Abstract
Objective
The results of studies on the relation between Mannose-binding lectin gene (mbl2) polymorphism and HBV infection were contradictory and inconclusive. In order to shed a light on these inconsistent findings and to clarify the role of mbl2 polymorphisms in susceptibility or progression of chronic hepatitis B (CHB), a meta-analysis was performed.
Methods
PubMed and Embase were searched for available articles. A meta-analysis was performed to examine the association between mbl2 polymorphisms and chronicity or progression of hepatitis B infection. Odds ratio (OR) and its 95% confidence interval (CI) served as indexes.
Results
A total of 17 eligible studies were involved, including 2151 healthy controls (HC), 1293 spontaneous recovered (SR) patients with acute infection, 2337cases with chronic hepatitis B (CHB) and 554 cases with progressive hepatitis B. There was no evidence of significant association between mbl2 exon1 polymorphisms and CHB risk in any genetic model or pairwise comparisons when compared with HC group or SR group. In the stratified analysis of ethnic groups, also no obvious relation between mbl2 polymorphism and CHB risk was identified. There was still no significant association between the complete mbl2 genotypic profile (including both the exon1 and the promoter gene) polymorphisms and CHB risk, as compared with SR group. However, it was found that there was an association between the mbl2 AO/OO genotype and severe hepatitis B (SHB) or liver cirrhosis (LC) (LC vs. HC:OR=3.66, 95%CI, 2.38-5.63; SHB vs. HC, OR=3.88, 95%CI, 2.26–6.64), but there was no relationship between the mbl2 AO/OO genotype and hepatocellular carcinoma (HCC) (OR=1.26, 95%CI, 0.82-1.94).
Conclusion
The present meta-analysis indicated that mbl2 exon1 polymorphisms might not significantly associate with chronicity of HBV infection, but might be significantly related to the progressive HBV such as SHB and LC.
Introduction
Hepatitis B virus (HBV) infection leads to a wide spectrum of clinical presentations from inapparent infection to self-limiting acute hepatitis, chronic infection, fulminant hepatic failure (FHF), liver cirrhosis (LC) and hepatocellular carcinoma (HCC) [1]. The mechanism for persistent and progressive HBV infection is still unclear, but host immune factors and genetic factors may play important roles [2].
Mannose-binding lectin (MBL) is an important constituent of the human innate immune system, which, as an acute-phase reactant, is secreted by the liver. MBL is a calcium-dependent C-type lectin with a structural analogy of complement component C1q. MBL can bind through multiple lectin domains to the carbohydrate moieties expressed on the surface of many microbial organisms, and activate macrophages and the complement system cascade [3]. It is also reported that serum MBL play an important role in regulating the production of proinflammatory cytokines such as TNF-α, IL-6 and IL-1βby monocytes in response to microbial infection [4], hence may affect the inflammation severity or disease progression.
Polymorphisms in the MBL gene (mbl2) have been shown to affect the oligomer formation and circulating levels of MBL [5]. Three single nucleotide polymorphisms (SNPs) in exon1 (codon52, codon54, and codon57) of mbl2 gene give rise to amino acid substitutions within the collagen-like region of MBL. These three polymorphic alleles are collectively designated as allele O, while the wild-type structural allele is described as allele A [6]. The wild-type A/A, heterogeneous-type A/O and homologous-type O/O are generally associated with high, intermediate and low (or absent) MBL levels, respectively. SNPs have also been found in the promoter region of mbl2 at positions 221 Y/X [7], and the X variant also significantly reduces the serum MBL levels. In recent years, a number of clinical and genetic studies demonstrated the association between low serum MBL levels due to SNPs in mbl2 and CHB [8-24]. Some studies reported that patients with mbl2 mutations are prone to developing persistent HBV infection and that the MBL plays an important role in the clinical outcome after HBV infection [8,11,18]. Moreover, it was reported that the mbl2 polymorphisms may be an important factor in determining the prognosis in patients with hepatitis B virus infection [13]. However, the data from several studies argue against a role of MBL in chronicity and progression of HBV [9,17]. For reason given above, a meta-analysis in a large population was fulfilled in present study.
Materials and Methods
Search strategy
In order to collect all papers involved in mbl2 polymorphisms and HBV, PubMed and Embase (up date to 31 March 2013) were searched independently by two investigators (Hangdi Xu and Mingfei Zhao). The search terms were the following key words: (“Mannose binding lectin” or “Mannose binding protein” or “Mannose-binding lectin” or “Mannose-binding protein” or “MBL2” or “MBP2” or “MBL-2” or “MBP-2”), and (“HBV” or “Hepatitis B”). The search strategy was shown in Table 1. The reference lists of reviews and retrieved articles were searched at the same time by hand. Data were only recruited from the full published paper, and did not include any conference abstract or unpublished report. When the same patient population was included in several publications, only the one with the biggest size sample or one in the most recent study was used in present meta-analysis.
Table 1. Search strategy for electronic databases.
| PubMed |
|---|
| ("mannose-binding lectin"[MeSH Terms] OR "mannose-binding lectin"[All Fields] OR "mannose binding lectin"[All Fields] OR "mannose-binding protein"[All Fields] OR "mannose binding protein"[All Fields] OR "mbl2"[All Fields] OR "mbl-2"[All Fields] OR "mbp-2"[All Fields] OR "mbp2"[All Fields]) AND (hbv[All Fields] OR "hepatitis b"[MeSH Terms] OR "hepatitis b"[All Fields]) |
| Embase |
| ('mannose-binding lectin'/exp OR 'mannose-binding lectin' OR 'mannose binding lectin'/exp OR 'mannose binding lectin' OR 'mannose-binding protein'/exp OR 'mannose-binding protein' OR 'mannose binding protein'/exp OR 'mannose binding protein' OR 'mbl2' OR 'mbl-2' OR 'mbp-2' OR 'mbp2') AND ('hbv’/exp OR hbv OR 'hepatitis b'/exp OR 'hepatitis b') |
Criteria of inclusion and exclusion
The criteria of collecting the published studies were following: (1) the evaluation of the association between mbl2 polymorphisms and HBV infection; (2) a case-control or cohort studies; (3) sufficient genotype data for calculating the odds ratio (OR) with 95% confidence interval (CI). The criteria of excluding studies were following: (1) the studies with overlapped data; (2) abstracts, comments, reviews or editorial letters.
Data extraction
The following information was extracted from each study: the first author’s name, years of publication, ethnicity of population, numbers of cases and controls, genotyping methods, genotyping results of cases and controls, and the Hardy-Weinberg equilibrium (HWE) results. The control groups included healthy control (HC) groups and spontaneous recovered (SR) groups with acute infection.
Definitions
HC were negative for hepatitis B surface antigen (HBsAg) and HBV DNA. The spontaneously recovered (SR) individuals were positive for both hepatitis B surface antibody and hepatitis B core antibody, but definitely negative for HBsAg; or positive for HBsAg and IgM anti-HBc on presentation and clearing HBsAg within 6 months. CHB individuals were with persistent HBsAg positive for more than 6 months. SHB patients had severe liver disease symptoms, including obvious clinical manifestations and conspicuous changes in their biochemical parameters, such as significant serum alanine aminotransferase (ALT) elevation (more than 5 folds of normal levels) and plasma prothrombin activity (PTA) <60%. LC subjects were defined as HBsAg carriers whose liver ultrasound scan or liver function test confirmed cirrhosis. HCC patients were diagnosed by serum α-fetoprotein levels and hepatic arteriography or histology.
Statistical analysis
First of all, HWE was assessed in the HC groups using the goodness-of-fit test (χ2 or Fisher’s exact test) and a P < 0.05 was considered as significant disequilibrium. OR and the corresponding 95% CI were utilized to evaluate the strength of association of mbl2 polymorphisms with CHB. Quantitative meta-analysis was performed using STATA version 10.0 (STATA Corporation, College Station, TX, USA). Heterogeneity was assessed for each study using Cochrane’s Q-test and I 2 measurement (I 2 is defined as the proportion of total variations across studies, which are due to heterogeneity rather than chance). P ≤ 0.10 or I2 ≥ 50% indicated that the heterogeneity was significant. A random effect model was used in the presence of substantial heterogeneity. The potential risk of publication bias was examined by the Egger test, and P ≤ 0.10 indicates the presence of a publication bias. Subgroup analysis was also performed for ethnicity and the types of progressive hepatitis B virus infection. Sensitivity analysis was conducted to evaluate the validity and reliability of primary meta-analysis. Nonsuperiority test was used to confirm the absence of association between mbl2 exon 1 polymorphism and persistence of HBV infection.
Results
Characteristics of studies included in the meta-analysis
After searching the databases, 81 potential eligible reports were identified (Figure 1), and 29 records were excluded due to duplicate data, and 21 records, which belonged to abstracts, editorials, comments or reviews, were excluded. Also 13 studies not related to polymorphisms or HBV were excluded. One record, a non-case control study, was excluded. Finally, a total of 17 studies were involved, which included 2151 HC, 1293 SR patients with acute infection, 2337cases with CHB and 554 cases (175 cases with severe hepatitis B (SHB), 223 cases with LC and 156 cases with HCC). Table 2 showed the general characteristics of the included studies.
Figure 1. Flow chart for selection of studies.
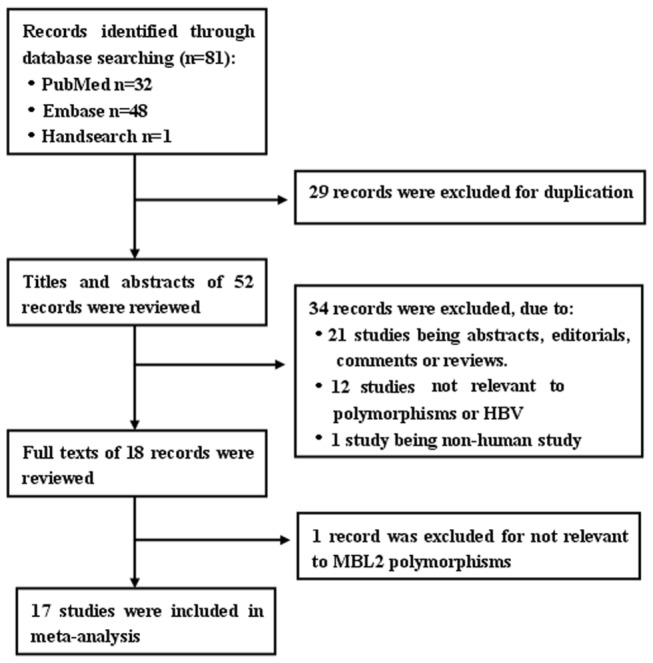
Table 2. Characteristics of studies for association between mbl2 polymorphisms and HBV infection.
| Study | Ethnicity | Control | Cases | Polymorphisms included | Genotyping Methods | HWE |
|---|---|---|---|---|---|---|
| Thomas HC 1996 [8] | Caucasian | HC/SR | CHB | Condon52, 54 and57 | SSOP; sequencing | No |
| Asian | HC | CHB | Condon52, 54 and57 | SSOP; sequencing | Yes | |
| Bellamy R 1998 [9] | African | HC/SR | CHB | Condon52, 54 and57 | SSOP | Yes |
| Höhler T 1998 [10] | Caucasian | HC/SR | CHB | Condon52 and 54 | PCR-RFLP | Yes |
| Yuen MF 1999 [11] | Asian | HC | CHB/LC/HCC | Condon54 | PCR-RFLP | − |
| Shi H 2001 [12] | Asian | HC | CHB | Condon52, 54 and 57 | PCR-RFLP | Yes |
| Hakozaki Y 2002 [13] | Asian | HC | SHB | Condon52, 54 and 57; -221Y/X | sequencing | No |
| Song le H 2003[1] | Asian | HC | CHB/LC/HCC | Condon52, 54 and57 | sequencing | Yes |
| Cheong JY 2005 [14] | Asian | SR | CHB | Condon54 | SBE | Yes |
| Thio CL 2005[15] | A mixed population | SR | CHB | Mbl2 promoter and exon1 combined polymorphisms | Real time PCR | − |
| Chong WP 2005 [16] | Asian | HC/SR | CHB/LC/HCC | Mbl2 promoter and exon1 combined polymorphisms | TaqMan PCR | Yes |
| Segat L 2008 [17] | Caucasian | HC | HCC | Condon52, 54 and57 | Real time PCR | Yes |
| Tong FY 2008 [18] | Asian | HC | CHB/SHB | Condon52, 54 and 57 | sequencing | − |
| Filho RM 2010 [19] | A mixed population | HC | CHB | Condon52, 54 and 57; -221Y/X | Taqman PCR | Yes |
| Fletcher GJ 2010 [20] | A mixed population | SR | CHB | Condon52, 54 and 57; -221Y/X | PCR-SSP | − |
| Chen DQ 2010 [21] | Asian | SR | CHB | Condon54 and -221Y/X | PCR-RFLP | Yes |
| Chatzidaki V 2012 [22] | Caucasian | HC/SR | CHB | Condon54 and 57; -221Y/X | PCR-RFLP | Yes |
| Zheng RD 2012 [23] | Asian | HC/SR | CHB/SHB/LC | Condon54 | PCR-RFLP | Yes |
RFLP: restriction fragment length polymorphisms; SSOP: sequence specific oligonucleotide probes; PCR-SSP: polymerase chain reaction-sequence specific primer; SBE: Single Base Primer Extension Assay. HWE: Hardy-Weinberg equilibrium; − not available. HC: healthy control; SR: spontaneous recovered control; CHB: chronic hepatitis B; LC: liver cirrhosis; HCC: hepatocellular carcinoma; SHB: severe hepatitis B.
Association between HBV persistence and MBL exon1 polymorphisms
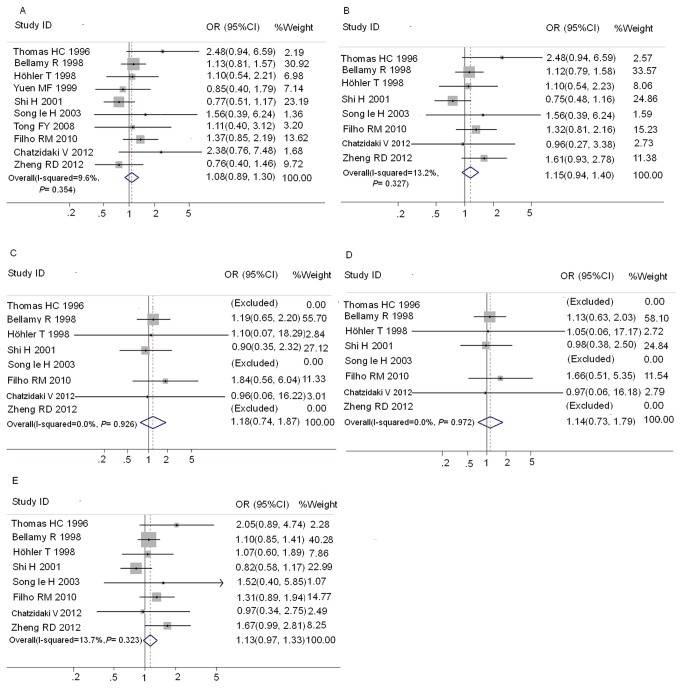
Ten studies [1,8-12,18,19,22,23] contained sufficient data for analysis of wild-type (AA) versus any MBL2 variant allele (OA/OO) genotype between HC groups and CHB groups (Table S1). The distribution of genotypes in HC of Caucasian subjects from the study of Thomas HC et al [8] was not consistent with HWE, so only the Asian subjects from this study were included in the meta-analysis between HC and CHB. Eight studies [1,8-10,14,20-22] contain sufficient data for analysis of AA versus OA/OO between SR groups and CHB groups (Table S1). Results of pooled analysis on the associations between MBL exon1 polymorphisms and the risk of CHB were shown in Figure 2 and Figure 3. Overall, no significant association between exon1 gene polymorphisms and the risk of CHB was observed as compared with both HC (Figure 2) and SR groups (Figure 3). There was no significant heterogeneity in these analyses (I2≤23.9%). The publication bias of these studies was assessed by Egger’s test. In HC groups, the funnel plot showed the asymmetry in mbl2 exon1 OO vs. AA and AO+OO vs. AA models to some extent, and Egger’s test proved the existence of publication bias in these two models (P= 0.020 and 0.007, respectively). No significant publication bias was detected in other comparisons.
Figure 2. Forest plot of chronic hepatitis B virus infection risk associated with mbl2 exon1 polymorphisms as compared with HC.
A: AO/OO vs. AA; B: AO vs. AA; C:OO vs. AA; D:OO vs. AO/AA; E: O allele vs. A allele.
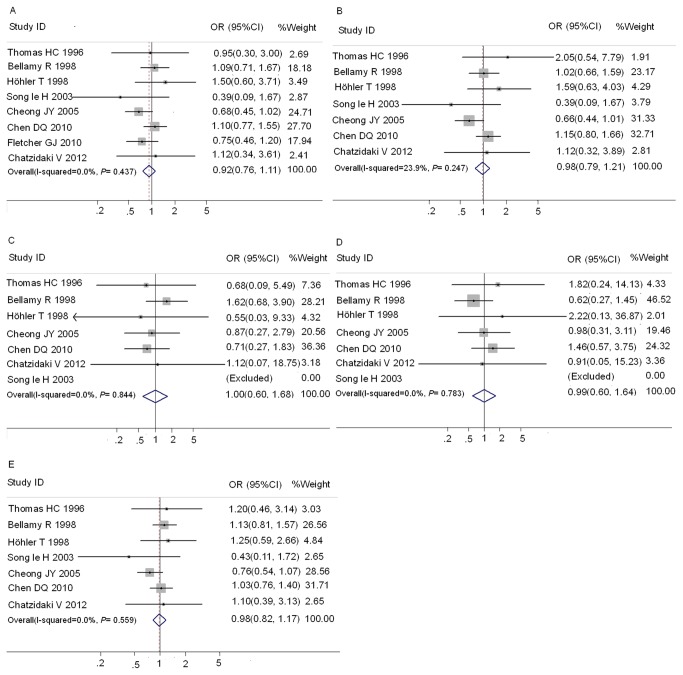
Figure 3. Forest plot of chronic hepatitis B virus infection risk associated with mbl2 exon1 polymorphisms as compared with SR group.
A: AO/OO vs. AA; B: AO vs. AA; C:OO vs. AA; D:OO vs. AO/AA; E: O allele vs. A allele.
The results of subgroup analysis based on ethnicity indicated that mbl2 O allele carriers (AO and / or OO) in both Asian populations and non-Asian populations were not associated with the increased risk of chronic HBV infection as compared with HC or SR groups, respectively (Table 3).
Table 3. Association between mbl2 exon1 polymorphisms and the persistence of HBV infection in different ethnicity-subgroups.
| No. studies |
OR* for variant genotype (95% CI)
|
Test for heterogeneity P value | I2 | ||
|---|---|---|---|---|---|
| Healthy controls | |||||
| Asian | |||||
| AO/OO vs AA | 6 | 1.08 | 0.82-1.42 | 0.156 | 37.6% |
| AO vs AA | 4 | 1.13 | 0.83-1.55 | 0.054 | 60.7% |
| OO vs AA | 4 | 0.90 | 0.35-2.32 | − | − |
| OO vs AO/AA | 4 | 0.98 | 0.38-2.50 | − | − |
| Non-Asian | |||||
| AO/OO vs AA | 5 | 1.18 | 0.92-1.51 | 0.899 | 0.0% |
| AO vs AA | 4 | 1.16 | 0.90-1.50 | 0.934 | 0.0% |
| OO vs AA | 4 | 1.28 | 0.76-2.16 | 0.927 | 0.0% |
| OO vs AO/AA | 4 | 1.20 | 0.72-1.99 | 0.947 | 0.0% |
| Spontaneous recovered controls | |||||
| Asian | |||||
| AO/OO vs AA | 3 | 1.15 | 0.88-1.49 | 0.120 | 52.9% |
| AO vs AA | 3 | 0.89 | 0.68-1.16 | 0.080 | 60.5% |
| OO vs AA | 3 | 0.77 | 0.37-1.59 | 0.788 | 0.0% |
| OO vs AO/AA | 3 | 0.80 | 0.39-1.64 | 0.604 | 0.0% |
| Non-Asian | |||||
| AO/OO vs AA | 5 | 1.00 | 0.75-1.32 | 0.561 | 0.0% |
| AO vs AA | 4 | 1.17 | 0.81-1.68 | 0.691 | 0.0% |
| OO vs AA | 4 | 1.32 | 0.63-2.76 | 0.807 | 0.0% |
| OO vs AO/AA | 4 | 1.27 | 0.62-2.60 | 0.688 | 0.0% |
OR: odds ratio; CI: confidence interval; −: can’t be calculated. “AO/OO vs. AA”: “Dominant model”; “OO vs. AO/AA”: “Recessive model”; “AO vs. AA” and “OO vs. AA”: “Co-dominant model.
Association between mbl2 promoter -221 Y/X polymorphisms and CHB
Four studies with SR groups and two studies with HC groups about association between mbl2 promoter -221 Y/X polymorphisms and CHB were reported (Table S2). The results of pooled analysis demonstrated no significant association between mbl2 promoter polymorphisms and the risk of CHB as compared with SR group (XY/XX vs. YY:OR=1.10, 95%CI 0.90-1.35; XY vs. YY:OR=0.93, 95%CI 0.71-1.21; XX vs. YY:OR=0.81, 95%CI 0.43-1.54; XX vs. XY/Y: OR=0.80, 95%CI 0.42-1.51) (Table 4). When analyzing the association between promoter polymorphisms and the risks of CHB as compared with the HC group, it was found that there was the significant relation between -221 Y/X polymorphisms and the risk of CHB under XY vs. YY contrast (OR=1.61, 95%CI 1.01-2.57), but no significant relation under other contrasts (XY/YY vs. YY:OR=1.42, 95%CI 0.91-2.20; XX vs YY:OR=0.65, 95%CI 0.23-1.90; XX vs. XY/Y: OR=0.56, 95%CI 0.20-1.61) was observed (Table 4).
Table 4. Meta-analysis for the association between mbl2 promoter -221Y/X polymorphisms and the persistence of HBV infection.
| No. of studies |
OR* for variant genotype (95% CI)
|
Test for heterogeneity P value | I2 | ||
|---|---|---|---|---|---|
| Healthy controls | |||||
| XY/XX vs YY | 2 | 1.42 | 0.91-2.20 | 0.775 | 0.0% |
| XY vs YY | 2 | 1.61 | 1.01-2.57 | 0.743 | 0.0% |
| XX vs YY | 2 | 0.65 | 0.23-1.90 | 0.524 | 0.0% |
| XX vs XY/YY | 2 | 0.56 | 0.20-1.61 | 0.488 | 0.0% |
| Spontaneous recovered controls | |||||
| XY/XX vs YY | 4 | 1.10 | 0.90-1.35 | 0.193 | 36.6% |
| XY vs YY | 3 | 0.93 | 0.71-1.21 | 0.640 | 0.0% |
| XX vs YY | 3 | 0.81 | 0.43-1.54 | 0.517 | 0.0% |
| XX vs XY/YY | 3 | 0.80 | 0.42-1.51 | 0.573 | 0.0% |
OR: odds ratio; CI: confidence interval. “XY/XX vs. YY”: “Dominant model”; “XX vs. XY/YY”: “Recessive model”; “XY vs. YY” and “XX vs. YY”: “Co-dominant model.
Association between mbl2 promoter and exon1 combined polymorphisms and CHB
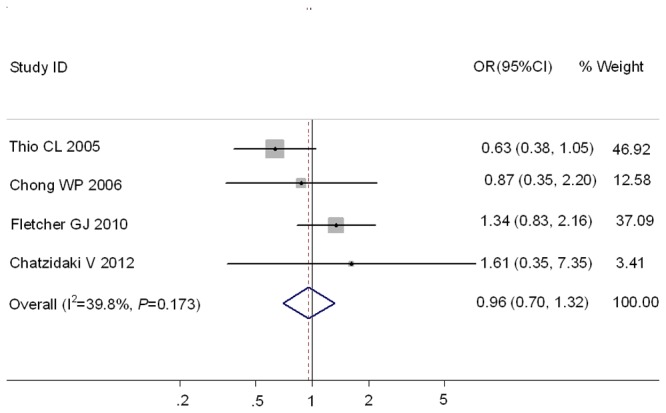
Subsequently, a meta-analysis was performed on the studies that reported a complete mbl2 genotypic profile including both the exon1 and the promoter polymorphisms. Although only four studies reported such data (Table S3), it was proposed that such genotype profiles were associated much more strongly with serum MBL levels than exon1 genotypes or promoter genotypes alone [24,25]. In this subset, the individuals with O/O and XA/O were considered as the subjects with low MBL production, whereas the individuals with other genotypes were demonstrated as the subjects with high MBL production [26]. However, it was found in Figure 4 that there was no significant association between mbl2 genotypes and the susceptibility of CHB, as compared with SR subjects (OR=0.96, 95%CI 0.70-1.32).
Figure 4. Forest plot of chronic hepatitis B virus infection risk associated with mbl2 promoter and exon1 combined polymorphisms as compared with SR group.
Association between mbl2 exon1 polymorphisms and the progression of CHB
There were six studies on hepatitis B progression with the sufficient data of mbl2 wild type versus mbl2 variant (AO/OO) genotype. As the HC group in the study of Hakozaki Y et al [13] was not consisting with HWE, so it was excluded from the meta-analysis. At last, a total of 511 cases and 545 controls were included in this analysis. As shown in Figure 5A, mbl2 genotypes AO/OO were significantly related to HBV progressive liver diseases (OR=2.29; 95%CI 1.29-4.09). As the progressive liver disease contains three types of diseases (SHB, LC and HCC), the association between mbl2 exon1 polymorphisms and the risks of each hepatic disease was investigated. The results (Figure 5B) demonstrated that there was the significant relation between mbl2 AO/OO genotypes and LC and SHB risks (LC vs. HC:OR=3.66, 95%CI 2.38-5.63; SHB vs. HC, OR=3.88, 95%CI, 2.26–6.64). However, the significant association between mbl2 AO/OO genotypes and HCC was not observed (OR=1.26, 95%CI 0.82-1.94).
Figure 5. Forest plot of progressive hepatitis B disease risk associated with mbl2 exon1 polymorphisms as compared with HC.
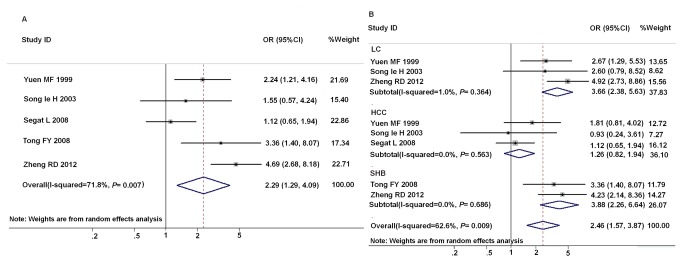
A: association between mbl2 genotypes AO/OO and HBV progressive liver diseases; B: subgroup analysis based on types of diseases.
Equivalence-based analysis to confirm the lack of relationship between MBL2 polymorphism and persistence of HBV infection
Non-superiority test was performed to confirm the absence of association between MBL2 polymorphism and persistence of HBV infection. The null hypothesis is that the frequency of MBL2 exon1 O alleles in CHB patients is greater by Δ as compared with the frequency in controls. The specified amount Δ may be arbitrary, and it may be taken from the lower boundary of the difference estimated in the initial report of the genetic association [27,28]. The difference of MBL2 O allele between CHB and HC patients first reported by Thomas HC were 10.1% [8]. Thus, a 5% excess in cases represents a fairly stringent cut-off.
The corresponding nonsuperiority P-values for MBL2 exon1 was 0.0005 and 0.0012, as compared with HC or SR respectively, which support the absence of association between MBL2 exon1 polymorphism and persistence of HBV (Table S4 and Table S5).
Discussion
MBL is an innate immune system pattern recognition protein which can kill the different pathogenic microbes through complement activation. The mbl2 polymorphisms could result in the MBL deficiency among a certain percentage of human, which potentially increases susceptibility to infectious disease [29,30]. Our meta-analysis has demonstrated no significant relation between mbl2 genotype and CHB. In Asia, the infection usually occurs at birth, and the infecting inoculums may be larger. About 90% of newborns, whose mothers are positive for HBeAg, will become persistently infected as a result of the induction of T-cell tolerance by secreted HBe, which crosses the placenta [31]. Thus, the genetic factors will be less important in these patients. However, among Caucasian and African patients, the infection usually occurs in adulthood and childhood, and 5% and 20% of cases become persistently infected, respectively [8]. The results of subgroup analysis based on ethnicity were shown in table 3. There was still no significant association between the chronicity of hepatitis B and mbl2 polymorphisms in Asian and non-Asian, respectively. However, there was the significant relation between -221 X/Y polymorphisms and the risk of CHB, when compared with HC under XY vs. YY contrast. As the studies included was limited (only two), more studies should been taken to verified this result in the future.
In order to investigate these alternative results, the additional ad hoc meta-analysis was performed for the studies that reported complete mbl2 genotypic profile, including promoter region and structural region. No significant association between complete mbl2 genotype and CHB susceptibility was found, as compared with SR group.
Our meta-analysis demonstrated that there was the significant relation between mbl2 variant genotypes (AO/OO) and SHB or LC. It was concluded that MBL would not appear to be involved significantly in host susceptibility to CHB, but might play an important role in exacerbation and progression of hepatitis B. These genotypes (AO/OO) might be associated with the natural wound healing process, which may develop the necroinflammation, rather than carcinogenesis [16,17].
The progression of HBV infections resulted from MBL mutations may be explained by two possible mechanisms. Firstly, MBL may have a direct effect on HBV infection through complement activation. The amannose-rich oligosaccharide was detected in the preS2 region of the hepatitis B surface protein, so MBL could bind HBV in theory [16,32]. Moreover, Chong WP et al [16] have studied the binding of MBL to HBsAg and found that MBL could bind HBsAg via its multiple carbohydrate recognition domains. It was suggested that MBL might function as an opsonin for HBV. Also MBL plays an important role in the complement system by acting as the recognition molecule of the lectin pathway and that MBL activates complement on HBsAg-MBL complexes through the lectin complement pathway and therefore may be involved in HBV clearance [16]. Besides the possibility of direct clearance of HBV, MBL-mediated complement activation might also be involved in immune complex removal during HBV infection. It was reported that immune complexes levels increased in chronic liver disease, which might be associated with the increased inflammatory responses in liver damage [33]. Thus, low MBL levels secondary to mutations may lead to defective complement activation, and poor clearance of immune complexes with subsequent deposition in the liver, which may subsequently predispose the patients to greater immunological damage of the liver [11]. The second possible mechanism is that MBL may regulate the production of some inflammatory cytokines. The high concentrations of MBL profoundly decreased the interleukin (IL)-6, IL-1β, and tumor necrosis factor-α produced by monocytes in response to pathogen, whereas lower concentrations of MBL could enhance the production of IL-6 and IL-1β [34]. So MBL not only is involved in complement activation but also is a potent regulator of inflammatory pathways, as such, MBL may affect the severity of HBV. These two mechanisms would explain why patients with mbl2 AO/OO genotypes were more likely to develop the symptomatic cirrhosis and severe hepatitis.
In spite of providing the most comprehensive assessment of the association between the outcome of HBV infection and the mbl2 polymorphisms, there are still some limitations that should be taken in to account when interpreting our results. Heterogeneity and confounding factors may have affected the meta-analysis. The moderate and high heterogeneity existed in the analyses, especially in the subgroup of Asians or the SHB group. Publication bias may be other influence factor of this meta-analysis. It is possible that the studies with negative results have not been published, and Egger’s test indicated that the publication bias existed in two contrasts when CHB group was compared with the HC group. Furthermore, there was only a small amount of data in present meta-analysis, especially in some subgroup analysis.
In summary, the association between the mbl2 polymorphisms and the HBV infection was systematically analyzed in present meta-analysis. The combined results showed that mbl2 exon1 polymorphisms might be not significantly associated with chronicity of HBV infection, but might be significantly associated with the progressive HBV such as SHB and LC. Moreover, mbl2 polymorphisms might be not significantly associated with carcinogenesis of CHB. Since the potential confounders could not be ruled out completely and the studies in subgroup analysis were limited, it is necessary to confirm these results with more studies in future.
Supporting Information
Distribution of MBL2 exon1 genotypes among HBV cases and controls in the meta-analysis.
(DOC)
Distribution of MBL2 promoter genotypes among HBV cases and controls in the meta-analysis.
(DOC)
Distribution of polymorphisms of MBL2 promoter plus exon1 among HBV cases and controls in the meta-analysis.
(DOC)
Non-superiority tests of mbl2 polymorphism in CHB as compared with HC.
(DOC)
Non-superiority tests of mbl2 polymorphism in CHB as compared with SR.
(DOC)
PRISMA 2009 Checklist.
(DOC)
Funding Statement
This work was funded by the 12-5 State S&T Projects of China (2012ZX10002007). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Song le H, Binh VQ, Duy DN, Jüliger S, Bock TC et al. (2003) Mannose-binding lectin gene polymorphisms and hepatitis B virus infection in Vietnamese patients. Mutat Res 522: 119-125. doi: 10.1016/S0027-5107(02)00284-1. PubMed: 12517417. [DOI] [PubMed] [Google Scholar]
- 2. Thursz M (2001) Genetic susceptibility in chronic viral hepatitis. Antiviral Res 52: 113-116. doi: 10.1016/S0166-3542(01)00175-9. PubMed: 11672820. [DOI] [PubMed] [Google Scholar]
- 3. Lee YH, Witte T, Momot T, Schmidt RE, Kaufman KM et al. (2005) The mannose-binding lectin gene polymorphisms and systemic lupus erythematosus. Arthritis Rheum 52: 3966–3974. doi: 10.1002/art.21484. PubMed: 16320344. [DOI] [PubMed] [Google Scholar]
- 4. Jack DL, Read RC, Tenner AJ, Frosch M, Turner MW et al. (2001) Mannose-binding lectin regulates the inflammatory response of human professional phagocytes to Neisseria meningitidis serogroup B. J Infect Dis 184: 1152-1162. doi: 10.1086/323803. PubMed: 11598838. [DOI] [PubMed] [Google Scholar]
- 5. Larsen F, Madsen HO, Sim RB, Koch C, Garred P (2004) Diseaseassociated mutations in human mannose-binding lectin compromise oligomerization and activity of the final protein. J Biol Chem 279: 21302–21311. doi: 10.1074/jbc.M400520200. PubMed: 14764589. [DOI] [PubMed] [Google Scholar]
- 6. Garred P, Larsen F, Madsen HO, Koch C (2003) Mannosebinding lectin deficiency-revisited. Mol Immunol 40: 73–84. doi: 10.1016/S0161-5890(03)00104-4. PubMed: 12914814. [DOI] [PubMed] [Google Scholar]
- 7. Madsen HO, Garred P, Thiel S, Kurtzhals JA, Lamm LU et al. (1995) Interplay between promoter and structural gene variants control basal serum level of mannan-binding protein. J Immunol 155: 3013–3020. PubMed: 7673719. [PubMed] [Google Scholar]
- 8. Thomas HC, Foster GR, Sumiya M, McIntosh D, Jack DL et al. (1996) Mutation of gene of mannose-binding protein associated with chronic hepatitis B viral infection. Lancet 348: 1417-1419. doi: 10.1016/S0140-6736(96)05409-8. PubMed: 8965590. [DOI] [PubMed] [Google Scholar]
- 9. Bellamy R, Ruwende C, McAdam KP, Thursz M, Sumiya M et al. (1998) Mannose binding protein deficiency is not associated with malaria, hepatitis B carriage nor tuberculosis in Africans. QJM 91: 13-18. doi: 10.1093/qjmed/91.1.13. PubMed: 9519208. [DOI] [PubMed] [Google Scholar]
- 10. Höhler T, Wünschel M, Gerken G, Schneider PM, Meyer zum Büschenfelde KH et al. (1998) No association between mannose-binding lectin alleles and susceptibility to chronic hepatitis B virus infection in German patients. Exp Clin Immunogenet 15: 130-133. doi: 10.1159/000019064. PubMed: 9813410. [DOI] [PubMed] [Google Scholar]
- 11. Yuen MF, Lau CS, Lau YL, Wong WM, Cheng CC et al. (1999) Mannose binding lectin gene mutations are associated with progression of liver disease in chronichepatitis B infection. Hepatology 29: 1248-1251. doi: 10.1002/hep.510290417. PubMed: 10094971. [DOI] [PubMed] [Google Scholar]
- 12. Shi H, Wang F, Jin L, Liu M, Hong W et al. (2001) [Genotype polymorphism and its implications of mannose-binding protein allele in 5 Chinese nationalities]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 18: 202-205. PubMed: 11402450. [PubMed] [Google Scholar]
- 13. Hakozaki Y, Yoshiba M, Sekiyama K, Seike E, Iwamoto J et al. (2002) Mannose-binding lectin and the prognosis of fulminant hepatic failure caused by HBV infection. Liver 22: 29-34. doi: 10.1046/j.0106-9543.2001.01516.x. PubMed: 11906616. [DOI] [PubMed] [Google Scholar]
- 14. Cheong JY, Cho SW, Lim SK, Shin DH, Yoon SK et al. (2005) Lack of association between hepatitis B virus infection and polymorphism of mannose-binding lectin gene in Korean population. J Korean Med Sci 20: 65-69. doi: 10.3346/jkms.2005.20.1.65. PubMed: 15716605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thio CL, Mosbruger T, Astemborski J, Greer S, Kirk GD et al. (2005) Mannose binding lectin genotypes influence recovery from hepatitis B virus infection. J Virol 79: 9192-9196. doi: 10.1128/JVI.79.14.9192-9196.2005. PubMed: 15994813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chong WP, To YF, Ip WK, Yuen MF, Poon TP et al. (2005) Mannose-binding lectin in chronic hepatitis B virus infection. Hepatology 42: 1037-1045. doi: 10.1002/hep.20891. PubMed: 16231358. [DOI] [PubMed] [Google Scholar]
- 17. Segat L, Fabris A, Padovan L, Milanese M, Pirulli D et al. (2008) MBL2 and MASP2 gene polymorphisms in patients with hepatocellular carcinoma. J Viral Hepat 15: 387-391. doi: 10.1111/j.1365-2893.2008.00965.x. PubMed: 18221301. [DOI] [PubMed] [Google Scholar]
- 18. Tong FY, Gan JH, Lu Q, Cao WG, Shen XJ (2008) [Relationship between the gene mutations of mannose binding protein and the progression ofhepatitis B infection]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 25: 331-333. PubMed: 18543229. [PubMed] [Google Scholar]
- 19. Filho RM, Carmo RF, Catsman C, Souza C, Silva A et al. (2010) High frequency of variant alleles of the mannose-binding lectin 2 (MBL2) gene are associated with patients infected by hepatitis B virus. Viral Immunol 23: 449-453. doi: 10.1089/vim.2009.0105. PubMed: 20712490. [DOI] [PubMed] [Google Scholar]
- 20. Fletcher GJ, Gnanamony M, Samuel P, Ismail AM, Kannangai R et al. (2010) Association of mannose-binding lectin polymorphisms and HBV outcome in a South Indian population. Int J Immunogenet 37: 177-184. doi: 10.1111/j.1744-313X.2010.00908.x. PubMed: 20193030. [DOI] [PubMed] [Google Scholar]
- 21. Chen DQ, Zeng Y, Zhou J, Yang L, Jiang S et al. (2010) Association of candidate susceptible loci with chronic infection with hepatitis B virus in a Chinese population. J Med Virol 82: 371-378. doi: 10.1002/jmv.21716. PubMed: 20087947. [DOI] [PubMed] [Google Scholar]
- 22. Chatzidaki V, Choumerianou D, Dimitriou H, Kouroumalis E, Galanakis E (2012) Genetic variants associated with susceptibility to mother-to-child transmission of hepatitis B virus. Eur J Gastroenterol Hepatol 24: 1185-1190. doi: 10.1097/MEG.0b013e328356440f. PubMed: 22772094. [DOI] [PubMed] [Google Scholar]
- 23. Zheng RD, Chen JN, Gao JP, Zhuang QY, Zhu QC et al. (2012) [Relationship between mannose-binding protein gene polymorphisms and disease progression and HBV DNA in patients with chronic HBV infection]. Zhonghua Shi Yan He Lin Chuang Bing Xue Za Zhi 26: 90-92. [PubMed] [Google Scholar]
- 24. Garred P, Larsen F, Madsen HO, Koch C (2003) Mannose-binding lectin deficiency—revisited. Mol Immunol 40: 73–84. doi: 10.1016/S0161-5890(03)00104-4. PubMed: 12914814. [DOI] [PubMed] [Google Scholar]
- 25. Steffensen R, Thiel S, Varming K, Jersild C, Jensenius JC (2000) Detection of structural gene mutations and promoter polymorphisms in the mannan-binding lectin (MBL) gene by polymerase chain reaction with sequence-specific primers. J Immunol Methods 241: 33–42. doi: 10.1016/S0022-1759(00)00198-8. PubMed: 10915847. [DOI] [PubMed] [Google Scholar]
- 26. Denholm JT, McBryde ES, Eisen DP (2010) Mannose-binding lectin and susceptibility to tuberculosis: a meta-analysis. Clin Exp Immunol 162: 84-90. doi: 10.1111/j.1365-2249.2010.04221.x. PubMed: 20636396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhong R, Tian Y, Liu L, Qiu Q, Wang Y et al. (2012) HBV-related hepatocellular carcinoma susceptibility gene KIF1B is not associated with development of chronic hepatitis B. PLOS ONE 7: e28839. doi: 10.1371/journal.pone.0028839. PubMed: 22363396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gourraud PA, International Multiple Sclerosis Genetics Consortium (IMSGC). When is the absence of evidence, evidence of absence? Use of equivalence-based analyses in genetic epidemiology and a conclusion for the KIF1B rs10492972*C allelic association in multiple sclerosis. Genet Epidemiol 35:568-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Neth O, Jack DL, Dodds AW, Holzel H, Klein NJ et al. (2000) Mannose-binding lectin binds to a range of clinically relevant microorganisms and promotes complement deposition. Infect Immun 68: 688–693. doi: 10.1128/IAI.68.2.688-693.2000. PubMed: 10639434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saifuddin M, Hart ML, Gewurz H, Zhang Y, Spear GT (2000) Interaction of mannose-binding lectin with primary isolates of human immunodefi ciency virus type 1. J Gen Virol 81: 949–955. PubMed: 10725420. [DOI] [PubMed] [Google Scholar]
- 31. Milich DR, Jones HE, Hughes JL, Price J, Raney AK et al. (1990) Is a function of secreted HBe antigen to induce immunologic tolerance in utero. Proc Natl Acad Sci U S A 87: 6599–6603. doi: 10.1073/pnas.87.17.6599. PubMed: 2395863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gerlich WH, Lu X, Heermann KH (1993) Studies on the attachment and penetration of hepatitis B virus. J Hepatol 17: S10-S14. doi: 10.1016/S0168-8278(05)80448-9. PubMed: 8509625. [DOI] [PubMed] [Google Scholar]
- 33. Miyaike J, Iwasaki Y, Takahashi A, Shimomura H, Taniguchi H et al. (2002) Regulation of circulating immune complexes by complement receptor type 1 on erythrocytes in chronic viral liver diseases. Gut 51: 591-596. doi: 10.1136/gut.51.4.591. PubMed: 12235086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jack DL, Read RC, Tenner AJ, Frosch M, Turner MW et al. (2001) Mannose-binding lectin regulates the inflammatory response of human professional phagocytes to Neisseria meningitidis serogroup B. J Infect Dis 184: 1152-1162. doi: 10.1086/323803. PubMed: 11598838. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of MBL2 exon1 genotypes among HBV cases and controls in the meta-analysis.
(DOC)
Distribution of MBL2 promoter genotypes among HBV cases and controls in the meta-analysis.
(DOC)
Distribution of polymorphisms of MBL2 promoter plus exon1 among HBV cases and controls in the meta-analysis.
(DOC)
Non-superiority tests of mbl2 polymorphism in CHB as compared with HC.
(DOC)
Non-superiority tests of mbl2 polymorphism in CHB as compared with SR.
(DOC)
PRISMA 2009 Checklist.
(DOC)