Abstract
Background
The ganglioside GD2 is an attractive target for immunotherapy of neuroectodermal tumors. We tested a unique bispecific antibody anti-CD3 × anti-GD2 (3F8BiAb) for its ability to redirect activated T cells (ATC) to target GD2-positive neuroblastomas.
Procedure
ATC were generated from normal human peripheral blood mononuclear cells (PBMC) by stimulating the PBMC with OKT3 and expanding the T cells in the presence of interleukin 2 (IL-2) for 14 days. ATC were armed with 3F8BiAb (100 ng/106 cells) or Her2BiAb (50 ng/106 cells) prior to use. 3F8 BiAb were tested for its dual-binding specificity to GD2 expressed on cancer cell lines and CD3 expressed on ATC. 3F8BiAb-armed ATC were further tested ex vivo for their cytotoxicity against GD2 positive tumor targets and its ability to induce cytokine response upon binding to targets.
Results
GD2 expression in neuroblastoma cells was confirmed by FACS analysis. Specific binding of 3F8BiAb to the tumor targets as well as to ATC was confirmed by FACS analysis. 3F8BiAb-armed ATC exhibited specific killing of GD2 positive neuroblastoma cell lines significantly above unarmed ATC (P < 0.001). GD2BiAb-armed ATC secreted significantly higher levels of Th1 cytokines and chemokines compared to unarmed ATC (P < 0.001).
Conclusions
These preclinical findings support the potential of a novel immunotherapeutic approach to target T cells to neuroblastoma.
Keywords: bispecific antibody, GD-2, immunotherapy, neuroblastoma, T cells
Introduction
Gangliosides are glycosphingolipids that are expressed on the surface of mammalian cells, and are concentrated in nervous tissues [1]. The ganglioside GD2 is expressed in human neuroblastoma, melanoma, and osteosarcoma as well as certain brain tumors [2,3]. Because of its tumor-specific and persistent surface expression, GD2 is an attractive target for cancer immunotherapy [1]. GD2 has been identified as an important target antigen for antibody-dependent cellular cytotoxicity (ADCC) for neuroblastoma and malignant melanoma cells [4].
Neuroblastoma is the most common extra-cranial tumor in children. Stage 4 disease in children more than 18 months of age at diagnosis is uniformly aggressive and often recurrent following successful induction therapy. Despite the use of intensive regimens, the survival rates for such patients have remained unacceptable for more than two decades. Since the first phase I study of anti-GD2 monoclonal antibodies (mAb) [5] to the most recent randomized trial, GD2 is accepted as a viable tumor target for immunotherapy [6]. The recent Children's Oncology Group trial of anti-GD2 ch14.18 antibody, IL-2, and granulocyte–macrophage colony stimulating factor combination, following autologous stem cell transplant in patients with high-risk neuroblastoma was one of the few rare randomized studies demonstrating the clinical benefit of antibody immunotherapy in metastatic solid tumors among children [6].
A number of mAbs specific for the GD2 (including 14.G2a, ch14.18, and 3F8) have been used in phase I–II clinical trials [7–9]. Anti-GD2 mouse mAb 3F8 [10] has shown highly specific tumor targeting in preclinical studies [11,12] and has shown objective tumor responses in patients with primary chemotherapy resistant bone marrow disease [9].
Despite the recent success of anti-GD2 mAb, there is a need for a further development of anti-GD2 therapeutic strategy. Although survival rates increased in high-risk patients treated with naked anti-GD2 mAb in combination with cytokines, only 63% of Stage 4 patients remained free of disease at 2 years [6]. Long-term survival analysis showed that more than 50% of the patients with Stage 4 neuroblastoma developed recurrent disease after treatment with anti-GD2 mAb alone [13]. The factors limiting the clinical utility and efficacy of naked anti-GD2 mAb are mostly unknown; however, the deficiencies in number and activity of effector cells mediating ADCC observed in patients with post-chemotherapy immunosuppression may play a role. We therefore hypothesized that arming of ex vivo activated and expanded cytotoxic T cells with anti-CD3 × anti-GD2 bispecific antibody (BiAb) will redirect them to GD2 positive tumors and result in enhanced cytotoxicity.
In this study, we exploit the non-MHC restricted, perforin/granzyme-mediated cytotoxic ability of activated T cells (ATC) by redirecting their cytotoxicity using a BiAb approach. BiAb was produced by chemically heteroconjugating anti-CD3 (OKT3) and anti-GD2 (3F8) to generate OKT3 × 3F8 BiAb (3F8BiAb). The first antibody is directed at CD3 on T cells and the second targets GD2 expressed on the surface of the tumor cells. Binding of ex vivo expanded and BiAb coated (armed) T cells to the tumor targets, through the tumor-specific portion of the BiAb molecule, reactivates the T cells. This approach has been used to redirect ATC toward Her2/neu+ [14,15], EGFR+ [16], and CD20+ [17] targets. Multiple infusions of targeted T cells in phase I clinical trials have been shown to be safe in patients with breast cancer [15].
Since 3F8 mAb treatment showed promising clinical outcomes in children with high risk neuroblastoma [18], we produced 3F8BiAb to investigate whether 3F8BiAb-armed ATC can mediate specific cytotoxicity directed at GD2-positive neuroblastoma cell lines. In this study, we showed that ATC armed with 3F8BiAb could engage and lyse GD2-positive neuroblastoma targets, providing preclinical rationale for immunotherapy using 3F8BiAb-armed ATC in children with neuroblastoma.
Materials and Methods
Cell Lines and mAb
The neuroblastoma cell lines (LAN-1, LAN-6, KCNR, and LHN) were all grown in RPMI-1640 media (Lonza, Walkersville, MD) supplemented with 10% fetal bovine serum (FBS; Valley Biomedical, Winchester, VA). The cell lines were a kind gift from Dr. Leonid Metelitsa (Texas Children's Cancer Center, Houston, TX). OKT3 is an anti-CD3 murine IgG2a (Janssen Biotech, Horsham, PA) that is available commercially. 3F8 is also a murine anti-GD2 mAb of the IgG3 subclass previously described [10]. Herceptin (trastuzumab) is an anti-HER2/neu, humanized IgG1 (Genentech, Inc., San Francisco, CA) antibody that is available commercially.
Preparation of Bispecific Antibodies
Both Anti-CD3 × Anti-GD2 BiAb and Anti-CD3 × Anti-Her2/neu BiAb was prepared by chemical heteroconjugation as previously described by Sen et al. [14]. Anti-CD3 (OKT3; Centrocor Ortho-Biotech, Raritan, NJ) was cross-linked with Traut's reagent (2-iminothiolane HCl; Pierce, Rockford, IL) and Anti-GD2 was cross-linked with sulphosuccinimidyl 4-(N-maleimidomethyl) cyclohexane-1-carboxylate (Sulpho-SMCC). Cross-linked mAb were desalted on PD-10 columns (Pharmacia, Uppsala, Sweden) to remove unbound cross-linker. The cross-linked OKT3 and anti-GD2 were heteroconjugated overnight. The heteroconjugated product was analyzed by non-reducing SDS–PAGE (4–20% gradient; Lonza Inc., Walkersville, MA) and quantified by densitometry using Quantity One software (Bio-Rad Lab., Hercules, CA).
Generation and Arming of ATC With the BiAb
Armed ATC were prepared as described previously [14]. Briefly, peripheral blood mononuclear cells (PBMC) purified from whole heparinized blood of healthy donors by ficoll-hypaque density gradient centrifugation were activated in culture with 20 ng/ml OKT3, and cultured for 14 days in complete RPMI 1640 media containing 100 IU/ml of IL-2 (Chiron Corp., Emeryville, CA). The harvested ATC were armed with Her2BiAb or 3F8BiAb at indicated doses/106 ATC for 15 minutes at room temperature. We wash armed ATC at least twice after arming in complete medium to eliminate any unbound dimer (BiAb), monomers and multimer before using them in vitro experiments. Blood collection and use of human blood products for research were conducted under protocols approved by the Internal Review Board at Wayne State University. Signed consents for blood draws were obtained from normal healthy donors.
Flow Cytometry
Tumor cell lines were stained with monoclonal mouse anti-GD2 IgG2a (clone 14.G2a; BD Pharmingen, San Diego, CA) and monoclonal mouse anti-human c-erbB-2 IgG1 (clone 9G6; BD Pharmingen) followed by a goat anti-mouse-F(ab')2 Phycoerythrin (PE) secondary antibody (Biosource International, Camarillo, CA) and analyzed with a EPICS-XL-MCL flow cytometer (Beckman Coulter, Brea, CA) equipped with an Argon laser. More than 10,000 events were analyzed in all experiments. The percentage of positive cells was determined from markers set with matched isotype control antibodies. Analysis was performed using Coulter System II Software.
Flow Cytometry for 3F8 BiAb-Binding Assays
Binding of 3F8 mAb and 3F8BiAb to ATC was evaluated by incubating 106 ATC with either 1 or 10 μg of 3F8 mAb or 3F8BiAb for 1 h at 4°C. A goat anti-mouse IgG3-fluorescein isothiocyanate (FITC; eBioscience, San Diego, CA) was used to label the 3F8 mAb and the 3F8 fragment of 3F8BiAb. Similarly, binding of 3F8 BiAb to LAN-6 and KCNR targets was determined by incubating 106 LAN-6 or KCNR cells with either 1 or 10 μg of 3F8 BiAb for 1 h at 4°C. The cells were washed and incubated with goat anti-mouse IgG2a-PE to measure the OKT3 fragment of the BiAb (Becton Dickinson, San Diego, CA). Cells were analyzed by flow cytometry.
Specific Cytotoxicity Assay
Tumor cell lines were plated into 96-well flat-bottom plates at cell concentration of 4 × 104 cells/well and incubated overnight at 37°C. The following day, targets were washed once and labeled with 51Cr (2 μCi/well; MP Biomedicals, Irvine, CA) for 4 hours at 37°C. The plates were washed three times with medium after 51Cr labeling and ATC or armed ATC were plated with the target cells in duplicates at different effector to target (E:T) ratios and incubated overnight for 18 hours at 37°C. 3F8 mAb-mediated ADCC was evaluated by exposing target cells to PBMC or ATC plus 100 ng of 3F8 mAb. Her2 BiAb (50 ng/106 cells)-armed ATC were used in some experiments as an irrelevant antibody control. 0.1 ml aliquots of the supernatants were harvested for quantification of chromium release. Spontaneous release was determined by incubation of targets with media alone, and maximum release was determined by lysing the targets with 2% sodium dodecyl sulfate (SDS) solution. Specific lysis of target cells was calculated by measuring 51Cr release in supernatants using the formula: [% specific lysis = (test − spontaneous release)/(maximum release − spontaneous release) × 100]. Results are expressed as a percentage of specific cytotoxicity (mean ± SD) from duplicate wells.
Cytokine Profiling of Co-Cultures
Cytokines were quantitated in culture supernatants following binding of 3F8BiAb armed ATC to GD2 expressing tumor cells. Unarmed ATC, armed ATC and GD2 positive tumor targets were co-cultured in a 96-well plate for 24 hours. Tumor cells alone, unarmed ATC and armed ATC without tumor cells were run as controls using a 25-plex human cytokine Luminex Array (Invitrogen, Carlsbad, CA) on a Bio-Plex system (Bio-Rad Lab.). The limit of detection for these assays is <10 pg/ml based on detectable signal of greater than twofold above background (Bio-Rad Lab.). Cytokine concentrations were automatically calculated by the BioPlex Manager Software (Bio-Rad Lab.).
Statistical Analysis
All statistical analysis were performed using GraphPad Prism 5 for windows (GraphPad software, San Diego,CA). Paired t-test was used to assess the optimal dose of the BiAb needed to exhibit maximum cytotoxicity with P < 0.05 considered as significant. Two-way analysis of variance (ANOVA) was used to analyze results from cytotoxcity and cytokine/chemokine assays between different groups.
Results
Heteroconjugation of 3F8 Bispecific Antibody
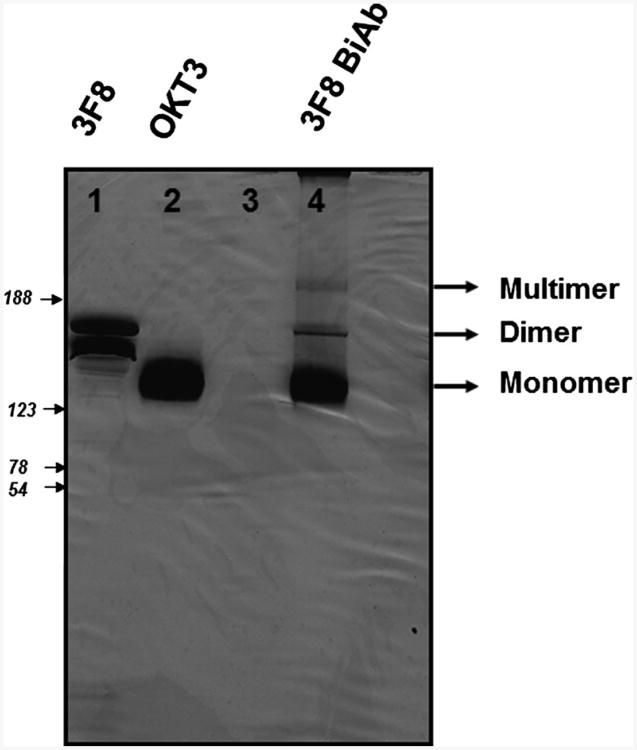
The heteroconjugated product of equimolar concentrations of OKT3 and 3F8 mAb was quantified by Coomassie blue staining of SDS-gel as shown in Figure 1. Densitometric quantitation of Lane 4 of the gel showed 73.5% monomer, 17.5% dimer, and 9% multimer fractions.
Fig. 1.
Production of 3F8BiAb. The 3F8BiAb was generated following chemical heteroconjugation of OKT3 and 3F8 mAb's as described in materials and methods. The product was resolved by SDS– non-reducing polyacrylamide gradient (4–20%) gel electrophoresis and detected by Coomassie blue staining. Lane 1, 8 μg of 3F8 mAb; lane 2, 8 μg of OKT3 mAb; lane 3, no sample; lane 4, 8 μg of 3F8 BiAb. The unconjugated OKT3 and 3F8 (monomers), conjugated 3F8 BiAb dimers and multimers are indicated in the figure.
GD2 and Her2 Expression in Neuroblastoma Cells
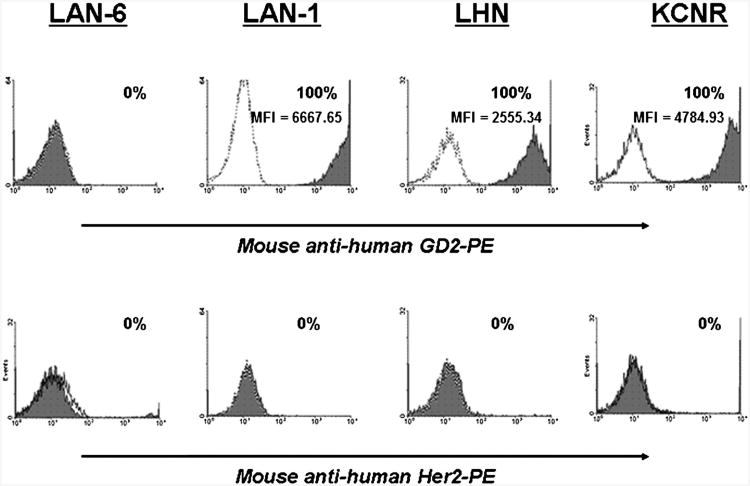
The expression of GD2 and Her2 proteins on neuroblastoma cells was quantitated by flow cytometric analysis (Fig. 2). All neuroblastoma cell lines expressed high levels of GD2 except for LAN-6 [LAN-1, 100% (MFI = 6667.6); LHN, 100% (MFI = 2555.3); and KCNR, 100% (MFI = 4784.9)], which did not show any detectable GD2 expression. There was no detectable expression of Her2 on any of the neuroblastoma cell lines tested.
Fig. 2.
GD2 and Her2 expression in neuroblastoma cell lines. The tumor cells were examined for expression of GD2 and Her2 proteins by flow cytometry, using anti-GD2 and anti-c-erbB2 mAb. Data are presented as histograms with their matched IgG isotype antibodies (open dotted curves) to account for background fluorescence. Solid gray histograms represent cells that are positive for GD2. Percentage of positive cells is shown as well.
Dual-Binding Specificity of 3F8 BiAb
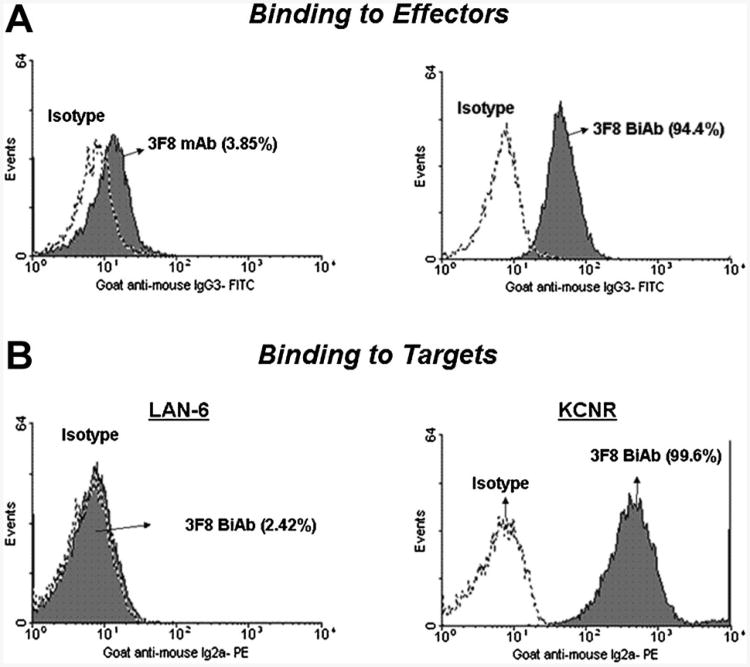
Binding of 3F8 mAb and 3F8BiAb to ATC was determined by arming ATC with 1 μg of the above antibodies, followed by staining with a FITC-conjugated anti-mouse IgG3 to measure the amount of 3F8BiAb bound on the ATC (Fig. 3A). Approximately, ∼95% of ATC stained positive for 3F8BiAb binding. In order to determine the binding of 3F8BiAb to the target cells, GD2-negative LAN-6 cells and GD2-positive KCNR cells were stained with the 3F8BiAb. The relative amount of OKT3 parental mAb associated with the 3F8BiAb was quantitated using PE-conjugated IgG2a (Fig. 3B). KCNR cells showed 100% positive staining for binding of 3F8BiAb. In contrast, LAN-6 cells did not stain with 3F8BiAb, confirming the absence of GD2 expression on LAN-6.
Fig. 3.
Binding of 3F8BiAb to ATC and target cells. A: Binding of 3F8BiAb to ATC. 1 ×106 ATC were armed with 1 μg each of 3F8mAb and 3F8BiAb and the amount of antibody bound to the surface of the cells was measured by flow cytometry as described in materials and methods. Solid gray histograms represent cells that have OKT3 bound to their surface via 3F8 (3F8BiAb). Percentage positive cells shown in parenthesis were obtained by setting the markers using appropriate isotype control antibodies (open dotted lines). B: Binding of 3F8BiAb to tumor cells. LAN-6 (106 cells) and KCNR (106 cells) were incubated with 1 μg of 3F8BiAb and analyzed by flow cytometry. The solid grey histograms show the binding of 3F8BiAb to the target cells. Matched isotype controls were used to quantitate the positive cells shown in parenthesis.
Specific Cytotoxicity With Increasing Arming Doses of the 3F8 BiAb
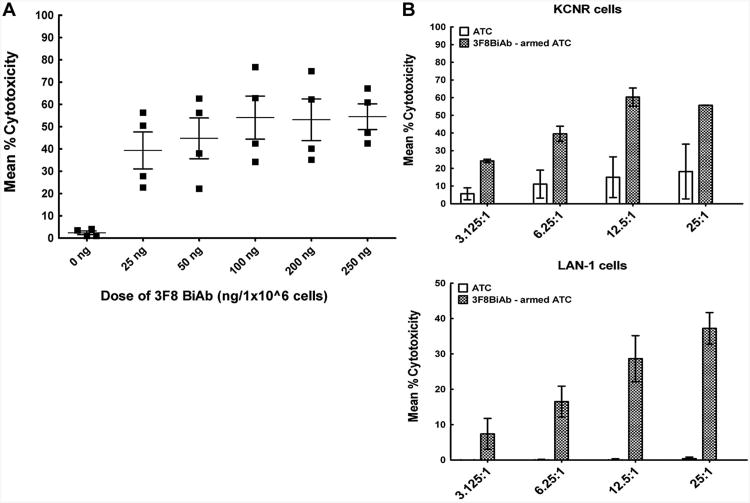
To determine the optimal arming dose of 3F8BiAb, a dose titration of 3F8BiAb using 51Cr release cytotoxicity assay was done against KCNR neuroblastoma target cells. ATC obtained from four normal donors were armed with increasing doses of 3F8BiAb ranging from 25 to 250 ng/106 ATC. Unarmed ATC from the same donors were used as controls. Figure 4A shows the mean % specific cytotoxicity for each donor ATC armed with increasing doses of the 3F8BiAb targeted against KCNR tumor cells at an E:T ratio of 25:1 for 18 hours. Increasing the arming doses of 3F8BiAb up to 100 ng/106 ATC showed dose-dependent increase in specific cytotoxicity against the KCNR neuroblastoma cell line. However, arming doses above 100 ng/106 ATC did not result in any further increase in specific cytotoxicity. Therefore, the dose of 100 ng/106 AT C (P < 0.007) was selected as an optimal dose for all subsequent experiments. The unarmed ATC control showed the expected low levels of non-MHC restricted cytotoxicity against the same targets.
Fig. 4.
A: Arming dose titration of 3F8BiAb. Cytotoxicity mediated by ATC armed with the 3F8BiAb at doses of 25, 50, 100, 200, and 250 ng per 1 × 106 cells was measured in 51Cr-release assay against KCNR neuroblastoma cells. Controls included unarmed ATC (0 ng). E:T of 25:1 was used for these experiments. Mean % cytotoxicity ± SEM for four healthy donors (■) is shown for each dose of the BiAb. *P < 0.01 and **P < 0.007 as analyzed by paired t-test. B: Cytotoxicity of 3F8BiAb armed ATC increases with E:T ratio. ATC generated from healthy donors (n = 2) were armed with 100 ng/1 × 106 cells of 3F8BiAb (
 ) and co-cultured at four different E:T ratio; overnight at 37°C in 96-well flat-bottom plates containing 51Cr-labeled KCNR (upper panel) or LAN-1 (lower panel) target cells. Unarmed ATC were used as controls (□). Mean % cytotoxicity was calculated and error bars represent SD from two experiments.
) and co-cultured at four different E:T ratio; overnight at 37°C in 96-well flat-bottom plates containing 51Cr-labeled KCNR (upper panel) or LAN-1 (lower panel) target cells. Unarmed ATC were used as controls (□). Mean % cytotoxicity was calculated and error bars represent SD from two experiments.
Effect of Effector:Target Ratio on Specific Cytotoxicity by 3F8BiAb Armed ATC
51Cr labeled KCNR and LAN-1 neuroblastoma cell lines and 3F8BiAb-armed ATC were plated at various E:T ratios ranging from 3.125:1 to a maximum of 25:1. Figure 4B shows mean % specific cytotoxicity of armed ATC against KCNR (upper panel) and LAN-1 (lower panel) targets. Increasing the E:T ratio led to increasing mean % specific cytotoxicity from 24.2% at 3.125:1 to 55.7% at 25:1 for KCNR specific lysis and from 7.4% at 3.125:1 to 37.2% at 25:1 for LAN-1 specific lysis. Unarmed ATC were run as controls for the same ratios.
Influence of ADCC on 3F8BiAb-Induced Cytotoxicity
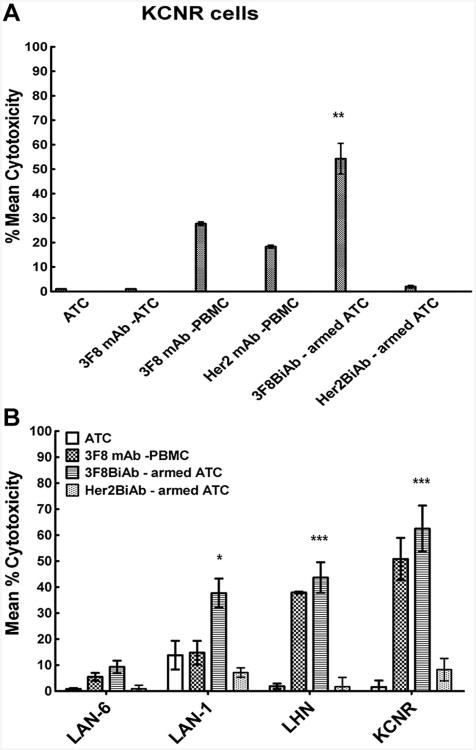
In order to assess the contribution of ADCC to 3F8BiAb-armed ATC and naked 3F8 mAb-mediated killing, both ATC and PBMC were tested as potential sources of effector cells mediating ADCC in KCNR neuroblastoma cell line (Fig. 5A). PBMC with Her2 mAb were used as an irrelevant antibody control and Her2BiAb armed ATC as irrelevant BiAb control. The dose of 3F8 mAb and 3F8BiAb applied to the targets with ATC was 100 ng/well and 100 ng/106 cells, respectively. While we detected a substantial amount of ADCC-mediated killing of neuroblastoma cells with 3F8 mAb plus PBMC (Fig. 5A,B), the cytotoxicity exhibited by 3F8 mAb plus ATC was not detected suggesting that ATC were not a significant source of effector cells mediating ADCC. Also, the cytotoxicity mediated by 3F8BiAb-armed ATC was higher than ADCC mediated by 3F8 mAb plus PBMC against neuroblastoma cell lines (P < 0.01; Fig. 5A,B).
Fig. 5.
A: 3F8 mAb dependent ADCC against neuroblastoma cells. 51Cr labeled target cells were co-cultured in the presence of unarmed ATC, 3F8 mAb plus ATC, 3F8 mAb plus PBMC and armed ATC with 3F8 BiAb, controls comprised of Her2 mAb in the presence of PBMC and Her2BiAb-armed ATC. A standard 18-hour chromium release cytotoxicity assay was performed as described in materials and methods. E:T of 25:1 was used. Representative donor for neuroblastoma (KCNR) cells is shown. Error bars represent SD of two replicates for each condition. **P < 0.01 for KCNR cells versus 3F8mAb plus PBMC control. B: 3F8BiAb-armed ATC kill GD2-expressing neuroblastoma cells in co-culture. LAN-6, LAN-1, LHN, and KCNR neuroblastoma cell lines were exposed to ATC armed with 100 ng/1 × 106 cells of 3F8BiAb (
 ) in a standard 51Cr release assay. ATC alone (□), 100 ng/well of 3F8 mAb combined with PBMC (
) in a standard 51Cr release assay. ATC alone (□), 100 ng/well of 3F8 mAb combined with PBMC (
 ) and Her2Bi-armed ATC (
) and Her2Bi-armed ATC (
 ) were used as controls. E:T was 25:1. Mean % cytotoxicity ± SEM of six donors with each condition done in duplicates is shown. *P < 0.05 and ***P < 0.001 versus the unarmed ATC control.
) were used as controls. E:T was 25:1. Mean % cytotoxicity ± SEM of six donors with each condition done in duplicates is shown. *P < 0.05 and ***P < 0.001 versus the unarmed ATC control.
Specific Cytotoxicity of 3F8BiAb-Armed ATC Against Neuroblastoma Cell Lines
ATC derived from six normal subjects were armed with 100 ng of 3F8BiAb per 106 cells and co-cultured for 18 hours with 51Cr labeled neuroblastoma cells at a E:T ratio of 25:1. Control conditions consisted of unarmed ATC alone and 3F8 mAb (100 ng/well) with PBMC. Her2BiAb armed ATC from the same donors were tested on neuroblastoma as irrelevant antibody control. 3F8BiAb-armed ATC effectively killed GD2-positive neuroblastoma cell lines (KCNR and LHN (P < 0.001), LAN-1 (P < 0.05), whereas the killing of GD2-negative neuroblastoma (LAN-6) was minimal (Fig. 5B). Her2Bi-armed ATC did not kill the neuroblastoma cell lines, further verifying the specificity of 3F8BiAb mediated killing of GD2-positive neuroblastoma cells.
Enhanced Secretion of Cytokines Following Binding of 3F8BiAb-Armed ATC to GD2 Positive Tumors
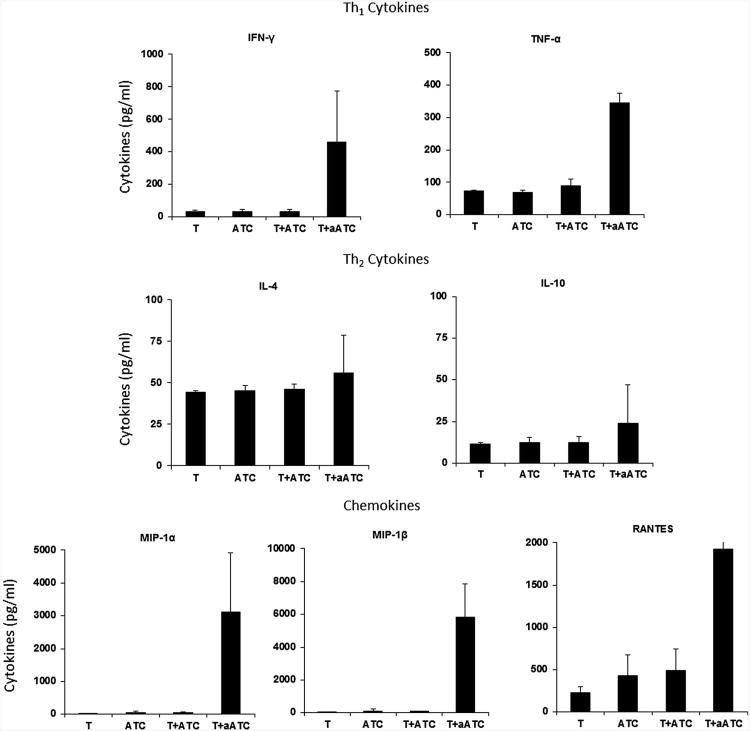
Since binding of armed ATC to Her2/neu on SK-BR-3 cells triggered cytokine and chemokine secretion [15], we tested if armed or unarmed ATC stimulated with KCNR tumor cells for 24 hours could trigger cytokine and chemokine production (Fig. 6). As expected, stimulation of 3F8BiAb armed ATC with target cells induced higher levels of two key cytokines, IFN-γ and TNF-α which were known to play a key role in cytotoxicity compared to unarmed ATC. Interestingly, no change in the levels of IL-4, IL-10 was detected between tumor cells alone or tumor cell stimulated with ATC or armed ATC (n = 4). The most striking up-regulation was seen in the chemokine secretion levels of MIP-1α, MIP-1β and RANTES with significantly higher levels (P < 0.001) in culture supernatant of 3F8BiAb armed-ATC stimulated with tumor cells compared to co-cultures of unarmed ATC and tumor cells or tumor cells alone.
Fig. 6.
Cytokine profile of culture supernatants detected by multiplex luminex system. Co-culture supernatants of ATC alone (ATC), 3F8BiAb armed ATC with tumor (T + aATC), tumor alone (T), and tumor + ATC (T + ATC) obtained from four normal donors were tested for Th1 cytokines IFN-γ and TNF-α (upper panel), Th2 cytokines IL-4 and IL-10 (middle panel), and chemokines MIP-1α, MIP-1β, and RANTES (lower panel).
Discussion
In this study, we produced and functionally characterized a unique BiAb 3F8BiAb that recognizes the tumor-associated ganglioside GD2 and the T-cell receptor antigen CD3. Our data show that 3F8BiAb can activate and redirect non-MHC restricted cytotoxic activity of ATC toward GD2-positive neuroblastoma cell lines. The tumor cell killing was GD2-specific. To the best of our knowledge, the strategy of targeting neuroblastoma with 3F8 BiAb-armed ATC has not been reported.
Studies using anti-GD2 BiAb with a different design had been reported. Thus, Bernhard et al. [19] used Fab' dimer anti-CD3 × anti-GD2 BiAb to target melanoma cell lines, and Manzke et al. [20] used 14G2a in a tetradoma-based anti-GD2 × anti-CD3 BiAb in preclinical experiments targeting the human neuroblastoma cell line IMR5. Compared to ours, these BiAb production methods are labor intensive and require multiple steps of production and purification of BiAb. Both groups did not coat T cells with BiAb, but used co-injection of BiAb with effectors. In our opinion, this may result in less effective arming and subsequent redirection of T cells to targets.
Most clinical studies used direct intravenous injection of BiAb and were limited by cytokine storm induced by BiAb binding to Fc-receptor bearing cells [21]. Recently, the development of T-cell engager BiAb (BiTE) and trifunctional bispecific antibodies (TriFAb) with engineered Fc have met with clinical success in the treatment of mantle cell lymphoma and ascites in ovarian cancer trials [22–26]. There are ongoing phase I/II clinical trials that are promising using a number of different BiAb that target solid tumor and hematologic malignancies (reviewed in [21]). Studies from our group have used BiAb armed ATCs to increase cytotoxicity directed at both solid tumors and hematologic malignancies [16,17,21,27]. However, none of our studies have targeted neuroblastoma cells.
Our in vitro data showed that 3F8BiAb armed ATC secreted increased levels of IFN-γ and TNF-α when they specifically engaged tumor targets but there was no change in IL-4 and IL-10 secretion by 3F8BiAb-armed ATC stimulated with tumor cells. Infusions of armed ATC may reverse tumor tolerance by polarizing the tumor microenvironment towards a Th1 condition rich in IFN-γ and TNF-α, which are known to be tumoricidal and capable of inducing local immunization and systemic anti-tumor responses [28][29]. Shifting the in vivo immune responses to Th1 was consistent with our observations of PBMC from women who received multiple infusions of armed ATC and developed specific cytotoxicity directed at breast cancer cell lines that persisted at least 4 months after immunotherapy [15]. In addition to cytokines, chemokines such as RANTES, MIP-1α, and MIP-1β were significantly upregulated (P < 0.001) by 3F8BiAb-armed ATC upon engagement with tumor cells. RANTES, MIP-1α, and MIP-1β are known to modulate T-cell migration, degranula-tion, co-stimulation, and tumor-specific cytolytic activity [30,31]. In addition, these soluble factors can recruit antigen-presenting cells and naive T cells to the site, and may facilitate antigen presentation and endogenous T-cell activation [30–32].
The use of GD2 as an immunological target was confirmed in clinical trials that reported objective clinical responses and improved survival in children with neuroblastoma who received infusions of anti-GD2 mAbs [6,8,9,13]. In vitro studies show that ADCC is the major mechanism of tumor cell killing mediated by the anti-GD2 mAb with NK-cells and granulocytes being the key effector cells [4,33,34]. In the current study, we addressed the hypothesis whether the cytotoxicity mediated by 3F8BiAb armed ATC is comparable or better than 3F8 mAb-mediated ADCC.
The potential clinical advantage of 3F8BiAb-armed ATC over naked 3F8 mAb includes a significantly lower dose of 3F8BiAb to arm ATC compared to intravenously injected dose of 3F8 mAb (10 mg/m2/dose × 5 days). GD2 expression in normal human tissues is restricted to the CNS, peripheral nerves, and skin melanocytes [1]. While CNS neurons are protected from anti-GD2 mAb (3F8) by blood brain barrier, their binding to peripheral nerve fibers causes pain, which represents one of the major dose-limiting toxicities of 3F8. All patients receiving 3F8 require morphine administration. Fevers, urticarial rash, hypotension, and development of human anti-mouse antibodies (HAMA) responses have been reported as the other common side effects of 3F8 [5]. The actual dose of 3F8 required to arm 2.5 × 109 ATC (a targeted cell dose per one weekly infusion in 20 kg child) would be 0.25 mg, but with a 20% arming efficiency, the actual amount of antibody bound to the ATC after washing would be only 0.05 mg/2.5 × 109 ATC. This is 200 times less than a single dose of naked 3F8 used in phase II–III trials. Since most of the 3F8 side effects were dose dependent, and rarely noted at dosages of <1 mg/m2 [5], we do not expect any significant toxicities related to 3F8 component of BiAb-armed ATC therapy.
In summary, our results showed that (i) 3F8BiAb-armed ATC induced cytotoxic activity directed at GD2 positive neuroblastoma cells; and (ii) 3F8BiAb-armed ATC secreted higher levels of tumoricidal cytokines IFN-γ and TNF-α and chemokines MIP-1α, MIP-1β, and RANTES compared to tumor cells alone or ATC stimulated with tumor cells. This approach may provide a viable alternative or synergistic addition to mAb therapies alone for the pediatric population with high risk neuroblastomas. The in vitro targeting studies provide a strong rationale for the use of 3F8BiAb armed ATC for the initiation of phase I/II clinical trials in patients with GD2 positive tumors. This approach with potentially low toxicity profile may be used in combination with adjuvant surgery, chemotherapy, or high dose chemotherapy and stem cell transplant to enhance overall survival and quality of life.
Acknowledgments
This study was supported in part by R01 CA 092344 (L.G.L.), R01 CA 140314 (L.G.L.). M.Y. is a Hyundai Hope on Wheels Scholar.
Footnotes
conflicts of interest: Nothing to report.
Sri Vidya Kondadasula and Maxim Yankelevich contributed equally to this work.
References
- 1.Modak S, Cheung NK. Disialoganglioside directed immunotherapy of neuroblastoma. Cancer Invest. 2007;25:67–77. doi: 10.1080/07357900601130763. [DOI] [PubMed] [Google Scholar]
- 2.Wu ZL, Schwartz E, Seeger R, et al. Expression of GD2 ganglioside by untreated primary human neuroblastomas. Cancer Res. 1986;46:440–443. [PubMed] [Google Scholar]
- 3.Longee DC, Wikstrand CJ, Mansson JE, et al. Disialoganglioside GD2 in human neuroectodermal tumor cell lines and gliomas. Acta Neuropathol. 1991;82:45–54. doi: 10.1007/BF00310922. [DOI] [PubMed] [Google Scholar]
- 4.Honsik CJ, Jung G, Reisfeld RA. Lymphokine-activated killer cells targeted by monoclonal antibodies to the disialogangliosides GD2 and GD3 specifically lyse human tumor cells of neuroectodermal origin. Proc Natl Acad Sci USA. 1986;83:7893–7897. doi: 10.1073/pnas.83.20.7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung NKV, Lazarus H, Miraldi FD, et al. Ganglioside Gd2 specific monoclonal antibody-3F8—A phase-I study in patients with neuroblastoma and malignant-melanoma. J Clin Oncol. 1987;5:1430–1440. doi: 10.1200/JCO.1987.5.9.1430. [DOI] [PubMed] [Google Scholar]
- 6.Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frost JD, Hank JA, Reaman GH, et al. A phase I/IB trial of murine monoclonal anti-GD2 antibody 14 G2a plus interleukin-2 in children with refractory neuroblastoma: A report of the Children's Cancer Group. Cancer. 1997;80:317–333. doi: 10.1002/(sici)1097-0142(19970715)80:2<317::aid-cncr21>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 8.Cheung NK, Kushner BH, Yeh SD, et al. 3F8 monoclonal antibody treatment of patients with stage 4 neuroblastoma: A phase II study. Int J Oncol. 1998;12:1299–1306. doi: 10.3892/ijo.12.6.1299. [DOI] [PubMed] [Google Scholar]
- 9.Kushner BH, Kramer K, Cheung NK. Phase II trial of the anti-G(D2) monoclonal antibody 3F8 and granulocyte-macrophage colony-stimulating factor for neuroblastoma. J Clin Oncol. 2001;19:4189–4194. doi: 10.1200/JCO.2001.19.22.4189. [DOI] [PubMed] [Google Scholar]
- 10.Cheung NK, Saarinen UM, Neely JE, et al. Monoclonal antibodies to a glycolipid antigen on human neuroblastoma cells. Cancer Res. 1985;45:2642–2649. [PubMed] [Google Scholar]
- 11.Cheung NK, Berger N, Coccia P, et al. Murine monoclonal-antibody (Mab) specific for Gd2 Ganglioside—A phase-I trial in patients with neuroblastoma, melanoma and osteogenic-sarcoma. Proc Am Assoc Cancer Res. 1986;27:318. [Google Scholar]
- 12.MiraldiFD, Nelson AD, Kraly C, et al. Diagnostic-imaging of human neuroblastoma with radiolabeled antibody. Radiology. 1986;161:413–418. doi: 10.1148/radiology.161.2.3763911. [DOI] [PubMed] [Google Scholar]
- 13.Simon T, Hero B, Faldum A, et al. Long term outcome of high-risk neuroblastoma patients after immunotherapy with antibody ch14.18 or oral metronomic chemotherapy. BMC Cancer. 2011;11:21–28. doi: 10.1186/1471-2407-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sen M, Wankowski DM, Garlie NK, et al. Use of anti-CD3 × anti-HER2/neu bispecific antibody for redirecting cytotoxicity of activated T cells toward HER2/neu Tumors. J Hematother Stem Cell Res. 2001;10:247–260. doi: 10.1089/15258160151134944. [DOI] [PubMed] [Google Scholar]
- 15.Grabert RC, Cousens LP, Smith JA, et al. Human T cells armed with Her2/neu bispecific antibodies divide, are cytotoxic, and secrete cytokines with repeated stimulation. Clin Cancer Res. 2006;12:569–576. doi: 10.1158/1078-0432.CCR-05-2005. [DOI] [PubMed] [Google Scholar]
- 16.Reusch U, Sundaram M, Davol PA, et al. Anti-CD3 × anti-epidermal growth factor receptor (EGFR) bispecific antibody redirects T-cell cytolytic activity to EGFR-positive cancers in vitro and in an animal model. Clin Cancer Res. 2006;12:183–190. doi: 10.1158/1078-0432.CCR-05-1855. [DOI] [PubMed] [Google Scholar]
- 17.Gall JM, Davol PA, Grabert RC, et al. T cells armed with anti-CD3 × anti-CD20 bispecific antibody enhance killing of CD20+ malignant B-cells and bypass complement-mediated Rituximab-resistance in vitro. Exp Hematol. 2005;33:452–459. doi: 10.1016/j.exphem.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Cheung NKV, Kushner BH, Cheung IY, et al. Anti-GD(2) antibody treatment of minimal residual stage 4 neuroblastoma diagnosed at more than 1 year of age. J Clin Oncol. 1998;16:3053–3060. doi: 10.1200/JCO.1998.16.9.3053. [DOI] [PubMed] [Google Scholar]
- 19.Bernhard H, Karbach J, Strittmatter W, et al. Induction of tumor-cell lysis by bi-specific antibody recognizing ganglioside Gd2 and T-cell antigen Cd3. Int J Cancer. 1993;55:465–470. doi: 10.1002/ijc.2910550324. [DOI] [PubMed] [Google Scholar]
- 20.Manzke O, Russello O, Leenen C, et al. Immunotherapeutic strategies in neuroblastoma: Antitumoral activity of deglycosylated ricin A conjugated anti-GD2 antibodies and anti-CD3×anti-GD2 bispecific antibodies. Med Pediatr Oncol. 2001;36:185–189. doi: 10.1002/1096-911X(20010101)36:1<185::AID-MPO1044>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 21.Thakur A, Lum LG. Cancer therapy with bispecific antibodies: Clinical experience. Curr Opin Mol Ther. 2010;12:340–349. [PMC free article] [PubMed] [Google Scholar]
- 22.Wolf E, Hofmeister R, Kufer P, et al. BiTEs: Bispecific antibody constructs with unique anti-tumor activity. Drug Discov Today. 2005;10:1237–1244. doi: 10.1016/S1359-6446(05)03554-3. [DOI] [PubMed] [Google Scholar]
- 23.Molhoj M, Crommer S, Brischwein K, et al. CD19-/CD3-bispecific antibody of the BiTE class is far superior to tandem diabody with respect to redirected tumor cell lysis. Mol Immunol. 2007;44:1935–1943. doi: 10.1016/j.molimm.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 24.Topp MS, Kufer P, Gokbuget N, et al. Targeted therapy with the T-cell-engaging antibody blinatumomabof chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29:2493–2498. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- 25.Burges A, Wimberger P, Kumper C, et al. Effective relief of malignant ascites in patients with advanced ovarian cancer by a trifunctional anti-EpCAM × anti-CD3 antibody: A phase I/II study. Clin Cancer Res. 2007;13:3899–3905. doi: 10.1158/1078-0432.CCR-06-2769. [DOI] [PubMed] [Google Scholar]
- 26.Sebastian M, Passlick B, Friccius-Quecke H, et al. Treatment of non-small cell lung cancer patients with the trifunctional monoclonal antibody catumaxomab (anti-EpCAM × anti-CD3): A phase I study. Cancer Immunol Immunother. 2007;56:1637–1644. doi: 10.1007/s00262-007-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lum LG, Thakur A. Bispecific antibodies for arming activated T cells and other effector cells for tumor therapy. In: Kontermann RE, editor. Bispecific antibodies. Berlin, Heidelberg, Stuttgart: Springer-Verlag; 2011. pp. 243–271. [Google Scholar]
- 28.Brunda MJ, Luistro L, Hendrzak JA, et al. Role of interferon-gamma in mediating the antitumor efficacy of interleukin 12. J Immunother. 1995;17:71–77. doi: 10.1097/00002371-199502000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Thakur A, Schalk D, Al-Khadimi Z, et al. A Th1 cytokine-enriched microenvironment enhances tumor killing by activated T cells armed with bispecific antibodies and inhibits the development of myeloid-derived suppressor cells. Cancer Immunol Immunother. 2012;61:497–509. doi: 10.1007/s00262-011-1116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schall TJ, Bacon K, Toy KJ, et al. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 31.Schall TJ, Bacon K, Camp RD, et al. Human macrophage inflammatory protein alpha (MIP-1 alpha) and MIP-1 beta chemokines attract distinct populations of lymphocytes. J Exp Med. 1993;177:1821–1826. doi: 10.1084/jem.177.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taub DD, Conlon K, Lloyd AR, et al. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1 alpha and MIP-1 beta. Science. 1993;260:355–358. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- 33.Munn DH, Cheung NK. Interleukin-2 enhancement of monoclonal antibody-mediated cellular cytotoxicity against human melanoma. Cancer Res. 1987;47:6600–6605. [PubMed] [Google Scholar]
- 34.Barker E, Mueller BM, Handgretinger R, et al. Effect of a chimeric anti-ganglioside GD2 antibody on cell-mediated lysis of human neuroblastoma cells. Cancer Res. 1991;51:144–149. [PubMed] [Google Scholar]
- 35.Chan JK, Hamilton CA, Cheung MK, et al. Enhanced killing of primary ovarian cancer by retargeting autologous cytokine-induced killer cells with bispecific antibodies: A preclinical study. Clin Cancer Res. 2006;12:1859–1867. doi: 10.1158/1078-0432.CCR-05-2019. [DOI] [PubMed] [Google Scholar]