Abstract
Information about the anti-inflammatory activity and metabolism of α-mangostin (α-MG), the most abundant xanthone in mangosteen fruit, in human cells is limited. On the basis of available literature, we hypothesized that α-MG will inhibit the secretion of pro-inflammatory mediators by control and activated macrophage-like THP-1, hepatic HepG2, enterocyte-like Caco-2, and colon HT-29 human cell lines, as well as primary human monocyte-derived macrophages (MDM), and that such activity would be influenced by the extent of metabolism of the xanthone. α-MG attenuated TNF-α and IL-8 secretion by the various cell lines but increased TNF-α output by both quiescent and LPS-treated MDM. The relative amounts of free and phase II metabolites of α-MG and other xanthones present in media 24 h after addition of α-MG was shown to vary by cell type and inflammatory insult. Increased transport of xanthones and their metabolites across Caco-2 cell monolayers suggests enhanced absorption during an inflammatory episode. The anti-inflammatory activities of xanthones and their metabolites in different tissues merit consideration.
Keywords: α-mangostin, xanthones, mangosteen, inflammation, metabolism, human cells
INTRODUCTION
Garcinia mangostana is a tree native to Southeast Asia that produces a fruit referred to as mangosteen. The aril portion of mangosteen fruit has an acidic, sweet taste that is enjoyed by many, whereas extracts of the pericarp have been used in traditional medicine. The proposed health-promoting properties of pericarp from mangosteen have been associated with a family of compounds referred to as xanthones.1 These hydrophobic compounds have a tricyclic aromatic ring system possessing various mixtures of isoprenyl, hydroxyl, and methoxyl substitutions.2 α-Mangostin (α-MG, Figure 1) and γ-mangostin (γ-MG) are the most abundant xanthones in the pericarp of mangosteen fruit.3,4 In vitro studies have consistently shown xanthones to possess antioxidant,5,6 antiproliferative,7 proapoptotic,8,9 antimicrobial,10 anti-inflammatory,11,12 and anticarcinogenic activities.8,9,13 Anti-inflammatory11,14–16 and anticarcinogenic17–19 activities have also been demonstrated in rodents. As a result of the aggressive marketing of health-promoting activities observed in cellular and rodent models, numerous supplements, beverages, and food products containing mangosteen fruit have become available with sales of beverages alone in 2008 exceeding $200 million in the United States.20
Figure 1.
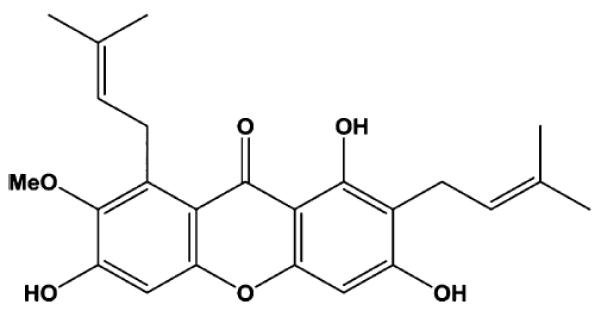
Structure of α-mangostin.
In order for xanthones to exert their proposed health-promoting activities, these compounds or their active metabolites must be delivered to target tissues. We previously reported that α-MG and its phase II metabolites were transported across the basolateral membrane of Caco-2 human intestinal cells, suggesting that a portion of xanthones in mangosteen products likely were bioavailable.21 Indeed, low concentrations of xanthones and their phase II metabolites have been identified in the plasma and urine of healthy adults after consumption of mangosteen juice.22,23 Xanthones and phase II metabolites also have been detected in the plasma and liver of athymic Balb/c nu/nu mice fed an AIN-93G diet containing 900 mg of xanthones/kg,18 as well as in plasma from C57BL/6J mice orally dosed with α-MG.24 Moreover, the presence of α-MG and other xanthones in the HT-29 human colon cell xenografts in mice fed the diet containing α-mangostin was associated with decreased tumor growth and reduced tumor expression of the mitogenic Wnt protein and antiapoptotic bcl-2 protein.18 These data suggest that xanthones and/or their metabolites are absorbed and delivered to various tissues, where they may be accumulated, further metabolized, and modulate cellular processes.
The antiproliferative and proapoptotic activities of xanthones have been demonstrated in numerous in vitro studies using rodent cell lines.7–9,11,12 However, the reported anti-inflammatory activity of xanthones in cells of human origin has been limited to primary cultures of adipocytes25 and the U937 macrophage-like cell line.26 To the best of our knowledge, the uptake and metabolism of these compounds by cells of animal or human origin have not been examined with the exception of differentiated cultures of Caco-2 human intestinal cells.21 Similarly, the effect that the pro-inflammatory condition may have on the metabolism of α-MG has not been addressed.
Although it has been generally assumed that xanthones are stable in cell culture media, many polyphenols degrade spontaneously in vitro and generate products such as hydrogen peroxide, which is known to induce transcriptional activity associated with the anti-inflammatory response.27,28 The first objective of the present study was to investigate the anti-inflammatory activity of α-MG in prototypical human immune cell types and in other human cells originating from tissues responsive to inflammatory insult. This initially required developing appropriate delivery systems to ensure the stability of the xanthone in the different culture media used for the proliferation and activities of the tested cell types. The second objective was to study the metabolism of this xanthone by these cell types maintained in normal and pro-inflammatory environments. To the best of our knowledge, this work is the first to systematically compare the anti-inflammatory activity of α-MG for human cells with various tissue origins, as well as the metabolism of this xanthone under normal and inflammatory conditions.
MATERIALS AND METHODS
Chemicals and Reagents
α-, γ-, and β-mangostins, 9-hydroxycalabaxanthone, gartanin, and garcinones D and E were purified (>98% as assessed by NMR spectroscopy and ESIMS) as described previously.3,5 All solvents (acetonitrile, ethyl acetate, acetic acid, n-butanol, 2-propanol) and water were HPLC grade from Fisher Scientific (Pittsburgh, PA). Cell culture reagents were from Sigma-Aldrich (St. Louis, MO) and Gibco (Grand Island, NY). IL-8 and TNF-α ELISA DuoSet Kits were purchased from R&D Systems (Minneapolis, MN). MCS-F was obtained from Peprotech, Inc. (Rocky Hill, NJ). Fluorochrome-conjugated anti-CD14 and anti-CD11b were purchased from BD Biosciences (San Jose, CA). Ficoll-Paque PLUS was obtained from GE Healthcare (Uppsala, Sweden). Transwell inserts and filters were from Millipore (Billerica, MA). All other reagents and materials were from Sigma-Aldrich.
Cell Cultures
The following human cell lines were purchased from American Type Culture Collection (ATCC, Manassas, VA) for use in this study: THP-1 (monocyte-like leukemia), HepG2 (hepatocellular carcinoma), Caco-2 HTB-37 (colorectal adenocarcinoma cells that spontaneously differentiate to enterocyte-like phenotype), and HT-29 (colorectal adenocarcinoma). Since α-MG has been reported to exert anti-inflammatory activity in murine RAW 264.7 macrophage-like cells,11,12 this cell line was included for comparison with the responses of the human cell types. HepG2 cells (passages 5–15), HT-29 cells (passages 135–139), and RAW 264.7 (unknown passage) were maintained as per ATCC recommendations and used for experiments 2 days after monolayers reached confluency. THP-1 monocytic cells (unknown passage) were differentiated to macrophage-like cells by treatment with 100 nM phorbol myristate acetate (PMA) for 48 h before initiating experiments. Caco-2 cells (passages 20–40) spontaneously differentiated to a phenotype resembling the intestinal epithelium by culturing by 21–25 day postconfluency as previously described.29 Primary cultures of monocytes were prepared from buffy coats obtained from the American Red Cross (Columbus, OH). Mononuclear cells were separated by centrifugation through a Ficoll-Paque PLUS and further purified by the clumping method.30 Monocytes were differentiated to macrophages by treatment with 50 ng/mL M-CSF for 6 days. Monocyte-derived macrophages (MDM) were 97% pure as estimated by flow cytometry after staining cells with anti-CD14 and anti-CD11b as cellular markers. All cultures were maintained at 37 °C in a humidified atmosphere of 95% air/5% CO2.
Stability of α-MG during Incubation in the Cell Culture Environment
As many polyphenolic compounds spontaneously degrade in cell culture media,27,28 we first examined the stability of α-MG solubilized in DMSO and added to the various media lacking FBS. Stability was assessed by incubation in a cell free system for 24 h. Aliquots were collected at 0, 4, 8, and 24 h before extraction and analyzed as described below. Recovery of α-MG from all basal media decreased in proportion to duration of incubation in the absence of cells (Figure 2A). The extent of loss after 24 h ranged from 55 to 97%, depending on the composition of the medium. The calculated rate of α-MG loss per hour during the 24 h incubation was 2.7, 4.0, 3.2, and 2.3% in RPMI, MEM, DMEM, and McCoy's 5A, respectively.
Figure 2.
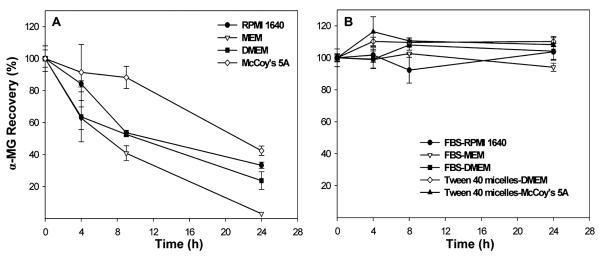
The lack of stability of α-MG in basal media in cell-free dishes (panel A) was offset by addition of the xanthone solubilized in DMSO to media containing 10% FBS or by addition of the xanthone incorporated in Tween 40 to basal media (panel B). Data are mean ± SD for n = 3 dishes with media.
On the basis of the above observation, the influence of delivery vehicle and the presence of FBS in media were tested as possible stabilizers of α-MG during incubation in cell-free media. Dimethyl sulfoxide (DMSO), Tween 40, and FBS were used to prepare stock solutions of the xanthone for addition to media. A stock solution of α-MG was prepared in DMSO before dilution in MEM, DMEM, and RPMI media containing 10% FBS and filter sterilized (0.22 μm pores). The final amount of DMSO in the test media was <0.03%. To prepare Tween 40 micelles containing α-MG, the compound was solubilized in acetonitrile and mixed with 20% Tween 40:80% acetone before evaporating the solvent under a stream of nitrogen gas. After addition of basal media (either DMEM or McCoy's 5A), the mixture was sonicated for 30 min to facilitate incorporation of the xanthone in the Tween micelles. Media were filtered (0.22 μm pores) and further diluted to obtain indicated concentrations of the xanthone. The final content of Tween 40 in test media was <0.02%. FBS was also used as delivery vehicle by incubating pure α-MG with 100% FBS for 48 h at 37 °C in a shaking water bath (85 rpm). This stock solution was then filtered (0.22 μm pores) and diluted 1:10 with RPMI 1640. The stability of α-MG during the 24 h incubation period was increased to 101 ± 6% (p < 0.05) when DMSO containing the xanthone was added to media (RPMI 1640, MEM, and DMEM) with 10% (v/v) FBS. Similarly, recovery of α-MG from DMEM and McCoy's media without FBS after 24 h incubation was 110 ± 3 and 108 ± 4, respectively, when the xanthone was incorporated in Tween 40 micelles. The stability of α-MG was 95 ± 8% after 24 h when the xanthone was added to 100% FBS that was diluted 1:10 (v:v) into RPMI 1640 (Figure 2B). Subsequently, α-MG solubilized in DMSO was delivered to medium containing 10% FBS for macrophage-like THP-1, macrophage-like RAW 264.7, and HepG2 cells, and α-MG was solubilized in Tween 40 micelles added to medium for enterocyte-like Caco-2 cells and HT-29 colonic cells. For MDM cells, α-MG was delivered in 100% FBS to a final concentration of 10% FBS in RPMI 1640 medium.
Cytotoxicity of α-MG
Cell cultures were exposed to increasing concentrations of α-MG to determine cytotoxicity. Viability of RAW 264.7, MDM, HepG2, and HT-29 was assessed after 24 h, whereas that of THP-1 and Caco-2 cells was determined after 8 and 4 h, respectively. Times of exposure matched those used in experiments addressing anti-inflammatory activity and metabolism described below. Examination of cell morphology by phase contrast microscopy and reduction of MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] to formazan were used as indicators of cytotoxicity as this reaction is dependent on mitochondrial respiration.31
Anti-Inflammatory Activity of α-MG
The anti-inflammatory activity of α-MG was studied by measuring its inhibitory effects on the secretion of pro-inflammatory mediators. Preliminary studies were performed for each cell type to establish nontoxic concentrations and appropriate periods for preincubation with α-MG, as well as the concentrations and duration of exposure to pro-inflammatory stimuli. Cells were incubated in media containing concentrations of α-MG well below the IC50 to determine the effect, if any, on responsiveness to pro-inflammatory insult. Experimental conditions for determining the anti-inflammatory activity of α-MG are presented in Table 1. As Caco-2 cells were grown on Transwell membrane inserts, α-MG was added to the apical compartment before addition of IL-1β to the basolateral compartment. IL-8 was selected as biomarker of inflammation in Caco-2 and HT-29 cells given its essential role as a chemoattractant during intestinal inflammation.32 IL-8 also served as the marker for THP-1 macrophage-like cells as it is the predominant chemokine in supernatants of LPS-treated cells.33 TNF-α is a key mediator of hepatic physiology and pathology, and its secretion was selected as the marker for PMA-stimulated HepG2 cells.34 Because TNF-α secretion by tissue macrophages has been proposed as a key mediator of LPS-triggered septic shock, this inflammatory cytokine was selected as a marker in MDM cells.35 Finally, medium nitrite concentration was used as a surrogate for the induction of iNOS (inducible nitric oxide synthase) activity in RAW 264.7 cells. IL-8 and TNF-α were quantified by ELISA as instructed by the manufacturer. Nitrite concentration in medium was measured by the Griess reaction.36
Table 1.
Experimental Conditions for Assessing the Anti-Inflammatory Activity of α-Mangostin
| α-MG pretreatment |
pro-inflammatory stimulus |
||||||
|---|---|---|---|---|---|---|---|
| cell type | delivery vehicle | IC50 (μM) | μM | h | insult (ng/mL) | h | inflammatory marker |
| RAW 264.7 | 10% FBS | 23 | 10 | 2 | LPSa (5) | 16 | nitric oxide |
| THP-1 | 10% FBS | 23 | 10 | 4 | LPS (0.1) | 4 | IL-8 |
| MDM | 10% FBS | >12 | 4.5 | 4 | LPS (100) | 10 | TNF-α |
| HepG2 | 10% FBS | 25 | 7 | 16 | PMAb (50) | 4 | TNF-α |
| Caco-2 | Tween 40 | >80 | 15 | 4 | IL-1β (5) | 16 | IL-8c |
| HT-29 | Tween 40 | 180 | 10 | 1 | LPS (100) | 16 | IL-8 |
Lipopolysaccharide (LPS) from Escherichia coli 0111:B4.
Phorbol 12-myristate 13-acetate.
Measured in basolateral medium.
Cellular Uptake and Metabolism of α-MG
Cells were exposed to nontoxic concentrations of α-MG (5.2, 3.3, 7.7, 9.0, 12.0, and 8.5 μM, for THP-1, MDM, RAW 264.7, HepG2, Caco-2, and HT-29 cells, respectively), using previously established delivery systems (Figure 2B) to ensure stability of the xanthone in culture. After 24 h, spent medium and cells were collected separately for analysis, except for RAW 264.7 cells, where both cells and medium were collected in the same tube, as some cells were nonadherent at confluency. For cultures with Caco-2 cells, medium with α-MG was added to the apical compartment, and medium from both the basolateral and apical compartments along with cell monolayer were collected separately for analysis. Spent medium and cells were extracted as previously described21 prior to reverse-phase high-performance liquid chromatography (RP-HPLC).3 Glucuronidated/sulfated metabolites of α-MG were determined as previously described.21 Identification and quantification of xanthones was based on retention time, UV spectrum, and five-point standard curves using pure (>98%) α-, γ-, and β-mangostins; 9-hydroxycalabaxanthone; gartanin; and garcinones D and E. When pure compounds were not available, identification was made by comparison with the retention time and UV spectrum reported in the literature3,4 and the concentration estimated as α-MG equivalents. Peaks with a xanthone-like spectra (λmax = 240–300 and 310–370 nm)4 that could not be matched to one of the standards were labeled as unknown.
Effect of Inflammation on Uptake and Metabolism
To study the effect of inflammatory conditions on the metabolism of α-MG, cells were exposed to the following inflammatory insults: 5 ng/mL LPS for THP-1 and RAW 264.7 cells, 100 ng/mL LPS for MDM, 10 ng/mL TNF-α for HepG2 cells, 5 ng/mL IL-1β for Caco-2 cells, and 800 ng/mL LPS for HT-29. LPS was used at higher concentrations in cultures with THP-1 and HT-29 cells than those used for the anti-inflammatory experiments in order to ensure a robust proinflammatory state. Since HepG2 cells secrete TNF-α in response to PMA treatment, we used this cytokine to induce a pro-inflammatory state. After 4 h, media containing inflammatory stimuli were aspirated and cells washed with sterile PBS. Cultures were then incubated in medium containing α-MG at concentrations similar to those used in cultures under control conditions. Spent media and washed cells were collected after 24 h for analysis as above.
Statistical Analysis
The number of independent cultures tested per cell line for each experiment was ≥5 and each experiment was repeated at least twice. Data are reported as mean ± standard deviation (SD). For analysis, one-way ANOVA was performed followed by Tukey post hoc in order to determine differences between treatments. A p-value of less than 0.05 was considered statistically significant. Statistical analyses were performed using Minitab 16.2.2.
RESULTS
Anti-Inflammatory Activity of α-MG
α-MG was previously reported to exert an anti-inflammatory effect on murine RAW 264.7 macrophage-like cells.11,12 We initially monitored the effect of treatment with α-MG on LPS-induced generation of nitric oxide (NO) in RAW 264.7 cultures for comparison with its effect on the response of various cell lines to pro-inflammatory insult. Exposure to LPS increased NO production 36-fold above that in control cultures. Concentrations of 1, 3, and 10 μM α-MG inhibited LPS-induced production of NO by 50, 61 and 78%, respectively, significantly (p < 0.05) exceeding the extent of inhibition (30%) mediated by 200 μM L-NAME, a widely used nonspecific inhibitor of iNOS (data not shown).
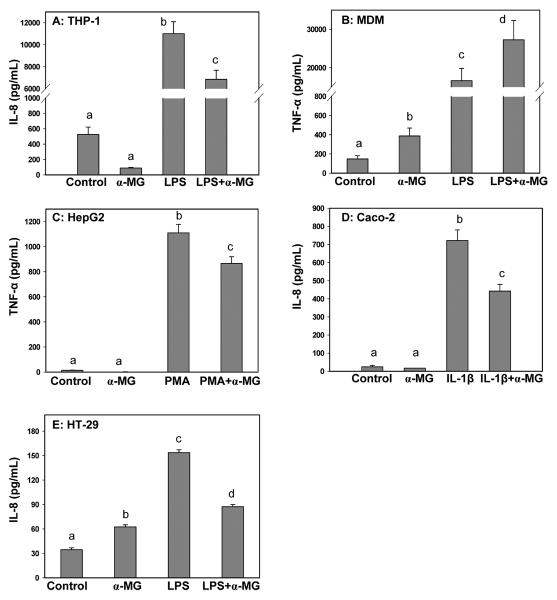
The effect of α-MG on the secretion of inflammatory mediators was dependent on human cell type and state of cellular activation (Figure 3). Pretreatment with α-MG significantly (p < 0.05) attenuated IL-8 secretion by activated macrophage-like THP-1 (Figure 3A), enterocyte-like Caco-2 (Figure 3D) and colonic HT-29 (Figure 3E) cells, and TNF-α secretion by PMA-activated HepG2 cells (Figure 3C). In contrast, pretreatment of MDM cells with α-MG increased LPS-stimulated secretion of TNF-α more than 50% (Figure 3B). When added to nonactivated cultures, α-MG also more than doubled basal secretion of TNF-α by MDM cells (Figure 3B) and IL-8 by HT-29 cells (Figure 3E), suggesting proinflammatory activity. Concentrations of α-MG as low as 0.7 μM also significantly (p < 0.05) increased TNF-α secretion by nonactivated MDM cells, although secretion of this cytokine was similar (p > 0.05) in LPS-treated cultures in the absence and presence of 0.7 μM α-MG (data not shown). Secretion of TNF-α in nonactivated cultures of HepG2 (Figure 3C) and secretion of IL-8 by nonactivated cultures of THP-1 (Figure 3A) and Caco-2 cells (Figure 3D) was not altered by the presence of α-MG in medium.
Figure 3.
α-MG attenuates secretion of inflammatory mediators in activated human cell lines but exacerbates TNF-α secretion by LPS-treated cultures of human monocyte-derived macrophages (MDM). THP-1 and MDM cells were differentiated to macrophage-like phenotype and Caco-2 cells differentiated to an enterocyte-like phenotype. Data are mean ± SD, for n ≥ 5. Different letters above bars in a panel indicate significant differences at p < 0.05.
Cell Uptake and Metabolism of α-MG in Nonactivated Cultures
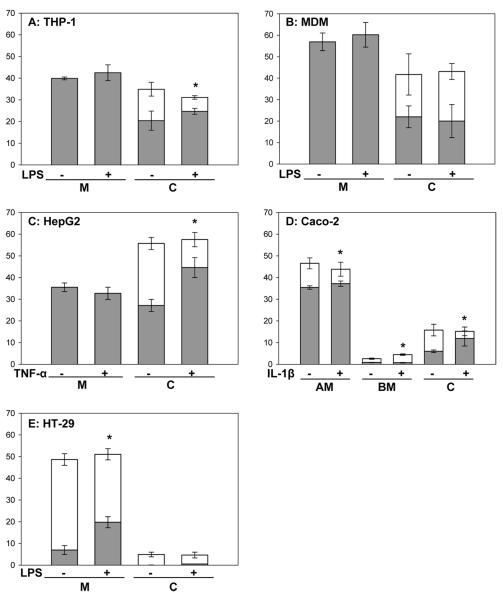
The reported anti-inflammatory activities of α-mangostin have assumed that the compound added to cell culture media or administered either by diet, gastric gavage, or injection in preclinical models is responsible for the observed effects. Although limited, recent data suggests that dietary xanthones are poorly absorbed.22–24,37 There is increased awareness that metabolites of “bioactive” dietary compounds often are the modulators of observed biological effects.38–40 Thus, we tested whether the observed anti-inflammatory activity of α-MG with the human cell lines was associated with the extent of its metabolism and profile of metabolites. Analysis of both media and cells indicated that α-MG was taken up by all tested cell lines. Glucuronide/sulfate conjugates of α-MG were identified in either both medium and cells or only in cells as described below. Xanthones other than α-MG also were present despite their absence in medium added to cultures or in medium incubated for the same period of time in the absence of cells. These xanthones were detected in cultures of RAW 264.7, THP-1, HepG2, Caco-2, and HT-29 cells, but not in MDM.
Analysis of combined medium and cells revealed that RAW 264.7 murine macrophage-like cultures, our reference cell line, internalized and metabolized α-MG. Glucuronidated/sulfated conjugates (61 ± 6%) were the primary metabolites, with free α-MG accounting for 18 ± 3% of the initial concentration present in cultures. In addition, relatively low amounts of garcinone C and an unknown xanthone were detected. Recovery of α-MG was 80 ± 6% after 24 h incubation.
Free xanthones (20 ± 4% of initial α-MG) and phase II conjugates (14 ± 3%) were present in THP-1 cells, whereas only free xanthones (40 ± 1%) were present in spent medium (Figure 4A). Garcinone D and three unknown xanthones also were detected in the cells (Table 3). Recovery of xanthones after 24 h incubation was 75 ± 6% of the amount of α-MG added to cultures. Only free xanthones also were detected in spent medium from MDM and HepG2 cultures and accounted for 57 ± 4% and 36 ± 2% of initial amounts of α-MG, respectively. Both free and glucuronidated/sulfated conjugates of α-MG were present in MDM cells (22 ± 5% and 20 ± 10% of total, respectively) (Figure 4B) and HepG2 cells (27 ± 3% and 29 ± 3% of total, respectively) (Figure 4C). Other xanthones including 9-hydroxycalabaxanthone, a calabaxanthone-like compound, and several unknown xanthones also were detected in spent medium and cells in HepG2 cultures (Tables 2 and 3), but not in cultures of MDM cells. Recovery of α-MG equivalents added to cultures of MDM and HepG2 was 99 ± 12% and 91 ± 4%, respectively.
Figure 4.
Metabolism of α-MG in control and activated cell cultures. Data (mean ± SD, n ≥ 5) represent the amounts of free xanthones and their glucuronidated/sulfated metabolites in medium and cells as a percentage of the initial amount of α-MG added to each culture. Dark bars represents free xanthones and open bars indicate glucuronidated/sulfated metabolites. M: medium, C: cells, AM: apical medium, BM: basolateral medium. THP-1 and MDM cells were differentiated to macrophage-like cells and Caco-2 cells differentiated to an enterocyte-like phenotype. *Denotes statistically significant difference in free:conjugated ratio in activated cultures when compared to control cultures (p < 0.05).
Table 3.
Intracellular Profile of Xanthones 24 h after Addition of α-MG to Control Cultures of Human Cell Typesa
| xanthone | THP-1 (pmol/dish) | HepG2 (pmol/dish) | Caco-2 (pmol/dish) | HT-29 |
|---|---|---|---|---|
| α-MG (0 h) | 33710 ± 161 | 17834 ± 115 | 20741 ± 261 | 16517 ± 373 |
| α-MG (24 h) | 11439 ± 995 | 9344 ± 298 | 3000 ± 551 | 807 ± 178 |
| garcinone D | 66 ± 2 | ndb | nd | nd |
| calabaxanthone derivativec | nd | 56 ± 10 | nd | nd |
| 9-hydroxycalabaxanthone | nd | 44 ± 5 | 100 ± 17 | nd |
| unknownsd | 355 ± 57 | 732 ± 174 | 170 ± 12 | nd |
Data are mean ± SD for n ≥ 5 cultures per cell type.
nd: not detected.
Quantity estimated by using a 9-hydroxycalabaxanthone calibration curve.
Quantity estimated as α-MG equivalents.
Table 2.
Profile of Xanthones in Media of Human Cell Cultures 24 h after Addition of α-MGa
| xanthone | THP-1 (pmol/dish) | HepG2 (pmol/dish) | Caco-2 (pmol/dish) | HT-29 |
|---|---|---|---|---|
| α-MG (0 h) | 33710 ± 161 | 17834 ± 115 | 20741 ± 261 | 16517 ± 373 |
| α-MG (24 h) | 13215 ± 212 | 4688 ± 354 | 9366 ± 510 | 7512 ± 410 |
| garcinone Cb | ndc | nd | 339 ± 41 | 273 ± 80 |
| garcinone D | nd | nd | nd | 461 ± 115 |
| calabaxanthone derivatived | nd | 173 ± 17 | nd | nd |
| 9-hydroxycalabaxanthone | nd | 44 ± 10 | 80 ± 7 | nd |
| unknownse | nd | 1190 ± 127 | 407 ± 18 | 502 ± 170 |
Data are mean ± SD for n ≥ 5 cultures per cell type.
Quantity estimated by using a garcinone D calibration curve.
nd: not detected.
Quantity estimated by using a 9-hydroxycalabaxanthone calibration curve.
Quantities estimated as α-MG equivalents.
Apical medium containing 12 μM α-MG was added to cultures of differentiated Caco-2 cells on Transwell inserts and incubated for 24 vh. Xanthones in apical medium, basolateral medium, and cells accounted for 47 ± 3% (35 ± 1% free and 11 ± 2% conjugates), 3 ± 0.3% (0.8 ± 0.1% free and 1.7 ± 0.3% conjugated), and 16 ± 3% (6 ± 0.6% free and 10 ± 3% conjugated) of the initial amount of α-mangostin, respectively. In addition, garcinone C, 9-hydroxycalabaxanthone, and two unknown xanthones were detected in Caco-2 cultures (Tables 2 and 3). Recovery of xanthones represented approximately 65% of the amount of α-MG added to the cultures, suggesting the presence of other unknown metabolites (Figure 4D).
HT-29 cells metabolized the majority of α-MG to phase II conjugates that were present in both medium and cells, accounting for 42 ± 3% and 5 ± 1%, respectively, of α-MG added to the cultures. Garcinones C and D and an unknown xanthone also were detected in spent medium (Table 2). Free xanthones accounted for 7 ± 2% of initial amount of α-MG in the medium, but only traces of these nonconjugated xanthones were detected in cells (Figure 4E). Recovery of α-MG after incubation in cultures of HT-29 cells was only 54 ± 4%.
The above data generally show that α-MG was transported and metabolized to phase II compounds by all human cell types and converted into other xanthones by the transformed human cell lines but not by the primary cultures of MDM cells.
Uptake and Metabolism of α-MG in Response to Inflammatory Insult
As α-MG was shown to attenuate secretion of IL-8 and TNF-α by the activated cell lines, we next examined whether the metabolism of this xanthone was altered in a pro-inflammatory environment. Cultures were exposed to cell-specific inflammatory stimuli before addition of α-MG to media. After 24 h, media and cells were analyzed for xanthones and their metabolites. The total amounts of xanthones in media and cells were similar to that in nonactivated cultures (Figure 4). However, the inflammatory environment was associated with an increased intracellular ratio of free to conjugated xanthones in THP-1 (Figure 4A), HepG2 (Figure 4C), and Caco-2 cells (Figure 4D), as well as in medium of HT-29 cultures (Figure 4E) and apical medium in Caco-2 cell cultures (p < 0.05). In contrast, the amount of xanthones and the ratio of conjugated to free xanthones transported into the basolateral compartment by Caco-2 cells were increased by treatment with IL-1β (p < 0.05) (Figure 4D). The ratio of free to conjugated xanthones in RAW 264.7 cultures also was increased by LPS treatment (data not shown). Treatment of MDM cells with LPS did not significantly alter the relative amounts of free xanthones and phase II metabolites of xanthones either in medium or intracellularly (Figure 4B).
DISCUSSION
The goal of this study was to examine the in vitro anti-inflammatory activity and metabolism of α-MG in cells of human origin. Stability of α-MG was maintained by delivering the xanthone into media containing FBS or by solubilizing the xanthone in Tween 40 micelles for addition to serum-free media. Noncytotoxic concentrations of α-MG attenuated secretion of IL-8 and TNF-α by the various human cell lines. Influx and metabolism of α-MG to phase II metabolites and/or other xanthone products occurred with all cell lines. The extent of metabolism and types of products depended on cell type. In marked contrast with the results from the various cell lines, α-MG stimulated TNF-α secretion in both control and activated primary cultures of human MDM cells, and only glucuronidated/sulfated metabolites were detected in these cells. The results suggest that metabolic conversion of α-MG to other xanthones may be necessary for anti-inflammatory activity. As Caco-2 cells have been shown to take up and transport metabolites of α-MG across the basolateral membrane21 and metabolites of xanthones were present in sera soon after ingesting mangosteen juice,23 it is expected that peripheral tissues are exposed to both free and conjugated xanthones.
Epidemiological evidence suggests that a diet rich in fruit and vegetables prevents or may delay onset of cardiovascular disease, diabetes, and cancer.41–44 These effects have been attributed, in part, to plant-derived polyphenolic compounds.45 Xanthones from mangosteen fruit, for instance, exhibit anti-inflammatory11,12 and anticarcinogenic8,9,13 activities in vitro. Consideration of the in vitro stability of phytochemicals when assessing bioactivities is often neglected, despite the fact that many polyphenolic compounds readily react with components of cell culture media to generate H2O2, quinones, and semiquinones capable of inducing alterations in cellular activities.27,28 As part of our preliminary analysis, we found that α-MG was unstable when introduced into different basal media in the commonly used DMSO vehicle. Previous cellular studies reporting the in vitro anti-inflammatory activity of α-MG bioactivities used DMSO to deliver the xanthone to medium.11,12 It is unclear if FBS was present, and the stability of α-MG in these studies was not reported. The report that addition of catalase to medium inhibited γ-mangostin-mediated apoptosis in human malignant glioma cells supports the likelihood that spontaneous degradation of xanthones in medium can induce alterations in cellular activities.46 Thus, our first goal was to stabilize α-MG in cell culture in order to test its in vitro anti-inflammatory activity and cellular metabolism. Previous investigators have used Tween 40 to solubilize and stabilize β-carotene47 and fetal bovine serum for delivery of lycopene48 to cultured cells. We found that degradation of α-MG in media was prevented by incorporating α-MG in Tween 40 micelles, in media containing FBS, and by direct addition to FBS prior to its dilution in media (Figure 2). As lipophilic compounds are incorporated into micelles during digestion before being transported into enterocytes, Tween 40 micelles were used to deliver α-MG to enterocyte-like Caco-2 cells and colonic HT-29 cells. Because liver and immune cells are exposed to plasma components in vivo, fetal bovine serum was selected as the delivery vehicle for HepG2, THP-1, MDM, and RAW 264.7 cells.
Previous studies examining the anti-inflammatory activity of α-MG in human cells have been limited to primary adipocytes25 and macrophage-like U937 lymphoma cells,26 where α-MG decreased LPS-induced expression of inflammatory genes. As the focus of our study was the anti-inflammatory activity of α-MG, we selected the human immune macrophage-like THP-1 cell line and primary monocyte derived macrophages (MDM) from human peripheral blood for investigation. HepG2 cells were included in our study because hepatic epithelial cells synthesize and secrete greater amounts of cytokines and acute phase proteins in response to pro-inflammatory signals, and our lab has identified xanthones and their metabolites in hepatic tissue of mice fed a diet containing 900 mg of α-MG/kg.18 Differentiated cultures of Caco-2 human intestinal cells possess enterocyte-like characteristics, including a variety of host defense activities such as antigen-processing and presentation and secretion of a battery of cytokines and chemokines in response to pro-inflammatory insult.49 Furthermore, we recently reported that α-MG inhibits colon HT-29 tumorigenicity in vitro and in vivo.18 Thus, these two colonic cell lines also were included in the study. Finally, because α-MG has been reported to inhibit the LPS-induced synthesis of NO by murine RAW 264.7 macrophage-like cells,11,12 the metabolism of the xanthone by these cells was considered for comparison with that by the human cells.
Our results showed that the impact of α-MG on the human cell lines differed from that on the primary MDM. Treatment of activated THP-1, Caco-2, and HT-29 cells with α-MG inhibited IL-8 chemokine secretion by 30–40%. Similarly, α-MG attenuated TNF-α secretion by 22% in PMA-activated cultures of HepG2 cells. In contrast, α-MG enhanced TNF-α secretion by both quiescent and activated primary cultures of MDM. This stimulatory effect with quiescent cultures was observed even when the concentration of the xanthone was less than 1 μM. This lower concentration is more in line with those we found in serum of healthy adults following ingestion of a mangosteen juice product23 and in mice after oral administration of α-MG in cottonseed oil.24 Possible explanations for different responses of the cell lines and the primary MDM cells to α-MG may include differences in phenotype, tissue origin, and metabolism by transformed versus normal cells. Our experimental design is limited by the selection of only two inflammatory markers, viz., secretion of IL-8 and TNF-α, to assess the activity of α-MG. More detailed studies, and particularly in vivo studies, are needed to further characterize the pro- and anti-inflammatory effects of xanthones. In this regard, increased concentrations of inflammatory cytokines IL-1α and IL-1β, as well as higher T helper cell frequency and increased levels of complement C3 and C4, were found after healthy human subjects consumed 59 mL of a blended juice product containing mangosteen for 30 days.50 The possibility that the proposed health-promoting effects of dietary xanthones are mediated by induction of an adaptive stress or “hormetic” response in nontransformed cells merits investigation,51,52 as does the possibility that xanthones may be detrimental for some inflammatory disorders.
Although there is extensive literature demonstrating the health-promoting properties of phytochemicals in vitro, many of these compounds undergo extensive first-pass metabolism to glucuronidated, sulfated, and methylated conjugates in vivo. Although such metabolites are generally assumed to be inactive,53,54 recent evidence suggests that phase II metabolites exert some of the activities attributed to their parent compounds.33–35,55 We previously reported the presence of glucuronidated/sulfated metabolites of α-MG as early as 1 h after incubation of cells with the xanthone.21 We also observed these metabolites in cultures of RAW 264.7 cells 8 h after addition of α-MG (data not shown). These observations suggest that metabolites and/or the parent compound α-MG may affect early events in signaling pathways involved in synthesis of pro-inflammatory mediators. The present results agree with our recent reports that Caco-2 cells,21 mice,18,24 and human subjects23 metabolize α-MG to phase II products. In addition to phase II metabolites, other xanthones such as garcinones C and D, 9-hydroxycalabaxanthone, a calabaxanthone derivative, and several unknown xanthone compounds were detected in our analysis with the human cell lines. Many of these identified metabolites of α-MG are also present in mangosteen pericarp, suggesting that mixtures of xanthones may have greater efficacy than individual compounds. Furthermore, recovery of α-MG in cultures for several of the human cell types was incomplete, suggesting the presence of additional metabolites. It also is possible that we underestimated xanthone conjugates due to incomplete enzymatic hydrolysis by the mollusc preparation due to the nature and position of substitutions on the tricyclic aromatic compounds, binding of conjugated xanthones to proteins, localization within organelles, and the presence of natural inhibitors.56 More robust analyses are required to further characterize xanthone metabolism and the bioactivities of the various metabolites.
During an inflammatory insult, the ratio of free to conjugated xanthones increased in THP-1, HepG2, and Caco-2 cells and in the media of HT-29 and Caco-2 cell cultures. LPS has been reported to increase intracellular and secreted β-glucuronidase activity in RAW 267.4 cells, causing enhanced hydrolysis of quercetin glucuronides.57 Similarly, increased serum β-glucuronidase activity in LPS-treated rats was associated with an increase in deconjugation of luteolin glucuronide.58 Glucuronidase activity was increased in media of LPS-activated THP-1 and RAW 264.7 cells (data not shown). These observations may explain the greater concentration of free xanthones in cells and media when α-MG was added to activated cultures. Also, transport of xanthones (mainly conjugates) across the Caco-2 monolayer was increased when cells were pretreated with IL-1β, supporting the possibility that xanthones may be more bioavailable during inflammatory episodes. The possible use of xanthones as coadjuvants for the treatment of chronic inflammatory conditions merits consideration.
The results for the present investigation show that α-MG attenuates the secretion of pro-inflammatory mediators by activated human cell lines of diverse tissue origin. However, this xanthone stimulates the secretion of TNF-α by primary cultures of quiescent and activated monocyte-derived human macrophages. We also show that α-MG was transported into cells, where it undergoes phase II metabolism and other metabolic processes in a manner that is dependent on cell type and inflammatory status. We speculate that these metabolites may be immunomodulatory either by stabilizing the parent compound or by directly exerting anti- or pro-inflammatory activity. Finally, we also show that inflammatory conditions may alter cellular metabolism of α-MG and possibly increase its bioavailability.
Acknowledgments
Funding This research was partially funded by The Ohio State University Comprehensive Cancer Center/Molecular Carcinogenesis and Chemoprevention Program and Food Innovation Center. F.G-O was supported in part by CONACyT (Mexico) doctoral fellowship.
ABBREVIATIONS USED
- α-MG
α-mangostin
- MDM
monocyte-derived macrophages
- LPS
lipopolysaccharide
- RP-HPLC
reverse-phase high-performance liquid chromatography
- MCS-F
macrophage colony stimulating factor
- PMA
phorbol myristate acetate
- LC–MS
liquid chromatography–mass spectroscopy
- NMR
nuclear magnetic resonance
- DMSO
dimethyl sulfoxide
- FBS
fetal bovine serum
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NO
nitric oxide
- iNOS
inducible nitric oxide synthase
- PBS
phosphate-buffered saline
- TNF-α
tumor necrosis factor-α
- IL-β
interleukin-1β
- IL-8
interleukin-8
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Pedraza-Chaverri J, Cárdenas-Rodríguez N, Orozco-Ibarra M, Pérez-Rojas JM. Medicinal properties of mangosteen (Garcinia mangostana) Food Chem. Toxicol. 2008;46:3227–39. doi: 10.1016/j.fct.2008.07.024. [DOI] [PubMed] [Google Scholar]
- (2).Obolskiy D, Pischel I, Siriwatanametanon N, Heinrich M. Garcinia mangostana L.: A phytochemical and pharmacological review. Phytother. Res. 2009;23:1047–1065. doi: 10.1002/ptr.2730. [DOI] [PubMed] [Google Scholar]
- (3).Chaivisuthangkura A, Malaikaew Y, Chaovanalikit A, Jaratrungtawee A. Prenylated xanthone composition of Garcinia mangostana (mangosteen) fruit hull. Chromatographia. 2009;69:315–318. [Google Scholar]
- (4).Walker EB. HPLC analysis of selected xanthones in mangosteen fruit. J. Sep. Sci. 2007;30:1229–1234. doi: 10.1002/jssc.200700024. [DOI] [PubMed] [Google Scholar]
- (5).Jung HA, Su BN, Keller W, Mehta RG, Kinghorn AD. Antioxidant xanthones from pericarp of Garcinia mangostana (Mangosteen) J. Agric. Food Chem. 2006;54:2077–2082. doi: 10.1021/jf052649z. [DOI] [PubMed] [Google Scholar]
- (6).Williams P, Ongsakul M, Proudfoot J, Croft K, Beilin L. Mangostin inhibits the oxidative modification of human low density lipoprotein. Free Radical Res. 1995;23:175–184. doi: 10.3109/10715769509064030. [DOI] [PubMed] [Google Scholar]
- (7).Akao Y, Nakagawa Y, Iinuma M, Nozawa Y. Anti-cancer effects of xanthones from pericarps of mangosteen. Int. J. Mol. Sci. 2008;9:355–370. doi: 10.3390/ijms9030355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Matsumoto K, Akao Y, Kobayashi E, Ohguchi K, Tanaka T, Iinuma M, Nozawa Y. Induction of apoptosis by xanthones from mangosteen in human leukemia cell lines. J. Nat. Prod. 2003;66:1124–1127. doi: 10.1021/np020546u. [DOI] [PubMed] [Google Scholar]
- (9).Nakagawa Y, Ilinuma M, Naoe T, Nozawa Y, Akao Y. Characterized mechanism of α-mangostin-induced cell death: Caspase-independent apoptosis with release of endonuclease-G from mitochondria and increased miR-143 expression in human colorectal cancer DLD-1 cells. Bioorg. Med. Chem. 2007;15:5620–5628. doi: 10.1016/j.bmc.2007.04.071. [DOI] [PubMed] [Google Scholar]
- (10).Suksamrarn S, Suwannapoch N, Phakhodee W, Thanuhiranlert J, Ratananukul P, Chimnoi N, Suksamrarn A. Antimycobacterial activity of prenylated xanthones from the fruits of Garcinia mangostana. Chem. Pharm. Bull. 2003;51:857–859. doi: 10.1248/cpb.51.857. [DOI] [PubMed] [Google Scholar]
- (11).Chen LG, Yang LL, Wang CC. Anti-inflammatory activity of mangostins from Garcinia mangostana. Food Chem. Toxicol. 2008;46:688–693. doi: 10.1016/j.fct.2007.09.096. [DOI] [PubMed] [Google Scholar]
- (12).Tewtrakul S, Wattanapiromsakul C, Mahabusarakam W. Effects of compounds from Garcinia mangostana on inflammatory mediators in RAW264.7 macrophage cells. J. Ethnopharmacol. 2009;121:379–382. doi: 10.1016/j.jep.2008.11.007. [DOI] [PubMed] [Google Scholar]
- (13).Lee YB, Ko KC, Shi MD, Liao YC, Chiang TA, Wu PF, Shih YX, Shih YW. α-Mangostin, a novel dietary xanthone, suppresses TPA-mediated MMP-2 and MMP-9 expressions through the ERK signaling pathway in MCF-7 human breast adenocarcinoma cells. J. Food Sci. 2010;75:H13–23. doi: 10.1111/j.1750-3841.2009.01407.x. [DOI] [PubMed] [Google Scholar]
- (14).Shankaranarayan D, Gopalak C, Kameswaran L. Pharmacological profile of mangostin and its derivatives. Arch. Int. Pharmacodyn. 1979;239:257–269. [PubMed] [Google Scholar]
- (15).Nakatani K, Yamakuni T, Kondo N, Arakawa T, Oosawa K, Shimura S, Inoue H, Ohizumi Y. γ-Mangostin inhibits inhibitor-κB kinase activity and decreases lipopolysaccharide-induced cyclooxygenase-2 gene expression in C6 rat glioma cells. Mol. Pharmacol. 2004;66:667–674. doi: 10.1124/mol.104.002626. [DOI] [PubMed] [Google Scholar]
- (16).Jang H, Kwon O, Oh S, Lee H, Ahn K. Mangosteen xanthones mitigate ovalbumin-induced airway inflammation in a mouse model of asthma. Food Chem. Toxicol. 2012;50:4042–4050. doi: 10.1016/j.fct.2012.08.037. [DOI] [PubMed] [Google Scholar]
- (17).Shan T, Ma Q, Guo K, Liu J, Wang F, Wu E. Xanthones from mangosteen extracts as natural chemopreventive agents: Potential anticancer drugs. Curr. Mol. Med. 2011;11:666–677. doi: 10.2174/156652411797536679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Chitchumroonchokchai C, Thomas-Ahner J, Li J, Riedl KM, Nontakham J, Suksumrarn S, Clinton SK, Kinghorn AD, Failla ML. Anti-tumorigenicity of dietary α-mangostin in a HT-29 colon cell xenograft model and the tissue distribution of xanthones and their phase II metabolites. Mol. Nutr. Food Res. 2013;57:203–211. doi: 10.1002/mnfr.201200539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Johnson JJ, Petiwala SM, Syed DN, Rasmussen JT, Adhami VM, Sliddiqui IA, Kohl AM, Mukhtar H. α-Mangostin, a xanthone from mangosteen fruit, promotes cell cycle arrest in prostate cancer and decreases xenograft tumor growth. Carcinogenesis. 2012;33:413–419. doi: 10.1093/carcin/bgr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Sloan EW. Getting ahead of the curve: Phytochemicals. Nutraceutical World. 2010;13:16–17. [Google Scholar]
- (21).Bumrungpert A, Kalpravidh R, Suksamrarn S, Chaivisuthangkura A, Chitchumroonchokchai C, Failla ML. Bioaccessibility, biotransformation, and transport of α-mangostin from Garcinia mangostana (Mangosteen) using simulated digestion and Caco-2 human intestinal cells. Mol. Nutr. Food Res. 2009;53:S54–S61. doi: 10.1002/mnfr.200800260. [DOI] [PubMed] [Google Scholar]
- (22).Kondo M, Zhang L, Hongping J, Kou Y, Ou B. Bioavailability and antioxidant effects of a xanthone-rich mangosteen (Garcinia mangostana) products in humans. J. Agric. Food Chem. 2009;57:8788–8792. doi: 10.1021/jf901012f. [DOI] [PubMed] [Google Scholar]
- (23).Chitchumroonchokchai C, Riedl KM, Suksumrarn S, Clinton SK, Kinghorn DA, Failla ML. Xanthones in mangosteen juice are absorbed and partially conjugated by healthy adults. J. Nutr. 2012;142:675–680. doi: 10.3945/jn.111.156992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Ramaiya A, Li G, Petiwala SM, Johnson JJ. Single dose oral pharmacokinetic profile of a-mangostin in mice. Curr. Drug Targets. 2012;13:1698–1704. doi: 10.2174/138945012804545524. [DOI] [PubMed] [Google Scholar]
- (25).Bumrungpert A, Kalpravidh RW, Chitchumroonchokchai C, Chuang C, West T, Kennedy A, McIntosh M. Xanthones from mangosteen prevent lipopolysaccharide-mediated inflammation and insulin resistance in primary cultures of human adipocytes. J. Nutr. 2009;139:1185–1191. doi: 10.3945/jn.109.106617. [DOI] [PubMed] [Google Scholar]
- (26).Bumrungpert A, Kalpravidh RW, Chuang C, Overman A, Martinez K, Kennedy A, McIntosh M. Xanthones from mangosteen inhibit inflammation in human macrophages and in human adipocytes exposed to macrophage-conditioned media. J. Nutr. 2010;140:842–847. doi: 10.3945/jn.109.120022. [DOI] [PubMed] [Google Scholar]
- (27).Halliwell B. The wanderings of a free radical. Free Radical Biol. Med. 2009;46:531–542. doi: 10.1016/j.freeradbiomed.2008.11.008. [DOI] [PubMed] [Google Scholar]
- (28).Long LH, Hoi A, Halliwell B. Instability of, and generation of hydrogen peroxide by, phenolic compounds in cell culture media. Arch. Biochem. Biophys. 2010;501:162–169. doi: 10.1016/j.abb.2010.06.012. [DOI] [PubMed] [Google Scholar]
- (29).Chitchumroonchokchai C, Schwartz SJ, Failla ML. Assessment of lutein bioavailability from meals and a supplement using simulated digestion and Caco-2 human intestinal cells. J. Nutr. 2004;134:2280–2286. doi: 10.1093/jn/134.9.2280. [DOI] [PubMed] [Google Scholar]
- (30).Shen L, Guyre PM, Ball ED, Fanger MW. Glucocorticoid enhances gamma interferon effects on human monocyte antigen expression and ADCC. Clin. Exp. Immunol. 1986;65:387–395. [PMC free article] [PubMed] [Google Scholar]
- (31).Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- (32).Schuerer-Maly CC, Eckman L, Kagnoff MF, Falco MT, Maly FE. Colonic epithelial cell lies as a source of interleukin-8: Stimulation by inflammatory cytokines and bacterial lipopolysaccharide. Immunol. 1994;81:85–91. [PMC free article] [PubMed] [Google Scholar]
- (33).Chanput W, Mes J, Vreeburg RMV, Savelkoul HFJ, Wichers HJ. Transcription profiles of LPS-stimulated THP-1 monocytes and macrophages: A tool to study inflammation modulating effects of food derived compounds. Food Funct. 2010;1:254–261. doi: 10.1039/c0fo00113a. [DOI] [PubMed] [Google Scholar]
- (34).Bauer I, Al Sarraj J, Vinson C, Larsen R, Thiel G. Interleukin-1β and tetradecanoylphorbol acetate-induced biosynthesis of tumor necrosis factor α in human hepatoma cells involves the transcription factors ATF2 and c-Jun and stress-activated protein kinases. J. Cell Biochem. 2007;100:242–255. doi: 10.1002/jcb.21075. [DOI] [PubMed] [Google Scholar]
- (35).Xaus J, Comalada M, Valledor AF, Lloberas J, Lopez-Soriano F, Argiles JM, Bogdan C, Celada A. LPS induces apoptosis in macrophages mostly through the autocrine production of TNF-α. Blood. 2000;95:3823–3831. [PubMed] [Google Scholar]
- (36).Wang CC, Lai JE, Chen LG, Yen KY, Yang LL. Inducible nitric oxide synthase inhibitor of Chinese herbs part II: Naturally occurring furanocoumarins. Bioorg. Med. Chem. 2000;8:2701–2707. doi: 10.1016/s0968-0896(00)00200-5. [DOI] [PubMed] [Google Scholar]
- (37).Li L, Brunner I, Han A, Hamburger M, Kinghorn AD, Frye R, Butterweck V. Pharmacokinetics of α-mangostin in rats after intravenous and oral application. Mol. Nutr. Food Res. 2011;55:S67–74. doi: 10.1002/mnfr.201000511. [DOI] [PubMed] [Google Scholar]
- (38).Terao J, Murota K, Kawai Y. Conjugated quercetin glucuronides as bioactive metabolites and precursors of aglycone in vivo. Food Funct. 2011;2:11–17. doi: 10.1039/c0fo00106f. [DOI] [PubMed] [Google Scholar]
- (39).Li S, Sang S, Pan M, Lai C, Lo C, Yang C, Ho C. Anti-inflammatory property of urinary metabolites of nobiletin in the mouse. Bioorg. Med. Chem. Lett. 2007;17:5177–5181. doi: 10.1016/j.bmcl.2007.06.096. [DOI] [PubMed] [Google Scholar]
- (40).Williamson G, Barron D, Shimoi K, Terao J. In vitro biological properties of flavonoid conjugates found in vivo. Free Radical Res. 2005;39:457–469. doi: 10.1080/10715760500053610. [DOI] [PubMed] [Google Scholar]
- (41).Hung H, Joshipura K, Jiang R, Hu F, Hunter D, Smith-Warner S, Colditz G, Rosner B, Spiegelman D, Willett W. Fruit and vegetable intake and risk of major chronic disease. J. Natl. Cancer Inst. 2004;96:1577–1584. doi: 10.1093/jnci/djh296. [DOI] [PubMed] [Google Scholar]
- (42).Riboli E, Norat T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am. J. Clin. Nutr. 2003;78:559–569S. doi: 10.1093/ajcn/78.3.559S. [DOI] [PubMed] [Google Scholar]
- (43).Carter P, Gray L, Troughton J, Khunti K, Davies M. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: Systematic review and meta-analysis. Br. Med. J. 2010;341:4229–4236. doi: 10.1136/bmj.c4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Dauchet L, Amouyel P, Hercberg S, Dallongeville J. Fruit and vegetable consumption and risk of coronary heart disease: A meta-analysis of cohort studies. J. Nutr. 2006;136:2588–2593. doi: 10.1093/jn/136.10.2588. [DOI] [PubMed] [Google Scholar]
- (45).Soto-Vaca A, Gutierrez A, Losso JN, Xu Z, Finley JW. Evolution of phenolic compounds from color and flavor problems to health benefits. J. Agric. Food Chem. 2012;60:6658–6677. doi: 10.1021/jf300861c. [DOI] [PubMed] [Google Scholar]
- (46).Chang H, Huang W, Chen H, Yang L. Apoptotic effects of γ-mangostin from the fruit hull of Garcinia mangostana on human malignant glioma cells. Molecules. 2010;15:8953–8966. doi: 10.3390/molecules15128953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).During A, Hussain MM, Morel DW, Harrison EH. Carotenoid uptake and secretion by Caco-2 cells: β-Carotene isomer selectively and carotenoid interactions. J. Lipid Res. 2002;43:1086–1095. doi: 10.1194/jlr.m200068-jlr200. [DOI] [PubMed] [Google Scholar]
- (48).Lin C, Huang C, Hu M. The use of fetal bovine serum as delivery vehicle to improve the uptake and stability of lycopene in cell culture studies. Br. J. Nutr. 2007;98:226–232. doi: 10.1017/S0007114507691752. [DOI] [PubMed] [Google Scholar]
- (49).Van de Walle J, Hendrickx A, Romier B, Larondelle Y, Schneider Y. Inflammatory parameters in Caco-2 cells: Effect of stimuli nature, concentration, combination and cell differentiation. Toxicol. In Vitro. 2010;24:1441–1449. doi: 10.1016/j.tiv.2010.04.002. [DOI] [PubMed] [Google Scholar]
- (50).Tang Y, Li P, Kondo M, Ji H, Kou Y, Ou B. Effect of a mangosteen dietary supplement on human immune function: A randomized double-blind-controlled trial. J. Med. Food. 2009;12:755–763. doi: 10.1089/jmf.2008.0204. [DOI] [PubMed] [Google Scholar]
- (51).Siow RC, Mann GE. Dietary isoflavones and vascular protection: Activation of cellular antioxidant defenses by SERMs or hormesis? Mol. Aspects Med. 2010;31:468–77. doi: 10.1016/j.mam.2010.09.003. [DOI] [PubMed] [Google Scholar]
- (52).Speciale A, Chirafisi J, Saija A, Cimino F. Nutritional antioxidants and adaptive cell responses: An update. Curr. Mol. Med. 2011;9:770–89. doi: 10.2174/156652411798062395. [DOI] [PubMed] [Google Scholar]
- (53).Spencer J, Abd-el-Mohsen M, Rice-Evans C. Cellular uptake and metabolism of flavonoids and their metabolites: Implications for their bioactivity. Arch. Biochem. Biophys. 2004;423:148–161. doi: 10.1016/j.abb.2003.11.010. [DOI] [PubMed] [Google Scholar]
- (54).Lotito S, Zhang W, Yang C, Crozier A, Frei B. Metabolic conversion of dietary flavonoids alters their anti-inflammatory and antioxidant properties. Free Radical Biol. Med. 2011;51:454–463. doi: 10.1016/j.freeradbiomed.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Larrosa M, González-Sarrías A, Yáñez-Gascón M, Selma M, Azorín-Ortuño M, Toti S, Tomás-Barberán F, Dolara P, Espín J. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J. Nutr. Biochem. 2010;21:717–725. doi: 10.1016/j.jnutbio.2009.04.012. [DOI] [PubMed] [Google Scholar]
- (56).Gu L, Laly M, Chang HC, Prior RL, Fang N, Ronis MJJ, Badger TM. Isoflavones conjugates are underestimated in tissues using enzymatic hydrolysis. J. Agric. Food Chem. 2005;53:6858–6863. doi: 10.1021/jf050802j. [DOI] [PubMed] [Google Scholar]
- (57).Kawai Y, Nishikawa T, Shiba Y, Saito S, Murota K, Shibata N, Kobayashi M, Kanayama M, Uchida K, Terao J. Macrophage as a target of quercetin glucuronides in human atherosclerotic arteries: Implication in the anti-atherosclerotic mechanism of dietary flavonoids. J. Biol. Chem. 2008;283:9424–34. doi: 10.1074/jbc.M706571200. [DOI] [PubMed] [Google Scholar]
- (58).Shimoi K, Saka N, Nozawa R, Sato I, Amano T, Nakayama T, Kinae N. Deglucuronidation of a flavonoid, luteolin monoglucuronide, during inflammation. Drug Metab. Dispos. 2001;29:1521–1524. [PubMed] [Google Scholar]