Abstract
Background
Vancomycin (VCM) treatment outcomes depend on the characteristics of the patient, and it is well known that hypoalbuminemia is a risk factor for poor treatment outcomes, as reported in a previous study. However, the reason that severe hypoalbuminemia has an influence on the treatment outcome of VCM remains unknown.
Objective
To elucidate the association between severe hypoalbuminemia and VCM treatment outcomes, we examined pharmacokinetic/pharmacodynamic (PK/PD) parameters in elderly patients with severe hypoalbuminemia.
Methods
We conducted a retrospective observational study of 94 patients with methicillin-resistant Staphylococcus aureus (MRSA) hospital-acquired pneumonia who had been treated with VCM between January 2006 and December 2012. The 94 patients were divided into severe hypoalbuminemia and non-severe hypoalbuminemia groups. The PK/PD parameters and treatment outcomes of VCM were compared between the two groups.
Results
The half-life of VCM in the severe hypoalbuminemia group was significantly longer than in the non-severe hypoalbuminemia group (33.2 + 5.4 vs 24.9 + 1.6; P = 0.049). Area under the concentration curve (AUC)/minimum inhibitory concentration (MIC) values of 250–450 and >450 μg × h/mL were significantly associated with 28-day mortality in the severe hypoalbuminemia group (P < 0.001), whereas AUC/MIC values of <250 μg × h/mL were not associated. We also detected a significant difference in the increased percentage of nephrotoxicity in the severe hypoalbuminemia group (6 of 23 patients [26%]) compared with the non-severe hypoalbuminemia group (6 of 71 patients [8%]; P < 0.001).
Conclusion
These findings indicate that severe hypoalbuminemia influences the half-life of VCM and treatment outcomes in elderly patients (≥75 years of age). To establish a more effective and safer treatment protocol, the issue of malnutrition in elderly patients needs to be addressed and improved.
Keywords: methicillin-resistant Staphylococcus aureus, elderly patients, vancomycin, severe hypoalbuminemia, pharmacokinetics, pharmacodynamics
Introduction
Senior citizens aged 75 years and older represent the largest and most frequent users of health care facilities such as hospitals and long-term skilled nursing and residential homes. Since immune systems are often affected by aging and underlying diseases, incidences of hospital-acquired pneumonia (HAP) induced by methicillin-resistant Staphylococcus aureus (MRSA) are more frequent in the elderly. 1 Improving treatment outcomes for elderly patients with MRSA infections will therefore increase survival and quality of life, and reduce health care expenditure burdens.2
MRSA HAP is primarily treated via the intravenous administration of vancomycin (VCM), and the dosage is determined by pharmacokinetic/pharmacodynamic (PK/PD) parameters. However, a previous study involving adult patients with MRSA pneumonia or sepsis failed to show an association between the PK/PD parameters for VCM and treatment outcome.3 Martin et al4 recommend a target area under the concentration curve (AUC)/minimum inhibitory concentration (MIC) for VCM of >400 μg × h/mL, while we previously reported that AUC/MIC values of 250–450 μg × h/mL were acceptable for elderly patients.5 The reason for this discrepancy is currently unclear.
VCM treatment outcomes depend on the characteristics of the patient, and it is well known that hypoalbuminemia is a risk factor for poor treatment outcomes, as reported in a previous study.6 Hayashi et al7 reported that hypoalbuminemia was a risk factor with the long-term administration of VCM. However, the reason that hypoalbuminemia has an influence on the treatment outcome of VCM remains unknown.
Since the potency of VCM is dependent on the free unbound form, albumin levels may have an influence on VCM treatment outcomes. In addition, body fluid volume and renal function are frequently decreased in elderly patients, and such patients show large individual differences in PK/PD parameters. Nevertheless, these parameters in elderly patients with hypoalbuminemia have not been clarified. To elucidate the association between severe hypoalbuminemia and VCM treatment outcomes, we examined PK/PD parameters in elderly patients with severe hypoalbuminemia.
Methods
Study location and patients
The study was conducted at the National Center for Geriatrics and Gerontology Hospital, Obu, Japan. This 320-bed hospital oversees general (including emergency) services, except pediatrics, and admits approximately 5,000 patients per year (more than 50% of whom are aged over 75 years). The average number of HAP-MRSA patients is 10–20 per year. MRSA is endemic in this hospital, and the ratio of MRSA isolates per total S. aureus isolates is approximately 70%.
Over a 7-year period (from January 2006 through December 2012), all hospitalized patients aged 75 years or older with MRSA pneumonia that had been microbiologically confirmed by sputum or blood cultures, and who had been treated with VCM therapy, were identified using the Clinical Pharmacokinetics Department computer database.
Study design and data collection
We conducted a retrospective observational study with 28-day mortality as the primary outcome for 94 elderly patients with MRSA pneumonia who had been treated with VCM during the 7-year study period. The secondary outcomes were nephrotoxicity and liver dysfunction. The baseline characteristics of the study patients were age, gender, body weight, serum creatinine level, Charlson Comorbidity Index, albumin level, combination antibiotic therapy, diagnosis of pneumonia, and infection severity. In addition, we assessed the relationship between the effect of VCM PK indices, including serum peak and trough concentrations, AUC/MIC values, volume of distribution, half-life, clearance of VCM, single and daily dose of VCM, and dose interval of VCM. The clinical characteristics of the study patients were retrieved from the hospital medical records. For patients with multiple episodes, only the first episode was counted. This study was approved by the Ethics Committee of the National Center for Geriatrics and Gerontology Hospital.
Definitions
The definition of HAP was based on American Thoracic Society guidelines for the management of adults with hospital-acquired, ventilator-associated, and health care-associated pneumonia.2 For the purposes of this study, HAP was defined as pneumonia that occurred 48 hours or more after hospitalzation for an acute lung infection characterized by a cough, fever, purulent sputum, and an abnormal chest X-ray that was not deemed to be incubating at the time of admission. Among the HAP cases, those with MRSA isolated from blood cultures or sputum that showed no signs of improvement after treatment with broad-spectrum antibiotics, such as carbapenem, for more than 3 days were defined as MRSA HAP.
Severe hypoalbuminemia was defined as a serum albumin level of <2.5 g/dL. The severity rating of pneumonia was defined according to the Japanese Respiratory Society guidelines for the management of HAP8 and was used to allocate the patients into severe, moderate, and mild groups. The severe group was defined as patients with three or more of the following risk factors or conditions: malignancy or immunocompromized status, impaired consciousness, requiring a fraction of inspired oxygen (FiO2) >35% to maintain a saturated oxygen (SaO2) level >90%, men aged 70 years or older, or women aged 75 years or older, and oliguria or dehydration. The moderate group was defined as patients with any two of the above risk factors, and in addition, at least one of the following secondary risk factors: C-reactive protein (CRP) ≥20.0 mg/L or extent of infiltration on a chest X-ray (CXR) covering at least two-thirds of one lung. The mild group was defined as all other patients who did not fit the severe or moderate criteria. The patients were divided into a severe hypoalbuminemia and a non-severe hypoalbuminemia group before VCM administration. Nephrotoxicity to VCM was defined as an increase in serum creatinine of 0.5 g/dL or a 50% increase over pretreatment levels.2 Liver dysfunction was defined according to International Consensus Meeting criteria.9
Pharmacokinetic data
The initial treatment schedule for VCM was simulated to achieve a trough concentration 10–15 μg/mL, with therapeutic drug monitoring (TDM) software using patient characteristics, including age, body weight and serum creatinine (VCM-TDM Microsoft® Excel Version 3.0, Shionogi and Co, Ltd, Osaka, Japan). The serum concentrations of VCM were determined from samples collected during the fifth day from the start of administration. The blood samples were obtained twice: before VCM administration (trough) and 1 hour after VCM administration (peak). The predicted 24-hour AUC values for VCM were calculated using the TDM software based on peak and trough concentrations.
Statistical analysis
All comparisons were unpaired, and all tests for significance were two-tailed. Continuous variables were compared using the Student’s t-test for normally distributed variables and the Welch test for non-normally distributed variables. Categorical variables were compared with the chi-square (χ2) test. In these tests, a two-sided P-value of <0.05 was considered to be significant. SPSS version 20.0 software (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis.
Results
The 94 patients were divided into severe hypoalbuminemia (n = 23) and non-severe hypoalbuminemia (n = 71) groups. Demographic data for these groups are summarized in Table 1.
Table 1.
Characteristics of patients with Methicillin-resistant Staphylococcus aureus pneumonia in this study
| Characteristic | Severe hypoalbuminemia (n=23) | Non-severe hypoalbuminemia (n=71) | P-value |
|---|---|---|---|
| Age (years) | 82.7 ± 1.4 (75–97) | 82.6 ± 0.7 (75–99) | 0.933a |
| Male (%) | 17 (74%) | 46 (65%) | 0.161b |
| Body weight (kg) | 41.8 ± 2.2 (28–72) | 43.3 ± 1.1 (27–67) | 0.526a |
| Serum creatinine (mg/dL) | 0.8 ± 0.1 (0.2–2.1) | 0.7 ± 0.1 (0.2–2.5) | 0.348a |
| Charlson Comorbidity Index | 2.9 ± 0.5 (1–10) | 2.5 ± 0.2 (0–10) | 0.475a |
| Albumin (g/dL) | 2.1 ± 0.1 (1.4–2.4) | 3.0 ± 0.1 (2.5–3.6) | <0.001a |
| Combination antibiotic therapy | 12 (52%) | 40 (56%) | 0.570b |
| Diagnosis | |||
| Pneumonia/pneumonia and sepsis | 21/2 | 55/16 | 0.143b |
| Infection severity | |||
| Mild | 4 (17%) | 9 (13%) | 0.428b |
| Moderate | 3 (13%) | 25 (35%) | <0.001b |
| Severe | 16 (70%) | 37 (52%) | 0.009b |
Notes: Data are presented as the mean ± standard (range) error or the number of subjects (%).
P-values determined using the Student’s t-test;
P-values determined using the χ2 test.
The PK/PD parameters for VCM in the severe hypoalbuminemia and non-severe hypoalbuminemia groups are also summarized in Table 2. Notably, no significant difference was found between the two groups for the mean trough and peak concentrations, clearance of VCM, and whether a single and daily dose of VCM was administered (Table 2). The percentage of AUC values of >450 μg × h/mL in the severe hypoalbuminemia group was significantly higher than in the non-severe hypoalbuminemia group (9 of 23 patients [39%] vs 6 of 71 patients [9%]; P < 0.001). The half-life of VCM in the severe hypoalbuminemia group was significantly longer than in the non-severe hypoalbuminemia group (33.2 ± 5.4 hours [15–135] vs 24.9 ± 1.6 hours [10–68]; P = 0.049).
Table 2.
Vancomycin pharmacokinetic/pharmacodynamic parameters between severe hypoalbuminemia and non-severe hypoalbuminemia patients with methicillin-resistant Staphylococcus aureus pneumonia
| PK/PD parameters | Severe hypoalbuminemia (n=23) | Non-severe hypoalbuminemia (n=71) | P-value |
|---|---|---|---|
| Cmax (μg/mL) | 26.8 ± 1.8 (11–44) | 25.7 ± 1.0 (12–40) | 0.606a |
| Trough concentration (μg/mL) | 10.9 ± 1.3 (2.7–23) | 9.0 ± 0.7 (1.6–23) | 0.179a |
| AUC/MIC (μg × h/mL) | 426.3 ± 43 (150–789) | 340.1 ± 14.0 (167–784) | 0.066b |
| <250 (μg × h/mL) | 6 (26%) | 11 (15%) | 0.113c |
| 250–450 (μg × h/mL) | 8 (35%) | 54 (76%) | <0.001c |
| >450 (μg × h/mL) | 9 (39%) | 6 (9%) | <0.001c |
| Vd (L) | 64.0 ± 1.1 (51–71) | 62.3 ± 0.7 (50–95) | 0.223a |
| T1/2 (hours) | 33.2 ± 5.4 (15–135) | 24.9 ± 1.6 (10–68) | 0.049a |
| CLr (mL/minute) | 33.7 ± 3.7 (4.8–58.7) | 40.7 ± 2.1 (7.9–91.2) | 0.101a |
| Single dose (mg) | 774 ± 51 (400–1,250) | 815 ± 27 (350–1,250) | 0.456a |
| Daily dose (mg/kg/day) | 19.5 ± 1.6 (6.9–34.5) | 20.4 ± 1.2 (3.4–63.1) | 0.658a |
| Dose interval (hours) | 25.0 ± 1.7 (12–48) | 26.5 ± 1.5 (12–72) | 0.509b |
Notes: Data are presented as the mean ± standard error (range) or the number of subjects (%).
P-values determined using the Student’s t-test;
P-values determined using the Welch test;
P-values determined using the χ2 test.
Abbreviations: AUC, area under the concentration curve; CLr, renal clearance; Cmax, peak concentration; MIC, minimum inhibitory concentration; PD, pharmacodynamic; PK, pharmacokinetic; T1/2, half-life of VCM; VCM, vancomycin; Vd, volume of distribution.
AUC/MIC values of 250–450 and >450 μg × h/mL were significantly associated with 28-day mortality as a primary outcome in the severe hypoalbuminemia patients (P < 0.001, Figure 1); statistical power was 0.968 and 0.778, respectively. AUC/MIC values of <250 μg × h/mL were not associated (P = 0.143, Figure 1).
Figure 1.
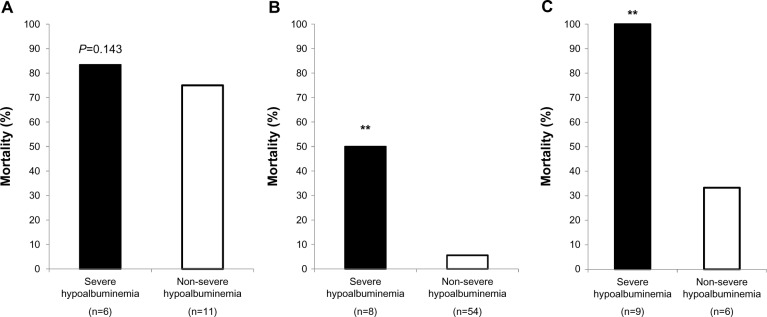
Twenty-eight-day mortality according to stratification of the vancomycin AUC/MIC values in severe hypoalbuminemia and non-severe hypoalbuminemia groups. (A) AUC/MIC <250 μg × h/ml, (B) AUC/MIC = 250–450 μg × h/ml, and (C) AUC/MIC >450 μg × h/ml. P-values were determined using χ2 tests. The AUC values of 250–450 and >450 μg × h/mL were significantly associated with 28-day mortality in patients with severe hypoalbuminemia (**P < 0.001), while AUC values of <250 μg × h/ml were not (P = 0.143).
Abbreviations: AUC, area under the concentration curve; MIC, minimum inhibitory concentration.
We also examined the adverse effects of VCM treatment among the elderly patients and found that 12 of 94 (13%) developed nephrotoxicity after VCM administration. We detected a significant difference in increasing the percentage of nephrotoxicity in the severe hypoalbuminemia group (6 of 23 patients [26%]) compared with the non-severe hypoalbuminemia group (6 of 71 patients [8%]; P < 0.001) (Table 3).
Table 3.
Adverse effects of vancomycin between severe hypoalbuminemia and non-severe hypoalbuminemia patients
| Characteristic | Severe hypoalbuminemia (n=23) | Non-severe hypoalbuminemia (n=71) | P-value |
|---|---|---|---|
| Nephrotoxicity | 6 (26%) | 6 (8%) | <0.001 |
| Liver dysfunction | 0 (0%) | 3 (4%) | 0.245 |
Notes: Data are presented as the number of subjects (%). P-values were determined using the χ2 test.
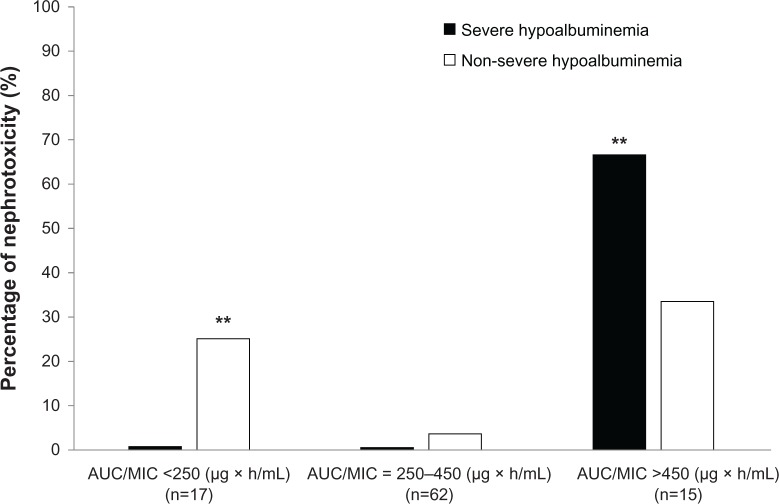
In patients with AUC/MIC values of >450 μg × h/mL, the percentage of nephrotoxicity after VCM administration was significantly higher in the severe hypoalbuminemia group than in the non-severe hypoalbuminemia group (67% vs 33%; P < 0.001) (Figure 2), whereas the percentage of nephrotoxicity in the non-severe hypoalbuminemia group was significantly higher than in the severe hypoalbuminemia group for AUC/MIC values of <250 μg × h/mL.
Figure 2.
Percentage of nephrotoxicity after VCM administration in the severe hypoalbuminemia and non-severe hypoalbuminemia groups.
Notes: P-values were determined using χ2 tests. The percentage of nephrotoxicity in severe hypoalbuminemia group was significantly higher than that in the non-severe hypoalbuminemia group for AUC/MIC values of >450 μg × h/ml (**P < 0.001), whereas the percentage of nephrotoxicity in the non-severe hypoalbuminemia group was significantly higher than in the severe hypoalbuminemia group for AUC/MIC values of <250 μg × h/ml (**P < 0.001).
Abbreviations: AUC, area under the concentration curve; MIC, minimum inhibitory concentration; VCM, vancomycin.
Discussion
This is the first study to have shown that severe hypoalbuminemia influences the half-life of VCM and treatment outcome. Furthermore, patients with severe hypoalbuminemia with AUC/MIC values of >450 μg × h/mL showed evidence of nephrotoxicity.
The volume of distribution and clearance of other highly protein-bound antibacterials, such as teicoplanin, aztreonam, fusidic acid, or daptomycin, were increased in critically ill patients with hypoalbuminaemia.10 Since the protein-binding rate of VCM is lower than that of teicoplanin,11 few studies have investigated whether severe hypoalbuminemia influences the PK/PD parameters of VCM in elderly patients. In the present study, the half-life of VCM was longer in elderly patients with severe hypoalbuminemia than in the patients with non-severe hypoalbuminemia. Furthermore, the percentage of AUC/MIC values of >450 μg × h/mL was increased in the patients with severe hypoalbuminemia (Table 2). Although VCM has a relatively low protein-binding rate, these results indicate that severe hypoalbuminemia might affect the concentration of the free form, and prolong the half-life of VCM in elderly patients.
VCM therapeutic guidelines recommend a target AUC/MIC for VCM of >400 μg × h/mL;3 however, our previous report suggested that AUC/MIC values of 250–450 μg × h/mL were suitable for the treatment of MRSA pneumonia with VCM in elderly patients.4 The reason for this discrepancy is currently unclear. In the present study, the half-life of VCM was extended in the patients with severe hypoalbuminemia, and the AUC/MIC values of VCM were also higher. Moreover, with an increase in AUC/MIC values, the percentage of nephrotoxicity was also increased in patients with severe hypoalbuminemia (Figure 2). Interestingly, AUC/MIC values of <250 μg × h/mL did not influence 28-day mortality in patients with severe hypoalbuminemia, whereas the mortality rate was high in patients with severe hypoalbuminemia with AUC/MIC values of ≥250 μg × h/mL (Figure 1). These results suggest that elderly patients with severe hypoalbuminemia had a high risk of developing nephrotoxicity and 28-day mortality in the case of target AUC/MIC values of >400 μg × h/mL. Elderly patients with infectious diseases are associated with a high incidence of severe hypoalbuminemia, Moreover, hypoalbuminemia can be acute in severely infected patients, and can be entirely independent of baseline nutritional state. Since malnutrition leads to poor outcomes for therapy with VCM, pre-VCM and post-VCM nutrition therapy is important for elderly patients. Although we did not recommend a target AUC/MIC in patients with severe hypoalbuminemia, we consider that elderly patients with low body weight and severe hypoalbuminemia should have their doses individually adjusted by the pharmacist according to those parameters.
A few limitations of our study need to be mentioned here. First, the targeted VCM trough concentration in this study is about 10 μg/mL, which is lower than the guidelines recommended by the Infectious Diseases Society of America/American Society of Health-System Pharmacists: 15–20 μg/mL.12 Moreover, the average weight of patients in the present study was very low. It was necessary to limit the dosage, because study patients had disuse syndrome or low muscle tone. We consider that elderly patients with low body weight and severe hypoalbuminemia should have their dose individually adjusted by the pharmacist according to those parameters. Second, a relatively small number of patients with MRSA pneumonia were enrolled in this study. Third, this study was retrospective. New, large prospective studies are needed to investigate whether nutritional treatment might be useful for VCM therapy in elderly patients.
Conclusion
This is the first study to indicate that severe hypoalbuminemia influences the half-life of VCM and VCM-related treatment outcomes in patients aged 75 years or older with MRSA pneumonia. To establish more effective and safer treatments, the issue of nutritional status of elderly patients needs to be addressed.
Footnotes
Disclosure
The authors reports no conflicts of interest in this work.
References
- 1.Rubinstein E, Kollef MH, Nathwani D. Pneumonia caused by methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2008;46(Suppl 5):S378–S385. doi: 10.1086/533594. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society. Infectious Diseases Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 3.Jeffres MN, Isakow W, Doherty JA, et al. Predictors of mortality for methicillin-resistant Staphylococcus aureus health-care-associated pneumonia: specific evaluation of vancomycin pharmacokinetic indices. Chest. 2006;130:947–955. doi: 10.1378/chest.130.4.947. [DOI] [PubMed] [Google Scholar]
- 4.Martin JH, Norris R, Barras M, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society Of Infectious Diseases Pharmacists. Clin Biochem Rev. 2010;31:21–24. [PMC free article] [PubMed] [Google Scholar]
- 5.Mizokami F, Shibasaki M, Yoshizue Y, Noro T, Mizuno T, Furuta K. Pharmacodynamics of vancomycin in elderly patients aged 75 years or older with methicillin-resistant Staphylococcus aureus hospital-acquired pneumonia. Clin Interv Aging. 2013;8:1015–1021. doi: 10.2147/CIA.S50238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sato S, Saito Y. Evaluation of the usefulness of vancomycin dosage design based on pharmacokinetics/pharmacodynamics theory. Jpn J Chemother. 2007;55:220–224. [Google Scholar]
- 7.Hayashi H, Matsuzaki T, Mizuno Y, et al. Analysis of factors affecting long-term administration of anti-methicillin resistant Staphylococcus aureus (MRSA) drugs. Yakugaku Zasshi. 2009;129:347–352. doi: 10.1248/yakushi.129.347. Japanese. [DOI] [PubMed] [Google Scholar]
- 8.Seki M, Watanabe A, Mikasa K, Kadota J, Kohno S. Revision of the severity rating and classification of hospital-acquired pneumonia in the Japanese Respiratory Society guidelines. Respirology. 2008;13:880–885. doi: 10.1111/j.1440-1843.2008.01348.x. [DOI] [PubMed] [Google Scholar]
- 9.Danan G, Benichou C. Causality assessment of adverse reactions to drugs: I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–1330. doi: 10.1016/0895-4356(93)90101-6. [DOI] [PubMed] [Google Scholar]
- 10.Ulldemolins M, Roberts JA, Rello J, Paterson DL, Lipman J. The effects of hypoalbuminaemia on optimizing antibacterial dosing in critically ill patients. Clin Pharmacokinet. 2011;50:99–110. doi: 10.2165/11539220-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Bailey EM, Rybak MJ, Kaatz GW. Comparative effect of protein binding on the killing activities of teicoplanin and vancomycin. Antimicrob Agents Chemother. 1991;35:1089–1092. doi: 10.1128/aac.35.6.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66:82–98. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]