Abstract
Helicobacter pylori VacA is a secreted protein toxin that may contribute to the pathogenesis of peptic ulcer disease and gastric adenocarcinoma. When added to cultured mammalian cells in the presence of weak bases (e.g., ammonium chloride), VacA induces the formation of large cytoplasmic vacuoles. Here, we report a previously unrecognized capacity of VacA to induce clustering and perinuclear redistribution of late endocytic compartments. In contrast to VacA-induced cell vacuolation, VacA-induced clustering and redistribution of late endocytic compartments are not dependent on the presence of weak bases and are not inhibited by bafilomycin A1. VacA mutant toxins defective in the capacity to form anion-selective membrane channels fail to cause clustering and redistribution. VacA-induced clusters of late endocytic compartments undergo transformation into vacuoles after the addition of ammonium chloride. VacA-induced clustering and redistribution of late endocytic compartments occur in cells expressing wild-type or constitutively active Rab7, but not in cells expressing dominant-negative mutant Rab7. In VacA-treated cells containing clustered late endocytic compartments, overexpression of dominant-negative Rab7 causes reversion to a nonclustered distribution. Redistribution of late endocytic compartments to the perinuclear region requires a functional microtubule cytoskeleton, whereas clustering of these compartments and vacuole formation do not. These data provide evidence that clustering of late endocytic compartments is a critical mechanistic step in the process of VacA-induced cell vacuolation. We speculate that VacA-induced alterations in late endocytic membrane traffic contribute to the capacity of H. pylori to persistently colonize the human gastric mucosa.
INTRODUCTION
Helicobacter pylori is a Gram negative bacterium that inhabits the mucus layer of the human gastric mucosa. Infection with H. pylori is a strong risk factor for the development of peptic ulcer disease as well as adenocarcinoma of the distal stomach (Dunn et al., 1997; Cover et al., 2001; Suerbaum and Michetti, 2002). An important H. pylori virulence factor is a secreted protein toxin known as VacA (Atherton et al., 2001; Papini et al., 2001; Montecucco and de Bernard, 2003). Expression of VacA contributes to the capacity of H. pylori to colonize the gastric mucosa (Salama et al., 2001). Specific subtypes of VacA cause gastric mucosal injury and may contribute to the pathogenesis of peptic ulceration and gastric cancer (Telford et al., 1994; Atherton et al., 1995; Figueiredo et al., 2002; Fujikawa et al., 2003).
The H. pylori vacA gene encodes a 140-kDa precursor protein, which is cleaved at both the amino terminus and carboxyl terminus to yield a mature 88 kDa secreted toxin (Cover and Blaser, 1992; Cover et al., 1994; Telford et al., 1994). Monomeric forms of VacA assemble into flower-shaped oligomeric structures with a molecular mass of ∼1000 kDa (Lupetti et al., 1996; Cover et al., 1997). On exposure to acidic or alkaline pH conditions, VacA oligomers dissociate into monomeric components (Cover et al., 1997; Yahiro et al., 1999). Acid- or alkaline-activation markedly enhances the capacity of VacA to enter cells and to cause cytotoxic effects (de Bernard et al., 1995; McClain et al., 2000).
One of the most extensively studied activities of VacA is its capacity to cause formation of large cytoplasmic vacuoles in cultured mammalian cells (Atherton et al., 2001; Papini et al., 2001; Montecucco and de Bernard, 2003). The first step in this process is presumed to be binding of VacA to the plasma membrane of mammalian cells. VacA binds to multiple components on the surface of eukaryotic cells, including RPTPβ(PTPrz) (Yahiro et al., 1999; Fujikawa et al., 2003), RPTPα (Yahiro et al., 2003), and lipid raft membrane microdomains (Patel et al., 2002; Schraw et al., 2002), and is subsequently internalized by cells (Garner and Cover, 1996; McClain et al., 2000; Ricci et al., 2000; Patel et al., 2002). Intracellular expression of VacA in cells transiently transfected with VacA-encoding plasmids results in cell vacuolation, which suggests that vacuole formation is the consequence of VacA action in an intracellular site (de Bernard et al., 1997; Ye et al., 1999). Indirect immunofluorescence studies have shown that the vacuoles formed in response to VacA contain Rab7, Lgp110, and vacuolar ATPase as components of the vacuolar membranes (Papini et al., 1994; Papini et al., 1996; Molinari et al., 1997), which suggests that the vacuolar membranes are derived from late endosomal and/or lysosomal compartments.
The formation of vacuoles in response to purified VacA is dependent on the presence of supplemental weak bases such as ammonium chloride in the tissue culture medium (Cover and Blaser, 1992; Cover et al., 1992; Ricci et al., 1997; Morbiato et al., 2001) and is inhibited by bafilomycin A1, an inhibitor of the vacuolar ATPase (Cover et al., 1993; Papini et al., 1993). One model for the mechanism of VacA-induced vacuole formation proposes that VacA inserts into endosomal membranes to form anion-selective membrane channels (Czajkowsky et al., 1999; Iwamoto et al., 1999; Szabo et al., 1999). Channel formation is proposed to result in an influx of anions into the endosomes, which stimulates increased proton pumping by the vacuolar ATPase (Papini et al., 2001; Montecucco and de Bernard, 2003). Protonated membrane-permeant weak bases, such as ammonium chloride, then accumulate inside endosomes, and osmotic swelling results in vacuole formation. In support of this model, mutational studies have provided evidence that the channel-forming activity of VacA is essential for vacuole formation (McClain et al., 2003).
Previous studies have reported that purified VacA does not produce any detectable alterations in cellular morphology if the toxin is added to cells in the absence of supplemental weak bases (Cover and Blaser, 1992; Cover et al., 1992; Ricci et al., 1997; Morbiato et al., 2001). We reasoned that if VacA were added to cells under conditions that are not permissive for vacuole formation (e.g., the absence of supplemental weak bases), it might be possible to detect subtle effects of the toxin on endocytic processes that are obscured by the extensive changes in cellular architecture associated with VacA-induced vacuolation. The identification of any such effects would potentially be helpful for understanding the mechanism of VacA action. We report here a previously unrecognized capacity of VacA to induce clustering and redistribution of late endocytic compartments. We propose that these effects of VacA on late endocytic membrane traffic are critical mechanistic steps in the process of VacA-induced cell vacuolation.
MATERIALS AND METHODS
Purification of VacA
VacA was purified from broth culture supernatant of H. pylori strain 60190 (ATCC 49503) as described previously (Cover et al., 1997; McClain et al., 2000). Acid activation of purified VacA was performed by adding 200 mM HCl dropwise until the pH was reduced to 3.0 (de Bernard et al., 1995; Cover et al., 1997; McClain et al., 2000).
Antibodies and Reagents
Mouse anti-lysosomal associated membrane protein (LAMP-1) and anti-early endosome antigen 1 (EEA1) monoclonal antibodies were from BD Biosciences (Franklin Lakes, NJ). The rabbit anti-cathepsin D polyclonal antibody was from Biomeda (Foster City, CA). The mouse anti-VacA mAb 5E4 was described previously (Vinion-Dubiel et al., 2001). Affinity-purified rabbit anti-Rab7 and anti-Rab9 polyclonal antibodies were obtained from Dr. M. Zerial (Papini et al., 1994). The anti-giantin antibody was obtained from Dr. Ed Chan (University of Florida, Gainesville, FL). Secondary Cy3- or Alexa 488-conjugated anti-mouse and anti-rabbit antibodies were from Molecular Probes (Eugene, OR). Cycloheximide, bafilomycin A1, nocodazole, colchicine, and cytochalasin D were from Sigma-Aldrich (St. Louis, MO). LysoTracker Red DND-99 and 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate low-density lipoprotein (DiI LDL) were from Molecular Probes (Eugene, OR).
Transient Transfection Methodology
HeLa cells were grown in Eagle's medium supplemented with 10% fetal bovine serum, 2 mM glutamine, and an antibiotic mixture (Sigma-Aldrich) at 37°C in a 5% CO2 incubator. AGS cells (ATCC CRL-1739) were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum. In experiments with fixed cells, cells were grown on 18-mm circular cover glasses (VWR, Suwanee, GA). In experiments with live cells, cells were grown on cover glasses mounted at the bottom of 35-mm tissue culture dishes (MatTek, Ashland, MA). The cells were transfected using Effectene transfection reagent (QIAGEN, Valencia, CA) according to the manufacturer's instructions. Enhanced green fluorescent protein (pEGFP)-Rab plasmids used in this study, including pEGFP-Rab5, pEGFP-Rab7, pEGFP-Rab11a, pEGFP-Rab7T22N, and pEGFP-Rab7Q67L, have been described previously (Chen and Wandinger-Ness, 2001; Feng et al., 2001). Rab7Q67L is a constitutively active mutant of Rab7 that is in a GTP-locked state. Rab7T22N is a dominant-negative mutant that lacks the capacity to bind GTP and become activated (Feng et al., 1995).
VacA-induced Vacuolation of HeLa Cells
Purified VacA was acid-activated as described above, diluted and neutralized in tissue culture medium, and then added to cells (5 μg/ml final concentration, unless stated otherwise). Cells were incubated with VacA for varying time intervals at 37°C either in the presence or absence of supplemental ammonium chloride (5 mM), as described in the text. Cell vacuolation was visualized by using an inverted light microscope or by differential interference contrast with a confocal laser scanning microscope.
Indirect Immunofluorescence Methodology
Indirect immunofluorescence studies were performed using previously described methods (Garner and Cover, 1996). In brief, cells were fixed with 3.7% formaldehyde for 10 min at room temperature. Then, cells were permeabilized with 100% methanol at -20°C for 20 min. Cells were subsequently blocked with 5% goat serum (Sigma-Aldrich) at room temperature for 15 min before incubation with primary and secondary antibodies for 1 h, respectively, at room temperature. The anti-VacA antibody 5E4 was used at a concentration of 0.2 μg/ml. The anti-Rab7 antibody was diluted 1:80, anti-EEA1 1:1000, anti-Rab9 1:150, and anti-cathepsin D and anti-LAMP-1 1:50. The secondary Cy3- or Alexa 488-conjugated anti-mouse or anti-rabbit antibodies were diluted 1:500. For double labeling studies, fixed cells were incubated with primary and secondary antibodies against the first target antigen for 1 h, respectively, and then incubated with primary and secondary antibodies against the second target antigen for 1 h, respectively. Cover glasses were washed with phosphate-buffered saline, mounted on slides with Aqua-Polymount (Polysciences, Warrington, PA), and viewed with a confocal laser scanning microscope.
Uptake of DiI LDL and LysoTracker Red DND-99
For experiments with DiI LDL, HeLa cells growing on cover glasses mounted to the bottom of tissue culture dishes (MatTek) were incubated at 37°C with 10 μg/ml DiI LDL for 30 min. Then, cells were washed and incubated with Eagle's medium for 1 h before live cell imaging by using a confocal laser scanning microscope. For experiments with LysoTracker Red DND-99, HeLa cells were incubated with 100 nM LysoTracker Red DND-99 for 30 min before live cell imaging.
Microscopic Imaging Methodology
Cells were visualized with LSM 410 or LSM 510 confocal laser scanning inverted microscopes (Carl Zeiss, Jena, Germany) equipped with an internal He/Ne laser and an external Ar/Kr laser, using a 100×/1.30 Plan-neofluor oil lens. Green fluorescent protein (GFP) and Alexa 488 were excited at 488 nm. Cy3, LysoTracker Red DND-99, and DiI LDL were excited at 568 nm. All microscopic images represent analyses of fixed cells, except for images of live cells treated with DiI LDL or LysoTracker Red DND-99. Images were edited using Adobe PhotoShop 7.0.
RESULTS
Formation of Cellular Vacuoles in Response to VacA
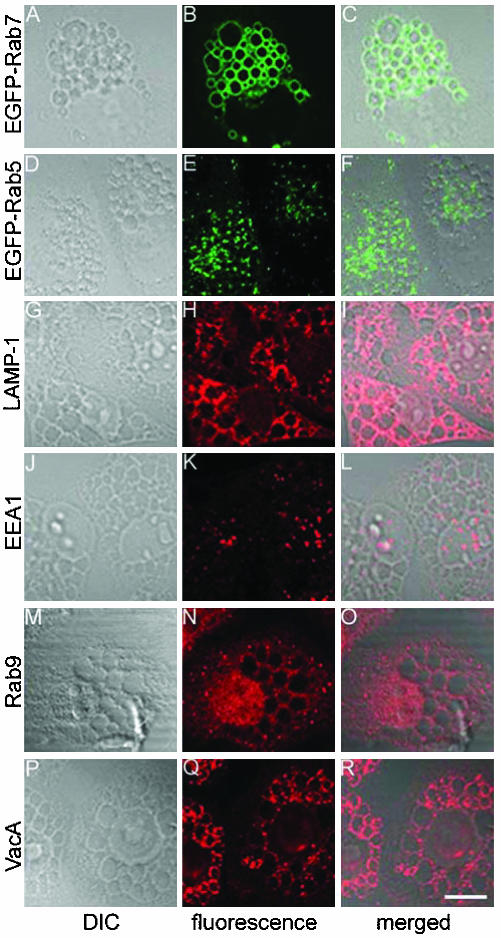
When added to HeLa cells in the presence of supplemental ammonium chloride, purified acid-activated VacA induces formation of large cytoplasmic vacuoles (Atherton et al., 2001; Papini et al., 2001; Montecucco and de Bernard, 2003). We confirmed a previous report (Papini et al., 1994) indicating that endogenous Rab7 (detected by indirect immunofluorescence methodology with an anti-Rab7 antibody) localizes to the vacuolar membranes in VacA-treated cells (our unpublished data). Similarly, in cells that had been transfected with plasmids encoding EGFP-Rab7, VacA treatment caused the formation of vacuoles, and the membranes of these vacuoles were labeled with EGFP-Rab7 (Figure 1, A-C). In contrast, EGFP-Rab5 did not localize to vacuole membranes (Figure 1, D-F). The vacuolar membranes in VacA-treated cells were enriched in LAMP-1 (Figure 1, G-I), although in contrast to Rab7, LAMP-1 seemed to be adherent to the external surface of vacuoles rather than an integral component of the vacuole membranes. The early endosomal marker EEA1 as well as EGFP-Rab11a (located on recycling early endosomes) (Chen and Wandinger-Ness, 2001) and Rab9 (cycling between late endosomes and Golgi apparatus) (Barbero et al., 2002) were distributed in a punctate pattern throughout the cytoplasm in vacuolated cells instead of associated with vacuolar membranes (Figure 1, J-O; our unpublished data). VacA was internalized by cells and localized in association with vacuolar membranes (Figure 1, P-R), in a distribution similar to that of Rab7 and LAMP-1. The enrichment of Rab7 and LAMP-1 in the membranes of VacA-induced vacuoles suggests that the vacuolar membranes are derived from late endocytic compartments.
Figure 1.
Analysis of intracellular vacuoles formed in response to VacA. (A-F) HeLa cells were transiently transfected with pEGFP-Rab7 or pEGFP-Rab5. Twenty-four hours after transfection, cells were treated with 5 μg/ml acid-activated VacA and 5 mM ammonium chloride for 20 h. Cells were then fixed and the localization of EGFP-Rab7 (A-C) and EGFP-Rab5 (D-F) was analyzed using confocal microscopy. (G-R) Nontransfected HeLa cells were treated with VacA and ammonium chloride as described above. The localization of LAMP-1 (G-I), EEA1 (J-L), Rab9 (M-O), and VacA (P-R) was analyzed using indirect immunofluorescence methodology and confocal microscopy. C, F, I, L, O, and R show superimposed differential interference contrast and fluorescence images. EGFP-Rab7, LAMP-1, and VacA localized to the vacuolar membranes, whereas EGFP-Rab5, EEA1, and Rab9 did not. Bar, 10 μm.
VacA-induced Redistribution of Markers for Late Endocytic Compartments
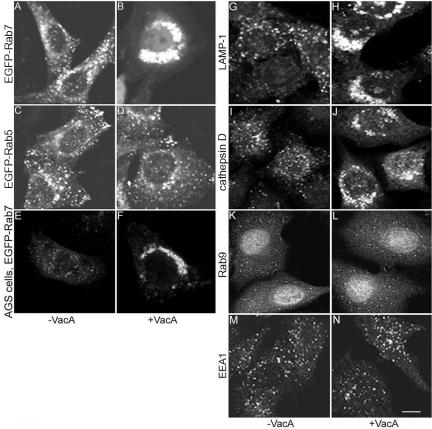
In the next series of experiments, we sought to determine whether treatment of cells with VacA in the absence of supplemental ammonium chloride results in any detectable alterations in endocytic compartments. HeLa cells were transiently transfected with pEGFP-Rab7 or pEGFP-Rab5, and then incubated either with VacA or medium alone for 20 h. In control cells that were not treated with VacA, each of these Rab GTPases was localized in a punctate distribution, which is consistent with the expected distribution of endosomal compartments (Figure 2, A and C). As expected, when acid-activated VacA was added to cells in the absence of supplemental ammonium chloride, no large vacuoles were seen and the cells seemed morphologically identical to untreated cells when examined by light microscopy (Cover and Blaser, 1992; Cover et al., 1992; Ricci et al., 1997; Morbiato et al., 2001). However, EGFP-Rab7 fluorescent signals in the VacA-treated cells were localized in dense perinuclear aggregates, indicating that the intracellular localization of Rab7 was markedly altered in response to VacA (Figure 2B). Indirect immunofluorescence microscopy studies using an anti-Rab7 antibody indicated that the localization of endogenous Rab7 in nontransfected cells was similarly altered in response to VacA (our unpublished data). In contrast, there was no apparent change in the intracellular localization of EGFP-Rab5 (Figure 2D) after VacA treatment. In a dose-response study, we found that the degree of compactness of the perinuclear EGFP-Rab7-labeled compartments was dependent on the concentration of VacA. VacA concentrations of at least 1 μg/ml were required to induce detectable redistribution of EGFP-Rab7 in more than half of transfected cells after treatment for 20 h. The intensity of the EGFP-Rab7 signal seemed to be enhanced in VacA-treated cells compared with untreated control cells, but no significant change in the total cellular level of EGFP-Rab7 was detected in immunoblot analyses by using an anti-GFP antibody (our unpublished data). Treatment of EGFP-Rab7-expressing cells with 20 μg/ml cycloheximide, a protein synthesis inhibitor, did not block the capacity of VacA to induce redistribution of EGFP-Rab7, indicating that the dense aggregates of EGFP-Rab7 do not result from enhanced synthesis of EGFP-Rab7, but instead represent a redistribution of preexisting EGFP-Rab7. Redistribution of EGFP-Rab7 was consistently observed when acid-activated VacA was added to transfected cells, but it was not seen after incubation of cells with native (nonactivated) VacA or acidified buffer control. Acid-activated VacA also caused redistribution of EGFP-Rab7 in transfected AGS cells, a cell line that was originally derived from a human gastric adenocarcinoma, similar to the redistribution observed in HeLa cells (Figure 2, E and F).
Figure 2.
Redistribution of late endocytic markers in response to VacA. (A-D) HeLa cells were transiently transfected with pEGFP-Rab7 (A and B) or pEGFP-Rab5 (C and D). Twenty-four hours after transfection, cells were either treated with 5 μg/ml acid-activated VacA in the absence of supplemental ammonium chloride (B and D) or left untreated (A and C) for 20 h. (E and F) AGS cells were transiently transfected with pEGFP-Rab7 and then treated with VacA (F) or left untreated (E). (G-N) Nontransfected HeLa cells were treated with 5 μg/ml acid-activated VacA (H, J, L, and N) or left untreated (G, I, K, and M). Twenty hours later cells were fixed, permeabilized, and stained with antibodies to LAMP-1 (G and H), cathepsin D (I and J), Rab9 (K and L), or EEA1 (M and N). VacA induced redistribution of EGFP-Rab7, LAMP-1, and cathepsin D from a scattered distribution to perinuclear aggregates, but it did not affect the localization of EGFP-Rab5, Rab9, or EEA1. Bar, 10 μm.
We next investigated whether the distribution of other endocytic markers in HeLa cells was altered in response to VacA treatment, by using indirect immunofluorescence methodology. After overnight VacA treatment (18-20 h), two lysosomal markers, LAMP-1 and cathepsin D, each became concentrated in the perinuclear area instead of scattered throughout the cytoplasm (Figure 2, G-J), similar to the observed redistribution of Rab7. In contrast, endogenous Rab9 and the early endosomal marker EEA1 did not change localization after VacA treatment (Figure 2, K-N). A double-labeling study of VacA-treated HeLa cells showed that EGFP-Rab7 colocalized with LAMP-1 in the perinuclear aggregates (our unpublished data). Perinuclear aggregates containing Rab7 and LAMP-1 were located in close proximity to the Golgi apparatus, but there was no colocalization of Rab7 with the trans-Golgi marker giantin in double-labeling studies. These data indicate that VacA changes the localization of multiple late endocytic markers, but it does not alter the distribution of early endosomal markers or Rab9.
Functional Characterization of VacA-induced Perinuclear Vesicle Aggregates
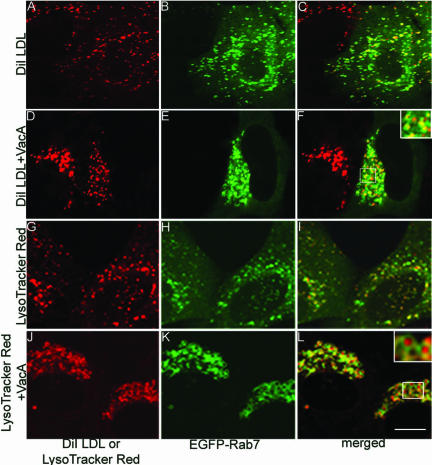
To determine whether the VacA-induced perinuclear structures are endocytically active or sequestered from the endocytic pathway, we analyzed the intracellular trafficking of DiI LDL, which is known to undergo endocytosis, dissociation from its receptor, and transport to lysosomes (Dunn and Maxfield, 1992). After incubation with control cells, DiI LDL colocalized with EGFP-Rab7, indicating arrival in late endocytic vesicles through endocytic trafficking (Figure 3, A-C). In cells that had been treated with VacA for 20 h in the absence of ammonium chloride, vesicles labeled with EGFP-Rab7 were clustered in the perinuclear area as expected (Figure 3E); after incubation of these cells with DiI LDL, DiI LDL was detected within the clustered EGFP-Rab7-labeled perinuclear compartments (Figure 3, D-F). These data indicate that the VacA-induced perinuclear vesicle aggregates communicate with the endocytic pathway and receive endocytosed substances.
Figure 3.
Uptake of DiI LDL and accumulation of LysoTracker Red in aggregated late endocytic compartments. (A-F) After transfection of HeLa cells with pEGFP-Rab7 for 24 h, cells were incubated with 5 μg/ml acid-activated VacA for 20 h (D-F) or left untreated (A-C). Cells then were incubated with 10 μg/ml DiI LDL for 30 min, washed, and incubated for an additional 1 h in the absence of DiI LDL. Left, localization of DiI LDL; middle, EGFP-Rab7; and right, merged images. (G-L) After transfection of cells with pEGFP-Rab7 for 24 h, cells were incubated with 5 μg/ml acid-activated VacA for 20 h (J-L) or left untreated (G-I). Cells were then incubated with 100 nM LysoTracker Red DND-99 for 30 min. Left, localization of LysoTracker Red; middle, EGFP-Rab7; and right, merged images. Bar, 10 μm.
We also examined the uptake into cells of LysoTracker Red, a fluorescent acidotropic probe that is permeant to cell membranes at neutral pH and localizes within acidic organelles in live cells. In control cells not treated with VacA, LysoTracker-labeled vesicles were scattered throughout the cytosol (Figure 3, G-I). In cells treated with VacA for 20 h in the absence of ammonium chloride, LysoTracker Red accumulated in the perinuclear-aggregated compartments surrounded by a rim of EGFP-Rab7 (Figure 3, J-L), indicating that the intracompartmental pH of the aggregated vesicles is acidic.
Analysis of Mutant VacA Toxins
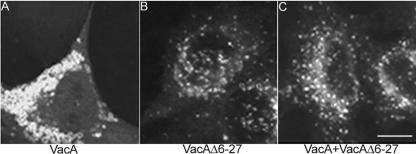
We next tested the capacity of a mutant VacA protein, VacAΔ6-27, to alter the distribution of Rab7. In previous studies, we have shown that this mutant VacA protein assembles into oligomeric structures and is internalized by HeLa cells, but it is defective in the capacity to form membrane channels and lacks the capacity to induce cell vacuolation (Vinion-Dubiel et al., 1999). In addition, VacAΔ6-27 exhibits a dominant-negative phenotype, i.e., it blocks the vacuolating activity of the wild-type toxin (Vinion-Dubiel et al., 1999). When added to HeLa cells that had been transiently transfected with pEGFP-Rab7, acid-activated VacAΔ6-27 failed to induce any redistribution of either Rab7 (compare Figure 4, A and B) or LAMP-1 (our unpublished data). In addition, when added to cells together with wild-type VacA in a 1:1 ratio, VacAΔ6-27 inhibited the capacity of wild-type VacA to induce Rab7 redistribution (Figure 4C). We also tested the capacity of three additional mutant toxins (VacAP9A, VacAG14A, and VacAG18A) to alter the distribution of Rab7. In previous studies, we have shown that these three toxins with single amino acid substitutions assemble into oligomeric structures but are defective in the capacity to form membrane channels and are unable to cause cell vacuolation (McClain et al., 2003). When added to HeLa cells at a concentration of 5 μg/ml, each of the mutant toxins failed to cause Rab7 redistribution (our unpublished data). These data indicate that an intact N-terminal region of VacA is essential not only for anion-selective membrane channel formation and cell vacuolation but also for clustering and redistribution of late endocytic compartments.
Figure 4.
Rab7 redistribution in response to wild-type VacA or VacAΔ6-27. HeLa cells were transiently transfected with pEGFP-Rab7. Twenty-four hours later, cells were treated for 18 h with either 5 μg/ml wild-type VacA (A), 5 μg/ml VacAΔ6-27 (B), or a mixture of wild-type VacA and VacAΔ6-27 in a 1:1 ratio (C). All VacA preparations were acid-activated before addition to cells. Wild-type VacA induced the redistribution of EGFP-Rab7 from dispersed endocytic vesicles to perinuclear aggregates (A), whereas neither VacAΔ6-27 (B) nor the 1:1 mixture (C) caused such a change. Bar, 10 μm.
Bafilomycin Blocks VacA-induced Vacuolation but Fails to Inhibit VacA-induced Rab7 Redistribution
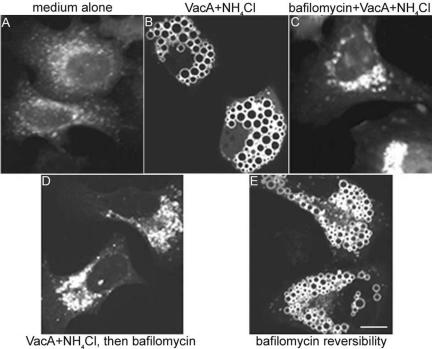
In previous studies, it has been shown that bafilomycin A1, an inhibitor of the vacuolar ATPase, blocks the capacity of VacA to induce cell vacuolation (Cover et al., 1993; Papini et al., 1993). This inhibitory action of bafilomycin A1 has been attributed to neutralization of the acidic pH of endosomal compartments and consequent failure of weak bases to accumulate and cause osmotic swelling (Cover et al., 1993; Papini et al., 1993); there is no evidence that bafilomycin directly inhibits VacA activity. Therefore, we tested whether bafilomycin could block the capacity of VacA to induce redistribution of Rab7. For these experiments, VacA was added to HeLa cells in the presence of supplemental ammonium chloride, and in the presence or absence of 10 nM bafilomycin. When cells were pretreated with bafilomycin, VacA-induced vacuolation was blocked as expected, whereas perinuclear aggregation of EGFP-Rab7-positive compartments still occurred (Figure 5, A-C). This result indicates that activity of the V-type ATPase is not required for VacA-induced clustering and redistribution of these compartments. One interpretation of these results is that cell vacuolation and redistribution of late endocytic compartments might represent two distinct consequences of VacA intoxication. Alternatively, we hypothesized that VacA-induced redistribution of endocytic compartments might represent an early step in the process of vacuole formation.
Figure 5.
Effect of bafilomycin A1 on VacA-induced Rab7 redistribution. HeLa cells were transiently transfected with pEGFP-Rab7. Twenty-four hours after transfection, cells were incubated with medium alone (A), 5 μg/ml acid-activated VacA in the presence of 5 mM supplemental ammonium chloride (B), or 5 μg/ml acid-activated VacA in the presence of 5 mM ammonium chloride and 10 nM bafilomycin (C) for 20 h. Bafilomycin A1, which blocks VacA-induced vacuolation, did not block VacA-induced redistribution of EGFP-Rab7 (C). Alternatively, transfected cells were incubated with 5 μg/ml acid-activated VacA in the presence of 5 mM ammonium chloride for 20 h (similar to B), and vacuolated cells were then treated with 10 nM bafilomycin for 1 h in the presence of VacA and ammonium chloride (D). Vacuoles disappeared in VacA-treated cells after treatment with bafilomycin, and EGFP-Rab7 signals were localized in perinuclear vesicle aggregates (D). Then, bafilomycin was removed from wells containing cells treated under the same conditions as in D, and cells were treated with VacA and ammonium chloride for 5 h. Vacuoles reappeared, with EGFP-Rab7 localized to the vacuolar membranes (E). Bar, 10 μm.
Relationship between VacA-induced Vesicle Aggregation and Cell Vacuolation
To investigate further whether there is a relationship between VacA-induced aggregation of late endocytic compartments and VacA-induced vacuolation, we performed additional experiments with bafilomycin A1. In agreement with previous reports (Cover et al., 1993; Papini et al., 1993), we found that VacA-induced vacuoles disappeared after the addition of bafilomycin to vacuolated cells. Concurrently, the EGFP-Rab7-positive vacuoles disappeared and were replaced by punctate perinuclear clustered structures (Figure 5D). Bafilomycin is a reversible inhibitor of vacuolar ATPase (Papini et al., 1993), and therefore we removed bafilomycin from cells that had been treated as shown in Figure 5D, and incubated the cells with medium containing VacA and ammonium chloride. Vacuoles reappeared in these cells and EGFP-Rab7 was detected as a component of the vacuolar membranes (Figure 5E). These results suggest that it is possible for Rab7-labeled perinuclear aggregates to undergo a transition into Rab7-labeled vacuoles, and vice versa.
Dynamics of Late Endocytic Compartment Clustering and Vacuole Formation
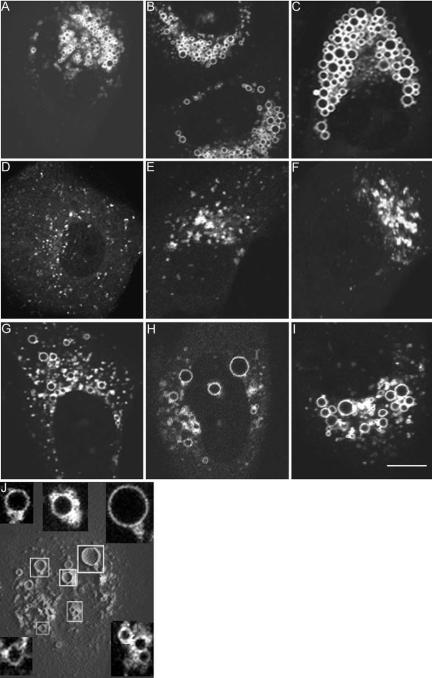
Next, we analyzed the temporal dynamics of late endocytic compartment clustering and vacuole formation. HeLa cells were transfected with EGFP-Rab7 and then treated with VacA for 20 h to induce perinuclear vesicle aggregation (as shown in Figure 2B). Subsequently, at different time points (15-60 min) after the addition of ammonium chloride to these cells, the localization of EGFP-Rab7 was monitored by confocal microscopy. Within 15 min after addition of ammonium chloride to these VacA-treated cells, the compact EGFP-Rab7-labeled vesicles in the perinuclear region began to undergo a transformation into vacuoles (Figure 6A). The vacuolar compartments were initially very small and barely visible by light microscopy. Over time, the vacuolar structures increased in size and expanded toward the periphery of the cells, until they eventually filled the entire cytoplasm (Figure 6, B and C). Addition of ammonium chloride to control cells (not previously treated with VacA) failed to induce this extensive vacuolation. These data provide strong evidence of a structural relationship between VacA-induced perinuclear endosomal aggregates and VacA-induced vacuoles.
Figure 6.
Dynamics of endosomal clustering and vacuole formation. (A-C) HeLa cells transiently transfected with pEGFP-Rab7 were incubated with VacA for 20 h, resulting in perinuclear aggregation of Rab7-positive vesicles. The medium overlying VacA-treated cells then was supplemented with 5 mM ammonium chloride. The formation of EGFPRab7-labeled vacuoles is shown at 15 min (A), 30 min (B), and 1 h (C) after addition of ammonium chloride. (D-F) Cells transfected with pEGFP-Rab7 were treated with 5 μg/ml acid-activated VacA in the absence of supplemental ammonium chloride. Intracellular localization of EGFP-Rab7 was analyzed before VacA treatment (D), at 3 h (E), and 8 h (F) after addition of VacA. (G-I) EGFP-Rab7-expressing cells were incubated with VacA for 3 h, resulting in formation of cytoplasmic clusters of Rab7-positive vesicles similar to E. The medium then was supplemented with 5 mM ammonium chloride. The formation of EGFP-Rab7-labeled, progressively enlarging vacuoles is shown at 15 min (G), 30 min (H), and 1 h (I) after addition of ammonium chloride. J, three-dimensional reconstructed image of H. Insets are magnified to show the development of vacuoles from the vesicle clusters, and the attachment of small vesicles to the nascent enlarging vacuoles within the clusters. Bar, 10 μm.
We next analyzed early kinetic events in the process by which VacA induces redistribution of late endocytic compartments. Clustering of these compartments was detectable within 3 h after addition of VacA to HeLa cells transiently transfected with EGFP-Rab7 (compare Figure 6D with E). Small fluorescent EGFP-Rab7 clusters were detected dispersed throughout the cytoplasm at this time point (Figure 6E), whereas almost all of the EGFP-Rab7-containing late endocytic vesicles were isolated from one another before VacA treatment (Figure 6D). After incubation of cells with VacA for longer periods of time, there was a progressive increase in the size of EGFP-Rab7-positive clusters and a tendency for the clusters to localize near the nucleus. By 8 h after addition of VacA, perinuclear fluorescent aggregates were readily observed in more than one-half of the cells, and nonclustered fluorescent signals in the periphery were markedly diminished in abundance (Figure 6F). Addition of VacA to cells induced clustering and redistribution of LAMP-1-labeled and LysoTracker-labeled endocytic compartments, in a pattern and time course similar to what was observed for Rab7 (our unpublished data). These findings suggest that VacA initially induces clustering of the highly dynamic late endocytic compartments in the periphery of the cell and that the clustered compartments thereafter become sequestered in the perinuclear region. When HeLa cells are simultaneously treated with 5 μg/ml acid-activated VacA and 5 mM ammonium chloride, cytoplasmic vacuoles typically occur within ∼3-4 h. That a similar time period is required for formation of late endosomal clusters in response to VacA in the absence of ammonium chloride (Figure 6E) suggests that late endosomal clustering may be a requisite early event in the process of vacuole formation.
To further dissect early events in the process of VacA-induced clustering of late endocytic compartments and cell vacuolation, cells that had been treated with VacA alone for 3 h (similar to Figure 6E) were subsequently incubated in medium containing 5 mM ammonium chloride. Within 15 min after exposure to ammonium chloride, EGFP-Rab7-labeled clusters began to undergo a transformation into vacuoles (Figure 6G), and over time these vacuoles enlarged until filling the cytoplasm (Figure 6, H and I). Addition of 5 mM ammonium chloride to control cells (not previously treated with VacA) failed to induce such vacuolation. Analysis of three-dimensional reconstructed images confirmed that most of the vacuoles formed within EGFP-Rab7-positive vesicle clusters soon after addition of ammonium chloride; small vesicles were frequently attached to the nascent enlarging vacuoles within the clusters (Figure 6J). These results indicate that vacuoles can form directly from VacA-induced clusters of late endocytic compartments and provide evidence that clustering of late endocytic compartments is an important step in the process of VacA-induced vacuolation.
Role of Rab7 in VacA-induced Aggregation of Late Endocytic Compartments
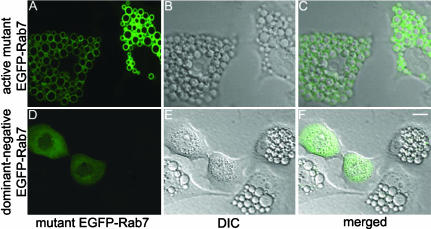
A previous study reported that Rab7 function is required for VacA-induced vacuolation, based on experiments involving transfection of cells with plasmids encoding mutant forms of Rab7 and indirect immunofluorescence methodology (Papini et al., 1997). Using EGFP-tagged Rab7 mutants, we confirmed that overexpression of a dominant-negative Rab7 mutant (Rab7T22N) renders HeLa cells resistant to the vacuolating activity of VacA in the presence of ammonium chloride (Figure 7, D-F), whereas overexpression of an active Rab7 mutant (Rab7Q67L) does not interfere with VacA-induced vacuolation (Figure 7, A-C). To investigate a potential role of Rab7 in VacA-induced clustering of endocytic vesicles, we next analyzed the effects of VacA on cells that had been transfected with these mutant forms of Rab7. In HeLa cells transfected with pEGFP-Rab7Q67L, VacA treatment in the absence of ammonium chloride induced the same pattern of Rab7 redistribution as that which occurred in VacA-treated cells expressing wild-type EGFP-Rab7 (Figure 8, A and B). In contrast, VacA treatment had no detectable effects on the localization of the dominant-negative mutant Rab7, EGFP-Rab7T22N, which remained distributed in a diffuse pattern throughout the cytosol (Figure 8, C and D). In addition to studying the localization of Rab7T22N itself, we studied the localization of LAMP-1 in cells that had been transfected with pEGFP-Rab7T22N. After treatment of such cells for 20 h with VacA, LAMP-1-positive vesicles remained in a punctate distribution instead of forming perinuclear aggregates (our unpublished data).
Figure 7.
Cells expressing dominant-negative Rab7 are resistant to VacA-induced vacuolation. HeLa cells were transiently transfected with pEGFP-Rab7Q67L (constitutively active mutant) (A-C) or pEGFP-Rab7T22N (dominant-negative mutant) (D-F). Twenty-four hours after transfection, HeLa cells were treated with 5 μg/ml acid-activated VacA in the presence of 5 mM ammonium chloride for 8 h. Cells expressing the dominant-negative Rab7 protein (Rab7T22N) did not become vacuolated in response to VacA (F). Bar, 10 μm.
Figure 8.
Effect of VacA on distribution of constitutively active or inactive Rab7. HeLa cells were transiently transfected with pEGFP-Rab7Q67L (encoding constitutively active mutant Rab7) (A and B) or pEGFP-Rab7T22N (encoding dominant-negative mutant Rab7) (C and D). Twenty-four hours after transfection, HeLa cells were treated with 5 μg/ml acid-activated VacA for 20 h without supplemental ammonium chloride (B and D) or left untreated (A and C). VacA induced redistribution of the active mutant EGFP-Rab7Q67L (B), whereas the dominant-negative mutant EGFP-Rab7T22N maintained its diffuse distribution in the cytosol (D). Bar, 10 μm.
Because Rab7 regulates trafficking from early endosomes to late endosomes (Feng et al., 1995), it is possible that the absence of detectable VacA effects (vacuolation and late endosomal clustering) in cells expressing dominant-negative Rab7 might result from disruption of intracellular trafficking of VacA, i.e., VacA may not be able to reach its intracellular target(s). To test this possibility, we examined and compared the internalization, trafficking, and localization of VacA in cells transfected with wild-type EGFP-Rab7 versus in cells transfected with dominant-negative EGFP-Rab7.
When cells expressing wild-type EGFP-Rab7 were incubated with acid-activated VacA for 4 h, most internalized VacA was localized in a punctate distribution throughout the cytoplasm (Figure 9A). With increasing time, the toxin became localized in the perinuclear region (Figure 9, D and G). At early time points the majority of intracellular VacA signals were separated from EGFP-Rab7-associated late endosomes based on double color imaging, although rare colocalization was detected in small clusters of late endosomes (Figure 9C). At later time points, there was a progressive increase in the colocalization of VacA with EGFP-Rab7 (Figure 9F). By 12 h, most VacA colocalized with EGFP-Rab7 in the perinuclear region where late endocytic compartments aggregated (Figure 9I). The mutant toxin VacAΔ6-27 entered cells and was targeted to EGFP-Rab7-containing compartments (Figure 9, J-L), similar to wild-type VacA. However, in cells treated with VacAΔ6-27, neither the toxin nor EGFP-Rab7-positive compartments clustered in the perinuclear region. Thus, both wild-type VacA and VacAΔ6-27 localize in association with Rab7-containing compartments, but the mutant toxin fails to cause clustering and redistribution of late endocytic compartments.
Figure 9.
Time course of VacA trafficking in HeLa cells expressing wild-type Rab7 or dominant-negative Rab7. HeLa cells were transiently transfected with either pEGFP-Rab7 (encoding wild-type Rab7) (A-L), or pEGFP-Rab7T22N (encoding dominant-negative mutant Rab7) (M-O) for 24 h. Then, cells were treated with 5 μg/ml acid-activated wild-type VacA (A-I and M-O) or VacAΔ6-27 (J-L). Intracellular localization of VacA was analyzed by indirect immunofluorescence methodology. The localization of VacA (left), EGFP-Rab7 (B, E, H, and K), and EGFP-Rab7T22N (N) at the indicated time points after VacA treatment is shown. Right, merged images of left and middle. In cells expressing wild-type EGFP-Rab7, wild-type VacA was internalized, clustered in the perinuclear region, and was colocalized with Rab7 (A-I). VacAΔ6-27 was targeted to Rab7-containing compartments in a manner similar to wild-type VacA, but it did not cause clustering of these compartments (J-L). In cells expressing EGFP-Rab7T22N, wild-type VacA did not cluster in the perinuclear region and did not colocalize with Rab7T22N (M-O). Bar, 10 μm.
In cells expressing dominant-negative Rab7 (EGFP-Rab7T22N), wild-type VacA was internalized and localized in a punctate distribution after 3- to 4-h incubation (our unpublished data). However, at later time points, internalized VacA in pEGFP-Rab7T22N-transfected cells remained localized in a punctate distribution throughout the cytoplasm, in sharp contrast to the perinuclear aggregated toxin in nearby nontransfected cells (Figure 9, M-O). These data suggest that there may be differences in the intracellular trafficking of VacA in cells expressing wild-type Rab7 versus in cells expressing dominant-negative Rab7. Therefore, the impaired capacity of VacA to induce vacuolation and late endocytic compartment clustering and redistribution in cells expressing dominant-negative Rab7 may be due in part to disrupted VacA trafficking.
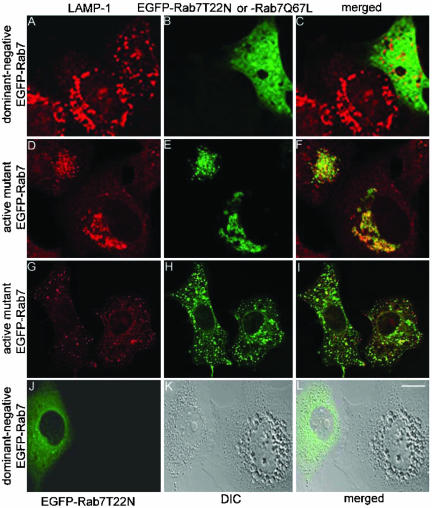
To circumvent a possible disruption of VacA trafficking in cells expressing dominant-negative Rab7, we first treated nontransfected HeLa cells with VacA for 20 h to allow normal internalization and trafficking of the toxin and then cells were transfected with pEGFP-Rab7T22N. In almost all of the VacA-treated cells expressing dominant-negative Rab7, LAMP-1-labeled vesicles were in a dispersed distribution, whereas LAMP-1-labeled perinuclear aggregates were observed in adjacent nontransfected cells (Figure 10, A-C). In contrast, in VacA-treated cells expressing the active mutant Rab7, LAMP-1-labeled vesicles formed perinuclear aggregates and colocalized with EGFP-Rab7Q67L (Figure 10, D-F). When cells were incubated with VacAΔ6-27 and then transfected with pEGFP-Rab7Q67L, no clustering of LAMP-1-labeled vesicles was detected (Figure 10, G-I). Similarly, if cells were treated with an equimolar mixture of wild-type VacA and VacAΔ6-27 and then transfected with pEGFP-Rab7Q67L, no clustering of LAMP-1-labeled vesicles was detected (our unpublished data). Thus, expression of dominant-negative Rab7 in VacA-treated cells results in a nonclustered distribution of LAMP-1-labeled vesicles (Figure 10, A-C). Similarly, expression of EGFP-Rab7T22N in cells that were already vacuolated in response to VacA and ammonium chloride resulted in disappearance of vacuoles in most cells (Figure 10, J-L). These data indicate that a functional form of Rab7 is essential for VacA-induced late endocytic compartment aggregation and also for VacA-induced vacuolation.
Figure 10.
Essential role of Rab7 for VacA-induced clustering of late endocytic compartments and vacuolation. (A-F) HeLa cells were treated with 5 μg/ml acid-activated wild-type VacA for 20 h. Cells were then transiently transfected with either pEGFP-Rab7T22N (A-C) or pEGFP-Rab7Q67L (D-F) for 24 h. Cells were fixed, permeabilized, and stained with an anti-LAMP-1 antibody. In cells expressing the active mutant EGFP-Rab7Q67L or nontransfected cells, perinuclear aggregates of late endocytic compartments formed in response to VacA (A-F). In contrast, in cells expressing the dominant-negative mutant EGFP-Rab7T22N, late endocytic compartments remained localized throughout the cytoplasm without evidence of clustering after treatment with VacA (A-C). (G-I) HeLa cells were treated with 5 μg/ml acid-activated VacAΔ6-27 for 20 h. Cells were then transiently transfected with pEGFP-Rab7Q67L (encoding active mutant Rab7) for 24 h. Cells were fixed, permeabilized, and stained with an anti-LAMP-1 antibody. No clustering or redistribution of late endocytic compartments was detected. (J-L) Cells were treated with 5 μg/ml acid-activated wild-type VacA in the presence of 5 mM ammonium chloride for 18 h. Then, vacuolated cells were transfected with pEGFP-Rab7T22N (encoding dominant-negative mutant Rab7). Vacuolated cells reverted to a nonvacuolated appearance after expression of the dominant-negative Rab7. Bar, 10 μm.
VacA-induced Redistribution of Late Endocytic Compartments Requires Microtubules
The microtubule and actin cytoskeleton have been implicated in vesicular transport and microtubules are important for late endosomal and lysosomal perinuclear aggregation (Matteoni and Kreis, 1987; Apodaca, 2001; Cantalupo et al., 2001). We hypothesized that VacA-induced clustering and redistribution of late endocytic compartments might require a functional microtubule or actin cytoskeleton. To test this hypothesis, we studied the effects of nocodazole and cytochalasin D, agents known to disrupt either the microtubule or actin cytoskeleton, respectively. As a first step, we tested whether either of these agents interfered with the internalization of VacA. HeLa cells were pretreated with 2 μg/ml nocodazole for 1 h or 0.5 μg/ml cytochalasin D for 2 h, incubated with acid-activated VacA for 18 h at 37°C, and then analyzed by indirect immunofluorescence and confocal microscopy. Consistent with the results of a previous report (Ricci et al., 2000), we found that cytochalasin D blocked the internalization of VacA (our unpublished data). Therefore, as expected, treatment of cells with cytochalasin D blocked VacA-induced clustering of late endocytic compartments and perinuclear redistribution of these compartments, as well as VacA-induced vacuolation. In contrast, nocodazole had no detectable effect on VacA internalization.
To determine whether nocodazole had any effect on VacA-induced vacuolation, VacA and ammonium chloride were simultaneously added to cells pretreated with nocodazole. VacA induced the formation of large cytoplasmic vacuoles in cells pretreated with nocodazole, albeit at a rate slightly slower than observed in control cells (our unpublished data). To test whether nocodazole blocked VacA-induced clustering or redistribution of late endocytic compartments, transfected cells expressing pEGFP-Rab7 were treated with nocodazole and then incubated with VacA. As shown in Figure 11, A and B, VacA treatment induced clustering of late endocytic compartments in nocodazole-treated cells, but it failed to induce perinuclear redistribution of these clustered compartments. As a result, clusters of EGFP-Rab7-containing vesicles were visualized throughout the cytoplasm, rather than concentrated in the perinuclear region (compare Figure 11, B and C). Similar results were obtained in experiments in which cells were pretreated with 20 μM colchicine (our unpublished data). Thus, the microtubule cytoskeleton is required for translocation of clustered late endosomes from a scattered distribution in the cytoplasm to the perinuclear region, but it is not required for clustering of these compartments in response to VacA. When ammonium chloride was added to nocodazole-treated cells containing clustered late endocytic compartments (similar to those shown in Figure 11B), vacuoles formed at the site of clustered compartments (Figure 11D). Thus, VacA-induced vacuolation is dependent on clustering of late endocytic compartments, but it does not require perinuclear redistribution of these compartments.
Figure 11.
Effect of nocodazole on VacA-induced redistribution of late endocytic compartments. HeLa cells were transiently transfected with pEGFP-Rab7. (A and B) After pretreatment with 2 μg/ml nocodazole for 1 h, cells were either treated with acid-activated VacA (5 μg/ml) (B) or left untreated (A), and incubated at 37°C for 16 h. (C) Transfected HeLa cells were incubated with VacA for 16 h in the absence of nocodazole. (D) Nocodazole-pretreated cells were incubated with VacA for 16 h and then incubated with 5 mM ammonium chloride for 1 h. Nocodazole did not inhibit VacA-induced clustering of Rab7-containing compartments or development of vacuoles from clustered compartments upon ammonium chloride supplementation, but it did prevent redistribution of clustered compartments to the perinuclear region. Bar, 10 μm.
DISCUSSION
VacA causes multiple cellular effects, including vacuole formation, disruption of endosomal/lysosomal function, membrane channel formation, apoptosis, epithelial monolayer permeabilization, detachment of primary gastric epithelial cells from the basement membrane, and inhibition of T lymphocyte activation (Satin et al., 1997; Papini et al., 1998; Atherton et al., 2001; Papini et al., 2001; Cover et al., 2003; Fujikawa et al., 2003; Gebert et al., 2003; Montecucco and de Bernard, 2003). One of the most intensively studied activities of VacA is its capacity to cause cell vacuolation, but the molecular mechanisms underlying this phenomenon remain incompletely understood. Multiple cellular factors, including vacuolar ATPase, Rab7, RPTPα, RPTPβ, Rac1, dynamin, and syntaxin 7, have been reported to be essential for vacuole formation in response to VacA (Cover et al., 1993; Papini et al., 1993; Papini et al., 1997; Hotchin et al., 2000; Suzuki et al., 2001; Suzuki et al., 2003), and PIKfyve overexpression has been reported to inhibit VacA-induced vacuolation (Ikonomov et al., 2002). However, the exact roles that most of these factors play in the process of vacuole formation have not yet been determined.
Whether VacA-induced vacuole formation involves vesicle fusion events remains controversial and unresolved. Osmotic swelling of lysosomes would be expected to result in rupture of these compartments rather than vacuole formation, unless an additional source of membrane was provided. Therefore, it seems likely that the formation of VacA-induced vacuoles may involve fusion of preexisting membrane-bound compartments. In support of this hypothesis, a recent study reported that syntaxin 7 is required for VacA-induced vacuole formation (Suzuki et al., 2003). In contrast, another study reported that microinjection of anti-syntaxin 7 antibodies into cells did not inhibit vacuole formation and concluded that VacA-induced vacuole formation does not depend on late endosomal soluble N-ethylmaleimide-sensitive factor attachment protein receptors (de Bernard et al., 2002). Based on electron microscopy studies, it was proposed that the vacuoles may form from expansion of the extensive intracompartmental membranes within late endosomes (de Bernard et al., 2002).
Previously, we and other investigators have found that addition of purified VacA to cells in the absence of supplemental weak bases does not result in any morphological changes that are detectable by light microscopy (Cover and Blaser, 1992; Cover et al., 1992; Ricci et al., 1997; Morbiato et al., 2001). However, in the current study, we show that in fact, under these conditions VacA causes clustering and redistribution of several markers for late endocytic compartments. The membrane markers that undergo clustering and redistribution in response to VacA are the same as those that localize to the vacuole membrane in vacuolated cells. Experiments involving addition of ammonium chloride or bafilomycin A1 (Figures 5 and 6) to VacA-treated cells indicate that there is a structural relationship between VacA-induced endosomal clusters and VacA-induced vacuoles. Detectable clustering of Rab7-positive vesicles occurs as early as 3 h after addition of 5 μg/ml VacA to cells (Figure 6E), which is consistent with the minimal time period required for VacA-induced vacuole formation in the presence of ammonium chloride. As shown in Figure 6J, when ammonium chloride is added to VacA-treated cells, the majority of nascent vacuoles arise as expansions of endosomal clusters and rarely arise from single isolated vesicles. These data provide strong evidence suggesting that clustering of late endocytic compartments is a prerequisite for vacuole formation. Clustered vesicles presumably serve as an abundant membrane source for the expanding vacuoles. Thus, we propose that VacA-induced clustering of late endocytic compartments facilitates vesicle fusion and that the combined processes of clustering and fusion are important steps in the process of VacA-induced vacuole formation.
How does VacA induce selective clustering and redistribution of late endocytic compartments? One possible explanation is that VacA itself may become localized on the surface of late endocytic compartments and function to promote docking or fusion of these compartments. In this scenario, VacA may function in a manner analogous to some viral proteins that promote fusion of membranes, such as paramyxoviral envelope proteins or the fusion protein gp41 of human immunodeficiency virus (Colman and Lawrence, 2003). In support of this hypothesis, intracellular VacA is found localized in association with clustered late endosomes (Figure 9). An alternative explanation is that VacA may cause alterations in an intracellular pathway that is normally used for fusion or fission of late endocytic compartments.
Rab GTPases play an important role in vesicle trafficking and fusion events by recruiting tethering and docking factors and soluble N-ethylmaleimide-sensitive factor attachment protein receptors to target membranes. Only two Rab proteins, Rab7 and Rab9, have been localized to late endosomes and lysosomes (Chavrier et al., 1990; Lombardi et al., 1993). Rab7 is present on the membranes of VacA-induced vacuoles, and we demonstrate in the current study that Rab7-positive compartments undergo clustering and redistribution in response to VacA treatment. Cells expressing dominant-negative Rab7 are resistant to VacA-induced redistribution of late endocytic compartments, and expression of dominant-negative Rab7 in VacA-treated cells results in a nonclustered distribution of late endocytic compartments (Figure 10). In contrast, Rab9 does not localize to the membranes of VacA-induced vacuoles (Figure 1), VacA treatment does not change the distribution of Rab9 (Figure 2), and expression of dominant-negative Rab9 does not inhibit VacA-induced vacuole formation (Papini et al., 1997). Thus, Rab7, but not Rab9, plays an important role in the process of VacA-induced clustering and fusion of late endocytic compartments. We speculate that VacA may selectively affect a select group of late endosomes that are Rab7 positive and Rab9 negative, and/or promote the fission of late endosome domains containing Rab7 segregated from Rab9 (Barbero et al., 2002).
Current evidence indicates that Rab7 regulates homotypic and heterotypic fusion events in the late endocytic pathway (Bucci et al., 2000; Stein et al., 2003). Notably, VacA-induced clustering of late endocytic compartments resembles the clustering of late endocytic compartments observed after overexpression of constitutively active Rab7, RILP (a putative Rab7 effector), or Vam6p in mammalian cells (Bucci et al., 2000; Cantalupo et al., 2001; Caplan et al., 2001). We speculate that there may be similarities in the processes by which VacA and these other proteins cause clustering and fusion of late endocytic compartments. Potentially VacA will prove to be a useful tool in further elucidating the processes underlying late endocytic trafficking in mammalian cells.
Finally, it is intriguing to speculate about what potential benefits may arise for H. pylori as a result of VacA-induced alterations in the late endocytic pathway of mammalian cells. Previous studies have reported that VacA causes mistargeting of nascent lysosomal enzymes and a delay in epidermal growth factor receptor degradation in HeLa cells (Satin et al., 1997), and disruption of the Ii-dependent pathway of antigen presentation in B cells (Molinari et al., 1998). In addition, it has been reported that VacA disrupts phagosome maturation in macrophages (Zheng and Jones, 2003) and contributes to the intracellular survival of H. pylori within gastric epithelial cells (Petersen et al., 2001). It seems likely that several of these phenomena are attributable to VacA-induced alterations in the trafficking of late endocytic compartments. Thus, effects of VacA on late endocytic trafficking and function may contribute to the capacity of H. pylori to establish persistent infection in the human gastric mucosa.
Acknowledgments
We thank Chris Aiken, Mark Denison, Luc Van Kaer, Robert Coffey, and members of the Cover laboratory for helpful discussions. This work was supported by National Institutes of Health grants R01 AI39657 and DK-53623 (to T.C.), National Science Foundation MCB9982161 (to A.W.N.), and by the Medical Research Service of the Department of Veterans Affairs (to T.C.).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-08-0618. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-08-0618.
References
- Apodaca, G. (2001). Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Traffic 2, 149-159. [DOI] [PubMed] [Google Scholar]
- Atherton, J.C., Cao, P., Peek, R.M., Jr., Tummuru, M.K., Blaser, M.J., and Cover, T.L. (1995). Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J. Biol. Chem. 270, 17771-17777. [DOI] [PubMed] [Google Scholar]
- Atherton, J.C., Cover, T.L., Papini, E., and Telford, J.L. (2001). Vacuolating cytotoxin. In: Helicobacter pylori: Physiology and Genetics, ed. H.L.T. Mobley, G.L. Mendz, and S.L. Hazell, Washington, DC.: ASM Press. [PubMed]
- Barbero, P., Bittova, L., and Pfeffer, S.R. (2002). Visualization of Rab9-mediated vesicle transport from endosomes to the trans-Golgi in living cells. J. Cell Biol. 156, 511-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci, C., Thomsen, P., Nicoziani, P., McCarthy, J., and van Deurs, B. (2000). Rab 7, a key to lysosome biogenesis. Mol. Biol. Cell 11, 467-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantalupo, G., Alifano, P., Roberti, V., Bruni, C.B., and Bucci, C. (2001). Rab-interacting lysosomal protein (RILP): the Rab7 effector required for transport to lysosomes. EMBO J. 20, 683-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan, S., Hartnell, L.M., Aguilar, R.C., Naslavsky, N., and Bonifacino, J.S. (2001). Human Vam6p promotes lysosome clustering and fusion in vivo. J. Cell Biol. 154, 109-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier, P., Parton, R.G., Hauri, H.P., Simons, K., and Zerial, M. (1990). Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell 62, 317-329. [DOI] [PubMed] [Google Scholar]
- Chen, W., and Wandinger-Ness, A. (2001). Expression and functional analyses of Rab8 and Rab11a in exocytic transport from trans-Golgi network. Methods Enzymol. 329, 165-175. [DOI] [PubMed] [Google Scholar]
- Colman, P.M., and Lawrence, M.C. (2003). The structural biology of type I viral membrane fusion. Nat. Rev. Mol. Cell. Biol. 4, 309-319. [DOI] [PubMed] [Google Scholar]
- Cover, T.L., Berg, D.E., Blaser, M.J., and Mobley, H.L.T. (2001). Helicobacter pylori pathogenesis. In: Principles of Bacterial Pathogenesis, ed. E.A. Groisman, San Diego: Academic Press, 510-558.
- Cover, T.L., and Blaser, M.J. (1992). Purification and characterization of the vacuolating toxin from Helicobacter pylori. J. Biol. Chem. 267, 10570-10575. [PubMed] [Google Scholar]
- Cover, T.L., Hanson, P.I., and Heuser, J.E. (1997). Acid-induced dissociation of VacA, the Helicobacter pylori vacuolating cytotoxin, reveals its pattern of assembly. J. Cell Biol. 138, 759-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover, T.L., Krishna, U.S., Israel, D.A., and Peek, R.M., Jr. (2003). Induction of gastric epithelial cell apoptosis by Helicobacter pylori vacuolating cytotoxin. Cancer Res. 63, 951-957. [PubMed] [Google Scholar]
- Cover, T.L., Reddy, L.Y., and Blaser, M.J. (1993). Effects of ATPase inhibitors on the response of HeLa cells to Helicobacter pylori vacuolating toxin. Infect. Immun. 61, 1427-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover, T.L., Tummuru, M.K.R., Cao, P., Thompson, S.A., and Blaser, M.J. (1994). Divergence of genetic sequences for the vacuolating cytotoxin among Helicobacter pylori strains. J. Biol. Chem. 269, 10566-10573. [PubMed] [Google Scholar]
- Cover, T.L., Vaughn, S.G., Cao, P., and Blaser, M.J. (1992). Potentiation of Helicobacter pylori vacuolating toxin activity by nicotine and other weak bases. J. Infect. Dis. 166, 1073-1078. [DOI] [PubMed] [Google Scholar]
- Czajkowsky, D.M., Iwamoto, H., Cover, T.L., and Shao, Z. (1999). The vacuolating toxin from Helicobacter pylori forms hexameric pores in lipid bilayers at low pH. Proc. Natl. Acad. Sci. USA 96, 2001-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bernard, M., Arico, B., Papini, E., Rizzuto, R., Grandi, G., Rappuoli, R., and Montecucco, C. (1997). Helicobacter pylori toxin VacA induces vacuole formation by acting in the cell cytosol. Mol. Microbiol. 26, 665-674. [DOI] [PubMed] [Google Scholar]
- de Bernard, M., Moschioni, M., Habermann, A., Griffiths, G., and Montecucco, C. (2002). Cell vacuolization induced by Helicobacter pylori VacA cytotoxin does not depend on late endosomal SNAREs. Cell Microbiol. 4, 11-18. [DOI] [PubMed] [Google Scholar]
- de Bernard, M., Papini, E., de Filippis, V., Gottardi, E., Telford, J., Manetti, R., Fontana, A., Rappuoli, R., and Montecucco, C. (1995). Low pH activates the vacuolating toxin of Helicobacter pylori, which becomes acid and pepsin resistant. J. Biol. Chem. 270, 23937-23940. [DOI] [PubMed] [Google Scholar]
- Dunn, B.E., Cohen, H., and Blaser, M.J. (1997). Helicobacter pylori. Clin. Microbiol. Rev. 10, 720-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn, K.W., and Maxfield, F.R. (1992). Delivery of ligands from sorting endosomes to late endosomes occurs by maturation of sorting endosomes. J. Cell Biol. 117, 301-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, Y., Press, B., Chen, W., Zimmerman, J., and Wandinger-Ness, A. (2001). Expression and properties of Rab7 in endosome function. Methods Enzymol. 329, 175-187. [DOI] [PubMed] [Google Scholar]
- Feng, Y., Press, B., and Wandinger-Ness, A. (1995). Rab 7, an important regulator of late endocytic membrane traffic. J. Cell Biol. 131, 1435-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo, C., et al. (2002). Helicobacter pylori and interleukin 1 genotyping: an opportunity to identify high-risk individuals for gastric carcinoma. J. Natl. Cancer Inst. 94, 1680-1687. [DOI] [PubMed] [Google Scholar]
- Fujikawa, A., et al. (2003). Mice deficient in protein tyrosine phosphatase receptor type Z are resistant to gastric ulcer induction by VacA of Helicobacter pylori. Nat. Genet. 33, 375-381. [DOI] [PubMed] [Google Scholar]
- Garner, J.A., and Cover, T.L. (1996). Binding and internalization of the Helicobacter pylori vacuolating cytotoxin by epithelial cells. Infect. Immun. 64, 4197-4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebert, B., Fischer, W., Weiss, E., Hoffman, R., and Haas, R. (2003). Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science 301, 1099-1102. [DOI] [PubMed] [Google Scholar]
- Hotchin, N.A., Cover, T.L., and Akhtar, N. (2000). Cell vacuolation induced by the VacA cytotoxin of Helicobacter pylori is regulated by the rac1 GTPase. J. Biol. Chem. 275, 14009-14012. [DOI] [PubMed] [Google Scholar]
- Ikonomov, O.C., Sbrissa, D., Yoshimori, T., Cover, T.L., and Shisheva, A. (2002). PIKfyve Kinase and SKD1 AAA ATPase define distinct endocytic compartments. Only PIKfyve expression inhibits the cell-vacuolating activity of Helicobacter pylori VacA toxin. J. Biol. Chem. 277, 46785-46790. [DOI] [PubMed] [Google Scholar]
- Iwamoto, H., Czajkowsky, D.M., Cover, T.L., Szabo, G., and Shao, Z. (1999). VacA from Helicobacter pylori: a hexameric chloride channel. FEBS Lett. 450, 101-104. [DOI] [PubMed] [Google Scholar]
- Lombardi, D., Soldati, T., Riederer, M.A., Goda, Y., Zerial, M., and Pfeffer, S.R. (1993). Rab9 functions in transport between late endosomes and the trans Golgi network. EMBO J. 12, 677-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupetti, P., Heuser, J.E., Manetti, R., Massari, P., Lanzavecchia, S., Bellon, P.L., Dallai, R., Rappuoli, R., and Telford, J.L. (1996). Oligomeric and subunit structure of the Helicobacter pylori vacuolating cytotoxin. J. Cell Biol. 133, 801-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoni, R., and Kreis, T.E. (1987). Translocation and clustering of endosomes and lysosomes depends on microtubules. J. Cell Biol. 105, 1253-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain, M.S., Iwamoto, H., Cao, P., Vinion-Dubiel, A.D., Li, Y., Szabo, G., Shao, Z., and Cover, T.L. (2003). Essential role of a GXXXG motif for membrane channel formation by Helicobacter pylori vacuolating toxin. J. Biol. Chem. 278, 12101-12108. [DOI] [PubMed] [Google Scholar]
- McClain, M.S., Schraw, W., Ricci, V., Boquet, P., and Cover, T.L. (2000). Acid-activation of Helicobacter pylori vacuolating cytotoxin (VacA) results in toxin internalization by eukaryotic cells. Mol. Microbiol. 37, 433-442. [DOI] [PubMed] [Google Scholar]
- Molinari, M., Galli, C., Norais, N., Telford, J.L., Rappuoli, R., Luzio, J.P., and Montecucco, C. (1997). Vacuoles induced by Helicobacter pylori toxin contain both late endosomal and lysosomal markers. J. Biol. Chem. 272, 25339-25344. [DOI] [PubMed] [Google Scholar]
- Molinari, M., Salio, M., Galli, C., Norais, N., Rappuoli, R., Lanzavecchia, A., and Montecucco, C. (1998). Selective inhibition of Ii-dependent antigen presentation by Helicobacter pylori toxin VacA. J. Exp. Med. 187, 135-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecucco, C., and de Bernard, M. (2003). Molecular and cellular mechanisms of action of the vacuolating cytotoxin (VacA) and neutrophil-activating protein (HP-NAP) virulence factors of Helicobacter pylori. Microbes Infect. 5, 715-721. [DOI] [PubMed] [Google Scholar]
- Morbiato, L., Tombola, F., Campello, S., Del Giudice, G., Rappuoli, R., Zoratti, M., and Papini, E. (2001). Vacuolation induced by VacA toxin of Helicobacter pylori requires the intracellular accumulation of membrane permeant bases, Cl(-) and water. FEBS Lett. 508, 479-483. [DOI] [PubMed] [Google Scholar]
- Papini, E., Bugnoli, M., De Bernard, M., Figura, N., Rappuoli, R., and Montecucco, C. (1993). Bafilomycin A1 inhibits Helicobacter pylori-induced vacuolization of HeLa cells. Mol. Microbiol. 7, 323-327. [DOI] [PubMed] [Google Scholar]
- Papini, E., de Bernard, M., Milia, E., Bugnoli, M., Zerial, M., Rappuoli, R., and Montecucco, C. (1994). Cellular vacuoles induced by Helicobacter pylori originate from late endosomal compartments. Proc. Natl. Acad. Sci. USA 91, 9720-9724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papini, E., Gottardi, E., Satin, B., de Bernard, M., Massari, P., Telford, J., Rappuoli, R., Sato, S.B., and Montecucco, C. (1996). The vacuolar ATPase proton pump is present on intracellular vacuoles induced by Helicobacter pylori. J. Med. Microbiol. 45, 84-89. [DOI] [PubMed] [Google Scholar]
- Papini, E., Satin, B., Bucci, C., de Bernard, M., Telford, J.L., Manetti, R., Rappuoli, R., Zerial, M., and Montecucco, C. (1997). The small GTP binding protein rab7 is essential for cellular vacuolation induced by Helicobacter pylori cytotoxin. EMBO J. 16, 15-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papini, E., Satin, B., Norais, N., de Bernard, M., Telford, J.L., Rappuoli, R., and Montecucco, C. (1998). Selective increase of the permeability of polarized epithelial cell monolayers by Helicobacter pylori vacuolating toxin. J. Clin. Investig. 102, 813-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papini, E., Zoratti, M., and Cover, T.L. (2001). In search of the Helicobacter pylori VacA mechanism of action. Toxicon 39, 1757-1767. [DOI] [PubMed] [Google Scholar]
- Patel, H.K., Willhite, D.C., Patel, R.M., Ye, D., Williams, C.L., Torres, E.M., Marty, K.B., MacDonald, R.A., and Blanke, S.R. (2002). Plasma membrane cholesterol modulates cellular vacuolation induced by the Helicobacter pylori vacuolating cytotoxin. Infect. Immun. 70, 4112-4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, A.M., Sorensen, K., Blom, J., and Krogfelt, K.A. (2001). Reduced intracellular survival of Helicobacter pylori vacA mutants in comparison with their wild-types indicates the role of VacA in pathogenesis. FEMS Immunol. Med. Microbiol. 30, 103-108. [DOI] [PubMed] [Google Scholar]
- Ricci, V., Galmiche, A., Doye, A., Necchi, V., Solcia, E., and Boquet, P. (2000). High cell sensitivity to Helicobacter pylori VacA toxin depends on a GPI-anchored protein and is not blocked by inhibition of the clathrin-mediated pathway of endocytosis. Mol. Biol. Cell 11, 3897-3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci, V., Sommi, P., Fiocca, R., Romano, M., Solcia, E., and Ventura, U. (1997). Helicobacter pylori vacuolating toxin accumulates within the endosomal-vacuolar compartment of cultured gastric cells and potentiates the vacuolating activity of ammonia. J. Pathol. 183, 453-459. [DOI] [PubMed] [Google Scholar]
- Salama, N.R., Otto, G., Tompkins, L., and Falkow, S. (2001). Vacuolating cytotoxin of Helicobacter pylori plays a role during colonization in a mouse model of infection. Infect. Immun. 69, 730-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satin, B., Norais, N., Telford, J., Rappuoli, R., Murgia, M., Montecucco, C., and Papini, E. (1997). Effect of Helicobacter pylori vacuolating toxin on maturation and extracellular release of procathepsin D and on epidermal growth factor degradation. J. Biol. Chem. 272, 25022-25028. [DOI] [PubMed] [Google Scholar]
- Schraw, W., Li, Y., McClain, M.S., van der Goot, F.G., and Cover, T.L. (2002). Association of Helicobacter pylori vacuolating toxin (VacA) with lipid rafts. J. Biol. Chem. 277, 34642-34650. [DOI] [PubMed] [Google Scholar]
- Stein, M.P., Feng, Y., Cooper, K.L., Welford, A.M., and Wandinger-Ness, A. (2003). Human VPS34 and p150 are rab7 interacting partners. Traffic 4, 1-18. [DOI] [PubMed] [Google Scholar]
- Suerbaum, S., and Michetti, P. (2002). Helicobacter pylori infection. N. Engl. J. Med. 347, 1175-1186. [DOI] [PubMed] [Google Scholar]
- Suzuki, J., et al. (2001). Dynamin is involved in human epithelial cell vacuolation caused by the Helicobacter pylori-produced cytotoxin VacA. J. Clin. Invest. 107, 1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, J., et al. (2003). Involvement of syntaxin 7 in human gastric epithelial cell vacuolation induced by the Helicobacter pylori-produced cytotoxin VacA. J. Biol. Chem. 278, 25585-25590. [DOI] [PubMed] [Google Scholar]
- Szabo, I., Brutsche, S., Tombola, F., Moschioni, M., Satin, B., Telford, J.L., Rappuoli, R., Montecucco, C., Papini, E., and Zoratti, M. (1999). Formation of anion-selective channels in the cell plasma membrane by the toxin VacA of Helicobacter pylori is required for its biological activity. EMBO J. 18, 5517-5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford, J.L., et al. (1994). Gene structure of the Helicobacter pylori cytotoxin and evidence of its key role in gastric disease. J. Exp. Med. 179, 1653-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinion-Dubiel, A.D., McClain, M.S., Cao, P., Mernaugh, R.L., and Cover, T.L. (2001). Antigenic diversity among Helicobacter pylori vacuolating toxins. Infect. Immun. 69, 4329-4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinion-Dubiel, A.D., et al. (1999). A dominant negative mutant of Helicobacter pylori vacuolating toxin (VacA) inhibits VacA-induced cell vacuolation. J. Biol. Chem. 274, 37736-37742. [DOI] [PubMed] [Google Scholar]
- Yahiro, K., Niidome, T., Kimura, M., Hatakeyama, T., Aoyagi, H., Kurazono, H., Imagawa, K., Wada, A., Moss, J., and Hirayama, T. (1999). Activation of Helicobacter pylori VacA toxin by alkaline or acid conditions increases its binding to a 250-kDa receptor protein-tyrosine phosphatase beta. J. Biol. Chem. 274, 36693-36699. [DOI] [PubMed] [Google Scholar]
- Yahiro, K., et al. (2003). Protein-tyrosine phosphatase alpha, RPTP alpha, is a Helicobacter pylori VacA receptor. J. Biol. Chem. 278, 19183-19189. [DOI] [PubMed] [Google Scholar]
- Ye, D., Willhite, D.C., and Blanke, S.R. (1999). Identification of the minimal intracellular vacuolating domain of the Helicobacter pylori vacuolating toxin. J. Biol. Chem. 274, 9277-9282. [DOI] [PubMed] [Google Scholar]
- Zheng, P.Y., and Jones, N.L. (2003). Helicobacter pylori strains expressing the vacuolating cytotoxin interrupt phagosome maturation in macrophages by recruiting and retaining TACO (coronin 1) protein. Cell Microbiol. 5, 25-40. [DOI] [PubMed] [Google Scholar]