Abstract
p38 MAPK and nuclear factor-κB (NF-κB) signaling pathways have been implicated in the control of skeletal myogenesis. However, although p38 is recognized as a potent activator of myoblast differentiation, the role of NF-κB remains controversial. Here, we show that p38 is activated only in differentiating myocytes, whereas NF-κB activity is present both in proliferation and differentiation stages. NF-κB activation was found to be dependent on p38 activity during differentiation, being NF-κB an effector of p38, thus providing a novel mechanism for the promyogenic effect of p38. Activation of p38 in C2C12 cells induced the activity of NF-κB, in a dual way: first, by reducing IκBα levels and inducing NF-κB-DNA binding activity and, second, by potentiating the transactivating activity of p65-NF-κB. Finally, we show that interleukin (IL)-6 expression is induced in C2C12 differentiating myoblasts, in a p38- and NF-κB-dependent manner. Interference of IL-6 mRNA reduced, whereas its overexpression increased, the extent of myogenic differentiation; moreover, addition of IL-6 was able to rescue significantly the negative effect of NF-κB inhibition on this process. This study provides the first evidence of a crosstalk between p38 MAPK and NF-κB signaling pathways during myogenesis, with IL-6 being one of the effectors of this promyogenic mechanism.
INTRODUCTION
The development of skeletal muscle is an ordered multistep process in which mesodermal cells give rise to myoblasts that subsequently withdraw from the cell cycle, align, elongate, differentiate, and fuse into multinucleated myotubes. Each of these stages is subjected to positive and negative regulatory mechanisms triggered by environmental cues, requiring the sequential activation of two groups of myogenic transcription factors: the myogenic regulatory factors (MRFs), including Myf5, MyoD, myogenin, and MRF4, and the myocyte enhancer binding factor 2 (MEF2) proteins (A-D). Muscle-specific gene transcription driven by the MRFs can be synergistically stimulated by MEF2 proteins, leading to the transcriptional activation of muscle-specific genes, such as α-actin, muscle creatine kinase (MCK), or myosin heavy chain (MHC), and eventually to the production of mature muscle fibers (Tapscott et al., 1988; Davis et al., 1990; Lassar and Munsterberg, 1994; Olson and Klein, 1994; Black and Olson, 1998).
Different intracellular signaling pathways, including p38 MAPK and nuclear factor-κB (NF-κB) pathways, have been implicated in the control of myogenic progression (reviewed in Puri and Sartorelli, 2000). p38 MAPK pathway is recognized as a critical and positive regulator of myogenic differentiation, whereas the role of the NF-κB pathway in this process is unresolved, because both positive and negative effects have been reported. p38 activity increases during muscle differentiation (Cuenda and Cohen, 1999; Zetser et al., 1999; Wu et al., 2000), and it was shown to phosphorylate and increase the transcriptional activity of specific MEF2 isoforms (Han et al., 1997; Zetser et al., 1999; Zhao et al., 1999), providing a potential explanation for the promyogenic effect of p38. In addition, recent studies showed that p38 stimulates MyoD activity through an unknown indirect mechanism (Wu et al., 2000). Contrary to this, NF-κB was shown to induce cyclin D1 expression and pRb hyperphosphorylation during proliferation of myoblasts, thus inhibiting their differentiation (Guttridge et al., 1999). Alternative studies, however, showed that insulin-like growth factor-II induced a transient activation of NF-κB in L6E9 rat myoblasts, with activation required for insulin-like growth factor-II-mediated differentiation (Kaliman et al., 1999; Canicio et al., 2001). Thus, the role of NF-κB during myoblast differentiation deserves further analysis.
NF-κB belongs to the Rel family of transcription factors that regulate the expression of genes involved in immune and inflammatory responses (Baeuerle and Baltimore, 1996; Ghosh and Karin, 2002). In mammals, the Rel family is composed of RelA/p65, c-Rel, p50 (NF-κB1), and p52 (NF-κB2). Although all Rel members bind DNA, only RelA/p65 (thereafter referred to as p65), c-Rel, and RelB have extended carboxy termini harboring transactivation function. NF-κB subunits are able to homo- or heterodimerize to form transcription factor complexes with a wide range of DNA binding and activation potentials (Ghosh and Karin, 2002). Prototypical NF-κB is a p50/p65 heterodimer that is usually retained in the cytoplasm of unstimulated cells in an inactive form by IκBα, one of the most important members of the IκBα inhibitory family. In response to various stimuli, IκBα is phosphorylated at Ser32 and Ser36 by IκBα kinases α and β. Once phosphorylated, IκBα is rapidly ubiquitinated and subsequently degraded by the 26S proteasome complex (DiDonato et al., 1997; Mercurio et al., 1997; Regnier et al., 1997; Zandi et al., 1997). The degradation of IκBα unmasks the nuclear localization signals of the NF-κB heterodimer, which then translocates into the nucleus where it directly binds to the cognate DNA sequence to regulate gene transcription. Although the induced nuclear translocation of NF-κB has been highly regarded as the principal method to activate NF-κB-dependent gene expression, alternate mechanisms of NF-κB activation are emerging that involve the phosphorylation of the RelA/p65 transactivation subunit (Bird et al., 1997; Wang and Baldwin, 1998; Zhong et al., 1998; Sizemore et al., 1999; Zhong et al., 2002; Vermeulen et al., 2003).
Many signaling pathways can interact with each other through biochemical cross talk. p38 and NF-κB are known to be activated in response to similar stimuli, including inflammatory cytokines, phorbol esters, bacterial toxins, viruses or UV light, in different cell types; however, such interactions between p38 and NF-κB are not well understood. Furthermore, their potential interaction during skeletal myogenesis has never been investigated.
In the present study, we examined the existence, and the nature, of a cross-talk between the NF-κB and p38 MAPK pathways during skeletal muscle differentiation. We show that NF-κB is activated in proliferating as well as in differentiating myoblasts; however, p38 MAPK is activated only in C2C12 myoblasts exposed to differentiation-promoting conditions, with the activation of p38 preceding that of NF-κB. We demonstrate that ectopic activation of p38 induces NF-κB transcriptional activity in C2C12 myoblasts, mimicking the effect of differentiation-promoting conditions, in a dual way: first, by stimulating IκBα degradation and subsequent nuclear translocation and DNA binding of NF-κB; and second, by inducing p65 transactivation, via CBP/p300. Moreover, we provide evidence that inhibition of NF-κB activity abrogates the advanced differentiation induced by ectopic activation of p38 in C2C12 myocytes. The finding that NF-κB is a downstream effector of p38 MAPK during muscle cell differentiation constitutes a novel mechanism explaining, at least in part, the promyogenic effects of p38. Finally, we show that interleukin (IL)-6 gene expression is induced during C2C12 myogenesis, in a p38- and NF-κB-dependent manner, being this expression necessary for full differentiation. This study provides the first evidence of a cross talk between p38 MAPK and NF-κB signaling pathways during myogenic differentiation, with IL-6 being one of the effectors of this promyogenic mechanism.
MATERIALS AND METHODS
Cell Culture
The C2C12 cell line was obtained from the American Type Culture Collection (Manassas, VA) and cultured in growth medium (GM) composed of DMEM containing 15% fetal bovine serum. To induce differentiation of C2C12 cells, GM was replaced by differentiation medium (DM) composed by DMEM supplemented with 2% horse serum and 174 nM insulin. Very low rates of differentiation were achieved when GM was replaced by DMEM containing no serum (DMEM). All media were supplemented with 100 u/ml penicillin, 100 μg/ml streptomycin, and 2 mM glutamine.
Reagents
The p38 kinase inhibitor SB203580 (Calbiochem, San Diego, CA) and the NF-κB inhibitors pyrrolidinedithiocarbamate (PDTC) (Alexis Biochemicals) and BAY11-7085 (BIOMOL Research laboratories) were dissolved in dimethyl sulfoxide and used at a final concentration of 10, 100, and 5 μM, respectively. Recombinant mouse IL-6 (R & D Systems, Minneapolis, MN) was dissolved in water and used at a final concentration of 2 ng/ml. Rabbit polyclonal antibodies were used against the following molecules: p38 MAP kinase (sc-535, 1:1000; Santa Cruz Biotechnology, Santa Cruz, CA), phospho-p38 MAP kinase Thr180/Tyr182 (1:1000; Cell Signaling Technology, Beverly, MA), α-tubulin (Sigma-Aldrich, St. Louis, MO), IκBα (sc-371, 1:1000; Santa Cruz Biotechnology), myosin heavy chain (MHC) (1:100; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), p65 (sc-109, 1:1000; Santa Cruz Biotechnology), p50 (sc-7178, 1:1000; Santa Cruz Biotechnology), p52 (1:1000, sc-848; Santa Cruz Biotechnology), c-Rel (sc-71, 1:1000; Santa Cruz Biotechnology), and RelB (sc-226, 1:1000; Santa Cruz Biotechnology).
Plasmid Constructs
The following expression plasmids were used: pcDNAIII-MKK6(E), pc-DNAIII-MKK6(A), RcβactinHA-IκBα(S32,36A), Rc/CMVHA-p65, pCEVZ9-ΔMEKK1, pM-AKT, and pBM6DraA6 (IL-6) (Han et al., 1996; Dickens et al., 1997; Vanden Berghe et al., 1998; Madrid et al., 2001; Purcell et al., 2001) and were generous gifts from Drs. J. Han (The Scripps Research Institute, La Jolla, CA), A. Lin (University of Chicago, Chicago, IL), M. Karin (University of California, San Diego, CA), G. Evan (Imperial Cancer Research Fund, London), and G. Haegeman (University of Ghent, Ghent, Belgium), respectively. The following luciferase reporter plasmids were used: p6 × NF-κB-Luc, pMCK-Luc, and GAL4-Luc (Pahl and Baeuerle, 1995; Guo and Walsh, 1997; Madrid et al., 2001) and were kindly provided by Drs. PA. Baeuerle (Tularik, San Francisco, CA), K. Walsh (St. Elizabeth's Medical Center, Boston, MA), and A.S. Baldwin (University of North Carolina, Chapel Hill, NC). The luciferase IL-6 reporter plasmid used (p1168huIL-6P-Luc) (Vanden Berghe et al., 1998) was provided by Dr. G. Haegeman. The expression plasmids pGAL4-p65 and pGAL4-MEF2C (Vanden Berghe et al., 1998; Zhao et al., 1999) were generously provided by Drs. L. Schmitz (Albert-Ludwig University, Freiburg, Germany) and E. Olson (University of Texas Southwestern Medical Center, Dallas, TX).
Transfections
C2C12 myoblasts were transiently transfected with LipofectAMINE (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. After transfection either with luciferase reporters (p6×NF-κB-Luc, pMCK-Luc, p5×GAL4-Luc, and p1168huIL-6P-Luc), with or without expression plasmids, or only with expression plasmids, cells were shifted to DM, or DMEM alone, for 24-36 h in the absence or presence of SB203580, PDTC, BAY11-7085, and recombinant IL-6. All transfections included constant amounts of β-galactosidase reporter plasmid, as control for transfection efficiency. The total amount of DNA for each transfection was kept constant using an empty expression vector. Cells were then harvested to measure luciferase activity (Promega, Madison, WI). Luciferase activities were normalized by dividing luciferase activity by β-galactosidase activity. For each experimental group, a minimum of four independent transfections (in triplicate) were performed.
Northern Blot Analysis
RNA was obtained from C2C12 cells cultured in GM, DM, or DMEM alone, in the absence or presence of specific p38 and NF-κB inhibitors and recombinant IL-6, for the indicated times, by using the commercial Ultraspec RNA isolation system (Biotecx, Houston, TX). Northern blot analysis was performed with 20 μg of total RNA. The membranes were hybridized with murine p21, myogenin, α-actin, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) radiolabeled cDNA probes (Amersham Biosciences, Piscataway, NJ), following standard protocols (Miralles et al., 1998).
Western Blot Analysis
Cells were lysed in ice-cold immunoprecipitation buffer (50 mM Tris-Hcl pH 7.5, 150 mM NaCl, 1% NP-40, 5 mM EGTA, 5 mM EDTA, and 20 mM NaF) with the corresponding amount of the Complete mini protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). Analyses were performed on 10% SDS-PAGE. After electrophoretic transfer of proteins from SDS-PAGE gels to polyvinylidene difluoride membranes, the membranes were blocked with phosphate-buffered saline-0.1% Tween 20 containing 5% milk for 1 h and incubated overnight at 4°C with primary antibodies. Membranes were then washed and incubated 1 h with a peroxidase-conjugated secondary antibody (1:2000; DAKO, Glostrup, Denmark), and developed using the enhanced chemiluminescence system (Amersham Biosciences).
Electrophoretic Mobility Shift Assay (EMSA)
Nuclear extracts (NEs) were obtained from C2C12 cells cultured in GM, DM, or DMEM, in the absence or presence of specific p38 and NF-κB inhibitors, or from C2C12 cells transfected with various plasmids. The extraction of nuclear proteins was performed as described by De Cesare et al. (1995). Briefly, cells were washed twice in cold phosphate-buffered saline and scraped, and the cellular pellet was resuspended in 10 mM HEPES, pH 7.9, 10 mM KCl, 1.5 mM MgCl2, 0.1 mM EGTA, and 0.5 mM dithiothreitol (DTT) on ice. Cells were passed five times through a 26-gauge needle and centrifuged to collect nuclei, which were subsequently resuspended in an equal volume of 10 mM HEPES, pH 7.9, 0.4 M NaCl, 1.5 mM MgCl2, 0.1 mM EGTA, 0.5 mM DTT, and 5% glycerol, to allow elution of nuclear proteins by gentle shaking at 4°C for 30 min. Nuclei were pelleted at 14,000 rpm for 5 min at 4°C, and the supernatant was aliquoted, snap-frozen in liquid nitrogen, and stored at -80°C until use. All solutions contained protease inhibitors leupeptin and aprotinin at 1 μg/ml, phenylmethylsulfonyl fluoride (0.5 mM) and benzamidine (1 mM). A protein assay (Bio-Rad, Hercules, CA) was used to determine protein concentration. For electrophoretic mobility shift assays, 10 μg of nuclear extracts was incubated in 50 mM Tris-HCl, pH 7.9, 12.5 mM MgCl2, 1 mM EDTA, 1 mM DTT, 20% glycerol, 0.5 mM phenylmethylsulfonyl fluoride, and 2 μg of poly dI-dC for 10 min at room temperature to titrate out nonspecific binding before the addition of 15,000-20,000 cpm labeled oligonucleotide and the reaction was further incubated for 20 min at 30°C. When unlabeled competing oligonucleotides or antibodies were added, nuclear extracts were preincubated for 30 min or 1 h at room temperature, respectively, before the addition of the labeled probe. Samples were loaded on a prerun polyacrylamide gel (29:1 in 0.25× Tris borate-EDTA) and electrophoresed at 200 V. Gels were dried and autoradiographed at -80°C.
The sequences of the sense strands of the oligonucleotides used in EMSAs are as follows: NF-κB, 5′-AGTTGAGGGGACTTTCCCAGGC-3′; and p53, 5′-ACACACATGCCTCAGCAAGTCCCAGA-3′.
Reverse Transcription-Polymerase Chain Reaction (RTPCR) Analysis
C2C12 cells were transfected with the indicated expression plasmids and then shifted to DMEM alone for 24 h. Alternatively, cells were cultured either in DMEM or DM, in the absence or presence of the indicated inhibitors (SB203580, PDTC, or BAY11-7085) and recombinant IL-6. Total RNA was extracted from cells as described above. For RT-PCR analysis, 3 μg of total RNA was reverse transcribed using the first strand cDNA synthesis kit (Pharmacia, Buckinghamshire, United Kingdom), in a 15-μl reaction. Amplification parameters were denaturation for 30 s at 94°C; annealing for 30 s at 57°C (myogenin), 55°C (GAPDH, IL-6, and luciferase), and 50°C (skeletal α-actin); and extension for 30 s at 72°C. PCRs were performed for 30 cycles, and for each PCR reaction 8% of the cDNA pool was amplified using Taq polymerase. Primers for the detection of reverse transcriptase products were derived from different exons to distinguish RT-PCR products from genomic DNA contaminations. Primer sequences were as follows: myogenin, 5′-GAGCGCGATCTCCGCTACAGAGG-3′ and 5′-CTGGCTTGTGGCAGGCCCAGG-3′; α-actin, 5′-GAGATG TCTCTCTCTTAGCCTACC-3′ and 5′-CGGTTGGCAAGTCCTGGTCTGG-3′; GAPDH, 5′-ACTCCCACTCTTCCACCTTC-3′ and 5′-TCTTGCTCAGTGTCCTTGC-3′; luciferase, 5′-TACAGATGCACATATCGAGGTG-3′ and 5′-ATGAGATGTGACGAACGTGTAC-3′; and IL-6, 5′-GAGACTTCCATCCAGTTGCC-3′ and 5′-CTGATTATATCCAGTTTGGTAGCATC-3′. Expected products' sizes were myogenin, 379 base pairs; α-actin, 440 base pairs, GAPDH, 185 base pairs; luciferase, 400 base pairs; and IL-6, 300 base pairs.
RNA Interference
The following 21-mer oligoribonucleotide pairs were used: IL-6(1) from nt 317-337 5′-UGAUGGAUGCUACCAAACUGG-3′ and 3′-AGUUUGGUAGCAUCCAUCAUU-5′ and IL-6(2) from nt 51-71 5′-GAGACUUCCAUCCAGUUGCCU-3′ and 3′-GCAACUGGAUGGAAGUCUCUU-5′. Designed RNA oligonucleotides were “blasted” against the GenBank/EMBL database to ensure gene specificity. The two complementary strands (each at 20 μM) in 200 μl of annealing buffer (100 mM potassium acetate, 30 mM HEPES/KOH, pH 7.4, and 2 mM magnesium acetate) were heated for 1 min at 90°C and then incubated for 1 h at 37°C. Small interfering RNA (siRNA) corresponding to nt 153-173 of the firefly luciferase mRNA was used as a negative control.
RESULTS
p38 MAPK and NF-κB Are Activated at Different Stages of Myogenesis
Our main goal in this study was to investigate the role of p38 and NF-κB activities in skeletal muscle differentiation and the existence of a potential crosstalk between both pathways during this process. Different studies have reported that p38 MAPK and NF-κB signaling pathways are involved in myogenic differentiation in vitro, although the implication of the latter remains controversial (reviewed in Puri and Sartorelli, 2000). Therefore, first we performed a simultaneous analysis of both activities in proliferation and in differentiation-promoting conditions. For this analysis, we used the C2C12 murine myoblast cell line (a widely used model for myogenesis in vitro): C2C12 myoblasts were grown to confluence and the serum concentration switched from 15% (GM, growth medium) to 2% (DM, differentiation medium) to induce the expression of muscle-specific gene products. As shown in Figure 1A, the muscle specific protein MHC was induced after 24 h in DM, whereas its expression was undetectable in GM. To study in parallel the implication of p38 and NF-κB in this process, protein extracts were prepared from C2C12 cells cultured either in GM or in DM and both activities analyzed comparatively. The activity of p38 (detected by immunoblotting with an antibody specific for its activated form) was induced during the onset of differentiation, whereas no phosphorylation of p38 was detected in cells cultured in GM (Figure 1B), confirming a correlation between p38 activation and muscle differentiation. In contrast, the activity of NF-κB was high in GM, decreased at early time points after switching the cells to DM (6 h), and reached higher levels at later stages of differentiation (24-48 h in DM), as demonstrated by EMSA (Figure 1C, top). The composition of the GM- and DM-induced NF-κB activities binding to DNA corresponded to the p65/p50 heterodimer, because its formation was prevented by specific antibodies against these subunits (Figure 1D); antibodies against other NF-κB subunits had no effect on the NF-κB-DNA complex formation in GM or DM (our unpublished data). These results indicated that p38 is activated exclusively during C2C12 cell differentiation, whereas NF-κB activity is induced in a dual phase: in proliferating myoblasts and in differentiating myocytes. Accordingly, the potential cross talk of these pathways in myogenesis may only take place during the differentiation process.
Figure 1.
NF-κB is activated in a dual phase during myogenesis, whereas p38 activity is only detected during differentiation. C2C12 myoblasts were cultured in GM until 80% confluence and then switched to lowserum DM. (A) Myogenic differentiation is induced in cells cultured in DM but not in GM. Protein lysates were obtained from C2C12 cells grown in GM and DM for the indicated time points (h) and analyzed by Western blotting with antibodies against MHC and α-tubulin (as loading control), respectively. (B) p38 MAPK phosphorylation is induced during C2C12 myoblast differentiation. Cell lysates were obtained from C2C12 myoblasts in GM or DM (as in A) and activation of p38 was determined by immunoblotting, by using a specific anti-phospho-p38 antibody (P-p38). An anti-p38 antibody was used to confirm that the amount of p38 protein was unchanged in all lanes. (C) NF-κB-DNA binding activity shows a biphasic profile during C2C12 myogenesis. NEs were obtained from cells cultured in GM or DM and incubated with a radiolabeled double-stranded oligonucleotide containing the NF-κB binding site, and DNA-protein binding activity was analyzed by EMSA. (D) NEs from C2C12 cells in GM or DM (24 h) were incubated in the absence or presence of specific antibodies for p65 and p50, respectively; an unspecific antibody was used as control. The specific NF-κB-DNA complex induced in DM is indicated by an arrow.
p38 MAPK and NF-κB Activities Are Necessary for Myoblast Differentiation
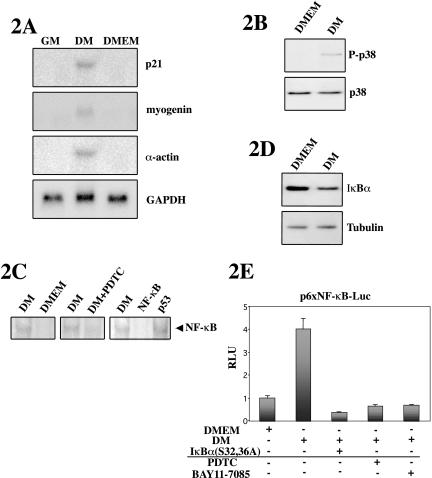
Based on the above-mentioned results, we investigated further the implication of both pathways during myoblast differentiation. As control for the differentiation-promoting conditions of DM, cells were also switched from GM to serum-free DMEM, because the complete absence of serum in the media had been shown by others to result in delayed and nonefficient myoblast differentiation (Wang and Walsh, 1996; Kaliman et al., 1999; Conejo et al., 2001). Total RNA was prepared from cells cultured in GM, DM, and DMEM, and as expected, the expression of p21, myogenin, and α-actin mRNA was induced in C2C12 cells cultured in DM for 24 h, whereas it was undetectable in cells cultured in GM, or in DMEM alone, for an identical period of time (Figure 2A). This result confirmed that DM, but not serum-free DMEM, promotes C2C12 myogenic differentiation in vitro. To analyze in parallel the implication of p38 and NF-κB during muscle differentiation, protein extracts were prepared from C2C12 cells cultured either in DM or in DMEM for 24 h, and both activities were analyzed comparatively. The activity of p38 was induced in DM, whereas no phosphorylation of p38 was detected in cells cultured in DMEM (Figure 2B). The activation of NF-κB in C2C12 cells cultured in DM or DMEM was analyzed by EMSA and NF-κB-dependent/luciferase reporter assays. Figure 2C (left) shows that the NF-κB-DNA binding activity was induced after 24 h in DM with respect to DMEM, correlating with the induction of p38 activity and of myogenic differentiation in DM (Figure 2, A and B). As expected, the DM-induced NF-κB-DNA binding activity was specific, because it was competed by excess of labeled NF-κB oligonucleotide, but not by excess of an unrelated competitor (Figure 2C, right), and in agreement with the composition of the NF-κB activity (p65/p50 heterodimer) shown in Figure 1D. The NF-κB-DNA binding activity in DM was sensitive to inhibition of IκBα phosphorylation, by treatment of the cells with PDTC (Figure 2C, middle), suggesting that DM might be promoting the degradation of the cytoplasmic NF-κB inhibitor. In agreement with this, Western blot analysis using an antibody against IκBα revealed that the levels of the inhibitor were lower in extracts from cells cultured for 24 h in DM than in DMEM (Figure 2D). This result suggested that the decrease in IκBα protein levels in DM might be causing an increase in NF-κB nuclear translocation and DNA binding in differentiation promoting culture conditions. The functionality of the NF-κB-DNA binding activity was confirmed by transient transfection of C2C12 cells with a plasmid containing a luciferase reporter gene driven by multimerized NF-κB consensus sequences (p6 × NF-κB-Luc). As shown in Figure 2E, NF-κB-dependent luciferase activity was induced in cells cultured in DM versus DMEM. Furthermore, the DM-induced luciferase activity was inhibited by cotransfection of an expression vector for the superepressor IκBα(S32,36A), coding for the NF-κB inhibitor IκBα with serines 32 and 36 replaced by alanines, or by treatment with PDTC or BAY11-085 (NF-κB inhibitors) (Figure 2E). Together, these results demonstrate that both p38 MAPK and NF-κB signaling pathways are activated in C2C12 myoblasts cultured in differentiation promoting conditions (DM), but not in conditions not favoring this process (DMEM).
Figure 2.
p38 MAPK and NF-κBare both activated during myogenic differentiation. C2C12 myoblasts were cultured in GM until 80% confluence and then switched to lowserum DM or to serum-free DMEM, used as control for nondifferentiation conditions. (A) Myogenic differentiation is induced in cells cultured in DM but not in serum-free DMEM. Total RNA obtained from C2C12 cells grown in GM, DM, or DMEM for 24 h was analyzed by Northern blotting by using p21, myogenin, α-actin, and GAPDH cDNA probes, respectively. (B) p38 MAPK phosphorylation is induced during C2C12 myoblast differentiation. C2C12 myoblasts proliferating in GM were shifted to DM or DMEM for 24 h, and protein lysates obtained. Activation of p38 was determined by immunoblotting, by using an anti-P-p38 antibody (P-p38). An anti-p38 antibody was used to confirm that the amount of p38 protein was unchanged in all lanes. (C) NF-κB-DNA binding activity is induced during C2C12 myoblast differentiation. Left, NE were obtained from cells cultured in DM or DMEM as in Figure 1, A and B, and incubated with a radiolabeled double-stranded oligonucleotide containing the NF-κB binding site, and DNA-protein binding activity was analyzed by EMSA. Middle, EMSA of cells cultured in DM containing or not the NF-κB inhibitor PDTC. Right, NE from C2C12 cells in DM were incubated with the labeled NF-κB oligonucleotide in the absence or presence of a 150-fold molar excess of unlabeled competitors: NF-κB (specific competitor) and p53 (unspecific competitor) and analyzed by EMSA. The specific NF-κB-DNA complex induced in DM is indicated by an arrow. (D) IκBα protein level is reduced during C2C12 differentiation. Total cellular extracts were obtained from C2C12 cells cultured in DM or DMEM as described above, and IκBα protein levels were analyzed by immunoblotting by using an anti-IκBα antibody. Bottom, immunoblotting with an anti-α-tubulin antibody to confirm equal loading in all lanes. (E) NF-κB transcriptional activity is induced during C2C12 myoblast differentiation. C2C12 cells were transiently transfected with a luciferase reporter gene construct controlled by olimerized κB-binding sites, p6×NF-κB-Luc, with or without an expression plasmid for the superepressor IκBα(S32,36A). Cells were maintained in DMEM or DM for 36 h after transfection, in the absence or presence of PDTC or BAY11-7085. Luciferase activities are expressed relative to the activity of the reporter construct in cells cultured in DMEM, which was given a value of 1. Error bars represent the SE of the mean value.
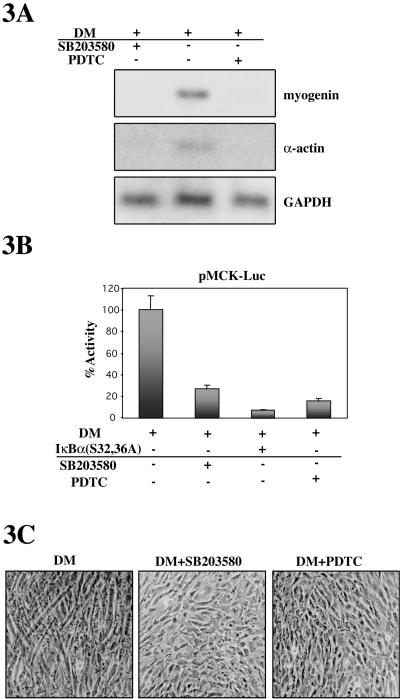
The role of p38 in myoblast differentiation is well established (Cuenda and Cohen, 1999; Zetser et al., 1999; Wu et al., 2000); however, whereas some results suggested that NF-κB inhibits differentiation, others proposed that NF-κB is a positive regulator of myogenesis (Guttridge et al., 1999; Kaliman et al., 1999; Conejo et al., 2001). To establish a causal relationship between C2C12 myoblast differentiation and NF-κB activation (as well as with activation of p38, in parallel), specific inhibitors of each pathway were used. Treatment of cells with either the NF-κB inhibitor PDTC or with the specific p38 MAPK inhibitor SB203580 completely precluded myogenin and α-actin expression after 24 h in DM (Figure 3A). We also examined the effect of NF-κB and p38 inhibitors on a luciferase reporter gene controlled by the MCK promoter (pMCK-Luc), which is transcriptionally activated only by MRFs during muscle differentiation. As shown in Figure 3B, MCK-dependent luciferase activity in DM was inhibited by SB203580 and PDTC treatments, respectively, as well as by cotransfection of the IκBα(S32,36A) expression plasmid (Figure 3B). Most importantly, C2C12 cells cultured in DM containing either SB203580 or PDTC did not fuse into myotubes, whereas cells cultured in DM formed plurinucleated myotubes after 3 d (Figure 3C). Collectively, these data clearly indicate that both NF-κB and p38 MAPK activities are necessary for myoblast fusion and differentiation.
Figure 3.
p38 and NF-κB activities are required for C2C12 myoblast differentiation and myotube formation. (A) Inhibition of p38 or NF-κB activity abrogates myoblast differentiation. C2C12 cells were cultured in DM for 24 h, in the absence or presence of the p38 inhibitor SB203580 or the NF-κB-inhibitor PDTC. Myogenin and α-actin expression was analyzed by Northern blotting by using the corresponding cDNA probes. Also, GAPDH mRNA expression was analyzed as internal loading control. (B) Muscle-specific promoter activity is abrogated by inhibition of p38 or NF-κB activity. C2C12 myoblasts were transiently transfected with the muscle-specific promoter-reporter plasmid MCK-Luc, together with an expression plasmid for the superepressor IκBα or with an empty vector and cultured in DM for 36, in the presence or absence of SB203580 or PDTC, respectively. Luciferase activities are expressed relative to the activity of MCK-Luc in DM, which was given a value of 100. Error bars represent the SE of the mean value. (C) Myoblast fusion is inhibited by p38 or NF-κB inhibitors. C2C12 cells were cultured for 3 d in the absence or presence of SB203580 or PDTC, and myoblast fusion was analyzed by examining the presence of plurinucleated myotubes.
Inhibition of p38 MAPK Prevents Activation of NF-κB during Myogenic Differentiation
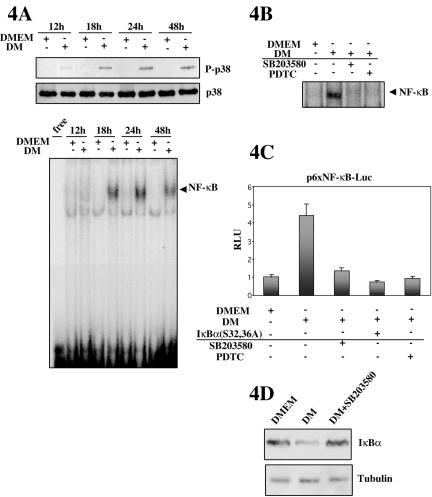
We have shown above that both p38 and NF-κB activities are induced in differentiation promoting conditions (DM) and are required for completion of the myogenic program. However, the existence of a potential crosstalk between both pathways during skeletal myogenesis has never been investigated. We set out to analyze whether NF-κB might function downstream of the p38 MAPK pathway during muscle differentiation. This issue is pertinent because, as shown in Figure 4A, a time course of NF-κB and p38 activities revealed that activation of p38 preceded that of NF-κB during differentiation in DM. Phosphorylation of p38 was readily detected in C2C12 cells by 12 h in DM (but not in DMEM), continuing to be observed thereafter (Figure 4A, top). In contrast, NF-κB activation was first detected after 18 h in DM (as assessed by EMSA), reaching a maximum after 24 h (Figure 4A, bottom), suggesting that NF-κB activation occurs later than that of p38 during DM-induced differentiation, and in agreement with the results shown in Figure 1, B and C. Accordingly, to investigate whether NF-κB functions downstream of p38 during myoblast differentiation, the activity of NF-κB in DM was analyzed in the absence or presence of the p38 inhibitor SB203580. As shown in Figure 4, B and C, both the NF-κB-DNA binding activity and the NF-κB-dependent luciferase activity of C2C12 cells cultured in DM were reduced in the presence of SB203580. As control for NF-κB specificity, we showed that treatment with PDTC or cotransfection of IκBα (S32/36A) inhibited both DM-induced NF-κB-DNA binding and luciferase activities, respectively (Figure 4, B and C). These results demonstrated that the activation of NF-κB during C2C12 cell differentiation could be blocked by inhibition of p38 activity. Moreover, as shown in Figure 4D, the lower levels of IκBα in DM versus DMEM were increased upon addition of SB203580, suggesting that p38 was involved in the degradation of IκBα and therefore in the subsequent nuclear localization of NF-κB during myogenic differentiation. Together, these results indicate that activation of NF-κB during C2C12 myoblast differentiation requires functional p38 MAPK activity.
Figure 4.
NF-κB activation in differentiating C2C12 cells is dependent on p38 MAPK activity. (A) p38 activation precedes activation of NF-κB during myoblast differentiation. C2C12 myoblasts were cultured in GM and then shifted to DM or DMEM for the indicated periods of time (12-48 h). Total cellular extracts or NEs were prepared, as detailed in MATERIALS AND METHODS, and p38 or NF-κB activity analyzed by Western blotting (top) or EMSA (bottom), respectively, as in Figure 1. (B) Induction of NF-κB-DNA binding activity in C2C12 differentiating cells requires functional p38 MAPK. NEs were prepared from C2C12 cells cultured in DM (or DMEM) for 24 h, in the absence or presence of 10 μM SB203580, or PDTC (used as control), and binding of nuclear proteins to a consensus labeled NF-κB oligonucleotide analyzed by EMSA. (C) Induction of NF-κB-dependent transcription during C2C12 myoblast differentiation requires functional p38 MAPK. C2C12 cells were transiently transfected with the p6×NF-κB-Luc reporter plasmid, with or without coexpression of IκBα(S32,36A), used as control for NF-κB activity. After transfection, cells were maintained in DM (or DMEM) for 36 h in the absence or presence of SB203580 or PDTC. Luciferase activities are expressed relative to the activity of MCK-Luc in DMEM, which was given a value of 1. Error bars represent the SE of the mean value. (D) Inhibition of p38 activity increases IκBα protein levels in C2C12 differentiating cells. Whole cell extracts were obtained from C2C12 cells cultured in DMEM, DM, or DM containing SB203580, and IκBα levels analyzed by immunoblotting by using an anti-IκBα antibody. Bottom, immunoblotting with an anti-α-tubulin antibody to confirm equal loading.
Ectopic Activation of p38 Induces NF-κB-dependent Transcription in C2C12 Cells
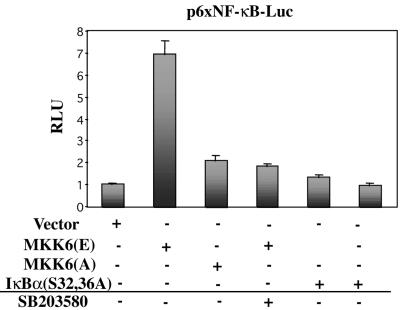
The above-mentioned results suggest that NF-κB acts downstream of p38 during myoblast differentiation. If DM-induced p38 activity is causally involved in activation of NF-κB in myocytes, then its deliberate activation should be sufficient for induction of NF-κB activity in C2C12 cells in DMEM (where neither NF-κB nor p38 activities can be detected; Figure 4A). Accordingly, experiments were carried out to determine the effect of a constitutively active form of MKK6, MKK6(E), a p38 activating kinase, on NF-κB-mediated transcription in C2C12 cells cultured in DMEM. As shown in Figure 5, MKK6(E) was able to induce significantly NF-κB-dependent luciferase activity, whereas a kinase-dead form of MKK6, MKK6(A), had practically no effect. SB203580 treatment inhibited the MKK6(E)-induced NF-κB transcriptional activity in C2C12 cells, indicating that p38 was mediating this effect. As a control, overexpression of IκBα(S32,36A) also blocked MKK6(E)-induced NF-κB transcriptional activity. Collectively, these data indicate that 1) p38 activity is required for activation of NF-κB during C2C12 cell differentiation in DM; and conversely, 2) deliberate activation of p38 is sufficient for triggering NF-κB activation in C2C12 cells cultured in DMEM, mimicking the effects of DM on NF-κB activation.
Figure 5.
Deliberate activation of p38 induces NF-κB-dependent transcription in C2C12 myoblasts cultured in serum-free medium. The reporter construct p6×NF-κB-Luc was transiently transfected into C2C12 cells, in the presence or absence of expression plasmids for MKK6(E), MKK6(A), IκBα(S32,36A) or empty vector, respectively, and luciferase activities were analyzed after 36 h in DMEM. When indicated, SB203580 was added to DMEM. pRSV-lacZ plasmid was used as a transfection control, and the corresponding β-galactosidase activities were used to normalize the luciferase values. Luciferase activities are expressed relative to the activity of the reporter plasmid (cotransfected with empty vector) in DMEM, which was given a value of 1. Error bars represent the SE of the mean value.
IκBα Degradation and NF-κB-DNA Binding Activity Are Augmented in C2C12 Cells Overexpressing Activated p38
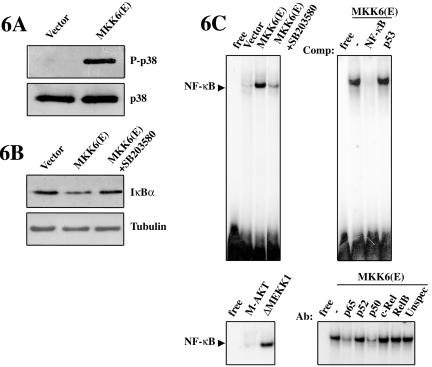
The results presented above indicate that p38 is capable of inducing NF-κB-dependent transcription in serum-deprived C2C12 myoblasts. This p38-dependent effect could be the result of 1) increased cytoplasmic-nuclear translocation of NF-κB leading to increased DNA binding, 2) of increased activity of the NF-κB activating subunit p65, or 3) both. To address these possibilities, C2C12 cells were transfected with the activated form of MKK6, or with test plasmids, and transferred to DMEM, and NF-κB activities were analyzed subsequently. First, we confirmed that overexpression of MKK6(E), but not of an empty vector, in C2C12 cells cultured in DMEM resulted in constitutive phosphorylation of p38 (Figure 6A). Next, we investigated the effect of deliberate p38 activation on IκBα levels and on NF-κB-DNA binding activity in C2C12 cells cultured in DMEM. As shown in Figure 6B, the levels of IκBα where reduced in cells overexpressing MKK6(E), with respect to cells transfected with an empty vector, whereas SB203580 treatment restored the levels of IκBα. These results indicated that MKK6 was able to promote the degradation of the NF-κB inhibitor, in a p38-dependent manner. Similarly, analysis of nuclear extracts from C2C12 cells expressing MKK6(E) showed a significantly higher NF-κB-DNA binding activity than cells transfected with an empty vector, and this activity was abrogated by SB203580 treatment (Figure 6C, top left). This p38-induced NF-κB-DNA binding activity was specific because it could be inhibited by excess of cold NF-κB oligonucleotide but not by excess of unrelated p53 oligonucleotide (Figure 6C, top right). As experimental controls, cells transfected with a plasmid coding for a constitutively active form of MEKK1 showed increased DNA-NF-κB binding activity, whereas overexpression of an activated form of PKB/AKT had no significant effect (Figure 6C, bottom left), in agreement with previous reports demonstrating that MEKK1 activity, but not PKB/AKT, could stimulate nuclear translocation and DNA binding of NF-κB in other cell types (Lee et al., 1997; Madrid et al., 2001). Our next aim was to investigate the composition of the NF-κB transcription factor. Using specific antibodies for the different NF-κB subunits, we were able to demonstrate that ectopic activation of p38 induced the formation of a DNA-NF-κB heterodimer containing p65 and p50 (Figure 6D, bottom right). Antibodies against other NF-κB subunits such as p52, c-Rel, and RelB had no effect on the NF-κB-DNA binding activity induced by MKK6(E). These results indicate that deliberate activation of p38 induces a NF-κB-DNA binding activity composed of the p65/p50 heterodimer in DMEM-cultured C2C12 cells, mimicking the NF-κB activity induced in DM (compare with Figure 1D).
Figure 6.
NF-κB-DNA binding activity is induced by ectopic activation of p38 MAPK in C2C12 cells. (A) MKK6(E)-expressing C2C12 cells show constitutive p38 phosphorylation. C2C12 cells were transfected with either empty vector or an expression plasmid for MKK6(E) and cultured for 24 h in DMEM. p38 activation was analyzed by immunoblotting by using an anti-P-p38 antibody. An anti-p38 antibody was used to ensure equal p38 levels. (B) Constitutive p38 activation reduces IκBα protein levels. C2C12 cells were transfected with either empty vector or an expression plasmid for MKK6(E) and cultured for 24 h in DMEM, in the absence or presence of SB203580. IκBα protein levels were analyzed by immunoblotting by using an anti-IκBα antibody. An anti-α-tubulin antibody was used as loading control. (C) Deliberate activation of p38 MAPK induces NF-κB (p65/p50) binding to DNA. C2C12 cells were transfected with MKK6(E) or with an empty vector, in the absence or presence of SB203580 (top left), or plasmids encoding constitutively active forms of MEKK1 or AKT (bottom/left panel). NE were prepared after 24 h in DMEM, in the absence or presence of SB203580, and analyzed by EMSA. The specificity of NF-κBDNA binding activity was analyzed by incubating NE from MKK6(E)-transfected C2C12 cells with the labeled NF-κB double-stranded oligonucleotide in the absence or presence of a 150-fold molar excess of unlabeled competitors: NF-κB (specific competitor) and p53 (unspecific competitor) (top right). The composition of the NF-κB heterodimer was analyzed by EMSA, after incubating NE from MKK6-transfected C2C12 cells in the absence or presence of different antibodies directed against the indicated members of the Rel/NF-κB family, respectively (bottom right).
p38 MAPK Induces the Transactivating Potential of the p65 Subunit of NF-κB in C2C12 Cells: Role of CBP/p300
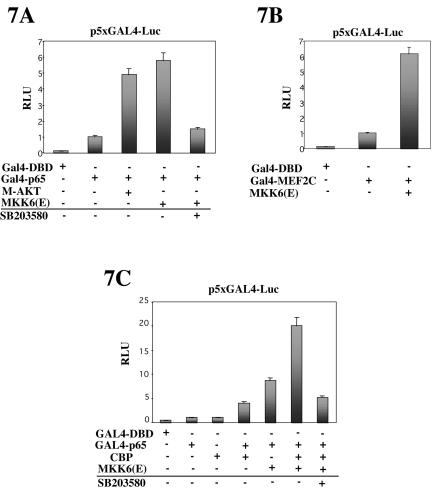
Various signaling pathways activate NF-κB-dependent transcription, at least in part, through mechanisms independent of NF-κB nuclear translocation, presumably by stimulating the transactivation domain (TAD) of the p65 subunit (Wang and Baldwin, 1998; Zhong et al., 1998; Ghosh and Karin, 2002; Vermeulen et al., 2003). We were interested in determining whether MKK6-activated p38, in addition to inducing NF-κB-DNA binding activity (as shown in Figure 6), could activate NF-κB by targeting the C-terminal TAD of the p65 subunit in C2C12 differentiating myoblasts. To address this question, we used a plasmid bearing DNA encoding the GAL4-p65 fusion protein, where sequences encoding the DNA binding domain of GAL4 have been joined with sequences encoding the TAD of p65. C2C12 cells were cotransfected with a GAL4-Luc reporter and the GAL4-p65 expression plasmids, and with plasmids bearing genes encoding activated forms of MKK6, or AKT (known to induce p65 transactivation by phosphorylating the TAD of p65; Madrid et al., 2001), or with an expression plasmid containing only the DNA binding domain of GAL4, and cells were further incubated for 24 h in DMEM. As shown in Figure 7A, MKK6(E) was able to activate p65 TAD, and this activation was inhibited by SB203580 treatment, suggesting that this effect was mediated by p38; similar results were obtained by overexpression of M-AKT (Figure 7A), as expected. Moreover, as a control for p38 activity in this assay, we observed that overexpression of MKK6(E) induced the MEF2C transactivating activity of a GAL4-MEF2 fusion protein (Figure 7B), in agreement with previous reports (Zetser et al., 1999; Wu et al., 2000). Together, these results indicated that MKK6 induced the transactivating potential of the p65 subunit of NF-κB via p38 in C2C12 myoblasts.
Figure 7.
p65-NF-κB transactivating activity is induced by ectopic activation of p38 MAPK: role of CBP. (A) C2C12 cells were transiently cotransfected with a GAL4 responsive-luciferase reporter (p5×GAL4-Luc) and GAL4-p65 or GAL-DBD, together with expression plasmids for MKK6(E) or MAKT (as positive control for GAL4-p65 activity). (B) p5×GAL4-Luc was cotransfected with a GAL4-MEF2C expression plasmid (as positive control for p38 activity), with or without MKK6(E). (C) p5×GAL4-Luc was cotransfected with GAL-DBD or GAL4-p65, and with MKK6(E) and/or CBP. Luciferase activities were analyzed after 36 h in DMEM. pRSV-lacZ plasmid was used as a transfection control, and the corresponding β-galactosidase activities were used to normalize the luciferase values. Luciferase activities are expressed relative to the activity of the GAL4-p65 or pGAL4-MEF2C plasmids, respectively, which were given a value of 1. Error bars represent the SE of the mean value.
Next, we aimed to analyze the mechanism(s) of p38-mediated activation of the p65 subunit of NF-κB in C2C12 cells. Because we were unable to detect phosphorylation of GST-p65 by MKK6-activated p38 in an in vitro kinase assay (our unpublished data), we hypothesized that activation of p65 by p38 is most likely unrelated to direct modification of this subunit. It is well known that NF-κB interacts with the basal transcriptional machinery and requires coactivators such as CBP and p300 (Gerritsen et al., 1997; Zhong et al., 1998). Therefore, we hypothesized that p38 may use CBP/p300 to stimulate NF-κB transcription in C2C12 cells. To pursue this idea, constructs encoding CBP or activated MKK6 were transiently cotransfected into C2C12 cells along with GAL4-Luc and GAL4-p65. As shown in Figure 7C, CBP stimulated p65 in conjunction with activated MKK6 in C2C12 cells cultured in DMEM. Treatment with SB203580 significantly reduced this cooperation, consistent with the idea that p38 indirectly stimulates the transactivating activity of p65 through a mechanism involving CBP.
MKK6-activated p38 Induces Muscle Differentiation in a NF-κB-dependent Manner
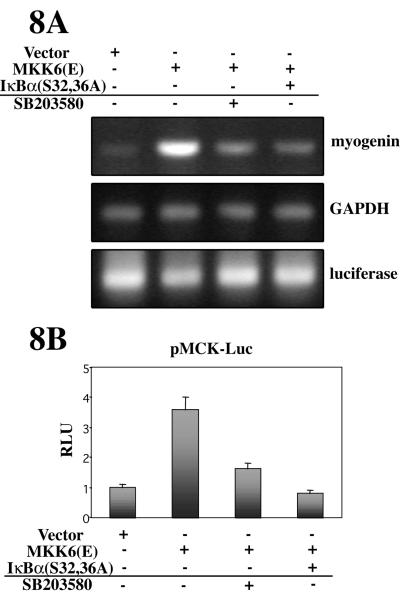
Ectopic activation of p38 has been shown to induce muscle differentiation in growth promoting conditions (GM) (Wu et al., 2000). Here, we analyzed whether overexpression of MKK6(E) could induce muscle differentiation in culture conditions that do not favor differentiation (DMEM alone). As shown in Figure 8A, myogenin mRNA was induced in MKK6(E)-transfected C2C12 cells cultured in DMEM, but not in cells transfected with an empty vector (Figure 8A, lanes 1 and 2). Because ectopic activation of p38 is able to induce NF-κB-dependent transcription in C2C12 cells in DMEM (Figures 4, 5, 6), we tested whether the p38-induced myogenic differentiation was mediated, at least in part, through NF-κB activation. As shown in Figure 8A, the MKK6(E)-induced expression of myogenin in C2C12 cells cultured in DMEM was reduced by coexpression of IκBα(S32,36A), or by treatment with SB203580. Similarly, the luciferase activity of the muscle differentiation-specific reporter plasmid MCK-Luc was induced by cotransfection of MKK6(E) in C2C12 cells, and this induction was reduced by coexpression of IκBα(S32,36A) or by SB203580 treatment (Figure 8B). Together, these results demonstrate that the induction of myogenic differentiation by deliberate activation of p38 MAPK is mediated, at least in part, by activation of NF-κB.
Figure 8.
Constitutive activation of p38 accelerates myogenic differentiation in DMEM in a NF-κB-dependent manner. (A) p38-induced muscle-specific gene expression is reduced by inhibition of NF-κB activity. C2C12 cells were transfected with an empty vector or with the MKK6(E) plasmid, with or without IκBα(S32/36A) coexpression, and maintained in DMEM for 24 h, in the absence or presence of SB203580. Myogenin mRNA expression was analyzed by RT-PCR as an index of myogenic differentiation. GAPDH and luciferase expression were analyzed as controls for RT-PCR and transfection efficiency, respectively. (B) p38-induced muscle-specific promoter activity is reduced by inhibition of NF-κB activity. C2C12 myoblast were transiently transfected with MCK-Luc, together with expression plasmids for MKK6(E), IκBα(S32/36A) or an empty vector, and cultured in DMEM, in the presence or absence of SB203580. Luciferase activities are expressed relative to the activity of MCK-Luc, which was given a value of 1. pRSV-lacZ plasmid was used as a transfection control, and the corresponding β-galactosidase activities were used to normalize the luciferase values. Error bars represent the SE of the mean value.
IL-6 Gene Expression Is Induced during Myoblast Differentiation, in a p38- and NF-κB-dependent Manner
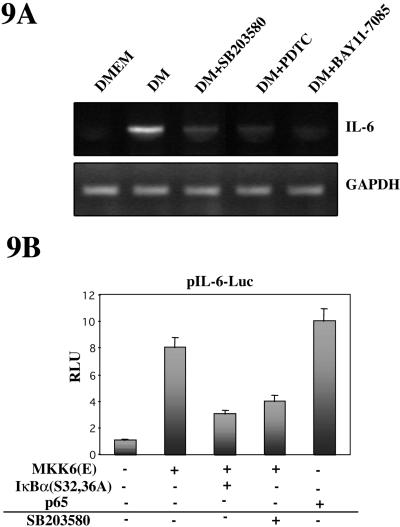
No consensus NF-κB responsive sequences have been reported in promoters of muscle-specific genes; therefore, we hypothesized that NF-κB might be promoting myogenic differentiation by inducing the expression of nonmuscle specific genes, whose protein products may, nevertheless, be involved in the differentiation process. As shown in Figure 9A, the expression of IL-6 mRNA was induced in C2C12 cells cultured in DM, but not in DMEM for 24 h, based on RT-PCR analysis, whereas the expression of the GAPDH gene remained constant in both culture conditions. Because the expression of IL-6 is known to depend on activation of p38 and NF-κB in other cell types (Vanden Berghe et al., 1998; Craig et al., 2000), we analyzed the effect of inhibiting these pathways on IL-6 mRNA expression during myoblast differentiation. As shown in Figure 9A, addition of either the p38 inhibitor SB203580 or the NF-κB inhibitors PDTC or BAY11-7085 abrogated the expression of IL-6 mRNA during C2C12 cell differentiation in DM.
Figure 9.
IL-6 expression is induced during myoblast differentiation in a p38- and NF-κB-dependent manner. (A) Analysis of IL-6 expression in myoblast differentiation. C2C12 cells were cultured in DMEM or DM for 24 h, in the absence or presence of SB203580, PDTC, or BAY11-7085. IL-6 mRNA was analyzed by RT-PCR using the corresponding cDNA probe. Also, GAPDH mRNA expression was analyzed as internal control. (B) IL-6 promoter activity is induced by p38 and NF-κB. C2C12 myoblasts were transiently transfected with an IL-6 promoter-reporter plasmid (see MATERIALS AND METHODS), together with expression plasmids for MKK6(E), IκBα(S32/36A), or p65 (or with an empty vector, as control), and cultured in DMEM for 36, in the presence or absence of SB203580, respectively. Luciferase activities are expressed relative to the activity of pIL-6-Luc transfected with an empty vector, which was given a value of 1. Error bars represent the SE of the mean value.
Based on our results that p38 and NF-κB inhibition affects IL-6 gene expression, we hypothesized that ectopic expression of activated MKK6 or p65 may affect transcription from the IL-6 promoter in C2C12 cells. To test this prediction, C2C12 cells were transiently cotransfected with the promoter/reporter plasmid IL-6-Luc with or without expression plasmids for MKK6(E) or p65, in the absence or presence of specific inhibitors, and cell extracts were prepared and analyzed for luciferase activity. As shown in Figure 9B, MKK6 overexpression resulted in an increase of luciferase activity from the IL-6-Luc reporter, which was reduced by SB203580 treatment or by cotransfection of IκBα(S32/36A). Moreover, the maximal induction of IL-6-dependent luciferase activity was obtained upon cotransfection of a p65-NF-κB expression vector. These results indicate that the IL-6 gene promoter (which contains functional NF-κB sites) is a final target of the NF-κB transcription factor in C2C12 cells.
Interference with IL-6 mRNA Expression Reduces Myoblast Differentiation
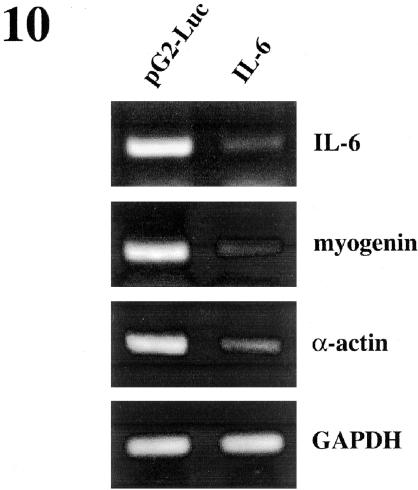
To confirm the involvement of IL-6 in regulation of myogenic differentiation, the effect of loss of function of IL-6 was examined using siRNA specific to IL-6. Transfection of C2C12 cells with specific IL-6 siRNAs resulted in a decrease in myogenin and α-actin mRNA levels after 24 h in DM, as detected by RT-PCR, in comparison with C2C12 cells transfected with unspecific siRNAs (Figure 10). This result indicates that IL-6 expression is necessary for efficient muscle differentiation.
Figure 10.
Ablation of IL-6 results in down-regulation of muscle differentiation-specific genes. C2C12 cells were transfected with control firefly luciferase or with IL-6 siRNAs, respectively. After transfection, cells were cultured for 24 h in DM and the expression of IL-6, myogenin, α-actin, and GAPDH (used as control) mRNA was analyzed by RT-PCR. As shown in the figure, IL-6 mRNA levels were strongly decreased when cells were transfected with IL-6 siRNAs. Myogenin and α-actin expression levels decreased significantly, whereas no change in GAPDH expression was observed in cells transfected with IL-6 siRNAs.
IL-6 Promotes Myogenic Differentiation
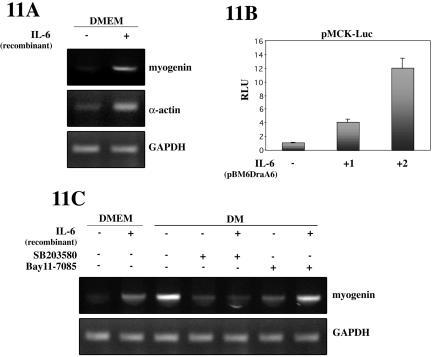
If IL-6 contributes positively to myogenic differentiation, it is conceivable that addition of the soluble cytokine (or overexpression) be sufficient for inducing this process in conditions not favoring differentiation. For this purpose, C2C12 cells were switched to DMEM and treated with recombinant murine IL-6 for 24 h, and myogenic differentiation analyzed subsequently. As shown in Figure 11A, myogenin and α-actin mRNA levels were induced in C2C12 cells upon IL-6 treatment, whereas GAPDH expression levels remained unchanged. These results indicate a promyogenic role of IL-6 in C2C12 cell differentiation. To confirm this further, we examined the effects of IL-6 on MCK-dependent luciferase activity. C2C12 cells were transiently transfected with pMCK-Luc with or without an expression plasmid for IL-6, and switched to DMEM for 48 h (conditions that do not favor differentiation). As shown in Figure 11B, IL-6 overexpression increased luciferase activity driven by the MCK promoter in a dose (plasmid)-dependent manner. Similar results were obtained after adding recombinant IL-6 to MCK-Luc-transfected C2C12 cells (our unpublished data). These results confirmed that myogenic differentiation is positively regulated by IL-6. Our next aim was to analyze whether exogenous IL-6 could bypass the negative effect of p38 and NF-κB inhibitors on myogenic differentiation. As shown in Figure 11C, addition of IL-6 induced myogenin and mRNA expression in C2C12 cells cultured in DMEM for 24 h (where levels of p38 and NF-κB are almost undetectable; Figures 2 and 4). Furthermore, the level of myogenin mRNA in C2C12 myoblasts cultured in differentiation promoting conditions (DM) was reduced by addition of SB203580 and BAY11-785, inhibitors of p38 and NF-κB, respectively. Addition of recombinant IL-6 to DM-containing SB203580 had very little effect on the expression of this myogenic marker (Figure 11C). In contrast, IL-6 was able to reverse significantly the reduction of myogenin mRNA expression in the presence of BAY11-785. This indicated that ectopic IL-6 was able to bypass, at least in part, the effect of NF-κB, but not p38, inhibition on myogenic differentiation. This suggests that p38 may affect multiple pathways required for muscle differentiation; therefore, the solely overexpression of one factor, IL-6, cannot reverse the complete differentiation block imposed by the p38 inhibitor SB203580; more importantly, this result reinforces the idea that IL-6 is an important direct target of NF-κB in myogenesis.
Figure 11.
Exogenous IL-6 promotes myoblast differentiation. (A) Addition of IL-6 increases the expression of muscle differentiation-specific genes. C2C12 cell were cultured in DMEM for 24 h in the absence or presence of recombinant murine IL-6. Myogenin, skeletal α-actin and GAPDH mRNA expression was analyzed by RT-PCR. (B) Ectopic overexpression of IL-6 increases MCK-dependent transcription. C2C12 myoblasts were transiently transfected with an MCK promoter-reporter plasmid, together with an expression plasmid for murine IL-6 (1:1 and 1:2 ratio MCK-Luc/IL-6), and cultured in DMEM for 36 h. Luciferase activities are expressed relative to the activity of pMCK-Luc transfected with an empty vector, which was given a value of 1. Error bars represent the SE of the mean value. (C) Effect of exogenous IL-6 on the negative action of p38 and NF-κB inhibitors on myogenin expression. C2C12 cells were cultured in DMEM or DM for 24 h, containing or not the p38 inhibitor SB203580 or the NF-κB-inhibitor BAY11-7085, and in the absence or presence of recombinant IL-6. Myogenin and GAPDH expression was analyzed by RT-PCR.
DISCUSSION
In this study, we demonstrate that p38 MAPK and NF-κB signaling pathways are induced during skeletal muscle differentiation, being both pathways required for completion of the myogenic program. We provide several lines of evidence indicating that p38 and NF-κB are elements of a common myogenic cascade: 1) activation of p38 MAPK during differentiation preceded in time the activation of NF-κB, 2) NF-κB activation was blocked by p38 MAPK inhibition, and 3) induction of differentiation by ectopic activation of p38 could be blocked by NF-κB inhibitors. Therefore, we propose a pathway by which NF-κB acts as a downstream effector of p38 MAPK to induce muscle differentiation. Previous reports had shown that p38 induced the phosphorylation of the myogenic coactivator MEF2 and increased MyoD-mediated transcription, resulting in promotion of myogenic differentiation. Here, we show that NF-κB, whose role in myogenesis has been considered controversial, is activated by p38 during differentiation, providing a novel mechanism for the promyogenic effect of this MAPK. We propose that the positive effect of p38/NF-κB on myoblast differentiation involves the activation of NF-κB-dependent genes, such as IL-6, constituting a functional consequence of the p38-mediated activation of NF-κB during myogenesis.
Requirement of p38 MAPK and NF-κB Activities for Myoblast Differentiation: NF-κB, an Effector of p38 MAPK
Our results show that NF-κB is activated during myoblast proliferation, in a p38-independent manner; NF-κB activity is low during early differentiation, increasing at later stages through a p38-dependent mechanism. We further show that inhibition of p38 activity abrogated both myoblast fusion and differentiation, confirming the requirement of p38 for completion of the myogenic program, as previously reported by other groups (Cuenda and Cohen, 1999; Zetser et al., 1999; Wu et al., 2000). The involvement of the NF-κB pathway in skeletal muscle differentiation has remained for some time controversial, because some reports have proposed an inhibitory role on differentiation, whereas others proposed a positive regulatory function (Guttridge et al., 1999; Kaliman et al., 1999; Canicio et al., 2001). Here, we demonstrate, by using inhibitors of the NF-κB pathway, that the activation of NF-κB is necessary for myogenic differentiation. These apparently conflicting results may be unified. We propose that NF-κB activity may be subject to biphasic regulation and may have a dual function during myogenesis. This hypothesis is supported by results from Guttridge et al. (1999), showing that NF-κB activity is elevated in proliferating C2C12 myoblasts leading to the transcriptional induction of the cyclin D1 gene. The decline in NF-κB activity observed in our study by switching the cells from high to low serum may facilitate a reduction in cyclin D1 levels at early stages, thereby allowing cell cycle withdrawal. Once cells have exited permanently from the cell cycle, the stimulation of p38 and NF-κB may induce the expression of myogenic markers such as α-actin. NF-κB will no longer be repressive and may collaborate with p38 in promoting differentiation (see below).
Similar stimuli had been shown to activate p38 and NF-κB pathways in different cell systems, although their interaction remained controversial. In particular, their interaction in differentiation of skeletal myoblasts had never been investigated. Here, we demonstrate that activation of NF-κB during muscle differentiation is dependent on functional p38, because treatment of myoblasts with SB203580 abrogated activation of NF-κB in differentiation promoting conditions (DM). Moreover, overexpression of MKK6-activated p38 in conditions that do not favor differentiation, such as serum-free medium, was able to induce myogenic differentiation as well as NF-κB activity, whereas specific inhibition of NF-κB abrogated the p38 promyogenic effect. Together, these results indicate that both p38 and NF-κB signaling pathways are required for myogenesis, being NF-κB a downstream effector of p38 action during myogenic differentiation. p38 had been shown to control the initiation of myogenic differentiation by increasing the activity of MEF2 and MyoD, through direct and indirect mechanisms, respectively (Zetser et al., 1999; Wu et al., 2000). We propose that p38 may also contribute to myogenic differentiation by inducing the activity of the nonmuscle-specific transcription factor NF-κB.
The requirement of p38 and/or NF-κB for cell differentiation is not exclusive of skeletal myoblasts. Activation of p38 MAPK has been shown to be required for several other differentiation systems: nerve growth factor-induced PC12 cell differentiation (Iwasaki et al., 1999; Pugazhenthi et al., 1999), erythropoietin-mediated cell formation (Nagata et al., 1998; Nagata and Todokoro, 1999), and the conversion of 3T3L fibroblasts into adipocytes (Wang and Ron, 1996; Engelman et al., 1998). A role for NF-κB in regulating cellular differentiation has also been demonstrated. For instance, mice with deletions of both p50 and p52 subunits exhibited defects in osteoclast maturation (Franzoso et al., 1997; Iotsova et al., 1997); also, the induction of c-Rel expression and DNA binding activity in mature B cells implicates this NF-κB subunit in the development of hematopoietic cells (Grumont and Gerondakis, 1994); moreover, the inhibition of NF-κB activity in keratinocytes, using a similar IκBα transdominant mutant to that used in the present study, prevented the maturation process of these cells (Seitz et al., 1998). These data, in conjunction with our results, suggest that p38 and NF-κB function in multiple tissues to regulate their differentiation.
Induction of IL-6 Gene Expression during Muscle Differentiation: a Target of p38/NF-κB Signaling Pathway
The precise mechanism by which NF-κB regulates differentiation of skeletal myoblasts has yet to be determined. As a transcription factor, NF-κB may regulate muscle-specific gene expression directly. However, to our knowledge, no classical NF-κB binding sites have been described in the promoter regions of muscle specific genes, although NF-κB may bind to atypical binding sites in those promoters. Because NF-κB can induce synthesis of contractile proteins such as myosin heavy chain in terminally differentiated smooth muscle cells (Guttridge et al., 1999), it is possible that in skeletal myoblasts NF-κB may regulate the expression of myosin heavy chain and myosin light chain proteins, whose accumulation is involved in cell enlargement during differentiation (Chien et al., 1991). Moreover, NF-κB can induce hypertrophic growth in differentiating cardiomyocytes (Purcell et al., 2001) supporting a potential role for NF-κB in controlling enlargement of skeletal myocytes during differentiation and fusion. Another possibility is that activation of NF-κB may induce the expression of nonmuscle-specific genes whose protein products may contribute to efficient myogenesis. Our results show for the first time the induction of the IL-6 gene during differentiation of C2C12 myoblasts. Moreover, IL-6 expression was dependent on p38 and NF-κB activities, respectively, demonstrating that a functional consequence of p38-mediated NF-κB activation in muscle differentiation is the induction of the IL-6 gene. Furthermore, we demonstrated that ablation of IL-6 mRNA expression reduced the extent of myoblast differentiation; conversely, overexpression or addition of IL-6 augmented the expression of muscle-specific genes, supporting its promyogenic function. Furthermore, IL-6 was able to bypass significantly the abrogation of myogenic differentiation imposed by the inhibition NF-κB activity, but not of p38, in differentiating myocytes, suggesting that IL-6 is a direct target of NF-κB, and that p38 affects muscle differentiation by controlling multiple signaling pathways.
IL-6 belongs to the superfamily of trophic factors, including cardiotrophin (CT-1) and ciliary neurotrophic factor (Pennica et al., 1996), which foster the growth and survival of different cell types. In particular, IL-6 plays an important growth-promoting and antiapoptotic role in cardiac myocytes (Kanda et al., 1996; Mann, 1996; Gwechenberger et al., 1999; Saito et al., 1999; Craig et al., 2000). Interestingly, skeletal muscle is a major source of IL-6 and can be elevated by muscle injury, a process involving myofiber regeneration and formation and growth of new muscle fibers (Kami and Senba, 1998; Kami et al., 2000; Keller et al., 2001; Frost et al., 2002). Therefore, IL-6 may play a role in myofiber growth and/or survival in vivo. Preliminary experiments from our laboratory indicate that IL-6 overexpression may partially rescue the cell death effect observed in C2C12 cells after 48 h in serum-free DMEM (Baeza-Raja and Muñoz-Cánoves, unpublished data), suggesting that IL-6 may also be a cytoprotective agent during myogenesis. This effect, however, deserves further investigation. In summary, we propose that p38 and NF-κB regulate muscle differentiation, at least in part, via activation of NF-κB target genes such as IL-6. Interestingly, a recent study by Pavlath and coworkers has identified another cytokine, IL-4, as a myoblast recruitment factor during mammalian muscle growth (Horsley et al., 2003).
Dual Effect of p38 in the Regulation of NF-κB Activity during Myogenic Differentiation
We have demonstrated that p38 activity controls the activation of NF-κB during myoblast differentiation in a dual way: first, activated p38 induces the dissociation of IκBα from NF-κB, thereby allowing NF-κB nuclear translocation and DNA binding; and second, p38 induces the transactivating potential of nuclear p65, probably indirectly, via CBP/p300.
Our results show that overexpression of MKK6-activated p38 reduced IκBα levels and increased DNA-NF-κB binding activity in C2C12 cells cultured in DMEM, mimicking the effect of the DM. These results are in contrast with those of Vanden Berghe et al. (1998) showing that tumor necrosis factor-α-induced p38 activated NF-κB transcription independently of NF-κB nuclear accumulation and DNA binding mechanisms in L929 fibroblasts) and are in agreement with others showing that overexpression of p38 induced the cytoplasmic-nuclear translocation of p65 in cardiomyocytes (Craig et al., 2000). Furthermore, our results indicate that p38 not only induces the NF-κB-DNA binding activity in C2C12 differentiating myoblasts but also the transactivating activity of the p65 NF-κB subunit, as demonstrated by GAL4-p65(TAD) reporter assays. In agreement with this, p38 was shown to increase the transcriptional activation potential of p65/NF-κB in L929 cells, whereas Craig et al. (2000) were unable to demonstrate such transactivation of p65 in cardiac myocytes. These authors proposed an alternative cross talk at the level of MKK6 and IKKβ, adding more complexity to the mechanisms of interaction of p38 and NF-κB (Craig et al., 2000). These apparently contradictory results may be due to cell-specific differences as well as to the different stimuli or culture conditions used in these studies. It is well established that phosphorylation of p65 augments its ability to bind DNA (Naumann and Scheidereit, 1994) and is apparently required for its transcriptional activity (Schmitz et al., 1995). However, we failed to observe phosphorylation of the transactivating C-terminal half of p65 by p38, whereas p38 was able to phosphorylate ATF2 by using in vitro kinase assays (our unpublished data), in agreement with results from other groups (Wesselborg et al., 1997; Carter et al., 1999). The failure to phosphorylate p65 excludes the possibility that p38 directly modulates the transactivation potential of NF-κB; however, it remains possible that a kinase downstream in the p38 pathway, such as a member of the MK2 family, may phosphorylate NF-κB subunits.
The transcriptional activity of the p65 subunit has recently been connected with the coactivator/cointegrator proteins p300 and CBP (Gerritsen et al., 1997; Zhong et al., 1998). Extensive protein-protein interactions have been mapped between the N- and C-terminal regions of CBP/p300, and the C terminus of p65 (Zhong et al., 1998). Interestingly, because our results with the Gal4-p65 fusion proteins demonstrate an important role of the p65 TAD domain for p38 inducibility, the possible phosphorylation status of this domain in p65-CBP interaction may be of particular interest. Furthermore, the coactivator proteins CBP/p300 might be subjected themselves to phosphorylation control by p38, as has been described for S6 kinase pp90rsk and for cyclin-dependent kinases (Lee et al., 1998). Because RNA polymerase II is constitutively associated with CBP/p300 (Ohta et al., 1994), and because p38 has recently been shown to interact with RNA polymerase II (Alepuz et al., 2003), the interaction of the coactivator with NF-κB on specific promoter targets may efficiently recruit the polymerase complex (which may include p38 MAPK), to trigger subsequent NF-κB-dependent gene expression in differentiating myoblasts.
The described role of the p38 and NF-κB pathways in mediating myogenic differentiation might also have implications in therapeutic strategies aimed at stimulating the activation and differentiation of satellite cells after muscle injury or in muscle degenerative diseases. Recruitment of satellite cells followed by fusion into multinucleated myofibers is regulated by endocrine and/or paracrine growth factors, possibly one of them being IL-6, whose synthesis may be regulated by multiple signal transduction pathways including p38 and NF-κB. Therefore, the proper modulation of these pathways, and/or the expression of their target genes, can be used to stimulate muscle regeneration in patients with different myopathies.
Acknowledgments
We thank Drs. F. Ventura, A. Lin, H. Han, F. Mercurio, J. DiDonato, M. Karin, A. Baldwin, K. Walsh, P. Baeuerle, G. Evan, E. Olson, L. Schmitz, and G. Haegeman for generously providing us with various reagents. We are especially grateful to Drs. M. Jardí and M. Suelves for technical help, and to F. Miralles, F. Lluís and M. Parra for helpful comments throughout this work. This study was supported by Marató-TV3, SAF2001-0482, MDA3665, FIS01/1474, and PI021873.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-08-0585. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-08-0585.
References
- Alepuz, P.M., de Nadal, E., Zapater, M., Ammerer, G., and Posas, F. (2003). Osmostress-induced transcription by Hot1 depends on a Hog1-mediated recruitment of the RNA Pol II. EMBO J. 22, 2433-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeuerle, P.A., and Baltimore, D. (1996). NF-kB: ten years after. Cell 87, 13-20. [DOI] [PubMed] [Google Scholar]
- Bird, T.A., Schooley, K., Dower, S.K., Hagen, H., and Virca, G.D. (1997). Activation of nuclear transcription factor NF-kappaB by interleukin-1 is accompanied by casein kinase II-mediated phosphorylation of the p65 subunit. J. Biol. Chem. 272, 32606-32612. [DOI] [PubMed] [Google Scholar]
- Black, B.L., and Olson, E.N. (1998). Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu. Rev. Cell Dev. Biol. 14, 167-196. [DOI] [PubMed] [Google Scholar]
- Canicio, J., Ruiz-Lozano, P., Carrasco, M., Palacin, M., Chien, K., Zorzano, A., and Kaliman, P. (2001). Nuclear factor kappa B-inducing kinase and Ikappa B kinase-alpha signal skeletal muscle cell differentiation. J. Biol. Chem. 276, 20228-20233. [DOI] [PubMed] [Google Scholar]
- Carter, A.B., Knudtson, K.L., Monick, M.M., and Hunninghake, G.W. (1999). The p38 mitogen-activated protein kinase is required for NF-kappaB-dependent gene expression. The role of TATA-binding protein (TBP). J. Biol. Chem. 274, 30858-30863. [DOI] [PubMed] [Google Scholar]
- Chien, K.R., Knowlton, K.U., Zhu, H., and Chien, S. (1991). Regulation of cardiac gene expression during myocardial growth and hypertrophy: molecular studies of an adaptive physiologic response. FASEB J. 5, 3037-3046. [DOI] [PubMed] [Google Scholar]
- Conejo, R., Valverde, A.M., Benito, M., and Lorenzo, M. (2001). Insulin produces myogenesis in C2C12 myoblasts by induction of NF-kappaB and downregulation of AP-1 activities. J. Cell. Physiol. 186, 82-94. [DOI] [PubMed] [Google Scholar]
- Craig, R., Larkin, A., Mingo, A.M., Thuerauf, D.J., Andrews, C., McDonough, P.M., and Glembotski, C.C. (2000). p38 MAPK and NF-kappa B collaborate to induce interleukin-6 gene expression and release. Evidence for a cytoprotective autocrine signaling pathway in a cardiac myocyte model system. J. Biol. Chem. 275, 23814-23824. [DOI] [PubMed] [Google Scholar]
- Cuenda, A., and Cohen, P. (1999). Stress-activated protein kinase-2/p38 and a rapamycin-sensitive pathway are required for C2C12 myogenesis. J. Biol. Chem. 274, 4341-4346. [DOI] [PubMed] [Google Scholar]
- Davis, R.L., Cheng, P.F., Lassar, A.B., and Weintraub, H. (1990). The MyoD DNA binding domain contains a recognition code for muscle-specific gene activation. Cell 60, 733-746. [DOI] [PubMed] [Google Scholar]
- De Cesare, D., Vallone, D., Caracciolo, A., Sassone-Corsi, P., Nerlov, C., and Verde, P. (1995). Heterodimerization of c-Jun with ATF-2 and c-Fos is required for positive and negative regulation of the human urokinase enhancer. Oncogene 11, 365-376. [PubMed] [Google Scholar]
- Dickens, M., Rogers, J.S., Cavanagh, J., Raitano, A., Xia, Z., Halpern, J.R., Greenberg, M.E., Sawyers, C.L., and Davis, R.J. (1997). A cytoplasmic inhibitor of the JNK signal transduction pathway. Science 277, 693-696. [DOI] [PubMed] [Google Scholar]
- DiDonato, J.A., Hayakawa, M., Rothwarf, D.M., Zandi, E., and Karin, M. (1997). A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature 388, 548-554. [DOI] [PubMed] [Google Scholar]
- Engelman, J.A., Lisanti, M.P., and Scherer, P.E. (1998). Specific inhibitors of p38 mitogen-activated protein kinase block 3T3-L1 adipogenesis. J. Biol. Chem. 273, 32111-32120. [DOI] [PubMed] [Google Scholar]
- Franzoso, G., Carlson, L., Xing, L., Poljak, L., Shores, E.W., Brown, K.D., Leonardi, A., Tran, T., Boyce, B.F., and Siebenlist, U. (1997). Requirement for NF-kappaB in osteoclast and B-cell development. Genes Dev. 11, 3482-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost, R.A., Nystrom, G.J., and Lang, C.H. (2002). Lipopolysaccharide regulates proinflammatory cytokine expression in mouse myoblasts and skeletal muscle. Am. J. Physiol. 283, R698-R709. [DOI] [PubMed] [Google Scholar]
- Gerritsen, M.E., Williams, A.J., Neish, A.S., Moore, S., Shi, Y., and Collins, T. (1997). CREB-binding protein/p300 are transcriptional coactivators of p65. Proc. Natl. Acad. Sci. USA 94, 2927-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh, S., and Karin, M. (2002). Missing pieces in the NF-kappaB puzzle. Cell 109, S81-96. [DOI] [PubMed] [Google Scholar]
- Grumont, R.J., and Gerondakis, S. (1994). The subunit composition of NF-kappa B complexes changes during B-cell development. Cell Growth Differ. 5, 1321-1331. [PubMed] [Google Scholar]
- Guo, K., and Walsh, K. (1997). Inhibition of myogenesis by multiple cyclin-Cdk complexes. Coordinate regulation of myogenesis and cell cycle activity at the level of E2F. J. Biol. Chem. 272, 791-797. [DOI] [PubMed] [Google Scholar]
- Guttridge, D.C., Albanese, C., Reuther, J.Y., Pestell, R.G., and Baldwin, A.S., Jr. (1999). NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol. Cell. Biol. 19, 5785-5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwechenberger, M., Mendoza, L.H., Youker, K.A., Frangogiannis, N.G., Smith, C.W., Michael, L.H., and Entman, M.L. (1999). Cardiac myocytes produce interleukin-6 in culture and in viable border zone of reperfused infarctions. Circulation 99, 546-551. [DOI] [PubMed] [Google Scholar]
- Han, J., Jiang, Y., Li, Z., Kravchenko, V.V., and Ulevitch, R.J. (1997). Activation of the transcription factor MEF2C by the MAP kinase p38 in inflammation. Nature 386, 296-299. [DOI] [PubMed] [Google Scholar]
- Han, J., Lee, J.-D., Jiang, Y., Li, Z., Feng, L., and Ulevitch, R.J. (1996). Characterization of the structure and function of a novel MAP kinase kinase (MKK6). J. Biol. Chem. 271, 2886-2891. [DOI] [PubMed] [Google Scholar]
- Horsley, V., Jansen, K.M., Mills, S.T., and Pavlath, G.K. (2003). IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell 113, 483-494. [DOI] [PubMed] [Google Scholar]
- Iotsova, V., Caamano, J., Loy, J., Yang, Y., Lewin, A., and Bravo, R. (1997). Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nat. Med. 3, 1285-1289. [DOI] [PubMed] [Google Scholar]
- Iwasaki, S., Iguchi, M., Watanabe, K., Hoshino, R., Tsujimoto, M., and Kohno, M. (1999). Specific activation of the p38 mitogen-activated protein kinase signaling pathway and induction of neurite outgrowth in PC12 cells by bone morphogenetic protein-2. J. Biol. Chem. 274, 26503-26510. [DOI] [PubMed] [Google Scholar]
- Kaliman, P., Canicio, J., Testar, X., Palacin, M., and Zorzano, A. (1999). Insulin-like growth factor-II, phosphatidylinositol 3-kinase, nuclear factor-kappaB and inducible nitric-oxide synthase define a common myogenic signaling pathway. J. Biol. Chem. 274, 17437-17444. [DOI] [PubMed] [Google Scholar]
- Kami, K., Morikawa, Y., Sekimoto, M., and Senba, E. (2000). Gene expression of receptors for IL-6, LIF, and CNTF in regenerating skeletal muscles. J. Histochem. Cytochem. 48, 1203-1213. [DOI] [PubMed] [Google Scholar]
- Kami, K., and Senba, E. (1998). Localization of leukemia inhibitory factor and interleukin-6 messenger ribonucleic acids in regenerating rat skeletal muscle. Muscle Nerve. 21, 819-822. [DOI] [PubMed] [Google Scholar]
- Kanda, T., Sakamoto, H., McManus, B.M., Sakamaki, T., Nagai, R., Suzuki, T., and Kobayashi, I. (1996). Interleukin-6 secreted from human myxoma reduces murine viral myocarditis. Life Sci. 58, 1705-1712. [DOI] [PubMed] [Google Scholar]
- Keller, C., Steensberg, A., Pilegaard, H., Osada, T., Saltin, B., Pedersen, B.K., and Neufer, P.D. (2001). Transcriptional activation of the IL-6 gene in human contracting skeletal muscle: influence of muscle glycogen content. FASEB J. 15, 2748-2750. [DOI] [PubMed] [Google Scholar]
- Lassar, A., and Munsterberg, A. (1994). Wiring diagrams: regulatory circuits and the control of skeletal myogenesis. Curr. Opin. Cell Biol. 6, 432-442. [DOI] [PubMed] [Google Scholar]
- Lee, C.W., Sorensen, T.S., Shikama, N., and La Thangue, N.B. (1998). Functional interplay between p53 and E2F through co-activator p300. Oncogene 16, 2695-2710. [DOI] [PubMed] [Google Scholar]
- Lee, F.S., Hagler, J., Chen, Z.J., and Maniatis, T. (1997). Activation of the IkappaB alpha kinase complex by MEKK1, a kinase of the JNK pathway. Cell 88, 213-222. [DOI] [PubMed] [Google Scholar]
- Madrid, L.V., Mayo, M.W., Reuther, J.Y., and Baldwin, A.S., Jr. (2001). Akt stimulates the transactivation potential of the RelA/p65 Subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38. J. Biol. Chem. 276, 18934-18940. [DOI] [PubMed] [Google Scholar]
- Mann, D.L. (1996). Stress activated cytokines and the heart. Cytokine Growth Factor Rev. 7, 341-354. [DOI] [PubMed] [Google Scholar]
- Mercurio, F., et al. (1997). IKK-1 and IKK-2, cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science 278, 860-866. [DOI] [PubMed] [Google Scholar]
- Miralles, F., Parra, M., Caelles, C., Nagamine, Y., Felez, J., and Munoz-Canoves, P. (1998). UV irradiation induces the murine urokinase-type plasminogen activator gene via the c-Jun N-terminal kinase signaling pathway: requirement of an AP1 enhancer element. Mol. Cell. Biol. 18, 4537-4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata, Y., Takahashi, N., Davis, R.J., and Todokoro, K. (1998). Activation of p38 MAP kinase and JNK but not ERK is required for erythropoietin-induced erythroid differentiation. Blood 92, 1859-1869. [PubMed] [Google Scholar]
- Nagata, Y., and Todokoro, K. (1999). Requirement of activation of JNK and p38 for environmental stress-induced erythroid differentiation and apoptosis and of inhibition of ERK for apoptosis. Blood 94, 853-863. [PubMed] [Google Scholar]
- Naumann, M., and Scheidereit, C. (1994). Activation of NF-kappa B in vivo is regulated by multiple phosphorylations. EMBO J. 13, 4597-4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta, H., Yatomi, Y., Sweeney, E.A., Hakomori, S., and Igarashi, Y. (1994). A possible role of sphingosine in induction of apoptosis by tumor necrosis factor-alpha in human neutrophils. FEBS Lett. 355, 267-270. [DOI] [PubMed] [Google Scholar]
- Olson, E.N., and Klein, W.H. (1994). bHLH factors in muscle development: dead lines and commitments, what to leave in and what to leave out. Genes Dev. 8, 1-8. [DOI] [PubMed] [Google Scholar]
- Pahl, H.L., and Baeuerle, P.A. (1995). A novel signal transduction pathway from the endoplasmic reticulum to the nucleus is mediated by transcription factor NF-kappa B. EMBO J. 14, 2580-2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennica, D., Wood, W.I., and Chien, K.R. (1996). Cardiotrophin-1, a multi-functional cytokine that signals via LIF receptor-gp 130 dependent pathways. Cytokine. Growth Factor. Rev. 7, 81-91. [DOI] [PubMed] [Google Scholar]
- Pugazhenthi, S., Boras, T., O'Connor, D., Meintzer, M.K., Heidenreich, K.A., and Reusch, J.E. (1999). Insulin-like growth factor I-mediated activation of the transcription factor cAMP response element-binding protein in PC12 cells. Involvement of p38 mitogen-activated protein kinase-mediated pathway. J. Biol. Chem. 274, 2829-2837. [DOI] [PubMed] [Google Scholar]
- Purcell, N.H., Tang, G., Yu, C., Mercurio, F., DiDonato, J.A., and Lin, A. (2001). Activation of NF-kappa B is required for hypertrophic growth of primary rat neonatal ventricular cardiomyocytes. Proc. Natl. Acad. Sci. USA 98, 6668-6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri, P.L., and Sartorelli, V. (2000). Regulation of muscle regulatory factors by DNA-binding, interacting proteins, and post-transcriptional modifications. J. Cell. Physiol. 185, 155-173. [DOI] [PubMed] [Google Scholar]
- Regnier, C.H., Song, H.Y., Gao, X., Goeddel, D.V., Cao, Z., and Rothe, M. (1997). Identification and characterization of an IkB kinase. Cell 90, 373-383. [DOI] [PubMed] [Google Scholar]
- Saito, S., Aikawa, R., Shiojima, I., Nagai, R., Yazaki, Y., and Komuro, I. (1999). Endothelin-1 induces expression of fetal genes through the interleukin-6 family of cytokines in cardiac myocytes. FEBS Lett. 456, 103-107. [DOI] [PubMed] [Google Scholar]
- Schmitz, M.L., dos Santos Silva, M.A., and Baeuerle, P.A. (1995). Transactivation domain 2 (TA2) of p65 NF-kappa B. Similarity to TA1 and phorbol ester-stimulated activity and phosphorylation in intact cells. J. Biol. Chem. 270, 15576-15584. [DOI] [PubMed] [Google Scholar]
- Seitz, C.S., Lin, Q., Deng, H., and Khavari, P.A. (1998). Alterations in NF-kappaB function in transgenic epithelial tissue demonstrate a growth inhibitory role for NF-kappaB. Proc. Natl. Acad. Sci. USA 95, 2307-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizemore, N., Leung, S., and Stark, G.R. (1999). Activation of phosphatidylinositol 3-kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-kappaB p65/RelA subunit. Mol. Cell. Biol. 19, 4798-4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapscott, S.J., Davis, R.L., Thayer, M.J., Cheng, P.F., Weintraub, H., and Lassar, A.B. (1988). MyoD 1, a nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts. Science 242, 405-411. [DOI] [PubMed] [Google Scholar]
- Vanden Berghe, W., Plaisance, S., Boone, E., De Bosscher, K., Schmitz, M.L., Fiers, W., and Haegeman, G. (1998). p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-kappaB p65 transactivation mediated by tumor necrosis factor. J. Biol. Chem. 273, 3285-3290. [DOI] [PubMed] [Google Scholar]
- Vermeulen, L., De Wilde, G., Van Damme, P., Vanden Berghe, W., and Haegeman, G. (2003). Transcriptional activation of the NF-kappaB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1). EMBO J. 22, 1313-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, D., and Baldwin, A.S., Jr. (1998). Activation of nuclear factor-kappaB-dependent transcription by tumor necrosis factor-alpha is mediated through phosphorylation of RelA/p65 on serine 529. J. Biol. Chem. 273, 29411-29416. [DOI] [PubMed] [Google Scholar]
- Wang, J., and Walsh, K. (1996). Resistance to apoptosis conferred by Cdk inhibitors during myocyte differentiation. Science 273, 359-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X.Z., and Ron, D. (1996). Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP kinase. Science 272, 1347-1348. [DOI] [PubMed] [Google Scholar]
- Wesselborg, S., Bauer, M.K., Vogt, M., Schmitz, M.L., and Schulze-Osthoff, K. (1997). Activation of transcription factor NF-kappaB and p38 mitogen-activated protein kinase is mediated by distinct and separate stress effector pathways. J. Biol. Chem. 272, 12422-12429. [DOI] [PubMed] [Google Scholar]
- Wu, Z., Woodring, P., Bhakta, K., Tamura, K., Wen, F., Feramisco, J.R., Karin, M., Wang, J.Y., and Puri, P.L. (2000). p38 and extracellular signal-regulated kinases regulate the myogenic program at multiple steps. Mol. Cell. Biol. 20, 3951-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandi, E., Rothwarf, D.M., Delhase, M., Hayakawa, M., and Karin, M. (1997). The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell 91, 243-252. [DOI] [PubMed] [Google Scholar]
- Zetser, A., Gredinger, E., and Bengal, E. (1999). p38 mitogen-activated protein kinase pathway promotes skeletal muscle differentiation. Participation of the Mef2c transcription factor. J. Biol. Chem. 274, 5193-5200. [DOI] [PubMed] [Google Scholar]
- Zhao, M., New, L., Kravchenko, V.V., Kato, Y., Gram, H., Di Padova, F., Olson, E.N., Ulevitch, R.J., and Han, J. (1999). Regulation of the MEF2 family of transcription factors by p38. Mol. Cell Biol. 19, 21-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, H., May, M.J., Jimi, E., and Ghosh, S. (2002). The phosphorylation status of nuclear NF-kappa B determines its association with CBP/p300 or HDAC-1. Mol. Cell 9, 625-636. [DOI] [PubMed] [Google Scholar]
- Zhong, H., Voll, R.E., and Ghosh, S. (1998). Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol. Cell 1, 661-671. [DOI] [PubMed] [Google Scholar]