Abstract
Protein targeting is essential for domain specialization in polarized cells. In photoreceptors, three distinct membrane domains exist in the outer segment: plasma membrane, disk lamella, and disk rim. Peripherin/retinal degeneration slow (rds) and rom-1 are photoreceptor-specific members of the transmembrane 4 superfamily of transmembrane proteins, which participate in disk morphogenesis and localize to rod outer segment (ROS) disk rims. We examined the role of their C termini in targeting by generating transgenic Xenopus laevis expressing green fluorescent protein (GFP) fusion proteins. A GFP fusion containing residues 317-336 of peripherin/rds localized uniformly to disk membranes. A longer fusion (residues 307-346) also localized to the ROS but exhibited higher affinity for disk rims than disk lamella. In contrast, the rom-1 C terminus did not promote ROS localization. The GFP-peripherin/rds fusion proteins did not immunoprecipitate with peripherin/rds or rom-1, suggesting this region does not form intermolecular interactions and is not involved in subunit assembly. Presence of GFP-peripherin/rds fusions correlated with disrupted incisures, disordered ROS tips, and membrane whorls. These abnormalities may reflect competition of the fusion proteins for other proteins that interact with peripherin/rds. This work describes novel roles for the C terminus of peripherin/rds in targeting and maintaining ROS structure and its potential involvement in inherited retinal degenerations.
INTRODUCTION
Although numerous membrane proteins have sequences that target them to intracellular compartments (e.g., endoplasmic reticulum, lysosomes, and nuclei), the molecular interactions governing the distribution of proteins to the plasma membrane of polarized cells are less clearly defined because of the diversity of unique proteins and membrane domains. Our group is particularly interested in the mechanisms governing the targeting of proteins to the specialized functional domains of rod photoreceptors. Rods are highly polarized and intricately organized neuronal cells, which reside in the outer retina. Perturbations of their complex cellular architecture lead to retinal degeneration (RD) by triggering apoptosis (Chang et al., 1993; Papermaster and Nir, 1994). It is therefore important to study how these cells create and maintain their unique morphology. Like other neurons, rods possess a synaptic terminal and cell body (named the inner segment or RIS). They also possess a unique organelle, called the outer segment (ROS), which is attached to the RIS by a nonmotile (9 + 0) connecting cilium. The ROS consists of a large stack of flattened membranous disks ensheathed within a plasma membrane (Figure 1A) and is the site of light capture and signal transduction. The distal ROS tips are shed daily, requiring concomitant synthesis and addition of new disks to the base of the ROS (Young and Droz, 1968).
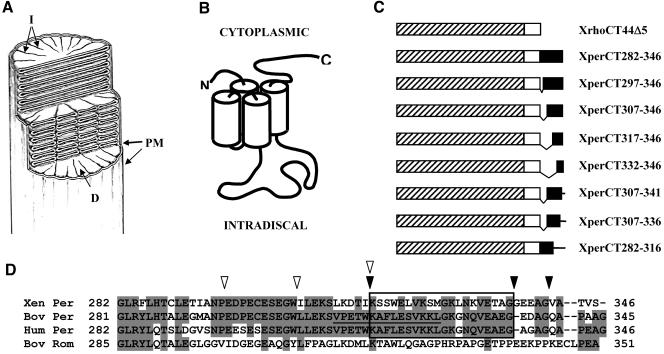
Figure 1.
Peripherin/rds, rom-1, and the GFP fusion proteins. (A) Diagram depicting ROS structure. A cut-away view of a rod outer segment showing the organization of the plasma membrane (PM), disks (D), and incisures (I), reproduced from The Journal of Cell Biology, 1978, 78, 415-425, by copyright permission of The Rockefeller University Press. (B) Diagram of the membrane topology of peripherin/rds and rom-1, members of the TM4 superfamily. (C) Diagram of the GFP fusion proteins. GFP, hatched bar; membrane-association domain derived from the X. laevis rhodopsin C terminus, white bar; and X. laevis peripherin/rds C terminus, black bar. (D) Sequence alignments of the cytoplasmic C terminus of X. laevis, bovine, and human peripherin/rds and bovine rom-1. The region identified as containing the outer segment (OS) targeting signal is boxed. The peptide associated with membrane fusion activity is underlined. Shading indicates identical residues. N-terminal truncations are indicated by white arrowheads and C-terminal truncations by black arrowheads.
Nascent disks begin as evaginations of the plasma membrane but eventually seal and separate from it. Mature disks have two distinct domains, the lamellar surfaces and the highly curved rim. Rod disks also have clefts called incisures that give the disks a scalloped or lobed appearance when viewed transversely (Cohen, 1960, 1961, 1963; Wald et al., 1962; Nilsson, 1965; Dunn, 1966; Pedler and Tilley, 1967; Kroll and Machemer, 1968). Incisure structure varies somewhat with species, and the reason for the variations are not known. Frog and human rods have multiple incisures, whereas in other species there is only a single incisure. The depth of the incisures also varies; in frogs they are deep, whereas in human rods they are shallow. Hundreds of adjacent disks are oriented such that their incisures are longitudinally aligned. The details of this process of disk morphogenesis (reviewed by Boesze-Battaglia and Goldberg, 2002) are not understood.
Proteins destined for the ROS are further compartmentalized into distinct subdomains during disk morphogenesis (Molday, 1998), reflecting a highly ordered system of protein targeting. Rhodopsin, the protein responsible for light capture, is present at high concentrations in the disk lamella but also at low concentrations in the ROS plasma membrane (Papermaster et al., 1978b; Molday and Molday, 1987). In contrast, peripherin/retinal degeneration slow (rds) (also known as rds/peripherin and peripherin-2) (Molday et al., 1987; Arikawa et al., 1992), rom-1 (Bascom et al., 1992; Moritz and Molday, 1996), and ABCR (Papermaster et al., 1978a; Azarian and Travis, 1997; Illing et al., 1997) reside only at disk rims. Still other proteins such as the Na/Ca exchanger (Reid et al., 1990) and the cGMP-gated channel (Cook et al., 1989) reside exclusively in the ROS plasma membrane. ROS proteins are synthesized in the cell body, delivered to the apical surface of the RIS, inserted into the plasma membrane, and transported along the connecting cilium to nascent disk membranes (Young and Droz, 1968; Peters et al., 1983; Papermaster et al., 1985; Papermaster, 2002). Before disk closure, disk proteins are segregated from those destined for the ROS plasma membrane. The distribution of these proteins may be a consequence of unique peptide sequences which confer “addresses” to the proteins and direct them to the correct membrane domains.
Peripherin/rds and rom-1 are transmembrane proteins known to be involved in disk morphogenesis and maintenance of ROS structure but their specific functions have not been elucidated. They share 35% homology with each other and are members of the tetraspanin or transmembrane 4 superfamily (TM4SF). Both proteins have the characteristic topology of a tetraspanin (Connell et al., 1991); their N and C termini face the cytoplasm and they have a large extracellular loop (the D2 loop) joining the third and fourth transmembrane regions (Figure 1B). The D2 loop is highly conserved among the TM4SF members and is a hot spot for mutations causing retinitis pigmentosa (RP). Peripherin/rds and rom-1 interact with themselves and each other via their D2 loops to form noncovalent core homo and heterotetramers (Goldberg and Molday, 1996; Loewen and Molday, 2000; Loewen et al., 2001). Disulfide bond formation between tetramers results in octamers and higher order complexes. In a proposed model of disk morphogenesis (Loewen and Molday, 2000), tetrameric peripherin/rds-rom-1 complexes on opposing membranes of a nascent disk interact via disulfide bonds to create the curved disk rim. Alternatively, disk rims may be formed by lateral oligomerization of tetramers (Loewen and Molday, 2000; Boesze-Battaglia and Goldberg, 2002). A functional role has also been ascribed to the C terminus of peripherin/rds. A peptide analogue of this domain inhibited peripherin/rds-mediated fusion of ROS plasma membrane vesicles with disk membranes but promoted membrane fusion and content mixing in a pure vesicle system, suggesting involvement of the C terminus in membrane fusion during disk shedding and/or morphogenesis (Boesze-Battaglia et al., 1998).
Despite their similarities and interactive nature, peripherin/rds and rom-1 do not play equivalent roles. In the rds mouse, the peripherin/rds gene is disrupted resulting in total ablation of ROS formation (Sanyal and Jansen, 1981; Travis et al., 1991). In contrast, rom-1 knockout mice exhibited a less severe phenotype, initially forming ROS, albeit disordered (Clarke et al., 2000). Also, although many peripherin/rds mutations are associated with inherited human RDs, a causal link with rom-1 mutations has only been established in digenic cases involving both a rom-1 null allele and a peripherin/rds missense mutation (Kajiwara et al., 1994). Furthermore, rom-1 may not exist in all species. In Xenopus laevis, a peripherin/rds ortholog, xrds38, was identified based on sequence identity and conservation of key amino acids, yet no rom-1 counterpart was found (Kedzierski et al., 1996). Two other peripherin-like molecules were also identified (xrds35 and xrds36) but are more distantly related. Like mammalian peripherin/rds, xrds35, xrds36, and xrds38 also localize exclusively to disk rims and incisures (Kedzierski et al., 1996; Loewen et al., 2003).
The highly restricted distribution of peripherin/rds and rom-1 suggests that their localization and function are intimately associated. However, little is known about how either protein is targeted to the ROS or why they are constrained to the disk rims. We have previously shown that the last eight amino acids of the C terminus of rhodopsin is both necessary and sufficient for the localization of rhodopsin to ROS (Tam et al., 2000; Moritz et al., 2001). In this study, we investigated whether the C terminus of peripherin/rds and rom-1 promote ROS targeting. We expressed GFP-peripherin/rds and GFP-rom-1 fusion proteins in transgenic X. laevis rod photoreceptors and examined their distribution and their effects on ROS structure.
MATERIALS AND METHODS
Molecular Biology
All X. laevis expression constructs are based on peGFP-C1 (BD Biosciences Clontech, Palo Alto, CA), which was modified to contain the proximal X. laevis opsin promoter (XOP1.3GFP-C1) as described previously (Tam et al., 2000). X. laevis peripherin/rds DNA sequences were amplified by polymerase chain reaction (PCR) from genomic DNA. Bovine rom-1 and bovine peripherin/rds DNA sequences were amplified by PCR from cDNA (gift of Dr. R. Molday, University of British Columbia, Vancouver, BC, Canada). Sense oligonucleotides incorporated X. laevis rhodopsin sequences so that they could be cloned in frame into the HindIII site within the rhodopsin sequence of XOP1.3GFP-CT44del5 (Tam et al., 2000). Antisense oligonucleotides introduced a unique BamHI restriction site downstream of the stop codon for cloning into the multiple cloning site. The final constructs were verified by DNA sequencing. The resulting plasmids encoded GFP, followed by the C terminus of X. laevis rhodopsin lacking the terminal five amino acids, followed by various regions of peripherin/rds or rom-1. Expression vectors were linearized by digestion with NotI and purified using the GeneClean kit (Q-BIOgene, Carlsbad, CA). Restriction endonucleases were obtained from Invitrogen (Carlsbad, CA) and New England Biolabs (Beverly, MA).
pcPer1D4 (encoding bovine peripherin/rds) and pcROM (encoding bovine rom-1) used for cell culture transfections have been described previously (Moritz and Molday, 1996; Loewen et al., 2001) and were a gift of Dr. R. Molday. For expression of XrhoCT44Δ5 and BperCT in cell culture, the cDNAs were ligated into the XhoI and BamHI sites of peGFP-C1 (BD Biosciences Clontech).
Transgenesis, GFP Screening, and Tadpole Rearing
Transgenic tadpoles were generated using a modified protocol (Moritz et al., 1999) based on that of Kroll and Amaya (1996). X. laevis sperm nuclei were incubated with 0.3 × high speed egg extract, 0.05 U of restriction enzyme, and 100-200 ng of linearized vector DNA in a total volume of 18 μl. After 10 min, the reaction mixture was diluted to 0.3 nuclei/nl and 10 nl was injected per egg. The resulting embryos were reared in 0.1 × Marc's modified Ringer containing 6% Ficoll and 50 μg/ml gentamicin for 48 h and then transferred to 0.1 × Gerhart's Ringer solution (Wu and Gerhart, 1991). At 5-6 d postfertilization, roughly corresponding to stages 40-42 (Nieuwkoop and Faber, 1994), tadpoles were screened for GFP expression by using a Leica MZ8 dissecting microscope equipped with epifluorescence optics and a GFP filter set (Kramer, Valley Cottage, NY). Tadpoles expressing GFP were identified by green fluorescence emitted from their eyes. All animals were raised at 18°C on a 12:12-h light cycle (7:00 AM to 7:00 PM). Wild-type adult X. laevis were obtained from Nasco (Fort Atkinson, WI).
Immuno-electron Microscopy (EM)
Transgenic tadpoles were sacrificed at 14 d postfertilization (stage 47/48) and fixed in 4% paraformaldehyde, 0.1 M sodium phosphate buffer, pH 7.5. Eyes were excised and embedded in LR White or LR Gold resins (Ted Pella Inc., Redding, CA). Thin sections were labeled with a rabbit anti-GFP polyclonal antibody (Abcam, Cambridge, United Kingdom) diluted 1:1000 in 1% goat serum, 0.1 M Tris, pH 7.4, followed by incubation with an anti-rabbit secondary antibody conjugated to 10-nm colloidal gold (Amersham Biosciences, Piscataway, NJ) diluted 1:5 in 0.1 M Tris, pH 8.2, 1% goat serum. Images were obtained on a Philips CM10 transmission electron microscope.
Immunocytochemistry and Confocal Microscopy
Stage 48-62 transgenic tadpoles were sacrificed between 4:00 and 7:00 PM (i.e., near the end of the light cycle) and fixed in 4% paraformaldehyde, O.1 M sodium phosphate buffer, pH 7.5. Fixed eyes were embedded in OCT tissue embedding medium (Ted Pella Inc., Redding, CA) and cryosectioned. Frozen sections (14 μM) were stained with Texas Red-conjugated wheat germ agglutinin (Molecular Probes, Eugene, OR) and Hoescht 33342 (Sigma-Aldrich) to label Golgi/post-Golgi membranes and nuclei, respectively, as described previously (Tam et al., 2000). Labeled sections were mounted in Mowiol (Calbiochem, San Diego, CA).
A Zeiss 510 scanning confocal microscope, equipped with Plan-Apochromat 100× oil, numerical aperture 1.4 and C-Apochromat 40× W Korr, numerical aperture 1.2 objectives, and Zeiss LSM software were used to acquire images of labeled sections. At least three transgenic animals were examined by confocal microscopy for each construct.
Due to technical considerations of confocal microscopy, different criteria were required for capturing images depicting RIS versus ROS localization (Figures 2, 3, and 4) than for those depicting disk lamella versus incisure localization (Figure 5). Because the ROS is extremely membrane dense, we often saturated the outer segment fluorescent signal to better capture inner segment detail (i.e., the absence of fluorescence in the RIS plasma membrane or the presence of fluorescence in the Golgi membranes). In contrast, when distinguishing between disk lamellar and disk rim localization, outer segment fluorescence was imaged at subsaturating levels.
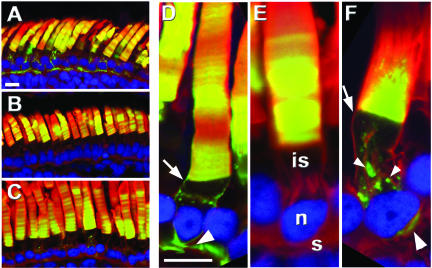
Figure 2.
The C terminus of peripherin/rds contains an ROS localization signal. Confocal micrographs of transgenic retinas expressing XrhoCT44Δ5 (A and D) or XperCT282-346 (B, C, E, and F) (green). XrhoCT44Δ5 was found in all post-Golgi membranes, including the ROS disks, RIS plasma membrane (arrow), and synapse (large arrowhead). XperCT282-346 localized primarily to the ROS (E) but in more highly expressing rods (F), it also occurred in puncta (small arrowhead) adjacent to the Golgi and the synapse. XperCT282-346 was not seen in the plasma membrane. Hoescht 33342 (blue), Texas Red-wheat germ agglutinin (red), and GFP fluorescence (green). Outer segment (os), inner segment (is), nucleus (n), synapse (s). Bars, 10 μm.
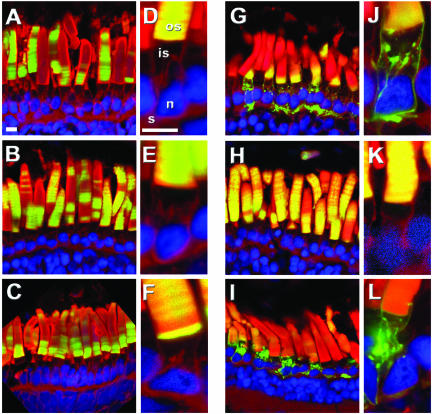
Figure 3.
The peripherin/rds ROS targeting signal is located between residues 317 and 336. Panels show confocal micrographs of transgenic retinas expressing GFP fused with truncated forms of the peripherin/rds C terminus. XperCT297-346 (A and D), XperCT307-346 (B and E), XperCT317-346 (C and F), XperCT332-346 (G and J), XperCT307-336 (H and K), or XperCT282-316 (I and L). XperCT297-346, XperCT307-346, XperCT317-346, XperCT 307-336 all localized exclusively to the ROS. XperCT332-346 and XperCT282-316 were present in the ROS but also in the RIS plasma membrane, Golgi, and synapse. Hoescht 33342 (blue), Texas red-wheat germ agglutinin (red), GFP fluorescence (green). Outer segment (os), inner segment (is), nucleus (n), synapse (s). Bars, 10 μm.
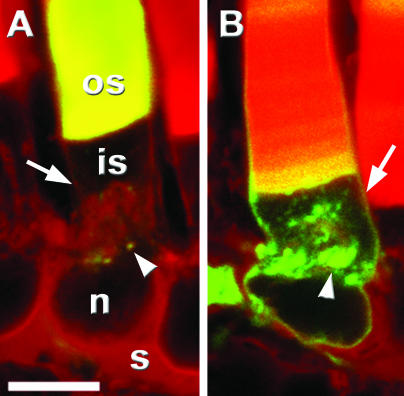
Figure 4.
The C terminus of bovine rom-1 does not promote ROS localization in X. laevis. Confocal micrographs of transgenic retinas expressing BperCT (A) and BromCT (B). BperCT (green) localized primarily to the ROS but also at low levels in puncta (arrowhead) adjacent to the Golgi. In contrast, BromCT distributed throughout the ROS, RIS plasma membrane (arrow), Golgi, and synapse. Texas Red-wheat germ agglutinin (red) and GFP fluorescence (green). Outer segment (os), inner segment (is), nucleus (n), synapse (s). Bar, 10 μm.
Figure 5.
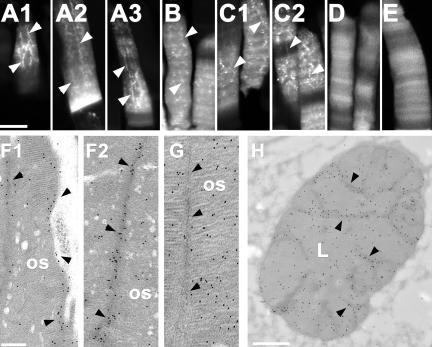
XperCT282-346, XperCT297-346, and XperCT307-346 are more concentrated at the disk rims/incisures than in the disk lamella. Confocal (A-E) and EM (F-H) micrographs of transgenic rod photoreceptors. XperCT282-346 (A), XperCT297-346 (B), and XperCT307-346 (C) were found throughout the ROS, but exhibited greater affinity for incisures (arrowheads) as indicated by intense longitudinal stripes of fluorescence superimposed over the fainter fluorescence in the disk lamella. In contrast, XrhoCT44Δ5 (D) and XperCT317-346 (E) showed no such affinity. EM micrographs of plastic embedded transgenic ROS were labeled with anti-GFP antibodies and gold-conjugated secondary antibodies. Sections perpendicular to (F and G) and in the plane of (H) the disks are shown. In rods expressing XperCT282-346 (F and H), disk rims/incisures (arrowheads) were more heavily decorated with gold particles than the lamellar regions (L). In rods expressing XrhoCT44Δ5 (G), gold particles were distributed randomly throughout the ROS. Bars, 10 μm (A-E), 0.5 μm (F and G), and 2 μm (H).
Cell Culture and Immunoprecipitation
COS-7 cells were transiently transfected with various plasmids, either singly or in combination, by using calcium phosphate (Chen and Okayama, 1987). The plasmids used for transfections encoded bovine peripherin/rds (BperFL), bovine rom-1 (BromFL), X. laevis rhodopsin C terminus fused to GFP (XrhoCT44Δ5), bovine peripherin/rds C terminus fused to Xrho CT44Δ5 (BperCT), or empty vector. BperCT contained a mutation (Q341P) that abolishes the binding of anti-bovine peripherin/rds antibody, monoclonal antibody (mAb) 2B6 (Loewen et al., 2001). Cells were harvested 48 h after transfection and lysed with phosphate-buffered saline (PBS) containing 1% Triton X-100. Cell lysates were immunoprecipitated with 2B6 (Molday et al., 1987) conjugated to Sepharose beads. The beads were washed with PBS 1% Triton X-100, and the bound proteins were eluted with a peptide (gift of Dr. R. Molday) corresponding to the 2B6 epitope (1 μg/ml in PBS 1% Triton X-100) (Connell et al., 1991). Cell lysates and immunoprecipitates were electrophoresed on SDS polyacrylamide gels and transferred to nylon membranes. Western blots were probed with 2B6, anti-GFP (Zymed Laboratories, South San Francisco), and anti-rom-1 1C6 mAb (gift of Dr. R. Molday) and anti-mouse secondary antibody conjugated to horseradish peroxidase (Amersham Biosciences). Western blots were developed using ECL substrate and Hyperfilm ECL film (Amersham Biosciences).
RESULTS
The C Terminus of X. laevis Peripherin/rds Contains a ROS Localization Signal
Like rhodopsin, peripherin/rds and rom-1 possess long cytoplasmic C-terminal tails and are specifically localized to the ROS. Previously, we showed that the 44-amino acid cytoplasmic tail of rhodopsin contains an ROS localization signal within its last eight amino acids (Tam et al., 2000). We therefore investigated whether peripherin/rds and rom-1 also contain C-terminal ROS targeting signals.
GFP-CT44del5 is a previously characterized membrane bound carrier molecule in which the cytoplasmic C terminus of rhodopsin lacking the last five amino acids is fused to GFP (Tam et al., 2000). It is membrane associated via two palmitoyl groups and, when expressed in rods, nonspecifically localizes throughout ROS and RIS membranes (Figure 2, A and D). To avoid confusion in this article, we have renamed this GFP fusion protein XrhoCT44Δ5. To identify peripherin/rds sequences containing targeting information, we fused regions of the cytoplasmic tail of X. laevis peripherin/rds (xrds38) to XrhoCT44Δ5 (Figure 1C). The GFP-peripherin/rds fusion proteins were expressed in X. laevis rod photoreceptors, and their subcellular distributions were analyzed by confocal microscopy of transgenic retinal sections.
We observed two different distribution patterns when we fused the 65-amino acid cytoplasmic C terminus of peripherin/rds to XrhoCT44Δ5 (XperCT282-346). At low expression levels, XperCT282-346 was detected only in the ROS (Figure 2, B and E), whereas at higher expression levels it was also present in discrete puncta within the RIS and at low levels in the synaptic terminal, but was conspicuously absent from the RIS plasma membrane (Figure 2, C and F). When fusion proteins containing only the C-terminal 50, 40, and 30 amino acids of peripherin/rds (XperCT297-346, XperCT307-346, and XperCT317-346, respectively) were expressed in rods, the fusion proteins were present almost exclusively in the ROS (Figure 3, A-F), even at very high expression levels. However, when only the C-terminal 15 amino acids of peripherin/rds were fused (XperCT332-346), polarized distribution was lost (Figure 3, G and J). XperCT332-346 was present in the ROS but also in the RIS plasma membrane, Golgi apparatus and synaptic terminal. This localization pattern is essentially identical to that of XrhoCT44Δ5. Truncations were subsequently made from the extreme C terminus toward the juxtamembrane region. When peptides corresponding to amino acids 307-336 were fused to XrhoCT44Δ5 (XperCT307-336), the fusion protein trafficked exclusively to the ROS (Figure 3, H and K). Identical results were obtained from a similar fusion (XperCT307-341), which incorporated amino acids 307-341 of peripherin/rds (our unpublished data). Finally, expression of a GFP fusion protein that lacked the terminal 30 amino acids (XperCT282-316) resulted in nonspecific localization (Figure 3, I and L). Together, these results indicate that peripherin/rds contains a ROS localization signal located near but not at the distal C terminus, between amino acids 317-336.
We did not make transgenic X. laevis expressing a full-length peripherin/rds in which amino acids 317-336 were deleted. Such a deletion mutant could still oligomerize (via its intradiscal D2 loop) with endogenous wild-type peripherin/rds that contain the ROS targeting signal. In this way, a peripherin/rds deletion mutant lacking its ROS targeting signal could still be cotransported to the ROS. Thus, the results of such an experiment would be uninterpretable.
The C Terminus of Bovine rom-1 Does Not Function as an ROS Localization Signal in X. laevis Rods
Peripherin/rds and rom-1 are similar in structure and sequence, but they do not play equivalent roles in ROS viability. To determine whether the C terminus of rom-1 also contains an ROS localization signal, we used bovine rom-1 because X. laevis does not have a rom-1 homologue (Kedzierski et al., 1996). We first ascertained whether the C terminus of bovine peripherin/rds also has an ROS localization signal and whether it is recognized by X. laevis rods. We analyzed frozen sections of transgenic retinas expressing BperCT, a fusion protein analogous to XperCT282-346 but containing bovine peripherin/rds sequences. BperCT localized almost exclusively in the ROS (Figure 4A). Minor amounts of protein were seen as faint puncta in the RIS and at the synaptic terminal when expression levels were high. These results are almost identical to those of XperCT282-346. Thus, X. laevis rods were able to recognize and properly sort the bovine peripherin/rds ROS targeting signal. However, when the C terminus of rom-1 was fused to GFP (BromCT), the fusion protein was distributed throughout the ROS, RIS plasma membrane, Golgi, and synaptic terminal at all expression levels (Figure 4B), indicating the absence of an ROS localization signal in this domain.
The C Terminal of Peripherin/rds targets GFP Fusion Proteins to the Disk Rim via a Distinct Domain
To test whether the peripherin/rds C-terminal region also contributes to disk rim and incisure localization, we analyzed the ROS of fixed transgenic retinas by confocal microscopy and immunoelectron microscopy. Frog rod photoreceptors have multiple deep incisures that are longitudinally aligned (Figure 1A). In confocal micrographs of cryosections and EM micrographs of antibody-labeled plastic sections, XperCT282-346, 297-346, and 307-346 exhibited the longitudinally striped ROS labeling pattern similar to that of disk rim proteins (e.g., peripherin/rds and ABCR), indicating increased affinity for the disk rims/incisures (Figure 5, A-C, F1, F2, and H). However, these fusion proteins were also found in the disk lamella and colocalization with incisures was not complete (i.e., colocalization was not seen in all ROS nor in all incisures within a single ROS). In contrast, neither XrhoCT44Δ5 (Figure 5, D and G) nor XperCT317-346 (Figure 5E) showed any affinity for disk rims/incisures. These combined results indicate that a peptide, minimally involving amino acids 307-317, exhibits affinity for incisures, possibly through interaction with other disk rim components. Moreover, affinity for rims/incisures and ROS localization are distinct properties because ROS localization can be uncoupled from incisure localization (c.f. XperCT307-346 to XperCT317-346).
XperCT Does Not Form Stable Interactions with Full-Length Peripherin/rds
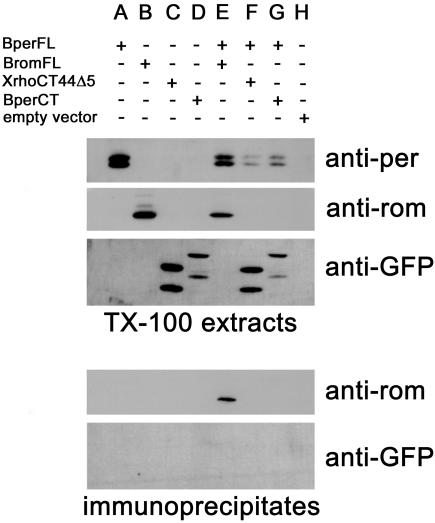
Peripherin/rds-peripherin/rds and peripherin/rds-rom-1 interactions similar to those present in ROS can be replicated in heterologous expression systems. When coexpressed in COS cells, homo- and heterotetrameric complexes can be immunoprecipitated with antibodies to either subunit (Goldberg et al., 1995). To test whether the XperCT fusion proteins trafficked to the ROS or showed enhanced affinity for the disk rim because of an unidentified peripherin/rds-peripherin/rds interaction involving the C terminus, we performed transfection and immunoprecipitation experiments. COS cells coexpressing BperCT and BperFL were lysed with Triton X-100. An anti-bovine peripherin/rds mAb (2B6) was used to immunoprecipitate BperFL from the soluble fraction. BperCT contained a point mutation (Q341P) that abolishes 2B6 binding and thus is not recognized by 2B6. Because proline is normally present at residue 341 in murine peripherin/rds, the amino acid change is unlikely to disrupt any conserved function. When analyzed by Western blot, the immunoprecipitate did not contain detectable levels of BperCT (Figure 6). However, in control experiments rom-1 coprecipitated with BperFL, as expected. Identical results were obtained when XperCT282-346 was coexpressed with BperFL (our unpublished data). These results indicate that the C terminus of peripherin/rds does not form stable interactions with itself or any other region of peripherin/rds.
Figure 6.
The C terminus of peripherin/rds does not form stable interactions with full-length peripherin/rds. COS cells were transfected with plasmids encoding full-length bovine peripherin/rds (BperFL) (A), full-length bovine rom-1 (BromFL) (B), XrhoCT44Δ5 (C), bovine peripherin/rds C terminus (BperCT) (D), vector alone (H) or cotransfected with BperFL and BromFL (E), BperFL and XrhoCT44Δ5 (F), or BperFL and BperCT (G). BperFL was immunoprecipitated from solubilized COS cell membranes with an anti-Bper antibody (2B6). Western blots of cell lysates (top) and immunoprecipitates (bottom) were probed with 2B6, an anti-GFP antibody, or an anti-rom-1 antibody (1C6). BromFL coprecipitated with BperFL (E), but BperCT did not (G).
The GFP-XperCT Fusion Proteins Disrupt ROS Structure
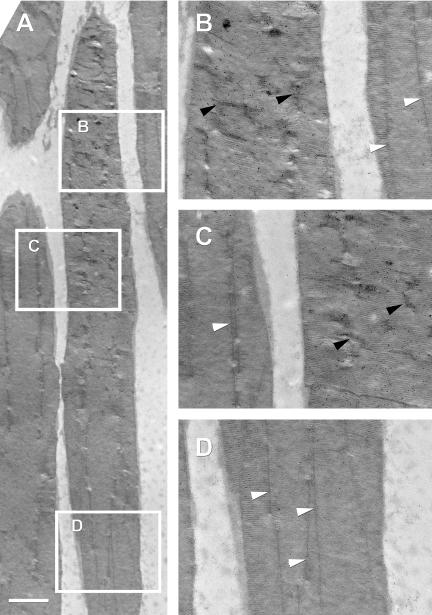
To determine whether the expression of XperCT fusion proteins had any effect on disk or overall ROS structure in transgenic tadpoles, we examined thin sections of plastic embedded retinas by electron microscopy. Detection of the fusion protein with anti-GFP antibodies revealed that at high concentrations, XperCT297-346 (Figure 7) and XperCT307-346 (our unpublished data) caused X. laevis rod incisures to look disordered. In areas of dense gold label, incisures were short and tortuous compared with regions with little or no anti-GFP label, where the incisures were long and straight. These results parallel those obtained by confocal microscopy where XperCT297-346 and XperCT307-346 fluorescence exhibited an irregular “zig-zag” appearance in the ROS.
Figure 7.
XperCT fusion proteins induce loss of incisure organization. EM micrograph of the outer segments of rod photoreceptors expressing XperCT297-346 (A). Thin sections were labeled with anti-GFP and a secondary antibody conjugated to 10-nm gold particles. The boxed regions are magnified to show detail. In areas of high gold label (B and C), incisures looked short and tortuous (black arrowheads). In contrast, incisures in areas of low or no gold label (B-D) were comparatively long and straight (white arrowheads). Bar, 2 μm.
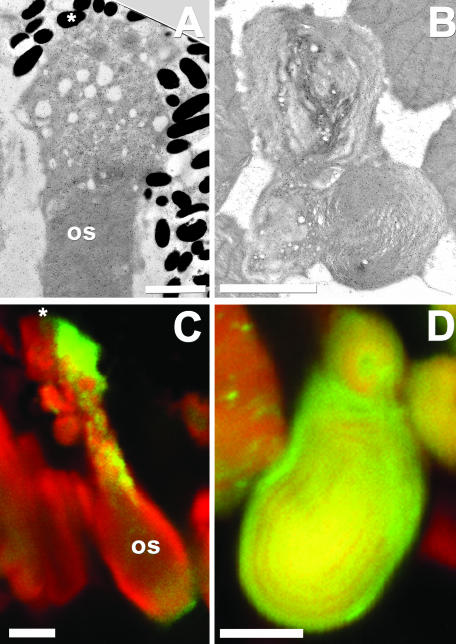
At the distal tips (adjacent to the retinal pigment epithelium) of rods that expressed XperCT282-346 or XperCT297-346, the disks were often highly disordered and appeared vesiculated (Figure 8, A and C). Whorls of disk membranes were also present in transgenic retinas (Figure 8, B and D). These gross structural abnormalities occurred only when accompanied by high levels of fusion proteins and were not observed in control retinas.
Figure 8.
The XperCT fusion proteins disrupt outer segment (OS) structure. EM and confocal micrographs of rods expressing XperCT282-346 (A and C) and XperCT297-346 (B and D). Distal ROS tips (adjacent to the retinal pigment epithelium, asterisks) containing high levels of fusion protein were extremely disordered (A and C). Dysplastic ROS membranes in the form of whorls (B and D) were also observed and correlated with the presence of high levels of fusion protein. Texas Red-wheat germ agglutinin (red), GFP fluorescence (green). Bars, 2.5 μm (A) and 5 μm (B-D).
DISCUSSION
Inherited retinal degenerative diseases often result from alterations in photoreceptor specific proteins such as peripherin/rds. To understand the pathogenic mechanism of such mutations, we sought to understand the structure and function of the affected gene product. In this study, we have identified two functional domains within the C terminus of peripherin/rds: a ROS localization signal and an incisure affinity sequence. The corresponding regions of rom-1 did not contain these functional domains. Localization of the XperCT fusion proteins to the ROS and incisures was not a result of interactions with peripherin/rds. Rather, our results suggest an interaction between the C terminus of peripherin/rds and another disk rim component. Finally, we provide evidence for a role for peripherin/rds in incisure organization.
Our discovery of a ROS targeting signal in the C terminus of peripherin/rds constitutes only the second such signal identified thus far. We have previously identified a ROS targeting signal in the cytoplasmic C terminus of rhodopsin (Tam et al., 2000). Cytoplasmic sorting signals may be a general ROS targeting mechanism in photoreceptors as is seen in polarized epithelial cells, where basolateral sorting signals are found on the cytoplasmic faces of proteins (Gu et al., 2001). Our results provide a potential explanation for the observation of Kedzierski et al. (1999) that a crucial functional domain must exist outside of the D2 loop of peripherin/rds and that this domain does not exist in rom-1. They showed that in transgenic mice, rom/D2 (a chimeric protein in which the D2 loop of rom-1 is replaced with the D2 loop of peripherin/rds) does not rescue the rds (per-/-) phenotype. Peripherin/rds' sorting signal constitutes a second functional domain located outside the D2 loop and absent from rom-1. It should be noted that the putative fusogenic role of the peripherin/rds C terminus also fulfills these criteria (Boesze-Battaglia and Gretzula, 2002). Strikingly, the ROS localization signal of peripherin/rds overlaps significantly with the region identified as having membrane fusion activity (Figure 1D). Although this region is proposed to participate in disk morphogenesis or shedding (Boesze-Battaglia et al., 1998), it is possible that the fusion activity is instead involved in targeting, for example by directing fusion of post-Golgi vesicles containing peripherin/rds with a specific RIS target membrane domain (e.g., the base of the connecting cilium). However, further refinement of the ROS localization signal may simply resolve these as two overlapping functional domains.
Although the C terminus of peripherin/rds functions as an autonomous ROS localization signal when directing membrane-bound GFP to the ROS, export of multimeric peripherin/rds complexes is subject to additional control mechanisms. Loewen et al. (2003) have recently shown that peripherin/rds containing the mutation C214S is unable to form tetramers and is retained in the RIS as homodimers despite having an intact C-terminal ROS targeting signal. Thus, rods seem to possess a quality control mechanism by which only properly assembled tetramers are allowed to exit the RIS (Loewen et al., 2003). Possibly, improper subunit assembly results in the exposure of peptide sequences, causing incomplete complexes to be retained in the RIS.
Given its overall similarity to peripherin/rds, it is interesting that the rom-1 C-terminal did not promote ROS localization in our assay. Because rom-1 primarily exists in the ROS as multimeric complexes with peripherin/rds, it is possible to rely solely on its interaction with peripherin/rds for targeting. A small fraction of rom-1 has been reported to exist as homotetramers in the ROS (Loewen and Molday, 2000), but these studies do not address how rom-1 homotetramers arrive at the ROS. The presence of a protein-disulfide isomerase in disks (Loewen and Molday, 2000) suggests that rom-1 homotetramers might be formed after transport to the disks by reduction of peripherin/rds-containing octameric complexes. The lack of a rom-1 targeting signal may provide a mechanism for regulating peripherin/rds:rom-1 stoichiometry (i.e., by restricting rom-1 access to the ROS in the absence of peripherin/rds). It may also explain the absolute requirement for peripherin/rds but not rom-1 for ROS formation. It would be interesting to examine whether a chimeric rom-1 molecule that included the peripherin/rds ROS localization signal could partially rescue the rds phenotype. We cannot exclude the possibility that X. laevis rods may not have recognized the bovine rom-1 ROS localization signal or that a signal exists outside of the C terminus.
The existence of a unique peripherin/rds ROS targeting signal suggests that the trafficking pathway of peripherin/rds is different from that of rhodopsin. In X. laevis rhodopsin, the ROS localization signal resides in the terminal eight amino acids SSSQVSPA (Tam et al., 2000). Comparison of this region across species produces the consensus sequence QVA/SPA. The penultimate P is crucial, because mutations to T, A, S, Q, L, or R are all linked to human autosomal dominant RP (Dryja et al., 1990; Gal et al., 1991; Rodriguez et al., 1994; Vaithinathan et al., 1994; Macke et al., 1995). Amino acids 317-336 of peripherin/rds do not contain this consensus sequence and therefore represent a second and distinct ROS targeting signal. The C terminus of rom-1 did not promote ROS localization and not surprisingly, this region shows little sequence identity to either peripherin/rds or rhodopsin. These results do not reveal where, in the trafficking pathway, peripherin/rds and rhodopsin segregate (i.e., before or after reaching the ROS). Studies by Fariss et al. (1997) support the hypothesis of separate RIS transport vesicles. In detached cat retinas, peripherin/rds accumulated in intracellular vesicles, whereas rhodopsin was transported to the plasma membrane. Furthermore, when we labeled plastic thin sections of retinas expressing XperCT282-346 with anti-GFP and anti-opsin antibodies, we detected GFP fusion protein in discrete puncta in the Golgi, separate from the labeling of rhodopsin (our unpublished data). This also suggests that peripherin/rds and rhodopsin segregate in the Golgi.
The lower prevalence of dominant RD-causing mutations in the ROS targeting signal of peripherin/rds compared with rhodopsin may reflect different mechanisms of toxicity. Studies in primary retinal cell cultures indicate that the presence of rhodopsin in the RIS plasma membrane may cause cell death by activating a normally inaccessible G protein-coupled cascade (Alfinito and Townes-Anderson, 2002). In transgenic rats, mislocalization of a mutant rhodopsin (Q334ter) causes rod death when expressed at only 10% of endogenous rhodopsin (Green et al., 2000). Thus, mutations in a single rhodopsin allele may cause autosomal dominant disease via a deleterious gain of function. Insufficiency of functional peripherin/rds in the ROS (rather than abnormal presence in the RIS) has been proposed to cause rod death (Kedzierski et al., 2001). Because peripherin/rds minimally forms tetramers before transport to the ROS in photoreceptors (Loewen et al., 2003), we predict that mutations in its ROS localization signal are recessive. In a heterozygote, peripherin/rds molecules lacking the localization signal can tetramerize via the D2 loop with wild-type peripherin/rds containing an intact localization signal and be cotransported to the ROS as part of a complex. Given the 2.5-fold excess of peripherin/rds over rom-1 (Kedzierski et al., 1999), ∼78% of tetramers would contain a peripherin/rds ROS targeting signal. If octamers are formed before transport, the percentage would be even greater. Because the critical threshold of combined peripherin/rds and rom-1 for photoreceptor viability was determined to be ∼60% (Kedzierski et al., 2001), insufficiency of peripherin/rds would only occur if both alleles were mutated. Mutations causing deletions of the C terminus at residues 285 and 316 have been reported in two cases of human RP (Felbor et al., 1997; Kohl et al., 1997). However, these pedigrees are too small to establish conclusively whether these mutations are causative for RP. Loss of the putative fusogenic activity of peripherin/rds may contribute in these particular RP cases (Muller-Weeks et al., 2002). Further experiments will be required to determine whether the presence of mislocalized peripherin/rds in the RIS (as opposed to deficiency in the ROS) also contributes to RD.
The incisure localization of the GFP-XperCT fusion proteins as well as the disruption of incisure organization observed in this study indicates that one function of peripherin/rds is to stabilize and/or align incisures. Incisure disruption may reflect competition of the fusion proteins for the normal interaction partners of peripherin/rds. What are these interaction partners? Endogenous peripherin/rds is not a likely candidate, because the XperCT fusion proteins did not interact with full-length peripherin/rds complexes in coimmunoprecipitation assays. We cannot, however, rule out the possibility that the XperCT fusion proteins were interacting with two other peripherin/rds-like molecules that exist in X. laevis, xrds35 and xrds36 (Kedzierski et al., 1996). Xrds35 and xrds36 are more distantly related to mammalian peripherin/rds than X. laevis peripherin/rds (xrds38). Xrds35, xrds36, and xrds38 all form homodimers and are all part of a larger oligomer within X. laevis rod outer segment disks. Although nothing is known about the nature of the interactions between xrds35, xrds 36, and xrds 38, the residues identified in tetramerization of mammalian peripherin/rds (all of which reside in the D2 loop) are absolutely conserved among them. Thus, it is probable that xrds35, 36, and 38 also interact via their D2 loops and not their C termini. Moreover, xrds 35 and 36 are expressed only in rods, not in cone photoreceptors. The localization of xrds 38 to cone outer segments, therefore, cannot be dependent on xrds 35 or xrds 36 and so it is unlikely that xrds38 requires xrds35 or xrds36 for localization in rods.
Peripherin/rds has been shown to interact with glutamic acid rich proteins (GARPs) but the domains of peripherin/rds that account for this binding have not yet been identified (Poetsch et al., 2001). Three GARPs exist in the ROS: the β-subunit of the cGMP-gated channel located in the plasma membrane (Colville and Molday, 1996) and two soluble splice variants (GARP-1 and GARP-2). GARP-1 and GARP-2 are localized to the rim region of disks (Korschen et al., 1999) presumably via interactions with other rim proteins. Four repeats of a proline rich domain (motifs associated with protein-protein interactions) are common to all three forms of GARP, making them potentially multivalent. The XperCT fusion proteins may colocalize with incisures by interacting with GARP as part of a larger multiprotein complex. This same interaction could also explain the observed disruption of incisures. Tethering of the disks to the plasma membrane, via an interaction between peripherin/rds and the GARP domain of the β-subunit of the cGMP channel, is one means of restricting disk rotation and maintaining incisure alignment. Adjacent disks might also associate through peripherin/rds-GARP-peripherin/rds interactions to maintain their position relative to each other. Either or both of these mechanisms could be disrupted by high concentrations of XperCT fusion proteins. XperCT282-346 was not as disruptive to incisure alignment (c.f. Figure 5A to 5B, and 5C and c.f. Figure 5F, Figure 6, Figure 7). The reasons for this difference are not clear; possibly, the additional upstream sequence contains another interaction domain, allowing XperCT282-346 to mimic more closely the endogenous peripherin/rds C terminus.
High levels of XperCT fusion proteins resulted in severe structural disorder of distal rod tips as well as whorls of ROS disk membranes, similar to those reported in rds+/- mice (Hawkins et al., 1985). It has been proposed that the whorls result from insufficiency of peripherin/rds resulting in grossly enlarged disks (Kedzierski et al., 1997). The membrane whorls seen in this study may mimic peripherin/rds insufficiency if the XperCT fusion proteins competitively inhibited interactions (such as membrane fusion) involved in disk morphogenesis. Similarly, membrane fusion may have been disrupted during shedding resulting in dysmorphic ROS tips. Alternatively, these disordered membrane structures may reflect the disruption of other interactions required for maintenance of ROS structure. A small percentage of transgenic tadpoles (<5%) exhibited severe RD. However, because of the inconsistent expression levels throughout the retina, and even within individual rods, caused by frequent transgene silencing (Moritz et al., 2001), it was difficult to demonstrate a dose dependent relationship between expression levels and RD.
Finally, our results demonstrate that the signals governing trafficking of photoreceptor membrane proteins may behave differently in other cell types. Truncation of the last 10 amino acids from the C terminus of peripherin/rds did not affect the targeting of the GFP-peripherin/rds fusion proteins to the ROS (i.e., XperCT307-336). However, truncation of the same region results in loss of delivery of peripherin/rds to the plasma membrane in polarized Madin-Darby canine kidney cells (Stefano et al., 2002). This truncated peripherin/rds is retained in intracellular membranes, a distribution pattern similar to that of peripherin/rds expressed in non-polarized COS cells (Goldberg et al., 1995). Furthermore, although truncation of rhodopsin's ROS localization signal results in loss of ROS specificity in photoreceptors (Tam et al., 2000), it does not affect apical sorting of rhodopsin in MDCK cells (Chuang and Sung, 1998). These results highlight the importance of conducting protein targeting studies in appropriate cell types.
The mechanisms underlying the polarized distribution of membrane proteins in photoreceptors are largely uncharacterized, but their disruptions are associated with RD. In this study, we have begun to differentiate the pathways involved in the targeting of the TM4SF members peripherin/rds and rom-1 and the G protein-coupled receptor rhodopsin. Studies of peripherin/rds targeting, as well as the targeting of other photoreceptor proteins, will provide insights into the pathological mechanisms associated with disruptions in protein trafficking.
Acknowledgments
We thank Dr. R.S. Molday for the generous gifts of monoclonal antibodies 2B6, 1C6, and 2B6 peptide, and for plasmids containing the bovine peripherin/rds and rom-1 cDNAs. We also appreciate the work of Dr. L. B. Hurd (electron microscopy), Dr. J. Hewett (COS cell transfections), and T. Clarke and K. Biddle (animal care). This research was supported by National Institutes of Health grant EY-06891, an award from the Foundation Fighting Blindness, and endowment of a chair by John A. and Florence Mattern Solomon.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-09-0650. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-09-0650.
Abbreviations used: GARP, glutamic acid rich protein; rds, retinal degeneration slow; RIS, rod inner segment; ROS, rod outer segment; TM4SF, transmembrane 4 superfamily.
References
- Alfinito, P.D., and Townes-Anderson, E. (2002). Activation of mislocalized opsin kills rod cells: a novel mechanism for rod cell death in retinal disease. Proc. Natl. Acad. Sci. USA 99, 5655-5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arikawa, K., Molday, L.L., Molday, R.S., and Williams, D.S. (1992). Localization of peripherin/rds in the disk membranes of cone and rod photoreceptors: relationship to disk membrane morphogenesis and retinal degeneration. J. Cell Biol. 116, 659-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarian, S.M., and Travis, G.H. (1997). The photoreceptor rim protein is an ABC transporter encoded by the gene for recessive Stargardt's disease (ABCR). FEBS Lett. 409, 247-252. [DOI] [PubMed] [Google Scholar]
- Bascom, R.A., Manara, S., Collins, L., Molday, R.S., Kalnins, V.I., and McInnes, R.R. (1992). Cloning of the cDNA for a novel photoreceptor membrane protein (rom-1) identifies a disk rim protein family implicated in human retinopathies. Neuron 8, 1171-1184. [DOI] [PubMed] [Google Scholar]
- Boesze-Battaglia, K., and Goldberg, A.F. (2002). Photoreceptor renewal: a role for peripherin/rds. Int. Rev. Cytol. 217, 183-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia, K., and Gretzula, C. (2002). A role for rom-1 in peripherin/rds dependent membrane fusion processes. Investig. Ophthalmol. Vis. Sci. 44, E-abstract 4259.
- Boesze-Battaglia, K., Lamba, O.P., Napoli, A.A., Jr., Sinha, S., and Guo, Y. (1998). Fusion between retinal rod outer segment membranes and model membranes: a role for photoreceptor peripherin/rds. Biochemistry 37, 9477-9487. [DOI] [PubMed] [Google Scholar]
- Chang, G.Q., Hao, Y., and Wong, F. (1993). Apoptosis: final common pathway of photoreceptor death in rd, rds, and rhodopsin mutant mice. Neuron 11, 595-605. [DOI] [PubMed] [Google Scholar]
- Chen, C., and Okayama, H. (1987). High-efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell. Biol. 7, 2745-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang, J.Z., and Sung, C.H. (1998). The cytoplasmic tail of rhodopsin acts as a novel apical sorting signal in polarized MDCK cells. J. Cell Biol. 142, 1245-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, G., et al. (2000). Rom-1 is required for rod photoreceptor viability and the regulation of disk morphogenesis. Nat. Genet. 25, 67-73. [DOI] [PubMed] [Google Scholar]
- Cohen, A.I. (1960). The ultrastructure of the rods of the mouse retina. Am. J. Anat. 107, 23-48. [DOI] [PubMed] [Google Scholar]
- Cohen, A.I. (1961). Some preliminary electron microscopic observations of the outer receptor segments of the retina of the Macaca rhesus. In: Smelser, G.K., ed. Structure of the Eye. London: Academic Press; 151-158.
- Cohen, A.I. (1963). The fine structure of the visual receptors of the pigeon. Exp. Eye Res. 2, 88-87. [DOI] [PubMed] [Google Scholar]
- Colville, C.A., and Molday, R.S. (1996). Primary structure and expression of the human beta-subunit and related proteins of the rod photoreceptor cGMP-gated channel. J. Biol. Chem. 271, 32968-32974. [DOI] [PubMed] [Google Scholar]
- Connell, G., Bascom, R., Molday, L., Reid, D., McInnes, R.R., and Molday, R.S. (1991). Photoreceptor peripherin is the normal product of the gene responsible for retinal degeneration in the rds mouse. Proc. Natl. Acad. Sci. USA 88, 723-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, N.J., Molday, L.L., Reid, D., Kaupp, U.B., and Molday, R.S. (1989). The cGMP-gated channel of bovine rod photoreceptors is localized exclusively in the plasma membrane. J. Biol. Chem. 264, 6996-6999. [PubMed] [Google Scholar]
- Dryja, T.P., McGee, T.L., Hahn, L.B., Cowley, G.S., Olsson, J.E., Reichel, E., Sandberg, M.A., and Berson, E.L. (1990). Mutations within the rhodopsin gene in patients with autosomal dominant retinitis pigmentosa. N. Engl. J. Med. 323, 1302-1307. [DOI] [PubMed] [Google Scholar]
- Dunn, R.F. (1966). Studies on the retina of the Gecko Coleonyx variegatus I. The visual cell classification. J. Ultrastr. Res. 16, 651-671. [DOI] [PubMed] [Google Scholar]
- Fariss, R.N., Molday, R.S., Fisher, S.K., and Matsumoto, B. (1997). Evidence from normal and degenerating photoreceptors that two outer segment integral membrane proteins have separate transport pathways. J. Comp. Neurol. 387, 148-156. [DOI] [PubMed] [Google Scholar]
- Felbor, U., Schilling, H., and Weber, B.H. (1997). Adult vitelliform macular dystrophy is frequently associated with mutations in the peripherin/RDS gene. Hum. Mutat. 10, 301-309. [DOI] [PubMed] [Google Scholar]
- Gal, A., Artlich, A., Ludwig, M., Niemeyer, G., Olek, K., Schwinger, E., and Schinzel, A. (1991). Pro-347-Arg mutation of the rhodopsin gene in autosomal dominant retinitis pigmentosa. Genomics 11, 468-470. [DOI] [PubMed] [Google Scholar]
- Goldberg, A.F., and Molday, R.S. (1996). Subunit composition of the peripherin/rds-rom-1 disk rim complex from rod photoreceptors: hydrodynamic evidence for a tetrameric quaternary structure. Biochemistry 35, 6144-6149. [DOI] [PubMed] [Google Scholar]
- Goldberg, A.F., Moritz, O.L., and Molday, R.S. (1995). Heterologous expression of photoreceptor peripherin/rds and Rom-1 in COS-1 cells: assembly, interactions, and localization of multisubunit complexes. Biochemistry 34, 14213-14219. [DOI] [PubMed] [Google Scholar]
- Green, E.S., Menz, M.D., LaVail, M.M., and Flannery, J.G. (2000). Characterization of rhodopsin mis-sorting and constitutive activation in a transgenic rat model of retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci. 41, 1546-1553. [PubMed] [Google Scholar]
- Gu, F., Crump, C.M., and Thomas, G. (2001). Trans-Golgi network sorting. Cell Mol. Life Sci. 58, 1067-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins, R.K., Jansen, H.G., and Sanyal, S. (1985). Development and degeneration of retina in rds mutant mice: photoreceptor abnormalities in the heterozygotes. Exp. Eye Res. 41, 701-720. [DOI] [PubMed] [Google Scholar]
- Illing, M., Molday, L.L., and Molday, R.S. (1997). The 220-kDa rim protein of retinal rod outer segments is a member of the ABC transporter superfamily. J. Biol. Chem. 272, 10303-10310. [DOI] [PubMed] [Google Scholar]
- Kajiwara, K., Berson, E.L., and Dryja, T.P. (1994). Digenic retinitis pigmentosa due to mutations at the unlinked peripherin/RDS and ROM1 loci. Science 264, 1604-1608. [DOI] [PubMed] [Google Scholar]
- Kedzierski, W., Lloyd, M., Birch, D.G., Bok, D., and Travis, G.H. (1997). Generation and analysis of transgenic mice expressing P216L-substituted rds/peripherin in rod photoreceptors. Investig. Ophthalmol. Vis. Sci. 38, 498-509. [PubMed] [Google Scholar]
- Kedzierski, W., Moghrabi, W. N., Allen, A. C., Jablonski-Stiemke, M. M., Azarian, S. M., Bok, D., and Travis, G. H. (1996). Three homologs of rds/peripherin in Xenopus laevis photoreceptors that exhibit covalent and noncovalent interactions. J. Cell Sci. 109, 2551-2560. [DOI] [PubMed] [Google Scholar]
- Kedzierski, W., Nusinowitz, S., Birch, D., Clarke, G., McInnes, R.R., Bok, D., and Travis, G.H. (2001). Deficiency of rds/peripherin causes photoreceptor death in mouse models of digenic and dominant retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 98, 7718-7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedzierski, W., Weng, J., and Travis, G.H. (1999). Analysis of the rds/peripherin.rom1 complex in transgenic photoreceptors that express a chimeric protein. J. Biol. Chem. 274, 29181-29187. [DOI] [PubMed] [Google Scholar]
- Kohl, S., Christ-Adler, M., Apfelstedt-Sylla, E., Kellner, U., Eckstein, A., Zrenner, E., and Wissinger, B. (1997). RDS/peripherin gene mutations are frequent causes of central retinal dystrophies. J. Med. Genet. 34, 620-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korschen, H.G., Beyermann, M., Muller, F., Heck, M., Vantler, M., Koch, K.W., Kellner, R., Wolfrum, U., Bode, C., Hofmann, K.P., and Kaupp, U.B. (1999). Interaction of glutamic-acid-rich proteins with the cGMP signalling pathway in rod photoreceptors. Nature 400, 761-766. [DOI] [PubMed] [Google Scholar]
- Kroll, A.J., and Machemer, R. (1968). Experimental retina detachment in the owl monkey. III. Electron microscopy of retina and pigment epithelium. Am. J. Ophthal. 66, 410-427. [DOI] [PubMed] [Google Scholar]
- Kroll, K.L., and Amaya, E. (1996). Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development 122, 3173-3183. [DOI] [PubMed] [Google Scholar]
- Loewen, C.J., and Molday, R.S. (2000). Disulfide-mediated oligomerization of Peripherin/Rds and Rom-1 in photoreceptor disk membranes. Implications for photoreceptor outer segment morphogenesis and degeneration. J. Biol. Chem. 275, 5370-5378. [DOI] [PubMed] [Google Scholar]
- Loewen, C.J., Moritz, O.L., and Molday, R.S. (2001). Molecular characterization of peripherin-2 and rom-1 mutants responsible for digenic retinitis pigmentosa. J. Biol. Chem. 276, 22388-22396. [DOI] [PubMed] [Google Scholar]
- Loewen, C.J., Moritz, O.L., Tam, B.M., Papermaster, D.S., and Molday, R.S. (2003). The role of subunit assembly in peripherin-2 targeting to rod photoreceptor disk membranes and retinitis pigmentosa. Mol. Biol. Cell 14, 3400-3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macke, J.P., Hennessey, J.C., and Nathans, J. (1995). Rhodopsin mutation proline347-to-alanine in a family with autosomal dominant retinitis pigmentosa indicates an important role for proline at position 347. Hum. Mol. Genet. 4, 775-776. [DOI] [PubMed] [Google Scholar]
- Molday, R.S. (1998). Photoreceptor membrane proteins, phototransduction, and retinal degenerative diseases. The Friedenwald Lecture. Investig. Ophthalmol. Vis. Sci. 39, 2491-2513. [PubMed] [Google Scholar]
- Molday, R.S., Hicks, D., and Molday, L. (1987). Peripherin. A rim-specific membrane protein of rod outer segment discs. Investig. Ophthalmol. Vis. Sci. 28, 50-61. [PubMed] [Google Scholar]
- Molday, R.S., and Molday, L.L. (1987). Differences in the protein composition of bovine retinal rod outer segment disk and plasma membranes isolated by a ricin-gold-dextran density perturbation method. J. Cell Biol. 105, 2589-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz, O.L., and Molday, R.S. (1996). Molecular cloning, membrane topology, and localization of bovine rom-1 in rod and cone photoreceptor cells. Investig. Ophthalmol. Vis. Sci. 37, 352-362. [PubMed] [Google Scholar]
- Moritz, O.L., Tam, B.M., Knox, B.E., and Papermaster, D.S. (1999). Fluorescent photoreceptors of transgenic Xenopus laevis imaged in vivo by two microscopy techniques. Investig. Ophthalmol. Vis. Sci. 40, 3276-3280. [PubMed] [Google Scholar]
- Moritz, O.L., Tam, B.M., Papermaster, D.S., and Nakayama, T. (2001). A functional rhodopsin-green fluorescent protein fusion protein localizes correctly in transgenic Xenopus laevis retinal rods and is expressed in a time-dependent pattern. J. Biol. Chem. 276, 28242-28251. [DOI] [PubMed] [Google Scholar]
- Muller-Weeks, S., Boesze-Battaglia, K., and Fitzgerald, C. (2002). Deletional analysis of the rod photoreceptor cell peripherin/RDS carboxy-terminal region. Exp. Eye Res. 75, 143-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop, P.D., and Faber, J. (1994). Normal table of Xenopus laevis (Daudin): a systematical and chronological survey of the development from the fertilized egg till the end of metamorphosis, In: Nieuwkoop, P.D., and Faber, J., eds. Normal Table of Xenopuslaevis (Daudin). New York: Garland Publishers.
- Nilsson, S.E.G. (1965). The ultrastructure of the receptor outer segments in the retina of the leopard frog (Rana pipiens). J. Ultrastr. Res. 12, 207-231. [DOI] [PubMed] [Google Scholar]
- Papermaster, D.S. (2002). The birth and death of photoreceptors: the Friedenwald Lecture. Investig. Ophthalmol. Vis. Sci. 43, 1300-1309. [PubMed] [Google Scholar]
- Papermaster, D.S., and Nir, I. (1994). Apoptosis in inherited retinal degenerations. In: Apoptosis, ed. E. Mihich and R.H. Schimke, New York: Plenum Press, 15-30.
- Papermaster, D.S., Schneider, B.G., and Besharse, J.C. (1985). Vesicular transport of newly synthesized opsin from the Golgi apparatus toward the rod outer segment. Ultrastructural immunocytochemical and autoradiographic evidence in Xenopus retinas. Investig. Ophthalmol. Vis. Sci. 26, 1386-1404. [PubMed] [Google Scholar]
- Papermaster, D.S., Schneider, B.G., Zorn, M.A., and Kraehenbuhl, J.P. (1978a). Immunocytochemical localization of a large intrinsic membrane protein to the incisures and margins of frog rod outer segment disks. J. Cell Biol. 78, 415-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papermaster, D.S., Schneider, B.G., Zorn, M.A., and Kraehenbuhl, J.P. (1978b). Immunocytochemical localization of opsin in outer segments and Golgi zones of frog photoreceptor cells. An electron microscope analysis of cross-linked albumin-embedded retinas. J. Cell Biol. 77, 196-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedler, C., and Tilley, R. (1967). The fine structure of photoreceptor discs. Vision Res. 7, 829-836. [DOI] [PubMed] [Google Scholar]
- Peters, K.R., Palade, G.E., Schneider, B.G., and Papermaster, D.S. (1983). Fine structure of a periciliary ridge complex of frog retinal rod cells revealed by ultrahigh resolution scanning electron microscopy. J. Cell Biol. 96, 265-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poetsch, A., Molday, L.L., and Molday, R.S. (2001). The cGMP-gated channel and related glutamic acid-rich proteins interact with peripherin-2 at the rim region of rod photoreceptor disc membranes. J. Biol. Chem. 276, 48009-48016. [DOI] [PubMed] [Google Scholar]
- Reid, D.M., Friedel, U., Molday, R.S., and Cook, N.J. (1990). Identification of the sodium-calcium exchanger as the major ricin-binding glycoprotein of bovine rod outer segments and its localization to the plasma membrane. Biochemistry 29, 1601-1607. [DOI] [PubMed] [Google Scholar]
- Rodriguez, J.A., Gannon, A.M., Birch, D.G., Heckenlively, J.R., and Daiger, S.P. (1994). Screening for mutations in rhodopsin and peripherin/RDS in patients with autosomal dominant retinitis pigmentosa. Am. J. Hum. Genet. (suppl), A239.
- Sanyal, S., and Jansen, H.G. (1981). Absence of receptor outer segments in the retina of rds mutant mice. Neurosci. Lett. 21, 23-26. [DOI] [PubMed] [Google Scholar]
- Stefano, F.P., Krouse, J., Marta, P., and Boesze-Battaglia, K. (2002). Heterologous expression of WT and mutant photoreceptor peripherin/rds in Madin Darby canine kidney cells: an assessment of fusogenic function. Exp. Eye Res. 74, 267-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam, B.M., Moritz, O.L., Hurd, L.B., and Papermaster, D.S. (2000). Identification of an outer segment targeting signal in the COOH terminus of rhodopsin using transgenic Xenopus laevis. J. Cell Biol. 151, 1369-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis, G.H., Sutcliffe, J.G., and Bok, D. (1991). The retinal degeneration slow (rds) gene product is a photoreceptor disc membrane-associated glycoprotein. Neuron 6, 61-70. [DOI] [PubMed] [Google Scholar]
- Vaithinathan, R., Berson, E.L., and Dryja, T.P. (1994). Further screening of the rhodopsin gene in patients with autosomal dominant retinitis pigmentosa. Genomics 21, 461-463. [DOI] [PubMed] [Google Scholar]
- Wald, G., Brown, P.K., and Gibbons, I.R. (1962). The problem of visual excitation. J. Opt. Soc. Am. 53, 20-35. [DOI] [PubMed] [Google Scholar]
- Wu, M., and Gerhart, J. (1991). Raising Xenopus in the laboratory. Methods Cell Biol. 36, 3-18. [DOI] [PubMed] [Google Scholar]
- Young, R.W., and Droz, B. (1968). The renewal of protein in retinal rods and cones. J. Cell Biol. 39, 169-184. [DOI] [PMC free article] [PubMed] [Google Scholar]