G. (1998). The Xenopus Chk1 protein kinase mediates a caffeine-sensitive pathway of checkpoint control in cell-free extracts. J. Cell Biol. 142, 1559-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai, A., and Dunphy, W.G. (2000). Claspin, a novel protein required for the activation of Chk1 during a DNA replication checkpoint response in Xenopus egg extracts. Mol. Cell 6, 839-849. [DOI] [PubMed] [Google Scholar]
- Kumagai, A., and Dunphy, W.G. (2003). Repeated phosphopeptide motifs in Claspin mediate the regulated binding of Chk1. Nat. Cell Biol. 5, 161-165. [DOI] [PubMed] [Google Scholar]
- Liu, Q., et al. (2000). Chk1 is an essential kinase that is regulated by Atr and required for the G2/M DNA damage checkpoint. Genes Dev. 14, 1448-1459. [PMC free article] [PubMed] [Google Scholar]
- Lopez-Girona, A., Tanaka, K., Chen, X.B., Baber, B.A., McGowan, C.H., and Russell, P. (2001). Serine-345 is required for Rad3-dependent phosphorylation and function of checkpoint kinase Chk1 in fission yeast. Proc. Natl. Acad. Sci. USA 98, 11289-11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund, E., and Paine, P.L. (1990). Non-aqueous isolation of transcriptionally active nuclei from Xenopus oocytes. Meth. Enzymol. 181, 36-43. [DOI] [PubMed] [Google Scholar]
- Matsuoka, S., Huang, M., and Elledge, S.J. (1998). Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science 282, 1893-1897. [DOI] [PubMed] [Google Scholar]
- Mattaj, I.W., and Englmeier, L. (1998). Nucleocytoplasmic transport: the soluble phase. Annu. Rev. Biochem. 67, 265-306. [DOI] [PubMed] [Google Scholar]
- Nakajo, N., Oe, T., Uto, K., and Sagata, N. (1999). Involvement of Chk1 kinase in prophase I arrest of Xenopus oocytes. Dev. Biol., 207, 432-444. [DOI] [PubMed] [Google Scholar]
- Nurse, P. (1997). Checkpoint pathways come of age. Cell 91, 865-867. [DOI] [PubMed] [Google Scholar]
- Oe, T., Nakajo, N., Katsuragi, Y., Okazaki, K., and Sagata, N. (2001). Cytoplasmic occurrence of the Chk1/Cdc25 pathway and regulation of Chk1 in Xenopus oocytes. Dev. Biol. 229, 250-261. [DOI] [PubMed] [Google Scholar]
- Okamoto, K., Nakajo, N., and Sagata, N. (2002). The existence of two distinct Wee1 isoforms in Xenopus: implications for the developmental regulation of the cell cycle, EMBO J. 21, 2472-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhind, N., and Russell, P. (2000). Chk1 and Cds 1, linchpins of the DNA damage and replication checkpoint pathways. J. Cell Sci. 113, 3889-3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez, Y., Wong, C., Thoma, R.S., Richman, R., Wu, Z., Piwnica-Worms, H., and Elledge, S.J. (1997). Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science 277, 1497-1501. [DOI] [PubMed] [Google Scholar]
- Sarkaria, J.N., Busby, E.C., Tibbetts, R.S., Roos, P., Taya, Y., Karnitz, L.M., and Abraham, R.T. (1999). Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 59, 4375-4382. [PubMed] [Google Scholar]
- Shann, Y., and Hsu, M. (2001). Cloning and characterization of liver-specific isoform of Chk1 gene from rat. J. Biol. Chem. 276, 48863-48870. [DOI] [PubMed] [Google Scholar]
- Shiloh, Y. (2001). ATM and ATR: networking cellular responses to DNA damage. Curr. Opin. Genet. Dev. 11, 71-77. [DOI] [PubMed] [Google Scholar]
- Shimuta, K., Nakajo, N., Uto, K., Hayano, Y., Okazaki, K., and Sagata, N. (2002). Chk1 is activated transiently and targets Cdc25A for degradation at the Xenopus midblastula transition. EMBO J. 21, 3694-3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen, C.S., Syljuåsen, R.G., Flack, J., Schroeder, T., Rönnstrand, L., Zhou, B.-B., Bartek, J., and Lukas, J. (2003). Chk1 regulates the S-phase checkpoint by coupling the physiological turnover and ionizing radiation-induced accelerated proteolysis of Cdc25A. Cancer Cell 3, 247-258. [DOI] [PubMed] [Google Scholar]
- Walworth, N.C. (2000). Cell-cycle checkpoint kinases: checking in on the cell cycle. Curr. Opin. Cell Biol. 12, 697-704. [DOI] [PubMed] [Google Scholar]
- Walworth, N.C., and Bernards, R. (1996). Rad-dependent response of the chk1-encoded protein kinase at the DNA damage checkpoint. Science 271, 353-356. [DOI] [PubMed] [Google Scholar]
- Walworth, N.C., Davey, S., and Beach, D. (1993). Fission yeast chk1 protein kinase links the rad checkpoint pathway to cdc2. Nature 363, 368-371. [DOI] [PubMed] [Google Scholar]
- Wang, S.X., and Dunphy, W.G. (2000). Activation of Xenopus Chk1 by mutagenesis of threonine-377. FEBS Lett. 487, 277-281. [DOI] [PubMed] [Google Scholar]
- Xu, X., Tsvetkov, L.M., and Stern, D.F. (2002). Chk2 activation and phosphorylation-dependent oligomerization. Mol. Cell. Biol. 22, 4419-4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden, R.I., Pardo-Reoyo, S., Sgagias, M., Cowan, K.H., and Brody, L.C. (2002). BRCA1 regulates the G2/M checkpoint by activating Chk1 kinase upon DNA damage. Nat. Genet. 30, 285-289. [DOI] [PubMed] [Google Scholar]
- Zhao, H., and Piwnica-Worms, H. (2001). ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol. Cell. Biol. 21, 4129-4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, B.B., and Elledge, S.J. (2000). The DNA damage response: putting checkpoints in perspective. Nature 408, 433-439. [DOI] [PubMed] [Google Scholar]