Abstract
The polo-box domain of the budding yeast polo kinase Cdc5p plays an essential role for targeting the catalytic activity of Cdc5p to spindle pole bodies (SPBs) and cytokinetic neck-filaments. Here, we report the isolation of Bbp1p as a polo-box interacting protein by a yeast two-hybrid screen. Bbp1p localizes to the periphery of the central plaque of the SPB and plays an important role in SPB duplication. Similarly, Cdc5p localized to the cytoplasmic periphery of the SPB. In vitro binding studies showed that Cdc5p interacted with the N-terminal domain of Bbp1p (Bbp1pΔC), but apparently not with Mps2p, a component shown to form a stable complex with Bbp1p. In addition, Bbp1p, but likely not Mps2p, was required for proper localization of Cdc5p to the SPB. The C-terminal coiled-coil domain of Bbp1p (Bbp1p243–385), which is crucial for both the homodimerization and the SPB localization, could target the localization-defective Cdc5pΔC to the SPB and induce the release of Cdc14p from the nucleolus. Consistent with this observation, expression of CDC5ΔC-BBP1243–385 under CDC5 promoter control partially complemented the cdc5Δ defect. These data suggest that Bbp1pΔC interacts with the polo-box domain of Cdc5p, and this interaction is critical for the subcellular localization and mitotic functions of Cdc5p.
INTRODUCTION
Polo protein kinases seem to play pivotal roles in regulating various cellular and biochemical events at multiple stages of M phase. Members of the polo subfamily of protein kinases have been isolated from species as divergent as budding yeast and mammals. They are characterized by the presence of a distinct region of homology in the C-terminal noncatalytic domain, termed the polo-box (Clay et al., 1993). Studies in budding yeast have shown that mutations in the polo-box domain of both the mammalian polo-like kinase Plk1p or the budding yeast polo kinase homologue Cdc5p disrupt the ability of these enzymes to localize to the spindle pole body (SPB) (the functional equivalent of the mammalian centrosomes) and cytokinetic neck-filaments (Lee et al., 1998; Song et al., 2000). Subsequent studies in cultured mammalian cells have shown that the polo-box domain of Plk1p is required for the localization of this enzyme to centrosomes, kinetochores, and midbody (Seong et al., 2002). These data suggest that the polo-box is critical in targeting the catalytic activity of the polo kinases to specific subcellular locations and that the role of the polo-box is likely conserved between budding yeast and mammalian cells.
The SPB of budding yeast is a multiple-layered structure with the central plaque embedded in the nuclear envelope. The outer plaque is important for organizing the cytoplasmic microtubules, whereas the inner plaque structure is important for the assembly of nuclear microtubules. Both direct biochemical purification of the SPB components and various genetic analyses of mutants defective in SPB function have led to the identification of many SPB components (Adams and Kilmartin, 2000; Schramm et al., 2001). BBP1 has been isolated as a dosage suppressor of the growth defect associated with temperature-sensitive mutations in SPC29 (Schramm et al., 2000), whose encoded protein plays a critical role in SPB duplication (Elliott et al., 1999). Immunoelectron microscopy (EM) studies have shown that Bbp1p localizes to the central plaque periphery and the cytoplasmic side of the SPB (Schramm et al., 2000). Studies with the temperature-sensitive bbp1–1 mutant revealed that these cells are often defective in inserting a duplication plaque into the nuclear envelope at the restrictive temperature, whereas cells with already duplicated SPBs exhibit one nonfunctional SPB with defective microtubule structures (Schramm et al., 2000). Coimmunoprecipitation and two-hybrid analyses suggest that Bbp1p forms a heterodimeric complex with Mps2p (Schramm et al., 2000; Winey et al., 1991), a protein that localizes to the SPB periphery and nuclear envelope (Munoz-Centeno et al., 1999).
In addition to the role of SPB in microtubule organization and chromosome segregation, it is now apparent that the SPB also plays an important role in recruiting many regulatory components critical for mitotic exit (for reviews, see Bardin and Amon, 2001; Bettignies and Johnston, 2003). These regulatory components form an intricate signaling network, termed mitotic exit network, which leads to release of Cdc14p phosphatase from the nucleolus. Released Cdc14p dephosphorylates the Cdh1/Hct1 of the anaphase promoting complex (APC) to stimulate APC-dependent degradation of mitotic cyclins (Visintin et al., 1998), resulting in the inactivation of the Cdc28/Clb2 complex. Tem1 GTPase plays a critical role in mediating this process by interacting with the downstream effector Cdc15p (Asakawa et al., 2001; Ro et al., 2002). It has been shown that Cdc5p functions upstream of Tem1 by phosphorylating and negatively regulating Bfa1p (Geymonat et al., 2003; Hu et al., 2001), which forms a two-component GTPase-activating protein (GAP) with Bub2p to negatively regulate Tem1 (Geymonat et al., 2002). In addition, Cdc5p has been shown to trigger Cdc14p release from the nucleolus during early anaphase through the FEAR pathway (Stegmeier et al., 2002; Yoshida et al., 2002), leading to early release of Cdc14p to promote mitotic exit. As with Cdc5p, Tem1p, Bfa1, and Bub2p are also shown to localize to the SPB (Pereira et al., 2000), emphasizing the importance of the SPB in regulating mitotic exit. In addition, it has been shown that Nud1p, an SPB component important for cytoplasmic microtubule organization, also plays a role in mitotic exit by promoting the Tem1–Cdc15 interaction (Gruneberg et al., 2000). This observation suggests that components at the SPB can also contribute to other cellular events by interacting with other SPB-associating proteins.
In this article, we demonstrate that, in a polo-box–dependent manner, Cdc5p interacts with Bbp1p. Bbp1p-dependent localization of Cdc5p to the SPB can induce mitotic exit and rescue the growth defect associated the cdc5Δ mutation. Reexamination of the bbp1-1 mutant revealed that, in addition to the role of Bbp1p in SPB duplication, Bbp1p is required for proper mitotic progression. Our data suggest that Bbp1p contributes to the Cdc5p-dependent mitotic events by promoting the Cdc5p localization to the SPB.
MATERIALS AND METHODS
Strain and Plasmid Construction
Yeast strains and plasmids used in this study are listed in Tables 1 and 2. All deletion and epitope-tagged strains constructed in this study were confirmed by PCR. To perform immuno-EM studies, a BamHI-SphI fragment containing GAL1 promoter-controlled 2 × (EGFP)-CDC5ΔDB (Song et al., 2000) was first cloned into a pUC19 derivative bearing URA1 (pSK906) at the corresponding sites. The resulting acentromeric URA1:GAL1–2×(EGFP)-CDC5ΔDB plasmid (pSK910) was integrated into the strain KKY921-2B (a gift of A. Sugino, Osaka University, Osaka, Japan) to generate strain KLY961. Complete deletions of the BBP1 (bbp1Δ::KanMX6) and CDC5 (cdc5Δ::KanMX6) open reading frames (ORFs) were generated by the one-step gene disruption method (Longtine et al., 1998). To generate strains expressing full-length or truncated forms of HA-GST-BBP1 fusion proteins under the GAL1 promoter control, EcoRI-SphI fragments containing indicated GAL1-HA-GST-BBP1 fusions were inserted into the corresponding sites of an acentromeric ADE2-bearing plasmid pASZ11 (Stotz and Linder, 1990). The resulting constructs were digested with BstXI and then integrated into strain IAY18 (a gift of J. Kilmartin, Medical Research Council, Cambridge, United Kingdom) at the ADE2 locus. To generate strains expressing a CDC5-GFP fusion under endogenous CDC5 promoter control (strains KLY3546, KLY3791, and KLY3729), a GFP::KanMX6 fragment obtained by PCR by using pFA6a-KanMX6 (Longtine et al., 1998) as a template was integrated into strains KLY1546 (wild-type), KLY2761 (bbp1-1), or KLY2770 (mps2-1), respectively. Strains KLY2761 and KLY2770 were derived from strain YCS64 (a gift of E. Schiebel, The Paterson Institute for Cancer Research, Manchester, United Kingdom) and strain Wx193-7b (Winey et al., 1991), respectively, by backcrossing the respective alleles repeatedly into the KLY1546 background. Cdc5p-GFP seemed to be fully functional, because strain KLY3546 did not exhibit any detectable defects (our unpublished data). Strains KLY5336 and KLY5334 were created by C-terminally tagging the BBP1 locus and the bbp1-1 gene in the TRP1 locus, respectively, with a GFP::KanMX6 fragment. Strains KLY3685 and KLY3692 were generated by integrating pRS306 (Sikorski and Hieter, 1989) or pRS305 (Sikorski and Hieter, 1989)-based SPC42-GFP plasmid into strains KLY1546 (wild-type) and KLY2761 (bbp1-1), respectively. Both pRS305- and pRS306-based SPC42-GFP constructs were generated by inserting an EcoRI-NotI or a XhoI-NotI fragment bearing SPC42-GFP into the corresponding sites in pRS306 or pRS305, respectively. To generate strain KLY4323, a pRS304-based CDC14–5×GFP plasmid (a gift of A. Toh-e, University of Tokyo, Tokyo, Japan) digested with StuI was integrated into the CDC14 locus of strain KLY1546. To generate strain KLY4426, a PCR fragment containing URA3 was first inserted into the SnaBI site of pRS304-CDC14–5×GFP. The resulting plasmid pCJ259 was digested with EcoNI and integrated into strain KLY2761 at the CDC14 locus. To study the ability of Cdc5p, Cdc5pΔC-Bbp1p243–385, and Cdc5pΔC to induce delocalization of Cdc14p-GFP5 from the nucleolus, DNA fragments containing GAL1-CDC5, GAL1-CDC5ΔC-BBP1243–385, or GAL1-CDC5ΔC were first cloned into an URA3-based, GAL1 promoter-controlled, acentromeric plasmid pCJ238 (GAL1:URA3) at the BamHI and SphI sites. The resulting constructs were digested with StuI to integrate into strain SAY801 (a gift of A. Toh-e) at the URA3 locus. Cells were cultured under induction conditions in the presence of 15 μg/ml nocodazole for 3 h, fixed with 3.7% formaldehyde, and then subjected to fluorescent microscopy to determine the localization of Cdc14p-GFP5 in the nucleolus.
Table 1.
Strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| KLY1546 | MATahis3-11,15 leu2-3, 112 trp1-1 ura3 | Laboratory stocka |
| KLY969 | MATaleu2-3, 112, trp1-289, ura3-52 | Laboratory stockb |
| JB811 | MATaprb1 pep4-3 trp1 leu2-3,112 ura3-52 | D. Kellogg |
| K699 | MATaade2-1 trp1-1 leu2-3, 112 his3-11,15 ura3 can1-100 ssd1-d | K. Nasmyth |
| KKY921-2B | MATacdc5-1, ura1, leu2-3, 112 trp1-289 | A. Sugino |
| KLY961 | KKY921-2B URA1::GAL1-2xEGFP-CDC5ΔDB | See text |
| SKY1710 | K699 bbp1Δ::KanMX6 + YCplac33-BBP1 | See text |
| SKY1716 | SKY1710 + YCplac22 vector | See text |
| SKY1717 | SKY1710 + YCplac22-BBP1 | See text |
| SKY1718 | SKY1710 + YCplac22-BBP1(1-295) | See text |
| SKY1719 | SKY1710 + YCplac22-BBP1(243-385) | See text |
| SKY1720 | SKY1710 + YCplac22-BBP1(295-385) | See text |
| SKY1721 | SKY1710 + YCplac22-BBP1(1-243) | See text |
| IAY18 | MATaspc42Δ::LEU2 TRP1::SPC42-GFPx3 ade2-1 trp1-1 leu2-3, 112 his3-11, 15 ura3, GAL psi+ ssd1-d2 | J. Kilmartin |
| KLY1989 | IAY18 ADE2::GAL1-HA-GST-BBP1(1-295) | See text |
| KLY1991 | IAY18 ADE2::GAL1-HA-GST-BBP1 | See text |
| KLY1993 | IAY18 ADE2::GAL1-HA-GST-BBP1(243-385) | See text |
| KLY1995 | IAY18 ADE2::GAL1-HA-GST-BBP1(1-243) | See text |
| KLY1997 | IAY18 ADE2::GAL1-HA-GST-BBP1(295-385) | See text |
| ESM988-1 | MATaura3-52 leu2Δ1 trp1Δ63 his3Δ200 SPC42-RFP::KanMX6 | E. Schiebel |
| KLY2761 | KLY1546 bbp1Δ::HIS3 TRP1::bbp1-1 | See text |
| KLY2770 | KLY1546 mps2-1 | See text |
| KLY3546 | KLY1546 CDC5-GFP::HIS3MX | See text |
| KLY3791 | KLY2761 CDC5-GFP::KanMX6 | See text |
| KLY3729 | KLY2770 CDC5-GFP::HIS3MX | See text |
| KLY5336 | KLY1546 BBP1-GFP::KanMX | See text |
| KLY5334 | KLY2761 bbp1-1-GFP::KanMX | See text |
| KLY3685 | KLY1546 URA3::SPC42-GFP | See text |
| KLY3692 | KLY2761 LEU2::SPC42-GFP | See text |
| KLY4323 | KLY1546 TRP1::CDC14-5xGFP | See text |
| KLY4426 | KLY2761 URA3::CDC14-5xGFP | See text |
| KLY3721 | KLY969 cdc5Δ::KanMX6 + YCplac33-GAL1-GFP-PLK1 | See text |
| SMY6-4b | MATα mps2-1 ura3-52 his3Δ200 leu2-3, 112 | M. Winey |
| SAY801 | MATaCDC14-5xGFP:TRP1 | A. Toh-e |
| KLY4079 | SAY801 URA3::GAL1-CDC5 | See text |
| KLY4085 | SAY801 URA3::GAL1-CDC5 | See text |
| KLY4091 | SAY801 URA3::GAL1-CDC5ΔC-BBP1(243-385) | See text |
| KLY4096 | SAY801 URA3::GAL1-CDC5ΔC | See text |
KLY1546 is in W303-1A genetic background
KLY969 is a segregant of KKY902 (Kitada et al., 1993)
Table 2.
Plasmids used in this study
| Name | Descriptiona | Source |
|---|---|---|
| pEG202-NLS | 2μ, HIS3, LexA DBD | Origene Technologies |
| pJG4-5 | 2μ, TRP1, Transcriptional AD | Ausubel et al. (1995) |
| pET21b | T7, His6 | Promega |
| pGEX-KG | GST fuson expression vector | Guan and Dixon (1991) |
| pRS305 | LEU2 | Sikorski and Hieter (1989) |
| pRS306 | URA3 | Sikorski and Hieter (1989) |
| pRS313 | CEN, HIS3 | Sikorski and Hieter (1989) |
| YCplac111 | CEN, LEU2 | Gietz and Sugino (1988) |
| YCplac22 | CEN, TRP1 | Gietz and Sugino (1988) |
| YCplac33 | CEN, URA3 | Gietz and Sugino (1988) |
| pSK910 | URA1, GAL1-2xEGFP-CDC5ΔDB | This study |
| pSK1405 | pEG202-NLS, CDC5ΔDB | This study |
| pSK1408 | pEG202-NLS, CDC5ΔDBΔC | This study |
| pSK1390 | pEG202-NLS, CDC5ΔN | This study |
| pSK1403 | pEG202-NLS, CDC5ΔN/FAA | This study |
| pSK1883 | pJG4-5, BBP1(1-385) | This study |
| pSK1882 | pJG4-5, BBP1(1-295) | This study |
| pSK1884 | pJG4-5, BBP1(243-385) | This study |
| pSK1901 | pEG202-NLS, BBP1(1-385) | This study |
| pSK1900 | pEG202-NLS, BBP1(1-295) | This study |
| pSK1902 | pEG202-NLS, BBP1(243-385) | This study |
| pKL2497 | pGEX-KG, BBP1(1-385) | This study |
| pKL2498 | pGEX-KG, BBP1(1-295) | This study |
| pKL2500 | pGEX-KG, BBP1(1-243) | This study |
| pKL2499 | pGEX-KG, BBP1(243-385) | This study |
| pKL2501 | pGEX-KG, BBP1 (295-385) | This study |
| pKL2496 | pGEX-KG, MPS2 | This study |
| pKL1053 | pET21b, CDC5 | This study |
| pSK1868 | YCplac111, GAL10-FLAG-YFP-BBP1(1-385) | This study |
| pSK1867 | YCplac111, GAL10-FLAG-YFP-BBP1(1-295) | This study |
| pSK1870 | YCplac111, GAL10-FLAG-YFP-BBP1(1-243) | This study |
| pSK1869 | YCplac111, GAL10-FLAG-YFP-BBP1(243-385) | This study |
| pSK1871 | YCplac111, GAL10-FLAG-YFP-BBP1(295-385) | This study |
| pSK1873 | YCplac22, GAL1-HA-GST-BBP1(1-385) | This study |
| pSK1874 | YCplac22, GAL1-HA-GST-BBP1(243-385) | This study |
| pSK1875 | YCplac22, GAL1-HA-GST-BBP1(1-243) | This study |
| pSK1878 | YCplac22, BBP1(1-385) | This study |
| pSK1877 | YCplac22, BBP1(1-295) | This study |
| pSK1880 | YCplac22, BBP1(1-243) | This study |
| pSK1879 | YCplac22, BBP1(243-385) | This study |
| pSK1881 | YCplac22, BBP1(295-385) | This study |
| pSK1910 | ADE2, GAL1-HA-GST-BBP1(1-385) | This study |
| pSK1909 | ADE2, GAL1-HA-GST-BBP1(1-295) | This study |
| pSK1912 | ADE2, GAL1-HA-GST-BBP1(1-243) | This study |
| pSK1911 | ADE2, GAL1-HA-GST-BBP1(243-385) | This study |
| pSK1913 | ADE2, GAL1-HA-GST-BBP1(295-385) | This study |
| pCJ159 | pRS306, SPC42-GFP | This study |
| pCJ204 | pRS305, SPC42-GFP | This study |
| pCJ259 | pUC19-URA3, CDC14-5xGFP | This study |
| pKL2701 | YCplac111, EGFP-CDC5 | This study |
| pKL2078 | YCplac111, YFP-CDC5ΔC-BBP1243-385 | This study |
| pKL2422 | pRS315, YFP-CDC5ΔC-MPS2 | This study |
| pCJ232 | YCplac111, YFP-CDC5ΔC | This study |
| pKL2420 | pRS315, YFP-CDC5ΔC-BBP1 | This study |
| pCJ107 | pRS313, MPS2 | This study |
| pCJ238 | YCplac33-GAL1, ΔCEN4 | This study |
| pCJ241 | pCJ238, GAL1-CDC5 | This study |
| pCJ240 | pCJ238, GAL1-CDC5ΔC-BBP1243-385 | This study |
| pCJ242 | pCJ238, GAL1-CDC5ΔC | This study |
| pKL2071 | YCplac111, GAL10-YFP-CDC5ΔC-BBP1243-385 | This study |
| pCJ231 | YCplac111, GAL10-YFP-CDC5ΔC | This study |
2μ indicates high-copy plasmids; CEN indicates low-copy plasmids
To construct plasmids for two-hybrid analyses, genes were amplified by PCR by using genomic DNA from strain S288C as template. For the tests of interaction between Cdc5p and Bbp1p, full-length BBP1 was fused to the B42 transcriptional activation domain (AD) in pJG4-5 (Ausubel et al., 1995) as a hemagglutinin (HA)-fusion protein (HA-tag derived from the vector), whereas CDC5, CDC5ΔC, CDC5ΔN, and CDC5ΔN/FAA were cloned in-frame to the LexA DNA-binding domain (DBD) in pEG202-NLS (Origene Technologies, Rockville, MD) (Song and Lee, 2001). For the tests of intramolecular interaction in Bbp1p, full-length or partial genes digested with BspEI and XhoI were cloned into pJG4-5 digested with the corresponding enzymes. The same fragments were also cloned into pEG202-NLS digested with BspEI and NcoI (end-filled) after the XhoI site was end-filled to allow blunt-end ligation. To construct plasmid pKL1053, which expresses full-length Cdc5p fused to both N-terminal T7 and C-terminal 6×His (His6) epitope tags, a BamHI-HindIII fragment comprising the entire CDC5 ORF was ligated into pET21b (Novagen, Madison, WI) after digesting with the corresponding enzymes. The baculovirus His6-HA-Cdc5p-Flag expression construct (Y.-W.C and K.S.L., unpublished data) will be described elsewhere. To construct full-length or partial BBP1 fused to glutathione S-transferase (GST), various BBP1 fragments digested with BspEI (end-filled) and HindIII were cloned into pGEX-KG (Guan and Dixon, 1991) digested with XbaI (end-filled) and HindIII. To construct plasmid pKL2496, an MPS2 fragment digested with XbaI and SalI was inserted into pGEX-KG digested with the corresponding enzymes. Constructs expressing yellow fluorescent protein (YFP)-fused BBP1 under control of the GAL1 promoter control were generated by inserting full-length or truncated BBP1 fragments digested with BspEI and SphI into a YCplac111-GAL1-Flag-YFP (pSK913) vector digested with the corresponding enzymes. To generate pSK1873, pSK1874, and pSK1875, the pSK865 construct bearing GAL1-HA-GST-CDC5 was first digested with BssHII and SphI to eliminate CDC5 and then ligated with the respective BBP1 fragments digested with the corresponding enzymes. To investigate the ability of full-length or truncated forms of BBP1 to complement the bbp1Δ defect, EcoRI-SphI fragments containing various BBP1 ORF sequences were inserted into the corresponding sites in YCplac22. These constructs bear the same endogenous BBP1 promoter and 3′-untranslated region sequences flanking the inserted fragments. To express full-length CDC5 as an enhanced green fluorescence protein (EGFP) fusion, a PpuMI fragment containing the EGFP ORF was inserted into the PpuMI site of the CDC5 genomic DNA cloned at the XbaI site of YCplac111. To express Flag-YFP-CDC5ΔC-BBP1243–385 (pKL2078), Flag-YFP-CDC5ΔC-BBP1 (pKL2420), or Flag-YFP-CDC5Δ-MPS2 (pKL2422) under endogenous CDC5 promoter control, a BssHII-NheI fragment containing BBP1243–385, BBP1, or MPS2 was C-terminally fused to Flag-YFP-CDC5ΔC (aa 1–500) after digesting YCplac111-Flag-YFP-CDC5ΔC or pRS315-Flag-YFP-CDC5ΔC, respectively, with BssHII and NheI. pCJ107 was generated by inserting the BamHI-XbaI fragment containing the full-length MPS2 into pRS313 (Sikorski and Hieter, 1989) digested with the corresponding enzymes. To construct plasmids pCJ241, pCJ240, and pCJ242, BamHI-SphI fragments bearing GAL1-CDC5, GAL1-CDC5ΔC-BBP1243–385, or GAL1-CDC5ΔC were cloned into an URA3-based acentromeric vector (pCJ238) digested with the corresponding enzymes. Detailed maps for the constructs described here can be provided upon request.
Growth Conditions and Media
Yeast cell culture and transformations were carried out by standard methods (Sherman et al., 1986). For cell cycle synchronization, MATa cells were arrested with 5 μg/ml α mating pheromone (Sigma-Aldrich, St. Louis, MO) for 2.5 h at 23°C, and then released into fresh growth medium. To select against cells containing URA3 plasmids, cells were streaked onto synthetic minimal medium (SDM) supplemented with 1 g/l 5-fluoro-orotic acid (FOA) (Boeke et al., 1984).
Two-Hybrid Assays
Quantitative β-galactosidase assays were performed as described previously (Ausubel et al., 1995) according to manufacturer's protocol (Origin Technologies, Rockville, MD).
Immunoblotting
Cell lysates were prepared in TED buffer [40 mM Tris-Cl, pH 7.5, 0.25 mM EDTA, 1 mM diethiothreitol, 1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride (Pefabloc; Boehringer Mannheim, Indianapolis, IN), 10 mg/ml pepstatin A (Sigma-Aldrich), 10 mg/ml leupeptin (Sigma-Aldrich), and 10 mg/ml aprotinin (Sigma-Aldrich)] with an equal volume of glass beads (Sigma-Aldrich) as described previously (Song et al., 2000). Total cellular proteins were separated by 10% SDS-PAGE (Ausubel et al., 1995). Western blot analyses of total lysates were carried out with anti-HA.11 (Babco, Richmond, CA), anti-LexA (Santa Cruz Biotechnology, Santa Cruz, CA), anti-FLAG (Sigma-Aldrich), anti-T7 (Novagen), anti-GST (BD Biosciences Clontech, Palo Alto, CA), anti-Cdc5p (Santa Cruz Biotechnologies), and anti-Cdc28p (a gift of R. Deshaies, California Institute of Technology, Pasadena, CA) as described previously (Song and Lee, 2001) using the enhanced chemiluminescence detection system (Pierce Chemical, Rockford, IL).
Preparation of Recombinant Proteins and In Vitro Protein–Protein Interaction Studies
Recombinant T7-Cdc5p-His6, GST, GST-Bbp1p, GST-Bbp1p1–295, GST-Bbp1p1–245, GST-Bbp1p243–385, GST-Bbp1p295–385, and GST-Mps2p fusion proteins were expressed from plasmids pKL1053, pGEX-KG, pKL2497, pKL2498, pKL2500, pKL2499, pKL2501, and pKL2496 in the Escherichia coli BL21 strain. T7-Cdc5p-His6 was partially purified using a Ni-NTA column (QIAGEN, Valencia, CA) according to the manufacturer's protocol, and GST or GST-fused proteins were purified using glutathione-Sepharose beads (Sigma-Aldrich). To investigate the interaction between Cdc5p and Bbp1p or Mps2p, cellular lysates prepared from Sf9 cells expressing His6-HA-Cdc5p-Flag were added to either bead-bound GST- or bead-bound GST-fusion proteins and then incubated in a binding buffer (1× phosphate-buffered saline containing 0.5% NP-40) for 1 h at 4°C. The resin was then washed five times with the binding buffer. Bound proteins were eluted by boiling in SDS-PAGE sample buffer and then analyzed by immunoblotting after SDS-PAGE. The same membrane was stained with Coomassie to detect GST and GST-fused proteins. To further investigate the interaction between Cdc5p and Bbp1p or Mps2p, T7-Cdc5p-His6 partially purified from E. coli was added to either bead-bound GST-Bbp1p or bead-bound GST-Mps2p and then incubated in a binding buffer as described above. Bound Cdc5p was separated by SDS-PAGE and detected by immunoblotting using anti-T7 (Novagen). Ligands precipitated were detected with anti-GST (BD Biosciences Clontech) antibody.
Cell Staining and Immunofluorescence Microscopy
Indirect immunofluorescence was performed as described previously (Lee et al., 1998). Briefly, cells were fixed with 3.7% formaldehyde, and microtubules were visualized using YOL1/34 rat anti-tubulin antibody (Accurate Chemical & Scientific, Westbury, NY) and goat anti-rat CY3 antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). Localization of GFP- or YFP-fused proteins was examined after fixing cells as described above. Similar localization patterns were observed with unfixed cells (our unpublished data). DNA was stained with 4′,6′-diamidino-2-phenylindole (DAPI).
Immuno-EM
Immuno-EM was performed using high-pressure frozen and freeze-substituted cells as described by Giddings et al. (2001). Serial thin sections were viewed on a Philips CM10 electron microscope (Philips Electronic Instruments, Mahwah, NJ), and images were captured on film or with a Gatan digital camera and viewed with the Digital Micrograph Software package (Gatan, Pleasanton, CA). GFP-fused Cdc5p was detected with a polyclonal anti-GFP antibody (Zeng et al., 1999) and 10-nm colloidal gold-conjugated secondary antibodies (Ted Pella, Redding, CA).
RESULTS
Localization of GFP-Cdc5p
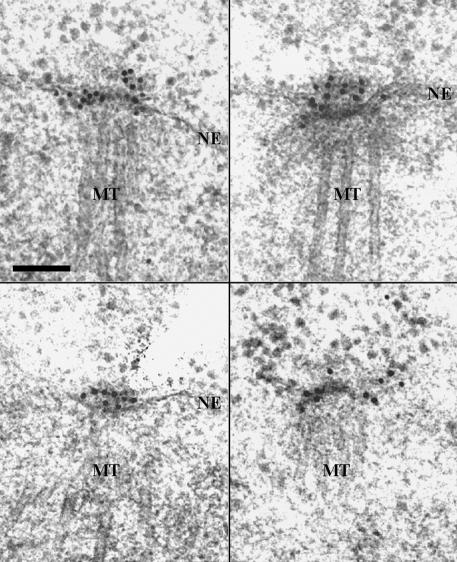
Studies have shown that Cdc5p localizes to the SPBs and the cytokinetic neck-filaments (Shirayama et al., 1998; Song et al., 2000). To develop a better understanding of the localization and function of Cdc5p at the SPB, we attempted to localize the GFP-fused Cdc5p within the SPB by carrying out immuno-EM with affinity-purified anti-GFP antibody. The expression level of a Cdc5p-GFP fusion protein under the endogenous CDC5 promoter control did not yield reliable signals (our unpublished data). Thus, strain KLY961, expressing GAL1–2×(EGFP)-fused CDC5ΔDB lacking the destruction box (Song et al., 2000) in the cdc5-1 background, was cultured under induction conditions for 1 h before fixation. Strong EGFP2-Cdc5pΔDB signals were manifest at or near the SPB under these conditions. Among 13 cells examined, EGFP2-Cdc5pΔDB was most commonly detected at the cytoplasmic side or the periphery of the central plaque or over the outer plaque (11/13 cells; Figure 1). In two cases, however, the EGFP2-Cdc5pΔDB signal was also evident at the inner plaque of the spindle pole body (our unpublished data). These observations suggest that Cdc5p primarily localizes to the cytoplasmic periphery of the SPB. Whether the less frequent EGFP2-Cdc5pΔDB signals at the nuclear side of the SPB are suggestive of a fraction of Cdc5p localizing to this location or are due to the artifact of overexpressed Cdc5p is not clear at present.
Figure 1.
Immuno-EM localization of GFP-Cdc5p. A cdc5-1 mutant strain integrated with a GAL1-promoter-controlled 2×(EGFP)-CDC5 at the URA1 locus (KLY961) was cultured in YEP-raffinose over-night and then shifted to YEP-galactose for 1 h to induce the expression of 2×(EGFP)-CDC5. The cells were harvested and prepared for immuno-EM by high-pressure freezing and freeze-substitution (see MATERIALS AND METHODS). Shown are four representative images of ∼13 SPBs examined. The 10-nm gold particles (black dots) indicate the EGFP2-Cdc5p signals. NE, nuclear envelope; MT, nuclear microtubule. Bar, 0.1 μm.
Isolation of Bbp1p as a Polo-Box–interacting Protein
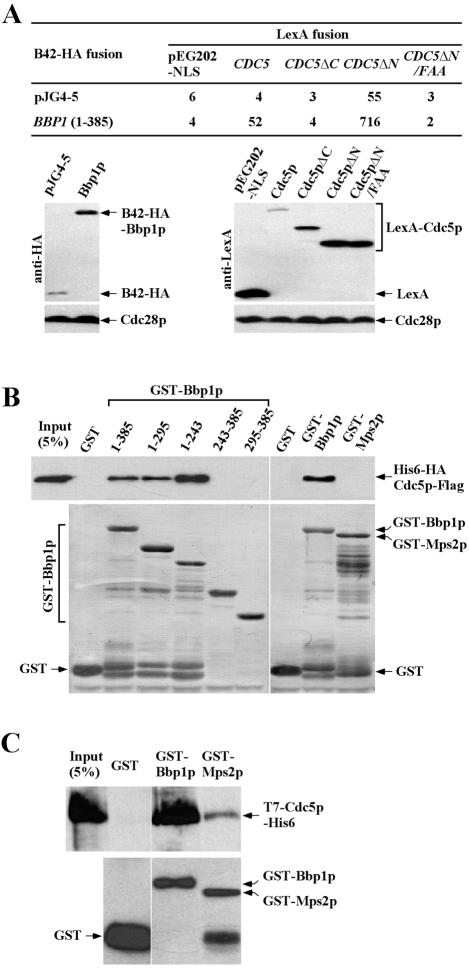
We have previously shown that the C-terminal domain of Cdc5p (Cdc5pΔN), which contains the polo-box, plays a critical role in targeting the catalytic activity of Cdc5p to the SPB and the bud-neck (Song et al., 2000). In an effort to identify the polo-box–binding proteins at these subcellular structures, we carried out a yeast two-hybrid screening by using a DBD fused with the C-terminal domain of Cdc5p (Cdc5pΔN; pSK1390) as the bait. Strain EGY48 (α-type), which bears the bait and a LexAop-LEU2 reporter, was transformed with GAL1-B42-HA-fused cDNA library constructed in pJG4-5 (TRP1) vector (Origene Technologies). Approximately 2 × 106 transformants were selected on a minimal medium lacking Trp, His, and Leu. Putative Cdc5p-interacting clones were retransformed into strain EGY48 and then mated with strain RFY206 (a-type) harboring a lacZ reporter plasmid (pSH18-34) for quantitative β-galactosidase assays. One of these clones encoding Bbp1p exhibited a strong interaction with Cdc5pΔN, and also with the full-length Cdc5p (Figure 2A). Consistent with the polo-box–dependent localization of Cdc5p to the SPB, the N-terminal polo-box domain (Cdc5pΔC) or Cdc5pΔN bearing conserved FAA triple mutations in the polo-box (Song et al., 2000) failed to interact with Bbp1p under the same conditions (Figure 2A).
Figure 2.
Physical interactions between Cdc5p and Bbp1p. (A) Two-hybrid assays were conducted as described in MATERIALS AND METHODS with plasmids that expressed full-length or truncated forms of BBP1 or CDC5 as AD or DBD fusions, respectively. Cdc5pΔN/FAA possesses the FAA mutations, which disrupt the function of the polo-box domain of Cdc5p (Song et al., 2000). Numbers indicate the Miller units of β-galactosidase activity averaged from two independent experiments. Immunoblotting (bottom) indicated the expression levels of various constructs with Cdc28p as loading control. CDC5, pSK1405; CDC5ΔC, pSK1408; CDC5ΔN, pSK1390; CDC5ΔN/FAA, pSK1403; and BBP1 (1-385), pSK1883. (B and C) In vitro interaction studies were carried out using various full-length or truncated forms of GST-Bbp1p or GST-Mps2p as ligands. Sf9 cell lysates expressing recombinant His6-HA-Cdc5p-Flag (B) or bacterially expressed, purified, recombinant T7-Cdc5p-His6 (C) were incubated with various ligands as described in MATERIALS AND METHODS. After SDS-PAGE, the amounts of bound His6-HA-Cdc5p-Flag or T7-Cdc5p-His6 were determined by immunoblotting with either an anti-FLAG antibody (B, top) or an anti-T7 antibody (C, top), whereas the amounts of GST, GST-Bbp1p, or GST-Mps2p ligands were determined by Coomassie staining (B, bottom) or immunoblotting with an anti-GST antibody (C, bottom). Input, 5% of His6-HA-Cdc5p-Flag or T7-Cdc5p-His6 that was incubated with GST, GST-Bbp1p, or GST-Mps2p. 1–385, pKL2497; 1–295, pKL2498; 1–243, pKL2500; 243–385, pKL2499; 295–385, pKL2501; GST-Bbp1p, pKL2497; and GST-Mps2p, pKL2496.
BBP1 is predicted to encode a protein of 45 kDa, which contains no currently recognizable functional motifs except for two predicted coiled-coil regions (for review, see Lupas, 1996) at amino acids 243–290 and 305–385. Bbp1p localizes at the cytoplasmic side of the central plaque periphery of the SPB (Schramm et al., 2000) and plays an important role in inserting a duplication plaque into the nuclear envelope and assembling a functional inner plaque (Schramm et al., 2000). Similar subcellular localizations and also the observed polo-box–dependent binding between Cdc5p and Bbp1p in two-hybrid suggest that they may directly interact. To test this possibility, bead-bound full-length or truncated forms of GST-Bbp1p purified from bacterial cells were incubated with Sf9 cell lysates containing His6-HA-Cdc5p-Flag and then bound Cdc5p was analyzed as described in MATERIALS AND METHODS. To investigate the specificity of the Cdc5p–Bbp1p interaction, Mps2p (Winey et al., 1991), which was shown to form a stable complex with Bbp1p (Schramm et al., 2000), was also examined. GST-Bbp1p1–385, GST-Bbp1p1–295, and GST-Bbp1p1–245 interacted with Cdc5p, whereas GST alone, GST-Bbp1p243–385, and GST-Bbp1p295–385 did not (Figure 2B). In addition, GST-Mps2p did not interact with Cdc5p under the same conditions (Figure 2B). In a second experiment, we observed that purified GST-Bbp1p, but not GST-Mps2p, could also interact with bacterially expressed, partially purified T7-Cdc5p-His6 (Figure 2C). These data together with the two-hybrid results suggest that the polo-box domain of Cdc5p interacts directly with the N-terminal domain of Bbp1p and that this interaction is likely specific. However, we failed to coimmunoprecipitate Cdc5p and Bbp1p from growing yeast cell lysates under various conditions (our unpublished data), suggesting that the interaction between Cdc5p and Bbp1p is likely transient.
The C-Terminal Coiled-Coil Domain of Bbp1p Is Sufficient for Localization and Homo-dimerization but Not for Function
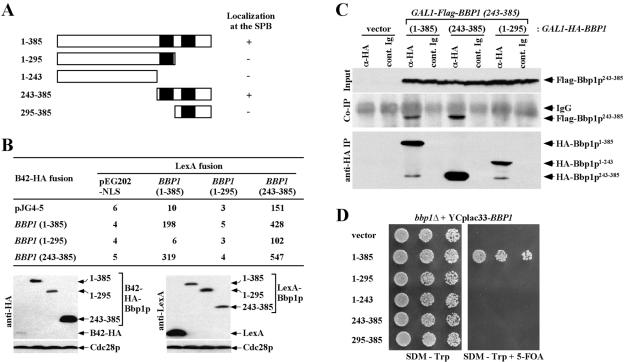
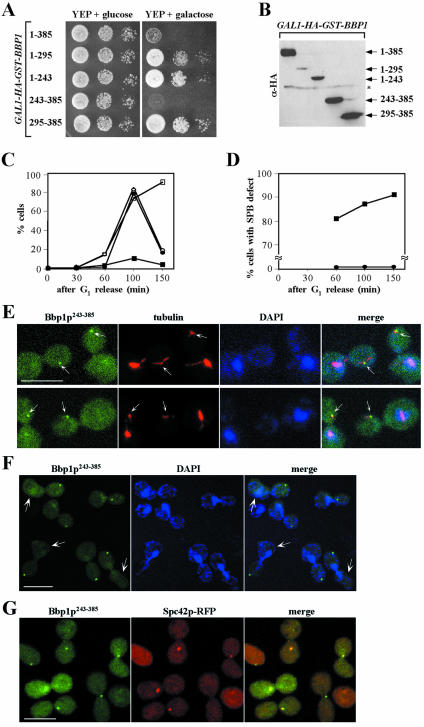
Coiled-coil domain has been implicated in protein–protein interactions (Lupas, 1996). Because the coiled-coil domain of Bbp1p (Bbp1p243–385) does not seem to interact with the C-terminal domain of Cdc5p, we tested whether it is responsible for the localization of Bbp1p to the SPB by using various GFP-fused Bbp1p constructs. Similar to the endogenous Bbp1p tagged with GFP at its C-terminal end (Figure 5B), expression of both the full-length YFP-Bbp1p1–385 and YFP-Bbp1p243–385 (C-terminal domain containing the two predicted coiled-coil regions of Bbp1p) under the GAL10 promoter control efficiently localized to the SPB. In contrast, both YFP-Bbp1p1–295 bearing the first coiled-coil region or YFP-Bbp1p295–385 containing only the second coiled-coil region failed to localize to the SPB (Figure 3A). These data indicate that the C-terminal domain of Bbp1p (Bbp1p243–385) is sufficient for the localization of Bbp1p to the SPB and that both of the predicted coiled-coil regions most likely are required to form a functional localization domain of Bbp1p.
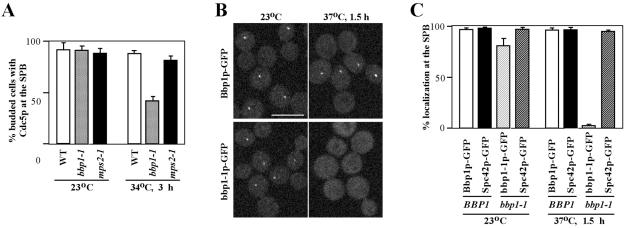
Figure 5.
Requirement of Bbp1p, but not Mps2p, for proper localization of Cdc5p at the SPB. (A) Strain KLY3546 (BBP1), KLY3791 (bbp1-1), or KLY3729 (mps2-1), which expresses Cdc5p-GFP under endogenous CDC5 promoter control was cultured overnight at 23°C and then shifted to 34°C in the presence of 15 μg/ml of nocodazole for 3 h. Among the large-budded cells, the fraction of cells with Cdc5p-GFP signals at the SPB were determined by counting >200 cells for each sample. These results are obtained from three independent experiments. Error bars indicate SD. WT, strain KLY3546; bbp1-1, strain KLY3791; mps2-1, strain KLY3729. (B and C) To examine the temperature-dependent localization of Bbp1p and bbp1-1p to the SPB, strains KLY5336 and KLY5334, which expresses Bbp1p-GFP or bbp1-1p-GFP under endogenous BBP1 promoter control, respectively, were arrested with nocodazole for 2.5 h. Cells were then shifted to 37°C for 1.5 h, fixed, and then examined by confocal microscopy (B). Localization of Spc42p-GFP in the BBP1 wild-type (KLY3685) or the bbp1-1 mutant (KLY3692) was also examined under the same conditions. More than 200 cells were counted in three independent experiments. Error bars indicate SD. Bar, 5 μm.
Figure 3.
Functional domain analysis of Bbp1p. (A) Structures of the Bbp1p truncations used in these analyses (see MATERIALS AND METHODS) and the ability of these constructs to localize to the SPB. To determine the domain of Bbp1p responsible for the SPB localization, N-terminally YFP-tagged full-length and various truncated forms of Bbp1p as indicated were expressed under control of the GAL10 promoter for 1 h. Filled box, the predicted coiled-coil domain; +, SPB localization; -, no detectable localization. 1–385, pSK1868; 1–295, pSK1867; 1–243, pSK1870; 243–385, pSK1869; and 295–385, pSK1871. (B) Intramolecular interactions of Bbp1p in two-hybrid assays. Two-hybrid assays were carried out as described in Figure 2A with full-length or truncated forms of BBP1 as indicated. Numbers indicate the Miller units of β-galactosidase activity. Immunoblotting (bottom) indicate the expression levels of various constructs with Cdc28p as loading control. LexA fusion: 1–385, pSK1901; 1–295, pSK1900; 243–385, pSK1902. B42-HA fusion: 1–385, pSK1883; 1–295, pSK1882; and 243–385, pSK1884. (C) Bbp1243–385 is sufficient for homo-dimerization. Equivalent amounts of protein from cells expressing indicated constructs under induction conditions for 2 h were subjected to immunoprecipitation with rabbit polyclonal anti-HA antibody to precipitate HA-Bbp1p1–385, HA-Bbp1p243–385, or HA-Bbp1p1–243. The immunoprecipitates were resolved by SDS-PAGE and analyzed by immunoblotting with anti-FLAG antibody to detect coprecipitated Flag-Bbp1p243–385 or with mouse monoclonal anti-HA.11 antibody to determine the level of immunoprecipitated HA-Bbp1p. Control Ig, rabbit control Ig. (D) Both N-terminal and C-terminal domains of Bbp1p are required for the function of Bbp1p. Strain SKY1710 (bbp1Δ + YCplac33-BBP1) was transformed with full-length or various truncated forms of BBP1. Transformants selected on SDM lacking tryptophan were cultured overnight, serially diluted, and spotted onto either SDM-Trp or SDM-Trp supplemented with 5-FOA to select against URA3-containing YCplac33-BBP1 plasmid. Plates were incubated at 30°C for 3 d. Vector, SKY1716; 1–385, SKY1717; 1–295, SKY1718; 1–243, SKY1721; 243–385, SKY1719; and 295–385, SKY1720.
It has been suggested that Bbp1p forms a homo-dimer (Schramm et al., 2000). Thus, we carried out two-hybrid analysis to investigate whether the C-terminal coiled-coil regions of Bbp1p are important for homo-dimerization. Both full-length Bbp1p1–385 and Bbp1p243–385 exhibited interactions with Bbp1p1–385 or Bbp1p243–385. Under the same conditions, Bbp1p1–295 lacking the second coiled-coil region did not interact (Figure 3B). In addition, immunoprecipitation of either Bbp1p1–385 or Bbp1p243–385, but not Bbp1p1–243, from yeast cellular lysates coprecipitated Bbp1p243–385 (Figure 3C). These results suggest that the C-terminal two coiled-coil repeats in Bbp1p243–385 are necessary and sufficient for homo-dimerization of Bbp1p. Because Bbp1p243–385 is sufficient to localize to the SPB, the homo-dimerization of Bbp1p may likely be a critical step for the subcellular localization of Bbp1p to the SPB.
We then examined whether the C-terminal coiled-coil regions of Bbp1p are sufficient for the function of Bbp1p. A bbp1Δ mutant, kept viable by the presence of a URA3-based centromeric BBP1 (SKY1710), was additionally transformed with full-length or truncated forms of BBP1. Transformants were then streaked on a minimal selection medium supple-mented with 5-FOA to select against the URA3-based BBP1 plasmid. Cells expressing full-length Bbp1p1–385 grew well on the 5-FOA–containing plate (Figure 3D). In contrast, cells expressing any of the truncated forms of Bbp1p did not support the viability of the bbp1Δ mutant (Figure 3D). These data suggest that Bbp1p243–385-dependent localization is not sufficient for the function of Bbp1p, and that the N-terminal domain of Bbp1p may have an uncharacterized role critical for fulfilling the function of Bbp1p.
Bbp1p243–385 Functions Dominant-Negatively to Induce a Cell Cycle Arrest
We then examined the phenotype associated with overexpression of full-length and various truncated forms of Bbp1p. Expression of either full-length Bbp1p1–385 or Bbp1p243–385 under the GAL1 promoter control strongly inhibited cell growth (Figure 4A). Expression of Bbp1p1–295 or Bbp1p1–243 also induced a weak but significant growth inhibition (Figure 4A), even though the expression levels of these proteins were much lower than those of others (Figure 4B). These observations suggest that overexpression of either the full-length or the truncated forms of BBP1 is detrimental for cell growth.
Figure 4.
Overexpression of BBP1243–385 results in a cell cycle arrest with an aberrant daughter-side SPB. (A) Inhibition of cell growth by overexpression of full-length BBP1 (Bbp1p1–385) or BBP1243–385. Wild-type strain K699 expressing GST-Bbp1p1–385 or GST-Bbp1p243–385 under control of the GAL1 promoter was cultured in YEP-raffinose overnight, serially diluted, and spotted onto either YEP-galactose (YEPG) or YEP-glucose (YEPD) plates. Plates were then incubated at 30°C for 3 d. 1–385, strain KLY1991; 1–295, strain KLY1989; 1–243, strain KLY1995; 243–385, strain KLY1993; and 295–385, strain KLY1997. (B) To determine the expression levels of GST-Bbp1p1–385 or GST-Bbp1p243–385 used in A, total protein prepared from cells cultured under induction conditions for 2 h was subjected to immunoblotting with anti-HA antibody. Asterisk indicates a nonspecific cross-reacting protein. (C and D) To determine the arresting phenotype induced by overexpression of YFP-BBP1243–385, wild-type strain KLY1546 was transformed with a centromeric GAL10-YFP-BBP1243–385 plasmid (pSK1869) or control YCplac111 vector. Transformants were arrested in G1 by α-factor treatment in YEP-raffinose for 4 h and then released into YEP-galactose to induce the expression of YFP-BBP1243–385. (C) Percentages of large-budded cells (open circle, control vector; open square, YFP-BBP1243–385) and cells exhibiting divided nuclear morphology (closed circle, control vector; closed square, YFP-BBP1243–385) were determined at the indicated time points after induction. Approximately 200 cells were counted at each time point. (D) Among the large-budded cells, the percentages of cells with single YFP-Bbp1p243–385 fluorescent dot signals at the daughter-side SPB were determined at the indicated time points. Closed circle, YCplac111; closed square, pSK1869. (E) Representative spindle morphologies of cells with GAL1-YFP-BBP1243–385 expression. Cells harvested at the 100-min time point in C were subjected to indirect immunofluorescence microscopy, after visualizing tubulin (red) with an anti-tubulin antibody as described in MATERIALS AND METHODS. DNA was stained with DAPI. Arrows indicate YFP-Bbp1p243–385 signals with apparently defective SPB with significantly weakened microtubule structures. Bar, 5 μm. (F) Localization of YFP-Bbp1p243–385 at the daughter-side of the SPB. Cells bearing GAL1-YFP-BBP1243–385 were treated with α-factor to mark the mother cells and then released into YEP-galactose medium for 1.5 h to examine the YFP-Bbp1p243–385 fluorescent signals. Arrows indicate cells with remnants of the shmoo morphology. Bar, 5 μm. (G) Colocalization of YFP-Bbp1p243–385 with Spc42-RFP. The Spc42-RFP cells (ESM988-1) harboring GAL1-YFP-BBP1243–385 were cultured under induction conditions for 1.5 h, fixed, and then subjected to fluorescence microscopy. Bar, 5 μm.
To closely monitor the arresting phenotype associated with Bbp1p1–385 or Bbp1p243–385 expression, strain KLY1546 bearing control vector, GAL10-YFP-BBP11–385 or GAL10-YFP-BBP1243–385 were cultured in YEP-raffinose, arrested with α-factor, and then released into YEP-galactose to induce the protein expression. Cells harboring the control vector proceeded through the cell cycle normally (Figure 4C). Expression of GAL10-YFP-BBP11–385 resulted in a delayed cell cycle progression without a uniformed arresting phenotype (our unpublished data), suggesting that overexpression of YFP-BBP11–385 is detrimental for growth. Under the same conditions, expression of GAL10-YFP-BBP1243–385 induced a drastic arrest with a large-budded morphology (Figure 4C). Greater than 90% of these cells (n = 110) possessed one nucleus in the mother cell 150 min after α-factor release (Figure 4D), suggesting an arrest before nuclear division. Interestingly, in most of these cells, a single YFP-Bbp1p243–385 fluorescent dot was apparent in the bud, and these dots were not associated with DAPI-stained DNA mass (Figure 4, D and E). When the cells were treated with α-factor to mark the mother cells and then released into YEP-galactose medium as described above, the localization of YFP-Bbp1p243–385 to the daughter-side of the SPB was manifest in >91% (n = 180) of the population (Figure 4F). In addition, most of these fluorescent signals (87%; n = 123) were colocalized with Spc42-RFP (Figure 4G), a daughter-side SPB marker (Pereira et al., 2001). These observations suggest that the newly synthesized YFP-Bbp1p243–385 has been incorporated into the daughter-side of the SPB, and this SPB is impaired in properly segregating sister chromatids. Consistent with this view, microtubule structures emanating from the daughter-side of the SPB were much weaker than those from the mother cells (Figure 4E). A similar phenotype has been observed in the bbp1-1 mutant (Schramm et al., 2000). These observations suggest that expression of the localization-competent Bbp1p243–385 interfered with the function of Bbp1p at the daughter-side SPB and that this interference led to a failure in bipolar spindle formation and chromosome segregation.
Bbp1p Is Required for Proper Localization of Cdc5p at the SPB
Our data suggest that Bbp1p interacts with Cdc5p directly and that Bbp1p localizes to the SPB through the coiled-coil region. Interestingly, the expression level of BBP1 peaks during the G1 phase of the cell cycle (Spellman et al., 1998), whereas CDC5 is expressed at the late stages of the cell cycle (Kitada et al., 1993; Hardy and Pautz, 1996). These temporal regulations of Bbp1p and Cdc5p during the cell cycle suggest that Cdc5p may require Bbp1p to localize to the SPB. To test this possibility, the temperature-sensitive bbp1-1 mutant that expresses Cdc5p-GFP under endogenous CDC5 promoter control was used to examine the efficiency of Cdc5p-GFP localization at the nonpermissive temperature. As a comparison, Cdc5p localization was also determined in an isogenic wild-type and the temperature-sensitive mps2-1 mutant. To enrich Cdc5p and to eliminate the cell cycle-dependent alterations in Cdc5p localization, these strains were arrested in M phase with nocodazole at 23°C for 3 h before shifting the temperature to 34°C, a nonpermissive temperature for both the bbp1-1 and mps2-1 mutants, for 3 h. At 23°C, Cdc5p-GFP efficiently localized to the SPB in all three strains. At 34°C, however, Cdc5p-GFP localization was markedly decreased in the bbp1-1 mutant, whereas both the wild-type and the mps2-1 mutant exhibited an efficient Cdc5p-GFP localization to the SPB (Figure 5A). These data indicate that Bbp1p is required for the localization of Cdc5p to the SPB. These observations further suggest that bbp1-1p, but not Bbp1p, incorporated into the SPB may have become nonfunctional upon exposure to a restrictive temperature. To examine this possibility, cells expressing Bbp1p-GFP or bbp1-1p-GFP under endogenous BBP1 promoter control (KLY5336 or KLY5334, respectively) were arrested with nocodazole at 23°C for 2.5 h before shifting the cultures to nonpermissive temperatures. The localization of bbp1-1p-GFP, but not Bbp1p-GFP, to the SPB was almost completely disrupted upon culturing at 37°C for 1.5 h (Figure 5, B and C); localization of bbp1-1p-GFP to the SPB was greatly diminished at 34°C, but weak fluorescent signals were still detectable in ∼25% of the population. In contrast, the localization efficiency of Spc42p-GFP in either the BBP1 wild-type or the bbp1-1 mutant remained unchanged under the same conditions (Figure 5C), suggesting that, unlike Cdc5p, Spc42p does not require Bbp1p for its localization to the SPB. We were not able to examine the Mps2p localization under these conditions because of a failure in generating a detectable Mps2p-GFP fluorescent signal (our unpublished data). Thus, even though we cannot rule out the possibility that Mps2p also contributes to the Cdc5p localization to the SPB, a drastic delocalization of bbp1-1p from the SPB may have resulted in delocalization of Cdc5p. Because Cdc5p interacts with Bbp1p in yeast two-hybrid and in vitro binding analyses, we speculate that the Bbp1p-dependent localization of Cdc5p to the SPB is likely through a direct protein–protein interaction.
Bbp1p Is Required for Proper M-Phase Progression
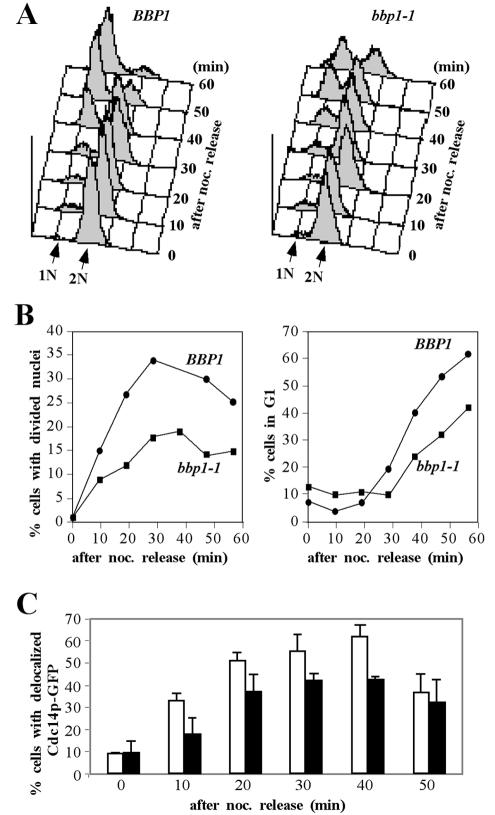
Proper subcellular localization of Cdc5p is critical for the mitotic functions of Cdc5p (Lee et al., 1998). Because the bbp1-1 mutant is impaired in Cdc5p localization to the SPB, it may have a previously uncharacterized mitotic defect. To examine this possibility, strains KLY3685 (isogenic wild-type) and KLY3692 (bbp1-1) arrested with nocodazole for 3 h at 23°C were shifted to 37°C to impair the bbp1-1p function (Figure 5, B and C) and then released into fresh medium at 37°C to monitor the mitotic progression. Flow cytometry analyses revealed that strain KLY3685 regenerated a distinct G1 population 40 min after nocodazole release (Figure 6A). Under the same conditions, strain KLY3692 exhibited an ∼10-min delay in generating G1 population (Figure 6A). Consistent with this observation, the bbp1-1 mutant was delayed in both achieving the nuclear division and generating unbudded G1 cells (Figure 6B). In comparison with the isogenic wild-type strain KLY3685, delocalization of Cdc14p-GFP5 from the nucleolus was also delayed in the bbp1-1 mutant (Figure 6C). Together, these data suggest that, in addition to the role of Bbp1p in SPB duplication, Bbp1p may also be required for proper mitotic progression. Because Cdc5p localization to the SPB is partially impaired in bbp1-1, the improper localization of Cdc5p may have contributed to the delayed mitotic progression observed in the bbp1-1 mutant.
Figure 6.
Loss of BBP1 function leads to a delayed mitotic progression. (A) Strains KLY3685 (BBP1) and KLY3692 (bbp1-1) were arrested in G1 with 5 μg/ml of α-factor for 3 h at 23°C and then released into 15 μg/ml of nocodazole-containing YEP-glucose at 23°C for 120 min to rearrest cells in early M phase. The resulting cells were shifted to 37°C for 1.5 h to cripple the bbp1-1 function before releasing into prewarmed YEP-glucose medium containing α-factor at 37°C. Samples were harvested at the indicated time points after nocodazole release and then subjected to flow cytometry analysis. 1N, cells with 1N DNA content; 2N, cells with 2N DNA content. (B) Aliquots of the same samples in A were fixed with formaldehyde and stained with DAPI to determine the percentages of cells with divided nuclei morphologies (left) or unbudded G1 cells (right) after nocodazole release. Approximately 200 cells were counted at each time point. (C) BBP1 is required for normal release of Cdc14p from the nucleolus. Strain KLY4323 (BBP1) and KLY4426 (bbp1-1) expressing a CDC14–5×GFP fusion protein under endogenous CDC14 promoter control were arrested with 15 μg/ml nocodazole for 2.5 h at 23°C. Cultures were then shifted to 37°C for 1.5 h before release into prewarmed YEP-glucose medium at 37°C. Percentages of cells with delocalized Cdc14p-GFP5 were determined at the indicated time points after nocodazole-release. Approximately 200 cells were counted at each time point. Error bars indicate SD. White bars, strain KLY4323; black bars, strain KLY4426.
Bbp1p243–385, but Not Mps2p, Can Replace the Function of the C-Terminal Domain of Cdc5p
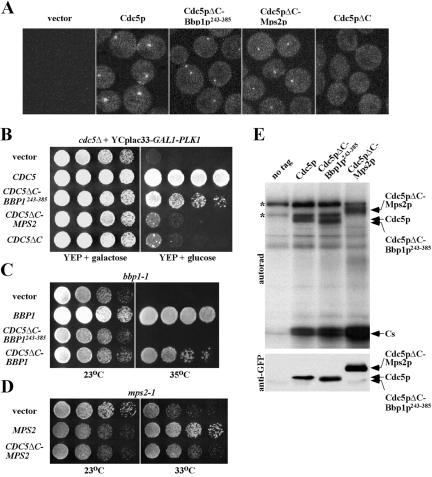
To further investigate whether the Bbp1p-dependent localization of Cdc5p to the SPB is important for the mitotic functions of Cdc5p, we asked whether the localization domain of Bbp1p (Bbp1p243–385) would substitute the C-terminal domain of Cdc5p, a region critical for subcellular localization of Cdc5p. To this end, Bbp1p243–385 or MPS2p was C-terminally fused to the localization-defective, nonfunctional GFP-Cdc5pΔC and expressed under endogenous CDC5 promoter control. In the wild-type strain KLY1546, GFP-Cdc5p localized to the SPB and weakly to the bud-neck, whereas GFP-Cdc5pΔC lacking the polo-box domain did not yield any distinct localization signals (Figure 7A). As expected, both GFP-Cdc5pΔC-Bbp1p243–385 and GFP-Cdc5pΔC-Mps2p localized to the SPB at similar levels, but they failed to localize to the bud-neck under the same conditions (Figure 7A). These observations suggest that either Bbp1p243–385 or Mps2p can effectively target the Cdc5pΔC to the SPB.
Figure 7.
Bbp1p243–385-dependent localization of Cdc5pΔC to the SPB remedies the cdc5Δ defect. (A) Bbp1p243–385-dependent targeting of the localization-defective Cdc5pΔC to the spindle poles. Wild-type strain KLY1546 transformed with control YCplac111 vector, GFP-CDC5 (pKL2701), GFP-CDC5ΔC-BBP1243–385 (pKL2078), GFP-CDC5ΔC-MPS2 (pKL2422), or GFP-CDC5ΔC (pCJ232) was grown in the presence of 15 μg/ml of nocodazole for 3 h at 23°C, fixed, and then subjected to fluorescent microscopy to examine the localization of the GFP-fused proteins. (B) To examine the ability of various constructs in A to complement the cdc5Δ defect, strain KLY3721 (cdc5Δ + GAL1-EGFP-PLK1) transformed with each plasmid was cultured overnight, serially diluted, and spotted onto either YEP-galactose (YEPG) or YEP-glucose (YEPD) plates, and then incubated at 30°C for 3 d. (C and D) To examine the ability of CDC5ΔC-BBP1, CDC5ΔC-BBP1243–385, or CDC5ΔC-MPS2 to complement the bbp1-1 or mps2-1 defect, strains KLY2761 (bbp1-1) or SMY6-4b (mps2-1) were transformed with the indicated plasmids. The resulting transformants were cultured overnight, serially diluted, spotted onto YEP-glucose plates, and then incubated at the indicated temperatures for 3 d. BBP1, pSK1878; CDC5ΔC-BBP1243–385, pKL2078; CDC5ΔC-BBP1, pKL2420; MPS2, pKL1135; and CDC5ΔC-MPS2, pKL2422. (E) To measure the kinase activities associated with Flag-YFP-Cdc5p, Flag-YFP-Cdc5pΔC-Bbp1p243–385, or Flag-YFP-Cdc5pΔC-Mps2p, a protease-negative JB811 strain transformed with pKL2772, pKL2078, or pKL2422, respectively, were arrested with nocodazole for 3 h, and then harvested. Equal amounts of protein prepared from each transformant were subjected to anti-FLAG immunocomplex kinase assays. Reaction mixtures were separated in SDS-PAGE, transferred onto a polyvinylidene difluoride membrane, and then exposed to detect the kinase activities (top). The same blot was subjected to immunoblotting with anti-GFP antibody to determine the amount of Flag-YFP-Cdc5p or the corresponding chimera proteins in each immunoprecipitate (bottom). Asterisks indicate nonspecific phosphorylation bands. Cs, casein.
Next, we asked whether these chimera constructs could rescue the growth defect associated with the cdc5Δ mutation when expressed under the CDC5 promoter control. Introduction of CDC5 fully complemented the growth defect of the cdc5Δ mutant, whereas introduction of CDC5ΔC led to the generation of nonviable microcolonies (Figure 7B). Interestingly, CDC5ΔC-BBP1243–385 rescued the cdc5Δ defect significantly, whereas CDC5ΔC-MPS2 did not (Figure 7B). As with BBP1243–385, provision of CDC5ΔC-BBP1243–385 did not rescue the bbp1-1 defect (Figure 7C). However, provision of CDC5ΔC-BBP1 or CDC5ΔC-MPS2 partially complemented the bbp1-1 or the mps2-1 defect, respectively (Figure 7, C and D). When expressed in a wild-type background, none of these constructs seemed to induce a dominant-negative growth defect (our unpublished data), suggesting that the encoded fusion proteins do not interfere with the function of Cdc5p, Bbp1p, or Mps2p in vivo. In addition, both Cdc5pΔC-Bbp1p243–385 and Cdc5pΔC-Mps2p immunoprecipitated from yeast cellular lysates exhibited autophosphorylation and transphosphorylation activities similar to those of Cdc5p (Figure 7E). Together, these data suggest that the C-terminal coiled-coil domain of Bbp1p (Bbp1p243–385) can substitute for the function of the C-terminal domain of Cdc5p by targeting the catalytic activity of Cdc5p (Cdc5pΔC) to the SPB and that Bbp1p, but not Mps2p, can direct Cdc5p to the proper locations at the SPB.
Cdc5pΔC-Bbp1p243–385 Promotes the Mitotic Exit
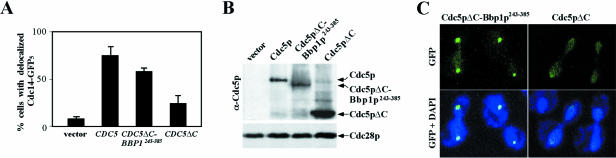
One of the critical mitotic events that requires the function of Cdc5p is mitotic exit, a late mitotic event that requires the inactivation of Cdc28p/Clb2p activity. Thus, we examined whether Bbp1p243–385-dependent targeting of Cdc5pΔC to the SPB is sufficient for inducing the Cdc14p release from the nucleolus. Strain SAY801 expressing CDC14-5×GFP under endogenous CDC14 promoter control was additionally integrated with the control GAL1 vector, GAL1-CDC5, GAL1-CDC5ΔC-BBP1243–385, or GAL1-CDC5ΔC at the URA3 locus. The resulting transformants were cultured under induction conditions for 2.5 h in the presence of nocodazole and then the percentages of cells with released Cdc14p from the nucleolus were determined. Expression of CDC5 resulted in Cdc14p release in 75% of the population, whereas expression of control vector did not yield any significant Cdc14p release. Under the same conditions, expression of CDC5ΔC-BBP1243–385 induced Cdc14 release in 58% of the population (Figure 8A), whereas expression of CDC5ΔC induced it in only 24% of the population even with a higher protein expression level (Figure 8, A and B). As with the localization of GFP-Cdc5pΔC-Bbp1p243–385 or GFP-Cdc5pΔC under endogenous CDC5 promoter control, expression of GAL10-GFP-CDC5ΔC-BBP1243–385 exhibited strong fluorescent signals at the SPB, whereas expression of GAL10-GFP-CDC5ΔC yielded a largely diffused signal in the nucleus (Figure 8C). These observations indicate that Bbp1p243–385-dependent targeting of Cdc5p to the SPB is sufficient to induce the Cdc14p release from the nucleolus and promote the mitotic exit.
Figure 8.
Cdc5pΔC-Bbp1p243–385 can induce the Cdc14p release from the nucleolus. (A) Strain SAY801 (Cdc14p-GFP5) integrated with GAL1 vector (pCJ238), GAL1-CDC5 (pCJ241), GAL1-CDC5ΔC-BBP1243–385 (pCJ240), or GAL1-CDC5ΔC (pCJ242) was cultured in YEP-raffinose overnight and then transferred to YEP-galactose medium containing 15 μg/ml of nocodazole for 2.5 h before fixation with formaldehyde to assess the subcellular localization of Cdc14p-GFP5 by GFP fluorescence. The percentages of cells with Cdc14p-GFP5 at the nucleolus were determined by counting >200 cells for each sample. Data were obtained from three independent experiments. Error bars indicate SD. Vector, strain KLY4079; CDC5, strain KLY4085; CDC5ΔC-BBP1243–385, strain KLY4091; and CDC5ΔC, strain KLY4096. (B) Expression levels of the above constructs were examined after transforming each plasmid into the protease-negative JB811 strain. Total cellular protein prepared from each transformant was subjected to immunoblotting with an anti-Cdc5p antibody. The levels of Cdc28p are shown as loading controls. (C) To determine the localization of Cdc5pΔC-Bbp1p243–385 or Cdc5pΔC, strain KLY1546 transformed with GAL10-YFP-CDC5ΔC-BBP1243–385 (pKL2071) or GAL10-YFP-CDC5ΔC (pCJ231) was cultured under induction conditions for 2.5 h, fixed, and then subjected to fluorescent microscopy.
DISCUSSION
Bbp1p as a Polo-Box–binding Protein at the SPB
It has been suggested that the polo-box plays an essential role for protein–protein interactions to target the catalytic activity of Cdc5p to specific subcellular structures. In an attempt to identify the polo-box–interacting proteins, we carried out a yeast two-hybrid screening and isolated Bbp1p as a potential polo-box–binding protein at the SPB. In vitro binding and in vivo localization studies showed that Cdc5p interacted with Bbp1p, but not with Mps2p, a protein suggested to form a heterodimer with Bbp1p (Schramm et al., 2000). These observations suggest that Cdc5p specifically interacts with Bbp1p, and likely that this interaction is direct. In support of this opinion, a Cdc5pΔC fused with Bbp1p243–385, but not with Mps2p, could support the SPB localization-dependent Cdc5p function in inducing Cdc14p release from the nucleolus. This observation suggests that Bbp1p243–385 can function as a substitute for the polo-box by targeting the catalytic domain of Cdc5p (Cdc5pΔC) to Cdc5p substrates at the SPB, which are essential for mitotic exit. It has been shown that Nud1p interacts with Bfa1p and Bub2p and influences the interactions between Tem1p and Cdc15p, a critical step in activating the mitotic exit network (Gruneberg et al., 2000). Because Cdc5p interacts with Bfa1p (Hu et al., 2001), Nud1p may also be important for targeting the Cdc5p to the SPB. However, as with the nonfunctional Cdc5pΔC-Mps2p fusion, Cdc5pΔC-Nud1p also failed to complement the cdc5Δ defect, even though it could efficiently target the Cdc5pΔC to the SPB (J.-E.P. and K.S.L., unpublished data). These observations suggest that interactions between Cdc5p and its specific binding partner(s) at the SPB most likely are critical to carry out Cdc5p-dependent mitotic functions.
Bbp1p localized to the SPB through the C-terminal coiled coil domain (Bbp1p243–385), and this domain was sufficient for homo-dimerization in yeast two-hybrid and coimmunoprecipitation studies. These data suggest that the C-terminal domain-dependent homodimerization of Bbp1p is most likely a prerequisite for the SPB localization. However, Bbp1p243–385 is not sufficient to rescue the bbp1Δ mutation, suggesting that the N-terminal domain of Bbp1p (Bbp1pΔC) is also critical for the function of Bbp1p. Our data showed that Bbp1pΔC interacts with the polo-box domain of Cdc5p in both yeast two-hybrid and in vitro binding studies. Thus, it is possible that at least one of the important roles that Bbp1pΔC plays is to target Cdc5p to the SPB, thus contributing to the Cdc5p-dependent mitotic functions at the SPB (Figure 9). In this scenario, besides its role in SPB duplication, Bbp1p plays an additional role in Cdc5p-dependent mitotic events. Because SPB duplication must precede the mitotic events, the timely interaction between Bbp1p and Cdc5p later in the cell cycle may ensure the order of these two events.
Figure 9.
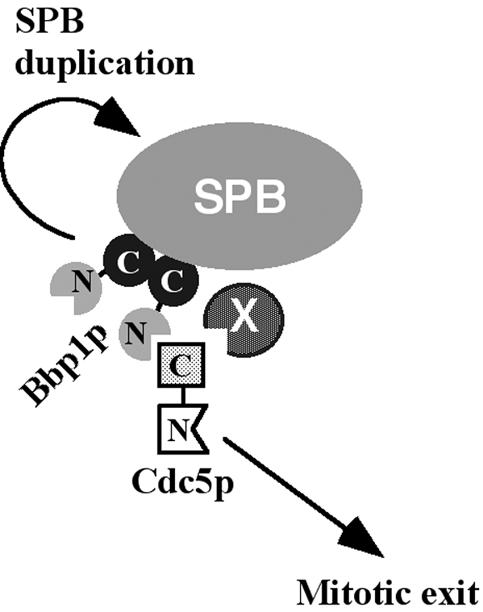
Model proposing the dual role of Bbp1p at the SPB. Bbp1p localizes to the SPB through the homo-dimerized C-terminal domain. In an early stage of the cell cycle, Bbp1p plays a critical role in SPB duplication. When Cdc5p is expressed late in the cell cycle, the N-terminal domain of Bbp1p interacts with the polo-box domain of Cdc5p, thus promoting the localization and mitotic functions of Cdc5p at the SPB. X indicates a hypothetical component, which may also contribute to the Cdc5p localization to the SPB (see text).
In the bbp1-1 mutant, Cdc5p-GFP localization to the SPB was diminished, but not eliminated, at a nonpermissive temperature. In addition, these cells exhibited a mitotic delay, but still continued to progress through mitosis at 37°C. These observations suggest that the bbp1-1 mutant is not deprived of the Cdc5p function at the SPB under these conditions. Several explanations can account for these observations. One possibility is that because the SPB structure is already matured in mitosis, the temperature sensitivity of the bbp1-1 allele could not have been rapidly exhibited. Alternatively, it is possible that SPB component(s) other than Bbp1p play a role in localizing Cdc5p to the SPB (Figure 9). These components may either cooperate with Bbp1p to localize Cdc5p to the periphery of the central plaque, or independently target Cdc5p to other parts of the SPB to dictate specific functions at those sites.
The Role of Cdc5p at the SPB
Our data showed that Cdc5p primarily localizes to the outer plaque of the SPB, and a fraction of Cdc5p may also localize to the nuclear side of the SPB. Many components functioning in the mitotic exit network localize to the SPB, suggesting that Cdc5p localization to the SPB is probably important for promoting mitotic exit. Consistent with this view, we observed that targeting Cdc5pΔC to the SPB by tethering with the Bbp1p localization domain (Bbp1p243–385) was sufficient to induce mitotic exit.
Does Cdc5p play any other roles at the SPB? Close examination of Cdc5p localization during the cell cycle revealed that Cdc5p localizes to the SPB as early as G1 phase (C.J.P. and K.S.L., unpublished data). Because mitotic exit occurs late in M phase, this finding suggests that Cdc5p may play additional roles earlier in the cell cycle. In other eucaryotic organisms, it has been shown that polo is required for centrosome maturations and bipolar spindle formations (Sunkel and Glover, 1988; Llamazares et al., 1991; Ohkura et al., 1995; Lane and Nigg, 1996). In addition, polo seems to provide sufficient activity to induce microtubule nucleation in salt-stripped centrosomes in vitro (de Carcer et al., 2001). Thus, it may be of interest to investigate whether Cdc5p activity is required for proper microtubule function, perhaps through the interaction with SPB component(s) critical for this event. However, because of the multiplicity of Cdc5p function, it is difficult to study specific Cdc5p functions without interfering with other Cdc5p-dependent processes. Identification of additional Cdc5p-interacting proteins and generation of cdc5 mutants defective in SPB localization or a specific protein–protein interaction at the SPB will likely be a critical step in dissecting the roles of Cdc5p at the SPB.
Acknowledgments
We are grateful to Doug Kellogg, John Kilmartin, Kim Nasmyth, Elmar Schiebel, Akio Sugino, and Akio Toh-e for strains. We also thank Geum-Yi Kim, Young-Wook Cho, and Satoshi Asano for technical support, and Susan Garfield for helping with confocal microscopy. This work was supported in part by National Institutes of Health grant R01 GM-51312 (to M.W.).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–07–0461. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-07-0461.
References
- Adams, I.R., and Kilmartin, J.V. (2000). Spindle pole body duplication: a model for centrosome duplication? Trends Cell Biol. 10, 329-335. [DOI] [PubMed] [Google Scholar]
- Asakawa, K., Yoshida, S., Otake, F., and Toh-E.A. (2001). A novel functional domain of Cdc15 kinase is required for its interaction with Tem1 GTPase in Saccharomyces cerevisiae. Genetics 157, 1437-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel, F.M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A., and Struhl, K. (1995). Current Protocols in Molecular Biology, New York: John Wiley & Sons Ltd.
- Bardin, A.J., and Amon, A. (2001). Men and sin: what's the difference? Nat. Rev. Mol. Cell. Biol. 2, 815-826. [DOI] [PubMed] [Google Scholar]
- Bettignies, G., and Johnston, L.H. (2003). The mitotic exit network. Curr. Biol. 13, R301. [DOI] [PubMed] [Google Scholar]
- Boeke, J.D., Croute, F.L., and Fink, G.R. (1984). A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol. Gen. Genet. 197, 345-346. [DOI] [PubMed] [Google Scholar]
- Clay, F.J., McEwen, S.J., Bertoncello, I., Wilks, A.F., and Dunn, A.R. (1993). Identification and cloning of a protein kinase-encoding mouse gene, Plk, related to the polo gene of Drosophila. Proc. Natl. Acad. Sci. USA 90, 4882-4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carcer, G., DoCarmo-Avides, M., Lallena, M.J., Glover, D.M., and Gonzalez, C. (2001). Requirement of Hsp90 for centrosomal function reflects its regulation of polo kinase stability. EMBO J. 20, 2878-2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott, S., Knop, M., Schlenstedt, G., and Schiebel, E. (1999). Spc29p is a component of the Spc110p subcomplex and is essential for spindle pole body duplication. Proc. Natl. Acad. Sci. USA 96, 6205-6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geymonat, M., Spanos, A., Smith, S.J., Wheatley, E., Rittinger, K., Johnston, L.H., and Sedgwick, S.G. (2002). Control of mitotic exit in budding yeast. In vitro regulation of Tem1 GTPase by Bub2 and Bfa1. J. Biol. Chem. 277, 28439-28445. [DOI] [PubMed] [Google Scholar]
- Geymonat, M., Spanos, A., Walker, P.A., Johnston, L.H., and Sedgwick, S.G. (2003). In vitro regulation of budding yeast Bfa1/Bub2 GAP activity by Cdc5. J. Biol. Chem. 278, 14591-14594. [DOI] [PubMed] [Google Scholar]
- Giddings, Jr., T.H., O'Toole, E.T., Morphew, M., Mastronarde, D.N., McIntosh, J.R., and Winey, M. (2001). Using rapid freeze and freeze-substitution for the preparation of yeast cells for electron microscopy and three-dimensional analysis. Methods Cell Biol. 67, 27-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz, R.D., and Sugino, A. (1988). New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74, 527-534. [DOI] [PubMed] [Google Scholar]
- Gruneberg, U., Campbell, K., Simpson, C., Grindlay, J., and Schiebel, E. (2000). Nud1p links astral microtubule organization and the control of exit from mitosis. EMBO J. 19, 6475-6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, K.L., and Dixon, J.E. (1991). Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal. Biochem. 192, 262-267. [DOI] [PubMed] [Google Scholar]
- Hardy, C.F.J., and Pautz, A. (1996). A novel role for Cdc5p in DNA replication. Mol. Cell. Biol. 16, 6775-6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, F., Wang, Y., Liu, D., Li, Y., Qin, J., and Elledge, S.J. (2001). Regulation of the Bub2/Bfa1 GAP complex by Cdc5 and cell cycle checkpoints. Cell 107, 655-665. [DOI] [PubMed] [Google Scholar]
- Kitada, K., Johnson, A.L., Johnston, L.H., and Sugino, A. (1993). A multicopy suppressor gene of the Saccharomyces cerevisiae G1 cell cycle mutant gene dbf4 encodes a protein kinase and is identified as CDC5. Mol. Cell. Biol. 13, 4445-4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane, H.A., and Nigg, E.A. (1996). Antibody microinjection reveals an essential role for human polo-like kinase 1 (Plk1) in the functional maturation of mitotic centrosomes. J. Cell Biol. 135, 1701-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, K.S., Grenfell, T.Z., Yarm, F.R., and Erikson, R.L. (1998). Mutation of the polo-box disrupts localization and mitotic functions of the mammalian polo kinase Plk. Proc. Natl. Acad. Sci. USA 95, 9301-9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llamazares, S., Moreira, A., Tavares, A., Girdham, C., Spruce, B.A., Gonzalez, C., Karess, R.E., Glover, D.M., and Sunkel, C.E. (1991). polo encodes a protein kinase homolog required for mitosis in Drosophila. Genes Dev. 5, 2153-2165. [DOI] [PubMed] [Google Scholar]
- Longtine, M.S., McKenzie, A., Demarini, D.J., Shah, N.G., Wach, A., Brachat, A., Philippsen, P., and Pringle, J.R. (1998). Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953-961. [DOI] [PubMed] [Google Scholar]
- Lupas, A. (1996). Coiled coils: new structures and new functions. Trends Biochem. Sci. 21, 375-382. [PubMed] [Google Scholar]
- Munoz-Centeno, M.C., McBratney, S., Monterrosa, A., Byers, B., Mann, C., and Winey, M. (1999). Saccharomyces cerevisiae MPS2 encodes a membrane protein localized at the spindle pole body and the nuclear envelop. Mol. Biol. Cell 10, 2393-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkura, H., Hagan, I.M., and Glover, D.M. (1995). The conserved Schizosaccharomyces pombe kinase plo1, required to form a bipolar spindle, the actin ring, and septum, can drive septum formation in G1 and G2 cells. Genes Dev. 9, 1059-1073. [DOI] [PubMed] [Google Scholar]
- Pereira, G., Hofken, T., Grindlay, J., Manson, C., and Schiebel, E. (2000). The Bub2p spindle checkpoint links nuclear migration with mitotic exit. Mol. Cell 6, 1-10. [PubMed] [Google Scholar]
- Pereira, G., Tanaka, T.U., Nasmyth, K., and Schiebel, E. (2001). Modes of spindle pole body inheritance and segregation of the Bfa1p-Bub2p checkpoint protein complex. EMBO J. 20, 6359-6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro, H.S., Song, S., and Lee, K.S. (2002). Bfa1 can regulate Tem1 function independently of Bub2 in the mitotic exit network of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 99, 5436-5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm, C., Elliott, S., Shevchenko, A., and Schiebel, E. (2000). The Bbp1p-Mps2p complex connects the SPB to the nuclear envelope and is essential for SPB duplication. EMBO J. 19, 421-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm, C., Janke, C., and Schiebel, E. (2001). Molecular dissection of yeast spindle pole bodies by two hybrid, in vitro binding, and co-purification. Methods Cell Biol. 67, 71-94. [DOI] [PubMed] [Google Scholar]
- Seong, Y.S., Kamijo, K., Lee, J.S., Fernandez, E., Kuriyama, R., Miki, T., and Lee, K.S. (2002). A spindle checkpoint arrest and a cytokinesis failure by the dominant-negative polo-box domain of Plk1 in U-2 OS cells. J. Biol. Chem. 277, 32282-32293. [DOI] [PubMed] [Google Scholar]
- Sherman, F., Fink, G.R., and Hicks, J.B. (1986). Methods in Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Shirayama, M., Zachariae, W., Ciosk, R., and Nasmyth, K. (1998). The Polo-like kinase Cdc5p and the WD-repeat protein Cdc20p/fizzy are regulators and substrates of the anaphase promoting complex in Saccharomyces cerevisiae. EMBO J. 17, 1336-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski, R.S., and Hieter, P. (1989). A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, S., Grenfell, T.Z., Garfield, S., Erikson, R.L., and Lee, K.S. (2000). Essential function of the polo box of Cdc5 in subcellular localization and induction of cytokinetic structures. Mol. Cell. Biol. 20, 286-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, S., and Lee, K.S. (2001). A novel function of Saccharomyces cerevisiae CDC5 in cytokinesis. J. Cell Biol. 152, 451-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellman, P.T., Sherlock, G., Zhang, M.Q., Iyer, V.R., Anders, K., Eisen, M.B., Brown, P.O., Botstein, D., and Futcher, B. (1998). Comprehensive identification of cell cycle-regulated genes of the yeast Saccharomyces cerevisiae by microarray hybridization. Mol. Biol. Cell 9, 3273-3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmeier, F., Visintin, R., and Amon, A. (2002). Separase, polo kinase, the kinetochore protein Slk19, and Spo12 function in a network that controls Cdc14 localization during early anaphase. Cell 108, 207-220. [DOI] [PubMed] [Google Scholar]
- Stotz, A., and Linder, P. (1990). The ADE2 gene from Saccharomyces cerevisiae: sequence and new vectors. Gene. 95, 91-98. [DOI] [PubMed] [Google Scholar]
- Sunkel, C.L., and Glover, D.M. (1988). polo, a mitotic mutant of Drosophila displaying abnormal spindle poles. J. Cell Sci. 89, 25-38. [DOI] [PubMed] [Google Scholar]
- Visintin, R., Craig, K., Hwang, E.S., Prinz, S., Tyers, M., and Amon, A. (1998). The phosphatase Cdc14 triggers mitotic exit by reversal of Cdk-dependent phosphorylation. Mol. Cell 2, 709-718. [DOI] [PubMed] [Google Scholar]
- Winey, M., Goetsch, L., Baum, P., and Byers, B. (1991). MPS1 and MPS2: novel yeast genes defining distinct steps of spindle pole body duplication. J. Cell Biol. 114, 745-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida, Y., Asakawa, K. and Toh-e, A. (2002). Mitotic exit network controls the localization of Cdc14 to the spindle pole body in Saccharomyces cerevisiae. Curr. Biol. 12, 944-950. [DOI] [PubMed] [Google Scholar]
- Zeng, X., Kahana, J.A., Silver, P.A., Morphew, M.K., McIntosh, J.R., Fitch, I.T., Carbon, J., and Saunders, W.S. (1999). Slk19p is a centromere protein that functions to stabilize mitotic spindles. J. Cell Biol. 146, 415-425. [DOI] [PMC free article] [PubMed] [Google Scholar]