Preface
BAP1 is a deubiquitylase that is found associated with multi-protein complexes that regulate key cellular pathways, including the cell cycle, cellular differentiation, cell death, gluconeogenesis and the DNA damage response (DDR). Recent findings indicate that germline BAP1 mutations cause a novel cancer syndrome, characterized, at least in the affected families studied so far, by the onset at an early age of benign melanocytic skin tumours with mutated BAP1, and later in life by a high incidence of mesothelioma, uveal melanoma, cutaneous melanoma and possibly additional cancers.
For the past 15 years we have investigated a mesothelioma epidemic in Cappadocia, Turkey, where we discovered that, in some families, susceptibility to mesothelioma, and possibly to mineral fibre carcinogenesis, was transmitted genetically in an autosomal dominant manner1-5. These studies led us to search for a putative mesothelioma susceptibility gene or genes. To isolate these putative genes, we worked with two unrelated US families, referred as “L” and “W”, for their Louisiana and Wisconsin residency, that experienced a high incidence of mesothelioma and had only minimal potential exposure to asbestos6. Our first important clinical observation was that two members in one of these families developed uveal melanoma (UVM): one of them died of the disease and the other was treated at an early stage and cured, but subsequently developed mesothelioma4, 6. There are about 3000 mesotheliomas and a similar or smaller number of UVMs diagnosed annually in the US. The likelihood of both malignancies occurring in more than one individual in the same family was estimated at 36 per trillion6; this linkage suggested a common genetic denominator. Chromosome microarray and genetic linkage analyses on the L and W families implicated chromosome region 3p216, a locus frequently altered in both UVM7 and mesotheliomas8, 9. Sequencing this region of chromosome 3 led to the identification of BAP1 as the gene mutated and associated with high rates of mesothelioma in the L and W families (experiments are in progress to address whether BAP1 also causes the high incidence of mesothelioma in Cappadocia)6.
BAP1 is a member of the ubiquitin C-terminal hydrolases (UCH) subfamily of deubiquitylating enzymes (DUBs) and was mutated in each family member who had developed mesothelioma, UVM and other cancers. None of these tumour types was detected in family members who did not carry BAP1 mutations6. Moreover, among several cases of sporadic mesotheliomas, we found that 2/26 patients had germline BAP1 mutations6 and importantly we found that both of these patients had been previously diagnosed with UVM. None of the 24 patients with sporadic mesothelioma who had wild-type germline BAP1 had been diagnosed with UVM. On the basis of these findings, we have proposed that germline BAP1 mutations cause a new cancer syndrome characterized by mesothelioma and UVM6.
In this Progress article, we discuss this potential inherited cancer syndrome, the possible mechanisms through which mutations in BAP1 might lead to tumour development and the clinical implications raised by detection of germline BAP1 mutations.
Benign melanocytic tumours in BAP1 families
In parallel with our paper6, Wiesner et al.10 reported that germline BAP1 mutations caused benign atypical melanocytic tumours. In a subsequent paper11, they noted that these melanocytic lesions resembled histologically “atypical Spitz tumours (ASTs)”. ASTs are a heterogeneous group of melanocytic tumours with overlapping histological characteristics between benign Spitz nevi and malignant melanoma and are formed by large melanocytes with mitotic activity not only in the superficial but also in the deep part of the lesion. They studied 32 sporadic ASTs and found that 9 had loss of BAP1 expression and 8 of these had concomitant BRAF mutations. Only 1 of 23 ASTs that expressed BAP1 had a BRAF mutation (P< 0.0001) 11. BRAF mutations are common in melanocytic nevi (80%) and in cutaneous melanoma (65%) but are rare in ASTs12. Njauw et al.13 noted the same type of skin tumours arising in families with germline BAP1 mutations and referred to them as nevoid melanoma-like melanocytic proliferations (NEMMPs). Thus, pathologists have used different names to identify these pink to tan skin tumors of about 0.2-1.0 cm in diameter, which macroscopically resemble dermal nevi. Together with a team of dermatologists, pathologists and dermatopathologists at 3 different US National Cancer Institute (NCI)-designated Cancer Centers we reviewed the published morphology and histology of all of these melanocytic tumours, including some of the original tissue sections kindly provided by Weisner and colleagues10. In addition, we examined the skin tumours we detected in the members of the L and W families. We found that all the melanocytic tumors in these BAP1 mutant patients had the same histological and molecular characteristics. In our review of the skin tumours in patients with mutant BAP1 we found that these are nevus-like lesions usually formed by a conventional junctional, compound or dermal nevus composed of small melanocytes expressing BAP1. Next to these conventional nevus cells there is a dermal lesion formed by large epithelioid BAP1 negative and most frequently BRAF mutated melanocytes with virtually no mitotic activity. This intradermal component with these unique features and the nearby conventional nevus-like lesion, to our minds, clearly sets these tumours apart from ASTs and other melanocytic lesions. Acknowledging that these lesions are characteristic of, although possibly not unique to, BAP1 mutation carriers, we proposed naming them melanocytic BAP1-mutated atypical intradermal tumours (MBAITs)12.
Malignant tumours in BAP1 families
Wiesner and colleagues noted that in the families that they studied there was a possible increased risk of cutaneous melanoma and UVM10 and subsequently they confirmed the association with mesothelioma in 2 out of the 3 European families they studied14. Such cancer clustering has been noted in other studies. A 1972 case report described an individual who developed UVM, mesothelioma and meningioma15. Harbour et al.7 reported inactivating mutations of BAP1 in 26 of 31 aggressive (class 2) UVMs, among which a germline mutation was found in 1 of 26 patients.
Abdel-Rahman et al.16 investigated BAP1 status in 53 unrelated patients with UVM who had a high risk of hereditary cancer. One of the 53 patients had a germline truncating BAP1 mutation that segregated in other family members. In this family, there were 7 BAP1 mutation carriers (5 tested and two inferred) and only one was cancer free at age 55; the other six had developed UVM (2 cases), cutaneous melanoma, mesothelioma, meningioma, lung and other types of carcinoma. The two patients treated for UVM subsequently developed a second malignancy16. Njauw et al.13 sequenced BAP1 from 50 patients with metastatic UVM and 50 patients with non-metastatic UVM. BAP1 was mutated in the germline of 8% of patients with metastatic UVM: none of the patients with non-metastatic UVM had BAP1 germline mutations. Also, they found that 2 of 7 probands from families with UVM-cutaneous melanoma carried germline BAP1 mutations, compared to 1 of 193 probands from families with a history of cutaneous melanoma and no UVMs (p=0.003). Aoude et al.17 reported that 2 of 66 (3%) patients with UVM unselected for family history carried germline BAP1 mutations. Additional tumour types, including renal, breast and lung carcinomas were also found among BAP1 mutation carriers6, 13, 16. Very recently, BAP1 mutations have been reported in sporadic renal carcinoma18. Moreover, a novel inactivating germline splice mutation was reported in a family with UVM and cutaneous melanoma. Members of this family were also affected by multiple myeloma, paraganglioma and breast cancer19.
Table 1 summarizes the reports on families with BAP1 mutations as of January 17, 2013. To date, 53 of 76 BAP1 germline mutation carriers have developed malignancies; 13 of these 53 developed two or more cancers. Table 1 and our meta-analysis12, indicate that mesothelioma, UVMs and cutaneous melanomas are clearly associated with the putative BAP1 cancer syndrome (Box 1). In addition, 35 BAP1 mutation carriers were investigated for the lesions that we call MBAITs, and 19 of them were found to have one or more (Table 1). These benign skin lesions may occasionally progress to cutaneous melanoma14.
Table 1.
Description of tumors rates and age at diagnosis in the BAP1-mutated cohort and 2005-2009 US cancer incidence and median age at diagnosis (source: SEER)
| Age at diagnosis | US statistics | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tumor | N total |
N cases |
Ref. | % (95%CI*) | N age known |
Range (years) |
Mean (s.d.) (years) |
Incidence per 100,000 p.yr |
Median age (years) |
| Malignant mesothelioma | 76 | 22 | 6,10,14,16,19 | 28.95 (19.96-39.96) | 22 | 37-85 | 55.2 (15.5) | 0.1-1 | 74 |
| Uveal melanoma | 76 | 21 | 6,7,10,12,16,19 | 27.63 (18.84-38.37) | 20 | 18-72 | 52.8 (12.8) | 0.6 | 56 |
| Cutaneous melanoma | 76 | 11 | 10,12,16,19 | 14.47 ( 7.79-24.84) | 10 | 21-57 | 41.7 (10.0) | 21.9 | 61 |
| Lung cancer | 76 | 5 | 12,16,19 | 6.58 (2.45-15.34) | 4 | 46-57 | 52.0 (5.35) | 64.5 | 70 |
| Breast cancer | 43 | 4 | 6,12,19 | 9.30 (3.68-21.16) | 3 | 37-75 | 51.7 (20.4) | 124.3 | 61 |
| Ovarian cancer | 43 | 2 | 6,16 | 4.65 (1.28-15.45) | 2 | 59-69 | 64.0 (7.1) | 12.7 | 63 |
| Renal carcinoma | 76 | 2 | 6,12 | 2.63 (0.72-9.09) | 2 | 46-57 | 51.5 (7.8) | 15.1 | 64 |
| Non-melanoma skin c. | 76 | 2 | 6 | 2.63 (0.72-9.09) | 0 | - | - | - | - |
| Meningioma | 76 | 1 | 16 | 1 | 59 | 3 | - | ||
| Cholangiocarcinoma | 76 | 1 | 12 | 1 | 47 | <1 | 8th decade | ||
| Leiomyosarcoma | 76 | 1 | 6 | 1 | 33 | <1 | 5th decade | ||
| Neuroendocrine tumors | 76 | 1 | 16 | 1 | 52 | 4.4 to 6.5 | - | ||
| Pancreatic cancer | 76 | 1 | 6 | 1 | 72 | 12.3 | 71 | ||
| Paraganglioma | 76 | 1 | 19 | 1 | 42 | 0.2 to 0.8 | 3rd to 5th decade | ||
| Malignant Fibrous Histiocytoma | 76 | 1 | 19 | 1 | 45 | 3 | 6rth to 7th decade | ||
| Cancer non specified | 76 | 1 | 16 | ||||||
|
| |||||||||
| Patients with at least 1 malignancy | 76 | 53 | 6,7,10,12,14,16, | 69.74 (57.99-79.48) | 47 | 18-85 | 51.7 (14.1) | 477 | 66 |
| Patients with 2 or more malignancies | 76 | 13** | 19 | 17.11 (10.28-27.10) | 8% of all cancer survivors | ||||
|
| |||||||||
| MBAITs | 35 | 19 | 10,12 | 54.29 (38.19-70.78) | |||||
The confidence intervals were calculated using the Wilson method, without correction for continuity.
13 persons among the 53 with malignancies had 2 or more malignant tumors; that is 24.53%, 95%CI: (14.94%-37.57%), compared with 8% in US.
Abbreviations: N: number; 95% CI: 95% confidence interval; s.d.: standard deviation; p.yr: person year; - data not available.
Criteria of inclusion: Published data of individuals tested genetically and found to carry BAP1 germline mutations.
Box 1 Clinical considerations.
Wiesner et al10 noted that in BAP1 mutation carriers benign melanocytic tumors develop during the second decade of life and their number increases with age, a finding confirmed in the BAP1 mutant families we are studying12. The occurrence of cutaneous melanoma in some BAP1 mutation carriers, including one that appeared to originate from these melanoctytic tumors14, suggests that occasionally these benign tumors may undergo malignant transformation. This finding may account, at least in part, for the overall high risk of cutaneous melanoma detected among BAP1 germline mutation carriers (Table 1). We propose that these melanocytic tumors that for the reasons described in the text and in ref. 12 we term MBAITs, could be viewed as a specific phenotypic marker of BAP1 mutation carriers. Other autosomal dominant cancer syndromes also have phenotypic markers, such as adenomas in familial adenomatous polyposis families and Lisch nodules and café-au-lait spots in families with neurofibromatosis type 1. Suspected MBAITs could be removed and tested to identify patients with BAP1 mutations. If this approach was found to have clinical relevance it could allow for the close monitoring of these individuals and enable early detection of cutaneous melanoma, uveal melanoma (UVM), mesothelioma and possibly other cancers12. Both cutaneous melanoma and UVM can be cured when detected at an early stage and are fatal when they have metastasized. Early diagnosis also benefits mesothelioma patients because when they are diagnosed and treated at a very early stage, survival for 5 or more years is not uncommon12, 49. Thus, the detection of families with inherited mutations of BAP1 offers opportunities for early cancer detection and more effective therapeutic approaches.
In the BAP1 mutation families, there is an exceptionally high incidence of malignancy overall (69.74% cancer incidence among 76 mutation carriers). Some of these malignancies developed at an earlier age than observed in the general population (Table 1), as expected in familial cancer syndromes. All of the mutation carriers unaffected by cancer are younger than 55 years old and may develop malignancies later in life. However, the relatively high frequency in the general population of the carcinomas that develop in BAP1 mutation carriers does not allow us yet to distinguish with certainty if the association with carcinomas is causal. It should also be noted that population-based studies have shown that mutation carriers in the general population may have a lower risk than suggested by estimates obtained from families with multiple tumours, as for example observed for CDKN2A mutations20. Therefore, large population based studies will be required to validate the association of germline BAP1 mutations with the exceptionally high incidence of malignancies detected in the BAP1 mutant families.
Most autosomal dominant cancer syndromes cause tumours in specific sites and tissues, and do not result in an overall increase in cancer risk. However, the Li-Fraumeni syndrome, caused by inherited mutations of the TP53 tumour suppressor gene, is an autosomal dominant cancer syndrome characteristically associated with the development of multiple tumour types 21. Is the BAP1 cancer syndrome, similarly to the Li-Fraumeni syndrome, associated with an overall general increase of cancer risk? If so, why do mesotheliomas, UVMs, cutaneous melanomas predominate? As we expand our studies to include additional families that have mutant BAP1 genes we will address these questions. In the section below we discuss the putative functions of BAP1 and how they might contribute to cancer. Most data about BAP1 function were published in the past 2 years, following a renewed interest in this gene caused by the discovery of its association with human mesothelioma and melanomas, and possibly with other cancer types. Therefore, some of the most recent studies have still to be independently verified. Overall the data indicate that BAP1 can influence multiple cellular pathways, at times suppressing and at times promoting cell growth, possibly in a cell type and species specific fashion.
BAP1 has multifaceted functions
Protein ubiquitylation was initially seen as a mechanism to label proteins for degradation; however, this notion has evolved as we have come to understand that ubiquitylation and DUBs regulate various cellular processes, including DNA repair, gene transcription, cell membrane trafficking, cell cycle progression, stress response, cell communication, differentiation and apoptosis, and that they have a role in cancer development22, 23.
The BAP1 gene is located at chromosome region 3p21.124, a genomic region that is deleted in several human malignancies, including approximately 30-60% of mesotheliomas 6, 8, 9, 25 and 85% of metastasizing UVMs10. BAP1 was discovered in a yeast two-hybrid screen owing to its interaction with the RING finger domain of the tumour suppressor BRCA1. Initial data suggested that BAP1 was a tumour suppressor gene because BAP1 suppressed the growth of MCF7 human breast cancer cells in soft agar24. However, despite the fact that auto-ubiquitylated BRCA1 is a DUB substrate, subsequent experiments have shown that BAP1 does not influence the deubiquitylation of BRCA126. BRCA1 forms a heterodimer through its RING domain with BRCA1 associated RING domain 1 (BARD1), and this BRCA1–BARD1 tumour-suppressor complex has E3 ubiquitin ligase activity that regulates the DNA damage response (DDR)27. BAP1 binds and deubiquitylates BARD1, thus modulating the E3 ligase activity of BRCA1–BARD128. Indeed, inhibition of BAP1 by short hairpin RNAs (shRNAs) impaired the DDR and caused HeLa cells to become hypersensitive to ionizing radiation resulting in S-phase retardation, a phenotype similar to BRCA1 deficiency. Therefore, BRCA1-mediated ubiquitylation and BAP1-mediated deubiquitylation may coordinately regulate these cellular processes28.
Ventii et al. reported that in human NCI-H226 non-small cell lung cancer cells that do not express BAP1, its exogenous expression through lentivirus infection accelerated passage through the G1/S checkpoint. This effect might have impaired DNA repair leading to DNA damage and in the induction of cell death both by apoptosis and necrosis29. Accordingly, NCI-H226 cells expressing exogenous wild-type BAP1 grew poorly in cell culture and when injected into athymic nude mice, they formed tumours that were about 10-15-fold smaller than those obtained with cells infected with vector alone or expressing BAP1 mutants lacking deubiquitylating activity (C91A substitution) or the second nuclear localization signal, NLS2, that is required for the nuclear compartmentalization of BAP129. BAP1-mediated growth suppression in this in vivo model was BRCA1-independent. These data were interpreted as further evidence that BAP1 is a tumour suppressor gene, and that both nuclear localization and deubiquitylating activity are required for BAP1-mediated tumour suppressor activity29.
When in the nucleus, BAP1 interacts with several proteins including host cell factor 1 (HCF1; also known as HCFC1). HCF1 is a cell cycle regulator that contains a Kelch domain that binds to a conserved peptide sequence known as the HCF1 binding motif (HBM) common to several transcription factors. Although HCF1 does not bind DNA directly, transcription factors that contain a HBM domain recruit HCF1 to specific promoters, where it regulates transcription. HCF1 binds BAP1 through a HBM sequence present in the middle portion of BAP130, 31. HCF1 is ubiquitylated on lysine residues, preferentially through K48-linked and K63-linked chains. BAP1 hydrolyzes K48-linked chains30, 31. Overexpression of mutated, catalytically inactive BAP130 and BAP1 silencing31 resulted in a modest accumulation of HCF1, indicating that HCF1 K48 ubiquitylation does not necessarily label HCF1 for degradation.
A critical role of HCF1 is to sustain the formation of complexes between chromatin modifying enzymes and transcription factors. For example, by recruiting histone methyltransferases to the E2F1 transcription factor, HCF1 allows the transcription of E2F1 target genes, including genes that control the cell cycle and cellular proliferation32. It has been proposed that ubiquitylation of HCF1 blocks E2F-responsive promoter activity, and that HCF1 deubiquitylation by BAP1 would remove this inhibition and promote cell proliferation33. In addition, co-immunoprecipitation experiments have shown that BAP1 directly associates with E2F family members, suggesting a possible additional direct control of these transcriptional factors by BAP133. Therefore, the ability of BAP1 to influence progression through the cell cycle may be mediated, at least in part, by E2F family members33.
The Drosophila Polycomb group (PcG) gene calypso is homologous to the human BAP1 gene. PcG proteins are transcriptional repressors that control cellular differentiation and development34. These proteins form multiprotein complexes that bind to chromatin and together repress gene expression. The two main families of PcG protein complexes are Polycomb repressive complex 1 (PRC1) and PRC2. Compaction of nucleosomes and ubiquitylation of histone 2A (H2A) are two of the mechanisms through which PRC1 silences gene expression35. Mono-ubiquitylation of H2A is a critical mechanism in the control of transcription initiation and elongation, transcription silencing and DNA repair36. In Drosophila, Calypso binds additional sex combs (ASX) to form the Polycomb repressive deubiquitinase (PR-DUB) complex that specifically removes mono-ubiquitin from H2A. Similarly, in humans BAP1 binds ASXL1 through its carboxy-terminus and deubiquitylates H2A34. It appears possible that PR-DUB may help fine-tune gene expression levels by preventing the hyperubiquitylation of H2A by PRC1 and BRCA1. BRCA1 maintains the ubiquitylated status of H2A, a mechanism that is critical for BRCA1 tumour suppressor activity because it antagonizes the transcriptional de-repression of satellite DNA repeats that contribute to breast cancer growth37.
Thus, PcG activity may not simply be ascribed to its gene suppressive functions, but may rather result from the delicate balance between H2A ubiquitylation by PRC1 and BRCA1 (which suppress transcription) and H2A deubiquitylation by PR-DUB (which should re-activate transcription). PRC2 might also be important in tumours that have mutant BAP1. The PRC2 proteins EZH2 and EED are frequently overexpressed in mesothelioma and Kemp et al. proposed inhibiting PRC2 as a possible therapeutic strategy for treating mesothelioma38.
Yu et al.39 reported that almost all cellular BAP1 is found in high-molecular weight multi-protein complexes, which include HCF1, ASXL1 and ASXL2, O-linked N-acetylglucosamine transferase (OGT) and the forkhead transcription factors FOXK1 and FOXK2, in what they called “the BAP1 core complex” (Figure 1a). This core complex, in turn, associates with additional regulators and transcription factors to form specific functional complexes that may be cell type specific39. In addition, recent studies have shown that BAP1, HCF-1 and OGT (which is essential for Polycomb mediate gene repression in Drosophila40) exist as a multiprotein complex that regulates itself and also regulates several targets (Figure 1b). For example, BAP1 deubiquitylates and thus stabilizes OGT, which O-GlcNacylates HCF1 and activates it41, 42. Activated HCF1 recruits OGT to O-GlcNacylate peroxisome proliferator activator receptor γ coactivator 1α (PGC-1α. This modification enables BAP1 to bind and deubiquitylate PGC-1α which is stabilized and can promote gluconeogenesis42 (Figure 1b). Moreover, chromatin immunoprecipitation (ChIP) assays identified a total of 9128 BAP1 peaks (5926 located near the transcription start sites of 5731 genes); 85% of promoters occupied by BAP1 also contained HCF1, and BAP1, HCF-1 and OGT were recruited as a complex on 1827 different promoters41.
Figure 1a. BAP1 protein partners.

Putative BAP1 protein partners, identified by co-immunoprecipitation and/or affinity-capture mass spectrometry. Proteins forming the putative BAP1 core complex are shown in blue39. Other putative BAP1 partners of hypothetical new complexes are shown in beige28, 39, 41. Protein partners shown may vary in different cell types and under different conditions.
Figure 1b. Possible mechanisms of BAP1 function.
I) Epigenetic regulation of Polycomb target genes is achieved via the cooperation of multiple protein complexes. The signature of gene silencing mediated by PRC2 is the trimethylation of H3K27. PRC1 can be recruited to H3K27me3 sites and contributes to gene silencing via monoubiquitylation of H2AK11935. The BAP1-ASXL1 complex (PR-DUB) has H2A-K119ub deubiquitylase activity opposite to PRC134. OGT contributes to the fine tuning of these epigenetic marks via O-GlcNacylation of PHCs40. Hypothesis: PR-DUB might be recruited to Polycomb target genes because of the interaction between BAP1, HCF1 and OGT.
II) BAP1 regulates gene transcription via association with other protein partners, e.g. HCF1 and YY139 or HCF1 and OGT41. Other putative BAP1 partners are shown in grey. Hypothesis: BAP1, HCF1, YY1 and OGT might also be part of one large protein complex.
III) BAP1-HCF1-OGT complex increases the stability of PGC-1α, regulating gluconeogenesis and possibly mitochondrial biogenesis42.
ASXL1/2, Additional sex combs like 1/2; BAP1, BRCA1-assiciated protein 1; BARD1, BRCA1-associated RING domain protein 1; BRCA1, breast cancer type 1 susceptibility protein; CBXs, mammalian chromobox protein homologues; EZHs, enhancer of zeste homologs; FOXK1/2, Forkhead box protein K1/2; HAT1, Histone acetyltransferase 1; HCF1, Host cell factor 1; KDM1B, lysine (K)-specific demethylase 1B; OGT, UDP-glucose-dependent O-glucosyltransferase; PCLs, Polycomb Like proteins; PGC-1α, Peroxisome proliferator-activated receptor gamma coactivator 1α; PHCs, Polyhomeotic-like proteins; PRC1, Polycomb repressive complex 1; PRC2, Polycomb repressive complex 2; PR-DUB, Polycomb repressive deubiquitinase; RING1, Really interesting new gene 1; RNF2, RING finger protein 2; YY1, Ying-Yang 1.
BAP1 has also been shown to form a ternary complex with HCF1 and the transcription factor Yin Yang 1 (YY1) that controls the transcription of genes involved in cell proliferation. Specifically, BAP1 interacts with the zinc fingers of YY1 through its coiled-coil motif and it is recruited together with HCF1 to the promoter of the COX7C gene that encodes a component of the mitochondrial respiratory chain39.
Thus, it can be expected that BAP1 influences a wide array of cellular functions, and consistent with this hypothesis, BAP1 depletion by RNA inhibition induced significant changes in the expression of many genes that control various cellular pathways39.
In summary, despite the findings implicating BAP1 in suppression of proliferation24, 29, some groups have reported that BAP1 promotes cell proliferation25, 30, 33. These conflicting results suggest that BAP1 effects may be cell-type specific and/or that they could be influenced by the experimental approach used. BAP1 activity could vary in different cells because the BAP1 core complex39 is likely to associate with different transcription factors and thus influence different pathways in a cell-type specific manner. Also, some of the proteins that normally bind BAP1 may have different activities in its absence. For example, YY1 acts as a repressor or an activator of COX7C depending on whether it is bound to the BAP1–HCF1 complex39.
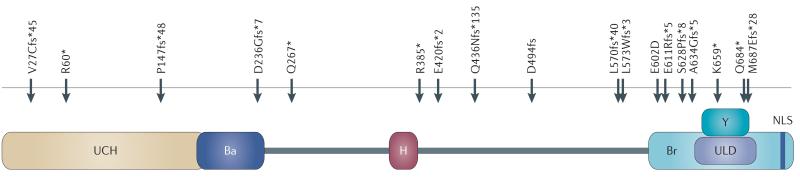
All the germline mutations found in the BAP1 cancer syndrome encode BAP1 proteins lacking the nuclear localization sequence and most of them have an intact UCH domain (Figure 2). In the tumours that develop in BAP1 mutant carriers, BAP1 expression is either absent because of loss of heterozygosity (LOH), resulting in biallelic inactivation, or the BAP1 protein is localized in the cytoplasm6, 16 where it may retain DUB activity, with as yet unknown possible effects on the cellular ‘ubiquitinome’.
Figure 2. Schematic representation of BAP1 domains and locations of the reported BAP1 germline mutations.
All the germline BAP1 mutations found in families with UVM and mesothelioma encode BAP1 proteins lacking the nuclear localization sequence. Most of them have an intact ubiquitin carboxyl-terminal hydrolase (UCH) domain6, 7, 10, 13, 14, 16, 17, 19.
Ba, BARD1 binding domain; H, HCF-1 binding domain; Br, BRCA1 binding domain; Y, YY1 binding domain; ULD, UCH37-like domain; NLS, nuclear localization signal; Red arrows, germline mutations.
What do BAP1 associated cancers have in common?
Because germline BAP1 mutations are associated with mesotheliomas, UVMs and cutaneous melanomas, there should be some common pathways controlled by BAP1 that are of particular importance to the development of these malignancies. The finding that somatic BAP1 mutations are common in these cancers supports this interpretation.
Somatic BAP1 mutations were found in 84% of metastasizing UVM biopsies7 and in 22%6 and 23%25 of sporadic US mesothelioma biopsies. Instead, Yoshikawa et al. found somatic inactivating BAP1 mutations in 61% of Japanese mesothelioma biopsies43. To address this discrepancy, in collaboration with our Japanese colleagues we are currently investigating whether the higher incidence of BAP1 mutations that they detected was caused by ethnic differences or by the different technical approach used, that is multiplex ligation-dependent probe amplification (MLPA)43 compared with PCR6.
Studies in mice41 revealed that Bap1 deletion is lethal during embryogenesis. When Bap1 was deleted in adult mice that expressed the tamoxifen-inducible recombinase cre–ERT2 ubiquitously and had Bap1 exons 4 and 5 flanked by lox sites, these mice developed splenomegaly caused by extramedullary hematopoiesis, monocytosis, neutrophilia, and progressive anemia41, findings comparable to the human myelodysplastic syndrome (MDS). The absence of mesothelioma, uveal and cutaneous melanoma in these mice is somewhat puzzling, and may be related to species differences. In addition, at the level of disease causation, the common denominator among mesotheliomas and melanomas is a well-defined role of environmental carcinogens. We are presently testing the hypothesis that germline Bap1 mutations increase the susceptibility to mineral fibre-induced and UV light-induced carcinogenesis; this may explain the absence of mesothelioma and melanoma in mice that were not exposed to these carcinogens. Mineral fibres are well-documented causes of mesothelioma1-5. Deposition of these fibres in tissues induces a chronic inflammatory reaction that is started by the release of high mobility group box 1 (HMGB1) by mesothelial cells undergoing programmed cell necrosis2, 44. Extracellular HMGB1 starts the inflammatory process by recruiting macrophages and other immune cells that in turn actively secrete HMGB1 and other cytokines, and release mutagenic oxygen and nitric radicals, promoting carcinogenesis and mesothelioma growth (reviewed in2). In such an environment, an impaired DDR caused by BAP1 deletion, might be expected to favour the accumulation of genetic damage that eventually gives rise to a malignant clone. However, Bott et al.25 found no consistent differences in RAD51 or BRCA1 complex formation between BAP1 wild-type and BAP1 deficient cell lines exposed to ionizing radiation, suggesting no critical role for BAP1 in the formation of these DNA repair complexes. Moreover, we6 found very minimal and Wiesner et al14, found no evidence of exposure to asbestos or erionite among the patients who developed mesothelioma in the families with BAP1 mutations, findings that do not support gene-environment interaction in causing mesothelioma, at least in these families. A separate mechanism, by which HMGB1 might influence mesothelioma especially in cells lacking BAP1, is the capacity of HMGB1 to induce nucleosome assembly45. High levels of HMGB1 may increase gene repression by PRC1 in a mutated BAP1 background.
Ultraviolet (UV) light exposure increases the risk of cutaneous melanoma. The role of UV light exposure in causing UVM is still debated46 however, it seems possible that BAP1 mutation carriers are at an increased risk because of impaired DDR. Because BAP1 mutation carriers represent only a fraction of patients who develop UVM (3-4%)13, 17, the overall association of UVM with UV light exposure should be re-evaluated in BAP1 mutations positive versus BAP1 mutation negative patients.
Moreover, BAP1 is a UV-inducible substrate of the ataxia telangiectasia mutated (ATM) and ataxia telangiectasia and Rad3-related (ATR) kinases. ATM and ATR regulate the DNA damage checkpoint that inhibits cell cycle progression in response to genomic insults – such as UV – to allow time to damage repair or, if the damage cannot be repaired, to enter apoptosis47.
We anticipate that as more studies uncover the precise mechanisms by which BAP1 mutations cause mesothelioma and melanomas, specific preventive and therapeutic approaches for these malignancies will be developed (Box 1). It is worth noting that a recent paper showed that histone deacetylase inhibitors (HDACIs) impair the in vitro and in vivo growth of UVM metastatic tumours with BAP1 inactivating mutations48. Specifically, HDAC inhibitors block H2A ubiquitylation through inhibition of PRC1 and so reverse H2A hyperubiquitylation caused by BAP1 loss. HDACIs also induce differentiation of Class II UVM to Class I UVM48, which is less aggressive.
Conclusions
The apparent ability of BAP1 mutations to cause multiple tumour types and the high tumour phenotype penetrance (Table 1) suggests that this gene has a major role in influencing cancer cell growth. The pleiotropic effects of BAP1 can account for this finding. Not only is BAP1 the human homologue of the Drosophila PcG gene calypso, but it also contains a HBM domain, which is absent in Calypso, that allows BAP1 to bind HCF1, a chromatin associated protein that similar to PcG regulates the expression of a plethora of different genes. Thus, by regulating the activities of PcG and HCF1 target genes, BAP1 has a central role in regulating gene expression in mammalian cells.
The clinical implications of these recent findings have yet to be fully established12. The presence of specific skin lesions that we have called MBAITs is an important clinical feature that might help to identify BAP1 mutation carriers. We think that these patients require close monitoring for early detection and curative resection of uveal and cutaneous melanoma, and of mesothelioma and other malignancies.
Acknowledgments
We thank Drs. Francine Baumann, Erin Flores, Shreya Kanodia, Andrea Napolitano, Janet D. Rowley and David C. Ward for critical reading of this manuscript. We thank Dr. T. Wiesner for sharing with us MBAITs tissue specimens for our review. NCI PO1 CA 1140047 to MC supported this work.
Glossary
- Uveal melanoma [UVM]
Uveal melanomas are the most common primary intraocular malignancy and they account for about 13% of melanoma deaths.
- Proband
Individual that, by seeking medical/scientific attention, allows the detection of a genetic disorder in a family.
- Autosomal dominant
A genetic disease inherited as a result of having a single copy of the mutated gene, located on one of the 22 non-sex chromosomes.
- Asbestos
This term identifies 6 different fibrous minerals – among about 400 present in nature – that were used commercially. Exposure to asbestos as well as to other mineral fibres, such as erionite, can cause mesothelioma.
Footnotes
Competing Interests Statement. MC and JRT have a pending patent application on BAP1.
Contributor Information
Michele Carbone, University of Hawaii Cancer Center, 701 Ilalo Street, Honolulu, Hawaii 96813, USA; Department of Pathology at the University of Hawaii JABSOM Medical School, Honolulu, Hawaii 96813, USA.
Haining Yang, University of Hawaii Cancer Center, 701 Ilalo Street, Honolulu, Hawaii 96813, USA; Department of Pathology at the University of Hawaii JABSOM Medical School, Honolulu, Hawaii 96813, USA.
Harvey I. Pass, Department of Cardiothoracic Surgery, New York University, New York 10016, USA
Thomas Krausz, Department of Pathology, University of Chicago, Illinois 60637, USA.
Joseph R. Testa, Cancer Biology Program at the Fox Chase Cancer Center, Philadelphia, Pennsylvania 19111, USA
Giovanni Gaudino, University of Hawaii Cancer Center, 701 Ilalo Street, Honolulu, Hawaii 96813, USA.
References
- 1.Carbone M, et al. A mesothelioma epidemic in Cappadocia: scientific developments and unexpected social outcomes. Nat Rev Cancer. 2007;7:147–54. doi: 10.1038/nrc2068. [DOI] [PubMed] [Google Scholar]
- 2.Carbone M, Yang H. Molecular pathways: targeting mechanisms of asbestos and erionite carcinogenesis in mesothelioma. Clin Cancer Res. 2012;18:598–604. doi: 10.1158/1078-0432.CCR-11-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carbone M, et al. Erionite exposure in North Dakota and Turkish villages with mesothelioma. Proc Natl Acad Sci U S A. 2011;108:13618–23. doi: 10.1073/pnas.1105887108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roushdy-Hammady I, Siegel J, Emri S, Testa JR, Carbone M. Genetic-susceptibility factor and malignant mesothelioma in the Cappadocian region of Turkey. Lancet. 2001;357:444–5. doi: 10.1016/S0140-6736(00)04013-7. [DOI] [PubMed] [Google Scholar]
- 5.Dogan AU, et al. Genetic predisposition to fiber carcinogenesis causes a mesothelioma epidemic in Turkey. Cancer Res. 2006;66:5063–8. doi: 10.1158/0008-5472.CAN-05-4642. [DOI] [PubMed] [Google Scholar]
- 6.Testa JR, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet. 2011;43:1022–5. doi: 10.1038/ng.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harbour JW, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010;330:1410–3. doi: 10.1126/science.1194472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu YY, Jhanwar SC, Cheng JQ, Testa JR. Deletion mapping of the short arm of chromosome 3 in human malignant mesothelioma. Genes Chromosomes Cancer. 1994;9:76–80. doi: 10.1002/gcc.2870090114. [DOI] [PubMed] [Google Scholar]
- 9.Zeiger MA, Gnarra JR, Zbar B, Linehan WM, Pass HI. Loss of heterozygosity on the short arm of chromosome 3 in mesothelioma cell lines and solid tumors. Genes Chromosomes Cancer. 1994;11:15–20. doi: 10.1002/gcc.2870110104. [DOI] [PubMed] [Google Scholar]
- 10.Wiesner T, et al. Germline mutations in BAP1 predispose to melanocytic tumors. Nat Genet. 2011;43:1018–21. doi: 10.1038/ng.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiesner T, et al. A Distinct Subset of Atypical Spitz Tumors is Characterized by BRAF Mutation and Loss of BAP1 Expression. Am J Surg Pathol. 2012 doi: 10.1097/PAS.0b013e3182498be5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carbone M, et al. BAP1 cancer syndrome: malignant mesothelioma, uveal and cutaneous melanoma, and MBAITs. J Transl Med. 2012;10:179. doi: 10.1186/1479-5876-10-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Njauw CN, et al. Germline BAP1 Inactivation Is Preferentially Associated with Metastatic Ocular Melanoma and Cutaneous-Ocular Melanoma Families. PLoS One. 2012;7:e35295. doi: 10.1371/journal.pone.0035295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiesner T, et al. Toward an Improved Definition of the Tumor Spectrum Associated With BAP1 Germline Mutations. J Clin Oncol. 2012 doi: 10.1200/JCO.2011.41.2965. [DOI] [PubMed] [Google Scholar]
- 15.Cagianut B. [Melanoma of the choroid and ciliary body, malignant meningioma and mesothelioma of the pleura (triple-malignoma) in a 63-year old female] Klin Monbl Augenheilkd. 1972;161:407–11. [PubMed] [Google Scholar]
- 16.Abdel-Rahman MH, et al. Germline BAP1 mutation predisposes to uveal melanoma, lung adenocarcinoma, meningioma, and other cancers. J Med Genet. 2011;48:856–9. doi: 10.1136/jmedgenet-2011-100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aoude LG, Vajdic CM, Kricker A, Armstrong B, Hayward NK. Prevalence of germline BAP1 mutation in a population-based sample of uveal melanoma cases. Pigment Cell Melanoma Res. 2012 doi: 10.1111/pcmr.12046. [DOI] [PubMed] [Google Scholar]
- 18.Pena-Llopis S, et al. BAP1 loss defines a new class of renal cell carcinoma. Nat Genet. 2012 doi: 10.1038/ng.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wadt K, et al. A cryptic BAP1 splice mutation in a family with uveal and cutaneous melanoma, and paraganglioma. Pigment Cell Melanoma Res. 2012 doi: 10.1111/pcmr.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Begg CB, et al. Lifetime risk of melanoma in CDKN2A mutation carriers in a population-based sample. J Natl Cancer Inst. 2005;97:1507–15. doi: 10.1093/jnci/dji312. [DOI] [PubMed] [Google Scholar]
- 21.Li FP, Fraumeni JF., Jr. Soft-tissue sarcomas, breast cancer, and other neoplasms. A familial syndrome? Ann Intern Med. 1969;71:747–52. doi: 10.7326/0003-4819-71-4-747. [DOI] [PubMed] [Google Scholar]
- 22.Bheda A, Shackelford J, Pagano JS. Expression and functional studies of ubiquitin C-terminal hydrolase L1 regulated genes. PLoS One. 2009;4:e6764. doi: 10.1371/journal.pone.0006764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang Y, Fu D, Shen XZ. The potential role of ubiquitin c-terminal hydrolases in oncogenesis. Biochim Biophys Acta. 2010;1806:1–6. doi: 10.1016/j.bbcan.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Jensen DE, et al. BAP1: a novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene. 1998;16:1097–112. doi: 10.1038/sj.onc.1201861. [DOI] [PubMed] [Google Scholar]
- 25.Bott M, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet. 2011;43:668–72. doi: 10.1038/ng.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mallery DL, Vandenberg CJ, Hiom K. Activation of the E3 ligase function of the BRCA1/BARD1 complex by polyubiquitin chains. EMBO J. 2002;21:6755–62. doi: 10.1093/emboj/cdf691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenberg RA, et al. Multifactorial contributions to an acute DNA damage response by BRCA1/BARD1-containing complexes. Genes Dev. 2006;20:34–46. doi: 10.1101/gad.1381306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishikawa H, et al. BRCA1-associated protein 1 interferes with BRCA1/BARD1 RING heterodimer activity. Cancer Res. 2009;69:111–9. doi: 10.1158/0008-5472.CAN-08-3355. [DOI] [PubMed] [Google Scholar]
- 29.Ventii KH, et al. BRCA1-associated protein-1 is a tumor suppressor that requires deubiquitinating activity and nuclear localization. Cancer Res. 2008;68:6953–62. doi: 10.1158/0008-5472.CAN-08-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Machida YJ, Machida Y, Vashisht AA, Wohlschlegel JA, Dutta A. The deubiquitinating enzyme BAP1 regulates cell growth via interaction with HCF-1. J Biol Chem. 2009;284:34179–88. doi: 10.1074/jbc.M109.046755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Misaghi S, et al. Association of C-terminal ubiquitin hydrolase BRCA1-associated protein 1 with cell cycle regulator host cell factor 1. Mol Cell Biol. 2009;29:2181–92. doi: 10.1128/MCB.01517-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tyagi S, Chabes AL, Wysocka J, Herr W. E2F activation of S phase promoters via association with HCF-1 and the MLL family of histone H3K4 methyltransferases. Mol Cell. 2007;27:107–19. doi: 10.1016/j.molcel.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 33.Eletr ZM, Wilkinson KD. An emerging model for BAP1’s role in regulating cell cycle progression. Cell Biochem Biophys. 2011;60:3–11. doi: 10.1007/s12013-011-9184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scheuermann JC, et al. Histone H2A deubiquitinase activity of the Polycomb repressive complex PR-DUB. Nature. 2010;465:243–7. doi: 10.1038/nature08966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simon JA, Kingston RE. Mechanisms of polycomb gene silencing: knowns and unknowns. Nat Rev Mol Cell Biol. 2009;10:697–708. doi: 10.1038/nrm2763. [DOI] [PubMed] [Google Scholar]
- 36.Gutierrez L, et al. The role of the histone H2A ubiquitinase Sce in Polycomb repression. Development. 2012;139:117–27. doi: 10.1242/dev.074450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu Q, et al. BRCA1 tumour suppression occurs via heterochromatin-mediated silencing. Nature. 2011;477:179–84. doi: 10.1038/nature10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kemp CD, et al. Polycomb repressor complex-2 is a novel target for mesothelioma therapy. Clin Cancer Res. 2012;18:77–90. doi: 10.1158/1078-0432.CCR-11-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu H, et al. The ubiquitin carboxyl hydrolase BAP1 forms a ternary complex with YY1 and HCF-1 and is a critical regulator of gene expression. Mol Cell Biol. 2010;30:5071–85. doi: 10.1128/MCB.00396-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gambetta MC, Oktaba K, Muller J. Essential role of the glycosyltransferase sxc/Ogt in polycomb repression. Science. 2009;325:93–6. doi: 10.1126/science.1169727. [DOI] [PubMed] [Google Scholar]
- 41.Dey A, et al. Loss of the tumor suppressor BAP1 causes myeloid transformation. Science. 2012;337:1541–6. doi: 10.1126/science.1221711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruan HB, et al. O-GlcNAc transferase/host cell factor C1 complex regulates gluconeogenesis by modulating PGC-1alpha stability. Cell Metab. 2012;16:226–37. doi: 10.1016/j.cmet.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshikawa Y, et al. Frequent inactivation of the BAP1 gene in epithelioid-type malignant mesothelioma. Cancer Sci. 2012;103:868–74. doi: 10.1111/j.1349-7006.2012.02223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jube S, et al. Cancer cell secretion of the DAMP protein HMGB1 supports progression in malignant mesothelioma. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-11-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Celona B, et al. Substantial histone reduction modulates genomewide nucleosomal occupancy and global transcriptional output. PLoS Biol. 2011;9:e1001086. doi: 10.1371/journal.pbio.1001086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horn EP, Hartge P, Shields JA, Tucker MA. Sunlight and risk of uveal melanoma. J Natl Cancer Inst. 1994;86:1476–8. doi: 10.1093/jnci/86.19.1476. [DOI] [PubMed] [Google Scholar]
- 47.Stokes MP, et al. Profiling of UV-induced ATM/ATR signaling pathways. Proc Natl Acad Sci U S A. 2007;104:19855–60. doi: 10.1073/pnas.0707579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Landreville S, et al. Histone deacetylase inhibitors induce growth arrest and differentiation in uveal melanoma. Clin Cancer Res. 2012;18:408–16. doi: 10.1158/1078-0432.CCR-11-0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flores RM, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg. 2008;135:620–6. 626, e1–3. doi: 10.1016/j.jtcvs.2007.10.054. [DOI] [PubMed] [Google Scholar]