Abstract
The case is made for the potential importance of compartmentalization in redox signaling with new data on the transporters that may be involved.
The nucleophilic properties of reduced glutathione (GSH) can be harnessed to produce glutathione S- (GS) conjugates or to reduce oxidants such as peroxides or dehydroascorbate (Dixon et al., 2009). When GSH is used as a reductant, glutathione disulfide (GSSG) is produced as a stable product from which the reduced form can be regenerated by NADPH-dependent glutathione reductase. In plants and some fungi, GS conjugates are imported into vacuoles, where they are degraded (Rea, 2007). Structurally, GSSG can be considered to be a glutathione S-autoconjugate, and this compound could also be transported into vacuoles. Indeed, in vitro studies show that isolated barley (Hordeum vulgare) vacuoles can take up GSSG and that certain Arabidopsis (Arabidopsis thaliana) tonoplast-localized proteins are competent in both GS conjugate and GSSG transport when expressed in yeast (Martinoia et al., 1993; Tommasini et al., 1993; Lu et al., 1997, 1998).
Despite these observations, it remained unclear whether GSSG accumulation in the vacuole is ever a significant phenomenon in vivo. Evidence that this is the case comes from a study of a catalase-deficient Arabidopsis mutant (cat2; Queval et al., 2011). In this system, the decreased capacity for catalase-dependent hydrogen peroxide (H2O2) metabolism increases the oxidative burden on the cellular reducing system, triggering well-defined changes in tissue glutathione status that are qualitatively similar to those that can be driven by certain external stresses. In cat2 leaf extracts, the GSH-GSSG ratio is close to 1 (compared with over 20 in wild-type leaves) and glutathione is typically increased about 3-fold, so that leaf GSSG contents are much higher than in the wild type (Mhamdi et al., 2010). To date, in plants, as in other organisms, the cytosolic redox potential of glutathione, estimated using thiol-dependent redox-sensitive green fluorescent protein (roGFP), implies a very low concentration of GSSG in optimal conditions. Here, we propose that compartmentalization of GSSG can explain some of these apparently conflicting observations. Although the cytosolic glutathione pool is significantly increased in cat2, much more marked changes are observed in the chloroplast and, especially, the vacuole, where concentrations are increased at least 10 times compared with ecotype Columbia (Col-0; Queval et al., 2011).
CONSEQUENCES OF GSSG SEQUESTRATION FOR GLUTATHIONE REDOX POTENTIAL
Reliable measurements of glutathione in leaf extracts from unstressed plants generally indicate that the extractable pool is represented by 90% to 95% GSH. Although 90% to 95% GSH may seem highly reduced, even these values are likely to underestimate the reduction state in metabolically active compartments, where the oxidation of protein thiols must be regulated. Even if great care is taken in measuring glutathione, some artifactual oxidation during extraction and sample preparation may be inevitable. In vitro measurements of glutathione in whole tissue extracts may also include GSSG that is sequestered in more metabolically inert compartments.
Quantification of the glutathione redox potential using roGFP techniques has consistently yielded values of lower than −300 mV (Meyer et al., 2007; Jubany-Mari et al., 2010; Maughan et al., 2010). By contrast, deriving this factor from glutathione measured in whole leaf extracts (95% glutathione tripeptides present as GSH and 5% as GSSG) gives much more oxidizing values (Table I, row A). This calculation assumes a homogenous distribution of GSH and GSSG pools in different (sub)cellular compartments. To approach the lower redox potentials reported in compartments such as the cytosol using roGFP, it has to be assumed that the GSSG found in extracts is generated artifactually or that an overwhelming proportion is sequestered in other compartments (Table I, row B). Using the GSSG-accumulating cat2 mutant as a model system for oxidative stress, we have attempted to assess the potential impact of compartmentalization in determining the redox potential of the metabolically important glutathione pools that may be crucial in relaying oxidative stress signals. Assuming no sequestration, and so a homogenous distribution of GSH and GSSG throughout the cell, the GSSG accumulated in cat2 would cause the glutathione redox potential to collapse to values well above −200 mV, representing a dramatic increase (about 130 mV) from measured values determined in wild-type Arabidopsis in the absence of stress (Table I, compare rows B and C). Such large changes contrast with the more modest adjustments that have been reported during stress using roGFP (Meyer et al., 2007; Jubany-Mari et al., 2010). One way to account for this discrepancy is that most of the accumulated GSSG is not found in compartments such as the cytosol but is sequestered in locations like the vacuole (Queval et al., 2011). This sequestration can significantly modulate the effective GSSG concentration and, therefore, the glutathione redox potential, allowing it to remain more reduced and so compatible with continued cell function.
Table I. Potential importance of GSSG sequestration in oxidative stress-triggered changes in glutathione redox potential.
In cat2, as little as 33% of the glutathione is present as GSH. In these conditions, the total pool is typically 3-fold higher than in Col-0, in which glutathione is typically 95% GSH (Mhamdi et al., 2010). Because each GSSG is formed from two GSH, these values correspond to whole leaf GSH-GSSG ratios of 38 and 1 in Col-0 and cat2, respectively. Based on this, GSH and GSSG concentrations were calculated assuming a total cytosolic glutathione concentration of 5 mm in Col-0 (Queval et al., 2011). Glutathione redox potential is calculated assuming a midpoint potential of −240 mV and calculated according to −240 – (29.6 log ([GSH]2/[GSSG]). Effective GSSG refers to the fraction that is not sequestered in compartments such as the vacuole. The very low value shown for Col-0 in row B is required to achieve cytosolic redox potentials that approximate those measured in vivo by roGFP (Meyer et al., 2007; Maughan et al., 2010).
| Condition | GSH | GSSG | Effective GSSG | Redox Potential |
|---|---|---|---|---|
| mm | mV | |||
| Col-0 (no oxidative stress) | ||||
| A. No sequestration of GSSG | 4.75 | 125 µm | 125 µm | −218 |
| B. With 99.9% sequestration of GSSG | 4.75 | 125 µm | 0.125 µm | −307 |
| cat2 (oxidative stress) | ||||
| C. No sequestration of GSSG | 5.0 | 5.0 mm | 5.0 mm | −172 |
| D. With 99% sequestration of GSSG | 5.0 | 5.0 mm | 50 µm | −231 |
| E. With 99.9% sequestration of GSSG | 5.0 | 5.0 mm | 5.0 µm | −261 |
One implication of the above discussion is that sequestration-dependent maintenance of very low basal levels of GSSG in the vicinity of sensitive components will confer the possibility of oxidative signaling through susceptible proteins. If the GSSG that can be detected in extracts from leaves or cells in unstressed conditions is largely excluded from compartments that are susceptible to signaling, the sensitivity that can be achieved by a relatively modest increase in GSSG in such compartments during oxidative stress is greater. Chief candidates to mediate glutathione-related redox signaling are glutaredoxins (GRX) such as those on which the roGFP signal is dependent (Meyer et al., 2007). While there are several types of GRX, and their redox characteristics and dependence on glutathione remain to be fully characterized, some may have midpoint potentials as low as −230 mV (Rouhier et al., 2008). At a redox potential of −307 mV, glutathione-dependent GRX should be very highly reduced, whereas at −218 mV, they could already be significantly oxidized (Table I, compare rows A and B).
In systems such as cat2, the in vivo accumulation of GSSG clearly occurs. Even if we assume that only 0.1% to 1% of the accumulated GSSG is found in compartments containing glutathione-sensitive components, this increase would still drive less reducing redox potentials (Table I, compare row B with rows D and E) and so possibly drive H2O2-triggered thiol-mediated signaling pathways. If the basal level of GSSG is maintained very low, then even efficient sequestration of 99% of the oxidative stress-induced GSSG would still cause the redox potential to climb by about 70 mV, enough to promote significant GRX oxidation and, therefore, changes in the structure or activity of sensitive target proteins.
TRANSPORTERS RESPONSIBLE FOR VACUOLAR ACCUMULATION
Vacuolar concentrations of glutathione are low in unstressed conditions (Rennenberg, 1982; Zechmann et al., 2008; Queval et al., 2011), and this organelle is considered to have lower levels of redox metabolism than many other cell compartments. Therefore, the most likely explanation of oxidative stress-triggered vacuolar GSSG accumulation is direct transfer from the cytosol. Based on current concepts, the best candidates to perform this transfer are members of the multidrug resistance-associated protein (MRP) family, now denoted subclass C of the ATP-binding cassette (ABC) translocators (Klein et al., 2006; Rea, 2007). In Arabidopsis, 15 ABCC genes (for subclass C of the ABC transporters) have been described (ABCC1–ABCC15; Wanke and Kolukisaoglu, 2010). The corresponding proteins are probably localized at the tonoplast, although some may also be found at the plasmalemma (Wanke and Kolukisaoglu, 2010). Of them, ABCC1 and ABCC2 are the best studied. Genetic evidence has been presented that these two proteins act together in phytochelatin transport in the response to heavy metals (Song et al., 2010). Heterologous expression in yeast has shown that both are also competent in GSSG transport, although ABCC2 has a higher affinity (Lu et al., 1998).
An early analysis of three ABCC (MRP) transcripts pointed to specificity in the response of their expression to pharmacologically induced oxidative stress (Sánchez-Fernández et al., 1998). In our hands, quantification of transcripts for ABCC genes in the GSSG-accumulating cat2 mutant revealed that nine out of 15 were significantly induced above wild-type values (Table II). There is evidence that the expression of paralogous ABCC genes is coregulated to some extent (Wanke and Kolukisaoglu, 2010), and this may contribute to the coordinated up-regulation of multiple genes by oxidative stress.
Table II. Induction of multiple ABCC genes by oxidative stress in cat2.
Transcripts were measured using gene-specific primers (Supplemental Table S1) for quantitative reverse transcription-PCR analysis of three biological repeats of leaf extracts from Col-0 and cat2 grown for 3 weeks in air in a 16-h/8-h light/dark regime at an irradiance of 200 µmol m−2 s−1. Shown in boldface are genes whose mean expression values were significantly different between cat2 and Col-0 at P < 0.05 (values indicated by asterisks).
| Gene | Fold Change, cat2/Col-0 |
|---|---|
|
ABCC1 |
1.7* |
|
ABCC2 |
2.3* |
|
ABCC3 |
1.1 |
|
ABCC4 |
0.8 |
|
ABCC5 |
1.5* |
|
ABCC6 |
1.9* |
|
ABCC7 |
1.0 |
|
ABCC8 |
1.6* |
|
ABCC9 |
0.9 |
|
ABCC10 |
1.7* |
|
ABCC11 |
1.0 |
|
ABCC12 |
2.1* |
|
ABCC13 |
2.3* |
|
ABCC14 |
1.5* |
| ABCC15 | 0.9 |
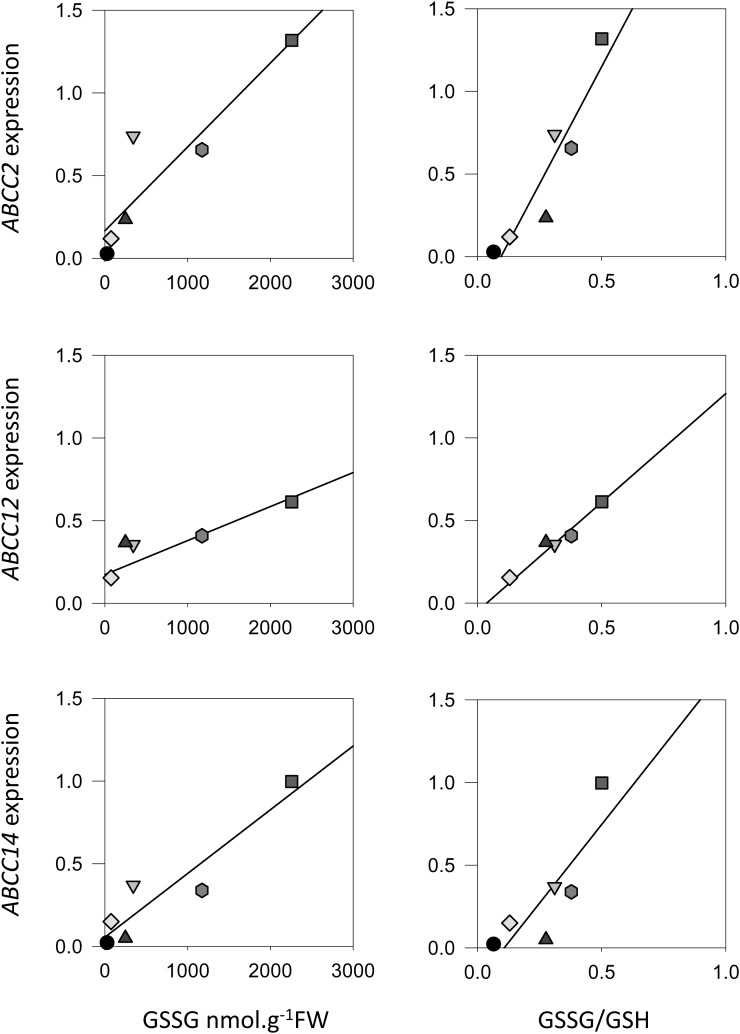
From the physiological point of view, it is unclear whether the induction of ABCC genes is linked to GSSG accumulation, to the formation of GS conjugates, or to other metabolic functions triggered by oxidative stress. In a first step to analyze the relationship between GSSG accumulation and ABCC expression, we mined an available microarray data set for mutants that accumulate GSSG to differing extents. Loss of GLUTATHIONE REDUCTASE1 (GR1) function causes a smaller increase in leaf GSSG than in cat2, while double cat2 gr1 mutants show about three times higher accumulation of GSSG than cat2 (Mhamdi et al., 2010). The accumulation of GSSG in these lines is further modulated by the duration of the photoperiod, since it is generally stronger in short days than in long days. The analyzed data set includes probe sets for 12 of the 15 AtABCC transcripts: probes for ABCC9, ABCC13, and ABCC15 were not present on the chip. When fold induction of transcript for the 12 ABCCs relative to the wild type were plotted against GSSG and, particularly, GSSG-GSH ratios in the three mutants growing in these two conditions, positive correlations were observed for several genes. Correlations were particularly evident for ABCC2, ABCC12, and ABCC14 (Fig. 1).
Figure 1.
Correlation between the expression of specific ABCCs and glutathione status in Arabidopsis lines. ABCC2, ABCC12, and ABCC14 transcript fold change (relative to Col-0) is plotted against GSSG (left) and GSSG-GSH ratio (right) in gr1, cat2, and cat2 gr1 lines. Glutathione was measured and microarray analysis was conducted as described by Mhamdi et al. (2010). FW, Fresh weight.
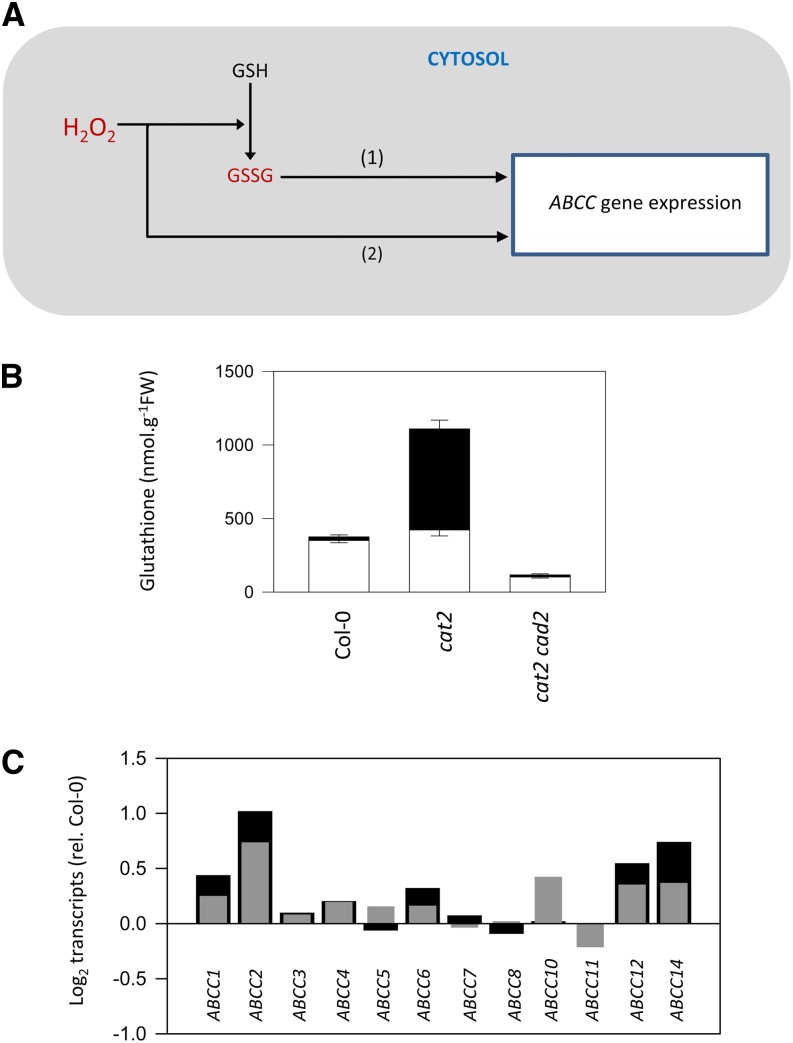
These correlations raise the possibility that the expression of certain ABCCs is under the control of GSSG accumulation and the resulting change in glutathione redox status. Alternatively, they could be induced by oxidative stress through a glutathione-independent pathway that operates in parallel with and/or to anticipate GSSG accumulation (Fig. 2A). A useful tool to analyze such questions has recently been reported. Introduction of the cad2 mutation in glutathione synthesis into the cat2 background produces an effective block over cat2-triggered GSSG accumulation (Han et al., 2013a). Whereas cat2 accumulates GSSG to high levels, in cat2 cad2, the total pool and GSSG remains at below Col-0 values (Fig. 2B). Despite this, several ABCCs that are up-regulated in cat2 (ABCC1, ABCC2, ABCC12, and ABCC14) are even more strongly induced in cat2 cad2 (Fig. 2C). This observation argues against regulation of the expression of these ABCCs by GSSG concentration. It should be noted, however, that glutathione redox potential values in cat2 cad2 relative to cat2 are not known and are difficult to predict, despite the dramatic difference in the status of extractable glutathione shown in Figure 2B (for further discussion of this point, see Han et al., 2013b). Consequently, the possibility that the expression of oxidative stress-inducible ABCCs is regulated by the glutathione redox potential cannot be excluded by the data shown in Figure 2C.
Figure 2.
Control of oxidative stress-triggered ABCC expression by GSSG levels? A, Scheme showing oxidative stress induction of ABCCs by possible GSSG-dependent (1) and GSSG-independent (2) pathways. B, Glutathione status in Col-0, cat2, and cat2 cad2, in which H2O2-triggered glutathione synthesis is blocked (Han et al., 2013a). White bars, GSH; black bars, GSSG. Data are means ± se of three biological replicates. FW, Fresh weight. C, Comparison of ABCC induction in cat2 (gray bars) and cat2 cad2 (black bars). Data show fold change relative to Col-0.
ACCUMULATION OF GSSG IN THE CHLOROPLAST
Based on immunolocalization studies, there is a marked specificity in the compartmentalization of oxidative stress-triggered glutathione accumulation. Little or no accumulation is observed in the mitochondria (Queval et al., 2011). This is in agreement with the stability of the mitochondrial pool during stress that has been reported in other studies (Zechmann et al., 2008). Slight but significant accumulation is observed in the cytosol, but after the vacuole, the chloroplast is the compartment in which glutathione accumulates most strongly in cat2 (Queval et al., 2011). Marked H2O2-triggered accumulation of GSSG in the Arabidopsis chloroplast is in agreement with an earlier study of barley catalase mutants using nonaqueous fractionation of leaf extracts (Smith et al., 1985). This phenomenon could have consequences for photosynthetic and respiratory functions, which are known to be regulated by thiol-disulfide status. For example, an enhanced GSSG concentration could interfere with enzyme activation by the chloroplast thioredoxin system (Michelet et al., 2005). Moreover, a large number of proteins that have so far been identified as potential targets of Cys S-glutathionylation reside in the chloroplast (Zaffagnini et al., 2012).
Whereas there are good reasons to think that vacuolar accumulation of glutathione is largely the result of GSSG import, this is less clear for the chloroplast, where the accumulation of GSSG may be driven by in situ oxidation of GSH. Nevertheless, cytosol-to-chloroplast transport of glutathione during oxidative stress cannot be completely discounted. Isolated wheat (Triticum aestivum) chloroplasts can take up glutathione from the surrounding medium (Noctor et al., 2002). Based on the results of a compartment-specific manipulation of glutathione synthesis, this phenomenon is able to occur in vivo (Pasternak et al., 2008). Chloroplast envelope components able to transport glutathione include the recently identified CLT family, composed of three genes (CLT1, CLT2, and CLT3; Maughan et al., 2010), although we are not aware of any data that show that they transport GSSG at high rates. Unlike the ABCC genes described above, none of the CLT-encoding genes were found to show significant differences in expression between Col-0 and cat2 (data not shown). Based on current knowledge, neither the chloroplast internal envelope membrane nor the inner mitochondrial membrane is able to transport GSSG at high rates. We consider that the most significant factor explaining GSSG accumulation in the chloroplast is probably in situ oxidation of newly synthesized GSH.
GLUTATHIONE COMPARTMENTATION: CONSEQUENCES FOR THE CYTOSOL AND NUCLEUS
Despite the induction of some ABCCs in cat2 cad2, GSSG accumulation triggered by oxidative stress is no longer observed in this line (Fig. 2B), consistent with its low, highly reduced glutathione status. Together, these observations suggest that during oxidative stress, wild-type plants activate vacuolar GSSG sequestration alongside glutathione synthesis to avoid an excessively oxidizing cytosolic redox potential (Fig. 3A). Based on the above data, these processes could be part of several coordinated responses that are induced, in parallel, in response to increased H2O2 and other oxidants.
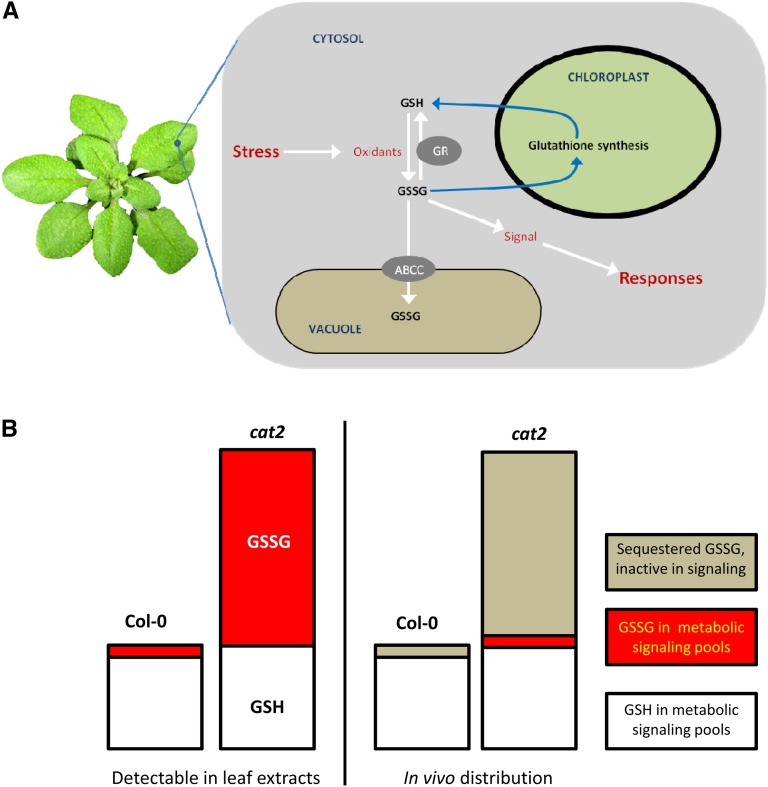
Figure 3.
The role of vacuolar sequestration in regulating the redox gatekeeper (glutathione redox potential). A, Increased availability of reactive oxygen species is a general feature of plants exposed to stress. Reactive oxygen species promote the oxidation of glutathione (GSH) to glutathione disulfide (GSSG), and this is followed by the activation of GSH synthesis, possibly driven by a change in the GSH-GSSG ratio. At least part of the GSSG that accumulates in oxidatively stressed plants is shipped to the vacuole, a response that could be important in regulating appropriate signaling to stress. For simplicity, GSSG is shown as a signal, but the sensed factor could be the redox potential or other related factor. B, How glutathione compartmentalization could determine the concentration of GSSG active in signaling in optimal and oxidative stress conditions. Left bars, cartoon of typical GSH and GSSG contents in Col-0 and cat2; right bars, hypothetical restriction of GSSG available for signaling by sequestration in the two conditions.
For the following reasons, we consider that the redox potential of glutathione might be a key redox gatekeeper and sensor for signaling during stress: (1) thiol-disulfide regulation is known to be an important factor in determining protein structure and/or function; (2) oxidative stress-induced changes in glutathione status are well documented; and (3) catalysts exist that could relay changes in glutathione status to sensitive proteins. We suggest that the sequestration of GSSG in vacuoles and other compartments is a key feature of any such gatekeeping and sensing role. This concept envisages that even under optimal conditions, sequestration keeps GSSG very low in sensitive compartments while ensuring appropriate but not excessive accumulation during oxidative stress (Fig. 3B).
Vacuolar accumulation of glutathione is not limited to catalase-deficient plants: it has also recently been described in some types of yeast subjected to oxidative stress (Zechmann et al., 2011; Morgan et al., 2013), suggesting that the phenomenon may be widespread among eukaryotes. The Km GSSG values obtained for heterologously expressed ABCC1 and ABCC2 were 219 and 73 µm, respectively (Lu et al., 1998). We note that the very low GSSG concentrations implied by the available redox potential data (Table I, row B) mean that, in addition to induction at the transcript level, the activities of the encoded proteins should be greatly stimulated in oxidative stress conditions. This could be important to limit the accumulation of GSSG in the cytosol and, perhaps, the nucleus. It is also important to note that GSH may be differentially compartmentalized under conditions such as those that initiate cell proliferation (García-Giménez et al., 2013). In this case, cytosolic oxidation and hence an increase in the redox potential of the cytosol could be achieved in the absence of GSSG formation by the movement of GSH into the nucleus. Although this has not yet been demonstrated in plants, oxidation of the cytosol in mammalian cells is required to activate the transcription of cyclin A that initiates the cell cycle (García-Giménez et al., 2013).
CONCLUSION
The above discussion highlights the key concepts that oxidative stress both drives the oxidation of glutathione and reconfigures its subcellular distribution. We consider that this process is an important facet of redox homeostasis and signaling, key factors in determining the outcome of plant responses to stress (Foyer and Noctor, 2009). Given the emerging evidence that oxidative signals could be partly transmitted by the modulation of glutathione status (Han et al., 2013a, 2013b), our hypothesis is that GSSG transport to vacuoles is important both for sensitization and for regulation of signaling through glutathione-dependent systems. While sequestration in other compartments could also contribute, we consider the vacuole to be a key player based on both the relative and absolute increases in glutathione in this compartment during oxidative stress (from 5% to at least 25% of cell glutathione in the case of cat2 growing at moderate irradiance; Queval et al., 2011).
Despite the operation of sequestration processes, GSSG accumulation caused by glutathione reductase deficiency in Arabidopsis is sufficient to impact signaling through phytohormone pathways (Mhamdi et al., 2010, 2013). Loss of GR1 function also affects phytohormone signaling induced by ozone (Dghim et al., 2013). Sequestration of GSSG may be required to clear the cytosol of active signal under basal conditions, to avoid excessive oxidative stimulation under stress conditions, and to allow the termination of signaling. Experiments are currently under way to explore these questions and to establish which transporters may be physiologically most important in these functions.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. Olignonucleotide sequences used for quantitative reverse transcription-PCR analysis.
Glossary
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- GS
glutamine synthetase
- H2O2
hydrogen peroxide
- roGFP
redox-sensitive green fluorescent protein
- Col-0
ecotype Columbia
- GRX
glutaredoxins
- GS
glutathione S-
- GSSG
glutathione disulfide
References
- Dghim AA, Mhamdi A, Vaultier MN, Hasenfratz-Sauder MP, LE Thiec D, Dizengremel P, Noctor G, Jolivet Y. (2013) Analysis of cytosolic isocitrate dehydrogenase and glutathione reductase 1 in photoperiod-influenced responses to ozone using Arabidopsis knockout mutants. Plant Cell Environ (in press) [DOI] [PubMed] [Google Scholar]
- Dixon DP, Hawkins T, Hussey PJ, Edwards R. (2009) Enzyme activities and subcellular localization of members of the Arabidopsis glutathione transferase superfamily. J Exp Bot 60: 1207–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. (2009) Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxid Redox Signal 11: 861–905 [DOI] [PubMed] [Google Scholar]
- García-Giménez JL, Markovic J, Dasí F, Queval G, Schnaubelt D, Foyer CH, Pallardó FV. (2013) Nuclear glutathione. Biochim Biophys Acta 1830: 3304–3316 [DOI] [PubMed] [Google Scholar]
- Han Y, Chaouch S, Mhamdi A, Queval G, Zechmann B, Noctor G. (2013a) Functional analysis of Arabidopsis mutants points to novel roles for glutathione in coupling H2O2 to activation of salicylic acid accumulation and signaling. Antioxid Redox Signal 18: 2106–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Mhamdi A, Chaouch S, Noctor G. (2013b) Regulation of basal and oxidative stress-triggered jasmonic acid-related gene expression by glutathione. Plant Cell Environ 36: 1135–1146 [DOI] [PubMed] [Google Scholar]
- Jubany-Mari T, Alegre-Batlle L, Jiang K, Feldman LJ. (2010) Use of a redox-sensing GFP (c-roGFP1) for real-time monitoring of cytosol redox status in Arabidopsis thaliana water-stressed plants. FEBS Lett 584: 889–897 [DOI] [PubMed] [Google Scholar]
- Klein M, Burla B, Martinoia E. (2006) The multidrug resistance-associated protein (MRP/ABCC) subfamily of ATP-binding cassette transporters in plants. FEBS Lett 580: 1112–1122 [DOI] [PubMed] [Google Scholar]
- Lu YP, Li ZS, Drozdowicz YM, Hörtensteiner S, Martinoia E, Rea PA. (1998) AtMRP2, an Arabidopsis ATP binding cassette transporter able to transport glutathione S-conjugates and chlorophyll catabolites: functional comparisons with AtMRP1. Plant Cell 10: 267–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YP, Li ZS, Rea PA. (1997) AtMRP1 gene of Arabidopsis encodes a glutathione S-conjugate pump: isolation and functional definition of a plant ATP-binding cassette transporter gene. Proc Natl Acad Sci USA 94: 8243–8248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinoia E, Grill E, Tommasini R, Kreuz K, Amrhein N. (1993) An ATP-dependent glutathione S-conjugate “export” pump in the vacuolar membrane of plants. Nature 364: 247–249 [Google Scholar]
- Maughan SC, Pasternak M, Cairns N, Kiddle G, Brach T, Jarvis R, Haas F, Nieuwland J, Lim B, Müller C, et al. (2010) Plant homologs of the Plasmodium falciparum chloroquine-resistance transporter, PfCRT, are required for glutathione homeostasis and stress responses. Proc Natl Acad Sci USA 107: 2331–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AJ, Brach T, Marty L, Kreye S, Rouhier N, Jacquot JP, Hell R. (2007) Redox-sensitive GFP in Arabidopsis thaliana is a quantitative biosensor for the redox potential of the cellular glutathione redox buffer. Plant J 52: 973–986 [DOI] [PubMed] [Google Scholar]
- Mhamdi A, Hager J, Chaouch S, Queval G, Han Y, Taconnat Y, Saindrenan P, Issakidis-Bourguet E, Gouia H, Renou JP, et al (2010) Arabidopsis GLUTATHIONE REDUCTASE 1 plays a crucial role in leaf responses to intracellular hydrogen peroxide and in ensuring appropriate gene expression through both salicylic acid and jasmonic acid signaling pathways. Plant Physiol 153: 1144–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhamdi A, Han Y, Noctor G. (2013) Glutathione-dependent phytohormone responses: teasing apart signaling and antioxidant functions. Plant Signal Behav 8: (in press) http://www.ncbi.nlm.nih.gov/pubmed/23470721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelet L, Zaffagnini M, Marchand C, Collin V, Decottignies P, Tsan P, Lancelin JM, Trost P, Miginiac-Maslow M, Noctor G, et al. (2005) Glutathionylation of chloroplast thioredoxin f is a redox signaling mechanism in plants. Proc Natl Acad Sci USA 102: 16478–16483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan B, Ezeriņa D, Amoako TN, Riemer J, Seedorf M, Dick TP. (2013) Multiple glutathione disulfide removal pathways mediate cytosolic redox homeostasis. Nat Chem Biol 9: 119–125 [DOI] [PubMed] [Google Scholar]
- Noctor G, Gomez L, Vanacker H, Foyer CH. (2002) Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signalling. J Exp Bot 53: 1283–1304 [DOI] [PubMed] [Google Scholar]
- Pasternak M, Lim B, Wirtz M, Hell R, Cobbett CS, Meyer AJ. (2008) Restricting glutathione biosynthesis to the cytosol is sufficient for normal plant development. Plant J 53: 999–1012 [DOI] [PubMed] [Google Scholar]
- Queval G, Jaillard D, Zechmann B, Noctor G. (2011) Increased intracellular H2O2 availability preferentially drives glutathione accumulation in vacuoles and chloroplasts. Plant Cell Environ 34: 21–32 [DOI] [PubMed] [Google Scholar]
- Rea PA. (2007) Plant ATP-binding cassette transporters. Annu Rev Plant Biol 58: 347–375 [DOI] [PubMed] [Google Scholar]
- Rennenberg H. (1982) Glutathione metabolism and possible biological roles in higher plants. Phytochemistry 21: 2771–2781 [Google Scholar]
- Rouhier N, Lemaire SD, Jacquot JP. (2008) The role of glutathione in photosynthetic organisms: emerging functions for glutaredoxins and glutathionylation. Annu Rev Plant Biol 59: 143–166 [DOI] [PubMed] [Google Scholar]
- Sánchez-Fernández R, Ardiles-Díaz W, Van Montagu M, Inzé D, May MJ. (1998) Cloning and expression analyses of AtMRP4, a novel MRP-like gene from Arabidopsis thaliana. Mol Gen Genet 258: 655–662 [DOI] [PubMed] [Google Scholar]
- Smith IK, Kendall AC, Keys AJ, Turner JC, Lea PJ. (1985) The regulation of the biosynthesis of glutathione in leaves of barley (Hordeum vulgare L.). Plant Sci 41: 11–17 [Google Scholar]
- Song WY, Park J, Mendoza-Cózatl DG, Suter-Grotemeyer M, Shim D, Hörtensteiner S, Geisler M, Weder B, Rea PA, Rentsch D, et al. (2010) Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proc Natl Acad Sci USA 107: 21187–21192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommasini R, Martinoia E, Grill E, Dietz K-J, Amrhein N. (1993) Transport of oxidized glutathione into barley vacuoles: evidence for the involvement of the glutathione-S-conjugate ATPase. Z Naturforsch 48c: 867–871 [Google Scholar]
- Wanke D, Kolukisaoglu HU. (2010) An update on the ABCC transporter family in plants: many genes, many proteins, but how many functions? Plant Biol (Stuttg) (Suppl 1) 12: 15–25 [DOI] [PubMed] [Google Scholar]
- Zaffagnini M, Bedhomme M, Marchand CH, Morisse S, Trost P, Lemaire SD. (2012) Redox regulation in photosynthetic organisms: focus on glutathionylation. Antioxid Redox Signal 16: 567–586 [DOI] [PubMed] [Google Scholar]
- Zechmann B, Liou LC, Koffler BE, Horvat L, Tomašić A, Fulgosi H, Zhang Z. (2011) Subcellular distribution of glutathione and its dynamic changes under oxidative stress in the yeast Saccharomyces cerevisiae. FEMS Yeast Res 11: 631–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechmann B, Mauch F, Sticher L, Müller M. (2008) Subcellular immunocytochemical analysis detects the highest concentrations of glutathione in mitochondria and not in plastids. J Exp Bot 59: 4017–4027 [DOI] [PMC free article] [PubMed] [Google Scholar]