Abstract
Objective
Thymosin beta 4 (Tβ4) is a peptide with 43 amino acids that is critical for repair and remodeling tissues on the skin, eye, heart, and neural system following injury. To fully realize its utility as a treatment for disease caused by injury, the authors constructed a cost-effective novel Tβ4 dimer and demonstrated that it was better able to accelerate tissue repair than native Tβ4.
Methods
A prokaryotic vector harboring two complete Tβ4 genes with a short linker was constructed and expressed in Escherichia coli. A pilot-scale fermentation (10 L) was performed to produce engineered bacteria and the Tβ4 dimer was purified by one-step hydrophobic interaction chromatography. The activities of the Tβ4 dimer to promote endothelial cell proliferation, migration, and sprouting were assessed by tetramethylbenzidine (methylthiazol tetrazolium), trans-well, scratch, and tube formation assays. The ability to accelerate dermal healing was assessed on rats.
Results
After fermentation, the Tβ4 dimer accounted for about 30% of all the bacteria proteins. The purity of the Tβ4 dimer reached 98% after hydrophobic interaction chromatography purification. An average of 562.4 mg/L Tβ4 dimer was acquired using a 10 L fermenter. In each assay, the dimeric Tβ4 exhibited enhanced activities compared with native Tβ4. Notably, the ability of the dimeric Tβ4 to promote cell migration was almost two times higher than that of Tβ4. The rate of dermal healing in the dimeric Tβ4-treated rats was approximately 1 day faster than with native Tβ4-treated rats.
Conclusion
The dimeric Tβ4 exhibited enhanced activity on wound healing than native Tβ4, and the purification process was simple and cost-effective. This data could be of significant benefit for the high pain and morbidity associated with chronic wounds disease. A better strategy to develop Tβ4 as a treatment for other diseases caused by injuries such as heart attack, neurotrophic keratitis, and multiple sclerosis was also described.
Keywords: thymosin beta 4, dimer, wound healing, tissue repair, peptide, genetic engineering
Introduction
Thymosin beta 4 (Tβ4) is a peptide with 43 amino acids and is the main component of fraction 5 of beta thymosin.1 It is present in all cells except red blood cells and in all body fluid.2,3 Tβ4 has been found to have considerable and broad biological activities, including promoting the migration of endothelial cells,4 accelerating angiogenesis,5,6 downregulating inflammatory responses,7,8 and inhibiting apoptosis and oxidative damage.9,10 Tβ4 also can organize the distribution of collagen by reducing the presence of myofibroblasts in the wound area.11 Angiogenesis is deemed as the most important mechanism for Tβ4-induced cutaneous wound healing and cardioprotection.12,13 Angiogenesis is the physiological process through which new blood vessels form from pre-existing vessels. It is vital for tissue repair as cell survival and regeneration depends on a sufficient supply of nutrients and oxygen. In endothelial cells, proliferation, migration, and then sprouting to become a vessel lumen are well-characterized stages of angiogenesis.14
Two independent randomized, double-blind clinical trials proved that a Tβ4 topical gel accelerates dermal healing approximately 1 month faster than a placebo in pressure ulcers (full thickness) and venous stasis ulcers patients.15,16 Some Stage IV wounds that had been present as long as 2 years only healed completely in the Tβ4 gel treatment group.17 RegeneRx Biopharmaceuticals, Inc. (Rockville, MD, USA) acquired an exclusive worldwide license for the use of Tβ4 in wound healing from the National Institutes of Health (Bethesda, MD, USA). The applications of Tβ4 in the treatment of other diseases caused by injury such as myocardial infarction or reperfusion injury,12,18,19 corneal injury,20 and brain traumatic or ischemia injuries21,22 have also entered into clinical stage in recent years.
Currently, Tβ4 products used in clinical and preclinical studies are chemically synthesized. Industrial-scale peptide synthesis is a costly, time-consuming, and highly polluting process especially for long peptides like Tβ4. On the other hand, the genetic engineering production of intact Tβ4 failed in several reports,23,24 which was attributed to the susceptibility of small peptides to proteolytic degradation.25 Protein tag fusion strategies and excess amino acids to resist degradation have been used23,24 to mitigate the susceptibility. However, the multiple purification steps and potential immunogenicity have not been cost-effective and satisfactory for therapeutic applications. Since the theoretical molecular weight (MW) of the Tβ4 dimer is over 9,500.0 Da, which satisfies the recombinant technology, and evidence that the dimerization of some cytokines or hormones strongly increase their activities and lifespan,25–27 it was hypothesized that the fusing of two Tβ4 molecules might confer an increase in its intrinsic activities and successful production using genetic engineering.
To prove this hypothesis, a prokaryotic vector harboring two entire Tβ4 genes with a small linker was constructed and expressed in Escherichia coli in the present study. After a large-scale cultivation of the engineered bacteria, the Tβ4 dimer (DTβ4) accounted for about 30% of all the proteins. The purity of DTβ4 reached 98% after a simple hydrophobic interaction chromatography (HIC) purification. An average of 562.4 mg/L DTβ4 was acquired using a 10 L fermenter. This dimer showed enhanced abilities to promote the proliferation, migration, and tube formation of the endothelial cells in vitro. Since the applications of Tβ4 in wound healing is more mature than in other diseases, and chronic cutaneous wounds are associated with significant morbidity especially in elderly, diabetes, and disability patients,28 the activities of DTβ4 to promote wounding healing were assessed in rats. The DTβ4 exhibited stronger biological activity on wound healing than the native Tβ4 monomer, which demonstrates the possibilities of clinical applications for the genetically engineered DTβ4. In the future, the authors hope to prove the potential uses of this molecule in the treatment of other diseases caused by injuries.
Supplementary materials
Materials and methods
Construction of DTβ4 expression vector
Two entire complementary DNA sequences of Tβ4 (UniProt: P62328) optimized with E. coli-preferred codons were synthesized by Bio Asia Diagnostics, Inc., (Shanghai, People’s Republic of China) with a small DNA sequence linker (GGTTCT) between them, and were constructed into prokaryotic expression plasmid pET22b(+) (Promega Corporation, Fitchburg, WI, USA) by NdeI and SalI restriction enzymes. The resulting expression plasmid was designated pET22b-DTβ4 and verified by agarose electrophoresis and DNA sequencing.
Expression of DTβ4 and large-scale cultivation of engineered bacteria
The pET22b-DTβ4 plasmid was transformed into E. coli BL21 (DE3; Promega) using the calcium chloride method. Successfully transformed BL21 clones were used for recombinant DTβ4 expression. Large-scale cultivation was performed using a 10 L fermenter (CT5-2; B. Braun Biotech International GmbH, Melsungen, Germany). Extended details are shown in Supplementary materials.
Purification of DTβ4
A 10% (weight/volume) bacteria suspension in 50 mmol/L tris(hydroxymethyl)aminomethane–hydrogen chloride with 100 mmol/L sodium chloride and 5 mmol/L ethylenediaminetetraacetic acid (buffer A, pH 8.0) was prepared for the purification. The cells were lysed by sonification using an ultrasonic processor (JY92-2D; Xinzhi Biotech Inc., Ningbo, People’s Republic of China). The cell debris was removed by centrifugation (12,000 rpm, 20 minutes, 4°C). Ammonium sulfate precipitation and HIC were used to purify DTβ4. The details are shown in Supplementary materials.
Basic quality determinations of DTβ4
Protein quantitation of the purified sample was determined by the standard Bradford method. The purity was determined by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and high-performance liquid chromatography (Alliance® HPLC; Waters Corporation, Milford MA, USA). The actual MW was determined by matrix-assisted laser desorption/ionization time-of-flight mass spectroscopy (Applied Biosystems® 4800 Plus; Life Technologies, Carlsbad CA, USA). Simultaneously, N-terminal sequencing was finished by ProtTech, Inc., (Phoenixville, PA, USA). The structure of DTβ4 was confirmed by Western blot using the Tβ4 monoclonal antibody (Abcam plc, Cambridge, UK).
Bioactivities of DTβ4 in vitro and in vivo
Cells and animals
Human umbilical vein endothelial cells (HUVEC) were maintained in Gibco® RPMI 1640 (Life Technologies) supplemented with Gibco® 10% fetal bovine serum (Life Technologies), 100 U/mL penicillin, and 100 μg/mL streptomycin (Thermo Fisher Scientific, Waltham, MA, USA). All cells were incubated at 37°C in 5% carbon dioxide. The cells used for all studies were taken from passage two. Sprague Dawley rats weighing about 220 g were purchased from Experimental Animal Center of Fourth Military Medical University (Xian, People’s Republic of China). All protocols were approved by the Committee for the Care and Use of Experimental Animals of the University.
The effect of DTβ4 on the proliferation of HUVEC
HUVEC were grown to 70% confluence in a 96-well plate (3,000 cells/well initially) and incubated at 37°C with DTβ4, Tβ4, or phosphate-buffered saline (PBS) in triplicate. The Tβ4 monomer was synthesized in the authors’ lab and used as the control. The final concentrations of 0.5, 1, 2, 4, and 8 μg/mL were applied. Forty-eight hours later, the wells were developed with tetramethylbenzidine (methylthiazol tetrazolium) (Sigma-Aldrich, St Louis, MO, USA), and the absorbance (490 nm) was determined by a microplate reader (ELx800; BioTek Instruments, Inc., Winooski, VT, USA).
The effect of DTβ4 on the migration of HUVEC
The effect of DTβ4 to promote the migration of HUVEC was tested by trans-well assay and scratch assay. Two concentrations were applied (1 μg/mL and 10 μg/mL). Extended details are shown in Supplementary materials.
The effect of DTβ4 on angiogenesis
The effect of DTβ4 to stimulate angiogenesis was evaluated by endothelial tube formation assay. Two concentrations were applied (1 μg/mL and 10 μg/mL). Extended details are shown in Supplementary materials.
The effects of DTβ4 on the wound healing in rats
DTβ4 hydrogel was prepared at three concentrations: 0.5 mg/mL, 0.25 mg/mL, and 0.125 mg/mL. Tβ4 hydrogel was prepared at 0.25 mg/mL and used as the control. The methods for wound repair evaluation in rats are described in Supplementary materials.
Statistics
All results are presented as mean ± standard deviation. For all of the biological activity validations in vitro and in vivo, a comparison of the difference in the mean findings between two groups was performed using a two-sided Student’s t-test with unequal variance. Statistical significance was assessed at the 0.05 level.
Results
Cloning and expression of DTβ4
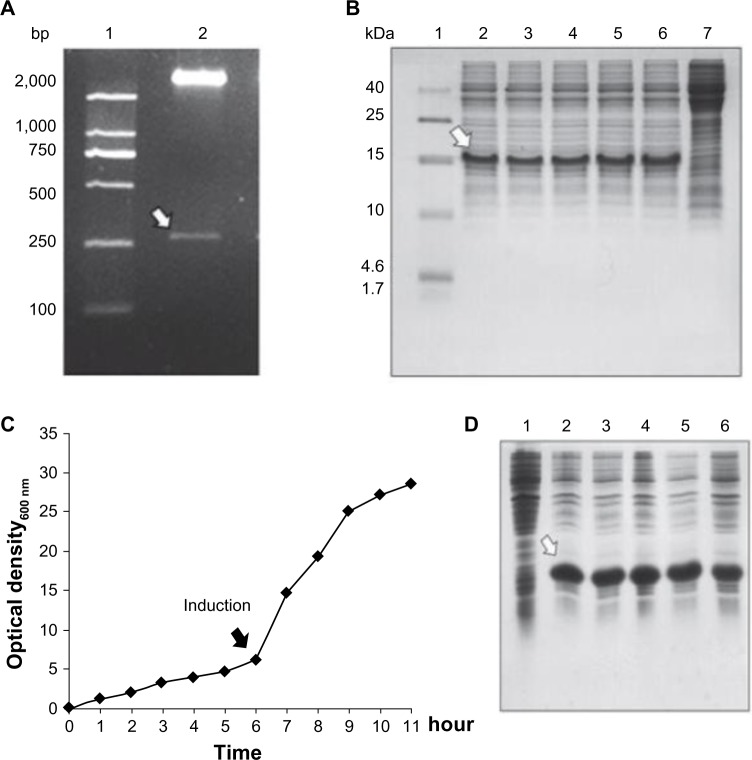
The DNA sequence encoding two identical Tβ4 with a short linker was synthesized and cloned into expression vector pET22b(+). The recombinant plasmid pET22b-DTβ4 was confirmed by NdeI/SalI digestion and sequencing. The results show a 267 bp fragment with correct sequence as expected (Figure 1A). Five colonies obtained after the transformation of BL21 with pET22b-DTβ4 were randomly picked to test the protein expression on a small scale. After isopropyl β-D-1-thiogalactopyranoside induction, a new protein appeared in each culture pellet (Figure 1B) and accounted for over 15% of all the bacteria proteins. The clone with the highest expression level of DTβ4 was selected for large-scale cultivation.
Figure 1.
Cloning, expression, and large-scale cultivation of dimeric thymosin beta 4 (DTβ4) engineered bacteria. (A) Two entire complementary DNA sequences of thymosin beta 4 (Tβ4) were constructed into a prokaryotic expression plasmid with a small DNA linker (GGTTCT). The results show a 267 bp fragment with correct sequence as expected (arrow). (B) Five colonies obtained after the transformation of Escherichia coli were randomly picked to test the protein expression on a small scale with sodium dodecyl sulfate polyacrylamide gel electrophoresis. After isopropyl β-D-1-thiogalactopyranoside (IPTG) induction, a new protein (arrow) appeared in each culture pellet and accounted for over 15% of all the bacteria proteins (1: molecular ladder; 2–6: protein expression of picked five clones; 7: protein expression without IPTG induction). (C) Bacterial growth curve of BL21/DTβ4 in a 10 L fermenter (the arrow indicates the induction time). (D) DTβ4 expression (arrow) during fermentation (1: DTβ4 expression without IPTG induction; 2–6: DTβ4 expression every hour after IPTG induction).
Abbreviation: DNA, deoxyribonucleic acid.
Large-scale cultivation of BL21/pET22b-DTβ4
In a 10 L fermenter, recombinant BL21 entered into log phase 6 hours after inoculation, and isopropyl β-D-1-thiogalactopyranoside induction was initiated at this time (Figure 1C). Optical density at 600 nm was about 6 at induction initiation. The cultivation was stopped when the growth entered into a stationary phase which was about 5 hours after induction. The expression of DTβ4 at this stage accounted for about 30% of all the bacteria proteins (Figure 1D) and optical density at 600 nm was >25. The fermentation was repeated three times, an average of 185.5 g cell paste was harvested each time. The parameters each time were similar, indicating that the condition of fermentation was appropriate for recombinant BL21 growth and DTβ4 expression.
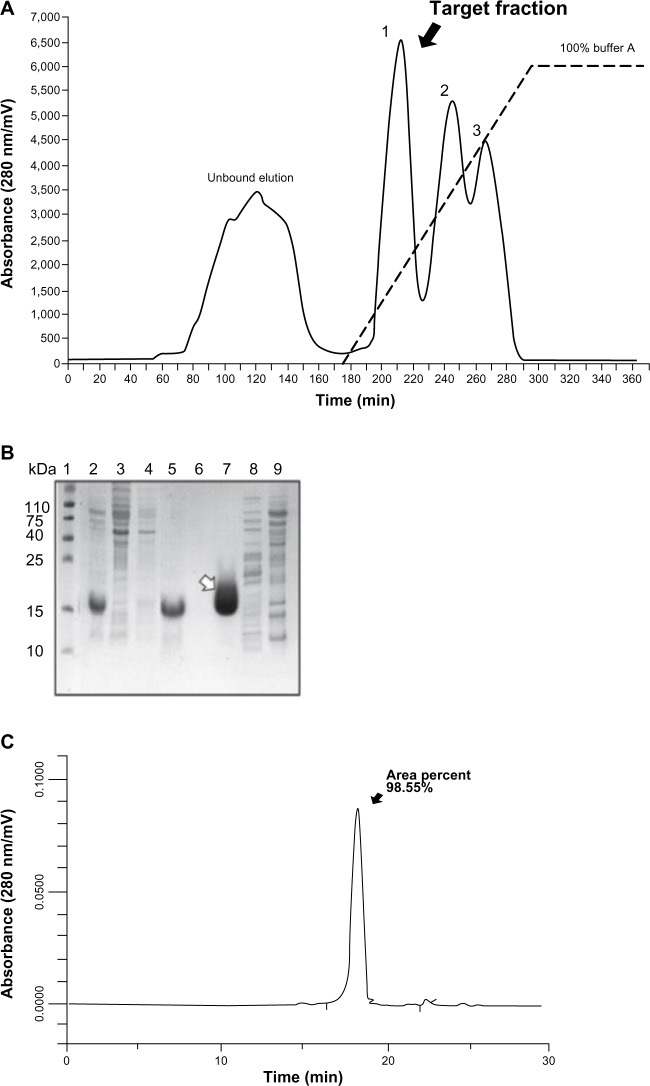
Purification and identification of DTβ4
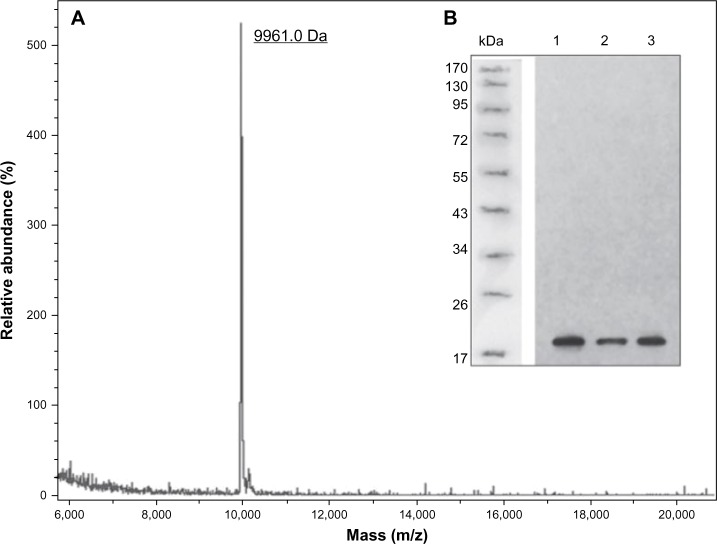
As DTβ4 has a good solubility, most of DTβ4 was in the buffered supernatant after sonification. Fifty percent ammonium sulfate precipitated most of the bacteria protein in the supernatant, which was a crude purification step. The resulting supernatant was further separated by HIC, and DTβ4 was detected in the first elution fraction (Figure 2A and B). The DTβ4 purity in this fraction was over 98% determined by high-performance liquid chromatography (Figure 2C). The actual MW of purified DTβ4 detected by mass spectroscopy was 9,961.0 Da, which was consistent with the theoretical MW despite the apparent MW of DTβ4 being about 15 kDa in SDS-PAGE (Figure 3A). The seven amino acids at the N-terminal of DTβ4 were methionine-serine-aspartic acid-lysine-proline-aspartic acid-methionine (MSDKPDM) as expected. DTβ4 was recognized by Tβ4 monoclonal antibody as shown by Western blot (Figure 3B). The whole purification procedure and identifications were repeated three times. Table 1 shows the yields of three batches of fermentation and purification. On average, 562.4 mg of DTβ4 could be obtained from 1 L of cultivation. The results of each replication were consistent, indicating that the purification procedure of DTβ4 was stable and reliable.
Figure 2.
Chromatogram of dimeric thymosin beta 4 (DTβ4) purification and high-performance liquid chromatography identification. (A) Chromatogram of hydrophobic interaction chromatography purification. DTβ4 was detected in the first elution fraction. (B) Sodium dodecyl sulfate polyacrylamide gel electrophoresis identification of each step during purification (1: molecular ladder; 2: DTβ4 expression after isopropyl β-D-1-thiogalactopyranoside induction; 3: without isopropyl β-D-1-thiogalactopyranoside; 4: the precipitation of 50% ammonium sulfate; 5: the supernatant of 50% ammonium sulfate [loading sample of hydrophobic interaction chromatography]; 6: unbounded elution; 7–9: elution fractions). The arrow indicates purified DTβ4 in the first elution. (C) High-performance liquid chromatography identification of the first elution.
Figure 3.
The molecular weight and structure validations of dimeric thymosin beta 4 (DTβ4) with matrix-assisted laser desorption/ionization time-of-flight mass spectroscopy and Western blot. (A) The actual molecular weight of purified DTβ4 detected by mass spectroscopy was 9,961.0 Da, which was consistent with its theoretical molecular weight. (B) DTβ4 was recognized by thymosin beta 4 (Tβ4) monoclonal antibody (1–3: samples of three batches).
Table 1.
The results of three batches of fermentation and purification of recombinant dimeric thymosin beta 4
| Batch | OD600 nm
|
Yield of bacteria (g/L) | Yield of DTβ4 (mg/L) | Purity (%) | Average
|
||
|---|---|---|---|---|---|---|---|
| Induction | Harvest | Yield (mg/L) | Purity (%) | ||||
| 1 | 6.20 | 25.1 | 32.56 | 570.35 | 98.46 | ||
| 2 | 6.14 | 27.9 | 34.44 | 552.75 | 98.55 | 562.4 | 98.38 |
| 3 | 6.21 | 29.1 | 33.88 | 564.35 | 98.13 | ||
Abbreviations: DTβ4, dimeric thymosin beta 4; OD600 nm, optical density at 600 nm.
Activities of DTβ4 and Tβ4 on HUVEC
DTβ4 as well as Tβ4 promoted the proliferation of HUVEC in a dose-dependent manner in the detected concentration range (Figure 4). At each concentration, DTβ4 exhibited significantly more promotion on HUVEC than Tβ4 (P<0.01). Compared with PBS, DTβ4 showed promotion at 0.5 µg/mL, whereas Tβ4 showed similar effects at 2 µg/mL.
Figure 4.
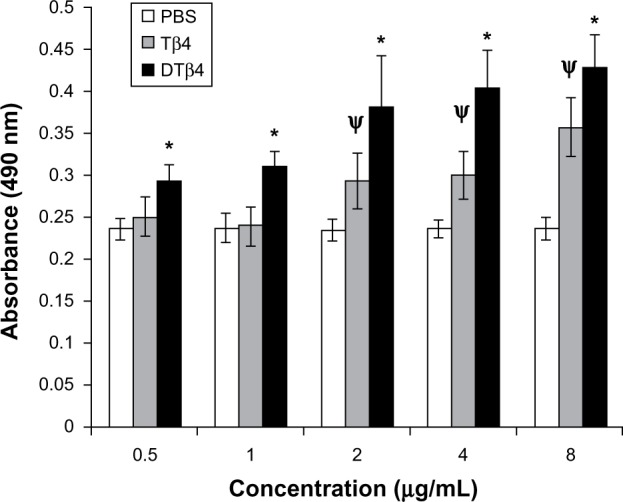
The proliferation of human umbilical vein endothelial cells under different concentrations of dimeric thymosin beta 4 (DTβ4), thymosin beta 4 (Tβ4), and phosphate-buffered saline (PBS). DTβ4 as well as Tβ4 promoted the proliferation of human umbilical vein endothelial cells in a dose-dependent manner in the 0–10 μg/ml concentration range. At each concentration, DTβ4 exhibited significant promotion compared with Tβ4. Compared with PBS, DTβ4 showed a promotion effect at 0.5 μg/mL, whereas Tβ4 showed a similar effect at 2 μg/mL.
Notes: *P<0.01 versus Tβ4; ψP<0.01 versus PBS.
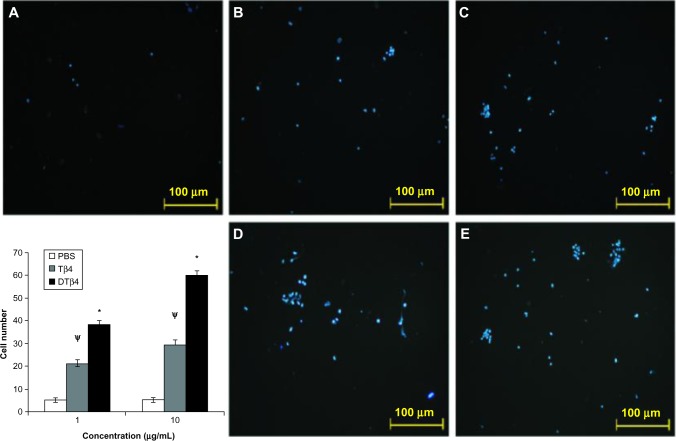
In the trans-well assay, DTβ4 exhibited more chemotactic effects on HUVEC (Figure 5). At 1 μg/mL, an average of 38.5 ± 1.5 cells migrated to the filter in response to DTβ4 compared with 21.3 ± 1.5 for Tβ4 (P<0.01). At 10 μg/mL, 59.8 ± 2.5 cells migrated to DTβ4 compared with 29.6 ± 1.8 for Tβ4 (P<0.01). The number of cells on the PBS treatment filter was 5.2 ± 1.1.
Figure 5.
Dimeric thymosin beta 4 (DTβ4) exhibited more chemotactic effects on human umbilical vein endothelial cells than thymosin beta 4 (Tβ4) and phosphate-buffered saline (PBS) in the trans-well assay. At 1 μg/mL, an average of 38.5 ± 1.5 cells migrated onto the filter in response to DTβ4 compared with 21.3 ± 1.5 in response to Tβ4. At 10 μg/mL, 59.8 ± 2.5 cells migrated in response to DTβ4 compared with 29.6 ± 1.8 in response to Tβ4. The number of cells on the PBS treatment filter was 5.2 ± 1.1. (A) PBS; (B) Tβ4 (1 μg/mL); (C) Tβ4 (10 μg/mL); (D) DTβ4 (1 μg/mL); (E) DTβ4 (10 μg/mL).
Notes: *P<0.01 versus Tβ4; ψP<0.01 versus PBS.
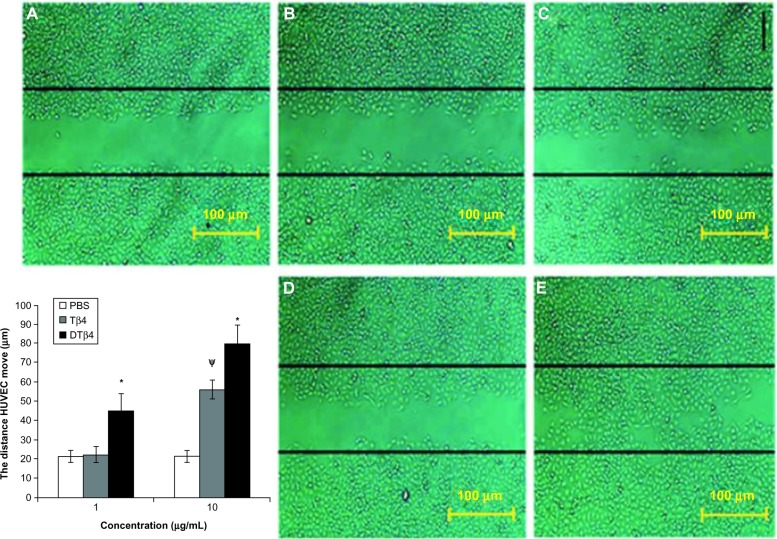
In the scratch assay, DTβ4 exhibited more promotions on the scratch closure than Tβ4. Figure 6 shows the representative images of scratched HUVEC after 12-hour treatments with DTβ4 or Tβ4. The average distance that HUVEC moved from the edge of the scratch toward the center with 1 μg/mL DTβ4 treatment was 45.32 ± 8.8 μm compared with 22.45 ± 4.2 μm for Tβ4 (P<0.01). After 10 μg/mL DTβ4 treatment, HUVEC moved 80.2 ± 9.8 compared with 56.5 ± 4.8 for Tβ4 (P<0.01). The distance HUVEC moved without any treatment was 21.38 ± 3.2 μm, which is a significantly smaller distance than that observed with 1 μg/mL DTβ4 but not 1 μg/mL Tβ4.
Figure 6.
Dimeric thymosin beta 4 (DTβ4) exhibited more promotions than thymosin beta 4 (Tβ4) and phosphate-buffered saline (PBS) in the scratch assay. After 12 hours of treatment, the average distance that human umbilical vein endothelial cells moved from the edge of the scratch towards the center with 1 μg/mL DTβ4 treatment was 45.32 ± 8.8 μm compared with 22.45 ± 4.2 μm for Tβ4. After 10 μg/mL DTβ4 treatment, the human umbilical vein endothelial cells moved 80.2 ± 9.8 μm compared with 56.5 ± 4.8 μm for Tβ4. The human umbilical vein endothelial cells without any treatment moved 21.38 ± 3.2 μm, which was a significantly smaller distance than that of 1 μg/mL DTβ4 but not Tβ4. (A) PBS; (B) Tβ4 (1 μg/mL); (C) Tβ4 (10 μg/mL); (D) DTβ4 (1 μg/mL); (E) DTβ4 (10 μg/mL).
Notes: *P<0.01 versus Tβ4; ψP<0.01 versus PBS.
Abbreviation: HUVEC, human umbilical vein endothelial cells.
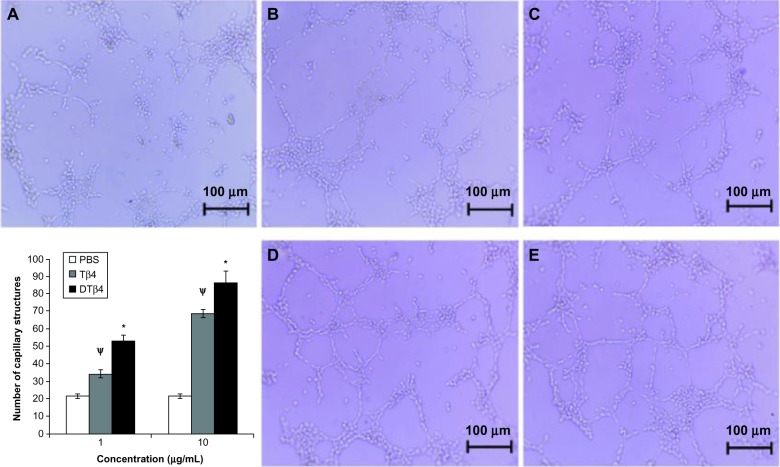
In the tube formation assay, the stimulation of DTβ4 on capillary-like structures caused a stronger formation of HUVEC than with Tβ4 (Figure 7). At 1 μg/mL, an average of 52.67 ± 3.2 capillary-like structures were observed in DTβ4 wells compared with 34.33 ± 2.3 for Tβ4 (P<0.01). At 10 μg/mL, the numbers increased to 86.34 ± 6.7 and 68.67 ± 2.4, respectively (P<0.01). The average number of structures observed in the PBS control was 21.67 ± 1.2, which is significantly lower than that of DTβ4 and Tβ4 (P<0.01).
Figure 7.
The stimulation of dimeric thymosin beta 4 (DTβ4) on the capillary-like structure formation of human umbilical vein endothelial cells was stronger than thymosin beta 4 (Tβ4) and phosphate-buffered saline (PBS). At 1 μg/mL, an average of 52.67 ± 3.2 capillary-like structures was observed in DTβ4 wells compared with 34.33 ± 2.3 for Tβ4. At 10 μg/mL, the average increased to 86.34 ± 6.7 and 68.67 ± 2.4, respectively. The average number of structures observed in PBS wells was 21.67 ± 1.2, which was significantly lower than that of DTβ4 and Tβ4 (P<0.01). (A) PBS; (B) Tβ4 (1 μg/mL); (C) Tβ4 (10 μg/mL); (D) DTβ4 (1 μg/mL); (E) DTβ4 (10 μg/mL).
Notes: *P<0.01 versus Tβ4; ψP<0.01 versus PBS.
All results on HUVEC indicate the multiple functions of DTβ4 and Tβ4. The ability of DTβ4 to promote endothelial cells growth, migration, and angiogenesis was stronger than Tβ4.
Promotions of wound repair in rats
During the study, no abnormalities were observed in the general condition and behavior of rats in all treatment cohorts, including asitia, nausea, diarrhea, cough, or weight loss, and no symptoms of allergic response such as hydroposia or localized rash occurred in animals receiving DTβ4 or Tβ4. The final autopsy did not show any pathological changes in various organs.
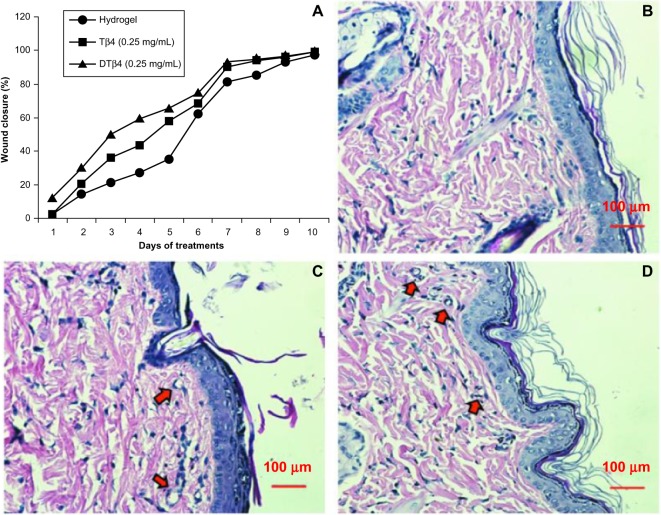
Wound repair evaluation proved either DTβ4 or Tβ4 increased wound healing, and a 50% wound closure was observed as early as day three after wound creation in the mid-dose DTβ4 (0.25 mg/mL) treatment cohort. The rates of 50% healing in mid-dose DTβ4-treated rats were approximately 3 days faster than the plain hydrogel and 1 day faster than Tβ4-treated rats. The healing of the plain hydrogel and Tβ4 eventually caught up with the mid-dose DTβ4 treatment at days nine and seven, respectively (Figure 8A). Table 2 shows the wound closure percentages of each cohort during the study. The final histological sections of each cohort show good re-epithelialization and angiogenesis in subcutaneous tissue (Figures 8B–D). Compared with plain hydrogel, both DTβ4 and Tβ4 resulted in more capillary growth. All of these data demonstrate that DTβ4 is most effective in the mid-dose range and accelerates wound healing, which resembles previous conclusions about Tβ4.17 At the same time, these data also suggest DTβ4 is more effective than Tβ4 in wound repair.
Figure 8.
Wound repair evaluation proved either dimeric thymosin beta 4 (DTβ4) or thymosin beta 4 (Tβ4) increased the healing of punch wounds in rats at 0.25 mg/mL. (A) The rates of 50% healing in mid-dose DTβ4-treated (0.25 mg/mL) rats were approximately 3 days faster than the plain hydrogel or 1 day faster than Tβ4-treated rats. The healing of the plain hydrogel and Tβ4-treated rats eventually caught up with the mid-dose DTβ4 treatment at days nine and seven, respectively. Histological sections of (B) plain hydrogel, (C) DTβ4, and (D) Tβ4 show good re-epithelialization and angiogenesis in subcutaneous tissues. Compared with plain hydrogel, DTβ4 and Tβ4 resulted in more capillary growth (arrows).
Table 2.
Wound closure percentage during 10 days treatment with dimeric thymosin beta 4 or thymosin beta 4 hydrogel in normal rats
| Cohorts (n=8) | Days
|
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Plain hydrogel | 2.3 ± 1.4 | 14.7 ± 4.1 | 21.5 ± 14.4 | 27.4 ± 6.8 | 35.3 ± 10.8 |
| Tβ4 (0.25 mg/mL) | 2.5 ± 1.8 | 20.7 ± 2.2 | 36.0 ± 13.9* | 43.2 ± 8.2** | 57.8 ± 10.7** |
| DTβ4 (0.125 mg/mL) | 7.6 ± 2.1 | 24.4 ± 3.8* | 40.3 ± 19.3* | 50.4 ± 9.5** | 58.3 ± 11.8** |
| DTβ4 (0.25 mg/mL) | 12.3 ± 3.6* | 30.2 ± 2.8**,ψ | 50.0 ± 18.0**,ψ | 59.2 ± 11.8**,ψ | 65.5 ± 13.3**,ψ |
| DTβ4 (0.5 mg/mL) | 12.6 ± 1.7* | 27.5 ± 1.6* | 46.3 ± 8.9** | 52.4 ± 13.8** | 60.5 ± 11.3** |
|
Days
|
|||||
| 6 | 7 | 8 | 9 | 10 | |
|
| |||||
| Plain hydrogel | 62.3 ± 6.2 | 81.0 ± 5.2 | 85.3 ± 4.2 | 93.1 ± 2.2 | 97.0 ± 2.1 |
| Tβ4 (0.25 mg/mL) | 68.4 ± 5.3* | 90.0 ± 6.0** | 93.6 ± 3.6* | 96.1 ± 1.5 | 98.3 ± 2.7 |
| DTβ4 (0.125 mg/mL) | 70.4 ± 2.6* | 90.8 ± 4.3** | 91.3 ± 2.7* | 93.2 ± 4.3 | 96.8 ± 2.1 |
| DTβ4 (0.25 mg/mL) | 74.6 ± 7.2**,ψ | 92.8 ± 3.5** | 94.7 ± 4.1* | 96.4 ± 2.9 | 99.0 ± 1.5 |
| DTβ4 (0.5 mg/mL) | 71.2 ± 6.4* | 88.0 ± 6.7* | 91.3 ± 3.8* | 94.0 ± 4.2 | 96.5 ± 3.3 |
Notes: Data are presented as mean ± standard deviation and were analyzed by student’s t-test.
P<0.05 versus plain hydrogel;
P<0.01 versus plain hydrogel;
P<0.05 versus Tβ4.
Abbreviations: DTβ4, dimeric thymosin beta 4; Tβ4, thymosin beta 4.
Discussion
As the genetic engineering production of intact Tβ4 failed, and protein tag fusion strategies and excess amino acids to resist degradation23,24 were complicated in the purification process and have potential immunogenicity, better methods for recombination production of Tβ4 are required. The recombined DTβ4 does not introduce a foreign sequence, exhibits a more enhanced activity than the Tβ4 monomer, and has a cheap, simple, and time-saving production process. Therefore, recombinant DTβ4 is a superior means to produce Tβ4 for medicine applications.
The simplicity of the purification process of DTβ4 is attributed to the hydrophilicity and good solubility of Tβ4. Ammonium sulfate precipitation and HIC produced a >98% purity of DTβ4. There were no dialysis and buffer changes between the purification steps. After triplicate pilot-scale fermentation (10 L) and purification steps, an average yield of 560 mg/L was obtained. The data of DTβ4 fermentation and purification indicate that the recombinant DTβ4 is a cost-effective means to enable commercial enterprises to produce it on a large scale.
In this study, the apparent MW of DTβ4 in SDS-PAGE was not consistent with its theoretical value. This deviation was reproducible. As the MW determined by mass spectroscopy was correct and the monoclonal antibody could recognize DTβ4, it is speculated that this is due to the properties of the protein itself. In fact, the MW deviations of native Tβ4 and DTβ4 were found in SDS-PAGE. Tβ4 is a highly hydrophilic protein, but most amino acids are polar proteins, which may result in a less negative charge after SDS treatment. In this situation, mobility would decrease. Further research is required to determine if there are other reasons for this discrepancy, for example, structure change.
Understanding why a dimeric protein has enhanced activity is always based on the study of the original monomer. For example, the reason recombinant erythropoietin (Epo) dimer has enhanced erythropoietic activity is speculated to be Epo–Epo fusion bridges, where two adjacent Epo receptors trigger the active state of Epo receptor.26,29,30 However, the exact mechanism of Tβ4 is still ambiguous, especially when the multiple activities and several active sites located on the molecule are considered.31 The widely accepted theory is that Tβ4 binds to/releases the G-actin monomer with its actin-binding site (amino acids 17–22) and coordinates F-actin depolymerization/polymerization to promote cell motility.32 Other studies insist Tβ4 functions through receptors such as the adenosine receptors.33 Tβ4 action is also believed to activate protein kinase B in an integrin-linked kinase-dependent manner.18 Various results on the mechanism of Tβ4 actions suggest it exerts functions via different mechanisms in different situations. In such a case, it is hard to speculate the exact reasons for the enhanced activity of DTβ4. Even though the mechanism is not clear, it is a fact that the dimerization of some cytokines or hormones increased their activities and lifespan. Thus, the construction of dimers or multimers represents a new method for the genetic engineering production of many small bioactive peptides.
In pre-experiments, it was found that Tβ4 and DTβ4 exhibited the highest activity at a relatively low concentration (10 µg/mL). When the concentration was over 10 µg/mL, the activities of Tβ4 and DTβ4 decreased greatly, and disappeared completely at 100 µg/mL. In rats, DTβ4 was found to be more effective at a mid dose (0.25 mg/mL) than at a high dose (0.5 mg/mL). In previous preclinical and clinical studies on dermal healing, Tβ4 has the highest activities at mid doses (0.2 mg/mL or 0.3 mg/mL) too.17 It is not clear why the lower and higher doses of Tβ4 and DTβ4 were less effective. It could be related to the bell curve of activity associated with many receptor-mediated biological responses.34 Such bioactive molecules can have a narrow effective dose range and differing responses seen at different concentrations are common.35
Apart from the activities to promote repair of various tissues, Tβ4 stimulates significant outgrowth from quiescent adult epicardial explants, restoring pluripotency and triggering differentiation of fibroblasts, smooth muscle cells, and endothelial cells.12 This suggests its applications in regenerative medicine. Regenerative medicine is a new field that uses stem cells to regenerate biological tissues and improve tissue functions.36 To help the engineered tissue survive and maintain function, some angiogenic molecules such as basic fibroblast growth factor and vascular endothelial growth factor are incorporated into a protein or peptide scaffold.36,37 Tβ4 or DTβ4 should be a better alternative for basic fibroblast growth factor or vascular endothelial growth factor considering the multiple activities of Tβ4. In addition, it is vital to establish the side effect and toxicity profile when testing new pharmacological agents. From the clinical trials, monomer Tβ4 was safe and well tolerated when applied topically to the skin or administered intravenously.15,38 However, given the previously described metastasis promotion of Tβ4 on some cancer cells and the upregulation in certain types of malignancy,39 it will be essential to establish in early trials that Tβ4 does not induce cancerous growth. This cannot be ignored either in the studies of DTβ4 as its ability to promote cell motility is even stronger. Another problem faced by Tβ4 application in wounding healing is that the response of the patients is inconsistent. Ways to identify Tβ4 healers and non-healers are required in the future. This should be considered in DTβ4 applications too. Briefly, any problems occurring in the clinical studies of Tβ4 should be paid attention to in DTβ4 studies.
Conclusion
Taken together, these data indicate that the recombinant DTβ4 represents a cost-effective means to produce Tβ4 compared with chemical synthesis. The resulting DTβ4 exhibited a more enhanced activity than the Tβ4 monomer in the promotion of endothelial cell proliferation, migration, capillary formation, and dermal wound repair. It could be of significant medical, economic, and social benefit given the extensive applications of Tβ4 in the diseases caused by injury. At the same time, the genetic engineering construction of the dimer represents a new method for the production of many small bioactive peptides.
Materials and methods
Expression of dimeric Tβ4 (DTβ4) and large-scale cultivation of engineered bacteria
The pET22b-DTβ4 plasmid was transformed into Escherichia coli BL21 (DE3; Promega Corporation, Fitchburg, WI, USA) using the calcium chloride method. Five BL21 clones successfully transformed by pET22b-DTβ4 were picked for recombinant DTβ4 expression. The initial expression was performed in lysogeny broth supplemented with ampicillin (100 mg/L) at 200 mL scale (37°C, 200 rpm). A final concentration of 0.01 mmol/L isopropyl β-D-1-thiogalactopyranoside (Sigma-Aldrich, St Louis MO, USA) was used for induction when the optical density at 600 nm reached 0.4–0.5. After 5 hours induction, 10 μg bacteria pastes were boiled and separated by 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis. The expression of the target protein was evaluated by gel documentation system (GDS-8000; UVP, Inc, Upland CA, USA). The clone which had the highest DTβ4 expression was used for large-scale cultivation.
Large-scale cultivation was performed using a 10 L fermenter (CT5-2; B. Braun Biotech International GmbH, Melsungen, Germany) with 8.5 L optimized 2YT medium at 500 rpm, air gassing (dissolved oxygen >30%), and pH 7.0. An initial overnight pre-culture of the selected clone was 5 mL. After two successional inoculations, the pre-culture was amplified to 400 mL and optical density at 600 nm was >2. The amplified pre-culture was then inoculated into the fermenter. After 6 hours growth, the optical density at 600 nm reached 6 and induction was initiated by 0.2 mmol/L isopropyl β-D-1-thiogalactopyranoside. The cultivation was sampled every hour and the expression of DTβ4 was examined by sodium dodecyl sulfate polyacrylamide gel electrophoresis. All bacteria were harvested 5 hours after induction by centrifugation.
Purification of DTβ4
A 10% (weight/volume) bacteria suspension in 50 mmol/L tris(hydroxymethyl)aminomethane–hydrogen chloride with 100 mmol/L sodium chloride and 5 mmol/L ethylenediaminetetraacetic acid (buffer A, pH 8.0) was prepared for the purification of DTβ4. The cells were lysed by sonification using an ultrasonic processor (JY92-2D; Xinzhi Biotech Co., Ningbo, People’s Republic of China). The cell debris was removed by centrifugation (12,000 rpm, 20 minutes, 4°C). Grinded ammonium sulfate was added to the cell-free extract to 50% saturation. After precipitation at 4°C overnight, the supernatant was collected and loaded to a hydrophobic interaction chromatography column (Phenyl Sepharose™ 6 Fast Flow; GE Healthcare Bio-Sciences AB, Uppsala, Sweden) using a protein purification system (AKTApurifer 10 plus; GE Healthcare). The column was equilibrated by buffer A supplemented with 50% ammonium sulfate. The target protein was eluted with a linear gradient of ammonium sulfate from 50% to 0%. Fraction containing target protein was collected and dialyzed into 20 mmol/L phosphate-buffered saline (pH 7.4) or normal saline according for later use.
Bioactivities of DTβ4 in vitro and in vivo
The effect of DTβ4 on the migration of human umbilical vein endothelial cells (HUVEC)
The effect of DTβ4 to promote the migration of HUVEC was tested by trans-well assay and scratch assay. Each assay was repeated at least three times. For trans-well assay, HUVEC (3 × 104/well) were plated evenly in upper chambers of the Transwell® plates (Corning Incorporated Tewksbury, MA, USA) and incubated at 37°C for 4 hours. DTβ4 or Tβ4 was applied to the bottom chambers at 1 μg/mL and 10 μg/mL. Another 4 hours later, the filter of each well was fixed with 4% paraformaldehyde and stained with Invitrogen® 4, 6-diamidino-2-phenylindole (Life Technologies, Carlsbad CA, USA). The cells on each filter were counted on a fluorescence microscope (Axioskop 2 plus; Carl Zeiss AG, Oberkochen, Germany).
For the scratch assay, HUVEC were grown to a confluent monolayer in 60 mm dishes (Corning). Similar size straight lines were created by scraping the cell monolayer with a pipette tip in all dishes. The cells were washed with phosphate-buffered saline and then incubated with serum-free medium supplemented with 1 μg/mL and 10 μg/mL of DTβ4 or Tβ4 for 12 hours. The images of each sample were collected on a phase-contrast microscope (CX31; Olympus Corporation, Tokyo, Japan) every 2 hours, matching the reference point every time. The scratch closure was measured by quantifying the total distance that the cells moved from the edge of the scratch toward the center of the scratch using ImageJ (National Institutes of Health, Bethesda, MD, USA).
The effect of DTβ4 on angiogenesis
The effect of DTβ4 to stimulate angiogenesis was evaluated by endothelial tube formation assay as follows. The wells of a 96-well plate were coated with Matrigel™ (Cultrex® BME; BD Biosciences, San Jose, CA, USA) according to the manufacturer. HUVEC were plated into the coated wells (2 × 104 cells/well) after the Matrigel jellied. DTβ4 or Tβ4 were applied to the cells at 1 μg/mL and 10 μg/mL in triplicate. Eight hours later, the images were collected using a phase-contrast microscope (CX31). The capillary-like structures featured as a central lumen surrounded by endothelial cells were counted in each view using Image J.
The effects of DTβ4 on the wound healing in rats
DTβ4 hydrogel were prepared at three concentrations: 0.5 mg/mL, 0.25 mg/mL, and 0.125 mg/mL. Tβ4 hydrogel was prepared at 0.25 mg/mL and used as the control. Two full-thickness 8 mm punch wounds were made on the dorsal surface of each rat as previously described.1 Eight rats (four females and four males) were used for each data point. The hydrogels were applied topically using cotton Q-tip swabs two times every day (0.5 mL/wound/time) for 10 days. The rats were bandaged with petroleum jelly-impregnated gauze. Control animals received plain hydrogel alone. The general condition, behavior, and wound repair of all animals were checked daily. For wound repair evaluation, a transparent acetate sheet (Sigma) was placed on the wound, its perimeter was traced and then sheared along the wound tracing, and the sheared part was weighed. Data are expressed as the percentage of wound closure (100 – weight of sheared acetate/weight of sheared acetate of the initial day × 100). Wound tissues were collected after euthanasia at day eleven and fixed in 10% buffered formalin (Sigma). The tissues were then sectioned from the middle of the wounds, stained with hematoxylin–eosin, and histopathologic interpretation of each section was performed by a pathologist blinded to treatment. Meanwhile, all animals underwent autopsy for examination.
Reference
- 1.Malinda KM, Sidhu GS, Mani H, et al. Thymosin beta4 accelerates wound healing. J Invest Dermatol. 1999;113(3):364–368. doi: 10.1046/j.1523-1747.1999.00708.x. [DOI] [PubMed] [Google Scholar]
Acknowledgments
The work in this study was supported by the National Science and Technology Major Project of China (New Drug Innovation Number: 2011ZXJ09104-01B) and National Natural Science Foundation of China (Number: 81272517). The authors thank Dr Ming-Gao Zhao (School of Pharmacy, Fourth Military Medical University, Xian, People’s Republic of China) for the wound repair experiment in rats. The authors also thank Mr Christopher Lanza (Santa Clara University, Santa Clara, CA, USA) and Mr Mat Caseres (University of Kansas, Kansas City, KS, USA) for critical reading and English editing of the manuscript.
Footnotes
Disclosure
Fourth Military Medical University has acquired an invention patent on behalf of Dr Ying-Qi Zhang on the construction and purification of recombinant DTβ4 presented in this study (ZL 200810232709.9). The other authors disclose no conflicts of interest in this work.
References
- 1.Goldstein AL, Slater FD, White A. Preparation, assay, and partial purification of a thymic lymphocytopoietic factor (thymosin) Proc Natl Acad Sci U S A. 1966;56(3):1010–1017. doi: 10.1073/pnas.56.3.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badamchian M, Damavandy AA, Damavandy H, Wadhwa SD, Katz B, Goldstein AL. Identification and quantification of thymosin beta4 in human saliva and tears. Ann N Y Acad Sci. 2007;1112:458–465. doi: 10.1196/annals.1415.046. [DOI] [PubMed] [Google Scholar]
- 3.Urso E, Le Pera M, Bossio S, Sprovieri T, Qualtieri A. Quantification of thymosin beta(4) in human cerebrospinal fluid using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal Biochem. 2010;402(1):13–19. doi: 10.1016/j.ab.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 4.Malinda KM, Goldstein AL, Kleinman HK. Thymosin beta 4 stimulates directional migration of human umbilical vein endothelial cells. FASEB J. 1997;11(6):474–481. doi: 10.1096/fasebj.11.6.9194528. [DOI] [PubMed] [Google Scholar]
- 5.Grant DS, Kinsella JL, Kibbey MC, et al. Matrigel induces thymosin beta 4 gene in differentiating endothelial cells. J Cell Sci. 1995;108(Pt 12):3685–3694. doi: 10.1242/jcs.108.12.3685. [DOI] [PubMed] [Google Scholar]
- 6.Koutrafouri V, Leondiadis L, Avgoustakis K, et al. Effect of thymosin peptides on the chick chorioallantoic membrane angiogenesis model. Biochim Biophys Acta. 2001;1568(1):60–66. doi: 10.1016/s0304-4165(01)00200-8. [DOI] [PubMed] [Google Scholar]
- 7.Qiu P, Wheater MK, Qiu Y, Sosne G. Thymosin beta4 inhibits TNF-alpha-induced NF-kappaB activation, IL-8 expression, and the sensitizing effects by its partners PINCH-1 and ILK. FASEB J. 2011;25(6):1815–1826. doi: 10.1096/fj.10-167940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young JD, Lawrence AJ, MacLean AG, et al. Thymosin beta 4 sulfoxide is an anti-inflammatory agent generated by monocytes in the presence of glucocorticoids. Nat Med. 1999;5(12):1424–1427. doi: 10.1038/71002. [DOI] [PubMed] [Google Scholar]
- 9.Ho JH, Tseng KC, Ma WH, Chen KH, Lee OK, Su Y. Thymosin beta-4 upregulates anti-oxidative enzymes and protects human cornea epithelial cells against oxidative damage. Br J Ophthalmol. 2008;92(7):992–997. doi: 10.1136/bjo.2007.136747. [DOI] [PubMed] [Google Scholar]
- 10.Sosne G, Siddiqi A, Kurpakus-Wheater M. Thymosin-beta4 inhibits corneal epithelial cell apoptosis after ethanol exposure in vitro. Invest Ophthalmol Vis Sci. 2004;45(4):1095–1100. doi: 10.1167/iovs.03-1002. [DOI] [PubMed] [Google Scholar]
- 11.Ehrlich HP, Hazard SW., 3rd Thymosin beta4 enhances repair by organizing connective tissue and preventing the appearance of myofibroblasts. Ann N Y Acad Sci. 2010;1194:118–124. doi: 10.1111/j.1749-6632.2010.05483.x. [DOI] [PubMed] [Google Scholar]
- 12.Smart N, Risebro CA, Melville AA, et al. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445(7124):177–182. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- 13.Schreml S, Szeimies RM, Prantl L, Landthaler M, Babilas P. Wound healing in the 21st century. J Am Acad Dermatol. 2010;63(5):866–881. doi: 10.1016/j.jaad.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 14.Flamme I, Frölich T, Risau W. Molecular mechanisms of vasculogenesis and embryonic angiogenesis. J Cell Physiol. 1997;173(2):206–210. doi: 10.1002/(SICI)1097-4652(199711)173:2<206::AID-JCP22>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 15.Crockford D, Turjman N, Allan C, Angel J. Thymosin beta4: structure, function, and biological properties supporting current and future clinical applications. Ann N Y Acad Sci. 2010;1194:179–189. doi: 10.1111/j.1749-6632.2010.05492.x. [DOI] [PubMed] [Google Scholar]
- 16.Guarnera G, DeRosa A, Camerini R. The effect of thymosin treatment of venous ulcers. Ann N Y Acad Sci. 2010;1194:207–212. doi: 10.1111/j.1749-6632.2010.05490.x. [DOI] [PubMed] [Google Scholar]
- 17.Treadwell T, Kleinman HK, Crockford D, Hardy MA, Guarnera GT, Goldstein AL. The regenerative peptide thymosin β4 accelerates the rate of dermal healing in preclinical animal models and in patients. Ann N Y Acad Sci. 2012;1270:37–44. doi: 10.1111/j.1749-6632.2012.06717.x. [DOI] [PubMed] [Google Scholar]
- 18.Bock-Marquette I, Saxena A, White MD, Dimaio JM, Srivastava D. Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature. 2004;432(7016):466–472. doi: 10.1038/nature03000. [DOI] [PubMed] [Google Scholar]
- 19.Rossdeutsch A, Smart N, Riley PR. Thymosin beta4 and Ac-SDKP: tools to mend a broken heart. J Mol Med (Berl) 2008;86(1):29–35. doi: 10.1007/s00109-007-0243-9. [DOI] [PubMed] [Google Scholar]
- 20.Sosne G, Qiu P, Kurpakus-Wheater M, Matthew H. Thymosin beta4 and corneal wound healing: visions of the future. Ann N Y Acad Sci. 2010;1194:190–198. doi: 10.1111/j.1749-6632.2010.05472.x. [DOI] [PubMed] [Google Scholar]
- 21.Morris DC, Chopp M, Zhang L, Lu M, Zhang ZG. Thymosin beta4 improves functional neurological outcome in a rat model of embolic stroke. Neuroscience. 2010;169(2):674–682. doi: 10.1016/j.neuroscience.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, Zhang ZG, Morris D, et al. Neurological functional recovery after thymosin beta4 treatment in mice with experimental auto encephalomyelitis. Neuroscience. 2009;164(4):1887–1893. doi: 10.1016/j.neuroscience.2009.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Che YK, Yang H, Lu F, Pu Q, Li RD, Zhao ZL. Cloning expression in E.coli and biological activity of human thymosin beta(4) Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai) 2002;34(4):502–505. [PubMed] [Google Scholar]
- 24.Li X, Zheng L, Peng F, et al. Recombinant thymosin beta 4 can promote full-thickness cutaneous wound healing. Protein Expr Purif. 2007;56(2):229–236. doi: 10.1016/j.pep.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Bommarius B, Jenssen H, Elliott M, et al. Cost-effective expression and purification of antimicrobial and host defense peptides in Escherichia coli. Peptides. 2010;31(11):1957–1965. doi: 10.1016/j.peptides.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dalle B, Henri A, Rouyer-Fessard P, et al. Dimeric erythropoietin fusion protein with enhanced erythropoietic activity in vitro and in vivo. Blood. 2001;97(12):3776–3782. doi: 10.1182/blood.v97.12.3776. [DOI] [PubMed] [Google Scholar]
- 27.Li W, Song L, Wu S, et al. Expression, purification and characterization of a novel soluble human thymosin alpha1 concatemer exhibited a stronger stimulation on mice lymphocytes proliferation and higher anti-tumor activity. Int J Biol Sci. 2011;7(5):618–628. doi: 10.7150/ijbs.7.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menke NB, Ward KR, Witten TM, Bonchev DG, Diegelmann RF. Impaired wound healing. Clin Dermatol. 2007;25(1):19–25. doi: 10.1016/j.clindermatol.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Livnah O, Stura EA, Middleton SA, Johnson DL, Jolliffe LK, Wilson IA. Crystallographic evidence for preformed dimers of erythropoietin receptor before ligand activation. Science. 1999;283(5404):987–990. doi: 10.1126/science.283.5404.987. [DOI] [PubMed] [Google Scholar]
- 30.Remy I, Wilson IA, Michnick SW. Erythropoietin receptor activation by a ligand-induced conformation change. Science. 1999;283(5404):990–993. doi: 10.1126/science.283.5404.990. [DOI] [PubMed] [Google Scholar]
- 31.Sonse G, Qiu P, Goldstein AL, Wheater M. Biological activities of thymosin beta4 defined by active sites in short peptide sequences. FASEB J. 2010;24(7):2144–2151. doi: 10.1096/fj.09-142307. [DOI] [PubMed] [Google Scholar]
- 32.Fan Y, Gong Y, Ghosh PK, Graham LM, Fox PL. Spatial coordination of actin polymerization and ILK-Akt2 activity during endothelial cell migration. Dev Cell. 2009;16(5):661–674. doi: 10.1016/j.devcel.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bednarek R, Boncela J, Smolarczyk K, Cierniewska-Cieslak A, Wyroba E, Cierniewski CS. Ku80 as a novel receptor for thymosin beta4 that mediates its intracellular activity different from G-actin sequestering. J Biol Chem. 2008;283(3):1534–1544. doi: 10.1074/jbc.M707539200. [DOI] [PubMed] [Google Scholar]
- 34.Occleston NL, O’Kane S, Laverty HG, et al. Discovery and development of avotermin (recombinant human transforming growth factor beta 3): a new class of prophylactic therapeutic for the improvement of scarring. Wound Repair Regen. 2011;19(Suppl 1):s38–s48. doi: 10.1111/j.1524-475X.2011.00711.x. [DOI] [PubMed] [Google Scholar]
- 35.Freeman KW, Bowman BR, Zetter BR. Regenerative protein thymosin beta-4 is a novel regulator of purinergic signaling. FASEB J. 2011;25(3):907–915. doi: 10.1096/fj.10-169417. [DOI] [PubMed] [Google Scholar]
- 36.Hosseinkhani H, Hong PD, Yu DS. Self-assembled proteins and peptides for regenerative medicine. Chem Rev. 2013;113(7):4837–4861. doi: 10.1021/cr300131h. [DOI] [PubMed] [Google Scholar]
- 37.Hosseinkhani H, Hosseinkhani M, Khademhosseini A, Kobayashi H, Tabata Y. Enhanced angiogenesis through controlled release of basic fibroblast growth factor from peptide amphiphile for tissue regeneration. Biomaterials. 2006;27(34):5836–5844. doi: 10.1016/j.biomaterials.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Ruff D, Crockford D, Girardi G, Zhang Y. A randomized, placebo-controlled, single and multiple dose study of intravenous thymosin beta4 in healthy volunteers. Ann N Y Acad Sci. 2010;1194:223–229. doi: 10.1111/j.1749-6632.2010.05474.x. [DOI] [PubMed] [Google Scholar]
- 39.Chen C, Li M, Yang H, Chai H, Fisher W, Yao Q. Roles of thymosins in cancers and other organ systems. World J Surg. 2005;29(3):264–270. doi: 10.1007/s00268-004-7817-2. [DOI] [PubMed] [Google Scholar]