Abstract
We have previously shown that c-Fos activates phospholipid synthesis through a mechanism independent of its genomic AP-1 activity. Herein, using PC12 cells induced to differentiate by nerve growth factor, the genomic effect of c-Fos in initiating neurite outgrowth is shown as distinct from its nongenomic effect of activating phospholipid synthesis and sustaining neurite elongation. Blocking c-Fos expression inhibited differentiation, phospholipid synthesis activation, and neuritogenesis. In cells primed to grow, blocking c-Fos expression determined neurite retraction. However, transfected cells expressing c-Fos or c-Fos deletion mutants with capacity to activate phospholipid synthesis sustain neurite outgrowth and elongation in the absence of nerve growth factor. Results disclose a dual function of c-Fos: it first releases the genomic program for differentiation and then associates to the endoplasmic reticulum and activates phospholipid synthesis. Because phospholipids are key membrane components, we hypothesize this latter phenomenon as crucial to support membrane genesis demands required for cell growth and neurite elongation.
INTRODUCTION
Relevant progress has been achieved in discerning the regulatory processes involved in the expression of genes controlling cell growth, a temporarily advanced phenomenon with respect to growth. However, how cells fulfill their demands of membrane components required for cell growth and morphological differentiation is still unclear, and progress in the comprehension of the biochemical mechanisms that coordinate bulk membrane component production in the course of cell growth remains fragmentary.
Among genes involved in the control of the cell cycle, the early response genes fos and jun have been thoroughly investigated. fos is a multigene family encoding five proteins, c-Fos, Fos-B, Δ Fos-B, Fra-1 and Fra-2, whereas the jun family has three members, c-Jun, JunB, and JunD (Hai and Curran, 1991; Shaulian and Karin, 2002). Fos and Jun proteins are usually not constitutive components of cells; rather they are rapidly and transiently induced in many cell types as a response to diverse stimuli such as growth factors, sensorial stimulation, and neurotransmitters (Curran and Morgan, 1987; Hughes and Dragunow, 1995; reviewed in Caputto and Guido, 2000). The Fos proteins cross-dimerize through leucine zipper interactions with protein products of the jun genes but do not homodimerize (Angel and Karin, 1991). The resulting heterodimers are the highly charged, basic, DNA-binding AP-1 transcription factors. These AP-1 dimers typically affect key events of cell division and differentiation by regulating downstream target genes. Identification of the transcription factor activity of these early response genes directed outcoming research toward establishing the regulatory constraints that determine their expression and the target genes that respond to their induction (Kovary and Bravo, 1992; Lallemand et al., 1997). There is now a considerable body of information concerning AP-1 induction pathways and expression patterns of various inducible early response genes.
On the other hand, data are fragmentary regarding the concerted metabolic response elicited in cells in response to induction of genes controlling growth. In this regard, the possibility that expression of their protein products in cytoplasm may have regulatory activities independent of, or in addition to those they exert in the nucleus had not been investigated in detail. In the present report, we examined the role of c-Fos in regulating the synthesis of phospholipids required for the genesis of membrane associated with cell growth and morphological differentiation of rat pheochromocytoma PC12 cells. Our results suggest that once the c-Fos-mediated genomic events required for neurite outgrowth have been triggered by nerve growth factor (NGF), cytoplasmic c-Fos is sufficient to sustain neurite elongation through a mechanism that involves c-Fos association to the ER and phospholipid synthesis activation.
MATERIALS AND METHODS
Cell Culture
PC12 cells were grown at 37°C in 5% CO2 in DMEM (Sigma-Aldrich, St. Louis, MO) supplemented with 0.04 mM glutamine and 5% fetal bovine serum (Invitrogen, Carlsbad, CA) plus 5% horse serum (Invitrogen). For establishing quiescence, cells grown for 72 h in DMEM supplemented with 5% horse serum plus 5% fetal bovine serum had their medium changed to DMEM supplemented with 1% horse serum for an additional 36 h. After this time in culture, the cells depleted the medium of serum growth factors and were growth arrested (Araki and Wurtman, 1997). To stimulate cells to differentiate, fresh medium containing 100 ng/ml NGF (Invitrogen) replaced medium in each well. Sister control cultures received the same volume of fresh medium without NGF.
For cotransfection of PC12 cells, cells were seeded onto acid-washed coverslips coated with poly-lysine (1 g/ml) in 35-mm petri dishes containing 2 ml of DMEM, and attached cells were transiently transfected with 5 μg of total DNA by using FuGENE 6 transfection reagent (Roche Diagnostics, Indianapolis, IN) according to manufacturer instructions. pcDNA3Fos (4 μg) (or the corresponding vector for controls) was cotransfected with 1 μg of pEYFP-N1 (BD Biosciences Clontech, Palo Alto, CA) or of lip33-pEYFP (Giraudo and Maccioni, 2003). More than 60% of the cells were pEYFP-N1 or lip33-pEYFP positive. Only these positive cells were considered for morphometric quantification of neurite formation. For establishing quiescence, transfected cells were treated as described above. For transfection with c-Fos or c-Jun antibody, 100 μg/ml c-Fos (Ab4) or c-Jun (N-G) antibody, as indicated (Santa Cruz Biotechnology, Santa Cruz, CA), was delivered at the indicated time to quiescent PC12 cells with BioPORTER QuickEasy delivery kit (Sigma-Aldrich) as indicated by the manufacturer, and cells were cultured to complete 4 d. Sister control cells (mock) received the same volume of the delivery system.
In the experiments with cellular effectors such as NGF, oligonucleotides (5′-GSA ACA TCA TGG TCG ST-3′ c-fos mRNA antisense oligonucleotide [ASO] and corresponding sense or random c-fos mRNA oligonucleotide [SO]) (Biosynthesis, Lewisville, TX), acetyl calpastatin (A-Calp) (Sigma-Aldrich), and a peptide carrying the AP-1 nuclear localization signal peptide (NLSP) (Biosynthesis), these were added to cultures in 5 μl of medium at the times and concentrations indicated for each experiment. Sister control cells received the same volume of medium alone, or in the case of experiments with ASO, the same volume of medium containing the corresponding SO. Medium was replaced with fresh medium containing the corresponding cellular effector as indicated for each experiment at 48 and 72 h after initiation of cell treatment.
Cell Phospholipid Labeling
To determine 32P-phospholipid labeling capacity, PC12 cells were grown as indicated above. Control and treated cells were pulsed with 45 μCi/ml 32Pi (PerkinElmer Life Sciences, Boston, MA) 2 h before harvesting. 32P-Phospholipid labeling was determined using the trichloroacetic acid-phosphotungstic acid method described previously (Guido and Caputto, 1990). Lipid extracts in chloroform:methanol [2:1 (vol/vol)] were dried for radioactivity quantification or used for chromatographic separation of individual phospholipids.
Immunofluorescence Analysis
Cells grown on round, acid-washed coverslips coated with poly-lysine (1 g/ml) and treated as indicated were rinsed with cool phosphate-buffered saline (PBS) and fixed for 10 min with 3% paraformaldehyde (wt/vol) plus 4% sucrose in PBS at 37°C. Cells were then permeabilized by treatment with 0.25% Triton X-100 in PBS for 10 min, washed three times with PBS, and blocked with PBS containing 1% bovine serum albumin plus Tween 20 [0.1% (vol/vol)] (blocking buffer) for 2 h at room temperature in a humid chamber. The coverslips were incubated overnight at 4°C with blocking buffer containing a polyclonal antibody to c-Fos (Ab4 or antibody 125, as indicated; Santa Cruz Biotechnology) and/or a polyclonal antibody to calnexin (Santa Cruz Biotechnology) for endoplasmic reticulum (ER) immunolabeling and/or a monoclonal antibody to α-tubulin (α-Tub) (DM1-A; Sigma-Aldrich). For viable cells transfected and cultured for 4 d with c-Fos or c-Jun antibody, only α-tubulin (DM1-A) or calnexin antibodies were present in the blocking buffer; c-Fos or c-Jun antibodies were omitted because they were previously transfected into the cells. Cells were washed with PBS plus Tween 20 [0.1% (vol/vol)] and then incubated for 2 h at room temperature in blocking buffer in a humid chamber with Alexa 546 and/or Alexa 488 and/or phalloidin red (Molecular Probes, Eugene, OR) as necessary. Coverslips were washed, mounted in Prolong Antifade (Molecular Probes), and visualized on a confocal laser scanning microscope LSM 510 (Carl Zeiss, Thornwood, NY) by using LSM 510 software for image analysis. When was necessary tetramethylrhodamine B isothiocyanate-conjugated anti-rabbit IgG and fluorescein isothiocyanate-conjugated anti-mouse IgG (Santa Cruz Biotechnology) was used for each case, mounted as described above, and then observed on an Axioplan epifluorescence microscope (Carl Zeiss) equipped with a Princeton Instrument Micromax camera controlled with MetaMorph Imaging software (Universal Imaging, Downingtown, PA).
Expression and Purification of c-Fos and c-Jun Full-Length and Deletion Mutants
c-Fos and c-Jun full-length and c-Fos deletion polypeptides containing the indicated amino acids were synthesized as histidine-tagged proteins in Escherichia coli as described previously (Borioli et al., 2001). The additional truncated genes were constructed from full-length c-Fos cDNA (a gift of T. Curran, St. Jude Childrens Hospital, Memphis, TN) with oligonucleotides corresponding to the 5′ and 3′ ends of the coding sequence to be amplified, cloned in pGEM-T-easy (Promega), and then subcloned in the prokaryote expression vector pET15b (Novagen) by using the NdeI and EcoR1 sites of the polylinker sequence of the vector. The hexahistidine-tagged proteins were synthesized in E. coli and purified from cell lysates by nickel affinity chromatography by running through HisBin resin (Novagen, Madison, WI).
For PC12 cell transfections, the pcDNA 3.1 eukaryotic expression vector containing the coding sequence of the corresponding deletion polypeptide obtained from the full length c-Fos dDNA (kindly supplied by J. Blenis, Harvard Medical School, Boston, MA) inserted in the EcoR1 site of the polylinker sequence of the vector was used.
Morphometric Analysis
To determine number of neurites per cell or length of neurites present in individual cells, antibody-labeled, Prolong Antifade-mounted coverslips were examined under epifluorescence microscope with filters adjusted for visualization of the antibody against α-Tub, by using the Morphometric menu of the MetaMorph Imaging software. For statistical purposes, double-blind quantification was performed as follows: 1) a cell prolongation was considered a neurite when its length was at least the diameter of the cell body; 2) when neurites were branched, only the longest ramification was considered to establish the total length of the neurite; 3) when a given cell had two or more neurites, only the longest neurite was considered; and 4) calculation of percentage of cells with neurites was only for viable cells. Consequently, differences between neurite-bearing and neurite-devoid cells are usually larger than those reflected in the final numbers of the figures. At least 300 cells for a given experimental condition were examined.
Protein Analysis
Cells were harvested in Protease Inhibitor Cocktail (Sigma-Aldrich); SDS-PAGE and Western blotting were performed as described previously (Bussolino et al., 2001) by using anti c-Fos polyclonal antibody (Santa Cruz Biotechnology). Immunoreactive bands were detected as described by Nakane (1968) with protein A-conjugated peroxidase (Sigma-Aldrich) or by ECL Plus (Amersham Biosciences, Piscataway, NJ) by using biotinylated antibodies (Vector Laboratories, Burlingame, CA) and streptavidin-horseradish peroxidase conjugate (Amersham Biosciences).
In Vitro Labeling of Phospholipids
In vitro phospholipid labeling capacity of PC12 cells was assayed by incubation for 60 min at 37°C in medium containing, in a final volume of 80 μl, 140 mM NaCl, 4.5 mM KCl, 0.5 mM MgCl2, 5.6 mM glucose, HEPES buffer, pH 7.4, 64 mM, 3 μCi of [32P]ATP (specific activity 3000 Ci/mmol; PerkinElmer Life Sciences), and indicated amounts of c-Fos or c-Jun protein suspended in 3 μl of 300 mM imidazole/8 M urea. Control incubates contained only 3 μl of 300 mM imidazole/8 M urea. Reactions were initiated by addition of 300 μg of PC12 cell homogenate and stopped by addition of TCA-PTA (5-0.5% respectively, final concentration). Phospholipid labeling was quantified by the TCA-PTA method (Guido and Caputto, 1990). For assays in the absence of nuclear fraction, PC12 homogenates were fractionated according to Neri et al. (1998). Nucleus contamination in homogenates devoid of nuclei was monitored by dot blot in final preparations by using an anti-Histone 1 antibody (Santa Cruz Biotechnology). Contamination was <5% of the DNA present in the starting homogenates.
Statistics
Statistical analysis of all morphometric quantifications was performed by one-way analysis of variance (ANOVA) with Newman-Keuls post hoc tests, when appropriate. Statistical analysis of radiolabeled phospholipids was performed by two-tailed Student's t test.
RESULTS
Nuclear and Cytoplasmic c-Fos Are Required at Different Stages of Cell Differentiation and Growth
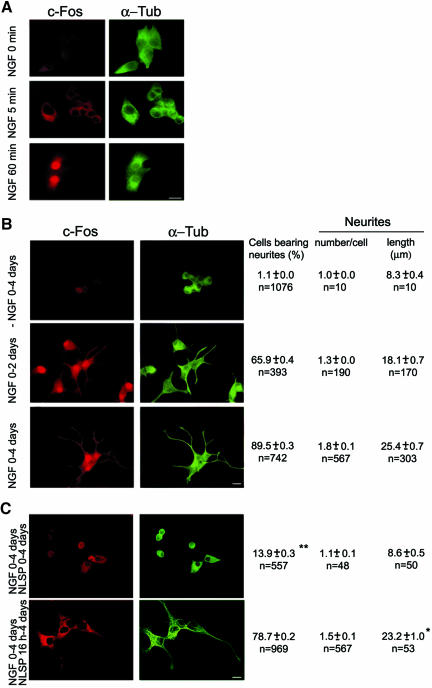
PC12 cells were examined for c-Fos immunocytochemical expression and subcellular localization at 5 and 60 min after feeding NGF (Figure 1A). Coimmunostaining for α-Tub served to delineate the cell body and neurites. No c-Fos immunostaining was observed at zero time of NGF treatment but by 5 min, NGF induced early and significant expression of c-Fos in the cytoplasm. After 60 min, c-Fos was detected both in the cytoplasm and the nucleus. Figure 1B shows that the nuclear and cytoplasmic distribution was maintained at longer periods of NGF treatment (2 and 4 d in culture). As already reported (Greene and Tischler, 1976; Araki and Wurtman, 1997) after 2 d in culture with NGF (Figure 1B, middle row), c-Fos expression is induced (Greenberg et al., 1985; Kruijer et al., 1985), and cells developed toward a sympathetic-neuron-like phenotype, which was fully attained at ∼4 d (Figure 1B, bottom row). This process was absolutely dependent on the presence of NGF (Figure 1B, top row). The quantitative data corresponding to each row show that after 4 d with NGF, 89% of the cells bear neurites, with a mean of 1.8 neurites per cell and a mean neurite length of 25.4 μm.
Figure 1.
NGF induces c-Fos expression and neuritogenesis in PC12 cells. Blocking nuclear import of c-Fos at initiation of NGF treatment blocks neuritogenesis. (A) Cells at the times of NGF treatment indicated at left were double immunostained for c-Fos (left) and α-Tub (right). (B) Cells cultured without NGF during 4 d (top row) or with NGF during 2 d (middle row) or 4 d (bottom row) were immunostained as in A and morphometrically quantified double blind. Results of the quantitative morphometric analyses of cells in each condition are posted on the right of the corresponding row; they are the mean ± SEM of the number of cells analyzed (n). Bar, 10 μm. All morphometric quantifications were subjected to one-way ANOVA with Newman-Keuls post hoc test. (C) Cells treated with NGF + NLSP for 4 d (top row) or with NGF for 16 h and then with NGF + NLSP to complete 4 d (bottom row) were double immunostained and morphometrically quantified as in B. *, neurite length was not significantly different (p > 0.1) from that of cells treated 4 d with NGF (B, bottom row) as determined by ANOVA with Newman-Keuls post hoc test. **, percentage of cells with neurites was calculated only for viable cells at 4 d (∼10% of the cells initially seeded).
c-Fos Is Imported into the Cell Nucleus before Triggering Neuritogenesis
To assess the importance of the early cytoplasmic c-Fos expression on NGF-mediated PC12 neurite outgrowth shown in Figure 1B, cells were fed NGF in the presence of the NLSP AAVALLPAVLLALLAPVQRK RQKLMP (Torgerson et al., 1998). This peptide, which carries the AP-1 signal-dependent nuclear import sequence (underlined) preceded by a cell-permeable sequence to deliver the functional domain (Lin et al., 1995), blocks the nuclear import of c-Fos as an AP-1 dimer (Torgerson et al., 1998; Bussolino et al., 2001). Examination of cells after 4 d in culture in the presvcence of NGF + NLSP showed that nuclear c-Fos is under the limit of detection (Figure 1C, top row) and clear abrogation of neuritogenesis had occurred (compare with bottom row of B), in spite that c-Fos was expressed at 1 and 4 d of NGF + NLSP treatment as assayed by Western blot (Figure 2, lanes c and e). At 4 d, these NLSP-treated cells remained round shaped as at seeding and ∼90% had died, as estimated by the erythrocin B exclusion assay (our unpublished data). However, if NLSP was added 16 h after onset of NGF treatment (Figure 1C, bottom row), neurite elongation was not significantly different from that of sister cells not treated with NLSP (Figure 1B, bottom row) or treated with peptide vehicle alone (neurite length in micrometers for vehicle-treated cells was 26.1 ± 0.71, n = 55; and for NLSP-treated cells, 23.5 ± 1.0, n = 53, p >0.1 as determined by one-way ANOVA with Newman-Keuls post hoc test). In fact, a time course of the effects of NLSP addition showed that after priming cells during 4 h with NGF, these can be fed NLSP and neurite elongation proceeds at the same rate as in cells cultured in the absence of NLSP (see Figure 1S of Supplementary Material). These results indicate that nuclear AP-1-c-Fos is required to trigger neuritogenesis at early stages of differentiation but not at later stages of neurite elongation.
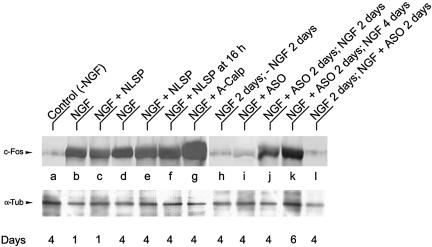
Figure 2.
Western blot analyses of c-Fos induction in PC12 cells cultured under different experimental conditions. PC12 cells were treated with the indicated effectors and harvested at the indicated days. For each condition, 60 μg of homogenate protein was subjected to SDS-PAGE and Western blotting with anti-c-Fos polyclonal antibody (top row) or with anti-α-Tub antibody (bottom row) to control protein loading. Results are from one representative experiment of three performed.
Cytoplasmic c-Fos Is Required for Differentiation to Proceed
To examine whether cytoplasmic c-Fos is necessary for sustaining neurite growth, c-Fos expression was specifically impaired at different stages of NGF-induced differentiation by feeding cells the c-fos mRNA antisense oligonucleotide ASO. When cells were fed ASO at the initiation of NGF treatment, they extended no or short neurites (Figure 3A, bottom row) relative to NGF-treated (Figure 3A, top row) or SO-treated cells (Figure 3A, middle row) or NGF + random scrambled oligonucleotide-treated cells (our unpublished data). Western blot analysis showed that there was essentially no c-Fos expression after 4 d in culture of ASO-treated cells (Figure 2i).
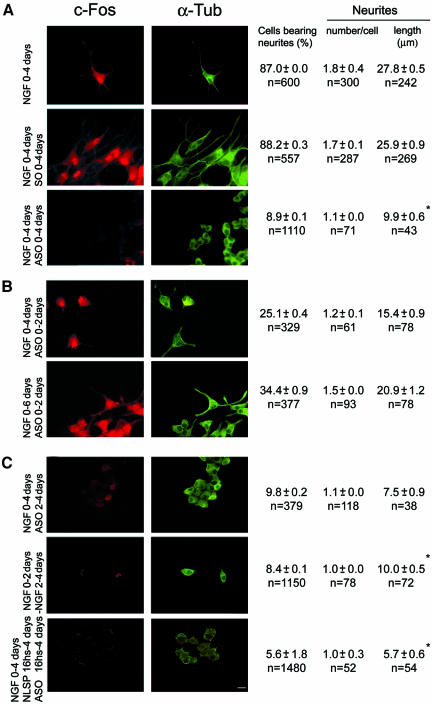
Figure 3.
Blocking c-Fos expression also blocks neuritogenesis. Cells cultured with NGF or NGF + SO or NGF + ASO for 4 d (A) or subjected to different temporal additions or withdrawals of NGF or ASO or NLSP as indicated at left (B and C) were immunostained for c-Fos (left) and α-Tub (right). Morphometric quantification data obtained as in Fig. 1 are posted to the right of the corresponding row. Bar, 10 μm. *, significantly shorter that cells treated 4 d with NGF at p < 0.00001 (by ANOVA test as in Figure 1).
The halting of differentiation by ASO was reversible for some cells, i.e., after treating cells for 2 d with NGF + ASO and culture medium replaced by fresh medium containing only NGF (Figure 3B, top row), ∼35% of the cells recovered c-Fos expression (Figure 2J) and extended neurites that, after 2 d, reached a mean length (15.4 μm) comparable with that of cells at 2 d with NGF alone (18.1 μm) (Figure 1B, middle row). In these cells, c-Fos expression (Figure 2K) and neurite elongation (Figure 3B, bottom row) continued increasing in the period from 2 to 4 d after changing to ASO-free medium (days 4-6 of the cultures).
To confirm that growth depends on the cellular content and localization of c-Fos, PC12 cells at 2 d with NGF were depleted of c-Fos either by addition of ASO (Figure 3C, top row) or by NGF withdrawal (Figure 3C, middle row) and examined after two additional days in culture. Western blot determination for the presence of c-Fos showed that c-Fos formed during the first 2 d with NGF disappeared during the following 2 d if cultured in the absence of NGF (Figure 2H) or in the presence of ASO (Figure 2l), returning to the level found in control cells, not treated with NGF (Figure 2A). Immunocytochemical analysis and morphometric quantification showed that the mean number of cells bearing neurites at 2 d with NGF decreased from 65.9% (Figure 1B, middle row) to 9.8 or 8.4%, respectively, in cells treated with ASO (Figure 3C, top row) or deprived of NGF (Figure 3C, middle row) during the additional 2 d in culture. This was so even though cells were not dying as determined by the erythrocin B exclusion assay (percentage of viable cells was of 95.2 ± 1, n = 1550 after culturing cells 2 d in the presence and then 2 d in the absence of NGF, compared with 99.2 ± 1.0, n = 1445 for cells cultured 4 d in the presence of NGF). In addition, no neurite elongation was observed after 4 d in culture when cells primed to grow by treatment with NGF during 16 h were then fed NLSP + ASO (Figure 3C, bottom row). These results, in combination with those of Figure 1C in which neurite elongation was observed after the differentiation program was triggered and cells were fed NLSP, support that cytoplasmic c-Fos is required to sustain neurite elongation.
In every experiment in which ASO was used, controls were performed with sister cultures that received an equivalent amount of SO. No differences were observed between these cultures and those in the absence of SO, either in the number of cells, the cellular content of c-Fos or in cell growth as determined by immunocytochemical examination and morphometric quantification (Figure 3A, middle row) or by Western blot (our unpublished data). Furthermore, no differences were observed in the number of viable cells after 4 d of ASO treatment as controlled by the erythrocin B exclusion assay (percentage of viable cells at 4 d in culture was 97.9 ± 1.5, n = 1090 for ASO-treated cells and 99.3 ± 0.8, n = 1045 for NGF-treated cells).
Increasing Cellular Content of c-Fos Stimulates Neuritogenesis
To further investigate the dependence of neuritogenesis on c-Fos expression, PC12 cells were treated with A-Calp, an inhibitor of calpain-dependent, cytoplasmic c-Fos degradation (Pariat et al., 2000). c-Fos content increased in NGF + A-Calp-treated cells at 4 d in culture (Figure 2G) with respect to cells grown with NGF alone (Figure 2D). Under these experimental conditions, the amount of cells extending neurites increased to near 95%, the neurites were 42% longer and the mean number of neurites per cell was 66% higher compared with cells grown in NGF alone (compare middle and top rows of Figure 4).
Figure 4.
Increasing c-Fos levels by inhibiting its degradation with A-Calp increases neuritogenesis. PC12 cells cultured 4 d with NGF (top row) or NGF + A-Calp (middle row) or NGF + A-Calp + ASO (bottom row), as indicated at left, were double immunostained for c-Fos (left) and for α-Tub (right). Double blind morphometric quantifications of each experimental condition are posted on the right of the corresponding row. Bar, 10 μm. *, neurites in cells treated with NGF + A-Calp (middle row) were significantly longer than NGF-treated (top row) cells at p < 0.000001 (by ANOVA test as in Figure 1).
The stimulating effect of A-Calp was dependent on c-Fos expression because it was abrogated upon the simultaneous treatment of cells with ASO + A-Calp + NGF (Figure 4, bottom row). A-Calp or ASO alone (-NGF) had no effect on c-Fos expression or neuritogenesis (our unpublished data).
PC12 Transfected Cells Expressing c-Fos Support Neurite Elongation in the Absence of NGF
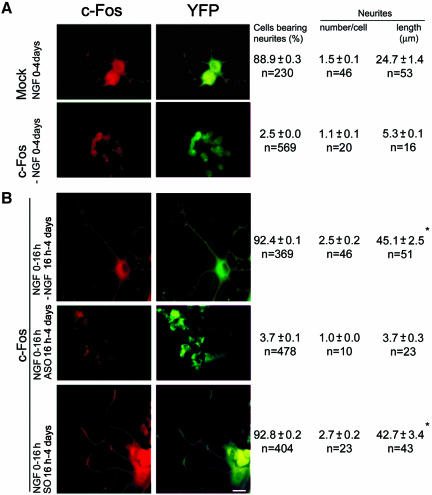
To confirm the need of cytoplasmic c-Fos to sustain neurite elongation, this process was examined in PC12 cells cotransfected to constitutively overexpress c-Fos and to express YFP to delineate the cell. In the absence of NGF, no neuritogenesis was observed in the c-Fos-overexpressing cells at 4 d in culture (Figure 5 A, bottom row), confirming the need of genomic AP-1-c-Fos to trigger neuritogenesis. To induce the expression of partner genes required for c-Fos heterodimerization and nuclear import to trigger the differentiation program, c-Fos-transfected cells were primed with NGF for 16 h, and then medium was replaced by fresh medium without NGF and cells cultured up to 4 d without NGF. c-Fos overexpression was observed in these cells both by immunocytochemistry (Figure 5B, top row) and Western blot (Figure 10B, lane c) compared with mock-transfected cells at 4 d of treatment with NGF (Figure 5A, top row; Figure 10B, lane d). Morphometric quantification showed a >50% increase in neurite number and a >80% increase in neurite length (Figure 5B, top row) in c-Fos-overexpressing cells compared with mock-transfected cells treated the 4 d with NGF (Figure 5A, top row). If medium replacement at 16 h contained ASO, cells failed to grow (Figure 5B, middle row) as occurred in c-Fos-transfected cells (Figure 5A, bottom row) or in mock-transfected cells (our unpublished data) when not primed for 16 h with NGF to trigger neuritogenesis. SO had no effect on neurite elongation in the c-Fos-overexpressing cells primed to grow with NGF (Figure 5B, bottom row). These results indicate that once the nuclear program for cell differentiation has been triggered by NGF priming, cytoplasmic c-Fos is capable of sustaining neurite elongation.
Figure 5.
Withdrawal of NGF does not impair neurite outgrowth in c-Fos-overexpressing cells. (A) Mock- or c-Fos-transfected PC12 cells were cotransfected to express YFP and cultured 4 d in the absence (c-Fos-expressing cells, bottom row) or presence of NGF (mock-transfected, top row). (B) c-Fos- and YPF-expressing cells were primed to grow by culturing 16 h with NGF. Then medium was replaced with fresh medium without NGF and cultured up to 4 d with no addition (top row) or in the presence of ASO (middle row) or SO (bottom row). Cells were immunostained with anti c-Fos antibody and morphometrically quantified as in Figure 1. *, differences in neurite length between c-Fos-expressing transfected cells (B, top and bottom rows) and mock-transfected cells treated with NGF (A, top row) were significant at p < 0.00002, whereas Fos-transfected cells cultured in the absence of NGF (A, bottom row) were not significantly different (p > 0.1) from Fos-transfected cells treated with ASO (B, middle row) as determined by ANOVA test as in Figure 1. Bar, 10 μm.
Figure 10.
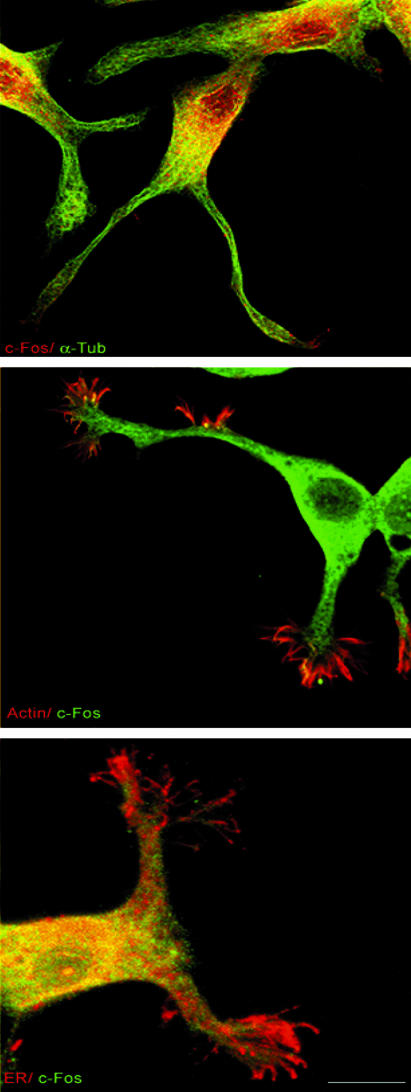
c-Fos colocalizes with ER membrane markers. Cells were double immunostained for c-Fos/α-Tub (top row), c-Fos/actin (middle row), or c-Fos/calnexin (bottom row) and analyzed after confocal microscopy. c-Fos is present in the cell nucleus and cytoplasm (top row) and in the growth cone (middle row) and colocalizes with ER membranes in the cell body and to a much lesser extent in growth cones (bottom row) as determined by comparison with α-Tub and actin immunostaining.
Activation of Phospholipid Synthesis Is Associated with Cell Growth
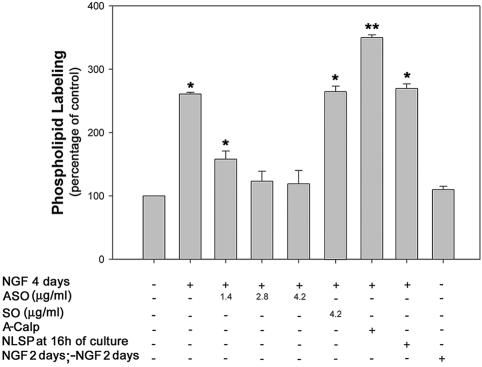
We have previously shown that c-Fos activates phospholipid synthesis in several cell types (Guido et al., 1996; Bussolino et al., 1998, 2001). To know whether phospholipid synthesis activation occurs in conditions of c-Fos-mediated neurite elongation, the 32P-labeling of phospholipids was determined in cells at 4 d in culture in the presence or the absence of NGF. A 2.5-fold activation of 32P-phospholipid synthesis was observed in NGF-treated cells (Figure 6) in accordance with previous results (Akari and Wurtman, 1997; Carter et at., 2003). This activation was dependent on c-Fos expression because it was specifically abolished in a dose-dependent manner by the addition of ASO. By contrast, A-Calp significantly increased phospholipid labeling ∼3.2-fold relative to cells treated with NGF alone. Blocking c-Fos import into the nucleus by feeding cells with NLSP 16 h after initiation of NGF treatment did not modify NGF-activation of 32P-phospholipid synthesis. The continuous presence of NGF was necessary for phospholipid activation because withdrawal of NGF after 2 d of culture with NGF abrogated the activation of phospholipid synthesis measured at day 4 (Figure 6). Because conditions that increase or decrease phospholipid synthesis activation also increase or decrease neurite elongation, results of Figure 6 are compatible with a phospholipid synthesis activation phenomenon participating in the c-Fos-regulated neurite elongation shown in Figures 3 and 4.
Figure 6.
32P-Phospholipid labeling in PC12 cells subjected to different culture conditions Cells cultured 4 d under the indicated experimental conditions were pulsed with [32P]orthophosphate 2 h before harvesting and 32P-phospholipid labeling determined as described in MATERIALS AND METHODS. Results are the mean ± SEM of eight independent experiments performed in triplicate. *p < 0.005; **p < 0.0001 with respect to -NGF-treated cells as determined by Student's t test.
All individual phospholipid species showed essentially the same activated 32P-labeling in NGF-treated compared with control (-NGF) cells at 4 d, whereas treatment of cells with increasing concentrations of ASO promoted a general decrease in labeling of all phospholipid species (our unpublished data).
c-Fos Activates Phospholipid Synthesis In Vitro; c-Jun Does Not
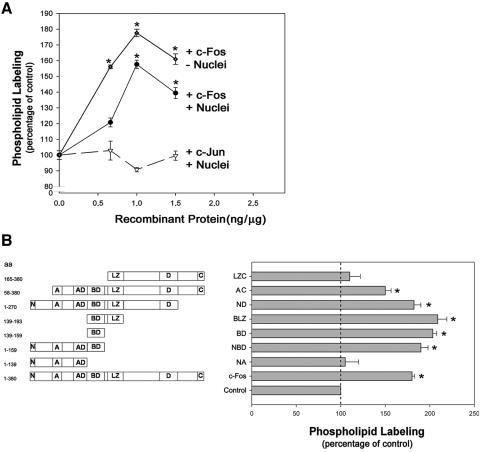
Direct confirmation that c-Fos content affects the rate of phospholipid synthesis was obtained by adding recombinant c-Fos in vitro to homogenates prepared from PC12 cells cultured in the absence of NGF and measuring incorporation of 32P from [γ-32P]ATP into phospholipids (Figure 7). Recombinant c-Fos activates phospholipid synthesis in a concentration-dependent manner, with maximal activation at 1 ng/μg homogenate protein which corresponds to a concentration of ∼105 molecules of c-Fos/cell. This c-Fos concentration is comparable with that calculated by Kovary and Bravo (1992) to be present in fibroblasts when c-Fos expression is induced (103-105 molecules of c-Fos/cell).
Figure 7.
Phospholipid labeling activation in vitro by c-Fos and c-Fos deletion mutants. (A) Phospholipid labeling was determined in the presence of increasing concentrations of recombinant c-Fos (full line) or c-Jun (dashed line) in total (closed circles, triangles) or nuclei free (diamonds) homogenates of PC12 cells cultured 4 d in the absence of NGF. Results are the mean ± SEM of 10 experiments performed in triplicate. *p < 0.0001 with respect to the corresponding control assayed in the absence of recombinant protein, as determined by Student's t test. (B) Phospholipid labeling was determined in the presence of the c-Fos deletion mutants depict (left), which were added at 1 ng/μg protein to homogenates of PC12 cells cultured 4 d in the absence of NGF. Results are the mean ± SEM of five experiments performed in triplicate. *p < 0.0001 with respect to the corresponding control assayed in the absence of recombinant protein, as determined by Student's t test. The deletion mutants (Abate et al., 1991) are named according to the schematic drawings to the left: LZ, consists of a heptad repeat of leucines that align on one face of an α-helix forming a dimerization interface required to form a bimolecular DNA-binding domain; A, corresponds to a region rich in praline residues; D, a region rich in acidic residues, mainly aspartic acid; C, C terminus; N, N terminus; AD, acidic domain containing four glutamic acid residues; BD, basic domain containing 12 basic amino acids, mostly arginine residues, and forms the bimolecular DNA-binding domain upon heterodimerization. Amino acids comprised in each mutant are depicted on the far left.
c-Fos has the same ability to activate phospholipid synthesis in vitro in total cell homogenates and in homogenates devoid of nuclei, further indicating that this activation is a cytoplasmic event (Figure 7A). By contrast, c-Jun, the common partner of c-Fos in AP-1 transcription factor complexes (Angel and Karin, 1991) had no effect on phospholipid synthesis when assayed in vitro at the same concentration (Figure 7A) or at concentrations up to threefold higher than c-Fos (our unpublished data).
c-Fos Deletion Mutants Activate Phospholipid Synthesis, Providing They Contain the Basic Domain Corresponding to Amino Acids 139-159 of Full-Length c-Fos
To advance in the knowledge of the molecular constraints to the activating capacity of c-Fos on phospholipid synthesis, diverse c-Fos deletion mutants (Figure 7B, left) were tested in vitro for their capacity to activate phospholipid synthesis. It was found that all mutants containing the basic domain of c-Fos (aa 139-159) were capable of activating phospholipid synthesis in vitro when tested at 1 ng μg PC12 cell homogenate protein (Figure 7B). Moreover, a 20-aa peptide containing only this basic domain was able to activate phospholipid synthesis to comparable levels as full-length c-Fos. Mutants containing either the first 139 aa (NA) or the last 215 aa (LZC), which includes the leucine zipper (LZ) domain required for c-Fos heterodimerization in AP-1 formation but lacks the basic domain, failed to activate phospholipid synthesis (Figure 7B), even when tested at a concentration range of up to 3 ng/μg cell homogenate (our unpublished data).
Transfected c-Fos Deletion Mutants That Activate Phospholipid Synthesis In Vitro, Associate to the ER and Support Neurite Elongation in the Absence of NGF
To further investigate the relationship between c-Fos-mediated phospholipid synthesis and neurite elongation, this process was examined in PC12 transfected to constitutively overexpress c-Fos or its deletion mutants. Cells were cotransfected with lip33 fused to the YFP to delineate the ER (Giraudo and Maccioni, 2003). lip33 is a type II membrane protein that contains an ER retention signal consisting of three R residues at positions 3-5 in its exposed cytoplasmic N-terminal domain (Schutze et al., 1994). The experimental protocol followed was as in Figure 5: the differentiation program was triggered in the transfected cells by priming with NGF during the first 16 h and then medium replaced by fresh medium without NGF and cultures prolonged to complete 4 d. c-Fos overexpression or expression of its deletion mutants were confirmed by Western blot (Figure 10B). By immunocytochemical examination, abundant cytoplasmic expression of c-Fos or of its deletion mutants was observed by confocal microscopy in these primed cells compared with mock-transfected cells at 4 d in the absence of NGF (compare top row of Figure 8 with the rest of the figure). Morphometric quantification showed that NA-(third row) and LZC (last row)-transfected cells produce scarce neurites as occurred in mock-transfected cells cultured in the absence of NGF. On the other hand, NBD-(N-terminus to basic domain) (fourth row) and NLZ (fifth row)-transfected cells closely resemble the stimulatory effect of full length c-Fos (second row) on the morphometric parameters of primed cells (compare rows 4 and 5 with 3 and 6 of Figure 8).
Figure 8.
c-Fos deletion mutants sustain growth upon NGF withdrawal providing they contain a 20-aa basic domain. PC12 cells expressing the ER marker lip33-YFP and mock transfected (first row) or expressing c-Fos (second row) or the corresponding c-Fos deletion mutants as depicted on Fig. 7B (rows 3-6) were cultured 16 h in the presence of NGF. Then, medium was replaced by fresh medium in the absence of NGF and culturing continued up to 4 d. Cells were immunostained with anti c-Fos antibody (Santa Cruz Biotechnology; USA antibody 4 c-Fos antibody for rows 1 through 5 and antibody 125 for row 6) and examined by confocal microscopy for colocalization with lip33-YFP. Right, merging of these images. Morphometrically quantification was performed as in Figure 1. *p < 0.00001 for differences in neurite length between mock transfected cells and c-Fos- or NBD- or NLZ-expressing transfected cells. No significant differences (p > 0.1) were found between mock transfected cells and NA- or LZC-expressing cells. Bar, 10 μm.
Comparison of immunostaining of c-Fos or its mutants with lip33-YFP as an ER marker showed abundant immunostaining in the perikarya with no detectable nuclear immunostaining. In addition, when immunostaining for c-Fos or its mutants was merged with lip33-YFP fluorescence, abundant colocalization was found only for c-Fos and for the deletion mutants NBD and NLZ (Figure 8, right), mutants that also sustain neurite elongation. These results are in line with our hypothesis that c-Fos and its deletion mutants associate to the ER and activate phospholipid synthesis to sustain neurite elongation in cells primed to grow.
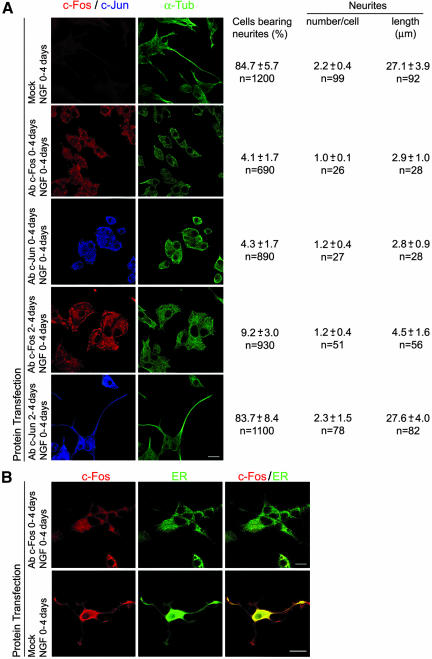
To obtain a better understanding of the c-Fos-mediated neurite elongation phenomenon, cells were fed a lipid-mediated delivery system (Zelphati et al., 2001) to introduce c-Fos or c-Jun antibody into the PC12 cells at different times of NGF treatment. As can be observed in Figure 9, both c-Fos and c-Jun antibodies completely blocked neurite formation after 4 d in culture when delivered to the cells at 0 h, together with NGF (Figure 9A, second and third rows) supporting that dimeric AP-1 c-Fos and c-Jun are required to trigger neuritogenesis. However, if the antibody was delivered to cells after 2 d in culture in the presence of NGF, c-Fos antibody blocked neurite extension and preformed neurites retracted (Figure 9A, fourth row), whereas c-Jun antibody was without effect (Figure 9A, bottom row). Furthermore, by immunocytochemical examination of cells containing the c-Fos antibody, c-Fos protein was not found colocalizing with the ER (Figure 9B, bottom row). These results support our hypothesis that nuclear-dimeric c-Fos is required to trigger neuritogenesis, whereas cytoplasmic c-Fos associates to the ER and sustains neurite elongation.
Figure 9.
Blocking c-Fos with a specific antibody blocks neuritogenesis, whereas blocking c-Jun only affects the triggering of neuritogenesis. (A) Cells cultured with NGF for 4 d were fed a lipid-mediated delivery system to transfect c-Fos or c-Jun antibody into PC12 cells at 0 h of NGF treatment (second and third rows) or after 2 d of culturing in the presence of NGF (forth and fifth rows). At 4 d in culture, cells were immunostained without addition of antibody for c-Fos or c-Jun (left) and with the addition of α-Tub antibody (right). The remaining protocol for immunostaining was as indicated for Figure 1. Morphometric quantification data obtained as in Fig. 1 are posted to the right of the corresponding row. (B) Cells mock transfected or transfected to deliver c-Fos antibody were single (top row) or double (bottom row) immunostained with c-Fos antibody (left) and the ER marker calnexin (middle). c-Fos colocalized with the ER marker only when cultures were performed in the absence of intracellular c-Fos antibody (right). Note that no additional c-Fos antibody was added during the immunocytochemical staining procedure of cells transfected with antibody to evidence the presence of antibody delivered during cell culture. Bar, 10 μm.
To further examine possible c-Fos/ER association in PC12 cells after 4 d with NGF, subcellular localization of c-Fos immunostaining was compared with immunostaining for α-Tub (Figure 10, top row), with phalloidin-stained actin (Figure 9, middle row), and with calnexin immunostaining for the ER (Figure 10, bottom row). Confocal microscopy showed, in addition to nuclear c-Fos, abundant c-Fos immunostaining in the cell perikarya (Figure 10, top row). In the growth cone, c-Fos immunostaining advances on the lamellipodia-localized actin filaments although at a much lower concentration than that observed for α-Tub-labeled neurites (Figure 10, middle row). In addition, abundant colocalization of c-Fos and ER immunostaining were observed in the cell body and in neurites (Figure 10, bottom row) consistent with results of Figure 8 showing colocalization of c-Fos immunostaining with lip33-YFP and with previous results in NIH 3T3 fibroblasts (Bussolino et al., 2001).
Only the Deletion Mutants That Sustain Growth in Primed Cells Activate Phospholipid Synthesis
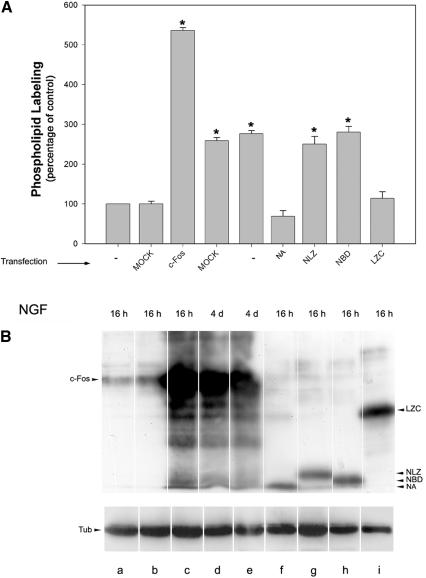
To confirm that c-Fos-dependent phospholipid synthesis activation is linked to the growth process, 32P-phospholipid synthesis was examined in cultures of transfected cells overexpressing c-Fos or expressing its deletion mutants and compared with mock-transfected cells. Figure 11A shows that full-length c-Fos and those deletion mutants that sustain growth are the only proteins capable of activating phospholipid synthesis in culture, i.e., those containing the 139-159 basic domain, activate phospholipid synthesis in cells primed to grow (Figure 11A, lanes g and h). Deletion mutants not containing the basic domain do not activate either phospholipid synthesis in vitro (Figure 7B) or in culture (Figure 11A, lanes f and i), and they cannot sustain neurite elongation in primed cells (Figure 8).
Figure 11.
32P-Phospholipid labeling activation in PC12 cells expressing c-Fos or c-Fos deletion mutants. (A) c-Fos or the corresponding c-Fos deletion mutants depicted on Fig. 7B or mock transfected PC12 cells were cultured for the indicated time in the presence of NGF, then medium was replaced by fresh medium and cultured up to 4 d. Cells were pulsed with [32P]orthophosphate 2 h before harvesting, and 32P-phospholipid labeling was determined. Results are the mean ± SEM of three independent experiments performed in triplicate. *p < 0.0001 as determined by Student's t test. (B) Ten micrograms of homogenate protein corresponding to each experimental condition depicted in A was subjected to SDS-PAGE and Western blotting with the corresponding anti-c-Fos polyclonal antibody as described in Figure 8 (top blots) or with anti-α-Tub antibody (bottom blots) to control protein loading and visualized by enhanced chemiluminescence. Results are from one representative experiment of three performed.
DISCUSSION
The c-Fos-containing heterodimer AP-1 was among the first inducible transcription factors identified. However, the physiological functions of c-Fos are still being unraveled (Curran and Morgan, 1987; Angel and Karin, 1991; Shaulian and Karin, 2002). c-Fos, as an AP-1 protein, was originally thought to be involved in transducing short-term mitogen-promoting signals into longer lived changes by regulating the expression of target genes important for proliferation. Later studies also implicated AP-1 dimers, and consequently c-Fos, in growth, differentiation, and apoptosis (Smeyne et al., 1993; Shaulian and Karin, 2002). However, the growing understanding of the multiple and complex genomic events in which c-Fos participates underscores our lack of knowledge of the precise role of AP-1, and more specifically, of c-Fos. Attempts have been made to elucidate the role of c-Fos through blocking its function by diverse in vivo and in culture experimental strategies that ranged from antisense constructs (Holt et al., 1986; Mercola et al., 1987, 1988; Nishikura and Murray, 1987; Edwards et al., 1988; Levi and Ozato, 1988; Ledwith et al., 1990), ribozyme-mediated c-fos mRNA cleavage (Scanlon et al., 1991), and nonfunctional heterodimers (Okuno et al., 1991) to c-fos(-/-) knockout mice (Johnson et al., 1992). Data from these experiments generally indicated that absence of c-Fos slows or inhibits cell growth (Johnson et al., 1992).
Results of the present study are not only consistent with the participation of c-Fos in triggering the genomic program for differentiation but also disclosed a participation of cytoplasmic c-Fos in sustaining growth by up-regulating the synthesis of phospholipids, the quantitatively most important components of all cell membranes. At the beginning of NGF-treatment of PC12 cells, it is critical that c-Fos reaches its nuclear localization to initiate the differentiation program: in cells in which nuclear import of AP-1-c-Fos was blocked at the initiation of NGF treatment, no differentiation occurred (Figure 1C). This differentiation activity of c-Fos is most probably related to its conventional transcription factor activity because the importin that the NLSP-peptide blocks, recognizes the bipartite nuclear localization signal formed in the AP-1 dimeric form of c-Fos rather than its monomeric form (Torgerson et al., 1998). In addition, an antibody blocking c-Jun also blocks the differentiation process. Furthermore, cytoplasmic c-Fos is unable to compensate its early genomic effect in cell differentiation. Likewise, because neurite extension occurs whenever cytoplasmic c-Fos expression occurs, blocking nuclear import of c-Fos once neurite growth has been initiated by priming cells with NGF has no significant effect on neurite elongation (Figure 1C). However, a decrease in cytoplasmic c-Fos by blocking its synthesis with ASO or by placing cells in a culture medium free of NGF, results in neurites that not only stop growing but also retract (Figure 3C). Furthermore, hampering the association of c-Fos to the ER with a specific antibody has an effect comparable to that of decreasing its expression in the cytoplasm (Figure 9).
In view of the above-mentioned considerations, it seems reasonable to expect that once a cell is primed to grow, in this case by NGF, growth, in terms of number and extension of neurites, will be significantly slowed down if any step of the c-Fos induction pathway is impaired, or accelerated if c-fos expression is exacerbated. Two lines of experiments support the dependence of growth on c-Fos levels: one in which cellular content of c-Fos is increased either by transfecting cells to constitutively express c-Fos (Figure 5B) or by inhibiting its degradation with A-Calp (Figure 4), which results in cells with increased growth; and the other in which growth decreases concomitantly with a decrease in c-Fos content due to treatment of cells with ASO (Figure 3). Results with cells expressing c-Fos deletion mutants confirm this hypothesis: deletion mutants lacking the leucine zipper domain required for heterodimerization and consequently for nuclear AP-1 localization retain both their phospholipid synthesis-activating capacity (Figure 7B) and their capacity to sustain growth in the absence of NGF in cells primed to grow, provided they contain the basic domain present amino acids 139-159 of full-length c-Fos (Figure 8). Moreover, these mutants associate to the ER, whereas those not containing this basic domain were not found associated with the ER after confocal microscopy examination (Figure 8). In view of the fact that c-Fos does not form homodimers (Angel and Karin, 1991) and that the sole addition of recombinant c-Fos or its deletion mutants containing the basic 139-159 aa domain can reproduce the activation of phospholipid synthesis in vitro, we also conclude that c-Fos associates with ER membranes and activates phospholipid synthesis in its monomeric form. This is further sustained by the finding that in cells primed to grow, blocking c-Fos with a specific antibody completely blocks neurite extension, whereas blocking c-Jun, an immediate early gene that can form homodimeric AP-1 transcription factors, has no effect on neurite elongation (Figure 9). However, these results do not discard the possibility that domains of the c-Fos protein other than the basic domain may be important in establishing, for example, the ER/c-Fos association. In addition, c-Fos-mediated genomic events such as the recently reported increase in the mRNA of the rate limiting enzyme in phosphatidylcholine formation, CTP:phosphocholine cytidylyl transferase β2 (Carter et al., 2003) may also contribute to phospholipid synthesis activation.
The phenomenon of cell growth is very complex and stringent control mechanisms must operate to coordinate the production of the different cellular components in synchrony both with cell cycle and with differentiation events. Having a few proteins participating in the growth process at different subcellular sites seems a reasonable mechanism to facilitate this coordination. The very precise control mechanisms that regulate c-fos expression may ensure that a cell will trigger its nuclear growth program and will support growth by activating the production of the cellular components it requires. It is important to note that c-Fos expression is not a required factor but an activator for phospholipid synthesis; abolition of c-Fos expression in vivo does not inhibit phospholipid synthesis; it only slows down the rate of production of these lipids (Guido et al., 1996; deArriba Zerpa et al., 1999; Bussolino et al., 1999). In accordance, c-fos(-/-) knockout mice are not lethal; these animals merely grow more slowly than their wild-type or heterozygous littermates (Johnson et al., 1992).
Elongation of neurites requires a constant supply of plasma membrane components and its insertion into the growing tip of the neurite. The common perception that phospholipids are synthesized in the cell body and that assembled membranes organized as vesicles are then distributed to their insertion sites has been revised after it was reported that significant phospholipid synthesis occurs in the growing axon, in addition to that occurring in the soma (Vance et al., 1991, 1994). In the present study, confocal microscopy shows colocalization of c-Fos and ER immunolabeling both in the cell body and in neurites. This points to a possible direct effect of c-Fos on the ER and opens the possibility that the diverse subcellular sites of phospholipid synthesis be regulated by c-Fos.
c-Fos activation of phospholipid synthesis is not peculiar to PC12 cells. It reflects a physiological regulatory phenomenon that has been observed in vivo in retinal ganglion (Guido et al., 1996) and photoreceptor cells (Bussolino et al., 1999) and in culture in NIH 3T3 fibroblasts (Bussolino et al., 2001). This cytoplasmic regulatory role of c-Fos is not the only AP-1-independent activity of an early response gene. c-Jun was shown to effectively protect PC12 cells from apoptosis by an unconventional mechanism that is also separable from its well-known AP-1 activity (Leppa et al., 2001). Future research is expected to reveal an increasingly complex but more coordinated mechanism through which all the players of cell proliferation, growth, differentiation, and death act in synchrony with one another. In addition to the multiple functions of a single induced protein, another key issue in cell growth is how signaling pathways common to many systems integrate signals from molecules having a wide spectrum of activities to deliver distinct and specific biological outcomes (Murphy et al., 2002; Vaudry et al., 2002). Improved understanding of the molecular mechanisms involved in cell growth regulation will open the possibility of beneficially intervening in selective normal and pathological growth processes such as those occurring in transformed cells.
Supplementary Material
Acknowledgments
We thank S. Deza and G. Schachner for excellent technical assistance, T. Santa Coloma (Instituto deInvestigaciones Bioquímicas Luis F. Leloir, Buenos Aires, Argentina) for support with confocal microscopy; and H. Maccioni for helpful discussions. The plasmids were generous gifts of T. Curran (p6hisFos and p6hisJun), John Blenis (pcDNA3Fos) and C.G. Giraudo (pEYFPlip33). This work was supported by the James S. McDonnell Foundation, Consejo Nacional de Investigaciones Científicas y Técnicas, Fondo para la Investigación Científica y Tecnológica, Agencia Córdoba Ciencia and SeCyT, Universidad Nacional de Córdoba. B.L.C. and M.E.G. are Career Investigators and D.F.B, M.L.R., and M.M.P. fellows of Consejo Nacional de Investigaciones Científicas y Técnicas. G.A.G. is a Carrillo-Oñativia fellow.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-09-0705. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-09-0705.
Online version is available at www.molbiolcell.org.
Abbreviations used: A-Calp, acetyl calpastatin; ASO, antisense oligonucleotide; NLSP, nuclear localization signal peptide; SO, sense oligonucleotide.
Online version of this article contains supplementary material.
References
- Abate, C., Luk, D., and Curran, T. (1991). Transcriptional regulation by Fos and Jun in vitro: interaction among multiple activator and regulatory domains. Mol. Cell. Biol. 11, 3624-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel, P., and Karin, M. (1991). The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim. Biophys. Acta 1072, 129-157. [DOI] [PubMed] [Google Scholar]
- Araki, W., and Wurtman, R.J. (1997). Control of membrane phosphatidylcholine biosynthesis by diacylglycerol levels in neuronal cells undergoing neurite outgrowth. Proc. Natl. Acad. Sci. USA 94, 11946-11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borioli, G.A., Caputto, B.L., and Maggio, B. (2001). c-Fos is surface active and interacts differentially with phospholipid monolayers. Biochem. Biophys. Res. Commun. 280, 9-13. [DOI] [PubMed] [Google Scholar]
- Bussolino, D.F., de Arriba Zerpa, G.A., Grabois, V.R., Conde, C.B., Guido, M.E., and Caputto, B.L. (1998). Light affects c-fos expression and phospholipid synthesis in both retinal ganglion cells and photoreceptor cells in an opposite way for each cell type. Mol. Brain Res. 58, 10-15. [DOI] [PubMed] [Google Scholar]
- Bussolino, D.F., Guido, M.E., Gil, G.A., Borioli, G.F., Renner, M.L., Grabois, V.R., Conde, C.B., and Caputto, B.L. (2001). c-Fos associates with the endoplasmic reticulum and activates phospholipid metabolism. FASEB J. 15, 556-558. [DOI] [PubMed] [Google Scholar]
- Caputto, B.L., and Guido, M.E. (2000). Immediate early gene expression within the visual system: light and circadian regulation in the retina and the suprachiasmatic nucleus. Neurochem. Res. 25, 153-162. [DOI] [PubMed] [Google Scholar]
- Carter, J.M., Waite, K.A., Campenot, R.B., Vance, J.E., and Vance, D.E. (2003). Enhanced expression and activation of CTP:phosphocholine cytidylyltransferase β2 during neurite outgrowth. J. Biol. Chem., 278, 44988-44994. [DOI] [PubMed] [Google Scholar]
- Curran, T., and Morgan, J.I. (1987). Memories of fos. Bioessays 7, 255-258. [DOI] [PubMed] [Google Scholar]
- de Arriba Zerpa, G.A., Guido, M.E., Bussolino D.F., Pasquaré, S.J., Castagnet, P.I., Giusto, N.M., and Caputto, B.L. (1999). Light exposure activates retina ganglion cell lysophosphatidic acid acyl transferase and phosphatidic acid phosphatase by a c-Fos dependent mechanism. J. Neurochem. 73, 1228-1235. [DOI] [PubMed] [Google Scholar]
- Edwards, S.A., Rundell, A.Y., and Adamson, E.D. (1988). Expression of c-fos antisense RNA inhibits the differentiation of F9 cells to parietal endoderm. Dev. Biol. 129, 91-102. [DOI] [PubMed] [Google Scholar]
- Giraudo, C.G., and Maccioni, H.J.F. (2003). Endoplasmic reticulum export of glycosyltransferases depends on interaction of cytoplasmic dibasic motif with Sar1. Mol. Biol. Cell. 14, 3753-3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg, M.E., Greene, L.A., and Ziff, E.B. (1985). Nerve growth factor and epidermal growth factor induce rapid transient changes in proto-oncogene transcription in PC12 cells. J. Biol. Chem. 260, 14101-14110. [PubMed] [Google Scholar]
- Greene, L.A., and Tischler, A.S. (1976). Establishment of a nonadrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. USA 73, 2424-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guido, M.E., and Caputto, B.L. (1990). Labeling of retina and optic tectum phospholipids in chickens exposed to light or dark. J. Neurochem. 55, 1855-1860. [DOI] [PubMed] [Google Scholar]
- Guido, M.E., de Arriba Zerpa, G.A., Bussolino, D.F., and Caputto, B.L. (1996). The immediate early gene c-fos regulates the synthesis of phospholipids but not of gangliosides. J. Neurosci. Res. 43, 93-98. [DOI] [PubMed] [Google Scholar]
- Hai, T., and Curran, T. (1991). Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc. Natl. Acad. Sci. USA 88, 3720-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt, J.T., Gopal, T.V., Moulton, A.D., and Nienhuis, A.W. (1986). Inducible production of c-fos antisense RNA inhibits 3T3 cell proliferation. Proc. Natl. Acad. Sci. USA 83, 4794-4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, P., and Dragunow, M. (1995). Induction of immediate-early genes and the control of neurotransmitter-regulated gene expression within the nervous system. Pharmacol. Rev. 47, 133-178. [PubMed] [Google Scholar]
- Johnson, R.S., Spiegelman, B.M., and Papaioannou, V. (1992). Pleiotropic effects of a null mutation in the c-fos proto-oncogene. Cell 71, 577-586. [DOI] [PubMed] [Google Scholar]
- Kovary, K., and Bravo, R. (1991). The JUN and FOS protein families are both required for cell cycle progression in fibroblasts. Mol. Cell. Biol. 11, 4466-4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovary, K., and Bravo, R. (1992). Existence of different Fos/Jun complexes during the G0-to G1 transition and during exponential growth in mouse fibroblasts: differential role of Fos proteins. Mol. Cell. Biol. 12, 5015-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijer, W., Shubert, D., and Verma, I.M. (1985). Induction of the proto-oncogene fos by nerve growth factor. Proc. Natl. Acad. Sci. USA 82, 7330-7334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemand, D., Spyrou, G., Yaniv, M., and Pfarr, C.M. (1997). Variations in Jun and Fos protein expression and the AP-1 activity in cycling, resting and stimulated fibroblasts. Oncogene 14, 819-830. [DOI] [PubMed] [Google Scholar]
- Ledwith, B.J., Manam, S., Kraynak, A.R., Nichols, W.W., and Bradley, M.O. (1990). Antisense-fos RNA causes partial reversion of the transformed phenotypes induced by the c-Ha-ras oncogene. Mol. Cell. Biol. 10, 1545-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppa, S., Eriksson, M., Saffrich, R., Ansorge, W., and Bohmann, D. (2001). Complex functions of AP-1 transcription factors in differentiation and survival of PC12 cells. Mol. Cell. Biol. 13, 4369-4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi, B.Z., and Ozato, K. (1988). Constitutive expression of c-fos antisense RNA blocks c-fos induction by interferon and by phorbol ester and reduces c-myc expression in F9 embryonal carcinoma cells. Genes Dev. 2, 554-566. [DOI] [PubMed] [Google Scholar]
- Lin, Y-Z., Yao, S.Y., Veach, R.A., Torgerson, T.R., and Hawiger, J. (1995). Inhibition of nuclear translocation of transcription factor NF-K B by a synthetic peptide containing a cell/membrane permeable motif and nuclear localization sequence. J. Biol. Chem. 270, 14255-14265. [DOI] [PubMed] [Google Scholar]
- Mercola, D., Rundell, A., Westwick, J., and Edwards, S.A. (1987). Antisense RNA to the c-fos gene: restoration of density-dependent growth arrest in a transformed cell line. Biochem. Biophys. Res. Commun. 147, 288-294. [DOI] [PubMed] [Google Scholar]
- Mercola, D., Westwick, J., Rundell, A.Y., Adamson, E.D., and Edwards, S.A. (1988). Analysis of a transformed cell line using antisense c-fos RNA. Gene. 72, 253-265. [DOI] [PubMed] [Google Scholar]
- Murphy, L.O., Smith, S., Chen, R.-H., Fingar, D.C., and Blenis, J. (2002). Molecular interpretation of ERK signal duration by immediate early gene products. Nat. Cell Biol. 4, 556-64. [DOI] [PubMed] [Google Scholar]
- Nakane, P.K. (1968). Simultaneous localization of multiple tissue antigens, using the peroxidase-labeled antibody method; a study on pituitary gland of the rat. Histochem. Cytochem. 16, 557-570. [DOI] [PubMed] [Google Scholar]
- Neri, L.M., Borgatti, P., Capitan, S., and Martelli, A. (1998). Nuclear diacylglycerol produced by phosphoinositide-specific Phospholipase C is responsible for nuclear translocation of Protein Kinase C-α. J. Biol. Chem., 45, 29738-29744. [DOI] [PubMed] [Google Scholar]
- Nishikura, K., and Murray, J.M. (1987). Antisense RNA of proto-oncogene c-fos blocks renewed growth of quiescent 3T3 cells. Mol. Cell. Biol. 7, 639-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno, H., Suzuki, T., Yoshida, T., Hashimoto, Y., Curran, T., and Iba, H. (1991). Inhibition of jun transformation by a mutated fos gene: design of an anti-oncogene. Oncogene 9, 1491-1497. [PubMed] [Google Scholar]
- Pariat, M., Salvat, C., Bébien, M., Brockly, F., Altieri, E., Carillo, S., Jariel-Encontre, I., and Piechaczyk, M. (2000). The sensitivity of c-Jun and c-Fos proteins to calpains depends on conformational determinants of the monomers and not on formation of dimers. Biochem. J. 345, 129-138. [PMC free article] [PubMed] [Google Scholar]
- Scanlon, K.J., Jiao, L., Funato, T., Wang, W., Tone, T., Rossi, J.J., and Kashani-Sabet, M. (1991). Ribozyme-mediated cleavage of c-fos mRNA reduces gene expression of DNA synthesis enzymes and metallothionein. Proc. Natl. Acad. Sci. USA 88, 10591-10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutze, M.P., Peterson, P.A., and Jackson, M.R. (1994). An N-terminal double-arginine motif maintains type II membrane proteins in the endoplasmic reticulum. EMBO J. 13, 1696-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaulian, E., and Karin, M. (2002). AP-1 as regulator of cell life and death. Nat. Cell Biol. 4, 131-136. [DOI] [PubMed] [Google Scholar]
- Smeyne, R.J., Vendrell, M., Hayward, M., Baker, S.J., Miao, G.G., Schilling, K., Robertson, L.M., Curran, T., and Morgan, J.L. (1993). Continuous c-fos expression precedes programmed cell death in vivo. Nature 363, 166-169. [DOI] [PubMed] [Google Scholar]
- Torgerson, T.R., Colosia, A., Donahue, P., Yao-Zhong, L., and Hawiger, J. (1998). Regulation of NF-KB, AP-1, N-FAR and STAT1 nuclear import in T lymphocytes by noninvasive delivery of peptide carrying the nuclear localization sequence of NF-KB p50. J. Immunol. 161, 6084-6092. [PubMed] [Google Scholar]
- Vance, J.E., Pan, D., Campenot, R.B., Bussière, M., and Vance, D.E. (1994). Evidence that the major membrane lipids, except cholesterol, are made in axons of cultured rat sympathetic neurons. J. Neurochem. 62, 329-337. [DOI] [PubMed] [Google Scholar]
- Vance, J.E., Pan, D., Vance, D.E., and Campenot, R.B. (1991). Biosynthesis of membrane lipids in rat axons. J. Cell Biol. 115, 1061-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry, D., Stork, P.J.S., Lazarovici, P., and Eiden, L.E. (2002). Signaling pathways for PC12 cell differentiation: making the right connections. Science 296, 1648-1649. [DOI] [PubMed] [Google Scholar]
- Zelphati, O., Wang, Y., Kitada, S., Reed, J.C., Felgner, P.L., and Corbeil, J. (2001). Intracellular delivery of proteins with a new lipid-mediated delivery system. J. Biol. Chem., 276, 35103-35110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.