Abstract
Background
With the establishment of minimally invasive surgery in society, the robot has been increasingly widely used in the urologic field, including in partial nephrectomy. This study aimed to comprehensively summarize the currently available evidence on the feasibility and safety of robotic partial nephrectomy for renal tumors of >4 cm.
Method and Findings
An electronic database search of PubMed, Scopus, Web of Science, and the Cochrane Library was performed. This systematic review and meta-analysis was based on all relevant studies that assessed robotic partial nephrectomy for renal tumors of >4 cm. Five studies were included. The meta-analysis involved 3 studies from 11 institutions including 154 patients, while the narrative review involved the remaining 2 studies from 5 institutions including 64 patients. In the meta-analysis, the mean ischemic time, operation time, and console time was 28, 319, and 189 minutes, respectively. The estimated blood loss and length of stay was 317 ml and 3.8 days, respectively. The rates of conversion, positive margins, intraoperative complications, postoperative complications, hilar clamping, and collecting system repair were 7.0%, 3.5%, 7.0%, 9.8%, 93.9%, and 47.5%, respectively. The narrative review showed results similar to those of the meta-analysis.
Conclusions
Robotic partial nephrectomy is feasible and safe for renal tumors of >4 cm with an acceptable warm ischemic time, positive margin rate, conversion rate, complication rate, operation time, estimated blood loss, and length of stay.
Introduction
Partial nephrectomy (PN) is the gold standard for treatment of small renal masses and selected T1b tumors for which removal is technically feasible [1]. Evolution has progressed from open radical nephrectomy (RN) through open PN to minimally invasive PN, including laparoscopic PN (LPN) and robotic PN (RPN) [2]. For small renal masses, RPN provides benefits similar to those provided by LPN with acceptable safety [3], [4].
PN for larger tumors (>4 cm) is reportedly similar to RN by a laparoscopic approach in terms of oncologic and functional outcomes [5], [6], providing evidence of the feasibility of this minimally invasive procedure. Since the first introduction of RPN in 2004 [7], renal tumors of >4 cm have reportedly been removed by this technique in some large intuitions [8], [9]. However, only a limited number of cases have been reported.
We performed a systematic review and meta-analysis of the available literature on RPN for renal tumors of >4 cm and herein discuss its feasibility and safety in terms of perioperative and early oncologic outcomes.
Materials and Methods
A prospective protocol of objectives, literature-search strategies, inclusion and exclusion criteria, outcome measurements, and methods of statistical analysis was prepared a priori according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [10].
Literature-search Strategy
A literature search was performed using the electronic databases of PubMed, Web of Science, Scopus, and the Cochrane Library in April 2013. The following terms and their combinations were searched: robot or robotic, partial or nephron-sparing, nephrectomy, and 4 cm. The Related Articles function was also used to broaden the search. Additional studies were manually searched in the reference lists of all retrieved articles. When multiple reports describing the same population were published, the most recent or complete report was used in the meta-analysis. However, it was not applicable if the outcome measures were mutually exclusive or measured in different time periods. The studies excluded from the meta-analysis underwent a narrative synthesis.
Inclusion and Exclusion Criteria
All articles and meeting abstracts that reported the performance of RPN for renal tumors of >4 cm in all age groups and that had at least one of the quantitative outcomes mentioned in the next section of this paper were included.
Data Extraction and Outcomes of Interest
Two authors (Li and Bi) independently extracted and summarized the data for the following parameters: authors, publication year, country, number of institutions, instruments for diagnosis, number of patients, tumor size, age, gender, body mass index, American Society of Anesthesiologists score, nephrometry score, and outcomes of interest. Any disagreement was resolved by the adjudicating senior authors (Huang and Lin).
The primary outcomes were warm ischemic time, conversion rate, positive margin rate, and complication rate. The secondary outcomes were operation room time, console time, estimate blood loss, hilar clamping rate, collecting system repair rate, blood transfusion rate, and length of stay.
Statistical Analysis
The meta-analysis was performed using Meta-Analyst [11]. The DerSimonian and Laird random method was used to combine dichotomous variables to rates. Continuous variables were combined to weighted mean with a random method. For studies that presented continuous data as medians and ranges, the means and standard deviations were calculated using statistical algorithms described by Hozo et al [12]. Statistical heterogeneity between studies was assessed using the chi-square test with significance set at p<0.10, and heterogeneity was quantified using the I2 statistic with significance set at I2>50% [13]. The use of Egger’s funnel plots was initially planned, but were eventually not used to assess the possibility of publication bias because of either the limited number of studies included for the meta-analysis or the significant heterogeneity among studies [14]. Studies not used for the meta-analysis were reviewed and underwent a narrative synthesis.
Results
Literature Search and Study Characteristics
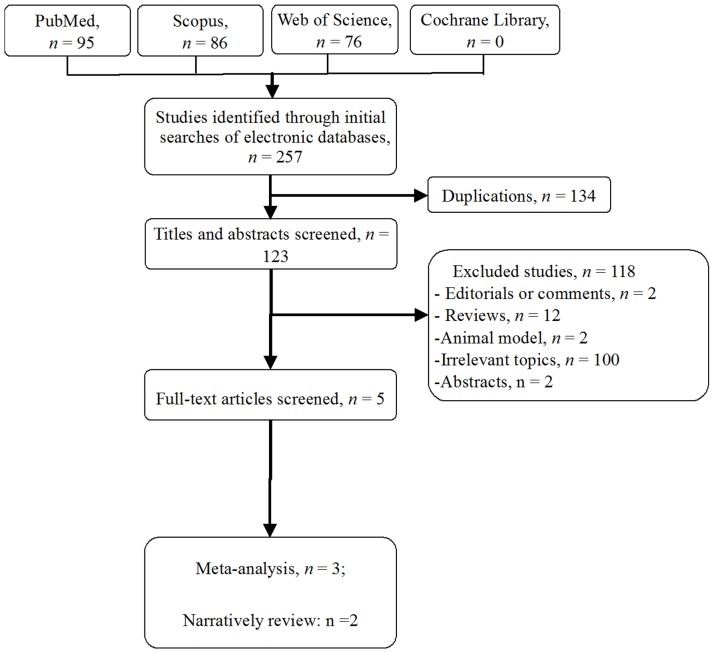
Five studies [8], [9], [15]–[17] fulfilled the predefined inclusion criteria and were included in the final analysis (Fig. 1). Table 1 shows the characteristics of the included studies. Two studies [9], [17] may have had some overlapping data as reported by Petros et al [16]. They were reviewed by a narrative synthesis. The other 3 studies [8], [15], [16] from 11 institutions including 154 patients were included in the meta-analysis.
Figure 1. Flow diagram of studies identified, included, and excluded.
Table 1. Characteristics of the studies included.
| First Author, Yearof Publication | Country | Study Design | Institutions, no. | Instrument for Diagnosis | Patients, no. | Age, years, Mean(Standard Deviation)/Median(Range) | Male, no. | Nephrometry RENAL Score, Mean(Standard Deviation)/Median(Range) | Clinical Tumor Size, Median (Range), cm |
| Petros2012 [16] | USA | R | 4 | Radiography | 83 | 61 (12) | 52 | 8.0 (4–11) | 5.0(4.1–11) |
| Lecomte2013 [8] | France | P | 6 | US+ CT | 54 | 62 (31–81) | 35 | 7.0 (1.5) | 4.5(4–7)* |
| Gupta2013 [15] | USA | R | 1 | Radiography | 17/19¶ | 47 (26–76) | 9 | 8.9 (1.3) | 5.0(4.1–15) |
| Ficarra2012 [9] | USA+ Italy | R | 4 | MR or CT | 49 | 60 (52–66)# | NA | 10 (8–10)#, † | 5.0(4.4–5.5)# |
| Patel2010 [17] | USA | P | 1 | MR or CT | 15 | 59 (44–76) | 9 | NA | 5.0(4.1–7.9) |
P = prospectively collected data; R = retrospectively; US = ultrasonography; CT = computerized tomography; MR = magnetic resonance; NA = data not available;
pathological tumor size;
median (interquartile range);
17 patients with 19 operations;
PAUDA score.
Primary Outcomes
Warm ischemic time
The median warm ischemic time was 22 to 36 minutes [8], [9], [15]–[17]. Pooling of the data of 11 institutions [8], [15], [16] showed a mean of 28 minutes (95% confidence interval [CI], 21–34 minutes) (Table 2).
Table 2. Perioperative information in meta-analysis.
| Variables | No. of Institutions | No. of Procedures | Mean | 95% Confidence Interval | |
| Primary Outcomes | |||||
| Warm ischemic time, min | 11 | 153 | 28 | 21–34 | |
| Conversion#, % | 11 | 156 | 7.0 | 2.6–17.7 | |
| Conventional laparoscopic PN | 2.9 | 1.2–7.2 | |||
| Open PN | 4.7 | 0.9–21 | |||
| Positive margin, % | 11 | 153 | 3.5 | 1.1–10.5 | |
| Intraoperative complication#, % | 11 | 156 | 7.0 | 2.6–17.7 | |
| Postoperative complication, % | 11 | 153 | 9.8 | 4.3–20.8 | |
| Major complication*, % | 11 | 153 | 4.7 | 2.3–9.5 | |
| Secondary Outcomes | |||||
| Operation room time, min | 5 | 70 | 319 | 193–445 | |
| Console time, min | 10 | 137 | 189 | 176–202 | |
| Estimate blood loss, ml | 11 | 153 | 317 | 43–591 | |
| Hilar clamping, % | 11 | 153 | 93.9 | 88.7–96.8 | |
| Collecting system repair, % | 7 | 99 | 47.5 | 37.9–57.3 | |
| Length of stay, days | 10 | 137 | 3.8 | 1.9–5.7 | |
PN = partial nephrectomy;
Clavien-Dino classification grade >3;
Conversion was treated as intraoperative complications according to Clavien-Dino classification.
Conversion
No conversion was reported by Petal et al [17] or Ficarra et al [9] (Table 3). However, the combined conversion rate of 11 institutions [8], [15], [16] as estimated by the random-effects model was 7.0% (95% CI, 2.6%–17.7%). Reported conversions were grouped into conventional laparoscopic PN and open PN with estimated rates of 2.9% and 4.7% (95% CI, 1.2%–7.2% and 0.9%–21%), respectively (Table 2).
Table 3. Comparison between meta-analysis and narrative review.
| Variables | Petal 2010 [17] | Ficarra2012 [9] | Meta-analysis |
| Cases, N | 15 | 49 | 156 |
| Median warm ischemic time, min (IQR) | 25 (20–30) | 22 (18–28) | 28(21–34)* |
| Conversion, % | 0 | 0 | 7.0 |
| Positive margin, % | 0 | 5.1 | 3.5 |
| Intraoperative complication, % | 0 | 4 | 7.0 |
| Postoperative complication, % | 26.6 | 26.5 | 9.8 |
| Major complication † , % | 19.8 | 8 | 4.7 |
| Median operation room time, min (IQR) | 275 (229–344) | 177 (138–200) | 319(193–445)* |
| Median console time, min (IQR) | NA | 145 (112–177) | 189(176–202)* |
| Median estimated blood loss, ml (IQR) | 100 (75–200) | 120 (62–237) | 317(43–591)* |
| Hilar clamping, % | 86.7 | NA | 93.9 |
| Collecting system repair, % | 71 | 57 | 47.5 |
| Median length of stay, days (IQR) | 2 (2–4) | NA | 3.8(1.9–5.7)* |
NA = data not available; IQR = interquartile range;
mean(95% confidence interval);
Clavien-Dino classification grade >3.
Positive margin
The positive margin rate was reported as 5.1% by Ficarra et al [9] and 0% by Petal et al [17] (Table 3). Pooling of the data of 11 institutions [7], [14], [15] indicated a rate of 3.5% (95% CI, 1.1%–10.5%) (Table 2).
Complications
The intraoperative complication rate was reported as 4% by Ficarra et al [9] and 0% by Petal et al [17] (Table 3). No intraoperative complications were declared among the 11 institutions [7], [14], [15]. However, conversions were treated as complications according to the Clavien-Dindo classification. The estimated rate was 7.0% (95% CI, 2.6%–17.7%) (Table 2).
The postoperative complication rate was reported as 26.5% by Ficarra et al [9] and 26.6% by Petal et al [17] (Table 3). Nonetheless, the combined rate from 11 institutions [7], [14], [15] was lower at 9.8% (95% CI, 4.3%–20.8%) (Table 3). The major complication rate was reported as 8% by Ficarra et al [9] and 19.8% by Petal et al [17] (Table 3). The combined major complication rate [7], [14], [15] was 4.7% (95% CI, 2.3%–9.5%) (Table 2). All reported major complications necessitating intervention were urine leakage and bleeding/pseudoaneurysm. One and two cases of urine leakage were reported by two [8], [15] and the remaining three studies [9], [16], [17], respectively. One and two cases of bleeding/pseudoaneurysm were reported by two [16], [17] and one study [8], respectively.
Secondary Outcomes
Operative room time and console time
The median operative room and console times were 177 to 275 minutes [9], [17] and 145 minutes [9], respectively (Table 3). Pooling of the operative room time data from six institutions [8], [15] showed a mean of 319 minutes (95% CI, 193–445 minutes), and console time data from 10 institutions [8], [16] showed a mean of 189 minutes (95% CI, 176–202 minutes) (Table 2).
Estimated blood loss and length of stay
The median estimated blood loss was 100 to 120 ml [9], [17]. The combined data from 11 institutions [8], [15], [16] showed a mean estimated blood loss of 317 ml (95% CI, 43–591 ml). The median length of stay was 2 days as reported by Petal et al [17]. The combined data from 10 institutions [8], [16] showed a mean length of stay of 3.8 days (95% CI, 1.9–5.7 days) (Tables 2 and 3).
Hilar clamping and collecting system repair
The hilar clamping rate was 86.7% as reported by Petal et al [17]. The combined data from 11 institutions [8], [15], [16] showed a rate of 93.9% (95% CI, 88.7%–96.8%). The collecting system repair rate was 57% to 71% [9], [17]. The combined data from seven institutions [15], [16] showed a repair rate of 47.5% (95% CI, 37.9%–57.3%) (Tables 2 and 3).
Discussion
The present systematic review provided a comprehensive overview of the current evidence on the feasibility and safety of RPN for renal tumors of >4 cm. It showed an acceptable warm ischemic time, conversion rate, complication rate, operation time, estimated blood loss, and length of stay.
In the treatment of renal masses of <4 cm, LPN as a minimally invasive technique has significantly evolved to the point at which the short- and long-term safety rivals that of open PN [18], [19]. RPN was recently introduced as a feasible and safe alternative to LPN in terms of perioperative outcomes. A meta-analysis comparing RPN with LPN for T1a tumors concluded that there exists no significant difference in perioperative variables between the two techniques [4]. Another meta-analysis of the treatment of tumors with a mean size of <4 cm indicated that RPN may be more suitable than LPN in terms of decreased warm ischemic times [3]. However, detailed comparisons of long-term outcomes should be performed.
The treatment of renal tumors of >4 cm may be complicated. However, some studies have been dedicated to the demonstration of the feasibility of LPN for large tumors. In a comparison of laparoscopic PN with laparoscopic RN, the overall mortality, cancer-specific mortality, and recurrence rates were equivalent [6]. With median follow-up periods of 15 and 21 months for the laparoscopic PN and RN cohorts, Deklaj et al indicated that LPN for T1b renal tumors provides superior preservation of renal function [5]. All of these intermediate-term data support the possibility of wide application of LPN to large renal tumors in future studies [5], [6].
LPN has been successfully applied to selected renal tumors of >4 cm for which removal is technically feasible [20]–[24]. Table 4 compares data reported in previous studies of LPN [20]–[24] and data analyzed in the present meta-analysis. A prolonged warm ischemic time, higher risk of perioperative complications, and higher rate of positive surgical margins may represent the most important concerns potentially limiting the diffusion of RPN for renal tumors of >4 cm. In this meta-analysis, the pooled mean warm ischemic time of RPN was 28 minutes, which is comparable with that of LPN (21.9–38 minutes) [20]–[24]. However, the rates of positive margins and postoperative complications were 3.5% and 9.8%, respectively. These rates seemed lower than those of LPN (3.8%–6.5% and 24%–42%, respectively) [20]–[24]. All other outcomes were acceptable (Table 4). Given the well-established technique and widespread application of LPN, RPN may be a feasible alternative to LPN for large renal tumors.
Table 4. Comparison between meta-analysis and other minimally invasive studies on partial nephrectomy for renal tumors >4 cm.
| Variables | Rais2008 [21] | Eng2009[22] | Simmons2009[23] | Porpiglia2010[24] | Sprenkle2012[20] | Meta-analysis |
| Procedure | Laparoscopic | Laparoscopic | Laparoscopic | Laparoscopic | Robotic andLaparoscopic | Robotic |
| Cases, N | 34 | 26 | 58 | 63/41¶ | 54 | 156 |
| Mean warm ischemic time, min (SD) | 21.9(13.7) | 30.3 (10.9) | 38 (11.9) | 25.7 (8.3) | 37 (31–41)# | 28(21–34)* |
| Conversion, % | NA | 15 | 4 | 7.3 | 13 | 7.0 |
| Positive margin, % | 5.3 | 3.8 | 6.5 | 6.5 | 4 | 3.5 |
| Intraoperative complication, % | 4.0 | NA | 7 | 7.3 | 19 | 7.0 |
| Postoperative complication, % | 37.0 | 42 | 24 | 26 | 33 | 9.8 |
| Major complication † , % | NA | 15 | 8.6 | 14.6 | 15 | 4.7 |
| Mean operation room time, min (SD) | NA | NA | NA | NA | NA | 319(138–200)* |
| Mean console time, min (SD) | 199.2(57.2) | 234(111) | 228 (78) | 154 (62) | NA | 189(176–202)* |
| Mean estimated blood loss, ml (SD) | 406.3(354.3) | 247(252) | 284 (302) | 230 (143) | 300 (144–438)# | 317(43–591)* |
| Hilar clamping, % | NA | NA | NA | 100 | 93 | 93.9 |
| Collecting system repair, % | NA | 88.5 | 90 | 43 | NA | 47.5 |
| Mean length of stay, day (SD) | 4.1(2.7) | NA | 3.5 (1.5) | 7 (3.5) | 3 (2–5)# | 3.8(1.9–5.7)* |
NA = data not available; SD = standard deviation;
Complete data on complications were available for 41 patients;
mean(95% confidence interval);
median(interquartile range);
Clavien-Dino classification grade >3.
The most important finding in this study is that the success of LPN can be rapidly transited to RPN. Lavery et al [25] focused on one experienced surgeon and highlighted the quick learning curve associated with the transition from LPN to RPN. There were no significant differences in warm ischemic time, estimated blood loss, or length of hospital stay when comparing the first 20 RPN and the last 18 LPN procedures. RPN achieves an operation time similar to that of LPN after five procedures. Similarly, Pierorazio et al [26] concluded that the transition from LPN to RPN can be undertaken without an additional learning curve and is associated with immediate benefits after approximately 25 LPN procedures. When performed by a surgeon with extensive robotic experience, RPN has a short learning curve to reach a warm ischemic time of <20 min, console time of <100 min, limited blood loss, and an acceptable overall complication rate [27]. Kaouk et al [28] showed that once past the learning curve, a significantly decreased estimated blood loss, transfusion rate, conversion rate, complication rate, operative time, and length of stay was obtained in the largest reported series comparing early and later experiences of RPN. Nevertheless, further high-quality studies are needed to determine whether the learning curve of RPN for renal tumors of >4 cm can be easily passed.
Despite the feasibility of RPN, cost might be an important factor impacting the choice of operation procedure. Mir et al [29] compared the costs of PN carried out by laparoscopic and robotic procedures. They performed a systematic review and meta-analysis and indicated that RPN is associated with higher costs than LPN because of maintenance and instrumentation. Yu et al [27] found that robotic surgery costs significantly more than laparoscopic procedures, although it is associated with a significantly shorter hospital stay, fewer complications, and a lower transfusion rate. However, there has been no social cost analysis of factors involved in quicker recovery and shorter convalescence by robotic procedures in urologic surgery [30]. It is estimated that the total costs of RPN are about $1600 more per person [29], [30]. A significant decrease in robotic costs is required for RPN to be cost-effective.
The present systematic review and meta-analysis has some limitations that must be considered. The main limitation is that it relied on a minority of eligible studies. Only five studies were included, and just three of them [8], [15], [16] were used for the meta-analysis. Because of some potentially overlapping data, the other two studies were reviewed narratively. The sample size of some studies was small, limiting the statistical power. In addition, because most studies originated from high-volume institutions or centers of excellence, the results may be difficult to transfer to community-based practice. Finally, the follow-up period was generally short, so long-term outcomes remained to be evaluated.
However, the procedure of RPN for renal tumors of >4 cm has only been applied for a short period of time, in limited institutions, and in small sample sizes. This present meta-analysis with 11 institutions including 153 patients and narrative review of 5 institutions including 64 patients may provide better evidence for the feasibility of RPN for renal tumors of >4 cm.
Conclusions
This systematic review and meta-analysis indicates that RPN is feasible and safe for renal tumors of >4 cm with an acceptable warm ischemic time, conversion rate, complication rate, operation time, estimated blood loss, and length of stay. Nevertheless, future large-volume, well-designed prospective and randomized studies comparing PN for renal tumors of >4 cm by robotic, laparoscopic, or open procedures and that compare PN and RN for renal tumors of >4 cm by robotic procedures are needed to confirm and update the findings of this analysis.
Supporting Information
PRISMA 2009 Checklist.
(DOC)
Take Home Message.
(DOC)
Funding Statement
This work was supported by The National Natural Science Foundation of China (81071688, 30972983, 81172431, 91029742, and 81101519); Guangdong Province Natural Scientific Foundation (07117366, 6104605); the Yat-sen Scholarship for Young Scientists (to Tianxin Lin); the Clinical Key Project of Public Health Ministry (to Jian Huang); the Program for New Century Excellent Talents in University (NCET-10-0852, to Tianxin Lin); the Natural Science Foundation of Guangdong Province (S2011040003777); the Fundamental Research Funds for the Central Universities 2011(to Liangkuan Bi); New Teacher Foundation of Ministry of Education (200805581124); Doctor Start Fund of Natural Science Foundation of Guangdong Province (9451008901003001); the Youth Funds of National Natural Science Foundation of China (30901488); and Science and technology planning projection of Guangdong Province (2012B031800109). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.European Association of Urology WebSite. Available: http://www.uroweb.org/gls/pdf/10_Renal_Cell_Carcinoma_LRV2.pdf. Accessed 2013 Sep 10.
- 2. Minervini A, Siena G, Carini M (2011) Robotic-assisted partial nephrectomy: the next gold standard for the treatment of intracapsular renal tumors. Expert Rev Anticancer Ther 11: 1779–1782. [DOI] [PubMed] [Google Scholar]
- 3. Aboumarzouk OM, Stein RJ, Eyraud R, Haber GP, Chlosta PL, et al. (2012) Robotic versus laparoscopic partial nephrectomy: a systematic review and meta-analysis. Eur Urol 62: 1023–1033. [DOI] [PubMed] [Google Scholar]
- 4. Froghi S, Ahmed K, Khan MS, Dasgupta P, Challacombe B (2013) Evaluation of robotic and laparoscopic partial nephrectomy for small renal tumours (T1a). BJU Int. 112: E322–33. [DOI] [PubMed] [Google Scholar]
- 5. Deklaj T, Lifshitz DA, Shikanov SA, Katz MH, Zorn KC, et al. (2010) Laparoscopic radical versus laparoscopic partial nephrectomy for clinical T1bN0M0 renal tumors: comparison of perioperative, pathological, and functional outcomes. J Endourol 24: 1603–1607. [DOI] [PubMed] [Google Scholar]
- 6. Simmons MN, Weight CJ, Gill IS (2009) Laparoscopic radical versus partial nephrectomy for tumors >4 cm: intermediate-term oncologic and functional outcomes. Urology 73: 1077–1082. [DOI] [PubMed] [Google Scholar]
- 7. Gettman MT, Blute ML, Chow GK, Neururer R, Bartsch G, et al. (2004) Robotic-assisted laparoscopic partial nephrectomy: technique and initial clinical experience with DaVinci robotic system. Urology 64: 914–918. [DOI] [PubMed] [Google Scholar]
- 8.Masson-Lecomte A, Yates DR, Bensalah K, Vaessen C, de la Taille A, et al.. (2013) Robot-assisted laparoscopic nephron sparing surgery for tumors over 4 cm: Operative results and preliminary oncologic outcomes from a multicentre French study. Eur J Surg Oncol. [DOI] [PubMed]
- 9. Ficarra V, Bhayani S, Porter J, Buffi N, Lee R, et al. (2012) Robot-assisted partial nephrectomy for renal tumors larger than 4 cm: results of a multicenter, international series. World J Urol 30: 665–670. [DOI] [PubMed] [Google Scholar]
- 10. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, et al. (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wallace BC, Schmid CH, Lau J, Trikalinos TA (2009) Meta-Analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Med Res Methodol 9: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JPT Green S (2011) Cochrane handbook for systematic reviews of interventions Version 5.1.0 [updated March 2011]: The Cochrane Collaboration.
- 14. Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I (2006) The case of the misleading funnel plot. BMJ 333: 597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gupta GN, Boris R, Chung P, Linehan WM, Pinto PA, et al. (2013) Robot-assisted laparoscopic partial nephrectomy for tumors greater than 4 cm and high nephrometry score: feasibility, renal functional, and oncological outcomes with minimum 1 year follow-up. Urol Oncol 31: 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Petros F, Sukumar S, Haber GP, Dulabon L, Bhayani S, et al. (2012) Multi-institutional analysis of robot-assisted partial nephrectomy for renal tumors >4 cm versus </ = 4 cm in 445 consecutive patients. J Endourol 26: 642–646. [DOI] [PubMed] [Google Scholar]
- 17. Patel MN, Krane LS, Bhandari A, Laungani RG, Shrivastava A, et al. (2010) Robotic partial nephrectomy for renal tumors larger than 4 cm. Eur Urol 57: 310–316. [DOI] [PubMed] [Google Scholar]
- 18. Permpongkosol S, Bagga HS, Romero FR, Sroka M, Jarrett TW, et al. (2006) Laparoscopic versus open partial nephrectomy for the treatment of pathological T1N0M0 renal cell carcinoma: a 5-year survival rate. J Urol 176: 1984–1988. [DOI] [PubMed] [Google Scholar]
- 19. Gill IS, Kavoussi LR, Lane BR, Blute ML, Babineau D, et al. (2007) Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol 178: 41–46. [DOI] [PubMed] [Google Scholar]
- 20. Sprenkle PC, Power N, Ghoneim T, Touijer KA, Dalbagni G, et al. (2012) Comparison of open and minimally invasive partial nephrectomy for renal tumors 4–7 centimeters. Eur Urol 61: 593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rais-Bahrami S, Romero FR, Lima GC, Kohanim S, Permpongkosol S, et al. (2008) Elective laparoscopic partial nephrectomy in patients with tumors >4 cm. Urology 72: 580–583. [DOI] [PubMed] [Google Scholar]
- 22. Eng MK, Bernstein AJ, Katz MH, Shikanov S, Zorn KC, et al. (2009) Impact of renal lesion size on perioperative and pathologic outcomes in patients undergoing laparoscopic partial nephrectomy. J Endourol 23: 439–443. [DOI] [PubMed] [Google Scholar]
- 23. Simmons MN, Chung BI, Gill IS (2009) Perioperative efficacy of laparoscopic partial nephrectomy for tumors larger than 4 cm. Eur Urol 55: 199–207. [DOI] [PubMed] [Google Scholar]
- 24. Porpiglia F, Fiori C, Piechaud T, Gaston R, Guazzoni G, et al. (2010) Laparoscopic partial nephrectomy for large renal masses: results of a European survey. World J Urol 28: 525–529. [DOI] [PubMed] [Google Scholar]
- 25. Lavery HJ, Small AC, Samadi DB, Palese MA (2011) Transition from laparoscopic to robotic partial nephrectomy: the learning curve for an experienced laparoscopic surgeon. JSLS 15: 291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pierorazio PM, Patel HD, Feng T, Yohannan J, Hyams ES, et al. (2011) Robotic-assisted versus traditional laparoscopic partial nephrectomy: comparison of outcomes and evaluation of learning curve. Urology 78: 813–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mottrie A, De Naeyer G, Schatteman P, Carpentier P, Sangalli M, et al. (2010) Impact of the learning curve on perioperative outcomes in patients who underwent robotic partial nephrectomy for parenchymal renal tumours. Eur Urol 58: 127–132. [DOI] [PubMed] [Google Scholar]
- 28. Kaouk JH, Hillyer SP, Autorino R, Haber GP, Gao T, et al. (2011) 252 robotic partial nephrectomies: evolving renorrhaphy technique and surgical outcomes at a single institution. Urology 78: 1338–1344. [DOI] [PubMed] [Google Scholar]
- 29. Mir SA, Cadeddu JA, Sleeper JP, Lotan Y (2011) Cost comparison of robotic, laparoscopic, and open partial nephrectomy. J Endourol 25: 447–453. [DOI] [PubMed] [Google Scholar]
- 30. Yu HY, Hevelone ND, Lipsitz SR, Kowalczyk KJ, Hu JC (2012) Use, costs and comparative effectiveness of robotic assisted, laparoscopic and open urological surgery. J Urol 187: 1392–1398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA 2009 Checklist.
(DOC)
Take Home Message.
(DOC)