Abstract
Patient: Female, 59
Final Diagnosis: Paraneoplastic limbic encephalitis
Symptoms: Seizure • memory changes • decreased concentration
Medication: Chemotherapy
Clinical Procedure: Cerebral images
Specialty: Hematology • Oncology
Objective:
Challenging differential diagnosis
Background:
Paraneoplastic neurological disorders (PND) are defined as remote effects on the nervous system that are not caused directly by the tumor, its metastases, or metabolic disruptions. This syndrome occurs in less than 1 per 10,000 patients diagnosed with a malignancy. Many antibodies are found in the central nervous system in PND, the most well known are Anti-Hu, Tr, CV2 Ta, Yo, Ri and amphiphysin. Paraneoplastic limbic encephalitis occurs due to involvement of the limbic system secondary to an autoimmune response to neurons of the brain provoked by the antibodies. Patients, thus, present with seizures, changes in mood, memory, and personality.
Case Report:
Fifty-nine years-old female patient presented with seizures, decreased concentration and memory changes. Laboratory workup was remarkable for hyponatremia. Further workup included brain computerized tomography (CT) and magnetic resonance imaging (MRI), which suggested a diagnosis of encephalitis for limbic encephalitis. Anti-Hu, anti-Ma and NMDA-receptor antibodies were requested of which Anti Hu antibodies were positive. Transbronchial biopsy was obtained which confirmed the diagnosis of small cell lung cancer.
Conclusions:
A very high index of suspicion should thus be present when patients present with paraneoplastic abnormalities. It must be emphasized that limbic encephalitis (LE) occurs at an early stage of the disease development and therefore the detection of paraneoplastic LE can lead to a quicker identification of the underlying malignancy and a better outcome.
Keywords: paraneoplastic disorder, seizures and memory changes, limbic system, small cell lung cancer
Background
Paraneoplastic neurological disorders (PND) is a rare disorder that affects less than 1/10,000 patients with cancer. In the majority of cases, PND will occur before the malignancy progresses to an overt stage. Several studies in the last few years have shown that some of the PNDs are associated with antibodies directed against antigens expressed by both the tumor and the nervous system. This discovery suggests the likelihood that these disorders are immune-mediated [1–4].
The presence or the absence of paraneoplastic antibodies and the type of antibodies present may help to diagnose the different forms of PND. The International panel of neurologists has now established new diagnostic criteria for PND [3].The “Classical syndromes” of PND usually are associated with a documented malignancy. These include encephalomyelitis, subacute cerebellar degeneration, opsoclonus-myoclonus, subacute sensory neuropathy, Lambert-Eaton myasthenic syndrome, and paraneoplastic limbic encephalitis (LE). These are thought to be the result of immune mechanisms unrelated to the direct effect of the tumor, metastases, or metabolites.
LE is characterized by the subacute onset of confusion with marked reduction of short-term memory. Seizures are not uncommon and may antedate the onset of cognitive deficit by months [5]. Among LE patients, 50% have small-cell lung cancer (SCLC), 20% have a testicular tumors and 8% have breast cancer; the rest have other cancers. Anti-Hu antibody (Hu-Ab) is present in up to 50% of patients with LE and lung cancer [5,6]. A minority of patients with LE and lung cancer may harbour CV2-Ab or amphiphysin-Ab [7,8].
Case Report
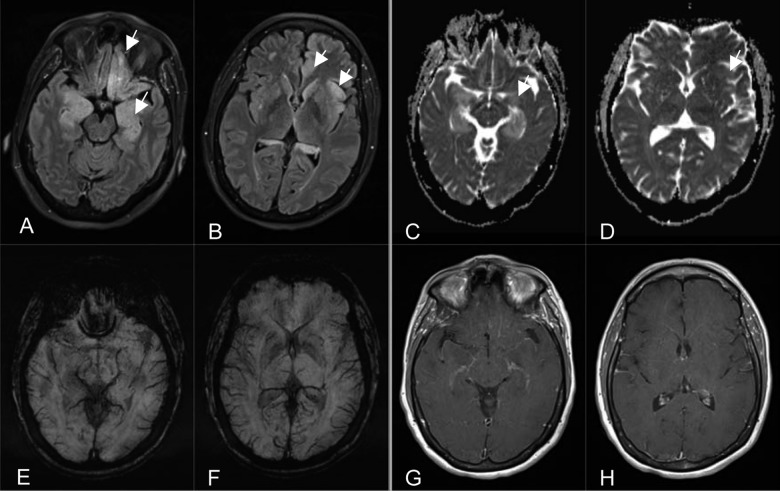
A 59 years old, Hispanic woman first presented to the Emergency Department with intermittent episodes of headaches, anxiety, confusion, agitation and memory changes over a period of one month. Her previous medical history was significant for a stage II right sided breast cancer treated with a partial mastecopmy and chemotherapy seven years prior to this admission. She has a negative history of smoking, alcohol or illegal drug use. Physical examination revealed altered mental status and agitation. There was no other evidence of any focal or global neurologic deficit like numbness, weakness, or cranial nerve abnormalities. Work-up included brain computerized tomography (CT) and magnetic resonance imaging (MRI), which suggested a diagnosis of encephalitis – viral encephalitis or limbic encephalitis (Figure 1). Laboratory workup revealed mild hyponatremia (Na=133). A lumbar puncture was performed for cerebrospinal (CSF) biochemistry, cytology and serology. The patient was started on empiric treatment for herpes encephalitis.
Figure 1.
(A, B) Axial Inversion Recovery MR images (TR/TE/TI=9000 ms/81 ms/2500 ms) demonstrate cortical hyperintensity of the limbic system involving the mesial temporal lobes, hippocampi, amygdalae, left cingulate and orbitofrontal gyri and left insular cortex (arrows), note the minimal white matter involvement disproportional to cortical involvement with loss of gray-white matter interfaces. (C, D) Axial Apparent Diffusion Coefficient maps (TR/TE=7200 ms/104 ms) show hyperintense signal indicating facilitated diffusion on corresponding inversion recovery cortical hyperintensity from T2 shine-through effect involving the limbic system (arrows) without diffusion restriction indicating absence of acute infarction, acute hyponatremic encephalopathy or pyogenic encephalitis. (E, F) Axial susceptibility-weighted MR images (TR/TE=27 ms/20 ms) demonstrate no evidence for hemorrhage, excluding hemorrhagic encephalitides. (G, H) Axial contrast-enhanced T1-weighted MR images (TR/TE=650 ms/8.4 ms) show no abnormal enhancement of the limbic structures.
The patient was hospitalized and subsequently, she developed severe hyponatremia (Na of 118) with onset of intractable seizures and was transferred to the Intensive Care Unit (ICU). After being stabilized the patient was transferred back to the regular medical floor. The likelihood of an underlying malignancy was entertained and workup was initiated including a bilateral mammogram in view of the history of breast cancer (which was negative) and a chest CT due to concerns about lung cancer with hyponatremia and limbic encephalitis. Chest CT showed right paratracheal lymphadenopathy. Anti-Hu, anti-Ma and NMDA-receptor antibodies were requested of which Anti Hu antibodies were positive and the serologies for viral encephalitis were negative. Transbronchial biopsy was performed and indicated the diagnosis of small cell lung cancer. The diagnosis of limbic encephalitis as a presentation of PND due to small cell lung cancer was thus established.
The patient was treated with intravenous immunoglobulin 25 gm daily for 4 days, and methylprednisolone (1000 mg) as well levetiracetam (1000 mg) and received the first cycle of chemotherapy with etoposide and cisplatin for the small cell lung cancer. Patient’s mental status and hyponatremia improved and she was discharged home six days after finishing the chemotherapy cycle on oral prednisone and anti-seizure medications with follow up with oncology for successive chemotherapy cycles. However the patient was readmitted in 2 weeks with febrile neutropenia and bilateral streptococcus pneumonia with influenza. The patient was intubated due to worsening pneumonia and as the patient’s condition worsened, the family opted to withdraw care. The patient passed away 3 days after her second admission.
Discussion
The incidence of malignancy in patients with potential PND varies depending on the disorder and ranges from 5 to 60% [9]. In almost 80% of patients, the PND antedates the diagnosis of cancer by several months, as in the case presented here. Most tumors are diagnosed within 4–6 months of the presentation of PND.
It has been shown that on several occasions, PND is associated with onconeural antibodies. Unfortunately, almost 50% of patients with true PND do not have any of the well-characterized onconeural antibodies [3]. In these patients, early diagnosis of the tumor is frequently difficult, resulting in a significant delay in the treatment of the cancer [10]. In our patient, the Anti Hu antibodies were positive and so the diagnosis was made promptly. The associated LE typically manifests with seizures, mood changes with effect on memory and personality. In PND, the neurologic abnormalities often precede the diagnosis of malignancy. When presenting with unexplained neurologic manifestations, patients with and without risk factors of malignancy, should be investigated for LE and malignancy.
According to the International panel of neurologist, four components are essential for the diagnosis of paraneoplastic syndrome [3,11]. These are the clinical symptoms, a diagnosis of cancer detected within 4 years after the onset of the neurologic symptoms, exclusion of other neurologic causes and one of the following: Cerebrospinal fluid analysis showing the presence of inflammation with negative cytology, Brain-MRI indicating a lesion in the temporal lobe, and/or epileptic activity in the temporal lobes by electroencephalography analysis.
Patients with neurologic abnormalities must be tested for PND by screening anti-neural antibodies in the serum. Since the sensitivity or specificity of these antibodies is not 100%, their absence should not dissuade a clinician from further investigation for possible malignancies [3,12,13]. Anti-HU is the most commonly occurring antibody, found in approximately 50% of cases of LE [14].
To our knowledge, studies have not proven that paraneoplastic antibodies are pathogenic; nevertheless, these antibodies are useful diagnostic markers that help classify the different subtypes of PND. Studies suggest that Yo-Ab, Ma-Ab or Hu-Ab, directed against Yo, Ma and Hu antigens respectively, may be due to T cell mediated destruction of neurons and thus have autoimmune etiology. Further studies are however required to characterize the mechanisms leading to neuronal death in PND.
Radiologic finding of the Brain MRI, characterized by hyperintensity signals on T2-weighted or fluid attenuation inversion recovery (FLAIR) images involving one or both medial temporal lobes,when present, are specific for LE but they are not found in every patient [11]. Twenty-four patients with LE were studied at the Mayo clinic [15]; 13 of them had a diagnosis of SCLC. In 23 patients, cognitive dysfunction was described, 13 patients had seizures and 12 had psychiatric symptoms. Serum paraneoplastic neuronal antibodies were found in 64% of these patients. In our patient the brain MRI showed diffusely increased T2 and FLAIR and cortical hyperintensity of the entire limbic system including the mesial temporal lobes, hippocampus, amygdala, fornix and mammillary bodies. It also demonstrated loss of normal gray-white interfaces of the mesial temporal lobe structures and mildly increased T2 FLAIR hyperintense signal intensity of the orbitofrontal, insular and cingulate cortex on the left. These findings along with the serological confirmation of anti-HU-antibodies are consistent with the diagnosis of limbic encephalitis.
Treatment consists of treating the underlying cancer with surgery, radiotherapy, hormonal medications or chemotherapy that removes the origin of the antibodies and may alleviate the symptoms. Studies have shown that the effect on LE may however be less successful since this is considered to be an immune response to the neurons [16]. Thus immunosuppression with steroids, IVIG, plasma exchange or cyclophosphamide has shown to be useful. Although, the data is limited, in most observed cases the outcome is not satisfactory [11].
Conclusions
This case brings to our attention, the uncommon presentation of some of the common cancers like the lung, breast and testicles. These tumors may present with neurologic manifestations which are due to autoimmune response to the neurons of the brain. Early diagnosis and treatment is very vital as it may lead to improvement of the PND. Diagnostic criteria by Graus and Saiz [17] for paraneoplastic limbic encephalitis were revised in 2005. This includes 1) subacute onset (<12 weeks) of seizure or confusion, 2) neuropathologic or radiologic evidence of involvement of the limbic system, 3) exclusion of other etiologies of limbic dysfunction, and 4) demonstration of cancer within 5 years of the diagnosis of neurologic symptoms in association with a well-characterized paraneoplastic antibody (Hu, Ma2, CV2, amphiphysin, Ri). All these diagnostic inclusion criteria were available in our patient. She had good cognitive improvement with the combination of steroids, IVIG and chemotherapy. A delayed establishment of LE is unfortunately common. A very high index of suspicion should thus be present when patients present with such paraneoplastic abnormalities. It must be emphasized that LE occurs at an early stage of the disease development and therefore the detection of paraneoplastic LE can lead to a quicker identification of the underlying malignancy and a better outcome.
References:
- 1.Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349:1543–54. doi: 10.1056/NEJMra023009. [DOI] [PubMed] [Google Scholar]
- 2.Vianello M, Vitaliani R, Pezzani R, et al. The spectrum of antineuronal autoantibodies in a series of neurological patients. J Neurol Sci. 2004;220:29–36. doi: 10.1016/j.jns.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Graus F, Delattre JY, Antoine JC, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. 2004;75:1135–1140. doi: 10.1136/jnnp.2003.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheid R, Honnorat J, Delmont E, et al. A new anti-neuronal antibody in a case of paraneoplastic limbic encephalitis associated with breast cancer. J Neurol Neurosurg Psychiatry. 2004;75:338–40. [PMC free article] [PubMed] [Google Scholar]
- 5.Alamowitch S, Graus F, Uchuya M, et al. Limbic encephalitis and small cell lung cancer. Clinical and immunological features. Brain. 1997;120:923–28. doi: 10.1093/brain/120.6.923. [DOI] [PubMed] [Google Scholar]
- 6.Sculier JP, Feld R, Evans WK, et al. Neurologic disorders in patients with small cell lung cancer. Cancer. 1987;60:2275–83. doi: 10.1002/1097-0142(19871101)60:9<2275::aid-cncr2820600929>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Honnorat J, Antoine JC, Derrington E, et al. Antibodies to a subpopulation of glial cells and a 66 kD developmental protein in patients with paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatr. 1996;61:270–78. doi: 10.1136/jnnp.61.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antoine JC, Absi L, Honnorat J, et al. Antiamphiphysin antibodies are associated with various paraneoplastic neurological syndromes and tumors. Arch Neurol. 1999;56:172–77. doi: 10.1001/archneur.56.2.172. [DOI] [PubMed] [Google Scholar]
- 9.Posner JB. Neurologic complications of cancer. Philadelphia: FA: Davis Company; 1995. [Google Scholar]
- 10.Graus F, Keime-Guibert F, Rene R, et al. Anti-Hu-associated paraneoplastic encephalomyelitis: analysis of 200 patients. Brain. 2001;124:1138–48. doi: 10.1093/brain/124.6.1138. [DOI] [PubMed] [Google Scholar]
- 11.Gultekin SH, Rosenfeld MR, Voltz R, et al. Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain. 2000;123:1481–94. doi: 10.1093/brain/123.7.1481. [DOI] [PubMed] [Google Scholar]
- 12.Graus F, Dalmou J, Reñé R, et al. Anti-Hu antibodies in patients with small-cell lung cancer: association with complete response to therapy and improved survival. J Clin Oncol. 1997;15:2866–72. doi: 10.1200/JCO.1997.15.8.2866. [DOI] [PubMed] [Google Scholar]
- 13.Dalmau J, Graus F, Rosenblum MK, Posner JB. Anti-Hu associated paraneoplastic encephalomyelitis/sensory neuronopathy: a clinical study of 71 patients. Medicine (Baltimore) 1992;71:59–72. doi: 10.1097/00005792-199203000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Alamowitch S, Graus F, Uchuya M, et al. Limbic encephalitis and small cell lung cancer. Clinical and immunological features. Brain. 1997;120(6):923–28. doi: 10.1093/brain/120.6.923. [DOI] [PubMed] [Google Scholar]
- 15.Lawn ND, Westmoreland BF, Kiely MJ, et al. Clinical, magnetic resonance imaging, and electroencephalographic findings in paraneoplastic limbic encephalitis. Mayo Clin Proc. 2003;78(11):1363–68. doi: 10.4065/78.11.1363. [DOI] [PubMed] [Google Scholar]
- 16.Sabin TD, Jednacz JA, Staats PN. Case records of the Massachusetts General hospital. Case 26-2008: A 26-year old woman with headache and behavioural changes. N Engl J Med. 2008;359(8):842–53. doi: 10.1056/NEJMcpc0804644. [DOI] [PubMed] [Google Scholar]
- 17.Graus F, Saiz A. Limbic encephalitis: a probably under-recognized syndrome. Neurologia. 2005;20:24–30. [PubMed] [Google Scholar]