Abstract
Purpose
Emerging evidence clearly suggests the potential chemopreventive and anti-tumor activity of a well known “natural agent” curcumin. However, studies have shown that curcumin is not readily bioavailable, and thus the tissue bioavailability of curcumin is also poor except for gastrointestinal track. Because of the potential biological activity of curcumin, many studies have attempted for making a better analog of cucumin that is equally effective or better with increased bioavailability, which was the purpose of our current study.
Methods
We have designed and synthesized new difluoro Knoevenagel condensates of curcumin and Schiff bases along with their copper (II) complexes and evaluated their biological activities with respect to the inhibitory effects on purified rabbit 26S proteasome, and growth inhibition and induction of apoptosis in colon and pancreatic cancer cell lines.
Results
All copper complexes possess distorted square planar geometries with 1:1 metal to ligand stoichiometry with reversible copper redox couple. The difluoro compound CDF exhibited inhibitory effects on purified rabbit 20S proteasome or cellular 26S proteasome, and caused both growth inhibition of cancer cell lines and induced apoptotic cell death in our preliminary assessment.
Conclusion
Our results suggest that our newly synthesized classes of curcumin analogs could be useful as chemopreventive and/or therapeutic agents against cancers.
Keywords: apoptosis, cancer cells, cell growth, curcumin, difluoro Knoevenagel condensates of curcumin
INTRODUCTION
Curcumin (diferuloylmethane, 1) is the active phenolic compound extracted from the rhizome of the plant Curcuma longa (Linn) grown in tropical Southeast Asia (1–3). The compound has been used as a spice and coloring agent in Indian cuisine as well as a chemopreventive agent in traditional Indian Ayurvedic Medicine for treating various health disorders including respiratory conditions, inflammation, liver disorders, diabetic wounds, cough and certain tumors (4–25). Recent investigations have provided evidence that Curcumin indeed prevents a variety of carcinogen-induced cancers in rodents in addition to suppressing the mutagenic effects of various chemical carcinogens such as tobacco, cigarette smoke condensates, benzo (a) pyrene, 1, 2-dimethyl-benz(a)anthracene(DMBA) and aflatoxin B1 (4–25).
Curcumin exhibits wide ranging anticancer activities both in vitro and in vivo through a variety of mechanisms. It inhibits proliferation and induces apoptosis in a wide array of cancer cells including bladder, breast, lung, pancreas, prostate, cervix, head and neck, ovary, kidney, brain and skin (16–18). These effects have been shown to arise by its interaction with numerous biochemicals and molecular targets (transcription factors, growth factors and their receptors, cytokines, enzymes) either through direct interaction or through modulation of gene expression, although the underlying mechanisms are not fully understood (18). It has also been found to potentiate the effects of some of the therapeutic agents in the clinics such as genistein, celecoxib, gemcitabine, 5-flurouracil and oxaliplatin (26).
The bioavailability of Curcumin is a major concern which limits its therapeutic utility since as much as 75% of Curcumin gets excreted in the feces indicating poor absorption from the gut (27). However, when injected intravenously, the majority of the drug is metabolized and actively transported into bile, suggesting that Curcumin has poor absorption and rapid metabolism. Metabolic transformations through sulfation and glucuronidation at various tissue sites especially in liver and intestine can be blocked through administrations of adjutants such as Piperine, a known inhibitor of hepatic and intestinal glucuronidation, which has been shown to increase the bioavailability of Curcumin (28,29). Different drug delivery systems including liposomes, micelles, phospholipid complexes and nanoparticles have also been employed with some success for improving bioavailability (2,30).
Since the chemical structure of Curcumin plays a crucial role in its biological activity as observed from the isomerization process influencing the antioxidant activity of curcumin (31), it is anticipated that enhanced absorption of Curcumin without any loss in its activity can be achieved by evolving appropriate Curcumin analogs (32,33). Snyder and coworkers have reviewed studies carried out on symmetrical 1,5-diaryl pentadienones which has indicated that central methylene group of Curcumin can be replaced without much loss in activity (34). Li and coworkers have extended this work to include cyclopentanone and cyclohexanone analogs and have evaluated their antibacterial properties against ampicillin-resistant bacteria showing heteroaryl and long chain substituents may enhance the activity of these compounds (32,33). More recently pyrazolic and isoxaxolic analogs of Curcumin have been shown to possess neuroprotective action (35).
Another strategy which has been employed to improve the biological activity of Curcumin is through its complexation with metal ions (8,36) and anti-tumor activity has been reported for such metal complexes by John et al. (37). However, none of these strategies have yielded compounds having better antitumor activity than Curcumin. In our group we have been attempting to slow down the rapid metabolism of Curcumin by preparing its Knoevenagel condensates and their copper complexes which were found to be more potent in inhibiting TNF-α induced NF-κB activation and proliferation of human leukemic KBM-5 cells, suggesting that this approach may yield potent curcumin analogs (38). Since metabolic stability of C–F bond is much higher than that of the C–H or C–OH bond, we were attracted to examine the fluoro analogs of the Knoevenagel condensates of Curcumin and their Schiff bases along with their copper complexes. Such substitution does not introduce any major steric changes allowing recognition at the macromolecular sites and hence maintaining the enhanced activities of the compounds (39,40). The rationale behind fluorine substitution in drug design has been discussed in the literature and several examples of the effectiveness of this strategy have been provided, and it has been suggested that fluoro substituents has a role in enhancing metabolic stability of anticancer agents (39,40).
In the present communication we describe synthesis and characterization of new fluoro Knoevenagel condensates of Curcumin and their Schiff bases along with their copper complexes which were evaluated for their proteasome inhibitory activity against a purified rabbit 20S proteasome, which was based on our recent observation that curcumin is a potent proteasome inhibitor as documented in colon cancer (HCT-116 and metastatic SW-480) cell lines (41). The results of our studies indicate that some of the new fluorocurcumin analogs are potent proteasome inhibitors as tested in vitro and in HCT116 cells in vivo, and one of these compounds (CDF) also induced cell growth inhibition in both colon and pancreatic cancer cells. We also found that CDF is somewhat better in inducing apoptosis in BxPC-3 pancreatic cancer cells in our initial screening. These preliminary findings suggest that CDF could be further developed by assessing its pharmacokinetics, tissue bioavailability and its mechanism of action for establishing the role of CDF as a chemopreventive and/or therapeutic agent against cancers.
EXPERIMENTAL DESIGN, MATERIALS AND METHODS
All reagents and solvents were of Analytical Grade. The 1H NMR spectra were recorded on FT-NMR Varian Mercury 300 MHz instrument. The electronic absorption spectra were recorded on a Spectronic Genesys-2 spectrophotometer while IR spectra were recorded in KBr pellets on FTIR 3400 Shimadzu spectrophotometer. Magnetic susceptibility was measured at 300K on Faraday Balance having field strength of 7000 KG. Electron Paramagnetic Resonance (EPR) spectra were recorded as the polycrystalline sample on Varian X-band spectrophotometer using 1,1-diphenyl-2-picrylhydrazy (DPPH) as calibrant.
Synthesis
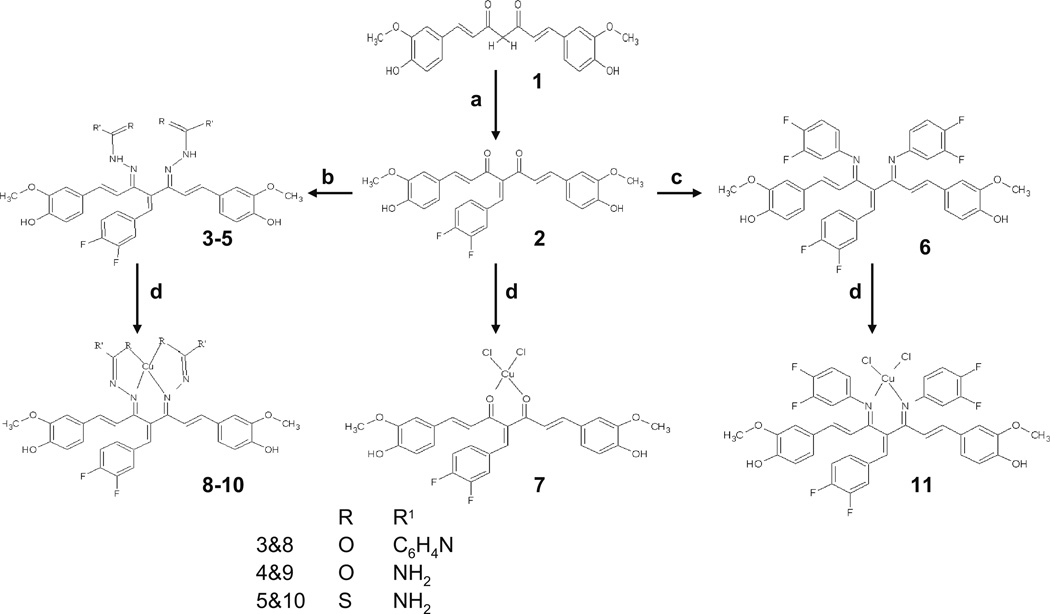
Curcumin was separated from the commercial curcuminoid sample by column chromatography over silica gel using chloroform: methanol (9:1) as eluting solvent. The purified Curcumin was dissolved in minimum amount of methanol to which respective flouroaldehyde (in stoichiometric ratio) was added in methanol slowly with continuous stirring. The reaction mixture was stirred for 48 h and then set aside for product separation. The precipitated product was washed with adequate quantities of n-Hexane and recrystallized from chloroform-hexane mixture to yield pure dark brown microcrystalline product. These condensates were reacted with different hydrazides/amines to yield corresponding bis-Schiff base ligands following a procedure described earlier (38) which upon interaction with copper chloride yielded mono-ligand copper complexes, as shown in Scheme 1 where we have coded the compounds 2, 3, 4, 5 and 6 as CDF, CDFI, CDFS, CDFT and CDFA, respectively.
Scheme 1.
Synthetic steps used in the preparation of copper conjugates of Knoevenagel condensates and Schiff bases of 1. The specific conditions followed for various steps include: (a) 3,4 diflouroaldehyde, piperidine, 48 h, methanol; (b) hydrazides, 24 h, piperidine, methanol, room temp. (1:2); (c) 3,4 diflouroamine, 24 h, piperidine, methanol, room temp; (d) CuCl2. 2H2O, methanol, piperidine (1:1).
Materials
Curcumin was obtained from Sigma-Aldrich (St. Louis, MO) and dissolved in ethanol (Sigma; St. Louis, MO) at a stock concentration of 10 mM, aliquoted and stored at −20°C. Purified rabbit 20S proteasome and fluorogenic substrate Suc-LLVY-AMC for the proteasomal chymotrypsin-like (CT-like) activity were obtained from Calbiochem Inc. (San Diego, CA). Fetal bovine serum (FBS) was from Tissue Culture Biologicals (Tulare, CA). Penicillin and streptomycin were purchased from Invitrogen Co. (Carlsbad, CA). Dulbecco’s modified Eagle’s medium was purchased from Mediatech Inc (Herndon, VA). MTT (3–4, 5-dimethyltiazol-2-yl-2.5-diphenyl-tetrazolium bromide) was purchased from Sigma-Aldrich.
Cell culture
HCT116 human colon cancer cells and BxPC-3 pancreatic cancer cells were purchased from American Type Culture Collection (Manassas, VA) and grown in DMEM medium supplemented with 10% FBS, 100 units/ml of penicillin, and 100 µg/ml of streptomycin. Cells were maintained at 37°C and 5% CO2.
Inhibition of purified 20S proteasome activity by curcumin and its fluorine substituted analogs
A purified rabbit 20S proteasome (35 ng) was incubated with 20 µM of substrate Suc-LLVY-AMC in 100 µl assay buffer (20 mM Tris–HCl, pH 7.5), in the presence of curucmin or fluorine substituted curcumin analogs at different concentrations or the solvent ethanol for 2 h at 37°C, followed by measurement of hydrolysis of the fluorogenic substrates using a Wallac Victor3™ multi-label counter with 355-nm excitation and 460-nm emission wavelengths.
Inhibition of the proteasome activity in intact colon cancer cells by curcumin and its fluorine substituted analogs
To measure inhibition of the proteasome activity in living tumor cells by curcumin and its fluorine substituted analogs, 5,000~8,000 human colon caner HCT 116 cells were planted to each well of a 96-well plate and then treated with either ethanol or curcumin or fluorine substituted curcumin analogs at different concentrations for 24 h, followed by an additional 2 h of incubation with the proteasomal CT-like specific substrate at 20 µM. The proteasome activity was measured using the whole plate as described above.
MTT assay
Cells were grown in a 96-well plate. Triplicate wells of cells were treated with indicated concentrations of curcumin or fluorine substituted curcumin analogs for 24 h. After aspiration of medium, MTT (1 mg/ml) was then added to the cell cultures, followed by incubation for 3 h at 37°C. After cells were crystallized, MTT was removed and DMSO was added to dissolve the metabolized MTT product. The absorbance was then measured on a Wallac Victor3 1420 Multi-label counter at 540 nm.
Apoptosis Assay
The Cell Apoptosis ELISA Detection Kit (Roche, Palo Alto, CA) was used to detect apoptosis in BxPC-3 pancreatic cancer cells after 72 h of treatment with curcumin and CDF. After the treatment, the cytoplasmic histone/DNA fragments were extracted and bound to immobilized anti-histone antibody. Subsequently, the peroxidase-conjugated anti-DNA antibody was used for the detection of immobilized histone/DNA fragments. After addition of substrate for peroxidase, the spectrophotometric absorbance of the samples was determined by using ULTRA Multifunctional Microplate Reader (TECAN, Durham, NC) at 405 nm.
Statistical Analysis
The statistical significance of the data was calculated using a paired two-tailed t-test following the GraphPad Prism software program and a p value of <0.05 was considered statistically significant comparing treatment groups against control.
RESULTS AND DISCUSSIONS
The interaction of purified Curcumin with diflouroaldehyde in methanolic solvent resulted in good yields of Knoevenagel condensates. These condensates when reacted with different hydrazides/amines produce corresponding bis-Schiff base ligands as shown in Scheme 1. The interaction of these ligands with copper chloride yield mono-ligand copper complexes. The compositional analysis of the copper complexes indicate 1:1 metal to ligand stoichiometries for Knoevenagel condensates and their Schiff bases while conductivity measurements in DMSO solvent reveal non-electrolyte nature for them (42).
IR spectrum of 1 in its stable enolizable form exhibits the carbonyl stretching frequency at 1,620 cm−1 and an intramolecularly hydrogen bonded hydroxyl absorption at 3,379 cm−1 whereas in the case of Knoevenagel condensates the carbonyl stretch appears at 1,655–1,633 cm−1 while the hydroxyl absorption is found to be absent due to loss of enolizable hydrogen. In the Schiff bases of Knoevenagel condensates (5–7) the carbonyl frequency is replaced by strong absorptions at 1,595–1,600 cm−1 ascribed to azomethine stretching frequency and an additional band at 860 cm−1 due to the thiocarbonyl stretch (in case of the thiosemicarbazone ligand), respectively. Upon complexation with copper ions the carbonyl frequency exhibits an upward shift due to back coordination effects of oximino nitrogen donor indicating its involvement in metal complexation. The new bands observed in the spectra of the copper complexes in the regions 550–590 and 360–390 are ascribed to γ(M-O)and γ(M-Cl) stretching frequencies, respectively (38).
All ligands exhibit absorptions in the region 200–450 nm in their electronic spectra recorded in DMSO solvent which are due to intra-ligand electronic transitions. The intense band observed in the range 450–650 nm in copper complex is due to ligand to metal charge transfer (41), while the additional band observed in the region 650–850 nm is characteristic of Cu(II) (2B1g-→ 2Eg) transition in square planar copper compounds (43). The magnetic moments of these complexes (1.69–1.82BM) indicate their monomeric nature and support their planar geometries. The X-band EPR spectra of the copper compounds in DMSO glass are typical of axial symmetry with gII > g ⊥ > 2.0023, indicating the presence of unpaired electron in dx2–y2 ground state (43). The Distortion Factor Values in the range 110–120 are typical for planar complexes, while the higher values indicate extent of distortion as indicated in our earlier communication (43). These chemical characteristics are encouraging because these analogs may serve as new generation of compounds that could be biologically active and perhaps better than curcumin in terms of pharmacokinetics and tissue bioavailability, and thus these compounds could be useful as chemopreventive and/or therapeutic agents against human malignancies.
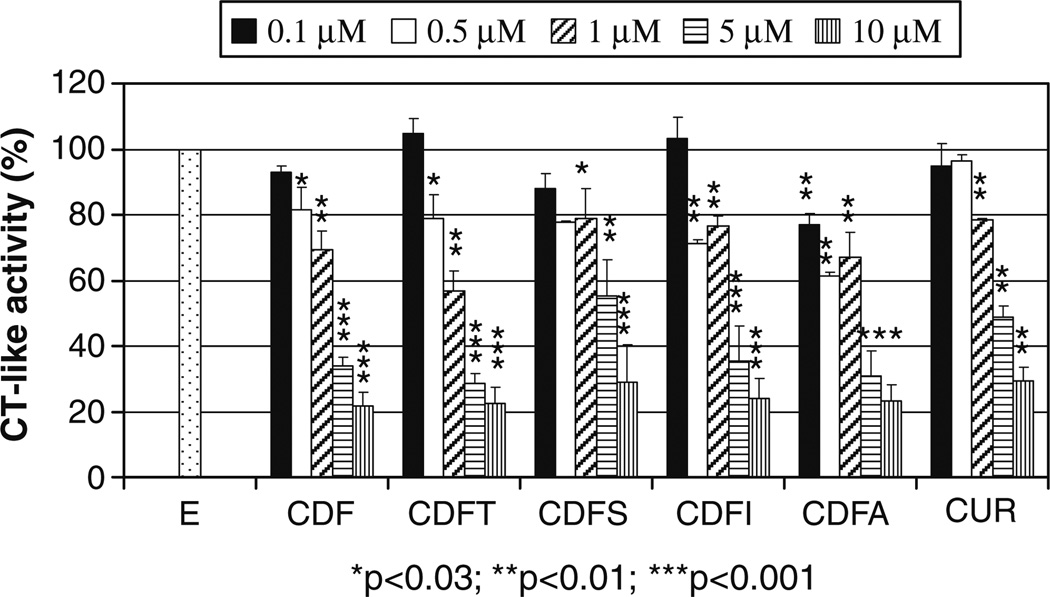
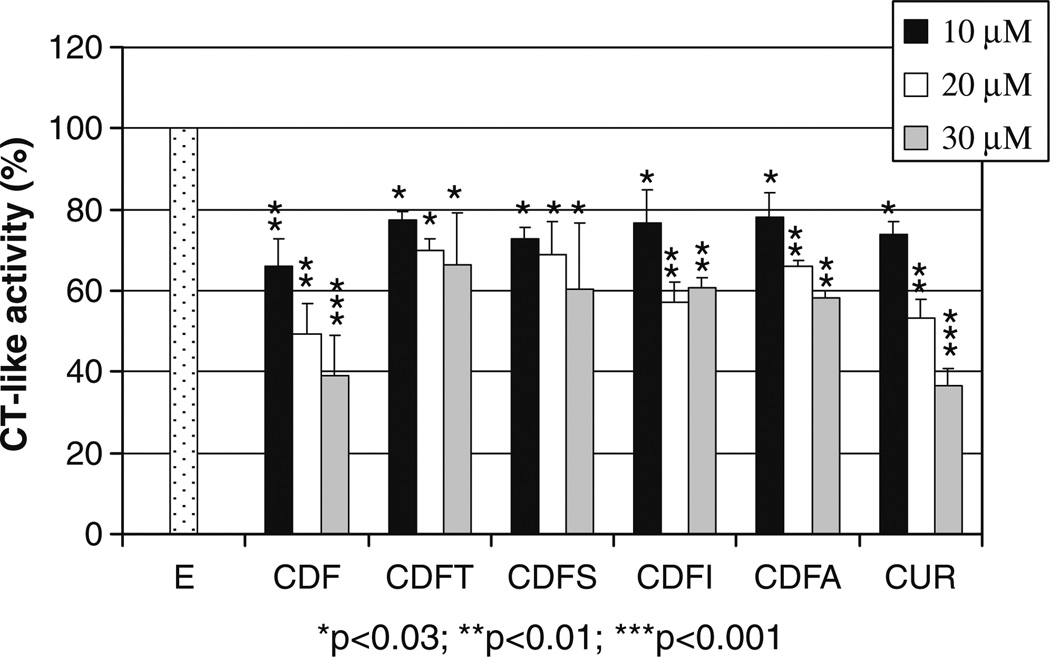
Since proteasome is one of the cellular targets of curcumin, and the inhibition of proteasome has been associated with the cell-killing activity of curcumin (17,41) as well as other proteasome inhibitors, in the current study we tested our hypothesis whether our newly synthesized fluorine substituted curcumin analogs could target the proteasome compared to curcumin as a control. As shown in Fig. 1, curcumin inhibited 20–70% chymotrypsin-like activity of the proteasome at 1–10 µM. At 0.5 µM, all fluorine substituted curcumin analogs reached 20–40% proteasome inhibition. At high concentration such as 10 µM, fluorine substituted curcumin analogs showed superior effects on proteasome inhibition compared to curcumin (70–78% vs. 70%). Next we tested whether fluorine substituted curcumin analogs could inhibit the cellular 26S proteasome as their parent compound curcumin does (41). Among these, CDF ligand exerted higher potency over other fluorine substituted curcumin analogs examined, showing 34%, 51% and 61% proteasome inhibition at 10, 20 and 30 µM, which was similar to Curcumin with 27%, 47% and 64% proteasome inhibition at 10, 20 and 30 µM (Fig. 2). Other fluorine substituted curcumin analogs, viz. CDFT, CDFS, CDFI, CDFA and CDFC, inhibited around 23–42% proteasome activity at 10–30 µM. These results suggest that CDF is biologically superior than curcumin, and we speculate that if CDF shows better bioavailability and tissue distribution than curcumin then it could be a much effective agent for further clinical development. Such pharmacokinetic and tissue distribution studies are currently being carried out in our laboratories.
Fig. 1.
20S proteasome inhibition by fluorine substituted curcumin analogs. Purified rabbit proteasome (35 ng) was incubated with or without (E) various concentrations of curcumin (Cur) and its fluorine substituted curcumin analogs for 2 h, followed by proteasomal chymotrypsin-like activity assay. Columns, mean values±SD. *p<0.03; **p<0.01; ***p<0.001.
Fig. 2.
Proteasome inhibition by fluorine substituted curcumin analogs in human colon cancer HCT 116 cells. Human colon cancer HCT 116 cells were treated with or without (E) various concentrations of curcumin (Cur) and its fluorine substituted curcumin analogs for 24 h, followed by proteasomal chymotrypsin-like activity assay. Columns, mean values±SD. *p<0.03; **p<0.01; ***p<0.001.
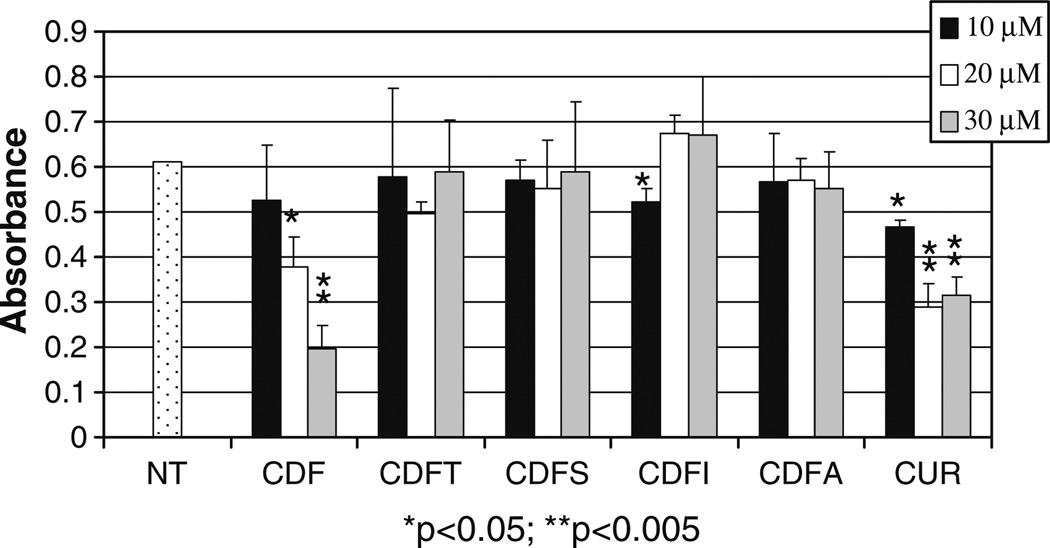
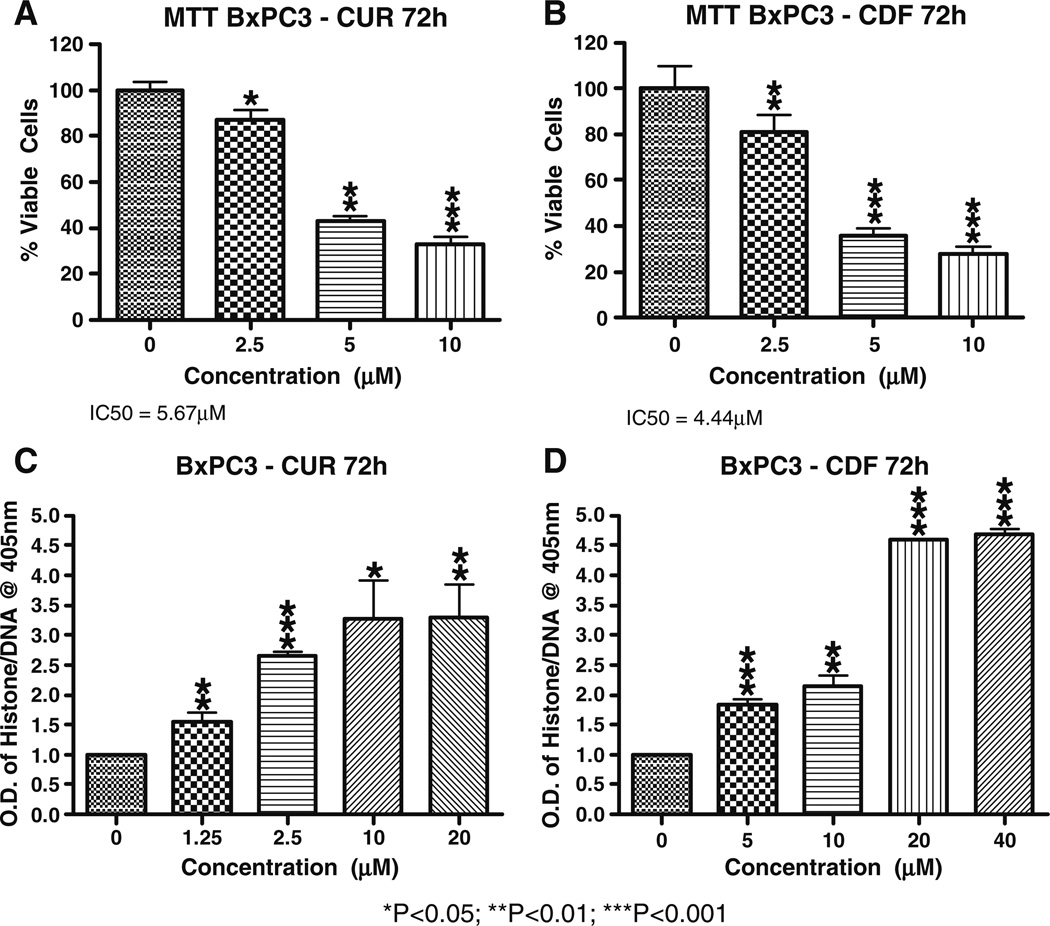
Next we determined the effects of fluorine substituted curcumin analogs on growth of HCT 116 cells and as anticipated, the CDF ligand was the most potent inhibitor compound showing 24%, 39% and 68% inhibition on cell proliferation at 10, 20 and 30 µM, respectively compared to curcumin with 24%, 53% and 49% inhibition at 10, 20 and 30 µM (Fig. 3). The inhibition of cell growth was also assessed in BxPC-3 pancreatic cancer cells and our data clearly show that CDF is superior compared to curcumin in inducing cell growth inhibition (Fig. 4, panels A and B). We subsequently tested whether the inhibition of cell growth could also be partly due to the induction of apoptosis in BxPC-3 pancreatic cancer cells. We found that both curcumin and CDF could induce apoptosis (Fig. 4, panels C and D) and that CDF was superior compared to curcumin. These results are consistent with the MTT results as discussed earlier.
Fig. 3.
Inhibition on cell growth by fluorine substituted curcumin analogs in HCT 116 cells. Human colon cancer HCT 116 cells were treated with or without (E) various concentrations of curcumin (Cur) and its fluorine substituted curcumin analogs for 24 h, followed by MTT assay. Columns, mean values±SD. *p<0.05; **p<0.005.
Fig. 4.
Inhibition on cell growth and induction of apoptotic cell death by fluorine substituted curcumin analogs in BxPC-3 pancreatic cancer cells. Human pancreatic cancer cells were treated with or without various concentrations of curcumin or its fluorine substituted curcumin analog CDF for 72 h, followed by MTT assay (panels A and B) or apoptosis assay (panels C and D). Statistical significance are shown *p<0.05; **p<0.01; ***p<0.001.
In summary, our studies indicate that CDF is the most potent fluoro analog of curcumin in the present series whose potency on the inhibition of the proteasome and cell growth and the induction of cell death was better than the parental curcumin, which is likely to be in part due to the metabolic stability afforded by the fluoro substituents. These results suggest that CDF could be further developed for pharmacokinetic and tissue distribution studies along with pre-clinical testing in animal models and based on those findings it could be evaluated in human patients in the future for the treatment of human malignancies.
REFERENCES
- 1.Abas F, Lajis NH, Shaari K, Israf DA, Stanslas J, Yusuf UK, Raof SM. A labdane diterpene glucoside from the rhizomes of Curcuma mangga. J Nat Prod. 2005;68:1090–1093. doi: 10.1021/np0500171. [DOI] [PubMed] [Google Scholar]
- 2.Bisht S, Feldmann G, Soni S, Ravi R, Karikar C, Maitra A, Maitra A. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): a novel strategy for human cancer therapy. J Nanobiotechnology. 2007;5:3. doi: 10.1186/1477-3155-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jagetia GC, Rajanikant GK. Role of curcumin, a naturally occurring phenolic compound of turmeric in accelerating the repair of excision wound, in mice whole-body exposed to various doses of gamma-radiation. J Surg Res. 2004;120:127–138. doi: 10.1016/j.jss.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci. 2009;30:85–94. doi: 10.1016/j.tips.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol Pharmacol. 2006;69:195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- 7.Ammon HP, Safayhi H, Mack T, Sabieraj J. Mechanism of antiinflammatory actions of curcumine and boswellic acids. J Ethnopharmacol. 1993;38:113–119. doi: 10.1016/0378-8741(93)90005-p. [DOI] [PubMed] [Google Scholar]
- 8.Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, Tharakan ST, Misra K, Priyadarsini IK, Rajasekharan KN, Aggarwal BB. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem Pharmacol. 2008;76:1590–1611. doi: 10.1016/j.bcp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Lett. 2008;267:133–164. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 10.Araujo CC, Leon LL. Biological activities of Curcuma longa L. Mem Inst Oswaldo Cruz. 2001;96:723–728. doi: 10.1590/s0074-02762001000500026. [DOI] [PubMed] [Google Scholar]
- 11.Babu PS, Srinivasan K. Hypolipidemic action of curcumin, the active principle of turmeric (Curcuma longa) in streptozotocin induced diabetic rats. Mol Cell Biochem. 1997;166:169–175. doi: 10.1023/a:1006819605211. [DOI] [PubMed] [Google Scholar]
- 12.Gilani AH, Shah AJ, Ghayur MN, Majeed K. Pharmacological basis for the use of turmeric in gastrointestinal and respiratory disorders. Life Sci. 2005;76:3089–3105. doi: 10.1016/j.lfs.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 13.Goel A, Jhurani S, Aggarwal BB. Multi-targeted therapy by curcumin: how spicy is it? Mol Nutr Food Res. 2008;52:1010–1030. doi: 10.1002/mnfr.200700354. [DOI] [PubMed] [Google Scholar]
- 14.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin": from kitchen to clinic. Biochem Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Jang EM, Choi MS, Jung UJ, Kim MJ, Kim HJ, Jeon SM, Shin SK, Seong CN, Lee MK. Beneficial effects of curcumin on hyperlipidemia and insulin resistance in high-fat-fed hamsters. Metabolism. 2008;57:1576–1583. doi: 10.1016/j.metabol.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67:3853–3861. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- 17.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Kunnumakkara AB, Diagaradjane P, Guha S, Deorukhkar A, Shentu S, Aggarwal BB, Krishnan S. Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-kappaB-regulated gene products. Clin Cancer Res. 2008;14:2128–2136. doi: 10.1158/1078-0432.CCR-07-4722. [DOI] [PubMed] [Google Scholar]
- 19.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Kunnumakkara AB, Diagaradjane P, Guha S, Deorukhkar A, Shentu S, Aggarwal BB, Krishnan S. Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-kappaB-regulated gene products. Clin Cancer Res. 2008;14:2128–2136. doi: 10.1158/1078-0432.CCR-07-4722. [DOI] [PubMed] [Google Scholar]
- 21.Shishodia S, Chaturvedi MM, Aggarwal BB. Role of curcumin in cancer therapy. Curr Probl Cancer. 2007;31:243–305. doi: 10.1016/j.currproblcancer.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Sidhu GS, Mani H, Gaddipati JP, Singh AK, Seth P, Banaudha KK, Patnaik GK, Maheshwari RK. Curcumin enhances wound healing in streptozotocin induced diabetic rats and genetically diabetic mice. Wound Repair Regen. 1999;7:362–374. doi: 10.1046/j.1524-475x.1999.00362.x. [DOI] [PubMed] [Google Scholar]
- 23.Singh S, Aggarwal BB. Activation of transcription factor NFkappa B is suppressed by curcumin (diferuloylmethane) [corrected] J Biol Chem. 1995;270:24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 24.Singh SV, Hu X, Srivastava SK, Singh M, Xia H, Orchard JL, Zaren HA. Mechanism of inhibition of benzo[a]pyrene-induced forestomach cancer in mice by dietary curcumin. Carcinogenesis. 1998;19:1357–1360. doi: 10.1093/carcin/19.8.1357. [DOI] [PubMed] [Google Scholar]
- 25.Xu YX, Pindolia KR, Janakiraman N, Noth CJ, Chapman RA, Gautam SC. Curcumin, a compound with anti-inflammatory and anti-oxidant properties, down-regulates chemokine expression in bone marrow stromal cells. Exp Hematol. 1997;25:413–422. [PubMed] [Google Scholar]
- 26.Verma SP, Salamone E, Goldin B. Curcumin and genistein, plant natural products, show synergistic inhibitory effects on the growth of human breast cancer MCF-7 cells induced by estrogenic pesticides. Biochem Biophys Res Commun. 1997;233:692–696. doi: 10.1006/bbrc.1997.6527. [DOI] [PubMed] [Google Scholar]
- 27.Wahlstrom B, Blennow G. A study on the fate of curcumin in the rat. Acta Pharmacol Toxicol (Copenh) 1978;43:86–92. doi: 10.1111/j.1600-0773.1978.tb02240.x. [DOI] [PubMed] [Google Scholar]
- 28.Bhutani MK, Bishnoi M, Kulkarni SK. Anti-depressant like effect of curcumin and its combination with piperine in unpredictable chronic stress-induced behavioral, biochemical and neurochemical changes. Pharmacol Biochem Behav. 2009;92:39–43. doi: 10.1016/j.pbb.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–356. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 30.Sahu A, Kasoju N, Bora U. Fluorescence study of the curcumin-casein micelle complexation and its application as a drug nanocarrier to cancer cells. Biomacromolecules. 2008;9:2905–2912. doi: 10.1021/bm800683f. [DOI] [PubMed] [Google Scholar]
- 31.Shen L, Ji HF. Theoretical study on physicochemical properties of curcumin. Spectrochim Acta Part A: Mol Biomol Spectrosc. 2007;67:619–623. doi: 10.1016/j.saa.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 32.Liang G, Yang S, Jiang L, Zhao Y, Shao L, Xiao J, Ye F, Li Y, Li X. Synthesis and anti-bacterial properties of mono-carbonyl analogues of curcumin. Chem Pharm Bull (Tokyo) 2008;56:162–167. doi: 10.1248/cpb.56.162. [DOI] [PubMed] [Google Scholar]
- 33.Liang G, Yang S, Zhou H, Shao L, Huang K, Xiao J, Huang Z, Li X. Synthesis, crystal structure and anti-inflammatory properties of curcumin analogues. Eur J Med Chem. 2008 doi: 10.1016/j.ejmech.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 34.Mosley CA, Liotta DC, Snyder JP. Highly active anticancer curcumin analogues. Adv Exp Med Biol. 2007;595:77–103. doi: 10.1007/978-0-387-46401-5_2. [DOI] [PubMed] [Google Scholar]
- 35.Poma P, Notarbartolo M, Labbozzetta M, Maurici A, Carina V, Alaimo A, Rizzi M, Simoni D, D'Alessandro N. The antitumor activities of curcumin and of its isoxazole analogue are not affected by multiple gene expression changes in an MDR model of the MCF-7 breast cancer cell line: analysis of the possible molecular basis. Int J Mol Med. 2007;20:329–335. [PubMed] [Google Scholar]
- 36.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 37.John VD, Kuttan G, Krishnankutty K. Anti-tumour studies of metal chelates of synthetic curcuminoids. J Exp Clin Cancer Res. 2002;21:219–224. [PubMed] [Google Scholar]
- 38.Zambre AP, Jamadar A, Padhye S, Kulkarni VM. Copper conjugates of knoevenagel condensates of curcumin and their schiff base derivatives: synthesis, spectroscopy, magnetism, ESR, and electrochemistry. Synthesis and Reactivity in Inorganic, Metal-Organic, and Nano-Metal Chemistry. 2007;37:19–27. [Google Scholar]
- 39.Smart BE. Introduction: fluorine chemistry. Chem Rev. 1996;96:1555–1556. doi: 10.1021/cr960075e. [DOI] [PubMed] [Google Scholar]
- 40.Kirk KL. Selective fluorination in drug design and development: an overview of biochemical rationales. Curr Top Med Chem. 2006;6:1447–1456. doi: 10.2174/156802606777951073. [DOI] [PubMed] [Google Scholar]
- 41.Milacic V, Banerjee S, Landis-Piwowar KR, Sarkar FH, Majumdar AP, Dou QP. Curcumin inhibits the proteasome activity in human colon cancer cells in vitro and in vivo. Cancer Res. 2008;68:7283–7292. doi: 10.1158/0008-5472.CAN-07-6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geary WJ. The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. 1971 Ref Type: Generic. [Google Scholar]
- 43.Wang YY, Shi Q, Shi QZ, Gao YC, Zhou ZY. Syntheses, characterization and crystal structure of copper(II) [alpha], [beta]-unsaturated carboxylate complexes with imidazole. Polyhedron. 1999;18:2009–2015. [Google Scholar]