Abstract
The microtubule cytoskeleton supports cellular morphogenesis and polar growth, but the underlying mechanisms are not understood. In a screen for morphology mutants defective in microtubule organization in the fungus Ustilago maydis, we identified eca1 that encodes a sarcoplasmic/endoplasmic calcium ATPase. Eca1 resides in the endoplasmic reticulum and restores growth of a yeast mutant defective in calcium homeostasis. Deletion of eca1 resulted in elevated cytosolic calcium levels and a severe growth and morphology defect. While F-actin and myosin V distribution is unaffected, Δeca1 mutants contain longer and disorganized microtubules that show increased rescue and reduced catastrophe frequencies. Morphology can be restored by inhibition of Ca2+/calmodulin-dependent kinases or destabilizing microtubules, indicating that calcium-dependent alterations in dynamic instability are a major cause of the growth defect. Interestingly, dynein mutants show virtually identical changes in microtubule dynamics and dynein-dependent ER motility was drastically decreased in Δeca1. This indicates a connection between calcium signaling, dynein, and microtubule organization in morphogenesis of U. maydis.
INTRODUCTION
Calcium (Ca2+) plays a key role in signal transduction in eukaryotic cells. A transient elevation in cytosolic Ca2+ stimulates several Ca2+ binding proteins that elicit downstream signaling pathways (Stull, 2001). Ubiquitous effectors of Ca2+ signaling are Ca2+/calmodulin-dependent protein kinases (CaMKs) and the Ca2+/CaM-dependent phosphatase calcineurin, which act by modulating the phosphorylation state of several proteins. The Ca2+ signal terminates when Ca2+-ATPases and Ca2+/H+ exchangers transport Ca2+ out of the cell or sequester it into organelles, thereby reducing cytosolic Ca2+ concentration to basal levels. The endoplasmic reticulum (ER) is the major Ca2+ store in most eukaryotic cells and sarco/ER-resident Ca2+-ATPases (SERCA; East, 2000) are required to pump Ca2+ into the ER so as to maintain low resting levels of cytosolic Ca2+ and to activate Ca2+ binding chaperones that assist the folding and modification of secretory proteins (Corbett and Michalak, 2000).
There is a growing body of evidence demonstrating a requirement for Ca2+-dependent signaling during the infection or invasion phase in diverse parasites of plants and animals, such as Histoplasma capsulatum (Sebghati et al., 2000) and Cryptococcus neoformans (Fox and Heitman, 2002). In particular, recent work in fungal pathogens reveals that inhibiting the function of calcineurin and Ca2+/CaMKs has major effects on fungal growth and leads to a loss in virulence (Joseph and Means, 2000; Cruz et al., 2002). Comparable pathways implicating Ca2+ signaling in the regulation of growth cone extension and filopodial motility in mammalian cells have been described (Gomez and Spitzer, 1999), suggesting that the role of Ca2+ in regulating morphogenesis is of widespread relevance. A potential target of Ca2+ signaling is the cytoskeleton that supports polar growth and determines the morphology of the cell. CaMKs are thought to regulate the activity of actin-based myosin V (Karcher et al., 2001). In addition, it has been shown that altered calcium homeostasis affects morphology and microtubule (MT) dynamics in fission yeast (Facanha et al., 2002), and CaMKs might also be involved, because it was shown that they regulate MT dynamics by phosphorylation of the MT regulator stathmin (Gardin et al., 1997).
Here, we describe a role of calcium in morphogenesis of the corn smut fungus, Ustilago maydis. This model pathogen is amenable to molecular genetics, its genome is published, and it is perfectly suited to analyze fungal dimorphism and pathogenicity (Bölker, 2001). Furthermore, it deserves attention because it shares similarities with vertebrate systems in several aspects of its cell biology, including unexpected gene conservation with higher eukaryotes (Kojic et al., 2002), a highly dynamic MT cytoskeleton (Steinberg et al., 2001; Straube et al., 2003), and the existence of kinesin motors, such as Kif1A- and a conventional kinesin (Lehmler et al., 1997; Wedlich-Söldner et al., 2002b), which are also crucial for axonal transport (Hirokawa, 1998). Moreover, similar to vertebrate cells intracellular motility of endosomes and ER depends on MTs and associated dynein (Wedlich-Söldner et al., 2000, 2002a).
As an initial step toward elucidating the role of Ca2+ signaling in morphogenesis in this fungus, we have characterized eca1, which was isolated by complementing a temperature-sensitive (ts) mutant. We show that Eca1 represents a true SERCA that is required for proper growth and cell survival. At standard growth conditions, mutants show a defective cell morphology that most likely results from altered MT dynamics and organization. Detailed analysis of parameters of MT dynamic instability, as well as ER motility suggests an involvement of cytoplasmic dynein in the Δeca1 phenotype. Both the morphology phenotype and MT defect can be rescued by inhibition of CaM-kinases, whereas inhibition of the Ca2+-dependent phosphatase calcineurin aggravated the phenotype of Δeca1 mutants.
MATERIALS AND METHODS
Generation of Mutants and Identification of eca1
Wild-type strain FB1 was UV-mutagenized to a survival rate of 4-5% and ts mutants were identified by growth at 24 and 34°C. One mutant was complemented with a genomic library on a self-replicating plasmid. Two plasmids contained a complementing 5.5-kb HindIII fragment that was cloned and sequenced with standard methods, revealing a single gene, named eca1. The accession number for eca1 is AJ577087.
Strains and Plasmids
All strains had the genetic background of FB1 (a1b1) or FB2 (a2b2; for details of plasmids and strains, see Table 1). In FB2ΔEca1 eca1 was deleted by a complete replacement with a phleomycin resistance cassette. For in vivo analysis of green fluorescent protein (GFP)-marked MTs, the a GFP-α-tubulin-encoding plasmid (potefGFPTub1; Table 1) was ectopically integrated into the succinate-dehydrogenase locus of strains FB1, FB2ΔEca1, and FB1Dyn2ts, resulting in FB1GT, FB1 and Dyn2tsGT, and FB2GT and FB2ΔEca1GT, respectively. For localization of U. maydis GFP-myosin V plasmid, pOG-Myo5 was introduced in FB2ΔMyo5 (Weber et al., 2003) and FB2ΔEca1. The ER network in FB2ΔEca1 was visualized by integration of plasmid pERGFP (Wedlich-Söldner et al. 2002a), and colocalization studies were done in strain FB2EYEC that was derived from FB2 transformed with plasmids pEca1YFP and pERCFP. Strain FB2ΔEca1Dyn2ts was generated by replacing dyn2 by the temperature-sensitive allele dyn2ts. In this strain, potef-GFP-Tub1 was introduced, resulting in FB2ΔEca1Dyn2tsGT. For detection of intracellular calcium, a GFP-based Ca2+ probe (Nakai et al., 2001) was put under the control of the otef-promoter and ectopically integrated in FB2 and FB2ΔEca1 cells. All integrations were confirmed by Southern blot analysis, and transformants were checked for proper morphology and doubling time to minimize possible defects due to the integration. For functional complementation of yeast strain K616 (Cunningham and Fink, 1994), eca1 was expressed under the control of the gal1-10-promoter (pGEca1, derived from plasmid YEplac195; kindly provided by Dr. H. Ulrich, MPI Marburg, Germany).
Table 1.
Strains and plasmids used in this study
| Strains/plasmids | Genotype | Reference |
|---|---|---|
| FB1 | a1b1 | Banuett and Herskowitz, 1989 |
| FB2 | a2b2 | Banuett and Herskowitz, 1989 |
| FB1Eca1ts | a1b1 eca1A1576T | This study |
| FB1Eca1tsEG | a1b1 eca1A1576T/pEca1GFP | This study |
| FB2EYEC | a2b2/pEca1YFP/pERCFP | This study |
| FB2ΔEca1 | a2b2 Δecal::bleR | This study |
| K616 | MATa pmr1::HIS3 pmc1::TRP1 cnb1::LEU2 ura3 | Cunningham and Fink, 1994 |
| K616pGal | MATa pmr1::HIS3 pmc1::TRP1 cnb1::LEU2 ura3/pGal | This study |
| K616Eca1 | MATa pmr1::HIS3 pmc1::TRP1 cnb1::LEU2 ura3/pGEca1 | This study |
| K616Eca1Y | MATa pmr1::HIS3 pmc1::TRP1 cnb1::LEU2 ura3/pGEca1YFP | This study |
| FB1YFP | a1b1/pOYFP | This study |
| FB2CMP | a2b2/pCaMP | This study |
| FB2ΔEca1CMP | a2b2 Δeca1::bleRpCaMP | This study |
| FB2ΔEca1GT | a2b2 Δecal::bleR/potefGFPTub1 | This study |
| FB1GT | a1b1/potefGFPTub1 | Steinberg et al., 2001 |
| FB2GT | a2b2/potefGFPTub1 | This study |
| FB1Dyn2ts | a1b1 Δdyn2::dynts, natR | Wedlich-Soldner et al. 2002a |
| FB1Dyn2tsGT | a1b1 Δdyn2::dyn2ts, natR/potefGFPTub1 | This study |
| FB2ΔEca1GM | a2b2 Δeca1::bleR/pOGmyo5C | This study |
| FB2EG | a2b2/pERGFP | This study |
| FB2ΔEca1EG | a2b2 Δecal::bleR/pERGFP | This study |
| FB1Dyn2tsEG | a1b1 Δdyn2::dyn2ts, natR/pERGFP | Wedlich-Söldner et al. 2002a |
| FB2ΔEca1Dyn2ts | a2b2 Δeca1::bleR, Δdyn2::dyn2ts, natR | This study |
| FB2ΔEca1Dyn2tsGT | a2b2 Δeca1::bleR, Δdyn2::dyn2ts, natR/potefGFPTub1 | This study |
| pEca1GFP | Peca1-eca1-egfp, cbxR | This study |
| pEca1YFP | Peca1-eca-yfp, bleR | This study |
| pERCFP | Potef-calS-cfp-HDEL, cbxR | Böhnert et al. unpublished |
| pGal | Pgal1-10, URA3 | H. Ullrich, unpublished |
| pGEca1 | Pgal1-10-ecal, URA3 | This study |
| pGEca1YFP | Pgal1-10-ecal-YFP, URA3 | This study |
| pOYFP | Potef-yfp, cbxR | This study |
| pCaMP | Potef-M13-cpEGFP-CaM, cbxR | This study |
| pOGmyo5C | Potef-egfp-myo5, cbxR | Weber et al., 2003 |
| pERGFP | Potef-calS-egfp-HDEL, cbxR | Wedlich-Söldner et al., 2002a |
| potefGFPTub1 | Potef-egfp-tub1, cbxR | Steinberg et al. 2001 |
a,b, mating type loci; Δ, deletion; P, promoter; ::, homologous replacement; -, fusion; bleR, phleomycine resistance; cbxR, carboxin resistance; natR, nourseothricin resistance; /, ectopically integrated; egfp, enhanced green fluorescent protein; yfp/cfp, yellow-shifted/cyan-shifted fluorescent protein; A1576T, point mutation at nucleotide residue + 1576; calS, signal sequence of calreticulin from rabbit (nt1-51); HDEL, ER retention signal; M13-cpEGFP-CaM, GFP-based Ca2+ probe (Nakai et al., 2001).
Growth Conditions
Strains were grown at 22 and 30°C in CM medium (Holliday, 1974) supplemented with 1% glucose (CM-G) or nitrate minimal medium supplemented with 5.6 mM Ca2+ (NM; Holliday, 1974) and without Ca2+ (NM/low Ca2+). Temperature shift experiments by using strains containing the dyn2ts-allele were done as described previously (Wedlich-Söldner et al., 2002a). For temperature shifts of Δeca1 mutant strains, 20 ml of liquid cultures was grown overnight in CM-G at 22°C to OD600 < 1, and 3-5 ml of this culture was supplemented with dimethyl sulfoxide, benomyl, KN-92, or KN-93 (Calbiochem, San Diego, CA) and shifted to 30°C in a water bath. Salt conditions were analyzed at 22°C. For low Ca2+ experiments, cells of 15-ml overnight cultures were washed twice in 15 ml of precooled water and resuspended in 5 ml of NM or NM/low Ca2+ medium, followed by incubation for 4 h at 30°C in culture flasks that were rinsed with ultrapure water. Colony growth of K616 derivatives was monitored on synthetic complete medium without uracil supplemented with 10 mM CaCl2 or 40 mM EGTA. Plate growth of U. maydis was analyzed on CM-G plates containing CaCl2 (1-1000 mM), EGTA (1-500 mM), LiCl (1-1000 mM), and NaCl (1-1000 mM). Inhibitor effects were analyzed on CM-G plates supplemented with the solvent dimethyl sulfoxide or ethanol, 0.5-3 μM benomyl, 10 μM cytochalasin D, 1-20 μM taxol, 1-10 μM tunicamycin, 1.5-3 mM dithiothreitol (DTT), 15-30 μg/ml cyclosporin A, and 1-4 μg/ml FK506 (kindly donated by Fujisawa GmbH, Munich, Germany). Unless otherwise noted, chemicals were purchased at Sigma Chemicals.
Southern, Northern, and Western Analysis
DNA isolation from U. maydis and transformation procedures were carried out as described previously (Krüger et al., 1998). RNA was isolated from strains grown in liquid culture for 3 h and prepared as described previously (Krüger et al., 1998). A 2005-bp SacII-KpnI fragment of eca1 was used as a probe. Western analysis was done as described previously (Straube et al., 2001) by using a monoclonal GFP antibody (Roche Diagnostics, Mannheim, Germany) and horseradish peroxidase-coupled anti-mouse IgG (Promega, Madison, WI).
Sequence Analysis
Phylogenetic dendograms were constructed using ClustalX and MEGA 2.1 (http://www.megasoftware.net) by using the minimum evolution or maximum parsimony algorithms and gap deletion option. Then 500 replicates were used for bootstrap support. Domain and motif analysis was carried out using BLAST (http://www.ncbi.nlm.nih.gov/blast/) and SMART (http://smart.embl-heidelberg.de) servers. Transmembrane helices were predicted with ISREC (http://www.ch.embnet.org). The genome of U. maydis was accessed at http://www.genome.wi.mit.edu/annotation/fungi/ustilago_maydis/index.html).
Light Microscopy and Image Processing
Microscopic analysis was performed using an Axioplan II microscope (Carl Zeiss, Jena, Germany). Frames were taken with a cooled charge-coupled device camera (C4742-95; Hamamatsu, Bridgewater, NJ). Epifluorescence of GFP was observed using the standard fluorescein isothiocyanate filter sets. Colocalization of YFP and CFP was analyzed with specific filter sets (YFP: BP500/20, FT515, and BP535/30; and CFP: BP436, FT455, and BP480-500). Quantification and image processing, including adjustment of brightness, contrast, and gamma values, was performed with ImageProPlus (Media Cybernetics, Gleichen, Germany), MetaMorph (Universal Imaging, Downing-town, PA), and Photoshop (Adobe Systems, Mountain View, CA).
Detection of Cytosolic Ca2+
Cells expressing a GFP-based calcium probe (Nakai et al., 2001) were grown in CM-G at 22°C. Cells were shifted incubated for 3 h at 30°C in a water bath, and samples were observed at 50% of lamp intensity (Atto Arc; Carl Zeiss) by using the standard fluorescein isothiocyanate filters and a cooled charge-coupled device camera (Coolsnap HQ; Photometrics, Tucson, AZ). To minimize the risk of artifacts due to oxygen depletion or radiation, only two to three cells per preparation were observed without embedding in agarose. Average cytoplasmic signal intensities were measured and corrected by the background noise and the autofluorescence of FB2 cells by using MetaMorph (Universal Imaging).
Quantification of Morphology Defects, ER Motility, and Microtubule Dynamics
For microscopic observation, cells from logarithmic cultures were embedded in 1% low melt agarose. Unless stated, morphology of control and Δeca1 mutant cells was analyzed after 4-5 h at 30°C. Cell morphology was considered “abnormal” if cells were multiple budded, branched, or irregularly shaped. For each experiment, at least 100 cells were analyzed. ER motility and MT dynamics was measured as described previously (Steinberg et al., 2001; Wedlich-Söldner et al., 2002a). All analysis was done using digital sequences of 30-60 frames that were taken with an exposure time of 500-1000 ms per frame at 40-60% lamp intensity. Microscopic preparations were observed no longer than 15 min to prevent defects due to oxygen depletion. All measurements were done using ImageProPlus (Universal Imaging). Statistical analysis by two-tailed t test at α = 0.05 was carried out using Prism (GraphPad Software Inc., San Diego, CA).
RESULTS
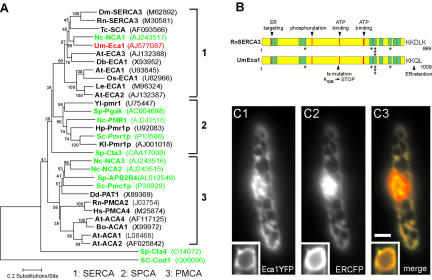
Eca1 Is Similar to Mammalian SERCAs and Localizes to the ER In a genetic screen for temperature-sensitive mutants, we obtained 63 strains that grew normally at 22°C but were unable to form colonies at 34°C. By complementing one of these mutant strains (FB1Eca1ts) with a genomic library, we identified an open reading frame of 3030 base pairs that fully restored growth at 34°C (our unpublished data). eca1 (accession no. AJ577087) encodes a putative protein of 1009 amino acids that shares highest sequence identity with animal type 3 PIIA SERCAs (47-58%) and with a putative Ca2+-transporter from Neurospora crassa (60%, NCA1; Benito et al., 2000; Figure 1A). The gene product Eca1 is predicted to contain 10 transmembrane domains and all motifs characteristic of P-type ATPases, including those implied in nucleotide binding and phosphorylation (Evans and Williams, 1998), as well as the evolutionary conserved Ca2+ binding sites (Clarke et al., 1989; Figure 1B). In addition, Eca1 contains an N-terminal ER targeting pentapeptide motif found in SERCAs (Magyar and Varadi, 1990) and a canonical dilysine C-terminal ER retention motif (KKQL; Andersson et al., 1999). To verify the predicted localization of Eca1 in the ER, we expressed an Eca1-GFP fusion protein in the eca1 mutant strain FB1Eca1ts. The fusion protein rescued the growth defect of the mutant and localized to a peripheral network and the nuclear envelope, a characteristic distribution of the ER (our unpublished data). To confirm this notion, we then coexpressed in wild-type cells a fusion protein of Eca1 and yellow fluorescent protein (Eca1-YFP; Figure 1C1) and an ER-targeted cyan fluorescent derivative (ER-CFP; Figure 1C2) that has been previously shown to localize to the ER in U. maydis (Wedlich-Söldner et al., 2002a). Both Eca1-YFP and ER-CFP colocalized in all areas (Figure 1C3), thereby confirming that Eca1 resides in the ER.
Figure 1.
Eca1 belongs to type 3 SERCAs and localizes to the ER. (A) Eca1 groups with other sacroplasmic/ER Ca2+ ATPases (SERCA) in a dendrogram. Fungal Ca2+ ATPases are highlighted in green. SPCA, secretory pathway Ca2+-ATPase; PMCA, plasma membrane Ca2+-ATPase. Bootstrap values are at each node and accession numbers are in parentheses. (B) Domain organization of Eca1 and SERCA3 from rat. Predicted transmembrane domains are indicated in green, and residues involved in Ca2+ binding are marked with an asterisk. Note that the temperature-sensitive mutation in Eca1ts results in a STOP codon. (C) An Eca1YFP fusion protein and an ER-targeted ER-CFP fusion protein (C2) colocalize within the peripheral ER network and the nuclear envelope (insets) of haploid U. maydis cells. Bar, 2 μm.
Eca1 Functions in Ca2+ Homeostasis
Sequencing of the mutated eca1 allele in FB1Eca1ts, revealed a single point mutation (A to T) at nucleotide +1576 that changed residue K526 to a stop codon, therefore making it likely that, regardless of temperature, only a nonfunctional Eca1 protein is expressed in this mutant. Consequently, we deleted the entire eca1 open reading frame. At 30°C, which is close to the optimum temperature of U. maydis, growth of the resulting strain FB2ΔEca1 was reduced (Figure 3A; wt, wild-type control; Δeca1, Δeca1 mutant) and cell morphology of mutants was heavily impaired (see below), whereas morphology and the ability to form colonies was restored at 22°C and abolished at 34°C (Figure 2A).
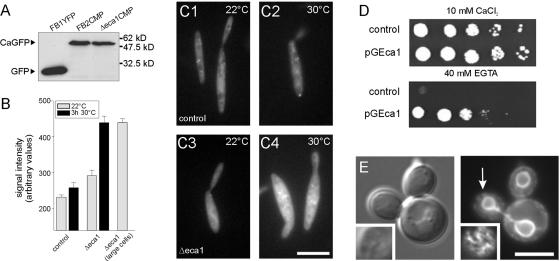
Figure 3.
Eca1 functions in Ca2+ homeostasis. (A) Western blots of cell extracts of strains that express a cytoplasmic GFP-based Ca2+ probe (Nakai et al., 2001). An anti-GFP antibody detects the M13-cpEGFP-CaM fusion protein in reference strain FB2CMP and mutant strain FB2ΔEca1CMP. Detection of YFP in extracts of FB1YFP is given as control. (B) Quantitative analysis of Ca2+ sensitive M13-cpEGFP-CaM signals at 22 and 30°C. At 22°C, cells of reference strain FB2CMP show faint cytoplasmic staining that slightly increases after shift to 30°C. In contrast, in normal shaped Δeca1 mutants the M13-cpEGFP-CaM-based fluorescence is already slightly evaluated, and growth at 30°C significantly increased the signal. A small portion of Δeca1 mutant cells show impaired morphology at 22°C that coincides with increased M13-cpEGFP-CaM fluorescence. Note that the graph was corrected for the background signal of U. maydis cells without GFP. Sample size is 18-34 cells and 4 for “Δeca1 large cells” at 22°C. (C) Examples of M13-cpEGFP-CaM signals in cells of reference strain FB2CMP and mutant strain FB2ΔEca1CMP. Bar, 10 μM. (D) Yeast strain K616 expressing a control plasmid (control) is unable to grow in the absence of Ca2+. Expression of eca1 (pGEca1) complements this defect, demonstrating that EcaI is involved in Ca2+ homeostasis. (E) Differential interference contrast image and corresponding fluorescence image of yeast strain K616Eca1YFP that expresses a functional Eca1-YFP fusion protein. Note that Eca1 localizes to the ER and the nuclear envelope (white arrow, insets show peripheral focal plane). Bar, 2 μm.
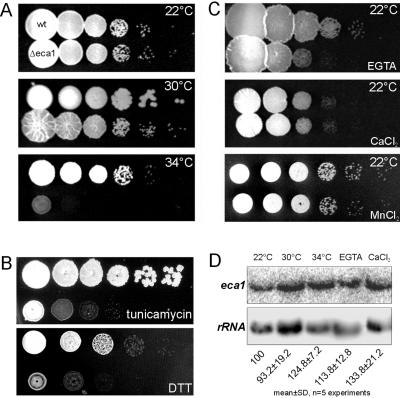
Figure 2.
Δeca1 mutants are sensitive to temperature, EGTA, and ER stress. (A) Growth of wild-type control (wt) and Δeca1 mutants (Δeca1) at 22, 30, and 34°C. Note that regular growth temperature for U. maydis is 28-30°C. (B) Growth of wild-type and Δeca1 mutants at 22°C on plates containing tunicamycin (2.5 μg/ml) and DTT (2 mM). Both inhibitors affect protein processing within the ER, suggesting that Ca2+-dependent protein folding in the ER is impaired in Δeca1 mutants. (C) Growth at 22°C on agar supplemented with EGTA (5 mM), Ca2+ (250 mM), or Mn2+ (10 mM). Note that treatment with CaCl2 did not affect colony formation, but had a negative effect on cell morphology and MT organization (Figure 4, C and H). (D) Northern analysis of eca1 expression in FB2 under temperature and Ca2+ stress conditions. The experiment was repeated five times and eca1 signals at 22°C, corrected by loading controls, were normalized to 100%. Note a significant increase under 50 mM Ca2+ and 34°C.
Because the localization and sequence of Eca1 indicated a role in Ca2+ transport into the ER, we compared intracellular Ca2+ levels in wild-type and Δeca1 cells by using a GFP-based Ca2+ probe that exhibits fluorescence upon binding to Ca2+ (Nakai et al., 2001). Western analysis showed that the reporter gene was expressed at similar levels in reference strain FB2CMP (Figure 3A) and Δeca1 mutant strain FB2ΔEca1CMP (Δeca1CMP). A quantitative analysis on the single cell level revealed a faint Ca2+ signal at 30°C in reference cells that was slightly decreased at 22°C (Figure 3B, not significantly different from 22°C, p = 0.1146; for example, see 3C1 and 3C2). In contrast, Ca2+ levels were strongly elevated in Δeca1 cells at 30°C and significantly decreased at 22°C (Figure 3B, 3C2, and 3C4; p < 0.0001), indicating that Eca1 is required to maintain normal cytosolic Ca2+ concentrations. In some cases, growth of Δeca1 mutants was not restored at 22°C, and this coincided with high Ca2+ levels, indicating that abnormal morphology and elevated Ca2+ levels are related (Figure 3B).
To confirm a role of Eca1 in Ca2+ transport, we complemented the yeast mutant strain K616 (Cunningham and Fink, 1994), an approach that was successfully used to confirm Ca2+ transport activity of SERCA pumps before (Sze et al., 2000). In this yeast mutant, two Ca2+ ATPases, Pmc1p and Pmr1p, are deleted, resulting in a requirement for external Ca2+ supplies. On plates supplemented with Ca2+ the mutant K616 transformed with empty vector (control; Figure 3D) or vector carrying the U. maydis eca1 gene (pGEca1; Figure 3D) formed colonies. However, growth of the control strain was abolished on a medium depleted for Ca2+ (control, 40 mM EGTA; Figure 3D), whereas expression of eca1 restored viability of K616 under these conditions (Figure 3D). Eca1-YFP expressed in K616 localized to the nuclear envelope and a peripheral network (arrow in Figure 3E), which suggests that Eca1 complements the defects of K616 by its Ca2+-pumping activity at the ER.
Because these data argue for a role of Eca1 in Ca2+ transport into the ER, where Ca2+ions are needed for ER-dependent folding of secretory proteins, we tested this ER function in Δeca1 cells. We generated ER stress by treatment with tunicamycin (2.5 μg/ml) and DTT (2 mM), which affect N-glycosylation and folding reactions in ER. Wild-type cells still grew under these conditions, but both agents inhibited growth of Δeca1 already at 22°C (Figure 2B). This hypersensitivity indicates that ER-based protein processing is compromised in Δeca1 mutants, which might be the cause of the lethal phenotype at 34°C. Growth of Δeca1 strains was also sensitive to EGTA (5 mM; Figure 2C), but neither high concentrations of Ca2+ (250 mM) nor Mn2+ (10 mM) inhibited colony formation, although most Δeca1 cells showed a pronounced morphology defect under these conditions (see below). Finally, Northern analysis indicated that eca1 was expressed at similar levels under various stress conditions, and a slight increase was only found at high temperature (p = 0.018) or 50 mM extracellular Ca2+ (p = 0.015; Figure 2D). In summary, these data strongly indicate that Eca1 regulates Ca2+ homeostasis by pumping cytosolic Ca2+ into the ER.
Eca1 Is Required for Interphase MT Organization and Polar Growth
At 30°C, Δeca1 cells showed growth at both poles and formed multiple septa (Figure 4A2, 4 h at 30°C; septa indicated by arrows). This resulted in large cell aggregates with rounded and apolar cells (Figure 4A3, 12 h at 30°C). Under these conditions, wild-type cells were unaffected (Figure 4A3, inset, and 4C). Low temperature (22°C) restored morphology of Δeca1 cells (Figure 4A1, for wild-type control, see inset in Figure 4A3). Interestingly, this temperature phenotype was dependent on external Ca2+ concentration, because low extracellular Ca2+ suppressed the temperature-dependent Δeca1 phenotype (Figure 4B, compare with 4A2; 4C; 4 h at 30°C in NM with low Ca2+). In addition, morphology of Δeca1 cells was more sensitive to high extracellular Ca2+ levels (250 mM; Figure 4C), which also affected the shape of wild-type cells but to a much smaller extent. Thus, Eca1 is required to maintain normal cell shape at 30°C and during Ca2+ stress.
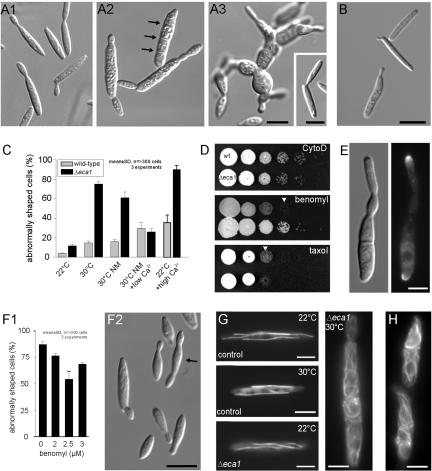
Figure 4.
Temperature-dependent morphology defect of Δeca1 mutants is associated with defects in microtubule organization. (A) Morphology of Δeca1 mutant cells. At 22°C, Δeca1 mutant cells are similar to control cells (A1; for control, see inset in A3). At 30°C, cells enlarge, start to grow at both cell poles, and fail to separate (A2, septa indicated by arrows; 4 h at 30°C). With extended time at 30°C, Δeca1 mutant cells form large structures of unseparated and often rounded cells (A3, 12 h). Under the same conditions, the control strain FB2 is unaffected (A3, inset). Bars, 5 μm. (B) Morphology of Δeca1 mutant cells shifted in low Ca2+ medium. Reduction of external Ca2+ suppressed the morphology phenotype of Δeca1 cells after 4 h at 30°C. Bar, 5 μm. (C) Quantitative analysis of morphology defects of Δeca1 mutants compared with wild-type control. Morphology of strain FB2 was virtually unaffected by temperature, whereas 75-80% aberrant mutant cells were observed after 4-5 h at 30°C. Similar results were obtained in minimal medium supplemented with Ca2+ (NM). However, no difference between control and mutant cells was found in minimal medium with reduced Ca2+ (NM, low Ca2+). eca1 mutants are much more sensitive to treatment with 250 mM Ca2+. All data points are based on three experiments with >100 cells each. For criteria of abnormal morphology, see MATERIALS AND METHODS. (D) Effect of cytoskeletal inhibitors on plate growth of wild-type and Δeca1 mutants strains. No difference between Δeca1 mutants and wild-type cells was found in the presence of the actin inhibitor cytochalasin D (10 μM). In contrast, Δeca1 cells are less sensitive to the MT destabilizing drug benomyl (1 μM, arrowhead) and slightly more sensitive to taxol (3 μM, arrowhead), indicating a link between MT stability and the growth phenotype of Δeca1 mutants. (E) Polar localization of a fusion of GFP and a class V myosin from U. maydis in Δeca1 mutants after 3h at 30°C. F-actin-dependent localization of GFP-Myo5 is not impaired in Δeca1, suggesting that Myo5 transport activity is not impaired. Bar, 5 μm. (F) Effect of low concentrations of the microtubule inhibitor benomyl on morphology of Δeca1. In liquid culture, 2.5 μM benomyl significantly restored morphology of Δeca1 (F1; 4-5 h at 30°C), with less cells showing separation defects or multiple budding. Note that even cells that were considered abnormal showed improved morphology (arrow in F2; for comparison, see 4A2 and 4E). Bar, 10 μm. (G) Organization of MTs in FB2GT and FB2 ΔEca1GT. At 22 and 30°C (4 h), control cells contained straight MTs, labeled with GFP-αtubulin. At 22°C, Δeca1 mutants contain normal MTs, but at 30°C MTs became longer and heavily disordered (4 h at 30°C). Note that MTs leave the focal plane, thereby looking shorter. Bar, 5 μm. (H) Reference strain FB2GT treated with high levels of Ca2+ (250 mM, 4 h). High external Ca2+ strongly affected MT organization in many cells, which is reminiscent of MT defects in Δeca1 mutants at 30°C and normal Ca2+ levels. Bar, 5 μm.
Morphology of fungal cells is based on the cytoskeleton. Therefore, we speculated that the Ca2+-dependent phenotype of FB2ΔEca1 was due to defects in cytoskeletal organization or function. Therefore, we first monitored the effect of cytoskeleton inhibitors on plate growth of wild-type control and Δeca1. In the presence of 10 μM of the actin inhibitor cytochalasin D, no difference between control and Δeca1 mutants was observed (Figure 4D), and this coincided with normal actin patch distribution in Δeca1 mutants at restrictive conditions (our unpublished data). Furthermore, in FB2Δeca1 transport along the actin cytoskeleton was apparently not impaired, because a functional GFP-myosin V fusion protein that requires F-actin for polar localization at the growth region (Weber et al., 2003) was correctly positioned at the cell poles (Figure 4E, 3 h 30°C). This suggested that elevated Ca2+ levels did not severely affect the actomyosin system. In contrast, Δeca1 mutant were more sensitive to the MT stabilizer taxol (5 μM) and less sensitive to low doses of destablizer benomyl (1 μM; Figure 4D). Moreover, 2.5 μM benomyl suppressed most of the temperature-dependent morphology defects of Δeca1 cells (Figure 4F1 and 4F2; 4 h at 30°C, compare with 4A2), indicating that growth and morphology defects of FB2Δeca1 are mainly due to unusually stable MTs. This notion was further supported by the observation that GFP-α tubulin-labeled MTs (Steinberg et al., 2001) in Δeca1 cells were much longer and irregular arranged (Figure 4G, Δeca1; see also 5D2) as in reference strain FB2GT (Figure 4G, control, 4 h at 30°C). Consistent with the restorative effect of lower temperature on morphology no obvious differences between the mutant and control cells were found at 22°C (Figure 4G). These results were confirmed by indirect immunofluorescence by using anti-tubulin antibodies in strains FB2 and FB2ΔEca1, indicating that they are not due to expression of GFP-α tubulin (our unpublished data). Interestingly, disturbed MT patterns were observed in wild-type cells exposed to high extracellular Ca2+ (250 mM; Figure 4H), suggesting that aberrant MTs and cell shape are generally associated with increased Ca2+ levels. Together, these results argue for an effect of high cytosolic Ca2+ levels on morphogenesis by influencing MT stability in Δeca1.
Ca2+ Signaling Is Involved in the Phenotype of Δeca1 Mutants
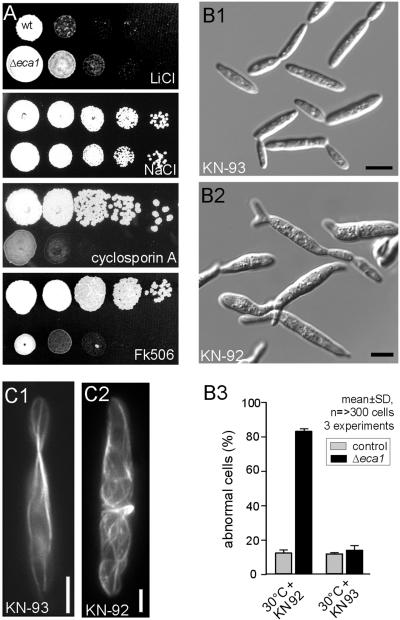
The effects of high Ca2+ levels on MTs might be mediated by effectors of Ca2+ signaling, Ca2+/CaM dependent-kinases, and the phosphatase calcineurin. Similar to Δeca1, calcineurin mutants in fungi show poor viability under ER stress conditions (Bonilla et al., 2002; Cruz et al., 2002). In addition, these mutants are sensitive to cations such as Li2+ and Na+. Consequently, we checked plate growth of Δeca1 mutants on LiCl (7.5 mM) and NaCl (0.5 M). No growth inhibition was observed, and Δeca1 cells grew even slightly better on LiCl, indicating that calcineurin was not inhibited in these cells (Figure 5A). Moreover, exposure of Δeca1 mutants to the specific calcineurin inhibitors cyclosporin A (15 μg/ml) or FK506 (4 μg/ml; Ho et al., 1996), led to a significant reduction in growth at 30°C (Figure 5A), whereas wild-type cells were not affected. In other words, the dephosphorylation activity of calcineurin is crucial for survival of Δeca1 on plates.
Figure 5.
Δeca1 mutants require the Ca2+-dependent phosphatase calcineurin for stress tolerance, and show increased CaMK activity. (A) Δeca1 mutants show normal growth at high levels of Li2+ (7.5 mM) and Na2+ (0.5M). Calcineurin inhibition with cyclosporin A (15 μg/ml) or FK506 (4 μg/ml), result in reduced viability of Δeca1 mutants at high temperature (30°C). This aggravation of the phenotype by inhibition of Ca2+-dependent protein dephosphorylation suggests that protein phosphorylation is involved in the observed defects of Δeca1 cells. (B) Inhibition of CaMKs restored the morphology phenotype of Δeca1 mutants at 30°C. Δeca1 mutants showed normal morphology at 30°C when grown in the presence of the specific CaMK inhibitor KN-93 (60 μM; B1). In contrast, the inactive analogue KN-92 has no restorative effect (B2 and B3). This observation argues for an involvement of Ca2+-dependent protein phosphorylation in the Δeca1 phenotype. Bars, 5 μm. (C) Inhibition of CaMKs rescues the MT defect of Δeca1 mutants at 30°C. In FB2ΔEca1GT, KN-93 (C1) restores organization of MTs, but the inactive analogue KN-92 was without effect (C2). Bars, 2 μm.
In contrast, the inhibition of CaMK by the potent inhibitor KN-93 (Sumi et al., 1991) had a positive effect on Δeca1 cells. Treatment with 60 μM KN-93, but not with its inactive structural analogue KN-92, restored near-normal morphology to Δeca1 mutants at 30°C (Figure 5B1 and 5B2), whereas this treatment did not affect morphology of wild-type cells (Figure 5B3). Restoration of morphology by KN-93 treatment was accompanied by the formation of nearly normal interphase MT arrays (Figure 5C1) in ∼70% of all cells after 3 h at 30°C, whereas MT arrays in KN-92-treated cells were disordered and resembled the characteristic Δeca1 phenotype (Figure 5C2). Thus, altered Ca2+-homeostasis in Δeca1 mutants seems to increase CaMK activity, which in turn deregulates MT dynamics and results in morphology defects.
Δeca1 and Dynein Mutants Share Virtually Identical Alterations in Microtubule Dynamics
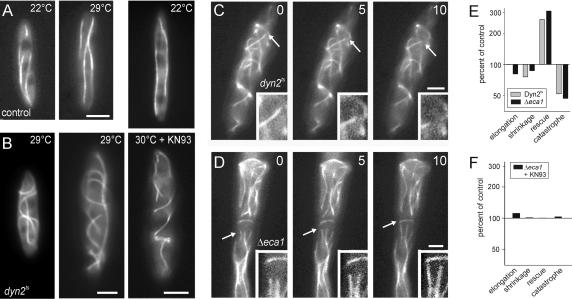
The defect in MT organization in FB2Δeca1 resembled that of dynein mutants (Straube et al., 2001). Therefore, we examined the relationship between eca1 and dynein effects on MT dynamics. We first analyzed the role of dynein in MT organization by using a temperature-sensitive allele of the dynein heavy chain (dyn2ts; Wedlich-Söldner et al. 2002a). At permissive temperature, MTs in dyn2ts and control cells were indistinguishable (Figure 6, A and B, 22°C), but inactivation of dynein at 29-30°C led to much longer, curved, and disorganized MTs (Figure 6B, 4-6 h at 29°C). This phenotype was reminiscent of Δeca1 mutants (compare Figures 6B and 4H or 5C2). In both mutants, MTs seemed less dynamic and more often growing MTs were observed (Figure 6C, dynts; 6D, Δeca1).
Figure 6.
Conditional dynein mutants and Δeca1 mutants share defects in MT dynamics and organization. (A) Reference strain FB1GT contained straight GFP-labeled MTs at 22 and 29°C. Bar, 3 μm. (B) MTs were normal in dynein mutants that contain a temperature-sensitive dynein allele (Wedlich-Söldner et al. 2002a), but severely altered at restrictive conditions (4-6 h at 29°C). Treatment with the CaMK inhibitor KN-93 was without effect on the dynein phenotype at restrictive conditions (3.5 h at 30°C). Note that this phenotype resembles that of Δeca1 mutants. Bars, 3 μm. (C and D) Dynamic behavior of MTs in dyn2ts (C) and Δeca1 (D) mutants. At restrictive temperature, both strains contain numerous curved MTs that show dynamic instability, including shrinkage due to depolymerization (arrow and inset in C) and polymerization-based elongation. Time in seconds is given in the upper right corner. Bar, 3 μm. (E) Parameters of MT dynamic instability in FB1Dyn2tsGT and FB2ΔEca1GT at restrictive conditions compared with reference strains FB1GT and FB2GT, respectively. Note the striking similarity between Δeca1 and dyn2ts mutants. For actual numbers, see Table 2. (F) CaMK inhibition by KN-93 restores most parameters of dynamic instability but did not affect the catastrophe value. Nevertheless, most mutant cells contained well-organized and shorter MTs.
MT length depends on the velocity of elongation and shrinkage, as well as the frequency by which MTs switch from growth to shrinkage (catastrophe) and vice versa (rescue; Verde et al., 1992). To gain deeper insight into the MT phenotype, we measured these parameters of dynamic instability in Δeca1 and dyn2ts mutant strains and compared the results to their reference strains. In both mutants, the velocity of elongation and shrinkage of MTs was almost unaltered, but in Δeca1 and dyn2ts cells rescue rate was threefold increased and catastrophe rates were twofold lowered (Figure 6E and Table 2). Thus, it seems that shrinking MTs more often restart growth, whereas elongating MTs less often switch to depolymerization, explaining the abnormally long MTs in dyn2ts and Δeca1 mutants. Inhibition of CaMKs by KN-93 resulted in almost normal MT organization of Δeca1 at 30°C (see above), and MT elongation and shrinkage rates, as well as rescue frequencies of Δeca1 mutants were restored to near normal levels (Figure 6F and Table 2). In contrast, MT defects in dynein mutants were not restored by the CaMK inhibitor KN-93 (Figure 6B), indicating that KN-93 acts upstream of the dynein complex in regulation of MT stability.
Table 2.
Dynamics of GFP-labeled MTs in Dyn2ts and Δeca1 mutants and reference strains
| Elongationa | Shrinkagea | Rescueb | Catastropheb | ||
|---|---|---|---|---|---|
| FBIGTc | 22°C | 10.3 ± 2.5 (19) | 38.1 ± 19.8 (75) | 0.48 | 2.64 |
| 29°Cc | 10.6 ± 2.3 (19) | 39.3 ± 20.9 (79) | 0.66 | 3.24 | |
| FB1Dyn2tsGTc | 22°C | 10.2 ± 2.2 (20) | 39.5 ± 19.1 (70) | 0.42 | 2.94 |
| 29°Cc | 10.6 ± 2.5 (24) | 29.7 ± 13.7 (80) | 1.80 | 1.68 | |
| FB2GTc | 22°C | 7.9 ± 2.7 (10) | 31.5 ± 17.1 (83) | 0.24 | 3.30 |
| 30°Cc | 8.4 ± 2.4 (13) | 34.7 ± 17.6 (40) | 0.42 | 2.82 | |
| FB2ΔEca1GTc | 22°C | 5.7 ± 2.5 (10) | 31.9 ± 16.9 (43) | 0.30 | 3.60 |
| 30°Cc | 6.8 ± 2.0 (17) | 30.3 ± 16.6 (44) | 1.38 | 1.32 | |
| FB2ΔEca1GT+KN93c | 30°Cc | 9.1 ± 2.1 (20) | 35.0 ± 12.6 (41) | 0.42 | 2.95 |
| FB2ΔEca1Dyn2tsGT | 30°Cc | 5.5 ± 21.4 (9) | 37.7 ± 18.8 (22) | 12.66 | 1.02 |
Rates given as mean ± S.D. (n) in micrometers per minute.
Transitions per minute.
Growth at 22°C, followed by 4-5 h of growth at 29°C or 30°C.
Δeca1 Mutants Are Defective in Dynein-dependent ER Motility
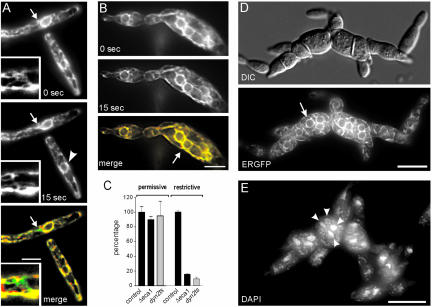
To gain further support for the hypothesis that the defects of Δeca1 mutants are due to altered dynein activity, we examined the effect of deletion of eca1 on another dynein-dependent process. The peripheral tubular ER network in U. maydis is highly dynamic (Figure 7A1, arrow; see inset for higher magnification) and this motility is solely dynein-dependent (Wedlich-Söldner et al., 2002a). To analyze ER organization and motility in Δeca1 cells, we generated a strain that expressed an ER-targeted GFP fusion protein (FB2ΔEca1EG). At 22°C, the ER was localized at the cell periphery of both Δeca1 and control cells (our unpublished data) and directed tubule motility in both strains was indistinguishable (Figure 7C; Δeca1: 4.12 ± 0.36 events/h × μm2; reference: 4.59 ± 0.60 events/h × μm2, n > 35 cells from three experiments; p = 0.3288). Temperature shift had no effect on the ER in control cells, but under these conditions Δeca1 mutants contained an irregular network of ER tubules that were no longer located at the cell periphery (Figure 7, B and D). At elevated temperature, ER motility in control cells increased, but directed ER tubule motion in Δeca1 was significantly reduced (Figure 7, B and C; Δeca1: 1.40 ± 0.14 events/h × μm2, reference: 8.94 ± 0.23 events/h × μm2; n = 3 experiments and >38 cells; p > 0.0001). This defect in ER-motility in Δeca1 corresponds well with that of dynein mutants (Figure 7C; not significantly different, p = 0.1340, values for dyn2ts from Wedlich-Söldner et al., 2002a). To analyze minor effects on ER motility, we did a quantitative comparison of digital images at two time points by using the software package MetaMorph. Again, we measured significantly less total ER displacement in Δeca1 than in control cells (p = 0.0059; 10-13 measurements, 28 cells). Finally, we set out to rescue the ER motility defect by the CaMK inhibitor KN-93. Although this CaMK inhibitor had no effect on ER in control cells at 30°C, KN-93 treatment led to fragmentation and disorganization of the ER networks in Δeca1 mutants that did not allow a study of motility. Interestingly, temperature shift led to a nuclear distribution defect in Δeca1. Control cells contained a single nucleus in the cell center that is detectable by ER-GFP in the nuclear envelope (Figure 7A, arrowhead). However, Δeca1 at 30°C often contained numerous nuclei (Figure 7D, overnight 30°C; see also arrow in Figure 7B) that were also detected by 4,6-diamidino-2-phenylindole staining of DNA (Figure 7E, arrowheads; maximum-Z-axis projection of cells grown overnight at 30°C). Such a defect is characteristic of dynein mutants (Straube et al., 2001), again supporting the notion that dynein function is impaired in Δeca1. Together, these results demonstrate that dynein-dependent motility of ER tubules is significantly impaired in Δeca1 mutants, again indicating that elevated Ca2+ levels in Δeca1 affect dynein activity.
Figure 7.
ER organization, ER motility, and nuclear migration in Δeca1 and dynein mutants. (A) ER motility in control cells. The network undergoes prominent motility (arrows; see inset for example at higher magnification) that is based on dynein activity (Wedlich-Söldner et al., 2002a). In pseudocolored overlay displacements reduce the regions of colocalization that are indicated in yellow. Arrowhead indicates the nuclear envelope. Time interval is given at the bottom. Bar, 5 μm. (B) ER motility in Δeca1. There is significantly reduced ER tubule motility over the 15-s observation period, as can be seen from the pseudocolor merge image. Note that Δeca1 mutants contain an irregular network of ER tubules. This organization is in contrast to reference strain FB2EG, in which the ER network is localized to the cell periphery. Arrow indicates a nuclear envelope. Time interval is given at the bottom. Bar, 5 μm. (C) Quantitative analysis of ER motility in control, Δeca1 and dynts cells. ER motility in Δeca1 mutants was measured as displacements per square micrometer and second according to Wedlich-Söldner et al. (2002a). Values for dyn2ts mutants are taken from the same reference. In both mutants ER motility is abolished at restrictive conditions. (D) Nuclear distribution defect in Δeca1 after overnight incubation at 30°C. Mutant cell form swollen cell chains that contained numerous nuclei, as indicated in corresponding fluorescent image of ER-GFP labeled nuclear envelopes (arrow). Bar, 10 μm. (E) DAPI staining of nuclei and fragmented mitochondria in Δeca1 at 30°C. Staining the nuclear DNA by DAPI (in one cell indicated by arrowheads) confirms the nuclear distribution defect of Δeca1. Bar, 10 μm.
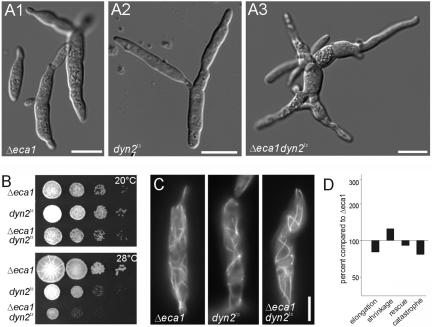
Dynein and Eca1 Double Mutants Show Synthetic Growth Phenotypes, but No Increase in MT Defects
To gain deeper insights in the functional relationship between Eca1 and dynein, we generated strain FB2ΔEca1Dyn2ts. Shifting this double mutant to 30°C simultaneously inactivated dynein and increased cytosolic Ca2+. Assuming that Eca1 and dynein act in the same pathway, inactivation of both should not increase the phenotype of the individual mutants. Growing mutants at 30°C in liquid culture resulted in a more severe morphological defect of the double mutant (Figure 8A, 11 h). In addition, at semipermissive conditions (Figure 8B, 28°C) the double mutant was clearly more impaired in plate growth, whereas growth of all mutants was the same at permissive temperature (Figure 8B, 22°C). However, mutations in eca1 and dynein showed no synthetic effect on MT organization (Figure 8C), and the alterations in MT dynamics in the double mutant were comparable with that of the Δeca1 single mutant strain (Figure 8D; changes in parameters compared with strain FB2ΔEca1GT). Therefore, we conclude that Eca1 and dynein are in the same pathway to regulate MT stability, which further supports the notion, an effect of Ca2+ signaling on MTs via the dynein complex. However, the increased growth defects of the double mutant argue for additional targets of Ca2+ signaling in morphology and cell separation.
Figure 8.
Analysis of a eca1 and dynein double mutant. (A) Morphology of Δeca1, dynts, and a Δeca1 dynts double mutant. At 30°C, Δeca1 cells grow irregular, branch, and display defects in cell separation, which results in cell chains (A1). At the restrictive temperature (30°C), dynts cells also show abnormal morphology, but cells tend to be longer and no cytokinesis defect was observed (A2). Under the same conditions, the double mutant showed an even stronger defect in cell shape (A3). Note that the morphology phenotype of the double mutant was very variable, ranging from branched cell chains to cells with long extensions. Bar, 10 μm. (B) Growth of Δeca1, dynts, and Δeca1 dynts mutants on agar plates. At permissive temperature (20°C), colony grow of all mutant strains was indistinguishable. However, at a semipermissive temperature (28°C), Δeca1 cells grow normal, whereas dynts mutants show impaired colony formation. Interestingly, these conditions are almost lethal for the Δeca1 dynts double mutant. This additive phenotype indicates that Δeca1 and dynein mutants are defective in different cellular processes and mutations in both confer synthetic lethality at restrictive temperature. (C) Microtubules in Δeca1, dynts, and Δeca1 dynts mutants. All mutant strains contained longer and irregular organized microtubules. Bar, 5 μm. (D) Parameters of MT dynamic instability in FB2ΔEca1Dyn2tsGT at restrictive conditions compared with the single mutant strain FB2ΔEca1GT (Δeca1, black bars). Note that rescue rates of the double mutant resemble that of the Δeca1 single mutant and only slight effects on catastrophe values, shrinkage, and growth velocities were found. These data demonstrate that inactivation of dynein does not increase the effect of Δeca1 on MTs, which argues for a role of EcaI and dynein in the same pathway to regulate microtubule dynamics. For measured numbers, see Table 2.
DISCUSSION
Eca1 Is a Fungal SERCA-type Ca2+ Pump
In a screen for morphology mutants, we identified eca1, a putative Ca2+ transporter that shares highest sequence identity with SERCA-type pumps from animals and plants. This sequence similarity, the presence of conserved Ca2+ binding motifs, phosphorylation and ATP binding sites, and the overall domain structure argue for Eca1 being a true SERCA-type pump. Moreover, the localization of Eca1 in the ER and the functional complementation of the yeast mutant K616 that is impaired in Ca2+ homeostasis (Cunningham and Fink, 1994), and elevated levels of cytosolic calcium in Δeca1 mutants implicate a function for Eca1 in Ca2+ transport into the ER. However, Δeca1 cells grow almost normally at 22°C, suggesting that other Ca2+ pumps partially compensate for the loss of Eca1 at this temperature. In addition to the vacuolar and Golgi-localized Ca2+-ATPases PMC1 and PMR1 in budding yeast (Cunningham and Fink, 1996), both budding and fission yeasts contain the V-subtype of ER-resident P-ATPases (Cronin et al., 2002; Facanha et al., 2002), which are also implicated in Ca2+ homeostasis. Surveys of the Ustilago genome (see MATERIALS AND METHODS) indicate that an orthologue of these proteins exists (47.7% identity and 64% similarity >1244 amino acids to Cta4p from Schizosaccharomyces pombe). Elucidating the role of this additional putative Ca2+ pump and their functional interplay with Eca1 will be a challenge for the nearer future.
Deregulated Ca2+ Signaling Is Responsible for the Defect of Δeca1 Mutants
Eca1 is dispensable at low temperature but is required to maintain morphology at 30°C. This indicates that a rise in temperature creates conditions under which transport of Ca2+ into the ER becomes crucial for the cell. In addition, treatment with tunicamycin and DTT, which targets protein modification and folding in the ER (Bonilla et al., 2002), is lethal to Δeca1 cells. A likely explanation for these observations is that higher temperatures increase the need for Ca2+-dependent protein folding in the ER and that Eca1 activity is required to provide Ca2+ as a cofactor for ER-resident chaperones (Corbett and Michalak, 2000). Obviously, this raises the possibility that misfolding of secretory proteins in the ER cause the observed morphology defect. However, the morphology phenotype of Δeca1 can be suppressed by lowering extracellular Ca2+ and by specific inhibition of cytosolic CaMK by using KN-93 (Sumi et al., 1991). Moreover, in Δeca1 cytosolic Ca2+ is increased. This implies that temperature rise induces influx of extracellular Ca2+ that cannot be stored in the ER in the absence of Eca1. In this model, this transport defect elevates cytosolic Ca2+ levels and leads to an increase of CaMK activity (Figure 9). The high sensitivity of Δeca1 mutants to inhibition of calcineurin further supports this notion, because it indicates that this Ca2+-dependent phosphatase counteracts the hyperactive CaMKs, thereby enabling Δeca1 to survive at 30°C. A link between calcineurin and CaMK-dependent signaling and cell morphology was also reported in fission yeast (Yoshida et al., 1994; Rasmussen, 2000) and in neurons (Chang et al., 1995). The accumulation of misfolded proteins in the ER of higher eukaryotes induces an entry of external Ca2+ into the cell (Putney et al., 2001), and SERCA activity is required to shuffle this Ca2+ into the ER (East, 2000). Therefore, we consider it most likely that similar mechanisms exist in U. maydis and that Eca1 is a key component facilitating survival and proper morphology under certain stress conditions.
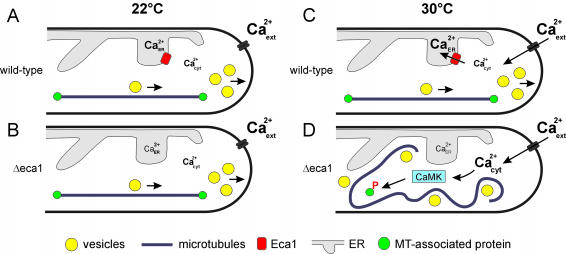
Figure 9.
Model for the role of Eca1 in microtubule organization in U. maydis. (A) In wild-type cells, Eca1 is an ER-resident Ca2+ pump that maintains Ca2+ in the ER high and holds cytosolic Ca2+ below a toxic threshold (Ca2+cyt). MTs run along the axis of the cell to support directed MT-dependent delivery of supplies to the growth region. A fine-tuning of parameters of dynamic instability, including elongation and shrinkage velocities and catastrophe and rescue frequencies, controls most likely MT-length. (B) At 22°C, Δeca1 mutants display normal MTs and morphology suggesting that other Ca2+ pumps, such as a Cta4-like ATPse (for sake of clarity, not included) are sufficient to keep cytosolic Ca2+ levels low. However, the high sensitivity of Δeca1 mutants to ER stress suggests that [Ca2+]ER is at a lower limit. (C) Increased temperature results in protein misfolding in the ER, which activates Ca2+-dependent chaperones. This might induce store-mediated entry of extracellular Ca2+ through unknown channels, and Eca1 is required to pump this incoming Ca2+ into the ER, thereby maintaining [Ca2+]cyt at resting levels. (D) In the absence of Eca1, incoming Ca2+ cannot be stored away, thus cytosolic Ca2+ increases, which activates CaMKs. These kinases phosphorylate MT-associated factors that regulate MT organization by influencing MT dynamic instability. A potential target of CaMK is cytoplasmic dynein that regulates MT dynamic instability and supports MT-dependent motility. These alterations in dynein activity and MT organization lead to defects in polar secretion and abnormal morphogenesis. Note that this model is supported by several key observations: 1) lowering external Ca2+ prevents the ts-phenotype of Δeca1, 2) inhibition of CaMK restores MT-rescue values and leads to normal MT organization and morphology, and 3) artificially induced destabilization of MTs by benomyl treatment leads to significant reduction in morphology defects.
Morphology Defects of Δeca1 Cells Are Due to Disordered MTs
Fungal growth and morphology depend on transport along the cytoskeleton, and a growing body of evidence indicates that MTs play a key role in this (Seiler et al., 1997; Sawin and Nurse, 1998; Wedlich-Söldner et al., 2000). Δeca1 mutants show severe defects in polar growth and cytokinesis, suggesting that cytoskeleton-based growth processes are affected. In agreement, the abnormal Δeca1 cells contained much longer and disorganized MTs, a defect that might be a consequence of deregulated dynamic instability of MTs. On the other hand, it is important to consider that elevated Ca2+ levels almost certainly affect numerous other cytoskeletal targets. For example, Myo5, a class V myosin involved in polar growth in U. maydis (Weber et al., 2003) contains a PEST site for Ca2+-regulated proteolysis and a putative CaMK phosphorylation site that is implied in Ca2+-dependent regulation of organelle traffic (Karcher et al., 2001). A functional GFP-myosinV fusion protein localized to the growing ends of Δeca1 cells, suggesting that Myo5 is not degraded and migrates along F-actin toward growth sites. Moreover, actin patch distribution was normal in Δeca1 (our unpublished data), indicating that the actin cytoskeleton is not severely affected by the deletion of eca1. On the other hand, high Ca2+ could interfere with other myosins or affect binding of Myo5 to secretory vesicles, thereby resulting in growth defects in Δeca1. However, the Δeca1 growth and morphology defect was significantly restored by benomyl, which is thought to destabilize MTs at low concentrations (Willins et al., 1995). Thus, altered morphology of Δeca1 cells is most likely a consequence of altered MT dynamics, although it cannot be excluded that additional but so far undetected defects add to the observed phenotype.
Is Dynein a Target of Ca2+ Signaling to Regulate MT Length and Organization?
MT-associated proteins control dynamic instability (Walczak, 2000), and these components are potential targets of CaMKs (Gardin et al., 1997). Evidence exists for a role of dynein in the control of MT dynamics in fungi (Carminati and Stearns, 1997; Han et al., 2001), and our data presented here support a role of dynein in modifying MT stability. Surprisingly, both dynein and Δeca1 mutants show strikingly similar defects in MT dynamics. In both strains, MTs were longer, curved, and this is accompanied by a threefold increase in rescue rates and a twofold decrease in the frequency of MT catastrophe events. It is important to note that the altered parameters in MT number and dynamics are specific signatures for the dynein phenotype, because mutants in other MT-associated proteins or motors have different effects on MT dynamics (Walczak, 2000; our unpublished data). Therefore, the striking similarity between both the Δeca1 and dynein mutant phenotype is remarkable and, together with the restorative effect of CaMK inhibition on MT organization, suggest a link between Ca2+ signaling and dynein activity. In addition, the inactivation of dynein in Δeca1 mutants (strain FB2Deca1Dyn2tsGT) did not significantly increase the defects in MT dynamics. In other words, MT defects in both Δeca1 and dyn2ts background do not add in the double mutant, which supports our model of Eca1 acting on MT stability via dynein (Figure 9). This notion is further supported by the analysis of ER motility, a process that solely depends on cytoplasmic dynein in U. maydis (Wedlich-Söldner et al., 2002a). In both Δeca1 and dyn2ts mutants, ER tubule motility was inhibited to the same extent, whereas ER motility was not affected by disruption of F-actin or deletion of kinesin (Wedlich-Söldner et al. 2002a). Therefore, dynein activity is apparently impaired in Δeca1 mutants, and this might be a result of CaMK-dependent phosphorylation. Consistent with this hypothesis, it was recently shown that dynein activity in flagellar axonemes is modulated by CaMKs (Smith, 2002). Although Ustilago dynein heavy chain contains several potential CaMK phosphorylation sites, we consider it unlikely that the heavy chain is directly regulated by CaMKs. A good candidate for this regulation is the conserved 8-kDa dynein light chain that associates with calmodulin (Yang et al., 2001). However, details of the composition of the dynein complex in U. maydis are presently lacking.
In summary, our data are consistent with the notion that deletion of eca1 that encodes a SERCA leads to deregulated Ca2+-dependent signaling, which in turn affects MT dynamics via impaired dynein activity in U. maydis. SERCA activity is indispensable for Notch receptor and secretory protein transport in Drosophila melanogaster (Periz and Fortini, 1999), and defects in calcium homeostasis affect MT catastrophe rates and growth of fission yeast (Facanha et al., 2002). Therefore, the described link between Ca2+ signaling and MT-dependent exocytosis might be of general importance for eukaryotic cells.
Acknowledgments
We are grateful to Dr. R. Kahmann for comments on the manuscript, Dr. K. Cunningham for strain K616, and Dr. H. Ulrich for yeast plasmids. We acknowledge Drs. J. Nakai and K. Imoto for providing the M13-cpEGFP-CaM construct, and Fujisawa USA Inc. for the gift of FK506. This work was supported by a grants from the Deutsche Forschungsgemeinschaft to G.S. (SP1111, STE799/4-1).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-09-0675. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-09-0675.
Abbreviations used: CaM, calmodulin; CaMK, Ca2+-calmodulin-dependent kinase; ER, endoplasmic reticulum; CFP, cyan fluorescent protein; GFP, green fluorescent protein; MT, microtubule; SERCA, sarcoplasmic/endoplasmic reticulum Ca2+ ATPase; ts, temperature sensitive; YFP, yellow fluorescent protein.
References
- Andersson, H., Kappeler, F., and Hauri, H.P. (1999). Protein targeting to endoplasmic reticulum by dilysine signals involves direct retention in addition to retrieval. J. Biol. Chem. 274, 15080-15084. [DOI] [PubMed] [Google Scholar]
- Bannuett, F., and Herskowitz, I. (1989). Different a alleles of Ustilago maydis are necessary for maintenance of filamentous growth but not for meiosis. Proc. Natl. Acad. Sci. USA 86, 5878-5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito, B., Garciadeblas, B., and Rodriguez-Navarro, A. (2000). Molecular cloning of the calcium and sodium ATPases in Neurospora crassa. Mol. Microbiol. 35, 1079-1088. [DOI] [PubMed] [Google Scholar]
- Bölker, M. (2001). Ustilago maydis - a valuable model system for the study of fungal dimorphism and virulence. Microbiology 147, 1395-1401. [DOI] [PubMed] [Google Scholar]
- Bonilla, M., Nastase, K.K., and Cunningham, K.W. (2002). Essential role of calcineurin in response to endoplasmic reticulum stress. EMBO J. 21, 2343-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carminati, J.L., and Stearns, T. (1997). Microtubules orient the mitotic spindle in yeast through dynein-dependent interactions with the cell cortex. J. Cell Biol. 138, 629-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, H.Y., Takei, K., Sydor, L.A., Born, T., Rusnak, F., and Jay, D.G. (1995). Asymmetric retraction of growth cone filopodia following focal inactivation of calcineurin. Nature 376, 686-690. [DOI] [PubMed] [Google Scholar]
- Clarke, D.M., Loo, T.W., Inesi, G., and MacLennan, D.H. (1989). Location of high affinity Ca2+-binding sites within the predicted transmembrane domain of the sarcoplasmic reticulum Ca2+-ATPase. Nature 339, 476-478. [DOI] [PubMed] [Google Scholar]
- Corbett, E.F., and Michalak, M. (2000). Calcium, a signalling molecule in the endoplasmic reticulum? Trends Biochem. Sci. 25, 307-311. [DOI] [PubMed] [Google Scholar]
- Cronin, S.R., Rao, R., and Hampton, R.Y. (2002). Cod1p/Spf1p is a P-type ATPase involved in ER function and Ca2+ homeostasis. J. Cell Biol. 157, 1017-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz, M.C., Goldstein, A.L., Blankenship, J.R., Del Poeta, M., Davis, D., Cardenas, M.E., Perfect, J.R., McCusker, J.H., and Heitman, J. (2002). Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J. 21, 546-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham, K.W., and Fink, G.R. (1994). Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J. Cell Biol. 124, 351-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham, K.W., and Fink, G.R. (1996). Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol. Cell. Biol. 16, 2226-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East, J.M. (2000). Sarco(endo)plasmic reticulum calcium pumps: recent advances in our understanding of structure/function and biology. Mol. Membr. Biol. 17, 189-200. [DOI] [PubMed] [Google Scholar]
- Evans, D.E., and Williams, L.E. (1998). P-type calcium ATPases in higher plants - biochemical, molecular and functional properties. Biochim. Biophys. Acta 1376, 1-25. [DOI] [PubMed] [Google Scholar]
- Facanha, A.L., Appelgren, H., Tabish, M., Okorokov, L., and Ekwall, K. (2002). The endoplasmic reticulum cation P-type ATPase Cta4p is required for control of cell shape and microtubule dynamics. J. Cell Biol. 157, 1029-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, D.S., and J. Heitman. (2002) Good fungi gone bad: the corruption of calcineurin. Bioessays 24, 894-903. [DOI] [PubMed] [Google Scholar]
- Gardin, H.M., Marklund, U., Larsson, N., Chatila, T.A., and Gullberg, M. (1997). Regulation of microtubule dynamics by Ca2+/Calmodulin-dependent kinase IV/Gr-dependent phosphorylation of oncoprotein 18. Mol. Cell. Biol., 17, 3459-3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez, T.M., and Spitzer, N.C. (1999). In vivo regulation of axon extension and pathfinding by growth-cone calcium transients. Nature 397, 350-355. [DOI] [PubMed] [Google Scholar]
- Han, G., Liu, B., Zhang, J., Zuo, W., Morris, N.R., and Xiang, X. (2001). The Aspergillus cytoplasmic dynein heavy chain and NUDF localize to microtubule ends and affect microtubule dynamics. Curr. Biol. 11, 719-724. [DOI] [PubMed] [Google Scholar]
- Hirokawa, N. (1998). Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science 279, 519-526. [DOI] [PubMed] [Google Scholar]
- Ho, S., Clipstone, N., Timmermann, L., Northrop, J., Graef, I., Fiorentino, D., Nourse, J., and Crabtree, G.R. (1996). The mechanism of action of cyclosporin A and FK506. Clin. Immunol. Immunopathol. 80, S40-S45. [DOI] [PubMed] [Google Scholar]
- Holliday, R. (1974). Ustilago maydis. In: Handbook of Genetics, ed. R.C. King, New YorK: Plenum Press.
- Joseph, J.D., and Means, A.R. (2000). Identification and characterization of two Ca2+/CaM-dependent protein kinases required for normal nuclear division in Aspergillus nidulans. J. Biol. Chem. 275, 38230-38238. [DOI] [PubMed] [Google Scholar]
- Karcher, R.L., Roland, J.T., Zappacosta, F., Huddleston, M.J., Annan, R.S., Carr, S.A., and Gelfand, V.I. (2001). Cell cycle regulation of myosin-V by calcium/calmodulin-dependent protein kinase II. Science 293, 1317-1320. [DOI] [PubMed] [Google Scholar]
- Kojic, M., Kostrub, C.F., Buchman, A.R., and Holloman, W.K. (2002). BRCA2 homolog required for proficiency in DNA repair, recombination, and genome stability in Ustilago maydis. Mol. Cell 10, 683-691. [DOI] [PubMed] [Google Scholar]
- Krüger, J., Loubradou, G., Regenfelder, E., Hartmann, A., and Kahmann, R. (1998). Crosstalk between cAMP and pheromone signalling pathways in Ustilago maydis. Mol. Gen. Genet. 260, 193-198. [DOI] [PubMed] [Google Scholar]
- Lehmler, C., Steinberg, G., Snetselaar, K.M., Schliwa, M., Kahmann, R., and Bolker, M. (1997). Identification of a motor protein required for filamentous growth in Ustilago maydis. Embo J. 16, 3464-3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magyar, A., and Varadi, A. (1990). Molecular cloning and chromosomal localization of a sarco/endoplasmic reticulum-type Ca2(+)-ATPase of Drosophila melanogaster. Biochem. Biophys. Res. Commun. 173, 872-877. [DOI] [PubMed] [Google Scholar]
- Nakai, J., Ohkura, M., and Imoto, K. (2001). A high signal-to-noise Ca(2+. probe composed of a single green fluorescent protein. Nat. Biotechnol. 19, 137-141. [DOI] [PubMed] [Google Scholar]
- Periz, G., and Fortini, M.E. (1999). Ca2+-ATPase function is required for intracellular trafficking of the Notch receptor in Drosophila. EMBO J. 18, 5983-5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney, J.W., Jr., Broad, L.M., Braun, F.J., Lievremont, J.P., and Bird, G.S. (2001). Mechanisms of capacitative calcium entry. J. Cell Sci. 114, 2223-2229. [DOI] [PubMed] [Google Scholar]
- Rasmussen, C.D. (2000). Cloning of a calmodulin kinase I homologue from Schizosaccharomyces pombe. J. Biol. Chem. 275, 685-690. [DOI] [PubMed] [Google Scholar]
- Sawin, K.E., and Nurse, P. (1998). Regulation of cell polarity by microtubules in fission yeast. J. Cell Biol. 142, 457-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebghati, T.S., Engle, J.T., and Goldman, W.E. (2000). Intracellular parasitism by Histoplasma capsulatum: fungal virulence and calcium dependence. Science 290, 1368-1372. [DOI] [PubMed] [Google Scholar]
- Seiler, S., Nargang, F.E., Steinberg, G., and Schliwa, M. (1997). Kinesin is essential for cell morphogenesis and polarized secretion in Neurospora crassa. EMBO J. 16, 3025-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, E.F. (2002). Regulation of flagellar dynein by calcium and a role for an axonemal calmodulin and calmodulin-dependent kinase. Mol. Biol. Cell. 13, 3303-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg, G., Wedlich-Soldner, R., Brill, M., and Schulz, I. (2001). Microtubules in the fungal pathogen Ustilago maydis are highly dynamic and determine cell polarity. J. Cell Sci. 114, 609-622. [DOI] [PubMed] [Google Scholar]
- Straube, A., Brill, M., Oakley, B.R., Horio, T., and Steinberg, G. (2003). Microtubule organization requires cell cycle dependent nucleation at dispersed cytoplasmic sites, polar and perinuclear MTOCs in the plant pathogen Ustilago maydis. Mol. Biol. Cell 14, 642-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube, A., Enard, W., Berner, A., Wedlich-Soldner, R., Kahmann, R., and Steinberg, G. (2001). A split motor domain in a cytoplasmic dynein. EMBO J. 20, 5091-5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stull, J.T. (2001). Ca2+-dependent cell signalling through calmodulin-activated protein phosphatase and protein kinases minireview series. J. Biol. Chem. 276, 2311-2312. [DOI] [PubMed] [Google Scholar]
- Sumi, M., Kiuchi, K., Ishikawa, T., Ishii, A., Hagiwara, M., Nagatsu, T., and Hidaka, H. (1991). The newly synthesized selective Ca2+/calmodulin dependent protein kinase II inhibitor KN-93 reduces dopamine contents in PC12h cells. Biochem. Biophys. Res. Commun. 181, 968-975. [DOI] [PubMed] [Google Scholar]
- Sze, H., Liang, F., Hwang, I., Curran, A.C., and Harper, J.F. (2000). Diversity and regulation of plant Ca2+ pumps: insights from expression in yeast. Annu. Rev. Plant Physiol. Plant Mol. Biol. 51, 433-462. [DOI] [PubMed] [Google Scholar]
- Verde, F., Dogterom, M., Stelzer, E., Karsenti, E., and Leibler, S. (1992). Control of microtubule dynamics by cyclin A- and cyclin B-dependent kinases in Xenopus egg extracts. J. Cell Biol. 118, 1097-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak, C.E. (2000). Microtubule dynamics and tubulin interacting proteins. Curr. Opin. Cell Biol. 12, 52-56. [DOI] [PubMed] [Google Scholar]
- Weber, I., Gruber, C., and Steinberg, G. (2003). A class-V myosin required for mating, hyphal growth, and pathogenicity in the dimorphic plant pathogen Ustilago maydis. Plant Cell 15, 2826-2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedlich-Söldner, R., Bölker, M., Kahmann, R., and Steinberg, G. (2000). A putative endosomal t-SNARE links exo- and endocytosis in the phytopathogenic fungus Ustilago maydis. EMBO J. 19, 1974-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedlich-Söldner, R., Schulz, I., Straube, A., and Steinberg, G. (2002a). Dynein supports motility of endoplasmic reticulum in the fungus Ustilago maydis. Mol. Biol. Cell. 13, 965-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedlich-Söldner, R., Straube, A., Friedrich, M.W., and Steinberg, G. (2002b). A balance of KIF1A-like kinesin and dynein organizes early endosomes in the fungus Ustilago maydis. EMBO J. 21, 2946-2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willins, D.A., Xiang, X., and Morris, N.J. (1995). An alpha tubulin mutation suppresses nuclear migration mutations in Aspergillus nidulans. Genetics 141, 1287-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, P., Diener, D.R., Rosenbaum, J.L., and Sale, W.S. (2001). Localization of calmodulin and dynein light chain LC8 in flagellar radial spokes. J. Cell Biol. 153, 1315-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida, T., Toda, T., and Yanagida, M. (1994). A calcineurin-like gene ppb1+ in fission yeast: mutant defects in cytokinesis, cell polarity, mating and spindle pole body positioning. J. Cell Sci. 107, 1725-1735. [DOI] [PubMed] [Google Scholar]