Abstract
Major changes have recently occurred in the epidemiology of myocardial infarction (MI) that could possibly affect outcomes such as heart failure (HF). Data describing trends in HF after MI are scarce and conflicting and do not distinguish between preserved and reduced ejection fraction (EF). We evaluated temporal trends in HF after MI. All residents of Olmsted County, Minnesota (n = 2,596) who had a first-ever MI diagnosed in 1990–2010 and no prior HF were followed-up through 2012. Framingham Heart Study criteria were used to define HF, which was further classified according to EF. Both early-onset (0–7 days after MI) and late-onset (8 days to 5 years after MI) HF were examined. Changes in patient presentation were noted, including fewer ST-segment–elevation MIs, lower Killip class, and more comorbid conditions. Over the 5-year follow-up period, 715 patients developed HF, 475 of whom developed it during the first week. The age- and sex-adjusted risk declined from 1990–1996 to 2004–2010, with hazard ratios of 0.67 (95% confidence interval (CI): 0.54, 0.85) for early-onset HF and 0.63 (95% CI: 0.45, 0.86) for late-onset HF. Further adjustment for patient and MI characteristics yielded hazard ratios of 0.86 (95% CI: 0.66, 1.11) and 0.63 (95% CI: 0.45, 0.88) for early- and late-onset HF, respectively. Declines in early-onset and late-onset HF were observed for HF with reduced EF (<50%) but not for HF with preserved EF, indicating a change in the case mix of HF after MI that requires new prevention strategies.
Keywords: cardiovascular diseases, community studies, ejection fraction, heart failure, myocardial infarction, population-based studies, secular trends, surveillance
Heart failure (HF) is a major health problem worldwide and a leading cause of hospital admissions and resource utilization in the United States (1, 2). Coronary artery disease, and in particular acute myocardial infarction (MI), is an important cause of HF (3, 4). The incidence of HF after MI is therefore a major clinical and public health concern, not only because of its frequency (5, 6) but also because of its substantial associated mortality rate (7, 8). HF is a syndrome that can occur with either reduced or preserved left ventricular ejection fraction (EF) (9). Both confer a comparable mortality risk (10), with the latter increasing in prevalence over time in the community (11). The respective proportion of HF with reduced or preserved left ventricular EF and how that proportion may have changed over time after MI is not known.
Important changes in the epidemiology of MI have occurred in the last 2 decades, characterized by increased proportion of non–ST-segment–elevation MI, improved treatment, reduced short-term case fatality rates, and an increased proportion of deaths from noncardiovascular causes (12–15). However, contemporary data on trends in the incidence of HF after MI, which may have been affected as well, are lacking. In the few studies on this topic that have been conducted to date, none of which extended beyond the early-to-mid 2000s, inconsistent results were reported, including increasing (5, 16), stable (17), and decreasing (18, 19) trends. Accordingly, Jhund et al. (20) called for the reevaluation of these trends using more recent data that better capture the consequences of the changes in MI epidemiology. Responding to this need, we evaluated trends in post-MI incidence of HF between 1990 and 2012 in a geographically defined population. Specifically, we examined trends in early versus late incidence and in HF with reduced versus preserved EF and assessed the extent to which these trends are attributable to observed changes in MI presentation and other clinical characteristics.
MATERIALS AND METHODS
Study design and setting
This research was conducted in Olmsted County, Minnesota, a location well suited for disease association studies because of its relative isolation from other urban centers and because comprehensive medical records from all sources of care for the local population are indexed and linked via the Rochester Epidemiology Project (21). Because nearly all Olmsted County residents are represented in this system, this data source provides a virtually complete enumeration of the source population for many decades (22). After approval by the appropriate institutional review boards, a follow-up study was carried out utilizing the above resources.
Cohort identification and validation
Residents admitted to Olmsted County hospitals with possible MI from 1990 to 2010 were identified with methods that have been described previously (13). Briefly, all events that were coded with International Classification of Diseases, 9th Revision, code 410 (acute MI) were reviewed. In addition, events with code 411 (other ischemic heart disease) were reviewed in a 50% random sample from 1987 to 1998, a 10% random sample from 1999 to 2002, and a 100% sample from 2003 to 2010. Additional codes were not included because they yielded a low number of results.
MIs were validated using standard epidemiologic criteria (13). Patients diagnosed with an MI prior to 1990 were excluded so that only incident (first-ever) cases were investigated. The diagnosis of MI was verified based on the presence of 2 of the following: cardiac pain, elevated biomarker levels, and electrocardiographic changes. Biomarkers used in clinical practice included creatine kinase (CK) and CK-MB until 2000 and troponin thereafter. However, CK-MB was still measured after 2000 as part of a surveillance study. Case reviews were performed to ensure that alternative causes for biomarker elevation were taken into consideration. Troponin T, CK, and CK-MB levels were measured with a sandwich electrochemiluminescence immunoassay on the Elecsys 2010 (Roche Diagnostics Corp, Indianapolis, Indiana) in the laboratories of the Department of Medicine and Pathology at Mayo Clinic.
Additional clinical data
Each medical record was reviewed to ascertain data on cardiovascular risk factors, comorbid conditions, MI characteristics, and acute treatment at the index date or at the closest time before hospital admission. Cigarette smoking was classified as current versus former/never smoking. Body mass index (measured as weight (kg)/height (m)²) was calculated using the current weight and earliest available adult height measurement. Clinical definitions were used to assess whether patients had hypertension or hyperlipidemia. Heart rate at the time of admission and data on the presence or absence of atrial fibrillation during the index hospitalization were obtained. The overall comorbidity burden was assessed using the Charlson index (23), which consists of 17 serious comorbid conditions weighted according to the degree to which they predict death. The Modification of Diet in Renal Disease equation (24) was used to estimate glomerular filtration rate, with a rate less than 60 mL/min regarded as evidence of impaired renal function. ST-segment elevation and Killip class were recorded. The latter was determined within 24 hours of the index MI and analyzed as a categorical variable (class >1 vs. class 1). Reperfusion/revascularization included thrombolytic therapy, coronary artery bypass grafting, and/or percutaneous coronary intervention during the index hospitalization. Recurrent MIs (occurrence and date) were recorded based on clinical diagnoses.
Outcome measure
The primary outcome was time to HF, overall and by type (according to EF measurement). Participants were followed up through their complete (inpatient and outpatient) medical records in the community from the index date to the occurrence of HF, death, or the most recent clinical contact. The study period extended from January 1990 through July 2012. Clinical diagnoses of HF were reviewed. HF was validated using the Framingham Heart Study criteria. These criteria require the presence of at least 2 major criteria or 1 major criterion in addition to 2 minor criteria (25) to confirm HF; the ascertainment process in the study setting has previously been described in detail (26). Echocardiograms in Olmsted County were performed at the Mayo Clinic throughout the study period. EF was measured using an approach that was recently described (9). The EF measurement that was closest to the HF diagnosis (applying a predefined maximum period of 60 days) was recorded for each participant. Reduced and preserved EFs were defined as less than 50% and 50% or more, respectively (10). Death was ascertained through multiple sources, including autopsy reports, death certificates filed in Olmsted County, obituary notices, and electronic death certificates obtained from the Section of Vital Statistics, Minnesota Department of Health.
Statistical analysis
Patients who had HF prior to the index MI were excluded from the analyses. For ease of interpretation and presentation, we used an approach similar to the one used in previous studies (16, 19). The year of the index MI (the primary exposure variable) was divided into 3 categories (1990–1996, 1997–2003, and 2004–2010). Baseline characteristics across year categories are presented as means and standard deviations for continuous variables and as frequencies for categorical variables.
Age- and sex-adjusted cumulative incidence curves for HF across year categories were projected for up to 5 years using the direct adjustment method (27). Because in the presence of competing risks, standard survival predictions may substantially overestimate the absolute risk of the event of interest (28), the cumulative incidence estimates were adjusted for death as a competing event (29), adopting the Fine and Gray subdistribution hazard model (30). On the basis of these computations, absolute risk differences and 95% confidence intervals between year categories at predefined follow-up intervals were evaluated.
Cox proportional hazards regression models (31) were constructed to estimate the hazard ratios and 95% confidence intervals for HF incidence between year categories. Adjustment was done sequentially for demographic variables, cardiovascular risk factors and comorbidities, MI characteristics and severity, acute interventions, and recurrent MI (which was modeled as a time-dependent covariate). Because we adjusted for the Charlson index as a measure of overall comorbidity burden, specific components of this index (e.g., diabetes mellitus and cerebrovascular disease) were not included individually in the models. The proportional hazards assumption was tested using the scaled Schoenfeld residuals and did not hold for several covariates, including the primary exposure variable. In light of previous findings that suggested a bimodal occurrence of HF after MI (32), follow-up was divided into 2 intervals (0–7 days (early risk period) and 8 days–5 years (late risk period)) so that the proportional hazards assumption was satisfied for all the variables considered.
A subsequent analysis was undertaken to assess temporal trends in the risk of HF by type. HF with reduced EF and HF with preserved EF were assessed individually. Data on EF were missing in 18% of the cases. A complete-case analysis was initially performed, followed by a multiple imputation analysis (33). Ten datasets were created, with missing values replaced by imputed values based on a model that incorporated various demographic and clinical variables and an indicator for HF along with the cumulative baseline hazard of HF approximated by the Nelson-Aalen estimator (34). The results of these datasets were then combined using Rubin's rules (33). Tests for linear trend were performed by using integer scores across year categories. Homogeneity in trends across subgroups was examined by including year-by-age and year-by-sex interaction terms. Analyses were performed using SAS statistical software, version 9.3 (SAS Institute Inc., Cary, North Carolina).
RESULTS
Changes in MI presentation
From January of 1990 to December of 2010, there were 2,943 residents hospitalized in Olmsted County with an initial MI, of whom 347 (12%) had a history of HF. The prevalence of HF before MI remained stable during the study period (10% in 1990–1996, 14% in 1997–2003, and 11% in 2004–2010; P for trend = 0.88). After excluding patients with HF that preceded the index MI, we were left with 2,596 participants to be analyzed in this study (mean age, 66.5 years; 60% men; 96% white). Baseline characteristics of the patients across categories of the year of diagnosis are shown in Table 1. The typical MI presentation changed over time, with fewer ST-segment–elevation and anterior MIs, lower Killip class, and more comorbid conditions. The overall utilization of reperfusion/revascularization therapy intensified.
Table 1.
Baseline Patient Characteristics by Index Myocardial Infarction Year Categories, Olmsted County, Minnesota, 1990–2010
| Characteristic | Index MI Year Category |
P Value | |||||
|---|---|---|---|---|---|---|---|
| 1990–1996 (n = 780) |
1997–2003 (n = 845) |
2004–2010 (n = 971) |
|||||
| No. | % | No. | % | No. | % | ||
| Age, years | 66.1 (13.9)a | 67.5 (14.8)a | 66.1 (14.8)a | 0.06 | |||
| Male sex | 441 | 57 | 509 | 60 | 616 | 63 | 0.01 |
| Body mass indexb | 27.9 (6.0)a | 28.3 (5.7)a | 28.9 (6.0)a | 0.01 | |||
| Hypertension | 407 | 52 | 511 | 61 | 653 | 67 | <0.001 |
| Hyperlipidemia | 255 | 33 | 460 | 54 | 636 | 66 | <0.001 |
| Current smoking | 246 | 32 | 202 | 24 | 222 | 23 | <0.001 |
| Heart rate on admission, bpm | 82 (22)a | 83 (23)a | 82 (23)a | 0.34 | |||
| Atrial fibrillation | 88 | 12 | 94 | 11 | 98 | 10 | 0.62 |
| Estimated GFR, <60 mL/min per 1.73 m2 | 383 | 49 | 468 | 56 | 352 | 36 | <0.001 |
| Charlson comorbidity index score | <0.001 | ||||||
| 0 | 368 | 47 | 329 | 39 | 412 | 42 | |
| 1–2 | 297 | 38 | 345 | 41 | 350 | 36 | |
| ≥3 | 114 | 15 | 171 | 20 | 209 | 22 | |
| ST-segment–elevation MI | 341 | 41 | 263 | 32 | 247 | 25 | <0.001 |
| Anterior MI | 279 | 36 | 312 | 38 | 316 | 33 | 0.03 |
| Troponin-only MI | 0 | 0 | 92 | 11 | 242 | 25 | <0.001 |
| Killip class >1 | 226 | 29 | 224 | 27 | 196 | 20 | <0.001 |
| Coronary artery bypass graft surgery | 80 | 10 | 77 | 9 | 82 | 8 | 0.43 |
| Percutaneous coronary intervention | 309 | 40 | 466 | 55 | 627 | 65 | <0.001 |
| Thrombolysis | 188 | 24 | 32 | 4 | 5 | 1 | <0.001 |
| Any reperfusion/revascularization therapy | 488 | 63 | 549 | 65 | 700 | 72 | <0.001 |
Abbreviations: GFR, glomerular filtration rate; MI, myocardial infarction.
a Values are expressed as mean (standard deviation).
b Weight (kg)/height (m)2.
Trends in HF incidence
Over 5 years of follow-up, 715 patients developed HF, 475 (66%) of whom did so during the first week after MI. The crude incidence rates of HF per 100 person-years were 10.8 in 1990–1996, 11.4 in 1997–2003, and 9.8 in 2004–2010. Accounting for death as a competing risk, the age- and sex-adjusted incidence rates of HF have declined over time both for the early-onset (0–7 days) and late-onset (8 days–5 years) HF categories (Figure 1). In absolute terms, the excess HF risk at 7 days in 1990–1996 versus 2004–2010 was 5.7 (95% confidence interval (CI): 2.5, 9.0) cases per 100 patients. Among patients who survived for 7 or more days, the excess risk at 5 years was 5.8 (95% CI: 2.1, 9.6) (Table 2).
Figure 1.
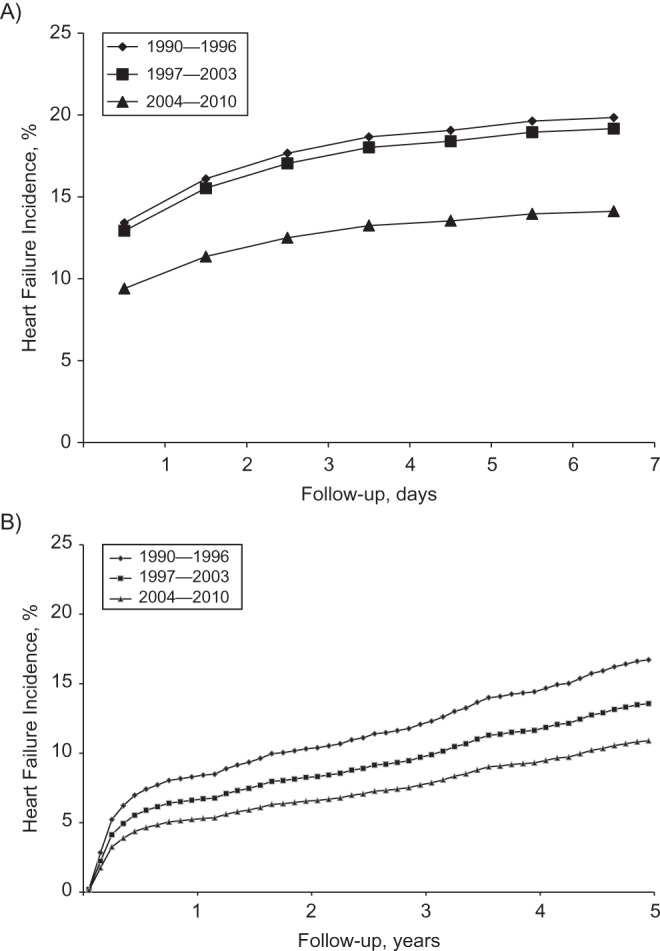
Temporal trends in the cumulative incidence rates of heart failure after myocardial infarction in Olmsted County, Minnesota, 1990–2010. The curves represent year categories of index myocardial infarction. Using the direct adjustment method, adjustment was made for age and sex, with death considered a competing event. Follow-up is divided into A) early risk (0–7 days after myocardial infarction) and B) late risk (0–5 years among those who survived 7 or more days after myocardial infarction) periods.
Table 2.
Trends in Age- and Sex-Adjusted Incidence of Heart Failure After Myocardial Infarction in Olmsted County, Minnesota, 1990–2010a
| Year Group Comparisonb | Entire Cohort (n = 2,596) |
7-Day Survivors (n = 2,041) |
||||||
|---|---|---|---|---|---|---|---|---|
| 0–1 Days After MI |
0–7 Days After MI |
0–1 Year After MI |
0–5 Years After MI |
|||||
| Absolute Risk Differencec | 95% CI | Absolute Risk Differencec | 95% CI | Absolute Risk Differencec | 95% CI | Absolute Risk Differencec | 95% CI | |
| 1990–1996 vs. 2004–2010 | 4.0 | 1.8, 6.3 | 5.7 | 2.5, 9.0 | 3.1 | 1.0, 5.1 | 5.8 | 2.1, 9.6 |
| 1997–2003 vs. 2004–2010 | 3.5 | 1.3, 5.7 | 5.0 | 1.9, 8.1 | 1.4 | −0.4, 3.2 | 2.7 | −0.8, 6.1 |
| 1990–1996 vs. 1997–2003 | 0.5 | −1.9, 2.9 | 0.7 | −2.7, 4.1 | 1.7 | −0.4, 3.8 | 3.1 | −0.8, 7.1 |
Abbreviations: CI, confidence interval; MI, myocardial infarction.
a Absolute risk differences (excess heart failure cases and 95% confidence intervals per 100 patients) between year groups for selected time intervals during follow-up.
b Values from the later period were subtracted from those from the earlier period.
c Risk differences were estimated using the direct adjustment method at the end of the intervals. Estimates were derived from the Fine and Gray subdistribution hazard regressions, with death treated as a competing event.
The age- and sex-adjusted hazard ratios for HF in the recent versus earliest time periods were 0.67 (95% CI: 0.54, 0.85) for early-onset HF and 0.63 (95% CI: 0.46, 0.86) for late-onset HF (Table 3). The temporal decline in the risk of early-onset HF was largely accounted for by changes in MI characteristics and severity (model 2) and was further reduced after adjustment for recurrent MI (model 4). Conversely, the temporal decline in late-onset HF was only minimally affected by these adjustments (Table 3). None of the trends differed significantly by age or sex (P for interaction > 0.05). In ancillary analyses, MIs that met only criteria based on troponin levels were excluded from the analyses; similar results were obtained (data not shown). In addition, further adjustment for heart rate on admission, atrial fibrillation, impaired renal function, and anterior MI yielded results virtually identical to those presented in the fully adjusted models in Table 3. Lastly, using a generalized additive model, a nonlinear trend in the relationship between year and early-onset HF was detected (P < 0.01 for the spline term). Accordingly, a quadratic term of year was tested in a Cox proportional hazards model and found to be significant (P = 0.001), indicating a more rapid decline in early HF risk after MI during recent years.
Table 3.
Temporal Trends in Incidence of Heart Failure After Myocardial Infarction in Olmsted County, Minnesota, 1990–2010
| Adjustment | Follow-up Period |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Earlya |
Lateb |
|||||||||||||
| 1990–1996 |
1997–2003 |
2004–2010 |
1990–1996 |
1997–2003 |
2004–2010 |
|||||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | PTrend | HR | 95% CI | HR | 95% CI | HR | 95% CI | PTrend | |
| Age and sex | 1.00 | Referent | 0.96 | 0.77, 1.19 | 0.67 | 0.54, 0.85 | 0.001 | 1.00 | Referent | 0.79 | 0.59, 1.07 | 0.63 | 0.46, 0.86 | 0.004 |
| Model 1c | 1.00 | Referent | 0.98 | 0.79, 1.22 | 0.70 | 0.55, 0.89 | 0.003 | 1.00 | Referent | 0.75 | 0.55, 1.02 | 0.56 | 0.40, 0.80 | 0.001 |
| Model 2d | 1.00 | Referent | 1.04 | 0.83, 1.30 | 0.81 | 0.63, 1.03 | 0.086 | 1.00 | Referent | 0.71 | 0.52, 0.98 | 0.54 | 0.38, 0.77 | 0.001 |
| Model 3e | 1.00 | Referent | 1.10 | 0.87, 1.38 | 0.78 | 0.61, 1.01 | 0.220 | 1.00 | Referent | 0.78 | 0.56, 1.09 | 0.60 | 0.42, 0.86 | 0.006 |
| Model 4f | 1.00 | Referent | 1.10 | 0.87, 1.39 | 0.86 | 0.66, 1.11 | 0.209 | 1.00 | Referent | 0.73 | 0.55, 0.99 | 0.63 | 0.45, 0.88 | 0.006 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a The early risk period was 0–7 days after myocardial infarction. Models were based on 475 heart failure cases among 2,596 patients with myocardial infarction.
b The late risk period was 8 days to 5 years after MI. Analyses were restricted to patients who survived 7 or more days without heart failure. Models were based on 240 heart failure cases among 2,041 patients with myocardial infarction.
c Model 1 was adjusted for age, sex, Charlson index category, body mass index (measured as weight (kg)/height (m)2), hypertension, hyperlipidemia, and smoking.
d Model 2 was adjusted for the factors in model 1 plus a Killip class greater than 1 and ST-segment–elevation myocardial infarction.
e Model 3 was adjusted for the factors in model 2 plus coronary artery bypass graft surgery, percutaneous coronary intervention, and thrombolysis.
f Model 4 was adjusted for the factors in model 3 plus recurrent myocardial infarction.
Trends by HF type
The observed (i.e., complete-case analysis) proportion of HF with reduced EF was 67% (276 of 415 incidents) for early-onset HF and 60% (103 of 172 incidents) for late-onset HF. Similar estimates were obtained using the multiple imputation analysis (67% (317 of 475 incidents) and 60% (144 of 240 incidents) for early- and late-onset HF, respectively). The observed proportion of patients who had HF with reduced EF declined from 1990–1996 to 2004–2010 for both early-onset (from 75% to 62%; P = 0.03) and late-onset (from 79% to 44%; P < 0.001) HF. These trends were again similar in the multiple imputation analysis (from 75% to 62% for early-onset HF and 76% to 44% for late-onset HF), illustrating the reducing prevalence of HF with impaired EF.
The association between year categories and HF types is summarized in Table 4 and Figure 2. For the multiple imputation analysis, the age- and sex-adjusted hazard ratios for HF with reduced EF in 2004–2010 versus 1990–1996 were 0.55 (95% CI: 0.41, 0.73) for early-onset HF and 0.36 (95% CI: 0.23, 0.56) for late-onset HF. The estimates for HF with preserved EF were 1.07 (95% CI: 0.69, 1.66) and 1.47 (95% CI: 0.85, 2.55), respectively, for early- and late-onset HF (Table 4). Accounting for death as a competing risk supported a steady decrease in the cumulative incidence of HF with reduced EF, with no evidence of a decline in HF with preserved EF, both for early-onset and late-onset HF (Figure 2). Multivariable adjustment for various prognostic factors accounted for some of the decline in the risk of early-onset HF with reduced EF (hazard ratio = 0.74, 95% CI: 0.54, 1.03) but did not materially change the risk estimate for late-onset HF with reduced EF (hazard ratio = 0.39, 95% CI: 0.25, 0.60). The hazard ratios for HF with preserved EF after similar adjustment were 1.22 (95% CI: 0.75, 1.98) and 1.36 (95% CI: 0.76, 2.43), respectively, for early- and late-onset HF. The results of the complete-case analysis were similar to those obtained from the multiple imputation analysis.
Table 4.
Temporal Trends by Heart Failure Type in Olmsted County, Minnesota, 1990–2010a
| Adjustmentb | Heart Failure With Reduced Ejection Fraction |
Heart Failure With Preserved Ejection Fraction |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1990–1996 |
1997–2003 |
2004–2010 |
PTrend | 1990–1996 |
1997–2003 |
2004–2010 |
PTrend | |||||||
| HRb | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |||
| Early riskc | ||||||||||||||
| Age and sex | 1.00 | Referent | 0.80 | 0.61, 1.04 | 0.55 | 0.41, 0.73 | 0.002 | 1.00 | Referent | 1.45 | 0.94, 2.23 | 1.07 | 0.69, 1.66 | 0.14 |
| Multivariable | 1.00 | Referent | 0.95 | 0.71, 1.28 | 0.74 | 0.54, 1.03 | 0.14 | 1.00 | Referent | 1.58 | 1.00, 2.50 | 1.22 | 0.75, 1.98 | 0.11 |
| Late riskd | 1.00 | 1.00 | ||||||||||||
| Age and sex | 1.00 | Referent | 0.58 | 0.39, 0.88 | 0.36 | 0.23, 0.56 | <0.001 | 1.00 | Referent | 1.44 | 0.78, 2.65 | 1.47 | 0.85, 2.55 | 0.35 |
| Multivariable | 1.00 | Referent | 0.58 | 0.39, 0.87 | 0.39 | 0.25, 0.60 | <0.001 | 1.00 | Referent | 1.38 | 0.75, 2.55 | 1.36 | 0.76, 2.43 | 0.53 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Hazard ratios and 95% CIs between year groups during early and late risk periods from the multiple imputation analysis.
b Multivariable adjustment was made for age, sex, comorbid conditions, body mass index (measured as weight (kg)/height (m)2), hypertension, hyperlipidemia, smoking, Killip class, ST-segment–elevation myocardial infarction, coronary artery bypass graft surgery, percutaneous coronary intervention, thrombolysis, and recurrent myocardial infarction (modeled as a time-dependent covariate).
c The early risk period was 0–7 days after myocardial infarction. Analyses were based on a total of 475 heart failure cases, 317 (67%) of whom had a reduced ejection fraction and 158 of whom had a preserved ejection fraction.
d The late risk period was 8 days–5 years after myocardial infarction. Analyses were based on a total of 240 heart failure cases, 144 (60%) of whom had a reduced ejection fraction and 96 of whom had a preserved ejection fraction.
Figure 2.
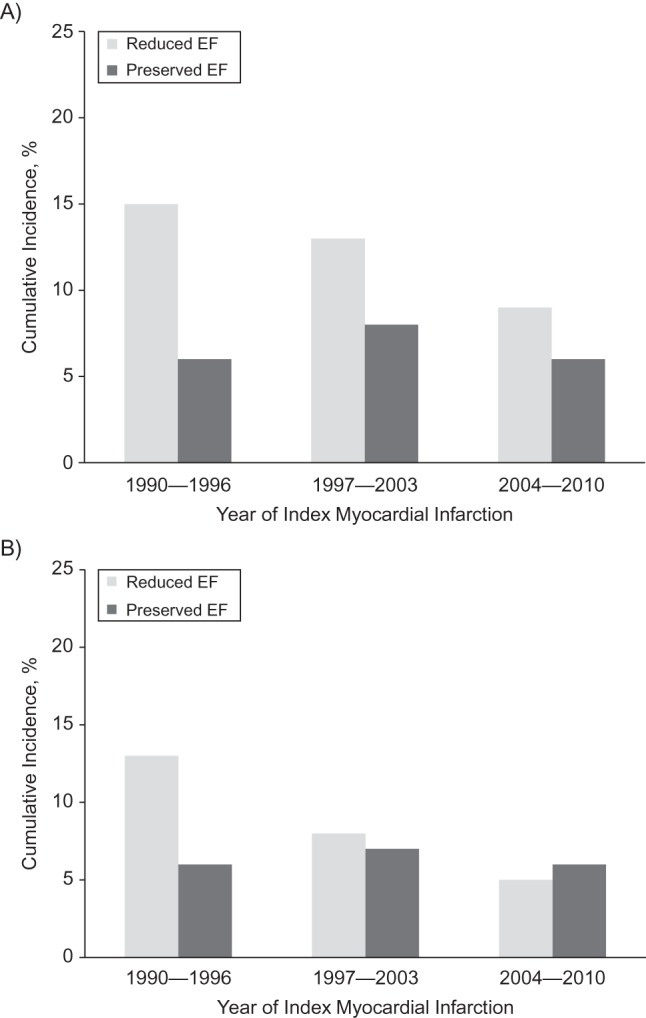
Temporal trends in the cumulative incidence rates of type of heart failure after myocardial infarction in Olmsted County, Minnesota, 1990–2010. The bars represent categories of index myocardial infarction year and consist of heart failure with either reduced or preserved ejection fraction (EF). Adjustment was made for age and sex using the direct adjustment method, with death considered a competing event. Rates are divided into A) early risk (0–7 days after myocardial infarction) and B) late risk (8 days–5 years after myocardial infarction) incidence periods.
DISCUSSION
Summary of findings
The present community surveillance study provides strong evidence for a temporal decline in HF risk after incident MI. Using data that spanned more than 2 decades (1990–2012) and originated from a carefully characterized cohort for whom we had complete ascertainment of outcomes, we detected a bimodal distribution of HF rates, with a high-risk stage during the first week after MI followed by a “reset period” at a lower rate. A temporal decline in incidence was observed for both early- and late-onset HF. Adjustment for several covariates of high clinical relevance, mainly MI characteristics and severity, accounted for the decline in the risk of early-onset HF but not the decline in the risk of late-onset HF. Among HF patients, the proportion of cases presenting with impaired EF declined over time for both early- and late-onset HF as a result of a lack of reduction in HF with preserved EF.
Challenges in monitoring trends in HF after MI
HF is among the most devastating outcomes after MI (7, 8). Although remarkable changes in the epidemiology of MI have taken place during the past decades that affected clinical presentation, treatment, and outcomes (12–14), the trends in HF after MI remain insufficiently characterized. This gap in knowledge likely reflects diverging and complex longitudinal changes in key determinants of HF after MI. On the one hand, the dramatic decline in case-fatality rates of MI (35) that reflect modern treatments and improved MI care have resulted in an increasing proportion of survivors at heightened risk of future nonfatal events, including HF (7). On the other hand, parallel developments in therapeutic strategies aimed at preventing the development of HF after MI (36, 37) and a decrease in overall MI severity (13, 38) have presumably contributed to reducing HF. Published data illustrate this controversy. In the Framingham Heart Study, an increase in the incidence of HF after MI was observed between 1970 and 1999 that paralleled the decrease in the death rate (16). These findings were supported in other settings with larger sample sizes (5, 17). In contrast, a decreasing trend was observed in Olmsted County, Minnesota, between 1979 and 1994 (39) and in the Worcester Heart Attack Study between 1975 and 2005 (18). Possible explanations for the conflicting results of previous studies may lie in differences in the case mix of the study samples (first vs. recurrent MI; inclusion vs. exclusion of patients with previous HF), variation in the duration of follow-up (in-hospital vs. long-term), quality of ascertainment of HF (International Classification of Diseases, 9th Revision codes vs. validated cases; inpatient vs. outpatient), and distinctions in the time periods of observation. Moreover, previous studies are now somewhat dated and were published in the midst of the HF epidemic (35); thus they do not fully capture the consequences of the aforementioned recent and major changes in the epidemiology of MI (20).
Interpretation of study findings
Community surveillance studies, which measure population trends in disease incidence and outcomes, are ideally suited to evaluate contemporary trends in HF after MI. Accordingly, the comprehensive population-based approach provided by the Rochester Epidemiology Project, along with a rigorous ascertainment of incident MI and the access to complete inpatient and outpatient data, offers a unique opportunity to conduct robust surveillance that addresses previous gaps in knowledge. We identified a decline in HF incidence after MI over the past 2 decades. The decline applied to both early (first week) and late (5 years) HF incidence. However, although the decline in early HF was largely attributable to observed changes in clinical variables (mainly MI characteristics), the decline in late HF was unaffected by these variables. Conceptually, early-onset HF after MI reflects extensive myocardial damage and is thus related to the severity of MI. By contrast, late-onset HF is thought to be related to several mechanisms, including progressive remodeling, recurrent MI, and subclinical ischemia (3, 7). The fact that clinical characteristics at the time of index hospitalization and recurrent MI could not account for the temporal decline in late-onset HF suggests that other factors play a role in this decline. The introduction of new HF treatments over the past decades, including angiotensin-converting enzyme inhibitors, aldosterone antagonists, angiotensin receptor blockers, and β-blockers, have been shown to improve morbidity and mortality in selected populations of patients with MI (36, 37), thereby providing a possible mechanism for the decreasing risk.
To the best of our knowledge, the present study provides one of the first longitudinal reports of the trends in HF after MI by type, as we were able to categorize HF from echocardiographic data as having either reduced of preserved EF, information that was lacking in previous publications (16). Although the rate of HF with reduced EF steadily declined for both the early-onset and late-onset periods, no reduction and even a possible increase was detected for the rate of HF with preserved EF, regardless of the timing of HF onset. This indicates that the case mix of HF after MI is shifting, raising the important question of changing mechanisms. It is often assumed that HF after MI is more likely to present with reduced EF (40); however, the present data challenges this dogma, underscoring the need for mechanistic studies on this matter.
Some limitations should be acknowledged in interpreting these data. These results emanate from a single Midwestern community that is predominantly white, and thus they may not be applicable to other populations. Yet, although no single community will be completely representative of the nation as a whole, comparisons of previous population-based studies of various chronic diseases in Olmsted County with those from other communities in the United States indicated that the results for the population of this area can be extrapolated to a large part of the population of the country (21, 22, 41). HF was ascertained by using the Framingham Heart Study criteria (25), which may result in some misclassification. Data on socioeconomic status or medical insurance were not routinely recorded until more recent years, precluding an adequate assessment of their intermediary role. Echocardiograms were not routinely performed during the index MI, which precluded modeling of baseline EF, and were missing in some of the HF cases, necessitating the use of multiple imputations in the analysis of HF type. In addition, beyond acute interventions, we did not model the use of specific treatments, so their potential mediating effect cannot be assessed in this study.
Potential implications
The American Heart Association recently announced a new 2020 strategic goal of improving cardiovascular health of all Americans by 20% (42). To demonstrate success, we must be able to monitor progress. Our ability to do so is hindered by the lack of a global approach to the surveillance of cardiovascular disease (43). Without a national system, the surveillance of cardiovascular disease relies on an integrated approach that leverages vital statistics, administrative datasets, community surveillance programs, and local registries (44). The present study illustrates the use of such an approach for surveillance of cardiovascular disease by reporting on contemporary trends in HF after MI in a well-characterized community for whom we had complete access to longitudinal follow-up data.
ACKNOWLEDGMENTS
Author affiliations: Department of Health Sciences Research, Mayo Clinic, Rochester, Minnesota (Yariv Gerber, Susan A. Weston, Cecilia Berardi, Sheila M. McNallan, Ruoxiang Jiang, Véronique L. Roger); Department of Epidemiology and Preventive Medicine, School of Public Health, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (Yariv Gerber); and Division of Cardiovascular Diseases (Margaret M. Redfield, Véronique L. Roger), Mayo Clinic, Rochester, Minnesota.
This work was supported by grants from the National Institutes of Health (R01 HL59205 and R01 HL72435) and made possible by the Rochester Epidemiology Project (grant R01 AG034676 from the National Institute on Aging).
Conflict of interest: none declared.
REFERENCES
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunlay SM, Shah ND, Shi Q, et al. Lifetime costs of medical care after heart failure diagnosis. Circ Cardiovasc Qual Outcomes. 2011;4(1):68–75. doi: 10.1161/CIRCOUTCOMES.110.957225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gheorghiade M, Bonow RO. Chronic heart failure in the United States: a manifestation of coronary artery disease. Circulation. 1998;97(3):282–289. doi: 10.1161/01.cir.97.3.282. [DOI] [PubMed] [Google Scholar]
- 4.He J, Ogden LG, Bazzano LA, et al. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161(7):996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 5.Ezekowitz JA, Kaul P, Bakal JA, et al. Declining in-hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J Am Coll Cardiol. 2009;53(1):13–20. doi: 10.1016/j.jacc.2008.08.067. [DOI] [PubMed] [Google Scholar]
- 6.Gerber Y, Jaffe AS, Weston SA, et al. Prognostic value of cardiac troponin T after myocardial infarction: a contemporary community experience. Mayo Clin Proc. 2012;87(3):247–254. doi: 10.1016/j.mayocp.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis EF, Moye LA, Rouleau JL, et al. Predictors of late development of heart failure in stable survivors of myocardial infarction: the CARE Study. J Am Coll Cardiol. 2003;42(8):1446–1453. doi: 10.1016/s0735-1097(03)01057-x. [DOI] [PubMed] [Google Scholar]
- 8.Adabag AS, Therneau TM, Gersh BJ, et al. Sudden death after myocardial infarction. JAMA. 2008;300(17):2022–2029. doi: 10.1001/jama.2008.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunlay SM, Roger VL, Weston SA, et al. Longitudinal changes in ejection fraction in heart failure patients with preserved and reduced ejection fraction. Circ Heart Fail. 2012;5(6):720–726. doi: 10.1161/CIRCHEARTFAILURE.111.966366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bursi F, Weston SA, Redfield MM, et al. Systolic and diastolic heart failure in the community. JAMA. 2006;296(18):2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 11.Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 12.Kostis WJ, Deng Y, Pantazopoulos JS, et al. Trends in mortality of acute myocardial infarction after discharge from the hospital. Circ Cardiovasc Qual Outcomes. 2010;3(6):581–589. doi: 10.1161/CIRCOUTCOMES.110.957803. [DOI] [PubMed] [Google Scholar]
- 13.Roger VL, Weston SA, Gerber Y, et al. Trends in incidence, severity, and outcome of hospitalized myocardial infarction. Circulation. 2010;121(7):863–869. doi: 10.1161/CIRCULATIONAHA.109.897249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeh RW, Sidney S, Chandra M, et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362(23):2155–2165. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 15.Gerber Y, Melton LJ, 3rd, Weston SA, et al. Association between myocardial infarction and fractures: an emerging phenomenon. Circulation. 2011;124(3):297–303. doi: 10.1161/CIRCULATIONAHA.110.007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Velagaleti RS, Pencina MJ, Murabito JM, et al. Long-term trends in the incidence of heart failure after myocardial infarction. Circulation. 2008;118(20):2057–2062. doi: 10.1161/CIRCULATIONAHA.108.784215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kostis WJ, Deng Y, Moreyra AE, et al. No decrease in the incidence of heart failure following acute myocardial infarction in the years 1994–2006. Circulation. 2011;124:A17546. [Google Scholar]
- 18.McManus DD, Chinali M, Saczynski JS, et al. 30-year trends in heart failure in patients hospitalized with acute myocardial infarction. Am J Cardiol. 2011;107(3):353–359. doi: 10.1016/j.amjcard.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shafazand M, Rosengren A, Lappas G, et al. Decreasing trends in the incidence of heart failure after acute myocardial infarction from 1993–2004: a study of 175,216 patients with a first acute myocardial infarction in Sweden. Eur J Heart Fail. 2011;13(2):135–141. doi: 10.1093/eurjhf/hfq205. [DOI] [PubMed] [Google Scholar]
- 20.Jhund PS, McMurray JJ. Heart failure after acute myocardial infarction: a lost battle in the war on heart failure? Circulation. 2008;118(20):2019–2021. doi: 10.1161/CIRCULATIONAHA.108.813493. [DOI] [PubMed] [Google Scholar]
- 21.Melton LJ., 3rd History of the Rochester epidemiology project. Mayo Clin Proc. 1996;71(3):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 22.St Sauver JL, Grossardt BR, Yawn BP, et al. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173(9):1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 25.McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failure: the Framingham Study. N Engl J Med. 1971;285(26):1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 26.Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292(3):344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Loberiza FR, Klein JP, et al. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88(2):95–101. doi: 10.1016/j.cmpb.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Wolbers M, Koller MT, Witteman JC, et al. Prognostic models with competing risks: methods and application to coronary risk prediction. Epidemiology. 2009;20(4):555–561. doi: 10.1097/EDE.0b013e3181a39056. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Zhang MJ. SAS macros for estimation of direct adjusted cumulative incidence curves under proportional subdistribution hazards models. Comput Methods Programs Biomed. 2011;101(1):87–93. doi: 10.1016/j.cmpb.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 31.Cox DR. Regression analysis and life table. J R Stat Soc (Series B) 1972;34(2):187–222. [Google Scholar]
- 32.Ali AS, Rybicki BA, Alam M, et al. Clinical predictors of heart failure in patients with first acute myocardial infarction. Am Heart J. 1999;138(6):1133–1139. doi: 10.1016/s0002-8703(99)70080-3. [DOI] [PubMed] [Google Scholar]
- 33.Rubin D. Multiple Imputations for Nonresponse in Surveys. New York, NY: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 34.White IR, Royston P. Imputing missing covariate values for the Cox model. Stat Med. 2009;28(15):1982–1998. doi: 10.1002/sim.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braunwald E. Shattuck lecture—cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337(19):1360–1369. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- 36.Kober L, Torp-Pedersen C, Carlsen JE, et al. A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril Cardiac Evaluation (TRACE) Study Group. N Engl J Med. 1995;333(25):1670–1676. doi: 10.1056/NEJM199512213332503. [DOI] [PubMed] [Google Scholar]
- 37.Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349(20):1893–1906. doi: 10.1056/NEJMoa032292. [DOI] [PubMed] [Google Scholar]
- 38.Myerson M, Coady S, Taylor H, et al. Declining severity of myocardial infarction from 1987 to 2002: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2009;119(4):503–514. doi: 10.1161/CIRCULATIONAHA.107.693879. [DOI] [PubMed] [Google Scholar]
- 39.Hellermann JP, Goraya TY, Jacobsen SJ, et al. Incidence of heart failure after myocardial infarction: is it changing over time? Am J Epidemiol. 2003;157(12):1101–1117. doi: 10.1093/aje/kwg078. [DOI] [PubMed] [Google Scholar]
- 40.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101(25):2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 41.St Sauver JL, Grossardt BR, Leibson CL, et al. Generalizability of epidemiological findings and public health decisions: an illustration from The Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(2):151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic impact goal through 2020 and beyond. Circulation. 2010;121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 43.Goff DC, Jr, Brass L, Braun LT, et al. Essential features of a surveillance system to support the prevention and management of heart disease and stroke: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Stroke, and Cardiovascular Nursing and the Interdisciplinary Working Groups on Quality of Care and Outcomes Research and Atherosclerotic Peripheral Vascular Disease. Circulation. 2007;115(1):127–155. doi: 10.1161/CIRCULATIONAHA.106.179904. [DOI] [PubMed] [Google Scholar]
- 44.Roger VL. Myocardial infarction outcomes: “the times, they are a-changin …”. Circ Cardiovasc Qual Outcomes. 2010;3(6):568–570. doi: 10.1161/CIRCOUTCOMES.110.959163. [DOI] [PMC free article] [PubMed] [Google Scholar]


