Abstract
The tumor suppressor protein, p53 is a transcription factor that not only activates expression of genes containing the p53 binding site but also can repress the expression of some genes lacking this binding site. Previous studies have shown that overexpression of wild-type p53 leads to apoptosis and cell cycle arrest. DNA damage, such as that caused by UV irradiation, results in p53 stabilization and nuclear localization that subsequently induces apoptosis. Recently, the level of calreticulin (CRT) has been correlated with the rate of apoptosis. Therefore, the aim of this study was to investigate the role of CRT in the regulation of apoptosis via modulating p53 function and expression. Here we show a significant decrease in both basal and DNA damage induced p53 functions in the CRT-deficient cells (crt-/-). This study is the first to demonstrate that CRT function is required for the stability and localization of the p53 protein. By using immuonocytochemical techniques, we showed that observed changes in p53 in the crt-/- cells are due to the nuclear accumulation of Mdm2 (murine double minute gene). These results, lead us to conclude that CRT regulates p53 function by affecting its rate of degradation and nuclear localization.
INTRODUCTION
p53 is a transcription factor important for both regulating the cell cycle progression and the induction of apoptosis in a variety of cells. In vivo, this protein functions as a tumor suppressor; indeed, >50% of all human cancers have been characterized with mutations in the p53 gene (Hollstein et al., 1991; Ko and Prives, 1996). Furthermore, gene targeted deletion of p53 in mice results in mice that have an increased susceptibility to the development of multiple tumors (Donehower et al., 1992, 1996). In normal proliferating cells, the p53 protein has a short half-life (Ashcroft and Vousden, 1999) and it continuously shuttles between the nucleus and the cytoplasm (Middeler et al., 1997). Under stresses such as UV irradiation, chemotherapeutic agents, or hypoxia, p53 is stabilized and accumulates in the nucleus (Lain et al., 1999a, 1999b; Ashcroft et al., 2000; Zhang and Xiong, 2001), where it activates expression of the stress response genes (Middeler et al., 1997), resulting in cell cycle arrest (Reich and Levine, 1984) and apoptosis (Cotton and Spandau, 1997; Reinke and Lozano, 1997). Therefore, the efficient regulation of p53 function is important for determining cell survival and proliferation.
Given the pivotal role of p53 in the cell, several mechanisms are involved in the regulation of its function, including regulation of its nuclear localization, regulation of protein stability, and posttranslational modifications (e.g., phosphorylation and acetylation; for review see Woods and Vousden, 2001). One of the proteins important in the regulation of p53 is Mdm2 (murine double minute gene). Mdm2 is a member of the RING-Finger protein family of E3 ubiquitin ligases (Honda et al., 1997; Fang et al., 2000) and is involved in ubiqutinylation of p53 (Fang et al., 2000). Interaction of Mdm2 with p53 mediates p53 export from the nucleus to the cytoplasm (Tao and Levine, 1999; Inoue et al., 2001), where p53 undergoes degradation via the proteasome pathway (Haupt et al., 1997; Yu et al., 2000). Interestingly, UV-induced activation of p53 has been shown to increase the expression level of Mdm2 in vivo (Wu and Levine, 1997; Saucedo et al., 1998). Furthermore, the activity of Mdm2 and its nuclear translocation has been shown to be regulated by phosphorylation via AKT (protein kinase B; Mayo and Donner, 2001; Mayo et al., 2002).
Calreticulin (CRT) is a ubiquitous eukaryotic protein that is localized to the endoplasmic/sarcoplasmic reticulum and to the nuclear envelope lumen. Several unique functions have been postulated for CRT (reviewed in Michalak et al., 1999), including chaperone activity (Nigam et al., 1994; Nauseef et al., 1995; Hebert et al., 1997) and regulation of Ca2+ homeostasis (Liu et al., 1994; Camacho and Lechleiter, 1995; Bastianutto et al., 1995; Mery et al., 1996; Coppolino et al., 1997). Recently, CRT has also been shown to be involved in regulation of nuclear transport (both import and export) of NFAT3 (Mesaeli et al., 1999), MEF2C (Li et al., 2002), and glucocorticoid receptor (Holaska et al., 2001, 2002). Interestingly, changes in the CRT expression have been shown to alter the response of cells to proapoptotic signals (Nakamura et al., 2000; Kageyama et al., 2002). CRT-overexpressing cells show increased sensitivity to drug-induced apoptosis (Nakamura et al., 2000; Kageyama et al., 2002), whereas CRT-deficient cells were more resistant to drug or UV-induced apoptosis (Nakamura et al., 2000). Therefore, the aim of this study was to investigate the functional role of CRT in regulating p53 expression, localization, and function.
MATERIALS AND METHODS
Plasmids
Vectors containing p53EGFP fusion protein (pp53-EGFP) and the Luciferase gene under control of the p53 response element (pp53-TA-Luc) were purchased from BD Biosciences CLONTECH (Palo Alto, CA). The cDNA encoding full-length CRT tagged with hemagglutinin (HA) epitope tag was a generous gift from Dr. Michalak (University of Alberta, Edmonton, Alberta, Canada). For generation of stably transfected cell line, the HA-CRT cDNA was cloned in the pcDNA3.1 plasmid containing the hygromycin resistance (Invitrogen, Burlington, ON, Canada). Plasmid containing the human p53 promoter (h-p53-luc) was a generous gift from Dr. Reisman (University of South Carolina, Columbia, SC). For the transfection experiments, plasmids were purified using DNA purification kits from QIAGEN (Santa Clarita, CA).
Cell Culture
Mouse embryonic fibroblast cells (MEF) were isolated from wild-type (wt) and calreticulin knockout (crt-/-) 14-day-old mouse embryos as described previously (Mesaeli et al., 1999). Briefly, embryos were washed in ice-cold PBS, dissociated, and trypsinized for 30 min. Dispersed cells were then cultured in DMEM containing 20% fetal calf serum. The primary MEF cells were immortalized by transfection with SV40 T-large antigen. To test that the observed effects were due to loss of CRT function, crt-/- MEF cells were stably transfected with pcDNA-CRT-HA using the Ca2+ phosphate transfection protocol as described previously (Waser et al., 1997). Transfected cells were cultured in the presence of 100 μg/ml hygromycin for 2 weeks, after which single colonies were isolated, expanded, and screened for the expression of CRT (hereafter will be referred to as CRT-crt-/- cells).
Immunoblotting and Immunocytochemistry
For Western blot analysis of p53 expression, cells were treated for 2 h with 20 μM ALLN (N-Acetyl-Leu-Leu-Nle-CHO, Calbiochem, La Jolla, CA). Cells were then washed with ice-cold PBS and lysed in RIPA buffer containing a protease inhibitor cocktail (Sigma, St. Louis, MO; Mesaeli et al., 1999). Monoclonal anti-p53 antibody was purchased from Oncogene (Boston, MA). CRT expression in wt, crt-/-, and CRT-crt-/- cells was detected by using goat anti-CRT (a gift from Dr. Michalak). For analysis of Mdm2 protein expression, western blot was carried out using a polyclonal anti-Mdm2 antibody (C-18) from Santa Cruz Biotechnology (Santa Cruz, CA).
To check the localization of endogenous p53 and Mdm2, cells were plated on coverslips at a density of 50,000 cells per well in six-well plates. Before treatment, media was changed with fresh media containing 20 μM ALLN. Coverslips were divided to three groups: nontreated and UV (25 J/m2)-treated followed by 2 or 6 h recovery. Cells were then fixed in 4% paraformaldehyde in PBS for 10 min. For localization of p53, monoclonal anti-p53 (Ab-1, Oncogene) was used at a 5 μg/ml concentration. Monoclonal anti-Mdm2 (Ab-4, Oncogene) was used at a concentration of 2 μg/ml for immunolocalization of Mdm2. FITC-conjugated rabbit anti-mouse antibody (Jackson ImmunoResearch, West Grove, PA) was used as a secondary antibody. The coverslips were mounted in Vectashield mounting media with DAPI (nuclear stain, Vector Laboratories Inc., Burlingame, CA). Fluorescent images were obtained using a Zeiss epi-fluorescent microscope (Axiovert 200M, Thornwood, NY)
To study the nuclear translocation of overexpressed p53 in wt, crt-/-, and CRT-crt-/- cells, equal number of cells were plated on coverslips and left overnight. The next day cells were transiently transfected with pp53-EGFP by the Ca2+ phosphate method. After 48 h coverslips were divided to three groups as described above. The EGFP signal was then colocalized with DAPI.
Apoptosis Assay
To assess the degree of apoptosis after UV treatment, an equal number of cells (2 × 106 in 10-cm2 plates) was plated on coverslips. The next day cells were exposed to UV and left to recover for either 2 or 6 h as described above. Apoptosis was then determined using a TUNEL-based assay, In Situ Cell Death Detection Kit, Fluorescein (Roche Applied Science, Laval, QC, Canada), following the manufacturer's protocol. In brief, after the recovery period (2 or 6 h), cells were fixed for 1 h in 2% paraformaldehyde in PBS. Cells were then permeablized and incubated with TUNEL reaction mixture for 1 h at 37°C, followed by three washes with PBS. The coverslips were then mounted using Vectashield mounting media containing DAPI (Vector Laboratories, Inc.). The FITC and DAPI images were obtained using a Zeiss epi-flourescent microscope. These images were superimposed to detect the apoptotic nuclei (FITC labeled).
Reporter Gene Assays
wt and crt-/- MEF cells were plated at a density of 50,000 per plate (in 3-cm2 plates) and transiently cotransfected with either pSV-β-gal and pp53-TA-luc, or pSV-β-gal and h-p53-luc using Lipofectamine (Invitrogen). Forty-eight hours after the transfection, cells were lysed and luciferase and β-galactosidase assays were carried out as described in Waser et al. (1997).
Reverse Transcriptase Assay
Total RNA was isolated from the wt, crt-/-, and CRT-crt-/- cells using Trizole reagent (Invitrogen). Full-length Mdm2 mRNA was amplified by RT-PCR using the QIAGEN OneStep RT-PCR kit (QIAGEN). The primers were chosen from the published Mdm2 cDNA with the following sequences: forward primer: 5′-AAAGAATTCATGTGCAATACCAACATG-3′ and reverse primer: 5′-AAACTCGAGGTTGAAGTAAGTTAGCAC-3′. To quantify the changes in the transcription of Mdm2 in different samples, specific primers for actin mRNA were used as internal control in the same RT-PCR tube. The actin-specific primers were as follows: forward 5′-GTTACCAACTGGGACGACATGG-3′ and reverse 5′-GATCTTGATCTTCATGGTGC-3′. An aliquot of the PCR reaction was resolved on a 1% agarose gel and bands corresponding to Mdm2 and actin were quantified using a Bio-Rad QuantityOne Program (Cambridge, MA). The data were then presented as the ratio of Mdm2 signal normalized to actin signal (mean ± SE).
RESULTS
p53 Function
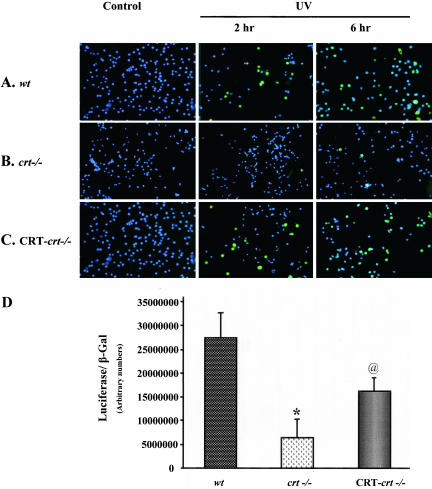
UV (25 J/m2) treatment induces significant apoptosis in the wild-type (wt) mouse embryonic fibroblast cells (MEF; Figure 1A). However, crt-/- MEF cells treated identically are resistant to UV-induced apoptosis (Figure 1B). As seen in Figure 1B, 2 or 6 h after UV exposure a very small number of crt-/- cells are positive for TUNEL activity (apoptosis). The crt-/- MEF cells stably transfected with CRT-HA (CRT-crt-/-) show a response to UV that is similar to what is seen in the wt cells (Figure 1C). These results are in agreement with previous reports on CRT-dependent changes in resistance to apoptosis (Nakamura et al., 2000; Kageyama et al., 2002). UVA and UVB have been shown to activate p53, resulting in its stabilization, nuclear localization, and increased transcriptional activity (Liu and Pelling, 1995; Bellamy et al., 1997). Activated p53 translocates to the nucleus where it binds to the p53 response element present in the promoter region of a number of genes, which are involved in cell fate determination (e.g., Mdm2, p21, Cyclin G, GADD45; for review see Levine, 1997). Therefore, we compared the transcriptional activity of p53 in CRT-deficient (crt-/-) cells with wt cells and CRT-deficient cells that were stably transfected with CRT (CRT-crt-/-; Figure 1D). wt, crt-/-, and CRT-crt-/- MEF cells were transiently transfected with a plasmid containing a p53 response element upstream of the luciferase reporter gene. There was a significant decrease (fivefold) in the p53-mediated reporter gene regulation in the crt-/- cells compared with that in wt cells (Figure 1D), a process that was partially reversed by transfecting the crt-/- cells with CRT (Figure 1D, CRT-crt-/-). These results emphasize that the increased resistance of crt-/- cells to UV-induced apoptosis could be due to impaired p53 function. To examine the role of CRT in regulation of p53 function, we studied the expression level and localization of p53 in the wt and crt-/- MEF cells.
Figure 1.
Impaired p53 function in calreticulin null (crt-/-) cells. (A) Wild-type (wt), (B) crt-/-, and (C) CRT-HA-transfected crt-/- (CRT-crt-/-) MEF cells were treated with UV (25 J/m2) followed by either 2 or 6 h of recovery at 37°C. Cells were then fixed and processed for TUNEL labeling as described in MATERIALS AND METHODS. (D) To test the p53-mediated transcriptional activation, wt crt-/-, and CRT-crt-/- cells were cotransfected with a luciferase reporter plasmid containing p53 response element and a plasmid containing β-gal reporter plasmid. Results are mean ± SE of five different experiments carried out in triplicates. *p < 0.001, significantly different from the wt. @p < 0.05, significantly different from both the wt and crt-/-.
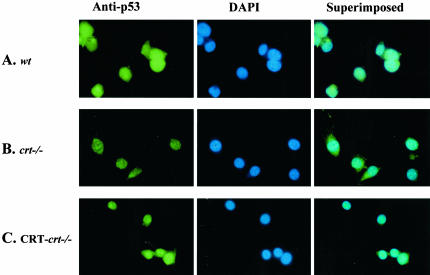
p53 Localization
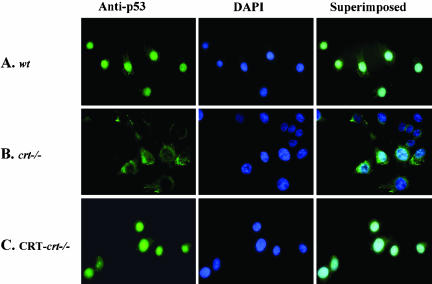
DNA damage, by chemicals such as doxorubicin or UV irradiation, results in the activation of p53, which is accompanied by its stabilization and nuclear accumulation. Increased nuclear p53 leads to cell cycle arrest and apoptosis. In many types of cancer, tumor development has been attributed to the failure of p53 translocation to the nucleus. Therefore we determined the subcellular localization of p53 protein in cells expressing different levels of CRT using immuonocytochemical techniques. Because of the short half-life of p53, we pretreated the cells with the calpain inhibitor, ALLN (as described in MATERIALS AND METHODS). In the absence of ALLN treatment p53 protein was undetectable (unpublished data). In MEF cells containing CRT (wt and CRT-crt-/-) p53 colocalizes predominantly with the DAPI nuclear stain (Figure 2, A and C). However, in CRT-deficient cells (Figure 2B) p53 fails to localize to the nucleus. This change in the nuclear localization of p53 can partially account for the observed alteration in p53 function. We (Mesaeli et al., 1999) and others (Li et al., 2002) have previously shown a defect in the nuclear transport of NF-AT3 and MEF2C (transcription factors) in the crt-/- cells. On the other hand, the nuclear transport of GATA4 (Li et al., 2002) and glucocorticoid receptor (Holaska et al., 2001) was not changed in the crt-/- cells, emphasizing that the defect in the nuclear transport is not a general phenomena in the absence of CRT.
Figure 2.
Changes in endogenous p53 localization with altered CRT expression. (A) wt, (B) crt-/-, and (C) CRT-crt-/- cells were treated with ALLN for 2 h, fixed, and labeled with a mAb to p53, followed by a FITC-labeled secondary antibody (anti-p53). To visualize the nuclei, cells were also stained with DAPI (middle panel). Images were taken using a Zeiss Axiovert epi-fluorescent microscope. The superimposed panel is merged images of the respective FITC and DAPI images.
p53 Protein Expression
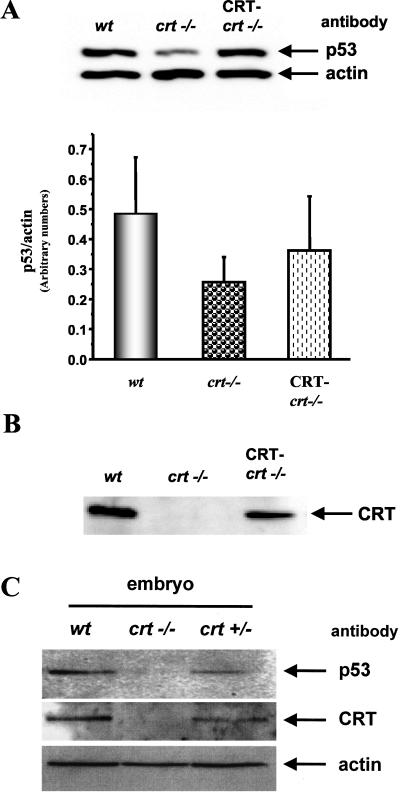
As mentioned above, p53 protein has a very short half-life (15-20 min), and its level is actively regulated by protein degradation by both the calpain and the proteasome pathways. Thus we examined the expression level of p53 protein with altered CRT expression. There was a significant decrease in the level of p53 protein in the crt-/- cells (49% of wt, Figure 3A). To determine if this observation was specific to loss of CRT function, we transfected crt-/- cells with CRT-HA and established stable cell lines (CRT-crt-/-). In these cells the CRT expression was restored to 60% of the wt levels of CRT (Figure 3B). Furthermore, these cells showed a partial recovery of the level of p53 protein (75% of wt) (Figure 3A, bar graph). To confirm that the decrease in the p53 protein level in the absence of CRT is not due to the use of established cell lines, we determined the level of p53 expression in the wt, heterozygous (crt+/-), and homozygous (crt-/-) CRT-deficient mouse embryos (Figure 3C). As shown previously (Mesaeli et al., 1999; also in Figure 3C), the level of CRT protein in the heterozygous (crt+/-) mouse embryos is half of that in wild-type mouse embryo. Figure 3C shows that the expression of p53 protein is significantly decreased in the heterozygous (crt+/-) mouse embryos, whereas in the homozygous CRT-deficient (crt-/-) mouse embryo the p53 protein is barely detectable.
Figure 3.
p53 protein level is correlated with CRT expression. (A) Western blot analysis showing the expression level of endogenous p53 in the different cell lines. The same blot was also reprobed for actin to normalize for loading. Cells were pretreated with ALLN (as described in MATERIALS AND METHODS) to inhibit p53 degradation. Bar graph shows the mean ± SE of the p53/actin protein expression from four different experiments. (B) Western blot analysis of CRT expression in the wt, crt-/-, and CRT-transfected crt-/- (CRT-crt-/-) MEF cells. (C) The expression level of p53, CRT, and actin proteins in tissue lysates from wt and CRT-deficient mouse embryos, homozygous (crt-/-), and heterozygous (crt+/-). The same blot was probed sequentially with antibodies to each p53 (monoclonal), then with CRT (goat polyclonal), and finally with actin (rabbit polyclonal).
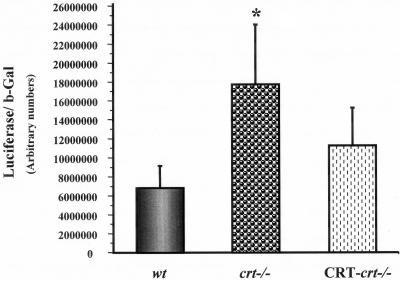
The decreased level of p53 protein in crt-/- cells could be due either to decreased transcription of p53 gene or to rapid degradation of the p53 protein. Thus we investigated whether p53 gene transcription was altered by different levels of cellular CRT. In these studies cells were transfected with a plasmid containing the luciferase reporter gene under control of the human p53 promoter. To normalize for transfection, efficiency cells were cotransfected with a control β-gal plasmid. Surprisingly, we observed a significant (2.5-fold) increase in the p53 promoter activation in the crt-/- cells as seen in Figure 4. In addition we showed that reintroduction of CRT in crt-/- cells resulted in a decrease in the p53 promoter activity (Figure 4). This finding illustrates that the decrease in the p53 protein in crt-/- cells is not due to inhibition of p53 gene transcription but due to a posttranscriptional mechanism such as ubiquitin mediated degradation.
Figure 4.
Activity of human p53 promoter in the wt, crt-/-, and CRT-crt-/- cells. The plasmid containing luciferase reporter gene under regulation of the human p53 promoter was cotransfected with β-gal reporter plasmid into the different cell lines. The transcriptional activity of human p53 promoter is represented as a ratio of luciferase activity to β-gal activity. Values are mean ± SE of four different experiments carried out in triplicates. *p < 0.05, significantly different from the wt.
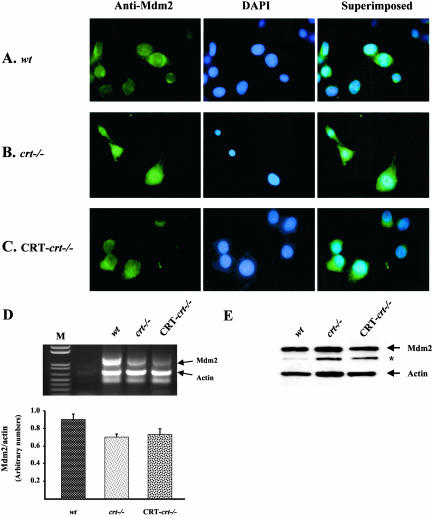
Mdm2 Localization
Activation of Mdm2 via Akt/PKB phosphorylation results in its translocation to the nucleus where it interacts with p53 (Tao and Levine, 1999; Inoue et al., 2001; Mayo and Donner, 2001). The process of p53 degradation is initiated by Mdm2-mediated ubiquitination and translocation to the cytoplasm, where p53 is degraded by the proteasome-dependent pathway (Haupt et al., 1997; Yu et al., 2000). Therefore, we tested whether the decrease in the p53 protein level in the crt-/- cells could be due to activation of the Mdm2 protein using immunocytochemical localization of Mdm2 protein (Figure 5). In the wt and CRT-crt-/- cells, Mdm2 is localized to the cytoplasm (Figure 5, A and C). By contrast, in the crt-/- cells, Mdm2 is predominantly nuclear (Figure 5B), colocalizing with the DAPI (Figure 5B, superimposed image). Previous reports have shown that p53 acts as a transcriptional regulator of Mdm2 (Wu and Levine, 1997; Saucedo et al., 1998). Thus, we tested changes in the level of Mdm2 mRNA by semiquantitative RT-PCR (Figure 5D). There was a 22% decrease in the Mdm2 mRNA in the crt-/- compared with the wt (Figure 5D, bar graph). This result supports our observations in Figure 1D showing a decrease in the p53-mediated function in the crt-/- cells. On the other hand, Western blot analysis using Mdm-2 antibody showed no significant changes in the Mdm2 protein expression in the wt, crt-/-, and CRT-crt-/- MEF cells (Figure 1E).
Figure 5.
Changes in Mdm2 protein localization with altered calreticulin expression (A) wt, (B) crt-/-, and (C) CRT-crt-/- cells were treated with ALLN (as described in MATERIALS AND METHODS), then fixed, and labeled with a mAb to Mdm2, followed by a FITC-labeled secondary antibody (anti-Mdm2 images). The nuclei were stained with DAPI (middle panel). Images were taken using an epi-fluorescent microscope. The superimposed images of the respective FITC and DAPI are shown in the right panel. (D) Top panel shows a representative photo of RTPCR product of Mdm2 and actin mRNA performed in the same tube. Bottom panel shows quantification of the bands corresponding to Mdm2 and actin signals using Bio-Rad QuantityOne Program. The data are presented as the ratio of Mdm2 signal normalized by actin signal. The values are mean ± SE of four separate RT-PCR reactions from four different total RNA isolations. M is the DNA ladder. (E) A representative Western blot showing the level of Mdm2 and actin protein expression in the wt, crt-/-, and CRT-crt-/- cells. *, indicates the presence of a non-specific band detected by antibody to Mdm2.
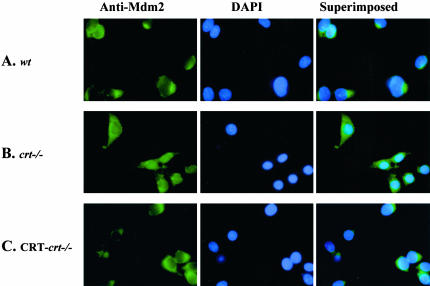
Does the localization of p53 and Mdm2 change after UV induction of apoptosis? To address this question we treated wt, crt-/-, and CRT-crt-/- MEF cells with UV, followed by immunocytochemical staining with specific antibodies to p53 or Mdm2 (Figures 6 and 7). UV treatment results in the accumulation of p53 protein in the nucleus of the wt and CRT-crt-/- MEF cells (Figure 6, A and C). However, in the crt-/- cells (Figures 6B) p53 is localized mainly in the cytoplasm and to a lesser extent in the nucleus. The nuclear signal of p53 in the crt-/- cells appears as a punctate pattern possibly associating with the nucleoli (Figure 6B). Conversely, UV treatment resulted in the cytosolic localization of the Mdm2 protein in all the three cell lines (Figure 7).
Figure 6.
UV-induced changes in p53 protein localization with altered calreticulin expression was studied using immunocytochemistry. Cells (wt, crt-/-, and CRT-crt-/-) were pretreated with ALLN (as described in MATERIALS AND METHODS) followed by exposure to UV (25 J/m2). Cells were incubated for 6 h at 37°C (in the presence of ALLN, recovery time) and then were fixed and labeled with a mAb to p53, followed by FITC-labeled secondary antibody (A-C). To visualize the nuclei, cells were also stained with DAPI. The FITC and DAPI images captured by an epi-fluorescent microscope were superimposed for colocalization.
Figure 7.
Changes in Mdm2 protein localization after UV exposure. (A) wt, (B) crt-/-, and (C) CRT-crt-/- were pretreated with ALLN (as described in MATERIALS AND METHODS), followed by exposure to UV (25 J/m2). Cells were incubated for 6 h at 37°C (recovery time) and then were fixed and labeled with mAb to Mdm2, followed by a FITC-labeled secondary antibody (anti-Mdm2). To visualize the nuclei cells were also stained with DAPI (DAPI images). The superimposed panel is merged images of the respective FITC and DAPI images.
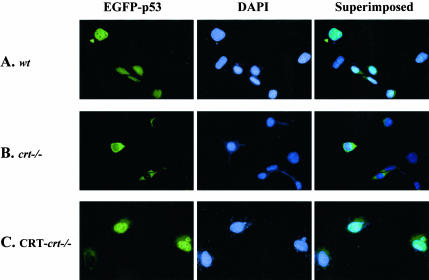
As seen in Figures 2 and 6 in the absence of CRT there is a defect in the nuclear localization of the endogenous p53. Thus, we transfected these cells with an expression plasmid expressing a p53-EGFP chimeric protein to investigate whether this observation was due to impaired p53 nuclear import or simply because of the decreased p53 protein level. Overexpression of exogenous wild-type p53 has been shown to induce apoptosis because of its accumulation in the nucleus (Rotter et al., 1983). As expected in the wt and CRT-crt-/- MEF cells, overexpression of EGFP-p53 resulted in the localization of EGFP-p53 in the nuclei (Figure 8, A and C). Furthermore, these cells showed evidence of apoptosis by the presence of the apoptotic bodies (Figure 8, A and C). Neither of the transfected wt or CRT-crt-/- MEF cells showed cytosolic EGFP signal (Figure 8, A and C). However, in the crt-/- MEF cells the EGFP signal was restricted to the cytoplasm (Figure 8B) with no nuclear signal. Our results in Figures 2, 3, 6, and 8 show that the increased resistance of the crt-/- cells to UV-induced apoptosis is due to both a decrease in p53 protein and impairment of its nuclear import.
Figure 8.
Altered nuclear import of EGFP-p53 with different levels of calreticulin expression. (A) wt, (B) crt-/-, and (C) CRT-crt-/- were plated on coverslips and transiently transfected with a plasmid encoding a p53-EGFP chimeric protein. Thirty-six hours after the transfection, cells were fixed (as described in MATERIALS AND METHODS). To visualize the nuclei, cells were stained with DAPI (middle panel). Images were taken using an epi-fluorescent microscope. The superimposed panel is merged images of the respective FITC and DAPI images.
DISCUSSION
In the present study, we have for the first time demonstrated a role for CRT in the regulation of p53 function. We have also shown that the increased resistance of crt-/- cells to UV-induced apoptosis could be attributed to an impaired nuclear localization of p53 protein. Our results using EGFP-p53 showed that the nuclear import of p53 is inhibited in the crt-/- cells, and this effect was specific to CRT because ectopic expression of CRT-HA in the crt-/- cells (CRT-crt-/-) was able to reverse this observation. Furthermore, we observed a significant decrease in the p53 protein level in the crt-/- cells and embryonic tissue, an effect that was not due to transcriptional regulation of p53 gene. In the present study we also presented evidence for activation of Mdm2, which can induce nuclear export of p53 and its degradation.
How Does CRT Regulate p53?
In the past two decades much emphasis has been placed on understanding the mechanism of regulation of p53 nuclear localization. This was due to the observation that defects in p53 localization to the nucleus can lead to tumor formation (Schlamp et al., 1997), whereas overexpression of wild-type p53 (or stimulation of this protein by hypoxia) results in accumulation of the protein in the nucleus and induces cell cycle arrest and apoptosis (Ko and Prives, 1996). The nuclear localization of p53 is dependent on nuclear localization signal (NLS) and nuclear export signal (NES). The p53 protein contains three putative NLS domains at its C-terminus (Shaulsky et al., 1990; Ko and Prives, 1996). The first NLS (aa 316-321) is the most conserved NLS across different species and has been shown to facilitate the translocation of p53 to the nucleus (Dang and Lee, 1989; Shaulsky et al., 1990). In our study, we showed impaired p53 nuclear localization (Figure 2). Recently, CRT has also been shown to be involved in the regulation of nuclear import of NF-AT3 (Mesaeli et al., 1999) and MEF2C (Li et al., 2002). Impaired nuclear export of the glucocorticoid receptor was also reported recently in the CRT-deficient cells (Holaska et al., 2001). These observed defects in nuclear transport have been attributed to altered Ca2+ homeostasis in the CRT-deficient cells (Mesaeli et al., 1999; Li et al., 2002; Holaska et al., 2001, 2002). However, not all nuclear import is impaired, as evident by normal nuclear transport of GATA4 (Li et al., 2002), dexamethasone-induced nuclear import of glucocorticoid receptor (Holaska et al., 2001), and Mdm2 (Figure 5).
Impaired nuclear localization of p53 could also be mediated by increased activity of nuclear export of p53. There are two NES sequences in p53, one at the N terminal domain of the protein and the second at the C-terminus located in the tetramerization site (Stommel et al., 1999; Lohrum et al., 2001; Zhang and Xiong, 2001). On activation of p53, it forms a tetramer that masks the latter NES and retains the protein in the nucleus (Stommel et al., 1999). The nuclear export of p53 involves binding of Mdm2 to the C-terminal part of p53 (Momand et al., 1992; Chen et al., 1995; Yu et al., 2000), which inhibits its tetramerization (Stommel et al., 1999) exposing the NES sequences to the nuclear export factor CRM1 (Tao and Levine, 1999; Inoue et al., 2001; Lohrum et al., 2001). Binding of Mdm2 to p53 mediates p53 ubiquitination and degradation via the proteasome pathway (Haupt et al., 1997; Yu et al., 2000). Our results showed a significant decrease in the p53 protein level in the crt-/- MEF cells, indicating the possibility for degradation of p53 protein in the absence of CRT. We also showed similar decrease in the p53 protein in the crt-/- mouse embryonic tissue, emphasizing that the observation in the MEF cells are not due to immortalization of these cells. We also observed accumulation of Mdm2 in the nuclei of the crt-/- cells. Mdm2 function and nuclear translocation is regulated by AKT/PKB-mediated phosphorylation (Mayo and Donner, 2001; Mayo et al., 2002). Indeed, inhibition of AKT/PKB kinase by PTEN protects p53 protein from ubiquitination by Mdm2 and degradation (Mayo et al., 2002). Recently, Kageyama et al. (2002) have shown inhibition of the AKT/PKB signaling pathway after overexpression of CRT in cardiomyocytes (H9c2). These cells are also more prone to apoptosis when cultured in the presence of 1% FBS and 10 nM retinoic acid (Kageyama et al., 2002). Therefore it is plausible that in the absence of CRT the AKT/PKB pathway can be activated, resulting in phosphorylation of Mdm2 and its nuclear translocation. Indeed our results show that in the absence of CRT, Mdm2 is activated and translocated to the nucleus. When in the nucleus, Mdm2 interacts with p53 and both proteins are exported from the nucleus and degraded by the proteasome pathway in the cytosol.
Under stress condition such as UV irradiation, chemotherapeutic agents, or hypoxia p53 is stabilized and accumulates in the nucleus (Lain et al., 1999a, 1999b; Ashcroft et al., 2000; Zhang and Xiong, 2001), where it activates expression of the stress response genes (Middeler et al., 1997). On the other hand, these stress conditions result in the localization of the Mdm2 protein in the cytoplasm and thus protect p53 protein from the Mdm2-mediated degradation (Wadgaonkar and Collins, 1999). UV treatment of the wt, crt-/-, and CRT-crt-/- cells resulted in redistribution of the p53 and Mdm2 proteins in these cells. As expected UV treatment resulted in cytoplasmic localization of Mdm2 protein in all the three cell types. On the other hand, UV treatment resulted in accumulation of p53 in the nuclei of the wt and CRT-crt-/- cells. Although the crt-/- cells showed a significantly lower p53 signal, the nuclear p53 observed was punctate in nature and seems to be associated with the nucleolus. This pattern of nuclear localization of p53 has been recently attributed to the latent form of p53 in HepG2 cell lines (Rubbi and Milner, 2000). This suggests that in crt-/- cells the UV-induced nuclear accumulation of p53 is perhaps due to decreased Mdm2 activity, but the stabilized p53 does not efficiently activate p53 downstream activities. Thus we conclude that the increased resistance of the crt-/- cells to UV-induced apoptosis could be due to both a decrease in the p53 protein stability and its impaired nuclear localization.
Acknowledgments
We thank Ms. Melanie Durston for superb technical assistance and Dr. Jeffrey Wigle for critically reading this manuscript. This work was supported by a grant to N.M. from the Heart and Stroke Foundation of Manitoba and the Canadian Institute of Health Research. N.M. is a New Investigator of the Heart and Stroke Foundation of Canada.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-04-0251. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-04-0251.
Abbreviations used: CRT, calreticulin; Mdm2, murine double minute protein; MEF, mouse embryonic fibroblast; NES, nuclear export signal; NLS, nuclear import signal; HA, hemagglutinin; crt-/- cells, calreticulin null cells; CRT-crt-/- cells, calreticulin null cells stably transfected with calreticulin-HA.
References
- Ashcroft, M., Taya, Y., and Vousden, K.H. (2000). Stress signals utilize multiple pathways to stabilize p53. Mol. Cell. Biol. 20, 3224-3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft, M., and Vousden, K.H. (1999). Regulation of p53 stability. Oncogene 18, 7637-7643. [DOI] [PubMed] [Google Scholar]
- Bastianutto, C., Clementi, E., Codazzi, F., Podini, P., De Giorgi, F., Rizzuto, R., Meldolesi, J., and Pozzan, T. (1995). Overexpression of calreticulin increases the Ca2+ capacity of rapidly exchanging Ca2+ stores and reveals aspects of their lumenal microenvironment and function. J. Cell Biol. 130, 847-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy, C.O., Prost, S., Wyllie, A.H., and Harrison, D.J. (1997). UV but not gamma-irradiation induces specific transcriptional activity of p53 in primary hepatocytes. J. Pathol. 183, 177-181. [DOI] [PubMed] [Google Scholar]
- Camacho, P., and Lechleiter, J.D. (1995). Calreticulin inhibits repetitive intracellular Ca2+ waves. Cell 82, 765-771. [DOI] [PubMed] [Google Scholar]
- Chen, J., Lin, J., and Levine, A.J. (1995). Regulation of transcription functions of the p53 tumor suppressor by the mdm-2 oncogene. Mol. Med. 1, 142-152. [PMC free article] [PubMed] [Google Scholar]
- Coppolino, M.G., Woodside, M.J., Demaurex, N., Grinstein, S., St-Arnaud, R., and Dedhar, S. (1997). Calreticulin is essential for integrin-mediated calcium signalling and cell adhesion. Nature 386, 843-847. [DOI] [PubMed] [Google Scholar]
- Cotton, J., and Spandau, D.F. (1997). Ultraviolet B-radiation dose influences the induction of apoptosis and p53 in human keratinocytes. Radiat. Res. 147, 148-155. [PubMed] [Google Scholar]
- Dang, C.V., and Lee, W.M. (1989). Nuclear and nucleolar targeting sequences of c-erb-A, c-myb, N-myc, p53, HSP70, and HIV tat proteins. J. Biol. Chem. 264, 18019-18023. [PubMed] [Google Scholar]
- Donehower, L.A. (1996). The p53-deficient mouse: a model for basic and applied cancer studies. Semin. Cancer Biol. 7, 269-278. [DOI] [PubMed] [Google Scholar]
- Donehower, L.A., Harvey, M., Slagle, B.L., McArthur, M.J., Montgomery, C.A., Jr., Butel, J.S., and Bradley, A. (1992). Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356, 215-221. [DOI] [PubMed] [Google Scholar]
- Fang, S., Jensen, J.P., Ludwig, R.L., Vousden, K.H., and Weissman, A.M. (2000). Mdm2 is a RING finger-dependent ubiquitin protein ligase for itself and p53. J. Biol. Chem. 275, 8945-8951. [DOI] [PubMed] [Google Scholar]
- Haupt, Y., Maya, R., Kazaz, A., and Oren, M. (1997). Mdm2 promotes the rapid degradation of p53. Nature 387, 296-299. [DOI] [PubMed] [Google Scholar]
- Hebert, D.N., Zhang, J.X., Chen, W., Foellmer, B., and Helenius, A. (1997). The number and location of glycans on influenza hemagglutinin determine folding and association with calnexin and calreticulin. J. Cell Biol. 139, 613-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holaska, J.M., Black, B.E., Love, D.C., Hanover, J.A., Leszyk, J., and Paschal, B.M. (2001). Calreticulin is a receptor for nuclear export. J. Cell Biol. 152, 127-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holaska, J.M., Black, B.E., Rastinejad, F., and Paschal, B.M. (2002). Ca-dependent nuclear export mediated by calretciulin. Mol. Cell. Biol. 22, 6286-6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollstein, M., Sidransky, D., Vogelstein, B., and Harris, C.C. (1991). p53 mutations in human cancers. Science 253, 49-53. [DOI] [PubMed] [Google Scholar]
- Honda, R., Tanaka, H., and Yasuda, H. (1997). Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 420, 25-27. [DOI] [PubMed] [Google Scholar]
- Inoue, T., Geyer, R.K., Howard, D., Yu, Z.K., and Maki, C.G. (2001). MDM2 can promote the ubiquitination, nuclear export, and degradation of p53 in the absence of direct binding. J. Biol. Chem. 276, 45255-45260. [DOI] [PubMed] [Google Scholar]
- Kageyama, K., Ihara, Y., Goto, S., Urata, Y., Toda, G., Yano, K., and Kondo, T. (2002). Overexpression of calreticulin modulates protein kinase B/Akt signaling to promote apoptosis during cardiac differentiation of cardiomyoblast H9c2 cells. J. Biol. Chem. 277, 19255-19264. [DOI] [PubMed] [Google Scholar]
- Ko, L.J., and Prives, C. (1996). p53: puzzle and paradigm. Genes Dev. 10, 1054-1072. [DOI] [PubMed] [Google Scholar]
- Lain, S., Midgley, C., Sparks, A., Lane, E.B., and Lane, D.P. (1999a). An inhibitor of nuclear export activates the p53 response and induces the localization of HDM2 and p53 to U1A-positive nuclear bodies associated with the PODs. Exp. Cell Res. 248, 457-472. [DOI] [PubMed] [Google Scholar]
- Lain, S., Xirodimas, D., and Lane, D.P. (1999b). Accumulating active p53 in the nucleus by inhibition of nuclear export: a novel strategy to promote the p53 tumor suppressor function. Exp. Cell Res. 253, 315-324. [DOI] [PubMed] [Google Scholar]
- Levine, A.J. (1997). p53, the cellular gatekeeper for growth and division. Cell 88, 323-331. [DOI] [PubMed] [Google Scholar]
- Li, J., Puceat, M., Perez-Terzic, C., Mery, A., Nakamura, K., Michalak, M., Krause, K.H., and Jaconi, M.E. (2002). Calreticulin reveals a critical Ca(2+) checkpoint in cardiac myofibrillogenesis. J. Cell Biol. 158, 103-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, M., and Pelling, J.C. (1995). UV-B/A irradiation of mouse keratinocytes results in p53-mediated WAF1/CIP1 expression. Oncogene 10, 1955-1960. [PubMed] [Google Scholar]
- Liu, N., Fine, R.E., Simons, E., and Johnson, R.J. (1994). Decreasing calreticulin expression lowers the Ca2+ response to bradykinin and increases sensitivity to ionomycin in NG-108-15 cells. J. Biol. Chem. 269, 28635-28639. [PubMed] [Google Scholar]
- Lohrum, M.A., Woods, D.B., Ludwig, R.L., Balint, E., and Vousden, K.H. (2001). C-terminal ubiquitination of p53 contributes to nuclear export. Mol. Cell. Biol. 21, 8521-8532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo, L.D., Dixon, J.E., Durden, D.L., Tonks, N.K., and Donner, D.B. (2002). PTEN protects p53 from Mdm2 and sensitizes cancer cells to chemotherapy. J. Biol. Chem. 277, 5484-5489. [DOI] [PubMed] [Google Scholar]
- Mayo, L.D., and Donner, D.B. (2001). A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc. Natl. Acad. Sci. USA 98, 11598-11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mery, L., Mesaeli, N., Michalak, M., Opas, M., Lew, D.P., and Krause, K.H. (1996). Overexpression of calreticulin increases intracellular Ca2+ storage and decreases store-operated Ca2+ influx. J. Biol. Chem. 271, 9332-9339. [DOI] [PubMed] [Google Scholar]
- Mesaeli, N., Nakamura, K., Zvaritch, E., Dickie, P., Dziak, E., Krause, K.H., Opas, M., MacLennan, D.H., and Michalak, M. (1999). Calreticulin is essential for cardiac development. J. Cell Biol. 144, 857-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak, M., Corbett, E.F., Mesaeli, N., Nakamura, K., and Opas, M. (1999). Calreticulin: one protein, one gene, many functions [In Process Citation]. Biochem. J. 344(Pt 2), 281-292. [PMC free article] [PubMed] [Google Scholar]
- Middeler, G., Zerf, K., Jenovai, S., Thulig, A., Tschodrich-Rotter, M., Kubitscheck, U., and Peters, R. (1997). The tumor suppressor p53 is subject to both nuclear import and export, and both are fast, energy-dependent and lectin-inhibited. Oncogene 14, 1407-1417. [DOI] [PubMed] [Google Scholar]
- Momand, J., Zambetti, G.P., Olson, D.C., George, D., and Levine, A.J. (1992). The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell 69, 1237-1245. [DOI] [PubMed] [Google Scholar]
- Nakamura, K. et al. (2000). Changes in endoplasmic reticulum luminal environment affect cell sensitivity to apoptosis. J. Cell Biol. 150, 731-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauseef, W.M., McCormick, S.J., and Clark, R.A. (1995). Calreticulin functions as a molecular chaperone in the biosynthesis of myeloperoxidase. J. Biol. Chem. 270, 4741-4747. [DOI] [PubMed] [Google Scholar]
- Nigam, S.K., Goldberg, A.L., Ho, S., Rohde, M.F., Bush, K.T., and Sherman, M. (1994). A set of endoplasmic reticulum proteins possessing properties of molecular chaperones includes Ca(2+)-binding proteins and members of the thioredoxin superfamily. J. Biol. Chem. 269, 1744-1749. [PubMed] [Google Scholar]
- Reich, N.C., and Levine, A.J. (1984). Growth regulation of a cellular tumour antigen, p53, in nontransformed cells. Nature 308, 199-201. [DOI] [PubMed] [Google Scholar]
- Reinke, V., and Lozano, G. (1997). Differential activation of p53 targets in cells treated with ultraviolet radiation that undergo both apoptosis and growth arrest. Radiat. Res. 148, 115-122. [PubMed] [Google Scholar]
- Rotter, V., Abutbul, H., and Ben-Ze'ev, A. (1983). P53 transformation-related protein accumulates in the nucleus of transformed fibroblasts in association with the chromatin and is found in the cytoplasm of non-transformed fibroblasts. EMBO J. 2, 1041-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubbi, C.P., and Milner, J. (2000). Non-activated p53 co-localizes with sites of transcription within both the nucleoplasm and the nucleolus. Oncogene 19, 85-96. [DOI] [PubMed] [Google Scholar]
- Saucedo, L.J., Carstens, B.P., Seavey, S.E., Albee, L.D., 2nd, and Perry, M.E. (1998). Regulation of transcriptional activation of mdm2 gene by p53 in response to UV radiation. Cell Growth Differ. 9, 119-130. [PubMed] [Google Scholar]
- Schlamp, C.L., Poulsen, G.L., Nork, T.M., and Nickells, R.W. (1997). Nuclear exclusion of wild-type p53 in immortalized human retinoblastoma cells [see comments]. J. Natl. Cancer Inst. 89, 1530-1536. [DOI] [PubMed] [Google Scholar]
- Shaulsky, G., Goldfinger, N., Ben-Ze'ev, A., and Rotter, V. (1990). Nuclear accumulation of p53 protein is mediated by several nuclear localization signals and plays a role in tumorigenesis. Mol. Cell. Biol. 10, 6565-6577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stommel, J.M., Marchenko, N.D., Jimenez, G.S., Moll, U.M., Hope, T.J., and Wahl, G.M. (1999). A leucine-rich nuclear export signal in the p53 tetramerization domain: regulation of subcellular localization and p53 activity by NES masking. EMBO J. 18, 1660-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao, W., and Levine, A.J. (1999). Nucleocytoplasmic shuttling of oncoprotein Hdm2 is required for Hdm2-mediated degradation of p53. Proc. Natl. Acad. Sci. USA 96, 3077-3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadgaonkar, R., and Collins, T. (1999). Murine double minute (MDM2) blocks p53-coactivator interaction, a new mechanism for inhibition of p53-dependent gene expression. J. Biol. Chem. 274, 13760-13767. [DOI] [PubMed] [Google Scholar]
- Waser, M., Mesaeli, N., Spencer, C., and Michalak, M. (1997). Regulation of calreticulin gene expression by calcium. J. Cell Biol. 138, 547-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods, D.B., and Vousden, K.H. (2001). Regulation of p53 function. Exp. Cell Res. 264, 56-66. [DOI] [PubMed] [Google Scholar]
- Wu, L., and Levine, A.J. (1997). Differential regulation of the p21/WAF-1 and mdm2 genes after high-dose UV irradiation: p53-dependent and p53-independent regulation of the mdm2 gene. Mol. Med. 3, 441-451. [PMC free article] [PubMed] [Google Scholar]
- Yu, Z.K., Geyer, R.K., and Maki, C.G. (2000). MDM2-dependent ubiquitination of nuclear and cytoplasmic P53. Oncogene 19, 5892-5897. [DOI] [PubMed] [Google Scholar]
- Zhang, Y., and Xiong, Y. (2001). A p53 amino-terminal nuclear export signal inhibited by DNA damage-induced phosphorylation. Science 292, 1910-1915. [DOI] [PubMed] [Google Scholar]