Abstract
Calcium signaling and reactive oxygen species signaling are directly connected, and both contribute to cell-to-cell signal propagation in plants.
Calcium (Ca2+) is an important second messenger with diverse functions not only in mammals but also in plants. It is released in response to a variety of stimuli like biotic and abiotic stresses and facilitates a tight regulation of response reactions as well as of developmental processes (Sanders et al., 2002; Steinhorst and Kudla, 2012). Ca2+ accumulation events are characterized by distinct temporal and spatial features, and they can vary in terms of amplitude, frequency, and duration (Webb et al., 1996; Scrase-Field and Knight, 2003; Dodd et al., 2010; Kudla et al., 2010). Spatially defined Ca2+ signals can be generated due to the especially slow diffusion rate of the Ca2+ ion in the cytoplasm in combination with tightly regulated release and uptake from and into different intracellular stores and the apoplast. Together, these characteristics encode information about particular stimuli, for example, drought stress that is presented to the cell as so-called Ca2+ signatures (Webb et al., 1996). This information has to be decoded and transmitted by a signaling machinery in order to initiate adequate response reactions, for example, stomatal closure (Allen et al., 2000, 2001; Sanders et al., 2002). Ca2+ signatures can be sensed by proteins that bind Ca2+ via helix-loop-helix EF-hand motifs. Arabidopsis (Arabidopsis thaliana) possesses at least 250 putative EF-hand proteins, 100 of which have been classified as Ca2+ sensor proteins (Day et al., 2002; Hashimoto and Kudla, 2011). Given that each member of this intricate set of Ca2+ sensor proteins can exhibit characteristic expression and subcellular localization profiles as well as distinct Ca2+ affinities, plants are equipped with a complex signal-decoding machinery to process a wide range of different Ca2+ signals (Batistič and Kudla, 2004; Batistič and Kudla, 2010). Ca2+ functions in concert with other important second messengers like reactive oxygen species (ROS). ROS can be generated in a controlled manner by several types of enzymes, such as NADPH oxidases, in order to contribute to pathogen defense and cell signaling. Recent findings point to direct connections between ROS and Ca2+ signaling pathways that enable cell-to-cell communication and thereby long-distance transmission of signals in plants. In this Update, we focus on new findings in the field of plant Ca2+ signaling during the past 3 years since the status of the field was discussed in comprehensive reviews (Dodd et al., 2010; Kudla et al., 2010; Mazars et al., 2011; Reddy et al., 2011) and put special emphasis on the contribution of a plant-specific Ca2+ signaling network to deciphering defined Ca2+ signals and its integration with ROS signaling.
GENERATION OF CALCIUM SIGNALS: NEW INSIGHTS
To form a Ca2+ signal, fluxes of Ca2+ across membranes have to occur in order to increase and subsequently decrease the cytosolic Ca2+ concentration ([Ca2+]cyt). In contrast to components responsible for Ca2+ extrusion out of the cytosol, like P-type Ca2+-ATPases and Ca2+/proton antiporters, the molecular identity of Ca2+-specific influx channels has remained unknown or controversial for a long time. However, recently, for two families of the group of nonspecific ligand-gated ion channels, increasing evidence is emerging that supports these candidates as being responsible for the generation of Ca2+ signals in response to pathogens, abiotic stresses, and during polar growth processes. These protein families are the glutamate-like receptor homologs (GLRs) and the cyclic nucleotide-gated ion channels (CNGCs; Fig. 1).
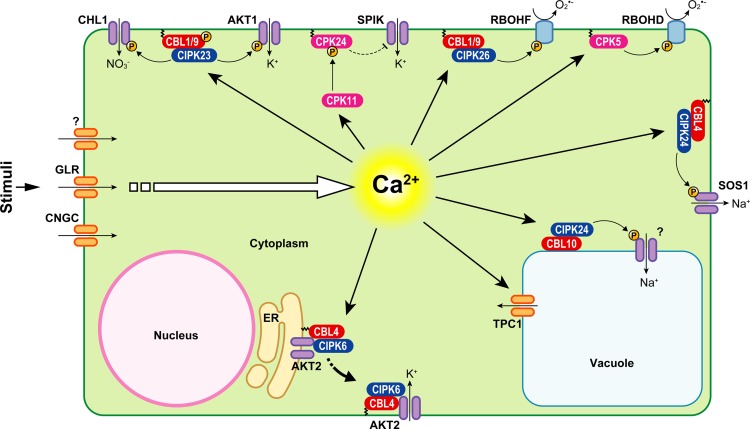
Figure 1.
Schematic model of Ca2+-mediated signal transduction processes in a plant cell. Examples of signal transduction processes mediated by CDPKs and CBL/CIPK complexes from Arabidopsis together with their respective target proteins are shown. For further details, see text. ER, Endoplasmic reticulum; P, target phosphorylation.
GLRs Function in Ca2+ Signaling
Members of the GLR family in plants are involved in many biological processes, such as carbon/nitrogen balance, photosynthesis, abscisic acid (ABA) sensing, responses to abiotic stresses, root morphogenesis, plant-pathogen interaction, and regulation of ionic currents including Ca2+ (Kim et al., 2001; Kang and Turano, 2003; Kang et al., 2004; Qi et al., 2006; Stephens et al., 2008; Cho et al., 2009; Price et al., 2012; Vincill et al., 2013). The importance of GLRs for the generation of Ca2+ currents during distinct cellular processes has recently been demonstrated for GLR1.2. GLR1.2 is a channel that facilitates Ca2+ influx across the plasma membrane in growing pollen tubes and thereby contributes to the modulation of the tip-focused Ca2+ gradient (Michard et al., 2011). Mutant analyses revealed that this channel is crucial for proper Ca2+ fluxes to ensure pollen tube growth and full fertility. This holds true not only with respect to tube morphogenesis but also to pollen tube guidance, since GLR1.2 activity is regulated through the abundance of d-Ser in the surrounding pistil tissue. Ca2+ conductance of pollen GLRs has been demonstrated with Ca2+-specific vibrating probe measurements combined with GLR agonists (d-Ser) or antagonists (Michard et al., 2011). However, direct evidence for amino acid-gated Ca2+ conductance of plant GLRs was still missing at that time, since the expression of plant GLRs in Xenopus laevis oocytes for voltage-clamp analyses was not successful, possibly due to wrong targeting (Li et al., 2006a; Price et al., 2012). Animal ionotropic glutamate receptors (iGluRs), however, could be analyzed with this method (Hollmann et al., 1989; Vincill et al., 2012), and it has been found that they form heterotetramers with varying conductances for sodium (Na+), potassium (K+), and Ca2+ (Kandel et al., 2000; Davenport, 2002; Furukawa et al., 2005; Traynelis et al., 2010). Therefore, they were classified as nonselective cation channels. Studies conducted thus far suggest that also plant GLRs are nonselective cation channels (Kim et al., 2001; Roy et al., 2008; Tapken and Hollmann, 2008; Price et al., 2012). However, none of these studies provided direct proof for amino acid-gated activity of a plant GLR.
Importantly, in a recent study, Vincill et al. (2012) established HEK cells as a system for studying GLR function. In their work, they found that AtGLR3.4 is an amino acid-gated channel that is suggested to be highly selective to Ca2+ and is capable of inducing cytosolic Ca2+ peaks in response to Asn, Gly, or Ser. In a more recent study, it was found that GLR3.4 together with GLR3.2 can form functional heteromers in planta and that this heteromerization is favored over homomerization of each of the two GLRs (Vincill et al., 2013). However, these heteromers exhibited the same Ca2+ conductance properties in response to Asn as GLR3.4 homomers. GLR3.2 did not form functional homomers in this study. In this regard, future research is needed to analyze in more detail the conductance properties of differently composed GLR heteromers to elucidate, for example, comprehensive agonist profiles or different kinetic properties (Vincill et al., 2013). Mutant analyses of GLR3.4 and GLR3.2, which are expressed in the apical root phloem of Arabidopsis, revealed that both GLRs are involved in the regulation of lateral root formation. This finding of GLR involvement in root developmental processes is especially interesting considering that primordium production induced by mechanical bending of primary roots is known to depend on Ca2+ signaling (Richter et al., 2009; Vincill et al., 2013). In order to extend our understanding about the function of GLRs in lateral root formation, precise subcellular localization analyses and analyses of expression patterns during developmental stages in combination with live-cell imaging of Ca2+ signals in response to amino acids are needed. Moreover, not much is known so far about the regulation of plant GLR at the posttranslational level, such as phosphorylation. Animal Glu receptors can be subject to regulation by phosphorylation that modulates receptor trafficking and receptor channel properties (Chen and Roche, 2007). Interestingly, several GLRs from Arabidopsis were identified as potential 14-3-3 client proteins in a proteomics study by Chang et al. (2009), which hints at interactions with other signaling proteins, including kinases and phosphatases (Price et al., 2012). Hence, activation or deactivation of GLRs by Ca2+-regulated kinases could represent a mechanism of positive or negative feedback regulation, respectively. Therefore, it would be most interesting to test whether plant GLRs are also subject to phosphorylation and if this phosphorylation can be brought about by Ca2+-regulated kinases, such as CBL-interacting protein kinases (CIPKs) or Ca2+-dependent protein kinases (CDPKs).
The Contribution of CNGCs to Ca2+-Regulated Processes
CNGCs are nonspecific cation channels that form a family of 20 members in Arabidopsis and are regulated by cyclic nucleotides such as cAMP or cyclic GMP (Mäser et al., 2001; Talke et al., 2003; Ward et al., 2009). Accumulating evidence suggests that CNGCs are required for Ca2+ fluxes across the plasma membrane and thereby contribute to Ca2+ signaling in the context of developmental processes, abiotic stress responses, pathogen defense responses, and regulation of pollen tube tip growth as well as in the establishment of thermotolerance (Frietsch et al., 2007; Chaiwongsar et al., 2009; Ma and Berkowitz, 2011; Tunc-Ozdemir et al., 2013a, 2013b). First evidence that linked CNGC function to polar growth processes in plants was provided by mutant analyses that revealed that CNGC18 function is required for pollen tube tip growth and consequently is crucial for male fertility (Frietsch et al., 2007). In addition to CNGC18, also CNGC7 and CNGC8 have been implicated in the regulation of pollen tube tip growth. Both proteins seem to fulfill overlapping functions, since mutant analyses of single and double mutant alleles revealed a reduced pollen transmission efficiency and bursting pollen tubes only for the double mutant (Tunc-Ozdemir et al., 2013a). Unfortunately, these studies did not address potential changes in Ca2+ dynamics of cngc mutant pollen. Therefore, possible alternative explanations for the observed pollen phenotypes could be, for example, a disrupted coordination of growth cycles or a disturbed turgor pressure regulation. The latter would be in agreement with the fact that CNGCs are also conductive for K+, as, for example, AtCNGC3 and AtCNGC10 (and other CNGCs) can compensate for K+ uptake in akt1 mutants (which lack the Shaker-like potassium channel AKT1; Caballero et al., 2012).
The importance of CNGC function for pollen fertility has been further corroborated lately in that AtCNGC16 was found to convey thermotolerance to (germinating) pollen by connecting cyclic nucleotide signaling to the transcriptional heat-stress response (Tunc-Ozdemir et al., 2013b). Pollen transmission of the cngc16 mutant was significantly reduced under temperature stress and also under drought stress conditions, and this phenotype appeared to be linked to an attenuated expression of key stress-responsive genes. The function of CNGCs in plant thermotolerance appears not to be restricted to pollen. The cation channel CNGC6 is ubiquitously expressed in Arabidopsis and mediates heat stress-induced Ca2+ influx, which affects the thermotolerance of vegetative plant tissues and also leads to induction of a heat-shock protein (HSP; Gao et al., 2012). cngc6 mutant plants exhibited decreased thermotolerance and reduced expression of several HSPs compared with the wild type. Furthermore, it was found that the level of cytosolic cAMP was increased by mild heat shock in the wild type. CNGC6 in turn was activated by cytosolic cAMP, and application of exogenous cAMP promoted the expression of HSP genes. Together, these findings support the concerted action of Ca2+ and cyclic nucleotide monophosphates (cNMPs) in plant thermotolerance. The role of CNGCs and Ca2+ signaling in establishing acquired thermotolerance appears to be quite complex and evolutionarily conserved. Mutants of Arabidopsis CNGC2 as well as the putative ortholog cngcb mutant of the moss Physcomitrella patens exhibited a hyperthermosensitive phenotype accompanied by a hyperthermoresponsive Ca2+ influx, giving rise to a heat stress response and acquired thermotolerance at significantly milder heat treatments than in wild-type plants (Finka et al., 2012). In these plants, the responsiveness to temperature was shifted by 5°C. Moreover, patch-clamp recordings on P. patens protoplasts revealed that deletion of one CNGC affected the open probability of remaining CNGC. This phenomenon points to complex compensatory and feedback regulatory mechanisms that may involve the regulated formation of CNGC heteromultimers and the modulation of cNMP and Ca2+-calmodulin (CaM) signaling. In all CNGCs studied so far, the binding site for CaM overlaps with the cNMP-binding site (Talke et al., 2003). Accordingly, it was well accepted that the binding of Ca2+-activated CaM to CNGCs interferes with cNMP-dependent activation. A new level of Ca2+-CaM-mediated CNGC regulation has been recently uncovered by the characterization of a conserved IQ domain, which is separated from the cyclic nucleotide-binding domain (Fischer et al., 2013). In CNGC20, this IQ domain interacts with CaM in a Ca2+-dependent manner. This novel finding represents an additional CaM-binding mode, increasing the complexity of CNGC regulation that deserves further studies.
In addition to GLRs and CNGCs, plant annexins have been found to be related to changes in cellular Ca2+ concentrations (Mortimer et al., 2008; Laohavisit et al., 2009). A recent study reported that Arabidopsis annexin1 mediates radical-activated plasma membrane Ca2+-permeable conductance in root cells (Laohavisit et al., 2012). Analyses of annexin1 loss-of-function mutants revealed a lack of the root hair and epidermal OH•-activated Ca2+ and K+ conductances, a finding that has so far not been obtained in studies of animal annexins (Laohavisit et al., 2012). Although plant annexins are without doubt involved in regulating Ca2+-related processes in plants, care should be taken to declare them as novel Ca2+-permeable transporters, and clearly, more research is needed to clarify their mode of function. The idea that annexins could be Ca2+ channels originated from early studies of mammalian annexins in artificial lipid bilayers (Rojas et al., 1992; Hawkins et al., 2000; Kourie and Wood, 2000). Additional studies revealed a second mode of function for animal annexins in that they modulate the activity of Ca2+ channels (Díaz-Muñoz et al., 1990; Gerke and Moss, 2002). While the modulatory function of animal annexins in regulating ion channels stood the test of time, today their function as bona fide Ca2+ channels has been severely challenged. In addition to conceptional problems with how peripherally membrane-associated annexins could conduct Ca2+ across the membrane, accumulating evidence indicated that annexin attachment to (artificial) membrane bilayers perturbs the organization of lipids in the bilayer to efficiently electroporate the membrane and, therefore, permit Ca2+ entry (Demange et al., 1994; Gerke and Moss, 2002; Gerke et al., 2005). These conclusions may be considered when interpreting experimental results on plant annexin function.
In contrast to the situation at the plasma membrane, much less is known about channels that could mediate Ca2+ release from internal stores. An abundant channel at the tonoplast is the slow vacuolar channel that was originally described as a voltage-dependent cation channel that is regulated by cytoplasmic Ca2+ concentrations (Hedrich and Neher, 1987). In addition, a Ca2+-release capability of this channel was reported (Allen and Sanders, 1994). This channel was molecularly identified as two-pore channel1 (TPC1; Fig. 1). A tpc1 knockout mutant lacks functional slow vacuolar channel activity and is defective in both the ABA-induced repression of germination and in the response of stomata to extracellular Ca2+ (Peiter et al., 2005). TPC1 is the only vacuolar Ca2+-permeable channel cloned to date. However, due to its complex regulation and its low selectivity among cations, the role of this channel in Ca2+ signaling is still debated (Peiter, 2011).
SENSING AND TRANSMISSION OF CALCIUM SIGNALS
Plants are equipped with an elaborate set of Ca2+ sensor proteins that sense defined Ca2+ signals and transmit them to initiate adequate responses to specific stimuli. In addition to the ubiquitous eukaryotic Ca2+ sensor calmodulin (CaM), they possess further families of EF-hand Ca2+ sensor proteins. These are the calmodulin-like proteins, the family of CDPKs, and the calcineurin B-like protein (CBL) family. CDPKs are Ca2+ sensor proteins that can bind Ca2+ via four EF-hand motifs in their C-terminus (Kudla et al., 2010). Binding Ca2+ leads to activation of the kinase domain located at the N-terminus and thereby facilitates direct sensing and transmission of a Ca2+ signal. In contrast, CBLs represent solely Ca2+ sensors without an enzymatic function. CBLs can bind Ca2+ with their four EF-hands, but in order to convert the signal into phosphorylation events, interaction with members of the CIPK family is required (Shi et al., 1999). Interaction between CBLs and CIPKs is mediated via the NAF domain, a conserved motif within the regulatory C-terminal domain of the kinase, and releases the kinase from autoinhibition (Albrecht et al., 2001). In the following, we will discuss the latest findings and new emerging mechanisms associated with CDPK and CBL-CIPK signaling networks as well as their interconnection in the context of ROS signaling.
New Emerging Functions and Mechanistic Principles of the CDPK Network
CDPKs (also abbreviated as CPKs) represent versatile Ca2+-sensing effector proteins that are involved in the regulation of many processes, such as abiotic and biotic stress signaling, pathogen defense, and hormone-regulated developmental processes in different plant cell types and in some protists (Cheng et al., 2002; Harper and Harmon, 2005; Liese and Romeis, 2013). In this section, we focus only on the most significant new findings on CDPK function and regulation in the context of their potential interplay with other Ca2+ signaling systems. In Arabidopsis, the family of CDPK protein kinases encompasses 34 members with both overlapping and distinct functions. Recently, substantial advances have been made in analyzing the characteristics and diverse functions of CDPKs. One example is CPK21, which was found to be involved in the regulation of responses to osmotic stress (Franz et al., 2011). Seedlings of a loss-of-function allele displayed increased stress responses when subjected to osmotic stress, and this phenotype could be rescued by expression of the active kinase. Considering that opposite phenotypes have been reported for mutants of CBLs and CIPKs like cbl1 and cipk1 (Albrecht et al., 2003; D’Angelo et al., 2006), this raises the possibility that, depending on the actual cellular Ca2+ concentration, both signaling systems may function antagonistically during osmotic stress conditions. Investigation of Ca2+-binding properties of the four EF-hands of CPK21 and the impact on kinase activity by analyzing different EF-hand mutant enzymes revealed differences between the two EF-hand pairs, suggesting that this feature could contribute to the specificity of CPK21 function (Franz et al., 2011).
Concerning the regulation of CDPKs by Ca2+ binding, an advanced model has recently been discussed (Liese and Romeis, 2013) that extends the hitherto existing model (Harper and Harmon, 2005) in terms of understanding the molecular mechanisms of autoinhibition and activation (Ojo et al., 2010; Wernimont et al., 2010, 2011). Accumulating evidence points to an important role of the N-terminal region of CDPKs as a targeting determinant that is crucial for establishing functional specificity in vivo (Asai et al., 2013). Membrane targeting of CDPKs appears to involve lipid modifications, since the majority of CDPKs contain potential N-terminal myristoylation and S-acylation sites that are required for membrane targeting of, for example, CPK2 and CPK3 from Arabidopsis (Martín and Busconi, 2000; Mehlmer et al., 2010). Also, the plasma membrane localization of StCPK5 depends on myristoylation and palmitoylation of the kinase N-terminus, and this targeting is essential for the phosphorylation of StRBOHB (for respiratory burst oxidase homolog B) in vivo (Asai et al., 2013). Similarly, myristoylation of Arabidopsis CPK5 seems to be a prerequisite for plasma membrane targeting of this CDPK (Lu and Hrabak, 2013). Since distinct lipid modification patterns could result in targeting to defined membrane subdomains, this targeting mechanism likely contributes to establishing specificity of CDPKs in plants. Moreover, since targeting of CBL-CIPK complexes, like the targeting of CDPKs, largely relies on similar lipid modification by myristoylation and S-acylation (see below), it appears conceivable that both signaling systems function simultaneously in the same membrane domains and may regulate the same target proteins.
A new mechanistic principle of CDPK function and regulation that may largely affect our concepts of Ca2+ signaling has very recently emerged from a study of the concerted regulation of a K+ channel by a pair of CDPKs in pollen (Zhao et al., 2013). So far, CDPKs (and CBL-CIPK complexes) have been considered as one-step relays that convey Ca2+ signals directly into phosphorylation events on substrate proteins. In the study by Zhao et al. (2013), the pollen-specific Shaker-type K+ channel SPIK was found to be negatively regulated by the concerted and subsequent action of CPK11 and CPK24. Disruption of CPK11 or CPK24 completely impaired the Ca2+-dependent inhibition of inward K+ currents and enhanced pollen tube growth. Moreover, the cpk11/cpk24 double mutant exhibited similar phenotypes to the corresponding single mutants, suggesting that these two CDPKs function in the same signaling pathway. Moreover, CPK11 interacted with CPK24 in vivo and phosphorylated the N-terminus of CPK24 in vitro, suggesting that these two CDPKs work together as part of a kinase cascade. It will be interesting to address the question of whether this mechanism of kinase cascades also applies for other CDPK-target combinations or even allows for cross-regulatory interconnections with CIPKs. In the past, there has been steadily increasing evidence that CDPKs directly contribute to the modulation of ROS signaling via the regulation of RBOH NADPH oxidases (Kudla et al., 2010). Previous studies in potato (Solanum tuberosum) suggested that two CDPKs (StCDPK4 and StCDPK5) phosphorylate NADPH oxidases and thereby positively regulate the production of ROS (Kobayashi et al., 2007). Accordingly, ectopic expression of a constitutively active mutant version of StCDPK5 provoked ROS production in Nicotiana benthamiana leaves, and this CDPK-mediated ROS production was disrupted by knockdown of StCDPK5. Moreover, a quadruple loss-of-function mutant of the Arabidopsis CDPKs CPK4, CPK5, CPK6, and CPK11 has been reported to exhibit impaired pathogen responsiveness involving a lack of ROS production, indicative of disturbed RBOH activation (Boudsocq et al., 2010). However, whether these CDPKs directly target RBOH proteins that mediate ROS production during pathogen responses remained to be addressed.
Subsequent integrated functional genomic and biochemical genetic screens identified six closely related CDPKs as contributing to immune signaling. Of these, CPK1/CPK2/CPK4/CPK11 were reported to phosphorylate NADPH oxidases in vitro and were implemented as crucial components for the production of ROS during immune signaling (Gao et al., 2013). In a most recent breakthrough study, Tina Romeis and colleagues discovered that the plasma membrane-localized CPK5 positively regulates the NADPH oxidase RBOHD during pathogen defense in Arabidopsis (Dubiella et al., 2013; Fig. 1). They demonstrated that activation of RBOHD occurs via CPK5-dependent phosphorylation of several Ser residues within the oxidase N-terminus in vivo. Most remarkably, the regulation of RBOHD by CPK5 enables signal propagation upon pathogen-associated molecular pattern (PAMP) stimulation to distal parts of the plant via an apoplastic ROS wave. Accordingly, CPK5 and RBOHD have been proposed to represent key components of a self-propagating activation circuit mediating cell-to-cell communication (Dubiella et al., 2013). Specifically, this work identified six Ser residues at which phosphorylation was increased in CPK5-overexpressing lines but was unaltered or reduced in cpk5 mutants. Interestingly, this work also provided evidence for the contribution of other protein kinases to RBOHD regulation, since phosphorylation of two additional Ser residues was enhanced in the cpk5 mutant and reduced in CPK5-overexpressing lines. These findings suggest that other protein kinases (e.g. CIPKs) may participate in the regulation of RBOHD phosphorylation in vivo (Dubiella et al., 2013). These exciting new facets of Ca2+ and ROS signaling will be discussed in more detail at the end of this review.
The CBL-CIPK Network: A Versatile Signaling Machinery
Evaluation of genome sequences identified a set of 10 CBLs and 26 CIPKs in Arabidopsis and a set of 10 CBLs and 30 CIPKs in rice (Oryza sativa; Kolukisaoglu et al., 2004; Weinl and Kudla, 2009). Phylogenetic analyses of the evolutionary development of CBLs and their interacting protein kinases revealed that the extensive network they build up in higher plants such as Arabidopsis is indeed restricted to higher plants. This complex network appears to have developed from a founder pair of a single CBL protein and a single CIPK that can still be detected in green algae like Ostreococcus tauri and the related species Ostreococcus lucimarinus and Chlorella spp. (Batistič and Kudla, 2009, 2012; Weinl and Kudla, 2009). In contrast, genomes of lower plants like the moss P. patens encode four CBLs and seven CIPKs (Batistič et al., 2010), and the lycopode Selaginella moellendorffii possesses a complement of four CBL proteins and five CIPKs (Weinl and Kudla, 2009). Evolution of the complex Ca2+ signal-decoding network of CBLs and CIPKs as it is found in Arabidopsis presumably occurred concurrently with the evolution of increasing morphological and developmental sophistication of land plants as a result of the colonization of new fluctuating habitats (Weinl and Kudla, 2009). Interestingly, the CBL-CIPK network is not limited to the plant lineage, since putative CBL and CIPK proteins were identified in the genomes of the protozoan species Trichomonas vaginalis and Naegleria gruberi (Batistič and Kudla, 2009, 2012; Weinl and Kudla, 2009). The discovery of these otherwise plant-specific components of the CBL-CIPK network in human pathogens, on the one hand, argues for an ancient CBL-CIPK network in nonplant species and, on the other hand, provides a potential starting point for medical research to combat human pathogens.
N-Terminal Lipid Modification Facilitates CBL Membrane Targeting
CBL proteins can be divided into two groups, according to their N-termini. CBL1, CBL4, CBL5, CBL8, and CBL9 constitute one group with a comparatively short N-terminus, whereas CBL2, CBL3, CBL6, CBL7, and CBL10 possess extended N-termini (Kolukisaoglu et al., 2004; Batistič et al., 2008, 2010). Except for CBL8, all members of the first group are subject to lipid modifications that determine their targeting to the plasma membrane. The N-termini of CBL1, CBL4, CBL5, and CBL9 harbor an MGXXX(S/T) motif that allows for the attachment of myristic acid to the Gly (Ishitani et al., 2000; Batistič et al., 2008). In addition to myristoylation, CBL1 has to be N-terminally S-acylated in order to be functional (Batistič et al., 2008). Further analyses conducted on CBL1 revealed that its N-terminus is S-acylated by both palmitate and stearate and that this dual lipid modification is crucial for plasma membrane targeting. CBL2, CBL3, CBL6, and CBL10, members of the second group of CBL proteins, possess extended N-termini that are responsible for targeting to the tonoplast (Batistič and Kudla, 2009; Batistič et al., 2010). CBL10 is an exception in this group, since its N-terminus contains a potential transmembrane domain that is thought to be important for localization at the tonoplast (Kim et al., 2007). In contrast, tonoplast targeting of CBL2 is brought about by S-acylation of three Cys residues in its N-terminus (Batistič et al., 2012). The vacuolar localization of CBL2 and CBL3 has also been confirmed in Arabidopsis (Tang et al., 2012). It seems likely that palmitoylation of CBLs is conducted by protein S-acyltransferases (PATs). In Arabidopsis, 24 PATs have been characterized, two of which have been detected at the tonoplast and nine at the plasma membrane (Batistič, 2012). These PATs represent candidates that could bring about the targeting of CBLs and lipid-modified CDPKs into defined membrane domains. The first evidence for the importance of PAT activity for CBL targeting has been recently presented by CBL localization analyses in a pat10 mutant line (Zhou et al., 2013) that suggested that the tonoplast-localized PAT10 is responsible for tonoplast targeting of CBL2, CBL3, and CBL6. Future research is needed to advance our understanding of the mechanisms by which PATs target CBL and CDPK proteins to membranes and how this might contribute to creating specific membrane microdomains that functionally combine different Ca2+ and hormone signaling proteins (Demir et al., 2013).
Subcellular Targeting of CBL-CIPK Complexes: A Mechanism for the Transmission of Spatially Defined Signals
While most CBL proteins are localized at membranes, their interacting kinases are mainly present in the cytosol and in the nucleus when expressed alone (Batistič et al., 2010). In planta protein-protein interaction analyses using the bimolecular fluorescence complementation (BiFC) system have greatly advanced our understanding of CBL and CIPK localization and targeting (Hu et al., 2002; Kerppola, 2006; Waadt and Kudla, 2008; Waadt et al., 2008; Batistič et al., 2010). BiFC interaction analyses of CIPK23 and CIPK24, for example, demonstrated that these two kinases both interact with CBL1 and CBL9 at the plasma membrane (Xu et al., 2006; Waadt et al., 2008). These results, among others, firmly established that CBL proteins determine the localization and site of action of CBL-CIPK complexes. A special case was observed for the interaction of the cytosolic calcium sensor CBL8 with the kinase CIPK14. Interaction between these two proteins was exclusively detected at the plasma membrane (Batistič et al., 2010). Considering that CBL8 is not lipid modified at its N-terminus and thereby differs from the lipid-modified plasma membrane-targeted CBL1, CBL4, CBL5, and CBL9, this points to another not yet identified targeting mechanism of CBL8-CIPK complexes. Other CIPK14 interactors are the tonoplast-localized CBL2 and CBL3, which target CIPK14 to the vacuole in planta, indicating that one and the same kinase can be recruited to different subcellular destinations by interacting with different CBLs located at different membranes. Further examples detected with the BiFC method that support the idea of alternative targeting routes for CIPKs are CBL-CIPK24 complexes at the plasma membrane such as CBL1-CIPK24 and CBL5-CIPK24 and at the tonoplast such as CBL2-CIPK24 and CBL10-CIPK24 (Waadt et al., 2008; Batistič et al., 2010). However, the BiFC method artificially stabilizes protein interactions and does not allow one to follow where the noninteracting fraction of proteins remains within the cell. Therefore, coexpression of fluorophore-tagged CBLs and CIPKs was applied as a complementary approach (Schlücking et al., 2013). Such studies revealed that GFP-tagged CIPK5 is no longer present in the cytosol but targeted to the tonoplast instead, when CBL3-mCherry is coexpressed in N. benthamiana. In this way, it became evident that CBL proteins are indeed able to efficiently trap interacting CIPKs out of the cytosol and recruit them to membranes.
This method of coexpression and colocalization analysis should allow us in the future to compare the interaction strengths of different CBL-CIPK pairs or preferential complex formation by expressing a CIPK together with two differently located interacting CBLs and monitoring competitive recruitment of the CIPK. Moreover, this should allow us to address the dependence of alternative CBL-CIPK interaction on the Ca2+ concentration in the cytosol. It appears likely that different CBL-CIPK complexes require individual Ca2+ concentrations to form and that this dependence relates to the decoding of specific Ca2+ signals. Moreover, the Ca2+-binding capacities of CBL proteins can change upon interaction with a distinct CIPK, as has been shown by crystallization studies of CBL4-CIPK24 as well as of CBL2-CIPK14 complexes (Sánchez-Barrena et al., 2013). This adds another layer of complexity to the dependence of CBL-CIPK complex formation on cytosolic Ca2+ concentrations. Taken together, all these variable parameters of CBL-CIPK complex formation appear to act in concert and thereby contribute to decoding distinct Ca2+ signals at defined locations within the cell, enabling the generation of specific and adequate responses.
Phosphorylation of CBLs Is Important for Target Regulation
A novel regulatory mechanism of CBL-CIPK complexes that has been recently uncovered is the phosphorylation of CBL proteins by their interacting CIPKs (Mahajan et al., 2006; Lin et al., 2009; Du et al., 2011; Hashimoto et al., 2012). Importantly, it was found that phosphorylation of AtCBL1 and AtCBL9 by their interacting kinase CIPK23 is absolutely required for the activation of the K+ channel AKT1 in X. laevis oocytes. It was shown that phosphorylation of the CBL protein increased the activity of the CBL-CIPK complex toward its substrate by an unknown mechanism. Most likely, phosphorylation of the flexible C-terminus of a CBL leads to conformational changes that in some way enhance the specificity and activity of the complex toward its target. Phosphorylation of the analyzed CBLs from Arabidopsis was found to occur at a Ser residue within a conserved C-terminal FPSF motif. This finding was also supported by another work in which the respective region was named the PFPF motif (Du et al., 2011). In some cases, the phosphorylation status of CBL proteins appears to impact on the stability of a CBL-CIPK complex (Du et al., 2011; Hashimoto et al., 2012). Clearly, this issue deserves further investigation to better understand its meaning and consequences.
CBL-CIPK Complexes Regulate a Broad Range of Targets
Targets regulated by CBL-CIPK complexes have so far mainly been identified at the plasma membrane, while not much is known about the functions of vacuolar CBL-CIPK complexes. However, in the past 2 years, major advances have been made linking tonoplast-localized CBL proteins to the regulation of vacuolar pH, K+ homeostasis, and ABA signaling (Batistič et al., 2012; Tang et al., 2012; Liu et al., 2013). Recently, it has been shown that CBL2 targeting to the tonoplast depends on N-terminal S-acylation (Batistič et al., 2012). Mutant analyses with a cbl2 knockout line revealed that CBL2 function is required for appropriate ABA responses during seed germination. A cbl2 mutant line was impaired in germination on ABA-containing medium compared with the wild type. Importantly, proper targeting to the tonoplast was absolutely required for full functionality of CBL2, pointing to a function of a tonoplast-localized Ca2+ sensor in regulating ABA responses by decoding Ca2+ signatures emanating from the vacuole. However, the mechanism by which CBL2 regulates ABA responses at the vacuole is still enigmatic. Since the cbl2 single mutant exhibits an ABA-hypersensitive phenotype, it is assumed that CBL3, the closest homolog of CBL2, is not redundant in this context, although these two proteins are 92% identical and exhibit overlapping expression patterns (Batistič et al., 2012). Similarly, it has been reported that CBL3 but not CBL2 is involved in the regulation of K+ homeostasis and that CBL3 together with CIPK9 functions in K+ homeostasis under low-K+ stress (Liu et al., 2013). Only cbl3 mutant plants exhibited a low-K+-tolerant phenotype and increased K+ content under low-K+ conditions, leading to the assumption that only CBL3 together with CIPK9 but not CBL2 is functionally included in this particular pathway. Thus, quite remarkably despite their high degree of similarity, CBL2 and CBL3 appear to fulfill distinct functions. Nevertheless, CBL2 and CBL3 can also have overlapping functions (Tang et al., 2012).
A recent work demonstrated that only a cbl2/cbl3 double mutant, but not the respective single mutants, exhibits several developmental defects related to a reduced vacuolar ATPase activity accompanied by compromised ionic tolerance and micronutrient accumulation. However, evidence for a direct connection between CBL2/CBL3 function and the regulation of vacuolar ATPase activity is still pending and requires further investigation. In contrast to the rather enigmatic situation at the tonoplast, several ion transport processes have been described at the plasma membrane that are subject to regulation by CBL-CIPK complexes, such as the salt overly sensitive (SOS) pathway for salt tolerance or K+ channel regulation and the modulation of NO3− uptake in plants. CBL4-CIPK24 (SOS3-SOS2) complexes at the plasma membrane, for example, can activate the H+/Na+ antiporter SOS1 and thereby contribute to the regulation of Na+ extrusion (Liu and Zhu, 1998; Halfter et al., 2000; Qiu et al., 2002; Quintero et al., 2002, 2011; Fig. 1). The kinase CIPK23, on the other hand, is targeted to the plasma membrane by the two highly related Ca2+ sensors CBL1 and CBL9 (Xu et al., 2006; Cheong et al., 2007), where these complexes can regulate the activity of the Shaker-like potassium channel AKT1 (Li et al., 2006b; Xu et al., 2006; Hashimoto et al., 2012; Fig. 1). Furthermore, CIPK23 can also regulate the activity of the plasma membrane nitrate transporter CHL1 (also named NRT1.1; Ho et al., 2009; Fig. 1). In addition to its function as a CBL1-CIPK23 complex in AKT1 and CHL1 regulation, CBL1 also plays a role in drought and osmotic stress responses together with CIPK1 (Albrecht et al., 2003; D’Angelo et al., 2006).
Recently, a proteomic analysis of a cbl1 mutant revealed that in response to salt stress, several protein expression levels are altered compared with the wild type, further strengthening CBL1-involvement in salt stress responses (Shi et al., 2011). The affected proteins are predicted to function in various processes, such as signal transduction, ROS scavenging, energy production, carbon fixation, metabolism, mRNA and protein processing, and structural stability. Furthermore, additional roles for CBL1 are beginning to emerge, such as regulation of cold response together with CIPK7 or modulation of glucose- and GA-mediated responses (Huang et al., 2011; Li et al., 2013a, 2013b). Another example for a plasma membrane CBL functioning in salt stress signaling is CBL5. Overexpression of CBL5 renders plants more resistant to salt and osmotic stresses during seed germination independent from ABA signaling, suggesting a role as a positive regulator for CBL5 in salt and drought stress responses (Cheong et al., 2010). Future research is needed to identify targets and to unravel the underlying mechanisms for this observation. CIPKs that in addition to SOS2/CIPK24 have recently been implicated in salt stress responses are CIPK16 and CIPK6 (Tripathi et al., 2009; Chen et al., 2013; Roy et al., 2013). CIPK16 was identified by quantitative trait locus mapping as being linked to Na+ exclusion, as it was up-regulated under salt stress conditions. Furthermore, overexpression of CIPK16 leads to an improved salt tolerance. Comparably, also overexpression of CIPK6 increases plant tolerance to salt stress, while a cipk6 knockdown line exhibited hypersensitive responses to salt stress in addition to several developmental defects as well as reduction of auxin transport processes (Tripathi et al., 2009; Chen et al., 2013). Moreover, CIPK26 has very recently been identified as an interactor of the RING-type E3 ligase Keep on Going (KEG), a negative regulator of ABA signaling (Lyzenga et al., 2013). Interaction with KEG probably leads to the degradation of CIPK26 via the 26S proteasome. Overexpression of CIPK26 induces an ABA-hypersensitive phenotype, suggesting CIPK26 as a positive regulator of ABA signaling, possibly by interaction with components of ABA signaling such as ABI1, ABI2, and ABI5. Interestingly, KEG targets ABI5 for degradation, while CIPK26 appears to phosphoregulate this ABA signaling component, suggesting that KEG is acting to counteract and balance ABA responses that are promoted by CIPK26 and ABI5. These findings point to a tight regulation of CBL and CIPK function at the protein level that clearly deserves further investigation.
An interesting new twist in the exploration of the CBL-CIPK network is the discovery of its participation in the regulation of polar growth processes such as pollen germination and tube elongation (Mähs et al., 2013). CBL1 and CBL9 function is crucially required for proper pollen germination as well as for pollen tube growth. It appears that these two plasma membrane CBLs have overlapping functions in this context and that they contribute to K+ homeostasis. Analyses of mutant alleles and overexpression lines suggested a positive regulatory function in the course of K+ uptake across the plasma membrane. CIPKs implicated in this process, as well as their targets, still have to be determined, but it seems likely that potential targets in pollen are represented by K+ channels, such as AKT5 or SPIK, considering that a comparable regulatory mechanism has been described for AKT1 in other cell types (Li et al., 2006b; Xu et al., 2006).
Mechanisms of Target Regulation
Targets such as ion channels can be subject to phosphorylation and thereby activated or deactivated by CIPKs that have been targeted by their interacting CBL partners to the respective membrane subdomain. This has been demonstrated, for example, for the potassium channel AKT1 and for the nitrate transporter CHL1 (Li et al., 2006b; Xu et al., 2006; Cheong et al., 2007; Ho et al., 2009). Both become phosphorylated and thereby activated by CIPK23 when the kinase is targeted to the plasma membrane by CBL1 or CBL9. Furthermore, AKT1 can be dephosphorylated by AKT1-INTERACTING PP2C1, which means that the action of CIPKs can be counteracted by phosphatases (Lee et al., 2007; Sánchez-Barrena et al., 2013). This observation points to an even more elaborate regulatory mechanism, taking into account that also CIPKs can directly interact with PP2Cs (Ohta et al., 2003). This has been shown for SOS2/CIPK24, which interacts with the PP2C-type protein phosphatase ABI2 through the C-terminal protein phosphatase interaction motif (Ohta et al., 2003). In this regard, two independent mechanisms of channel regulation that have already been postulated for slow anion channel1 (SLAC1) regulation by the protein kinase Open Stomata1 (OST1) and by the protein phosphatase PP2CA appear conceivable (Lee et al., 2009b). On the one hand, the phosphatase could block the kinase activity by interaction with the kinase. This would terminate the activation of the channel. Additionally, the channel could be dephosphorylated and thereby directly inactivated by the phosphatase. Indeed, biochemical data indicated direct dephosphorylation of SLAC1 by the PP2C ABI1 (Brandt et al., 2012). The anion channel SLAC1 appears to represent a hub of guard cell regulation and, consequently, is subject to regulation by multiple kinases that involve SNF1-related kinase2s (SnRK2s), CDPKs, and receptor-like kinases (Geiger et al., 2009, 2010; Brandt et al., 2012; Hua et al., 2012). In several of these studies, kinase interaction with PP2Cs appears to reduce SLAC1 channel activation. It will be interesting to elucidate if CIPKs also contribute to the regulation of this channel.
Interaction of CIPKs with CBLs via the NAF domain and with phosphatases via the protein phosphatase interaction domain are assumed to be mutually exclusive (Sánchez-Barrena et al., 2013). However, there is also the possibility that phosphatases might additionally interact with the kinase domain of CIPKs (Lan et al., 2011). Moreover, interactions between PP2C phosphatases and CBLs might even further increase the complexity of the mechanism of target phosphoregulation. Remarkably, in addition to the regulation of targets via phosphorylation, recent research uncovered that CBL-CIPK complexes are also able to modulate channel activity independently of phosphorylation events. This turned out to be the case for K+ channel AKT2 regulation by CBL4-CIPK6 complexes (Held et al., 2011). Here, the interaction with the kinase is required for channel activation, but instead of phosphorylating the channel, the CBL4-CIPK6 complex serves as a translocation mediator delivering the channel from the endoplasmic reticulum to the plasma membrane, where it can fulfill its function (Fig. 1). Whether this targeting mechanism also applies for other targets regulated by CBL-CIPK complexes remains to be explored. Another potential mechanism of target regulation has recently emerged for AKT1. It was found that AKT1 directly interacts with CBL10 (Ren et al., 2013). This interaction apparently does not rely on a kinase and may give rise to AKT1 inactivation by outcompeting activating CBL-CIPK23 complexes. Together, these examples show that many variations exist in the mechanisms that contribute to target regulation.
The CBL-CIPK Network Is Conserved in Many Plant Species
Several recent studies indicate that the function of the CBL-CIPK network in abiotic stress and ABA signaling is not restricted to Arabidopsis. The diverse spectrum of plants in which CBL and CIPK function was analyzed encompasses species such as rice, rape (Brassica napus), wheat (Triticum aestivum), sorghum (Sorghum bicolor), apple (Malus domestica), cotton (Gossypium hirsutum), and poplar (Populus spp.; Yang et al., 2008; Piao et al., 2010; Zhang et al., 2011, 2013; Chen et al., 2012; Wang et al., 2012; Deng et al., 2013; He et al., 2013). Moreover, lately, five novel CIPKs and two CBLs have been discovered in kidney bean (Phaseolus vulgaris) and 43 putative CIPK genes that are closely related to rice CIPKs have been identified in maize (Zea mays; Hamada et al., 2009; Chen et al., 2011). A function of a rice CIPK has been reported for OsCIPK31, which was found to modulate responses to abiotic stresses during seed germination and seedling growth (Piao et al., 2010), and OsCIPK23, which turned out to be a multistress-induced gene likely regulating signaling pathways during pollination and drought stress responses (Yang et al., 2008). Another example for a multifunctional rice CIPK is OsCIPK15, which has been implicated in mediating oxygen deficiency tolerance as well as being involved in various PAMP-induced immune responses together with OsCIPK14 (Lee et al., 2009a; Kurusu et al., 2010). Comparative structural analyses of CBLs and CIPKs from Arabidopsis, pea (Pisum sativum), and rice revealed a high degree of conservation, and additional functional analyses suggested comparable mechanisms underlying signal transduction via CBL-CIPK complexes from these species (Mahajan et al., 2006; Tuteja and Mahajan, 2007).
In terms of target regulation, it seems that findings from Arabidopsis can also be transferred to other plant species, such as the regulation of potassium channels. The Shaker potassium channel VvK1.1 from grape (Vitis vinifera), which is the counterpart of AtAKT1, could be stimulated by coexpression of AtCIPK23-AtCBL1 in X. laevis oocytes (Cuéllar et al., 2010). Accordingly, it appears likely that this channel is regulated by a related CBL-CIPK complex in grape. In a subsequent study, a second Shaker potassium channel (VvK1.2) was characterized and analyzed for conductance in oocytes together with CBL-CIPK pairs from grape (Cuéllar et al., 2013). It was found that VvK1.2 could be stimulated by the two different VvCBL-VvCIPK pairs VvCIPK04-VvCBL01 and VvCIPK03-VvCBL02 (Cuéllar et al., 2013). Moreover, the SOS pathway for salt tolerance that was originally identified in Arabidopsis is also conserved in rice (Martínez-Atienza et al., 2007). Taken together, these findings suggest that the CBL-CIPK network fulfills conserved functions in many different plant species. However, it will be interesting to study the function of CBL-CIPK complexes in lower plants that have less complex CBL-CIPK networks, like algae and mosses, to uncover their original function.
CALCIUM AND ROS SIGNALING DIRECTLY INTERACT
Elevation of [Ca2+]cyt and the synthesis of ROS are fundamental constituents of rapid immune responses to pathogen attacks and other signaling pathways in plants and animals. Both are prerequisite for establishing pathogen resistance (Schwessinger and Zipfel, 2008; Boller and Felix, 2009; Dubiella et al., 2013) but are also important for ABA signaling in guard cells, seed germination, pollen tube growth, and root elongation as well as in the regulation of plant Na+/K+ homeostasis upon salt stress (Kwak et al., 2003; Potocký et al., 2007, 2012; Liu et al., 2009; Ma et al., 2012). Moreover, several lines of evidence support a mutual interconnection between Ca2+ and ROS elevation, because it has been reported that ROS activate Ca2+ channels in guard and root cells (Pei et al., 2000; Demidchik et al., 2003; Hua et al., 2012), and vice versa, Ca2+ enhances cellular ROS accumulation (Takeda et al., 2008). The most-studied ROS-producing enzymes in plants are the RBOHs (Suzuki et al., 2011; Marino et al., 2012). RBOH enzymes are integral membrane proteins that can generate superoxide anions that are rapidly converted to hydrogen peroxide. RBOH proteins possess two Ca2+-binding EF-hands and multiple phosphorylation sites in their N-termini (Keller et al., 1998; Dubiella et al., 2013). The first evidence for phosphoregulation of an RBOH by a Ca2+-regulated kinase was provided in potato with the identification of StRBOHB phosphorylation by StCPK5 (Kobayashi et al., 2007; Asai et al., 2013). Studies conducted in HEK293T cells revealed that AtRBOH activity is modulated by Ca2+ binding to the EF-hands as well as by N-terminal phosphorylation (Ogasawara et al., 2008; Takeda et al., 2008; Kimura et al., 2012). In addition to these findings, the observation that Ca2+ binding alone does not lead to the activation of RBOHs when kinases are inhibited in HEK cells points to a fundamental importance of RBOH phosphoregulation (Kimura et al., 2012).
Recently, the activation of RBOHF by plasma membrane-localized CBL1/CBL9-CIPK26 complexes has been described (Drerup et al., 2013; Fig. 1). Analyses in HEK293T cells and in vitro phosphorylation assays uncovered that CBL1/CBL9-CIPK26 complexes phosphorylate and thereby activate RBOHF. Furthermore, this study was the first to demonstrate an interaction between a CIPK and an RBOH in planta using the BiFC system in N. benthamiana. Important insights for in planta regulation of RBOH proteins by phosphorylation were provided by the finding that activation of RBOHD by CPK5 enables signal propagation upon PAMP stimulation to distal parts of the plant via an apoplastic ROS wave (Dubiella et al., 2013). Previous studies by Mittler et al. (2011) had already established that ROS waves can propagate through plants with a speed of up to 8.4 cm min−1 and that these waves are crucial for long-distance signaling. Moreover, it has been reported that the NADPH oxidase RBOHD plays a critical role in the formation and propagation of such ROS waves (Miller et al., 2009). In addition, classical and newer studies have provided evidence that also Ca2+ waves occur in plants (Trewavas et al., 1996; Trewavas, 1999; Gilroy, 2013). Major insights into the mechanisms that interconnect Ca2+ and ROS waves have been provided by the recent study of Dubiella et al. (2013), who found a mechanistic link between Ca2+ and ROS signaling in the context of cell-to-cell signal propagation. In the model resulting from this work, Ca2+ activates Ca2+-dependent kinases that lead to RBOH activation that, in turn, forms apoplastic ROS. These ROS induce Ca2+ release in neighboring cells that, in turn, again activates Ca2+-dependent kinases. In this way, cell-to-cell propagation of a signal is achieved. Since accumulating evidence suggests that also other protein kinases than CPK5 contribute to the regulation of RBOHD, it is tempting to speculate that also the CBL-CIPK network is involved in generating ROS waves for long-distance signal propagation. This is supported by recent work that has shown that CBL1/CBL9-CIPK26 complexes can activate the NADPH oxidase RBOHF (Drerup et al., 2013), and also RBOHD appears to be subject to regulation by CBL-CIPK complexes (P. Köster and J. Kudla, unpublished data). Moreover, several other stimuli are known to induce RBOHD-generated apoplastic ROS waves as well, such as wounding and local heat stress application (Miller et al., 2009). Therefore, it appears likely that ROS waves forward signals not only in PAMP-triggered responses.
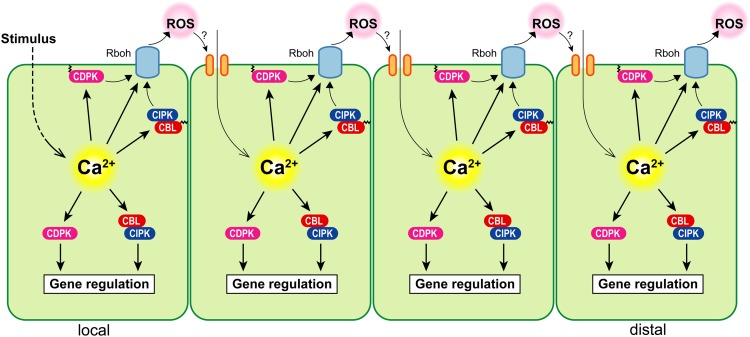
Taking this information together, all these data suggest a new model in which the cytosolic Ca2+ signal leads to the activation of RBOHD by Ca2+-regulated kinases, subsequent ROS generation, and propagation of the signal by the activation of Ca2+ channels of the neighboring cells (Fig. 2). Despite the convincing evidence for this model with regard to pathogen responses, it will be important to investigate the overall significance of the Ca2+-dependent phosphoregulation of ROS-producing NADPH oxidases for other physiological processes in the near future. As mentioned before, local heat stress application was found to induce systemic heat stress responses through ROS waves generated by the NADPH oxidase RBOHD, which is activated by Ca2+-regulated kinases (Miller et al., 2009). Combining this information with the novel finding that CNGCs can facilitate Ca2+ influx in response to heat stress (Finka et al., 2012; Gao et al., 2012; Tunc-Ozdemir et al., 2013b), it is tempting to speculate whether CNGCs might be responsible for Ca2+ influx in response to a local heat stress signal and thereby enable signal propagation through the activation of RBOHD, resulting in systemic heat stress tolerance. It will be interesting to investigate mechanistic details of this novel long-distance signal propagation in the future.
Figure 2.
Schematic model of Ca2+- and ROS-mediated cell-to-cell signal propagation in plants. For further details, see text.
CONCLUSION AND PERSPECTIVES
In recent years, the exploration of Ca2+ signal transduction in plants has significantly moved forward. Major advances have been made in the investigation of components contributing to Ca2+ signal generation, particularly in the field of GLRs, as well as components responsible for the translation of Ca2+ signals into physiological responses. Two of the latter components are represented by CDPKs and by the CBL-CIPK network, which both appear to fulfill a broad range of tasks crucial for plant survival. Recent studies uncovered new mechanisms influencing the CBL-CIPK-mediated signal transmission, such as PAT-mediated lipid modification of CBLs and phosphorylation of CBLs by their interacting kinases. These mechanisms require deeper analysis for better understanding. Likewise, the mechanism of differential CBL-CIPK complex formation and its dependence on [Ca2+]cyt deserves further investigation. In addition to target phosphoregulation by CBL-CIPK complexes, additional types of target regulation are now emerging, such as phosphorylation-independent translocation, adding another layer to the functional complexity of this regulatory network. A new direction for the investigation of the Ca2+ signal-decoding machinery has opened with the finding that the regulation of ROS-generating RBOHs by Ca2+-regulated kinases is an essential part of cell-to-cell communication and the establishment of systemic responses to local stimuli. Based on existing models for apoplastic ROS and cytosolic Ca2+ waves (Trewavas et al., 1996; Trewavas, 1999; Torres et al., 2005; Miller et al., 2009; Mittler et al., 2011; Dubiella et al., 2013), we here postulate a new potential model that combines these two important second messengers and also involves the CBL-CIPK network. Nevertheless, despite major advancements, we are merely beginning to understand how Ca2+ signals are generated and processed. One unsolved issue, for example, is the question of how apoplastic ROS signals are sensed and converted into cytoplasmic Ca2+ signals. Also, the identity of Ca2+ channels responsible for ROS-induced Ca2+ influx has to be uncovered. Future research is needed to address these kinds of fundamental questions and to elucidate the exact mechanisms underlying CBL-CIPK-mediated signal transduction and how these mechanisms are linked to generate specificity in Ca2+ signal-response coupling.
Acknowledgments
We thank Dr. Kenji Hashimoto for providing figure artwork and Anette Mähs for critical reading.
Glossary
- ROS
reactive oxygen species
- [Ca2+]cyt
cytosolic Ca2+ concentration
- GLR
glutamate-like receptor homolog
- CNGC
cyclic nucleotide-gated ion channel
- ABA
abscisic acid
- CIPK
CBL-interacting protein kinase
- CDPK
Ca2+-dependent protein kinase
- HSP
heat-shock protein
- CaM
calmodulin
- CBL
calcineurin B-like protein
- BiFC
bimolecular fluorescence complementation
References
- Albrecht V, Ritz O, Linder S, Harter K, Kudla J. (2001) The NAF domain defines a novel protein-protein interaction module conserved in Ca2+-regulated kinases. EMBO J 20: 1051–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht V, Weinl S, Blazevic D, D’Angelo C, Batistič O, Kolukisaoglu Ü, Bock R, Schulz B, Harter K, Kudla J. (2003) The calcium sensor CBL1 integrates plant responses to abiotic stresses. Plant J 36: 457–470 [DOI] [PubMed] [Google Scholar]
- Allen GJ, Chu SP, Harrington CL, Schumacher K, Hoffmann T, Tang YY, Grill E, Schroeder JI. (2001) A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature 411: 1053–1057 [DOI] [PubMed] [Google Scholar]
- Allen GJ, Chu SP, Schumacher K, Shimazaki CT, Vafeados D, Kemper A, Hawke SD, Tallman G, Tsien RY, Harper JF, et al. (2000) Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science 289: 2338–2342 [DOI] [PubMed] [Google Scholar]
- Allen GJ, Sanders D. (1994) Two voltage-gated, calcium release channels coreside in the vacuolar membrane of broad bean guard cells. Plant Cell 6: 685–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai S, Ichikawa T, Nomura H, Kobayashi M, Kamiyoshihara Y, Mori H, Kadota Y, Zipfel C, Jones JD, Yoshioka H. (2013) The variable domain of a plant calcium-dependent protein kinase (CDPK) confers subcellular localization and substrate recognition for NADPH oxidase. J Biol Chem 288: 14332–14340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batistič O. (2012) Genomics and localization of the Arabidopsis DHHC-cysteine-rich domain S-acyltransferase protein family. Plant Physiol 160: 1597–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batistič O, Kudla J. (2004) Integration and channeling of calcium signaling through the CBL calcium sensor/CIPK protein kinase network. Planta 219: 915–924 [DOI] [PubMed] [Google Scholar]
- Batistič O, Kudla J. (2009) Plant calcineurin B-like proteins and their interacting protein kinases. Biochim Biophys Acta 1793: 985–992 [DOI] [PubMed] [Google Scholar]
- Batistič O, Kudla J (2010) Calcium: not just another ion. In R Hell, RR Mendel, eds, Cell Biology of Metals and Nutrients. Plant Cell Monographs 17. Springer-Verlag, Berlin, pp 17–54 [Google Scholar]
- Batistič O, Kudla J. (2012) Analysis of calcium signaling pathways in plants. Biochim Biophys Acta 1820: 1283–1293 [DOI] [PubMed] [Google Scholar]
- Batistič O, Rehers M, Akerman A, Schlücking K, Steinhorst L, Yalovsky S, Kudla J. (2012) S-Acylation-dependent association of the calcium sensor CBL2 with the vacuolar membrane is essential for proper abscisic acid responses. Cell Res 22: 1155–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batistič O, Sorek N, Schültke S, Yalovsky S, Kudla J. (2008) Dual fatty acyl modification determines the localization and plasma membrane targeting of CBL/CIPK Ca2+ signaling complexes in Arabidopsis. Plant Cell 20: 1346–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batistič O, Waadt R, Steinhorst L, Held K, Kudla J. (2010) CBL-mediated targeting of CIPKs facilitates the decoding of calcium signals emanating from distinct cellular stores. Plant J 61: 211–222 [DOI] [PubMed] [Google Scholar]
- Boller T, Felix G. (2009) A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol 60: 379–406 [DOI] [PubMed] [Google Scholar]
- Boudsocq M, Willmann MR, McCormack M, Lee H, Shan L, He P, Bush J, Cheng SH, Sheen J. (2010) Differential innate immune signalling via Ca2+ sensor protein kinases. Nature 464: 418–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt B, Brodsky DE, Xue S, Negi J, Iba K, Kangasjärvi J, Ghassemian M, Stephan AB, Hu H, Schroeder JI. (2012) Reconstitution of abscisic acid activation of SLAC1 anion channel by CPK6 and OST1 kinases and branched ABI1 PP2C phosphatase action. Proc Natl Acad Sci USA 109: 10593–10598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero F, Botella MA, Rubio L, Fernández JA, Martínez V, Rubio F. (2012) A Ca2+-sensitive system mediates low-affinity K+ uptake in the absence of AKT1 in Arabidopsis plants. Plant Cell Physiol 53: 2047–2059 [DOI] [PubMed] [Google Scholar]
- Chaiwongsar S, Strohm AK, Roe JR, Godiwalla RY, Chan CW. (2009) A cyclic nucleotide-gated channel is necessary for optimum fertility in high-calcium environments. New Phytol 183: 76–87 [DOI] [PubMed] [Google Scholar]
- Chang IF, Curran A, Woolsey R, Quilici D, Cushman JC, Mittler R, Harmon A, Harper JF. (2009) Proteomic profiling of tandem affinity purified 14-3-3 protein complexes in Arabidopsis thaliana. Proteomics 9: 2967–2985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BS, Roche KW. (2007) Regulation of NMDA receptors by phosphorylation. Neuropharmacology 53: 362–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Ren F, Zhou L, Wang QQ, Zhong H, Li XB. (2012) The Brassica napus calcineurin B-like 1/CBL-interacting protein kinase 6 (CBL1/CIPK6) component is involved in the plant response to abiotic stress and ABA signalling. J Exp Bot 63: 6211–6222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Wang QQ, Zhou L, Ren F, Li DD, Li XB. (2013) Arabidopsis CBL-interacting protein kinase (CIPK6) is involved in plant response to salt/osmotic stress and ABA. Mol Biol Rep 40: 4759–4767 [DOI] [PubMed] [Google Scholar]
- Chen X, Gu Z, Xin D, Hao L, Liu C, Huang J, Ma B, Zhang H. (2011) Identification and characterization of putative CIPK genes in maize. J Genet Genomics 38: 77–87 [DOI] [PubMed] [Google Scholar]
- Cheng SH, Willmann MR, Chen HC, Sheen J. (2002) Calcium signaling through protein kinases: the Arabidopsis calcium-dependent protein kinase gene family. Plant Physiol 129: 469–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong YH, Pandey GK, Grant JJ, Batistič O, Li L, Kim BG, Lee SC, Kudla J, Luan S. (2007) Two calcineurin B-like calcium sensors, interacting with protein kinase CIPK23, regulate leaf transpiration and root potassium uptake in Arabidopsis. Plant J 52: 223–239 [DOI] [PubMed] [Google Scholar]
- Cheong YH, Sung SJ, Kim BG, Pandey GK, Cho JS, Kim KN, Luan S. (2010) Constitutive overexpression of the calcium sensor CBL5 confers osmotic or drought stress tolerance in Arabidopsis. Mol Cells 29: 159–165 [DOI] [PubMed] [Google Scholar]
- Cho D, Kim SA, Murata Y, Lee S, Jae SK, Nam HG, Kwak JM. (2009) De-regulated expression of the plant glutamate receptor homolog AtGLR3.1 impairs long-term Ca2+-programmed stomatal closure. Plant J 58: 437–449 [DOI] [PubMed] [Google Scholar]
- Cuéllar T, Azeem F, Andrianteranagna M, Pascaud F, Verdeil JL, Sentenac H, Zimmermann S, Gaillard I. (2013) Potassium transport in developing fleshy fruits: the grapevine inward K+ channel VvK1.2 is activated by CIPK-CBL complexes and induced in ripening berry flesh cells. Plant J 73: 1006–1018 [DOI] [PubMed] [Google Scholar]
- Cuéllar T, Pascaud F, Verdeil JL, Torregrosa L, Adam-Blondon AF, Thibaud JB, Sentenac H, Gaillard I. (2010) A grapevine Shaker inward K+ channel activated by the calcineurin B-like calcium sensor 1-protein kinase CIPK23 network is expressed in grape berries under drought stress conditions. Plant J 61: 58–69 [DOI] [PubMed] [Google Scholar]
- D’Angelo C, Weinl S, Batistič O, Pandey GK, Cheong YH, Schültke S, Albrecht V, Ehlert B, Schulz B, Harter K, et al. (2006) Alternative complex formation of the Ca2+-regulated protein kinase CIPK1 controls abscisic acid-dependent and independent stress responses in Arabidopsis. Plant J 48: 857–872 [DOI] [PubMed] [Google Scholar]
- Davenport R. (2002) Glutamate receptors in plants. Ann Bot (Lond) 90: 549–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day IS, Reddy VS, Shad Ali G, Reddy AS. (2002) Analysis of EF-hand-containing proteins in Arabidopsis. Genome Biol 3: RESEARCH0056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demange P, Voges D, Benz J, Liemann S, Göttig P, Berendes R, Burger A, Huber R. (1994) Annexin V: the key to understanding ion selectivity and voltage regulation? Trends Biochem Sci 19: 272–276 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Shabala SN, Coutts KB, Tester MA, Davies JM. (2003) Free oxygen radicals regulate plasma membrane Ca2+- and K+-permeable channels in plant root cells. J Cell Sci 116: 81–88 [DOI] [PubMed] [Google Scholar]
- Demir F, Horntrich C, Blachutzik JO, Scherzer S, Reinders Y, Kierszniowska S, Schulze WX, Harms GS, Hedrich R, Geiger D, et al. (2013) Arabidopsis nanodomain-delimited ABA signaling pathway regulates the anion channel SLAH3. Proc Natl Acad Sci USA 110: 8296–8301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Zhou S, Hu W, Feng J, Zhang F, Chen L, Huang C, Luo Q, He Y, Yang G, et al. (2013) Ectopic expression of wheat TaCIPK14, encoding a calcineurin B-like protein-interacting protein kinase, confers salinity and cold tolerance in tobacco. Physiol Plant (in press) [DOI] [PubMed] [Google Scholar]
- Díaz-Muñoz M, Hamilton SL, Kaetzel MA, Hazarika P, Dedman JR. (1990) Modulation of Ca2+ release channel activity from sarcoplasmic reticulum by annexin VI (67-kDa calcimedin). J Biol Chem 265: 15894–15899 [PubMed] [Google Scholar]
- Dodd AN, Kudla J, Sanders D. (2010) The language of calcium signaling. Annu Rev Plant Biol 61: 593–620 [DOI] [PubMed] [Google Scholar]
- Drerup MM, Schlücking K, Hashimoto K, Manishankar P, Steinhorst L, Kuchitsu K, Kudla J. (2013) The calcineurin B-like calcium sensors CBL1 and CBL9 together with their interacting protein kinase CIPK26 regulate the Arabidopsis NADPH oxidase RBOHF. Mol Plant 6: 559–569 [DOI] [PubMed] [Google Scholar]
- Du W, Lin H, Chen S, Wu Y, Zhang J, Fuglsang AT, Palmgren MG, Wu W, Guo Y. (2011) Phosphorylation of SOS3-like calcium-binding proteins by their interacting SOS2-like protein kinases is a common regulatory mechanism in Arabidopsis. Plant Physiol 156: 2235–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubiella U, Seybold H, Durian G, Komander E, Lassig R, Witte CP, Schulze WX, Romeis T. (2013) Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc Natl Acad Sci USA 110: 8744–8749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finka A, Cuendet AF, Maathuis FJ, Saidi Y, Goloubinoff P. (2012) Plasma membrane cyclic nucleotide gated calcium channels control land plant thermal sensing and acquired thermotolerance. Plant Cell 24: 3333–3348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer C, Kugler A, Hoth S, Dietrich P. (2013) An IQ domain mediates the interaction with calmodulin in a plant cyclic nucleotide-gated channel. Plant Cell Physiol 54: 573–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz S, Ehlert B, Liese A, Kurth J, Cazalé AC, Romeis T. (2011) Calcium-dependent protein kinase CPK21 functions in abiotic stress response in Arabidopsis thaliana. Mol Plant 4: 83–96 [DOI] [PubMed] [Google Scholar]
- Frietsch S, Wang YF, Sladek C, Poulsen LR, Romanowsky SM, Schroeder JI, Harper JF. (2007) A cyclic nucleotide-gated channel is essential for polarized tip growth of pollen. Proc Natl Acad Sci USA 104: 14531–14536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa H, Singh SK, Mancusso R, Gouaux E. (2005) Subunit arrangement and function in NMDA receptors. Nature 438: 185–192 [DOI] [PubMed] [Google Scholar]
- Gao F, Han X, Wu J, Zheng S, Shang Z, Sun D, Zhou R, Li B. (2012) A heat-activated calcium-permeable channel - Arabidopsis cyclic nucleotide-gated ion channel 6 - is involved in heat shock responses. Plant J 70: 1056–1069 [DOI] [PubMed] [Google Scholar]
- Gao X, Chen X, Lin W, Chen S, Lu D, Niu Y, Li L, Cheng C, McCormack M, Sheen J, et al. (2013) Bifurcation of Arabidopsis NLR immune signaling via Ca2+-dependent protein kinases. PLoS Pathog 9: e1003127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, Marten I, Ache P, Matschi S, Liese A, Wellmann C, Al-Rasheid KA, Grill E, et al. (2010) Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc Natl Acad Sci USA 107: 8023–8028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, Stange A, Marten I, Bauer H, Ache P, Matschi S, Liese A, Al-Rasheid KA, et al. (2009) Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proc Natl Acad Sci USA 106: 21425–21430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke V, Creutz CE, Moss SE. (2005) Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol 6: 449–461 [DOI] [PubMed] [Google Scholar]
- Gerke V, Moss SE. (2002) Annexins: from structure to function. Physiol Rev 82: 331–371 [DOI] [PubMed] [Google Scholar]
- Gilroy S (2013) Ca2+ waves and plant systemic signaling. In 16th International Workshop on Plant Membrane Biology, Abstract Book. Institute of Plant Science and Resources, Okayama University, Japan, p 58 [Google Scholar]
- Halfter U, Ishitani M, Zhu JK. (2000) The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3. Proc Natl Acad Sci USA 97: 3735–3740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S, Seiki Y, Watanabe K, Ozeki T, Matsui H, Ito H. (2009) Expression and interaction of the CBLs and CIPKs from immature seeds of kidney bean (Phaseolus vulgaris L.). Phytochemistry 70: 501–507 [DOI] [PubMed] [Google Scholar]
- Harper JF, Harmon A. (2005) Plants, symbiosis and parasites: a calcium signalling connection. Nat Rev Mol Cell Biol 6: 555–566 [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Eckert C, Anschütz U, Scholz M, Held K, Waadt R, Reyer A, Hippler M, Becker D, Kudla J. (2012) Phosphorylation of calcineurin B-like (CBL) calcium sensor proteins by their CBL-interacting protein kinases (CIPKs) is required for full activity of CBL-CIPK complexes toward their target proteins. J Biol Chem 287: 7956–7968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Kudla J. (2011) Calcium decoding mechanisms in plants. Biochimie 93: 2054–2059 [DOI] [PubMed] [Google Scholar]
- Hawkins TE, Merrifield CJ, Moss SE. (2000) Calcium signaling and annexins. Cell Biochem Biophys 33: 275–296 [DOI] [PubMed] [Google Scholar]
- He L, Yang X, Wang L, Zhu L, Zhou T, Deng J, Zhang X. (2013) Molecular cloning and functional characterization of a novel cotton CBL-interacting protein kinase gene (GhCIPK6) reveals its involvement in multiple abiotic stress tolerance in transgenic plants. Biochem Biophys Res Commun 435: 209–215 [DOI] [PubMed] [Google Scholar]
- Hedrich R, Neher E. (1987) Cytoplasmic calcium regulates voltage-dependent ion channels in plant vacuoles. Nature 329: 833–836 [Google Scholar]
- Held K, Pascaud F, Eckert C, Gajdanowicz P, Hashimoto K, Corratgé-Faillie C, Offenborn JN, Lacombe B, Dreyer I, Thibaud JB, et al. (2011) Calcium-dependent modulation and plasma membrane targeting of the AKT2 potassium channel by the CBL4/CIPK6 calcium sensor/protein kinase complex. Cell Res 21: 1116–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CH, Lin SH, Hu HC, Tsay YF. (2009) CHL1 functions as a nitrate sensor in plants. Cell 138: 1184–1194 [DOI] [PubMed] [Google Scholar]
- Hollmann M, O’Shea-Greenfield A, Rogers SW, Heinemann S. (1989) Cloning by functional expression of a member of the glutamate receptor family. Nature 342: 643–648 [DOI] [PubMed] [Google Scholar]
- Hu CD, Chinenov Y, Kerppola TK. (2002) Visualization of interactions among bZIP and Rel family proteins in living cells using bimolecular fluorescence complementation. Mol Cell 9: 789–798 [DOI] [PubMed] [Google Scholar]
- Hua D, Wang C, He J, Liao H, Duan Y, Zhu Z, Guo Y, Chen Z, Gong Z. (2012) A plasma membrane receptor kinase, GHR1, mediates abscisic acid- and hydrogen peroxide-regulated stomatal movement in Arabidopsis. Plant Cell 24: 2546–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Ding S, Zhang H, Du H, An L. (2011) CIPK7 is involved in cold response by interacting with CBL1 in Arabidopsis thaliana. Plant Sci 181: 57–64 [DOI] [PubMed] [Google Scholar]
- Ishitani M, Liu J, Halfter U, Kim CS, Shi W, Zhu JK. (2000) SOS3 function in plant salt tolerance requires N-myristoylation and calcium binding. Plant Cell 12: 1667–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM (2000) Principles of Neural Science, Ed 4. McGraw-Hill, New York [Google Scholar]
- Kang J, Mehta S, Turano FJ. (2004) The putative glutamate receptor 1.1 (AtGLR1.1) in Arabidopsis thaliana regulates abscisic acid biosynthesis and signaling to control development and water loss. Plant Cell Physiol 45: 1380–1389 [DOI] [PubMed] [Google Scholar]
- Kang J, Turano FJ. (2003) The putative glutamate receptor 1.1 (AtGLR1.1) functions as a regulator of carbon and nitrogen metabolism in Arabidopsis thaliana. Proc Natl Acad Sci USA 100: 6872–6877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller T, Damude HG, Werner D, Doerner P, Dixon RA, Lamb C. (1998) A plant homolog of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. Plant Cell 10: 255–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerppola TK. (2006) Visualization of molecular interactions by fluorescence complementation. Nat Rev Mol Cell Biol 7: 449–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BG, Waadt R, Cheong YH, Pandey GK, Dominguez-Solis JR, Schültke S, Lee SC, Kudla J, Luan S. (2007) The calcium sensor CBL10 mediates salt tolerance by regulating ion homeostasis in Arabidopsis. Plant J 52: 473–484 [DOI] [PubMed] [Google Scholar]
- Kim SA, Kwak JM, Jae SK, Wang MH, Nam HG. (2001) Overexpression of the AtGluR2 gene encoding an Arabidopsis homolog of mammalian glutamate receptors impairs calcium utilization and sensitivity to ionic stress in transgenic plants. Plant Cell Physiol 42: 74–84 [DOI] [PubMed] [Google Scholar]
- Kimura S, Kaya H, Kawarazaki T, Hiraoka G, Senzaki E, Michikawa M, Kuchitsu K. (2012) Protein phosphorylation is a prerequisite for the Ca2+-dependent activation of Arabidopsis NADPH oxidases and may function as a trigger for the positive feedback regulation of Ca2+ and reactive oxygen species. Biochim Biophys Acta 1823: 398–405 [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Ohura I, Kawakita K, Yokota N, Fujiwara M, Shimamoto K, Doke N, Yoshioka H. (2007) Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. Plant Cell 19: 1065–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolukisaoglu Ü, Weinl S, Blazevic D, Batistič O, Kudla J. (2004) Calcium sensors and their interacting protein kinases: genomics of the Arabidopsis and rice CBL-CIPK signaling networks. Plant Physiol 134: 43–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourie JI, Wood HB. (2000) Biophysical and molecular properties of annexin-formed channels. Prog Biophys Mol Biol 73: 91–134 [DOI] [PubMed] [Google Scholar]
- Kudla J, Batistič O, Hashimoto K. (2010) Calcium signals: the lead currency of plant information processing. Plant Cell 22: 541–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurusu T, Hamada J, Hamada H, Hanamata S, Kuchitsu K. (2010) Roles of calcineurin B-like protein-interacting protein kinases in innate immunity in rice. Plant Signal Behav 5: 1045–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JD, Schroeder JI. (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22: 2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan WZ, Lee SC, Che YF, Jiang YQ, Luan S. (2011) Mechanistic analysis of AKT1 regulation by the CBL-CIPK-PP2CA interactions. Mol Plant 4: 527–536 [DOI] [PubMed] [Google Scholar]
- Laohavisit A, Mortimer JC, Demidchik V, Coxon KM, Stancombe MA, Macpherson N, Brownlee C, Hofmann A, Webb AA, Miedema H, et al. (2009) Zea mays annexins modulate cytosolic free Ca2+ and generate a Ca2+-permeable conductance. Plant Cell 21: 479–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laohavisit A, Shang Z, Rubio L, Cuin TA, Véry AA, Wang A, Mortimer JC, Macpherson N, Coxon KM, Battey NH, et al. (2012) Arabidopsis annexin1 mediates the radical-activated plasma membrane Ca2+- and K+-permeable conductance in root cells. Plant Cell 24: 1522–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KW, Chen PW, Lu CA, Chen S, Ho TH, Yu SM. (2009a) Coordinated responses to oxygen and sugar deficiency allow rice seedlings to tolerate flooding. Sci Signal 2: ra61 [DOI] [PubMed] [Google Scholar]
- Lee SC, Lan W, Buchanan BB, Luan S. (2009b) A protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proc Natl Acad Sci USA 106: 21419–21424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Lan WZ, Kim BG, Li L, Cheong YH, Pandey GK, Lu G, Buchanan BB, Luan S. (2007) A protein phosphorylation/dephosphorylation network regulates a plant potassium channel. Proc Natl Acad Sci USA 104: 15959–15964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhu S, Song X, Shen Y, Chen H, Yu J, Yi K, Liu Y, Karplus VJ, Wu P, et al. (2006a) A rice glutamate receptor-like gene is critical for the division and survival of individual cells in the root apical meristem. Plant Cell 18: 340–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Kim BG, Cheong YH, Pandey GK, Luan S. (2006b) A Ca2+ signaling pathway regulates a K+ channel for low-K response in Arabidopsis. Proc Natl Acad Sci USA 103: 12625–12630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZY, Xu ZS, Chen Y, He GY, Yang GX, Chen M, Li LC, Ma YZ. (2013a) A novel role for Arabidopsis CBL1 in affecting plant responses to glucose and gibberellin during germination and seedling development. PLoS ONE 8: e56412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZY, Xu ZS, He GY, Yang GX, Chen M, Li LC, Ma Y. (2013b) The voltage-dependent anion channel 1 (AtVDAC1) negatively regulates plant cold responses during germination and seedling development in Arabidopsis and interacts with calcium sensor CBL1. Int J Mol Sci 14: 701–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liese A, Romeis T. (2013) Biochemical regulation of in vivo function of plant calcium-dependent protein kinases (CDPK). Biochim Biophys Acta 1833: 1582–1589 [DOI] [PubMed] [Google Scholar]
- Lin H, Yang Y, Quan R, Mendoza I, Wu Y, Du W, Zhao S, Schumaker KS, Pardo JM, Guo Y. (2009) Phosphorylation of SOS3-LIKE CALCIUM BINDING PROTEIN8 by SOS2 protein kinase stabilizes their protein complex and regulates salt tolerance in Arabidopsis. Plant Cell 21: 1607–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhu JK. (1998) A calcium sensor homolog required for plant salt tolerance. Science 280: 1943–1945 [DOI] [PubMed] [Google Scholar]
- Liu LL, Ren HM, Chen LQ, Wang Y, Wu WH. (2013) A protein kinase, calcineurin B-like protein-interacting protein kinase9, interacts with calcium sensor calcineurin B-like protein3 and regulates potassium homeostasis under low-potassium stress in Arabidopsis. Plant Physiol 161: 266–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Li RL, Zhang L, Wang QL, Niehaus K, Baluska F, Samaj J, Lin JX. (2009) Lipid microdomain polarization is required for NADPH oxidase-dependent ROS signaling in Picea meyeri pollen tube tip growth. Plant J 60: 303–313 [DOI] [PubMed] [Google Scholar]
- Lu SX, Hrabak EM. (2013) The myristoylated amino-terminus of an Arabidopsis calcium-dependent protein kinase mediates plasma membrane localization. Plant Mol Biol 82: 267–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyzenga WJ, Liu H, Schofield A, Muise-Hennessey A, Stone SL. (2013) Arabidopsis CIPK26 interacts with KEG, components of the ABA signalling network and is degraded by the ubiquitin-proteasome system. J Exp Bot 64: 2779–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Zhang H, Sun L, Jiao Y, Zhang G, Miao C, Hao F. (2012) NADPH oxidase AtrbohD and AtrbohF function in ROS-dependent regulation of Na+/K+ homeostasis in Arabidopsis under salt stress. J Exp Bot 63: 305–317 [DOI] [PubMed] [Google Scholar]
- Ma W, Berkowitz GA. (2011) Ca2+ conduction by plant cyclic nucleotide gated channels and associated signaling components in pathogen defense signal transduction cascades. New Phytol 190: 566–572 [DOI] [PubMed] [Google Scholar]
- Mahajan S, Sopory SK, Tuteja N. (2006) Cloning and characterization of CBL-CIPK signalling components from a legume (Pisum sativum). FEBS J 273: 907–925 [DOI] [PubMed] [Google Scholar]
- Mähs A, Steinhorst L, Han JP, Shen LK, Wang Y, Kudla J. (2013) The calcineurin B-like Ca2+ sensors CBL1 and CBL9 function in pollen germination and pollen tube growth in Arabidopsis. Mol Plant 6: 1149–1162 [DOI] [PubMed] [Google Scholar]
- Marino D, Dunand C, Puppo A, Pauly N. (2012) A burst of plant NADPH oxidases. Trends Plant Sci 17: 9–15 [DOI] [PubMed] [Google Scholar]
- Martín ML, Busconi L. (2000) Membrane localization of a rice calcium-dependent protein kinase (CDPK) is mediated by myristoylation and palmitoylation. Plant J 24: 429–435 [DOI] [PubMed] [Google Scholar]
- Martínez-Atienza J, Jiang X, Garciadeblas B, Mendoza I, Zhu JK, Pardo JM, Quintero FJ. (2007) Conservation of the salt overly sensitive pathway in rice. Plant Physiol 143: 1001–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäser P, Thomine S, Schroeder JI, Ward JM, Hirschi K, Sze H, Talke IN, Amtmann A, Maathuis FJ, Sanders D, et al. (2001) Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol 126: 1646–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazars C, Brière C, Bourque S, Thuleau P. (2011) Nuclear calcium signaling: an emerging topic in plants. Biochimie 93: 2068–2074 [DOI] [PubMed] [Google Scholar]
- Mehlmer N, Wurzinger B, Stael S, Hofmann-Rodrigues D, Csaszar E, Pfister B, Bayer R, Teige M. (2010) The Ca2+-dependent protein kinase CPK3 is required for MAPK-independent salt-stress acclimation in Arabidopsis. Plant J 63: 484–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michard E, Lima PT, Borges F, Silva AC, Portes MT, Carvalho JE, Gilliham M, Liu LH, Obermeyer G, Feijó JA. (2011) Glutamate receptor-like genes form Ca2+ channels in pollen tubes and are regulated by pistil D-serine. Science 332: 434–437 [DOI] [PubMed] [Google Scholar]
- Miller G, Schlauch K, Tam R, Cortes D, Torres MA, Shulaev V, Dangl JL, Mittler R. (2009) The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci Signal 2: ra45 [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F. (2011) ROS signaling: the new wave? Trends Plant Sci 16: 300–309 [DOI] [PubMed] [Google Scholar]
- Mortimer JC, Laohavisit A, Macpherson N, Webb A, Brownlee C, Battey NH, Davies JM. (2008) Annexins: multifunctional components of growth and adaptation. J Exp Bot 59: 533–544 [DOI] [PubMed] [Google Scholar]
- Ogasawara Y, Kaya H, Hiraoka G, Yumoto F, Kimura S, Kadota Y, Hishinuma H, Senzaki E, Yamagoe S, Nagata K, et al. (2008) Synergistic activation of the Arabidopsis NADPH oxidase AtrbohD by Ca2+ and phosphorylation. J Biol Chem 283: 8885–8892 [DOI] [PubMed] [Google Scholar]
- Ohta M, Guo Y, Halfter U, Zhu JK. (2003) A novel domain in the protein kinase SOS2 mediates interaction with the protein phosphatase 2C ABI2. Proc Natl Acad Sci USA 100: 11771–11776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo KK, Larson ET, Keyloun KR, Castaneda LJ, Derocher AE, Inampudi KK, Kim JE, Arakaki TL, Murphy RC, Zhang L, et al. (2010) Toxoplasma gondii calcium-dependent protein kinase 1 is a target for selective kinase inhibitors. Nat Struct Mol Biol 17: 602–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI. (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731–734 [DOI] [PubMed] [Google Scholar]
- Peiter E. (2011) The plant vacuole: emitter and receiver of calcium signals. Cell Calcium 50: 120–128 [DOI] [PubMed] [Google Scholar]
- Peiter E, Maathuis FJ, Mills LN, Knight H, Pelloux J, Hetherington AM, Sanders D. (2005) The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature 434: 404–408 [DOI] [PubMed] [Google Scholar]
- Piao HL, Xuan YH, Park SH, Je BI, Park SJ, Park SH, Kim CM, Huang J, Wang GK, Kim MJ, et al. (2010) OsCIPK31, a CBL-interacting protein kinase is involved in germination and seedling growth under abiotic stress conditions in rice plants. Mol Cells 30: 19–27 [DOI] [PubMed] [Google Scholar]
- Potocký M, Jones MA, Bezvoda R, Smirnoff N, Zárský V. (2007) Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytol 174: 742–751 [DOI] [PubMed] [Google Scholar]
- Potocký M, Pejchar P, Gutkowska M, Jiménez-Quesada MJ, Potocká A, Alché JdeD, Kost B, Žárský V. (2012) NADPH oxidase activity in pollen tubes is affected by calcium ions, signaling phospholipids and Rac/Rop GTPases. J Plant Physiol 169: 1654–1663 [DOI] [PubMed] [Google Scholar]
- Price MB, Jelesko J, Okumoto S. (2012) Glutamate receptor homologs in plants: functions and evolutionary origins. Front Plant Sci 3: 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z, Stephens NR, Spalding EP. (2006) Calcium entry mediated by GLR3.3, an Arabidopsis glutamate receptor with a broad agonist profile. Plant Physiol 142: 963–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu QS, Guo Y, Dietrich MA, Schumaker KS, Zhu JK. (2002) Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc Natl Acad Sci USA 99: 8436–8441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero FJ, Martinez-Atienza J, Villalta I, Jiang X, Kim WY, Ali Z, Fujii H, Mendoza I, Yun DJ, Zhu JK, et al. (2011) Activation of the plasma membrane Na/H antiporter Salt-Overly-Sensitive 1 (SOS1) by phosphorylation of an auto-inhibitory C-terminal domain. Proc Natl Acad Sci USA 108: 2611–2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero FJ, Ohta M, Shi H, Zhu JK, Pardo JM. (2002) Reconstitution in yeast of the Arabidopsis SOS signaling pathway for Na+ homeostasis. Proc Natl Acad Sci USA 99: 9061–9066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AS, Ali GS, Celesnik H, Day IS. (2011) Coping with stresses: roles of calcium- and calcium/calmodulin-regulated gene expression. Plant Cell 23: 2010–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren XL, Qi GN, Feng HQ, Zhao S, Zhao SS, Wang Y, Wu WH. (2013) Calcineurin B-like protein CBL10 directly interacts with AKT1 and modulates K+ homeostasis in Arabidopsis. Plant J 74: 258–266 [DOI] [PubMed] [Google Scholar]
- Richter GL, Monshausen GB, Krol A, Gilroy S. (2009) Mechanical stimuli modulate lateral root organogenesis. Plant Physiol 151: 1855–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas E, Arispe N, Haigler HT, Burns AL, Pollard HB. (1992) Identification of annexins as calcium channels in biological membranes. Bone Miner 17: 214–218 [DOI] [PubMed] [Google Scholar]
- Roy SJ, Gilliham M, Berger B, Essah PA, Cheffings C, Miller AJ, Davenport RJ, Liu LH, Skynner MJ, Davies JM, et al. (2008) Investigating glutamate receptor-like gene co-expression in Arabidopsis thaliana. Plant Cell Environ 31: 861–871 [DOI] [PubMed] [Google Scholar]
- Roy SJ, Huang W, Wang XJ, Evrard A, Schmöckel SM, Zafar ZU, Tester M. (2013) A novel protein kinase involved in Na+ exclusion revealed from positional cloning. Plant Cell Environ 36: 553–568 [DOI] [PubMed] [Google Scholar]
- Sánchez-Barrena MJ, Martínez-Ripoll M, Albert A. (2013) Structural biology of a major signaling network that regulates plant abiotic stress: the CBL-CIPK mediated pathway. Int J Mol Sci 14: 5734–5749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D, Pelloux J, Brownlee C, Harper JF. (2002) Calcium at the crossroads of signaling. Plant Cell (Suppl) 14: S401–S417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlücking K, Edel KH, Köster P, Drerup MM, Eckert C, Steinhorst L, Waadt R, Batistič O, Kudla J. (2013) A new β-estradiol inducible vector set that facilitates easy construction and efficient expression of transgenes reveals CBL3-dependent cytoplasm to tonoplast translocation of CIPK5. Mol Plant (in press) [DOI] [PubMed] [Google Scholar]
- Schwessinger B, Zipfel C. (2008) News from the frontline: recent insights into PAMP-triggered immunity in plants. Curr Opin Plant Biol 11: 389–395 [DOI] [PubMed] [Google Scholar]
- Scrase-Field SA, Knight MR. (2003) Calcium: just a chemical switch? Curr Opin Plant Biol 6: 500–506 [DOI] [PubMed] [Google Scholar]
- Shi J, Kim KN, Ritz O, Albrecht V, Gupta R, Harter K, Luan S, Kudla J. (1999) Novel protein kinases associated with calcineurin B-like calcium sensors in Arabidopsis. Plant Cell 11: 2393–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Chen W, Sun W. (2011) Comparative proteomic analysis of the Arabidopsis cbl1 mutant in response to salt stress. Proteomics 11: 4712–4725 [DOI] [PubMed] [Google Scholar]
- Steinhorst L, Kudla J. (2012) Calcium: a central regulator of pollen germination and tube growth. Biochim Biophys Acta 1833: 1573–1581 [DOI] [PubMed] [Google Scholar]
- Stephens NR, Qi Z, Spalding EP. (2008) Glutamate receptor subtypes evidenced by differences in desensitization and dependence on the GLR3.3 and GLR3.4 genes. Plant Physiol 146: 529–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Miller G, Morales J, Shulaev V, Torres MA, Mittler R. (2011) Respiratory burst oxidases: the engines of ROS signaling. Curr Opin Plant Biol 14: 691–699 [DOI] [PubMed] [Google Scholar]
- Takeda S, Gapper C, Kaya H, Bell E, Kuchitsu K, Dolan L. (2008) Local positive feedback regulation determines cell shape in root hair cells. Science 319: 1241–1244 [DOI] [PubMed] [Google Scholar]
- Talke IN, Blaudez D, Maathuis FJ, Sanders D. (2003) CNGCs: prime targets of plant cyclic nucleotide signalling? Trends Plant Sci 8: 286–293 [DOI] [PubMed] [Google Scholar]
- Tang RJ, Liu H, Yang Y, Yang L, Gao XS, Garcia VJ, Luan S, Zhang HX. (2012) Tonoplast calcium sensors CBL2 and CBL3 control plant growth and ion homeostasis through regulating V-ATPase activity in Arabidopsis. Cell Res 22: 1650–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapken D, Hollmann M. (2008) Arabidopsis thaliana glutamate receptor ion channel function demonstrated by ion pore transplantation. J Mol Biol 383: 36–48 [DOI] [PubMed] [Google Scholar]
- Torres MA, Jones JD, Dangl JL. (2005) Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat Genet 37: 1130–1134 [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. (2010) Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev 62: 405–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas A. (1999) How plants learn. Proc Natl Acad Sci USA 96: 4216–4218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas A, Read N, Campbell AK, Knight M. (1996) Transduction of Ca2+ signals in plant cells and compartmentalization of the Ca2+ signal. Biochem Soc Trans 24: 971–974 [DOI] [PubMed] [Google Scholar]
- Tripathi V, Parasuraman B, Laxmi A, Chattopadhyay D. (2009) CIPK6, a CBL-interacting protein kinase is required for development and salt tolerance in plants. Plant J 58: 778–790 [DOI] [PubMed] [Google Scholar]
- Tunc-Ozdemir M, Rato C, Brown E, Rogers S, Mooneyham A, Frietsch S, Myers CT, Poulsen LR, Malho R, Harper JF. (2013a) Cyclic nucleotide gated channels 7 and 8 are essential for male reproductive fertility. PLoS ONE 8: e55277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunc-Ozdemir M, Tang C, Ishka MR, Brown E, Groves NR, Myers CT, Rato C, Poulsen LR, McDowell S, Miller G, et al. (2013b) A cyclic nucleotide-gated channel (CNGC16) in pollen is critical for stress tolerance in pollen reproductive development. Plant Physiol 161: 1010–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuteja N, Mahajan S. (2007) Further characterization of calcineurin B-like protein and its interacting partner CBL-interacting protein kinase from Pisum sativum. Plant Signal Behav 2: 358–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincill ED, Bieck AM, Spalding EP. (2012) Ca2+ conduction by an amino acid-gated ion channel related to glutamate receptors. Plant Physiol 159: 40–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincill ED, Clarin AE, Molenda JN, Spalding EP. (2013) Interacting glutamate receptor-like proteins in phloem regulate lateral root initiation in Arabidopsis. Plant Cell 25: 1304–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waadt R, Kudla J. (2008) In planta visualization of protein interactions using bimolecular fluorescence complementation (BiFC). CSH Protoc 2008: pdb.prot4995 [DOI] [PubMed] [Google Scholar]
- Waadt R, Schmidt LK, Lohse M, Hashimoto K, Bock R, Kudla J. (2008) Multicolor bimolecular fluorescence complementation reveals simultaneous formation of alternative CBL/CIPK complexes in planta. Plant J 56: 505–516 [DOI] [PubMed] [Google Scholar]
- Wang RK, Li LL, Cao ZH, Zhao Q, Li M, Zhang LY, Hao YJ. (2012) Molecular cloning and functional characterization of a novel apple MdCIPK6L gene reveals its involvement in multiple abiotic stress tolerance in transgenic plants. Plant Mol Biol 79: 123–135 [DOI] [PubMed] [Google Scholar]
- Ward JM, Mäser P, Schroeder JI. (2009) Plant ion channels: gene families, physiology, and functional genomics analyses. Annu Rev Physiol 71: 59–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AA, McAinsh MR, Taylor JE, Hetherington AM. (1996) Calcium ions as intracellular second messengers in higher plants. Adv Bot Res 22: 45–96 [Google Scholar]
- Weinl S, Kudla J. (2009) The CBL-CIPK Ca2+-decoding signaling network: function and perspectives. New Phytol 184: 517–528 [DOI] [PubMed] [Google Scholar]
- Wernimont AK, Amani M, Qiu W, Pizarro JC, Artz JD, Lin YH, Lew J, Hutchinson A, Hui R. (2011) Structures of parasitic CDPK domains point to a common mechanism of activation. Proteins 79: 803–820 [DOI] [PubMed] [Google Scholar]
- Wernimont AK, Artz JD, Finerty P, Jr, Lin YH, Amani M, Allali-Hassani A, Senisterra G, Vedadi M, Tempel W, Mackenzie F, et al. (2010) Structures of apicomplexan calcium-dependent protein kinases reveal mechanism of activation by calcium. Nat Struct Mol Biol 17: 596–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Li HD, Chen LQ, Wang Y, Liu LL, He L, Wu WH. (2006) A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 125: 1347–1360 [DOI] [PubMed] [Google Scholar]
- Yang W, Kong Z, Omo-Ikerodah E, Xu W, Li Q, Xue Y. (2008) Calcineurin B-like interacting protein kinase OsCIPK23 functions in pollination and drought stress responses in rice (Oryza sativa L.). J Genet Genomics 35: 531–543 [DOI] [PubMed] [Google Scholar]
- Zhang C, Bian M, Yu H, Liu Q, Yang Z. (2011) Identification of alkaline stress-responsive genes of CBL family in sweet sorghum (Sorghum bicolor L.). Plant Physiol Biochem 49: 1306–1312 [DOI] [PubMed] [Google Scholar]
- Zhang H, Lv F, Han X, Xia X, Yin W. (2013) The calcium sensor PeCBL1, interacting with PeCIPK24/25 and PeCIPK26, regulates Na+/K+ homeostasis in Populus euphratica. Plant Cell Rep 32: 611–621 [DOI] [PubMed] [Google Scholar]
- Zhao LN, Shen LK, Zhang WZ, Zhang W, Wang Y, Wu WH. (2013) Ca2+-dependent protein kinase11 and 24 modulate the activity of the inward rectifying K+ channels in Arabidopsis pollen tubes. Plant Cell 25: 649–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou LZ, Li S, Feng QN, Zhang YL, Zhao X, Zeng YL, Wang H, Jiang L, Zhang Y. (2013) Protein S-acyl transferase10 is critical for development and salt tolerance in Arabidopsis. Plant Cell 25: 1093–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]