Abstract
Recent visual neuroscience investigations suggest that ventral occipito-temporal cortex is retinotopically organized, with high acuity foveal input projecting primarily to the posterior fusiform gyrus (pFG), making this region crucial for coding high spatial frequency information. Because high spatial frequencies are critical for fine-grained visual discrimination, we hypothesized that damage to the left pFG should have an adverse effect not only on efficient reading, as observed in pure alexia, but also on the processing of complex non-orthographic visual stimuli. Consistent with this hypothesis, we obtained evidence that a large case series (n = 20) of patients with lesions centered on left pFG: 1) Exhibited reduced sensitivity to high spatial frequencies; 2) demonstrated prolonged response latencies both in reading (pure alexia) and object naming; and 3) were especially sensitive to visual complexity and similarity when discriminating between novel visual patterns. These results suggest that the patients' dual reading and non-orthographic recognition impairments have a common underlying mechanism and reflect the loss of high spatial frequency visual information normally coded in the left pFG.
Keywords: foveal/parafoveal vision, fusiform gyrus, letter-by-letter reading, pure alexia, spatial frequency, ventral occipito-temporal cortex, visual recognition
Introduction
Lesions involving ventral occipito-temporal cortex (vOT) typically arise from cerebral ischemia in the territory of the posterior cerebral artery, head trauma, or surgical ablation. A classic presentation of patients with left vOT damage is a reading deficit without significant spelling or spoken language impairment other than anomia (Benson and Geschwind 1969; Capitani et al. 2009). This pattern is known as pure alexia or letter-by-letter reading (Dejerine 1892), while more severe patients also suffer from visual agnosia, optic aphasia (Humphreys et al. 1997), or visual semantic access disorders (Warrington and Shallice 1979, 1980). In alphabetic languages, the hallmark symptom of pure alexia is slowed, inefficient processing of letter strings with an exaggerated effect of word length on reading performance (Shallice and Saffran 1986; Roberts et al. 2010). This contrasts with normal word reading, where length exerts little influence on performance (Weekes 1997). Lesion-deficit correlation studies have provided evidence that the critical neural substrate of pure alexia is damage to the posterior fusiform gyrus (pFG; Damasio and Damasio 1983; Binder and Mohr 1992; Cohen et al. 2003; Leff et al. 2006). Cognitive theories, combining neuropsychological and functional neuroimaging data, have suggested that the left pFG is important for orthographic recognition either because this area has become specialized for this specific stimulus type (Cohen et al. 2000, 2002, 2004; Dehaene et al. 2001, 2004, 2005; Dehaene and Cohen 2011) or because this region is crucial in some way for rapid decoding of complex visual stimuli—a process that reading is especially reliant upon (Price et al. 1996; Behrmann et al. 1998a,b; Price and Devlin 2003, 2011; Devlin et al. 2006; Mycroft et al. 2009).
The primary purpose of this study was not to add to this ongoing, vibrant debate between the competing cognitive theories (Dehaene and Cohen 2011; Price and Devlin 2011) but was to initiate an investigation about why the left pFG region appears to be crucial for the recognition of visually complex orthographic and non-orthographic stimuli. More specifically, we explored the visual processing of patients with vOT damage in the context of potentially important insights offered by recent visual neuroscience studies of this region. On the basis of functional imaging studies in normal subjects, Malach and colleagues (Levy et al. 2001; Hasson et al. 2002, 2003; Malach et al. 2002) have proposed the notion of a retinotopically organized ventral occipito-temporal area (vOT; Malach et al. 2002) which runs from the pFG to the collateral sulcus. Within this region, specific areas respond maximally to different object categories (animals, objects, houses, faces, and words). Hasson et al. (2002) proposed that this graded functional separation reflects the visual demands of each type of stimuli and, in turn, the acuity variation across retinal eccentricity. Visual acuity (sensitivity to high spatial frequencies) is highest in the fovea and drops toward the parafoveal region (Fiset et al. 2006; Starrfelt et al. 2009). Foveal vision is delivered to the pFG and this region is maximally active for categories of visual stimuli that require fine visual discrimination/foveation (faces and words; Hasson et al. 2002). Other categories (e.g. houses) activate areas medial to the pFG where parafoveal vision is primarily projected. In effect, there is a graded division of labor across the vOT such that acuity-demanding visual categories dominate activation of the pFG, whereas categories less dependent on high-resolution foveal input can be processed by peripherally biased regions on the basis of low spatial frequency information (for a formal computational exploration, see Plaut and Behrmann 2011).
A recent fMRI study (Woodhead et al. 2011) investigated the relationship between object processing areas along the left and right occipito-temporal cortex (including specific subregions determined by functional localizer scans for words and faces) and the BOLD response to simple sine-wave gratings across a range of spatial frequencies. In keeping with Malach et al.'s (2002) hypothesis, Woodhead et al. (2011) found that early occipital regions were tuned toward mid-to-high spatial frequencies and this extended to the pFG. At this point along the ventral visual pathway, while the pFG was tuned to high spatial frequencies, more medial and lateral vOT structures responded to low spatial frequencies (Fig. 3; Woodhead et al. 2011). Woodhead et al. (2011) concluded that word recognition elicited a strongly left-lateralized activation. These results are consistent with the notion of vOT subregion specialization for the processing of different kinds of object, reflecting the availability of different spatial frequencies across this area (Levy et al. 2001; Hasson et al. 2002).
These visual neuroscience findings suggest that damage to foveally biased regions within the left pFG should result in reduced sensitivity to medium-to-high spatial frequencies and thus, in turn, give rise to both orthographic and non-orthographic visual processing impairments. However, as far as we are aware, despite offering a crucial link between basic visual processing and object recognition, this theory has yet to be tested in patients with focal brain damage. Lesion-deficit correlation studies are important because they can provide a critical test of the necessity of the visual processing carried out by the left pFG and also reveal novel insights about the basis of visual recognition deficits in pure alexia. To accomplish this goal, we investigated 3 hypotheses in a large case series of patients with left vOT damage. First, following the retinotopic account (Levy et al. 2001; Hasson et al. 2003) and the findings of Woodhead et al. (2011), individuals with vOT damage should show reduced sensitivity to medium-to-high spatial frequencies, which will cause a reading deficit consisting of the enhanced length effects that define pure alexia. Secondly, the severity of the reading deficit as reflected in the slope of the word length effect should correspond to the degree of damage to the left pFG. Thirdly, comparable deficits should be observed when patients are required to process complex visual stimuli (familiar or novel) that necessitate high acuity/spatial frequency information for differentiation.
Two additional features of the current study are also important to note. Previous investigations of visual processing in pure alexia have suffered from 2 limitations. In all but the most severely affected patients, the reading deficit is most apparent in speed (being both slow and characterized by abnormal length effects). In contrast, performance on non-reading visual tasks has been assessed using accuracy-based measures (for a review, see Behrmann et al. 1998b). This is important because patients may exhibit accurate object naming despite being abnormally slow (Davidoff and Warrington 1999) and so we measured reaction time (RT) and accuracy in all tasks across all patients. Secondly, the bulk of the neuropsychological literature is composed of intensive investigations of individual patients or studies with a small sample size (Behrmann et al. 1998b). It is, therefore, difficult to gauge the reliability of findings across this patient population and to establish the impact of severity on visual recognition of words and other non-verbal stimuli—2 problems that were overcome by our investigation of this large case series of patients.
Patients
Nine patients were recruited from local NHS speech and language therapy services in the United Kingdom and a further 11 patients through collaboration with the University of Arizona (AZ). The study was approved by the National Health Service Multicentre Research Ethics Committee and by the Institutional Review Board of the University of Arizona, and informed consent was provided in all cases. In order to explore the impact of severity upon performance, it was necessary to recruit a broad range of patients using both behavioral and lesion criteria. Therefore, inclusion was based on neuroradiological evidence of damage to left vOT and/or a reading deficit characterized by an abnormally strong effect of length on reading speed. As expected, there was a wide range of severity among the recruited patients as measured by reading speed on a subset of the 3, 4, 5, and 6 letter word lists developed by Weekes (1997). For measuring correct RTs in tasks requiring a spoken response (e.g. reading, naming, etc.), RTs were measured in the AZ patients using a voice key. For the (typically more severe) UK patients, RTs were established offline via a digital recording of each experimental trial using WavePad software (NCH, Swiftsound: www.nch.com.au/wavepad).
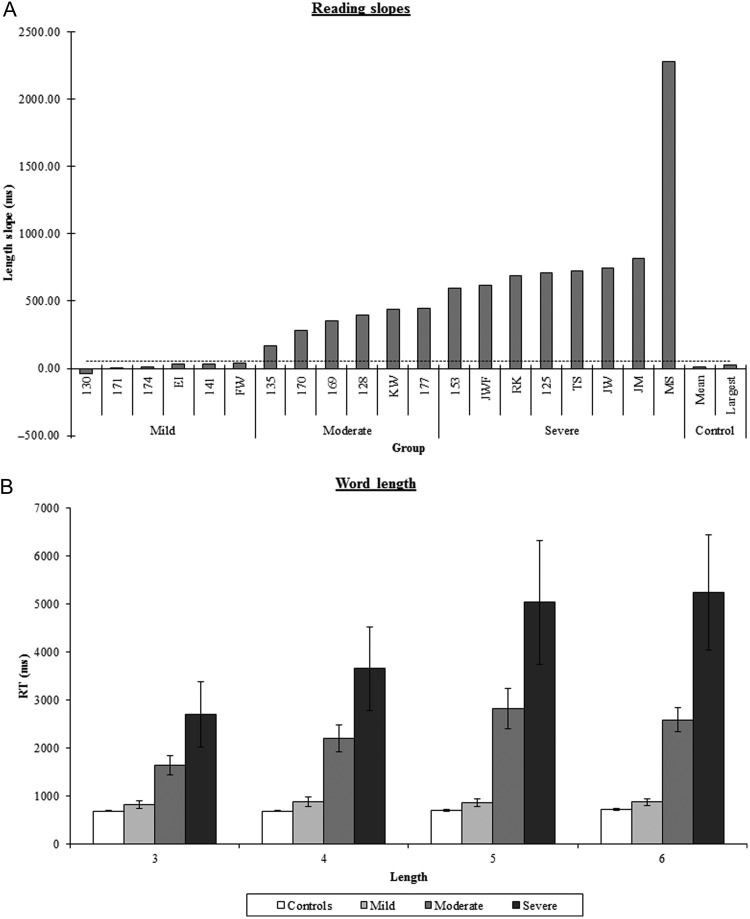
Given that pure alexia is characterized primarily by the abnormal length effect as well as slow reading times, the patients were stratified accordingly to the slope of their reading times. Slope was used as the severity measure as it captures both the overall slowed reading as well as the key defining feature of the disorder, which is an abnormal word length effect. The results are shown in Figure 1a. The full case series was split into 3 severity-based subgroups: 1) Mild—those 6 patients whose slope fell within the normal range (below the dotted line in Fig. 1a); and the remainder were split into 2) a moderate group of 6 patients and 3) a severe group of 8 patients. The average reading speed as a function of word length for each group is summarized in Figure 1b.
Figure 1.
Summary reading data for the 20 patients included in the study for (A) the reading regression slope and (B) the mean reading speed as a function of word length. Error bars indicate standard error. Dashed line in (A) is control mean plus 2 standard deviations.
Background Neuropsychological Assessment
Each patient completed a battery of background assessments to give a profile of their cognitive abilities. UK and AZ patients completed slightly different background tests (Tables 1 and 2). For UK patients, the Visual Object and Space Perception battery (VOSP; Warrington and James 1991) was used to test a range of visual and visuospatial skills such as identifying incomplete letters, naming progressively difficult silhouettes of common objects, and counting how many cubes a 3D block contains (for a detailed description of each task, see Warrington and James 1991). A further battery of assessments investigated semantic and phonological processing more generally.
Table 1.
Demographic and background neuropsychological assessment for the 9 UK patients ordered left to right, from mild to severe, according to the severity of the reading impairment (slope of the word length effect in RTs)
| Maximum | Normal cut-off | EI | FW | KW | JWF | RK | TS | JW | JM | MS | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | |||||||||||
| Age | — | — | 40 | 80 | 44 | 54 | 63 | 57 | 59 | 67 | 70 |
| Sex | — | — | F | M | M | F | M | M | M | M | F |
| Handedness | RH | RH | RH | LH | RH | RH | RH | RH | LH | ||
| Years of education | — | — | 13 | 11 | 10 | 10 | 10 | 10 | 11 | 10 | 10 |
| Lesion aetiology | Stroke | Stroke | Stroke | Stroke | Stroke | Tumor resection | Stroke | Tumor resection | Stroke | ||
| Lesion volume (cc) | 12.11 | No scan | No scan | 92.89 | 39.93 | 162.69 | 93.27 | 14.34 | 99.34 | ||
| Visual field loss | RUQ | RHH | RHH | RHH | RHH | RHH | RHH | RUQ | RHH | ||
| Working memory | |||||||||||
| Digit span | |||||||||||
| Forward (12) | — | 5 | 9 | 8 | 8 | 6 | NT | 8 | 7 | 12 | 10 |
| Backward (12) | — | 2 | 5 | 4 | 7 | 5 | NT | 4 | 4 | 7 | 6 |
| Visual processing | |||||||||||
| VOSP | |||||||||||
| Incomplete letters | 20 | 16 | 20 | 17 | 20 | 17 | 20 | 19 | 19 | 20 | 16 |
| Silhouettes | 30 | 15 | 21 | 21 | 19 | 24 | 20 | 22 | 25 | 18 | 19 |
| Object decision | 20 | 14 | 19 | 17 | 20 | 19 | 15 | 18 | 17 | 17 | 16 |
| Progressive silhouettes | 20 | 15 | 11 | 14 | 16 | 8 | 20 | 5 | 8 | 11 | 9 |
| Dot counting | 10 | 8 | 10 | 7 | 9 | 10 | 10 | 10 | 10 | 10 | 9 |
| Position discrimination | 20 | 18 | 20 | 19 | 20 | 16 | 20 | 18 | 20 | 20 | 19 |
| Number location | 10 | 7 | 9 | 10 | 10 | 8 | 9 | 10 | 10 | 10 | 10 |
| Cube analysis | 10 | 6 | 10 | 9 | 4 | 10 | 6 | 10 | 9 | 10 | 7 |
| Semantic processing | |||||||||||
| Naminga | 64 | 62 | 62 | 62 | 58 | 56 | 56 | 41 | 59 | 61 | 45 |
| Camel and Cactus (pictures)a | 64 | 52 | 61 | 59 | 44 | 61 | 52 | 24 | 52 | 61 | 47 |
| Word–picture matchinga | 64 | 62 | 64 | 64 | NT | NT | NT | 63 | 64 | 63 | 62 |
| 96 Synonyms | 96 | 90 | 91 | 96 | 74 | 94 | 90 | 83 | 93 | 93 | 81 |
| Phonological processing | |||||||||||
| PALPA 2: Phonological judgment | |||||||||||
| Total | 72 | 64 | 68 | 71 | 71 | 72 | 72 | 68 | 71 | 72 | 71 |
| Same | 36 | 34 | 32 | 35 | 35 | 36 | 36 | 36 | 36 | 36 | 36 |
| Different | 36 | 30 | 36 | 36 | 36 | 36 | 36 | 32 | 35 | 36 | 35 |
| PALPA 15: Rhyme judgment | 60 | 43 | 47 | 57 | 59 | 58 | 57 | 56 | 57 | 56 | 53 |
| Phoneme segmentationb | |||||||||||
| Total | 96 | 76 | 94 | 96 | 87 | 96 | 73 | 87 | 96 | 94 | 91 |
| Addition | 48 | 39 | 46 | 48 | 40 | 48 | 36 | 48 | 48 | 46 | 45 |
| Subtraction | 48 | 37 | 48 | 48 | 47 | 48 | 37 | 39 | 48 | 48 | 46 |
Note: Bold denotes abnormal performance. VOSP, Visual Object and Space Perception battery; PALPA, Psycholinguistic Assessment of Language Processing in Aphasia (Kay et al. 1992); NT, not tested; RHH, right homonymous hemianopia; RUQ, right upper quadrantanopia; ND, no field defect.
aTests from Bozeat et al. (2000).
bTests from Patterson and Marcel (1992).
Table 2.
Demographic and background neuropsychological assessment for the 11 AZ patients ordered left to right, from mild to severe, according to the severity of the reading impairment (slope of the word length effect in RTs)
| Maximum | Normal cut-off | 130 | 171 | 174 | 141 | 135 | 170 | 169 | 128 | 177 | 153 | 125 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | |||||||||||||
| Age | — | — | 80 | 78 | 63 | 72 | 80 | 60 | 72 | 54 | 62 | 69 | 65 |
| Sex | — | — | M | M | M | M | F | M | M | M | M | M | M |
| Handedness | — | — | R | R | R | R | R | R | R | R | L | R | R |
| Years of education | — | — | 18 | 14 | 18 | 14 | 12 | 14 | 14 | 18 | 10 | 11 | 12 |
| Lesion etiology | — | — | Stroke | Stroke | Stroke | Stroke | Stroke | Stroke | Stroke | Stroke | Stroke | Stroke | Stroke |
| Lesion volume (cc) | 37.23 | 38.33 | 5.15 | 29.8 | 29.46 | 56.82 | 74.42 | 97.69 | 51.91 | 42.11 | 2.19 | ||
| Visual field loss | ND | RUQ | RHH | RHH | RUQ | RUQ | RHH# | RUQ | RUQ | ND | ND | ||
| Working memory | |||||||||||||
| Digit span forward | 12 | 5.8 | 9 | 10 | 10 | 7 | 9 | 11 | 6 | 10 | 5 | 9 | 7 |
| Visual/orthographic processing | |||||||||||||
| Visual field lossa | — | — | |||||||||||
| Letter case matching (PALPA 19, 20) | 52 | 51 | 52 | 51 | 52 | 52 | 51 | 52 | 50 | 52 | 52 | 52 | 52 |
| Letter discrimination in words/non-words (PALPA 21) | 30 | 27 | 30 | 30 | 28 | 29 | 30 | 29 | 28 | 28 | 25 | 28 | 29 |
| Visual lexical decision (PALPA 25) | 60 | 58 | 58 | 59 | 60 | 58 | 59 | 58 | 48 | 59 | 38 | 37 | 51 |
| Semantic processing | |||||||||||||
| BNT | 60 | 53 | 32 | 58 | 58 | 53 | 20 | 46 | 42 | 57 | 39 | 55 | 43 |
| PPT (pictures) | 52 | 49 | 48 | 51 | 52 | 52 | 51 | 52 | 51 | 52 | 47 | 50 | 51 |
| Word–picture matching (PALPA 48) | 40 | 39 | 40 | 40 | 39 | 40 | 38 | 39 | 39 | 40 | 39 | 40 | 40 |
| Auditory synonym judgment (PALPA 49) | 20 | 19 | 20 | 19 | 20 | 20 | 20 | 20 | 17 | 20 | 19 | 20 | 20 |
| Phonological processing | |||||||||||||
| Rhyme judgment | 40 | 36 | 39 | 39 | 40 | 40 | 39 | 40 | 37 | 39 | 33 | 38 | 39 |
| Phoneme segmentation | 80 | 71 | 71 | 78 | 79 | 70 | 77 | 79 | 69 | 80 | 56 | 77 | 79 |
| Minimal pair discrimination | 40 | 38 | 39 | 40 | 38 | 40 | 40 | 40 | 40 | 40 | 36 | 39 | 40 |
Note: Bold denotes abnormal performance. PALPA, Psycholinguistic Assessment of Language Processing in Aphasia (Kay et al. 1992); BNT, Boston Naming Test (Goodglass and Kaplan 1983); PPT, Pyramids and Palm Trees Test (Howard and Patterson 1992); RHH, right homonymous hemianopia; RUQ, right upper quadrantanopia; ND, no field defect.
aIn addition to extensive left vOT damage, CT scan in this patient also indicated a right dorsomedial occipital lesion that was associated with a left inferior quadrant visual field defect.
Semantic tasks were taken from the Cambridge Semantic Memory (CSM) test battery (Bozeat et al. 2000; Adlam et al. 2010). The CSM battery is a collection of tests that use the same set of stimulus items to assess semantic knowledge systematically across different input and output modalities. The battery contains 64 items representing 3 subcategories of living things (animals, birds, and fruit) and 3 subcategories of artifacts (household items, tools, and vehicles) matched for psycholinguistic variables such as concept familiarity and age of acquisition. Knowledge of all items is assessed in both verbal and non-verbal modalities of stimulus and/or response, enabling the detection of differential impairments across these domains of input/output. The semantic memory tests administered include: 1) Oral picture naming (to the 64 line drawings) and 2) word comprehension (spoken word–picture matching, WPM). For WPM, the participant is presented with 64 picture arrays, one for each item, with each array consisting of 10 items from the same category (e.g. birds); the task is to point to the item named by the examiner. Across the arrays, within one category, the target and foil items move around to different positions; furthermore, 2 of the 10 items in each category are never targets. These design factors prevent participants who remember their own previous choices within a category from working out subsequent correct responses by a simple process of elimination. The test sequence is consistent across subjects and is arranged so that each item is followed by an item from a different category; 3) Camel and Cactus Test (CCT; Bozeat et al. 2000), designed along the principles of the Pyramids and Palm Trees test (PPT; Howard and Patterson 1992). For the CCT, participants are required to choose the correct 1 of 4 response-choice pictures that has an associative relationship with each of the 64 target items. For example, in one of the trials, the subject is asked to match the target camel to 1 of 4 types of vegetation: Tree, sunflower, cactus (the correct response), or rose. In addition, the synonym judgment test (Jefferies et al. 2009) was also administered in which patients had to decide which of 3 words has the closest meaning to the target.
Phonological tasks included: 1) Same–different phonological discrimination (PALPA 2; Kay et al. 1992); 2) rhyme judgment (PALPA 15; Kay et al. 1992); and 3) phonological segmentation and blending (Patterson and Marcel 1992).
On the more visually challenging Silhouettes and Progressive Silhouettes tests of the VOSP (Warrington and James 1991), the majority of UK patients showed evidence of general visual processing deficits. All patients, bar the mildest (FW, EI), were impaired in picture naming suggestive of a visual recognition deficit. The more severe patients also showed mild but measureable impairments on receptive semantic tests. All patients had preserved working memory and were in the normal range on the minimal pairs test (PALPA 2; Kay et al. 1992) and the rhyme judgment test (PALPA 15; Kay et al. 1992). Performance was also excellent on the more demanding tests of phonological segmentation and blending (Patterson and Marcel 1992), with the exception of RK (who suffered from significant age-related hearing loss).
Table 2 presents background neuropsychological data for the AZ patients who comprised most of the mild and moderate subgroups. Comparable tests were used between UK and AZ patients whenever possible (e.g. CCT UK = PPT AZ; CSM Naming UK = BNT AZ; analogous phonological processing tasks, etc.) Some patients showed mild impairments on orthographic letter matching and lexical decision tasks from the PALPA battery (Kay et al. 1992). Most patients were also impaired on one or more picture naming or semantic matching tasks. All patients, bar 177, were in the normal range on rhyme judgment, phoneme segmentation (169 and 141 scored 1 and 2 points below the normal cut-off) and minimal pair discrimination.
Inherent in large studies employing the case-series design, not all patients could complete the full set of tasks. This was due to further neurological events, demise, withdrawal, or medical illness. Table 3 presents each task and the corresponding number of patients who completed the task for the sections that follow.
Table 3.
Patients contributing to each of the analyses in this study
| Analysis | n | Patients |
|---|---|---|
| Lesion mapping | 18 | 130, 171, 174, EI, 141, 135, 170, 169, 128, 177, 153, JWF, RK, 125, TS, JW, JM, MS |
| Spatial frequency | 8 | JM, EI, FW, MS, JW, TS, KW, JWF |
| Naming | 17 | 130, 171, 174, EI, 135, 170, 169, 128, KW, 177, JWF, RK, 125, TS, JW, JM, MS |
| Word–picture matching | 14 | 130, 174, EI, 170, 169, 128, KW, JWF, RK, 125, TS, JW, JM, MS |
| Checkerboards | 20 | 130, 171, 174, EI, 141, FW, 135, 170, 169, 128, KW, 177, 153, JWF, RK, 125, TS, JW, JM, MS |
| Kanji characters | 17 | 130, 171, 174, EI, FW, 170, 169, 128, KW, 177, JWF, RK, 125, TS, JW, JM, MS |
Lesion Mapping
Lesions were reconstructed based on high-resolution research MRI or clinical MRI/computed tomography (CT) scans that were available for 18 of 20 participants. For each patient, a lesion region of interest (ROI) was created using MRIcron software (http://www.cabiatl.com/mricro/mricron/). For research MRI scans, lesions were manually drawn directly on the patients' T1-weighted structural brain images at 1 mm intervals and then normalized to the standard MNI template brain using the lesion volume as a mask during the normalization process (Brett et al. 2001; Andersen et al. 2010). For the clinical CT and MRI scans, lesions were manually drawn onto the standard MNI template brain oriented to match the alignment of the scans. In cases where the lesion was associated with compensatory ventricular dilatation, the right ventricle was traced and flipped to estimate the size of the ventricle in the damaged left hemisphere. Dilated ventricular spaces falling outside the tracing were included in the lesion volume, as they were considered to represent areas of tissue loss. For additional details of our lesion analysis methods, see Andersen et al. (2010). Individual ROIs were subsequently combined to generate lesion overlap maps. Lesion reconstructions were also used to conduct voxel-based lesion-symptom mapping (VLSM; Bates et al. 2003).
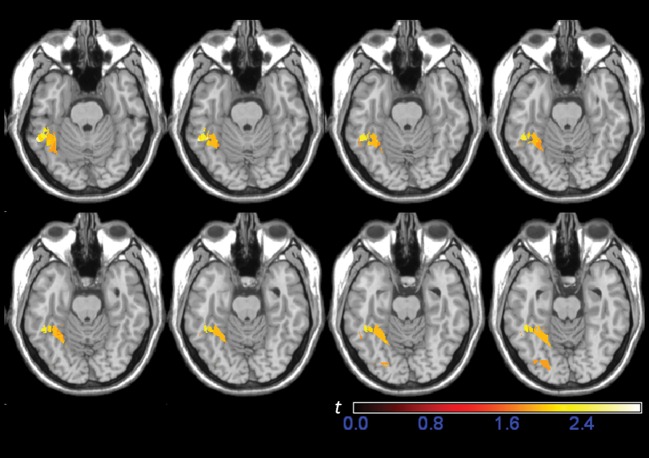
As displayed in Figure 2b, the lesion overlap in this patient group was centered on the left fusiform gyrus and aligns with the area that showed activation during reading in normal individuals (localized in an fMRI contrast of words minus checkerboards in 15 normal subjects; Fig. 2a). Figure 2c–e shows lesion overlap for each severity subgroup. The importance of the pFG in reading is underlined by these Figures, as it is in this region that the most pronounced differences between the severity subgroups are found. In particular, damage to the pFG was present in the majority of patients in the moderate and severe groups, whereas involvement of this region was infrequent in the mild group.
Figure 2.
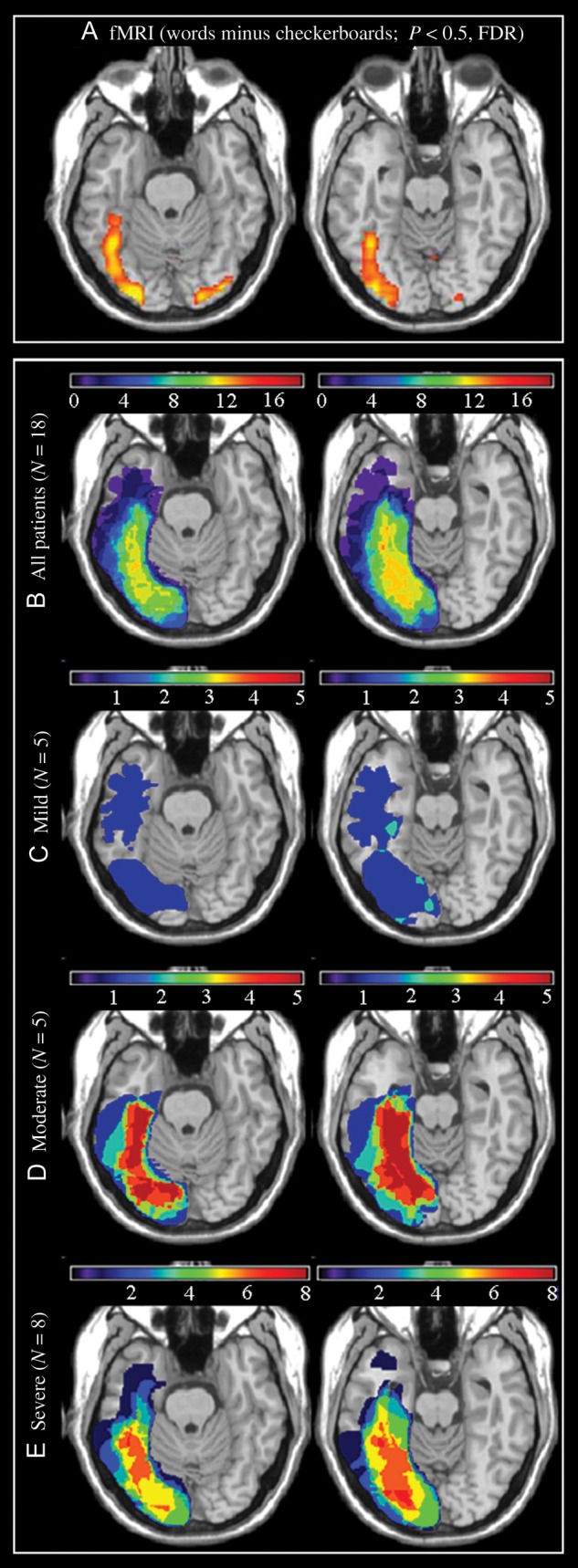
(A) fMRI activation during a reading task in 15 normal subjects (words—checkerboards, P < 0.05; FDR), (B) lesion overlap maps for the total group of 18 patients for whom neuroimaging studies were available, and (C) for the mild (n = 5), (D) moderate (n = 5), and (E) severe (n = 8) subgroups. Color bars indicate the number of patients with damage to each region. In order to display the course of the fusiform gyrus more clearly, the axial slices have been rotated −15 degrees from the AC-PC line.
The association between damage to the pFG and reading impairment was confirmed further by an exploratory VLSM analysis (Bates et al. 2003). The behavioral measure used in this analysis was length effect slope computed over RTs for each length normalized according to the overall RT. Using the normalized slope allowed us to focus on areas associated with the abnormal word length effect that defines pure alexia, while avoiding potential spurious correlations between overall reading speed and lesion size. As illustrated in Figure 2, the lesion distribution of the patients in this study was relatively homogenous in the sense that most shared lesion overlap in the left fusiform gyrus, and this, combined with a modest sample size, limits the power of a VLSM analysis to detect an association between reading performance and voxel lesion status in this region. Nevertheless, as can be seen in Figure 3, the results of the VLSM analysis confirm the link between the increased word length effect and damage to the left fusiform gyrus and adjacent occipito-temporal sulcus, including some of the cortical regions that showed activation in the fMRI study of reading in normal individuals (Fig. 2a).
Figure 3.
Result of the VLSM analysis showing the association between damage to the fusiform gyrus and an increased word length effect, as measured by the normalized slopes. Due to low statistical power attributable to relatively small sample size and spatially homogeneous lesion distribution, the maps are thresholded at P < 0.05 uncorrected for multiple comparisons.
Consistent with damage to left occipital lobe visual areas (Fig. 2), most though not all patients in our study had evidence of right homonymous hemianopia or right upper quadrantanopia (Tables 1 and 2). However, the presence of a right visual field defect did not show a systematic relationship with the severity of the reading impairment. For instance, the mild and severe subgroups both included patients with right homonymous hemianopia as well as patients without visual field defects. These observations are consistent with other evidence in the literature, suggesting that the exaggerated word length effect that characterizes letter-by-letter reading is not the result of visual field loss (e.g. Leff et al. 2001, 2006; Cohen et al. 2003; Pflugshaupt et al. 2009). (To investigate further whether visual field defects had an effect on performance, we asked a subset of the patients to read words and name objects presented in their left and right visual fields. No significant difference between performance in each hemifield was present for either task. The fact that patients could name images presented in their impaired visual field as easily as those presented in their intact visual field indicates that visual field defects are extremely unlikely to impact upon performance for centrally presented items [see Supplementary Material for further details].)
Spatial Frequency
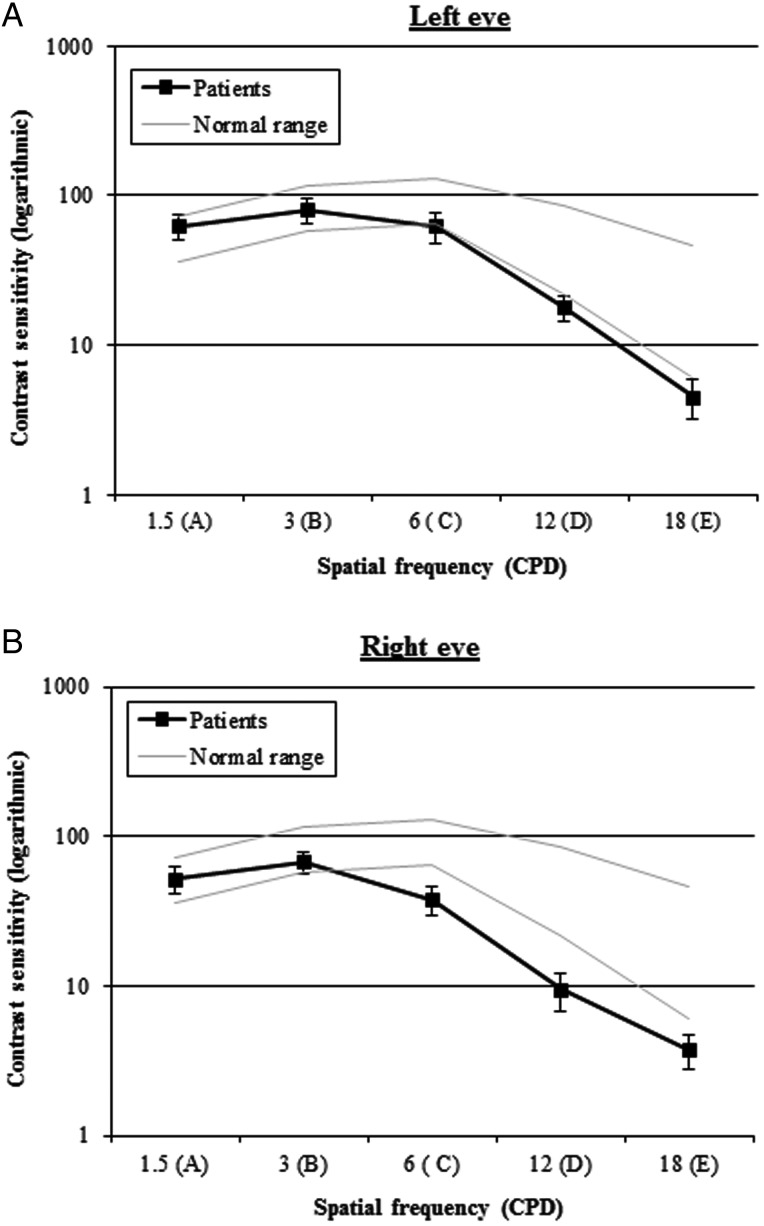
The retinotopic eccentricity account (Malach et al. 1995, 2002; Levy et al. 2001; Hasson et al. 2002, 2003) and the findings by Woodhead et al. (2011) predict that sensitivity to moderate-to-high spatial frequency should be impaired in patients with damage to the left pFG. The functional acuity contrast test (http://www.stereooptical.com/) is a diagnostic tool widely used among practitioners to evaluate real-world vision capabilities. It was used to assess 8 patients mainly from the moderate and severe subgroups. The test evaluates sensitivity across a range of spatial frequencies and contrast. The test comprises a progression of high-quality, sine-wave gratings that probe sensitivity to 1.5, 3, 6, 12, and 18 cycles per degree. The contrast step between each grating patch is 0.15 log units. The contrast range spans the variation of contrast sensitivity found in the normal population. Following the standard instructions, the patients were asked to decide whether each grating was tilted right, vertical, or left. Figure 4a,b displays average results from the patients, for left and right eyes, respectively. A healthy visual system is expected to have contrast sensitivity within the normal range of Figure 4 (between the grey lines). Normative limits which include 90% of the normal population are used to help minimize the potential for false positives. A functional impairment is indicated if the curve is below the normal range for either eye. All patients demonstrated abnormal contrast sensitivity profiles at the medium and high frequencies—a frequency range that psychophysical investigations have shown to be crucial for the recognition of letters, silhouettes, and faces (Owsley and Sloane 1987).
Figure 4.
Average spatial-frequency sensitivity curves for (A) left and (B) right eyes for the 8 patients tested.
Recognition of Familiar Objects
To the extent that the reading deficits observed in the present case series are underpinned by a more general visual processing deficit, we would expect to see deficits in the recognition of other familiar but non-orthographic visual objects. We examined, therefore, the relationship between reading and general visual processing by testing recognition of common objects. (Recognition of famous faces was also tested and found to be abnormal for the UK subset of patients; refer to Supplementary Material.) Given that pure alexia is defined on the basis of reading speed, RT as well as accuracy was measured, so that it was possible to compare recognition performance directly across orthographic and non-orthographic visual stimuli.
Materials
Visual recognition (see below for procedure) was probed with 2 tasks—naming and cross-modal (word–picture) matching, using the 64 black and white line drawings from the CSM battery (Bozeat et al. 2000; Adlam et al. 2010). A total of 17 and 14 patients completed the naming and WPM version of the task, respectively. Ten control participants (4 males) with no previous history of neurological problems also completed the tasks. They were comparable to patients with respect to age (mean = 68.4) and years of education (mean = 10). All control subjects passed a screening test for dementia (Addenbrooke's cognitive examination-revised [ACE]-R; Mioshi et al. 2006). Although passing the ACE-R, one control participant had to be removed from the analysis due to suspected early-onset dementia.
Procedure
In this and all subsequent tasks, stimuli were presented using E-prime 1.1 software (Schneider et al. 2002) on a laptop. Participants were seated approximately 50 cm from the screen. The administration of the set of materials began with 16 practice items, followed by 64 experimental items. For naming, items were presented centrally following a fixation cross and the participants were asked to name them. In the matching task, participants were presented with a target name in both spoken (by the experimenter) and written (for an unlimited duration) forms. This was followed by a backward pattern mask and a series of 4 objects from the same category to match the name to. The first 2 were presented left of fixation and the second 2 to the right. For example, the name “peacock” followed by a series of 4 pictures: owl, peacock, chicken, and swan. Participants indicated their choice by means of a key press. Targets were counterbalanced and distributed equally across the 4 positions across the trials. For both tasks, stimuli remained on the screen until a response was given. RT and accuracy data were recorded. For this and all subsequent experiments, RT data were taken from correct trials only, and any trials more than 2 standard deviations from that participant's overall mean were excluded from analysis.
Results
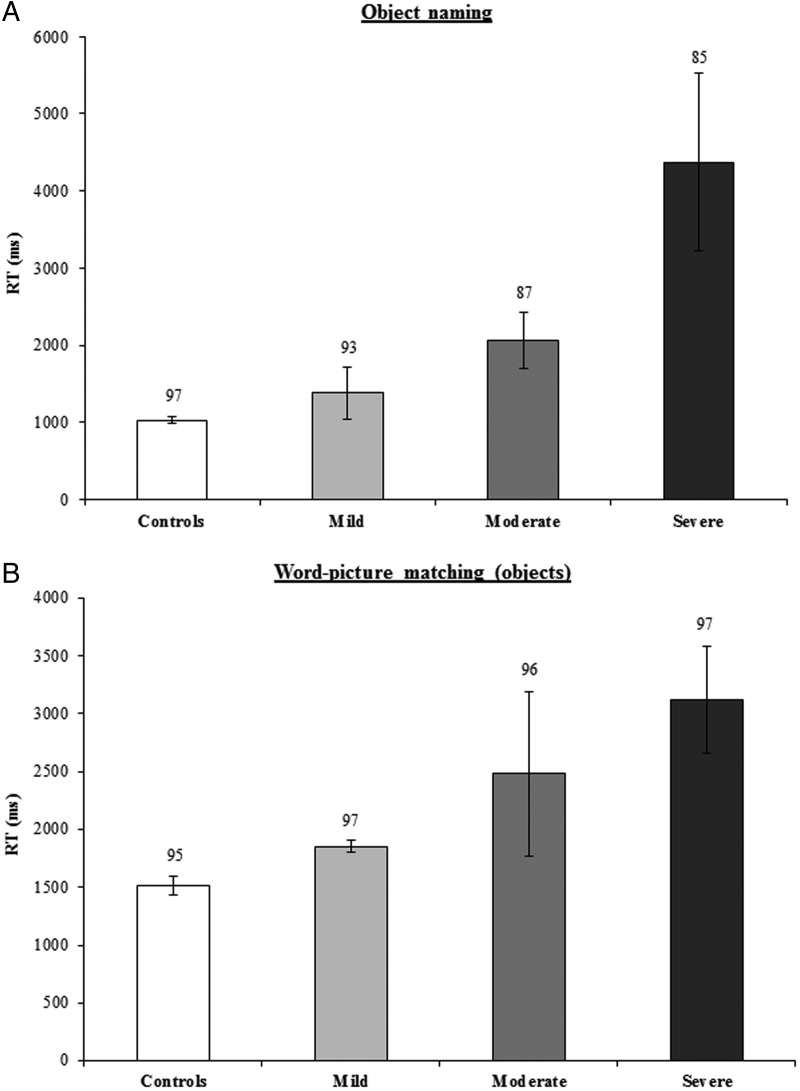
Figure 5 displays results for patient and control groups on 1) naming and 2) WPM. One-way ANOVAs were performed for RT and accuracy on each condition including severity (controls/mild/moderate/severe) as a between-subjects factor. For naming, ANOVA revealed a main effect of severity in RT (F3,25 = 5.71, P< 0.005) but no effect in accuracy (F3,25 = 2.43, P = 0.10). Comparative ANOVAs for WPM also revealed a main effect of severity for RT (F3,22 = 4.45, P< 0.01) but not for accuracy (F3,22 = 0.84, P = 0.49). These effects can be seen in Figure 5 where performance in RT for both conditions was slowest for the patients with the severest reading impairment. Further analysis were performed, revealing marginally significant correlations between the size of the length effect in reading RT and RT for naming (r(15) = 0.37, P = 0.07, one-tailed) and WPM (r(12) = 0.44, P = 0.06, one-tailed).
Figure 5.
Average correct RTs for (A) object naming (n = 17) and (B) WPM (n = 14) tasks for the patient subgroups split by severity (reading regression slope) and controls. Error bars indicate standard error. Numbers refer to accuracy rates (percent correct).
Processing of Novel Objects
In addition to their acquired reading impairment, the patients also had more general object recognition deficits which covaried with the severity of their alexia. These parallel reading and recognition deficits would not have been so apparent if only accuracy measures had been used. Instead, the patients' reading and non-reading visual recognition deficits are graded and reflected in terms of response times. This fits with previous observations that impairments are easily missed if only accuracy is considered (Davidoff and Warrington 1999).
The use of familiar objects to assess non-orthographic processing does however run the risk of underestimating the extent of deficits as intact top-down support from central object and semantic representations might boost impaired early processing. We therefore aimed to assess the patients' processing of non-orthographic visual stimuli using novel objects that have no intrinsic meaning or familiarity (for English-speaking participants), namely checkerboards. (We are indebted to Marlene Behrmann for suggesting these stimuli.) and kanji characters. The use of such stimuli also allowed us to explore the hypothesis that pure alexic patients tend to present with reading impairment because orthographic stimuli are especially visually demanding (Behrmann et al. 1998a,b; Mycroft et al. 2009) by manipulating the complexity of these novel non-orthographic stimuli. For the checkerboards, visual complexity was manipulated by varying the size of matrix and the number of constituent squares; for the kanji, visual complexity was manipulated by varying the number of strokes that comprise each character. It was predicted that performance would be disproportionately slower and less accurate for patients compared with matched controls, particularly for complex items. A total of 20 patients participated for the checkerboard task and 17 for the kanji test. The 2 tasks were also completed by the control group (n = 10).
Materials
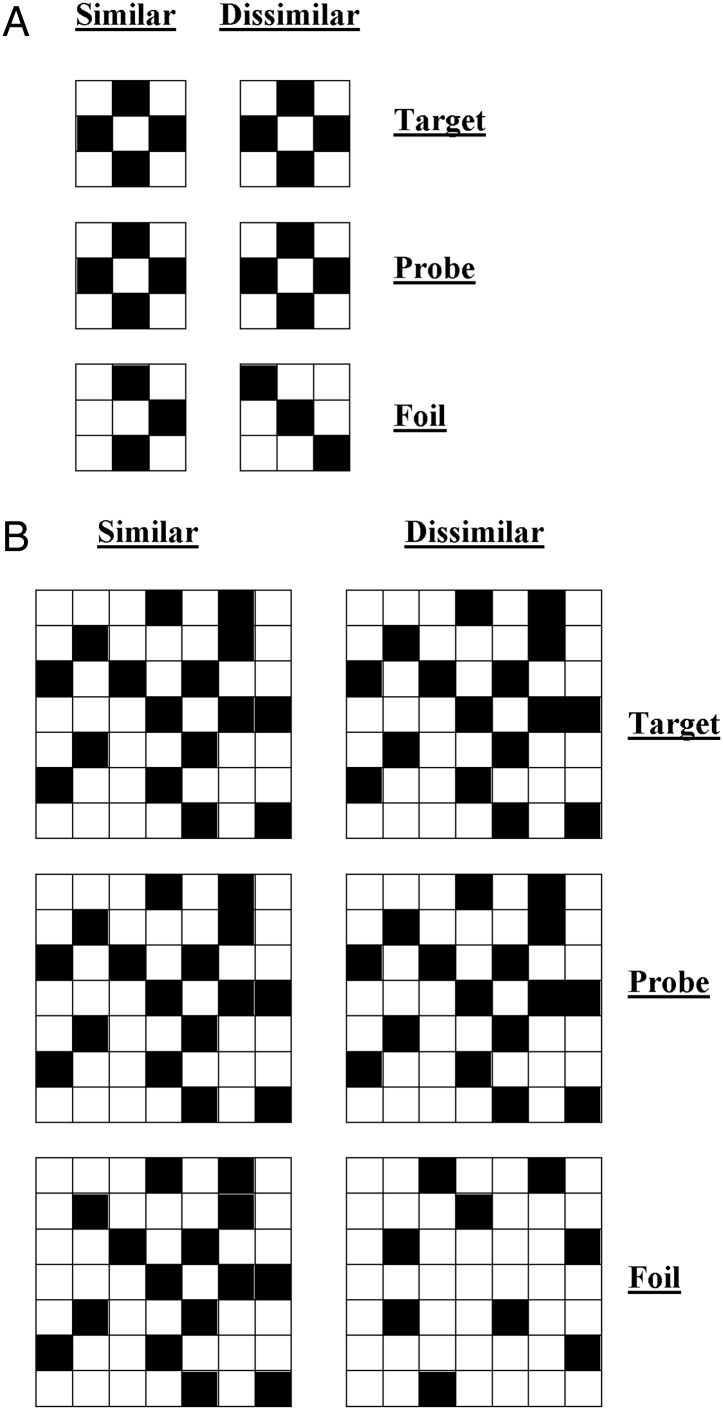
Checkerboards
A set of 32 black and white checkerboards were designed. The number of squares in each matrix was either 9 (3 × 3) or 49 (7 × 7), corresponding to simple and complex visual sets. Grids were constructed by avoiding placement of blocks of the same color together or any other regularity in the patterns (that might simplify visual processing). Stimuli were used to form a triad-based matching-to-sample task (Fig. 6), in which the probe was flanked above and below by the target and foil. The position (above/below) of target and foil was randomized. Two types of foil were created: The similar condition reflected foil patterns that differed by only one block from the target pattern. The dissimilar condition reflected foils that differed from the target considerably (by several blocks), such that each foil could be distinguished easily.
Figure 6.
Example checkerboard stimuli for (A) simple condition and (B) complex condition with similar and dissimilar foils.
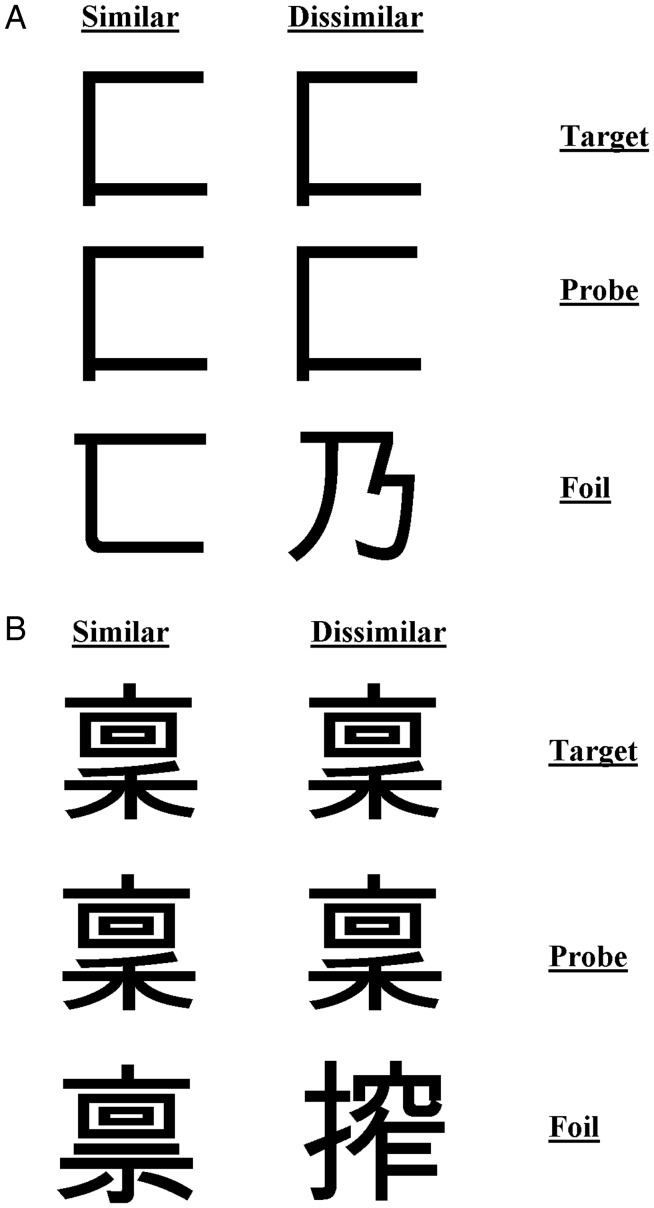
Kanji Characters
A set of 60 single kanji characters were selected. Complexity was defined in terms of the number of strokes in each character. Characters with 2–4 strokes constituted the simple items, and those with 13 strokes formed the complex set. Again, each target character appeared in a matching-to-sample triad (Fig. 7). The probe was placed in the center with the target and foil above or below. The position of the target was randomized across trials. In half the trials, the foil was a character differing only slightly from the target to give the similar condition; in the other half, a character differing from the target considerably was selected for the dissimilar condition.
Figure 7.
Example kanji stimuli for (A) simple condition and (B) complex condition with similar and dissimilar foils.
Procedure
Participants were seated approximately 50 cm from the screen and were asked to discriminate which of the 2 sample stimuli were the same as the central probe. Participants were asked to respond as quickly and as accurately as possible by means of a key press. Stimuli remained on the screen until a response was given. A block of 10 practice trials preceded the experiment so participants could familiarize themselves with the process. Depending on patient fatigue, both tasks were usually completed in the same session or counter-balanced across 2 testing sessions.
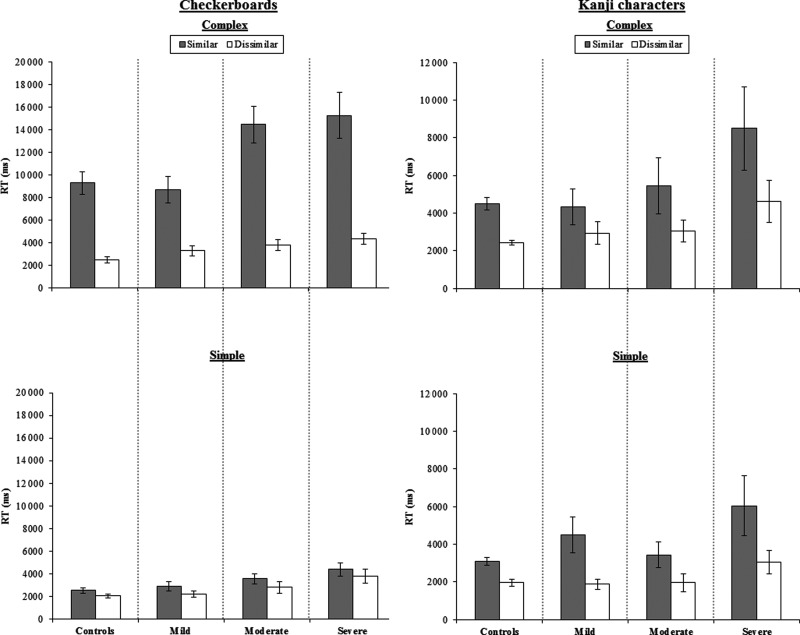
Results
Figure 8 displays the mean matching times for patient and control groups. Repeated-measures ANOVAs were conducted using severity (controls/mild/moderate/severe) as a between-subject factor and visual complexity (complex/simple) and similarity (similar/dissimilar) as within-subject factors. Figure 8 shows that RTs for checkerboards were modulated by complexity (F1,26 = 223.20, P < 0.001), similarity (F1,26 = 143.45, P< 0.001), and an interaction between the two factors (F1,26 = 126.59, P< 0.001). Interactions between severity and complexity (F3,26 = 4.87, P < 0.02) and severity and similarity (F3,26 = 3.45, P < 0.05), and a 3-way interaction between severity, complexity, and similarity (F3,26 = 3.79, P < 0.02) were present. Accuracy rates were also modulated by complexity (F1,26 = 8.00, P < 0.01), similarity (F1,26 = 24.00, P < 0.001), and an interaction between the two (F1,26 = 11.57, P < 0.005).
Figure 8.
Average correct RTs for the checkerboard (n = 20) and kanji (n = 17) tasks for patient subgroups split by severity (reading regression slope) and controls. Error bars represent standard error.
To further establish the relationship of deficits across tasks, ANOVA was conducted on patient RT using reading slope as a predictor, revealing significant interactions between slope and complexity (F1,18 = 13.98, P < 0.005), slope and similarity (F1,18 = 10.13, P < 0.005), and a 3-way interaction between slope, complexity, and similarity (F1,18 = 9.56, P < 0.01). As one would expect, parameter estimates for length slope revealed the strongest relationship in the high similar condition (β = 6.67, P < 0.001) compared with high different (β =1.00, P = 0.07), low similar (β =1.35, P = 0.03), and low different (β= 1.10, P = 0.09) conditions.
Comparable ANOVAs performed on the kanji RT data revealed identical main effects of complexity (F1,23 = 33.95, P < 0.001) and similarity (F1,26 = 51.60, P < 0.001). An interaction was approaching significance for severity and complexity (F3,23 = 2.60, P = 0.077) and a 3-way interaction between severity, complexity, and similarity (F3,23 = 3.06, P < 0.05) was present. There were no significant effects in the analysis of accuracy.
A further ANOVA was conducted on patient RT using length slope as a predictor, revealing a marginal interaction between slope and complexity (F1,15 = 3.08, P = 0.09) and a significant interactions between slope and similarity (F1,15 = 5.90, P < 0.05) but no 3-way interaction between slope, complexity, and similarity (F1,15 = 0.54, P = 0.48). Looking at the parameter estimates, this is because a similarly strong relationship for conditions high on similarity and on complexity was present in this task: High similar (β =4.26, P < 0.05), high different (β =2.03, P < 0.05), low similar (β =2.92, P = 0.05), and low different (β =1.30, P = 0.06).
As can be seen in Figure 8, the 3-way interactions common to both tasks in the RT data reflect the fact that the cost of visual complexity was limited to trials where the distractors were visually similar and that performance in this condition became slowest for the patients with the severest reading impairment.
Discussion
This investigation is the largest neuropsychological case series to be conducted to date on reading and general visual processing following left ventral occipito-temporal lesions centered on the pFG. We utilized this opportunity to explore why this region seems to be crucial for the recognition of orthography and other visually demanding stimuli, with damage to left vOT often leading to the clinical presentation of pure alexia in the context of other visual recognition deficits (see below). Specifically, we evaluated and found multiple lines of support for a novel hypothesis arising from recent visual neuroscience investigations—namely that the ventral occipito-temporal area (vOT) is retinotopically organized with foveal, high-acuity information projected to the pFG (Malach et al. 2002; Plaut and Behrmann, 2011), making it especially sensitive to high spatial frequencies (Woodhead et al. 2011).
Direct evidence in favor of this hypothesis was obtained on the spatial frequency sensitivity test. The vOT eccentricity hypothesis predicts that sensitivity to high spatial frequencies should be differentially disrupted in patients with damage to the left pFG, and this was exactly the pattern that we found in this patient case series. Our results are in agreement with findings from neurologically intact subjects, showing that the left pFG is involved to a greater extent in high than low spatial frequency processing (Woodhead et al. 2011). Low spatial frequencies are informative about the overall configuration of an image (Goffaux et al. 2005; Goffaux and Rossion 2006), whereas high frequencies support perceptual extraction of local features (Fiset et al. 2006) and thus efficient recognition of any visually complex stimulus relies upon the information carried by high spatial frequency components.
Evidence for the notion that efficient processing of both orthographic and non-orthographic complex visual stimuli relies upon the high spatial frequencies encoded in the left pFG was obtained from the assessment of performance with familiar and novel objects. We found a direct parallel between the patients' degree of reading deficit and their recognition of familiar objects. Thus, as their degree of reading impairment worsened, the patients' speed to recognize these non-orthographic stimuli also slowed significantly and was marginally correlated with the size of the length effect in reading RT. In comparison to controls, RTs were at least twice as slow for the moderate patients and 3 times as slow for the severe group. The same pattern of data was obtained for timed naming and recognition of faces in a subset (United Kingdom) of patients (see Supplementary Material). Overall, the results for these different types of familiar stimuli align directly with previous functional neuroimaging studies and computational modeling explorations, which have demonstrated that visually demanding stimuli such as words, faces, and complex objects generate greatest activation in the pFG, where foveally derived visual information is projected (Hasson et al. 2002, 2003; Plaut and Behrmann, 2011; Woodhead et al. 2011).
Unfamiliar stimuli were also employed to probe the patients' visual abilities (checkerboards and kanji characters). Such stimuli test the functioning of the visual system with minimal top-down support from central object and semantic representations, which might otherwise help to minimize the patients' underlying visual processing deficits. Moreover, novel stimuli can be constructed deliberately to vary key visual parameters—in this study, visual complexity and visual overlap/similarity. In keeping with the vOT eccentricity/spatial frequency hypothesis, we found that the patients' matching times were pathologically slow and were especially so when they were required to differentiate complex visual targets from perceptually similar foils. Among the patients, RT to process the most complex stimuli and those presented with the most similar foils was most strongly related to the size of the length effect in reading RTs, suggesting a common mechanism for impaired performance with both orthographic and non-orthographic stimuli.
Some past studies of individuals with pure alexia have noted that despite impaired reading (as measured by reading times), the patients have normal accuracy on non-reading visual recognition tests. Other investigations and literature reviews have found evidence for dual deficits (Behrmann et al. 1998a,b; Mycroft et al. 2009). By utilizing a case series of patients covering a broad range of impairment severity and several different types of visual task and stimuli, this study has gone some way to explain the disparity across previous investigations. Three key factors emerge from the current findings: 1) It is critically important to compare reading and non-reading abilities using the same dependent measure (RTs or accuracy), especially given that accuracy tends to be insensitive to the underlying visual impairments in this patient group; 2) impairment on non-reading tasks is most obvious when the stimuli and the required discrimination mirror the visually demanding nature of orthographic stimuli; and 3) impairment on both reading and non-reading tasks is modulated by the severity of the patient—such that only the severe patients have suppressed accuracy as well as extremely long RTs.
To conclude, we will briefly consider the relationship between these results, the vOT eccentricity/spatial frequency hypothesis and the differing cognitive interpretations of pure alexia. As noted in the Introduction section, the cognitive theories can be divided into 1) reading-specific accounts, attributing the deficit to damage to a brain region specialized for the identification and storage of orthographic strings, and 2) general visual accounts, suggesting that the deficit reflects a more general visual processing impairment. In terms of the reading specific accounts (Dehaene and Cohen 2011), if one assumes that orthographic-specific regions are formed during development, then one might expect the area most likely to take up orthographic recognition to be the region with the most appropriate form of visual input—namely, the left pFG area with its high spatial frequency, foveal bias. However, the concurrently impaired orthographic and non-reading visual behavior of the patients in the current study clearly fits most readily with the general visual deficit hypothesis, consistent with previous research (Friedman and Alexander 1984; Farah and Wallace 1991; Sekuler and Behrmann 1996; Behrmann et al. 1998a,b; Polk and Farah 2002; Hillls et al. 2005; Marsh and Hillis 2005; Joseph et al. 2006; Mycroft et al. 2009). Moreover, the link made here to the vOT eccentricity/spatial frequency hypothesis provides a powerful foundation for explaining why the processing of these different types of complex visual stimuli is simultaneously impaired in patients with lesions centered on the left pFG.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
The work reported here was supported by NIH-NIDCD grants 008286 and 007646, and an MRC programme grant (MR/J004146/1). D.J.R. was supported by funding from The University of Manchester Alumni. Funding to pay the Open Access publication charges for this article was provided by the RCUK block grant to the University of Manchester.
Supplementary Material
Notes
We thank all the patients and their carers for their continued support of our research. The authors also wish to thank Sarah Andersen for her assistance with the lesion maps. Conflict of Interest: None declared.
References
- Adlam ALR, Patterson K, Bozeat S, Hodges JR. The Cambridge Semantic Memory Test Battery: Detection of semantic deficits in semantic dementia and Alzheimer's disease. Neurocase. 2010;16:193–207. doi: 10.1080/13554790903405693. [DOI] [PubMed] [Google Scholar]
- Andersen SM, Rapcsak SZ, Beeson PM. Cost function masking during normalization of brains with focal lesions: Still a necessity? Neuroimage. 2010;53:78–84. doi: 10.1016/j.neuroimage.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF. Voxel-based lesion-symptom mapping. Nat Neurosci. 2003;6:448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Nelson J, Sekuler EB. Visual complexity in letter-by-letter reading: “Pure” alexia is not pure. Neuropsychologia. 1998a;36:1115–1132. doi: 10.1016/s0028-3932(98)00005-0. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Plaut DC, Nelson J. A literature review and new data supporting an interactive account of letter-by-letter reading. Cognitive Neuropsychology. 1998b;15:7–51. doi: 10.1080/026432998381212. [DOI] [PubMed] [Google Scholar]
- Benson DF, Geschwind N. The alexias. In: Vinken PJ, Bruyn GW, editors. Handbook of clinical neurology. Vol. 4. New York: John Wiley & Sons Inc; 1969. pp. 112–140. [Google Scholar]
- Binder JR, Mohr JP. The topography of callosal reading pathways: A case-control analysis. Brain. 1992;115:1807–1826. doi: 10.1093/brain/115.6.1807. [DOI] [PubMed] [Google Scholar]
- Bozeat S, Lambon Ralph MA, Patterson K, Garrard P, Hodges JR. Non-verbal semantic impairment in semantic dementia. Neuropsychologia. 2000;38:1207–1215. doi: 10.1016/s0028-3932(00)00034-8. [DOI] [PubMed] [Google Scholar]
- Brett M, Leff AP, Rorden C, Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. NeuroImage. 2001;14:486–500. doi: 10.1006/nimg.2001.0845. [DOI] [PubMed] [Google Scholar]
- Capitani E, Laiacona M, Pagani R, Capasso R, Zampetti P, Miceli G. Posterior cerebral artery infarcts and semantic category dissociations: A study of 28 patients. Brain. 2009;132:965–981. doi: 10.1093/brain/awp013. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehericy S, Dehaene-Lambertz G, Henaff MA, Michel F. The visual word form area: Spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123:291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Cohen L, Henry C, Dehaene S, Martinaud O, Lehericy S, Lemer C, Ferrieux S. The pathophysiology of letter-by-letter reading. Neuropsychologia. 2004;42:1768–1780. doi: 10.1016/j.neuropsychologia.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex functional properties of the visual word form area. Brain. 2002;125:1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Cohen L, Martinaud O, Lemer C, Lehericy S, Samson Y, Obadia M, Slachevsky A, Dehaene S. Visual word recognition in the left and right hemispheres: Anatomical and functional correlates of peripheral alexias. Cerebral Cortex. 2003;13:1313–1333. doi: 10.1093/cercor/bhg079. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Damasio H. The anatomic basis of pure alexia. Neurology. 1983;33:1573–1583. doi: 10.1212/wnl.33.12.1573. [DOI] [PubMed] [Google Scholar]
- Davidoff J, Warrington EK. The bare bones of object recognition: Implications from a case of object recognition impairment. Neuropsychologia. 1999;37:279–292. doi: 10.1016/s0028-3932(98)00076-1. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L. The unique role of the visual word form area in reading. Trends Cogn Sci. 2011;15:254–262. doi: 10.1016/j.tics.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L, Sigman M, Vinckier F. The neural code for written words: A proposal. Trends Cogn Sci. 2005;9:335–341. doi: 10.1016/j.tics.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Jobert A, Naccache L, Ciuciu P, Poline JB, Le Bihan D, Cohen L. Letter binding and invariant recognition of masked words—Behavioral and neuroimaging evidence. Psychol Sci. 2004;15:307–313. doi: 10.1111/j.0956-7976.2004.00674.x. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Naccache L, Cohen L, Le Bihan D, Mangin JF, Poline JB, Riviere D. Cerebral mechanisms of word masking and unconscious repetition priming. Nat Neurosci. 2001;4:752–758. doi: 10.1038/89551. [DOI] [PubMed] [Google Scholar]
- Dejerine J. Contribution a l'étude anatomo-pathologique et clinique des differentes variétés de cécité-verbale. Mémoires Société Biologique. 1892;4:61–90. [Google Scholar]
- Devlin JT, Jamison HL, Gonnerman LM, Matthews PM. The role of the posterior fusiform gyrus in reading. J Cogn Neurosci. 2006;18:911–922. doi: 10.1162/jocn.2006.18.6.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah MJ, Wallace MA. Pure alexia as a visual impairment: A reconsideration. Cogn Neuropsychol. 1991;8:313–334. [Google Scholar]
- Fiset D, Gosselin F, Blais C, Arguin M. Inducing letter-by-letter dyslexia in normal readers. J Cogn Neurosci. 2006;18:1466–1476. doi: 10.1162/jocn.2006.18.9.1466. [DOI] [PubMed] [Google Scholar]
- Friedman RB, Alexander MP. Pictures, images, and pure alexia—A case-study. Cogn Neuropsychol. 1984;1:9–23. [Google Scholar]
- Goodglass H, Kaplan E, Weintraub S. Boston Naming Test. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- Goffaux V, Hault B, Michel C, Vuong QC, Rossion B. The respective role of low and high spatial frequencies in supporting configural and featural processing of faces. Perception. 2005;34:77–86. doi: 10.1068/p5370. [DOI] [PubMed] [Google Scholar]
- Goffaux V, Rossion B. Faces are “spatial”—Holistic face perception is supported by low spatial frequencies. J Exp Psychol Hum Percept Perform. 2006;32:1023–1039. doi: 10.1037/0096-1523.32.4.1023. [DOI] [PubMed] [Google Scholar]
- Hasson U, Harel M, Levy I, Malach R. Large-scale mirror-symmetry organization of human occipito-temporal object areas. Neuron. 2003;37:1027–1041. doi: 10.1016/s0896-6273(03)00144-2. [DOI] [PubMed] [Google Scholar]
- Hasson U, Levy I, Behrmann M, Hendler T, Malach R. Eccentricity bias as an organizing principle for human high-order object areas. Neuron. 2002;34:479–490. doi: 10.1016/s0896-6273(02)00662-1. [DOI] [PubMed] [Google Scholar]
- Hillls AE, Newhart M, Heidler J, Barker P, Herskovits E, Degaonkar M. The roles of the “visual word form area” in reading. Neuroimage. 2005;24:548–559. doi: 10.1016/j.neuroimage.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson K. Pyramids and Palm Trees test. Hove: Erlbaum; 1992. [Google Scholar]
- Humphreys GW, Riddoch MJ, Price CJ. Top-down processes in object identification: Evidence from experimental psychology, neuropsychology and functional anatomy. Philos Trans R Soc Lond Ser B Biol Sci. 1997;352:1275–1282. doi: 10.1098/rstb.1997.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies E, Patterson K, Jones RW, Lambon Ralph MA. Comprehension of Concrete and Abstract Words in Semantic Dementia. Neuropsychology. 2009;23:492–499. doi: 10.1037/a0015452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JE, Cerullo MA, Farley AB, Steinmetz NA, Mier CR. fMRI correlates of cortical specialization and generalization for letter processing. Neuroimage. 2006;32:806–820. doi: 10.1016/j.neuroimage.2006.04.175. [DOI] [PubMed] [Google Scholar]
- Kay J, Lesser R, Coltheart M. Psycholinguistic Assessments of Language Processing in Aphasia (PALPA) Hove: Erlbaum; 1992. [Google Scholar]
- Leff AP, Crewes H, Plant GT, Scott SK, Kennard C, Wise RJ. The functional anatomy of single word reading in patients with hemianopic and pure alexia. Brain. 2001;124:510–521. doi: 10.1093/brain/124.3.510. [DOI] [PubMed] [Google Scholar]
- Leff AP, Spitsyna G, Plant GT, Wise RJS. Structural anatomy of pure and hemianopic alexia. J Neurol Neurosurg Psychiatry. 2006;77:1004–1007. doi: 10.1136/jnnp.2005.086983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy I, Hasson U, Avidan G, Hendler T, Malach R. Center-periphery organization of human object areas. Nat Neurosci. 2001;4:533–539. doi: 10.1038/87490. [DOI] [PubMed] [Google Scholar]
- Malach R, Levy I, Hasson U. The topography of high-order human object areas. Trends Cogn Sci. 2002;6:176–184. doi: 10.1016/s1364-6613(02)01870-3. [DOI] [PubMed] [Google Scholar]
- Malach R, Reppas JB, Benson RR, Kwong KK, Jiang H, Kennedy WA, Ledden PJ, Brady TJ, Rosen BR, Tootell RBH. Object-related activity revealed by functional magnetic-resonance-imaging in human occipital cortex. Proc Natl Acad Sci USA. 1995;92:8135–8139. doi: 10.1073/pnas.92.18.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh EB, Hillis AE. Cognitive and neural mechanisms underlying reading and naming: Evidence from letter-by-letter reading and optic aphasia. Neurocase. 2005;11:325–337. doi: 10.1080/13554790591006320. [DOI] [PubMed] [Google Scholar]
- Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges JR. The Addenbrooke's Cognitive Examination Revised (ACE-R): A brief cognitive test battery for dementia screening. Int J Geriatr Psychiatry. 2006;21:1078–1085. doi: 10.1002/gps.1610. [DOI] [PubMed] [Google Scholar]
- Mycroft RH, Behrmann M, Kay J. Visuoperceptual deficits in letter-by-letter reading? Neuropsychologia. 2009;47:1733–1744. doi: 10.1016/j.neuropsychologia.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Owsley C, Sloane ME. Contrast Sensitivity, Acuity, and the Perception of Real-World Targets. Br J Ophthalmol. 1987;71:791–796. doi: 10.1136/bjo.71.10.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K, Marcel AJ. Phonological ALEXIA or PHONOLOGICAL alexia? In: Alegria J, Holender D, Juncas de Morais J, Radeau M, editors. Analytic approaches to human cognition. Amsterdam: North-Holland; 1992. pp. 259–274. editor. [Google Scholar]
- Pflugshaupt T, Gutbrod K, Wurtz P, Wartburg RV, Nyffeler T, de Haan B, Karnath H-O, Mueri RM. About the role of visual field defects in pure alexia. Brain. 2009;132:1907–1917. doi: 10.1093/brain/awp141. [DOI] [PubMed] [Google Scholar]
- Plaut DC, Behrmann M. Complementary neural representations for faces and words: A computational exploration. Cogn Neuropsychol. 2011;28:251–275. doi: 10.1080/02643294.2011.609812. [DOI] [PubMed] [Google Scholar]
- Polk TA, Farah ML. Functional MRI evidence for an abstract, not perceptual, word-form area. J Exp Psychol Gen. 2002;131:65–72. doi: 10.1037//0096-3445.131.1.65. [DOI] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. The Interactive Account of ventral occipitotemporal contributions to reading. Trends Cogn Sci. 2011;15:246–253. doi: 10.1016/j.tics.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. The myth of the visual word form area. Neuroimage. 2003;19:473–481. doi: 10.1016/s1053-8119(03)00084-3. [DOI] [PubMed] [Google Scholar]
- Price CJ, Moore CJ, Humphreys GW, Frackowiak RSJ, Friston KJ. The neural regions sustaining object recognition and naming. Proc R Soc Lond Ser B Biol Sci. 1996;263:1501–1507. doi: 10.1098/rspb.1996.0219. [DOI] [PubMed] [Google Scholar]
- Roberts DJ, Lambon Ralph MA, Woollams AM. When does less yield more? The impact of severity upon implicit recognition in pure alexia. Neuropsychologia. 2010;48:2437–2446. doi: 10.1016/j.neuropsychologia.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-Prime user's guide. Pittsburgh: Psychology Software Tools; 2002. [Google Scholar]
- Sekuler EB, Behrmann M. Perceptual cues in pure alexia. Cogn Neuropsychol. 1996;13:941–974. [Google Scholar]
- Shallice T, Saffran E. Lexical processing in the absence of explicit word identification—Evidence from a letter-by-letter reader. Cogn Neuropsychol. 1986;3:429–458. [Google Scholar]
- Starrfelt R, Habekost T, Leff A. Too little, too late: Reduced visual span and speed characterize pure alexia. Cereb Cortex. 2009;19:2880–2890. doi: 10.1093/cercor/bhp059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington EK, James M. The Visual Object and Space Perception battery. Bury St. Edmunds, Suffolk: Thames Valley Test Company; 1991. [Google Scholar]
- Warrington EK, Shallice T. Semantic access dyslexia. Brain. 1979;102:43–63. doi: 10.1093/brain/102.1.43. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Shallice T. Word-form dyslexia. Brain. 1980;103:99–112. doi: 10.1093/brain/103.1.99. [DOI] [PubMed] [Google Scholar]
- Weekes BS. Differential effects of number of letters on word and nonword naming latency. Q J Exp Psychol. 1997;50A:439–456. [Google Scholar]
- Woodhead ZVJ, Wise RJS, Sereno M, Leech R. Dissociation of sensitivity to spatial frequency in word and face preferential areas of the fusiform gyrus. Cerebral Cortex. 2011;21:2307–2312. doi: 10.1093/cercor/bhr008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.