Abstract
Despite enormous efforts that have been made in the search for novel drugs and treatments, cancer continues to be a major public health problem. Moreover, the emergence of resistance to cancer chemotherapy often prevents complete remission. Researchers have thus turned to natural products mainly from plant origin to circumvent resistance. Pectin and pH- or heat-modified pectin have demonstrated chemopreventive and antitumoral activities against some aggressive and recurrent cancers. The focus of this review is to describe how pectin and modified pectin display these activities and what are the possible underlying mechanisms. The failure of conventional chemotherapy to reduce mortality as well as serious side effects make natural products, such as pectin-derived products, ideal candidates for exerting synergism in combination with conventional anticancer drugs.
Keywords: pectin, cancer, galectin-3, drug combination, apoptosis, chemoprevention
Despite enormous progress in oncology therapy during the last decade, especially regarding the development of “smart drugs,” cancer still remains one of the leading causes of death. Hence, the development of new therapeutic strategies remains a high priority. Natural compounds represent an important source of new “leads” with potent chemotherapeutic or chemopreventive activity. Structure-activity relationship studies have led to the development of natural molecules or of semi-synthetic analogs with higher activity or lower toxicity. Two of the best examples currently used in cancer therapy are paclitaxel and etoposide. In this review, we will describe what is known about one particular class of complex plant polysaccharides, pectin, and its potential anti-cancer activities.
DESCRIPTION OF PECTIN
In 1825, a French chemist and pharmacist, Henri Braconnot, who was an expert in the extraction of active components from plants, was the first to discover a heteropolysaccharide with gelling properties which he named “pectic acid” (in ancient Greek πηκτ ικóς meaning coagulant).
Pectin is a family of complex polysaccharides, which are found in high amounts in plant primary wall. The main role of the plant wall components is to give mechanical strength to plants, to maintain an extracellular water phase by imbibition and to provide a barrier from external environment.
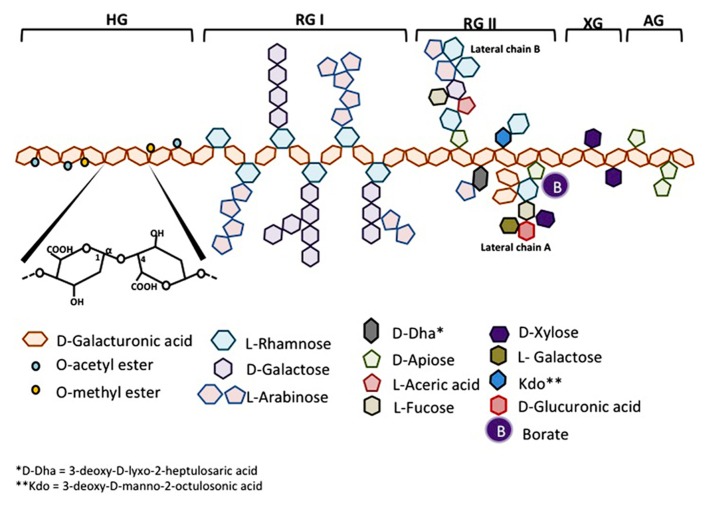
The exact chemical structure of pectin is still under debate. Pectins are a family of covalently linked galacturonic acid-rich polymers. Until now, three main pectic polysaccharides have been isolated from plant wall whose structure has been identified. They are homogalacturonan (HG), rhamnogalacturonan-I (RG-I) and substituted galacturonans (GS).
Homogalacturonan which constitutes about 65% of pectin molecule is a linear chain of D-galactopyranosyluronic acid (GalpA) bound in α-1,4. The carboxyl group of some residues can be methyl-esterified. According to the plant species, HGs may also be partly O-acetylated on C-3 or C-2 (Figure 1).
FIGURE 1.
Schematic representation of pectin structure. AG, arabinogalactan; HG, homogalacturonan; RG, rhamnogalacturonan; XG, xylogalacturonan.
Rhamnogalacturonan-I makes about 20–35% of pectin. RG-I is a family of pectic polysaccharides whose main chain is a repetition of disaccharides composed of galacturonic acid and rhamnosyl bound [→4)-α-D-GalpA-(1→2)-α-L-Rhap-(1→]. The Galp residues forming the main chain can be O-acetylated in C-3 or C-2 but are usually not linked with monomers or lateral chains. According to the plant species, about 20–80% of the rhamnosyl residues are substituted with neutral or acidic oligosaccharide chains on the carbon C4 of rhamnosyl residues. The most frequent lateral chains contain α-L-arabinofuranosyl (Araf) and/or galactopyranosyl (Galp). These lateral chains (arabinans, galactans or arabinogalactans) may be linear or branched (Figure 1).
Substituted galacturonans make a group of various polysaccharides whose linear chain is composed of D-GalpA residues linked in α-1,4 (as in HG) and on which are grafted other residues. Among these GS, is rhamnogalacturonan-II (RG-II). RG-II has nothing to do with RG-I, its main chain is not composed of GalA-Rhap disaccharide but of a HG chain. Four types of chains with structurally different oligosaccharides are linked to the main chain of RG-II, they are composed of 12 types of glycosyl residues bound together by at least 22 types of glycosidic bounds. One nonasaccharide (lateral chain B) and one octasaccharide (lateral chain A) are attached in C-2 of some GalA residues from the main chain and two different disaccharides are linked in C-3 of the main chain. The localization of these lateral chains one in relation to the other is not yet determined (Figure 1). RG-II is often found in dimers thanks to a borate ion located on chain A. This dimerisation seems essential for the integrity of the plant cell wall. Despite its complexity, the RG-11 structure is well conserved in vascularized plants. Very few mutants with modified RG-II have been identified until now, which indicates the importance to conserve its structure. Other GS have been described in a short number of plants. Xylogalacturonan contains β-D-xylosyl (Xylp) linked in C3 of the main chain and is present in reproductory tissues of plants like apple, carrot and cotton. Apiogalacturonan contains monomers or dimers of β-D-apioduranosyl (Apif) attached in C-2 and C-3 of the main chain. Apiogalacturonan is found in some monocotyledons (Ridley et al., 2001; Mohnen, 2008; Caffall and Mohnen, 2009; Harholt et al., 2010; Figure 1).
The most accepted model for pectin structure is a main backbone of HG in which are intercalated regions of RG-I, RG-II and GS (Caffall and Mohnen, 2009). There are linkages between pectin polysaccharides as well as to other wall molecules, combined to make a network that makes the primary cell wall.
FUNCTION OF PECTIN IN PLANT CELL WALL
As mentioned here above, the role of the components of the plant cell wall is first to give mechanical solidity and to form a barrier from the external environment. HGs and RGII are known to be responsible for the wall rigidification. HGs have the property to form structures which are named “egg boxes.” Two HG chains are bound one to the other through interactions including bivalent Ca2+ ions intercalated in between them (Liners et al., 1989). This process is important for the gelling of pectin.
The mechanical role of RG-I has been less studied but it seems that RG-I may play a role in cell wall plasticity, for example by preventing HG chains to interact with Ca2+ ions. Transgenic plants with decreased amounts of arabinans and galactans display a stiffening of their cell wall.
Pectin organization and composition in plant primary cell wall depend on the growth state of the plant, on the tissues and on the plant species. Its synthesis is a complex process involving numerous enzymes that are just becoming to be identified (Atmodjo et al., 2013).
BIOLOGICAL ACTIVITIES OF OLIGOGALACTURONIDES IN PLANTS
There are three different ways for a pathogen to enter into a plant: to go through a natural opening, such as stomata; to seep into a wound or to digest the cell wall. Pectin is then the first substrate. Pathogens are able to secrete endopolygalacturonases and endopectate lyases which degrade HGs present in the cell wall and then release oligogalacturonides (OGAs). OGAs are biologically active carbonhydrates which act as signal molecules initiating defense responses from the plant. The first defense response observed in response to OGA production is the production of reactive oxygen species such as H2O2 and . OGAs also initiate signaling pathways that activate defense systems in plants, like the production of protease inhibitors able to block the activity of proteases secreted by insects to digest plant cell wall. Finally OGAs are also responsible for wall reinforcement in response to pathogen infection. In addition to their roles in plant defense systems, OGAs also influence plant growth and development and they play a role in fruit ripening.
PECTIN ACTIVITIES IN HUMAN BEINGS
Since humans are able to extract pectin, they try to use its huge potential to their benefits. In addition to be used as a gelling agent in food industry, pectin displays properties useful in medicine (Lattimer and Haub, 2010). In humans, pectin, as a dietary fiber, is not enzymatically digested in the small intestine but is degraded by microbia in colon. It keeps its gelling action in the digestive track, so that it slows down digestion. This is very beneficial in patients with Dumping syndrome who have a too rapid digestion within their stomach (Lawaetz et al., 1983). Pectin is also capable of diminishing blood cholesterol level and of stimulating lipid excretion. However, the exact mechanisms underlying these effects are not known yet (Brown et al., 1999). Pectin is also investigated for its ability to increase 137Cs clearance (Nesterenko et al., 2004). 137Cs is a radio-isotope produced during uranium fission that is found in Tchernobyl area. PectaSol®, a modified form of pectin, when eaten during several days, allows a better clearance via the urinary track of toxic elements like arsenic or cadmium, which seem to be chelated by modified pectin and then eliminated in the urine (Eliaz et al., 2006). Finally, several studies have shown that orally taken pectin decreases the risk of intestinal infection and of diarrhea in children by favoring the growth of “good” bacteria in the colon (e.g., Biffidobacteria and Lactobacillus) to the detriment of pathogenic bacteria (Olano-Martin et al., 2002).
PECTIN AND CANCER, STATE OF THE ART
Pectin is known for its anti-tumor activities already since several decades. Because of its highly complex structure, it is not surprising that it displays so many different biological activities (Maxwell et al., 2012). In the literature, it is not easy to make the link between structure and pectin bioactivity, notably because the origin of the pectin used in the different studies and the possible chemical modifications that create molecular fragments it has undergone are not always well described. It has to be noted that differences in size of the fragments generated, in their degree of esterification (DE), in the nature of the sugar monomers present in the polysaccharide(s) and the extraction procedure are likely to have significant influence on the properties on these different types of pectin. However, six main issues will be highlighted hereunder.
EFFECT OF PECTIN AS A DIETARY FIBER
As a dietary fiber, pectin plays a role in preventing colon cancer. In 1979, Watanabe et al. (1979) have shown that rats treated with azoxymethane or methylnitrosourea develop less colon tumors if their diet was enriched in pectin. Heitman et al. (1992) similarly demonstrated less numerous colon tumors in rats treated with 1,2-dimethylhydrazine if they were given pectin. Ohkami et al. (1995) evidenced that citrus and apple pectin in the diet of rats exposed to azoxymethane decreased carcinogenesis. The two types of pectin decreased the number of tumors and apple pectin decreased the activity of β-glucuronidase, an enzyme from fecal bacteria whose activity is correlated to colon cancer development (Ohkami et al., 1995). Different types of carbohydrates have been studied for their antimutagenic activity. For example, Hensel and Meier (1999) showed that xyloglucans and rhamnogalacturonans decreased the mutagenic effect of 1-nitropyrene. This protection is dose-dependent and could come from a direct interaction between cells and polymers that would protect the cells from mutagenic effects of 1-nitropyrene.
Colon carcinogenesis is a multi-step process that results from disruption of the balance between proliferation of colonocytes at the base of the crypt and loss of colonocytes at the luminal surface due to apoptosis. Most colon cancer cells become resistant to apoptosis, hence promoting tumor growth. Chemoprotection may arise if luminal colonocyte sensitivity to apoptosis is restored. Schwartz’s team first showed that in rats, a pectin rich diet, compared to a standard diet, favored the expression of caspase-1 in luminal colonocytes from colon crypts and increased cleaved PARP level in basal and luminal colonocytes. The expression of the anti-apoptotic protein Bcl2 is on the other hand higher in rats with standard diet (Avivi-Green et al., 2000c). They then demonstrated that the activation of apoptosis due to the pectin rich diet had protective effects and diminished the number and the size of tumors in rats treated with 1,2-dimethylhydrazine. Colonocytes of rats nourished with pectin presented a high activity of caspase-1 and expressed pro-caspase-3 at a higher level, with a higher level of cleaved PARP. Pectin per se may induce apoptosis since the viability of cells exposed in culture to different pectin-derived oligosaccharides is decreased. Olano-Martin et al. (2003) evidenced that when colon adenocarcinoma HT29 cells were incubated in the presence of pectin oligosaccharides during 3 days, an increase in apoptosis, in DNA fragmentation and in caspase-3 activity was observed. This is also true for cells from other types of cancer: Attari et al. (2009) demonstrated that concentrations of 100 μg/ml to 1 mg/ml of pectic acids induced apoptosis in rat GH3/B6 pituitary tumor cells in a concentration dependent way while concentrations of 2.5 and 5.0 mg/ml induced necrosis. DNA fragmentation which was directly proportional to the number of apoptotic cells was observed (Attari et al., 2009). In combination with n-3 polyunsaturated fatty acid-rich fish oil, pectin also demonstrated chemoprevention in a colon cancer model of rats injected with azoxymethane. This was associated with a decrease in Bcl-2 expression due to promoter methylation (Cho et al., 2012) as well as to changes in the expression profile of mRNA implicated in and of miRNA targeting canonical oncogenic signaling pathway (Davidson et al., 2009; Cho et al., 2011; Shah et al., 2011).
On the other hand, colonocyte apoptosis activation in animals fed with pectin is also largely due to butyrate, a molecule coming from pectin fermentation by colon bacteria flora (Avivi-Green et al., 2000a,b). Indeed, intracolonic instillation of butyrate recapitulates the effect of orally administered pectin (Avivi-Green et al., 2000b). Butyrate is also able to induce apoptosis in colonocytes in vitro in a p53-independent manner (Kolar et al., 2007) and by inducing mitochondrial Ca2+ overload (Kolar et al., 2011). In parallel, both in vitro in rat intestinal epithelial cells exposed to butyrate and in mice fed with a diet supplemented with 20% pectin, TGF-ß signaling has been demonstrated to be enhanced, leading to colonocyte growth inhibition and apoptosis. Apoptosis seems to be induced via an increased expression of Id2 (inhibitor of differentiation 2), probably via inhibition of selective isoforms of HDACs (Cao et al., 2011).
ANTI-TUMOR ACTIVITY OF pH-MODIFIED PECTIN
Pectin can be modified by treatment at different pHs; the most studied pH-modified pectin is the one isolated from citrus (MCP, modified citrus pectin). pH-modification involves an alkaline treatment that causes ß-elimination reactions, which results in depolymerization of the polysaccharide backbone and de-esterification of the HG regions. This is followed by an acid treatment that cleaves neutral sugars, releases the branched regions of the pectin backbone and preferentially removes arabinose residues. Thus, arabinogalactans and galactans are generated in high amounts.
Modified citrus pectin was mainly studied in Avraham Raz’s laboratory and has shown strong anti-cancer activities. Injection of pectin increased the number of tumors detected in lung after B16-F1 melanoma cells implantation in C57BL/6 mice probably by increasing homotypic aggregation between tumor cells while MCP significantly diminished the number of metastases. MCP which is rich in galactoside residues seems to impair cell-cell interactions by competing with endogenous ligands of “galactoside binding proteins” and more particularly of galectin-3 (Platt and Raz, 1992; Inohara and Raz, 1994). Raz’s team also showed that orally administered MCP decreased the number of metastases in lung in rats injected with prostate cancer MAT-LyLu cells. This decrease was dose-dependent (Pienta et al., 1995). In 2002, they also evidenced that MCP decreased the growth of breast (MDA-MB-435) and colon (LSLiM6) tumors implanted in NRC nu/nu mice as well as the number of metastases in lung and lymph nodes. These effects were associated with anti-angiogenic effects since a decrease in the number of capillaries in vivo and an inhibition of tubulogenesis in vitro using HUVEC were observed (Nangia-Makker et al., 2002). Other works have also evidenced the anti-tumor activity of MCP. When added to the culture medium of prostate androgen-independent JCA-1 tumor cells, MCP diminished proliferation and tritiated thymidine incorporation. MCP decreased the expression of nm23, a protein whose expression is inversely correlated with metastasis in various cancers (Hsieh and Wu, 1995). Hayashi et al. (2000) showed that oral daily doses of 0.8 and 1.6 mg/ml MCP to Balb-C mice implanted with colon tumors decreased tumor size, of respectively 38 and 70%. GCS-100, which is a commercially available form of modified pectin, has been shown to be efficient against different lines of multiple myelomas some of them resistant to chemotherapy, by inducing caspase-3 and -8 activation as well as PARP cleavage. Modified pectin-induced apoptosis was partly inhibited by Z-VAD-fmk, a pan-caspase inhibitor (Chauhan et al., 2005). A phase II clinical study on prostate cancer patients showed that PectaSol® MCP significantly increased PSADT (PSA doubling time) in 7 out of the 10 cases included in this study (Guess et al., 2003). PectaSol® and its ameliorated version PectaSol-C® are cytotoxic for different cancer cell lines: LNCaP, PC3, CASP2.1, CASP1.1, and BPH-1. In CASP1.1 and PC3 cells, cytotoxicity was correlated with MAP kinase activation inhibition, increased Bim protein expression and caspase-3 cleavage (Yan and Katz, 2010). This product also inhibits the invasive behavior of human breast and prostate cancer cells in vitro (Jiang et al., 2013).
Galectin-3 seems to be a target of MCP. Galectin-3 protein can be found intra- and extracellularly and contains a lectin domain. It has pleiotropic functions, amongst which, it mediates cell-cell as well as cell-extracellular matrix adhesion, through binding to glycoconjugates. Indeed, this lectin-domain has a high affinity for ß-galactoside residues. Galectin-3 expression is dysregulated in transformed cells, being highly expressed in numerous different types of cancer cells (Newlaczyl and Yu, 2011). MCP has been shown to decrease liver metastasis in a mouse colon cancer model, in a dose-dependent manner. This effect may be linked to the higher expression of galactin-3 in the liver metastases (Liu et al., 2008). The relationship between MCP structure and its inhibitory activity on galectin-3 was investigated in several studies. One such example is the work by Sathisha et al. (2007) who compared the activation of pectins from different dietary plants. Pectins rich in galactose and arabinose and in arabinogalactan significantly inhibited galectin-3-dependent hemagglutination of MDA-MB-231 cells to erythrocytes (Sathisha et al., 2007). Pectin nearly mainly composed of RG-I isolated from okra, a tropical plant, arrested cell cycle of B16F10 cells in G2/M phase and induced apoptosis probably through interaction with galectin-3 (Vayssade et al., 2010). Gao et al. (2012) suggested that MCP ability to inhibit galectin-3 resides in its RG-I regions and more particularly from galactan, of which the nature of last residue is the most important. Gunning et al. (2013) confirmed that neutral galactan side chains did selectively bind to recombinant galectin-3. These active fragments can be obtained by enzymatic treatment of isolated RG-I regions from potato pectin (Gunning et al., 2009).
In conclusion, MCP displays many anti-metastatic properties demonstrated both in vitro and in vivo, in various malignancies. Many of them, if not all, are due to its binding to the pleiotropic galectin-3 protein which is overexpressed in cancer. Due to its well tolerance and among other plant-derived products, pectin-derived GCS-100 is being explored for the maintenance therapy of patient with B-chronic lymphocytic leukemia relapse (O’Brien and Kay, 2011).
ANTI-TUMOR ACTIVITY OF OTHER FORMS OF MODIFIED PECTIN
Jackson et al. (2007) investigated the apoptosis induction of different forms of modified pectin in prostate cancer cells which were either androgen-dependent (LNCaP) or androgen-independent and which were not expressing galactin-3 (LNCaP C4-2). In their work, citrus pectin and pH-modified pectin, PectaSol®, exerted no pro-apoptotic activity while two different forms of heat-modified pectins, one commercially available and the other one prepared in their laboratory, did markedly induce apoptosis in the two cell lines (Jackson et al., 2007). They showed HGs, RG-I, and RG-II taken separately had no cytotoxic activity. Treatment of heat-modified pectin with pectinmethylesterase to remove galacturonosyl carboxymethylesters and/or with endopolygalacturonase to cleave non-methylesterified HG did not result in loss of activity. On the other hand, mild base treatment that removed ester bounds destroyed the pro-apoptotic activity. Biological effectiveness thus requires a base-sensitive linkage in OGAs other than a carboxymethylester bound. Size analyses of the active fragments suggested low mass (10–20 kDa) oligosaccharides (Jackson et al., 2007).
Similar results were obtained by Cheng et al. (2011) who tested the anti-tumor activity of different polysaccharide fractions isolated from ginseng on colon cancer HT-29 cells. While fractions rich in HG stopped cell cycle in G2/M phase, fractions rich in HG and modified by heat treatment exerted a much higher anti-proliferative activity, which was accompanied by caspase-3 activation and apoptosis induction (Cheng et al., 2011). Similarly, potato pectin, rich in HG, inhibited in vitro HT-29 cell proliferation and provoked a cell cycle arrest in G2/M phase. This inhibition was due to a decrease in cyclin B1 expression and in CDK-1 activity (Cheng et al., 2013). It is important to note that Kang et al. (2006) also produced a citrus pectin-derived oligosaccharide, which was biologically active, by irradiation, i.e., without chemical treatment. Pectin irradiated with 20 kGy and then dialyzed (WT <10,000) inhibited cancer cell growth.
IMMUNOPOTENTIATING ACTIVITY OF PECTIN
Some of the components of pectin exert their anti-tumor activity in vivo by stimulating the immune system. Pectic polysaccharides named angelans and isolated from Angelica gigas Nakai, a Chinese medicinal plant, are immunopotentiators which increase immune functions of B lymphocytes, of macrophages and of “natural killer” cells and which directly activate T helper and cytotoxic lymphocytes. Angelans have also an anti-metastatic activity, it inhibits B16F10 cancer cell adhesion to the extracellular matrix as well as its invasion. In a urine model of colon cancer, apple oligogalactan (AOG) composed of five subunits showed preventive effects against the toxic and carcinogenic effects of 1,2-dimethylhydrazine and of sodium dextran sulfate. The underlying mechanisms included AOG targeting of the LPS/TLR4/NF-κB pathway by modifying TLR4 membrane distribution hence preventing LPS binding (Liu et al., 2010). HG-rich pectin polysaccharides isolated from red ginseng (Ginseng panax) exert both anti-tumor and immunomodulatory properties which derived from NO production by macrophages (Choi et al., 2008). In addition, this immunomodulatory effect ameliorates the paclitaxel-induced anti-cancer activity in mice with transplanted B16 melanoma tumors (Shin et al., 2004). As demonstrated by Chen et al. (2006) pectin effect on LPS-activated macrophages depends on its DE. Pectin esterified up to 90% (DE90) inhibits iNOS and COX2 expression in macrophages much more efficiently than pectin esterified to 30 or 60%. DE90 pectin also inhibits MAPK phosphorylation, IKK kinase activity as well as NF-κB and AP-1 activation. DE90 pectin binds LPS, which could modify LPS binding to its receptor (Chen et al., 2006). It has also been shown that PectaSol-C exerts immunostimulating activity in human blood, activating cytotoxic T cells, B cells and NK cells, inducing cytotoxicity toward chronic myeloid leukemia K562 cells (Ramachandran et al., 2011).
MODIFIED PECTIN TO OVERCOME CHEMORESISTANCE
Chemoresistance is a heavy burden in the treatment of cancer, especially since a large number of patients already display metastatic disease at the time of diagnosis. The vast majority of anti-cancer drugs currently used act by inducing apoptosis via the intrinsic pathway. Numerous mechanisms underlie cancer chemoresistance (Rebucci and Michiels, 2013), but it appears that galectin-3 which is overexpressed in numerous tumor types, suppresses cell apoptosis and hence, decreases sensitivity of cancer cells to chemotherapeutic drugs (Glinsky and Raz, 2009). Since MCP has been shown to target galectin-3, several works were dedicated to delineate MCP-induced possible re-sensitization of cancer cells to different cytotoxic molecules. Johnson et al. (2007) showed that galectin-3 targeting via MCP or via a more specific inhibitor, lactosyl-L-leucine (LL), decreased malignant endothelial cell proliferation by themselves and sensitized these cells to the cytotoxic effect of doxorubicin. These two compounds also increases metastatic-derived MDA-MB-435 cells sensitivity to taxol both in vitro and in vivo (Glinsky et al., 2009). GCS-100, a commercially form of pH-MCP, enhanced bortezomide and dexamethasone-induced apoptosis in multiple myeloma cells and decreased viability. The effect was accompanied by a marked decrease in galectin-3 protein level (Chauhan et al., 2005). GCS-100 also induced calpain activation in prostate cancer cells that led to their sensitization to cisplatin treatment (Wang et al., 2010). Combination of modified pectin with different anti-cancer agents may thus represent an efficient new strategy to overcome resistance in cancer patients.
USE OF PECTIN AS A VEHICLE FOR DRUG DELIVERY IN CANCER
Colon cancer is one of the most common cancers worldwide. Conventional chemotherapy is usually administered by intravenous injection to target tumor growth and metastases. However, severe side effects are observed. Oral administration using colon-specific delivery systems is expected to augment drug availability at tumor site while reducing systemic adverse effects. To this purpose, pectin-based vehicle is ideal since pectin is not digested in the gastrointestinal tract until it reaches colon, where it is fermented by residential bacteria, thus releasing the transported drug (for reviews, Chourasia and Jain, 2003; Patel et al., 2007; Wong et al., 2011). Different kinds of vehicles with different drugs and different chemistry binding or encapsulation have been designed and tested. Pectinate pellets or microspheres containing drugs led to prolonged dissolution and drug release in simulating colonic fluid (Zhang et al., 2011; Elyagoby et al., 2013). Other forms of vehicles like nanoparticles or pectin-based coated-based matrix tablets have been tested in vitro using colon cancer cells with efficient cell killing (Kanthamneni et al., 2010; Dev et al., 2011). Nanoparticles, capsules or microsponges have also been developed. Pharmacokinetics evaluation in rats and in rabbits provided evidence for colon delivery and delayed plasma appearance (Xu et al., 2005; Dev et al., 2011; Srivastava et al., 2012). However, actual demonstration for efficient treatment of colon cancer in animal models with pectin-based anti-cancer agent delivery vehicles is still lacking.
In parallel, pectin-derived biocompatible hydrogel loaded with different chemotherapeutic drugs have been produced. Doxorubicin-containing hydrogels displayed cytotoxicity toward HepG2 cells and inhibited homotypic aggregation of B16 melanoma cells, suggesting that it could also prevent metastasis in vivo (Takei et al., 2010). Similarly, pectin-coated chitosan gels encapsulating 5-fluorouracil showed controlled drug release and cytotoxicity against two cancer cell lines (Puga et al., 2013). Anti-cancer in vivo activity was also evidenced using doxorubicin-pectin hydrogel in subcutaneous B16 melanoma cell tumor in mice (Takei et al., 2013).
Finally, it is worth mentioning two other types of scaffolds including pectin- and fibrin-based nano-composites containing gemcitabine (Chandran et al., 2013) for the treatment of ovarian cancer and pectin nanoparticles loaded with methotrexate that displayed enhanced cytotoxicity toward HepG2 hepatocarcinoma cells in vitro (Chittasupho et al., 2013).
CONCLUSION
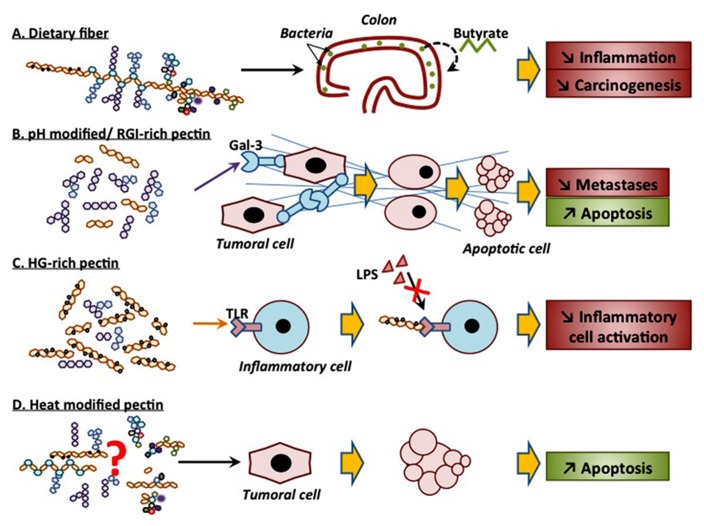
In conclusion, pectin seems to exert anti-tumor activity on different cell lines and in different mice models, and this probably through different effects. These mechanisms depend on the structure of pectin or on the modified form of pectin that is likely to yield to various active fragments. Differences in extraction methods, in the plant species from which the pectin is isolated, in fragmentation techniques as well as in the structural complexity of pectin itself make the characterization of the active molecule(s) very difficult. The different anti-cancer activities of different forms of pectin are summarized in Figure 2. As a dietary fiber, pectin is not digested in the upper digestive tract and could protect cells from mutagenic attacks. In colon, pectin is fermented, by bacteria, into butyrate that inhibits colon inflammation and prevents carcinogenesis. pH-modified pectin as well as galactan-rich pectin (RG-I) are capable of interacting with galectin-3, thus inhibiting cell-cell interactions and cancer cell metastasis. Furthermore, HG-rich pectin with a high DE competes with LPS for TLR4 binding, hence preventing inflammatory cell activation. Finally, heat-modified pectin initiates apoptosis in cancer cells, in a galectin-3-independent manner. Despite the fact that the exact structure of these modified molecules is not yet known and that neither is their mechanisms of action, modified pectin emerges as one of the promising anti-metastatic drugs, especially if used in combination with more conventional molecules.
FIGURE 2.
Schematic representation of the different anti-cancer activities of different forms of pectin.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHOR CONTRIBUTIONS
Lionel Leclere drafted the manuscript. Pierre Van Cutsem and Carine Michiels helped to write the final version of the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Lionel Leclere was a recipient of a FRIA fellowship (FNRS, Belgium).
REFERENCES
- Atmodjo M. A., Hao Z., Mohnen D. (2013). Evolving views of pectin biosynthesis. Annu. Rev. Plant Biol. 64 747–779 10.1146/annurev-arplant-042811-105534 [DOI] [PubMed] [Google Scholar]
- Attari F., Sepehri H., Delphi L., Goliaei B. (2009). Apoptotic and necrotic effects of pectic acid on rat pituitary GH3/B6 tumor cells. Iran Biomed J. 13 229–236 [PubMed] [Google Scholar]
- Avivi-Green C., Madar Z., Schwartz B. (2000a). Pectin-enriched diet affects distribution and expression of apoptosis-cascade proteins in colonic crypts of dimethylhydrazine-treated rats. Int. J Mol Med. 6 689–98 [DOI] [PubMed] [Google Scholar]
- Avivi-Green C., Polak-Charcon S., Madar Z., Schwartz B. (2000b). Apoptosis cascade proteins are regulated in vivo by high intracolonic butyrate concentration: correlation with colon cancer inhibition. Oncol. Res. 12 83–95 10.3727/096504001108747558 [DOI] [PubMed] [Google Scholar]
- Avivi-Green C., Polak-Charcon S., Madar Z., Schwartz B. (2000c). Dietary regulation and localization of apoptosis cascade proteins in the colonic crypt. J. Cell. Biochem. 77 18–29 [DOI] [PubMed] [Google Scholar]
- Brown L., Rosner B., Willett W. W., Sacks F. M. (1999). Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am. J. Clin. Nutr. 69 30–42 [DOI] [PubMed] [Google Scholar]
- Caffall K. H., Mohnen D. (2009). The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 344 1879–1900 10.1016/j.carres.2009.05.021 [DOI] [PubMed] [Google Scholar]
- Cao Y., Gao X., Zhang W., Zhang G., Nguyen A. K., Liu X., et al. (2011). Dietary fiber enhances TGF-beta signaling and growth inhibition in the gut. Am. J. Physiol. Gastrointest. Liver Physiol. 301 G156–G164 10.1152/ajpgi.00362.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran S., Praveen G., Snima K. S., Nair S. V., Pavithran K., Chennazhi K., et al. (2013). Potential use of drug loaded nano composite pectin scaffolds for the treatment of ovarian cancer. Curr. Drug Deliv. 10 326–335 10.2174/1567201811310030009 [DOI] [PubMed] [Google Scholar]
- Chauhan D., Li G., Podar K., Hideshima T., Neri P., He D., et al. (2005). A novel carbohydrate-based therapeutic GCS-100 overcomes bortezomib resistance and enhances dexamethasone-induced apoptosis in multiple myeloma cells. Cancer Res. 65 8350–8358 10.1158/0008-5472.CAN-05-0163 [DOI] [PubMed] [Google Scholar]
- Chen C. H., Sheu M. T., Chen T. F., Wang Y. C., Hou W. C., Liu D. Z., et al. (2006). Suppression of endotoxin-induced proinflammatory responses by citrus pectin through blocking LPS signaling pathways. Biochem. Pharmacol. 72 1001–1009 10.1016/j.bcp.2006.07.001 [DOI] [PubMed] [Google Scholar]
- Cheng H., Li S., Fan Y., Gao X., Hao M., Wang J., et al. (2011). Comparative studies of the antiproliferative effects of ginseng polysaccharides on HT-29 human colon cancer cells. Med. Oncol. 28 175–181 10.1007/s12032-010-9449-8 [DOI] [PubMed] [Google Scholar]
- Cheng H., Zhang Z., Leng J., Liu D., Hao M., Gao X., et al. (2013). The inhibitory effects and mechanisms of rhamnogalacturonan I pectin from potato on HT-29 colon cancer cell proliferation and cell cycle progression. Int. J. Food Sci. Nutr. 64 36–43 10.3109/09637486.2012.694853 [DOI] [PubMed] [Google Scholar]
- Chittasupho C., Jaturanpinyo M., Mangmool S. (2013). Pectin nanoparticle enhances cytotoxicity of methotrexate against hepG2 cells. Drug Deliv. 20 1–9 10.3109/10717544.2012.739214 [DOI] [PubMed] [Google Scholar]
- Cho Y., Kim H., Turner N. D., Mann J. C., Wei J., Taddeo S. S., et al. (2011). A chemoprotective fish oil- and pectin-containing diet temporally alters gene expression profiles in exfoliated rat colonocytes throughout oncogenesis. J. Nutr. 141 1029–1035 10.3945/jn.110.134973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y., Turner N. D., Davidson L. A., Chapkin R. S., Carroll R. J., Lupton J. R. (2012). A chemoprotective fish oil/pectin diet enhances apoptosis via Bcl-2 promoter methylation in rat azoxymethane-induced carcinomas. Exp. Biol. Med. (Maywood) 237 1387–1393 10.1258/ebm.2012.012244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H. S., Kim K. H., Sohn E., Park J. D., Kim B. O., Moon E. Y., et al. (2008). Red ginseng acidic polysaccharide (RGAP) in combination with IFN-gamma results in enhanced macrophage function through activation of the NF-kappaB pathway. Biosci. Biotechnol. Biochem. 72 1817–1825 10.1271/bbb.80085 [DOI] [PubMed] [Google Scholar]
- Chourasia M. K., Jain S. K. (2003). Pharmaceutical approaches to colon targeted drug delivery systems. J. Pharm. Pharm. Sci. 6 33–66 [PubMed] [Google Scholar]
- Davidson L. A., Wang N., Shah M. S., Lupton J. R., Ivanov I., Chapkin R. S. (2009). n-3 Polyunsaturated fatty acids modulate carcinogen-directed non-coding microRNA signatures in rat colon. Carcinogenesis 30 2077–2084 10.1093/carcin/bgp245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev R. K., Bali V., Pathak K. (2011). Novel microbially triggered colon specific delivery system of 5-fluorouracil: statistical optimization, in vitro, in vivo, cytotoxic and stability assessment. Int. J. Pharm. 411 142–151 10.1016/j.ijpharm.2011.03.057 [DOI] [PubMed] [Google Scholar]
- Eliaz I., Hotchkiss A. T., Fishman M. L., Rode D. (2006). The effect of modified citrus pectin on urinary excretion of toxic elements. Phytother. Res. 20 859–864 10.1002/ptr.1953 [DOI] [PubMed] [Google Scholar]
- Elyagoby A., Layas N., Wong T. W. (2013). Colon-specific delivery of 5-fluorouracil from zinc pectinate pellets through in situ intracapsular ethylcellulose-pectin plug formation. J. Pharm. Sci. 102 604–616 10.1002/jps.23388 [DOI] [PubMed] [Google Scholar]
- Gao X., Zhi Y., Zhang T., Xue H., Wang X., Foday A. D., et al. (2012). Analysis of the neutral polysaccharide fraction of MCP and its inhibitory activity on galectin-3. Glycoconj. J. 29 159–165 10.1007/s10719-012-9382-5 [DOI] [PubMed] [Google Scholar]
- Glinsky V. V., Kiriakova G., Glinskii O. V., Mossine V. V., Mawhinney T. P., Turk J. R., et al. (2009). Synthetic galectin-3 inhibitor increases metastatic cancer cell sensitivity to taxol-induced apoptosis in vitro and in vivo. Neoplasia 11 901–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinsky V. V., Raz A. (2009). Modified citrus pectin anti-metastatic properties: one bullet, multiple targets. Carbohydr. Res. 344 1788–1791 10.1016/j.carres.2008.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guess B. W., Scholz M. C., Strum S. B., Lam R. Y., Johnson H. J., Jennrich R. I. (2003). Modified citrus pectin (MCP) increases the prostate-specific antigen doubling time in men with prostate cancer: a phase II pilot study. Prostate Cancer Prostatic Dis. 6 301–304 10.1038/sj.pcan.4500679 [DOI] [PubMed] [Google Scholar]
- Gunning A. P., Bongaerts R. J., Morris V. J. (2009). Recognition of galactan components of pectin by galectin-3. FASEB J. 23 415–424 10.1096/fj.08-106617 [DOI] [PubMed] [Google Scholar]
- Gunning A. P., Pin C, Morris V. J. (2013). Galectin 3-beta-galactobiose interactions. Carbohydr. Polym. 92 529–533 10.1016/j.carbpol.2012.08.104 [DOI] [PubMed] [Google Scholar]
- Harholt J., Suttangkakul A, Vibe Scheller H. (2010). Biosynthesis of pectin. Plant Physiol. 153 384–395 10.1104/pp.110.156588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi A., Gillen A. C., Lott J. R. (2000). Effects of daily oral administration of quercetin chalcone and modified citrus pectin on implanted colon-25 tumor growth in Balb-c mice. Altern. Med. Rev. 5 546–552 [PubMed] [Google Scholar]
- Heitman D. W., Hardman W. E., Cameron I. L. (1992). Dietary supplementation with pectin and guar gum on 1,2-dimethylhydrazine-induced colon carcinogenesis in rats. Carcinogenesis 13 815–818 10.1093/carcin/13.5.815 [DOI] [PubMed] [Google Scholar]
- Hensel A., Meier K. (1999). Pectins and xyloglucans exhibit antimutagenic activities against nitroaromatic compounds. Planta Med. 65 395–399 10.1055/s-1999-14013 [DOI] [PubMed] [Google Scholar]
- Hsieh T. C., Wu J. M. (1995). Changes in cell growth, cyclin/kinase, endogenous phosphoproteins and nm23 gene expression in human prostatic JCA-1 cells treated with modified citrus pectin. Biochem. Mol. Biol. Int. 37 833–841 [PubMed] [Google Scholar]
- Inohara H., Raz A. (1994). Effects of natural complex carbohydrate (citrus pectin) on murine melanoma cell properties related to galectin-3 functions. Glycoconj. J. 11 527–532 10.1007/BF00731303 [DOI] [PubMed] [Google Scholar]
- Jackson C. L., Dreaden T. M., Theobald L. K., Tran N. M., Beal T. L., Eid M., et al. (2007). Pectin induces apoptosis in human prostate cancer cells: correlation of apoptotic function with pectin structure. Glycobiology 17 805–819 10.1093/glycob/cwm054 [DOI] [PubMed] [Google Scholar]
- Jiang J., Eliaz I., Sliva D. (2013). Synergistic and additive effects of modified citrus pectin with two polybotanical compounds, in the suppression of invasive behavior of human breast and prostate cancer cells. Integr. Cancer Ther. 12 145–152 10.1177/1534735412442369 [DOI] [PubMed] [Google Scholar]
- Johnson K. D., Glinskii O. V., Mossine V. V., Turk J. R., Mawhinney T. P., Anthony D. C., et al. (2007). Galectin-3 as a potential therapeutic target in tumors arising from malignant endothelia. Neoplasia 9 662–670 10.1593/neo.07433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H. J., Jo C., Kwon J. H., Son J. H., An B. J., Byun M. W. (2006). Antioxidant and cancer cell proliferation inhibition effect of citrus pectin-oligosaccharide prepared by irradiation. J. Med. Food 9 313–320 10.1089/jmf.2006.9.313 [DOI] [PubMed] [Google Scholar]
- Kanthamneni N., Chaudhary A., Wang J., Prabhu S. (2010). Nanoparticulate delivery of novel drug combination regimens for the chemoprevention of colon cancer. Int. J. Oncol. 37 177–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolar S., Barhoumi R., Jones C. K., Wesley J., Lupton J. R., Fan Y. Y., et al. (2011). Interactive effects of fatty acid and butyrate-induced mitochondrial Ca(2)(+) loading and apoptosis in colonocytes. Cancer 117 5294–5303 10.1002/cncr.26205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolar S. S., Barhoumi R., Callaway E. S., Fan Y. Y., Wang N., Lupton J. R., et al. (2007). Synergy between docosahexaenoic acid and butyrate elicits p53-independent apoptosis via mitochondrial Ca(2+) accumulation in colonocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 293 G935–G943 10.1152/ajpgi.00312.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattimer J. M., Haub M. D. (2010). Effects of dietary fiber and its components on metabolic health. Nutrients 2 1266–1289 10.3390/nu2121266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawaetz O., Blackburn A. M., Bloom S. R., Aritas Y., Ralphs D. N. (1983). Effect of pectin on gastric emptying and gut hormone release in the dumping syndrome. Scand. J. Gastroenterol. 18 327–336 10.3109/00365528309181602 [DOI] [PubMed] [Google Scholar]
- Liners F., Letesson J. J., Didembourg C, Van Cutsem P. (1989). Monoclonal antibodies against pectin: recognition of a conformation induced by calcium. Plant Physiol. 91 1419–1424 10.1104/pp.91.4.1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H. Y., Huang Z. L., Yang G. H., Lu W. Q., Yu N. R. (2008). Inhibitory effect of modified citrus pectin on liver metastases in a mouse colon cancer model. World J. Gastroenterol. 14 7386–7391 10.3748/wjg.14.7386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Li Y. H., Niu Y. B., Sun Y., Guo Z. J., Li Q., et al. (2010). An apple oligogalactan prevents against inflammation and carcinogenesis by targeting LPS/TLR4/NF-kappaB pathway in a mouse model of colitis-associated colon cancer. Carcinogenesis 31 1822–1832 10.1093/carcin/bgq070 [DOI] [PubMed] [Google Scholar]
- Maxwell E. G., Belsham N. J., Waldron K. W., Morris V. J. (2012). Pectin - an emerging new bioactive food polysaccharide. Trends Food Sci. Technol. 24 64–73 10.1016/j.tifs.2011.11.002 [DOI] [Google Scholar]
- Mohnen D. (2008). Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 11 266–277 10.1016/j.pbi.2008.03.006 [DOI] [PubMed] [Google Scholar]
- Nangia-Makker P., Hogan V., Honjo Y., Baccarini S., Tait L., Bresalier R., et al. (2002). Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. J. Natl. Cancer Inst. 94 1854–1862 10.1093/jnci/94.24.1854 [DOI] [PubMed] [Google Scholar]
- Nesterenko V. B., Nesterenko A. V., Babenko V. I., Yerkovich T. V., Babenko I. V. (2004). Reducing the 137Cs-load in the organism of “Chernobyl” children with apple-pectin. Swiss Med. Wkly. 134 24–27 [DOI] [PubMed] [Google Scholar]
- Newlaczyl A. U., Yu L. G. (2011). Galectin-3 – a jack-of-all-trades in cancer. Cancer Lett. 313 123–128 10.1016/j.canlet.2011.09.003 [DOI] [PubMed] [Google Scholar]
- O’Brien S., Kay N. E. (2011). Maintenance therapy for B-chronic lymphocytic leukemia. Clin. Adv. Hematol. Oncol. 9 22–31 [PubMed] [Google Scholar]
- Ohkami H., Tazawa K., Yamashita I., Shimizu T., Murai K., Kobashi K., et al. (1995). Effects of apple pectin on fecal bacterial enzymes in azoxymethane-induced rat colon carcinogenesis. Jpn. J. Cancer Res. 86 523–529 10.1111/j.1349-7006.1995.tb02429.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olano-Martin E., Gibson G. R., Rastell R. A. (2002). Comparison of the in vitro bifidogenic properties of pectins and pectic-oligosaccharides. J. Appl. Microbiol. 93 505–511 10.1046/j.1365-2672.2002.01719.x [DOI] [PubMed] [Google Scholar]
- Olano-Martin E., Rimbach G. H., Gibson G. R., Rastall R. A. (2003). Pectin and pectic-oligosaccharides induce apoptosis in in vitro human colonic adenocarcinoma cells. Anticancer Res. 23 341–346 [PubMed] [Google Scholar]
- Patel M., Shah T., Amin A. (2007). Therapeutic opportunities in colon-specific drug-delivery systems. Crit. Rev. Ther. Drug Carrier Syst. 24 147–202 10.1615/CritRevTherDrugCarrierSyst.v24.i2.20 [DOI] [PubMed] [Google Scholar]
- Pienta K. J., Naik H., Akhtar A., Yamazaki K., Replogle T. S., Lehr J., et al. (1995). Inhibition of spontaneous metastasis in a rat prostate cancer model by oral administration of modified citrus pectin. J. Natl. Cancer Inst. 87 348–353 10.1093/jnci/87.5.348 [DOI] [PubMed] [Google Scholar]
- Platt D., Raz A. (1992). Modulation of the lung colonization of B16-F1 melanoma cells by citrus pectin. J. Natl. Cancer Inst. 84 438–442 10.1093/jnci/84.6.438 [DOI] [PubMed] [Google Scholar]
- Puga A. M., Lima A. C., Mano J. F., Concheiro A., Alvarez-Lorenzo C. (2013). Pectin-coated chitosan microgels crosslinked on superhydrophobic surfaces for 5-fluorouracil encapsulation. Carbohydr. Polym. 98 331–340 10.1016/j.carbpol.2013.05.091 [DOI] [PubMed] [Google Scholar]
- Ramachandran C., Wilk B. J., Hotchkiss A., Chau H., Eliaz I., Melnick S. J. (2011). Activation of human T-helper/inducer cell, T-cytotoxic cell, B-cell, and natural killer (NK)-cells and induction of natural killer cell activity against K562 chronic myeloid leukemia cells with modified citrus pectin. BMC Complement. Altern. Med. 11:59 10.1186/1472-6882-11-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebucci M., Michiels C. (2013). Molecular aspects of cancer cell resistance to chemotherapy. Biochem. Pharmacol. 85 1219–1226 10.1016/j.bcp.2013.02.017 [DOI] [PubMed] [Google Scholar]
- Ridley B. L., O’Neill M. A., Mohnen D. (2001). Pectins: structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 57 929–967 10.1016/S0031-9422(01)00113-3 [DOI] [PubMed] [Google Scholar]
- Sathisha U. V., Jayaram S., Harish Nayaka M. A., Dharmesh S. M. (2007). Inhibition of galectin-3 mediated cellular interactions by pectic polysaccharides from dietary sources. Glycoconj. J. 24 497–507 10.1007/s10719-007-9042-3 [DOI] [PubMed] [Google Scholar]
- Shah M. S., Schwartz S. L., Zhao C., Davidson L. A., Zhou B., Lupton J. R., et al. (2011). Integrated microRNA and mRNA expression profiling in a rat colon carcinogenesis model: effect of a chemo-protective diet. Physiol. Genomics 43 640–654 10.1152/physiolgenomics.00213.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H. J., Kim Y. S., Kwak Y. S., Song Y. B., Kim Y. S., Park J. D. (2004). Enhancement of antitumor effects of paclitaxel (taxol) in combination with red ginseng acidic polysaccharide (RGAP). Planta Med. 70 1033–1038 10.1055/s-2004-832643 [DOI] [PubMed] [Google Scholar]
- Srivastava R., Kumar D., Pathak K. (2012). Colonic luminal surface retention of meloxicam microsponges delivered by erosion based colon-targeted matrix tablet. Int. J. Pharm. 427 153–162 10.1016/j.ijpharm.2012.01.036 [DOI] [PubMed] [Google Scholar]
- Takei T., Sato M., Ijima H., Kawakami K. (2010). In situ gellable oxidized citrus pectin for localized delivery of anticancer drugs and prevention of homotypic cancer cell aggregation. Biomacromolecules 11 3525–3530 10.1021/bm1010068 [DOI] [PubMed] [Google Scholar]
- Takei T., Sugihara K., Yoshida M., Kawakami K. (2013). Injectable and biodegradable sugar beet pectin/gelatin hydrogels for biomedical applications. J. Biomater. Sci. Polym. Ed. 24 1333–1342 10.1080/09205063.2012.757727 [DOI] [PubMed] [Google Scholar]
- Vayssade M., Sengkhamparn N., Verhoef R., Delaigue C., Goundiam O., Vigneron P., et al. (2010). Antiproliferative and proapoptotic actions of okra pectin on B16F10 melanoma cells. Phytother. Res. 24 982–989 10.1002/ptr.3040 [DOI] [PubMed] [Google Scholar]
- Wang Y., Nangia-Makker P., Balan V., Hogan V., Raz A. (2010). Calpain activation through galectin-3 inhibition sensitizes prostate cancer cells to cisplatin treatment. Cell Death Dis. 1 e101 10.1038/cddis.2010.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Reddy B. S., Weisburger J. H., Kritchevsky D. (1979). Effect of dietary alfalfa, pectin, and wheat bran on azoxymethane-or methylnitrosourea-induced colon carcinogenesis in F344 rats. J. Natl. Cancer Inst. 63 141–145 [PubMed] [Google Scholar]
- Wong T. W., Colombo G., Sonvico F. (2011). Pectin matrix as oral drug delivery vehicle for colon cancer treatment. AAPS PharmSciTech 12 201–214 10.1208/s12249-010-9564-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Zhang J. S., Mo Y., Tan R. X. (2005). Calcium pectinate capsules for colon-specific drug delivery. Drug Dev. Ind. Pharm. 31 127–134 10.1081/DDC-200046990 [DOI] [PubMed] [Google Scholar]
- Yan J., Katz A. (2010). PectaSol-C modified citrus pectin induces apoptosis and inhibition of proliferation in human and mouse androgen-dependent and- independent prostate cancer cells. Integr. Cancer Ther. 9 197–203 10.1177/1534735410369672 [DOI] [PubMed] [Google Scholar]
- Zhang L., Cao F., Ding B., Li Q., Xi Y., Zhai G. (2011). Eudragit(R) S100 coated calcium pectinate microspheres of curcumin for colon targeting. J. Microencapsul. 28 659–667 10.3109/02652048.2011.604436 [DOI] [PubMed] [Google Scholar]