Abstract
Chronic inflammation may play a role in ovarian carcinogenesis. We examined associations between 3 plasma biomarkers of inflammation—C-reactive protein (CRP), interleukin 6, and tumor necrosis factor α receptor 2—and risk of invasive epithelial ovarian cancer in prospectively collected samples from the Nurses' Health Study (NHS; 1989–2010), Nurses’ Health Study II (NHS II; 1996–2009), and the Women's Health Study (WHS; 1992–2011) and performed a meta-analysis including data from previous publications. Associations with ovarian cancer risk were calculated using logistic regression (NHS/NHS II; n = 217 cases) or Cox proportional hazards regression (WHS; n = 159 cases). Study-specific results were combined using random-effects meta-analysis. In the NHS/NHS II and WHS, we observed a 53% increased risk of invasive ovarian cancer when comparing women in the fourth quartile of CRP with women in the first quartile (95% confidence interval (CI): 1.05, 2.23). A CRP level of >10 mg/L versus a level of ≤1 mg/L was associated with a 2.16-fold increased risk (95% CI: 1.23, 3.78). In a meta-analysis of published studies, women in the third tertile of CRP had a 35% increased risk (95% CI: 1.10, 1.67) compared with women in the first tertile. There were no significant associations between interleukin 6 or tumor necrosis factor α receptor 2 and risk in the NHS/NHS II. Our results support the hypothesis that higher levels of circulating CRP are associated with increased risk of ovarian cancer, indicating that the role of inflammation in ovarian cancer requires further elucidation.
Keywords: C-reactive protein, interleukin 6, ovarian cancer, tumor necrosis factor α receptor 2
Ovarian cancer is highly fatal (1), partly because of an incomplete understanding of its etiology. One hypothesis regarding ovarian carcinogenesis is that of incessant ovulation, which may increase risk through repeated damage to and wound repair of the ovarian epithelium, a process that can induce inflammation (2, 3). Epidemiologic evidence supports this hypothesis, as events that interrupt ovulation (e.g., pregnancy, oral contraceptive use) or lower inflammation (e.g., tubal ligation) reduce risk while exposures that cause inflammation (e.g., talc use) increase risk (2, 4–6). Further, higher lifetime numbers of ovulations increase the risk of ovarian cancer (2, 7–9). Biological effects of inflammation include enhanced cellular proliferation and angiogenesis, an inability to adapt to oxidative stress, and inhibition of apoptosis (10, 11).
C-reactive protein (CRP) is a marker of global inflammation (12, 13) that is higher in women with malignant ovarian tumors than in women with benign ovarian tumors or healthy controls, and it is positively correlated with stage at diagnosis (14, 15). Prediagnostic CRP levels have been positively associated with ovarian cancer risk in 3 studies, with odds ratios ranging from 1.15 to 1.96 for the top tertile compared with the bottom tertile (16–18). Lundin et al. (17) observed that the increased risk was highest among women with very high CRP levels (for >10 mg/L vs. <1 mg/L, odds ratio = 4.4).
Other inflammatory markers also may be important in ovarian carcinogenesis. Interleukin 6 (IL-6), a proinflammatory cytokine involved in immune defense (19), is higher in women with malignant ovarian tumors than in women with benign ovarian tumors (20, 21) or healthy controls (14, 22), is detectable in ascites (23, 24), and is associated with increased tumor burden and worse survival (14, 25). Tumor necrosis factor α (TNF-α) stimulates production of various cytokines and induces expression of cellular adhesion molecules (26). Its levels are higher in ovarian cancer cases than in healthy controls (14, 23). In a recent prospective study, Clendenen et al. (27) reported that women in the highest quartile of IL-6 level versus women in the lowest quartile had a 70% increased risk of ovarian cancer; however, no associations were observed for TNF-α or its soluble receptors.
The goal of this study was to examine the relationship between circulating levels of CRP, IL-6, and TNF-α and risk of ovarian cancer in the Nurses' Health Study (NHS) and Nurses' Health Study II (NHS II). However, reliably assaying TNF-α in previously frozen samples is not possible. Therefore, we measured levels of tumor necrosis factor α receptor 2 (TNF-α-R2), one of two TNF-α receptors. TNF-α signaling though TNF-α-R2 results in T-cell proliferation and other proinflammatory responses (28). We also evaluated the association for CRP in the Women's Health Study (WHS) and conducted a meta-analysis of this association with data from 3 prior studies.
MATERIALS AND METHODS
Study population
This analysis was based on data from nested case-control studies in the NHS (29) and NHS II (30) and a prospective cohort study in the WHS (31–33), described below. The NHS was established in 1976 among 121,701 US female nurses aged 30–55 years, and NHS II was established in 1989 among 116,430 female nurses aged 25–42 years. Participants have been followed biennially by questionnaire to update information on exposure status and disease diagnoses. In 1989–1990, 32,826 NHS participants provided blood samples and completed a short questionnaire (29). Briefly, women arranged to have their blood drawn and shipped with an ice pack, via overnight courier, to our laboratory, where it was processed and separated into plasma, red blood cell, and white blood cell components. Follow-up of the NHS blood study cohort was 87.5% in 2010.
Between 1996 and 1999, 29,611 NHS II participants provided blood samples and completed a short questionnaire (30). Premenopausal women (n = 18,521) who had not taken hormones, been pregnant, or lactated within the past 6 months provided blood samples drawn 7–9 days before the anticipated start of their next menstrual cycle (luteal phase). Other women (n = 11,090) provided a single 30-mL untimed blood sample. Samples were shipped and processed identically to the NHS samples. Follow-up of the NHS II blood study cohort was 95% in 2009.
Cases had no previous history of cancer, except nonmelanoma skin cancer, before blood collection and were diagnosed with ovarian cancer between blood draw and June 1, 2010 (NHS), or June 1, 2009 (NHS II). A total of 217 cases of invasive epithelial ovarian or peritoneal cancer (182 in NHS and 35 in NHS II) with plasma were confirmed by medical record review. Cases were matched to 2 controls with intact ovaries at the time of the case diagnosis on menopausal status at blood draw and diagnosis (premenopausal, postmenopausal, or unknown), age (±1 year), month of blood collection (±1 month), time of day of blood draw (±2 hours), fasting status (>8 hours or ≤8 hours), and postmenopausal hormone use at blood draw (yes or no). For NHS II cases with timed samples, we additionally matched on date of the luteal blood draw (date of next menstrual cycle minus date of blood draw, ±1 day).
The WHS is a completed randomized trial, initiated in 1992, that examined low-dose aspirin and vitamin E supplementation in the primary prevention of cancer and cardiovascular disease (31–33). Blood samples were collected from 28,345 women prior to randomization and were processed into plasma, red blood cells, and white blood cells. We included women from the treatment and placebo groups. When the trial was completed in 2004, morbidity and mortality follow-up were 97.2% and 99.4% complete, respectively. Women were invited to continue in a follow-up study, and 88% agreed. Follow-up for morbidity among the observational follow-up participants is 93% complete. Cases were diagnosed with invasive ovarian cancer between blood collection and March 2, 2011. A total of 159 invasive epithelial ovarian cancer cases were identified. All 3 studies were approved by the Committee on the Use of Human Subjects in Research at Brigham and Women's Hospital (Boston, Massachusetts).
Laboratory assays
The NHS and NHS II samples were assayed for high-sensitivity CRP, IL-6, and TNF-α-R2; the WHS blood samples were assayed for high-sensitivity CRP. The assays were conducted by Dr. Nader Rifai's laboratory at the Children's Hospital Medical Center and Harvard Medical School. CRP levels were measured via a validated immunoturbidometric method (Denka Seiken, Tokyo, Japan) (34). IL-6 was measured via a quantitative sandwich enzyme immunoassay technique using a Quantikine HS kit (R&D Systems, Minneapolis, Minnesota), and TNF-α-R2 was assessed via an enzyme-linked immunosorbent assay kit utilizing immobilized monoclonal antibody to human TNF-α-R2 (Genzyme, Cambridge, Massachusetts). Case-control sets (for the NHS/NHS II) and samples from the same study were assayed together, ordered randomly, and labeled to mask case-control status and quality control status. The intraassay coefficient of variation from blinded, replicate quality control samples ranged from 1% to 10% for all assays. These biomarkers were largely unaffected by transport conditions, and levels were reproducible within persons over time (35, 36). We assayed TNF-α-R2 because it is measured more reproducibly than TNF-α when samples have been previously frozen (37).
Statistical analysis
Statistical outliers (0 for CRP, 1 for TNF-α-R2, 6 for IL-6) were excluded (38). In the NHS/NHS II, relative risks and 95% confidence intervals were determined using unconditional logistic regression comparing quartiles of biomarker concentrations, with cutpoints based on the control distribution. In the WHS, relative risks and 95% confidence intervals were determined using Cox proportional hazards models; quartile cutpoints were based on the entire population. Trend tests were assessed using natural log-transformed values. Results were combined using random-effects meta-analysis (39). Analyses were adjusted for oral contraceptive use (never use, <5 years, or ≥5 years), tubal ligation (yes or no), parity (continuous), and body mass index (weight (kg)/height (m)2) at blood draw (continuous). NHS and NHS II analyses were further adjusted for matching factors.
In secondary analyses, we mutually adjusted the biomarkers (NHS/NHS II only), excluded cases diagnosed within 2, 5, and 10 years of blood collection, and evaluated associations among serous cases; cases with other histological types were too few to evaluate separately. We also evaluated whether the associations were stronger for tumors that were rapidly fatal (i.e., death due to ovarian cancer occurring within 3 years of diagnosis vs. not (40)) using polytomous logistic regression (NHS/NHS II) or competing-risks Cox models (WHS). Further, we stratified by body mass index (<25 or ≥25) and age (<60 years or ≥60 years). Interaction terms were developed by multiplying variables using the above cutpoints by quartiles of biomarker levels. Likelihood ratio tests were used to determine the P value for interaction. We also conducted a sensitivity analysis excluding persons with inflammatory conditions, including cardiovascular disease and rheumatoid arthritis, and adjusting for diabetes.
For the meta-analysis including data from previously published studies, we searched for “CRP” or “C-reactive protein” along with the terms “ovarian cancer” or “ovarian carcinoma” and “prospective.” Only 1 other study assessed IL-6 and TNF-α-R2 levels; therefore, we did not conduct a meta-analysis for these markers. Details on the previously published studies are provided in Web Table 1 (available at http://aje.oxfordjournals.org/). In 1 study, the association was strongest among women with CRP levels above 10 mg/L; thus, we replicated this analysis in the NHS, NHS II, and WHS and conducted a meta-analysis. In the NHS/NHS II, we included both invasive and borderline cases for the meta-analysis to be consistent with previous publications. Individual study results were combined using random-effects meta-analysis (39).
RESULTS
A total of 217 invasive cases from the NHS/NHS II and 159 invasive cases from the WHS were available for analysis. On average, cases were aged 66 years at diagnosis in the NHS/NHS II and aged 64 years at diagnosis in the WHS (Table 1); serous tumors were the most common histological type. Among cases diagnosed at least 3 years prior to the end of follow-up, 29% (NHS/NHS II) and 27% (WHS) died of ovarian cancer within 3 years of diagnosis. The 3 inflammatory markers were modestly correlated: The Pearson correlation coefficient for correlation between CRP and IL-6 was 0.37. For CRP and TNF-α-R2, the coefficient was 0.34, and for IL-6 and TNF-α-R2, it was 0.36.
Table 1.
Characteristics of Ovarian Cancer Patients in a Study of Inflammatory Markers and Risk of Invasive Ovarian Cancer, Nurses' Health Study (1989–2010), Nurses' Health Study II (1996–2009), and Women's Health Study (1992–2011)
| Characteristic | NHS/NHS II (n = 217) | WHS (n = 159) |
|---|---|---|
| Mean age (SD) at diagnosis, years | 66 (10) | 64 (9) |
| Histological type, % | ||
| Serous | 65 | 58 |
| Endometrioid | 12 | 7 |
| Mucinous | 5 | 4 |
| Other/unknown | 18 | 31 |
| Tumor stage, % | ||
| 1 or 2 | 30 | NA |
| 3 or 4 | 68 | |
| Missing data | 2 | |
| Ovarian cancer death within 3 years of diagnosis, %a | 29 | 27 |
Abbreviations: NA, not available; NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II; SD, standard deviation; WHS, Women's Health Study.
a Excludes women who did not have a full 3 years of follow-up postdiagnosis.
Plasma levels of CRP were positively associated with invasive ovarian cancer risk in the NHS/NHS II and WHS, with no heterogeneity between studies (P-heterogeneity > 0.11; Table 2). Women in the fourth quartile of CRP versus the first quartile had a 53% increased risk of epithelial ovarian cancer (95% confidence interval (CI): 1.05, 2.23). For very high levels of CRP, we observed that women with CRP levels greater than 10 mg/L had a relative risk of 2.16 (95% CI: 1.23, 3.78) in comparison with women with levels less than or equal to 1 mg/L. Results were similar when we evaluated serous cases, when we added borderline cases in the NHS/NHS II, and among cases who died within 3 years of diagnosis. Further, we observed similar associations when we excluded cases diagnosed within the first 2, 5, and 10 years after blood draw (Web Table 2) or those with known inflammatory conditions, as well as when we analyzed the results by categories of body mass index and age (data not shown).
Table 2.
Relationship Between C-Reactive Protein Level and Risk of Invasive Ovarian Cancer, Overall and by Case Type, in the Nurses' Health Study (1989–2010), Nurses' Health Study II (1996–2009), and the Women's Health Study (1992–2011)
| Analysis and CRP Level | NHS/NHS II (n = 217) |
WHS (n = 159) |
Combined Data From All 3 Cohorts |
|||||
|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | No. of Cases | RRa | 95% CIa | P-heterogeneityb | |
| All invasive casesc | ||||||||
| Quartile of CRP level | ||||||||
| Q1 | 1.00 | Reference | 1.00 | Reference | 78 | 1.00 | Reference | |
| Q2 | 1.10 | 0.68, 1.78 | 1.65 | 1.02, 2.68 | 97 | 1.35 | 0.90, 2.01 | 0.24 |
| Q3 | 1.05 | 0.64, 1.74 | 1.86 | 1.13, 3.05 | 99 | 1.40 | 0.80, 2.45 | 0.12 |
| Q4 | 1.34 | 0.79, 2.27 | 1.76 | 1.03, 3.01 | 102 | 1.53 | 1.05, 2.23 | 0.48 |
| P-trend | 0.18 | 0.03 | 0.01 | 0.59 | ||||
| Category of CRP level, mg/L | ||||||||
| ≤1 | 1.00 | Reference | 1.00 | Reference | 120 | 1.00 | Reference | |
| >1–≤10 | 1.08 | 0.74, 1.57 | 1.61 | 1.08, 2.41 | 233 | 1.31 | 0.88, 1.94 | 0.15 |
| >10 | 2.33 | 1.00, 5.44 | 2.02 | 0.96, 4.27 | 23 | 2.16 | 1.23, 3.78 | 0.80 |
| Serous/poorly differentiated cases | (n = 117) | (n = 92) | ||||||
| Q1 | 1.00 | Reference | 1.00 | Reference | 41 | 1.00 | Reference | |
| Q2 | 1.16 | 0.64, 2.12 | 2.31 | 1.21, 4.40 | 60 | 1.62 | 0.83, 3.17 | 0.13 |
| Q3 | 1.26 | 0.67, 2.36 | 2.10 | 1.06, 4.16 | 58 | 1.60 | 0.97, 2.62 | 0.28 |
| Q4 | 1.25 | 0.63, 2.47 | 1.97 | 0.94, 4.12 | 50 | 1.54 | 0.93, 2.54 | 0.37 |
| P-trend | 0.38 | 0.18 | 0.12 | 0.75 | ||||
| Cases fatal within 3 years of diagnosis | (n = 59) | (n = 43) | ||||||
| Q1 | 1.00 | Reference | 1.00 | Reference | 15 | 1.00 | Reference | |
| Q2 | 2.03 | 0.83, 4.96 | 1.25 | 0.46, 3.42 | 28 | 1.64 | 0.84, 3.20 | 0.48 |
| Q3 | 1.47 | 0.57, 3.80 | 2.09 | 0.81, 5.39 | 33 | 1.75 | 0.90, 3.43 | 0.61 |
| Q4 | 1.35 | 0.50, 3.68 | 1.80 | 0.64, 5.07 | 26 | 1.55 | 0.76, 3.19 | 0.69 |
| P-trend | 0.78 | 0.35 | 0.66 | 0.94 | ||||
Abbreviations: CI, confidence interval; CRP, C-reactive protein; NHS, Nurses' Health Study; NHS II, Nurses’ Health Study II; Q, quartile; RR, relative risk; WHS, Women's Health Study.
a Determined by random-effects meta-analysis of estimates from the NHS/NHS II nested case-control study and the WHS Cox proportional hazards model and adjusted for matching factors (NHS/NHS II only), oral contraceptive use (never use, <5 years, or ≥5 years), tubal ligation (yes vs. no), parity (continuous), and body mass index (weight (kg)/height (m)2) at blood draw (continuous).
b Determined using random-effects meta-analysis comparing risk estimates across studies.
c For the NHS/NHS II, median CRP levels in the 4 quartiles were 0.33 mg/L, 0.88 mg/L, 2.11 mg/L, and 5.17 mg/L, respectively. For the WHS, median CRP levels in the 4 quartiles were 0.43 mg/L, 1.34 mg/L, 2.98 mg/L, and 6.93 mg/L.
In the NHS/NHS II, plasma levels of IL-6 and TNF-α-R2 were not statistically significantly associated with risk of invasive epithelial ovarian cancer (Table 3). The relative risk comparing the fourth quartile of IL-6 levels with the first quartile was 0.85 (95% CI: 0.52, 1.40; P-trend = 0.69). There was a suggestion of a positive association for TNF-α-R2, with a relative risk of 1.57 (95% CI: 0.92, 2.68) comparing the top quartile with the bottom quartile (P-trend = 0.08). In the NHS/NHS II, mutually adjusting the biomarkers had little impact on the relative risk estimates. For example, for TNF-α-R2 adjusted for CRP and IL-6, the relative risk for the top quartile versus the bottom quartile was 1.51. Results were similar for serous cases and cases who died within 3 years of diagnosis, as well as after excluding cases diagnosed within the first 2, 5, or 10 years after blood draw, after excluding cases and controls with inflammatory conditions, or when stratifying by body mass index and age (data not shown).
Table 3.
Associations Between Interleukin-6 and Tumor Necrosis Factor α Receptor 2 and Risk of Invasive Epithelial Ovarian Cancer in the Nurses' Health Study (1989–2010) and Nurses' Health Study II (1996–2009)
| Biomarker and Quartile | Median Level, pg/mL | No. of Cases | No. of Controls | Relative Riska | 95% Confidence Interval | P-trend |
|---|---|---|---|---|---|---|
| Interleukin-6 | ||||||
| 1 | 0.67 | 58 | 128 | 1.00 | Reference | 0.69 |
| 2 | 1.03 | 45 | 128 | 0.75 | 0.46, 1.21 | |
| 3 | 1.56 | 56 | 128 | 0.93 | 0.58, 1.50 | |
| 4 | 3.11 | 56 | 129 | 0.85 | 0.52, 1.40 | |
| TNF-α-R2 | ||||||
| 1 | 1,934.25 | 41 | 129 | 1.00 | Reference | 0.08 |
| 2 | 2,360.20 | 53 | 130 | 1.34 | 0.81, 2.22 | |
| 3 | 2,770.80 | 61 | 130 | 1.48 | 0.89, 2.45 | |
| 4 | 3,475.60 | 61 | 129 | 1.57 | 0.92, 2.68 |
Abbreviation: TNF-α-R2, tumor necrosis factor α receptor 2.
a Adjusted for matching factors, batch, oral contraceptive use (never use, <5 years, or ≥5 years), tubal ligation (yes vs. no), parity (continuous), and body mass index (weight (kg)/height (m)2) at blood draw (continuous).
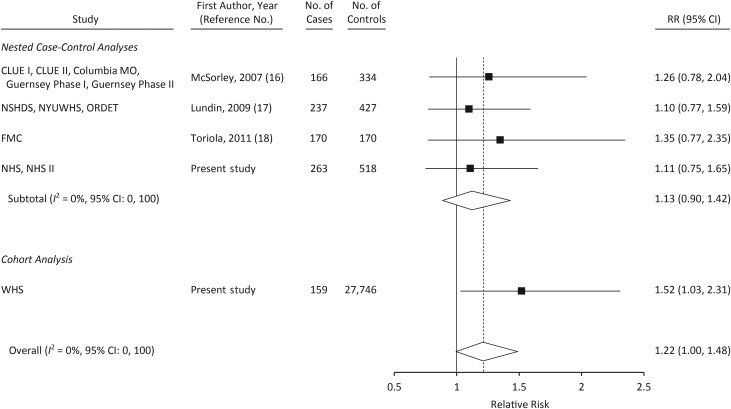
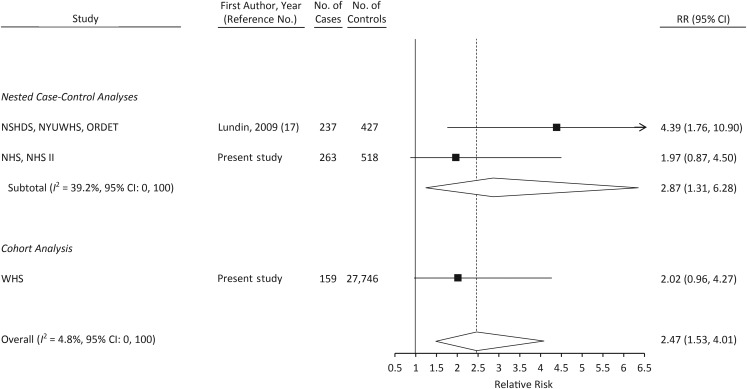
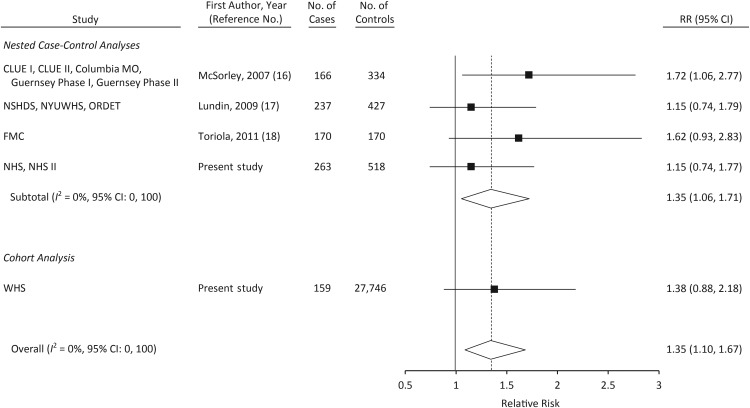
The results of meta-analyses including data from the 3 other prospective studies of CRP (16–18) are shown in Figures 1–3. When comparing women in the top tertile of CRP with those in the bottom tertile, the relative risk was 1.35 (95% CI: 1.10, 1.67; P-heterogeneity = 0.66; Figure 2). Only 1 other study reported on the association between CRP levels greater than 10 mg/L and ovarian cancer risk (17); in a meta-analysis of that study, NHS/NHS II, and WHS, a CRP level greater than 10 mg/L was associated with statistically significantly increased risk of ovarian cancer (relative risk = 2.47, 95% CI: 1.53, 4.01; Figure 3). In a meta-analysis restricted to cases that occurred more than 2 years after blood draw, the relative risk associated with the highest tertile of CRP was attenuated slightly (relative risk = 1.28, 95% CI: 1.01, 1.62).
Figure 1.
Relative risk (RR) of ovarian cancer among women in the second tertile of C-reactive protein level in a meta-analysis of published studies and in the present study. Numbers of cases and controls shown reflect the total number in each study rather than the number in the second tertile. CLUE I, Campaign Against Cancer and Stroke; CLUE II, Campaign Against Cancer and Heart Disease; Columbia MO, Columbia, Missouri, Serum Bank; FMC, Finnish Maternity Cohort; Guernsey, Island of Guernsey Prospective Study; NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II; NSHDS, Northern Sweden Health and Disease Study; NYUWHS, New York University Women's Health Study; ORDET, Hormones and Diet in the Etiology of Breast Cancer; WHS, Women's Health Study. Bars, 95% confidence interval (CI).
Figure 3.
Relative risk (RR) of ovarian cancer among women with a C-reactive protein level greater than 10 mg/L in a meta-analysis of published studies and in the present study. Numbers of cases and controls shown reflect the total number in each study rather than the number with a C-reactive protein level greater than 10 mg/L. CLUE I, Campaign Against Cancer and Stroke; CLUE II, Campaign Against Cancer and Heart Disease; Columbia MO, Columbia, Missouri, Serum Bank; FMC, Finnish Maternity Cohort; Guernsey, Island of Guernsey Prospective Study; NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II; NSHDS, Northern Sweden Health and Disease Study; NYUWHS, New York University Women's Health Study; ORDET, Hormones and Diet in the Etiology of Breast Cancer; WHS, Women's Health Study. Bars, 95% confidence interval (CI).
Figure 2.
Relative risk (RR) of ovarian cancer among women in the third tertile of C-reactive protein level in a meta-analysis of published studies and in the present study. Numbers of cases and controls shown reflect the total number in each study rather than the number in the third tertile. CLUE I, Campaign Against Cancer and Stroke; CLUE II, Campaign Against Cancer and Heart Disease; Columbia MO, Columbia, Missouri, Serum Bank; FMC, Finnish Maternity Cohort; Guernsey, Island of Guernsey Prospective Study; NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II; NSHDS, Northern Sweden Health and Disease Study; NYUWHS, New York University Women's Health Study; ORDET, Hormones and Diet in the Etiology of Breast Cancer; WHS, Women's Health Study. Bars, 95% confidence interval (CI).
DISCUSSION
Our results support the hypothesis of a positive association between CRP levels and risk of ovarian cancer. Across multiple prospective studies of European and US women (16–18), including our own data, comprising a total of 996 ovarian cancer cases, we observed a 35% increased risk of ovarian cancer when comparing women in the top tertile with women in the bottom tertile, an association that remained even after excluding cases diagnosed within 2 years of blood draw. Interestingly, in 3 of the cohort studies (the NHS/NHS II, the WHS, and the study by Lundin et al. (17)), this risk was even stronger when women with very high levels of CRP were considered. Conversely, in the NHS/NHS II, we did not observe an association between IL-6 and risk of ovarian cancer, although levels of TNF-α-R2 were suggestively positively associated with risk.
CRP is a global marker of inflammation that has been associated with cardiovascular disease, diabetes, and overall cancer risk (13, 41–45). Consistent evidence supports the hypothesis that CRP is also associated with an increased risk of ovarian cancer (16–18). In ovarian cancer patients, CRP levels are higher in cases than in healthy controls (14, 15, 46); however, this could be because the disease process itself increases CRP levels. Among cases, CRP has been associated with higher stage (14, 46) and found to be an independent predictor of survival (15). However, in the prospective studies, the associations with CRP level were similar by invasiveness, histological type, and, in our study, death within 3 years of diagnosis, although statistical power was limited. Further studies are needed to address whether CRP is associated specifically with more aggressive disease.
It is not clear whether CRP directly influences ovarian carcinogenesis or is an indirect marker of inflammatory exposure to the ovary, although one study suggested that high levels of CRP in ovarian cancer patients were correlated with an impaired T-cell response (14). Further, several small studies observed that circulating (47–50) or peritoneal (51) CRP levels were higher during postovulatory phases of the menstrual cycle. However, most of the women in the current and prior studies were postmenopausal at blood collection. In the current study, we also evaluated whether CRP levels were associated with known ovarian cancer risk factors, such as parity or oral contraceptive use, or with use of nonsteroidal anti-inflammatory drugs at the time of blood draw among controls in the NHS. In analyses adjusted for body mass index and age, none of these exposures were related to CRP levels (data not shown). These analyses suggest that CRP is not a marker of exposure to currently known risk factors for ovarian cancer, nor is it likely that the association with CRP levels is due to an inverse association with use of nonsteroidal antiinflammatory drugs.
Although IL-6 and TNF-α-R2 have also been implicated in ovarian cancer development (14, 21, 23, 25, 52–54), the results from our study and that of Clendenen et al. (27) did not yield consistent results. We did not observe an association between IL-6 and ovarian cancer risk, while Clendenen et al. (27) noted a 10% increase in risk with a doubling of IL-6 levels (P-trend = 0.03). Interestingly, Clendenen et al. also reported that women with high IL-6 levels but low soluble IL-6 receptor levels had a 65% increased risk of ovarian cancer in comparison with women with low levels of both (27). Our study did not measure IL-6 receptor. The intraclass correlation coefficient for IL-6 over 4 years was somewhat low (0.47); thus, it is possible that our study did not observe an association because we had a much longer duration of follow-up (median of 10.0 years) than did Clendenen et al. (median of 6.3 years). Conversely, our study observed a suggestive positive association for TNF-α-R2, while Clendenen et al. (27) did not observe an association with TNF-α or TNF-α-R2. However, both studies had somewhat limited statistical power, and different assay methods may have led to differing results across the studies. Given the increasing evidence that inflammation is important in ovarian cancer development, additional prospective studies are needed to evaluate associations for these markers, as well as other inflammatory markers/cytokines that may reflect inflammation in the ovary.
Our study had several strengths and limitations. First, our study was prospective, and we had 376 invasive cases for assessing CRP and 217 invasive cases for assessing IL-6 and TNF-α-R2. Despite this, we had limited power to assess potential interactions or evaluate less common subtypes of ovarian cancer. Second, we had only 1 blood draw per person; however, the intraclass correlation coefficient for these analytes over 4 years was 0.47 for IL-6, 0.67 for CRP, and 0.78 for TNF-α-R2 (35), suggesting that 1 sample is reflective of long-term exposure. Third, the studies used different types of anticoagulants for long-term storage of blood samples (NHS/NHS II: heparin; WHS: ethylenediaminetetraacetic acid); however, we used study-specific quantiles to reduce the influence of this factor on the results. Fourth, the WHS was a clinical trial of the use of aspirin for prevention of cardiovascular disease and cancer; women with a prior history of cardiovascular disease or cancer were excluded. Thus, CRP levels in WHS participants may not have represented those in the general population. However, as shown in Table 2, median CRP levels were similar in each of the WHS quartiles compared with the NHS/NHS II quartiles. This suggests that the WHS exclusions did not affect the distribution of CRP. Fifth, it is possible that the increased risk of ovarian cancer with high levels of CRP observed in this study was due to underlying disease in cases. However, as shown in Web Table 2, results were similar when restricted to cases diagnosed 2, 5, or 10 years after blood draw. This suggests that the observed associations were not due to reverse causation.
Overall, the results of the current and prior studies strongly support the hypothesis that CRP levels are positively and prospectively associated with risk of ovarian cancer. However, associations for other inflammatory markers, including IL-6 and TNF-α-R2, are less clear. Additional research pooling data from multiple studies to increase the sample size is needed to clarify these associations and to assess whether CRP levels are more strongly associated with certain subtypes of ovarian cancer. In addition, biological studies regarding the specific role of CRP in ovarian carcinogenesis are warranted. These data further support evidence on the importance of inflammation in ovarian cancer development.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts (Elizabeth M. Poole, Susan E. Hankinson, Shelley S. Tworoger); Division of Preventive Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts (I-Min Lee, Paul M. Ridker, Julie E. Buring); Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts (Elizabeth M. Poole, I-Min Lee, Paul M. Ridker, Julie E. Buring, Susan E. Hankinson, Shelley S. Tworoger); and Department of Epidemiology, School of Public Health and Health Sciences, Division of Biostatistics and Epidemiology, University of Massachusetts, Amherst, Massachusetts (Susan E. Hankinson).
This study was supported by National Cancer Institute grants T32 CA009001, P01 CA87969, R01 CA49449, R01 CA67262, R01 CA50385, R01 HL043851, R01 HL080467, R01 CA047988, and RC1 HL099355.
We thank the following state cancer registries for their help: Alabama, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, Washington, and Wyoming.
Conflict of interest: none declared.
REFERENCES
- 1.American Cancer Society. Cancer Facts & Figures 2011. Atlanta, GA: American Cancer Society; 2011. [Google Scholar]
- 2.Fleming JS, Beaugie CR, Haviv I, et al. Incessant ovulation, inflammation and epithelial ovarian carcinogenesis: revisiting old hypotheses. Mol Cell Endocrinol. 2006;247(1-2):4–21. doi: 10.1016/j.mce.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Ness RB, Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst. 1999;91(17):1459–1467. doi: 10.1093/jnci/91.17.1459. [DOI] [PubMed] [Google Scholar]
- 4.Beral V, Doll R, Hermon C, et al. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet. 2008;371(9609):303–314. doi: 10.1016/S0140-6736(08)60167-1. [DOI] [PubMed] [Google Scholar]
- 5.Permuth-Wey J, Sellers TA. Epidemiology of ovarian cancer. Methods Mol Biol. 2009;472:413–437. doi: 10.1007/978-1-60327-492-0_20. [DOI] [PubMed] [Google Scholar]
- 6.Riman T, Nilsson S, Persson IR. Review of epidemiological evidence for reproductive and hormonal factors in relation to the risk of epithelial ovarian malignancies. Acta Obstet Gynecol Scand. 2004;83(9):783–795. doi: 10.1111/j.0001-6349.2004.00550.x. [DOI] [PubMed] [Google Scholar]
- 7.Moorman PG, Schildkraut JM, Calingaert B, et al. Ovulation and ovarian cancer: a comparison of two methods for calculating lifetime ovulatory cycles (United States) Cancer Causes Control. 2002;13(9):807–811. doi: 10.1023/a:1020678100977. [DOI] [PubMed] [Google Scholar]
- 8.Pelucchi C, Galeone C, Talamini R, et al. Lifetime ovulatory cycles and ovarian cancer risk in 2 Italian case-control studies. Am J Obstet Gynecol. 2007;196(1):83.e1–83.e7. doi: 10.1016/j.ajog.2006.06.088. [DOI] [PubMed] [Google Scholar]
- 9.Terry KL, Titus-Ernstoff L, McKolanis JR, et al. Incessant ovulation, mucin 1 immunity, and risk for ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(1):30–35. doi: 10.1158/1055-9965.EPI-06-0688. [DOI] [PubMed] [Google Scholar]
- 10.Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology (Williston Park) 2002;16(2):217–226. 229. [PubMed] [Google Scholar]
- 11.Ziegler J. Cancer and arthritis share underlying processes. J Natl Cancer Inst. 1998;90(11):802–803. doi: 10.1093/jnci/90.11.802. [DOI] [PubMed] [Google Scholar]
- 12.Blake GJ, Ridker PM. Novel clinical markers of vascular wall inflammation. Circ Res. 2001;89(9):763–771. doi: 10.1161/hh2101.099270. [DOI] [PubMed] [Google Scholar]
- 13.Blake GJ, Ridker PM. C-reactive protein and other inflammatory risk markers in acute coronary syndromes. J Am Coll Cardiol. 2003;41(4 suppl S):37S–42S. doi: 10.1016/s0735-1097(02)02953-4. [DOI] [PubMed] [Google Scholar]
- 14.Maccio A, Lai P, Santona MC, et al. High serum levels of soluble IL-2 receptor, cytokines, and C reactive protein correlate with impairment of T cell response in patients with advanced epithelial ovarian cancer. Gynecol Oncol. 1998;69(3):248–252. doi: 10.1006/gyno.1998.4974. [DOI] [PubMed] [Google Scholar]
- 15.Hefler LA, Concin N, Hofstetter G, et al. Serum C-reactive protein as independent prognostic variable in patients with ovarian cancer. Clin Cancer Res. 2008;14(3):710–714. doi: 10.1158/1078-0432.CCR-07-1044. [DOI] [PubMed] [Google Scholar]
- 16.McSorley MA, Alberg AJ, Allen DS, et al. C-reactive protein concentrations and subsequent ovarian cancer risk. Obstet Gynecol. 2007;109(4):933–941. doi: 10.1097/01.AOG.0000257126.68803.03. [DOI] [PubMed] [Google Scholar]
- 17.Lundin E, Dossus L, Clendenen T, et al. C-reactive protein and ovarian cancer: a prospective study nested in three cohorts (Sweden, USA, Italy) Cancer Causes Control. 2009;20(7):1151–1159. doi: 10.1007/s10552-009-9330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toriola AT, Grankvist K, Agborsangaya CB, et al. Changes in pre-diagnostic serum C-reactive protein concentrations and ovarian cancer risk: a longitudinal study. Ann Oncol. 2011;22(8):1916–1921. doi: 10.1093/annonc/mdq694. [DOI] [PubMed] [Google Scholar]
- 19.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 20.Schroder W, Ruppert C, Bender HG. Concomitant measurements of interleukin-6 (IL-6) in serum and peritoneal fluid of patients with benign and malignant ovarian tumors. Eur J Obstet Gynecol Reprod Biol. 1994;56(1):43–46. doi: 10.1016/0028-2243(94)90152-x. [DOI] [PubMed] [Google Scholar]
- 21.Darai E, Detchev R, Hugol D, et al. Serum and cyst fluid levels of interleukin (IL)-6, IL-8 and tumour necrosis factor-alpha in women with endometriomas and benign and malignant cystic ovarian tumours. Hum Reprod. 2003;18(8):1681–1685. doi: 10.1093/humrep/deg321. [DOI] [PubMed] [Google Scholar]
- 22.Tempfer C, Zeisler H, Sliutz G, et al. Serum evaluation of interleukin 6 in ovarian cancer patients. Gynecol Oncol. 1997;66(1):27–30. doi: 10.1006/gyno.1997.4726. [DOI] [PubMed] [Google Scholar]
- 23.Moradi MM, Carson LF, Weinberg B, et al. Serum and ascitic fluid levels of interleukin-1, interleukin-6, and tumor necrosis factor-alpha in patients with ovarian epithelial cancer. Cancer. 1993;72(8):2433–2440. doi: 10.1002/1097-0142(19931015)72:8<2433::aid-cncr2820720822>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 24.van der Zee AG, de Cuyper EM, Limburg PC, et al. Higher levels of interleukin-6 in cystic fluids from patients with malignant versus benign ovarian tumors correlate with decreased hemoglobin levels and increased platelet counts. Cancer. 1995;75(4):1004–1009. doi: 10.1002/1097-0142(19950215)75:4<1004::aid-cncr2820750416>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 25.Plante M, Rubin SC, Wong GY, et al. Interleukin-6 level in serum and ascites as a prognostic factor in patients with epithelial ovarian cancer. Cancer. 1994;73(7):1882–1888. doi: 10.1002/1097-0142(19940401)73:7<1882::aid-cncr2820730718>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 26.Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994;43(11):1271–1278. doi: 10.2337/diab.43.11.1271. [DOI] [PubMed] [Google Scholar]
- 27.Clendenen TV, Lundin E, Zeleniuch-Jacquotte A, et al. Circulating inflammation markers and risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2011;20(5):799–810. doi: 10.1158/1055-9965.EPI-10-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med. 1996;334(26):1717–1725. doi: 10.1056/NEJM199606273342607. [DOI] [PubMed] [Google Scholar]
- 29.Hankinson SE, Willett WC, Michaud DS, et al. Plasma prolactin levels and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1999;91(7):629–634. doi: 10.1093/jnci/91.7.629. [DOI] [PubMed] [Google Scholar]
- 30.Tworoger SS, Sluss P, Hankinson SE. Association between plasma prolactin concentrations and risk of breast cancer among predominately premenopausal women. Cancer Res. 2006;66(4):2476–2482. doi: 10.1158/0008-5472.CAN-05-3369. [DOI] [PubMed] [Google Scholar]
- 31.Cook NR, Lee IM, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294(1):47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 32.Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294(1):56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 33.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352(13):1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 34.Roberts WL, Moulton L, Law TC, et al. Evaluation of nine automated high-sensitivity C-reactive protein methods: implications for clinical and epidemiological applications. Part 2. Clin Chem. 2001;47(3):418–425. [PubMed] [Google Scholar]
- 35.Pischon T, Hankinson SE, Hotamisligil GS, et al. Leisure-time physical activity and reduced plasma levels of obesity-related inflammatory markers. Obes Res. 2003;11(9):1055–1064. doi: 10.1038/oby.2003.145. [DOI] [PubMed] [Google Scholar]
- 36.Pai JK, Curhan GC, Cannuscio CC, et al. Stability of novel plasma markers associated with cardiovascular disease: processing within 36 hours of specimen collection. Clin Chem. 2002;48(10):1781–1784. [PubMed] [Google Scholar]
- 37.Fernandez-Real JM, Broch M, Ricart W, et al. Plasma levels of the soluble fraction of tumor necrosis factor receptor 2 and insulin resistance. Diabetes. 1998;47(11):1757–1762. doi: 10.2337/diabetes.47.11.1757. [DOI] [PubMed] [Google Scholar]
- 38.Rosner B. Percentage points for a generalized ESD many-outlier procedure. Technometrics. 1983;25(2):165–172. [Google Scholar]
- 39.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 40.Poole EM, Merritt MA, Jordan SJ, et al. Hormonal and reproductive risk factors for epithelial ovarian cancer by tumor aggressiveness. Cancer Epidemiol Biomarkers Prev. 2013;22(3):429–437. doi: 10.1158/1055-9965.EPI-12-1183-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cook NR, Buring JE, Ridker PM. The effect of including C-reactive protein in cardiovascular risk prediction models for women. Ann Intern Med. 2006;145(1):21–29. doi: 10.7326/0003-4819-145-1-200607040-00128. [DOI] [PubMed] [Google Scholar]
- 42.Ridker PM, Rifai N, Cook NR, et al. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA. 2005;294(3):326–333. doi: 10.1001/jama.294.3.326. [DOI] [PubMed] [Google Scholar]
- 43.Schulze MB, Hoffmann K, Manson JE, et al. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr. 2005;82(3):675. doi: 10.1093/ajcn.82.3.675. 715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heikkila K, Harris R, Lowe G, et al. Associations of circulating C-reactive protein and interleukin-6 with cancer risk: findings from two prospective cohorts and a meta-analysis. Cancer Causes Control. 2009;20(1):15–26. doi: 10.1007/s10552-008-9212-z. [DOI] [PubMed] [Google Scholar]
- 45.Allin KH, Bojesen SE, Nordestgaard BG. Baseline C-reactive protein is associated with incident cancer and survival in patients with cancer. J Clin Oncol. 2009;27(13):2217–2224. doi: 10.1200/JCO.2008.19.8440. [DOI] [PubMed] [Google Scholar]
- 46.Hefler-Frischmuth K, Hefler LA, Heinze G, et al. Serum C-reactive protein in the differential diagnosis of ovarian masses. Eur J Obstet Gynecol Reprod Biol. 2009;147(1):65–68. doi: 10.1016/j.ejogrb.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 47.Blum CA, Muller B, Huber P, et al. Low-grade inflammation and estimates of insulin resistance during the menstrual cycle in lean and overweight women. J Clin Endocrinol Metab. 2005;90(6):3230–3235. doi: 10.1210/jc.2005-0231. [DOI] [PubMed] [Google Scholar]
- 48.Capobianco G, de Muro P, Cherchi GM, et al. Plasma levels of C-reactive protein, leptin and glycosaminoglycans during spontaneous menstrual cycle: differences between ovulatory and anovulatory cycles. Arch Gynecol Obstet. 2010;282(2):207–213. doi: 10.1007/s00404-010-1432-2. [DOI] [PubMed] [Google Scholar]
- 49.Puder JJ, Blum CA, Mueller B, et al. Menstrual cycle symptoms are associated with changes in low-grade inflammation. Eur J Clin Invest. 2006;36(1):58–64. doi: 10.1111/j.1365-2362.2006.01591.x. [DOI] [PubMed] [Google Scholar]
- 50.Wander K, Brindle E, O'Connor KA. C-reactive protein across the menstrual cycle. Am J Phys Anthropol. 2008;136(2):138–146. doi: 10.1002/ajpa.20785. [DOI] [PubMed] [Google Scholar]
- 51.Bouckaert PX, Evers JL, Doesburg WH, et al. Patterns of changes in proteins in the peritoneal fluid of women during the periovulatory phase of the menstrual cycle. J Reprod Fertil. 1986;77(2):329–336. doi: 10.1530/jrf.0.0770329. [DOI] [PubMed] [Google Scholar]
- 52.Dobrzycka B, Terlikowski SJ, Garbowicz M, et al. Tumor necrosis factor-alpha and its receptors in epithelial ovarian cancer. Folia Histochem Cytobiol. 2009;47(4):609–613. doi: 10.2478/v10042-008-0117-1. [DOI] [PubMed] [Google Scholar]
- 53.Dobrzycka B, Terlikowski SJ, Kowalczuk O, et al. Circulating levels of TNF-alpha and its soluble receptors in the plasma of patients with epithelial ovarian cancer. Eur Cytokine Netw. 2009;20(3):131–134. doi: 10.1684/ecn.2009.0161. [DOI] [PubMed] [Google Scholar]
- 54.Kulbe H, Chakravarty P, Leinster DA, et al. A dynamic inflammatory cytokine network in the human ovarian cancer microenvironment. Cancer Res. 2012;72(1):66–75. doi: 10.1158/0008-5472.CAN-11-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.