Abstract
Human immunodeficiency virus type 1 (HIV-1) Vpr is a 15-kDa accessory protein that contributes to several steps in the viral replication cycle and promotes virus-associated pathology. Previous studies demonstrated that Vpr inhibits G2/M cell cycle progression in both human cells and in the fission yeast Schizosaccharomyces pombe. Here, we report that, upon induction of vpr expression, fission yeast exhibited numerous defects in the assembly and function of the mitotic spindle. In particular, two spindle pole body proteins, sad1p and the polo kinase plo1p, were delocalized in vpr-expressing yeast cells, suggesting that spindle pole body integrity was perturbed. In addition, nuclear envelope structure, contractile actin ring formation, and cytokinesis were also disrupted. Similar Vpr-induced defects in mitosis and cytokinesis were observed in human cells, including aberrant mitotic spindles, multiple centrosomes, and multinucleate cells. These defects in cell division and centrosomes might account for some of the pathological effects associated with HIV-1 infection.
INTRODUCTION
Lentiviruses possess a number of accessory genes in addition to the gag, pol, and env genes common to all retroviruses. Human immunodeficiency virus type 1 (HIV-1), the lentivirus most commonly associated with the acquired immunodeficiency syndrome, carries six accessory genes that are required either for full viral replication capacity or for pathogenicity. Vpr is a 15-kDa accessory protein that is virion-associated (Cohen et al., 1990; Yuan et al., 1990; Paxton et al., 1993) and transported to the nucleus as part of the viral preintegration complex (Heinzinger et al., 1994; Fassati and Goff, 2001). All primate immunodeficiency viruses possess either Vpr or the closely related protein Vpx (Tristem et al., 1992). The functional importance of Vpr is demonstrated by the fact that an intact vpr open reading frame is selected for in vivo: repair of a disrupted vpr open reading frame was observed in chimps, or the rare human, in which there was documented infection with a provirus bearing vpr mutations (Goh et al., 1998). Vpr variants have been correlated with long-term survival in HIV-1-infected individuals (Zhao et al., 2002). Vpr increases virus replication rates in macrophages in standard tissue culture systems (Connor et al., 1995; Vodicka et al., 1998) and in tonsil histoculture (Eckstein et al., 2001). Increased virus replication rates in macrophages may be related to effects of Vpr on nuclear transport of preintegration complexes upon entry into these nondividing cells (Heinzinger et al., 1994). Many laboratory strains of HIV viruses lack vpr, because Vpr inhibits growth of cells in culture (Rogel et al., 1995). Vpr thus contributes to HIV replication and pathogenesis.
Numerous effects of Vpr on the cell have been reported, including disruption of cell cycle control, enhancement of transcription, and induction of apoptosis. One relatively well characterized function is the arrest of proliferating CD4+ T cells in the G2 stage of the cell cycle (He et al., 1995; Jowett et al., 1995; Re et al., 1995; Rogel et al., 1995; Bartz et al., 1996). This provides a replication advantage for the virus because proviral transcription is highest during G2 (Forget et al., 1998; Goh et al., 1998). In human or fission yeast cells expressing vpr, cdc2p, the cyclin-dependent kinase that regulates entry into mitosis, is in the inactive phosphorylated state (He et al., 1995; Re et al., 1995; Zhao et al., 1996; Zhang et al., 1997). Vpr does not seem to be a stoichiometric inhibitor of the cdc2p-cyclin B complex, but rather, may exert effects on regulators of cdc2p, such as cdc25C, Wee1, or PP2A (He et al., 1995; Re et al., 1995; Zhao et al., 1996; Elder et al., 2000, 2001; Masuda et al., 2000).
A second major function of Vpr is on nuclear transport. Vpr promotes nuclear entry of the viral preintegration complex and colocalizes with nuclear pores on the nuclear envelope (Heinzinger et al., 1994; Vodicka et al., 1998). Recent studies show that Vpr expression in mammalian cells causes herniations and large holes in the nuclear envelope, which may facilitate nuclear entry of viral preintegration complexes and disrupt the localization of cell cycle regulatory proteins (de Noronha et al., 2001).
Here, we have characterized additional effects of Vpr in both fission yeast and human cells. Vpr caused multiple defects in cell division, including effects on the mitotic spindle, centrosome/spindle pole body (SPB), nuclear envelope, and cytokinesis. Because the centrosome/SPB acts not only as a microtubule-organizing center but also as a repository for many cell cycle regulatory factors (Lange, 2002), defects in the SPB/centrosome might explain some of the multiple effects of Vpr on the cell division cycle.
MATERIALS AND METHODS
Plasmid DNAs
HIV-1NL4-3 vpr was polymerase chain reaction (PCR) amplified using Pfu DNA polymerase (Stratagene, La Jolla, CA) and the following primers: GCGCGGATCCGAGAGATGGAACAAGCCCCAGAAGAC and GCGCGGATCCCTAGGATCTACTGGCTCCATTTC. To construct a hemagglutinin (HA) epitope tagged vpr, HIV-1NL4-3 vpr was PCR amplified using Pfu DNA polymerase (Stratagene) and the following primers: 5′-GCGCGGATCCGCCACCATGGGTTACCCATACGATGTTCCAGATTACGCTGAACAAGCCCCAGAAGACCAAGG-3′ (HA sequence underlined) and 5′-GCGCGGATCCCTAGGATCTACTGGCTCCATTTCTTGC-3′. The PCR products were digested with BamHI, cloned into the BamHI site of pBluescript II KS- (Stratagene) and sequenced. For thiamine-regulated expression in Schizosaccharomyces pombe, BamHI fragments containing either vpr or HA-vpr sequences were then cloned into the BamHI site of pREP1N (Maundrell, 1993). For expression in mammalian cells, a SpeI/XhoI fragment containing HA-vpr was inserted into the NheI and XhoI sites of pCep4 (Invitrogen, Carlsbad, CA). The bicistronic vector pVpr-IRES-EGFP was constructed by inserting the BamHI fragment containing the vpr sequence into the BglII site of pIRES2-EGFP (BD Biosciences Clontech, Palo Alto, CA).
Fission Yeast Methods
Standard methods for S. pombe media, genetic manipulations, immunofluorescence, and staining are described at http://www.bio.uva.nl/pombe/handbook/. S. pombe strains used were FC16 (h- leu1-32) and FC840 (h- leu1-32 plo1-GFP-kanMX) (Bahler et al., 1998). Yeast transformations were performed using a Frozen EZ yeast transformation kit (Zymo Research, Orange, CA). Fresh pnmt-vpr transformants were characterized within 1 or 2 wk after transformation, because cells that had been passaged repeatedly or frozen previously exhibited less consistent phenotypes. For induction of the nmt1 promoter, exponential phase liquid cultures of cells in EMM with thiamine were filtered, washed in water, and then resuspended in prewarmed Edinburgh minimal media (EMM) without thiamine and grown at 30°C.
To monitor nuclear envelope defects, we used a S. pombe strain in which an SV40 NLS-GFP-LacZ nuclear reporter and an Nsp1p-GFP nucleoporin that localizes to the nuclear envelope are expressed by the nmt1 gene promoter from constructs integrated into the chromosome (Yoshida and Sazer, 2004). For immunofluorescence, methanol-fixed cells were stained with a monoclonal anti-tubulin antibody TAT1 (Woods et al., 1989) (gift from K. Gull, University of Oxford, Oxford, United Kingdom) and rabbit anti-sad 1p antibody (Hagan and Yanagida, 1995) (gift from I. Hagan, University of Manchester, Manchester, United Kingdom) at a 1/100 dilution. Rhodamine-phalloidin (Molecular Probes, Eugene, OR) staining was performed as described previously (Chang et al., 1996a) using a 4-min formaldehyde fixation period.
Culture and Transfection of Human Cells
HeLa cells were obtained from American Type Culture Collection (Manassas, VA) and maintained in DMEM with 10% fetal calf serum. Pooled human umbilical vein endothelial cells (HUVECs) were purchased from Cambrex Bio Science Walkersville (San Diego, CA). HUVECs were grown on gelatin-coated petri dishes and maintained <10 passages in human endothelial-SFM basal growth medium (Invitrogen) supplemented with 20% fetal calf serum (Invitrogen), 10 ng/ml human recombinant epidermal growth factor (Intergen, Purchase, NY), 15 ng/ml human recombinant basic fibroblast growth factor (Intergen), and 1 μg/ml heparin (Sigma-Aldrich). For transfection, HeLa and HUVECs were briefly trypsinized, washed in DMEM with 10% fetal calf serum, and resuspended in ice-cold phosphate-buffered saline (PBS) with 1.25% dimethyl sulfoxide at a concentration of 2 × 107 cells/ml. Then, 300 μl of the cell suspension was electroporated with 30 μg of plasmid DNA at 250 V, 960 μF by using an electroporator (Bio-Rad, Hercules, CA). Hygromycin concentrations used for plasmid selection were 750 μg/ml for HeLa cells and 100 μg/ml for HUVECs. DNA content was measured by flow cytometry after stained with propidium iodide as described previously (Re et al., 1995).
Microscopy
Microscopy was performed using a wide field fluorescence microscope as described previously (Pelham and Chang, 2001) by using Open Lab 2 software (Improvision, Lexington, MA) for acquisition and analysis. In some cases, multiple focal planes were acquired and used to assemble deconvolved maximum projections.
Microscopic Examination of Human Cells
HeLa cells grown on glass coverslips were fixed in PBS with 4% paraformaldehyde for 30 min, permeabilized in PBS with 0.1% Triton X-100, and mounted in Vectashield (Vector Laboratories, Burlingame, CA) containing 1 μg/ml 4,6-diamidino-2-phenylindole (DAPI). HUVECs were photographed on an inverted phase-contrast microscope. For immunofluorescence staining, cells were fixed in methanol at -20°C for 20 min and permeabilized in PBS/0.1% Tween 20. Coverslips were incubated with primary antibody in PBS/3% bovine serum albumin for 1 h, washed three times, and incubated with secondary antibodies in PBS/3% bovine serum albumin/0.1% Tween 20 for 30 min. The following antibodies and dilutions were used: anti-γ-tubulin GT335, mouse IgG, 1:1000; and anti Centrin-2, rabbit serum, 1:2000. They were both kindly provided by Anne Paoletti (Institute Curie, Paris, France). The following secondary antibodies were used: Alexa Fluor 594 goat anti-mouse IgG (Molecular Probes) and phycoerythrin-conjugated goat anti-rabbit. Both were used at 1:1000 dilution. For images of mitotic spindles, images were acquired in multiple focal planes and then were deconvolved and maximum projected using OpenLab 3 image analysis software (Improvision, Lexington, MA).
RESULTS
vpr Expression Causes Mitotic Spindle Defects
The effects of HIV-1 Vpr on S. pombe were examined using a vector in which expression was under the control of the thiamine-repressible nmt1 promoter. In the presence of thiamine, cells showed little or no inhibition in growth rate (Figure 1A). In the absence of thiamine, vpr expression caused strong inhibition of colony formation (Figure 1A).
Figure 1.
Nuclear division defects in S. pombe expressing vpr. (A) Fission yeast cells carrying pREP1N vector or pREP-VPR were streaked onto minimal plates without thiamine (nmt1 promoter on) or with thiamine (nmt1 promoter off). (B) Cells stained with DAPI to visualize DNA and calcofluor to visualize septum. Left, wild-type cells. Right, cells with pREP-VPR 17 h after shift into media lacking thiamine. These panels show septated cells that have a failure in nuclear division.
Fission yeast expressing Vpr were analyzed in time courses 17-24 h after removal of thiamine. Previous studies showed that Vpr expression in S. pombe is associated with an accumulation of elongated cells that are delayed or arrested at the G2/M cell cycle transition (Zhao et al., 1996; Zhang et al., 1997). We found that cells began to exhibit elongated cell shapes by 19 h and lengthened progressively by 24 h. We noticed that after 17 h in thiamine-free media, before significant cell elongation, 95% of cells with a septum (7% of total cells; n = 100) exhibited a “cut” phenotype, in which the nucleus failed to divide and was sometimes cut by the septum or was dispersed unevenly between the two daughter cells (Figure 1B). Because this phenotype is indicative of mitotic defects, this finding suggested that Vpr may affect nuclear division before development of a G2 arrest.
To characterize these mitotic defects, we visualized mitotic spindles by staining cells for tubulin, DNA, and the spindle pole body marker sad1p. In cells carrying the vector only, bipolar spindles were organized from two discrete spindle pole bodies located at the nuclear membrane (Figure 2A). In vpr-expressing cells, many types of mitotic defects were seen, including monopolar spindles, V-shaped spindles, and lagging chromosomes during anaphase (Figure 2, B and C). At 19 h after thiamine removal, 20% of the cells were in mitosis (by tubulin staining), and of these (n = 113 mitotic cells), 59% exhibited an abnormal spindle, with 51% showing some defect in mitotic spindle assembly. Twenty-eight percent of mitotic cells had a monopolar spindle, in which a dot or a single bar of microtubules emanated from one sad1p-staining dot, which can be caused by a defect in spindle pole body duplication or separation. Twenty-three percent of the spindles were abnormal V-shaped spindles, in which two or more intranuclear microtubule bundles were associated with a single sad1p-staining dot, which can be caused by mitotic spindle collapse or by defects in spindle pole body separation. These patterns suggest defects in spindle pole body function, separation, and duplication. We also noted lagging chromosomes during anaphase, indicative of chromosomal segregation defects. There were also some SPB defects in interphase cells, notably premature duplication or separation of spindle pole bodies (Figure 2, cell 2). Similar defects in mitotic spindles were also seen after 17 h of induction (31% spindles were abnormal; n = 100). At both of these early time points, mitotic spindle abnormalities were present in cells before cell elongation, suggesting that G2 arrest did not contribute to these phenotypes.
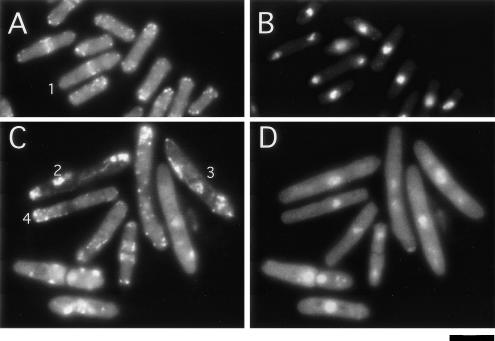
Figure 2.
Mitotic spindle defects in S. pombe expressing vpr. Cells carrying pREP1N (A) or pREP-VPR (B and C) were grown under inducing conditions for 17 h (B) or 24 h (A and C) and then fixed with methanol and stained by immunofluorescence for tubulin, sad1p, and DNA. Presented are projections of multiple focal planes. Panels are organized in sets of four, with the top (red) for tubulin, second (green) for sad1p, third (blue) for DNA, and fourth (multicolor) for merged image. Examples of particular phenotypes are indicated with numbers as follows: 1, abnormal V-shaped spindle; 2, interphase cell with two separate spindle pole bodies; 3, cell in anaphase with unequal separation of the chromosomes; 4, monopolar spindle; 5, cell in which spindle microtubules do not connect and spindle pole body is delocalized; 6, interphase cell with delocalized sad1p in a perinuclear pattern; 7 and 9, mitotic cells with perinuclear sad1p; and 8, mitotic cell with a double spindle.
One striking defect was that sad1p, a nuclear envelope transmembrane protein that is usually concentrated in one or two discrete dots (the spindle pole body) on the nuclear envelope, was sometimes delocalized in a punctuate pattern around the nuclear envelope (Figure 2C, cells 6, 7, and 9). This perinuclear distribution was present in 12% of cells at 17 h, and by 22 and 24 h, in >60% of cells (Figure 3). This sad1p pattern was seen in mitotic as well as interphase cells and occurred in nonelongated cells in the early time points. However, this phenotype was especially prevalent in elongated cells at the later time points: 92% of the elongated cells (>13 μm) at 24 h exhibited perinuclear sad1p. Many mitotic cells exhibited perinuclear sad1p staining even with a bipolar spindle (30-40% cells with a bipolar spindle at 17-19 h), suggesting that these additional sad1p dots did not represent extrafunctional spindle pole bodies. Cells in both interphase and mitosis exhibited this perinuclear pattern. Cells carrying the vector only or the vpr plasmid under repressing conditions did not exhibit these defects in mitosis or sad1p distribution. In contrast to sad1p, the distribution of the γ-tubulin complex protein alp4-GFP was not disrupted in Vpr-expressing cells, even at later time points, suggesting that only a subset of spindle pole body components were affected. Thus, Vpr disturbs mitosis in part by affecting the integrity of a portion of the spindle pole body.
Figure 3.
Progressive delocalization of the spindle pole body protein sad1p upon induction of vpr expression. Cells bearing pREP1 or pREP-VPR were grown under inducing conditions for 17, 19, 22, or 24 h. Cells were harvested, fixed, and examined by immunofluorescence for Sad1p, and imaged in three-dimensional projections; 78-200 cells were examined for each time point.
Vpr Causes Cytokinesis Defects
Cell division in S. pombe involves the formation of an actin-based contractile ring in early mitosis and then contraction of the ring and formation of the cell wall septum in late mitosis. At 17 h, vpr-expressing cells formed fairly normal septa and actin rings (Figure 1C). However, at later time points, severe defects in actin ring formation and septum formation were apparent (Figure 4; our unpublished observations). In some mitotic cells (cells with condensed chromosomes shown by DAPI staining or in cells with chromosomes in an anaphase configuration), F-actin was located in abnormal aggregates instead of a discrete ring (Figure 4, cells 2 and 3) (see also Chen et al., 1999). In some mitotic cells, actin was present as a disorganized medial web of actin. Some cells maintained normal interphase actin distribution of polarized actin patches (Figure 4, cell 4), suggesting that Vpr inhibits actin rings specifically and not other actin structures. These actin ring defects were accompanied by similar severe defects in the organization of the septum (Chen et al., 1999; our unpublished observations). Because actin ring assembly is not dependent on spindle assembly (Chang et al., 1996b), these types of cytokinesis defects were probably not a secondary effect of the mitotic defects.
Figure 4.
Vpr causes defects in the contractile actin ring. Cells bearing pREP-VPR were grown under inducing conditions for 21 h, fixed, and stained with Alexa Fluor-phalloidin (A and C) and DAPI (B and D). Cell 1 exhibits a normal actin ring. Cells 2 and 3 are mitotic cells (as evidenced by condensed chromatin) and exhibit very abnormal actin organization. Cell 4 has normal interphase actin distribution.
Vpr Causes Mislocalization of Polo Kinase
The polo kinase plo1p is a conserved regulator of multiple aspects of cell division, including mitotic entry, mitotic spindle assembly, centrosome maturation, mitotic exit, and cytokinesis (Ohkura et al., 1995; Glover et al., 1998; Bahler et al., 1998). Because many of the phenotypes of Vpr expression are similar to those of plo1-loss of function mutants, we tested the effect of Vpr on plo1-GFP localization. This plo1-GFP fusion is functional and expressed from the plo1+ chromosomal locus at endogenous levels (Bahler et al., 1998). In cells carrying the control vector, plo1-GFP was located on the spindle pole body, spindle and weakly in the nucleus during mitosis, with no detectable fluorescence in interphase cells (Figure 5A). In Vpr-expressing cells, plo1-GFP was delocalized in multiple, discrete cytoplasmic dots in some cells or concentrated in the nucleus (Figure 5B). Because abnormal plo1-GFP distribution occurred even in nonelongated cells, this effect is not secondary to G2 arrest. These results indicate that at least some of the effects of Vpr may be caused by abnormal localization of polo kinase.
Figure 5.
Vpr causes mislocalization of the polo kinase plo1p in S. pombe. Cells with a chromosomal plo1p-GFP construct and containing either pREP1 (A) or pREP-VPR (B) plasmids were grown under inducing conditions for 21 h and imaged for GFP fluorescence.
Vpr Causes Defects in the Nuclear Envelope
A recent study in mammalian tissue culture cells suggested that Vpr expression causes large herniations and holes in the nuclear envelope (de Noronha et al., 2001). We tested whether Vpr affects nuclear structure in fission yeast. We examined a strain that expressed both a nucleoporin-GFP, which marks the nuclear envelope, and a GFP-lacZ fusion containing a nuclear localization signal (NLS), a soluble marker that enters the nucleus from constructs integrated into the genome to ensure uniform expression throughout the population (Yoshida and Sazer, 2004). Expression of these fusions themselves does not affect nuclear structure or cell viability. In cells with broken nuclear envelopes, the soluble NLS-GFP leaks out of the nucleus, so that the nucleus is dimmer and exhibits the nucleoporin GFP outlining the nuclear envelope (Yoshida and Sazer, 2004). In wild-type cells, the NLS-GFP-lacZ was strongly concentrated in the nucleus (Figure 6A). In cells expressing Vpr, we observed multiple types of nuclear defects. First, the majority of cells exhibited odd nuclear shape: nuclei were often larger and were no longer round and smooth but had a lumpy shape with multiple small herniations or projections (Figure 6B) (84% abnormal nuclei at 19 h, 90% at 21 h, and 93% at 24 h; n ≥ 85). Second, a subset of nuclei was no longer stained brightly with NLS-GFP-lacZ. Some of these cells also had an uneven distribution of the GFP-labeled nuclear pores at the nuclear envelope (45% of nuclei at 21 h; n = 96) (Figure 6B, cells marked with arrows). In addition, in many of these cells, (11% cells at 21 h of induction), dots of nucleoporin staining were detected in the cytoplasm, consistent with a structural defect in nuclear envelope components (Figure 6B, cell on right with arrow). Some of these defective nuclei were cut by the septum, but others were in interphase cells without a septum. The loss of NLS-GFP-lacZ staining was consistent with breaks in the nuclear envelope or defects in nuclear transport. Time-lapse movies (>30-50 min) generally did not show sudden changes in the NLS-GFP intensity or large changes in nuclear shape. During the course of these movies, we documented nuclear breakage events (by the sudden loss of NLS-GFP-lacZ fluorescence) in <1% of cells, suggesting that in contrast to findings in mammalian cells, frequent breakage, and resealing of the nuclei did not occur. Thus, these images suggest that as in mammalian cells, Vpr induced nuclear envelope defects in fission yeast.
Figure 6.
Vpr causes defects in nuclear structure in S. pombe cells. Nuclei in cells carrying a pREP1N (A) or pREP-VPR (19-h induction time) (B) were imaged by fluorescence microscopy by coexpression of an NLS-GFP-lacZ, which assays for nuclear transport and breakage, and a nucleoporin-GFP fusion protein, which labels the nuclear envelope. (A) Wild-type nuclei just after or during nuclear division. The dots between one pair of nuclei represent nuclear pores that are present on nuclear envelope that surrounds the elongating mitotic spindle. (B) Dim nuclei (arrows) represent ones that lack NLS-GFP-lacZ staining and thus may have broken.
Vpr Causes Mitotic and Cytokinesis Defects in Human Cells
These results in fission yeast prompted reevaluation of the phenotype of human cells expressing Vpr. First, we examined the effect of Vpr expression in HeLa cells (Figures 7 and 8). To analyze the effect of vpr expression within hours of transfection, we electroporated HeLa cells with a bicistronic vector that expresses both vpr and GFP sequences. GFP positive cells (which can be presumed to express Vpr) were purified by flow cytometry and analyzed for nuclear abnormalities by DAPI staining. By 6 h posttransfection GFP expression was detectable. Staining of the mitotic apparatus in vpr-expressing cells with anti-tubulin antibodies and DAPI revealed abnormal mitotic spindles (Figure 7), such as monopolar spindles (6.4% of mitotic cells), multipolar spindles (26.8% of mitotic cells) and misaligned or lagging chromosomes (8% of mitotic cells). These defects were not apparent in cells transfected with the GFP expression vector alone (100 mitotic cells examined).
Figure 7.
Expression of vpr induces mitotic defects in HeLa cells. HeLa cells were electroporated with pVpr-IRES-EGFP. After 6 h, EGFP expressing cells were isolated by FACS sorting and replated on coverslips. Eighteen hours later, cells were fixed and stained with anti-γ-tubulin antibodies and DAPI. Shown are representative cells exhibiting monopolar spindle (A), multipolar spindle (B), or misaligned chromosomes (C).
Figure 8.
Expression of vpr induces cytokinesis defects in HeLa cells. HeLa cells were electroporated with pCEP4 (A) or pCEP4-HA-Vpr (B-E, G, and H). After 48 h of selection in hygromycin-containing medium, cells were trypsinized and replated. Five days after electroporation, cells were fixed and stained with DAPI (A-E) or with antibodies against pericentrin (G) or γ-tubulin (H) and examined by UV-fluorescence microscopy. Arrows indicate bridges of DNA that seem to connect separate nuclei within a given cell (B) or nuclei between two daughter cells (C-E). (F) Kinetics of the appearance of multinucleated cells in cultures electroporated with pCEP4-HA-Vpr.
After 3 d of Vpr expression (Figure 8F), large cells were apparent and the incidence of mitotic cells decreased. By 5 d posttransfection, the proportion of large, multinucleate cells steadily increased to 31% of vpr-expressing cells versus 3% of control cells (Figure 8F). Multinucleate vpr-expressing HeLa cells often contained micronuclei and incompletely separated nuclei still connected by bridges of DNA, resembling a “cut” phenotype indicative of a defect in nuclear division (Figure 8, B-E). Thus, as in fission yeast cells, vpr-expressing human cells also exhibited defects in nuclear division and cytokinesis.
To determine whether Vpr induces centrosome abnormalitites, we stained vpr-expressing cells with centriole markers pericentrin, γ-tubulin, and Cen2 (Figure 8; our unpublished data). Most cells, even ones with a single nucleus, contained multiple pairs of centrioles scattered in the perinuclear region (Figure 8, G and H). Thus, Vpr causes the accumulation of multiple centrosome-like structures.
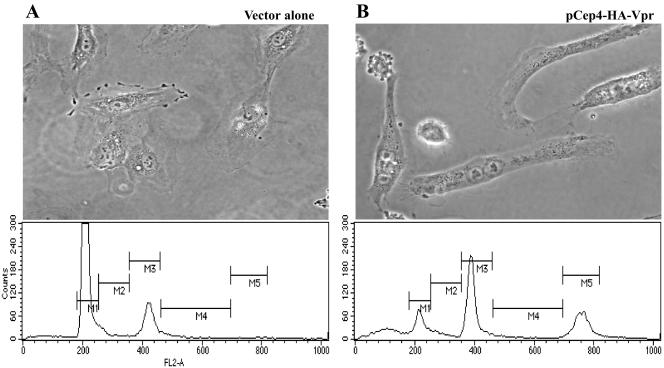
Next, we examined the effect of Vpr expression on primary HUVECs. Cells were electroporated with a vpr-expression plasmid or a control vector. After 48 h under selection, cells were trypsinized and replated. Five days postelectroporation, the morphology of cells was analyzed by phase contrast microscopy, and the DNA content was measured by flow cytometry. Cells electroporated with empty vector were observed to grow during this time period. No multinucleate cells were evident (Figure 9A, top), and FACS analysis showed the expected DNA profile of an asynchronously proliferating population (Figure 9A, bottom; 67.3% of the cells had 2N DNA and 16.9% had 4N DNA). In contrast, cells electroporated with the vpr-expression vector increased in size, did not divide, and became multinucleate (Figure 9B, top). DNA profiles showed increased number of cells with 4N (36.6% cells) and 8N (21.7% cells) DNA contents compared with the population control cells (Figure 9B, bottom). These findings indicate that Vpr also induces cell division defects in primary human cells.
Figure 9.
Expression of vpr induces G2 arrest and cytokinesis defects in primary human cells. HUVEC were electroporated with pCEP4 (A) or pCEP4-HA-Vpr (B). After 48 h of selection in hygromycin-containing medium, cells were trypsinized and replated. Five days after electroporation, cells were examined by phase-contrast microscopy at 400× magnification (top). Arrows indicate multinucleate cells. Bottom, flow cytometric analysis after staining with propidium iodide. M1 indicates 2N DNA content. M3 indicates 4N DNA content. M5 indicates 8N DNA content.
DISCUSSION
Multiple Effects of Vpr on Cell Division
Here, we reexamined the effect of Vpr expression in both fission yeast and human cells and found that, in addition to cell cycle arrest, Vpr also induces defects in nuclear morphology, mitotic spindle assembly and function, and actin ring assembly in cytokinesis. These additional effects are not secondary to cell cycle arrest. Spindle and nuclear abnormalities were clearly apparent well before development of elongated cells in fission yeast. Also, previous fission yeast studies have established that cell cycle delay in G2 phase alone (for instance in a cdc25 temperature-sensitive mutant) does not produce defects in mitosis or cytokinesis as severe as those reported here (Hagan et al., 1990; Naqvi et al., 1999).
Several different models can be imagined to explain how Vpr has so many effects on the cell division cycle. Vpr might disrupt multiple targets, each of which controls a different process or pathway. Alternatively, Vpr may target a single protein or organelle that affects these interdependent processes.
Vpr Effects on Centrosomes
Vpr seemed to disturb centrosome/SPB integrity and function. In fission yeast, characterization of mitotic defects indicated that SPB function or replication of the spindle pole body was disrupted. Furthermore, the distributions of sad1p and polo kinase plo1p, but not γ-tubulin complex, were perturbed, suggesting that only part of the SPB structure was affected. In human cells, Vpr expression led to the accumulation of multiple centrosome-like structures, as shown by pericentrin and γ-tubulin staining, although it is not yet clear whether the integrity of the centrosome was disrupted.
The centrosome abnormalities that we observed are unlikely to be artifacts of our experimental system because multiple centrosomes have been observed in cells infected with wild-type HIV-1 but not in cells infected with HIV-1 bearing a vpr mutation (Watanabe et al., 2000). Similar multiple centrosome phenotypes are commonly found in tumor cells, perhaps arising from multiple centrosome duplication cycles without proper cell division (Meraldi et al., 2002) or from abnormal de novo synthesis of centrosome-like structures after the centrosome laser ablation (Khodjakov et al., 2002). Vpr might cause the multiple centrosome phenotype by perturbing cytokinesis or via direct effects on the regulation of centrosome duplication or integrity. Once established, the multiple centrosome phenotype could then contribute to further abnormalities in mitosis and cytokinesis.
Vpr Effects on the Nuclear Envelope
Vpr has been shown to disrupt the integrity of the nuclear envelope in mammalian cells, causing the formation of large holes in the interphase nuclear envelope that may facilitate the entry of viral particles into the nucleus (de Noronha et al., 2001). One proposed candidate target for Vpr are nuclear lamins, which are responsible for nuclear structure and integrity. In fission yeast, we found that Vpr also causes abnormal nuclear morphology and DNA staining (Zhao et al., 1998). In addition, as with Vpr in mammalian cells, a GFP-Vpr fusion protein is localized in a perinuclear distribution in fission yeast (Chen et al., 1999). Thus, it is likely that the effect of Vpr on the nucleus is conserved. Because fission yeast do not contain nuclear lamins, Vpr must affect a nuclear envelope target separate from nuclear lamins.
Vpr at the nuclear envelope can potentially affect mitosis and centrosomes in multiple ways. First, both fission yeast SPB and mammalian centrosomes are often tightly associated with nuclear envelopes, but the possible effects of nuclear envelope integrity on the SPB and centrosome assembly and function are not yet known. Second, Vpr has been shown to bind to the nuclear transport factor importin α (Popov et al., 1998; Vodicka et al., 1998), which with ran GTPase, regulates both nuclear transport and mitotic spindle assembly (Dasso, 2001). Disruption of the Ran GTPase system in fission yeast also causes nuclear envelope fragmentation (Demeter et al., 1995) and spindle assembly defects (Fleig et al., 2000; Salus et al., 2002).
Vpr and Cytokinesis
Vpr induced strong defects in actin ring formation, and thus may affect actin indirectly or directly. In T cells, Vpr has been shown to modulate cell adhesion and actin organization (Matarrese et al., 2000), and it has been found to bind to F-actin directly (D. Schafer, personal communication). Centrosome defects can lead also to cytokinesis defects, although in fission yeast, mitotic spindle defects do not necessarily disrupt contractile actin ring formation (Chang et al., 1996a), Thus, Vpr may target directly or indirectly one or more of the proteins involved in actin ring assembly, such as actin.
Ways to Arrest the Cell Cycle
Much prior work has focused on the role of Vpr on its effect on the cell cycle. Nuclear envelope defects may affect cell cycle regulators by affecting their nuclear trafficking (de Noronha et al., 2001). Our findings lead to additional possible mechanisms for how Vpr causes cell cycle arrest. Disturbances in centrosome integrity caused by Vpr are likely to affect the function of many regulators of the cell cycle and cytokinesis present on the centrosome. Removal of the centrosome causes cells to go through M phase, elicits cytokinesis defects in a fraction of the cells, and then arrests cells with a 4N DNA content in the next cell cycle before S phase (Hinchcliffe et al., 2001; Khodjakov and Rieder, 2001). The centrosome/SPB contains many regulators of cell cycle progression (Doxsey, 2001), including factors required for G2/M transition (cdc2/cyclin B), mitotic spindle assembly (plo1p), contractile ring assembly (plo1p, rng2p), septation, and exit from mitosis (sid components). Here, we examined the effect of Vpr on one of these regulators, plo1p polo kinase. Polo kinase has multiple conserved functions in mitotic spindle function, cytokinesis, and G2/M transition through interactions with multiple proteins (Glover et al., 1998). In human cells, overexpression of polo kinase causes cell division defects and accumulation of multiple centrosomes (Meraldi et al., 2002). In S. pombe, loss of function of the polo kinase plo1p can lead to similar cell division defects as seen in vpr-expressing cells (Ohkura et al., 1995). The plo1p-GFP fusion protein normally localizes to the SPB and spindle but was grossly mislocalized in vpr-expressing cells. However, we currently have no evidence suggesting that Vpr interacts directly with plo1p.
CONCLUSION
This work leads to a new view that Vpr affects multiple aspects of cell division, including mitotic spindle function and cytokinesis. Interestingly, proteins encoded by other viruses have also recently been shown to affect centrosomal function (Duensing et al., 2000; Ploubidou et al., 2000; Gaillard et al., 2001). How these multiple Vpr effects relate to HIV infection in vivo is not yet clear, but it is likely that these effects contribute to cell killing or possibly to intracellular trafficking of viral components (McDonald et al., 2002). Genetic studies in S. pombe may allow the identification of Vpr cellular target(s), a prerequisite for understanding Vpr function in HIV-1 replication and pathogenesis.
Acknowledgments
We thank D. McCollum, A. Paoletti, and I. Hagan for reagents. This work was supported by Columbia-Rockefeller Center for AIDS Research AI42848 and by National Institutes of Health grants AI-41857 (to J.L.), GM-56836 (to F.C), and GM-49119 (to S.S.).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-09-0691. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-09-0691.
References
- Bahler, J., Steever, A.B., Wheatley, S., Wang, Y., Pringle, J.R., Gould, K.L., and McCollum, D. (1998). Role of polo kinase and Mid1p in determining the site of cell division in fission yeast. J. Cell Biol. 143, 1603-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz, S.R., Rogel, M.E., and Emerman, M. (1996). Human immunodeficiency virus type 1 cell cycle control: Vpr is cytostatic and mediates G2 accumulation by a mechanism which differs from DNA damage checkpoint control. J. Virol. 70, 2324-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, F., Woollard, A., and Nurse, P. (1996a). Isolation and characterization of fission yeast mutants defective in the assembly and placement of the contractile actin ring. J. Cell Sci. 109, 131-142. [DOI] [PubMed] [Google Scholar]
- Chang, F., Woollard, A., and Nurse, P. (1996b). Isolation and characterization of fission yeast mutants defective in the assembly and placement of the contractile actin ring. J. Cell Sci. 109, 131-142. [DOI] [PubMed] [Google Scholar]
- Chen, M., Elder, R.T., Yu, M., O'Gorman, M.G., Selig, L., Benarous, R., Yamamoto, A., and Zhao, Y. (1999). Mutational analysis of vpr-induced G2 arrest, nuclear localization, and cell death in fission yeast. J. Virol. 73, 3236-3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, E.A., Dehni, G., Sodroski, J.G., and Haseltine, W.A. (1990). Human immunodeficiency virus vpr product is a virion-associated regulatory protein. J. Virol. 64, 3097-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor, R.I., Chen, B.K., Choe, S., and Landau, N.R. (1995). Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206, 935-944. [DOI] [PubMed] [Google Scholar]
- Dasso, M. (2001). Running on Ran: nuclear transport and the mitotic spindle. Cell 104, 321-324. [DOI] [PubMed] [Google Scholar]
- de Noronha, C.M., Sherman, M.P., Lin, H.W., Cavrois, M.V., Moir, R.D., Goldman, R.D., and Greene, W.C. (2001). Dynamic disruptions in nuclear envelope architecture and integrity induced by HIV-1 Vpr. Science 294, 1105-1108. [DOI] [PubMed] [Google Scholar]
- Demeter, J., Morphew, M., and Sazer, S. (1995). A mutation in the RCC1-related protein pim1 results in nuclear envelope fragmentation in fission yeast. Proc. Natl. Acad. Sci. USA 92, 1436-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxsey, S.J. (2001). Centrosomes as command centres for cellular control. Nat. Cell Biol. 3, E105-E108. [DOI] [PubMed] [Google Scholar]
- Duensing, S., Lee, L.Y., Duensing, A., Basile, J., Piboonniyom, S., Gonzalez, S., Crum, C.P., and Munger, K. (2000). The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc. Natl. Acad. Sci. USA 97, 10002-10007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein, D.A., Sherman, M.P., Penn, M.L., Chin, P.S., de Noronha, C.M., Greene, W.C., and Goldsmith, M.A. (2001). HIV-1 Vpr enhances viral burden by facilitating infection of tissue macrophages but not nondividing CD4+ T cells. J. Exp. Med. 194, 1407-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder, R.T., Yu, M., Chen, M., Edelson, S., and Zhao, Y. (2000). Cell cycle G2 arrest induced by HIV-1 Vpr in fission yeast (Schizosaccharomyces pombe) is independent of cell death and early genes in the DNA damage checkpoint. Virus Res. 68, 161-173. [DOI] [PubMed] [Google Scholar]
- Elder, R.T., Yu, M., Chen, M., Zhu, X., Yanagida, M., and Zhao, Y. (2001). HIV-1 Vpr induces cell cycle G2 arrest in fission yeast (Schizosaccharomyces pombe) through a pathway involving regulatory and catalytic subunits of PP2A and acting on both Wee1 and Cdc25. Virology 287, 359-370. [DOI] [PubMed] [Google Scholar]
- Fassati, A., and Goff, S.P. (2001). Characterization of intracellular reverse transcription complexes of human immunodeficiency virus type 1. J. Virol. 75, 3626-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleig, U., Salus, S.S., Karig, I., and Sazer, S. (2000). The fission yeast ran GTPase is required for microtubule integrity. J. Cell Biol. 151, 1101-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget, J., Yao, X.J., Mercier, J., and Cohen, E.A. (1998). Human immunodeficiency virus type 1 vpr protein transactivation function: mechanism and identification of domains involved. J. Mol. Biol. 284, 915-923. [DOI] [PubMed] [Google Scholar]
- Gaillard, S., Fahrbach, K.M., Parkati, R., and Rundell, K. (2001). Overexpression of simian virus 40 small-T antigen blocks centrosome function and mitotic progression in human fibroblasts. J. Virol. 75, 9799-9807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover, D.M., Hagan, I.M., and Tavares, A.A. (1998). Polo-like kinases: a team that plays throughout mitosis. Genes Dev. 12, 3777-3787. [DOI] [PubMed] [Google Scholar]
- Goh, W.C., Rogel, M.E., Kinsey, C.M., Michael, S.F., Fultz, P.N., Nowak, M.A., Hahn, B.H., and Emerman, M. (1998). HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat. Med. 4, 65-71. [DOI] [PubMed] [Google Scholar]
- Hagan, I., and Yanagida, M. (1995). The product of the spindle formation gene sad1+ associates with the fission yeast spindle pole body and is essential for viability. J. Cell Biol. 129, 1033-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan, I.M., Riddle, P.N., and Hyams, J.S. (1990). Intramitotic controls in the fission yeast Schizosaccharomyces pombe: the effect of cell size on spindle length and the timing of mitotic events. J. Cell Biol. 110, 1617-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, J., Choe, S., Walker, R., Di Marzio, P., Morgan, D.O., and Landau, N.R. (1995). Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J. Virol. 69, 6705-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzinger, N.K., Bukinsky, M.I., Haggerty, S.A., Ragland, A.M., Kewalramani, V., Lee, M.A., Gendelman, H.E., Ratner, L., Stevenson, M., and Emerman, M. (1994). The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc. Natl. Acad. Sci. USA 91, 7311-7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchcliffe, E.H., Miller, F.J., Cham, M., Khodjakov, A., and Sluder, G. (2001). Requirement of a centrosomal activity for cell cycle progression through G1 into S phase. Science 291, 1547-1550. [DOI] [PubMed] [Google Scholar]
- Jowett, J.B., Planelles, V., Poon, B., Shah, N.P., Chen, M.L., and Chen, I.S. (1995). The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J. Virol. 69, 6304-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov, A., and Rieder, C.L. (2001). Centrosomes enhance the fidelity of cytokinesis in vertebrates and are required for cell cycle progression. J. Cell Biol. 153, 237-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov, A., Rieder, C.L., Sluder, G., Cassels, G., Sibon, O., and Wang, C.L. (2002). De novo formation of centrosomes in vertebrate cells arrested during S phase. J. Cell Biol. 158, 1171-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange, B.M. (2002). Integration of the centrosome in cell cycle control, stress response and signal transduction pathways. Curr. Opin. Cell Biol. 14, 35-43. [DOI] [PubMed] [Google Scholar]
- Masuda, M., Nagai, Y., Oshima, N., Tanaka, K., Murakami, H., Igarashi, H., and Okayama, H. (2000). Genetic studies with the fission yeast Schizosaccharomyces pombe suggest involvement of wee1, ppa2, and rad24 in induction of cell cycle arrest by human immunodeficiency virus type 1 Vpr. J. Virol. 74, 2636-2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarrese, P., Conti, L., Varano, B., Gauzzi, M.C., Belardelli, F., Gessani, S., and Malorni, W. (2000). The HIV-1 vpr protein induces anoikis-resistance by modulating cell adhesion process and microfilament system assembly. Cell Death Differ 7, 25-36. [DOI] [PubMed] [Google Scholar]
- Maundrell, K. (1993). Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene 123, 127-130. [DOI] [PubMed] [Google Scholar]
- McDonald, D., Vodicka, M.A., Lucero, G., Svitkina, T.M., Borisy, G.G., Emerman, M., and Hope, T.J. (2002). Visualization of the intracellular behavior of HIV in living cells. J. Cell Biol. 159, 441-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraldi, P., Honda, R., and Nigg, E.A. (2002). Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53-/- cells. EMBO J. 21, 483-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi, N.I., Eng, K., Gould, K.L., and Balasubramanian, M.K. (1999). Evidence for F-actin-dependent and -independent mechanisms involved in assembly and stability of the medial actomyosin ring in fission yeast. EMBO J. 18, 854-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkura, H., Hagan, I.M., and Glover, D.M. (1995). The conserved Schizosaccharomyces pombe kinase plo1, required to form a bipolar spindle, the actin ring, and septum, can drive septum formation in G1 and G2 cells. Genes Dev. 9, 1059-1073. [DOI] [PubMed] [Google Scholar]
- Paxton, W., Connor, R.I., and Landau, N.R. (1993). Incorporation of Vpr into human immunodeficiency virus type 1 virions: requirement for the p6 region of gag and mutational analysis. J. Virol. 67, 7229-7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham, R.J., and Chang, F. (2001). Role of actin polymerization and actin cables in actin-patch movement in Schizosaccharomyces pombe. Nat. Cell Biol. 3, 235-244. [DOI] [PubMed] [Google Scholar]
- Ploubidou, A., Moreau, V., Ashman, K., Reckmann, I., Gonzalez, C., and Way, M. (2000). Vaccinia virus infection disrupts microtubule organization and centrosome function. EMBO J. 19, 3932-3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov, S., Rexach, M., Zybarth, G., Reiling, N., Lee, M.A., Ratner, L., Lane, C.M., Moore, M.S., Blobel, G., and Bukrinsky, M. (1998). Viral protein R regulates nuclear import of the HIV-1 pre-integration complex. EMBO J. 17, 909-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re, F., Braaten, D., Franke, E.K., and Luban, J. (1995). Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J. Virol. 69, 6859-6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogel, M.E., Wu, L.I., and Emerman, M. (1995). The human immunodeficiency virus type 1 vpr gene prevents cell proliferation during chronic infection. J. Virol. 69, 882-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salus, S.S., Demeter, J., and Sazer, S. (2002). The Ran GTPase system in fission yeast affects microtubules and cytokinesis in cells that are competent for nucleocytoplasmic protein transport. Mol. Cell. Biol. 22, 8491-8505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tristem, M., Marshall, C., Karpas, A., and Hill, F. (1992). Evolution of the primate lentiviruses: evidence from vpx and vpr. EMBO J. 11, 3405-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodicka, M.A., Koepp, D.M., Silver, P.A., and Emerman, M. (1998). HIV-1 Vpr interacts with the nuclear transport pathway to promote macrophage infection. Genes Dev. 12, 175-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, N., Yamaguchi, T., Akimoto, Y., Rattner, J.B., Hirano, H., and Nakauchi, H. (2000). Induction of M-phase arrest and apoptosis after HIV-1 Vpr expression through uncoupling of nuclear and centrosomal cycle in HeLa cells. Exp. Cell Res. 258, 261-269. [DOI] [PubMed] [Google Scholar]
- Woods, A., Sherwin, T., Sasse, R., MacRae, T.H., Baines, A.J., and Gull, K. (1989). Definition of individual components within the cytoskeleton of Trypanosoma brucei by a library of monoclonal antibodies. J. Cell Sci. 93, 491-500. [DOI] [PubMed] [Google Scholar]
- Yoshida, M., and Sazer, S. (2004). Nucleocytoplasmic transport and nuclear envelope integrity in the fission yeast Schizosaccharomyces pombe. Methods (in press). [DOI] [PubMed]
- Yuan, X., Matsuda, Z., Matsuda, M., Essex, M., and Lee, T.H. (1990). Human immunodeficiency virus vpr gene encodes a virion-associated protein. AIDS Res. Hum. Retroviruses 6, 1265-1271. [DOI] [PubMed] [Google Scholar]
- Zhang, C., Rasmussen, C., and Chang, L.J. (1997). Cell cycle inhibitory effects of HIV and SIV Vpr and Vpx in the yeast Schizosaccharomyces pombe. Virology 230, 103-112. [DOI] [PubMed] [Google Scholar]
- Zhao, Y., Cao, J., O'Gorman, M.R., Yu, M., and Yogev, R. (1996). Effect of human immunodeficiency virus type 1 protein R (vpr) gene expression on basic cellular function of fission yeast Schizosaccharomyces pombe. J. Virol. 70, 5821-5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y., Chen, M., Wang, B., Yang, J., Elder, R.T., Song, X.Q., Yu, M., and Saksena, N.K. (2002). Functional conservation of HIV-1 Vpr and variability in a mother-child pair of long-term non-progressors. Virus Res. 89, 103-121. [DOI] [PubMed] [Google Scholar]
- Zhao, Y., Yu, M., Chen, M., Elder, R.T., Yamamoto, A., and Cao, J. (1998). Pleiotropic effects of HIV-1 protein R (Vpr) on morphogenesis and cell survival in fission yeast and antagonism by pentoxifylline. Virology 246, 266-276. [DOI] [PubMed] [Google Scholar]