Abstract
Executive dysfunction in fragile X-associated tremor/ataxia syndrome (FXTAS) has been suggested to mediate other cognitive impairments. In the present study, event-related potentials and neuropsychological testing were combined to investigate the brain mechanisms underlying the executive dysfunction in FXTAS. Thirty-two-channel electroencephalography was recorded during an auditory “oddball” task requiring dual responses. FXTAS patients (N= 41, mean age= 62) displayed prolonged latencies of N1 and P3 and reduced amplitudes of P2 and P3, whereas their N2 measures remained within the normal range, indicating relatively preserved early-stage auditory attention but markedly impaired late-stage attention and working memory updating processes (as indexed by P3). Topographical mapping revealed a typical parietal P3 peak preceded by a prominent fronto-central P3 in normal control subjects (N= 32), whereas FXTAS patients had decreased parietal P3 amplitude and diminished fronto-central positivities with a delayed onset (∼50 ms later than controls, P < 0.002). The P3 abnormalities were associated with lower executive function test (e.g., BDS-2) scores. Smaller P3 amplitudes also correlated with increased CGG repeat length of fragile X mental retardation 1 (FMR1) gene and higher FMR1 mRNA levels. These results indicate that abnormal fronto-parietal attentional network dynamics underlie executive dysfunction, the cardinal feature of cognitive impairment in FXTAS.
Keywords: attention, executive function, FMR1, P300, working memory
Introduction
Fragile X-associated tremor/ataxia syndrome (FXTAS) is a recently identified neurodegenerative disorder characterized by cerebellar ataxia, intention tremor, polyneuropathology, and cognitive impairment (Hagerman et al. 2001; Jacquemont et al. 2003). As one of the most common single-gene, late-onset neurodegenerative disorders, FXTAS mainly affects male carriers of a premutation expansions of CGG repeats (∼55–200 repeats) in a noncoding region of the fragile X mental retardation 1 (FMR1) gene on the X chromosome (the full mutation with over 200 CGG repeats results in gene silencing and fragile X syndrome, the leading cause of inherited intellectual disability) (Coffey et al. 2008; Rodriguez-Revenga et al. 2009). Individuals with premutation alleles have elevated FMR1 mRNA levels, but normal or slightly reduced fragile X mental retardation protein (FMRP) levels (Kenneson et al. 2001; Allen et al. 2004; Tassone et al. 2007). It has been suggested that FMR1 mRNA toxicity is the pathogenic molecular mechanism of neurodegeneration in FXTAS (Jacquemont et al. 2007; Garcia-Arocena and Hagerman 2010; Willemsen et al. 2011). FXTAS brains usually show multiple neuropathological abnormalities including brain atrophy, white matter disease, loss of axons and myelin, and intranuclear inclusions in neurons and astrocytes (Greco et al. 2006; Hashimoto et al. 2011a, 2011b).
Compared with normal elderly, FXTAS patients often show impairments on tests of general intelligence (IQ), executive function, memory, and visual spatial processing, whereas other cognitive domains usually remain within the normal range (Grigsby et al. 2008; Cornish et al. 2009; Sévin et al. 2009; Juncos et al. 2011). Executive dysfunction in FXTAS is a consistent finding, reported both in case studies (Hagerman et al. 2001; Peters et al. 2006) and group studies (Jacquemont et al. 2003; Grigsby et al. 2006, 2007, 2008; Cornish et al. 2008). FXTAS patients display executive function deficits including failure of initiating purposeful activity, disinhibition of irrelevant or inappropriate behavior, impaired attention control and working memory, and deficits in generating information actively. Brega et al. (2008) tested the contribution of executive dysfunction to other cognitive impairments in FXTAS and suggested that executive dysfunction to be the primary deficit that mediates the other “secondary” cognitive deficits (e.g., learning, memory, and visual-spatial) observed in FXTAS.
Executive function is generally thought of as a set of higher-level processes coordinated in order to effortfully direct and modulate behavior according to certain task demands (e.g., goal-directed), especially in nonroutine circumstances where automatic stimulus-response association is insufficient (Miyake et al. 2000; Gilbert and Burgess 2008; Banich 2009). Cognitive processes thought to be component factors of executive function include, but not limited to, maintaining and updating goal-relevant information in attention and working memory, inhibition of irrelevant processes, switching between alternative goals, and monitoring task performance. Although there has been controversy regarding the manner in which the frontal lobe supports executive function, neuroimaging and human lesion studies generally agree that prefrontal cortex plays a key role in executive function (Banich 2009). A functional magnetic resonance imaging (fMRI) study reported decreased activation in inferior frontal cortex of FXTAS patients during a working memory task (Hashimoto et al. 2011c), but the specific brain mechanisms underlying executive dysfunction in FXTAS remain to be elucidated.
In the present study, a well-established event-related potential (ERP) paradigm, the auditory “oddball” P300 task, was employed to quantitatively examine executive dysfunction, considered the primary cognitive impairment, in FXTAS. In a classic auditory oddball paradigm, 2 tones are presented with unequal probability and the infrequent target tone reliably elicits a prominent P300 (P3) component (a scalp-positive brain potential maximal over parietal sites between 300 and 400 ms poststimulus onset) in normal young subjects. The P3 (or P3b) is a widely recognized electrophysiological index of attention and working memory (see Herrmann and Knight 2001; Polich 2007, for review) and has been related to fluid intelligence which refers to reasoning and novel problem-solving abilities (e.g., Pang et al. 1990; McGarry-Roberts et al. 1992; Jausovec and Jausovec 2000).
Some prior studies have reported relationships between P3 measures and neuropsychological test scores of executive function. In a normal young control group, Dichter et al. (2006) observed a correlation between the auditory oddball P3 amplitude and the Wisconsin Card Sorting Test (WCST), as well as negative correlations between P3 latency and the WCST, the Trail Making test and Working Memory. A study using a 3-stimuli visual oddball task (Fjell et al. 2007) demonstrated significant correlation between the P3 (P3a) amplitude to infrequent nontarget stimuli and a composite score of 5 executive function tests. These authors showed that P3b latency was inversely associated with cortical thickness, especially in frontal areas. In another 3-stimuli visual oddball task, P3b latency was found to be negatively correlated with composite attention/executive test scores (Daffner et al. 2006). Furthermore, ERPs recorded during performance of executive function tasks revealed P3 activities related to cognitive control processes (e.g., Barceló et al. 1997, 2000; Kopp et al. 2006; Zurrón et al. 2009).
In our current study, we hypothesized that P3 measures would correlate with executive function abilities and that FXTAS patients would have reduced P3 amplitude and prolonged latency compared with age-matched controls. By analyzing ERP components related to both early and late-stage attentional processes and integrating the ERP findings with neuropsychological testing, we aimed to provide new insights into the brain mechanisms underlying executive dysfunction, thought to be fundamental in this disorder.
Materials and Methods
Participants
A group of 41 patients with mild FXTAS symptoms (mean FXTAS stage = 2.7, range: 2–4) and 32 normal elderly controls (NC) who were negative for the premutation participated in the study (see Table 1 for demographics and genetic-molecular data). FXTAS was diagnosed according to the criteria for probable or possible FXTAS (Jacquemont et al. 2003). FMR1 allele status was identified in all subjects by DNA testing. FMR1 CGG repeat lengths and FMR1 mRNA levels were quantified following procedures described elsewhere (Tassone et al. 2000, 2008). All subjects provided informed consent for a protocol approved by the Institutional Review Board of University of California at Davis. Five of the FXTAS participants and four in the NC group were left-handed. There were no significant group differences on age (t71 = 0.3, P = 0.76) or gender (χ2 = 1, P = 0.31). Both groups were highly educated, with the NC group having slightly higher educational attainment (t69 = 2.3, P = 0.02). Subjects with history of alcohol abuse/dependence, stroke, schizophrenia, traumatic brain injury, or other disorders of the central nervous system (CNS) were excluded. Because of the common use of primary CNS-active medications for depression and neuropathic pain in a representative sample of FXTAS, we did not exclude subjects on these medications: 15 were on antidepressants, usually selective serotonin-reuptake inhibitors (N = 7) or venlafaxine (N = 5), 4 were on neuropathic pain medications (N = 3 on gabapentin), and 2 patients were on oxybutynin.
Table 1.
Demographics and genetic-molecular measures
| NC (N= 32) | FXTAS (N= 41) | |
|---|---|---|
| Age | 61.3 ± 6.7 | 61.9 ± 8.1 |
| Female | 13 | 12 |
| Education | 17.2 ± 2.3 | 15.6 ± 3.0 |
| CGG repeats | 28.4 ± 4.7 | 91.9 ± 24.4 |
| FMR1 mRNA | 1.55 ± 0.25 | 2.84 ± 1.0 |
Neuropsychological Testing
The subjects' global/general cognitive abilities, executive functioning, attention abilities, and memory were evaluated with tests described below. The Mini-Mental State Examination (MMSE), Verbal and Performance IQ (VIQ and PIQ) from the Wechsler Adult Intelligence Scale (WAIS-III) were used as measures of global/general cognitive abilities.
Executive function was evaluated with 3 tests: Behavioral Dyscontrol Scale-2 (BDS-2), the Controlled Oral Word Association Test (COWAT), and the Stroop Test. The BDS-2 measures behavioral self-regulation in intentional control of simple voluntary motor behavior (Grigsby and Kaye 1996; Diesfeldt 2004) and consistently revealed impaired performance among individuals with FXTAS (e.g., Jacquemont et al. 2003; Grigsby et al. 2006, 2007, 2008). The COWAT is a highly reliable assessment of verbal fluency (DesRosiers and Kavanaugh 1987) and widely thought to be a measure of executive function, as it involves the ability to generate information with speed and accuracy. The Stroop Test (MacLeod 1991; Spreen and Strauss 1998), one of the most commonly used tests of frontal lobe function, provides a general index of cognitive flexibility and inhibition.
The subjects' attention abilities were evaluated with the Symbol Digit Modalities Test (SDMT; Smith 1982). Finally, memory abilities were assessed with the WAIS-III Working Memory Subscale, the Wechsler Memory Scale (WMS-III), and the California Verbal Learning Test (CVLT) (Delis et al. 1987).
ERP Paradigm
An auditory oddball paradigm with dual-response requirement (i.e., button press response and mental counting of targets) was given to subjects in a sound-attenuated recording chamber. While attending to a series of pure tones, participants were instructed to press a button in response to each infrequent target tone. Because of the often-observed high-frequency hearing loss in elderly (e.g., Gates et al. 1990), 2 types of low-frequency tones were used as stimuli: higher tone (200 Hz) and lower tone (113 Hz). Target tones were the higher tone in 2 blocks and the lower tone in the other 2 blocks. Across 4 blocks, 400 tones were presented, with 75% were “standard” tones (nontarget) and 25% were targets. All tones had a 200 ms duration and were presented at 40 dB above individual hearing threshold with a ∼1–1.5 s stimulus onset asynchrony, in a pseudorandomized manner. Hearing threshold was obtained using the titration method at the beginning of each experimental session. There was no group difference on individual hearing thresholds (NC = 28 ± 5 dB, FXTAS = 29.2 ± 6.4; t71 = 0.9, P = 0.38). The button press was counterbalanced between hands, both within and between subjects.
In addition to the button press, the subjects were also asked to count the number of target tones silently. The mental counting task was included to place larger demands on frontal lobe processing during oddball task performance (Brázdil et al. 2003; Linden 2005). The total number of counted target tones in each block was reported immediately after the block.
Electroencephalography Recording
Thirty-two-channel electroencephalogram (EEG) was recorded with tin disk electrodes mounted in an elastic electrode cap (Electro-Cap International Inc., Eaton, OH), referenced online to the left mastoid and subsequently rereferenced offline to the average of the left and right mastoids (see Olichney et al. 2010 for details of EEG montage and customized electrode locations). Electro-oculogram from vertical and horizontal eye movements was recorded with 4 electrodes, one beneath and one at the outer canthus of each eye. The signals were amplified by Nicolet SM 2000 amplifiers with a bandpass of 0.016–100 Hz and digitized at a sampling rate of 250 Hz. Interelectrode impedance was maintained at ≤5 kΩ.
Data Analysis
Continuous EEG was segmented into epochs of 1024 ms duration with 100 ms prestimulus period. Epochs contaminated with blinks, eye movements, excessive muscle activity, or amplifier blocking were rejected. Artifact-free epochs were averaged off-line according to stimulus type to produce the ERPs.
Reaction time (RT) of correct button responses to target tones (hits) was measured. Accuracy was calculated as target “hit rate”, that is, the percentage of target trials with a button press response. Count-hit discrepancy (discrepancy between the number of hits and the participant's count for number of target tones within each block) was calculated as a measure of working memory capacity during oddball task performance.
Based on the inspection of averaged ERP waveforms and the published literature (e.g., Picton et al. 2000; Nordin et al. 2011), time windows of 70–150 ms were applied for N100 (N1), 160–260 ms for P200 (P2), 170–290 ms for N200 (N2), and 300–650 ms for P3 measures. N1 was measured both in response to target and standard tones. P2 was measured to standards alone. N2 (or N2b) component was primarily measured from the difference waveforms (=targets − standards) because N2 to targets had large temporal overlap with P2 to standards. P3 was measured for target tones, which selectively elicit this component. Mean voltage during the 100 ms prestimulus period was defined as baseline. Local peak amplitude and local peak latency (Luck, 2005) were calculated for all the components. Low-pass filtering with a 30 Hz cutoff was performed on the data prior to the peak measurements.
Two-way repeated-measures analyses of variance (ANOVAs; SPSS 19, IBM) were performed with the between-subject factor of group and the within-subject factor of electrode. N1 and P2 analyses included four fronto-central electrodes (Fz, Cz, FC1/2). Five electrodes (Cz, FC1/2, CP1/2) were used to measure the N2 component because of the central distribution of auditory N2b. Three midline electrodes (Fz, Cz, Pz) were included in the main analyses for P3, with the Tukey–Kramer test applied to adjust for multiple comparisons in which group effects were tested for at individual electrode sites. Additional analyses were also performed on P3 data from 2 subsets of channels (8 parasagittal leads surrounding the midline: F3/4, FC1/2, CP1/2, and P3/4; and 10 lateral electrodes: F7/8, Bl, Br, 41l, 41r, Wl, Wr, and T5/6). These analyses utilized two within-subject factors for channel location: hemisphere (L/R) and an anterior/posterior factor (with 2 levels for parasagittal analyses contrasting frontal vs. parietal channels, and five levels for far lateral channels). The Greenhouse–Geiser correction (Geisser and Greenhouse 1959) was used where appropriate for violations of sphericity and adjusted P-values of ≤0.05 were considered as significant.
To examine the correlational relationships of the P3 with neuropsychological test scores and genetic-molecular measures, partial correlation tests, controlling for the covariates of age and/or education, were performed. The potential confounding effect of CNS-active medications on ERP measures was evaluated by comparing the FXTAS patients not taking CNS-active medication with those patients on CNS-active medications and with the NC group. Finally, main ERP peak amplitudes and latencies (N1 at Fz, P2 and N2 at Cz, and P3 at Fz/Pz) were used as input variables for a forward stepwise logistic regression model to explore the utility of auditory ERP measures to discriminate between FXTAS patients and NC subjects (probability in = 0.05, probability out = 0.10).
Results
Neuropsychological Test Results
Table 2 summarizes the neuropsychological test scores by group. FXTAS subjects had lower performance on the MMSE, VIQ, and PIQ than the NC group, indicating decreased global cognitive abilities in these patients. Consistent with previous findings, our FXTAS patients showed deficits on all of the 3 executive function tests (i.e., the BDS-2, COWAT, and Stroop) compared with NC.
Table 2.
Neuropsychological test scores (mean ± SD) in FXTAS and normal controls (NC)
| NC (N = 32) | FXTAS (N = 41) | |
|---|---|---|
| Global abilities | ||
| MMSE | 28.4 ± 1.1 | 27.3 ± 1.5*** |
| WAIS-VIQ | 121 ± 12.2 | 111.6 ± 12.6* |
| WAIS-PIQ | 121.9 ± 14.2 | 105.2 ± 15*** |
| Executive function | ||
| BDS-2 | 22.6 ± 3.1 | 16.6 ± 4.8*** |
| Stroop | 38.9 ± 7 | 30.9 ± 10.2*** |
| COWAT | 51.5 ± 13.2 | 37 ± 14*** |
| Attention and memory | ||
| SDMT | 54.7 ± 10.9 | 42 ± 10.4*** |
| WAIS Working Memory | 117.8 ± 14.3 | 104.3 ± 11.7*** |
| WMS Working Memory | 115.3 ± 12.8 | 104.3 ± 10.4* |
| CVLT Discriminability | 91.6 ± 7.8 | 89.4 ± 10.4 |
| CVLT Long Delay Cued Recall | 10.6 ± 4.5 | 9.7 ± 4.1 |
| CVLT Long Delay Free Recall | 9.5 ± 4.5 | 9.5 ± 3.8 |
| Other | ||
| WAIS Processing Speed | 111.4 ± 15.8 | 100.1 ± 14.1* |
| WAIS Comprehension | 14.1 ± 2.5 | 13.1 ± 2.2 |
| WAIS Information | 13 ± 2.4 | 12.2 ± 2.5 |
*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
FXTAS patients also displayed slower information processing speed as measured by WAIS and lower attention abilities as assessed by SDMT. The working memory performances, as measured by WAIS-III and WMS-III, were also decreased in FXTAS. In contrast, their performance was comparable to NC subjects on CVLT delayed recall subscales (t55 < 0.9, P > 0.36, in both cases) and WAIS-III subscales of Comprehension and Information (t59 < 1.7, P > 0.11, in both cases), indicating preserved language comprehension and long-term semantic memory in FXTAS.
Behavioral Performance (Auditory Oddball Counting Task)
FXTAS patients had longer mean RT to target tones (517 ± 80 ms) than NC (458 ± 65 ms) (t71 = 3.4, P < 0.001). Both groups performed the task with very high accuracy (NC = 97.5%, FXTAS = 96.1%; t71 = 1, P = 0.33), but the count-hit discrepancy was increased in FXTAS (mean = 2.3) compared with NC (mean = 1.4) (t71 = 2, P = 0.05), suggesting that the dual-response task placed high demands on the attention/working memory processing capacity of the patients.
ERP Results
The number of accepted trials after EEG artifacts rejection was comparable for the 2 groups (e.g., target tone trials: NC = 63.3 ± 18, FXTAS = 62.2 ± 20; t71 = 0.2, P = 0.81). For the group grand-average ERP waveforms, see Figure 1A.
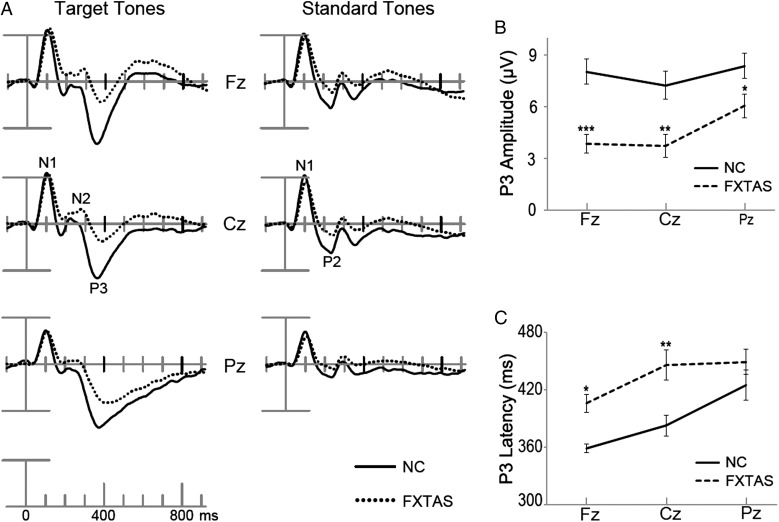
Figure 1.
Grand-average ERPs elicited by correct target tones and standard tones at 3 midline electrodes (A). Time 0 was stimulus onset and there was 100 ms prestimulus baseline. P3 peak amplitudes (B) and latencies (C) from 3 midline channels were plotted with significant group differences indicated (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001).
ANOVAs of the N1 revealed that the FXTAS group had a small (∼8 ms), but significant prolongation of N1 latencies compared with NC, both to target (N1 latency at Fz: NC = 104 ± 13 ms, FXTAS = 113 ± 14) and standard tones (NC = 100 ± 10 ms, FXTAS = 110 ± 14) (F(1,71) > 9.5, P < 0.003, in both cases). No intergroup differences were found for N1 amplitudes to either tone (F(1,71) < 1.3, P > 0.25, in both cases). FXTAS patients had smaller P2 amplitude compared with NC subjects (P2 amplitude at Fz: NC = 3.8 ± 1.8 μV, FXTAS = 1.8 ± 2.3) (F(1,71) = 17.8, P < 0.001), whereas there was no group difference detected on P2 latency (F(1,71) = 0.1, P = 0.92). For N2, the 2 groups had comparable amplitude (F(1,71) = 1.6, P = 0.21) and latency (F(1,71) = 0.2, P = 0.66).
Substantial intergroup differences were found in the P3 analyses. ANOVA on three midline channels showed that FXTAS patients had decreased P3 amplitude (F(1,71) = 13, P = 0.001) and delayed P3 latency (F(1,71) = 9.6, P = 0.003) compared with the NC group. As Figure 1B,C illustrates, pairwise comparisons with Tukey–Kramer adjustment on midline electrode ERPs (Fz, Cz, Pz) found that FXTAS group had reduced P3 amplitudes at all the 3 electrode sites (t > 2.3, adjusted P < 0.04, in all cases), as well as slower P3 latencies at Fz and Cz (t > 3.3, adjusted P < 0.02, in both cases), but there was no group difference for P3 latency at Pz (t = 1.2, adjusted P = 0.15). These group differences were replicated by ANOVAs on both 10 lateral electrodes (amplitude: F(1,71) = 7, P = 0.01; latency: F(1,71) = 16.4, P = 0.0001) and 8 parasagittal electrodes surrounding the midline (amplitude: F(1,71) = 11.8, P = 0.001; latency: F(1,71) = 10.5, P = 0.002). The ANOVA of midline P3 amplitudes also revealed a significant group× electrode interaction (F(2,142) = 5.2, P = 0.01), suggesting different scalp distribution of P3 for the 2 groups. As illustrated by the topographic distribution map across 300–550 ms window (Fig. 2), both a fronto-central P3 peak and a parietal P3 (P3b) peak were obtained in the NC group, but FXTAS patients displayed a reduced parietal P3b with a more severely diminished and delayed fronto-central P3. In fact, prior to 340 ms time window, FXTAS group did not show positive voltage at the fronto-central midline sites (see 300–340 ms time window in Fig. 2). The group× anterior/posterior interaction was obtained in the ANOVA on amplitudes of 8 parasagittal electrodes (F(1,71) = 6.7, P = 0.01), but not of the 10 lateral electrodes (F(4,284) = 0.5, P = 0.57), indicating that substantial P3 topographic distribution difference occurs along the midline area. Neither ANOVA (parasagittal or lateral channels) showed any significant effect of group× hemisphere interaction (F < 1.9, P > 0.17, in both cases), but there were main effects of hemisphere (F > 12.1, P < 0.001, in both cases), due to ∼10% larger P3 amplitudes over the right versus left side (see Fig. 2) in both groups.
Figure 2.
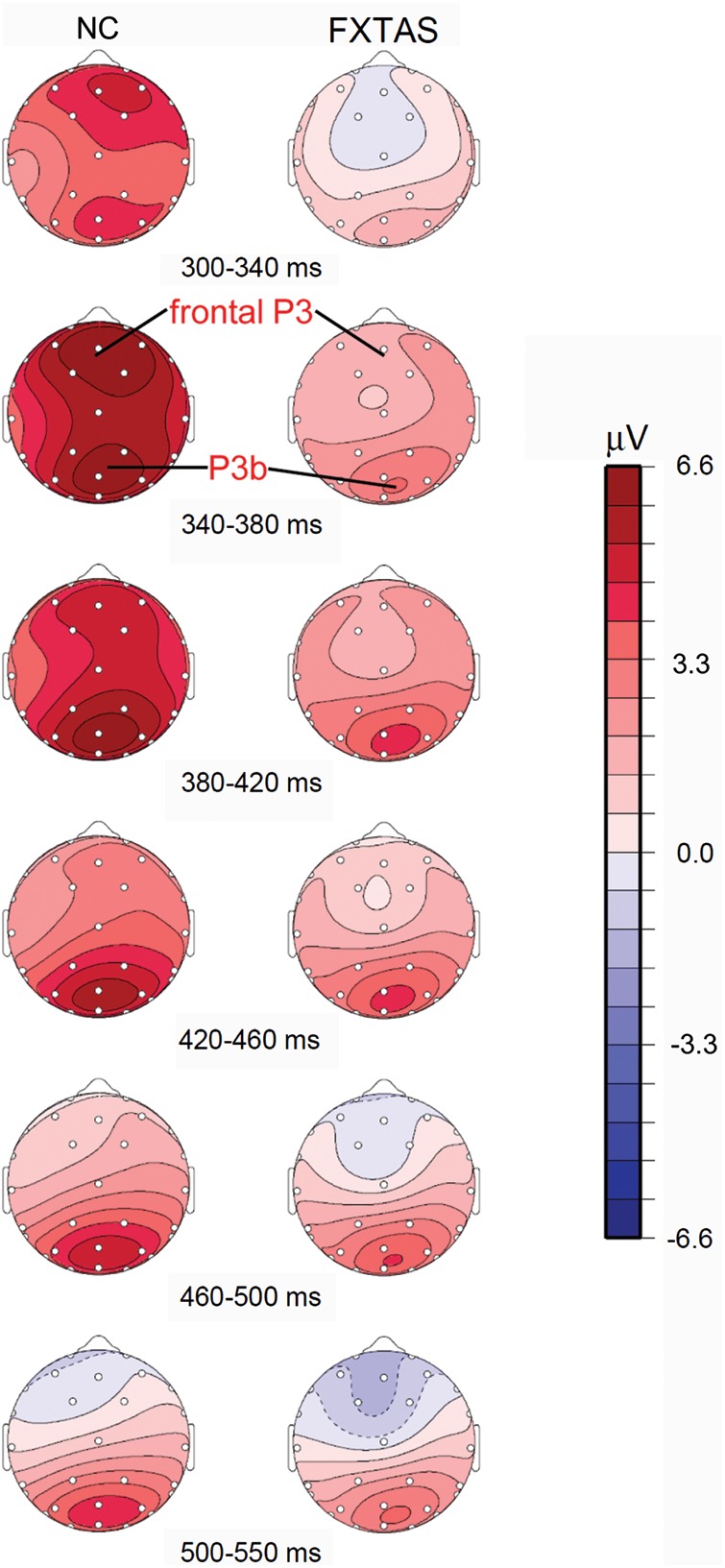
Topographical distribution of ERPs elicited by correct target tones (anterior = up, right = right, left = left). Color scale indicates mean voltage (μV).
ANOVA comparing the FXTAS patients who were (N = 21, mean age = 61 ± 9) and were not (N = 20, age = 63 ± 7; age t-test: t39 = 1, P = 0.31) on CNS-active medications demonstrated no significant medication effects on any of the P3, N2, and P2 measures, or N1 amplitudes (F < 0.5, P > 0.48, in all cases). There was a insignificant trend for longer N1 latencies in the subgroup taking CNS-active medications (to targets: F(1,39) = 2.1, P = 0.16; to standards: F(1,39) = 2.8, P = 0.10). Additional ANOVAs which compared the 20 FXTAS patients not taking CNS-active medications to the 32 NC subjects (age t-test: t50 = 1, P = 0.34) replicated the significant group differences on P3 amplitude (F(1,50) = 7.4, P = 0.009), P3 latency (F(1,50) = 7.5, P = 0.009), and P2 amplitude (F(1,50) = 11.1, P = 0.002). However, the N1 latencies of the FXTAS patients not on CNS-active medications were no longer significantly different from the NC group (F < 3.6, P > 0.06, in both cases), suggesting that the N1 latency prolongation observed in all 41 FXTAS patients might be partially influenced by CNS-active medications. As in the main ANOVA of all subjects described above, no group differences were found on N1 amplitudes (F < 1.7, P > 0.2, in both cases), P2 latency (F(1,50) = 0.2, P = 0.63), or neither of N2 measures (F < 0.71, P > 0.4, in both cases).
Correlation Results
Correlations were tested between P3 peak measures at 3 midline electrodes and executive function tests scores, with partial correlation tests that controlled for the covariate of age. Years of education was also controlled for analyses across all subjects from the 2 groups, since there was a group difference on educational attainment. Fisher's r-to-r′ transformation was conducted on correlation coefficients significant within one group, and between-group z-tests were then applied to assess potential group differences on the strength of those correlations.
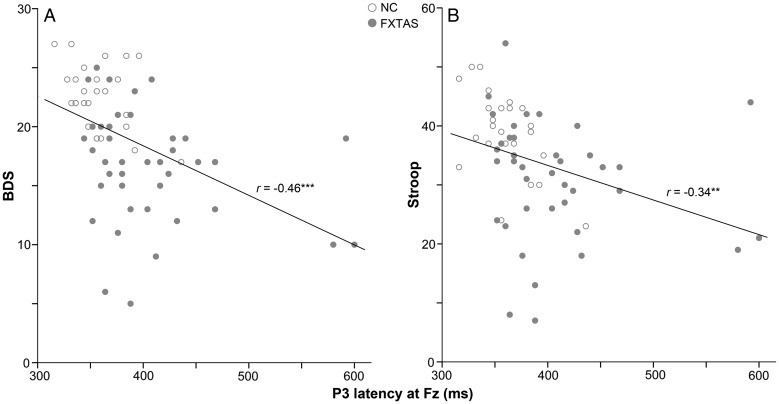
Table 3 presents a summary of the partial correlation coefficients across all participants. Both P3 amplitude and latency displayed significant correlations with executive function scores on the Stroop and BDS-2 tests (see Fig. 3 for scatterplots which illustrate correlations of these 2 tests with P3 latency at Fz). Larger P3 amplitude at Fz was also associated with better performance on the COWAT across all subjects. Within the NC group, P3 amplitude at Fz correlated with the BDS-2 (r = 0.47, r′ = 0.51, P = 0.02; FXTAS: r′ = 0.28), whereas P3 latency at Fz correlated with the Stoop test (r = − 0.48, r′ = − 0.52, P = 0.02; FXTAS: r′ = − 0.13). Between-group z-tests on these r-values found no group differences (z < 1.4, P > 0.16, in all cases). Within the FXTAS group, P3 latency at Pz showed significant correlation with the BDS-2 (r = − 0.33, r′ = − 0.34, P = 0.04; NC: r′ = − 0.08). The r-values were again not significantly different between the 2 groups (z = 1, P = 0.34).
Table 3.
Partial correlation coefficients, after controlling for age and education (across all subjects)
| P3 peak amplitude |
P3 peak latency |
|||||
|---|---|---|---|---|---|---|
| Fz | Cz | Pz | Fz | Cz | Pz | |
| BDS-2 | 0.52*** | 0.32** | 0.18 | −0.43*** | −0.36** | −0.27* |
| Stroop | 0.28* | 0.14 | 0.12 | −0.29* | −0.19 | −0.21 |
| COWAT | 0.30* | 0.21 | 0.16 | 0.04 | −0.10 | 0.09 |
| SDMT | 0.32** | 0.14 | 0.11 | −0.37** | −0.19 | −0.28* |
| WAIS Working Memory | 0.22 | 0.11 | 0.12 | −0.14 | −0.29* | −0.04 |
| WMS Working Memory | 0.13 | −0.01 | −0.03 | −0.25+ | −0.35** | −0.23+ |
| Count-hit discrepancy | −0.26* | −0.12 | −0.11 | 0.08 | 0.31** | 0.15 |
| RT | −0.23* | −0.10 | −0.16 | 0.07 | 0.03 | 0.08 |
| CVLT Long Delay Cued Recall | 0.12 | −0.06 | −0.08 | −0.07 | −0.02 | −0.21 |
| CVLT Long Delay Free Recall | 0.10 | −0.02 | 0.06 | 0.05 | −0.003 | −0.21 |
*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, + 0.07 ≤ p ≤ 0.10.
Figure 3.
Pearson correlations between P3 latency at Fz and executive function scores on the BDS-2 (panel A, regression equation: score = 35.2–0.04*latency) and the Stroop test (panel B, score = 56.8–0.06*latency) (across all subjects, **P ≤ 0.01, ***P ≤ 0.001).
P3 measures were also correlated with test scores of attention (SDMT) and working memory (WAIS-III and WMS-III), 2 cognitive abilities which are often considered fundamental for executive function. The count-hit discrepancy, an index of working memory capacity during the oddball task performance, correlated with P3 amplitude at Fz across the 2 groups and with P3 latency at Cz both across all subjects and within the FXTAS group (r = 0.37, r′ = − 0.39, P = 0.02; NC: r′ = 0.03; z-test: z = − 1.5, P = 0.15) Unlike the moderate correlations with working memory measures, the P3 measures showed no significant correlations with verbal memory performance on the CVLT delayed recall tests. Although RT correlated modestly with P3 amplitude at Fz, no significant association was found between P3 latency and RT across all subjects, nor within the NC (r < 0.34, P > 0.1, in all cases) or FXTAS (r < − 0.09, P > 0.28, in all cases) group.
Relationships between P3 and genetic-molecular measures in the FXTAS patients were examined with partial correlations that controlled for age effects. FMR1 mRNA level was inversely correlated with P3 amplitude at Cz (r = − 0.37, P = 0.03) and Fz (r = − 0.35, P = 0.049). After excluding 2 “outlier” patients with FMR1 premutation CGG repeats greater than 2 standard deviations above the mean (CGG > 141), significant inverse correlations were observed between CGG repeat length and P3 amplitude at Cz (r = − 0.41, P = 0.01) and Pz (r = − 0.34, P = 0.04). It is also worth noting that in this FXTAS patient group, FMR1 mRNA level correlated with both CGG repeat length (r = 0.64, P < 0.0001) and FXTAS disease stage (r = 0.38, P = 0.03), but that CGG repeat length was not associated with disease stage (r = 0.08, P = 0.63). This pattern of results is consistent with prior studies which have implicated mRNA toxicity as a likely mechanism for the neurodegeneration observed in FXTAS (Jacquemont et al. 2007; Garcia-Arocena and Hagerman 2010; Willemsen et al. 2011).
Group Classification
Two P3 measures from Fz (peak amplitude and latency) entered the forward stepwise logistic model, achieving an overall group discrimination accuracy of 79.5%, with sensitivity = 82.9% (34 of 41) and specificity = 75% (24 of 32) [χ2 = 32.2, df = 2, P < 0.0001).
Discussion
This is the first ERP study designed to measure and quantify executive dysfunction, the core cognitive impairment, in FXTAS (Brega et al. 2008). Our FXTAS patients showed impaired executive function performance and ERP abnormalities on an auditory oddball paradigm with dual-response requirements. Robust intergroup differences were found on measures of both P3 latency and amplitude. Significant correlations were found between executive function and P3 measures. Smaller P3 amplitude was also associated with higher FMRP mRNA level and longer FMR1 CGG repeat length.
FXTAS patients also displayed abnormal earlier ERP components (i.e., auditory N1 latency to all tones, and P2 amplitude elicited by the standard tones). The delayed N1 and reduced P2 amplitude in FXTAS implicate the primary and secondary auditory cortex (Mangun and Hillyard 1995; Crowley and Colrain 2004) as being affected in this neurodegenerative disorder with white matter and grey matter pathology (Greco et al. 2006; Hashimoto et al. 2011a, 2011b). However, the N2 (N2b) component, thought to reflect orientation to deviant/novel stimuli (e.g., Renault et al. 1982; Näätänen and Picton 1986) and/or stimulus categorization (e.g., Luck 2005) appeared to be generally normal in our FXTAS patient group.
Compared with the NC group, FXTAS patients had significantly prolonged latency and diminished amplitude of P3. Topographical mapping and comparisons among midline electrodes revealed that NC subjects had 2 prominent P3 components, showing a typical parietal P3 peak, preceded by a fronto-central P3 peak with similar amplitude. In contrast, the fronto-central P3 peak was barely detectable in our FXTAS patient group. FXTAS patients showed significantly diminished fronto-central positivities which peaked ∼50 ms later than controls and also displayed decreased parietal P3 (P3b) amplitude. P3 amplitude and latency measures at frontal channel Fz correlated with executive function test scores both in controls and across all subjects. These correlations did not achieve significance, however, within the FXTAS group alone.
These abnormalities of the auditory P3 component may provide further insights into the brain mechanisms underlying the executive dysfunction characteristic of FXTAS. Prior studies have demonstrated top-down attentional modulation by frontal brain regions during oddball task performance (e.g., Crottaz-Herbette and Menon 2006; Low et al. 2006). The substantially diminished and delayed fronto-central P3 in FXTAS thus provided the first electrophysiological evidence of frontal lobe dysfunction in this disorder. Moreover, the abnormal frontal and parietal P3 components in the patient group further implicate dysfunction of the fronto-parietal network believed to be critical for attentional (controlled) processing of external stimuli (e.g., Fox et al. 2005). It is plausible that the observed parietal P3 abnormalities were due to abnormal inputs/control from frontal brain regions. This interpretation is consistent with the high discriminability which P3 measures at Fz achieved in our logistic regression model and agrees with a conceptual model in which executive dysfunction mediates other secondary impairments of cognitive processes in FXTAS (Brega et al. 2008). A recent volumetric MRI study of FXTAS reported significant atrophy in several frontal brain regions, including dorsomedial and dorsolateral prefrontal cortex, orbitofrontal and anterior cingulate cortex, and also in medial parietal (precuneus) and superior parietal cortex (Hashimoto et al. 2011a). We have previously reported another electrophysiological abnormality in patients with mild FXTAS (Olichney et al. 2010). These FXTAS patients showed reduced N400 word repetition effects, thought to reflect impaired implicit verbal memory, but to which attentional impairment may also contribute.
As mentioned above, the P3 (or P3b) component obtained from the auditory oddball task commonly has a parietal scalp distribution in normal young subjects. But aging has been shown to affect the scalp topography of P3b, with an anterior (or frontal) shift observed in elderly populations (e.g., Iragui et al. 1993; Fabiani et al. 1998). This “anterior shift” of P3 has been suggested to reflect compensatory frontal processes necessary for elderly persons to successfully accomplish the task (e.g., Friedman et al. 1997; Daffner et al. 2006; West et al. 2010). In the present study, a prominent P3 anterior shift was obtained in our NC subjects, but not in the FXTAS group. The absence of the P3 anterior shift in FXTAS likely reflects decreased recruitment of frontal lobe processes helpful in performing our dual-response oddball task including the counting of target tones. In fact, prior studies requiring target counting during a 2-stimuli auditory oddball task have shown substantial target P3 at Fz in young normal subjects (e.g., Fjell and Walhovd 2001; Walhovd and Fjell 2003).
It has been recently proposed that every P3 component is composed of P3a and P3b (Polich 2007, 2012). In this framework, the anterior P3 (P3a) reflects attention orienting to salient or unexpected events and is generated by change in frontal lobe activation pattern, whereas the P3b is associated with working memory updating and mainly generated by temporal/parietal cortex (Soltani and Knight 2000; Polich 2003; also see Donchin 1981; Donchin and Coles 1988, for the context-updating theory of P3). Since selective attention and working memory are key components of executive functioning and are closely related to maintaining and updating of attentional set, this framework provides explanation for the frequently observed associations between P3 measures and executive function performance (e.g., Barceló et al. 1997, 2000; Daffner et al. 2006; Dichter et al. 2006; Kopp et al. 2006; Fjell et al. 2007). According to this view, the delayed and diminished fronto-central P3 in FXTAS patients could be interpreted as the impaired P3a indexing attention-related deficits, while the reduced amplitude of the parietal P3b indicated compromised working memory updating processes. The correlations between P3 measures and attention/working memory tests in our study lend support to this general interpretation in which the P3 is composed of both attention and working memory processes. It should also be noted that the fronto-central P3 elicited in NC subjects by our dual-response oddball task is likely distinct from the “classic” P3a, elicited by task-irrelevant stimuli. The classic P3a is thought to more directly reflect the CNS orienting response (Courchesne et al. 1975; Knight 1984).
In summary, the P3 abnormalities we observed in FXTAS demonstrate dysfunction of the fronto-parietal attentional network in these patients. The diminished and delayed fronto-central P3 associates with compromised task-related attention allocation. On the other hand, our main measures of working memory tended to correlate with more posterior measures of P3 latency, which may reflect the temporal dynamics of working memory updating. This impaired fronto-parietal brain dynamics observed on our P3 paradigm appeared to be central to the executive dysfunction in FXTAS.
With its sensitivity to the primary cognitive impairment, that is, executive dysfunction, in FXTAS, P3 could provide a promising electrophysiological marker to examine early or prodromal stage disease may or may not related to FXTAS, in FMR1 premutation carriers. Although FXTAS typically has a late onset of over 55–60 years of age, self-reported attention deficit was found to be associated with CGG repeat length within female premutation carriers under age of 50 (Hunter et al. 2008). Sévin et al. (2009) suggested that the cognitive impairments in FXTAS may develop prior to motor symptoms. The age when subtle abnormalities in both cognitive and motor function begin in FXTAS remains unknown and new findings suggest that the FMR1 gene premutation may affect early brain development (Farzin et al. 2006; Bailey et al. 2008), even in the embryonic stage (Chen et al. 2010; Garcia-Arocena and Hagerman 2010; Cunningham et al. 2011).
One limitation of the current study is that the correlation coefficients between P3 measures and executive function tests were only moderately strong. Although these are comparable to the correlation coefficients commonly observed between different executive function tests (Gilbert and Burgess 2008), stronger relationship may be obtained by recording simultaneous EEG while FXTAS patients are performing executive function tests such as the WCST (e.g., Barceló et al. 2000). Such concurrent ERP/EEG studies can also examine the neural mechanisms associated with planning, error detection, and other subcomponents of executive function in FXTAS.
Another limitation is that we did not measure the fragile X protein FMRP levels in most of this cohort. FMRP is an mRNA-binding protein thought to act as a master regulator of many synaptic proteins (e.g., Liu-Yesucevitz et al. 2011), including the translation of message RNA to produce FMRP itself. It is intriguing to note that the cognitive dysfunction most characteristic of FXTAS involves regulation, both of higher-level behavior and of competing task demands. We believe that the dual-response P3 paradigm is capable of detecting these aspects of frontal executive dysfunction in mild FXTAS.
The P3 oddball paradigm and other cognitive ERP paradigms may be particularly valuable in the early detection of cognitive slowing in FXTAS, because of the millisecond-level temporal resolution which ERPs offer (Nunez and Srinivasan 2006). Furthermore, many studies including the current report have confirmed that P3 latency is not determined by response selection and execution (e.g., Duncan-Johnson and Kopell 1981; Ilan and Polich 1999); rather, it is thought to measure central processing speed and allocation of cognitive resources without contamination by motor problems (e.g., tremor in FXTAS, motor slowing due to the cortical-spinal tract, or peripheral nerve disease). Finally, as a noninvasive and relatively simple task, this P3 paradigm could be useful in clinical applications such as tracking disease progression in FXTAS or response to targeted treatments for FXTAS.
Funding
This work was supported by the National Institutes of Health Roadmap Interdisciplinary Research Consortium Grant, by grant numbers UL1DE019583 (NIDCR), RL1AG032115 (National Institutes on Aging), and RL1AG032119 (National Institutes on Aging), and by National Institutes on Aging grant AG18442.
Notes
We thank Jamie Pak, Lillian Chi, and Christa Simon for help with data collection and data preprocessing, Kelsey Laird for help on an early draft of the manuscript, and Danielle Harvey for statistical consultation. Conflict of Interest: Dr Randi J. Hagerman receives research support from Neuropharm, Seaside therapeutics, Forest, Johnson and Johnson and Roche and consultation with Novartis for fragile X research studies.
References
- Allen E, He W, Yadav-Shah M, Sherman S. A study of the distributional characteristics of FMR1 transcript levels in 238 individuals. Hum Genet. 2004;114:439–447. doi: 10.1007/s00439-004-1086-x. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Jr, Raspa M, Olmsted M, Holiday DB. Co-occurring conditions associated with FMR1 gene variations: findings from a national parent survey. Am J Med Genet A. 2008;16:2060–2069. doi: 10.1002/ajmg.a.32439. [DOI] [PubMed] [Google Scholar]
- Banich MT. Executive function: the search for an integrated account. Curr Dir Psychol Sci. 2009;18:89–94. [Google Scholar]
- Barceló F, Muñoz-Céspedes JM, Pozo MA, Rubia FJ. Attentional set shifting modulates the target P3b response in the Wisconsin card sorting test. Neuropsychologia. 2000;38:1342–1355. doi: 10.1016/s0028-3932(00)00046-4. [DOI] [PubMed] [Google Scholar]
- Barceló F, Sanz M, Molina V, Rubia FJ. The Wisconsin Card Sorting Test and the assessment of frontal function: a validation study with event-related potentials. Neuropsychologia. 1997;35:399–408. doi: 10.1016/s0028-3932(96)00096-6. [DOI] [PubMed] [Google Scholar]
- Brázdil M, Roman R, Daniel P, Rektor I. Intracerebral somatosensory event-related potentials: effect of response type (button pressing versus mental counting) on P3-like potentials within the human brain. Clin Neurophysiol. 2003;114:1489–1496. doi: 10.1016/s1388-2457(03)00135-4. [DOI] [PubMed] [Google Scholar]
- Brega AG, Goodrich G, Bennett RE, Hessl D, Engle K, Leehey MA, Bounds LS, Paulich MJ, Hagerman RJ, Hagerman PJ, et al. The primary cognitive deficit among males with fragile X-associated tremor/ataxia syndrome (FXTAS) is a dysexecutive syndrome. J Clin Exp Neuropsychol. 2008;30:853–869. doi: 10.1080/13803390701819044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Tassone F, Berman RF, Hagerman PJ, Hagerman RJ, Willemsen R, Pessah IN. Murine hippocampal neurons expressing Fmr1 gene premutations show early developmental deficits and late degeneration. Hum Mol Genet. 2010;19:196–208. doi: 10.1093/hmg/ddp479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SM, Cook K, Tartaglia N, Tassone F, Nguyen DV, Pan R, Bronsky HE, Yuhas J, Borodyanskaya M, Grigsby J, et al. Expanded clinical phenotype of women with the FMR1 premutation. Am J Med Genet. 2008;146:1009–1016. doi: 10.1002/ajmg.a.32060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish KM, Kogan CS, Li L, Turk J, Jacquemont S, Hagerman RJ. Lifespan changes in working memory in fragile X premutation males. Brain Cogn. 2009;69:551–558. doi: 10.1016/j.bandc.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish KM, Li L, Kogan CS, Jacquemont S, Turk J, Dalton A, Hagerman RJ, Hagerman PJ. Age-dependent cognitive changes in carriers of the fragile X syndrome. Cortex. 2008;44:628–636. doi: 10.1016/j.cortex.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Hillyard SA, Galambos R. Stimulus novelty, task relevance and the visual evoked potential in man. Electroencephalogr Clin Neurophysiol. 1975;39:131–143. doi: 10.1016/0013-4694(75)90003-6. [DOI] [PubMed] [Google Scholar]
- Crottaz-Herbette S, Menon V. Where and when the anterior cingulate cortex modulates attentional response: combined fMRI and ERP evidence. J Cogn Neurosci. 2006;18:766–780. doi: 10.1162/jocn.2006.18.5.766. [DOI] [PubMed] [Google Scholar]
- Crowley KE, Colrain IM. A review of the evidence for P2 being an independent component process: age, sleep and modality. Clin Neurophysiol. 2004;115:732–744. doi: 10.1016/j.clinph.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Martínez Cerdeño V, Navarro Porras E, Prakash AN, Angelastro JM, Willemsen R, Hagerman PJ, Pessah IN, Berman RF, Noctor SC. Premutation CGG-repeat expansion of the FMR1 gene impairs mouse neocortical development. Hum Mol Genet. 2011;20:64–79. doi: 10.1093/hmg/ddq432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffner KR, Ryan KK, Williams DM, Budson AE, Rentz DM, Wolk DA, Holcomb PJ. Increased responsiveness to novelty is associated with successful cognitive aging. J Cogn Neurosci. 2006;18:1759–1773. doi: 10.1162/jocn.2006.18.10.1759. [DOI] [PubMed] [Google Scholar]
- Delis D, Kramer J, Kaplan E, Ober B. California Verbal Learning Test. San Antonio, TX: The Psychological Corporation. 1987. [Google Scholar]
- DesRosiers G, Kavanagh D. Cognitive assessment in closed head injury: stability, validity and parallel forms for two neuropsychological measures of recovery. Int J Clin Neuropsyc. 1987;9:162–173. [Google Scholar]
- Dichter GS, van der Stelt O, Boch JL, Belger A. Relations among intelligence, executive function, and P300 event related potentials in schizophrenia. J Nerv Ment Dis. 2006;194:179–187. doi: 10.1097/01.nmd.0000202490.97425.de. [DOI] [PubMed] [Google Scholar]
- Diesfeldt HF. Executive functioning in psychogeriatric patients: scalability and construct validity of the Behavioral Dyscontrol Scale (BDS) Int J Geriatr Psychiatry. 2004;19:1065–1073. doi: 10.1002/gps.1212. [DOI] [PubMed] [Google Scholar]
- Donchin E. Surprise! … Surprise? Psychophysiology. 1981;18:493–513. doi: 10.1111/j.1469-8986.1981.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Donchin E, Coles MGH. Is the P300 component a manifestation of context updating? Behav Brain Sci. 1988;11:357–374. [Google Scholar]
- Duncan-Johnson CC, Kopell BS. The Stroop effect: brain potentials localize the source of interference. Science. 1981;214:938–940. doi: 10.1126/science.7302571. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Friedman D, Cheng JC. Individual differences in P3 scalp distribution in older adults, and their relationship to frontal lobe function. Psychophysiology. 1998;35:698–708. [PubMed] [Google Scholar]
- Farzin F, Perry H, Hessl D, Loesch D, Cohen J, Bacalman S, Gane L, Tassone F, Hagerman P, Hagerman R. Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J Dev Behav Pediatr. 2006;27:S137–S144. doi: 10.1097/00004703-200604002-00012. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB. P300 and neuropsychological tests as measures of aging: scalp topography and cognitive changes. Brain Topogr. 2001;14:25–40. doi: 10.1023/a:1012563605837. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Fischl B, Reinvang I. Cognitive function, P3a/P3b brain potentials, and cortical thickness in aging. Hum Brain Mapp. 2007;28:1098–1116. doi: 10.1002/hbm.20335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D, Kazmerski V, Fabiani M. An overview of age-related changes in the scalp distribution of P3b. Electroencephalogr Clin Neurophysiol. 1997;104:498–513. doi: 10.1016/s0168-5597(97)00036-1. [DOI] [PubMed] [Google Scholar]
- Garcia-Arocena D, Hagerman PJ. Advances in understanding the molecular basis of FXTAS. Hum Mol Genet. 2010;19:R83–R89. doi: 10.1093/hmg/ddq166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates GA, Cooper JC, Jr, Kannel WB, Miller NJ. Hearing in the elderly: the Framingham cohort, 1983–1985. Part I. Basic audiometric test results. Ear Hear. 1990;11:247–256. [PubMed] [Google Scholar]
- Geisser S, Greenhouse S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. [Google Scholar]
- Gilbert SJ, Burgess PW. Executive function. Curr Biol. 2008;18:R110–R114. doi: 10.1016/j.cub.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Greco CM, Berman RF, Martin RM, Tassone F, Schwartz PH, Chang A, Trapp BD, Iwahashi C, Brunberg J, Grigsby J, et al. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS) Brain. 2006;129:243–255. doi: 10.1093/brain/awh683. [DOI] [PubMed] [Google Scholar]
- Grigsby J, Brega AG, Engle K, Leehey MA, Hagerman RJ, Tassone F, Hessl D, Hagerman PJ, Cogswell JB, Bennett RE, et al. Cognitive profile of fragile X premutation carriers with and without fragile X-associated tremor/ataxia syndrome. Neuropsychology. 2008;22:48–60. doi: 10.1037/0894-4105.22.1.48. [DOI] [PubMed] [Google Scholar]
- Grigsby J, Brega AG, Jacquemont S, Loesch DZ, Leehey MA, Goodrich GK, Hagerman RJ, Epstein J, Wilson R, Cogswell JB, et al. Impairment in the cognitive functioning of men with fragile X-associated tremor/ataxia syndrome (FXTAS) J Neurol Sci. 2006;248:227–233. doi: 10.1016/j.jns.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Grigsby J, Brega AG, Leehey MA, Goodrich GK, Jacquemont S, Loesch DZ, Cogswell JB, Epstein J, Wilson R, Jardini T, et al. Impairment of executive cognitive functioning in males with fragile X-associated tremor/ataxia syndrome (FXTAS) Mov Disord. 2007;22:645–650. doi: 10.1002/mds.21359. [DOI] [PubMed] [Google Scholar]
- Grigsby J, Kaye K. The behavioral dyscontrol scale: manual. 2nd ed. Denver, CO: BDS; 1996. [Google Scholar]
- Hagerman RJ, Leehey M, Heinrichs W, Tassone F, Wilson R, Hills J, Grigsby J, Gage B, Hagerman PJ. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology. 2001;57:127–130. doi: 10.1212/wnl.57.1.127. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Backer KC, Tassone F, Hagerman RJ, Rivera SM. An fMRI study of the prefrontal activity during the performance of a working memory task in premutation carriers of the fragile X mental retardation 1 gene with and without fragile X-associated tremor/ataxia syndrome (FXTAS) J Psychiatr Res. 2011c;45:36–43. doi: 10.1016/j.jpsychires.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto R, Javan AK, Tassone F, Hagerman RJ, Rivera SM. A voxel-based morphometry study of grey matter loss in fragile X-associated tremor/ataxia syndrome. Brain. 2011a;134:863–878. doi: 10.1093/brain/awq368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto R, Srivastava S, Tassone F, Hagerman RJ, Rivera SM. Diffusion tensor imaging in male premutation carriers of the fragile X mental retardation gene. Mov Disord. 2011b;26:1329–1336. doi: 10.1002/mds.23646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann CS, Knight RT. Mechanisms of human attention: event-related potentials and oscillations. Neurosci Biobehav Rev. 2001;25:465–476. doi: 10.1016/s0149-7634(01)00027-6. [DOI] [PubMed] [Google Scholar]
- Hunter JE, Allen EG, Abramowitz A, Rusin M, Leslie M, Novak G, Hamilton D, Shubeck L, Charen K, Sherman SL. No evidence for a difference in neuropsychological profile among carriers and noncarriers of the FMR1 premutation in adults under the age of 50. Am J Hum Genet. 2008;83:692–702. doi: 10.1016/j.ajhg.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilan AB, Polich J. P300 and response time from a manual Stroop task. Clin Neurophysiol. 1999;110:367–373. doi: 10.1016/s0168-5597(98)00053-7. [DOI] [PubMed] [Google Scholar]
- Iragui VJ, Kutas M, Mitchiner MR, Hillyard SA. Effects of aging on event-related brain potentials and reaction times in an auditory oddball task. Psychophysiology. 1993;30:10–22. doi: 10.1111/j.1469-8986.1993.tb03200.x. [DOI] [PubMed] [Google Scholar]
- Jacquemont S, Hagerman RJ, Hagerman PJ, Leehey MA. Fragile-X syndrome and fragile X-associated tremor/ataxia syndrome: two faces of FMR1. Lancet Neurol. 2007;6:45–55. doi: 10.1016/S1474-4422(06)70676-7. [DOI] [PubMed] [Google Scholar]
- Jacquemont S, Hagerman RJ, Leehey M, Grigsby J, Zhang L, Brunberg JA, Greco C, Des Portes V, Jardini T, Levine R, et al. Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am J Hum Genet. 2003;72:869–878. doi: 10.1086/374321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jausovec N, Jausovec K. Correlations between ERP parameters and intelligence: a reconsideration. Biol Psychol. 2000;55:137–154. doi: 10.1016/s0301-0511(00)00076-4. [DOI] [PubMed] [Google Scholar]
- Juncos JL, Lazarus JT, Graves-Allen E, Shubeck L, Rusin M, Novak G, Hamilton D, Rohr J, Sherman SL. New clinical findings in the fragile X-associated tremor ataxia syndrome (FXTAS) Neurogenetics. 2011;12:123–135. doi: 10.1007/s10048-010-0270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenneson A, Zhang F, Hagedorn CH, Warren ST. Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum Mol Genet. 2001;10:1449–1454. doi: 10.1093/hmg/10.14.1449. [DOI] [PubMed] [Google Scholar]
- Knight RT. Decreased response to novel stimuli after prefrontal lesions in man. Electroencephalogr Clin Neurophysiol. 1984;59:9–20. doi: 10.1016/0168-5597(84)90016-9. [DOI] [PubMed] [Google Scholar]
- Kopp B, Tabeling S, Moschner C, Wessel K. Fractionating the neural mechanisms of cognitive control. J Cogn Neurosci. 2006;18:949–965. doi: 10.1162/jocn.2006.18.6.949. [DOI] [PubMed] [Google Scholar]
- Linden DE. The p300: where in the brain is it produced and what does it tell us? Neuroscientist. 2005;11:563–576. doi: 10.1177/1073858405280524. [DOI] [PubMed] [Google Scholar]
- Liu-Yesucevitz L, Bassell GJ, Gitler AD, Hart AC, Klann E, Richter JD, Warren ST, Wolozin B. Local RNA translation at the synapse and in disease. J Neurosci. 2011;31:16086–16093. doi: 10.1523/JNEUROSCI.4105-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low KA, Leaver E, Kramer AF, Fabiani M, Gratton G. Fast optical imaging of frontal cortex during active and passive oddball tasks. Psychophysiology. 2006;43:127–136. doi: 10.1111/j.1469-8986.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- Luck SJ. An introduction to the event-related potential technique. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Hillyard SA. Mechanisms and models of selective attention. In: Rugg D, Coles MGH, editors. Electrophysiology of mind eventrelated potentials and cognition. New York, NY: Oxford University Press; 1995. pp. 86–131. [Google Scholar]
- McGarry-Roberts PA, Stelmack RM, Campbell KB. Intelligence, reaction time, and event-related potentials. Intelligence. 1992;16:289–313. [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Picton TW. N2 and automatic versus controlled processes. Electroencephalogr Clin Neurophysiol Suppl. 1986;38:169–186. [PubMed] [Google Scholar]
- Nordin S, Andersson L, Olofsson JK, McCormack M, Polich J. Evaluation of auditory, visual and olfactory event-related potentials for comparing interspersed- and single-stimulus paradigms. Int J Psychophysiol. 2011;81:252–262. doi: 10.1016/j.ijpsycho.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. Electric fields of the brain: the neurophysics of EEG. 2nd Ed. New York, NY: Oxford University Press; 2006. pp. 163–166. [Google Scholar]
- Olichney JM, Chan S, Wong LM, Schneider A, Seritan A, Niese A, Yang JC, Laird K, Teichholtz S, Khan S, et al. Abnormal N400 word repetition effects in fragile X-associated tremor/ataxia syndrome. Brain. 2010;133:1438–1450. doi: 10.1093/brain/awq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang S, Borod JC, Hernandez A, Bodis-Wollner I, Raskin S, Mylin L, Coscia L, Yahr MD. The auditory P300 correlates with specific cognitive deficits in Parkinson's disease. J Neural Transm Park Dis Dement Sect. 1990;2:249–264. doi: 10.1007/BF02252920. [DOI] [PubMed] [Google Scholar]
- Peters N, Kamm C, Asmus F, Holinski-Feder E, Kraft E, Dichgans M, Brüning R, Gasser T, Bötzel K. Intrafamilial variability in fragile X-associated tremor/ataxia syndrome. Mov Disord. 2006;21:98–102. doi: 10.1002/mds.20673. [DOI] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, Jr, Miller GA, Ritter W, Ruchkin DS, Rugg MD, et al. Guidelines for using human event-related potentials to study cognition: recording standards and publication criteria. Psychophysiology. 2000;37:127–152. [PubMed] [Google Scholar]
- Polich J. Neuropsychology of P300. In: Luck SJ, Kappenman ES, editors. Oxford handbook of event-related potential components. New York, NY: Oxford University Press; 2012. pp. 159–188. [Google Scholar]
- Polich J. Overview of P3a and P3b. In: Polich J, editor. Detection of change: eventrelated potential and fMRI findings. Boston, MA: Kluwer Academic Publishers; 2003. pp. 83–98. [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault B, Ragot R, Lesevre N, Remond A. Onset and offset of brain events as indices of mental chronometry. Science. 1982;215:1413–1415. doi: 10.1126/science.7063853. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Revenga L, Madrigal I, Pagonabarraga J, Xunclà M, Badenas C, Kulisevsky J, Gomez B, Milà M. Penetrance of FMR1 premutation associated pathologies in fragile X syndrome families. Eur J Hum Genet. 2009;17:1359–1362. doi: 10.1038/ejhg.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sévin M, Kutalik Z, Bergman S, Vercelletto M, Renou P, Lamy E, Vingerhoets FJ, Di Virgilio G, Boisseau P, Bezieau S, et al. Penetrance of marked cognitive impairment in older male carriers of the FMR1 gene premutation. J Med Genet. 2009;46:818–824. doi: 10.1136/jmg.2008.065953. [DOI] [PubMed] [Google Scholar]
- Smith A. Symbol Digit Modalities Test: manual. Los Angeles, CA: Western Psychological Services; 1982. [Google Scholar]
- Soltani M, Knight R. Neural origins of the P300. Crit Rev Neurobiol. 2000;14:199–224. [PubMed] [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests. 2nd ed. New York, NY: Oxford University Press; 1998. [Google Scholar]
- Tassone F, Beilina A, Carosi C, Albertosi S, Bagni C, Li L, Glover K, Bentley D, Hagerman PJ. Elevated FMR1 mRNA in premutation carriers is due to increased transcription. RNA. 2007;13:555–562. doi: 10.1261/rna.280807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Taylor AK, Gane LW, Godfrey TE, Hagerman PJ. Elevated levels of FMR1mRNAin carrier males: a new mechanism of involvement in the fragile-X syndrome. Am J Hum Genet. 2000;66:6–15. doi: 10.1086/302720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassone F, Pan R, Amiri K, Taylor AK, Hagerman PJ. A rapid polymerase chain reaction-based screening method for identification of all expanded alleles of the fragile X (FMR1) gene in newborn and highrisk populations. J Mol Diagn. 2008;10:43–49. doi: 10.2353/jmoldx.2008.070073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM. The relationship between P3 and neuropsychological function in an adult life span sample. Biol Psychol. 2003;62:65–87. doi: 10.1016/s0301-0511(02)00093-5. [DOI] [PubMed] [Google Scholar]
- West R, Schwarb H, Johnson BN. The influence of age and individual differences in executive function on stimulus processing in the oddball task. Cortex. 2010;46:550–563. doi: 10.1016/j.cortex.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Willemsen R, Levenga J, Oostra BA. CGG repeat in the FMR1 gene: size matters. Clin Genet. 2011;80:214–225. doi: 10.1111/j.1399-0004.2011.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurrón M, Pouso M, Lindín M, Galdo S, Díaz F. Event-related potentials with the Stroop colour-word task: timing of semantic conflict. Int J Psychophysiol. 2009;72:246–252. doi: 10.1016/j.ijpsycho.2009.01.002. [DOI] [PubMed] [Google Scholar]