Abstract
There is a wealth of evidence showing enhanced attention towards drug-related information (i.e. attentional bias) in substance abusers. However, little is known about attentional bias in deregulated behaviors without substance use such as abnormal gambling. This study examined whether problem gamblers (PrG, as assessed through self-reported gambling-related craving and gambling dependence severity) exhibit attentional bias for gambling-related cues.
Forty PrG and 35 control participants performed a change detection task using the flicker paradigm, in which two images differing in only one aspect are repeatedly flashed on the screen until the participant is able to report the changing item. In our study, the changing item was either neutral or related to gambling. Eye movements were recorded, which made it possible to measure both initial orienting of attention as well as its maintenance on gambling information.
Direct (eye-movements) and indirect (change in detection latency) measures of attention in individuals with problematic gambling behaviors suggested the occurrence of both engagement and of maintenance attentional biases towards gambling-related visual cues. Compared to non-problematic gamblers, PrG exhibited (1) faster reaction times to gambling-cues as compared to neutral cues, (2) higher percentage of initial saccades directed toward gambling pictures; (3) an increased fixation duration and fixation count on gambling pictures. In the PrG group, measures of gambling-related attentional bias were not associated with craving for gambling and gambling dependence severity. Theoretical and clinical implications of these results are discussed.
Keywords: Gambling, Attentional bias, Dependence, Eye-tracking, Craving
Introduction
The main goal of this study is to explore the time course of the deployment of attention in pathological gamblers as they process visual stimuli that are or are not related to their addiction.
Pathological gambling (PG), similar to other addictions, can be operationally defined as the continuation of maladaptive choices despite the occurrence of aversive consequences (e.g., relationship, job; APA). PG afflicts about 1.6% of the general population (Inserm, 2008). With growing availability of gambling opportunities, prevalence of PG is rising and beginning to pose a serious public health problem (Inserm, 2008).
Numerous studies have shown that addiction-related cues are processed more efficiently by addicted individuals, thus further reinforcing subsequent maladaptive cognition and behaviors (for a review see, Field and Cox, 2008; Field, Munafo, & Franken, 2009). According to the incentive-sensitization theory (Robinson and Berridge, 1993; 2003), compulsive gambling (as other states of addiction) might be caused primarily by repeated exposure to gambling-related stimuli that would induce gambling sensitization in the brain’s meso-limbic and meso-cortical dopamine systems that attribute incentive salience to reward-associated stimuli. In other terms, pathological motivation could arises from sensitization of brain circuits that mediate Pavlovian conditioned incentive motivational processes. Therefore, this sensitization might occur even in the absence of drug actions, such as in abnormal gambling. Once rendered hypersensitive, these systems generate pathological incentive motivation (i.e., wanting) for addictive behaviors. During wanting, incentive salience, which is a type of incentive motivation, plays a role in promoting approach towards, and consumption of, rewards. Wanting has distinct psychological and neurobiological features from liking. In this context, incentive sensitization could produce an attentional bias towards processing drug-associated stimuli and pathological motivation for drugs (compulsive wanting; Robinson and Berridge, 1993; 2003).
Recent theoretical models of addiction (e.g., Baker et al., 1987; Field & Cox, 2008; Franken, 2003; Kavanagh et al., 2005; Robinson & Berridge, 1993; Ryan, 2002) suggest that attentional biases for substance-related cues experienced by substance users could modulate aspects of subjective experience (e.g., craving) and influence addictive behaviors. A recent meta-analysis by Field, Munaro and Franken (2009) indicated a modest but statistically significant positive correlation between subjective craving, as assessed with self reported measures, and attentional bias. Moreover, this study highlighted a marginally significant effect for a larger association between craving and attentional bias when measures of the maintenance of attention from substance-related cues were compared with measures of the initial orienting response of attention. One possible explanation for this difference is that the attentional maintenance measure better reflects the specific attentional processes that are influenced by incentive mechanisms, namely, a bias to hold attention on motivationally salient cues (LaBerge, 1995). These insights led us to investigate the relationship between attention biases and craving in problem gamblers.
Although numerous studies have focused on the attentional biases in individuals who abuse substances such as alcohol, drugs and/or tobacco (for a recent review of attentional biases in addicts see, Field and Cox, 2008; Field et al., 2009), little is known about attentional bias in addictive disorders that do not necessarily involve the ingestion of exogenous substances, namely pathological gambling. For instance, using a modified Stroop paradigm, participants with compulsive gambling took longer to name the color of words relating to gambling compared to healthy controls or to low problem gamblers (Boyer and Dickerson, 2003; Molde et al., 2010). However, performance on the modified Stroop paradigm does not allow investigation of more specific attentional processes, such as the initial orienting component, which is typically followed by attentional capture or repulsion (Jones, Bruce, Livingstone, & Reed, 2006). Hence the main goal of this study was to investigate the effects of gambling on these specific processes of attention.
To shed further light on the nature of gambling-related attentional bias, we used a change detection task called “the flicker paradigm” (Rensink, O’Regan, & Clark, 1997; Simons & Rensink, 2005), which has often been used to demonstrate “change blindness”. This task consists of consecutive and repeated presentations of two identical visual scenes separated by a mask (typically a gray screen), that differ in only one element. The presentation of the visual scenes continues until the change is detected. With normal individuals, the number of presentations necessary for the change to be detected is much higher than what would be expected based on a direct comparison of the two alternating pictures — hence the expression “change blindness”, for participants are surprisingly found to be unable to detect changes that are typically obvious under normal viewing conditions. The number of repetitions required for the change to be detected thus constitutes the main dependent measure in this paradigm, and it has been shown to be influenced by specific conditions or with specific populations. For instance, some studies have reported faster change detection latency by problematic heavy drinkers for addiction-related changes compared to neutral ones (Jones, Jones, Smith and Copley, 2003; Jones, Bruce, Livingstone and Reed, 2006).
However, a main limitation of classic behavioral paradigms (such as the flicker paradigm but also modified Stroop and visual probe tasks) is that they do not make it possible to explore the time course of the allocation of attention. Tracking eye movements, by contrast, in addition to being ecologically valid, importantly enables the investigation of attentional biases not only at stimulus offset but also during the entire duration of the stimulus presentation (Schoonmaker, Wiers, & Field, 2008). Attentional biases as revealed by the pattern of eye movements in response to a visual stimulus (i.e., prolonged maintenance of gaze, or a higher proportion of initial eye movements directed toward addiction-related versus neutral cues) has so far been demonstrated only in individuals addicted to psychoactive substances such as tobacco, cannabis, alcohol and drugs (for a review see, Field, Munafo, & Franken, 2009).
In summary, we aimed to investigate the nature of gambling-related attentional bias in a group of problem gamblers (PrG) by using a flicker paradigm for induced change blindness with direct (i.e., eye movements recording) and indirect (i.e., change detection latency) measures of attentional processes. We test three primary hypotheses: compared to normal control (CONT), PrG would (1) detect a gambling-related change more rapidly than a neutral change; (2) direct their initial eye movement toward gambling-related cues, indicating facilitated attentional engagement towards gambling stimuli; (3a) show prolonged maintenance of gaze towards gambling-related elements compared to neutral stimuli and (3b) would exhibit a higher proportion of eye movements towards gambling-related elements, indicating attentional maintenance on gambling cues. In addition, we expect to find an association between gambling self-reported craving and maintenance of attention towards gambling cues.
Methods
Participants
Two groups participated in the study: (1) a control group (CONT) (n=35) and (2) a problem gamblers (PrG) group (n=40). All subjects were adults (>18 years old) and provided informed consent that was approved by the appropriate human subject committee at the Brugmann University Hospital. The demographic data on the two groups are presented in Table 1.
Table 1.
Demographical data and standard deviations for pathological gambling (PG), problem gambling (PrG) and normal control (CONT) groups.
|
normal
Control |
problem
Gambling |
Test Statistics |
Bonferroni-corrected
Pairwise Comparisons |
|
|---|---|---|---|---|
| n | 35 | 40 | ||
| Age | 32.78(9.77) | 31.00(10.20) | t(72) = .74, p = .46 | CONT = PrG |
| Male/Female | 20/15 | 22/18 | X2(1,75) = .04, p = .85 | CONT = PrG |
| BDI | 2.14(1,81) | 4.3(5,41) | t(73) = 2.11, p <.05 | CONT < PrG |
| Employed full time % (n) | 74.3(26) | 55.0(22) | X2(1,75) = 3.01, p = .097 | CONT = PrG |
| Education% (n) | X2(1,75) = .89, p = .48 | CONT = PrG | ||
| < 12th grade | 34.3(12) | 45.0(18) | ||
| 12th grade or higher | 65.7(23) | 55.0(22) | ||
| STAI-E | 38.62(7.66) | 43.25(10.07) | t(73) = −2.16, p < .05 | CONT < PrG |
| STAI-T | 30.08(7.31) | 34,64(10,23) | t(73) = −2.01, p <.05 | CONT < PrG |
| Cigarettes/Day | 3.61(6.67) | 8.87(9.33) | t(73) = 2.81,p < 01 | CONT < PrG |
| SOGS | 0.00(0.00) | 4.6(2.71) | ||
| Craving anticipation | / | 13,53(5,75) | ||
| Craving desire | / | 5,48(3,43) | F(2, 38) = 64,83,p < 01 | |
| Craving relief | / | 5,35(4,22) |
Note. Values shown are the mean and standard deviations on each measure. The South Oaks Gambling Screen was administered only in the PrG group. Degrees of freedom differ due to missing data. BDI = Beck Depression Inventory, STAI-E = State version of the State-Trait Anxiety Inventory, STAI-T = Trait version of the State-Trait Anxiety Inventory, SOGS = South Oaks Gambling Screen.
Recruitment and screening methods
Gambling dependence severity was assessed with the South Oaks Gambling screen (SOGS; Lesieur & Blume, 1987). Scores on the SOGS can vary between 0 and 20. An example of an item is: “Have you ever borrowed from someone and not paid them back as a result of your gambling?”.
All PrG (n=40) scored ≥3 (max = 8) on the SOGS, indicative of problem gambling, and 13 participants (32.5%) met the more stringent criteria for probable pathological gambling (SOGS ≥ 5). On the basis of Lawrence, Luty, Bogdan, Sahakian, & Clark (2009), we will refer to this combined group henceforth as PrG. Distribution of SOGS scores in the PrG group is presented in Table 1. CONT were recruited by word of mouth from the employees at the psychiatric unit of the Brugmann University Hospital. To avoid biases, resulting from inside knowledge of how these tasks operate, Psychiatrists, Psychologists and other personnel having had psychological training were excluded from participation. On the SOGS, only 6 CONT (17%) reported playing the numbers or betting on lotteries occasionally (i.e., less than once a week). All remaining control participants reported not gambling at all.
Current clinical status
Current clinical status of depression and anxiety was rated with French versions of the Beck Depression Inventory (Beck, Ward, Mendelson, Mock, & Erbaugh, 1967) and the Spielberger State-Trait Anxiety Inventory (STAI; Spielberger, 1993), respectively. The number of cigarettes per day was also included on the basis of previous studies (e.g., Heishman, 1998) that highlighted an effect of nicotine dependence on cognitive processing (e.g., sustained attention). We excluded any control subject who met an Axis I psychiatric diagnosis assessed by the Structured Clinical Interview for DSM–IV (First, Spitzer, Gibbon, & Williams, 2002), who had experienced a drug use disorder during the year before enrollment in the study, or who had consumed more than 54 g/day of alcohol for longer than 1 month. On the basis of the results of their medical history and physical examination, they were judged to be medically healthy. All participants were asked to avoid the use of drugs, including narcotic pain medication, for the 5 days prior to testing and to avoid alcohol consumption for the preceding 24 hr.
Self-report measure of gambling-related craving
We used the Gambling Craving Scale (GACS; Young and Wohl, 2009) to assess subjective craving toward gambling in PrG. The GACS contains 3 factors: Anticipation (e.g., “Gambling would be fun right now”), Desire (e.g., I crave gambling right now”) and Relief (e.g., “If I were gambling now, I could think more clearly”). There are 9 items (3 items for each of the 3 factors) assessed on a 7-point scale. For this study, the GACS was translated into French. Back translation method was used. For the present sample, using Cronbach’s alpha, the internal consistency reliability was .78, .84, .89 for the factors Anticipation, Desire, and Relief, respectively.
Paradigm and design
The original stimulus (OS) was presented for 250ms, followed by the mask (M) for 80 ms, then the changed stimulus (CS) for 250ms. The OS-M-CS-M series was continuously presented until change detection. Based upon previous research with the flicker paradigm (Jones et al., 2002; Jones et al., 2003, 2005), participants performed only 1 single-flicker task (in the current case, to detect either the gambling related or the neutral change). The dependent variable was change-detection latency, direction of first eye movement, proportion of eye fixation count and length.
We controlled for the possibility that information from the left hemispace might be processed more readily than information from the right hemispace in normal individuals (i.e., pseudoneglect; e.g., Nicholls, Orr, Okuba, & Loftus, 2006). Therefore, all participants were randomly assigned to one of four flicker conditions, leading to a 2 (CONT vs PrG group) × 2 (gambling-related vs neutral change) × 2 (bilateral organization of the stimulus; gambling stimuli on the left and neutral on the right, GN, vs neutral left and gambling right, NG) between-subjects design.
Apparatus and Stimuli
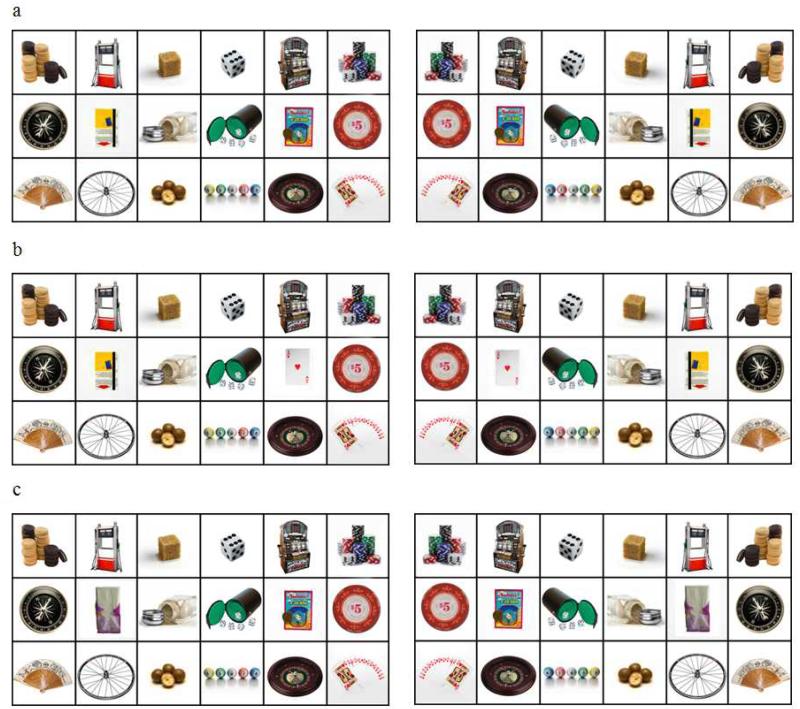
The OS consisted of a matrix of 18 full color photographs depicting 9 gambling related and 9 neutral objects on each side (see Figure 1). The 9 pairs of gambling and neutral objects were selected so that their physical properties (e.g., color, height, width, shape) were similar. The two sets of 9 photographs were arranged in two 3 × 3 matrices set in a 3 × 6 landscape matrix, with items of each matched pair occupying corresponding positions across their respective matrices. The CS with the gambling related change was identical to the OS except that the object at the center of the gambling matrix was substituted (see Figure 1b).
Figure 1.
The original stimuli (OS) and changed stimuli (CS) used in the flicker paradigm for induced change blindness. Panel 1a. Two OS (gambling-right, neutral-left, NG, and neutral-right, gambling-left, GN); Panel 1b. Two CS, CS-gambling-related-change (gambling-right, neutral-left, NG, and neutral-right, gambling-left, GN); Panel 1c. Two CS, CS-neutral-change (gambling-right, neutral-left, NG, and neutral-right, gambling-left, GN).
There was a second CS with a corresponding neutral substitution (Figure 1c). The two different CSs with their common OS represented the two levels of Factor 2 (nature of change). Finally, bilateral reversals of each of the OS and the two CSs were made for the two levels of Factor 3 (i.e., GN and NG). The single mask comprised rows of uppercase, 20-point Xs in Times New Roman font.
This task consists of consecutive and repeated presentations of two identical visual scenes separated by a mask (typically a gray screen), that differ in only one element. The presentation of the visual scenes continues until the change is detected.
A TOBII X120 eye tracker was used to measure participant’s eye movements. The TOBII X120 records the X and Y coordinates of participant’s eye position at 60 Hz by using corneal reflection techniques. Calibration procedures were run using Clearview software (TOBII Technology, Sweden) which allows an optimal accuracy of 0.5 degrees. Stimulus presentation and data output for the flicker task were programmed in E-Prime version 2.0 professional and appeared on a 17 inch CRT-monitor with a refresh rate of 85 Hz.
The eye tracking software and measures were run and recorded on an Intel Xenon based PC, which was linked to an Intel Core 2 based laptop through a local area network. E-Prime software was used on the Intel Core 2 based laptop, which also recorded the change-detection latency measure.
Procedure
Testing took place individually and in a quiet room, located at the Medical Psychology Laboratory of the Brugmann Hospital. Participants were invited to first complete the STAI-State (Spielberger, 1993). Participants were seated 60 cm in front of the TOBII monitor. The experimenter manipulated the monitor until the cameras detected participants’ corneal reflection. Participants were then shown a series of looming balls that appeared in a 5-point calibration sequence. Calibration accuracy was checked and repeated if necessary. Before performing the flicker task, participants were shown a preview of the flicker paradigm for induced change blindness, but with unrelated objects than those used in the following flicker paradigm and without the difference between OS and CS. This was made to accustom participants to the fast stimuli’s appearance rate. Participants then performed the flicker task with a gambling or neutral change. They were asked to watch a series of nearly identical pictures “flicked back and forth” on the screen and to detect the difference between them as quickly as possible. Participants had to indicate that they had detected a change by quickly saying “STOP” aloud, at which moment the experimenter pushed a dedicated button on a wireless gamepad to time-stamp the moment of change detection.
Immediately after the flicker task, PrG participants were required to fill out the GACS.
Data analysis
Change-detection latency
Change-detection latency was the total number of combined OS-M-CS-M presentations until change detection. We performed a univariate analysis of variance (ANOVA) with group (CONT vs. PrG), type of change to be detected (gambling-related vs. neutral) and bilateral organization of the stimulus (gambling stimuli on the left and neutral on the right, GN vs. neutral left and gambling right, NG) as between-subjects factors, and change detection latency as dependent variable.
Direction of first eye movement
The first eye movement was defined as the first fixation lasting at least 100 ms in the region of either the gambling or neutral stimulus, at least 100 ms after the first OS onset. This enabled us to calculate the percentage of initial eye movements that were directed at gambling-related vs control pictures during the task. To examine whether participants showed a bias in the first eye movement direction during the flicker task, the percentage of initial eye movements toward gambling pictures was compared with 50% (which indicates no bias).
Proportion of fixation count
Proportion of fixation count was the total number of eye-fixation directed toward gambling or neutral stimuli until change detection divided by the total amount of eye-fixations. Fixation count was analyzed using ANOVA with repeated measures, with group, type of change to be detected and bilateral organization of the stimulus as between-subjects factors; with type of stimulus (gambling, neutral) as a within subjects factor; and proportion of fixation count, as the dependent measure.
Proportion of fixation length
Proportion of fixation length was the total time (ms) of eye-fixation directed toward gambling or neutral stimuli until change detection divided by the total length of eye-fixation. Fixation length was analyzed using ANOVA with repeated measurements, with group, type of change to be detected and bilateral organization of the stimulus as between-subjects factors; type of stimulus (gambling, neutral) as a within subjects factor; and fixation length, as the dependent measure.
Association between gambling related attentional bias, self-reported gambling-related craving and gambling dependence severity in PrG
Correlation analyses were conducted between the gambling-related attentional bias measures, total score of the GACS, scores of the three factors of the GACS and score on the SOGS (n = 40). A univariate ANOVA was also conducted with direction of first eye movement (Neutral vs. Gambling), as between-subjects factors, and total score of the GACS, scores of the three factors of the GACS and score on the SOGS score as dependent variable.
Results
Demographics and current clinical status
A description of demographic variables, scores on the South Oaks Gambling Screen (SOGS), Beck Depression Inventory (BDI), the Trait and State version of the State-Trait Anxiety Inventory (STAI) and the average number of cigarettes smoked per day is presented in Table 2. Chi square analyses revealed no differences in the number of male and female participants. Depression was higher in PrG than in CONT, t(73) = 2.11, p < .05. State and trait anxiety was higher in the PrG group in comparison with the CONT group, t(73) = −2.16, p < .05; t(73) = −2.01, p <. 05, respectively. The average number of cigarettes smoked per day was higher in PrG than in CONT, t(73) = 2.81, p <.01. No other group differences were present. Because our sample of PrG included individuals who met the more stringent criteria for probable pathological gambling, the effect of gambling severity was controlled for the PrG group. In the absence of effect covariate effect of depression, trait-state anxiety, and number of cigarettes smoked per day on group comparisons, we performed ANOVAs.
Table 2.
Correlation (n = 40) between gambling related attentional bias, self-reported gambling-related craving and gambling dependence severity in PrG.
| Variables | SOGS | Craving total |
Craving anticipation |
Craving desire |
Craving relief |
|---|---|---|---|---|---|
| Gambling-related change detection latency |
.23 | .08 | .09 | .05 | .02 |
| Neutral change detection latency |
−.14 | −.31 | −.30 | −.09 | −.21 |
| Proportion of fixation count toward gambling cues |
.11 | .06 | .13 | .10 | .04 |
| Proportion of fixation length towards gambling cues |
.05 | .04 | .14 | .12 | .01 |
Change detection latency
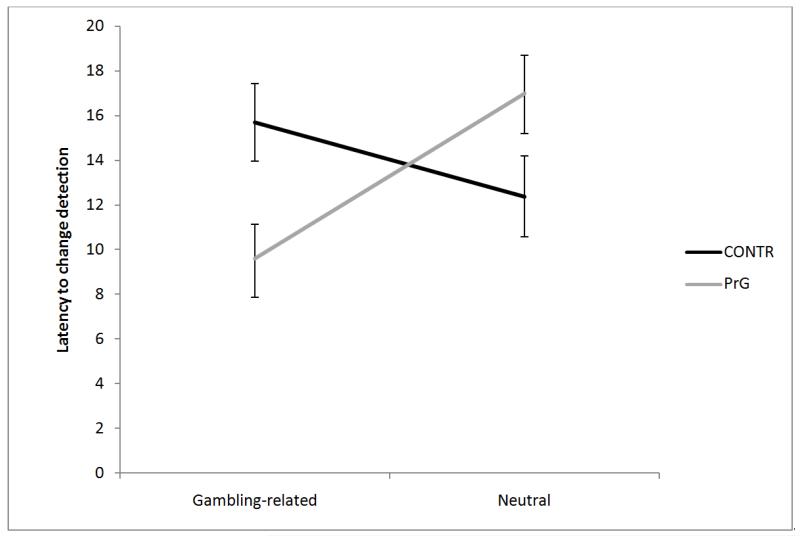
All participants detected all changes correctly. The ANOVA showed no main effects of Group, Type of Change, or Stimulus Orientation (all p > .05). There was no interaction except for the following one, which supported the gambling-related attentional bias hypothesis in problem gamblers. An interaction between groups and type of change was found, F(1, 67) = 10.57, p < .01, η2 = .13. This analysis showed that PrG’ change-detection latency for the gambling-related change was smaller than for the neutral change. Control participants’ change-detection latency for the gambling-related change and for the neutral change, however, were not different (see Figure 2).
Figure 2.
Latency to change-detection for CONT and PrG with gambling-related and neutral changes.
Direction of first eye movement
The percentage of first eye movements toward gambling pictures was significantly greater than 50% in the PrG group but not in the CONT group, t(39) = 2.73, p < .01 and t(34) = .12, ns, respectively. Also, a t test revealed that the first eye movement percentages toward gambling pictures differed significantly between groups, t(74) = 4.71, p < .05.
Proportion of fixation count
There were interactions between type of change and fixation count, F(1,67) = 16.68, p < .001, η2 = .19, and between group and fixation count, F(1,67) = 6.04, p < .05, η2 = .08. Analyses revealed that participants fixation on change-related stimuli occurred more frequently (M = .58, SD = .15) compared to stimuli not linked to the change (M = .42, SD = .15). The other interaction effect revealed that PrG group, but not CONT, fixated on gambling-related stimuli more frequently compared to neutral stimuli. Results of the group × type of stimulus interaction are presented in Figure 3.
Figure 3.
Proportion of fixation count for CONT and PrG with gambling-related and neutral stimuli.
Fixation length
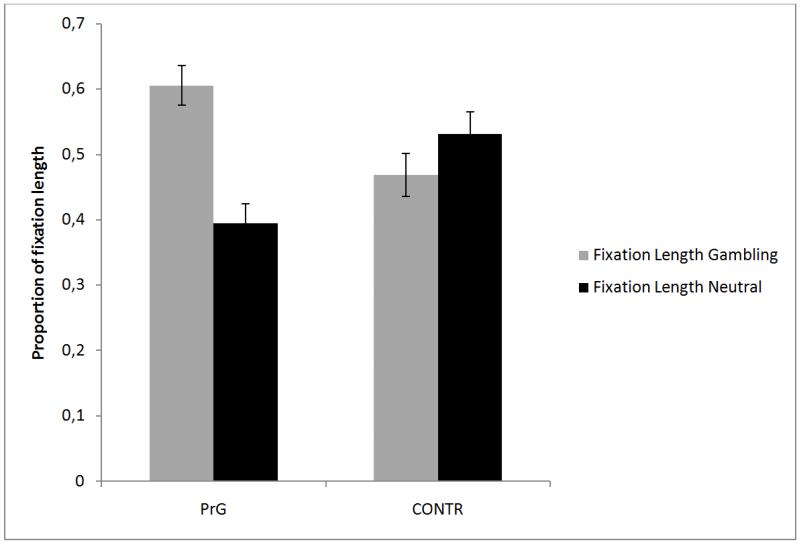
Analyses revealed a type of change × fixation length interaction, F(1,73) = 13.31, p < .001, η2 = .17, and a group × type of stimulus interaction, F(1,73) = 9.78, p < .001, η2 = .13. Analyses revealed that participants fixated longer change-related stimuli (M = .57, SD = .17) compared to stimuli not linked to the change (M = .43, SD = .17). For the other interaction, the analyses revealed that PrG group fixated much longer gambling-related stimuli compared to neutral stimuli (see Figure 4).
Figure 4.
Proportion of fixation length for CONT and PrG with gambling-related and neutral stimuli.
Association between gambling related attentional bias, self-reported gambling-related craving and gambling dependence severity in PrG
Correlation analyses (n = 40) revealed that there was no significant correlation between the gambling-related attentional bias measures, the total score of the GACS, scores on the three factors of the GACS and score on the SOGS (see Table 3). There was also no significant difference between the direction of first fixation on both GACS and SOGS scores (F < 1).
Discussion
The main findings of the present research could be summarized as follows: comparison of the PrG and the CONT showed that PrG are faster in detecting gambling-related changes in the flicker paradigm, exhibit more gaze fixation counts and longer fixation lengths toward gambling-related stimuli. In addition, unlike CONT, the percentage of first eye movements toward gambling cues was higher and significantly above chance level for the PrG group.
As hypothesized, behavioral data (indirect measure of attention) recorded during the flicker paradigm showed that, in comparison with CONT, PrG, all of whom met criteria for problem gambling based on their scores on the South Oaks Gambling Screen (SOGS), are faster to detect gambling-related change. This result suggests that PrG’s attention is captured by gambling related cues, i.e., attentional bias. This finding is in line with studies showing that on a modified version of the Stroop task, PrG’s take more time to name the color of the words related to gambling practices than neutral one(s) (Boyer and Dickerson, 2003; Molde et al., 2010).
We then set out to ascertain whether this attention bias was due to engagement or/and maintenance of attention. To do so, participants’ eye movements were monitored using eye-tracking technology (direct measure of attention). Compared to control participants, PrG directed their first eye movements more frequently toward gambling-related than toward neutral stimuli (bias of attentional engagement), exhibit more gaze fixation counts on gambling stimuli and spent more time looking at gambling-related (bias of attentional maintenance) than control stimuli. This pattern of eye-movements suggests that both initial engagement and maintenance of attention are parts of the problem that drive gambling cognition and behavior.
Contrary to our hypothesis, we found no significant correlation between the maintenance of attention and craving scores assessed with the Gambling Craving Scale (GACS). An explanation for the absence of a relationship between attentional bias and craving is that, like substance addictions, it may occur automatically and habitually in the absence of any conscious subjective experience of craving (Tiffany, 1990). As an alternative explanation, the absence of relationship between craving and attentional bias might be accounted for by a low subjective craving in PrG at the time of assessment. Indeed, scores on the GACS’ subscales revealed that PrG experienced an intention to gamble that was anticipated to be fun and enjoyable (the Anticipation scale) rather than a strong, urgent desire to gamble (the Desire scale) and an expectation that gambling would provide relief from negative affect (the Relief scale). Moreover, there was also no association between gambling-related attentional bias and gambling dependence severity. This was probably due to the relatively small variation of SOGS’ scores between PrG participants.
Findings related to the presence of attentional bias in PrG are consistent with the incentive-sensitization theory (Robinson and Berridge 1993, 2003). This model proposes that attentional and approach biases for addiction-related stimuli are an indication of incentive processes, and that incentive sensitization mechanisms play an important role in the development and the maintenance of an addiction state. The presence of attentional bias in PrG as well as in individuals addicted to substance (alcohol, cannabis, tobacco, heroin, & cocaine; for a review see, Field, Munafo, & Franken, 2009) suggests that this shared component may lead to poor self-regulation. Most importantly, it raises the possibility that gambling-related attentional bias might be a treatment target (van Holst, van den Brink, Veltman, & Goudriaan, 2009). Indeed, decreasing attentional biases with the help of behavioral therapy and modification paradigms may result in increasing likelihood to select alternative behaviors to have fun (or to feel less anxiety).
A limitation of this paper is that we cannot isolate the “problem gambling” component per se since problem gamblers have been compared to non-gamblers instead of healthy non-problem gamblers. This problem limits the generalizability of our results. Therefore, it is certainly important to extend this research to a larger sample of gamblers which has both extreme ends of the spectrum of gambling dependence well represented, including healthy non-problem gamblers (e.g., usual lottery players) as well as pathological gamblers who attempt to stop gambling. Furthermore, on the basis of Tiffany (1990), gamblers who want to stop gambling may experience extreme deprivation conditions that would elicit strong incentive effects (and associated intense craving) towards gambling-related stimuli, such that attentional bias for gambling cues may rise to ceiling levels. Finally, even if we did not seek in this experiment to investigate the relationship between the intensity of craving and attentional biases in control, the Gambling Craving Scale could also be administrated to these subjects in further studies. Such research might clarify the precise nature of the relationships between state and trait gambling-related variables (e.g., craving, gambling dependence severity) and the cognitive and behavioural indications of incentive salience processes (e.g., attentional biases), given that these incentive mechanisms are proposed to play a key role in maintaining addictive behaviors and in increasing the risk of relapse following quit attempts.
In summary, direct and indirect measures of attention in individuals with problematic gambling behaviors emphasized the presence of both attentional engagement and maintenance biases towards gambling-related pictorial cues during a flicker paradigm for induced change blindness. These attentional biases correspond well to those seen in substance addiction, including alcohol, tobacco, cannabis, heroin and cocaine. This research is consistent with models of addiction which suggest that addiction-related cues acquire incentive-motivational properties.
Acknowledgement
This research was supported by the Belgium National Lottery and the National Fund for Scientific Research, Belgium. We thank Michael Baker, Table Games Manager for the VIAGE casino complex (Brussels, Belgium), for his help in recruiting gamblers participants.
Contributor Information
Damien Brevers, Psychological Medicine Laboratory, CHU-Brugmann, Université Libre de Bruxelles, Belgium.
Axel Cleeremans, Consciousness, Cognition & Computation Group, Université Libre de Bruxelles, Belgium.
Antoine Bechara, Psychiatry Department and Faculty of Management, McGill University, Canada, and Department of Psychology, University of Southern California, USA.
Cédric Laloyaux, Psychological Medicine Laboratory, CHU-Brugmann, Université Libre de Bruxelles, Belgium
Charles Kornreich, Psychological Medicine Laboratory, CHU-Brugmann, Université Libre de Bruxelles, Belgium
Paul Verbanck, Psychological Medicine Laboratory, CHU-Brugmann, Université Libre de Bruxelles, Belgium
Xavier Noël, Psychological Medicine Laboratory, CHU-Brugmann, Université Libre de Bruxelles, Belgium
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental disorders - 4th Edition. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Baker TB, Morse E, Sherman JE. The motivation to use drugs: A psychobiological analysis of urges. In: Dienstbier RA, Rivers PC, editors. Nebraska Symposium on Motivation: Vol. 34. Alcohol and addictive behavior. University of Nebraska Press; Lincoln: 1987. pp. 257–323. [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Boyer M, Dickerson M. Attentional bias and addicitve behaviour: Automaticity in a gambling-specific modified Stroop task. Addiction. 2003;98(1):61–70. doi: 10.1046/j.1360-0443.2003.00219.x. [DOI] [PubMed] [Google Scholar]
- Expertise collective Inserm . Jeux de hasard et d’argent, contextes et addictions. INSERM; Paris: 2008. [Google Scholar]
- Field M, Cox WM. Attentional bias in addictive behaviors: A review of its development, causes, and consequences. Drug and Alcohol Dependence. 2008;97:1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Field M, Munafò MR, Franken IH. A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychological Bulletin. 2009;135(4):589–607. doi: 10.1037/a0015843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP) Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Franken IHA. Drug craving and addiction: Integrating psychological and neuropsychopharmacological approaches. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2003;27:563–579. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Heishman SJ. What aspects of human performance are truly enhanced by nicotine? Addiction. 1998;93(3):317–320. doi: 10.1080/09652149835864. [DOI] [PubMed] [Google Scholar]
- Jones BC, Jones BT, Blundell L, Bruce G. Social users of alcohol and cannabis who detect substance-related changes in a change blindness paradigm report higher levels of use than those detecting substance-neutral changes. Psychopharmacology. 2002;165:93–96. doi: 10.1007/s00213-002-1264-2. [DOI] [PubMed] [Google Scholar]
- Jones BT, Jones BC, Livingstone S, Reed E. Alcohol-related attentional bias in problem drinkers with the flicker change blindness paradigm. Psychology of Addictive Behaviors. 2006;20(2):171–177. doi: 10.1037/0893-164X.20.2.171. [DOI] [PubMed] [Google Scholar]
- Jones BT, Jones BC, Smith H, Copely N. A flicker paradigm for inducing change blindness reveals alcohol and cannabis information processing biases in social users. Addiction. 2003;98:235–244. doi: 10.1046/j.1360-0443.2003.00270.x. [DOI] [PubMed] [Google Scholar]
- Kavanagh DJ, Andrade J, May J. Imaginary relish and exquisite torture: The elaborated intrusion theory of desire. Psychological Review. 2005;112:446–467. doi: 10.1037/0033-295X.112.2.446. [DOI] [PubMed] [Google Scholar]
- Laberge D. Computational anatomical models of selective attention in object identification. In: Gazzaniga Michael S., editor. The cognitive neurosciences. The MIT Press; Cambridge, MA, US: 1995. pp. 649–663. [Google Scholar]
- Lawrence AJ, Luty J, Bogdan NA, Sahakian BJ, Clark L. Impulsivity and response inhibition in alcohol dependence and problem gambling. Psychopharmacology. 2009;207(1):163–172. doi: 10.1007/s00213-009-1645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesieur HR, Blume SB. The South Oaks Gambling Screen (SOGS): a new instrument for the identification of pathological gamblers. American Journal of Psychiatry. 1987;144:1184–8. doi: 10.1176/ajp.144.9.1184. [DOI] [PubMed] [Google Scholar]
- Molde H, Pallesen S, Saetrevik B, Hammerborg DK, Laberg JC, Johnsen BH. Attentional biases among pathological gamblers. International Gambling Studies. 2010;10(1):45–49. [Google Scholar]
- Nicholls ME, Orr CA, Okubo, Loftus A. Satisfaction Guaranteed: The effect of spacial biases on responses to Likert Scales. Psychological Science. 2006;17(12):1027–1028. doi: 10.1111/j.1467-9280.2006.01822.x. [DOI] [PubMed] [Google Scholar]
- Rensink RA, O’Regan JK, Clark JJ. To see or not to see: The need for attention to perceive changes in scenes. Psychological Science. 1997;8:368–373. [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annual Review of Psychololigy. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Ryan F. Detected, selected, and sometimes neglected: Cognitive processing of cues in addiction. Experimental and Clinical Psychopharmacology. 2002;10:67–76. doi: 10.1037//1064-1297.10.2.67. [DOI] [PubMed] [Google Scholar]
- Schoenmakers T, Wiers RW, Field M. Effects of a low dose of alcohol on cognitive biases and craving in heavy drinkers. Psychopharmacology. 2008;197:169–178. doi: 10.1007/s00213-007-1023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons DJ, Rensink RA. Change blindness: Past, present, and future. Trends in Cognitive Science. 2005;9:16–20. doi: 10.1016/j.tics.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Spielberger C. Manual for the State-Trait Anxiety Inventory: STAI (Eorm I) Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: Role of automatic and nonautomatic processes. Psychological Review. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- van Holst RJ, van den Brink W, Veltman DJ, Goudriaan AE. Why gamblers fail to win: a review of cognitive and neuroimaging findings in pathological gambling. Neuroscience & Biobehavioral Reviews. 2009;34(1):87–107. doi: 10.1016/j.neubiorev.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Young MM, Wohl MJ. The Gambling Craving Scale: Psychometric validation and behavioral outcomes. Psychology of Addictive Behaviors. 2009;23(3):512–522. doi: 10.1037/a0015043. [DOI] [PubMed] [Google Scholar]