Abstract
Neural cell adhesion molecules (CAMs) are important players during neurogenesis and neurite outgrowth as well as axonal fasciculation and pathfinding. Some of these developmental processes entail the activation of cellular signaling cascades. Pharmacological and genetic evidence indicates that the neurite outgrowth-promoting activity of L1-type CAMs is at least in part mediated by the stimulation of neuronal receptor tyrosine kinases (RTKs), especially FGF and EGF receptors. It has long been suspected that neural CAMs might physically interact with RTKs, but their activation by specific cell adhesion events has not been directly demonstrated. Here we report that gain-of-function conditions of the Drosophila L1-type CAM Neuroglian result in profound sensory axon pathfinding defects in the developing Drosophila wing. This phenotype can be suppressed by decreasing the normal gene dosage of the Drosophila EGF receptor gene. Furthermore, in Drosophila S2 cells, cell adhesion mediated by human L1-CAM results in the specific activation of human EGF tyrosine kinase at cell contact sites and EGF receptors engage in a physical interaction with L1-CAM molecules. Thus L1-type CAMs are able to promote the adhesion-dependent activation of EGF receptor signaling in vitro and in vivo.
INTRODUCTION
L1-type cell adhesion molecules (CAMs) are evolutionarily well-conserved members of the immunoglobulin domain superfamily (Hortsch, 2000). They usually exhibit a strong homophilic cell adhesion activity and have essential functions in the developing nervous system, especially during neurogenesis, neuronal migration, and axonal growth and guidance. Humans bearing a mutation in their L1-CAM gene display a phenotype, which includes a range of severe neurological dysfunctions, including agenesis of the cortico-spinal tract and the corpus callosum, spastic paraplegia, and mental retardation (Kenwrick et al., 2000). In Drosophila, mutations in the single L1-type gene, neuroglian, cause embryonic lethality and various axonal pathfinding defects (Hall and Bieber, 1997; Garcia-Alonso et al., 2000). In vitro, L1-proteins induce a strong neurite outgrowth response in neuronal cells (Lemmon et al., 1989). This process appears to be mediated by the activation of neuronal receptor and nonreceptor tyrosine kinases (Doherty et al., 2000; Panicker et al., 2003). Several lines of evidence indicate that the CAM- dependent activation of neuronal RTKs, especially of FGFRs, initiates a conserved phospholipase C signaling cascade, which causes a transient influx of extracellular Ca2+ ions and ultimately neurite outgrowth (Saffell et al., 1997; Kolkova et al., 2000). In support of this model, gain-of-function (GOF) conditions of the Drosophila FGFR and the EGFR genes are able to rescue the postembryonic axonal neuroglian loss-of-function (LOF) phenotype (Garcia-Alonso et al., 2000). These observations suggest that invertebrate and vertebrate L1-type CAMs initiate at least some of their axonal growth and pathfinding function by activating neuronal RTK activities. The involvement of FGFR in neurite outgrowth and its interactions with various types of neural CAMs has been studied in detail using vertebrate-based in vitro systems (Saffell et al., 1997; Kolkova et al., 2000). Although functional EGF receptor protein is widely expressed in the developing Drosophila and mammalian central nervous systems (Hortsch et al., 1983; Zak et al., 1990), less is known about its specific roles during neuronal development (Yamada et al., 1997). Several in vitro studies demonstrated that EGF supports cell survival and neurite outgrowth in various different preparations of primary cortical and cerebellar neurons (Morrison et al., 1988; Kornblum et al., 1990; Yamada et al., 1997). In addition, EGFR is also able to directly activate phospholipase C-mediated phosphatidyl-inositol turnover, which appears to be an important mechanism for initiating neurite outgrowth (Wahl et al., 1988; Margolis et al., 1989; Meisenhelder et al., 1989). Although it is generally assumed that L1-CAM and other neuronal CAMs directly interact with RTKs and thereby trigger their tyrosine kinase activities, a direct molecular interaction between these proteins or a direct activation of RTK function by cell adhesion has still not been demonstrated. Recently, Cavallaro et al. (2001) have presented evidence for a model, in which NCAM recruits FGFR and other signaling proteins into a multimolecular complex, which is crucial for inducing an FGFR-mediated signaling response. Despite of all this evidence, it is still unclear whether neuronal CAMs directly interact with RTKs to activate their tyrosine kinase activity and whether a similar mechanism also applies to the interaction with EGFR.
Here we report a GOF analysis of the Drosophila L1-family member neuroglian, which extends the previously published LOF study (Garcia-Alonso et al., 2000). Neuroglian protein is widely expressed during all developmental stages, with the Nrg180 isoform being restricted to neuronal cells, whereas the Nrg167 isoform is widely expressed by many nonneuronal cells (Hortsch et al., 1990). Our results demonstrate that the strong axonal pathfinding phenotype in the developing Drosophila wing, which is caused by neuroglian GOF conditions, only requires the extracellular part of the Neuroglian protein. Moreover, this phenotype is caused in part by the overactivation of the Drosophila EGF receptor. To test, whether the vertebrate orthologue of Nrg, L1-CAM, similarly interacts with and induces EGFR activity, we used an in vitro cell expression system. These experiments show that human L1-CAM specifically induces human EGFR tyrosine kinase activity at cell contact sites. In addition, both proteins engage in a direct, physical trans-interaction. However, this trans-interaction alone is not sufficient for effective EGFR activation. On the basis of these observations, we propose a model in which L1-type and EGFR proteins engage in a pattern of trans- and cis-interactions, which together result in the specific, L1-CAM adhesion-dependent activation of EGFR tyrosine kinase activity.
MATERIALS AND METHODS
Drosophila Strains
Transgenic lines were generated by microinjection of pUAST vector constructs into Drosophila embryos and suitable transgenic lines were selected to generate overexpression conditions. The torpedo (top) allele of the DER gene was used for generating flies with a reduced Drosophila EGFR activity (Price et al., 1989). The MS1075 GAL4 driver line has been previously characterized by Garcia-Alonso et al. (2000). Flies were maintained and all experiments were performed at 25°C. The temperature-sensitive nrg3 allele was maintained at 18°C and shifted to 27°C as the nonpermissive temperature (Hall and Bieber, 1997).
Overexpression Analysis and Immunostaining of Developing Wings
To induce ectopic and overexpression of Nrg, we used the Gal4/UAS system (Brand and Perrimon, 1993). The MS1075-Gal4 driver line (Garcia-Alonso et al., 2000) was combined with UAS-Nrg167 or UAS-NrgGPI in either a wild-type or nrg3 background and with either a DER wild-type, a homo-, or a heterozygous DER-mutant genotype. For experiments involving the nrg3 allele, 20 h before puparium formation the incubation temperature was raised to and subsequently maintained at 27°C, and male individuals were selected at the time of puparium formation. Pupae were fixed in 4% formaldehyde/PBS 30-40 h after onset of pupation and wings were processed for immunocytochemistry with the 22C10 MAb using the Vectastain ABC kit (Vector Laboratories Inc., Burlingame, CA). Stained wings were mounted in 50% glycerol and the phenotype was quantified and documented using a Nikon Optiphot 2 microscope (Nikon Corp., Tokyo, Japan) equipped with Nomarski optics and a Nikon DXM1200 digital camera system.
SDS-PAGE Electrophoresis and Western Blotting Analyses
Transfected S2 cells were pelleted and solubilized in SDS-containing buffer. Total cell proteins were separated by electrophoresis in 10% SDS-polyacrylamide gels and transferred onto nitrocellulose filters. Subsequently, the blots were probed with specific primary and HRP-conjugated secondary antibodies and developed with 3,3′-diaminobenzidine as described in Hortsch et al. (1985) or using the ECL Western blot detection kit from Amersham Pharmacia Biotech (Piscawataway, NJ). Goat polyclonal antisera recognizing human L1-CAM or EGFR were from Santa Cruz Biotechnology Inc. (Santa Cruz, CA) and the mouse monoclonal antiphosphotyrosine antibody PY20 was purchased from Zymed Laboratories (South San Francisco, CA). The mAb f5H7 against Drosophila Fasciclin I has been characterized previously (Hortsch and Goodman, 1990). All secondary antibodies were purchased from Jackson ImmunoReseach Laboratories Inc. (West Grove, PA).
Immunoprecipitations
Immunoprecipitations were performed using a modification of the protocol by Anderson and Blobel (1983). For each immunoprecipitation, 7.5 × 106 cells, which expressed in addition to human EGFR either L1-CAM or Drosophila Fasciclin I, were induced overnight with 0.7 mM CuSO4. After aggregation for 35 min at room temperature on an orbital shaking platform in the presence of activated sodium orthovanadate (Imbert et al., 1994), the cells were collected by centrifugation and solubilized in cold dilution buffer (60 mM Tris/HClm, pH 7.5, 80 mM NaCl, 1.25% Triton X-100, 6 mM EDTA, and a mixture of protease inhibitors). The soluble fraction was incubated overnight at 4°C with either goat anti-EGFR antiserum or a goat nonimmune serum. Supernatants were further incubated on a rotator with Protein G Sepharose beads (Amersham Pharmacia Biotech) for 2 h and immunoprecipitates were eluted with SDS gel electrophoresis buffer after three washing steps. After separation in 10% SDS PAGE gels, proteins were transferred to a nitrocellulose filter and then probed with an antiphosphotyrosine antibody, followed by an HRP-conjugated anti-IgG secondary antibody.
Generation of Transfected S2 Cell Lines
Drosophila S2 cells were grown at 25°C in Schneider's medium, which was supplemented with 10% FCS and penicillin/streptomycin (all reagents were from Life Technologies, Gaithersburg, MD). Using Lipofectin (Life Technologies) S2 cells were transfected with either pRmHa3 or pMT/V8-His constructs. Cotransfection with the pCOhygro plasmid was performed to confer hygromycin resistance as a selectable marker to transfected cells. Detailed methods for establishing cloned S2 cell lines using soft agar cloning and the pRmHa3 construct expressing the neuronal form of human L1-CAM has been described previously (Bieber, 1994). For human EGFR, a 3.8-kb XbaI cDNA fragment encoding the entire open reading frame was subcloned into the pMT/V5-His expression vector (Invitrogen, Carlsbad, CA) and a complete cDNA encoding Drosophila Fasciclin I was subcloned into pRmHa3 as a 2.9-kb EcoRI/XhoI fragment. cDNA expression from these vectors is under the control of the Drosophila metallothionein promoter (Bunch et al., 1988), and protein production was induced overnight by the addition of 0.7 mM CuSO4 to the cell culture medium. Individual cell clones were analyzed and selected using Western blot analysis for high expression levels of the transfected cDNAs.
Cell Aggregation and Immunostaining Procedures
Transfected S2 cells were induced with 0.7 mM CuSO4 at a concentration of 1 × 106 cells/ml for overnight in serum free medium. Induced cells were aggregated for 5 min at 200 rpm on a shaking platform in the presence or absence of 100 μM peroxidase-activated sodium orthovanadate. Aliquots of cells, 25 μl, were mounted on l-polylysine-coated glass slides and fixed with 2% paraformaldehyde. Subsequently the cells were incubated with mouse antiphosphotyrosine PY20 monoclonal antibodies, followed by FITC-labeled anti-mouse IgG secondary antibodies. The double immunofluorescence experiments were performed by using the goat anti-EGFR in combination with a rhodamine-labeled anti-goat Ig secondary antibody. The slides were inspected using a Bio-Rad MRC 600 confocal scanning laser microscope (Richmond, CA), which is housed, in the Microscope Imaging Laboratory of the University of Michigan or a Nikon Optiphot-2 microscope (Tokyo, Japan), which is equipped with FITC, and rhodamine epifluorescence filter sets. Cell mixing and coaggregation experiments of unlabeled and DiI-labeled S2 cells were performed as described elsewhere (Islam et al., 2003) and scored as outlined in Table 3.
Table 3.
Recruitment of human EGFR-expressing S2 cells into L1-CAM-expressing S2 cell aggregates
| Unlabeled S2 cells expressing | DiI-labeled S2 cells expressing | Average of cell clusters with ≥3 labeled cells ± SD (%) | p-Value |
|---|---|---|---|
| human L1-CAM | human L1-CAM | 99.6 ± 0.5 | <0.0001 |
| human L1-CAM | human EGFR | 30.1 ± 8.2 | 0.0004 |
| human L1-CAM | untransfected | 2.5 ± 3.4 | |
| Drosophila Fas I | human EGFR | 1.0 ± 1.0 | 0.9860 |
S2 cells that expressed either human L1-CAM or Drosophila Fasciclin I were mixed and incubated with an equal number of S2 cells, which were labeled with the fluorescent dye DiI and either expressed human L1-CAM, human EGFR, or no exogenous S2 cell protein (not transfected). The final total cell concentration was 1.5 × 106 cells/ml. Cells were aggregated on a shaking platform for 1 h, and cell aggregates, which consisted of at least 10 cells, were evaluated for the presence or absence of three or more DiI-labeled S2 cells. The numbers represent the averages and SDs of the percent of positive cell aggregates from five independent experiments. The statistical analysis was made by comparing the incorporation of DiI-labeled S2 cells to the number of untransfected, DiI-labeled S2 cells into L1-CAM-expressin cell clusters. The paired Student's t test was used and p-values <0.05 were considered to be significant.
RESULTS
Neuroglian GOF phenotype Is Mediated by the Nrg Extracellular Protein Domain and Can Be Suppressed by Genetically Decreasing Drosophila EGF-receptor Gene Dosage
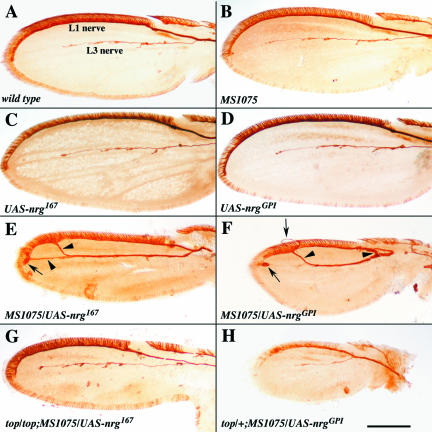
In the Drosophila ocellar sensory system, LOF conditions of the Drosophila FGFR (heartless) and the EGFR (DER) genes exhibit a phenotype similar to that of a temperature-sensitive mutation in the neuroglian gene (Garcia-Alonso et al., 2000). Although GOF conditions of both the FGFR and EGFR can rescue the neuroglian phenotype, it could be argued that this DER GOF capacity might reflect an altered, abnormally expanded range of DER specificity. In this work, we use a reciprocal approach, which involves nrg GOF and DER LOF conditions. During metamorphosis, sensory neurons in the wing epithelium of Drosophila extend their axons proximally along two major pathways toward the base of the wing, forming the L1 nerve along the anterior wing margin and the L3 nerve along the third longitudinal wing vein (Hertweck, 1931; Murray et al., 1984; Figure 1A). These sensory axons extend between the basal surfaces of the dorsal and ventral wing epithelium and their guidance mainly relies on their interactions with the wing epithelium, whereas glial cells in the wing migrate along the already established nerve pathways (Giangrande, 1994). Normally, the epithelium expresses the Nrg167 isoform and the sensory axons the Nrg180 isoform (Hortsch et al., 1990). Overexpression of the Nrg167 isoform in both wing sensory neurons and the two wing epithelial layers was induced in transgenic UAS-nrg167 animals using the MS1075 Gal4-enhancer trap line (Garcia-Alonso et al., 2000). These MS1075/UAS-nrg167 animals develop a number of different alterations, including an overall reduction of wing size, the appearance of ectopic neuronal cells and severe axonal pathfinding defects (Figure 1E). L1 axons often extend distally and connect with the distal end of the L3 nerve. The proximal projection, closer to the wing base, where the L1 and the L3 nerve normally join, is sometimes missing (Figure 1F). Occasionally, L1 axons projecting toward each other clump together and form axonal nodules. In some cases the L1 nerve penetrates the wing epithelium and extends outside the wing tissue (Figure 1F). Because the penetrance of these alterations depends on the expression level of the neuroglian transgene and therefore varies between different transgenic lines, we selected UAS- nrg lines exhibiting a moderate to severe axonal pathfinding phenotype for studying the involvement of EGFR in its generation (Table 1). The level of this GOF phenotype was reduced, but not completely eliminated by decreasing doses of the Drosophila EGFR. This was accomplished by using heterozygous and homozygous conditions of the DER allele torpedo (Price et al., 1989). Therefore, the nrg GOF phenotype is mediated in part by the overactivation of the endogenous EGFR, confirming the idea that in vivo Nrg functions through EGFR (Garcia-Alonso et al., 2000). Moreover, the Nrg167 GOF phenotype and its rescue by the torpedo allele do not depend on the presence of endogenous Neuroglian protein, because both are still observed in a functional neuroglian null background (Table 1).
Figure 1.
Axonal growth and pathfinding phenotypes in the developing Drosophila wing. (A-H) Each wing has a different genotype, as indicated in each panel. The L1 nerve at the anterior margin of the wing and the L3 nerve along the third wing vein were immunochemically visualized using the MAb 22C10. The wings in A-D exhibit a wild-type nerve pattern and represent controls for the various transgenic lines used in this experiment. (E and F) Typical examples of the axonal growth and pathfinding alterations, which are caused by the overexpression of the Nrg167 isoform (E) or of the artificial GPI- anchored isoform NrgGPI (F). Inappropriate axonal connections between the L1 and the L3 nerve are marked by arrowheads. Axonal nodules and axons that perforate the wing epithelium are indicated by arrows. (G-H) Wings from pupae with the hypomorphic DER mutation torpedo in either homo- or heterozygous configuration, respectively. Although these wings are still smaller than wild-type, they do not exhibit axonal growth or pathfinding alterations. Bar, 120 μm.
Table 1.
Quantification of nerve growth and pathfinding alterations in Drosophila wings
| Genotype | Total no. of wings analyzed | No. of wings with alterations | Percentage (%) of wings with alterations | p-Value |
|---|---|---|---|---|
| wt | 77 | 0 | 0.0 | |
| MS1075/wt | 87 | 0 | 0.0 | |
| UAS-nrg167/wt | 64 | 0 | 0.0 | |
| MS1075/UAS-nrg167 | 73 | 38 | 52.1 | |
| top/wt; MS1075/UAS-nrg167 | 115 | 33 | 28.7 | 0.0022 |
| top/top; MS1075/UAS-nrg167 | 68 | 14 | 20.6 | 0.0002 |
| UAS-nrgGPI/wt | 88 | 0 | 0.0 | |
| MS1075/UAS-nrgGPI | 81 | 74 | 91.4 | |
| top/wt; MS1075/UAS-nrgGPI | 76 | 54 | 71.1 | 0.0021 |
| nrg3; MS1075/wt (27°C) | 51 | 0 | 0.0 | |
| nrg3; MS1075/UAS-nrg167 (27°C) | 72 | 56 | 77.8 | |
| nrg3; top/wt; MS1075/UAS-nrg167 (27°C) | 32 | 14 | 43.8 | 0.0013 |
| nrg3; MS1075/UAS-nrgGPI (27°C) | 71 | 57 | 80.3 |
Wings were dissected from pupae with various genotypes and processed for immunocytochemistry using the 22C10 MAb. The axonal growth and pathfinding phenotype was scored, as indicated in Figure 1. We did not score wings that overexpressed NrgGPI in a homozygous torpedo background. These animals exhibited a significant loss of neuronal cells in the wing, which prevented the formation of a proper V3 nerve and made the evaluation of the axonal alterations unreliable. Instead of 25°C, the experiments using the nrg3 allele were performed at 27°C. p-values for the rescue of the Nrg167 and the NrgGPI overexpression phenotype by the DER top allele were calculated using the Chi square test with Yates corrections of the GraphPad program package.
Remarkably, the same wing phenotype was evident after overexpression of an artificial, GPI-anchored isoform of Neuroglian (NrgGPI; Figure 1F). Similar to the results found for the Nrg167 GOF, introduction of the DER torpedo allele into the genetic background of MS1075/UAS-nrgGPI animals resulted in a partial rescue of the axonal phenotype. Moreover, similar to the natural Nrg167 isoform, the GOF phenotype of the NrgGPI form does not require any endogenously expressed Nrg protein (Table 1). Thus a functional interaction of Drosophila EGFR with Neuroglian requires only the extracellular domain of the Neuroglian protein. In summary, our analysis of Nrg GOF conditions together with the previous Nrg LOF results (Garcia-Alonso et al., 2000) clearly demonstrate that the axonal pathfinding activity of Drosophila Nrg in part involves the activation of the Drosophila EGF receptor by the extracellular Nrg protein domain.
L1-mediated Cell Interactions Result in the Localized Stimulation of EGF-receptor Tyrosine Kinase Activity
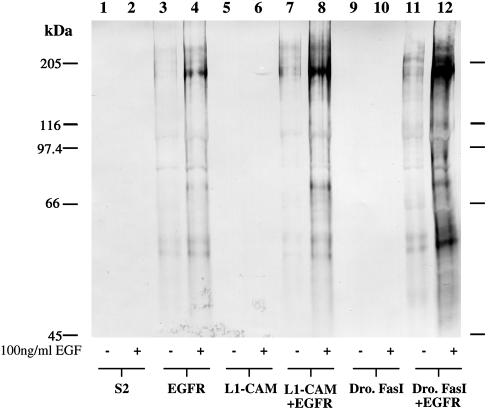
To investigate whether L1-mediated homophilic interaction triggers EGFR tyrosine kinase activity and whether vertebrate L1-type proteins are also able operate through vertebrate EGFR, we used Drosophila Schneider 2 (S2) as a heterologous cell expression system. S2 cells do not express endogenous Neuroglian, any other known Drosophila CAM or the Drosophila EGF receptor homologue (DER; Figure 2A). To further reduce the chance that endogenous S2 cell proteins might interfere with or modulate the postulated interaction between the L1-type CAM and the EGFR, we expressed human proteins in this Drosophila cell line. As demonstrated earlier, human L1-CAM is functionally competent when expressed in S2 cells (Hortsch et al., 1998). Human EGFR, human L1-CAM and Drosophila Fasciclin I cDNA constructs were transfected into S2 cells, and clonal cell lines expressing these proteins were isolated (Figure 2B). Drosophila Fasciclin I is a GPI-anchored, homophilic neural CAM with no structural or sequence homologies to L1-type CAMs (Elkins et al., 1990) and was therefore used as a control for the effect of generic adhesion on EGFR activity. Because high cell surface density of receptor protein can result in the nonspecific autoactivation of EGFR (Schweitzer et al., 1995), the ratio of CAM- to EGFR-expressing plasmid molecules was optimized to ensure maximal cell aggregation and minimal autoactivation of EGFR (Figure 3). The first step in the activation of EGFR after ligand binding is the dimerization and subsequent trans-tyrosine-phosphorylation of EGF receptor protein (Weiss and Schlessinger, 1998). Addition of EGF to the culture medium of induced, EGFR- expressing S2 cells resulted in a robust increase of tyrosine-phosphorylated EGFR protein (Figure 3). Thus these transfected S2 cell lines express functional human EGFR on their cell surface.
Figure 2.
Western blot analysis of transfected Schneider 2 cell lines. Panel A demonstrates that S2 cells do not express Drosophila EGFR. Shown is a Western blot that was incubated with a rat anti-Drosophila DER antiserum. Lane 1: the membrane protein from 2.5 × 105 S2 cells; lane 2: 30 μg of total Drosophila embryonic protein. Bound antibodies were visualized using the ECL kit from Amersham Pharmacia Biotech. Immunoblots B-D show that transfected S2 cells express human L1-CAM, human EGFR, and/or Drosophila Fasciclin I. Each lane contains the total protein from 2.5 × 105 induced S2 cells. Lanes 1: native S2 cells; lanes 2: S2 cells expressing human EGFR; lanes 3: S2 cells expressing human L1-CAM; lanes 4: S2 cells expressing human EGFR and human L1-CAM; lanes 5: S2 cells expressing Drosophila Fasciclin I; and lane 6: S2 cells expressing human EGFR and Drosophila Fasciclin I. Western blot B was incubated with a goat anti-EGFR antiserum, Western blot C with a goat anti-L1-CAM antiserum, and Western blot D with the f5H7 MAb against Drosophila Fasciclin I.
Figure 3.
Human EGFR which is expressed by Drosophila S2 cells can be activated by the addition of 100 ng/ml human EGF to the tissue culture medium. Native or transfected S2 cell lines were induced overnight with CuSO4 and recombinant human EGF (Sigma, St. Louis, MO) was added for 30 min to nonaggregated cells at a final concentration of 100 ng/ml. Subsequently, the cells were harvested for SDS-polyacrylamide electrophoresis and Western blot analysis with an antiphosphotyrosine antibody. Each lane contains the total protein from 2.5 × 105 cells.
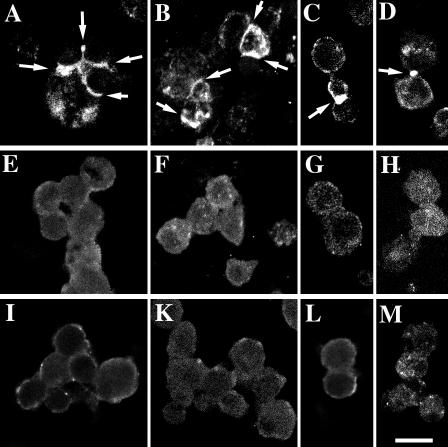
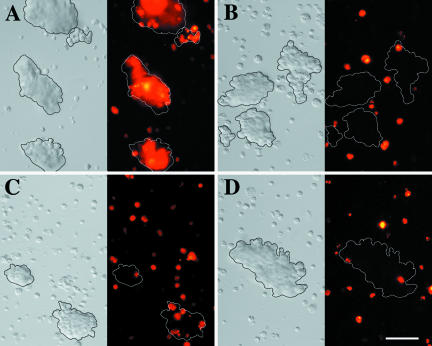
To analyze how homophilic L1-L1 interactions influence EGFR activity, transfected S2 cells were induced with Cu2+ ions, briefly aggregated and processed for immunocytochemistry with an antiphosphotyrosine MAb. Only cell contacts between cells expressing both human L1-CAM and human EGFR consistently exhibited a positive signal for phosphotyrosine (Figure 4, A-D). Aggregates of S2 cells expressing the Drosophila neural CAM Fasciclin I together with human EGFR showed no phosphotyrosine staining at cell contact sites (Figure 4, I-M), nor S2 cell aggregates, which expressed only human L1-CAM (Figure 4, E-H). Thus the activation of EGFR at cell contact sites is not the result of cell adhesion in general, but rather is specific for L1-mediated cell-cell interactions.
Figure 4.
Induction of L1-CAM-mediated EGFR tyrosine kinase activity in S2 cells. Protein expression in transfected S2 cell lines was induced overnight in serum-free medium. Cells were briefly aggregated and prepared for immunocytochemistry with antiphosphotyrosine antibodies. (A-D) Aggregates of S2 cells that coexpress human L1-CAM and human EGFR; (E-H) S2 cells that express human L1-CAM; (I-M) S2 cells that coexpress Drosophila Fasciclin I and human EGFR. Bar, 12 μm.
Although a significant fraction of cell contact sites was labeled by antiphosphotyrosine antibodies (Table 2), many other cell contacts exhibited no detectable signal. The addition of orthovanadate (a protein tyrosine phosphatase inhibitor) to the culture medium during aggregation and processing of induced S2 cells more than doubled the percentage of labeled cell contacts (Table 2). This result indicates that even in this heterologous cell system, endogenous S2 protein tyrosine phosphatases are able to deactivate the human EGFR and to render the L1-mediated EGFR autophosphorylation transient.
Table 2.
Quantification of phosphotyrosine immunostaining at S2 cell contact sites
| Percentage (%) of cell contacts stained ± SD
|
||
|---|---|---|
| Cell line expressing | Without Na3VO4 | With Na3VO4 |
| Human L1-CAM and human EGFR | 14.5 ± 0.9 | 34.8 ± 2.5 |
| Human L1-CAM | 0 | 0.2 ± 0.4 |
| Drosophila Fasciclin I and human EGFR | 0 | 0.4 ± 0.7 |
For the quantitative analysis of phosphotyrosine staining at S2 cell contact sites, only cell aggregates of 2 to 5 cells in which individual cell contacts were identifiable and could be individually scored, were analyzed. Cell aggregation was performed either in the absence or in the presence of 100 μM peroxidase-activated orthovanadate. The results represent the average ± SD of three independent experiments, in which at least 100 individual cell contact sites per experiment were analyzed for each of the different cell lines.
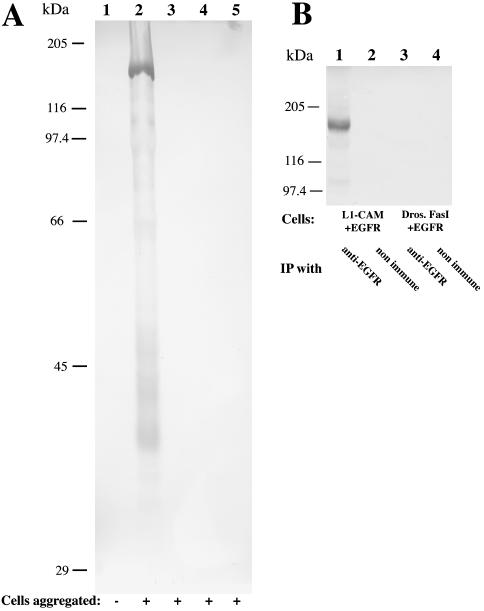
A Western blot analysis of aggregated vs. nonaggregated S2 cells indicates that in response to L1-mediated cell adhesion only one polypeptide with an apparent molecular weight of 170 kDa becomes phosphorylated at tyrosine residues (Figure 5A). Drosophila Fasciclin I-mediated cell adhesion did not elicit a similar response (Figure 5A, lane 3), nor did human L1-CAM induce the phosphorylation of any endogenous S2 cell proteins (Figure 5A, lane 4). An immunoprecipitation experiment using an anti-EGFR antiserum confirmed that the tyrosine-phosphorylated 170-kDa protein is human EGFR (Figure 5B) and that tyrosine-phosphorylated EGFR protein could only be immunoprecipitated from cell aggregates that coexpressed human L1-CAM and EGFR, but not from cell clusters coexpressing Drosophila Fasciclin I and human EGFR (Figure 5B).
Figure 5.
EGFR is phosphorylated at tyrosine residues in response to L1-CAM cell adhesion. (A) L1-mediated cell adhesion specifically induces the phosphorylation of human EGFR, which is expressed by Drosophila S2 cells. After protein induction, transfected S2 cells were either left nonaggregated at a low cell density in a tissue culture dish (lane 1) or were aggregated on a shaking platform for 20 min (lanes 2-5). Each lane represents the total protein extract from 3.5 × 105 cells, which were separated by SDS-PAGE and tested for their phosphotyrosine content by Western blot analysis. Cells, which were analyzed in A, coexpressed human L1-CAM and human EGFR (lanes 1 and 2), Drosophila Fasciclin I and human EGFR (lane 3), or expressed only human L1-CAM (lane 4) or human EGFR (lane 5). (B) Human EGFR, which is immunoprecipitated from S2 cell aggregates expressing human L1-CAM, contains phosphorylated tyrosine residues. Shown is an immunoprecipitation experiment from S2 cell aggregates expressing either human L1-CAM (lanes 1 and 2) or Drosophila Fasciclin I (lanes 3 and 4) in conjunction with human EGFR. Proteins were solubilized with Triton X-100 and immunoprecipitated with either an anti-EGFR antiserum (lanes 1 and 3) or with a goat nonimmune serum (lanes 2 and 4). Both Western blots were probed with antiphosphotyrosine antibodies.
Human L1-CAM and EGFR Engage in a Weak Heterophilic trans-Interaction
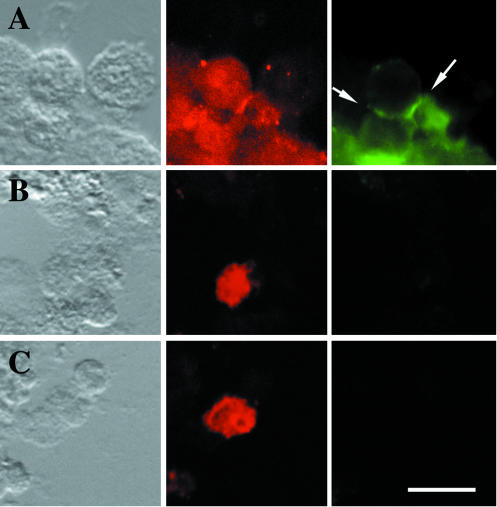
A coimmunoprecipitation approach for identifying the trans- or cis-interacting ligands of L1-type proteins is complicated by L1-CAM's interaction with the membrane skeleton. L1-type proteins, which participate in an adhesive interaction, bind tightly to cytoplasmic Ankyrins and become resistant to solubilization under nondenaturing conditions (Dubreuil et al., 1996). Using conditions that allow the coimmunoprecipitation of human L1-CAM with TAG-1 (Malhotra et al., 1998), we were unable to coimmunoprecipitate human L1-CAM and EGFR from induced S2 cells (unpublished data). A putative, direct interaction between the two membrane proteins might therefore be relatively unstable and of low affinity. We used S2 cell mixing experiments for testing whether L1-CAM and EGFR can engage in a direct trans-interaction with each other. In the current model of CAM-RTK interaction, activation of the tyrosine kinase activity by the CAM proteins occurs between molecules in the same plasma membrane (cis-interaction; Williams et al., 1994). However, as exemplified by the zipper model for cadherin adhesion (Shapiro et al., 1995), CAM-ligand and CAM-CAM interactions often rely on both cis-, as well as trans-interactions, depending on the specific topology of the binding sites involved. S2 cells, which express human EGFR, exhibit no homophilic adhesive activity (unpublished data). However, when mixed with S2 cells expressing human L1-CAM, they are specifically incorporated into L1-CAM- expressing cell aggregates (Figure 6 and Table 3). Untransfected S2 cells are not significantly trapped in L1-CAM cell clusters, nor do human EGFR-expressing S2 cells coaggregate with Fasciclin I-expressing cells (Table 3). When compared with a mixing experiment between two S2 cell populations, which both express human L1-CAM, the level of the heterophilic interaction between human EGFR and L1-CAM appears to be relatively low (see Table 3 and compare Figure 6A with 6C), although statistically highly significant. To test whether this weak trans-interaction is able to trigger the EGFR tyrosine kinase activity, we performed double immunofluorescence staining experiments (Figure 7). These experiments gave no indication that the EGFR phosphorylation activity is triggered in EGFR-expressing S2 cells, which are incorporated in L1-CAM- expressing S2 cell aggregates (Figure 7, B and C). The fact that the observed trans-interaction between L1-CAM and EGFR is not sufficient for inducing EGFR tyrosine kinase activity further supports the idea that RTK activation by L1-type CAMs also requires cis-interactions between the two types of molecules (Williams et al., 1994).
Figure 6.
S2 cells expressing human EGFR interact with S2 cell aggregates, which express L1-CAM. Shown are representative results of an S2 cell mixing experiment, in which unlabeled S2 cells that either expressed human L1-CAM (A-C) or Drosophila Fasciclin I (D) were coaggregated with DiI-labeled S2 cells, which either expressed human L1-CAM (A), no exogenous S2 cell protein (B), or human EGFR (C and D). Cell aggregates of 10 and more cells were counted as positive when they contained 3 or more DiI-labeled S2 cells; e.g., the larger cell aggregate in C was scored as positive, whereas the smaller aggregate as negative. Bar, 70 μm.
Figure 7.
The heterophilic trans-interaction between human EGFR and human L1-CAM does not induce EGFR tyrosine kinase activity. Shown are three series of overlapping micrographs. Each first image was taken using Nomarski optics. The second panels display the same area as shown by Nomarski optics using epifluorescence microscopy with a rhodamine filter set. This visualizes S2 cells, which express human EGFR. The third image of each series was obtained with an FITC filter set and represents phosphotyrosine staining. Set A shows a positive control of S2 cells, which coexpress both human EGFR and L1-CAM. Panel sets B and C are from a mixing experiment, in which S2 cells expressing human L1-CAM were coaggregated with S2 cells, which expressed only transfected human EGFR. Bar, 15 μm.
DISCUSSION
Our results demonstrate a specific, functional, and direct physical interaction between L1-type CAMs and EGFR at cell contact sites, which results in the induction of the receptor tyrosine kinase activity in the absence of classical receptor agonists. Based on the observation that the artificial, GPI-anchored isoform of Nrg maintains its ability to generate a GOF phenotype in the absence of endogenous Nrg protein, this L1-mediated EGFR activation only requires the extracellular domain of the L1-type protein. Therefore, intracellular L1-binding proteins, such as the membrane skeleton component Ankyrin, do not appear to be essential for L1-dependent EGFR activation, although they may contribute to its modulation. An alternative model may involve a specific, but indirect interaction through a hypothetical “bridging” molecule expressed by S2 cells. However, for such a Drosophila linker protein to function in our experimental system, its binding specificities to EGFR and to L1- CAM would have to be conserved between flies and humans. Therefore, considering the evolutionary distance between humans and Drosophila, the present data are more consistent with a direct molecular interaction between L1- CAM and EGFR.
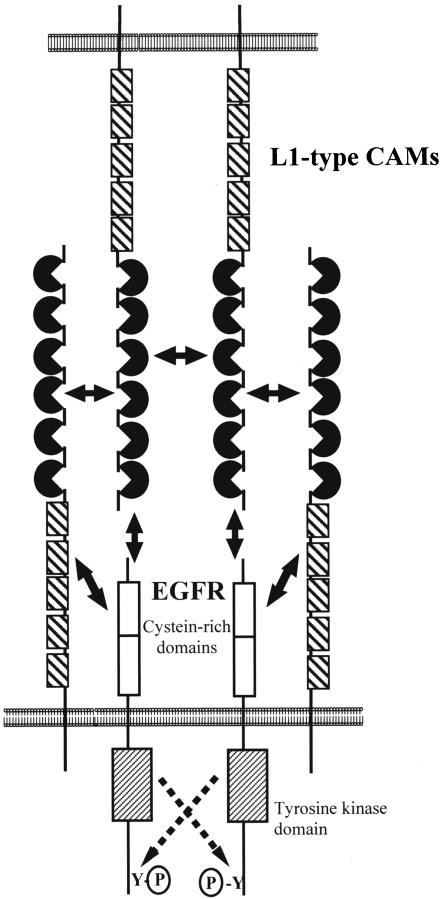
Our observation that the trans-interaction between L1- CAM and EGFR does not induce the RTK activity (Figure 7) suggests that the stimulation of EGFR autophosphorylation by L1-mediated cell adhesion requires additional cis-type interactions between the two proteins. We therefore postulate that a combination of trans- and cis-interactions between EGFR and L1-type CAMs functions in physically aligning RTK proteins and in inducing their tyrosine kinase activity in the absence of a traditional receptor ligand (Figure 8). It remains to be elucidated which of the L1 and EGFR protein domains are involved in this interaction. Williams et al. (1994) postulated that specific cis-interactions between neural CAMs and FGFR are responsible for the induction of FGFR signaling in neuronal cells. They provided some experimental evidence for the involvement of a specific, conserved amino acid motif (CAM homology domain) that is present in several neural CAMs and the vertebrate FGFR protein. However, structural studies of the FGFR ectodomain argue against the involvement of this particular amino acid motif in the activation by neural CAMs (Plotnikov et al., 1999).
Figure 8.
Model of the functional interactions between L1-type CAMs and EGF receptor proteins. L1-cell adhesion relies on trans-interactions between L1-type proteins, which are expressed on the surface of neighboring cells. The interaction between L1-type CAMs and neuronal EGFR involves cis-, as well as trans-binding events. Cis-interactions between the two molecules are required for the activation of EGFR tyrosine kinase activation. A current model of L1-CAM homophilic cell adhesion also involves both cis- and trans-interactions (Kamiguchi and Lemmon, 2000).
The current knowledge about neurite outgrowth, which was obtained mainly from in vitro studies, indicates that vertebrate FGFRs are also activated by a CAM-dependent mechanism that might be very similar to the one demonstrated here for EGFR. It remains to be determined whether EGFR and FGFR both act through the same phospholipase C-mediated pathway in neuronal cells or through different signaling cascades, which might result in differential effects on neuronal growth and differentiation. In Drosophila, the Heartless FGFR and the DER EGFR-homologues seem to have at least partially overlapping specificities in mediating Nrg functionality (Garcia-Alonso et al., 2000). Interestingly, Chen et al. (2001) demonstrated in vivo that the Caenorhabditis elegans L1-type protein, LAD-1, is a substrate of an FGFR-dependent tyrosine kinase. This observation suggests the existence of a feedback loop, in which FGFR activity modulates the function of L1-type proteins through its intracellular domain and its association with the membrane skeleton.
In the Drosophila nervous system, the importance of EGFR signaling is well established, especially during neurogenesis, eye disk, and midline development (Lage et al., 1997; Okabe and Okano, 1997; Dominguez et al., 1998; Dumstrei et al., 1998; Kumar et al., 1998). Our finding that the functional interaction between L1-type molecules and EGFR is conserved in the human proteins suggests that such interactions also play important roles during vertebrate development. EGFR is widely expressed by many neuronal and glial cell types in vertebrates and appears to be involved in neuronal proliferation, cell fate choices, chemotactic cell migration, and neuronal survival (Yamada et al., 1997). In addition, several studies report that EGF stimulates process outgrowth in primary cortical and cerebellar neurons (Morrison et al., 1988; Kornblum et al., 1990; Yamada et al., 1997). However, Kornblum et al. (1990) found that when compared with EGF, basic FGF appears to be the more effective trophic agent for rat neocortical neurons in vitro.
In addition to the afore mentioned phospholipase C-mediated signaling pathway, which is triggered by the CAM- dependent activation of RTKs, the oligomerization of L1- type proteins induces neurite outgrowth that is initiated by the activation of the neuronal MAPK pathway (Panicker et al., 2003). This mechanism appears to require the endocytosis of L1-CAM protein and the activation of nonreceptor tyrosine kinases, such as pp60 (c-src), and of small GTPases, such as Rac1 (Schaefer et al., 1999; Schmid et al., 2000). At least in the case of NCAM-mediated neuritogenesis, the activation of both receptor as well as nonreceptor tyrosine kinase pathways is required for a robust neurite outgrowth response (Niethammer et al., 2002). This suggests that a similar cosignaling mechanism might also be involved in L1-induced axonal extension.
The results presented here demonstrate that L1-type CAMs directly induce the first step in the activation of one classical signaling pathway. With the exception of the experiment shown in Figure 3, all S2 cell experiments were performed under serum-free conditions in the absence of EGF. Nevertheless, under in vivo conditions, classical vertebrate and Drosophila EGFR ligands (e.g., EGF, alpha-TGF, Spitz, Vein, and others) may still be required for achieving a full activation of neuronal EGFRs. In this case, neural CAMs might act in combination with these classical ligands to lower the activation threshold, rather than eliminating the requirement for them. In such a model, endogenous ligands and CAM-CAM interactions act synergistically during nervous system development and the activation of neuronal EGFR molecules would be the result of an integrative process, including different types of stimuli.
Acknowledgments
We are grateful to Dr. B. Shilo (Weizmann Institute of Science, Rehovot, Israel) for making anti-DER antibodies available to us and to Drs. Y. Yarden (Weizmann Institute of Science, Rehovot, Israel) and V. Lemmon (Case Western Reserve University, Cleveland, OH) for generously providing the cDNAs for human EGFR and human L1-CAM, respectively. We very much appreciate the helpful hands of N. Crisp for some of the genetic experiments and are indebted to Drs. P. Raymond and D. Engel for their useful comments on the manuscript. L. K. is grateful for his support by Drs. E. Bock and V. Berezin (University of Copenhagen, Denmark). This work was made possible in part by grants from Ministerio de Ciencia y Tecnologia (SAF2001-1628, in part FEDER) to L.G.-A. and the National Institute of Child Health and Human Development (RO1HD29388), the National Science Foundation (IBN-0132819), and the Spinal Cord Research Foundation (SCRF 1797) to M.H.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-05-0333. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-05-0333.
Abbreviations used: CAM, cell adhesion molecule; DER, Drosophila EGF receptor; EGFR, epidermal growth factor receptor; FGFR, fibroblast growth factor receptor; GOF, gain-of-function; GPI, glycosyl-phosphatidyl inositol; LOF, loss-of-function; Nrg, Neuroglian; RTK, receptor tyrosine kinase.
References
- Anderson, D.J., and Blobel, G. (1983). Immunoprecipitation of proteins from cell-free translations. Methods Enzymol. 96, 111-120. [DOI] [PubMed] [Google Scholar]
- Bieber, A.J. (1994). Analysis of cellular adhesion in cultured cells. In: Drosophila melanogaster: Practical Uses in Cell Biology, Vol. 44, ed. L. Goldstein and E. Fyrberg, San Diego: Academic Press, 683-696. [DOI] [PubMed] [Google Scholar]
- Brand, A.H., and Perrimon, N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401-415. [DOI] [PubMed] [Google Scholar]
- Bunch, T.A., Grinblat, Y., and Goldstein, L.S.B. (1988). Characterization and use of the Drosophila metallothionein promoter in cultured Drosophila melanogaster cells. Nucleic Acids Res. 16, 1043-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallaro, U., Niedermeyer, J., Fuxa, M., and Christofori, G. (2001). N-CAM modulates tumour-cell adhesion to matrix by inducing FGF-receptor signalling. Nat. Cell Biol. 3, 650-657. [DOI] [PubMed] [Google Scholar]
- Chen, L., Ong, B., and Bennett, V. (2001). LAD-1, the Caenorhabditis elegans L1CAM homologue, participants in embryonic and gonadal morphogenesis and is a substrate for fibroblast growth factor receptor pathway-dependent phosphotyrosine-based signalling. J. Cell. Biol. 154, 841-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty, P., Williams, G., and Williams, E.J. (2000). CAMs and axonal growth: a critical evaluation of the role of calcium and the MAPK cascade. Mol. Cell. Neurosci. 16, 283-295. [DOI] [PubMed] [Google Scholar]
- Dominguez, M., Wasserman, J.D., and Freeman, M. (1998). Multiple functions of the EGF receptor in Drosophila eye development. Curr. Biol. 8, 1039-1048. [DOI] [PubMed] [Google Scholar]
- Dubreuil, R.R., MacVicar, G., Dissanayake, S., Liu, C., Homer, D., and Hortsch, M. (1996). Neuroglian-mediated cell adhesion induces assembly of the membrane skeleton at cell contact sites. J. Cell Biol. 133, 647-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumstrei, K., Nassif, C., Abboud, G., Aryai, A., and Hartenstein, V. (1998). EGFR signaling is required for the differentiation and maintenance of neural progenitors along the dorsal midline of the Drosophila embryonic head. Development 125, 3417-3426. [DOI] [PubMed] [Google Scholar]
- Elkins, T., Hortsch, M., Bieber, A.J., Snow, P.M., and Goodman, C.S. (1990). Drosophila fasciclin I is a novel homophilic adhesion molecule that along with fasciclin III can mediate cell sorting. J. Cell Biol. 110, 1825-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alonso, L., Romani, S., and Jimenez, F. (2000). The EGF and FGF receptors mediate neuroglian function to control growth cone decisions during sensory axon guidance in Drosophila. Neuron 28, 741-752. [DOI] [PubMed] [Google Scholar]
- Giangrande, A. (1994). Glia in the fly wing are clonally related to epithelial cells and use the nerve as pathways for migration. Development 120, 523-534. [Google Scholar]
- Hall, S.G., and Bieber, A.J. (1997). Mutations in the Drosophila neuroglian cell adhesion molecule affect motor neuron pathfinding and peripheral nervous system patterning. J. Neurobiol. 32, 325-340. [PubMed] [Google Scholar]
- Hertweck, H. (1931). Anatomie und Variabilität des Nervensystems und der Sinnesorgane von Drosophila melanogaster (Meigen). Zeitschr. wiss. Zool. 139, 559-663. [Google Scholar]
- Hortsch, M. (2000). Structural and functional evolution of the L1-family: Are four adhesion molecules better than one? Mol. Cell. Neurosci. 15, 1-10. [DOI] [PubMed] [Google Scholar]
- Hortsch, M., Avossa, D., and Meyer, D.I. (1985). A structural and functional analysis of the docking protein. Characterization of active domains by proteolysis and specific antibodies. J. Biol. Chem. 260, 9137-9145. [PubMed] [Google Scholar]
- Hortsch, M., Bieber, A.J., Patel, N.H., and Goodman, C.S. (1990). Differential splicing generates a nervous system-specific form of Drosophila neuroglian. Neuron 4, 697-709. [DOI] [PubMed] [Google Scholar]
- Hortsch, M., and Goodman, C.S. (1990). Drosophila fasciclin I, a neural cell adhesion molecule, has a phosphatidylinositol lipid membrane anchor that is developmentally regulated. J. Biol. Chem. 265, 15104-15109. [PubMed] [Google Scholar]
- Hortsch, M., O'Shea, K.S., Zhao, G., Kim, F., Vallejo, Y., and Dubreuil, R.R. (1998). A conserved role for L1 as a transmembrane link between neuronal adhesion and membrane cytoskeleton assembly. Cell Adhes. Commun. 5, 61-73. [DOI] [PubMed] [Google Scholar]
- Hortsch, M., Schlessinger, J., Gootwine, E., and Webb, C.G. (1983). Appearance of functional EGF receptor kinase during rodent embryogenesis. EMBO J. 2, 1937-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbert, V., Peyron, J.F., Farahi Far, D., Mari, B., Auberger, P., and Rossi, B. (1994). Induction of tyrosine phosphorylation and T-cell activation by vanadate peroxide, an inhibitor of protein tyrosine phosphatases. Biochem. J. 297, 163-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam, R., Wei, S.-Y., Chiu, W.-H., Hortsch, M., and Hsu, J.-C. (2003). Neuroglian activates Echinoid to antagonize the Drosophila EGF receptor signaling pathway. Development 130, 2051-2059. [DOI] [PubMed] [Google Scholar]
- Kamiguchi, H., and Lemmon, V. (2000). IgCAMs: bidirectional signals underlying neurite growth. Curr. Opin. Cell Biol. 12, 598-605. [DOI] [PubMed] [Google Scholar]
- Kenwrick, S., Watkins, A., and Angelis, E.D. (2000). Neural cell recognition molecule L 1, relating biological complexity to human disease mutations. Hum. Mol. Genet. 9, 879-886. [DOI] [PubMed] [Google Scholar]
- Kolkova, K., Novitskaya, V., Pedersen, N., Berezin, V., and Bock, E. (2000). Neural cell adhesion molecule-stimulated neurite outgrowth depends on activation of protein kinase C and the Ras-mitogen-activated protein kinase pathway. J. Neurosci. 20, 2238-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblum, H.I., Raymon, H.K., Morrison, R.S., Cavanaugh, K.P., Bradshaw, R.A., and Leslie, F.M. (1990). Epidermal growth factor and basic fibroblast growth factor: effects on an overlapping population of neocortical neurons in vitro. Brain Res. 535, 255-263. [DOI] [PubMed] [Google Scholar]
- Kumar, J.P., Tio, M., Hsiung, F., Akopyan, S., Gabay, L., Seger, R., Shilo, B.Z., and Moses, K. (1998). Dissecting the roles of the Drosophila EGF receptor in eye development and MAP kinase activation. Development 125, 3875-3885. [DOI] [PubMed] [Google Scholar]
- Lage, P., Jan, Y.N., and Jarman, A.P. (1997). Requirement for EGF receptor signalling in neural recruitment during formation of Drosophila chordotonal sense organ clusters. Curr. Biol. 7, 166-175. [DOI] [PubMed] [Google Scholar]
- Lemmon, V., Farr, K.L., and Lagenaur, C. (1989). L1-mediated axon outgrowth occurs via a homophilic binding mechanism. Neuron 2, 1597-1603. [DOI] [PubMed] [Google Scholar]
- Malhotra, J.D., Tsiotra, P., Karagogeos, D., and Hortsch, M. (1998). Cis-activation of L1-mediated ankyrin recruitment by TAG-1 homophilic cell adhesion. J. Biol. Chem. 273, 33354-33359. [DOI] [PubMed] [Google Scholar]
- Margolis, B., Rhee, S.G., Felder, S., Mervic, M., Lyall, R., Levitzki, A., Ullrich, A., Zilberstein, A., and Schlessinger, J. (1989). EGF induces tyrosine phosphorylation of phospholipase C-II: a potential mechanism for EGF receptor signaling. Cell 57, 1101-1107. [DOI] [PubMed] [Google Scholar]
- Meisenhelder, J., Suh, P.G., Rhee, S.G., and Hunter, T. (1989). Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell 57, 1109-1122. [DOI] [PubMed] [Google Scholar]
- Morrison, R.S., Keating, R.F., and Moskal, J.R. (1988). Basic fibroblast growth factor and epidermal growth factor exert differential trophic effects on CNS neurons. J. Neurosci. Res. 21, 71-79. [DOI] [PubMed] [Google Scholar]
- Murray, M.A., Schubiger, M., and Palka, J. (1984). Neuron differentiation and axon growth in the developing wing of Drosophila melanogaster. Dev. Biol. 104, 259-273. [DOI] [PubMed] [Google Scholar]
- Niethammer, P., Delling, M., Sytnyk, V., Dityatev, A., Fukami, K., and Schachner, M. (2002). Cosignaling of NCAM via lipid rafts and the FGF receptor is required for neuritogenesis. J. Cell Biol. 157, 521-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe, M., and Okano, H. (1997). Two-step induction of chordotonal organ precursors in Drosophila embryogenesis. Development 124, 1045-1053. [DOI] [PubMed] [Google Scholar]
- Panicker, A.K., Buhusi, M., Thelen, K., and Maness, P.F. (2003). Cellular signalling mechanisms of neural cell adhesion molecules. Front. Biosci. 8, D900-911. [DOI] [PubMed] [Google Scholar]
- Plotnikov, A.N., Schlessinger, J., Hubbard, S.R., and Mohammadi, M. (1999). Structural basis for FGF receptor dimerization and activation. Cell 98, 641-650. [DOI] [PubMed] [Google Scholar]
- Price, J.V., Clifford, R.J., and Schüpbach, T. (1989). The maternal ventralizing locus torpedo is allelic to faint little ball, an embryonic lethal, and encodes the Drosophila EGF receptor homolog. Cell 56, 1085-1092. [DOI] [PubMed] [Google Scholar]
- Saffell, J.L., Williams, E.J., Mason, I.J., Walsh, F.S., and Doherty, P. (1997). Expression of a dominant negative FGF receptor inhibits axonal growth and FGF receptor phosphorylation stimulated by CAMs. Neuron 18, 231-242. [DOI] [PubMed] [Google Scholar]
- Schaefer, A.W., Kamiguchi, H., Wong, E.V., Beach, C.M., Landreth, G., and Lemmon, V. (1999). Activation of the MAPK signal cascade by the neural cell adhesion molecule L1 requires L1 internalization. J. Biol. Chem. 274, 37965-37973. [DOI] [PubMed] [Google Scholar]
- Schmid, R.S., Pruitt, W.M., and Maness, P.F. (2000). A MAP kinase-signaling pathway mediates neurite outgrowth on L1 and requires Src-dependent endocytosis. J. Neurosci. 20, 4177-4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer, R., Shaharabany, M., Seger, R., and Shilo, B.Z. (1995). Secreted Spitz triggers the DER signaling pathway and is a limiting component in embryonic ventral ectoderm determination. Genes Dev. 9, 1518-1529. [DOI] [PubMed] [Google Scholar]
- Shapiro, L., Fannon, A.M., Kwong, P.D., Thompson, A., Lehmann, M.S., Grubel, G., Legrand, J.F., Als-Nielsen, J., Colman, D.R., and Hendrickson, W.A. (1995). Structural basis of cell-cell adhesion by cadherins. Nature 374, 327-337. [DOI] [PubMed] [Google Scholar]
- Wahl, M.I., Daniel, T.O., and Carpenter, G. (1988). Antiphosphotyrosine recovery of phospholipase C activity after EGF treatment of A-431 cells. Science 241, 968-970. [DOI] [PubMed] [Google Scholar]
- Weiss, A., and Schlessinger, J. (1998). Switching signals on or off by receptor dimerization. Cell 94, 277-280. [DOI] [PubMed] [Google Scholar]
- Williams, E.J., Furness, J., Walsh, F.S., and Doherty, P. (1994). Activation of the FGF receptor underlies neurite outgrowth stimulated by L1, N-CAM, and N-cadherin. Neuron 13, 583-594. [DOI] [PubMed] [Google Scholar]
- Yamada, M., Ikeuchi, T., and Hatanaka, H. (1997). The neurotrophic action and signalling of epidermal growth factor. Prog. Neurobiol. 51, 19-37. [DOI] [PubMed] [Google Scholar]
- Zak, N.B., Wides, R.J., Schejter, E.D., Raz, E., and Shilo, B.Z. (1990). Localization of the DER/flb protein in embryos: implications on the faint little ball lethal phenotype. Development 109, 865-874. [DOI] [PubMed] [Google Scholar]