Abstract
BACKGROUND:
Musculocutaneous perforator flaps offer advantages over musculocutaneous flaps, including reduced donor site morbidity, more predictable reconstruction of soft tissue deformities, and a wider variety of flap options. Perforator flaps are becoming increasingly popular for many applications. In the present study, we set out to examine the various perforators of the thoracoacromial axis through the pectoralis major (PM) muscle with respect to their suitability for transfer to the head and neck region as a pedicled flap.
METHODS:
A series of 10 fresh cadavers were injected with lead oxide, gelatin and water (250 mL/kg) through the femoral vessels. The cadavers were cooled and the integument was removed. Perforating vessels from the underlying muscles were marked and the resulting angiograms of the integument and deep tissues were compared with the dissection notes describing the course, size and distribution of the perforating vessels.
RESULTS:
The perforators through the PM muscle to the overlying skin included three regional groups: perforators of the thoracoacromial axis; perforators of the medial intercostal vessels; and perforators of the lateral thoracic artery. The major group of perforators supplying the overlying skin was from the intercostal vessels. However, the thoracoacromial axis did consistently give rise to perforators in the upper portion of the PM muscle. In particular, there were reliable perforators from the clavicular and deltoid branches of the thoracoacromial artery.
DISCUSSION:
The present study illustrates the potential clinical applications of a series of perforator flaps based on the thoracoacromial axis, which may be useful in head and neck reconstructive surgery.
Keywords: Anterior chest wall, Pectoralis major, Perforator flaps, Thoracoacromial artery, Vascular territories
Abstract
CONTEXTE :
Les lambeaux musculo-cutanés à vaisseau perforant offrent plusieurs avantages sur les lambeaux musculo-cutanés ordinaires, dont une diminution de la morbidité de la zone donneuse, une reconstruction plus prévisible des tissus mous déformés et une plus grande variété de lambeaux. On a maintenant de plus en plus recours aux lambeaux à vaisseau perforant dans divers contextes. Dans la présente étude, nous nous penchons sur les différents vaisseaux perforants de l’axe acromio-thoracique qui traversent le muscle grand pectoral (GP) quant à leurs possibilités d’implantation dans la région de la tête et du cou sous forme de lambeau pédiculé.
MÉTHODE :
Une solution composée d’oxyde de plomb, de gélatine et d’eau (250 ml/kg) a été injectée par les vaisseaux fémoraux dans 10 cadavres frais. Après leur refroidissement, nous avons procédé à l’enlèvement du tégument. Ensuite, nous avons marqué les vaisseaux perforants qui provenaient des muscles sous-jacents, puis comparé les angiogrammes du tégument et des tissus profonds en consignant le trajet, la grosseur et le territoire de ces vaisseaux.
RÉSULTATS :
Les vaisseaux perforants qui traversaient le GP jusqu’à la peau sus-jacente se répartissaient en trois groupes régionaux : l’axe acromio-thoracique, les vaisseaux intercostaux internes et l’artère thoracique latérale. Le principal groupe irriguant la peau sus-jacente provenait des vaisseaux intercostaux. Toutefois, l’axe acromio-thoracique donnait toujours naissance à des vaisseaux perforants dans la partie supérieure du GP. En particulier, les rameaux claviculaires et deltoïdes de l’artère acromio-thoracique constituaient une source fiable de vaisseaux perforants.
DISCUSSION :
La présente étude montre les possibilités d’utilisation clinique d’une série de lambeaux à vaisseau perforant prélevés sur l’axe acromio-thoracique pour la chirurgie reconstructrice de la région de la tête et du cou.
The genesis of at least two major advances in plastic and reconstructive surgery has been directly related to the continuing challenge of head and neck reconstruction. The first advancement was the deltopectoral flap that was developed by Bakamjian (1) in 1968 to fully reconstruct the pharynx. This resulted in a surge of interest in axial pattern flaps that revolutionized the manner by which surgeons transferred tissues throughout the body. The second major advancement was the pectoralis major (PM) musculocutaneous flap described by Ariyan in 1979 (2,3). This flap has subsequently become the workhorse for head and neck reconstruction (4–6).
Although both flaps offer advantages, both the deltopectoral and PM flaps have obvious shortcomings. The deltopectoral flap pedicle is short, a skin graft may be needed to close the donor site, and a secondary minor procedure is often required to debulk the pedicle (2). Inclusion of muscle in the PM flap often creates unneeded bulk in the reconstruction and increases donor site morbidity with some functional loss (7).
A new advancement in free flap surgery is the perforator flap technique. Perforator flaps are based on musculocutaneous perforators that pass through the muscle, omitting the need to harvest a passive muscle carrier with the skin paddle. The advantages of perforator flaps include improved accuracy in reconstruction, reduced donor site morbidity and an increased choice of potential tissue donor sites. Perforator free flaps are increasing in popularity for many reconstructive procedures requiring only soft tissue for the reconstruction (8–13).
The overall objective of the current study was to review the vascular anatomy of the soft tissues of the anterior chest wall. The specific objective was to identify and describe all direct cutaneous and musculocutaneous perforators supplying the integument of the anterior chest.
METHODS
Ten fresh, adult, human cadavers were perfused with a radiopaque mixture of lead oxide, gelatin and water through catheters in the femoral vessels. The age and sex of each cadaver were recorded. Exclusion criteria for cadaver selection included all individuals with severe systemic vascular disease. The injection protocol was similar to that described by Rees and Taylor (14).
Sixteen-gauge Foley catheters were inserted cranially and caudally into both femoral arteries and 10 L of a 50°C isotonic saline solution was flushed through the arteries before the injection of the warm lead oxide and gelatin solution (60°C). Adequate perfusion of the cadaver was indicated by the presence of the orange injectate in the sclera, fingers and toes. The cadavers were cooled for 24 h at 4°C to allow the gelatin to solidify.
The borders of the anterior chest wall and abdomen were outlined with ink before dissection. The anterior chest was defined inferiorly by the margin of the costal cartilage, laterally by the midaxillary line and superiorly by the superior border of the clavicle and acromion. The integument was raised along the deep fascial plane and all cutaneous perforators, either septal or musculocutaneous, were marked with a lead bead and recorded in the dissection notes. The integument and the deep tissue with muscle were radiographed (Figure 1). The PM muscle was dissected away from the anterior chest wall, deep to the thoracoacromial axis, and was radiographed separately (Figure 2). The radiographs were assessed to determine the vascular supply to the integument and muscle, and the contributions of the major and minor vascular territories. Digital pictures were taken at each stage of the dissection to correlate with the dissection notes.
Figure 1).
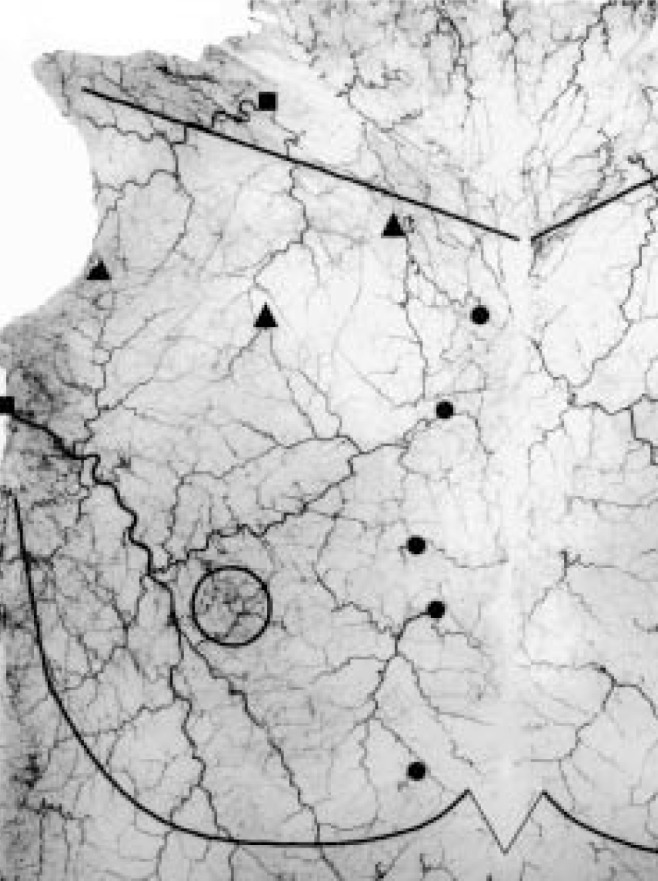
Angiogram of the skin from the right anterior chest wall. Costal margin, midclavicular line and nipple-areola complex are outlined with lead wire. Triangles – Musculocutaneous perforators from the deltoid, pectoral and clavicular branches of the thoracoacromial artery (from left to right); Circles – Musculocutaneous perforators from the internal mammary artery; Squares – Septocutaneous perforators from the lateral thoracic artery (inferolateral) and supraclavicular artery (superior)
Figure 2).
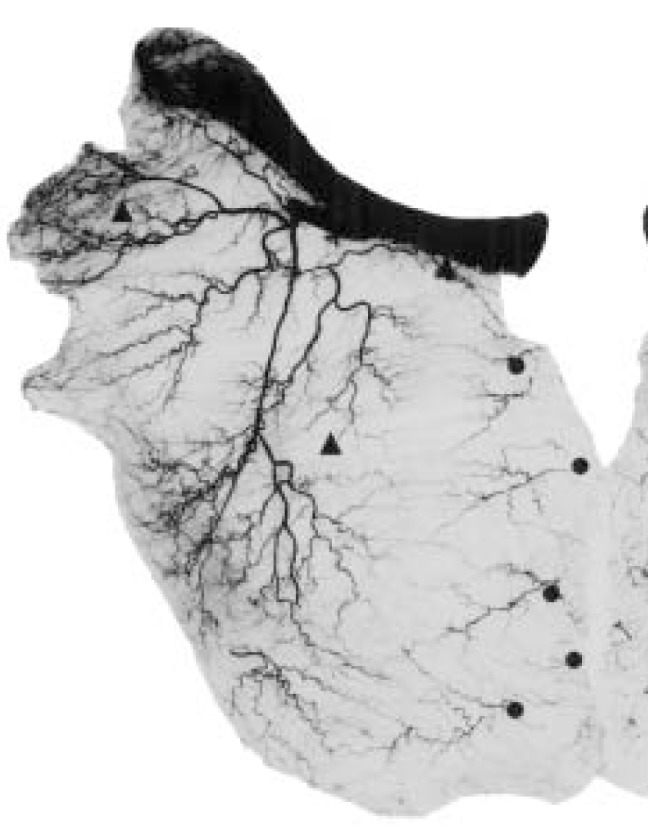
Angiogram of the right pectoralis major muscle and clavicle showing origin of musculocutaneous perforator vessels. Triangles – Musculocutaneous perforators from the deltoid, pectoral and clavicular branches of the thoracoacromial artery (from left to right); Circles – Musculocutaneous perforators from the internal mammary artery
RESULTS
Injection studies
In this series of injection studies (n=10), the lead oxide and gelatin injection technique provided excellent angiograms of the anterior torso. All 10 cadavers were white (nine men and one woman) and had a mean age of 71.4 years. The quality of each cadaver angiography did vary, however, likely due to the combination of factors such as the presence of peripheral vascular disease. Significant variations in the vascular anatomy were also noted between individuals and in opposite sides of the same individual.
The thoracoacromial artery
The thoracoacromial artery (TAA) originated as a single branch from the axillary artery in all 20 specimens. The average diameter of the TAA at the axillary artery was 2.5±0.5 mm. It is the major blood supply to the PM muscle and is coursed within the fat pad between the PM and minor muscles. Our results showed that the TAA gives rise to three major branches: the pectoral, clavicular and deltoid arteries (Figure 2). The acromial artery may also be considered a main branch; however, it originated from the deltoid artery in 18 of 20 dissections, and from the TAA axis in the remaining two dissections. The pectoral branch of the TAA supplied the largest vascular territory and had an average diameter of 1.7±0.6 mm. This artery predominantly (60%) gave rise to three secondary branches that traveled obliquely, medially and inferiorly on the deep surface of the PM muscle. The medial and inferior branches anastomosed within the PM muscle to the intercostal perforators of the internal mammary artery (IMA). The anastomoses occurred mainly in a line below the medial third of the clavicle. Only minor musculocutaneous perforators (<0.5 mm) were found to arise from these branches. The lateral branch also anastomosed with terminal branches of the lateral thoracic artery (LTA) within the PM muscle. The deltoid artery always originated at the thoracoacromial axis and had an average diameter of 1.8±0.4 mm. The clavicular branch of the thoracoacromial axis was more variable between individuals and between sides on the same individual. This artery varied in its origin from the TAA axis (66%), pectoral artery (24%) and deltoid artery (10%). The average diameter of the clavicular artery was 1.1±0.5 mm.
Blood supply of the PM muscle and overlying integument
Three major source arteries, the TAA, perforators from the IMA, and the LTA, supply the PM muscle (Figure 2). Each of these three blood vessels was present in all of our dissections; however, the size and distribution of the vascular territories within the muscle varied. The pectoral branch of the TAA supplied the largest muscular vascular territory, followed by the intercostal perforators of the IMA, and, lastly, the pectoral branch of the LTA.
The clavicular and sternocostal heads of the PM had independent blood supplies. The sternocostal head was supplied by the pectoral branch of the TAA (intermediate region), the muscular branch of the LTA (inferolateral region), and the intercostal perforators from the IMA (medial and inferior regions). The clavicular head was supplied medially by the clavicular branch, and laterally by the deltoid and acromial arteries.
The superficial thoracic artery (branch of the LTA) and the intercostal perforators of the internal thoracic artery supplied the majority of the integument of the lateral, medial and intermediate regions of the anterior chest wall (Figure 1). In the absence of a large superficial thoracic artery, there was an enlargement of the second, fourth, or both the second and fourth intercostal perforators. This primary perforator from either intercostal space anastomosed with the superficial thoracic artery around the nipple and areola. The superior integument was supplied by musculocutaneous perforators from the clavicular and deltoid arteries, and variable fasciocutaneous vessels from the transverse cervical, suprascapular and supraclavicular arteries.
Musculocutaneous perforators of the pectoral branch of the thoracoacromial axis were limited to small perforators in the inferolateral and intermediate zones of the PM. No perforators from the pectoral artery were found to pass below the inferolateral border of the sternocostal head. However, significant musculocutaneous perforators greater than 0.5 mm were consistently found emerging from the clavicular and deltoid branches of the TAA. The clavicular artery perforators appeared from the clavicular head of the PM as a musculocutaneous perforator and via the septum between the clavicular and sternal heads of the PM muscle. The average pedicle length of the clavicular perforators was 6.0±2.1 cm. The dominant perforator from the deltoid branch had an average length of 7.9±2.0 cm and arose from the PM muscle fibres close to the delto-pectoral groove. Each perforator was accompanied by paired venae comitantes.
Venous drainage of the PM was found to parallel the TAA. In addition, the lateral pectoral nerve was also found to course in close proximity to the TAA in 10 of 20 dissections.
DISCUSSION
Advancements in microsurgery have decreased the perioperative failure rate of free tissue transfers in the head and neck region to less than 5% (15). Subsequently, many centres around the world have adopted the use of a variety of free flaps for the reconstruction of soft-tissue defects of the head and neck. Despite the growing popularity of free-tissue transfers for the reconstruction of the head and neck region (12,16,17), reliable pedicled regional skin flaps are still indicated in some situations (6,18,19). Several authors have questioned the cost effectiveness, morbidity and suitability of free flap procedures for elderly patients or those with incurable disease as compared with a regional pedicled musculocutaneous flap such as the PM flap (19–23). Pedicled musculocutaneous perforator flaps have some advantages over conventional pedicled musculocutaneous flaps and may be advantageous for soft-tissue reconstruction of head and neck defects.
By combining the advantages of a musculocutaneous perforator flap with the reliability of a pedicled skin flap, we hope to improve the armamentarium of reconstructive surgeons by introducing a reliable pedicled perforator flap. Yet, to be of clinical use, a pedicled flap must have a reliable vascular basis (both arterial and venous) and a good arc of rotation. In our results, we were unable to show consistent major musculocutaneous perforators from the pectoral branch of the TAA. Our dissection and angiographic results confirmed the vascular variability of the PM muscle reported by Yang et al (24). Our anatomical findings are consistent with previous anatomical studies performed in our laboratory (24) and by others (25,26).
The pectoral branch is of the greatest clinical interest for a pedicled perforator flap due to its long pedicle, and, thus, superior arc of rotation. However, the inconsistent nature of this artery between individuals and different sides of the same individual would be a limitation to its use in this capacity. In contrast, two potential pedicled or free perforator flaps may be possible from consistently large musculocutaneous perforators from the clavicular and deltoid branches of the TAA.
Other groups have assessed the potential for musculocutaneous and osteocutaneous flaps based on the clavicular and deltoid arteries. A study by Williams et al (27) described the anatomical basis of a local rotation flap using the clavicular head of the PM muscle for soft tissue coverage of nonunion of fractures of the clavicle. Seikaly et al (25) reported their assessment of the anatomical variability, and independent vascular and nerve supply to the clavicular region for use as a clavipectoral osteomyocutaneous free flap. Yet, no author has described a perforator skin flap based on these vessels.
The proximity of these flaps to the head and neck gives them excellent skin matching qualities for skin tone and texture. In addition, the relatively hairless nature of this anatomical region is another desirable trait. Further assessment to determine the size of the skin paddle and the possible arc of rotation are needed.
The relationship between the size and course of the LTA and the intercostal perforators was consistent with Salmon’s Law of Equilibrium (28). In this law, Salmon describes the number of vascular pedicles for a certain anatomical area as relatively constant, while the anatomical territories of arteries within that area may vary significantly. Our study revealed that, although the arterial territories varied significantly, the same pattern of vessels was always present in both the skin and underlying muscle.
A pedicled fasciocutaneous skin flap for the reconstruction of the head and neck may be possible based on the superficial thoracic branch of the LTA. Surgical skin flaps from the lateral chest wall have been indicated by several authors for pedicled reconstruction of the axilla (29) and dorsum of the hand (30). We believe that the LTA may provide sufficient pedicle length and arc of rotation to cover the head and neck region, provided that it originates from the axillary artery {ok?}. In our 10 cadaver series, the superficial thoracic branch (lateral mammary branch) of the LTA provided a significantly large vascular territory in the inferolateral region of the integument of the anterior chest wall. The terminal branches of the artery anastomosed with the internal mammary perforators of the intercostal spaces. Thus, a skin flap that captures the adjacent territory of the internal mammary perforators should be viable according to experimental skin flap studies by Morris and Taylor (31). One potential disadvantage of this flap is the vascular variability of the origin of the superficial thoracic artery (32). Future assessment of this vessel is needed to elucidate its potential clinical use.
CONCLUSIONS
Due to the inconsistent size of the pectoral branch of the TAA and the small size of the musculocutaneous perforators, we believe that the pectoral branch of the TAA is not a good donor site for pedicled perforator flaps. However, musculocutaneous perforator flaps are possible from the clavicular and deltoid branches of the TAA. Further anatomical study of the arc of rotation and size of the skin paddle is needed to validate their potential as donor sites for pedicled or free-tissue transfer.
REFERENCES
- 1.Bakamjian VY. Total reconstruction of pharynx with medially based deltopectoral skin flap. N Y State J Med. 1968;68:2771–8. [PubMed] [Google Scholar]
- 2.Ariyan S. The pectoralis major myocutaneous flap. A versatile flap for reconstruction in the head and neck. Plast Reconstr Surg. 1979;63:73–81. doi: 10.1097/00006534-197901000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Ariyan S. Further experiences with the pectoralis major myocutaneous flap for the immediate repair of defects from excisions of head and neck cancers. Plast Reconstr Surg. 1979;64:605–12. [PubMed] [Google Scholar]
- 4.Dedivitis RA, Guimaraes AV. Pectoralis major musculocutaneous flap in head and neck cancer reconstruction. World J Surg. 2002;26:67–71. doi: 10.1007/s00268-001-0183-4. [DOI] [PubMed] [Google Scholar]
- 5.Ord RA. The pectoralis major myocutaneous flap in oral and maxillofacial reconstruction: a retrospective analysis of 50 cases. J Oral Maxillofac Surg. 1996;54:1292–5. doi: 10.1016/s0278-2391(96)90484-x. [DOI] [PubMed] [Google Scholar]
- 6.Liu R, Gullane P, Brown D, et al. Pectoralis major myocutaneous pedicled flap in head and neck reconstruction: retrospective review of indications and results in 244 consecutive cases at the Toronto General Hospital. J Otolaryngol. 2001;30:34–40. doi: 10.2310/7070.2001.21011. [DOI] [PubMed] [Google Scholar]
- 7.de Azevedo JF. Modified pectoralis major myocutaneous flap with partial preservation of the muscle: a study of 55 cases. Head Neck Surg. 1986;8:327–31. doi: 10.1002/hed.2890080503. [DOI] [PubMed] [Google Scholar]
- 8.Rajacic N, Gang RK, Krishnan J, et al. Thin anterolateral thigh free flap. Ann Plast Surg. 2002;48:252–7. doi: 10.1097/00000637-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Kimura N. A microdissected thin tensor fasciae latae perforator flap. Plast Reconstr Surg. 2002;109:69–77. doi: 10.1097/00006534-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Demirkan F, Chen HC, Wei FC, et al. The versatile anterolateral thigh flap: a musculocutaneous flap in disguise in head and neck reconstruction. Br J Plast Surg. 2000;53:30–6. doi: 10.1054/bjps.1999.3250. [DOI] [PubMed] [Google Scholar]
- 11.Koshima I, Hosoda M, Moriguchi T, et al. New multilobe “accordion” flaps for three-dimensional reconstruction of wide, full-thickness defects in the oral floor. Ann Plast Surg. 2000;45:187–92. doi: 10.1097/00000637-200045020-00017. [DOI] [PubMed] [Google Scholar]
- 12.Shieh SJ, Chiu HY, Yu JC, et al. Free anterolateral thigh flap for reconstruction of head and neck defects following cancer ablation. Plast Reconstr Surg. 2000;105:2349–57. doi: 10.1097/00006534-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Kimata Y, Uchiyama K, Sakuraba M, et al. Deep circumflex iliac perforator flap with iliac crest for mandibular reconstruction. Br J Plast Surg. 2001;54:487–90. doi: 10.1054/bjps.2001.3633. [DOI] [PubMed] [Google Scholar]
- 14.Rees MJ, Taylor GI. A simplified lead oxide cadaver injection technique. Plast Reconstr Surg. 1986;77:141–5. doi: 10.1097/00006534-198601000-00023. [DOI] [PubMed] [Google Scholar]
- 15.O’Brien CJ, Lee KK, Stern HS, et al. Evaluation of 250 free-flap reconstructions after resection of tumours of the head and neck. Aust N Z J Surg. 1998;68:698–701. doi: 10.1111/j.1445-2197.1998.tb04654.x. [DOI] [PubMed] [Google Scholar]
- 16.Koshima I, Fukuda H, Yamamoto H, et al. Free anterolateral thigh flaps for reconstruction of head and neck defects. Plast Reconstr Surg. 1993;92:421–8. [PubMed] [Google Scholar]
- 17.Germann G, Busching K, Wittemann M. Two modifications of the radial forearm flap for reconstruction of complex facial defects. J Reconstr Microsurg. 1999;15:489–93. doi: 10.1055/s-2007-1000127. [DOI] [PubMed] [Google Scholar]
- 18.Shindo ML, Sullivan MJ. Muscular and myocutaneous pedicled flaps. Otolaryngol Clin North Am. 1994;27:161–72. [PubMed] [Google Scholar]
- 19.Shektman A, Silver C, Strauch B. A re-evaluation of hypopharyngeal reconstruction: pedicled flaps versus microvascular free flaps. Plast Reconstr Surg. 1997;100:1691–6. doi: 10.1097/00006534-199712000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Talesnik A, Markowitz B, Calcaterra T, et al. Cost and outcome of osteocutaneous free-tissue transfer versus pedicled soft-tissue reconstruction for composite mandibular defects. Plast Reconstr Surg. 1996;97:1167–78. doi: 10.1097/00006534-199605000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Urken ML, Buchbinder D, Weinberg H, et al. Functional evaluation following microvascular oromandibular reconstruction of the oral cancer patient: a comparative study of reconstructed and nonreconstructed patients. Laryngoscope. 1991;101:935–50. doi: 10.1288/00005537-199109000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Kroll SS, Schusterman MA, Reece GP. Costs and complications in mandibular reconstruction. Ann Plast Surg. 1992;29:341–7. doi: 10.1097/00000637-199210000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Kudo K, Shoji M, Yokota M, et al. Evaluation of mandibular reconstruction techniques following resection of malignant tumors in the oral region. J Oral Maxillofac Surg. 1992;50:14–21. doi: 10.1016/0278-2391(92)90186-4. [DOI] [PubMed] [Google Scholar]
- 24.Yang D, Marshall G, Morris SF. Variability in the vascularity of the pectoralis major muscle. Can J Otolaryngol. doi: 10.2310/7070.2003.35357. (In press) [DOI] [PubMed] [Google Scholar]
- 25.Seikaly H, Calhoun K, Rassekh CH, et al. The clavipectoral osteomyocutaneous free flap. Otolaryngol Head Neck Surg. 1997;117:547–54. doi: 10.1016/S0194-59989770029-9. [DOI] [PubMed] [Google Scholar]
- 26.Reid CD, Taylor GI. The vascular territory of the acromiothoracic axis. Br J Plast Surg. 1984;37:194–212. doi: 10.1016/0007-1226(84)90010-9. [DOI] [PubMed] [Google Scholar]
- 27.Williams GR, Koffler K, Pepe M, et al. Rotation of the clavicular portion of the pectoralis major for soft-tissue coverage of the clavicle. An anatomical study and case report. J Bone Joint Surg Am. 2000;82:1736–42. doi: 10.2106/00004623-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Salmon M. Arteres de la Peau. Paris: Masson et Cie; 1936. [Google Scholar]
- 29.Schwabegger AH, Herczeg E, Piza H. The lateral thoracic fasciocutaneous island flap for treatment of recurrent hidradenitis axillaris suppurativa and other axillary skin defects. Br J Plast Surg. 2000;53:676–8. doi: 10.1054/bjps.2000.3443. [DOI] [PubMed] [Google Scholar]
- 30.Chandra R, Kumar P, Abdi SH. The subaxillary pedicled flap. Br J Plast Surg. 1988;41:169–73. doi: 10.1016/0007-1226(88)90046-x. [DOI] [PubMed] [Google Scholar]
- 31.Morris SF, Taylor GI. Predicting the survival of experimental skin flaps with a knowledge of the vascular architecture. Plast Reconstr Surg. 1993;92:1352–61. [PubMed] [Google Scholar]
- 32.Manchot C. Die hautarterien des menslichen korpers. Liepzig: FCW Vogel; 1889. [Google Scholar]


