Abstract
We have previously shown that targeting human CD34+ hematopoietic stem cells (HSC) with a bispecific antibody (BiAb) directed against myosin light chain (MLC) increases delivery of cells to the injured hearts and improves cardiac performance in the nude rat. In this study, we have sought to validate our previous observations and to perform more detailed determination of ventricular function in immunocompetent mice with myocardial infarction (MI) that were treated with armed CD34+ HSC. We examined whether armed CD34+ HSC would target the injured heart following MI and restore ventricular function in vitro. MI was created by ligation of the left anterior descending artery. After 48 h, adult ICR mice received either 0.5 × 106 human CD34+ HSC armed with anti-CD45 × anti-MLC BiAb or an equal volume of medium through a single tail vein injection. Two weeks after stem cell administration, ventricular function of hearts from mice receiving armed CD34+ HSC was significantly greater compared with the same parameters from control mice. Immunohistochemistry confirmed the accumulation of CD34+ HSC in MI hearts infused with stem cells. Angiogenesis was significantly enhanced in CD34+ HSC-treated heart as determined by vascular density per area. Furthermore, histopathological examination revealed that the retained cardiac function observed in CD34+ HSC-treated mice was associated with decreased ventricular fibrosis. These results suggest that peripheral administration of armed CD34+ HSC results in localization of CD34+ HSC to injured myocardium and restores myocardial function.
Keywords: CD34+ hematopoietic stem cell, bispecific antibody, mouse, myocardium
Adult Bone Marrow is a rich reservoir of tissue-specific stem and progenitor cells. Bone marrow stromal cells have many characteristics of mesenchymal stem cells (21). Indeed, transplantation of bone marrow from C57/MLacZ into immunodeficient mice has revealed that marrow-derived cells migrate into areas of injury and induce muscle regeneration (6). Furthermore, directly injected primitive bone marrow cells give rise to the formation of new myocytes, endothelial cells, and smooth muscle cells, which coincides with significant improvements in myocardial functional recovery (12, 17, 20). In support of this latter observation, investigators using a gender-mismatch strategy identified Y chromosome-positive cardiac myocytes in histological sections of the left ventricle, suggesting that bone marrow is a contributor to low level de novo cardiac myocyte formation (5). In addition, cytokines such as G-CSF or GM-CSF have been shown to enhance mobilization of endothelial progenitors to ischemic limbs and thereby augment re-endothelialization (1).
Conflicting observations, however, do exist. For example, using sensitive genetic markers, some investigators have observed results that led them to conclude that bone marrow-derived hematopoietic stem cells (HSC) do not contribute to nonhematopoietic tissues in the injured myocardium (3, 19). Nevertheless, recent clinical studies have pointed toward some modest beneficial effects of intracoronary infusion of bone marrow-derived progenitor cells into ischemic hearts following myocardial infarction (2, 16, 24, 25). The reason(s) for these contradictory findings remains unclear. Potential causes include differences between animal models, cell delivery approaches, or sensitivity of detection of the cell phenotype. Additionally, it has been suggested that the failure to deliver sufficient bone marrow stem cells into damaged hearts or lack of a consistently high number of progenitor cells in the site of injury could be the main reason for the inability to detect differentiated cardiac structures in damaged myocardium (10).
We have recently taken advantage of a cell targeting strategy developed for cancer immunotherapeutics (15, 22). In this approach, a monoclonal antibody directed against a stem cell antigen is covalently conjugated to a monoclonal antibody directed at an antigen expressed on the target tissue. We and colleagues have shown that bispecific antibody (BiAb) technology offers a viable means to improve the delivery of stem cells into injured myocardium and facilitate myocardial repair (11, 13, 14). In previous studies, arming CD34+ HSC with anti-CD45 × anti-myosin light chain (MLC) BiAb and intravenously administering them post-myocardial infarction significantly increased the accumulation of human CD34+ stem cells in the region of injury and improved in vivo cardiac function in a nude rat myocardial infarction model (11). It remained to be determined to what extent targeted stem cells improve myocardial function and prevent myocardial remodeling. Therefore, we used the Langendorff isovolumetrically perfused heart model to compare cardiac function of mice with myocardial infarction that are either untreated or treated with armed, human CD34+ HSC. Our findings indicate that armed CD34+ HSC administered intravenously 48 h after myocardial infarction localizes to the ischemic regions of the mouse heart. This localization was associated with improved ventricular function, enhanced angiogenesis, and reduced fibrosis. Furthermore, administration of armed stem cells prevented cardiac remodeling in hearts that received CD34+ HSC compared with those receiving vehicle alone.
Methods and Materials
Animals
Adult male outbred mice (ICR strain) were supplied by Harlan (Indianapolis, IN). All animal experiments were conducted under a protocol approved by our Institutional Animal Care and Use Committee and complied with the guidelines on humane use and care of laboratory animals for biomedical research published by the National Institutes of Health.
In vivo myocardial infarction
A mouse myocardial infarction model was created following thoracotomy by applying permanent ligation of the left anterior descending artery (LAD) as described by ourselves and others (8). Briefly, mice were anesthetized with an intraperitoneal (ip) injection of pentobarbital sodium (50 mg/kg); additional doses of pentobarbital were given as needed during the procedure to maintain an anesthetized state. Mice were placed in a supine position and were intubated with an endotracheal tube. Ventilation was maintained by connecting the endotracheal tube to a rodent ventilator (Harvard, Holliston, MA). Thoracotomy was performed with tenotomy scissors. A 7-0 nylon suture was passed with a tapered needle under the left anterior descending coronary artery. The suture was tied to create a coronary occlusion. On completion of the ligation, the chest was closed in a layered fashion, and air was evacuated to prevent a pneumothorax. Mice in the sham group were anesthetized and underwent thoracotomy without coronary ligation. Animals' body temperatures were maintained at 37°C during the procedure and postoperatively.
CD34+ HSC purification and characterization
The methodology for CD34+ HSC purification has been described previously (11, 13, 14). Briefly, unused, excess G-CSF-primed peripheral blood mononuclear cells were obtained from normal human stem cell donors by leukapheresis. Collection and use of human blood products for research were conducted under Institutional Review Board-approved transplant protocols at Roger Williams Medical Center, and signed consents were obtained from donors. Highly purified CD34+ HSC were obtained using an ISOLEX 300i Magnetic Cell Selection System and standard operating procedures compliant with good laboratory practices in the stem cell processing laboratory of the Blood and Stem Cell Transplant Program. Purified CD34+ HSC were cryopreserved in convenient aliquots and were later thawed and washed free of cryopreservative before their experimental use.
The purified CD34+ HSC population was characterized by double staining with anti-CD34-APC and various stem cell markers including anti-CD45-FITC, anti-CD133-PE, anti-CD117-PE, and anti-CD38-PE (BD Pharmingen, San Diego, CA). The population was then analyzed by flow cytometry on a FACSCalibur System using “CELLQuest” software (BD Biosciences, San Jose, CA). Cell populations for analysis were gated to exclude dead cells based on forward-scatter vs. side-scatter plots.
BiAb engineering, arming, and MLCBi-armed CD34+ HSC treatments
The anti-CD45 × anti-MLC BiAb (MLCBi) was prepared by chemically heteroconjugating mouse anti-human CD45 (BD Pharmingen, San Diego, CA) to mouse anti-human cardiac MLC as previously described (11). According to non-reducing SDS-PAGE analysis, MLCBi has a 0.18 dimer-to-monomer ratio (11). Previous binding studies of MLCBi (5–500 ng/106 CD34+ HSC), based on use of goat anti-mouse IgG2a-phycoerythrin for detection of the anti-MLC moiety of the BiAb and a goat IgG-phycoerythrin isotype control to determine nonspecific staining, have revealed an arming dose of 50 ng/106 CD34+ HSC for saturation of the CD45 binding sites on CD34+ HSC. Therefore, for these studies, we used 50 ng/106 CD34+ HSC to arm purified CD34+ HSC.
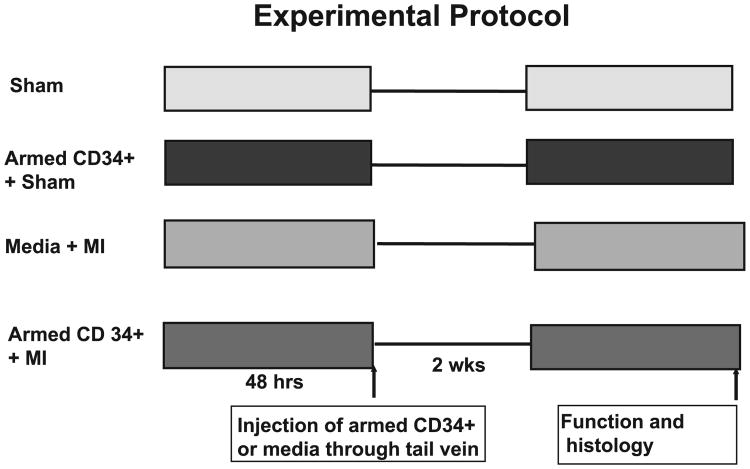
Two days after myocardial infarction, either 0.5 × 10 armed CD34+ HSC or 0.15 ml RPMI cell culture medium was administered to mice by intravenous injection via the tail vein. All animals receiving stem cells or RPMI medium were killed 2 wk later. Additionally, a control group was included in which 0.5 × 106 armed CD34+ HSC was administered to sham animals to rule out the potential impact of armed CD34+HSC on non-injured hearts. The experimental protocol is depicted in Fig. 1.
Fig. 1.
Experimental protocols and animal groups for study of the effects of the armed CD34+ HSC on myocardial functional recovery. See methods for detailed description of the groups.
Langendorff isolated heart perfusion
The methodology of Langendorff's perfused heart preparation and measurement of ventricular function has been described previously in detail (28). Briefly, mice were anesthetized with a lethal ip injection of pentobarbital sodium (120 mg/kg). Hearts were rapidly excised and arrested in ice-cold Krebs-Henseleit buffer. They were then cannulated via the ascending aorta for retrograde perfusion by the Langendorff method using Krebs-Henseleit buffer containing (in mM) 110 NaCl, 4.7 KCl, 1.2 MgSO4·7H2O, 2.5 CaCl2·2H2O, 11 glucose, 1.2 KH2PO4, 25 NaHCO3, and 0.5 EDTA. The buffer, aerated with 95% O2-5% CO2 to give a pH of 7.4 at 37°C, was perfused at a constant pressure of 55 mmHg. A water-filled latex balloon, attached to the tip of polyethylene tubing, was then inflated sufficiently to provide a left ventricular end-diastolic pressure (LVEDP) of ∼10 mmHg measured by means of a disposable Gould pressure transducer. Left ventricular functional analysis was recorded using software and a computer-based recording system (BIOPAC Systems, MP100A, Goleta, CA). These parameters included left ventricular systolic pressure (LVSP), LVEDP, heart rate, and cardiac contractile function. Rate pressure product (RPP) was calculated as the product of left ventricular developed pressure and heart rate, where developed pressure is systolic pressure minus LVEDP.
Immunohistology and immunofluorescent staining
Tissues were snap-frozen in super cooled 2-methylbutane embedded in Tissue-Tek OCT (Sakura Finetek, Torrance, CA). Then 10- μm-thick sections from the middle part of hearts were cut with a cryostat (Thermo Shandon, Pittsburgh, PA) at −22°C, mounted on plus-charged slides (Fisher Scientific) and air-dried at room temperature. Cryostat sections were fixed in ethanol for 15 min, dried for 30 min at 37°C, and then rehydrated in PBS. To detect human cells in the mouse myocardium, staining was carried out with mouse anti-human HLA-A,B,C (BD Biosciences) according to the manufacturer's instructions using the Animal Research Kit (ARK; DakoCytomation, Carpinteria, CA) following high pH target retrieval and endogenous biotin blocking (DakoCytomation). Anti-HLA (0.625 (μg/ml) was reacted with biotinylating and blocking reagents (ARK System) just before addition to tissue sections and then incubated for 30 min at room temperature. Primary antibody was detected with streptavidin-horseradish peroxidase (1:500 dilution; DAKOCytomation). For immunofluorescence staining, sections were fixed in 3.7% (vol/vol) paraformaldehyde for 15 min and permeabilized in 0.5% Triton X-100 in PBS for 10 min. The sections were then incubated with an anti α-smooth muscle actin (α-SMA) monoclonal antibody and anti-von Willebrand factor antibody (vWF; Sigma, St. Louis, MO) for 1 h. Secondary antibodies Texas Red horse anti-mouse IgG (H+L) and/or fluorescein anti-rabbit IgG (H+L) (Vector Laboratories, Burlingame, CA) were applied at room temperature. Fluorescent imaging was performed using a high-resolution Nikon E2000 fluorescence microscope equipped with MetaVue software for image analysis. The numbers of α-SMA- and vWF-positive vessels were counted in six randomized fields of the tissue sections, which were taken in the middle plane of each heart and contained infarct and border regions. The total number of vessels from each group was calculated and normalized to tissue area.
Statistics
All data are expressed as means ± SE. Differences among the groups were analyzed by one-way ANOVA, followed by post hoc Bonferroni comparison or Student's unpaired t-test for two groups. A probability P < 0.05 was considered a significant difference.
Results
Armed stem cells restore ventricular function of postmyo-cardial infarction in mouse heart
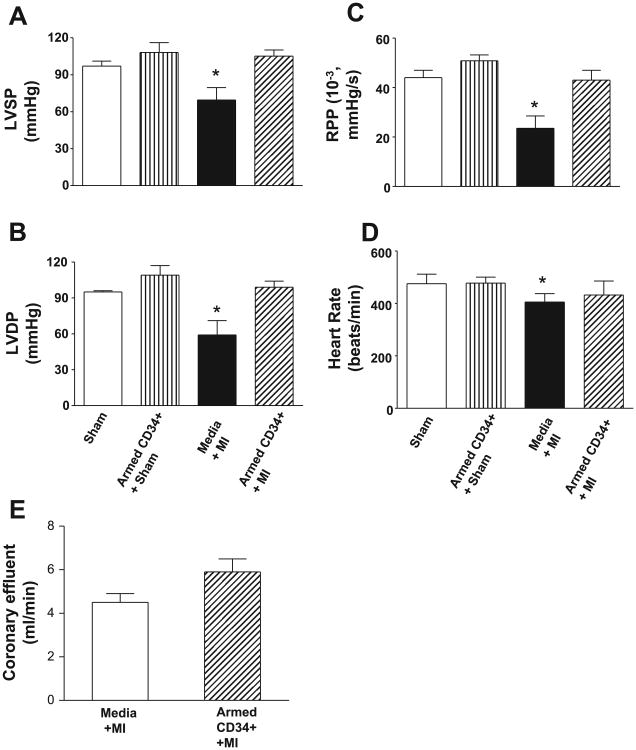
Ventricular function in the four groups of animals 2 wk after intravenous infusions is shown in Fig. 2, A–D. There was a significant reduction in left ventricular function, as measured by LVSP, LVDP, and RPP, following LAD ligation in the animals that received media alone compared with sham control hearts (P < 0.05). In contrast, administration of armed CD34+ HSC stem cells after LAD ligation restored myocardial function to a level near that of the sham thoracotomy control hearts. LVSP, LVDP, and RPP were all significantly better in the animals receiving armed stem cells and the sham controls than the animals receiving the media vehicle (Fig. 2, A–C; P < 0.05). Additionally, although heart rates were similar between all groups, coronary flow was significantly increased (P < 0.05) in the hearts of mice that received CD34+ HSC compared with those that received media alone (Fig. 2E).
Fig. 2.
The effects of infusion of the armed CD34+ on ventricular function in left ventricular systolic pressure (LVSP; A), left ventricular developed pressure (LVDP; B), rate pressure product (C), heart rate (D), and coronary flow (E). Values are means ± SE (n = 4–7). *Media less than sham or armed cells (P < 0.05).
Evidence of CD34+ HSC in infarcted regions of mouse hearts
We assessed the presence of human cells 2 wk post-myocardial injury. As shown in Fig. 3, numerous cells expressing human HLA class I antigen were detected solely in the ischemic regions of the myocardial tissues of animals that received MLCBi-armed CD34+ HSC. This was visualized by IHC staining with anti-HLA A, B, C (class I) antibody. However, no HLA class I-positive staining was detected in the hearts of animals that underwent LAD but did not receive stem cell infusions. These data, therefore, confirm the presence of human HLA class I cells localized to ischemic injury sites following intravenous infusion of CD34+ HSC armed with MLCBi antibody. These findings are consistent with our previous reports using a myocardial infarction model in nude rats whereby MLCBi-armed CD34+ HSC similarly homed to sites of ischemic injury. In our previous study, arming with MLCBi provided a significant increase in the number of CD34+ HSC that localized to the injured myocardium after intravenous injection compared with CD34+ HSC that were not armed (11).
Fig. 3.
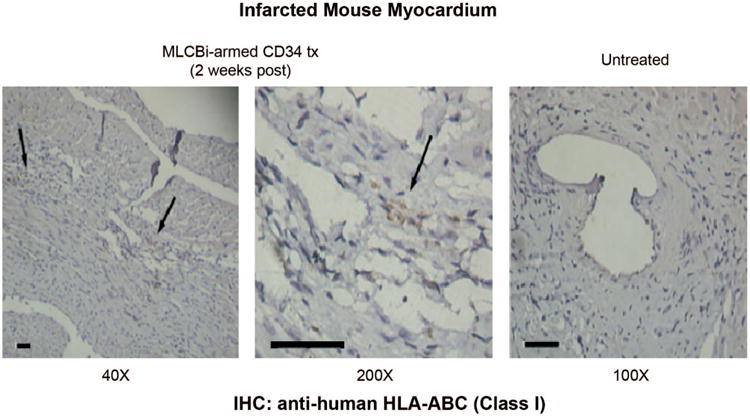
Immunohistochemical staining for human HLA-A,B,C class I cells in the infarcted myocardium 2 wk after tail vein infusion. Left and middle: positive staining at ×40 and ×200 in an animal that received armed CD34+ HSC. Right: no staining in a control animal. Bars = 100 μm
Prevention of myocardial remodeling and increase in angiogenesis
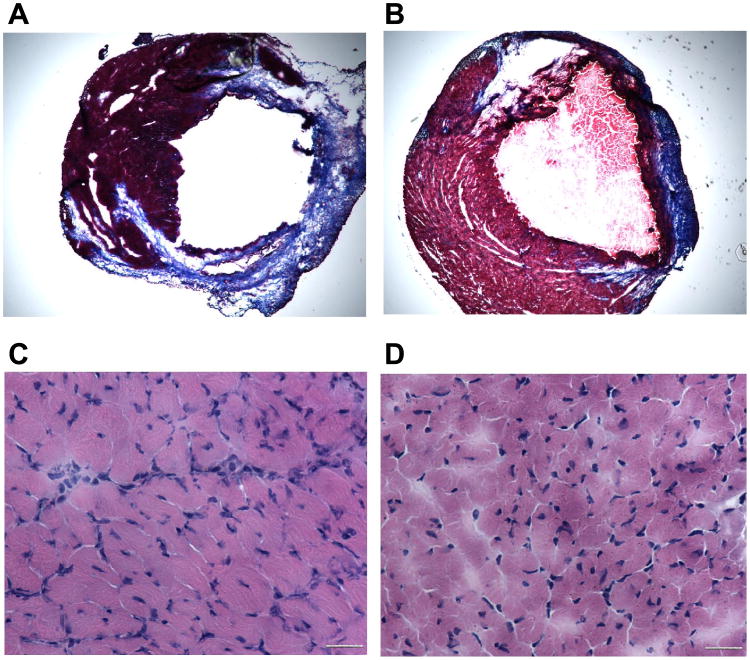
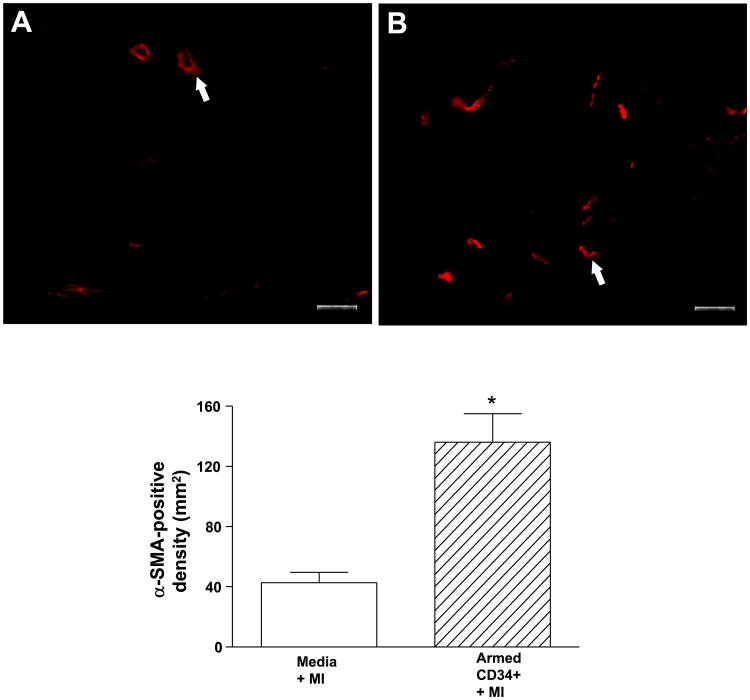
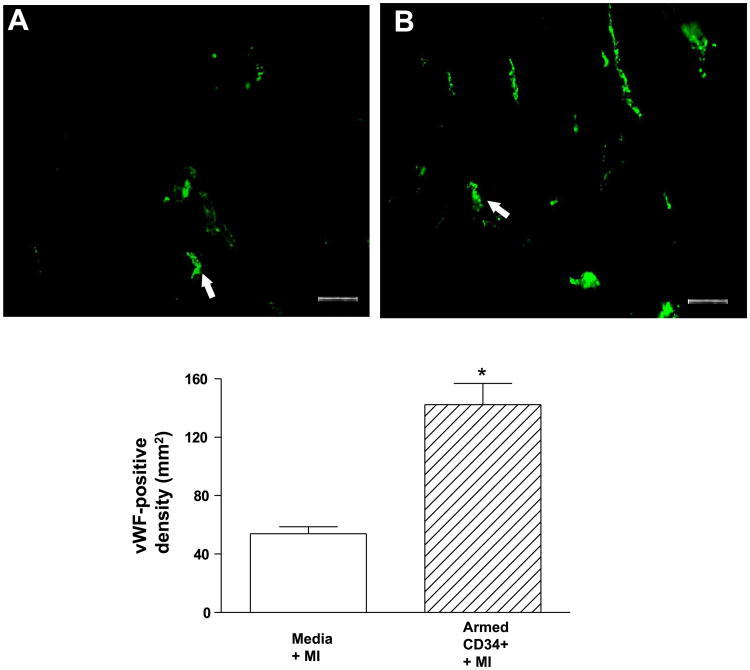
As shown in Fig. 4, A and B, animals with myocardial infarction alone and animals with myocardial infarction receiving the armed CD34+ HSC exhibited enlarged chamber diameters compared with noninfarcted control hearts. However, animals with myocardial infarction alone (Fig. 4A) exhibited a decrease in infarct wall thickness, suggesting characteristic postmyocardial infarction scar thinning and infarct expansion. In contrast, the hearts of animals receiving armed CD34+ HSC (Fig. 4B) had sustained wall thickness relative to myocardial infarction hearts. The infarcted myocardium displayed areas of concentrated fibrosis defined by Masson-trichrome staining, whereas noninfarcted control hearts appeared as continuous viable myocardium with homogenous thickness. Masson-trichrome staining revealed that administration of the armed CD34+ HSC reduced the content of fibrosis, although both infarcted hearts and myocardial infarction hearts receiving stem cells displayed an irregular arrangement in myocyte orientation. As shown in Fig. 4, C and D, hematoxylin and eosin staining showed that the compensatory hypertrophic enlarged cross-sectional myofibrillar response (Fig. 4C) was alleviated following treatment with armed stem cells (Fig. 4D). The angiogenic response was examined by immunofluorescent staining for α-SMA and vWF, which are shown in Figs. 5 and 6, respectively. In the border and center of the infarct zones, vascular SMA and von Willebrand factor-positive staining was observed. Infusion of armed CD34+ HSC following LAD ligation increased the number of α-SMA and vWF stained vessels and vascular density in the border zones of infarcted hearts compared with LAD ligation alone (Figs. 5 and 6).
Fig. 4.
Microscopic analysis in trichrome-stained (×20; A and B) and hematoxylin and eosin-stained (×400; C and D) heart section from infarcted mouse receiving vehicle (A and C) or CD34+ HSC (B and D). Bars = 50 μm
Fig. 5.
Immunofluorescent staining for alpha-smooth muscle actin-positive cells in the hearts receiving vehicle (A) or CD34+ HSC (B), ×200 (top), and average number for alpha-smooth muscle actin-positive cells (bottom). Values are means ± SE (n = 3). *P < 0.05. Bars = 50 μm.
Fig. 6.
Immunofluorescent staining for von Willebrand factor-positive cells in the hearts receiving vehicle (A) or CD34+ HSC (B), ×200 (top), and average number for von Willebrand factor-positive cells (bottom). Values are means ± SE (n = 3). *P < 0.05. Bar = 50 μm.
Discussion
This study demonstrates the beneficial effects of targeted stem cells on restoration of cardiac function following myocardial infarction. By adapting BiAb technology, we have developed a novel approach for increasing the delivery and persistence of stem cells to sites of injured cardiac target tissue (11, 13). We sought to validate this effect using an alternative model and to evaluate functional effects in greater detail. We examined whether similarly prepared human CD34+ HSC would target the injured heart following myocardial infarction in adult, immunocompetent ICR mice. Cardiac injury was created by ligation of the LAD in adult ICR mice. After 48 h, animals received either 0.5 × 106 human CD34+ HSC targeted with a BiAb directed against CD34 and MLC or an equal volume of cell culture medium through a single tail vein injection. We selected the MLC as the antigen for arming stem cells because ischemic injury of myocardium leads to an abundant release and local accumulation of MLC from myocyte into myocardium (26). In our previous studies, there was no significant difference between animals with myocardial infarction that received unarmed cells and placebo. There was also no effect of infusion the MLCBi alone following cardiac injury (11). As seen following administration of armed human CD34+ HSC to nude rats after myocardial infarction, in the present study, cells were detected with human class I antibodies in infarcted myocardium of immunocompetent mice infused with armed CD34+ HSC. These results indicate that peripheral administration of the armed HSC is a reliable approach to delivering stem cells to the injured myocardium (14).
Although infusion of armed stem cells significantly improved the myocardial chamber dimension as measured by echocardiographic analysis in the nude rat, we were still not certain to what extent administration of armed CD34+ HSC could improve ventricular functional recovery as assessed by detailed in vitro analysis and in fully immunocompetent adult mice. As such, using the isovolumetric, retrograde perfused heart, we assessed the ventricular functional recovery of post-myocardial infarcted hearts in adult mice. Administration of armed CD34+ HSC resulted in dramatic improvement in ventricular functional recovery of postinfarcted myocardium, whereas administration of the armed CD34+ HSC did not significantly affect ventricular function in sham mice. Because we did not administer stem cells to the control ischemic hearts, we reason that functional improvement is due in large measure to the biological function(s) of the armed CD34+ HSC.
There are several reports indicating that the beneficial effects of stem cells on damaged myocardium is due to the prevention of myocardial remodeling. Myocardial infarction is associated with an increase in fibrosis and irregular myocyte structure, which was ameliorated following intramyocardial injection of mesenchymal stem cells (18). In our study, negative myocardial remodeling was also significantly diminished following provision of CD34+ HSC. This may be due to paracrine secretory effects of stem cells, which have been regarded as important mechanisms whereby stem cells generate these protective effects (7). We observed significant restoration of coronary flow and increased staining for vascular SMA in the perinfarction region of animals that received armed stem cells. Increased nutrient and oxygen delivery may be another potential mechanism for prevention of cardiac remodeling.
We had considered using immunosuppression to sustain the fate of transplanted cells into recipients (11). It is unknown whether such a manipulation of immunofunction would produce detrimental effects on stem cell function(s) in recipient hearts. It has been shown that the marrow stromal cells have a unique immunological tolerance allowing their engraftment into a xenogeneic environment (23). Based on these observations, we tested the function of armed CD34+ HSC on outbred ICR mice. The apparent lack of a negative immune response, indicated by the absence of an inflammatory response despite the persistence of the armed CD34+ HSC in adult ICR mice, is intriguing. Although tolerance of xenogeneic cells in a fully immunocompetent recipient is demonstrated, our studies were completed within 2 wk. We do not yet know the long-term persistence of these cells in this model or their long-term functional effects.
Experiments in mice have shown that significant numbers of bone marrow-derived stem cells appear in liver, spleen, and bone marrow after intravenous injection (4, 9). Based on our earlier results, we reasoned that arming stem cells with MLC provided the best chance for stem cells to find a “niche” within the myocardium. As documented in our previous studies, a significantly greater number of armed cells reach the injured area compared with unarmed cells in ours and other models (11, 13, 27). Such a “niche” may provide an opportunity for stem cells to communicate with myocytes, which may coordinate a signal for stem cell-mediated modulation of repair and inflammation or even differentiation and regeneration. Homing of stem cells to the myocardium could considerably reduce the number of cells needed for cell-based repair in the infarct zone. We employed the arming approach, which has been validated in cancer immunotherapy to direct armed stem cells into the injury area and increase the persistence of their numbers. The increase in stem cell accumulation in the infarcted myocardium using armed cells has been well demonstrated by both internal jugular vein and direct injection of cells into infarcted myocardium compared with nonarmed stem cells (11, 15). Whether or not armed CD34+ HSC or any other stem cells develop into cardiac myocyte-like structures remains controversial.
It has been shown that angiogenesis following stem cell delivery contributes to the restoration/preservation of myocardial function following myocardial infarction (27). Similarly, we observed a more robust angiogenic response after administration of the armed CD34+ HSC. In addition to prevention of remodeling, this may constitute a pivotal mechanism by which the armed stem cells produce a beneficial effect on myocardial injury. Future experiments are needed to define the cellular mechanism(s) elicited by armed CD34+ HSC in the preservation of myocardial function following experimental ligation.
In summary, we used an established approach to target CD34+ HSC with MLC antigen and peripherally delivered the stem cells into fully immunocompetent adult mice. Using the isovolumetrically perfused heart model, our data demonstrated that the intravenous injection of armed CD34+ HSC resulted in restoration of ventricular function in postinfarcted myocardium, which was associated with the prevention of myocardial fibrosis and myocardial remodeling. The armed CD34+ HSC were present in the border area of injured hearts, documenting their presence in the area of injury, suggesting the lack of immunoresponse in immunocompetent recipients, and suggesting the lack of immunoresponse in immunocompetent recipients. The increase in angiogenic response may also indicate the contribution of CD34+ HSC to neovascularization and myocardial functional recovery. Our results further suggest that armed CD34+ HSC hold promise for clinical stem cell therapy in the future.
Acknowledgments
Grants: This work was supported by National Center for Research Resources Grant 1P20 RR-01872802 and Rhode Island Foundation 20052634; L. G Lum was supported in part by National Cancer Institute Grant R01 CA-92344.
References
- 1.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Assmus B, Honold J, Schächinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 3.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 4.Burnet FM. The Clonal Selection Theory of Acquired Immunity. Nashville, TN: Vanderbilt University Press; 1959. [Google Scholar]
- 5.Deb A, Wang S, Skelding KA, Miller D, Simper D, Caplice NM. Bone marrow-derived cardiomyocytes are present in adult human heart: a study of gender-mismatched bone marrow transplantation patients. Circulation. 2003;107:1247–1249. doi: 10.1161/01.cir.0000061910.39145.f0. [DOI] [PubMed] [Google Scholar]
- 6.Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 7.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 8.Guo Y, Jones WK, Xuan YT, Tang XL, Bao W, Wu WJ, Han H, Laubach VE, Ping P, Yang Z, Qiu Y, Bolli R. The late phase of ischemic preconditioning is abrogated by targeted disruption of the inducible NO synthase gene. Proc Natl Acad Sci USA. 1999;96:11507–11512. doi: 10.1073/pnas.96.20.11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hendrikx PJ, Martens CM, Hagenbeek A, Keij JF, Visser JW. Homing of fluorescently labeled murine hematopoietic stem cells. Exp Hematol. 1996;24:129–140. [PubMed] [Google Scholar]
- 10.Kajstura J, Rota M, Whang B, Cascapera S, Hosoda T, Bearzi C, Nurzynska D, Kasahara H, Zias E, Bonafe M, Nadal-Ginard B, Torella D, Nascimbene A, Quaini F, Urbanek K, Leri A, Anversa P. Bone marrow cells differentiate in cardiac cell lineages after infarction independently of cell fusion. Circ Res. 2005;96:127–137. doi: 10.1161/01.RES.0000151843.79801.60. [DOI] [PubMed] [Google Scholar]
- 11.Lee RJ, Fang Q, Davol PA, Gu Y, Sievers RE, Grabert RC, Gall JM, Tsang E, Yee MS, Fok H, Huang NF, Padbury JF, Larrick JW, Lum LG. Antibody targeting of stem cells to infarcted myocardium. Stem Cells. 2007;25:712–717. doi: 10.1634/stemcells.2005-0602. [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Hu Q, Wang Z, Xu C, Wang X, Gong G, Mansoor A, Lee J, Hou M, Zeng L, Zhang JR, Jerosch-Herold M, Guo T, Bache RJ, Zhang J. Autologous stem cell transplantation for myocardial repair. Am J Physiol Heart Circ Physiol. 2004;287:H501–H511. doi: 10.1152/ajpheart.00019.2004. [DOI] [PubMed] [Google Scholar]
- 13.Lum LG, Fok H, Sievers R, Abedi M, Quesenberry PJ, Lee RJ. Targeting of Lin-Sca+ hematopoietic stem cells with bispecific antibodies to injured myocardium. Blood Cells Mol Dis. 2004;32:82–87. doi: 10.1016/j.bcmd.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Lum LG, Padbury JF, Davol PA, Lee RJ. Virtual reality of stem cell transplantation to repair injured myocardium. J Cell Biochem. 2005;95:869–874. doi: 10.1002/jcb.20504. [DOI] [PubMed] [Google Scholar]
- 15.Lum LG, Davol PA, Lee RJ. The new face of bispecific antibodies: targeting cancer and much more. Exp Hematol. 2006;34:1–6. doi: 10.1016/j.exphem.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, Endresen K, Ilebekk A, Mangschau A, Fjeld JG, Smith HJ, Tarald-srud E, Grøgaard HK, Bjørnerheim R, Brekke M, Müller C, Hopp E, Ragnarsson A, Brinchmann JE, Forfang K. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 17.Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 19.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 20.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 21.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 22.Reusch U, Sundaram M, Davol PA, Olson SD, Davis JB, Demel K, Nissim J, Rathore R, Liu PY, Lum LG. Anti-CD3 × anti-epidermal growth factor receptor (EGFR) bispecific antibody redirects T-cell cytolytic activity to EGFR-positive cancers in vitro and in an animal model. Clin Cancer Res. 2006;12:183–190. doi: 10.1158/1078-0432.CCR-05-1855. [DOI] [PubMed] [Google Scholar]
- 23.Saito T, Kuang JQ, Bittira B, Al-Khaldi A, Chiu RC. Xenotransplant cardiac chimera: immune tolerance of adult stem cells. Ann Thorac Surg. 2003;74:19–24. doi: 10.1016/s0003-4975(02)03591-9. [DOI] [PubMed] [Google Scholar]
- 24.Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Süselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM. Intracoronary bone marrow-eerived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 25.Xu M, Uemura R, Dai Y, Wang Y, Pasha Z, Ashraf M. In vitro and in vivo effects of bone marrow stem cells on cardiac structure and function. J Mol Cell Cardiol. 2007;42:441–448. doi: 10.1016/j.yjmcc.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yazaki Y, Nagai R, Yamaoki K, Ueda S. Myocardial infarct size from serum cardiac myosin light chain. Clinical and experimental studies. Adv Myocardiol. 1983;4:489–495. doi: 10.1007/978-1-4757-4441-5_46. [DOI] [PubMed] [Google Scholar]
- 27.Yeh ET, Zhang S, Wu HD, Korbling M, Willerson JT, Estrov Z. Transdifferentiation of human peripheral blood CD34+-enriched cell population into cardiomyocytes, endothelial cells, and smooth muscle cells in vivo. Circulation. 2003;108:2070–2073. doi: 10.1161/01.CIR.0000099501.52718.70. [DOI] [PubMed] [Google Scholar]
- 28.Zhao TC, Kukreja RC. Protein kinase C-delta mediates adenosine A3 receptor-induced delayed cardioprotection in mouse. Am J Physiol Heart Circ Physiol. 2003;285:H434–H441. doi: 10.1152/ajpheart.00095.2003. [DOI] [PubMed] [Google Scholar]