Abstract
Aims
Left ventricular (LV) (dys)synchrony has an important impact on LV function and structure. Our study aimed to describe the distribution and determinants of LV mechanical delay indexes in the general population and to assess an association of different Doppler indexes reflecting LV diastolic function with LV mechanical delay indexes.
Methods and results
In 200 subjects enrolled in a family-based population study (46.5% women; mean age, 57.9; 48% hypertensive), we performed echocardiography with tissue synchronization imaging (TSI) and two-dimensional speckle tracking. We measured the maximum difference in time to peak systolic velocity between any 2 of 12 LV segments (Ts-max); the standard deviation of time to peak systolic velocity of 12 segments (Ts-sd); the difference in time to peak systolic velocity and strain between septal and lateral LV walls and the strain delay index in septal and lateral walls [septal and lateral (SDI)]. In univariable and multivariable regression analyses, TSI indexes and lateral SDI independently increased with age (P ≤ 0.027) and body mass index (P ≤ 0.010). Ts-max and Ts-sd also increased with female sex (P ≤ 0.0002) and decreased with heart rate (P ≤ 0.0004). Septal SDI only increased with female sex (P < 0.0001). Among the Doppler indexes of LV diastolic function, only E/e′ was significantly and positively associated with TSI indexes (P ≤ 0.037) and lateral SDI (P = 0.0026), but not with septal SDI (P = 0.69). In participants with advanced stage of LV diastolic dysfunction, TSI indexes were prolonged compare with subjects with normal LV diastolic function (P ≤ 0.002).
Conclusion
We demonstrated that in unselected subjects LV diastolic dysfunction was associated with mechanical LV dyssynchrony as assessed by echocardiography.
Keywords: Population, Left ventricular dyssynchrony, Tissue synchronization imaging, Strain, Diastolic dysfunction
Introduction
Left ventricular (LV) synchrony has an important impact on LV performance.1 Delayed electrical activation and/or impaired excitation–contraction coupling result in a dispersion of regional mechanical activation known as intraventricular dyssynchrony, which has a negative influence on cardiac performance.1 Intraventricular dyssynchrony can be assessed using different echocardiographic techniques, such as M-mode and tissue Doppler imaging (TDI) and two-dimensional (2D) speckle tracking.2 Newer echocardiographic techniques, such as tissue synchronization imaging (TSI) and 2D speckle tracking, allow analysing peak systolic velocity and strain delays of various LV walls and, therefore, provide estimates of LV mechanical dyssynchrony. However, by using these wall motion echocardiographic indexes, one cannot define whether LV mechanical dyssynchrony was caused by a delay in electrical activation or by the heterogeneity of the contractile properties of the LV wall. To our knowledge, no study described the distribution and determinants of the echocardiographic indexes reflecting LV mechanical dyssynchrony in the general population. Furthermore, the prevalence of LV dyssynchrony, as defined by echocardiography, in patients with symptomatic diastolic heart failure (HF) is as high as 18–45%.3,4 However, the prevalence of LV mechanical dyssynchrony in subjects with different stages of subclinical diastolic dysfunction is currently unknown. The objectives of the present research were, therefore, to describe the distributions and determinants of the echocardiographic indexes of LV mechanical dyssynchrony in a general population and to investigate the association of Doppler indexes of LV diastolic function with LV mechanical dyssynchrony.
Methods
Study participants
This study is nested in the Flemish Study on Environment, Genes and Health Outcomes (FLEMENGHO),5 a large family-based population resource on the genetic epidemiology of cardiovascular phenotypes, for which recruitment started in 1985 and continued through 2010. The selection criteria and study design are detailed in the Supplementary data online, Methods. From October 2010 to November 2011, 225 former FLEMENGHO participants were re-invited for a follow-up examination at the field centre, including echocardiography with TSI (participation rate, 81.3%).
We excluded seven subjects from analysis, because of atrial fibrillation (n = 5) or pacemaker (n = 2). We excluded a further 18 subjects, because the TSI or 2D echocardiogram was of insufficient quality. Thus, the number of participants statistically analysed totalled 200.
Echocardiography
Echocardiographic methods are detailed in the Supplementary data online, Methods. Echocardiographic examinations were performed by an experienced physician (T.K.) as previously described5,6 by using a Vivid E9 (GE Vingmed, Horten, Norway) ultrasound scanner, interfaced with a 2.5- to 3.5-MHz-phased array and a 3 V probes. The observer obtained standard 2D images along the parasternal long- and short-axis and from the apical four-chamber, two-chamber, and long-axis views, while simultaneously ECG was recorded. All the recordings lasted at least five cardiac cycles and were digitally stored for offline analysis. One experienced observer (T.K.) analysed the conventional echocardiographic images, averaging three heart cycles for statistical analysis, using a work station running the EchoPac software package, version BT11.0.0 (GE Vingmed).
Transmitral blood flow Doppler signals were used to measure peak early (E) and late (A) diastolic velocities, and A flow duration. The duration of the pulmonary vein (PV) reversal flow during atrial systole (AR) was measured from the PV flow signal. From the pulsed wave TDI recordings, we measured the early (e′) and late (a’) peak diastolic velocities of the mitral annulus displacement, and the e′/a‘ ratio at the four acquisition sites (septal, lateral, inferior, and posterior). We calculated the E/e′ ratio by dividing transmitral E peak by e′ averaged from the four acquisition sites.
We combined the mitral inflow and TDI velocities to classify the stages of LV diastolic dysfunction (LVDD), as previously described.5 Briefly, the first group of LVDD included subjects with an abnormally low age-specific E/A ratio indicative of impaired relaxation, but without evidence of increased LV filling pressures (E/e′ ≤ 8.5). The second group had mildly-to-moderately elevated LV filling pressure (E/e′ > 8.5), and the E/A ratio within the normal age-specific range. We used the differences in durations between the mitral A flow and the reverse PV flow during AR (Ad < ARd + 10) and/or left atrial volume index (≥28 mL/m2) to confirm possible elevation of the LV filling pressures in Group 2. Group 3 had an elevated E/e′ ratio (≥8.5) and an abnormally low age-specific E/A ratio.5
Offline analysis of multi-dimensional TDI
Digitally stored multi-dimensional tissue Doppler myocardial motion images were post-processed in duplicate by two observers (T.K. and P.B.). On the colour-coded tissue Doppler images, sample volumes were placed in 12 regions of six LV walls (septal, lateral, inferior, anterior, posterior, and anteroseptal segments of the basal and mid-portion of the LV wall). Myocardial velocity curves were generated using the TSI algorithm as implemented in EchoPac BT11. This algorithm determined automatically the time from the onset of the QRS complex to peak myocardial systolic velocities of each LV segment (see Supplementary data online, Figure S1). The time to peak velocity in 12 LV wall segments was measured simultaneously from the same cardiac cycle. The following TSI indexes of mechanical dyssynchrony were computed using the TSI analysis software: septal-lateral (Ts-sl) delay, maximal difference (Ts-max), and standard deviation (Ts-sd) of 12 time to peak myocardial systolic velocities.2
2D LV strain by speckle tracking
For the measurement of 2D strain, the endocardial borders were manually traced at the end-systolic frame of the standard greyscale 2D images from the three long-axis views. The 2D strain software (Q-analysis, GE Vingmed, Horten, Norway) automatically tracks myocardial speckle motion throughout the entire cardiac cycle, creating basal-, mid-, and apical regions of interest. The distance between speckles was measured as a function of time, and myocardial (deformation) strain was calculated. From the strain curves obtained in four segments of septal and lateral LV walls, indexes of LV mechanical dyssynchrony were derived. We calculated time delay between peak systolic strain of septal and lateral segments (Tε-sl) as well as the post-systolic strain delay index (SDI) as (peak-systolic strain – end-systolic strain)/end systolic strain × 100 for each LV segment strain curve.7
Inter-observer variability of TSI indexes and 2D strain
To determine inter-observer variability, time to peak systolic velocities were measured in duplicates by two observers in 23 subjects. The inter-observer variability was estimated using the Bland and Altman’s approach. The absolute bias was calculated as the mean difference between the repeated readings (x1 − x2) and was plotted against the average value of these readings (x1 + x2). The relative bias was calculated by taking the percentage of the pairwise difference between repeated readings (100 × (x1−x2)/averaged) and was plotted against the average value of repeated readings (x1 + x2). The absolute and relative differences between two observers for time to peak systolic velocity measured were 0.85 ms [95% limits of agreement (LA): −23.2–25.2 ms] and 1.06% (95% LA: −18.4 to 20.5%), respectively. The absolute and relative differences for 2D strain were −0.30% (95% LA: −1.72 to 2.32%) and −1.42% (95% LA: −11.7 to 8.87%), respectively.
Other measurements
Detailed information on other measurements is given in the Supplementary data online, Methods.
Statistical methods
For database management and statistical analysis, we used SAS software version 9.3 (SAS Institute, Car, NC, USA). Normality was evaluated by Kolmogorov–Smirnov’s statistic and skewness and kurtosis by the computation of their coefficients. The central tendency and the spread of the data are reported as mean ± SD or as median and 10–90% percentiles. We compared means and proportion by means of t-test and χ2 statistics, respectively. Significance was P < 0.05 on the two-sided test. We searched for variables associated with the LV mechanical dyssynchrony indexes (TSI and 2D strain) measured on a continuous scale using stepwise linear regression. P-values for independent variables to enter and to stay in the models were set at 0.15. The anthropometrics and haemodynamic variables considered for entry in the models were sex, age, body mass index, heart rate, and systolic and diastolic blood pressures. We also searched for variables associated with SDI using stepwise logistic regression. We ran regression diagnostics to exclude possible collinearity, which could influence the multivariable models. We tested the association between Doppler indexes reflecting diastolic function, such as E/e′ and LV mechanical dyssynchrony measures using mixed models. The multivariable-adjusted effect size was expressed for a 10 ms increase in the explanatory variables (TSI indexes).
Results
Characteristics of the participants
The 200 participants included 93 (46.5%) women and 96 (48.0%) hypertensive patients. The mean age (±SD) was 57.9 ± 14.2 years. In our study, 57 (28.5%) participants were on one or more antihypertensive drugs (beta-blockers, 36 patients; diuretics, 24 patients; calcium channel blockers, 10 patients; and ACE-inhibitors or angiotensin receptor blockers, 16 patients). Table 1 lists the clinical, electrocardiographic, and echocardiographic characteristics of the participants by sex. Women compared with men had a shorter QRS duration and smaller Cornell product, LA volume index, relative wall thickness, and LV mass index, whereas transmitral E peak were significantly lower in men (Table 1). Overall, 31 subjects (15.5%) had LV hypertrophy; 8 women (4.0%) and 23 men (11.5%). The echocardiographic measurements reflecting systolic function (Table 1) were greater in women than in men. Overall, 15 subjects (7.5%) had overt cardiac diseases, such as myocardial infarction or coronary revascularization (n = 7), or mild-to-moderate valvular abnormalities (n = 7), or EF of <50% (n = 1). The prevalence of any degree of LVDD was 32% (64 participants). The number of subjects in LVDD Groups 1, 2, and 3 were 24 (12.0%), 26 (13.0%), and 14 (7.0%), respectively (Table 1).
Table 1.
Clinical and echocardiographic characteristics of participants
| Characteristic | Men (n = 107) | Women (n = 93) |
|---|---|---|
| Anthropometrics | ||
| Age, y | 57.9±14.7 | 57.8±13.7 |
| Height, cm | 173.5±7.4 | 162.5±7.3*** |
| Weight, kg | 83.2±11.6 | 68.9±12.9*** |
| Body mass index, kg/m2 | 27.7±3.7 | 26.1±4.6** |
| Systolic pressure, mmHg | 133.8±15.5 | 129.7±19.3 |
| Diastolic pressure, mmHg | 84.2±9.0 | 80.0±8.5*** |
| Heart rate, bpm | 58.3±10.3 | 62.6±9.2** |
| Questionnaire data | ||
| Current smoking, n (%) | 12 (11.2) | 20 (21.5)* |
| Drinking alcohol, n (%) | 60 (56.1) | 22 (23.7)*** |
| Hypertensive, n (%) | 57 (53.3) | 39 (41.9) |
| Treated for hypertension, n (%) | 29 (27.1) | 28 (30.0) |
| Cardiac disease, n (%) | 10 (9.4) | 5 (5.4) |
| Electrocardiography | ||
| QRS duration, ms | 96.7±16.3 | 86.1±9.7*** |
| QTc, ms | 401±25.8 | 411±21.2** |
| Cornell product, mv × ms | 1133±731 | 877±450*** |
| Diastolic function | ||
| Normal function, n (%) | 69 (64.5) | 67 (72.0) |
| Group 1, n (%) | 14 (13.0) | 10 (10.8) |
| Group 2, n (%) | 16 (15.0) | 10 (10.7) |
| Group 3, n (%) | 8 (7.5) | 6 (6.5) |
| Conventional echocardiography | ||
| Left atrium volume index, mL/m2 | 28.4±7.3 | 24.6±7.6*** |
| Relative wall thickness | 0.40±0.06 | 0.37±0.05** |
| LV mass index, g/m2 | 104.5±22.6 | 85.7±17.0*** |
| Ejection fraction, % | 61.7±6.4 | 64.2±5.9** |
| Doppler data | ||
| E peak, cm/s | 63.6±14.6 | 69.7±15.5** |
| A peak, cm/s | 61.4±15.5 | 65.4±16.0 |
| E/A ratio | 1.12±0.46 | 1.15±0.47 |
| s’ peaka, cm/s | 8.27±1.29 | 7.85±1.61 |
| e′ peaka, cm/s | 9.44±3.54 | 9.91±3.62 |
| a’ peaka, cm/s | 10.3±2.4 | 9.58±2.44* |
| e′/a‘ ratioa | 1.03±0.64 | 1.16±0.65 |
| E/e′ ratioa | 7.26±1.99 | 7.71±2.87 |
| 2D Strain | ||
| End systolic septal, % | −18.2±2.77 | −19.6±2.88*** |
| Peak systolic septal, % | −18.7±2.68 | −20.4±2.80*** |
| End systolic lateral, % | −19.1±3.60 | −20.7±3.58** |
| Peak systolic lateral, % | −19.5±3.50 | −21.2±3.32** |
LV, left ventricle. Group 1, impaired relaxation or low E/A and normal E/e′; Group 2, possible elevated end-diastolic pressure or normal E/A and high E/e′; Group 3, combined dysfunction or low E/A and high E/e′.
Values are mean (±SD) or number of subjects (%).
TDI velocities were averaged of septum, lateral, inferior, and posterior mitral annulus sites.
Significance of the sex difference: P ≤ 0.05,
P ≤ 0.01,
P ≤ 0.001.
LV mechanical delay indexes
In all subjects, the distributions of the TSI indexes were positively skewed (P < 0.01) with the coefficients of skewness ranging between 0.48 and 0.79 (see Supplementary data online, Figure S2). In the entire population, Ts-max and Ts-sd averaged 75.3 ms (10–90% percentile, 22.3–135.5 ms) and 27.5 ms (6.52–54.1 ms), respectively. The opposing wall delay index (Ts-sl) averaged 5.24 ms (−73.2–97.9 ms). The distribution of Tε-sl was normal with a mean of −0.70 ms (280–80 ms) (P > 0.15; coefficient of skewness 0.21; see Supplementary data online, Figure S2). Table 2 lists the means and medians of the LV mechanical dyssynchrony indexes by sex. Ts-max and Ts-sd were significantly higher in women than in men (Table 2).
Table 2.
LV mechanical delay indexes of participants
| LV mechanical delay indexes | Men (n = 107) |
Woman (n = 93) |
||
|---|---|---|---|---|
| Mean (SD) | Median (10–90%) | Mean (SD) | Median (10–90%) | |
| TSI indexes | ||||
| Ts-max, ms | 69.0 (40.6) | 62.0 (22.2 to 127.1) | 82.6 (45.0) | 83.1* (23.3 to 139.3) |
| Ts-sd, ms | 24.5 (16.3) | 20.7 (6.52 to 47.6) | 30.8 (18.8) | 30.7* (6.36 to 56.2) |
| Ts-sl, ms | 5.28 (53.7) | −2.93 (−58.7 to 91.6) | 5.19 (67.5) | 0.0 (−82.2 to 108.8) |
| 2D Strain | ||||
| Tε-sl, ms | 4.77 (64.0) | 8.25 (−66.7 to 96.2) | −6.71 (59.0) | −9.0 (−89.0 to 71.5) |
Ts-max, maximal difference in time to peak myocardial systolic velocity between any 2 of 12 LV segments; Ts-sd, standard deviation of time to peak myocardial systolic velocity of all 12 LV segments; Ts-sl, difference in time to peak myocardial velocity between septal and lateral LV walls; Tε-sl, difference in time to peak strain between septal and lateral LV walls.
Values are mean (±SD) and median (10–90% percentiles).
Significance of the sex difference: P ≤ 0.05.
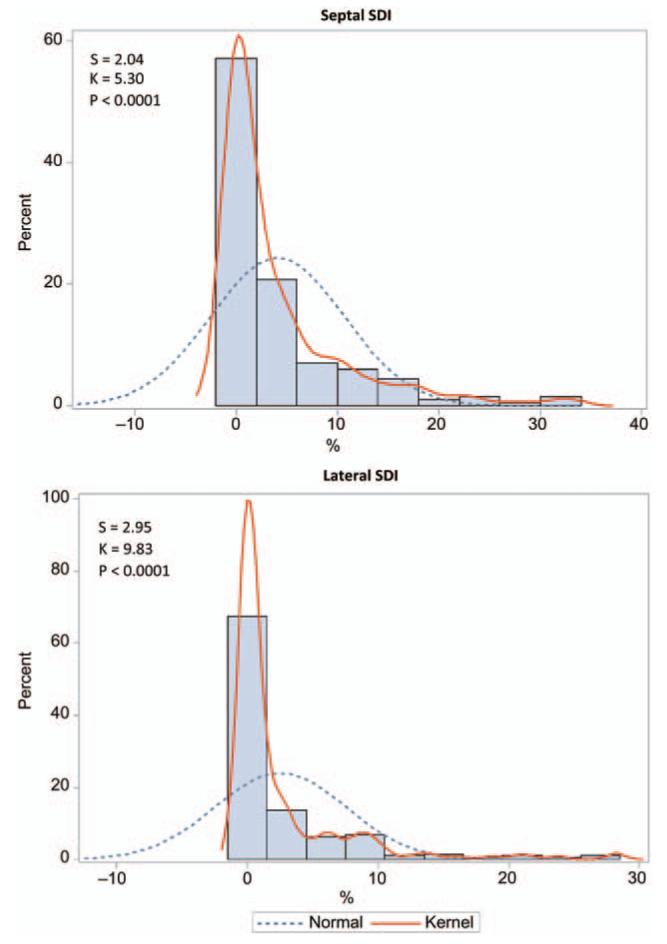
Figure 1 shows the distributions of the septal and lateral SDI. In the entire cohort, 43.5% (n = 87) and 51.5% (n = 103) of the participants did not have any post-systolic septal and lateral strain delays (0%), respectively. Therefore, in further statistical analyses, we treated SDI as a categorical variable and divided our study participants into groups with or without any post-systolic septal or lateral strain delays. Table 3 lists the clinical characteristics of the participants by the septal and lateral strain delay groups. Considering the septal site, the strain delay was associated with female sex (P < 0.0001). Otherwise, there were no other significant differences in the clinical characteristics between subjects with and without a septal strain delay. Considering the lateral site, participants with a lateral strain delay were older, had a higher body mass index (BMI), more often treated with antihypertensive medications, and had a higher prevalence of LV diastolic dysfunction indicative of elevated LV filling pressure (Table 3).
Figure 1.
Frequency distributions of septal (A) and lateral (B) strain delay indexes (SDI). S and K are the coefficients of skewness and kurtosis, respectively; the P-value is for departure of the actually observed distribution (Kernel distribution; full line) from normality (dotted line).
Table 3.
Clinical characteristics of participants by strain delay groups
| Characteristic | Septal SDI |
Lateral SDI |
||||
|---|---|---|---|---|---|---|
| No delay (n = 87) |
Any delay (n = 113) |
P-value | No delay (n = 103) |
Any delay (n = 97) |
P-value | |
| Anthropometrics | ||||||
| Women, n (%) | 25 (29.4) | 67 (59.3) | <0.0001 | 41 (43.6) | 50 (51.5) | 0.27 |
| Age, years | 58.3±15.0 | 57.7±13.6 | 0.77 | 54.3±13.0 | 61.3±14.5 | 0.0006 |
| Body mass index, kg/m2 | 27.1±4.02 | 26.8±4.34 | 0.70 | 26.0±3.67 | 27.7±4.56 | 0.005 |
| Systolic pressure, mmHg | 134.2±17.4 | 130.4±17.4 | 0.13 | 129.6±15.8 | 133.5±17.6 | 0.11 |
| Diastolic pressure, mmHg | 82.8±9.46 | 81.8±8.70 | 0.43 | 82.2±8.67 | 82.3±9.22 | 0.92 |
| Heart rate, bpm | 59.4±10.7 | 60.8±9.51 | 0.33 | 61.0±10.4 | 59.4±9.41 | 0.26 |
| Questionnaire data | ||||||
| Hypertensive, n (%) | 46 (52.9) | 50 (44.2) | 0.17 | 39 (37.9) | 53 (54.6) | 0.07 |
| Treated for hypertension, n (%) | 27 (31.0) | 30 (26.5) | 0.42 | 18 (17.5) | 36 (37.1) | 0.006 |
| Cardiac disease, n (%) | 6 (6.9) | 9 (7.96) | 0.83 | 4 (3.9) | 10 (10.3) | 0.11 |
| Diastolic function | ||||||
| Normal function, n (%) | 61 (70.1) | 75 (66.4) | 0.66 | 78 (75.7) | 58 (59.8) | 0.08 |
| Group 1, n (%) | 6 (6.9) | 18 (15.9) | 0.08 | 15 (14.6) | 9 (9.3) | 0.56 |
| Group 2, n (%) | 10 (11.5) | 16 (14.2) | 0.60 | 7 (6.8) | 19 (19.6) | 0.017 |
| Group 3, n (%) | 10 (11.5) | 4 (3.5) | 0.06 | 3 (2.9) | 11 (11.3) | 0.020 |
Values are mean (±SD), or number of subjects (%). SDI, strain delay index.
Covariables of LV mechanical delay indexes
In univariable and multivariable stepwise regression analyses, Ts-max and Ts-sd derived from 12 LV segments independently and significantly increased with female sex, age, BMI, and decreased with heart rate (P < 0.05, Table 4 and see Supplementary data online, Figure S3). The measurements of peak systolic velocity and strain delays in opposing walls (Ts-sl and Tε-sl) significantly and independently increased with age and BMI (P 0.05, Table 4 and see Supplementary data online, Figure S3). Age accounted for most of the explained variances in Ts-sl and Tε-sl (14.7 and 4.15%, respectively). The selected anthropometric and haemodynamic covariables explained from 17.9 to 23.5% of the total variance for TSI indexes. The explained variance for Tε-sl was only 6.2%.
Table 4.
Determinants of the LV mechanical delay indexes in stepwise regression
| Parameter | Ts-max, ms | Ts-sd, ms | Ts-sl, ms | Tε-sl, ms |
|---|---|---|---|---|
| R2 (%) | 17.9 | 19.3 | 23.5 | 6.23 |
| Adjusted R2 (%) | 16.2 | 17.6 | 22.7 | 5.23 |
| Partial regression coefficients | ||||
| Female (0, 1) | ||||
| β±SE | 22.6±5.87 | 10.21±2.39 | — | — |
| Partial r2 (%) | 4.19 | 5.18 | ||
| P-value | 0.0002 | <0.0001 | ||
| Age (+10 years) | ||||
| β±SE | 4.58±2.03 | 1.84±0.83 | 13.8±2.74 | 0.76±0.31 |
| Partial r2 (%) | 2.15 | 2.06 | 14.7 | 4.15 |
| P-value | 0.025 | 0.027 | <0.0001 | 0.047 |
| BMI (+1 kg/m2) | ||||
| β±SE | 2.79±0.70 | 1.21±0.29 | 4.30±0.92 | 2.15±1.05 |
| Partial r2 (%) | 5.54 | 6.02 | 8.71 | 2.08 |
| P-value | <0.0001 | <0.0001 | <0.0001 | 0.043 |
| HR (+10 bpm) | ||||
| β±SE | −10.5±2.9 | −4.29±1.18 | — | — |
| Partial r2 (%) | 6.03 | 5.99 | ||
| P-value | 0.0004 | 0.0003 |
Values are mutually adjusted partial regression coefficients ±SE. BMI, body mass index; HR, heart rate.
Variance inflation factor (VIF) did not exceed 1.12. Other abbreviations are listed in Table 2.
We tested in the multivariable-adjusted logistic regression models the odds of having septal or lateral strain delay. Higher age (OR: 1.032; P = 0.006) and BMI (OR: 1.11; P = 0.010) were significantly and independently associated with a higher risk of lateral strain delay. On the other hand, the odds of having a septal strain delay were higher in women than men (P < 0.0001), but not associated with any other covariables.
Association between Doppler diastolic function indexes and LV mechanical delay indexes
While accounting for sex, age, BMI, heart rate, pulse pressure, and antihypertensive drug treatment, we investigated the association of various Doppler indexes reflecting LV diastolic function with LV mechanical delay indexes. Significant positive correlations of the E/e′ ratio with all LV mechanical delay indexes were observed (Table 5). For a 10 ms increase in Ts-max and Ts-sd, the E/e′ ratio increased by 0.12 (P = 0.0005) and 0.26 (P = 0.002), respectively. The multivariable-adjusted associations between the E/e′ ratio and the opposing wall delay indexes (Ts-sl and Tε-sl) were weaker (P ≤ 0.037), but trends were similar (Table 5).
Table 5.
Multivariable-adjusted association between E/e′, its components and TSI indexes
| LV mechanical delay indexes |
Effect size |
95% CI | P-value |
|---|---|---|---|
| E/e′ Ratio | |||
| Ts-max, ms | 0.12 | 0.051 to 0.182 | 0.0005 |
| Ts-sd, ms | 0.26 | 0.094 to 0.42 | 0.0020 |
| Ts-sl, ms | 0.054 | 0.0034 to 0.10 | 0.037 |
| Tε-sl, ms | 0.064 | 0.020 to 0.11 | 0.0045 |
| Transmitral E velocity | |||
| Ts-max, ms | 0.34 | −0.090 to 0.76 | 0.12 |
| Ts-sd, ms | 0.79 | −0.25 to 1.83 | 0.14 |
| Ts-sl, ms | 0.21 | −0.11 to 0.53 | 0.20 |
| Tε-sl, ms | 0.25 | −0.030 to 0.53 | 0.080 |
| TDI e′ velocity | |||
| Ts-max, ms | −0.050 | −0.12 to 0.0016 | 0.13 |
| Ts-sd, ms | −0.10 | −0.27 to 0.063 | 0.22 |
| Ts-sl, ms | −0.013 | −0.064 to 0.038 | 0.61 |
| Tε-sl, ms | −0.0051 | −0.050 to 0.040 | 0.82 |
The effect sizes are expressed for a 10 ms increase in the explanatory variables. Estimates for E/e′, E and e′ are adjusted for sex, age, body mass index, heart rate, pulse pressure and anti-hypertensive treatment. Abbreviations are listed in Table 2.
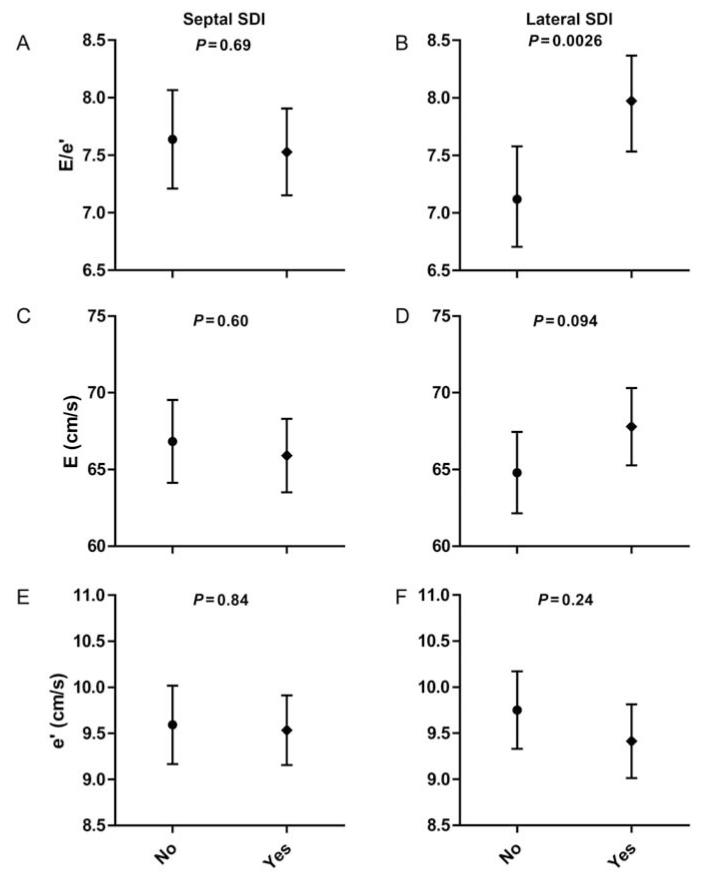
Figure 2 shows the adjusted E/e′ ratio and its components in the septal and lateral strain delay subgroups. The adjusted E/e′ ratio did not differ between subjects with and without a septal strain delay (P = 0.69), but was significantly higher in participants with a lateral strain delay compared with those without any lateral delay (7.97 vs. 7.12; P = 0.0026; Figure 2).
Figure 2.
An adjusted E/e′ ratio (A and B) and its components (transmitral E velocity, C and D; TDI e′ velocity, E and F) in subjects with and without the septal strain delay (left panel) or lateral strain delay (right panel). P-values are for difference between groups. E/e′ and components are adjusted for age, sex, body mass index, heart rate, pulse pressure, and antihypertensive drug treatment. SDI, strain delay index.
We did not observe any significant associations of LV mechanical delay indexes with other echocardiographic indexes of diastolic function, such as the transmitral E/A ratio or deceleration time (P ≥ 0.064; see Supplementary data online, Table S1 and Figure S4).
In additional sensitivity analyses, we excluded 15 subjects with overt cardiac diseases. As shown in Supplementary data online, Table S2 and Figure S5, our findings remained consistent. For a 10 ms increase in Ts-max, Ts-sd, and Tε-sl, the E/e′ ratio increased by 0.11 (P = 0.0002), 0.24 (P = 0.005), and 0.060 (P = 0.01), respectively (see Supplementary data online, Table S2). Furthermore, only lateral SDI was positively associated with the E/e′ ratio (P = 0.003; see Supplementary data online, Figure S4).
LV mechanical delay indexes in diastolic dysfunction groups
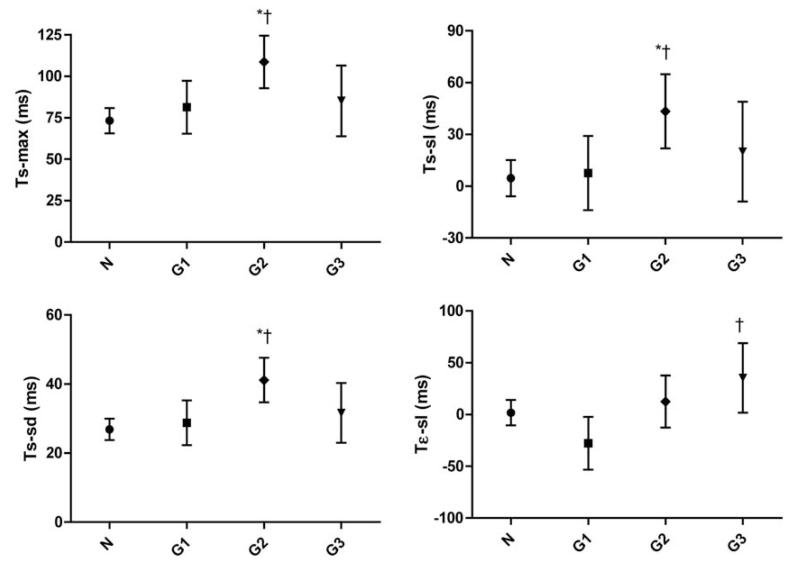
Figure 3 shows the adjusted LV mechanical delay indexes by diastolic function groups. Although accounting for covariables such as sex, age, BMI, heart rate, pulse pressure, and antihypertensive drug treatment, all the TSI indexes were significantly prolonged in diastolic dysfunction Group 2 (elevated filling pressure, n = 26) when compared with participants with normal diastolic function (n = 136; P ≤ 0.002) or with impaired relaxation (n = 24; P ≤ 0.02) (Figure 3). Finally, Tε-sl was also significantly different between subjects in Groups 1 and 2 (P = 0.03) and Groups 1 and 3 (combined dysfunction; n = 14) (−28 vs. 35 ms, P = 0.0024).
Figure 3.
Adjusted LV mechanical delay indexes by diastolic function groups. N, normal diastolic function, n = 136; G1, Group 1 (impaired relaxation), n = 24; G2, Group 2 (elevated end-diastolic pressure), n = 26; G3, Group 3 (combined dysfunction), n = 14. Significance between groups: *P < 0.05 between normal function and diastolic dysfunction groups; †P < 0.05 between diastolic dysfunction Group 1 and other diastolic function groups; LV, left ventricle. Abbreviations are listed in Table 2.
Discussion
The key finding of this study was that LV diastolic dysfunction was directly associated with LV mechanical systolic dyssynchrony as assessed by echocardiography in 200 participants randomly selected from the general population. In this population study, for the first time, we also described the distributions and determinants of LV mechanical delay indexes as measured by TSI and 2D speckle tracking. These indexes commonly used in the literature2 reflect mainly disparity in LV walls motion (either deformation or velocity) during the systole.
The population mean of TSI indexes reported in our study is in line with previous reports in selected healthy subjects.3,8 For instance, Conca et al.8 analysed LV mechanical synchronicity using different TSI indexes in 160 healthy volunteers (mean age 45.2) recruited at two institutions. Mean values ranged from 60.8 to 88.3 ms for Ts-max, from 33.1 ms to 43.9 ms for Ts-sd, and from 15.5 to −5.54 ms for Ts-sl. Yu et al.3 reported mean values of 54 ms for Ts-max and 17.6 ms for Ts-sd in a healthy control group (n = 100; mean age = 64.2). None of the previously published studies described the determinants of LV mechanical delay indexes. In our cohort, Ts-max and Ts-sd significantly and independently increased with female sex, age and BMI and decreased with heart rate. Ts-sl significantly and independently increased only with age and BMI. We did not observe any differences between women and men in the septal lateral delay. Thus, in our general population LV mechanical dyssynchrony increased with age and BMI, which are important risk factors for HF.
Lim et al.7 first proposed to use the longitudinal SDI averaged from 16 LV segments, as a measure for LV dyssynchrony based on the assumption that delayed LV segments do not equally contribute to end-systolic LV deformation. Therefore, in addition to the TSI indexes, we measured the end-systolic and peak longitudinal strain in septal and lateral LV walls by 2D speckle tracking and calculated the post-systolic time delay to peak strain.7 In our study population, women had more often septal peak strain delay than men. No other anthropometric or haemodynamic characteristics, or the prevalence of HF risk factors was significantly different between subjects with and without a post-systolic septal strain delay. In contrast, participants with any degree of a lateral strain delay were older, had a higher BMI, and more often were treated with antihypertensive medications and had an advanced stage of diastolic dysfunction. These findings might imply that the lateral strain delay was significantly associated with risk factors for HF, whereas the septal strain delay did not. Thus, we suggest that the lateral strain delay might characterize pathological LV dyssynchrony more accurately than septal.
The identification of risk factors and pathophysiological mechanisms leading to subclinical LV diastolic dysfunction is important to identify patients at risk for developing clinically overt HF. Our study was the first to estimate the association of LV mechanical dyssynchrony with subclinical diastolic dysfunction in the general population. The non-invasive estimation of LV diastolic function is currently based on conventional and tissue Doppler echocardiographic parameters. Commonly the transmitral E/A ratio is used as a measure of LV relaxation and pressure changes between LA and LV. The E/e′ ratio is considered as a measure of LV filling pressure.9 In multivariable adjusted models, we found that all TSI indexes and lateral SDI were positively associated with the E/e′ ratio. The current findings are consistent with the two studies in patients published so far. Ciampi et al.10 recently found that the higher E/e‘ in 81 congestive HF patients, associated with the presence of LV systolic dyssynchrony assessed by Ts-sl. Chang et al.11 also found an independent and positive association between the E/e′ ratio and TSI indexes in 110 asymptomatic hypertensive patients.
LV synchrony has an influence on LV structure and function. In long-term experimental models of isolate LV pacing, van Oosterhout et al.12 demonstrated that the pacing-induced LV dyssynchrony leads to changes in wall thickness, LV cavity volume and, in turn, increased LV end-systolic wall stress. Moreover, the strength and coordination (LV synchronicity) of the previous systole are major determinants of early diastolic filling and myocardial lengthening. Previous experimental studies13,14 suggested that LV systolic shortening and diastolic lengthening velocities are tightly coupled. Thus, an incomplete systole induced by mechanical dyssynchrony might lead to an increased LV filling pressure and eventually alter LV diastolic function.
To provide further evidence in support of the association between LV diastolic dysfunction and systolic dyssynchrony, we describe LV mechanical dyssynchrony indexes in subjects with different stages of subclinical LV diastolic dysfunction. In our study, all TSI indexes were prolonged in diastolic dysfunction Group 2 (elevated end-diastolic filling pressure) when compared with participants with normal diastolic function or with early stage of LV diastolic dysfunction. Studies in patients on LV dyssynchrony and diastolic symptomatic HF are also scarce. Yu et al.3 observed systolic dyssynchrony in 39.1% of diastolic HF patients. De Sutter et al.4 found that in symptomatic HF patients (n = 60), the prevalence of systolic intraventricular dyssynchrony was 18% in patients with HF with preserved EF and 36% in those with a low EF (<40%).
The present study must be interpreted within the context of its potential limitation. Echocardiographic measurements, such as TSI indexes and strain, are prone to measurements errors due to bad visualization, signal noise, and acoustic artefacts.2 In the present study, only one experienced observer recorded all echocardiographic images for offline analyses. Furthermore, all digitally stored TSI images were centrally post-processed using dedicated software by two observers in duplicate. We assessed SDI only in septal and lateral LV walls. We might therefore have underestimated the full spectrum of strain delays between the LV walls. On the other hand, LV dyssynchrony typically results from the delay in activation of the lateral LV free wall.1
In conclusion, we demonstrated that in unselected subjects subclinical diastolic LV dysfunction is associated with mechanical LV dyssynchrony as assessed by echocardiography. LV mechanical delay indexes were prolonged in participants with advanced stages of LV diastolic dysfunction compared with subjects with normal diastolic function or with early stage of LV diastolic dysfunction. Further longitudinal population studies are required to clarify the role of LV dyssynchrony in the transition from subclinical LV dysfunction to overt HF which is currently unknown.
Acknowledgments
Funding The European Union (grants IC15-CT98-0329-EPOGH, LSHMCT-2006-037093-InGenious HyperCare, HEALTH-F4-2007-201550-HyperGenes, HEALTH-2011-278249-EU-MASCARA, and ERC Advanced Grant-2011-294713-EPLORE) supported the Studies Coordinating Centre (Leuven, Belgium). The Studies Coordinating Centre also received grants from the Fonds voor Wetenschappelijk Onderzoek Vlaanderen, Ministry of the Flemish Community, Brussels, Belgium (grants G.057506 and G.0734.09).
Footnotes
Supplementary data Supplementary data are available at European Heart Journal – Cardiovascular Imaging online.
Conflict of interest: none declared.
References
- 1.Spragg DD, Kass DA. Pathobiology of left ventricular dyssynchrony and resynchronization. Prog Cardiovasc Dis. 2006;49:26–41. doi: 10.1016/j.pcad.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur J Echocardiogr. 2011;12:167–205. doi: 10.1093/ejechocard/jer021. [DOI] [PubMed] [Google Scholar]
- 3.Yu CM, Zhang Q, Yip GW, Lee PW, Kum LC, Lam YY, et al. Diastolic and systolic asynchrony in patients with diastolic heart failure: a common but ignored condition. J Am Coll Cardiol. 2007;49:97–105. doi: 10.1016/j.jacc.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 4.De Sutter J, Van de Veire NR, Muyldermans L, De Backer T, Hoffer E, Vaerenberg M, et al. Prevalence of mechanical dyssynchrony in patients with heart failure and preserved left ventricular function (a report from the Belgian Multicenter Registry on dyssynchrony) Am J Cardiol. 2005;96:1543–8. doi: 10.1016/j.amjcard.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 5.Kuznetsova T, Herbots L, López B, Jin Y, Richart T, Thijs L, et al. Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail. 2009;2:105–12. doi: 10.1161/CIRCHEARTFAILURE.108.822627. [DOI] [PubMed] [Google Scholar]
- 6.Kuznetsova T, Herbots L, Richart T, D’hooge J, Thijs L, Fagard RH, et al. Left ventricular strain and strain rate in a general population. Eur Heart J. 2008;29:2014–23. doi: 10.1093/eurheartj/ehn280. [DOI] [PubMed] [Google Scholar]
- 7.Lim P, Buakhamsri A, Popovic ZB, Greenberg NL, Patel D, Thomas JD, et al. Longitudinal strain delay index by speckle tracking imaging: a new marker of response to cardiac resynchronization therapy. Circulation. 2008;118:1130–7. doi: 10.1161/CIRCULATIONAHA.107.750190. [DOI] [PubMed] [Google Scholar]
- 8.Conca C, Faletra FF, Miyazaki C, Oh J, Mantovani A, Klersy C, et al. Echocardiographic parameters of mechanical synchrony in healthy individuals. Am J Cardiol. 2009;103:136–42. doi: 10.1016/j.amjcard.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 9.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–33. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 10.Ciampi Q, Petruzziello B, Della Porta M, Caputo S, Manganiello V, Astarita C, et al. Effect of intraventricular dyssynchrony on diastolic function and exercise tolerance in patients with heart failure. Eur J Echocardiogr. 2009;10:907–13. doi: 10.1093/ejechocard/jep094. [DOI] [PubMed] [Google Scholar]
- 11.Chang SA, Kim HK, Kim DH, Kim YJ, Sohn DW, Oh BH, et al. Left ventricular systolic and diastolic dyssynchrony in asymptomatic hypertensive patients. J Am Soc Echocardiogr. 2009;22:337–42. doi: 10.1016/j.echo.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 12.van Oosterhout MF, Prinzen FW, Arts T, Schreuder JJ, Vanagt WY, Cleutjens JP, et al. Asynchronous electrical activation induces asymmetrical hypertrophy of the left ventricular wall. Circulation. 1998;98:588–95. doi: 10.1161/01.cir.98.6.588. [DOI] [PubMed] [Google Scholar]
- 13.Opdahl A, Remme EW, Helle-Valle T, Lyseggen E, Vartdal T, Pettersen E, et al. Determinants of left ventricular early-diastolic lengthening velocity: independent contributions from left ventricular relaxation, restoring forces, and lengthening load. Circulation. 2009;119:2578–86. doi: 10.1161/CIRCULATIONAHA.108.791681. [DOI] [PubMed] [Google Scholar]
- 14.Remme EW, Opdahl A, Smiseth OA. Mechanics of left ventricular relaxation, early diastolic lengthening, and suction investigated in a mathematical model. Am J Physiol Heart Circ Physiol. 2011;300:H1678–87. doi: 10.1152/ajpheart.00165.2010. [DOI] [PubMed] [Google Scholar]