Abstract
Regional patterning of the cerebral cortex is initiated by morphogens secreted by patterning centers that establish graded expression of transcription factors within cortical progenitors. Here, we show that Dmrt5 is expressed in cortical progenitors in a high-caudomedial to low-rostrolateral gradient. In its absence, the cortex is strongly reduced and exhibits severe abnormalities, including agenesis of the hippocampus and choroid plexus and defects in commissural and thalamocortical tracts. Loss of Dmrt5 results in decreased Wnt and Bmp in one of the major telencephalic patterning centers, the dorsomedial telencephalon, and in a reduction of Cajal–Retzius cells. Expression of the dorsal midline signaling center-dependent transcription factors is downregulated, including Emx2, which promotes caudomedial fates, while the rostral determinant Pax6, which is inhibited by midline signals, is upregulated. Consistently, Dmrt5−/− brains exhibit patterning defects with a dramatic reduction of the caudomedial cortex. Dmrt5 is increased upon the activation of Wnt signaling and downregulated in Gli3xt/xt mutants. We conclude that Dmrt5 is a novel Wnt-dependent transcription factor required for early cortical development and that it may regulate initial cortical patterning by promoting dorsal midline signaling center formation and thereby helping to establish the graded expression of the other transcription regulators of cortical identity.
Keywords: choroid plexus, cortical hem, Emx2, telencephalon, Wnt/Bmp
Introduction
The cerebral cortex derives from the embryonic dorsal telencephalon. It can be subdivided into distinct regions. The most lateral region becomes the 3-layered paleocortex (olfactory piriform cortex). The most posterior and medial region develops into the archicortex (including the hippocampus). The neocortex, which is the larger region, is positioned between the two other regions. It has a very sophisticated architecture, being organized radially into 6 layers and tangentially into distinct areas that serve specialized functions.
Regionalization of the dorsal cortical primordium and arealization are thought to be initiated by signaling molecules secreted from localized inductive centers. Two major patterning centers are most directly implicated in telencephalon patterning. The first is the anterior neural ridge, which later becomes the commissural plate (CoP), which is located at the rostromedial pole of the telencephalon and secretes Fgfs. The second is the roof plate and cortical hem (CH) at the dorsal/caudal midline and immediate adjacent territories, which produce a variety of Wnt and Bmp ligands critical for hippocampus development. These signals are thought to establish in cortical progenitors graded expression of transcription factors that are crucial for the regionalization and subsequent arealization of the cortex (Hébert and Fishell 2008; O'Leary and Sahara 2008; Rakic 2009; Borello and Pierani 2010; Mallamaci 2011). These include Emx2, Lhx2, Pax6, Foxg1, Sp8, and COUP-TF1. How these secreted ligands and transcription factors function together is still not well understood. Whether additional transcription factors are implicated in the regionalization of the cortical primordium is unknown.
The Dmrt (doublesex and mab-3-related transcription factor) genes encode a large family of evolutionarily conserved transcription factors sharing the DM domain DNA-binding motif, whose function in sex determination and sexual dimorphism has been well studied in invertebrates and vertebrates (Ross et al. 2005; Kimura et al. 2008; Matson et al. 2010). In vertebrates, most Dmrt genes are expressed in the developing gonads. Like doublesex and mab-3, Dmrt1 has been shown to play a critical role in mammalian male sex differentiation (Raymond et al. 1998; Matson et al. 2010). However, some of them are also expressed and function in non-gonadal tissues. For example, Dmrt3–5 are strongly expressed in the embryonic forebrain (Hong et al. 2007). In the simple chordate Ciona, Dmrt1, the ortholog of vertebrate Dmrt4–5, is essential for the development of anterior neural plate derivatives (Tresser et al. 2010). In zebrafish, a recent analysis of a Dmrt5 (Dmrta2) mutant has revealed that it regulates neurogenesis in the telencephalon (Yoshizawa et al. 2011). The role of Dmrt genes in mammalian forebrain development is unknown.
In this study, we show that Dmrt5 is expressed within cortical progenitors in a high-caudomedial to low-rostrolateral gradient and that its inactivation disrupts the development of the caudomedial cerebral cortex. Together, our data suggest that Dmrt5 is essential for the specification of regional fate in dorsal telencephalic progenitors and that it may act through the promotion of Wnt and Bmp expression in the embryonic dorsal midline signaling center and through the modulation of the expression of the transcription factors that impart distinctive regional identities on cortical progenitors.
Materials and Methods
Generation and Genotyping Strategy of Dmrt5 Knockout Mice
To generate Dmrt5 null mice, a targeting vector containing a neomycin phosphotransferase resistance cassette (PGK-neo) was inserted by homologous recombination into the wild-type (WT) Dmrt5 allele, replacing the entire Dmrt5 ORF. The PGK-neo cassette was inserted into a pBKSII vector as a SacI–BamHI fragment in between 2 kb 5′ and 5 kb 3′ genomic Dmrt5 BamHI–SacI fragments isolated from PAC clone 403-P18. The DTA gene was also inserted at the 3′ extremity of the construct. The linearized targeting construct was electroporated in embryonic stem (ES) cells. After G418 selection, DNA from 650 resistant clones was analyzed by PCR using the external primer: 5′-AGGCTCAACGCCCCTTCTTA-3′ and the internal primer: 5′-AGCGCCTCCCCTACCCGGTA-3′. Three positive clones were identified. The Dmrt5 null mouse strain was generated by injection of one positive ES cell clone into 129/Sv blastocysts. The chimeric mice were mated to WT C57Bl/6 mice to generate heterozygous animals that were then intercrossed to generate F2 offspring for analysis.
To distinguish between the WT and Dmrt5 null allele, mice were genotyped by PCR using genomic DNA from the tail of postnatal and late embryonic stages or yolk sac from earlier embryos and primers: Fwd 5′-CGAATCTTTCGGACACTGTAGA-3′; Rev WT 5′-CCAGACCCTCAAGCACTCAA-3′; Rev knockout (KO) 5′-AGCGCCTCCCCTACCCGGTA-3′. Morning of the vaginal plug is E0.5 and the first 24 h after birth is P0. Animal care was in accordance with institutional guidelines.
Reverse transcription (RT)-PCR and Southern Analysis
RT–PCR analysis was performed on total RNA isolated from E15 WT and Dmrt5 null brain and from adult testis. RNAs were reverse transcribed with the “iScript cDNA Synthesis Kit” (Bio-Rad). The sequences of the primers used to detect Dmrt5 are the following: Fwd 5′-CTTACGAAGTCTTTGGCTCG-3′ and Rev 5′-TTCTTTGTCAGCCTCCGAAC-3′. Primers used to detect the expression of the Dmrt5 adjacent genes Faf1 and Elav4 are for Faf1: Fwd 5′-GGCGTCCAACATGGACCGGG-3′ and Rev 5′-CTGCAGGGCACCAGCATGGC-3′; and for Elav4: Fwd 5′-CGAAGCGCTGCGAGACCCAA-3′ and Rev 5′-GGCGGGCGTAGGACACCTT-3′. Amplification of HPRT (primers: Fwd 5′-CCTGCTGGATTACATTAAAGCACTG-3′ and Rev 5′-GTCAAGGGCATATCCAACAACAAAC-3′) was used as a control.
Southern blot analysis was performed on genomic DNA digested with HindIII (for the 5′ Southern) or EcoRV (for the 3′ Southern) using external probes (500 bp for the 5′ Southern and 600 bp for the 3′ Southern). In each case, the predicted fragments were observed (6.8 kb for the WT allele, 8.2 kb for the targeted allele in the 5′ Southern, 14.6 kb for the WT allele, and 11.7 kb for the targeted allele in the 3′ Southern). An internal Neo probe was also used with HindIII digests to detect a single 8.2 kb Dmrt5 targeted allele (see Supplementary Fig. 2; data not shown).
Histology, Immunohistochemistry, and Immunofluorescence Staining
Standard hematoxylin and eosin staining was performed on 6–8 µm sections of embryos fixed overnight in Bouin, dehydrated, and paraffin-embedded.
For immunohistochemistry (IHC), embryos or brains were fixed overnight in 4% paraformaldehyde, dehydrated in ethanol, paraffin-embedded, and sectioned (6–8 µm). Sections were washed in xylene and rehydrated in ethanol. Antigen retrieval was performed by boiling the sections in Target Retrieval Solution Citrate, pH 6.0 (DAKO). Sections were then treated with 0.3% H2O2, blocked in a 5% lamb serum solution, and incubated with primary antibodies overnight at 4°C. The following primary antibodies were used: Anti-2H3a (mouse, 1:500, DSHB), anti-L1 (rabbit, 1:500, Fritz G. Rathjen), anti-Lim1/2 (mouse, 1:10, DSHB), anti-Pax6 (rabbit, 1:200, PRB-278P, Covance), anti-Tbr2 (rabbit, 1:500, ab23345, Abcam), anti-Reelin (mouse, 1:750, A. Goffinet), anti-Calretinin (rabbit, 1:1000, AB5054, Millipore), anti-Ctip2 (rat, clone 25B6, 1:500, ab18465, Abcam), anti-phospho-histone H3 (PH3, rabbit, 1:200, 06-570, Millipore), and anti-cleaved Caspase 3 (rabbit, 1:1000, 9661, Cell Signaling). Sections were incubated with anti-rabbit (1:200, 2AB02B, AbD Serotec), anti-mouse (1:200, B0529, Sigma), or anti-rat (1:200, BA-9400, Vector) secondary antibodies. Detection was performed with the ABC Kit (Vectastain) according to the manufacturer's instructions and visualized by DAB (Sigma). Images were acquired with an Olympus SZX2-ILLB or an Olympus XC50 camera, using the Imaging software Cell*.
For immunofluorescence staining (IF), brains were fixed overnight in 4% paraformaldehyde, infused in 30% sucrose/phosphate-buffered saline (PBS) overnight, frozen in gelatin (7.5% gelatin, 15% sucrose/PBS), and sectioned in 20 µm cryostat sections. Sections were rinse in PBS. Antigen retrieval was performed by boiling the sections in Target Retrieval Solution Citrate, pH 6.0 (DAKO). Sections were then permeabilized in 0.3% Triton X-100, blocked in 5% goat serum, and incubated with primary antibodies overnight at 4°C. The following antibodies were used: Ctip2 (rat, clone 25B6, 1:250, ab18465, Abcam), Tbr1 (rabbit, 1:1000, ab31940, Abcam), Satb2 (mouse, 1:100, ab51502, Abcam), and Cux1 (rabbit, 1:100, sc-13024 Santa-Cruz). Sections were incubated with Alexa Fluor 488 goat anti-rabbit (1:400, A-11008, Invitrogen), Alexa Fluor 594 goat anti-rabbit (1:400, A-11012, Invitrogen), Alexa Fluor 488 goat anti-mouse (1:400, A-11017, Invitrogen), or Alexa Fluor 594 goat anti-rat (1:400, A-11007, Invitrogen) secondary antibodies. Wide-field images were acquired with a Leica DM 4000B equipped with an EBQ10 lamp and leica filters (i3, Tx2, and A).
DiI Labeling
Brains were fixed overnight in 4% paraformaldehyde. To retrogradely label neurons in the dorsal thalamus, brains were sagitally hemidissected. Crystals of 1.1′-Dioctadecyl-3,3,3′,3′-Tetramethylindocarbocyanine perchlorate (DiI, Molecular Probes, D282, Invitrogen) were placed with a fine tungsten wire in the thalamus. Samples were kept in PBS-azide 0.01% for 2 weeks at 37°C in the dark. Brains were then washed in PBS, embedded in gelatine, and cut at 100 µm on a vibroslice (M752 vibroslice, Campden Instruments). Sections were mounted onto slides and analyzed by fluorescence with an Olympus XC50 camera, using the Imaging software Cell*. For each genotype and for each cortical labeling point, at least 3 hemibrains were processed.
In Situ Hybridization
An antisense Dmrt5 probe was synthetized by linearizing EST AI592924 (GenBank) with EcoRI and transcribing it with T3. The Dmrt3 probe was synthesized by linearizing EST BU054807 with MluI and transcribing it with T3. The p75 probe was synthesized by linearizing EST BC038365 with SalI and transcribing it with T7. The other antisense probes were generated from the following previously described cDNA clones: Lef1, Fzd10, Id3, Emx2, Hes5 (Muzio et al. 2005), Axin2 (Bluske et al. 2009), Cux2, Nfib, Nurr1/Nr4a2, Rspo1, Rspo2, Rspo3 (Hasenpusch-Theil et al. 2012), Lhx2, Wn2b, Ttr, Wnt3a (Monuki et al. 2001), Bmp4 and Bmp7 (Furuta et al. 1997), Msx1, Wnt5a, Gli3 (Grove et al. 1998), Math2, Ngn2 (Schuurmans et al. 2004), Mash1 (Chou et al. 2009), Dbx1 (Causeret et al. 2011), Auts2, Tbr1 (Bedogni et al. 2010), COUP-TF1 (Zhou et al. 2001), EphrinA5, EphA7, RZRβ, Cad8 (Bishop et al. 2002), Sp8 (Zembrzycki et al. 2007), Fgf8, Fgf17, RORβ (Fukuchi-Shimogori and Grove 2003), ER81, Dlx2, Nrp2, Satb2 (Chou et al. 2009), Foxg1, Lmx1a (Imayoshi et al. 2008), Pax6 (Hamasaki et al. 2004), KA1, Scip1, EphB1 (Bulchand et al. 2001), Reelin (Yoshida et al. 2005), Prox1 (Xie et al. 2010), and Wnt8b (Lee et al. 2000).
Whole-mount embryo and section in situ hybridization (ISH) were performed as previously described (Wilkinson and Nieto 1993), using digoxigenin-labeled riboprobes, an alkaline phosphatase (AP)-conjugated anti-digoxigenin Fab fragment (1:2000, Roche), and NBT/BCIP substrates. For section ISH, 20 µm cryostat sections of 4% paraformaldehyde fixed, 30% sucrose/PBS-infused tissue frozen in gelatin (7.5% gelatin, 15% sucrose/PBS) were used. Double ISH was performed as previously described (Hauptmann and Gerster 1994), using digoxigenin-labeled Wnt3a and fluorescein-labeled Dmrt5 riboprobes, an AP-conjugated anti-fluorescein Fab fragment (1:2000, Roche), and a Magenta phosphate substrate (Sigma).
RNA Isolation and RT–qPCR
Total RNA was extracted from telencephalon using the “RNAspin mini” (GE Healthcare) according to the manufacturer's protocol. DNase treatment was performed using the “Rnase free—Dnase set” (Qiagen), followed by cDNA synthesis (“iScript cDNA Synthesis Kit,” Bio-rad) starting from equal amounts of RNA.
RT–qPCRs were performed on a StepOnePlus Real Time PCR system (Applied Biosystems) using SYBER Green MESA GREEN qPCR MasterMix Plus (Eurogentec) with a program optimized by the manufacturer. Gene expression was normalized to the expression of 2 reference genes: Hypoxanthine-guanine phosphoribosyltransferase (HPRT) and glyceraldehydes-3-phosphate dehydrogenase (GAPDH). Reference genes were validated using the Genorm program. The primers were designed using the PrimerBank (Spandidos et al. 2010) and Primer3 (Rozen and Skaletsky 2000). The primers were verified for specificity with Primer-Blast from NCBI. The PCR efficiency was estimated with the Applied Biosystems software using a calibration dilution curve for each primer set. Primers used were as follows: Dmrt3 forward primer GCGCAGCTTGCTAAACCAG, Dmrt3 reverse primer CCTCTGATCGGTGTCTTTGTCA; Dmrt4 forward primer CCAACTTTCGAGGTTTTCCA, Dmrt4 reverse primer GATGATCCCAGAGAATGGTGA; Dmrt5 forward primer GCGCCAACAGAGGAGGAG, Dmrt5 reverse primer ACGCTCACTGGTAGCGATG; Emx2 forward primer GTCCACCTTCTACCCCTGG, Emx2 reverse primer CCACCACGTAATGGTTCTTCTC; Lhx2 forward primer TGGCAGCATCTACTGCAAAG, Lhx2 reverse primer TGTGCATGTGAAGCAGTTGA; Pax6 forward primer AGGGCAATCGGAGGGAGTAA, Pax6 reverse primer CAGGTTGCGAAGAACTCTGTTT; Gli3 forward primer CACAGCTCTACGGCGACTG, Gli3 reverse primer CTGCATAGTGATTGCGTTTCT; Sp8 forward primer CACTCGACGATTTCGAAGGG, Sp8 reverse primer GGAAGCCTTTACCGAAGGAAG; COUP-TF1 forward primer AAAGTGGGCATGAGGCGGGA, COUP-TF1 reverse primer CCCGTTTGTGAGTGCATACTG.
Explant Culture
Organotypic slice cultures of E13.5 embryonic mouse telencephalon in the presence of either DMSO or 5, 25, or 50 µM Chir (Cambridge BioScience) were performed as described previously (Hasenpusch-Theil et al. 2012).
Statistical Analysis
To quantify the dorsal surface area of the cortex of Dmrt5 mutants and their littermate, brains from E18.5 embryos were dissected and fixed overnight in 4% paraformaldehyde. The brains were then photographed under a Nikon dissection microscope, slide images were digitized, and the dorsal surface measured using Imaging Software NIS-Elements. Measurements were made blind to genotype.
Quantification of the thickness of the cortical wall of Dmrt5 null mice and WT embryos has been done on coronal sections at the level of the caudomedial telencephalon based on hematoxylin and eosin histological images using the Imaging software Cell*.
Quantifications of cells expressing PH3 and Tbr2 have been done on 3 sections/embryo at caudal levels of the telencephalic vesicles (n = 2–4). Images were acquired with a Zeiss Axioskop microscope.
For quantification of cells expressing Tbr2, a rectangle of 100 µm width was fixed in medial regions and at caudal levels of the telencephalic vesicles. Quantification of apical PH3-positive cells at E11.5 and E14.5 was done similarly. Apical PH3-positive cells at E9.5 and basal PH3-positive cells at E11.5 and E14.5 were counted throughout the entire medial part of the telencephalon. At E18.5, PH3-positive cells were counted through the entire dorsal telencephalon (n = 3 for each stage analyzed). All counts performed without the use of a rectangle marquee were normalized with respect to the size of the vesicles. Positive cells were counted in Adobe CS5 Photoshop. All statistical analyses were done with Student's t-test; values of P < 0.05 were regarded as statistically significant.
Results
Dmrt5 is Expressed in Mouse Cortical Progenitors in a High-Caudomedial to Low-Rostrolateral Gradient
Dmrt5 was identified as a downregulated gene in a microarray screen comparing the transcriptome of WT and Mdm4 KO brains, which display a high level of apoptosis and a severe deficit in neurogenesis (Martoriati et al. 2005). Embryonic expression of Dmrt5 was studied from E9.5 by ISH on whole embryos and on sections. We found that at E9.5, Dmrt5 is strongly expressed in the developing dorsal telencephalon, except in the dorsal midline. Outside the telencephalon, Dmrt5 was also found in the optic stalk, the lateral head ectoderm, and in the maxillary and mandibular processes (Fig. 1A,B). At E10.5, Dmrt5 expression was also detected in the ventral midbrain, eyes, nasal placodes and hypophysis. From that stage, Dmrt5 was detected in a high-caudomedial to low-rostrolateral gradient in the telencephalic evagination and was restricted to progenitors. Dmrt5 staining in the dorsomedial part of the telencephalic evagination includes the primordia of the CH and choroid plexus. No expression was detected in the roof plate. From E11.5, Dmrt5 was also detected in the developing diencephalon. At E13.5, Dmrt5 expression in the choroid plexus has strongly decreased. From that stage, additional expression was detected in scattered cells located in the most marginal portion of the cerebral walls, most abundant in the medial area (Fig. 1C–H; see Supplementary Fig. 1A–H). The marginal zone of the cortex contains Cajal–Retzius (CR) cells, one of the earliest neuronal cell types in the mammalian telencephalon that express Reelin, a key molecule in the orchestration of the radial migration of neurons (Ogawa et al. 1995). We therefore asked whether these Dmrt5+ cells also express Reelin. Using ISH combined with immunohistochemistry (IHC), we detected colocalization of Dmrt5 transcripts and Reelin protein, indicating that Dmrt5 is expressed in CR cells (see Supplementary Fig. 1I,J). Together, these data identified Dmrt5 as a likely candidate for a cortical regulatory factor.
Figure 1.
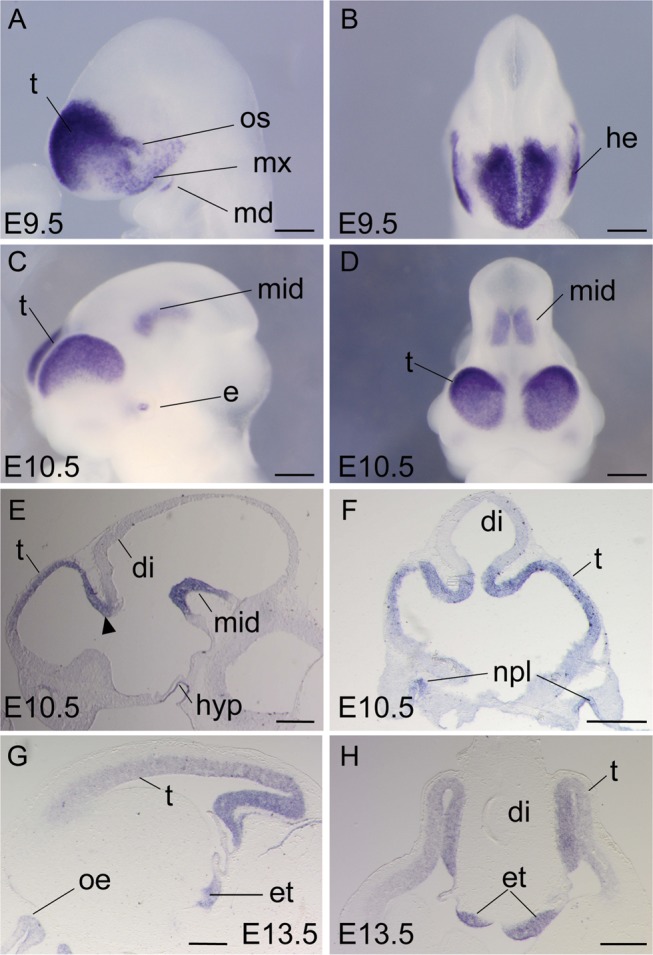
Dmrt5 expression in the mouse developing brain. (A–D) Whole-mount ISH of Dmrt5 at E9.5 (A and B) and E10.5 (C and D). (E–H) Sagittal (E and G) and coronal (F and H) brain sections. Arrowhead in (E) indicates Dmrt5 expression at the telen-diencephalic boundary. di, diencephalon; e, eye; et, eminentia thalami; he, head ectoderm; hyp, hypophysis; md, mandibular process; mid, midbrain; mx, maxillary process; npl, nasal placode; oe, olfactory epithelium; os, optic stalk; t, telencephalon. Scale bars: 200 μm in A and B; 500 μm in C–H.
Dmrt5 Deletion Leads to a Strong Reduction of Posterior Midline Cortical Structures
To elucidate Dmrt5 function, Dmrt5 null mice were generated (see Supplementary Fig. 2A–C). While heterozygous mice were healthy and fertile, about 95% of homozygous Dmrt5−/− mice died within a few hours after birth. Surviving animals were growth retarded and presented neurological abnormalities (see Supplementary Fig. 2D–F). While E18.5 Dmrt5+/− brains were indistinguishable from WT brains (data not shown), E18.5 Dmrt5−/− brains exhibited a strong reduction in the surface area of the cerebral hemispheres (−38.6 ± 5.9%, n = 16, P< 0.001) and olfactory bulbs (Fig. 2A). Hematoxylin and eosin staining of E18.5 sagittal brain sections revealed that the cortical wall of the mutants has a decreased thickness (−35.8 ± 10.1%, n = 3, P< 0.05) and a poorly differentiated structure. On average, the proportional size of the ventricular zone (VZ), subventricular zone (SVZ), and cortical plate was similar in mutant and control embryos. The subplate appeared much less well formed (Fig. 2B). Defects in the subplate were confirmed by ISH or IHC using EphA7, p75, and Calretinin (see below). Remarkably, the caudomedial portion of the cortex of Dmrt5 mutants including the hippocampus, dentate gyrus, and choroid plexus was missing. A strong impairment of commissural axonal tracts crossing the midline was also evident in mutant mice (Fig. 2C). Anteriorly, the limit of the neocortex with the olfactory bulbs was abnormal. The VZ often appeared disorganized, showing in some cases bulges protruding to the pial surface (Fig. 2D). Development of the efferent fibers from the hippocampus was also poor (Fig. 2E). L1 and 2H3 neurofilament IHC confirm these observations. These data revealed that mutant forebrains have also severe defects in the thalamocortical axon (TCA) and cortifugal axon (see Supplementary Fig. 3A and D). DiI injections into the dorsal thalamus confirm the TCA defects (see Supplementary Fig. 3E). The anlagen of the cerebral hemispheres was already smaller than normal at E10.5. Unexpectedly, the cortical wall, which is smaller in Dmrt5−/− embryos at E18.5, was thicker in Dmrt5−/− embryos at E11.5 and of about the same size as controls at E14.5 (see Supplementary Fig. 4 and see below). Thus, the loss of Dmrt5 causes early alterations in the development of the telencephalic vesicles leading to a strong reduction at E18.5 of the caudomedial cortex.
Figure 2.
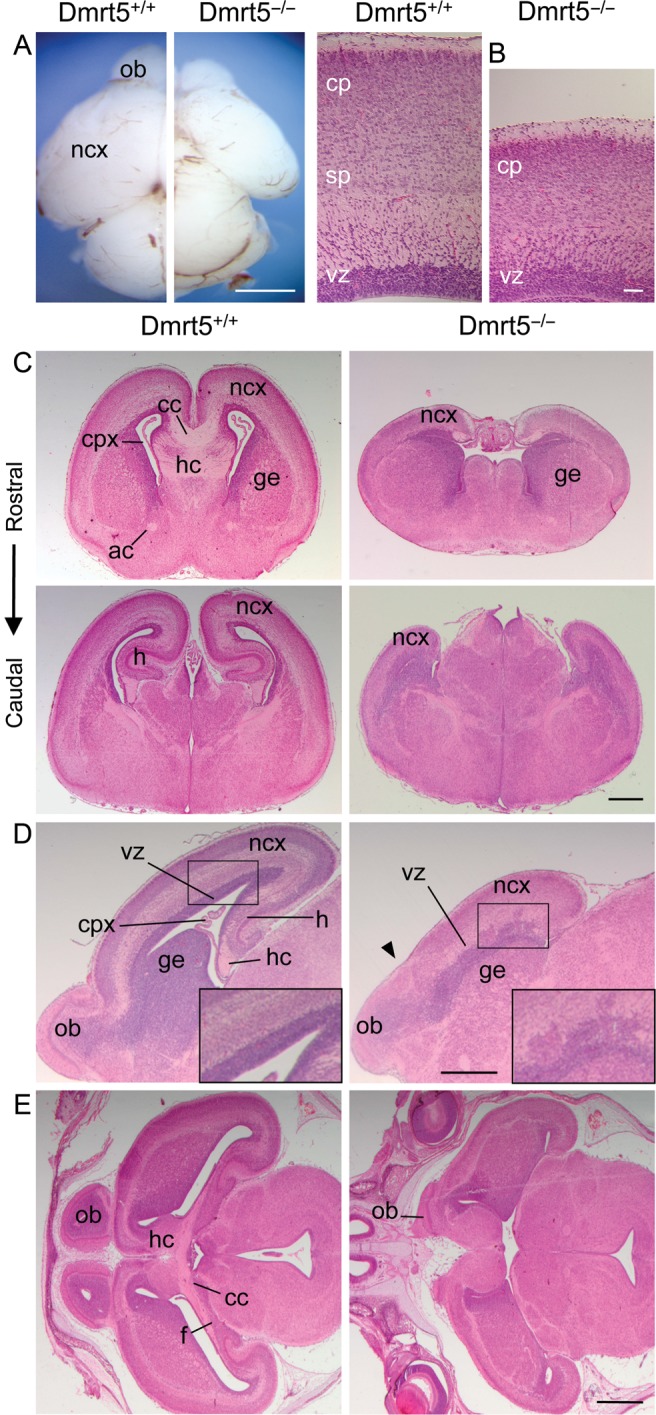
Reduced cerebral cortex with midline defects in Dmrt5−/− mice. (A) Dorsal views of P0 control and Dmrt5−/− brains. (B) High magnification view of sagittal sections of E18.5 control and mutant brains. (C–E) Hematoxylin and eosin staining of coronal (C, at the rostral level, upper panels, and the caudal level, lower panels), sagittal (D) and horizontal (E) sections of E18.5 control and mutant brains. Note the abnormal limit of the neocortex with the olfactory bulbs (arrowhead in D). ac, anterior commissure; cc, corpus callosum; cp, cortical plate; cpx, choroid plexus; f, fimbria; ge, ganglionic eminences; h, hippocampus; hc, hippocampal commisure; ncx, neocortex; ob, olfactory bulbs; sp, subplate; vz, ventricular zone. Scale bars: 200 μm in A and B; 500 μm in C and D; 1 mm in E.
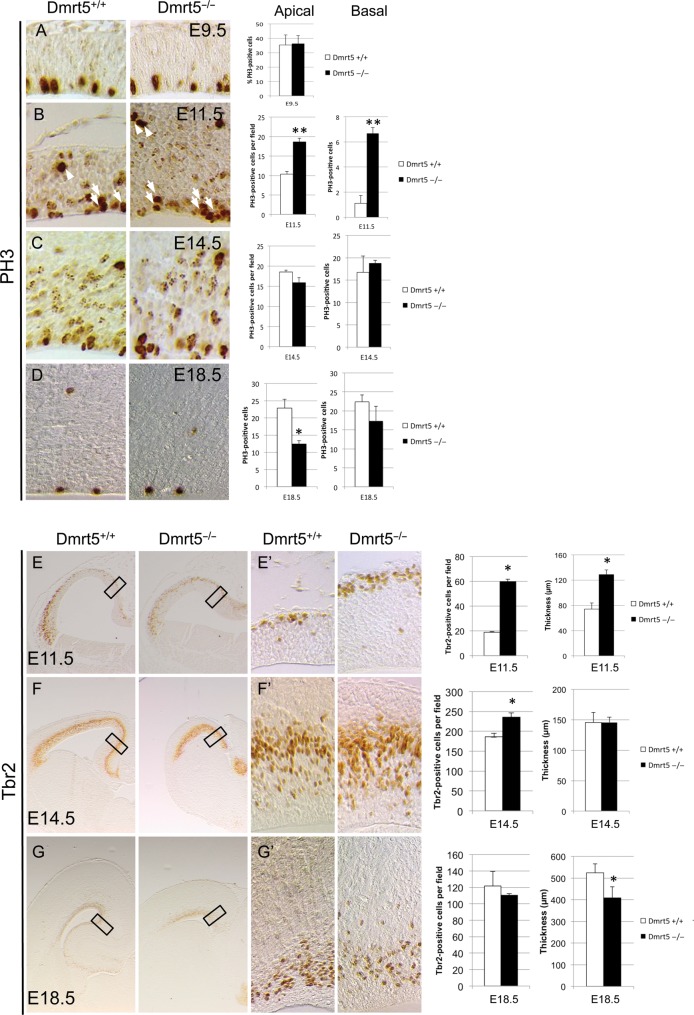
To understand the cause of the observed phenotype, we first examined the extent of apoptosis in the Dmrt5−/− neocortex by anti-cleaved Caspase-3 immunostaining at E9.5, E11.5, and E14.5. We found no increase in apoptosis in the entire cortex of Dmrt5 mutants compared with WT embryos (see Supplementary Fig. 5). Next, we quantified the mitotic progenitors labeled with an antibody against PH3 based on their location (apical in the VZ for the radial glial cell [RGCs] and basal in the SVZ for the intermediate progenitor cell [IPCs]) at the same stages and at E18.5. We counted the number of mitotic progenitors in medial regions and at caudal levels of the telencephalic vesicles in Dmrt5 mutants and wild types. We found that there is no significant difference in the total number of proliferating cells in the neuroepithelium of Dmrt5−/− embryos compared with controls at E9.5 (Fig. 3A). However, at E11.5, we observed a slight increase in the number of apical (1.86-fold) and a larger increase in basal (6-fold) mitotic progenitors in Dmrt5−/− embryos compared with controls (Fig. 3B). We thus also tested in E11.5 and later embryos the expression of Tbr2 which labels specifically basal progenitors. In accordance with our PH3 results, we found at E11.5 an increase in Tbr2-positive cells (3.15-fold) in the mutants with respect to the controls (Fig. 3E,E′). At E14.5, we observed that mitotic rates return to normal for both RGCs and IPCs in the Dmrt5 mutants. This normalization of the mitotic rate also comes with a smaller increase in Tbr2-positive cells (1.25-fold) in Dmrt5 mutants (Fig. 3C,F,F′). At E18.5, we observed a reduction in both apical (1.8-fold) and basal (1.28-fold) progenitors in Dmrt5−/− cortices. No differences in the number of Tbr2-positive cells could be detected anymore between mutants and controls (Fig. 3D,G,G′). Thus, in Dmrt5 mutant cortices, a transient burst of proliferation with an expansion of the population of basal progenitors occurs at E11.5, followed by a reduction later of apical progenitors. Those defects in cell proliferation are likely to contribute to the observed phenotype.
Figure 3.
Altered cell proliferation in the telencephalon of Dmrt5−/− mice. (A–D) PH3 IHC on coronal sections of Dmrt5−/− and control embryos at the indicated stages. Quantification of the results is shown on the right. Note the transient slight increased in apical and larger increase in basal PH3 cells at E11.5 and later slight decrease in PH3-positive cells in Dmrt5−/− embryos. (E–G) Tbr2 IHC on coronal sections of Dmrt5−/− and control embryos at the indicated stages. High magnifications of the rectangles in (E–G) are shown in (E′–G′). Quantification of the results and thickness of the cortical wall is shown on the right. Note the transient increase of the number of Tbr2-positive cells and of the thickness of the cortical wall at E11.5, and the decrease of the thickness at E18.5 in Dmrt5−/− embryos (*P< 0.05 and **P< 0.01). Error bars indicate standard deviation (SD).
Reduction of Wnt and Bmp Signals in the Caudomedial Cortex and Decrease in CR Cells in Dmrt5−/− Embryos
The CH region is a Wnt and Bmp-rich tissue that plays an essential role in the specification of the hippocampus (Lee et al. 2000; Muzio and Mallamaci 2005; Fernandes et al. 2007). The structural perturbations observed in the forebrain of Dmrt5−/− mice prompted us to investigate expression of the signaling molecules produced by the CH region. We first looked by in situ at the Wnt family members Wnt2b, Wnt3a, and Wnt5a, whose expression in the telencephalon is restricted to the CH and Wnt8b expressed in the eminentia thalami, in the CH, and in a high-medial to low-lateral gradient in the dorsomedial telencephalon (Grove et al. 1998). At E12.5, Wnt2b and Wnt5a were undetectable in Dmrt5−/− embryos (Fig. 4A,B). Wnt3a, which is the earliest Wnt selectively expressed in the CH (Lee et al. 2000), was still expressed but at a reduced level (Fig. 4C). Wnt8b expression in the dorsomedial telencephalon was strongly reduced in the Dmrt5 mutants but was still detected in the eminentia thalami visualized by immunostaining using the Lim1/2 marker (Fig. 4D). We also looked at the expression of Wnt receptors, nuclear effectors, and cytoplasmic modulators. The Wnt receptor gene Fzd10 expressed in the CH, as well as the Wnt nuclear effector Lef1, normally restricted to the dorsomedial cortex (Muzio et al. 2005), were downregulated in mutants (Fig. 4E,F). Several modulators including Axin2 which is upregulated in the area of strong canonical Wnt signaling (Jho et al. 2002) and Rspo1-3 strongly expressed in the CH (Hasenpusch-Theil et al. 2012) were also reduced in Dmrt5−/− embryos. The remaining Rspo1-3 signal detected in the caudal telencephalon of the mutants likely corresponds to the signal detected in the eminentia thalami in the controls (Fig. 4G–J). Thus, canonical Wnt signaling is strongly reduced in Dmrt5−/− caudomedial cortical progenitors.
Figure 4.
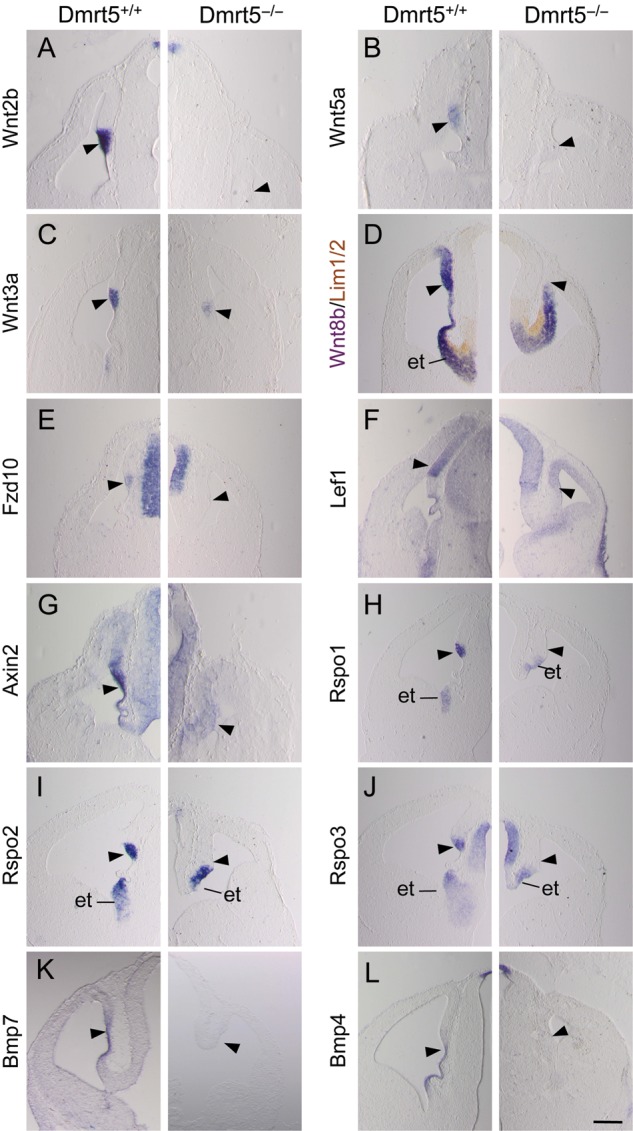
Reduction of Wnt and Bmp genes and genes encoding Wnt signaling components in the caudomedial cerebral cortical wall of Dmrt5 mutants. (A–C and E–L) Coronal forebrain sections of mutant and control mice at E12.5 analyzed by ISH for the indicated genes. (D) ISH for Wnt8b combined to immunoperoxidase staining for Lim1/2, a marker of diencephalic cells. Note that all tested genes were undetectectable or strongly reduced in the CH region (arrowheads) of E12.5 mutant mice. et, eminentia thalami. Scale bars: 500 μm.
Several Bmp family members that are required for the formation of the cortical plate and choroid plexus are also expressed within cortical dorsal midline cells (Furuta et al. 1997; Currle et al. 2005; Cheng et al. 2006; Fernandes et al. 2007). Two of these, Bmp4 and Bmp7, which mark the choroid plexus and the ventral portion of the CH, were also strongly reduced in mutants (Fig. 4K,L). Reduced Wnt3a and Bmp7 expression was already detectable in the abnormal telencephalic dorsal midline of mutants at E10.5 (see Supplementary Fig. 6A,B). We also investigated the expression of Fgfs produced by the CoP. By ISH on sections and in whole mounts, Fgf8 and Fgf17 expression in the bridge region between the two hemispheres in the mutants was similar in intensity to that of control embryos (see Supplementary Fig. 6C–F). Thus, while Fgfs are not dramatically affected in the CoP, Wnt and Bmp are deficient in the dorsal midline in the telencephalon of Dmrt5 mutants.
As the CH is a major source of CR cells (Yoshida et al. 2006), we examined the CR cell markers Reelin and Calretinin in Dmrt5−/− embryos. As expected, based on the observed CH defects, Reelin was severely reduced at E18.5. However, a few positive cells remained in the most medial portion of the cortex. Some Reelin+ cells also accumulated in the form of aggregates at the border between the neocortex and the piriform cortex. Similar results were obtained using Calretinin at E15.5 (see Supplementary Fig. 7). Thus, the differentiation of CR cells is severely impaired in Dmrt5 mutants. Together, these observations indicate that the loss of the caudomedial cortex is likely caused by the reduction of Wnt and Bmp signals at the dorsal midline signaling centers.
Altered Expression of Transcriptional Regulators of Cortical Development in Dmrt5−/− Embryos
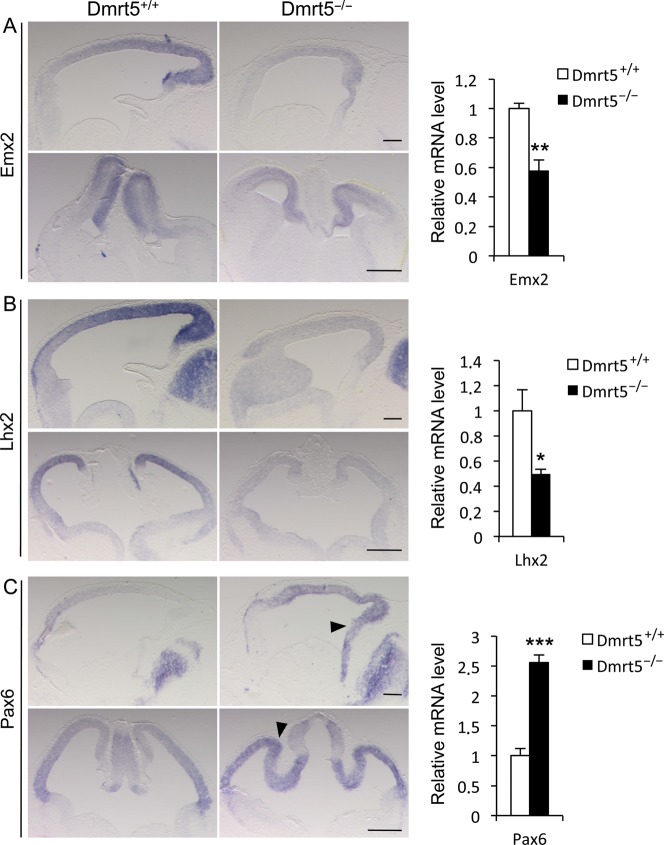
The expression of several cortical transcription factors is known to be dependent on signaling molecules from the CH and surrounding regions. Among them, Emx2, which promotes caudomedial fates and is expressed similarly to Dmrt5, is upregulated by Wnt and Bmp ligands (Theil et al. 2002). Lhx2 is a Bmp-regulated selector gene that is expressed similarly to Dmrt5 except that, unlike Dmrt5, Lhx2 is not expressed in the CH and choroid plexus. In mice in which Lhx2 is disrupted between E8.5 and E10.5, the neocortex and the hippocampal primordium are lost at the expense of an expanded CH and choroid plexus (Monuki et al. 2001; Mangale et al. 2008). When Lhx2 is deleted later, between E10.5 and E11.5, lateral cortical progenitors generate olfactory cortex rather than lateral neocortex (Chou et al. 2009). Genes encoding the homeodomain transcription factor Cux2, the orphan nuclear receptor Nurr1/Nr4A2, Nfib, and Dmrt3 are recently identified Wnt targets that show restricted expression in the dorsomedial telencephalon and may thus also function in early cortical regionalization (Hasenpusch-Theil et al. 2012). Given the deficiency of Wnt and Bmp in the caudomedial part of the telencephalon of Dmrt5 mutants, we suspected that expression of those genes would be altered and examined their expression by ISH at E12.5. We found that the Emx2 gradient is maintained in Dmrt5−/− cortices but at a lower level than in controls. Lhx2 was also reduced in the mutants to a low level similar to that detected in the ventral telencephalon and its normal graded pattern of expression was no longer apparent. RT–qPCR on RNA extracted from isolated E12.5 mutant or WT telencephalons confirmed the downregulation of Emx2 and Lhx2 in the mutants (Fig. 5A,B). As Lhx2 selector activity occurs only during early development, we analyzed its expression in earlier E9.5–E10.5 embryos. At E9.5, Lhx2 appeared similar in mutant and control embryos (data not shown). At E10.5, consistent with the decrease of Bmps observed at that stage in the mutants and its bimodal regulation and suppression by high levels of Bmps (Monuki et al. 2001), Lhx2 appeared slightly decreased and the negative domain of expression in the dorsal midline was reduced (see Supplementary Fig. 8A,B). Cux2, which is strongly expressed in the CH, was lost in the mutant. The low expression of Nurr1 in the CH and its higher expression in the choroid plexus and eminentia thalami were reduced in Dmrt5−/− embryos, the remaining signal likely corresponding to the eminentia thalami. Nfib, expressed in a high-medial to low-lateral gradient in dorsal telencephalic progenitors, was also strongly reduced in Dmrt5 mutants. In contrast, the level of Dmrt3 appeared similar in mutants compared with controls (see Supplementary Fig. 9A–D).
Figure 5.
Downregulation of Emx2 and Lhx2 and upregulation of Pax6 in the cortex of E12.5 Dmrt5 mutants. (A–C) ISH for the indicated markers on sagittal (upper panels) and coronal (lower panels) sections. Sagittal sections are shown with anterior to the left. The arrowheads in (C) indicate Pax6 upregulation in the medial and posterior part of the cortex of the mutants. Quantitative RT–qPCR analysis of Emx2, Lhx2, and Pax6 in the cortex of WT and mutant E12.5 embryos is shown on the right. Results are normalized to the level of expression in the WT forebrain. Error bars show SD of 3 independent experiments. Scale bars: 200 μm in upper panels; 500 μm in lower panels (*P< 0.05, **P< 0.01, and ***P< 0.001).
We also examined the expression of the other transcription factors playing crucial roles in cortical development: Pax6, Foxg1, COUP-TF1, Sp8, and Gli3. Pax6 is downregulated by Emx2 and inhibited by Wnt signaling (Machon et al. 2007; Ivaniutsin et al. 2009). It is expressed in an opposite pattern to Emx2 and promotes rostrolateral fates (Bishop et al. 2000; Hamasaki et al. 2004). Foxg1 is a forkhead gene downregulated by Wnt that suppresses hem fate. It is expressed in the entire telencephalon with the exception of the caudomedial regions (Muzio and Mallamaci 2005). COUP-TF1 represses frontal/motor cortical areas. It is expressed in a high-caudolateral to low-rostromedial gradient in the cortex, and hence, its expression is similar to Dmrt5 along the rostrocaudal axis but opposite to Dmrt5 along the mediolateral axis (Zhou et al. 2001; Armentano et al. 2007). Sp8 promotes frontal/motor areas. It is induced by Fgf8 and is expressed at highest levels in the medial and anterior part of the dorsal telencephalon, thus similarly to Dmrt5 along the L-M axis and in an opposite manner along the A-P axis (Sahara et al. 2007; Zembrzycki et al. 2007). Gli3 is detected from early stages of neurulation throughout the telencephalic anlagen and is then progressively downregulated in the ventral portion (Aoto et al. 2002; Fotaki et al. 2006). In Dmrt5 mutants, Pax6 was upregulated in the medial and posterior cortical anlage of Dmrt5 mutants where it is normally less intensively expressed. Similar changes were observed by RT–qPCR and at the protein level (Fig. 5C; see Supplementary Fig. 8C–E). The Foxg1 negative domain of expression was absent in the mutants (see Supplementary Fig. 9E). In the telencephalon of Dmrt5−/− mutants, COUP-TF1 was expressed in a gradient along both axes as in wild types with apparently no dramatic change in the level of its expression (see Supplementary Fig. 9F). In accordance with our observation that Fgf8 in the CoP appears not dramatically affected in the mutants, no significant changes in Sp8 expression were detected in the cortex between controls and mutants (see Supplementary Fig. 9G). Gli3 expression appears also unaffected in the mutants (see Supplementary Fig. 9H). COUP-TF1, Sp8, and Gli3 expression was also not significantly affected in E12.5 mutant embryos by RT–qPCR (see Supplementary Fig. 9I). Thus, among the known transcription factors controlling cortical identity, Emx2, Lhx2, and Pax6 are the most severely affected in Dmrt5 mutants. While Emx2 and Lhx2 are decreased, the rostral determinant Pax6 is upregulated in the cortex of Dmrt5 mutants. The alteration of their expression is likely to contribute to the observed cortical defects.
Mediolateral Patterning Defects and Laminar Expression of Cortical Markers in the Cortex of Dmrt5 Mutants
To investigate the role of Dmrt5 in mediolateral cortical patterning, we first examined the expression of Transthyretin (Ttr) and of the homeobox transcription factor Msx1, which are expressed in the choroid plexus. Neither gene was transcribed in Dmrt5 mutants. The homeobox gene Lmx1a, which is expressed in both the choroid plexus and CH, was also absent (Fig. 6A–C). EphB1, an early marker of the hippocampal primordium, KA1, a marker of the hippocampal CA3 field, and Prox1, a marker of the dentate gyrus, were also undetectable in mutants (Fig. 6D–F). Strikingly, a site of EphB1 expression in the lateral part of the cortex in WT animals was found more dorsally in the cortex of mutants, suggesting possible additional defects in the lateral telencephalon. We therefore investigated the expression of additional markers expressed regionally along the mediolateral axis of the dorsal telencephalon in Dmrt5−/− mice. Scip, which is expressed in the lateral neocortex, was detected, although reduced, throughout the cortex in the mutants (Fig. 6G). The neurogenic gene Ngn2, whose expression is activated initially in the lateral cortical field, was upregulated within the medial cortical wall (Fig. 6H). The antineural Id3 gene, which is confined to the medial pallium, and the dorsomedial subdomain of expression of the Notch mediator Hes5 (Muzio et al. 2005) were in contrast downregulated (Fig. 6I,J). ER81, expressed in a high-ventral to low-dorsal gradient in the cortical plate, Dbx1, expressed in the anti-hem, and Nrp2, expressed in the paleocortex, were all detected more medially in the lateral wall of the telencephalon (Fig. 6K–M). Ctip2 expression in layer II of the piriform cortex and olfactory tubercles appeared shifted medially, whereas expression in layer V of the neocortex extended less ventrally (Fig. 6N).
Figure 6.
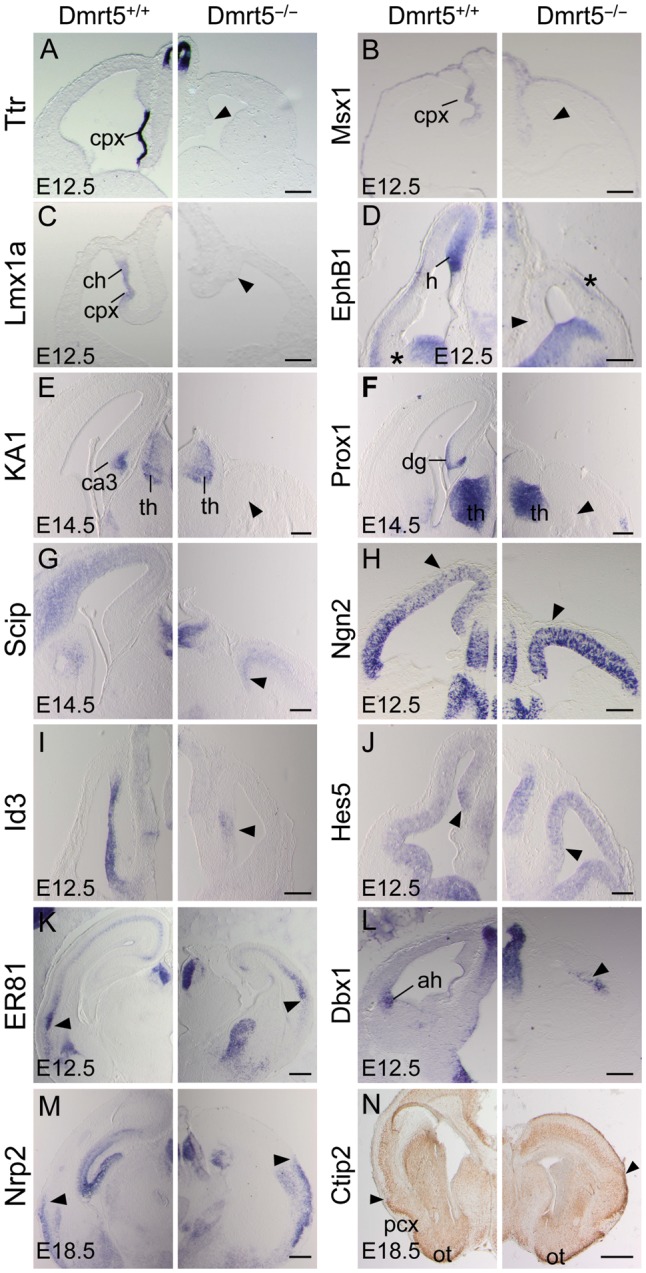
The Absence of choroid plexus and hippocampal markers and altered pattern of mediolateral regional markers in the dorsal telencephalon of Dmrt5−/− embryos. (A–M) Coronal forebrain sections processed by ISH for the indicated markers at the indicated stages. (A–J) While Ttr, Msx1, Lmx1a, EphB1, KA1, Prox1, Scip, Id3, and Hes5 medial markers were strongly reduced in the mutants (arrowheads), Ngn2 expression spreads from the lateral part of the cortex toward the dorsomedial cortex (arrowheads in H). (K–M) ER81, Dbx1 and Nrp2 as well as a site of EphB1 expression in the lateral part of the cortex (asterisk in D) appeared all shifted medially in the lateral cortical wall of mutant mice (arrowheads). (N) Olfactory cortex specific Ctip2 expression appears shifted medially while neocortical-specific expression extends less ventrally in the mutant. The limit between the neocortex and piriform cortex is indicated (arrowheads). ah, anti-hem; ch, cortical hem; cpx, choroid plexus; dg, dentate gyrus; h, hippocampal primordium; ot, olfactory tubercles; pcx, piriform cortex; th, thalamus. Scale bars: 500 μm in A–M; 1 mm in L.
To determine whether the different cortical cell types are specified in the Dmrt5 mutant, we first analyzed by ISH the expression of a panel of 6 layer-specific neocortical markers (Lhx2, Cux2, Satb2, RORβ, ER81, and Tbr1). In the mutants, expression of these markers appeared in a pial to ventricular order similar to that in wild types. The ventral border of their expression as well as that of the pan-neuronal marker Math2 was shifted medially, providing further evidence for mediolateral patterning defects. Their expression was in some cases more diffuse than in controls (see Supplementary Fig. 10A and B). Double immunofluorescence for Cux1 (layers II–IV) together with Ctip2 (layers V and VI) or for Satb2 (layers II–IV) and Tbr1 (layer VI and subplate) confirm the expression of markers of all layers and their more diffused distribution in the cortex of Dmrt5 mutants (see Supplementary Fig. 10C).
To determine the effects of Dmrt5 ablation on dorsoventral patterning of the telencephalon, we examined the expression of Mash1 and Dlx2, 2 markers of the ventral telencephalon. Those markers were unchanged, indicating that the telencephalon does not become ventralized in the mutants (see Supplementary Fig. 10D). Thus, genetic evidence confirms our histological observations, indicating that Dmrt5 ablation leads to dramatic cortical patterning defects, including agenesis of the hippocampus and choroid plexus, and a disorganization and reduction of the cortical plate.
Reduction of Caudal Neocortical Regions in Dmrt5 Mutants
We next investigated on sagittal sections of E18.5 control and mutant embryos the expression of genes with restricted or graded expression patterns across the embryonic neocortex that relate to its future organization into areas (EphrinA5, EphA7, Cad8, RZRβ, Auts2, and p75). EphrinA5 is expressed predominantly in multiple layers of the prospective somatosensory area located in between the frontal motor and caudal visual areas. In mutants, we found that EphrinA5 is expressed at a lower level and is restricted to the most caudal part of the cortex (Fig. 7A). EphA7 and Cad8 high expression domain mark the region of the motor cortex. In mutants, their domain of high expression appeared to occupy most of the reduced cortex. The domain of low EphA7 expression centered on the future somatosensory area was reduced and shifted caudally (Fig. 7B,C). RZRβ, Auts2, and p75 normally exhibit strongly graded patterns of expression across the neocortex. RZRβ and Auts2 are broadly expressed in the cortical plate in a high-rostral to low-caudal gradient. The low-affinity nerve growth factor receptor p75 is expressed in the caudal half of the cortical plate. In Dmrt5 mutants, while RZRβ and Auts2 were still detectable and appeared to expand to almost the caudal end of the cortex, p75 was severely reduced and confined to the caudal-most cortex (Fig. 7D–F). Therefore, a dramatic reduction of caudal neocortical areas occurs in Dmrt5−/− mice.
Figure 7.
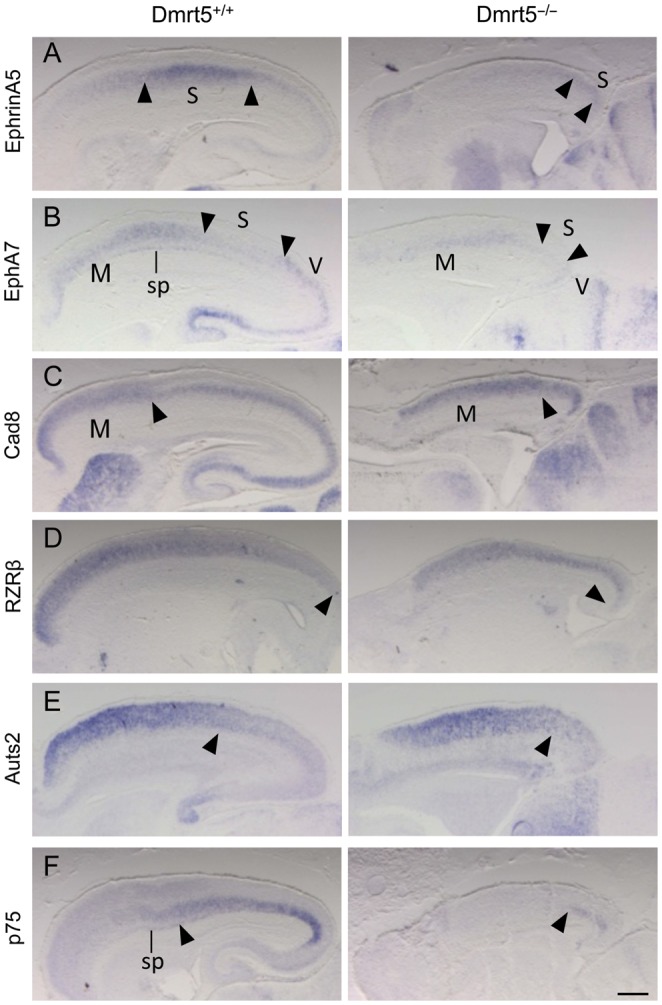
Reduction of caudomedial neocortical areas in Dmrt5 mutants. Sagittal brain sections were analyzed by ISH for the indicated genes. (A–C) EphrinA5 domain of high expression and EphA7 domain of low expression that demarcate the somatosensory areas (delimitated by arrowheads) is contracted and detected in the most caudal part of the cortex in Dmrt5−/− embryos. EphA7 and Cad8 high expression that mark the rostrally located motor area (with caudal limits indicated by arrowheads) occupies most of the reduced cortex in mutants. (D–F) RZRβ and Auts2 are expanded and p75 constricted caudally in mutants. Arrowheads mark the approximate caudal and rostral limit of their expression, respectively. Note the reduced EphA7 and p75 in the subplate. M, motor; S, somatosensory; sp, subplate; V, visual areas. Scale bars: 500 μm.
Activation of Wnt/β-Catenin Signaling Induces Dmrt5 Expression
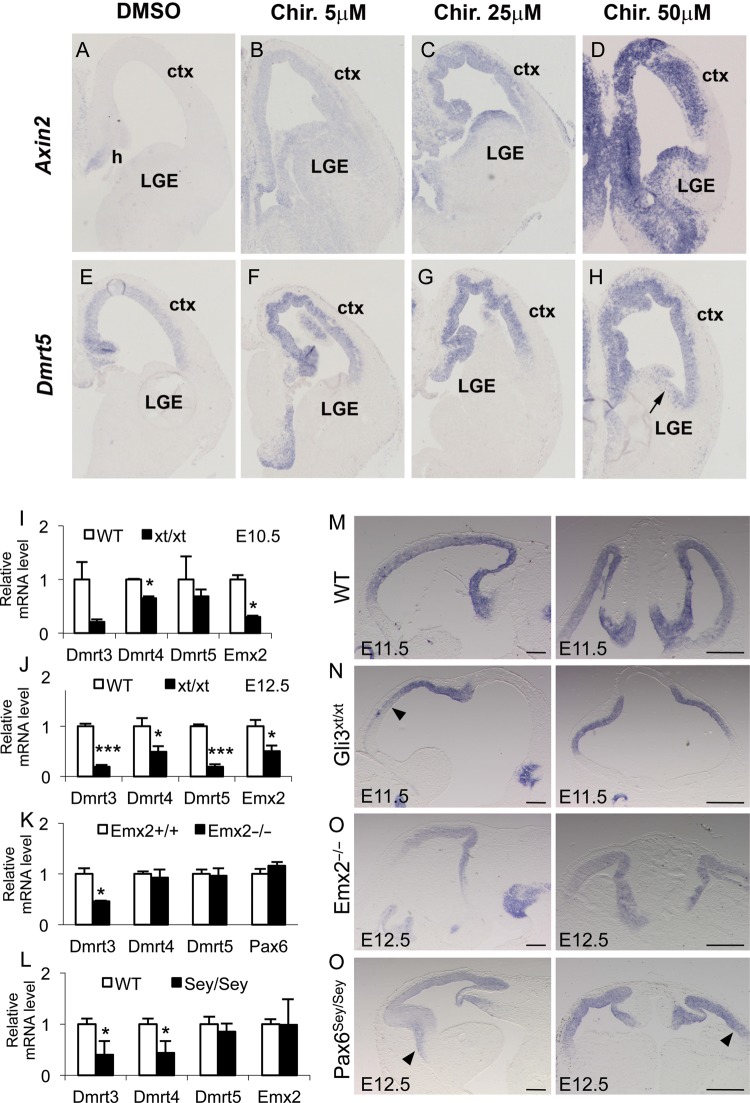
The high-caudomedial to low-rostrolateral Dmrt5 gradient in the dorsal telencephalon suggests that Dmrt5 might be regulated by midline signals. To determine whether it is regulated by Wnt signaling, we analyzed the consequences of ectopic activation of this pathway on Dmrt5 expression using organotypic slice cultures of E13.5 mouse telencephalon. Slices were cultured for 24 h in the presence of DMSO or various concentration of Chir, which selectively inhibits GSK3β and therefore activates Wnt signaling, and processed for ISH for Dmrt5 and Axin2 as a positive control. As previously reported, Axin2 was induced by Chir and at the highest dose was expressed through the whole telencephalic ventricular zone (Hasenpusch-Theil et al. 2012) (Fig. 8A–D). Dmrt5 expression was also induced at the lowest Chir concentration, and at the highest concentration, ectopic expression was detected in the ventricular zone of the lateral ganglionic eminence (Fig. 8E–H). These results indicate that Dmrt5 expression is controlled by Wnt signaling, suggesting the existence of a positive feedback loop between Wnt and Dmrt5.
Figure 8.
Dmrt5 expression in the cortex is upregulated by activation of Wnt/β-catenin, downregulated in Gli3xt/xt embryos, and unchanged in Emx2−/− a Pax6sey/sey embryo. (A–H) Dmrt5 expression in Chir-treated and control ex vivo cortical explants. Note the activation of Dmrt5 throughout the telencephalic ventricular zone similar to that observed for Axin2 used as a positive control after addition of Chir in a concentration-dependent manner. Note also the Dmrt5 ectopic expression detected at high dose in the ventricular zone of the lateral ganglionic eminence (arrow). (I–P) Dmrt5 expression in Gli3xt/xt, Emx2−/−, and Pax6sey/sey embryos. (I–L) Quantitative RT–qPCR analysis of Dmrt3, 4, and 5 in the cortex of WT and Gli3xt/xt at E10.5 (I) and E12.5 (J) and in Emx2−/− (K) and Pax6sey/sey (L) at E12.5. Results are normalized to the level of expression in the WT forebrain. Error bars show SD of 3 independent experiments (*P< 0.05 and ***P< 0.001). (M–P) Sagittal (left panels) and coronal (right panels) brain sections of WT (M), Gli3xt/xt (N), Emx2−/− (O), and Pax6sey/sey (P) embryos, processed by ISH for Dmrt5 at the indicated stage. Note in (N) the reduction anteriorly and in (P) the slight extension on Dmrt5 anteriorly and laterally (arrowheads). Ctx, cortex; h, hem; LGE, lateral ganglionic eminences. Scale bars in (M–P): 200 μm, left panels; 500 μm, rights panels.
Dmrt3–5 Expression in Gli3xt/xt, Emx2−/−, and Pax6sey/sey Mutants
Little is known about the genetic hierarchy underlying Wnt-regulated hippocampal development. Wnt gene expression is severely affected in Gli3xt/xt mutants (Grove et al. 1998; Theil et al. 1999) and is known to regulate the expression of the homeobox Emx2 gene (Theil et al. 2002; Suda et al. 2010). To determine whether Gli3 and Emx2 influence Dmrt5 expression, we investigated its expression in Gli3xt/xt and Emx2−/− mutants. In addition, we examined Dmrt5 expression in Pax6sey/sey mutants as Emx2 and Pax6 interact with each other to control regionalization of the cortical primordium (Muzio et al. 2002). In all 3 mutants, we used ISH on sections of E11.5–E12.5 embryos and RT–qPCR analysis of RNA extracted from dissected E10.5–E12.5 cortices to analyze Dmrt5 expression. In our RT–qPCR analysis, we also examined the expression of the closely related Dmrt3 and Dmrt4 genes that are coexpressed with Dmrt5 in the developing forebrain (Smith et al. 2002; Balciuniene et al. 2006). In Gli3xt/xt mutants, Dmrt3 and Dmrt4 but not Dmrt5 were downregulated at E10.5. Later at E12.5, all 3 genes were downregulated in Gli3xt/xt mutants. Emx2, used as control, was reduced as reported previously (Theil et al. 1999) (Fig. 8I,J). In Emx2−/− mutants, Dmrt3 was reduced while Dmrt5 and Dmrt4 and Pax6 used as controls (Muzio et al. 2002) were unchanged (Fig. 8K). In Pax6sey/sey mutants, Dmrt3 and Dmrt4 were reduced but Dmrt5 and Emx2 used as controls (Muzio et al. 2002) were not significantly changed (Fig. 8L). ISH revealed a graded Dmrt5 expression in Gli3xt/xt and Emx2−/− mutants similar to that in normal embryos but a downregulation in the rostral telencephalon of Gli3xt/xt mutants (Fig. 8M–O). In Pax6sey/sey mutants, the Dmrt5 graded expression appeared to spread slightly more anteriorly and laterally, thus flattening its gradient (Fig. 8P). These data indicate that Gli3 but not Emx2 is required for normal Dmrt5 expression in the cortex and that Pax6 may antagonize its expression. They also reveal that Dmrt3–5, although coexpressed in the developing forebrain, are differentially regulated by Gli3, Emx2, and Pax6.
Discussion
Regionalization of the cerebral cortex is initiated by morphogens secreted from patterning centers located at the perimeter of the dorsal telencephalon that establish within cortical progenitors differential expression of transcription factors that determine their area identity. In this study, we show that the transcription factor Dmrt5 is expressed in cortical apical progenitors in a high-caudomedial to low-rostrolateral gradient. We generated a Dmrt5 KO mouse and found that it exhibits significant brain abnormalities, including a severe reduction of the caudomedial cortex, a loss of CR cells, a decrease in Wnt and Bmp signaling in the embryonic dorsomedial telencephalon, and an alteration of the downstream transcription factors, including Emx2, Lhx2, and Pax6. Finally, we observed that Dmrt5 expression is activated by Wnt/β-catenin signaling and downregulated in Gli3 mutants. These findings establish Dmrt5 as a novel Wnt-dependent key transcriptional regulator of early cortical development.
Roles for Dmrt5 in the Gene Network Regulating Early Caudomedial Cortical Development
The earliest and most severe defect observed in Dmrt5 mutants is the reduction of the cortical surface, detectable as early as E10.5, associated with the loss of the caudomedial cortex, including the CH expressing a number of signals essential for cortical midline structure formation. Such a phenotype is reminiscent of that observed in roof plate ablated embryos (Currle et al. 2005; Cheng et al. 2006). As in Dmrt5 mutants, midline genes such as Wnt2b, Wnt3a, Bmp4, and Lmx1a and the graded cortical transcription factors Lhx2 and Emx2 are also reduced in those roof plate ablated mutants. Pax6, Foxg1, and Ngn2 are however relatively unaffected in those mutants, which contrast with their upregulation seen in Dmrt5 mutants. A similar shrinkage and disorganization of the caudomedial cortex has also been reported in hem-ablated embryos (Yoshida et al. 2006). These similarities highlight the importance of Wnt/Bmp expression at the dorsal midline for telencephalon development and strengthen arguments for the primacy of their defective expression in the Dmrt5 null phenotype.
In the dorsal telencephalon, both Emx2 and Emx1 have graded expression similar to that of Dmrt5. In the double Emx1;Emx2 mutants, but not in the single Emx1 or Emx2 mutants, the dorsomedial telencephalon fails to develop, indicating that the two genes cooperate in its formation (Shinozaki et al. 2002, 2004; Bishop et al. 2003). The similarity of the Dmrt5 and Emx1;Emx2 phenotypes and the reduction of Emx1 and Emx2 expression in the Dmrt5 mutant telencephalon (Fig. 5; data not shown) suggest that some of the telencephalic defects observed in the Dmrt5 mutants may be attributable to the deficiency of Emx gene expression. The Dmrt5 phenotype is also very similar to that observed in Gli3 mutants. Indeed, in Gli3 mutants, the dorsal telencephalon is abnormally small and fails to develop dorsomedial structures, including the hippocampus and CH. As in Dmrt5−/− embryos, loss of Gli3 results in a reduction and/or loss of Wnt/Bmp expression in the dorsomedial telencephalon (Grove et al. 1998; Theil et al. 1999; Tole et al. 2000; Fotaki et al. 2011). Gli3 mutants however also show dorsoventral patterning defects and an expansion of Fgf8 (Theil et al. 1999; Tole et al. 2000; Aoto et al. 2002; Kuschel et al. 2003), defects not detected in Dmrt5 mutants.
While Dmrt5 inactivation does not affect the global dorsoventral patterning of the telencephalon nor apoptosis, the size and decreased thickness of the cortical hemispheres, the disorganization of the cortical ventricular zone, and the frequency of PH3 positive cells are altered in Dmrt5 mutants. These results suggest that the Dmrt5 phenotype cannot be purely due to misspecification and that it might have a role in corticogenesis. As apical progenitors did not decrease in number at E11.5, it is unclear whether the transient increase in Tbr2-positive cells observed at that stage in Dmrt5−/− mice is due to apical progenitors exiting prematurely from the cell cycle and differentiating into IPCs. The decrease of Wnt signals known to control initiation of neurogenesis in the cortex (Machon et al. 2007), the reduction of Emx2 that is required for the proliferation of cortical progenitors (Heins et al. 2001; Muzio et al. 2005), the repression of the antineurogenic Id3 and Hes5 genes, and the upregulation of Pax6 and of its downstream target, the proneural gene Ngn2 (Scardigli et al. 2003), observed in the mutants support however this premature differentiation hypothesis. Additional studies will be required to provide further evidence for those changes in proliferation and determine rigorously the cell cycle kinetics in these mutants.
Interactions between secreted ligands and transcription factors controlling cortical regionalization are very complex. Emx2 promotes Wnt signaling, which in turn promotes its expression (Theil et al. 2002; Muzio et al. 2005). In this way, a robust positive feedback loop is established in the caudomedial cortex contributing to reinforcing Emx2 expression (Mallamaci 2011). Gli3 controls Wnt gene expression which in turn activates its expression and induces several Wnt targets, including direct targets such as Emx2 and Dmrt3 (Theil et al. 1999, 2002; Fotaki et al. 2011; Hasenpusch-Theil et al. 2012). Our data show that Dmrt5 which is required for Wnt gene expression in the midline is regulated by Wnt and that Gli3 is required at least for the maintenance of Dmrt5 in the forebrain. In contrast, the absence of Emx2 does not affect its expression. These data, together with the observation that Gli3 is unaltered in Dmrt5 mutants, suggest that Dmrt5 may act downstream of Gli3 and upstream of Emx2 genes in the Wnt/Bmp-dependent genetic cascade controlling development of the dorsomedial telencephalon (Fig. 9). Whether as in the case of Emx2 (Theil et al. 2002), Bmp signaling plays a role in the regulation of Dmrt5 is unknown. Whether Gli3 regulates directly Dmrt5 expression or whether its loss in Gli3 mutants is indirect, due to a blocking of Wnt signaling, as recently shown for Dmrt3 (Hasenpusch-Theil et al. 2012), remains also to be investigated. Whether Dmrt5 regulates directly or indirectly Wnt and Emx genes requires also further investigations.
Figure 9.
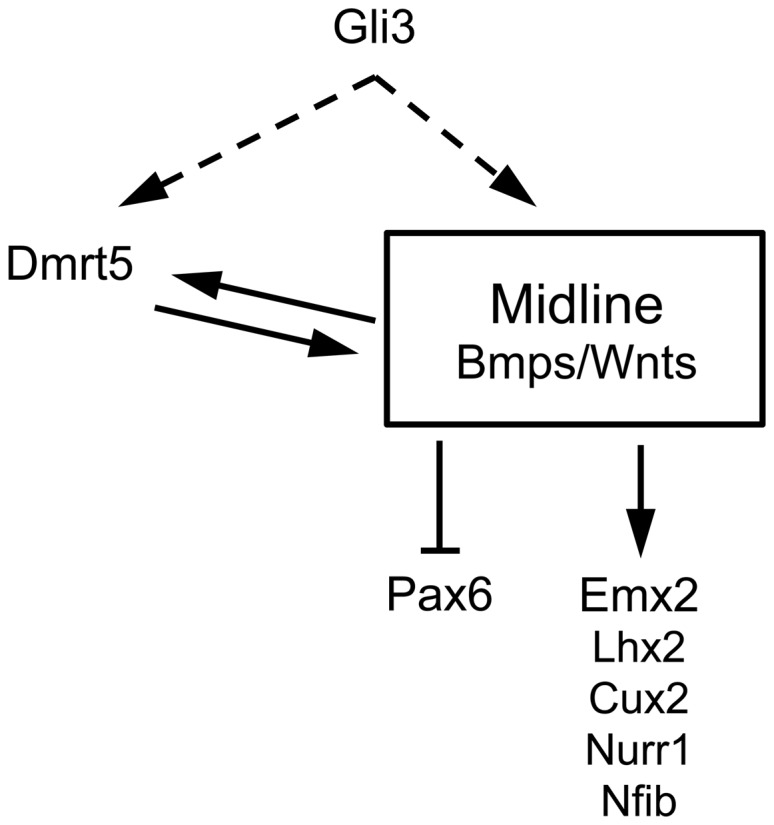
Summary of the interactions identified in this work between Dmrt5 and the secreted ligands and Wnt/Bmp regulated graded transcription factors expressed during early patterning of the cerebral cortex. Wnt and Gli3 control Dmrt5 expression. Direct or indirect action of Gli3 on Dmrt5, through regulation of the Wnt signaling pathway, is indicated by dashed lines. Dmrt5 in turn is required for Wnt and Bmp expression in the dorsal midline signaling center. In the absence of Dmrt5, this feedback loop is not maintained, which is likely responsible for the downregulation of Wnt target genes. Pax6 is upregulated in Dmrt5 mutants, presumably through its negative regulation by Wnts and Emx2.
We found that Dmrt3, like Dmrt5, is reduced in Gli3 mutants. In contrast to Dmrt5, Dmrt3 is also decreased in Pax6 and Emx2 mutants, suggesting that it may function downstream of the 3 factors. Dmrt4, like Dmrt5, is reduced in Gli3 mutants and unaltered in Emx2 mutants. In contrast to Dmrt5, Dmrt4 is downregulated in Pax6 mutants, suggesting a possible function downstream of this factor.
Reduction of CR and Subplate Cells in Dmrt5 Mutants
CR cell formation is dramatically impaired in Dmrt5 mutants, as previously reported in Emx2 mutants (Mallamaci et al. 2000; Shinozaki et al. 2002). This deficit of CR cells is probably a consequence of the reduction of the hem, which is a major source of CR cells (Yoshida et al. 2006). Hem-derived CR cells have been shown to predominantly populate the caudomedial and dorsolateral pallium, while CR cells found in the rostromedial and lateral pallium are derived from the septum and anti-hem, respectively (Bielle et al. 2005). It is thus likely that the remaining CR cells found mainly in the medial part of the cortex of Dmrt5 mutants derive from the rostromedial source. The few remaining cells found in the lateral neocortex may be derived from the anti-hem. Their abnormal clustering may be due to altered intrinsic properties, or alternatively, to non-cell autonomous modifications in the caudal telencephalon related to the loss of CH and choroid plexus territories. Aberrant clustering of CR cells has been previously reported in the Gli3 compound mutant Gli3Xt/Pdn (Friedrichs et al. 2008) and in embryos carrying mutant alleles of other genes involved in CR cells development (Miquelajauregui et al. 2010). As migrating CR neurons have been shown to express a repertoire of signaling factors and to signal to VZ progenitors (Borello and Pierani 2010), their absence likely contribute to the early regionalization defects observed in Dmrt5 mutants. We showed that from E13.5, Dmrt5 is expressed in CR cells. Whether it plays a role in the maintenance CR cells of the marginal zone requires further investigation.
Our analysis indicates that the subplate is reduced in the cortex of Dmrt5 mutants at E15.5 and E18.5 similar to Emx2 and Gli3 mutants (Mallamaci et al. 2000; Shinozaki et al. 2002; Friedrichs et al. 2008). The fact that this early neuronal population is affected in the mutant, despite the absence of Dmrt5 expression in subplate cells and main restriction of Dmrt5 expression in the VZ, further suggests a role for Dmrt5 in the control of the timing of the exit of apical progenitors from the cell cycle or the differentiation-survival program of their descendants.
Dmrt5 is Required for Cortical Regionalization
In addition to these early midline defects, our histological and molecular analyses at E18.5 have revealed that the caudal neocortex is dramatically reduced in Dmrt5 mutants. Although rostral regions are expanded in proportional sizes, their absolute sizes appeared rather similar in mutants and wild types. The contraction of the caudal neocortex of Dmrt5 mutants may be caused in part by its decreased growth. It is also possible to be due to alteration of positional identity of cortical progenitors. Those changes may be the consequence of the reduction of Emx2 which promotes caudomedial fates, as small changes in its expression have substantial effects on arealization (Hamasaki et al. 2004). Emx2 has been show to pattern the neocortex by regulating Fgf positional signaling (Fukuchi-Shimogori and Grove 2003; Cholfin and Rubenstein 2008). However, a direct cell-intrinsic mechanism has also been implicated (Hamasaki et al. 2004). As Fgf8 and Fgf17 appear not dramatically affected in the mutants, alteration of these intrinsic Emx2-dependent patterning mechanisms is likely to be responsible for the mutant phenotype. COUP-TF1 is another transcription factor that plays an essential direct role in cortical arealization by regulating Emx2 and Pax6 expression without apparently altering Fgf8 expression (Armentano et al. 2007). The contribution of the upregulation of the rostral determinant Pax6 to the Dmrt5 phenotype is also unclear, as its overexpression has no substantial effect on area patterning (Manuel et al. 2007). We also found that the loss of Dmrt5 has substantial effects on cortical mediolateral patterning. In its absence, choroid plexus, CH, and hippocampal markers are switched off, which is consistent with the observation that Foxg1, which restricts the CH to the dorsomedial-most telencephalon (Muzio and Mallamaci 2005b), is expressed in the entire cortex. Neocortical markers extend less laterally and paleocortical markers are detected slightly more medially in the reduced cortex of the Dmrt5−/− embryos. This phenotype fits with our observation that Lhx2 becomes downregulated in already specified cortical progenitors (at E10.5) in Dmrt5−/− embryos, during the critical period of time where it controls regional fate within telencephalic progenitors (Chou et al. 2009).
Mild Cortical Lamination Defects and Severe Forebrain Axonal Connection Abnormalities in Dmrt5 Mutants
The absence of Reelin expression in the neocortical marginal zone of Dmrt5−/− mice suggested that radial neuronal migration could be perturbed in these mice. We therefore investigated laminar ordering in E18.5 Dmrt5 mutants. Despite the massive loss of CR cells, we found that neocortical layers are ordered correctly, as previously observed in hem-ablated embryos (Yoshida et al. 2006). As in hem-ablated embryos, expression of layer-specific neuronal markers showed however a variable degree of diffuseness in Dmrt5 mutants relative to wild types. As in Emx2 and in Gli3Xt/Pdn mutants, βIII-tub positive neurons were also present in the cortical VZ/SVZ of the Dmrt5 mutants (data not shown) which may correspond to neuronal cells coming out of the cell cycle and migrating, slower than normal, from the VZ toward their final radial location (Mallamaci et al. 2000). In addition to potential migration abnormalities, post-migratory mechanisms involved in laminar consolidation could also explain these mild defects.
Dmrt5 mutants have major defects in the pathfinding of most cortical axons. Similar defects have been observed in Emx1;Emx2 double mutant mice and in the Gli3 hypomorphic mutant Polydactyly Nagoya (Pdn) (Shinozaki et al. 2002; Bishop et al. 2003; Magnani et al. 2010). The cortex appears to fail to send axons to the striatum. TCAs fail to enter the cortex and a large proportion of them are stopped at the border region between the diencephalon and telencephalon. The reason why cortical efferent axons are affected is not clear. Their inability to pathfind may be a cell autonomous defect or it may be secondary to a loss of instructive cues within the cortex. Because Dmrt5 is not expressed in the region of the thalamus that gives rise to the TCA projection neurons, it is likely that the TCA defects are non-cell autonomous to them and that these defects are secondary to the cortical alterations. Pioneer neurons from the subplate are well known to play an important guidance role in thalamocortical projections (Allendoerfer and Shatz 1994; Zhou et al. 1999; Kanold and Luhmann 2010). Their absence in Dmrt5 mutants is thus likely to play a role in the observed thalamocortical tract defects. As Dmrt5 is also expressed in the prethalamus and hypothalamus, in the eminentia thalami, and in a narrow strip of the medial ventral telencephalon (see Supplementary Fig. 1; data not shown), additional studies are also required to determine whether Dmrt5 in these regions influence the trajectory of TCA axons.
All major telencephalic commissures (corpus callosum, hippocampal commissure, and anterior commissure) are hypoplastic in Dmrt5−/− embryos. The origin of the commissure defects observed in Dmrt5−/− embryos, also found in Emx2 and Gli3 mutants (Naruse et al. 1990; Yoshida et al. 1997), is unknown. Disturbance of commissure formation is likely to result from intrinsic defects in cortical neurons or from failure of the environment to produce an extracellular environment that is suitable for navigation (Lindwall et al. 2007). Midline structures that are required for corpus callosum formation, glial wedge, induseum griseum neurons, and glia are most likely severely perturbed in the Dmrt5−/− embryos given the fact that the whole rostral midline structures are mostly absent. As their formation is dependent on appropriate Bmp and Wnt signaling levels (Wang et al. 2006; Sánchez-Camacho et al. 2011), we speculate that the absence of the corpus callosum in the mutant is a secondary consequence of the reduction of Bmp and Wnt from midline structures and the downregulation of transcription targets of midline signals such as Nfib known to be required for the development of midline glial populations (Piper et al. 2009). Proper patterning of the cortical septal boundary and hence accurate distribution of guidepost neurons at later stages has been demonstrated recently to be crucial for callosal development in Rfx3 mutants (Benadiba et al. 2012).
Recent studies using embryonic stem cells have shown that Dmrt5 is an important regulator of ventral mesencephalic neural fate specification (Gennet et al. 2011). Our analysis of the defects of Dmrt5−/− mice indicate that it also plays a crucial role in caudomedial cerebral cortex development, likely via the control of the production of midline signaling molecules during early forebrain development. In the future, it will be interesting to determine whether Dmrt5 also has a role in later forebrain development and whether Dmrt3 and Dmrt4 have redundant function with Dmrt5 during hippocampus formation.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This work was supported by grants from the Belgian Fonds de la Recherche Scientifique (FRFC 3.4635.06 to E.J.B.), the Belgian Queen Elisabeth Medical Foundation (to E.J.B.), the Fédération Wallonie-Bruxelles (Action de Recherche Concertée, to E.J.B.), the Interuniversity Attraction Poles Programme, Belgian State, Federal Office for Scientific, Technical and Cultural Affairs (IUAP-P5/35 to E.J.B.), the Walloon Region Excellence Programme (“CIBLES” to E.J.B.), the Medical Research Council (G0801359 to T.T.), the US National Institute of health (GM059152 to D.Z.). C.K.M. is a postdoctoral fellow from the US National Science Foundation. M.K., V.M., and D.P. are doctoral fellows from the Belgian Fonds de la Recherche Scientifique (Fonds pour la formation à la Recherche dans l'Industrie et dans l'Agriculture).
Supplementary Material
Notes
We thank A. Goffinet, L. Nguyen, and P. Vanderhaeghen for helpful discussions. We are grateful to Drs J. Boulter, A. Chedotal, H. Cremers, M. Dewerchin, P. Gruss, Y. Furuta, A. Goffinet, F. Guillemot, R. Hevner, R. Kageyama, G. Lemke, A. Mansouri, K. Millen, E. Monuki, D. O'Leary, A. Pierani, C. Ragsdale, J. Rubenstein, V. Tarabykin, P. Vanderhaeghen, and H. Westphal for generous sharing of antibodies and/or cDNAs, U. Rüther for Gli3 cDNA and Gli3xt/xt embryos and L. Delhaye, S. Ghogomu, and A. Wery for experimental help. Conflict of Interest: None declared.
References
- Allendoerfer KL, Shatz CJ. The subplate, a transient neocortical structure: Its role in the development of connections between thalamus and cortex. Annu Rev Neurosci. 1994;17:185–218. doi: 10.1146/annurev.ne.17.030194.001153. [DOI] [PubMed] [Google Scholar]
- Aoto K, Nishimura T, Eto K, Motoyama J. Mouse GLI3 regulates Fgf8 expression and apoptosis in the developing neural tube, face, and limb bud. Dev Biol. 2002;251:320–332. doi: 10.1006/dbio.2002.0811. [DOI] [PubMed] [Google Scholar]
- Armentano M, Chou SJ, Tomassy GS, Leingärtner A, O'Leary DD, Studer M. COUP-TF1 regulates the balance of cortical patterning between frontal/motor and sensory areas. Nat Neurosci. 2007;10:1277–1286. doi: 10.1038/nn1958. [DOI] [PubMed] [Google Scholar]
- Balciuniene J, Bardwell VJ, Zarkower D. Mice mutant in the DM domain gene Dmrt4 are viable and fertile but have polyovular follicles. Mol Cell Biol. 2006;26:8984–8991. doi: 10.1128/MCB.00959-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedogni F, Hodge RD, Elsen GE, Nelson BR, Daza RA, Beyer RP, Bammler TK, Rubenstein JL, Hevner RF. Tbr1 regulates regional and laminar identity of postmitotic neurons in developing neocortex. Proc Natl Acad Sci USA. 2010;107:13129–13134. doi: 10.1073/pnas.1002285107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benadiba C, Magnani D, Niquille M, Morlé L, Valloton D, Nawabi H, Ait-Lounis A, Otsmane B, Reith W, Theil T, et al. The ciliogenic transcription factor RFX3 regulates early midline distribution of guidepost neurons required for corpus callosum development. PLoS Genet. 2012;8:e1002606. doi: 10.1371/journal.pgen.1002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielle F, Griveau A, Narboux-Nême N, Vigneau S, Sigrist M, Arber S, Wassef M, Pierani A. Multiple origins of Cajal-Retzius cells at the borders of the developing pallium. Nat Neurosci. 2005;8:1002–1012. doi: 10.1038/nn1511. [DOI] [PubMed] [Google Scholar]
- Bishop KM, Garel S, Nakagawa Y, Rubenstein JL, O'Leary DD. Emx1 and Emx2 cooperate to regulate cortical size, lamination, neuronal differentiation, development of cortical efferents, and thalamocortical pathfinding. J Comp Neurol. 2003;457:345–360. doi: 10.1002/cne.10549. [DOI] [PubMed] [Google Scholar]
- Bishop KM, Goudreau G, O'Leary DD. Regulation of area identity in the mammalian neocortex by Emx2 and Pax6. Science. 2000;288:344–349. doi: 10.1126/science.288.5464.344. [DOI] [PubMed] [Google Scholar]
- Bishop KM, Rubenstein JL, O'Leary DD. Distinct actions of Emx1, Emx2, and Pax6 in regulating the specification of areas in the developing neocortex. J Neurosci. 2002;22:7627–7638. doi: 10.1523/JNEUROSCI.22-17-07627.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluske KK, Kawakami Y, Koyano-Nakagawa N, Nakagawa Y. Differential activity of Wnt/b-catenin signaling in the embryonic mouse thalamus. Dev Dyn. 2009;238:3297–3309. doi: 10.1002/dvdy.22167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borello U, Pierani A. Patterning the cerebral cortex: Traveling with morphogens. Curr Opin Genet Dev. 2010;20:408–415. doi: 10.1016/j.gde.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Bulchand S, Grove EA, Porter FD, Tole S. LIM-homeodomain gene Lhx2 regulates the formation of the cortical hem. Mech Dev. 2001;100:165–175. doi: 10.1016/s0925-4773(00)00515-3. [DOI] [PubMed] [Google Scholar]
- Causeret F, Ensini M, Teissier A, Kessaris N, Richardson WD, de Couville T Lucas, Pierani A. Dbx1-expressing cells are necessary for the survival of the mammalian anterior neural and craniofacial structures. PloS One. 2011;6:e19367. doi: 10.1371/journal.pone.0019367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Hsu CM, Currle DS, Hu JS, Barkovich AJ, Monuki ES. Central roles of the roof plate in telencephalic development and holoprosencephaly. J Neurosci. 2006;26:7640–7649. doi: 10.1523/JNEUROSCI.0714-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholfin JA, Rubenstein JL. Frontal cortex subdivision patterning is coordinately regulated by Fgf8, Fgf17 and Emx2. J Comp Neurol. 2008;509:144–155. doi: 10.1002/cne.21709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou SJ, Perez-Garcia CG, Kroll TT, O'Leary DD. Lhx2 specifies regional fate in Emx1 lineage of telencephalic progenitors generating cerebral cortex. Nat Neurosci. 2009;12:1381–1389. doi: 10.1038/nn.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currle DS, Cheng X, Hsu CM, Monuki ES. Direct and indirect roles of CNS dorsal midline cells in choroid plexus epithelia formation. Development. 2005;132:3549–3559. doi: 10.1242/dev.01915. [DOI] [PubMed] [Google Scholar]
- Fernandes M, Gutin G, Alcorn H, McConnell S, Hébert JM. Mutations in the BMP pathway in mice support the existence of two molecular classes of holoprosencephaly. Development. 2007;134:3789–3794. doi: 10.1242/dev.004325. [DOI] [PubMed] [Google Scholar]
- Fotaki V, Price DJ, Mason JO. Wnt/β-catenin is disrupted in the extra-toes (Gli3xt/xt) mutant from early stages of forebrain development, concomitant with anterior neural plate patterning defects. J Comp Neurol. 2011;519:1640–1657. doi: 10.1002/cne.22592. [DOI] [PubMed] [Google Scholar]
- Fotaki V, Yu T, Zaki PA, Mason JO, Price DJ. Abnormal positioning of diencephalic cell types in neocortical tissue in the dorsal telencephalon of mice lacking functional Gli3. J Neurosci. 2006;26:9282–9292. doi: 10.1523/JNEUROSCI.2673-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrichs M, Larralde O, Skutella T, Theil T. Lamination of the cerebral cortex is disturbed in Gli3 mutant mice. Dev Biol. 2008;318:203–214. doi: 10.1016/j.ydbio.2008.03.032. [DOI] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Grove EA. Emx2 patterns the neocortex by regulating FGF positional signaling. Nat Neurosci. 2003;6:825–831. doi: 10.1038/nn1093. [DOI] [PubMed] [Google Scholar]
- Furuta Y, Piston DW, Hogan LM. Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development. 1997;124:2203–2212. doi: 10.1242/dev.124.11.2203. [DOI] [PubMed] [Google Scholar]
- Gennet N, Gale E, Nan X, Farley E, Takacs K, Oberwallner B, Chambers D, Li M. Doublesex and Mab-3-related transcription factor 5 promotes midbrain dopaminergic identity in pluripotent stem cells by enforcing a ventral-medial progenitor fate. Proc Natl Acad Sci USA. 2011;108:9131–9136. doi: 10.1073/pnas.1016679108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove EA, Tole S, Limon J, Yip L, Ragsdale CW. The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development. 1998;125:2315–2325. doi: 10.1242/dev.125.12.2315. [DOI] [PubMed] [Google Scholar]
- Hamasaki T, Leingärtner A, Ringstedt T, O'Leary DD. EMX2 regulates sizes and positioning of the primary and motor areas in neocortex by direct specification of cortical progenitors. Neuron. 2004;43:359–372. doi: 10.1016/j.neuron.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Hasenpusch-Theil K, Magnani D, Amaniti EM, Han L, Amstrong D, Theil T. Transcriptional analysis of Gli3 mutants identifies Wnt target genes in the developing hippocampus. Cereb Cortex. 2012 doi: 10.1093/cercor/bhr365. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauptmann G, Gerster T. Two-color whole-mount in situ hybridization to vertebrate and drosolphilia embryos. Trends Genet. 1994;10:266. doi: 10.1016/0168-9525(90)90008-t. [DOI] [PubMed] [Google Scholar]
- Hébert JM, Fishell G. The genetics of early telencephalon patterning: Some assembly required. Nat Rev Neurosci. 2008;9:678–685. doi: 10.1038/nrn2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heins N, Cremisi F, Malatesta P, Gangemi RM, Corte G, Price J, Goudreau G, Gruss P, Gotz M. Emx2 promotes symmetrical cell divisions and a multipotential fate in precursors from the cerebral cortex. Mol Cell Neurosci. 2001;18:485–502. doi: 10.1006/mcne.2001.1046. [DOI] [PubMed] [Google Scholar]
- Hong CS, Park BY, Saint-Jeannet JP. The function of DMRT genes in vertebrate development: It is not just about sex. Dev Biol. 2007;310:1–9. doi: 10.1016/j.ydbio.2007.07.035. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Shimogori T, Ohtsuka T, Kageyama R. Hes genes and neurogenin regulate non-neural versus neural fate specification in the dorsal telencephalic midline. Development. 2008;135:2531–2541. doi: 10.1242/dev.021535. [DOI] [PubMed] [Google Scholar]
- Ivaniutsin U, Chen Y, Mason JO, Price DJ, Pratt T. Adenomatous polyposis coli is required for early events in the normal growth and differentiation of the developing cerebral cortex. Neural Dev. 2009;4:3. doi: 10.1186/1749-8104-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanold PO, Luhmann HJ. The subplate and early cortical circuits. Annu Rev Neurosci. 2010;33:23–48. doi: 10.1146/annurev-neuro-060909-153244. [DOI] [PubMed] [Google Scholar]
- Kimura K, Hachiya T, Koganezawa M, Tazawa T, Yamamoto D. Fruitless and doublesex coordinate to generate male-specific neurons that can initiate courtship. Neuron. 2008;59:759–769. doi: 10.1016/j.neuron.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Kuschel S, Rüther U, Theil T. A disrupted balance between Bmp/Wnt and Fgf signaling underlies the ventralization of Gli3 mutant telencephalon. Dev Biol. 2003;260:484–495. doi: 10.1016/s0012-1606(03)00252-5. [DOI] [PubMed] [Google Scholar]
- Lee SM, Tole S, Grove S, McMahon A. A local Wnt-3a signal is required for development of the mammalian hippocampus. Development. 2000;127:457–467. doi: 10.1242/dev.127.3.457. [DOI] [PubMed] [Google Scholar]
- Lindwall C, Fothergill T, Richards LJ. Commissure formation in the mammalian forebrain. Curr Opin Neurobiol. 2007;17:3–14. doi: 10.1016/j.conb.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Machon O, Backman M, Machonova O, Kozmik Z, Vacik T, Andersen L, Krauss S. A dynamic gradient of Wnt signaling controls initiation of neurogenesis in the mammalian cortex and cellular specification in the hippocampus. Dev Biol. 2007;311:223–237. doi: 10.1016/j.ydbio.2007.08.038. [DOI] [PubMed] [Google Scholar]
- Magnani D, Hasenpusch-Theil K, Jacobs EC, Campagnoni AT, Price DJ, Theil T. The Gli3 hypomorphic mutation Pdn causes selective impairment in the growth, patterning, and axon guidance capability of the lateral ganglionic eminence. J Neurosci. 2010;30:13883–13894. doi: 10.1523/JNEUROSCI.3650-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallamaci A. Molecular bases of cortico-cerebral regionalization. Prog Brain Res. 2011;189:37–64. doi: 10.1016/B978-0-444-53884-0.00017-8. [DOI] [PubMed] [Google Scholar]
- Mallamaci A, Mercurio S, Muzio L, Cecchi C, Pardini CL, Gruss P, Boncinelli E. The lack of Emx2 causes impairment of Reelin signaling and defects of neuronal migration in the developing cerebral cortex. J Neurosci. 2000;20:1109–1118. doi: 10.1523/JNEUROSCI.20-03-01109.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangale VS, Hirokawa KE, Satyaki PR, Gokulchandran N, Chikbire S, Subranian L, Shetty A, Martynoga B, Paul J, Mai MV, et al. Lhx2 selector activity specifies cortical identity and suppresses hippocampal organizer fate. Science. 2008;319:304–309. doi: 10.1126/science.1151695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel M, Georgala PA, Carr CB, Chanas S, Kleinjan DA, Martynoga B, Mason JO, Molinek M, Pinson J, Pratt T, et al. Controlled overexpression of Pax6 in vivo negatively autoregulates the Pax6 locus, causing cell-autonomous defects of late cortical progenitor proliferation with little effects on cortical arealization. Development. 2007;134:545–555. doi: 10.1242/dev.02764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martoriati A, Doumont G, Alcalay M, Bellefroid E, Pelicci PG, Marine JC. dapk1, encoding an activator of a p19ARF-p53-mediated apoptotic checkpoint, is a transcription target of p53. Oncogene. 2005;24:1461–1466. doi: 10.1038/sj.onc.1208256. [DOI] [PubMed] [Google Scholar]
- Matson CK, Murphy MW, Griswold MD, Yoshida S, Bardwell VJ, Zarkower D. The mammalian doublesex homolog DMRT1 is a transcriptional gatekeeper that controls the mitosis versus meiosis decision in male germ cells. Dev Cell. 2010;19:612–624. doi: 10.1016/j.devcel.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquelajauregui A, Vareal-Echavarria A, Ceci ML, Garcia F, Ricano I, Hoang K, Frade-Perez D, Portera-Cailliau C, Tamariz E, De Carlos JA, et al. LIM-homeobox gene Lhx5 is required for normal development of Cajal-Retzius cells. J Neurosci. 2010;30:10551–10562. doi: 10.1523/JNEUROSCI.5563-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monuki ES, Porter FD, Walsh CA. Patterning of the dorsal telencephalon and cerebral cortex by a roof plate-Lhx2 pathway. Neuron. 2001;32:591–604. doi: 10.1016/s0896-6273(01)00504-9. [DOI] [PubMed] [Google Scholar]
- Muzio L, DiBenedetto B, Stoykova A, Boncinelli E, Gruss P, Mallamaci A. Emx2 and Pax6 control regionalization of the pre-neurogenic cortical primordium. Cereb Cortex. 2002;12:129–139. doi: 10.1093/cercor/12.2.129. [DOI] [PubMed] [Google Scholar]
- Muzio L, Mallamaci A. FoxG1 confines Cajal-Retzius neurogenesis and hippocampal morphogenesis to the dorsomedial pallium. J Neurosci. 2005;25:4435–4441. doi: 10.1523/JNEUROSCI.4804-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzio L, Soria JM, Pannese M, Piccolo S, Mallamaci A. A mutually stimulating loop involving emx2 and canonical wnt signalling specifically promotes expansion of occipital cortex and hippocampus. Cereb Cortex. 2005;15:2021–2028. doi: 10.1093/cercor/bhi077. [DOI] [PubMed] [Google Scholar]
- Naruse I, Kato K, Asano T, Suzuki F, Kameyama Y. Developmental brain abnormalities accompanied with the retarded production of S-100 beta protein in genetic polydactyly mice. Brain Res Dev Brain Res. 1990;51:253–258. doi: 10.1016/0165-3806(90)90283-5. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Miyata T, Nakajima K, Yagyu K, Seike M, Ikenaba K, Yamamoto H, Mikoshiba K. The reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron. 1995;14:899–912. doi: 10.1016/0896-6273(95)90329-1. [DOI] [PubMed] [Google Scholar]
- O'Leary DD, Sahara S. Genetic regulation of arealization of the neocortex. Curr Opin Neurobiol. 2008;18:90–100. doi: 10.1016/j.conb.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper M, Moldrich RX, Lindwall C, Little E, Barry G, Mason S, Sunn N, Kurniawan ND, Gronostajski RM, Richards LJ. Multiple non-cell-autonomous defects underlie neocortical callosal dysgenesis in Nfib-deficient mice. Neural Dev. 2009;4:43. doi: 10.1186/1749-8104-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Evolution of the neocortex: A perspective from developmental biology. Nat Rev Neurosci. 2009;10:724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CS, Shamu CE, Shen MM, Seifert KJ, Hirsch B, Hodgkin J, Zarkower D. Evidence for evolutionary conservation of sex-dertermining genes. Nature. 1998;391:691–695. doi: 10.1038/35618. [DOI] [PubMed] [Google Scholar]
- Ross JM, Kalis AK, Murphy MW, Zarkower D. The DM domain protein MAB-3 promotes sex-specific neurogenesis in C. elegans by regulating bHLH proteins. Dev Cell. 2005;8:881–892. doi: 10.1016/j.devcel.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- Sahara S, Kawakami Y, Izpisua Belmonte JC, O'Leary DD. Sp8 exhibits reciprocal induction with Fgf8 but has an opposing effect on anterior-posterior cortical area patterning. Neural Dev. 2007;2:10. doi: 10.1186/1749-8104-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Camacho C, Ortega JA, Ocaña I, Alcántara S, Bovolenta P. Appropriate Bmp7 levels are required for the differentiation of midline guidepost cells involved in corpus callosum formation. Dev Neurobiol. 2011;71:337–350. doi: 10.1002/dneu.20865. [DOI] [PubMed] [Google Scholar]
- Scardigli R, Baumer N, Gruss P, Guillemot F, Le Roux I. Direct and concentration-dependent regulation of the proneural gene Neurogenin2 by Pax6. Development. 2003;130:3269–3281. doi: 10.1242/dev.00539. [DOI] [PubMed] [Google Scholar]
- Schuurmans C, Armant O, Nieto M, Stenman JM, Britz O, Klenin N, Brown C, Langevin LM, Seibt J, Tang H, et al. Sequential phases of cortical specification involve Neurogenin-dependent and -independent pathways. EMBO J. 2004;23:2892–2902. doi: 10.1038/sj.emboj.7600278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K, Miyagi T, Yoshida M, Miyata T, Ogawa M, Aizawa S, Suda Y. Absence of Cajal-Retzius cells and subplate neurons associated with defects of tangential migration from ganglionic eminence in Emx1/2 double mutant cerebral cortex. Development. 2002;129:3479–3492. doi: 10.1242/dev.129.14.3479. [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Yoshida M, Nakamura M, Aizawa S, Suda Y. Emx1 and Emx2 cooperate in initial phase of archipallium development. Mech Dev. 2004;121:475–489. doi: 10.1016/j.mod.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Smith CA, Hurley TM, McClive PJ, Sinclair AH. Restricted expression of DMRT3 in chicken and mouse embryos. Mech Dev. 2002;119(Suppl 1):S73–S76. doi: 10.1016/s0925-4773(03)00094-7. [DOI] [PubMed] [Google Scholar]
- Spandidos A, Wang X, Wang H, Seed B. PrimerBank: A resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res. 2010;38:D792–D799. doi: 10.1093/nar/gkp1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda Y, Kokura K, Kimura J, Kajikawa E, Inoue F, Aizawa S. The same enhancer regulates the earliest Emx2 expression in caudal forebrain primordium, subsequent expression in dorsal telencephalon and later expression in the cortical ventricular zone. Development. 2010;137:2939–2949. doi: 10.1242/dev.048843. [DOI] [PubMed] [Google Scholar]
- Theil T, Alvarez-Bolado G, Walter A, Rüther U. Gli3 is required for Emx gene expression during dorsal telencephalon development. Development. 1999;126:3561–3571. doi: 10.1242/dev.126.16.3561. [DOI] [PubMed] [Google Scholar]
- Theil T, Aydin S, Koch S, Grotewold L, Rüther U. Wnt and BMP signalling cooperatively regulate graded Emx2 expression in the dorsal telencephalon. Development. 2002;129:3045–3054. doi: 10.1242/dev.129.13.3045. [DOI] [PubMed] [Google Scholar]
- Tole S, Goudreau G, Assimacopoulos S, Grove EA. Emx2 is required for growth of the hippocampus but not for hippocampal field specification. J Neurosci. 2000;20:2618–2625. doi: 10.1523/JNEUROSCI.20-07-02618.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresser J, Chiba S, Veeman M, El-Nachef D, Newman-Smith E, Horie T, Tsuda M, Smith WC. Doublesex/mab3 related-1 (dmrt1) is essential for development of anterior neural plate derivatives in Ciona. Development. 2010;137:2197–2203. doi: 10.1242/dev.045302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang J, Mori S, Nathans J. Axonal growth and guidance defects in Frizzled3 knock-out mice: A comparison of diffusion tensor magnetic resonance imaging, neurofilament staining, and genetically directed cell labeling. J Neurosci. 2006;26:355–364. doi: 10.1523/JNEUROSCI.3221-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DG, Nieto MA. Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol. 1993;225:361–373. doi: 10.1016/0076-6879(93)25025-w. [DOI] [PubMed] [Google Scholar]
- Xie Z, Ma X, Ji W, Zhou G, Lu Y, Xiang Z, Wang YX, Zhang L, Hu Y, Ding YQ, et al. Zbtb20 is essential for the specification of CA1 field identity in the developing hippocampus. Proc Natl Acad Sci U S A. 2010;107:6510–6515. doi: 10.1073/pnas.0912315107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Assimacopoulos S, Jones K, Grove E. Massive loss of Cajal-Retzius cells does not disrupt neocortical layer order. Development. 2006;133:537–545. doi: 10.1242/dev.02209. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Suda Y, Matsuo I, Miyamoto N, Takeda N, Kuratani S, Aizawa S. Emx1 and Emx2 functions in development of dorsal telencephalon. Development. 1997;124:101–111. doi: 10.1242/dev.124.1.101. [DOI] [PubMed] [Google Scholar]
- Yoshizawa A, Nakahara Y, Izawa T, Ishitani T, Tsutsumi M, Kuroiwa A, Itoh M, Kikuchi Y. Zebrafish Dmrta2 regulates neurogenesis in the telencephalon. Genes Cells. 2011;16:1097–1109. doi: 10.1111/j.1365-2443.2011.01555.x. [DOI] [PubMed] [Google Scholar]
- Zembrzycki A, Griesel G, Stoykova A, Mansouri A. Genetic interplay between the transcription factors Sp8 and Emx2 in the patterning of the forebrain. Neural Dev. 2007;2:8. doi: 10.1186/1749-8104-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Qiu Y, Pereira FA, Crair MC, Tsai SY, Tsai MJ. The nuclear orphan receptor COUP-TFI is required for differentiation of subplate neurons and guidance of thalamocortical axons. Neuron. 1999;24:847–859. doi: 10.1016/s0896-6273(00)81032-6. [DOI] [PubMed] [Google Scholar]
- Zhou C, Tsai SY, Tsai MJ. COUP-TF1: An intrinsic factor for early regionalization of the neocortex. Genes Dev. 2001;15:2054–2059. doi: 10.1101/gad.913601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.