Abstract
A microarray (LungCaGxE), based on Illumina BeadChip technology, was developed for high-resolution genotyping of genes that are candidates for involvement in environmentally driven aspects of lung cancer oncogenesis and/or tumor growth. The iterative array design process illustrates techniques for managing large panels of candidate genes and optimizing marker selection, aided by a new bioinformatics pipeline component, Tagger Batch Assistant. The LungCaGxE platform targets 298 genes and the proximal genetic regions in which they are located, using ∼13,000 DNA single nucleotide polymorphisms (SNPs), which include haplotype linkage markers with a minimum allele frequency of 1% and additional specifically targeted SNPs, for which published reports have indicated functional consequences or associations with lung cancer or other smoking-related diseases. The overall assay conversion rate was 98.9%; 99.0% of markers with a minimum Illumina design score of 0.6 successfully generated allele calls using genomic DNA from a study population of 1873 lung-cancer patients and controls.
Keywords: genetic association, environmental exposures, Tagger Batch Assistant, LungCaGxE
INTRODUCTION
Lung cancer is the leading cause of cancer death for men and women in the United States. The American Cancer Society estimates that in 2013, there will be 228,190 new cases (118,080 in men; 110,110 in women) and 159,480 deaths.1 Many patients present with disease that is too advanced to treat successfully with surgery and the current portfolio of drugs. Identification of those at highest risk of disease would facilitate earlier diagnosis and therapeutic intervention, with consequent reduced mortality and longer survival time. Risk identification techniques would also support preventative screening and targeted interventions, such as smoking-cessation programs leading to reduced incidence. Given the huge number of new lung cancer cases that occur each year, the impact of such interventions would be significant even if applicable only to an etiologically distinct subset of all cases.
As the majority (up to 90%) of lung cancers occurs in smokers, but only a minority (∼10%) of smokers get the disease,2 it is likely that significant gene/phenotype/environment interactions exist.3 Although tobacco smoke is the main etiologic agent,4 the long latency between exposure and disease, the multistep nature of neoplastic transformation,5 and the low, 10-year lung-cancer risk of elderly, life-long heavy smokers (15%)6 suggest that factors other than tobacco-associated carcinogens modify risk. These likely include environmental variables,7 functional genetic polymorphisms,8,9 and differential expression of genes that interact with such variables.10
Strategies to identify associations between genetic variants and diseases, such as lung cancer, include genotyping sequence polymorphisms that are distributed throughout the genome or that occur in specifically targeted genes of interest. Compared with genome-wide approaches, genotyping a focused set of single nucleotide polymorphisms (SNPs) for high-resolution haplotype mapping boosts analysis power for identifying single gene and gene family effects with statistical significance. Targeted, redundant genotyping of candidate genes further enables the analysis of additional variables, such as environmental factors, without a requirement to sample extremely large populations. However, designing a genotyping assay that adequately covers each candidate gene with a sufficiently large number of markers poses a challenge for this approach, especially when interrogating many genes in parallel. Standard genome-wide platforms, such as Affymetrix (Affymetrix, Santa Clara, CA, USA) or Illumina microarrays (Illumina, San Diego, CA, USA), provide predesigned collections of genotyping assays but rarely include enough markers to approach saturation of any given target gene. Microarray vendors therefore offer custom manufacturing options to allow researchers to create comprehensive panels of assays that satisfy the requirements of high-resolution genotyping. We describe a process that connects publicly available SNP catalogs with commercial assay design interfaces, using a new bioinformatics tool that assists with the management of large collections of genes and their haplotype-tagging (HapTag) SNPs. This process was used to demonstrate the rapid and iterative design of a custom-genotyping microarray for studying lung cancer.
MATERIALS AND METHODS
Target Selection
Investigators in our consortium contributed prioritized lists of genes potentially relevant to environmentally mediated biological processes leading to lung cancer. Candidate genes included modulators of and checkpoints within pathways hypothesized to respond to tobacco toxins and environmental factors that may promote oncogenesis, as well as those that may act in concert with environmental factors to support tumor survival, progression, and growth. These genes fell into broad categories, including tobacco-specific nitrosamine [particularly nitrosaminoketone (NNK)] activation and detoxification, polycyclic aromatic hydrocarbon (PAH) activation and detoxification, repair of NNK- and PAH-attributable DNA damage, oxidative stress, inflammatory signaling and processes of immune regulation, steroid hormone metabolism and signaling, nicotine addiction and smoking behavior, and folate transport and metabolism. For each individual gene, HapTag SNPs and genetic polymorphisms known to affect function or shown previously to be associated with risk for lung cancer were sought and if found, incorporated into the final microarray design.
Target sources included extensive literature searches, Ingenuity Pathway Analysis (http://www.ingenuity.com), Database for Annotation, Visualization, and Integrated Discovery (DAVID) Bioinformatics Resources,11,12 and ongoing research in investigators' laboratories.
SNP Selection
All targeted genes/chromosomal regions were uploaded to the Assay Design Tool (http://support.illumina.com/tools.ilmn; Illumina) for retrieval of all iSelect Infinium database SNPs within each targeted region, as well as from 15 kb sequences flanking the gene-boundary coordinates. Known polymorphisms from the target-selection phase were also queried by reference SNP (rs) number from database of SNPs (dbSNP; http://www.ncbi.nlm.nih.gov/snp/), or uploaded as custom sequences if polymorphisms were unrecognized by iSelect or not annotated in dbSNP. Independently, the targeted genes and regions were analyzed using Tagger (http://www.broadinstitute.org/mpg/tagger/server.html, and International HapMap Project haplotype mapping databases therein)13 with the following parameters in all combinations: HapMap panels of Utah (U.S.A.) residents of northern and western European ancestry (CEU) and residents of Ibadan, Nigeria of Yoruban ancestry (YRI); SNP minimum allele frequencies 5% and 1%; Tagger mode pairwise and aggressive; SNP r2 threshold 0.8; and default settings for all other parameters. The Tagger online interface does not support batch queries using gene symbols, so we created the Tagger Batch Assistant (http://www.bioinformatics.upenn.edu/tagtool/batch.html) as a tool for automated processing of large query lists and management and formatting of the output data.
The retrieved iSelect SNPs were filtered to retain markers with an Infinium design score ≥0.6 (a 60% probability of conversion, i.e., successful genotyping assays for that SNP), and the subset corresponding to selected HapTag SNPs from Tagger was identified. No Infinium design score limits were imposed on functional SNPs from the target selection phase. A panel of 357 ancestry informative markers was included (http://support.illumina.com/array/array_kits/dna_test_panel.ilmn, Illumina catalog GT-17-222).
Genotyping
DNA was extracted from whole-blood samples or buffy-coat fractions using Chemagic DNA purification kits and a Chemagen Magnetic Separation Module I robot (Chemagen/PerkinElmer, Baesweiler, Germany). DNA quality-control checks included A260/280 and E-Gel electrophoresis (Invitrogen, Life Technologies, Grand Island, NY, USA), and DNA samples (n=1873) were normalized to 50 ng/ul and used for genotyping assays. Genotyping was conducted using the iScan system (Illumina), according to the manufacturer's protocols.14 The Infinium assay amplified and fragmented 200 ng genomic DNA, which was then hybridized to our LungCaGxE iSelect HD Custom BeadChips containing 24 arrays/BeadChip and 13,308 assayed SNPs/array. Four negative control (no DNA) arrays were processed, and 43 samples were processed twice to check assay consistency. Data from scanned BeadChips were processed in Illumina GenomeStudio for signal quantitation, quality control, and genotype assignments.
The research described does not involve animals. Blood samples from human subjects were collected with their informed consent for research use, including genetic analyses. This study was approved by Institutional Review Boards at the University of Pennsylvania, Pennsylvania State University, Temple University, and Fox Chase Cancer Center.
RESULTS
Tagger Batch Assistant
The online Tagger Batch Assistant tool was designed with two components: one for rapid retrieval of genomic coordinates for large lists of genes and another for managing Tagger output files that result from a batch query using genomic coordinates. Starting with a list of official National Center for Biotechnology Information gene symbols, the tool supports queries of several human genome-build versions, concatenation or separation of overlapping genes, and rules for flanking regions that allow the addition of sequences adjacent to gene coordinates. Multiple choices are available for the amount of flanking sequences added, and rules can be stacked to vary the flanking regions by gene length. The output file can be reviewed in text or spreadsheet formats and is configured for uploading to the Tagger query interface. After receiving compressed Tagger results files, the tool supports automated merging of the user's annotated gene query lists with the corresponding Tagger results.
Assembly of Target Gene Panel
Project investigators identified 298 genes in pathways for which genetically mandated differential interactions with environmental factors leading to lung cancer were deemed to be biologically plausible. These pathways included those supporting or mediating carcinogen effects (i.e., nitrosamine and PAH activation and detoxification), oxidative stress, DNA damage repair, inflammation or immune-system monitoring, estrogen, and other steroid hormone processes, nicotine addiction/smoking behavior, and folate metabolism. Target genes were chosen by examining previous literature, established molecular pathways, and gene interactions and sequence polymorphisms known to affect the functions of genes involved in lung tumor oncogenesis or responses to environmental factors that may impact lung cancer (Table 1). Confirmatory DAVID annotation analyses were performed on the final gene list to summarize the categories represented from Online Mendelian Inheritance in Man (OMIM) Disease, Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway, Gene Ontology (GO) Molecular Function, and GO Biological Process databases (Supplemental Table 1). As expected, the final target panel was confirmed as being enriched for genes associated with risk for lung cancer, folate-sensitive phenotypes, hormone synthesis and signaling, oxidative stress responses, DNA repair, detoxification and metabolism of complex molecules, and apoptosis. Cross-category annotation indicates that the panel is coincidentally enriched for genes involved in schizophrenia, trichothiodystrophy, myocardial infarction, reproductive development, and various neurological processes.
Table 1.
Targeted Genes, Annotations, and Number of HapTag SNPs Identified by Pairwise or Multimarker (multi) Algorithms at the Indicated Minor Allele Frequencies (MAFs) and Infinium (Inf) Design Scores ≥0.6
| Gene symbol | Genetic locus overlaps | All HapTags: pairwise, MAF1% | Inf 0.6+, pairwise, MAF1% | Inf 0.6+, pairwise MAF5% | Inf 0.6+, multi, MAF1% | Inf 0.6+, multi, MAF5% | Array coverage: multi HapTags, MAF1% | Gene name | Target categorya |
|---|---|---|---|---|---|---|---|---|---|
| A2BP1 | 1099 | 1046 | 914 | 710 | 599 | full | ataxin 2 binding protein 1 | tum | |
| ABCB1 | 91 | 81 | 62 | 62 | 44 | full | ATP binding cassette, subfamily B [multidrug resistance (MDR)/transporter associated with antigen processing (TAP)], member 1 | tum | |
| ABCC1 | 199 | 180 | 162 | 142 | 123 | full | ATP binding cassette, subfamily C [cystic fibrosis transmembrane conductance regulator (CFTR)/multidrug resistance-associated protein (MRP)], member 1 | mut/PAH | |
| ABCC2 | 51 | 44 | 33 | 40 | 29 | full | ATP binding cassette, subfamily C (CFTR/MRP), member 2 | fol | |
| ABCC4 | 355 | 324 | 274 | 244 | 199 | full | ATP binding cassette, subfamily C (CFTR/MRP), member 4 | mut/PAH | |
| ACHE | 12 | 12 | 10 | 12 | 10 | full | ACETYLCHOLINESTERASE (YT BLOOD GROUP) | nic | |
| ADAM19 | 110 | 101 | 78 | 81 | 61 | full | a disintegrin and metalloprotease domain (ADAM) metallopeptidase domain 19 (meltrin β) | adh | |
| ADH1B | 28 | 26 | 23 | 23 | 20 | full | alcohol dehydrogenase 1B (class I), β polypeptide | tox | |
| ADH7 | 49 | 47 | 40 | 40 | 33 | full | alcohol dehydrogenase 7 (class IV), μ or σ polypeptide | tox | |
| ADK | 120 | 98 | 80 | 74 | 55 | full | adenosine kinase | inf | |
| AGER | PPT2 | 23 | 22 | 20 | 22 | 17 | full | advanced glycosylation end product-specific receptor | inf/mut |
| AHCY | 17 | 12 | 12 | 8 | 8 | full | S-ADENOSYLHOMOCYSTEINE HYDROLASE | fol | |
| AHR | 27 | 27 | 24 | 25 | 21 | full | ARYL-HYDROCARBON RECEPTOR | PAH | |
| AHRR | 87 | 79 | 71 | 61 | 51 | full | ARYL-HYDROCARBON RECEPTOR REPRESSOR | PAH | |
| AKAP9 | 23 | 21 | 17 | 19 | 15 | full | A kinase (PRKA) anchor protein (yotiao) 9 | tum | |
| AKR1A1 | 17 | 13 | 10 | 12 | 7 | full | ALDO-KETO REDUCTASE FAMILY 1, MEMBER A1 (ALDEHYDE REDUCTASE) | PAH | |
| AKR1B10 | 30 | 25 | 24 | 22 | 21 | full | ALDO-KETO REDUCTASE FAMILY 1, MEMBER B10 (ALDOSE REDUCTASE-LIKE) | onc | |
| AKR1C1 | AKR1C2 | 18 | 15 | 15 | 22 | 21 | full | ALDO-KETO REDUCTASE FAMILY 1, MEMBER C1 [DIHYDRODIOL DEHYDROGENASE 1; 20-α (3-α)-HYDROXYSTEROID DEHYDROGENASE] | nit/PAH/str |
| AKR1C2 | AKR1C1 | 46 | 38 | 36 | 24 | 23 | full | ALDO-KETO REDUCTASE FAMILY 1, MEMBER C2 (DIHYDRODIOL DEHYDROGENASE 2; BILE ACID BINDING PROTEIN; 3-α HYDROXYSTEROID DEHYDROGENASE, TYPE III) | nit/PAH/str |
| AKR1C3 | 44 | 36 | 35 | 27 | 26 | full | ALDO-KETO REDUCTASE FAMILY 1, MEMBER C3 (3-α HYDROXYSTEROID DEHYDROGENASE, TYPE II) | PAH/str | |
| AKT1 | 16 | 13 | 12 | 13 | 12 | full | V-AKT MURINE THYMOMA VIRAL ONCOGENE HOMOLOG 1 | onc | |
| AKT2 | 15 | 14 | 11 | 13 | 10 | full | v-akt murine thymoma viral oncogene homolog 2 | tum | |
| AKT3 | 95 | 89 | 70 | 69 | 54 | full | v-akt murine thymoma viral oncogene homolog 3 (PKB, γ) | tum | |
| ALDH1L1 | 115 | 98 | 89 | 67 | 58 | full | aldehyde dehydrogenase 1 family, member L1 | fo1 | |
| ALOX5 | 65 | 57 | 48 | 46 | 36 | full | ARACHIDONATE 5-LIPOXYGENASE | inf/oxs | |
| ALPL | 100 | 96 | 88 | 79 | 70 | dropped for capacity | alkaline phosphatase, liver/bone/kidney | ||
| ANKK1 | DRD2 | 44 | 41 | 33 | 26 | 19 | full | ANKYRIN REPEAT AND KINASE DOMAIN CONTAINING 1 | nic |
| APEX1 | 30 | 29 | 26 | 26 | 22 | full | APEX NUCLEASE (MULTIFUNCTIONAL DNA REPAIR ENZYME) 1 | DNA | |
| AR | 15 | 5 | 5 | 12 | 12 | full | ANDROGEN RECEPTOR (DIHYDROTESTOSTERONE RECEPTOR; TESTICULAR FEMINIZATION; SPINAL AND BULBAR MUSCULAR ATROPHY; KENNEDY DISEASE) | str | |
| AREG | 31 | 14 | 12 | 10 | 7 | full | AMPHIREGULIN (SCHWANNOMA-DERIVED GROWTH FACTOR) | onc | |
| ARID1A | 10 | 10 | 7 | 10 | 7 | full | AT RICH-INTERACTIVE DOMAIN 1A (SWI-LIKE) | onc | |
| ARNT | 30 | 29 | 17 | 27 | 15 | full | ARYL-HYDROCARBON RECEPTOR NUCLEAR TRANSLOCATOR | PAH | |
| ARNTL | 103 | 98 | 88 | 84 | 75 | full | ARYL-HYDROCARBON RECEPTOR NUCLEAR TRANSLOCATOR-LIKE | PAH | |
| ATIC | 38 | 34 | 31 | 28 | 25 | full | 5-AMINOIMIDAZOLE-4-CARBOXAMIDE RIBONUCLEOTIDE FORMYLTRANSFERASE/IMP CYCLOHYDROLASE | fol | |
| BCL2 | 229 | 224 | 178 | 190 | 144 | full | B CELL chronic lymphocytic leukemia (CLL)/LYMPHOMA 2 | onc | |
| BDNF | 33 | 33 | 26 | 30 | 23 | full | BRAIN-DERIVED NEUROTROPHIC FACTOR | nic | |
| BHMT | 28 | 28 | 25 | 26 | 22 | full | BETAINE-HOMOCYSTEINE METHYLTRANSFERASE | fol | |
| BIRC5 | 21 | 19 | 18 | 17 | 16 | full | BACULOVIRAL inhibitor of apoptosis (IAP) REPEAT-CONTAINING 5 (SURVIVIN) | onc | |
| BMPR1B | 210 | 199 | 180 | 143 | 125 | full | BONE MORPHOGENETIC PROTEIN RECEPTOR, TYPE IB | onc | |
| BRCA2 | 88 | 82 | 59 | 68 | 47 | full | breast cancer 2, early onset | tum | |
| C3 | 63 | 59 | 49 | 55 | 45 | full | COMPLEMENT COMPONENT 3 | inf | |
| CAMKK1 | 41 | 36 | 33 | 35 | 32 | full | calcium/calmodulin-dependent protein kinase kinase 1, α | tum | |
| CBR1 | 19 | 17 | 15 | 13 | 11 | full | CARBONYL REDUCTASE 1 | nit/PAH | |
| CBR3 | 16 | 13 | 11 | 11 | 9 | full | CARBONYL REDUCTASE 3 | nit/PAH | |
| CBS | 60 | 57 | 53 | 47 | 43 | full | CYSTATHIONINE-β-SYNTHASE | fol | |
| CCL2 | 23 | 20 | 17 | 16 | 13 | full | chemokine (C–C motif) ligand 2 | inf | |
| CCL21 | 20 | 18 | 16 | 15 | 14 | full | chemokine (C–C motif) ligand 21 | inf | |
| CCL5 | 10 | 9 | 6 | 8 | 5 | full | chemokine (C–C motif) ligand | inf | |
| CCNA2 | 22 | 17 | 12 | 13 | 9 | full | cyclin A2 | onc | |
| CCND1 | 25 | 25 | 20 | 23 | 18 | full | CYCLIN D1 | onc | |
| CCND3 | 83 | 21 | 17 | 18 | 14 | full | cyclin D3 | onc | |
| CCR2 | 6 | 0 | 0 | 0 | 0 | nine non-HapTag SNPs | chemokine (C–C motif) receptor 2 | inf | |
| CD47 | 46 | 43 | 35 | 36 | 28 | full | CD47 ANTIGEN (RH-RELATED ANTIGEN, INTEGRIN-ASSOCIATED SIGNAL TRANSDUCER) | adh | |
| CDH1 | 66 | 56 | 53 | 50 | 47 | full | CADHERIN 1, TYPE 1, E-CADHERIN (EPITHELIAL) | adh | |
| CDKN2A | 33 | 31 | 27 | 27 | 24 | full | cyclin-dependent kinase inhibitor 2A (melanoma, p16, inhibits CDK4) | onc | |
| CES3 | 10 | 8 | 5 | 7 | 4 | full | CARBOXYLESTERASE 3 | tox | |
| CHAT | SLC18A3 | 85 | 98 | 81 | 112 | 93 | full | CHOLINE ACETYLTRANSFERASE | nic |
| CHRNA3 | CHRNA5 | 2 | 2 | 1 | 22 | 18 | full | CHOLINERGIC RECEPTOR, NICOTINIC, α 3 | nic |
| CHRNA4 | 28 | 25 | 24 | 21 | 20 | full | CHOLINERGIC RECEPTOR, NICOTINIC, α 4 | nic | |
| CHRNA5 | CHRNB4 | 28 | 26 | 21 | 13 | 10 | full | CHOLINERGIC RECEPTOR, NICOTINIC, α 5 | nic |
| CHRNA7 | 89 | 85 | 71 | 76 | 62 | full | CHOLINERGIC RECEPTOR, NICOTINIC, α 7 | nic | |
| CHRNB2 | 24 | 23 | 22 | 20 | 18 | full | CHOLINERGIC RECEPTOR, NICOTINIC, β 2 (NEURONAL) | nic | |
| CHRNB3 | 21 | 20 | 17 | 16 | 13 | full | CHOLINERGIC RECEPTOR, NICOTINIC, β 3 | nic | |
| CHRNB4 | CHRNA3 | 27 | 26 | 24 | 15 | 14 | full | CHOLINERGIC RECEPTOR, NICOTINIC, β 4 | nic |
| CHUK | 27 | 26 | 20 | 23 | 17 | full | CONSERVED HELIX-LOOP-HELIX UBIQUITOUS KINASE | onc | |
| CLOCK | 35 | 29 | 27 | 20 | 18 | full | CLOCK HOMOLOG (MOUSE) | onc | |
| COL3A1 | 55 | 54 | 46 | 46 | 37 | full | COLLAGEN, TYPE III, α 1 (EHLERS-DANLOS SYNDROME TYPE IV, AUTOSOMAL DOMINANT) | adh | |
| COMT | 56 | 49 | 43 | 39 | 34 | full | CATECHOL-O-METHYLTRANSFERASE | PAH/str | |
| CRP | 31 | 31 | 23 | 25 | 18 | full | C-REACTIVE PROTEIN, PENTRAXIN-RELATED | inf | |
| CRY1 | 51 | 45 | 38 | 36 | 28 | full | CRYPTOCHROME 1 (PHOTOLYASE-LIKE) | onc | |
| CSNK1D | 19 | 15 | 11 | 15 | 11 | full | CASEIN KINASE 1, δ | onc | |
| CTH | 38 | 34 | 31 | 30 | 27 | full | CYSTATHIONASE (CYSTATHIONINE γ-LYASE) | fol | |
| CTLA4 | 27 | 27 | 24 | 18 | 15 | full | CYTOTOXIC T-LYMPHOCYTE-ASSOCIATED PROTEIN 4 | inf | |
| CTSD | 9 | 8 | 7 | 8 | 7 | full | CATHEPSIN D (LYSOSOMAL ASPARTYL PEPTIDASE) | tum | |
| CYP17A1 | 24 | 19 | 15 | 16 | 12 | full | CYTOCHROME P450, FAMILY 17, SUBFAMILY A, POLYPEPTIDE 1 | str | |
| CYP19A1 | 112 | 104 | 94 | 75 | 65 | full | CYTOCHROME P450, FAMILY 19, SUBFAMILY A, POLYPEPTIDE 1 | str | |
| CYP1A1 | CYP1A2 | 13 | 11 | 11 | 11 | 10 | full | CYTOCHROME P450, FAMILY 1, SUBFAMILY A, POLYPEPTIDE 1 | PAH |
| CYP1A2 | CYP1A1 | 13 | 13 | 10 | 11 | 9 | full | CYTOCHROME P450, FAMILY 1, SUBFAMILY A, POLYPEPTIDE 2 | PAH |
| CYP1B1 | 41 | 38 | 34 | 33 | 28 | full | CYTOCHROME P450, FAMILY 1, SUBFAMILY B, POLYPEPTIDE 1 | PAH | |
| CYP21A2 | 15 | 7 | 5 | 7 | 5 | full | CYTOCHROME P450, FAMILY 21, SUBFAMILY A, POLYPEPTIDE 2 | str | |
| CYP2A13 | 11 | 7 | 6 | 7 | 6 | full | cytochrome P450, family 2, subfamily A, polypeptide 13 | nit | |
| CYP2A6 | 18 | 13 | 11 | 13 | 11 | full | CYTOCHROME P450, FAMILY 2, SUBFAMILY A, POLYPEPTIDE 6 | nit | |
| CYP2B6 | 43 | 34 | 31 | 27 | 23 | full | cytochrome P450, family 2, subfamily B, polypeptide 6 | nit/tum | |
| CYP2C9 | 32 | 28 | 20 | 26 | 18 | full | CYTOCHROME P450, FAMILY 2, SUBFAMILY C, POLYPEPTIDE 9 | PAH | |
| CYP2D6 | 11 | 5 | 5 | 5 | 5 | full | CYTOCHROME P450, FAMILY 2, SUBFAMILY D, POLYPEPTIDE 6 | nit | |
| CYP2E1 | 39 | 36 | 35 | 30 | 29 | full | cytochrome P450, family 2, subfamily E, polypeptide 1 | nit | |
| CYP3A4 | 30 | 24 | 18 | 23 | 17 | full | CYTOCHROME P450, SUBFAMILY IIIA (NIPHEDIPINE OXIDASE), POLYPEPTIDE 3 | str | |
| DBH | 88 | 85 | 75 | 73 | 63 | dropped for capacity | DOPAMINE β-HYDROXYLASE (DOPAMINE β-MONOOXYGENASE) | ||
| DDX54 | 10 | 10 | 9 | 9 | 8 | full | DEAD (ASP-GLU-ALA-ASP) BOX POLYPEPTIDE 54 | onc | |
| DHFR | 24 | 19 | 16 | 15 | 12 | full | DIHYDROFOLATE REDUCTASE | fol | |
| DMGDH | 75 | 69 | 64 | 58 | 50 | full | dimethylglycine dehydrogenase | fol | |
| DNMT1 | 18 | 16 | 12 | 14 | 10 | full | DNA (cytosine-5-)-methyltransferase 1 | fol | |
| DNMT3A | 56 | 54 | 44 | 45 | 35 | full | DNA (cytosine-5-)-methyltransferase 3 α | fol | |
| DNMT3B | 58 | 56 | 46 | 40 | 30 | full | DNA (cytosine-5-)-methyltransferase 3 β | fol | |
| DRD2 | ANKK1 | 53 | 53 | 45 | 44 | 36 | full | DOPAMINE RECEPTOR D2 | nic |
| DRD4 | 11 | 11 | 10 | 10 | 9 | full | DOPAMINE RECEPTOR D4 | nic | |
| EGF | 129 | 115 | 79 | 95 | 61 | full | epidermal growth factor | tum | |
| EGFR | 212 | 196 | 167 | 162 | 135 | full | EPIDERMAL GROWTH FACTOR RECEPTOR (ERYTHROBLASTIC LEUKEMIA VIRAL (V-ERB-B) ONCOGENE HOMOLOG, AVIAN) | onc | |
| EGLN2 | 22 | 20 | 18 | 17 | 15 | full | egl nine homolog 2 | oxs | |
| EPHX1 | 36 | 26 | 23 | 26 | 22 | full | EPOXIDE HYDROLASE 1, MICROSOMAL (XENOBIOTIC) | PAH | |
| ERCC1 | 20 | 18 | 14 | 16 | 12 | full | EXCISION REPAIR CROSS-COMPLEMENTING RODENT REPAIR DEFICIENCY, COMPLEMENTATION GROUP 1 (INCLUDES OVERLAPPING ANTISENSE SEQUENCE) | DNA | |
| ERCC2 | 34 | 33 | 22 | 30 | 20 | full | EXCISION REPAIR CROSS-COMPLEMENTING RODENT REPAIR DEFICIENCY, COMPLEMENTATION GROUP 2 (XERODERMA PIGMENTOSUM D) | DNA | |
| ERCC3 | 39 | 36 | 25 | 32 | 20 | full | excision repair cross-complementing rodent repair deficiency, complementation group 3 (xeroderma pigmentosum group B complementing) | DNA | |
| ERCC4 | 61 | 55 | 39 | 41 | 26 | full | EXCISION REPAIR CROSS-COMPLEMENTING RODENT REPAIR DEFICIENCY, COMPLEMENTATION GROUP 4 | DNA | |
| ERCC5 | 67 | 58 | 42 | 46 | 30 | full | EXCISION REPAIR CROSS-COMPLEMENTING RODENT REPAIR DEFICIENCY, COMPLEMENTATION GROUP 5 [XERODERMA PIGMENTOSUM, COMPLEMENTATION GROUP G (COCKAYNE SYNDROME)] | DNA | |
| ERCC6 | 64 | 58 | 37 | 46 | 25 | full | excision repair cross-complementing rodent repair deficiency, complementation group 6 | DNA | |
| ERCC8 | 38 | 33 | 21 | 28 | 17 | full | EXCISION REPAIR CROSS-COMPLEMENTING RODENT REPAIR DEFICIENCY, COMPLEMENTATION GROUP 8 | DNA | |
| ESR1 | 341 | 237 | 173 | 182 | 126 | full | ESTROGEN RECEPTOR 1 | str | |
| ESR2 | 68 | 61 | 52 | 43 | 34 | full | ESTROGEN RECEPTOR 2 (ER β) | str | |
| EYA2 | 342 | 325 | 293 | 247 | 217 | full | eyes absent homolog 2 (Drosophila) | DNA | |
| FAM13A | 165 | 156 | 138 | 113 | 93 | full | family with sequence similarity 13, member A | mut | |
| FCGR1A | 8 | 1 | 1 | 1 | 1 | full | Fc fragment of IgG, high-affinity Ia, receptor (CD64) | inf | |
| FKBP5 | 53 | 30 | 22 | 24 | 18 | full | FK506 BINDING PROTEIN 5 | inf/str | |
| FMO3 | 46 | 42 | 34 | 34 | 26 | full | Flavin containing monooxygenase 3 | tox | |
| FOLH1 | 22 | 14 | 10 | 13 | 9 | full | FOLATE HYDROLASE (PROSTATE-SPECIFIC MEMBRANE ANTIGEN) 1 | fol | |
| FOLR1 | FOLR2 | 5 | 5 | 4 | 8 | 6 | full | FOLATE RECEPTOR 1 (ADULT) | fol |
| FOLR2 | FOLR1 | 9 | 7 | 6 | 3 | 3 | full | FOLATE RECEPTOR 2 (FETAL) | fol |
| FOLR3 | 23 | 13 | 12 | 9 | 9 | full | FOLATE RECEPTOR 3 (γ) | fol | |
| FPGS | 23 | 20 | 17 | 19 | 16 | full | FOLYLPOLYGLUTAMATE SYNTHASE | fol | |
| FTCD | 51 | 50 | 46 | 43 | 39 | full | formiminotransferase cyclodeaminase | fol | |
| GAB1 | 72 | 68 | 58 | 56 | 46 | dropped for capacity | growth factor receptor-bound protein 2-associated binding protein 1 | ||
| GART | 22 | 19 | 14 | 19 | 14 | full | PHOSPHORIBOSYLGLYCINAMIDE FORMYLTRANSFERASE, PHOSPHORIBOSYLGLYCINAMIDE SYNTHETASE, PHOSPHORIBOSYLAMINOIMIDAZOLE SYNTHETASE | fol | |
| GATA3 | 63 | 62 | 50 | 58 | 45 | full | GATA BINDING PROTEIN 3 | tum | |
| GCLC | 85 | 76 | 69 | 66 | 59 | full | GLUTAMATE-CYSTEINE LIGASE, CATALYTIC SUBUNIT | oxs | |
| GCLM | 17 | 16 | 15 | 15 | 12 | full | GLUTAMATE-CYSTEINE LIGASE, MODIFIER SUBUNIT | oxs | |
| GDF15 | 18 | 16 | 15 | 15 | 14 | full | growth differentiation factor 15 | onc | |
| GGH | 27 | 21 | 13 | 16 | 9 | full | γ-glutamyl hydrolase (conjugase, folylpolygammaglutamyl hydrolase) | fol | |
| GHR | 93 | 84 | 72 | 66 | 55 | full | growth hormone receptor | tum | |
| GNMT | 17 | 16 | 13 | 15 | 12 | full | glycine N-methyltransferase | fol | |
| GPC5 | 969 | 889 | 739 | 666 | 528 | full | glypican 5 | mut | |
| GPER | 15 | 12 | 11 | 12 | 11 | full | GPCR 30 | str | |
| GPR126 | 70 | 63 | 52 | 54 | 44 | 85% | GPCR 126 | adh | |
| GPX1 | 14 | 10 | 8 | 10 | 8 | full | GLUTATHIONE PEROXIDASE 1 | oxs | |
| GPX3 | 53 | 51 | 47 | 43 | 39 | full | GLUTATHIONE PEROXIDASE 3 (PLASMA) | oxs | |
| GRPR | 13 | 13 | 13 | 22 | 22 | full | GASTRIN-RELEASING PEPTIDE RECEPTOR | onc | |
| GSK3B | 71 | 58 | 44 | 48 | 35 | full | glycogen synthase kinase 3 β | tum | |
| GSR | 49 | 42 | 32 | 37 | 27 | full | GLUTATHIONE REDUCTASE | oxs | |
| GSS | 23 | 20 | 19 | 18 | 17 | full | GLUTATHIONE SYNTHETASE | fol | |
| GSTA1 | 16 | 11 | 9 | 10 | 8 | full | GLUTATHIONE S-TRANSFERASE A1 | oxs/PAH | |
| GSTA4 | 37 | 34 | 24 | 27 | 17 | full | GLUTATHIONE S-TRANSFERASE A4 | oxs | |
| GSTCD | 39 | 37 | 27 | 29 | 18 | full | glutathione S-transferase, C-terminal domain containing | fol | |
| GSTM1 | GSTM2 | 7 | 4 | 6 | 7 | 7 | full | GLUTATHIONE S-TRANSFERASE M1 | oxs/PAH |
| GSTM2 | GSTM1 | 15 | 12 | 10 | 10 | 8 | full | GLUTATHIONE S-TRANSFERASE M2 (MUSCLE) | oxs/PAH |
| GSTM5 | GSTM1 | 25 | 14 | 12 | 11 | 10 | full | GLUTATHIONE S-TRANSFERASE M5 | oxs |
| GSTO1 | 33 | 30 | 24 | 28 | 23 | full | GLUTATHIONE S-TRANSFERASE ω 1 | oxs | |
| GSTP1 | 21 | 19 | 17 | 16 | 14 | full | GLUTATHIONE S-TRANSFERASE π 1 | oxs/PAH | |
| GSTT1 | 1 | 0 | 0 | 0 | 0 | four non-HapTag SNPs | GLUTATHIONE S-TRANSFERASE θ 1 | oxs/PAH | |
| HDC | 38 | 36 | 33 | 32 | 29 | full | HISTIDINE DECARBOXYLASE | inf | |
| HELQ | 40 | 37 | 31 | 26 | 20 | full | HELQ helicase, POLQ-like | DNA | |
| HFE | 24 | 23 | 20 | 19 | 17 | full | HEMOCHROMATOSIS | tox | |
| HGF | 83 | 78 | 59 | 64 | 46 | full | HEPATOCYTE GROWTH FACTOR (HEPAPOIETIN A; SCATTER FACTOR) | onc | |
| HHIP | 43 | 43 | 37 | 35 | 28 | dropped for capacity | Hedgehog-interacting protein | ||
| hsa-mir21 | 97 | full | HOMO SAPIENS MICRORNA 21 | onc | |||||
| HSD11B1 | 39 | 37 | 35 | 29 | 27 | full | HYDROXYSTEROID (11-β) DEHYDROGENASE 1 | nit/str | |
| HSD17B1 | 17 | 14 | 12 | 13 | 11 | full | HYDROXYSTEROID (17-β) DEHYDROGENASE 1 | str | |
| HSD17B12 | 84 | 77 | 63 | 64 | 51 | full | HYDROXYSTEROID (17-β) DEHYDROGENASE 12 | str | |
| HSD17B3 | 63 | 56 | 46 | 45 | 35 | full | HYDROXYSTEROID (17-β) DEHYDROGENASE 3 | str | |
| HSD17B7 | 24 | 20 | 19 | 17 | 16 | full | HYDROXYSTEROID (17-β) DEHYDROGENASE 7 | str | |
| HSD3B1 | 20 | 16 | 16 | 11 | 11 | full | HYDROXY-δ-5-STEROID DEHYDROGENASE, 3 β- AND STEROID δ-ISOMERASE 1 | str | |
| HTR3E | 24 | 22 | 18 | 20 | 16 | full | 5-hydroxytryptamine (serotonin) receptor 3, family member E | nic | |
| HTR4 | 126 | 115 | 105 | 89 | 81 | full | 5-hydroxytryptamine (serotonin) receptor 4 | nic | |
| ICAM1 | 26 | 25 | 21 | 25 | 21 | full | intercellular adhesion molecule 1 | inf | |
| ID2 | 13 | 13 | 11 | 12 | 11 | full | INHIBITOR OF DNA BINDING 2, DOMINANT NEGATIVE HELIX-LOOP-HELIX PROTEIN | onc | |
| IDH1 | 35 | 31 | 24 | 27 | 22 | full | ISOCITRATE DEHYDROGENASE 1 (NADP+), SOLUBLE | onc | |
| IER3 | 21 | 19 | 18 | 16 | 15 | full | immediate early response 3 | mut/inf | |
| IFNG | 40 | 38 | 30 | 31 | 23 | full | IFN-γ | inf | |
| IGF1 | 65 | 57 | 38 | 48 | 30 | full | insulin-like growth factor 1 (somatomedin C) | onc | |
| IGF1R | 318 | 306 | 272 | 239 | 206 | full | insulin-like growth factor 1 receptor | onc | |
| IGF2 | TH | 16 | 14 | 16 | 17 | 16 | full | insulin-like growth factor 2 (somatomedin A) | onc |
| IGF2R | 161 | 148 | 108 | 123 | 86 | full | insulin-like growth factor 2 receptor | onc | |
| IGFBP3 | 24 | 19 | 18 | 19 | 18 | full | INSULIN-LIKE GROWTH FACTOR BINDING PROTEIN 3 | onc | |
| IKBKB | 25 | 24 | 18 | 20 | 15 | full | inhibitor of κ light polypeptide gene enhancer in B-cells, kinase β | inf | |
| IL10 | 34 | 30 | 24 | 28 | 21 | full | INTERLEUKIN 10 | inf | |
| IL1B | 25 | 24 | 20 | 23 | 19 | full | interleukin 1, β | inf | |
| IL1RN | 66 | 66 | 58 | 54 | 46 | full | interleukin 1 receptor antagonist | inf | |
| IL4 | 49 | 46 | 40 | 39 | 33 | full | INTERLEUKIN 4 | inf | |
| IL6 | 44 | 39 | 32 | 34 | 26 | full | INTERLEUKIN 6 | inf | |
| IL8 | 30 | 28 | 24 | 21 | 17 | full | interleukin 8 | inf | |
| IRS1 | 46 | 43 | 33 | 37 | 28 | full | INSULIN RECEPTOR SUBSTRATE 1 | onc | |
| JUN | 19 | 18 | 16 | 17 | 15 | full | jun oncogene | onc | |
| KEAP1 | 11 | 9 | 9 | 9 | 9 | full | kelch-like ECH-associated protein 1 | oxs | |
| KLRK1 | 27 | 21 | 20 | 19 | 18 | full | KILLER CELL LECTIN-LIKE RECEPTOR SUBFAMILY C, MEMBER 4 | adh | |
| KRT18 | 15 | 10 | 7 | 10 | 7 | full | KERATIN 18 | adh | |
| KRT19 | 19 | 19 | 18 | 17 | 16 | full | KERATIN 19 | adh | |
| LTA | TNF | 10 | 9 | 10 | 19 | 10 | full | lymphotoxin α (TNF superfamily, member 1) | inf |
| LTC4S | 6 | 5 | 5 | 5 | 5 | full | LEUKOTRIENE C4 SYNTHASE | inf | |
| MAF | 48 | 47 | 43 | 45 | 41 | full | v-maf musculoaponeurotic fibrosarcoma oncogene homolog (avian) | onc | |
| MAOA | 19 | 19 | 19 | 22 | 22 | full | MONOAMINE OXIDASE A | nic | |
| MDM2 | 40 | 31 | 24 | 27 | 20 | full | MDM2, TRANSFORMED 3T3 CELL DOUBLE-MINUTE 2, P53 BINDING PROTEIN (MOUSE) | DNA | |
| MGMT | 249 | 238 | 212 | 179 | 152 | full | O-6-METHYLGUANINE-DNA METHYLTRANSFERASE | DNA/nit | |
| MGST3 | 62 | 56 | 53 | 48 | 45 | full | MICROSOMAL GST 3 | oxs | |
| MIF | 44 | 42 | 39 | 35 | 31 | full | macrophage migration inhibitory factor (glycosylation-inhibiting factor) | inf | |
| MSR1 | 99 | 94 | 81 | 79 | 68 | full | macrophage scavenger receptor 1 | inf | |
| MTAP | 64 | 59 | 52 | 44 | 37 | full | methylthioadenosine phosphorylase | fol | |
| MT-COI | 61 SNPs | mitochondrially encoded cytochrome c oxidase I | oxs | ||||||
| MTHFD1 | 36 | 32 | 29 | 31 | 28 | full | METHYLENETETRAHYDROFOLATE DEHYDROGENASE (NADP+ DEPENDENT) 1, METHENYLTETRAHYDROFOLATE CYCLOHYDROLASE, FORMYLTETRAHYDROFOLATE SYNTHETASE | fol | |
| MTHFR | 36 | 32 | 29 | 26 | 22 | full | 5,10-METHYLENETETRAHYDROFOLATE REDUCTASE (NADPH) | fol | |
| MTHFS | 57 | 53 | 47 | 35 | 29 | full | 5,10-METHENYLTETRAHYDROFOLATE SYNTHETASE (5-FORMYLTETRAHYDROFOLATE CYCLO-LIGASE) | fol | |
| MTR | 79 | 64 | 49 | 42 | 33 | full | 5-METHYLTETRAHYDROFOLATE-HOMOCYSTEINE METHYLTRANSFERASE | fol | |
| MTRR | 54 | 49 | 36 | 34 | 22 | full | 5-METHYLTETRAHYDROFOLATE-HOMOCYSTEINE METHYLTRANSFERASE REDUCTASE | fol | |
| MUTYH | 17 | 17 | 14 | 16 | 13 | full | MUTY HOMOLOG (Escherichia coli) | DNA/oxs | |
| MYBL2 | 34 | 32 | 26 | 24 | 18 | full | v-myb myeloblastosis viral oncogene homolog (avian)-like 2 | tum | |
| MYC | 40 | 38 | 27 | 36 | 24 | full | V-MYC MYELOCYTOMATOSIS VIRAL ONCOGENE HOMOLOG (AVIAN) | onc | |
| NAT1 | 115 | 99 | 86 | 80 | 68 | full | N-ACETYLTRANSFERASE 1 (ARYLAMINE N-ACETYLTRANSFERASE) | fol | |
| NAT2 | 56 | 50 | 43 | 39 | 32 | full | N-ACETYLTRANSFERASE 2 (ARYLAMINE N-ACETYLTRANSFERASE) | PAH | |
| NCOA6 | 19 | 17 | 16 | 14 | 13 | full | NUCLEAR RECEPTOR COACTIVATOR 6 | onc | |
| NFE2L2 | 30 | 30 | 24 | 28 | 21 | full | NUCLEAR FACTOR (ERYTHROID-DERIVED 2)-LIKE 2 | oxs | |
| NFKB1 | 74 | 72 | 54 | 56 | 39 | full | NUCLEAR FACTOR OF κ LIGHT POLYPEPTIDE GENE ENHANCER IN B CELLS 1 (P105) | inf/onc | |
| NFKBIA | 27 | 26 | 25 | 23 | 21 | full | NUCLEAR FACTOR OF κ LIGHT POLYPEPTIDE GENE ENHANCER IN B CELLS INHIBITOR, α | inf/onc | |
| NOS1 | 191 | 172 | 141 | 137 | 108 | full | NITRIC OXIDE SYNTHASE 1 (NEURONAL) | inf | |
| NOS2 | 56 | 53 | 51 | 47 | 44 | full | NITRIC OXIDE SYNTHASE 2A (INDUCIBLE, HEPATOCYTES) | inf | |
| NOS3 | 30 | 29 | 23 | 26 | 20 | full | NITRIC OXIDE SYNTHASE 3 (ENDOTHELIAL CELL) | inf | |
| NQO1 | 20 | 19 | 19 | 15 | 15 | full | NAD(P)H DEHYDROGENASE, QUINONE 1 | oxs/PAH | |
| NR1D2 | 27 | 22 | 18 | 21 | 17 | full | NUCLEAR RECEPTOR SUBFAMILY 1, GROUP D, MEMBER 2 | onc | |
| NR3C1 | 58 | 56 | 43 | 48 | 36 | full | nuclear receptor subfamily 3, group C, member 1 (glucocorticoid receptor) | onc | |
| NRIP1 | 85 | 33 | 30 | 30 | 26 | full | NUCLEAR RECEPTOR-INTERACTING PROTEIN 1 | onc | |
| NSD1 | 46 | 33 | 26 | 27 | 20 | full | NUCLEAR RECEPTOR BINDING SET DOMAIN PROTEIN 1 | onc | |
| OAS1 | 34 | 30 | 26 | 26 | 22 | full | 2′,5′-oligoadenylate synthetase 1, 40/46 kDa | inf | |
| OAS2 | 62 | 51 | 46 | 38 | 32 | full | 2′,5′-oligoadenylate synthetase 2, 69/71 kDa | inf | |
| OGG1 | 19 | 18 | 14 | 17 | 12 | full | 8-OXOGUANINE DNA GLYCOSYLASE | DNA/oxs | |
| OPRM1 | 209 | 174 | 149 | 139 | 115 | full | OPIOID RECEPTOR, μ 1 | nic | |
| PCDH7 | 179 | 174 | 143 | 135 | 109 | full | protocadherin 7 | adh | |
| PER1 | 14 | 14 | 11 | 14 | 11 | full | PERIOD HOMOLOG 1 (DROSOPHILA) | onc | |
| PGR | 91 | 71 | 56 | 53 | 39 | full | PROGESTERONE RECEPTOR | str | |
| PHB2 | 9 | 9 | 8 | 9 | 8 | full | PROHIBITIN 2 | adh/str | |
| PID1 | 234 | 221 | 200 | 173 | 153 | full | Phosphotyrosine-interaction domain containing 1 | inf | |
| PIK3CG | 47 | 45 | 36 | 31 | 22 | full | phosphoinositide-3-kinase, catalytic, γ polypeptide | onc | |
| PLA2G6 | 57 | 55 | 43 | 42 | 30 | full | phospholipase A2, group VI (cytosolic, calcium-independent) | tum | |
| PLEKHA6 | 134 | 126 | 120 | 90 | 85 | full | pleckstrin homology domain containing, family A member 6 | nic | |
| POLH | 17 | 14 | 12 | 14 | 11 | full | POLYMERASE (DNA-DIRECTED), η | DNA | |
| POLI | 19 | 18 | 10 | 18 | 10 | full | POLYMERASE (DNA-DIRECTED) ι | DNA | |
| POLK | 39 | 35 | 26 | 34 | 25 | full | POLYMERASE (DNA-DIRECTED) κ | DNA | |
| POLL | 20 | 19 | 15 | 18 | 13 | full | POLYMERASE (DNA-DIRECTED), λ | DNA | |
| PON1 | 67 | 66 | 59 | 56 | 48 | full | paraoxonase 1 | tox | |
| PPARG | 113 | 107 | 80 | 83 | 60 | full | PEROXISOME PROLIFERATIVE ACTIVATED RECEPTOR, γ | onc | |
| PPARGC1B | 150 | 141 | 132 | 112 | 102 | full | PEROXISOME PROLIFERATIVE ACTIVATED RECEPTOR, γ, COACTIVATOR 1, β | onc | |
| PPT2 | AGER | 18 | 18 | 14 | 15 | 13 | full | palmitoyl-protein thioesterase 2 | tox |
| PTCH1 | 20 | 20 | 20 | 18 | 18 | full | patched homolog 1 | tum | |
| PTEN | 30 | 28 | 20 | 27 | 18 | full | phosphatase and tensin homolog | onc | |
| PTGIS | 65 | 59 | 47 | 57 | 44 | dropped for capacity | PG I2 (prostacyclin) synthase | ||
| PTGS1 | 73 | 70 | 54 | 61 | 47 | full | PG-endoperoxide synthase 1 (PG G/H synthase and cyclooxygenase) | inf | |
| PTGS2 | 29 | 24 | 19 | 19 | 14 | full | PG-ENDOPEROXIDE SYNTHASE 2 (PG G/H SYNTHASE AND COX) | infl/oxs | |
| RELA | 13 | 13 | 11 | 12 | 10 | full | v-rel reticuloendotheliosis viral oncogene homolog A (avian) | onc | |
| RERGL | 23 | 19 | 15 | 17 | 13 | full | RAS-like, estrogen-regulated, growth inhibitor (RERG)/RAS-like | str | |
| RNASEL | 27 | 26 | 23 | 25 | 22 | full | ribonuclease L (2′,5′-oligoisoadenylate synthetase-dependent) | inf | |
| SELE | 49 | 45 | 32 | 38 | 25 | full | selectin E | inf | |
| SERPINA3 | 40 | 39 | 37 | 37 | 34 | full | SERPIN PEPTIDASE INHIBITOR, CLADE A (α-1 ANTIPROTEINASE, ANTITRYPSIN), MEMBER 3 | adh/onc | |
| SHMT1 | 29 | 23 | 20 | 18 | 14 | full | SERINE HYDROXYMETHYLTRANSFERASE 1 (SOLUBLE) | fol | |
| SHMT2 | 8 | 6 | 6 | 6 | 6 | full | SERINE HYDROXYMETHYLTRANSFERASE 2 (MITOCHONDRIAL) | fol | |
| SLC18A3 | CHAT | 16 | 32 | 36 | 6 | 10 | full | SOLUTE CARRIER FAMILY 18 (VESICULAR ACETYLCHOLINE), MEMBER 3 | nic |
| SLC19A1 | 31 | 29 | 26 | 25 | 22 | full | SOLUTE CARRIER FAMILY 19 (FOLATE TRANSPORTER), MEMBER 1 | fol | |
| SLC5A7 | 46 | 45 | 36 | 38 | 29 | full | SOLUTE CARRIER FAMILY 5 (CHOLINE TRANSPORTER), MEMBER 7 | nic | |
| SLC6A3 | 55 | 47 | 43 | 41 | 37 | full | SOLUTE CARRIER FAMILY 6 (NEUROTRANSMITTER TRANSPORTER, DOPAMINE), MEMBER 3 | nic | |
| SLC7A5 | 58 | 51 | 46 | 44 | 40 | full | SOLUTE CARRIER FAMILY 7 (CATIONIC AMINO ACID TRANSPORTER, Y+ SYSTEM), MEMBER 5 | onc | |
| SOD1 | 17 | 15 | 14 | 15 | 14 | full | SUPEROXIDE DISMUTASE 1, SOLUBLE [AMYOTROPHIC LATERAL SCLEROSIS 1 (ADULT)] | inf | |
| SOD2 | 17 | 15 | 13 | 13 | 11 | full | SUPEROXIDE DISMUTASE 2, MITOCHONDRIAL | oxs | |
| SOD3 | 25 | 21 | 18 | 20 | 16 | full | SUPEROXIDE DISMUTASE 3, EXTRACELLULAR | inf | |
| STC2 | 30 | 27 | 26 | 24 | 23 | full | STANNIOCALCIN 2 | onc | |
| SULT1A1 | 14 | 10 | 7 | 10 | 7 | full | SULFOTRANSFERASE FAMILY, CYTOSOLIC, 1A, PHENOL-PREFERRING, MEMBER 1 | PAH | |
| SULT1E1 | 54 | 49 | 28 | 43 | 22 | full | SULFOTRANSFERASE FAMILY 1E, ESTROGEN-PREFERRING, MEMBER 1 | str | |
| SULT2A1 | 28 | 26 | 24 | 21 | 18 | full | SULFOTRANSFERASE FAMILY, CYTOSOLIC, 2A, DEHYDROEPIANDROSTERONE (DHEA)-PREFERRING, MEMBER 1 | str | |
| TCN2 | 41 | 40 | 38 | 33 | 29 | full | TRANSCOBALAMIN II; MACROCYTIC ANEMIA | fol | |
| TEF | 32 | 21 | 20 | 18 | 17 | full | THYROTROPHIC EMBRYONIC FACTOR | onc | |
| TFF1 | 72 | 64 | 57 | 49 | 43 | full | TREFOIL FACTOR 1 (BREAST CANCER, ESTROGEN-INDUCIBLE SEQUENCE EXPRESSED IN) | onc | |
| TFF3 | 46 | 44 | 39 | 39 | 35 | full | TREFOIL FACTOR 3 (INTESTINAL) | onc | |
| TGFA | 136 | 129 | 107 | 109 | 87 | full | TRANSFORMING GROWTH FACTOR, α | onc | |
| TGFB1 | 19 | 19 | 17 | 18 | 16 | full | TRANSFORMING GROWTH FACTOR, β 1 (CAMURATI-ENGELMANN DISEASE) | onc | |
| TGFBR1 | 37 | 34 | 24 | 29 | 19 | full | TRANSFORMING GROWTH FACTOR, β RECEPTOR I (ACTIVIN A RECEPTOR TYPE II-LIKE KINASE, 53 KDA) | onc | |
| TH | IGF2 | 28 | 26 | 20 | 20 | 18 | full | TYROSINE HYDROXYLASE | nic |
| THSD4 | 594 | 558 | 498 | 428 | 364 | full | thrombospondin, type I, domain containing 4 | adh/inf | |
| TLR1 | TLR6 | 20 | 20 | 17 | 23 | 19 | full | Toll-like receptor 1 | inf |
| TLR10 | 35 | 30 | 24 | 21 | 17 | full | Toll-like receptor 10 | inf | |
| TLR2 | 23 | 20 | 20 | 19 | 19 | full | Toll-like receptor 2 | inf | |
| TLR4 | 58 | 56 | 43 | 50 | 38 | full | Toll-like receptor 4 | inf | |
| TLR5 | 14 | 12 | 12 | 0 | 0 | 13 pairwise HapTags | Toll-like receptor 5 | inf | |
| TLR6 | TLR1 | 32 | 12 | 9 | 9 | 6 | full | Toll-like receptor 6 | inf |
| TNF | LTA | 20 | 17 | 6 | 4 | 3 | full | tumor necrosis factor | inf |
| TNS1 | 154 | 151 | 133 | 135 | 114 | dropped for capacity | tensin 1 | ||
| TP53 | 19 | 17 | 17 | 15 | 15 | full | TUMOR PROTEIN P53 (LI-FRAUMENI SYNDROME) | onc | |
| TP53BP1 | 25 | 23 | 18 | 20 | 15 | full | tumor protein p53 binding protein 1 | onc | |
| TYMS | 31 | 28 | 26 | 24 | 22 | full | THYMIDYLATE SYNTHETASE | fol | |
| UGT1A1 | UGT1A8 | 70 | 58 | 46 | 53 | 37 | full | UDP glucuronosyltransferase 1 family, polypeptide A cluster | PAH |
| UGT1A8 | UGT1A1 | 151 | 113 | 78 | 79 | 53 | full | UDP GLUCURONOSYLTRANSFERASE 1 FAMILY, POLYPEPTIDE A8 | PAH |
| UGT2B10 | 21 | 10 | 9 | 9 | 8 | full | UDP GLUCURONOSYLTRANSFERASE 2 FAMILY, POLYPEPTIDE B10 | nit/PAH | |
| UGT2B11 | 18 | 10 | 9 | 10 | 9 | full | UDP GLUCURONOSYLTRANSFERASE 2 FAMILY, POLYPEPTIDE B11 | nit/PAH | |
| UGT2B15 | 1 | 0 | 0 | 0 | 0 | nine non-HapTag SNPs | UDP GLUCURONOSYLTRANSFERASE 2 FAMILY, POLYPEPTIDE B15 | nit/PAH | |
| UGT2B17 | 14 | 10 | 7 | 10 | 7 | full | UDP GLUCURONOSYLTRANSFERASE 2 FAMILY, POLYPEPTIDE B17 | nit/PAH | |
| UGT2B28 | 8 | 4 | 4 | 4 | 4 | full | UDP GLUCURONOSYLTRANSFERASE 2 FAMILY, POLYPEPTIDE B28 | nit/PAH | |
| UGT2B4 | 31 | 24 | 22 | 18 | 16 | full | UDP GLUCURONOSYLTRANSFERASE 2 FAMILY, POLYPEPTIDE B4 | nit/PAH | |
| UGT2B7 | 21 | 17 | 15 | 13 | 11 | full | UDP GLUCURONOSYLTRANSFERASE 2 FAMILY, POLYPEPTIDE B7 | PAH | |
| VCAM1 | 69 | 65 | 45 | 63 | 42 | full | vascular cell adhesion molecule 1 | adh/inf | |
| VEGFA | 45 | 45 | 36 | 43 | 33 | full | VASCULAR ENDOTHELIAL GROWTH FACTOR A | onc | |
| VEGFB | 15 | 15 | 15 | 12 | 12 | full | VASCULAR ENDOTHELIAL GROWTH FACTOR B | inf | |
| VEGFC | 73 | 71 | 63 | 54 | 45 | full | VASCULAR ENDOTHELIAL GROWTH FACTOR C | inf | |
| XIAP | 25 | 18 | 18 | 18 | 18 | full | BACULOVIRAL IAP REPEAT-CONTAINING 4 | onc | |
| XPA | 34 | 31 | 25 | 26 | 20 | full | XERODERMA PIGMENTOSUM, COMPLEMENTATION GROUP A | DNA | |
| XPC | 61 | 59 | 42 | 49 | 34 | full | XERODERMA PIGMENTOSUM, COMPLEMENTATION GROUP C | DNA | |
| XRCC1 | 47 | 46 | 30 | 42 | 27 | full | X-RAY REPAIR COMPLEMENTING DEFECTIVE REPAIR IN CHINESE HAMSTER CELLS 1 | DNA | |
| XRCC4 | 134 | 120 | 94 | 94 | 68 | full | X-ray repair complementing defective repair in Chinese hamster cells 4 | DNA | |
| Sum: | 17,797 | 15,961 | 13,474 | 12,926 | 10,511 | Count: | 298 |
adh, Adhesion molecules; DNA, repair of DNA damage; fol, folate transport and metabolism; inf, inflammatory signaling and processes or immune regulation; mut, mutagenic processes; nic, nicotine addiction and smoking behavior; nit, tobacco-specific nitrosamine (in particular, NNK) activation and detoxification; onc, oncogenesis; oxs, oxidative stress; str, steroid hormone metabolism and signaling; tox, other toxin or toxicity; tum, risk for lung cancer or related tumors.
Comparison of Pairwise and Multimarker Tagger Analyses
With the use of dbSNPs for the CEU and YRI populations, Tagger analysis was performed initially to predict marker HapTag SNPs that cover polymorphisms with minimum minor allele frequency (MAF) of 5% and then repeated for MAF >1%. Two Tagger algorithms were compared: pairwise modeling, in which a HapTag marker reports its own genotype and predicts the genotype of one linked SNP, and “aggressive” multimarker modeling, in which the combined genotypes of one to three HapTags report the local haplotype and predict the genotype(s) of one or more linked SNPs.13,15,16 The resulting number of HapTags calculated for each gene is shown in Table 1. At MAF >1%, pairwise modeling produced a g/h ratio of 1.92 (g=measured+predicted genotypes; h=HapTag markers), and multimarker modeling resulted in 2.38 g/h for the same number of genotypes.
Genotyping Array Design and Assay Performance
Tagger multimarker-predicted HapTags with MAF >1% were filtered for iSelect Infinium design scores ≥0.6. TLR5 had no multimarker HapTags, so pairwise HapTags were selected; CCR2, UGT2B15, and GSTT1 had no HapTags, so marker SNPs were manually identified. To avoid exceeding the marker capacity set by our microarray manufacturing budget, the low-priority genes, ALPL, TNS1, GAB1, HHIP, DBH, and PTGIS, were dropped, and HapTag coverage of GPR126 was reduced to 85%. With the addition of specifically targeted functional SNPs and published marker SNPs, 12,890 genomic SNPs were compiled for the final design of the LungCaGxE array with average and median intermarker distances of 5958 bp and 1093 bp, respectively. Sixty-one mitochondrial DNA SNPs were included to target MT-COI, as well as 357 ancestry informative markers for a total of 13,308 genotyping markers on the array. All markers and their sequences, coordinates, and targeted genes are provided in Supplemental Table 2.
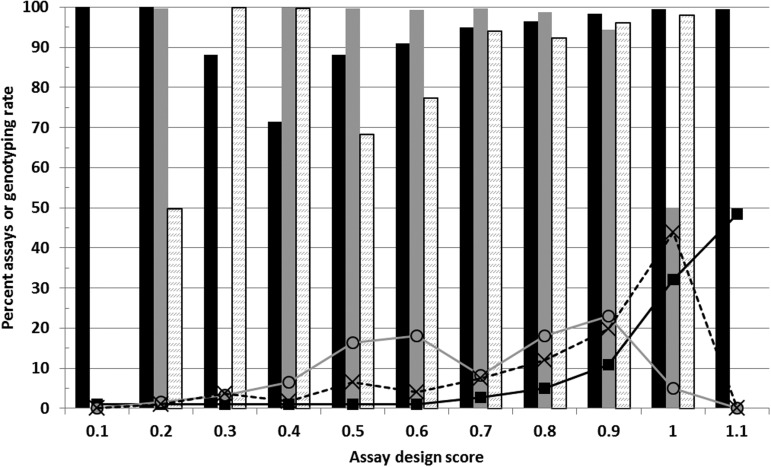
Genotyping assays were performed on 1873 DNA samples from lung-cancer patients and controls using LungCaGxE microarrays. Forty-seven samples had a SNP assay call rate <99.0%. If these samples are excluded, SNP assays with an Infinium design score of at least 0.6 produced unambiguous genotype calls in 99.03% of the attempted reactions (Fig. 1). Targeted functional SNPs with a design score <0.6 generated genotype calls in 84.96% of the attempted reactions; the average genotyping rate for SNPs with recognized rs numbers in the Illumina database was 99.09%, whereas the rate for SNPs submitted as custom sequences was 96.16% (design score >0.6 in both sets).
Figure 1.
Distribution of assay conversion rates for SNPs in various design categories. Assays were assigned to Infinium design score bins equal to or less than the indicated values. The percent of all assays in a bin that successfully generated genotypes (unambiguous SNP allele calls in at least 95% of DNA samples) is plotted for Infinium-eligible SNPs in the Illumina database (black bars), mitochondrial DNA SNPs (gray bars), and SNPs uploaded as custom sequences (dashed bars). The number of SNPs in each bin, as a percent of total SNPs in each category, is plotted with square line markers for Infinium database SNPs, circles for mitochondrial, and X for custom sequences.
DISCUSSION
The advancement of array-based SNP genotyping technologies has led to genome-wide association studies (GWAS), in which genetic markers distributed evenly throughout the genome17 (or covering predicted haplotypes throughout the genome14) are tested for statistically significant association with a phenotype. Arrays offer advantages for GWAS over current deep-sequencing methods, including lower cost, faster assay turnaround and sample throughput, and easier data processing. However, the success of proxy markers depends on linkage to causal but unmeasured genetic variants, and even the highest capacity arrays of over 5 million SNPs may not cover rare variants or diverse populations well. Whole-genome or exome sequencing directly detects causal variants and polymorphism types beyond bi-allelic single nucleotides and does not rely on linked markers for statistical analysis. Whether deployed on SNP arrays or deep sequencing platforms, the primary concern for whole-genome assays is statistical power. Rare variants, multiple causes for the same phenotype, intergenic and multigene effects, and genetically mandated differential interactions between genes and environmental variables can all combine with multiple testing correction requirements to drive study population sizes to thousands or tens of thousands of subjects to adequately power GWAS.18–21 Projects of this scale are an expensive proposition for arrays and would be extremely costly with deep sequencing even at the as-yet unattained goal of $1000/genome.
Comprehensive genotyping of targeted genes, by arrays or sequencing, takes advantage of high multiplex assay capacities to saturate targets with genetic markers. Hence, array data are less reliant on capturing a single, important linked marker while retaining rapid sample throughputs, and sequencing costs and efficiency are improved by focusing on a subset of genes rather than the whole genome. Depending on the size of the target panel and degree of saturation desired, custom arrays or sequencing can ease multiple testing penalties and reduce study population sizes necessary to achieve statistical power. Of course, the critical issue for this strategy is choosing which genes to assay. For the LungCaGxE panel, we chose genes involved in pathways relevant to responses to environmental stressors and saturated the resulting target panel with genetic markers as well as previously demonstrated functional and disease-associated variants.
The Illumina design score, whereas generally predictive of positive assay performance, underestimated the LungCaGxE genotype success rate achieved for Infinium-eligible tagSNPs and custom SNPs from the nuclear genome. The design scores were somewhat less positively predictive (i.e., further underestimated) of genotyping rates achieved for mitochondrial genome SNPs, which performed well over a wide range of design scores. The relatively high success rates for assays with design scores <0.6 indicate that for future targeted genotyping projects, failure to meet this overly stringent standard cutoff should not necessarily disqualify an assay if the specific SNP in question is important for the study goals.
In summary, the investigator tasked with designing a custom-targeted genotyping assay must balance several considerations. Given that the platform's multiplex capacity is often dictated by the project's budget, the investigator must select the marker types, thresholds for number of genes targeted, and MAF cutoffs that will provide the most efficient use of available assay resources. Several iterations of empirical design are usually needed to assess the impact of these parameters, and this process is aided by a streamlined bioinformatics workflow. Tagger Batch Assistant helps automate the retrieval of genetic coordinates for requested genes, managing genome build versions and providing an output format that easily interfaces with Tagger for marker prediction. The resulting Tagger files are then automatically processed to connect markers with the user's upstream gene annotations. We used this tool to optimize the LungCaGxE design through multiple versions, preserving sensitivity for marker MAFs as low as 1%, while reducing the number of SNPs required by using the Tagger multimarker haplotyping algorithm. This array enables rapid, cost-effective, and comprehensive genotyping of a panel of genes important for exploring genetic factors in lung cancer and the environmental influences that impact those factors.
ACKNOWLEDGMENTS
This work was funded by grant PA4100038714 from the Pennsylvania Department of Health and U.S. National Institutes of Health–National Institute of Environmental Health Sciences grant 5 P30 ES 013508-06 for the Center of Excellence in Environmental Toxicology. We thank David McGain and Kathakali Addya (Penn Molecular Profiling Facility) and Cecilia Kim (Children's Hospital of Philadelphia Center for Applied Genomics) for technical assistance.
DISCLOSURE
The authors have no associations or sources of financial support that pose a conflict of interest for conducting or interpreting the work presented in this manuscript.
Supplemental Data Section
REFERENCES
- 1. American Cancer Society Cancer Facts & Figures 2013. Atlanta, GA, USA: American Cancer Society, 2013. (http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845.pdf) [Google Scholar]
- 2. Cassidy A, Duffy SW, Myles JP, Liloglou T, Field JK. Lung cancer risk prediction: a tool for early detection. Int J Cancer 2007;120:1–6 [DOI] [PubMed] [Google Scholar]
- 3. Ihsan R, Chauhan PS, Mishra AK, et al. Multiple analytical approaches reveal distinct gene-environment interactions in smokers and non-smokers in lung cancer. PLoS One 2011;6:e29431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thomas L, Doyle LA, Edelman MJ. Lung cancer in women: emerging differences in epidemiology, biology, and therapy. Chest 2005;128:370–381 [DOI] [PubMed] [Google Scholar]
- 5. Braithwaite KL, Rabbitts PH. Multi-step evolution of lung cancer. Semin Cancer Biol 1999;9:255–265 [DOI] [PubMed] [Google Scholar]
- 6. Bach PB, Kattan MW, Thornquist MD, et al. Variations in lung cancer risk among smokers. J Natl Cancer Inst 2003;95:470–478 [DOI] [PubMed] [Google Scholar]
- 7. Bilello KS, Murin S, Matthay RA. Epidemiology, etiology, and prevention of lung cancer. Clin Chest Med 2002;23:1–25 [DOI] [PubMed] [Google Scholar]
- 8. Liu G, Zhou W, Christiani DC. Molecular epidemiology of non-small cell lung cancer. Semin Respir Crit Care Med 2005;26:265–272 [DOI] [PubMed] [Google Scholar]
- 9. Taioli E. Gene-environment interaction in tobacco-related cancers. Carcinogenesis 2008;29:1467–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gustafson AM, Soldi R, Anderlind C, et al. Airway PI3K pathway activation is an early and reversible event in lung cancer development. Sci Transl Med 2010;2:26ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2009;4:44–57 [DOI] [PubMed] [Google Scholar]
- 12. Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 2009;37:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Bakker PI, Yelensky R, Pe'er I, Gabriel SB, Daly MJ, Altshuler D. Efficiency and power in genetic association studies. Nat Genet 2005;37:1217–1223 [DOI] [PubMed] [Google Scholar]
- 14. Peiffer DA, Le JM, Steemers FJ, et al. High-resolution genomic profiling of chromosomal aberrations using Infinium whole-genome genotyping. Genome Res 2006;16:1136–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goode EL, Fridley BL, Sun Z, et al. Comparison of tagging single-nucleotide polymorphism methods in association analyses. BMC Proc 2007;1(Suppl 1):S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nam MH, Won HH, Lee KA, Kim JW. Effectiveness of in silico tagSNP selection methods: virtual analysis of the genotypes of pharmacogenetic genes. Pharmacogenomics 2007;8:1347–1357 [DOI] [PubMed] [Google Scholar]
- 17. Matsuzaki H, Dong S, Loi H, et al. Genotyping over 100,000 SNPs on a pair of oligonucleotide arrays. Nat Methods 2004;1:109–111 [DOI] [PubMed] [Google Scholar]
- 18. Becker T, Herold C, Meesters C, Mattheisen M, Baur MP. Significance levels in genome-wide interaction analysis (GWIA). Ann Hum Genet 2011;75:29–35 [DOI] [PubMed] [Google Scholar]
- 19. Park JH, Wacholder S, Gail MH, et al. Estimation of effect size distribution from genome-wide association studies and implications for future discoveries. Nat Genet 2010;42:570–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sale MM, Mychaleckyj JC, Chen WM. Planning and executing a genome wide association study (GWAS). Methods Mol Biol 2009;590:403–418 [DOI] [PubMed] [Google Scholar]
- 21. Spencer CC, Su Z, Donnelly P, Marchini J. Designing genome-wide association studies: sample size, power, imputation, and the choice of genotyping chip. PLoS Genet 2009;5:e1000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.