Phosphatidylethanolamine-binding proteins have a role in the control of annual growth rhythm in the conifer Norway spruce.
Abstract
The timing of bud set, as one determinant of the annual growth rhythm, is critical for local adaptation of the conifer Norway spruce (Picea abies). Previous gene expression and population genetic studies have suggested a role for P. abies FLOWERING LOCUS T/TERMINAL FLOWER1-Like2 (PaFTL2) in the control of growth cessation and bud set in Norway spruce as well as in local adaptation resulting in clinal variation for timing of bud set. Using transgenic plants with PaFTL2 driven by an inducible promoter, we found that PaFTL2 indeed induces bud set and most probably also growth cessation. PaFTL2 shows high expression around the procambium and vascular tissue and in the crown region in buds of both seedlings and older trees. Furthermore, PaFTL2 expression is induced in vegetative shoots and all bud types in late summer, when growth cessation occurs. This supports the notion that PaFTL2 is involved in growth cessation. A close paralog to PaFTL2, PaFTL1, is strongly expressed in meristems during the summer, possibly to repress meristem activity and the formation of needle primordia during this period. The temporal and spatial expression of PaFTL1 and PaFTL2 largely complement each other, which suggests that they act in concert to control perennial growth in Norway spruce.
Since plants are sessile, there is strong pressure to adapt to local abiotic and biotic environments. For perennials, an important aspect is the matching of the growing period to the seasonal changes in the environment, which often vary locally. In the gymnosperm Norway spruce (Picea abies) and other temperate trees, photoperiod is an important regulator of the annual growth cycle (Ekberg et al., 1979). Each year, when the days are shortened in the autumn, trees initiate growth cessation, bud set, and dormancy in order to achieve full cold hardiness before the winter (Rohde and Bhalerao, 2007). As early bud set reduces growth and late bud set increases the risk for frost damage, there is a tradeoff between the risk of frost damage and increased growth. Consequently, the timing of growth cessation and bud set is often strongly adapted to the local environment, despite high levels of gene flow. As a result, strong clinal variation is often observed for the timing of bud set in trees. Such clinal variation of a heritable trait is considered adaptive as a result of the action of natural selection. In Norway spruce, the clinal variation in growth cessation and bud set is coupled to a variation in critical night length, which steadily increases from about 2 h in the north to 6 to 7 h in the more southern populations (Ekberg et al., 1979).
In the angiosperm plant model species Arabidopsis (Arabidopsis thaliana), the photoperiodic control of flowering has been extensively studied. An important mediator of environmental signals (including photoperiod) in floral transition is FLOWERING LOCUS T (FT; Kobayashi et al., 1999). FT is expressed in the vascular tissue of cotyledons and young leaves but not in the shoot apical meristem (SAM), where the floral transition occurs (Takada and Goto, 2003). The FT protein is transported to the SAM via the phloem (Corbesier et al., 2007; Jaeger and Wigge, 2007). In the SAM, FT interacts with the basic leucine zipper transcription factor FLOWERING LOCUS D (FD), most likely to promote flowering and the activation of floral meristem identity genes (Abe et al., 2005; Wigge et al., 2005). By ectopic and/or overexpression of FT homologs in different species, the flowering-promoting FT function has been shown to be conserved in the angiosperm lineage in both monocot and dicot species (for review, see Pin and Nilsson, 2012). These species include day-neutral species as well as plants induced to flower by long days or short days, showing that FT genes can function as a universal florigenic signal. Furthermore, the function of FT homologs has been reported to extend beyond flowering and also affect growth cessation and bud set in poplar (Populus spp.; Böhlenius et al., 2006; Hsu et al., 2011), growth termination in tomato (Solanum lycopersicum; Lifschitz et al., 2006), and tuberization in potato (Solanum tuberosum; Navarro et al., 2011). Recent experiments also suggest that functional diversification of FT paralogs may be quite common. In sugar beet (Beta vulgaris), BvFT1 and BvFT2 have antagonistic functions in the control of flowering (Pin et al., 2010), and in poplar, FT1 regulates reproductive onset in response to winter temperatures, whereas vegetative growth and the inhibition of bud set are promoted by FT2 in response to warm temperatures and long days in the growing season (Hsu et al., 2011).
A homolog to FT is the floral inhibitor TERMINAL FLOWER1 (TFL1). TFL1 acts as a repressor of flowering and extends the vegetative growth state while maintaining the indeterminate state of inflorescences (Shannon and Meeks-Wagner, 1991; Alvarez et al., 1992; Bradley et al., 1997; Ratcliffe et al., 1998). TFL1 is expressed in the nucleus and cytoplasm but interacts with FD solely in the nucleus and represses genes activated by FT (Hanano and Goto, 2011). The antagonistic function of FT and TFL1 is partly controlled by a single amino acid exchange, even though the remaining protein sequence is also important for full protein function (Hanzawa et al., 2005). TFL1 mRNA is expressed in the central part of both lateral and main shoot meristems, but the TFL1 protein moves and spreads out over the whole meristem, allowing the repression of floral identity genes (Conti and Bradley, 2007). As for FT, TFL1 function appears to be at least partially conserved in angiosperms (Pnueli et al., 1998; Nakagawa et al., 2002; Carmona et al., 2007; Hou and Yang, 2009; Danilevskaya et al., 2010; Mohamed et al., 2010; Repinski et al., 2012; Tsaftaris et al., 2012). In the perennial Arabidopsis relative Arabis alpina, the TFL1 homolog AaTFL1 prevents flowering in young vernalized plants and prolongs the required vernalization period in older plants (Wang et al., 2011). Furthermore, AaTFL1 expression in axillary meristems ensures that the vegetative branches are preserved, to maintain a perennial growth habit (Wang et al., 2011). Besides a function through interaction with FD, TFL1 has been reported to be involved in the trafficking of proteins to the protein storage vacuoles (Sohn et al., 2007).
Angiosperms and gymnosperms diverged about 300 million years ago (Bowe et al., 2000), and the degree of conservation in pathways controlling the induction of flowering or bud set is so far unclear. We have previously shown that the antagonistically functioning paralogs FT and TFL1 likely arose after duplication in the angiosperm lineage (Karlgren et al., 2011). In the conifer Norway spruce, two FT/TFL1-like genes were identified (PaFTL1 and PaFTL2), with roughly equal similarity to FT and TFL1 (Supplemental Fig. S1). With the recent publication of the Norway spruce genome, four additional copies of FT/TFL1-like genes were identified that cluster close to PaFTL1 and PaFTL2 (Nystedt et al., 2013). Whether these newly identified genes are expressed and functional is, to our knowledge, unknown at present. When PaFTL1 and PaFTL2 were ectopically expressed in Arabidopsis, flowering time was delayed and flower morphology showed similarities with TFL1 overexpressors (Karlgren et al., 2011; Klintenas et al., 2012). Expressing PaFTL1 and PaFTL2 in the tfl1 mutant further showed that both genes can substitute for TFL1 (Klintenas et al., 2012). These data suggest that the flowering-promoting function of FT evolved after the split between angiosperms and gymnosperms (Karlgren et al., 2011).
The expression of PaFTL2 is induced by long nights, and its expression is strongly correlated with bud set under various photoperiodic treatments (Gyllenstrand et al., 2007). Furthermore, the expression of PaFTL2 in plants from Scandinavian natural populations shows a latitudinal cline again associated with the clinal variation in bud set (Chen et al., 2012). Chen et al. (2012) also identified single-nucleotide polymorphisms in the promoter of PaFTL2 with a clinal variation in allele frequency indicative of divergent selection in populations from northern and southern latitudes. These data suggest that PaFTL2 is important for the control of bud set and that the gene might be involved in local adaptation conferring clinal variation.
By controlled induction of PaFTL2 in transgenic Norway spruce, we show here that the gene does indeed regulate bud set. We also present a detailed localization of PaFTL2 expression in buds under inductive conditions and show that PaFTL1 and PaFTL2 appear to have complementary expression patterns indicating that they may act in concert to control the growth cycle in Norway spruce.
RESULTS
PaFTL2 Induces Bud Set in Transgenic Spruce
In order to test the role of PaFTL2 in growth cessation and bud set, we first aimed to constitutively overexpress the gene in Norway spruce. A construct with the strong cauliflower mosaic virus 35S promoter driving the expression of the coding region of PaFTL2 was cotransformed into embryogenic cell cultures together with the bar gene using particle bombardment (Clapham et al., 2000). Three independent experiments with approximately 40 bombardments each were performed, but all of them failed to produce viable callus. Similar results were also reported by Klintenas et al. (2012). Since it appeared impossible to produce viable transgenic Norway spruce lines constitutively expressing PaFTL2, we instead tested to activate PaFTL2 from an inducible heat shock promoter (hsp::PaFTL2). This promoter is activated when subjected to a fast and drastic increase in temperature (Baumann et al., 1987; Saidi et al., 2005). Several individually transformed calli were obtained, and the presence of the hsp::PaFTL2 transcript was confirmed by PCR. Plantlets of several different transgenic lines were tested with heat shock treatment (HS). Three lines showed a good response to a first HS and were selected for further studies.
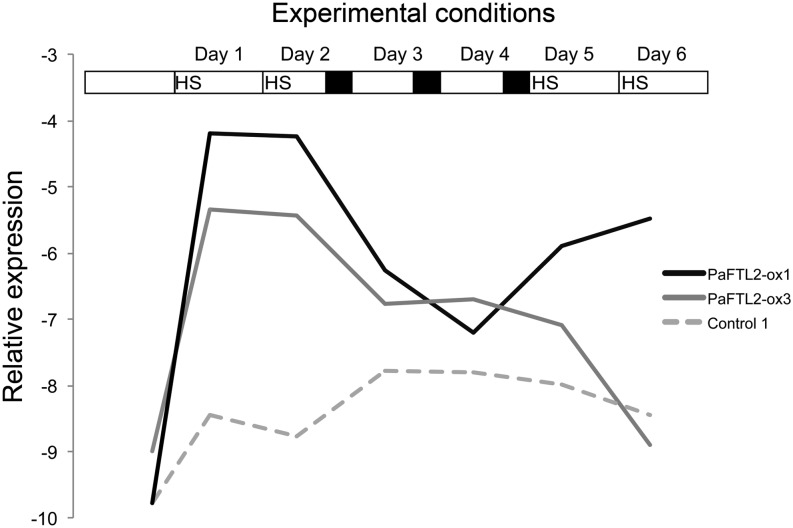
To obtain sufficient induction of PaFTL2, we optimized the treatment. High expression was induced by a 1-h HS at 40°C. Unfortunately, the induced expression of PaFTL2 declined quite quickly, and the PaFTL2 mRNA levels were almost back to normal after 24 h. If HS was given once every 24 h, one of the three selected transgenic lines, PaFTL2-ox3, responded only to the first two HS and the other two lines showed varying results. Successive HS given every 24 h did not result in a consistent increase in bud set in the PaFTL2-ox lines as compared with the controls. Since several nights, each inducing high PaFTL2 expression, are needed to induce bud set in the genotype used for transformation, we hypothesized that a certain amount of PaFTL2 over a period of several days is required for bud set. Therefore, we decided to use a treatment where HS was alternated with short nights (7 h) to partially replace the poor sequent HS induction with elevated endogenous expression of PaFTL2. The first 2 d (days 1 and 2), the plants were subjected to a 1-h heat shock at 40°C, followed by three short nights (days 2–4), and the treatment was ended with 2 d of heat shocks (days 5 and 6). The induced PaFTL2 expression for the first of three independent experiments is shown in Figure 1. PaFTL2-ox1 responded to all four HS, while PaFTL2-ox3 responded only to the first two HS. The control plants displayed a slight increase in endogenous PaFTL2 expression as a result of the short night treatment, but this was not sufficient to induce bud set.
Figure 1.
Expression of PaFTL2 in transgenic Norway spruce hsp::PaFTL2 and control plants after HS. The bar at top illustrates the experimental conditions. During a 6-d period, HS of 1 h (40°C) was given four times. After the second HS, plants were transferred to a photoperiod of 19 h of light/7 h of darkness for 3 d, after which they were returned to constant light before administration of the last two HS. The graph shows daily averages of expression levels of PaFTL2 (endogenous expression plus that produced by hsp::PaFTL2). Samples for qRT-PCR were taken just before HS or immediately after the dark period and 5 h later each day. In the graph, the two daily measurements are averaged to reveal general trends in expression.
Plant height and the frequency of bud set were measured before HS and during the following weeks. Although the bud set induction frequency differed between experiments, it is clear that HS-induced expression of PaFTL2 leads to a significant increase in bud set in the transgenic lines compared with control plants (Table I). However, the effect of hsp::PaFTL2 induction on growth cessation was weak, and only PaFTL2-ox1 showed a clear growth retardation (Supplemental Fig. S2). Still, the two most responsive transgenic lines (PaFTL2-ox1 and PaFTL2-ox2) displayed a generally reduced growth rate when grown in ambient temperatures, possibly as an effect of background activity from the hsp promoter (Wang et al., 2005). In conclusion, our data strongly support our hypothesis that PaFTL2 plays an important role in the control of bud set and growth arrest in Norway spruce.
Table I. Assessment of bud formation 25 d (experiments 1 and 2) or 32 d (experiment 3) after first HS.
Bud set was assessed on a scale from 0 to 3, where 0 = no sign of bud set, 1 = beginning of bud formation (needle extension reduced, but no white bud scales evident), 2 = bud scales formed, and 3 = bud burst and resumption of growth.
| Experiment | Transgenic Line | Bud Stage 0 | Bud Stage 1 | Bud Stage 2 | Bud Stage 3 | Percentage Bud Stage 1 to 3 |
|---|---|---|---|---|---|---|
| 1 | PaFTL2-ox1 | 0 | 8 | 1 | 0 | 100 |
| PaFTL2-ox3 | 10 | 2 | 1 | 0 | 23 | |
| Control 1 | 15 | 0 | 0 | 0 | 0 | |
| 2 | PaFTL2-ox1 | 0 | 7 | 4 | 1 | 100 |
| PaFTL2-ox2 | 1 | 8 | 1 | 7 | 94 | |
| Control 1 | 17 | 0 | 0 | 0 | 0 | |
| Control 2 | 11 | 1 | 0 | 0 | 8 | |
| 3 | PaFTL2-ox1 | 14 | 2 | 20 | 1 | 62 |
| PaFTL2-ox2 | 9 | 4 | 3 | 0 | 44 | |
| Control 1 | 59 | 0 | 0 | 0 | 0 |
PaFTL2 mRNA Is Localized beneath the Meristem and Procambium after Induction in Short Days
Previous studies have shown that PaFTL2 is expressed in needles, where the expression is induced by dark periods exceeding the critical night length for the genotype in question (Gyllenstrand et al., 2007). Expression was also detected in developing buds (Karlgren et al., 2011). To determine the temporal and spatial mRNA expression during induction of bud set, stem pieces approximately 5 mm long including the SAM were sampled for mRNA in situ hybridization. The material was obtained from seedlings that were raised under constant light and transferred to short-day (SD) conditions (8 h of light and 16 h of dark). This treatment is known to induce bud set in all genotypes of Norway spruce (Ekberg et al., 1979). Two sources of material were used: seeds originating from latitude 67° (SE-67) and seeds from latitude 47° (RO-47).
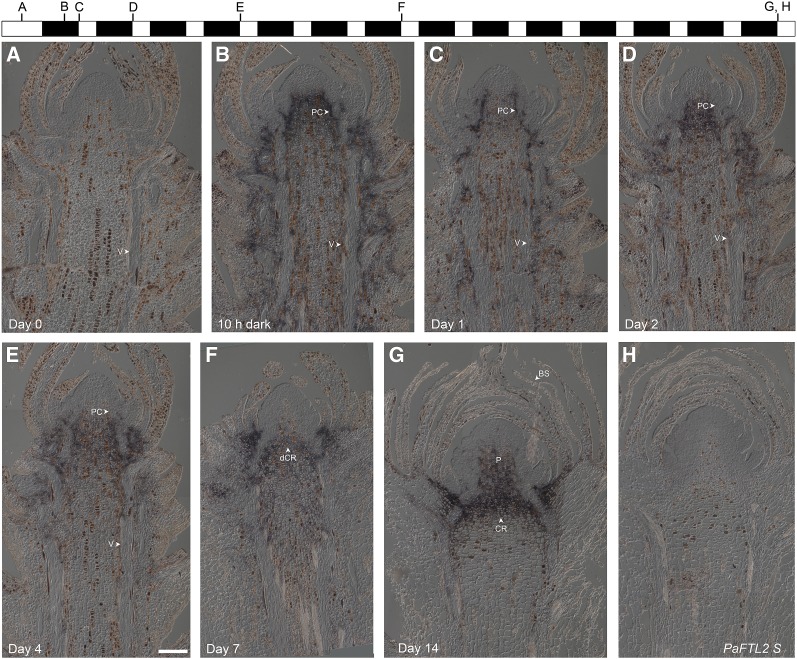
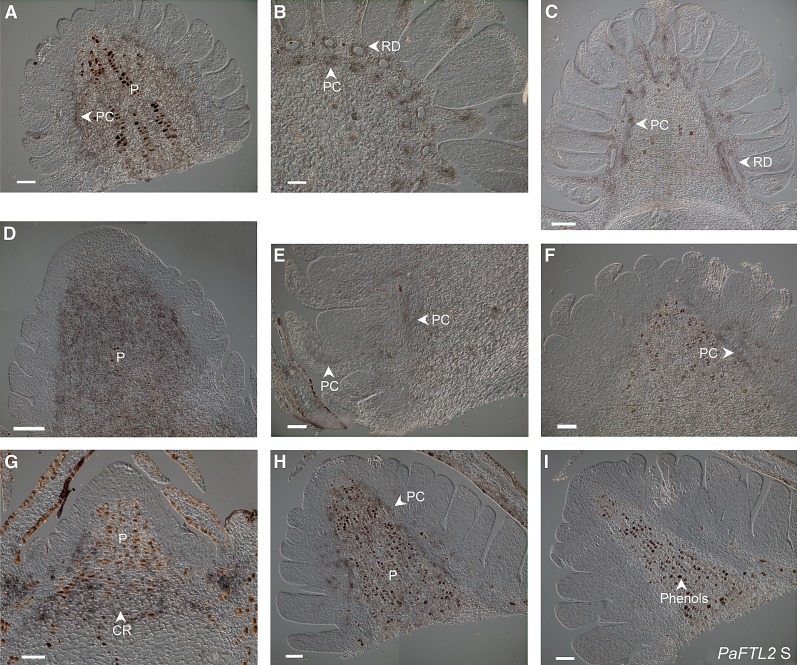
No PaFTL2 expression could be detected at day 0 (Fig. 2A; Supplemental Fig. S3A), but a strong signal below the meristem and around the procambium and vascular tissue could be observed already after 10 h in darkness (Fig. 2B; Supplemental Fig. S3B). A similar pattern was observed in all samples until day 4 (Fig. 2, B–F; Supplemental Fig. S3, B–F). At day 7, PaFTL2 expression became confined to the area underneath the developing bud, and at day 14, expression was detected in a region delineating the newly formed bud and the shoot (the crown region or colenchymatous plate) and extended into the pith of the bud (Fig. 2G; Supplemental Fig. S3G). Cross sections of RO-47 at day 2 confirmed that no expression was detectable at the tip of the meristem (Fig. 3A) and that the strongest signal was found below the meristem in the center of the shoot (Fig. 3B). Farther down the stem, a defined expression around the procambium and vascular bundles could be observed (Fig. 3C). A very similar expression pattern to that in top shoots was also observed in axillary shoots: no expression was observable at day 0, whereas PaFTL2 mRNA was detected around the procambium at days 4 and 7 and at the crown region and the pith of the bud at day 14 (Supplemental Fig. S4).
Figure 2.
In situ localization of PaFTL2 mRNA in top shoots from SE-67 Norway spruce seedlings. A to G, Antisense probe. H, Sense probe. Seedlings were grown in constant light until day 0, followed by transfer to short days with 8 h of light/16 h of dark. Shoots were collected at day 0 (A), after 10 h in darkness (B), and then directly before the lights were turned on at day 1 (C), day 2 (D), day 4 (E), day 7 (F), and day 14 (G and H). Before transfer to darkness, no PaFTL2 expression could be detected (A), but a strong induction of PaFTL2 mRNA was visible below the meristem and around the vascular tissue (V) and procambium (PC) after transfer to darkness (B–E). After approximately 7 d (F), PaFTL2 expression began to concentrate in the developing crown region (dCR) when the bud started to form needle primordia. At day 14 (G), buds with bud scales (BS) had formed and the expression was concentrated to the crown region (CR) and the pith (P) of the bud. Bar = 200 μm.
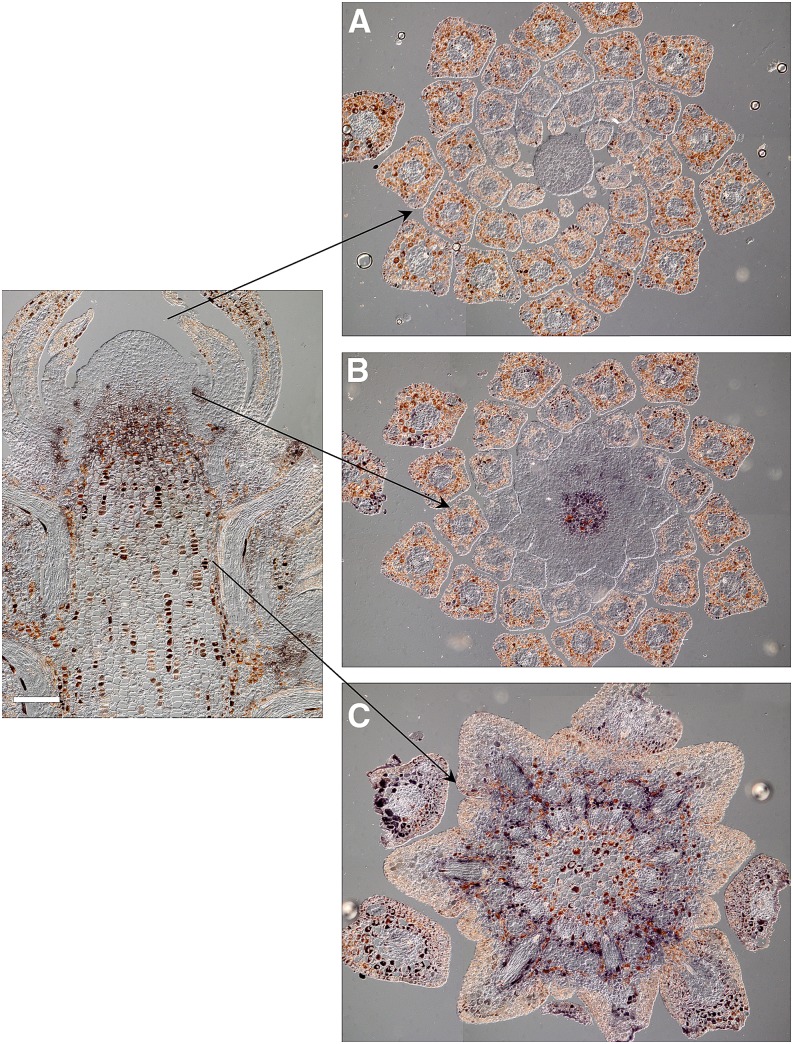
Figure 3.
In situ localization of PaFTL2 mRNA in transverse sections of a top shoot from a RO-47 Norway spruce seedling subjected to two short days of 8 h of light/16 h of dark (day 2). A, In the tip of the meristem, no signal could be observed. B, Just below the meristem, a strong signal could be observed in the center of the shoot. C, Farther down the stem, the signal was concentrated around the procambium and vascular tissue. Bar = 200 μm.
PaFTL2 Expression Differs between Genotypes from Different Latitudes
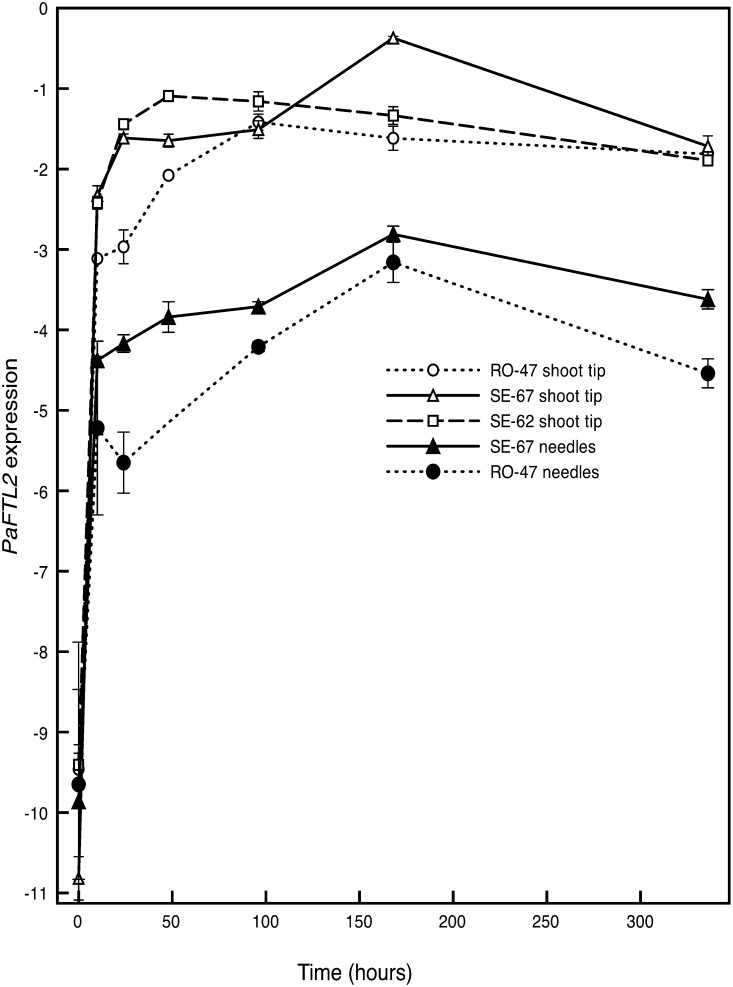
Recent data suggest that the expression level of PaFTL2 in needles displays a latitudinal cline and that single-nucleotide polymorphisms in the promoter might be responsible for part of the clinal variation (Chen et al., 2012). Our new in situ data indicated that PaFTL2 might display a stronger expression also in the shoot tip of the high-latitude genotype SE-67, compared with the low-latitude genotype RO-47, early after transfer to SD conditions (Fig. 2; Supplemental Fig. S3). To quantify the expression in the shoot tip in genotypes from different latitudes, seedlings from three populations, SE-67, SE-62, and RO-47, were sampled and subjected to quantitative reverse transcription (qRT)-PCR. Shoot tips containing the SAM and 1 to 2 mm of stem were obtained from all three populations, while needles closest to the SAM were available only for RO-47 and SE-67, and shoot tips from lateral branches were available only from RO-47.
PaFTL2 expression was very low in all tissues in constant light, but already after 10 h of darkness, a strong increase in expression could be observed in all tissues (Fig. 4). In needles, the expression levels steadily increased until day 7 but had declined at day 14 (Fig. 4). Expression levels were consistently lower in samples from RO-47. In shoot tips, a strong and largely stable expression was observed already from day 1 in SE-62 and SE-67, while samples from RO-47 reached a similar level around day 4 (Fig. 4). A conspicuously high expression was observed for SE-67 at day 7 in shoot tips. Shoot tips from lateral branches showed a similar increase in PaFTL2 expression as the top shoots, supporting that PaFTL2 function is not limited to the SAM (data not shown).
Figure 4.
Quantification of PaFTL2 expression in shoot tips and needles of Norway spruce seedlings. Norway spruce seedlings from two populations, SE-67 and RO-47, were raised in constant light and transferred to short days (8 h of light/16 h of dark). The expression of PaFTL2 was determined with qRT-PCR in needles closest to the SAM and in the shoot tip including the SAM and 1 to 2 mm of the stem. Relative expression values (y axis) were calculated as delta cycle threshold values (CtPaUBQ − CtPaFTL2). Error bars indicate se values of technical replicates. The x axis indicates hours after transfer to short days.
PaFTL2 Is Highly Expressed in the Autumn
To study the expression of PaFTL2 in older trees under natural conditions, shoot tips from basal branches were collected on several occasions during one growing season. Each sample contained the meristem and approximately 5 mm of stem. qRT-PCR data indicated a slow increase in PaFTL2 expression in June and early July and a sharp increase in expression in late July and early August (Fig. 5A). The sharp increase coincides with the time when the night length exceeds the critical night length for growth cessation at the study site in late July.
Figure 5.
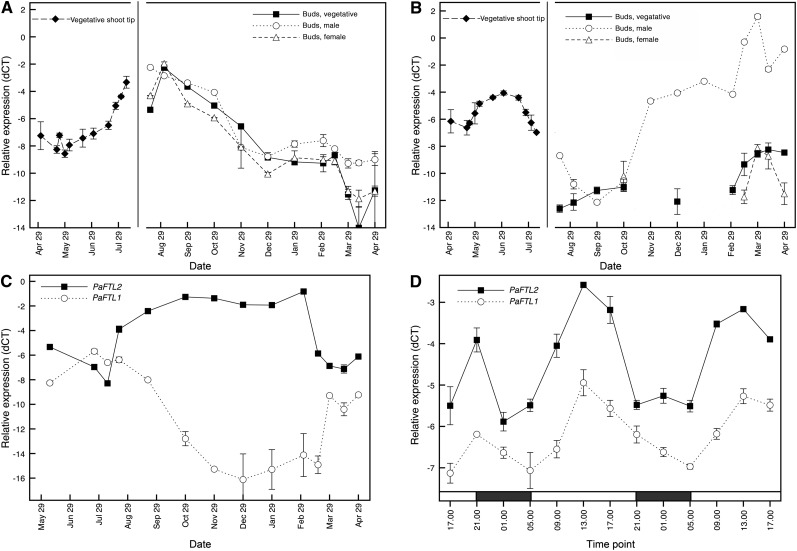
The expression of PaFTL1 and PaFTL2 in trees with predetermined growth was determined with qRT-PCR. A, Expression of PaFTL2 in vegetative shoot tips before growth cessation (left) and in vegetative, male, and female buds after growth cessation (right). B, Expression of PaFTL1 in vegetative shoot tips before growth cessation (left) and in vegetative, male, and female buds after growth cessation (right). C, Expression of PaFTL1 and PaFTL2 in needles as determined over 1 year. D, Diurnal expression of PaFTL1 and PaFTL2 in needles as estimated during 2 d in August. Relative expression refers to delta cycle threshold (dCT) values (CtPaUBQ − CtPaFTL). Vegetative shoots were sampled from three different trees, and error bars derive from biological replicates. Buds and needles were collected from a single tree, and error bars derive from technical replicates.
To investigate the expression of PaFTL2 in subsequent stages of developing buds, samples of vegetative, male, and female buds were collected from bud set until bud burst (August until April). qRT-PCR data showed that PaFTL2 displayed similar expression levels in all three bud types (Fig. 5A). Expression peaked at the time of cessation of shoot elongation and decreased steadily until late December, coincident with the transition from endodormancy to ecodormancy (Qamaruddin et al., 1993). A further decrease was observed at bud burst, in particular in vegetative and female buds, consistent with data from vegetative bud flush in forcing experiments (Gyllenstrand et al., 2007).
Data from shoot tips and buds were compared with the yearly PaFTL2 expression pattern in needles. Needles were collected monthly over 1 year, and in contrast to the decline in PaFTL2 expression in buds during the transition from endodormancy to ecodormancy, expression remained high in needles until March (Fig. 5C). Expression then rapidly declined in March and remained at a relatively steady level until the increase in August coinciding with the advent of the critical night length, as observed for shoot tips (Fig. 5, A and C). This suggests that the regulation of PaFTL2 expression in needles seems to be largely controlled by photoperiod (Gyllenstrand et al., 2007), while additional factors seem important for its expression in buds. To test if PaFTL2 showed a diurnal expression pattern in adult trees under natural conditions, as observed for seedlings grown under controlled conditions, needles were sampled from an adult tree every 4 h during 2 d in August. PaFTL2 showed a rhythmic expression with a peak in the middle of the day (Fig. 5D).
The spatial expression of PaFTL2 in female, male, and vegetative buds was investigated with in situ hybridization. In young developing male cones (collected in August), PaFTL2 expression was detected in the pith and the procambial region (Fig. 6A). One month later, PaFTL2 expression was concentrated around the procambium and the resin ducts, but some expression also remained in the pith (Fig. 6, B and C). In developing female cones, a similar expression pattern was observed (Fig. 6, D–F). In young vegetative buds, the expression was localized in the crown region delineating the actual bud and the stem (Fig. 6G), which is consistent with the expression observed in the shoots from seedlings (Fig. 2G). In September, when the vegetative buds had developed several leaf primordia, PaFTL2 expression had moved up into the pith and the procambial region as for the early reproductive cones (Fig. 6H). Vegetative buds develop later than the reproductive cones (Sundström, 2001), which may explain the delay of expression in the actual bud.
Figure 6.
In situ localization of PaFTL2 mRNA in vegetative and reproductive buds from an adult Norway spruce tree. A, In young male cones sampled in August, PaFTL2 mRNA were localized in the pith (P) and around the procambium (PC). B and C, When the male bud had developed all microsporophylls in late September (transverse section [B] and longitudinal section [C]), the expression was centered around the resin ducts (RD) and procambium, although some expression remained in the pith. D to F, A similar pattern can be observed in female cones, with high expression in the pith early in the development (August; D) and a more focused expression around the procambium later in September (E and F). G, Vegetative buds in August have PaFTL2 expression in the crown region (CR) and the pith. H, When more needle primordia have developed in September, the expression, as in male and female buds, is more concentrated around the procambium. I, Sections treated with sense probes showed no visible signal. Bars = 100 μm (A, B, and E–I) and 200 μm (C and D).
The Expression Pattern of PaFTL1 Is Largely Complementary to That of PaFTL2
As the protein sequence of PaFTL1 shows high similarity to PaFTL2 and both genes generate similar phenotypes when expressed in Arabidopsis (Karlgren et al., 2011), both genes might possess related functions in Norway spruce. Previous studies have revealed a high expression of PaFTL1 during male cone development (Karlgren et al., 2011), but its expression pattern in other tissues was not known.
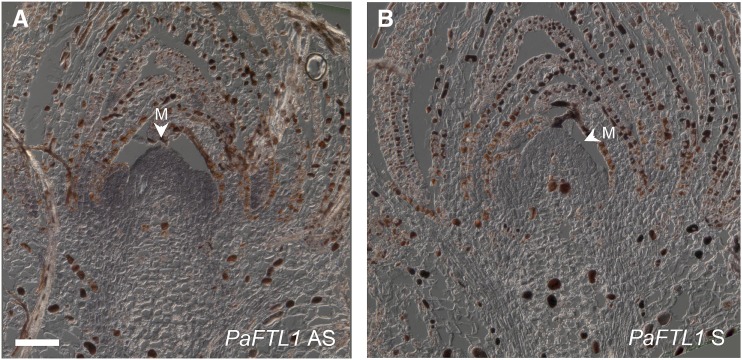
In shoot tips and needles, expression was relatively high during active shoot elongation in the summer, but it was low during winter in needles and vegetative and female buds (Fig. 5, B and C). Concordant with previous data, expression increased to very high levels in male buds from late November until bud burst (Fig. 5B; Karlgren et al., 2011). In needles sampled in August, PaFTL1, like PaFTL2, showed a rhythmic expression with a peak in the middle of the day, but the expression level of PaFTL1 was lower than for PaFTL2 (Fig. 5D). In situ localization of PaFTL1 mRNA suggested a high expression during the growing season at the top and flanks of the inactive SAM (Fig. 7). Thus, in many respects, the temporal and spatial expression pattern of PaFTL1 was complementary to that of PaFTL2.
Figure 7.
In situ localization of PaFTL1 mRNA in a vegetative shoot from a lateral branch of a Norway spruce tree sampled outdoor in the beginning of July. A, PaFTL1 was expressed in the meristem (M). B, No signal was visible with the sense probe. Bar = 100 μm.
DISCUSSION
PaFTL2 Induces Bud Set and Likely Functions as a Growth Repressor
Previous expression data and population genetic analyses have suggested a role for PaFTL2 in the control of growth cessation and bud set in Norway spruce as well as in local adaptation resulting in clinal variation for the timing of bud set (Gyllenstrand et al., 2007; Chen et al., 2012). In this study, we confirm, by manipulation of the expression of PaFTL2 in transgenic Norway spruce seedlings, that the gene does indeed have a role in the control of bud set. Analysis of PaFTL2 expression domains and temporal patterns further supported that role. Our data further suggest that PaFTL2 has a function in the repression of growth. We were unable to produce transgenic Norway spruce plants constitutively expressing PaFTL2. Likewise, Klintenas et al. (2012) reported that embryogenic spruce cultures expressing FTL2 stopped growing and died after a few months of tissue culture. Furthermore, an obvious phenotype of our hsp::PaFTL2 plant was a retarded growth, also when grown under ambient temperatures. The latter suggests increased background levels of PaFTL2 expression, and collectively, these results support that PaFTL2 functions as a growth repressor.
In angiosperm poplar trees, one FT homolog, FT2, has an important role in the control of annual growth (Hsu et al., 2011). FT2 is controlled by photoperiod and temperature and shows a high expression during the growing season. Transgenic experiments manipulating the expression levels of FT2 further showed that high expression prevents growth cessation (Hsu et al., 2011). Available data suggest that the protein function of PaFTL2 is more similar to TFL1 than to FT (Karlgren et al., 2011). Thus, the observation that a high expression of FT2 is associated with growth while a high expression of PaFTL2 is associated with growth cessation is compatible with the presumed opposite functions of the two corresponding proteins.
Genotypes from northern latitudes (SE-62 and SE-67) require only a single short day to set bud when raised in constant light, while those from lower latitudes (RO-47) need four short days (Qamaruddin et al., 1995). Still, similar spatial PaFTL2 expression was evident at the shoot tip 10 h after transfer to darkness in all genotypes. The rapid induction of PaFTL2 expression in shoot apices already 10 h into the first long night suggests that the transport of PaFTL2 protein from needles is not required for the induction of bud set. The different SD requirement for bud set in the studied genotypes is paralleled by the expression levels of PaFTL2 in shoot tips, which reached a high steady level already after 1 d in SE-62 and SE-67, in contrast to the gradual increase in RO-47, where expression reached a similar high level after 4 d. The similar spatial patterns of expression observed from 10 h until 4 d after induction in both southern and northern genotypes suggest that the accumulation of a threshold level of protein is required to induce bud set.
The strong expression of PaFTL2 evident at the shoot tip already after 10 h in darkness was concentrated around the vascular tissue and procambium. When buds were formed after 2 weeks, the spatial expression pattern of PaFTL2 had clearly changed. A strong expression was concentrated in the crown region (colenchymatous plate) at the base of the bud. The crown region is, in effect, a plate across the base of the bud, consisting of cells with thickened cell walls (Krasowski and Owens, 1989). The role of the crown region is unclear, but it is thought to physiologically isolate the bud from the shoot, although the plate is penetrated by procambial tissue (Krasowski and Owens, 1989). The plate also separates the region forming bud scales from the one forming needle primordia (Banasiak and Zagorska-Marek, 2006). When buds were formed and new needle primordia developed, the expression of PaFTL2 also extended into the pith of the bud. Studies of later stages of bud development from adult trees showed that expression was gradually focused to the procambial regions surrounding the pith.
The strong expression in the crown region and the pith of the bud is consistent with the hypothesis that PaFTL2 represses cell division or expansion in these regions. The crown region consists of layers of compact cells, where cell expansion seems to be conspicuously retarded. Likewise, elongation of the cells in pith of the bud must be repressed during the active formation of new needle primordia, within the bud, for the next growing season. As the crown region where PaFTL2 is strongly expressed coincides with the region where bud scales are formed instead of needles, the gene could potentially also be involved in the specification of identity of primordia formed at the flanks of the meristem.
The conspicuous expression of PaFTL2 around procambial tissue, both during the induction of bud set and later in developing buds, would, in analogy with the movement of FT in the phloem, suggest that PaFTL2 protein is transported in vascular tissue. Alternatively, PaFTL2 might repress the development or maturation of vascular tissue in order to prevent transport to the meristem. Isolation of the bud from growth-promoting substances seems to be important for developing and maintaining a dormant state of the bud (Rinne et al., 2001). Such a function would be consistent with a general function for the protein in repressing cell proliferation.
Annual Patterns of PaFTL2 Expression in Adult Trees
Expression patterns of PaFTL2 in older trees support its role in the control of bud set and bud burst also in adult trees. In contrast to seedlings, new bud scales in adult Norway spruce are formed already when the buds from the previous year are flushing (predetermined growth). The shoot then elongates the needle primordia and intervening internodes of last year’s bud. The meristem in the newly formed bud is then largely inactive until growth cessation in the autumn. At that time, the meristem is reactivated and starts to form needle primordia for the next growing season in vegetative meristems. In reproductive meristems, the reactivation of the meristem and the formation of primordia start somewhat earlier.
Expression data from needles and vegetative shoot tips of adult trees confirmed previous observations that PaFTL2 expression increases in the autumn, around the time of growth cessation and vegetative meristem reactivation. In needles, expression increases until late October, after which high expression is retained until spring, when it drops to an intermediate level concurrent with increasing daylength and temperature. In vegetative buds, a peak of expression is reached in late August, and the gene is expressed in the crown region and the pith of the expanding bud, when new needle primordia are observed. A steady decrease in PaFTL2 expression was then observed from September to January. Bud burst in Norway spruce requires a chilling period (several weeks of low subzero temperature, typically October to January) followed by an accumulation of high-temperature days and occurs well before the scotoperiod reaches the critical night length. As our data suggest that PaFTL2 represses growth, they support that bud burst occurs as a result of low PaFTL2 expression in the buds. The decline in PaFTL2 expression during winter coincides with the chilling period, and a further reduction occurs concurrently with bud burst. Chromatin modification is important for the regulation of FT expression, directly on FT chromatin as well as on its upstream regulators (He, 2012). It is tempting to speculate that the reduction in PaFTL2 expression, specifically in buds, during autumn and early winter is important for the transition from endodormancy to ecodormacy and that it is controlled in a similar way to the regulation of FT expression by vernalization.
PaFTL1 Is Highly Expressed in Inactive Meristems
Available data suggest that PaFTL1 and PaFTL2 proteins may confer similar functions (Karlgren et al., 2011), but their expression patterns seem to be largely complementary both spatially and temporally. We still lack access to Norway spruce plants with manipulated expression levels of PaFTL1, although expression data may give some hints on its putative function. The high expression during male cone development suggests a specific function in this process (Karlgren et al., 2011). An interesting observation from this study was that the gene displays a high expression in shoot tips during the growing season (during shoot elongation of previous year buds), where expression seems to be focused to the top and flanks of the SAM. During this time, the meristems are seemingly inactive, and when the meristems are reactivated and start producing needle primordia, PaFTL1 expression declines. These data are consistent with a hypothesis in which PaFTL1 represses meristem activity and the formation of needle primordia.
Concurrently with growth cessation (repression of stem elongation), reactivation of the meristem, and the decline in PaFTL1 expression, PaFTL2 expression increases at the base of the meristem. PaFTL2 expression then spreads into the pith and procambium of the newly formed buds. Thus, PaFTL1 and PaFTL2 both seem to be expressed in regions of repressed growth, but their spatial and temporal patterns of expression largely complement each other.
Potential Molecular Functions of PaFTL1 and PaFTL2
Besides its involvement in the induction of flowering, FT has been implicated in various developmental processes, such as tuberization, stomatal control, and vegetative growth (Gyllenstrand et al., 2007; Hsu et al., 2011; Kinoshita et al., 2011; Navarro et al., 2011). In angiosperm flower induction, both FT and TFL1 are hypothesized to interact with the transcription factor FD, and the resulting protein complex either promotes (FT/FD) or represses (TFL1/FD) the transcription of floral meristem identity genes, primarily through the induction of SOC1 and FUL (Hanano and Goto, 2011; Torti et al., 2012). No FD orthologs have been identified so far in gymnosperms (Tsuji et al., 2013), suggesting that interaction with other proteins might be important for PaFTL1 and PaFTL2 function.
The molecular functions of FT-like genes in other developmental processes are poorly known. In stomatal control, FT has been suggested to regulate proton-pumping ATPases (Kinoshita et al., 2011) and thus might act as a general growth regulator. Recent work in poplar suggests that AINTEGUMENTA-LIKE (AIL) transcription factors of the AP2 family act downstream of poplar FT2 in SD-induced growth cessation (Karlberg et al., 2011). The proposed function of AIL in this process is in the control of cell cycle regulators. Direct targets of FT2 in poplar are unknown, but available data support that the regulation of AIL genes by FT2 is dependent on intermediate genes (Karlberg et al., 2011). In poplar, the expression domain of AIL1 was mainly confined to the zone of dividing cells in the apex, provascular tissue, and leaf primordia (Karlberg et al., 2011), similar expression domains to those observed for PaFTL2 in Norway spruce.
Available data do not favor any of these proposed molecular functions for PaFTL1 or PaFTL2, and more data are clearly needed to unravel the molecular control of bud development and meristem activities during the annual growth cycle in conifers.
CONCLUSION
This study clearly supports a role for PaFTL2 in the control of growth cessation and bud set in first-year seedlings with free growth. Expression data from adult trees with predetermined growth further suggest a function during growth cessation and the development of new needle primordia for the coming season. Both these roles and a potential role of PaFTL1 in the inactivation of meristems are compatible with a function that represses cell proliferation. An attractive hypothesis is that PaFTL1 and PaFTL2 act in concert to control perennial growth in Norway spruce. According to this hypothesis, PaFTL1, which is expressed in the meristem, prevents proliferation of the meristem during active extension growth in the summer, while PaFTL2 attenuates extension growth during bud development in the autumn.
MATERIALS AND METHODS
Plasmids and Constructs for Transformation
Based on previous results (Gyllenstrand et al., 2007; Karlgren et al., 2011), PaFTL2 was chosen for transformation as the most likely Norway spruce (Picea abies) PEBP gene candidate involved in the control of growth cessation and bud set. The hsp::PaFTL2 construct was produced using Gateway technology (Invitrogen). The complete complementary DNA (cDNA) was amplified using gene-specific primers with attB sites as described (Karlgren et al., 2011), and the PCR product was cloned into the pDONR 221 vector (Invitrogen). The destination vector pMDC30 (Curtis and Grossniklaus, 2003), containing a heat-inducible promoter from soybean (Glycine max), was used to construct hsp::PaFTL2 plasmids. A 35S::PaFTL2 construct with the cauliflower mosaic virus 35S promoter generating constitutive expression was also created using the destination vector pMDC32 (Curtis and Grossniklaus, 2003).
The bar gene construct conferring resistance to phosphinothrycin and acting as a selectable marker for transformation was derived from plasmid pAHC25 (Christensen and Quail, 1996). The construct was partially cleaved from pAHC25 with EcoRI and cloned into pUC19 to give plasmid pUbi-bar. The bar gene was amplified from the plasmid pUbi-bar with the primers T3 (5′-AATTAACCCTCACTAAAG-3′) and T7 (5′-GTAATACGACTCACTATA-3′) using Phusion HF enzyme from Dynazyme. Conditions were 98°C for 30 s followed by 35 cycles of 98°C for 10 s, 50°C for 30 s, and 72°C for 1 min.
Plant Material for Transformation
The somatic embryogenic cell line 06:28:05 was induced by standard methods (von Arnold and Clapham, 2008) from seed resulting from a controlled cross made in 2006 between selected parents of Norway spruce as part of the Forestry Research Institute of Sweden’s breeding program for southern Sweden.
Transgenic sublines of Norway spruce 06:28:05 were created by a particle acceleration method as described (von Arnold and Clapham, 2008). Embryogenic cultures were bombarded with gold particles double coated with hsp::PaFTL2 and pAHC25 or 35S::PaFTL2 and the bar cassette in the molecular proportion 1:2. Control bombardments contained only pAHC25 or the bar cassette. In the coating procedure, 20 µg of DNA was precipitated over 10-mg gold particles (1.5–3 µm in diameter). Callus cultures resistant to phosphinothrycin at 0.2 mg L−1, supplied as the commercial preparation Basta, emerged 2 to 4 months after bombardment. Transformation was confirmed by PCR using the primers Forward (5′-TAATACGACTCACTATAGGGCCCCTGGTAGACCAATCCTA-3′) and Reverse (5′-TAATCCAAGGCCATTCATCTCT-3′).
Embryos were matured on BMI-SI (von Arnold and Clapham, 2008), and after collection, the embryos were left to dry at high humidity for at least 1 month before they were converted to plantlets on solid one-quarter-strength Schenk and Hildebrandt medium (von Arnold and Clapham, 2008). When the roots were 4 to 6 cm long, plantlets were transferred to pots of 8.0 × 8.0 × 6.5 cm containing mineral wool and watered with Ingestad’s nutrient solution (Ingestad, 1979). They were raised in a controlled growth room under continuous light from metal halogen lamps (250–300 µmol m−2 s−1) at 20°C and 75% relative humidity for 3 to 5 months depending on the rate of establishment of the sublines. At least 1 week before the HS experiments, the plants were removed to a growth cabinet with continuous light at 200 µmol m−2 s−1 at 20°C and 75% relative humidity in a randomized block design.
HS
The effectiveness of HS was tested by the isolation of total RNA from needles and PCR amplification of the PaFTL2 gene transcript from reverse-transcribed mRNA as described (Karlgren et al., 2011). To induce optimal expression of the PaFTL2 construct and find conditions that enabled repeated induction of expression, plants were placed in growth cabinets at 200 µmol m−2 s−1, at 38°C or 40°C, for 1 or 3 h. The HS was repeated on subsequent days. The best procedure was 1 h at 40°C on consecutive days, followed by 2 d without heat shock. More frequent treatments were ineffective, apparently owing to side effects of the heat shock. Repeated HS was effective in two transformed sublines, PaFTL2-ox1 and PaFTL2-ox2, whereas only the first HS was effective in PaFTL2-ox3.
The experimental regime adopted for the HS experiments (experiments 1–3) was as follows: days 1 and 2, continuous light, heat shock of 40°C from 9 to 10 am; days 2 to 4, plants were transferred to a light regime of 17 h of light from 9 am to 2 am and 7 h of dark from 2 am to 9 am at 20°C and 75% relative humidity; days 5 and 6, HS as for days 1 and 2. After the treatment, plants were transferred to continuous light at 20°C and 75% humidity, and they were examined over the next month for growth cessation and bud set. The purpose of the 7-h nights on days 2 to 4 was to prevent a decline of PaFTL2 expression to background levels in the absence of heat shock in the PaFTL2 transformants without inducing bud set in the controls; about seven 7-h nights are normally required to induce bud set in 3-month-old plants of the genotype utilized for transformation (data not shown).
To assess the effects of heat shock on PaFTL2 expression, needles were sampled from the plants of each transformed subline at 9 am and 3 pm on day 1 and at 3 pm on days 2 to 6. A total of eight to 12 needles were harvested from each transformed subline on each occasion. One needle was taken from each plant, randomly selected, where there were more than 12 plants per cell line.
Bud set was assessed on plants with the pot labels covered to allow unprejudiced judgment on a scale from 0 to 3, where 0 represents no sign of bud set; 1 represents beginning of bud formation, with needle extension reduced but no white bud scales evident; 2 represents bud scales formed; and 3 represents bud burst and resumption of growth.
Norway Spruce Plant Material
Female, male, and vegetative buds and needles were collected from adult trees of Norway spruce (more than 30 years old) growing at latitude 59°53'N (Uppsala, Sweden) during 2007, 2008, and 2009. Buds were collected on the first date after dissection on which they could be visually determined as female, male, or vegetative. Shoots from three several-year-old Norway spruce trees in different sizes were collected during May to August 2012. Seedlings from three populations were used to determine differences in expression levels of PaFTL1 and PaFTL2 in different tissues: a northern population from 66.68°N (Jock Valsjärv, Sweden) here named SE-67, another northern population from 61.57°N (Fulufjället, Sweden) named SE-62, and a southern population from 47.30°N (Frasin, Romania) named RO-47.
Quantitative Gene Expression Analysis
Total RNA was isolated with the Spectrum Plant Total RNA Kit (Sigma-Aldrich) using the On-Column DNase I Digestion Set (Sigma-Aldrich) according to the manufacturer’s recommendations or following the protocol described by Azevedo et al. (2003) with minor modifications. cDNA was synthesized from 0.5 μg of total RNA using SuperScript III reverse transcriptase and random hexamer primers. Real-time PCR was performed according to Karlgren et al. (2011) or with the Eco Real-Time PCR System (Illumina). For Eco, each reaction was performed in duplicate samples containing 5 µL of DyNAmo Flash SYBRGreen (Thermo Scientific), 0.5 mm of each primer, and 4 µL of cDNA (diluted 1:100) in a total volume of 10 µL. Alphabot (Alphahelix Technologie) performed all pipetting prior to runs in the Eco. The thermal conditions used were 95°C for 7 min followed by 40 cycles of 95°C for 10 s and 60°C for 30 s. For primers, see Gyllenstrand et al. (2007) or Karlgren et al. (2011).
In Situ Hybridization
In situ hybridization was performed according to Karlgren et al. (2009). For probe sequences, see Karlgren et al. (2011). After the experiment, the sections were photographed with Nomarski microscopy in 10× magnification. The photographs were merged in Adobe Photoshop CS4, and peripheral regions and background were, in some cases, modified to create a cohesive image. There has been no manipulation in regions containing signal or in crucial structures.
Phylogenetic Analyses
Amino acid sequences of Arabidopsis (Arabidopsis thaliana) and Norway spruce PEBP genes were analyzed using the Web service at www.Phylogeny.fr. This pipeline includes alignment with MUSCLE, curation with Gblocks, and phylogeny reconstruction with PhyML. The Web service was run with the “One Click” option and default settings.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Inferred phylogeny of Norway spruce and Arabidopsis PEBP genes.
Supplemental Figure S2. Height growth of transgenic Norway spruce with the hsp::PaFTL2 construct.
Supplemental Figure S3. In situ localization of PaFTL2 mRNA in top shoots of RO-47 Norway spruce seedlings.
Supplemental Figure S4. In situ localization of PaFTL2 mRNA in longitudinal sections of axillary shoots from RO-47 Norway spruce seedlings.
Acknowledgments
We thank Kerstin Jeppson for excellent laboratory assistance.
Glossary
- SAM
shoot apical meristem
- HS
heat shock treatment
- SD
short-day
- qRT
quantitative reverse transcription
- cDNA
complementary DNA
References
- Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, Ichinoki H, Notaguchi M, Goto K, Araki T. (2005) FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309: 1052–1056 [DOI] [PubMed] [Google Scholar]
- Alvarez J, Guli CL, Yu XH, Smyth DR. (1992) terminal flower: a gene affecting inflorescence development in Arabidopsis thaliana. Plant J 2: 103–116 [Google Scholar]
- Azevedo H, Lino-Neto T, Tavares R. (2003) An improved method for high-quality RNA isolation from needles of adult maritime pine trees. Plant Mol Biol Rep 21: 333–338 [Google Scholar]
- Banasiak A, Zagorska-Marek B. (2006) Signals flowing from mature tissues to shoot apical meristem affect phyllotaxis in coniferous shoot. Acta Soc Bot Pol 75: 113–121 [Google Scholar]
- Baumann G, Raschke E, Bevan M, Schöffl F. (1987) Functional analysis of sequences required for transcriptional activation of a soybean heat shock gene in transgenic tobacco plants. EMBO J 6: 1161–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhlenius H, Huang T, Charbonnel-Campaa L, Brunner AM, Jansson S, Strauss SH, Nilsson O. (2006) CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science 312: 1040–1043 [DOI] [PubMed] [Google Scholar]
- Bowe LM, Coat G, dePamphilis CW. (2000) Phylogeny of seed plants based on all three genomic compartments: extant gymnosperms are monophyletic and Gnetales’ closest relatives are conifers. Proc Natl Acad Sci USA 97: 4092–4097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D, Ratcliffe O, Vincent C, Carpenter R, Coen E. (1997) Inflorescence commitment and architecture in Arabidopsis. Science 275: 80–83 [DOI] [PubMed] [Google Scholar]
- Carmona MJ, Calonje M, Martínez-Zapater JM. (2007) The FT/TFL1 gene family in grapevine. Plant Mol Biol 63: 637–650 [DOI] [PubMed] [Google Scholar]
- Chen J, Källman T, Ma X, Gyllenstrand N, Zaina G, Morgante M, Bousquet J, Eckert A, Wegrzyn J, Neale D, et al (2012) Disentangling the roles of history and local selection in shaping clinal variation of allele frequencies and gene expression in Norway spruce (Picea abies). Genetics 191: 865–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen AH, Quail PH. (1996) Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgenic Res 5: 213–218 [DOI] [PubMed] [Google Scholar]
- Clapham D, Demel P, Elfstrand M, Koop HU, Sabala I, Von Arnold S. (2000) Gene transfer by particle bombardment to embryogenic cultures of Picea abies and the production of transgenic plantlets. Scand J For Res 15: 151–160 [Google Scholar]
- Conti L, Bradley D. (2007) TERMINAL FLOWER1 is a mobile signal controlling Arabidopsis architecture. Plant Cell 19: 767–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C, et al (2007) FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316: 1030–1033 [DOI] [PubMed] [Google Scholar]
- Curtis MD, Grossniklaus U. (2003) A Gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol 133: 462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilevskaya ON, Meng X, Ananiev EV. (2010) Concerted modification of flowering time and inflorescence architecture by ectopic expression of TFL1-like genes in maize. Plant Physiol 153: 238–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekberg I, Eriksson G, Dormling I. (1979) Photoperiodic reactions in conifer species. Holarct Ecol 2: 255–263 [Google Scholar]
- Gyllenstrand N, Clapham D, Källman T, Lagercrantz U. (2007) A Norway spruce FLOWERING LOCUS T homolog is implicated in control of growth rhythm in conifers. Plant Physiol 144: 248–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanano S, Goto K. (2011) Arabidopsis TERMINAL FLOWER1 is involved in the regulation of flowering time and inflorescence development through transcriptional repression. Plant Cell 23: 3172–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanzawa Y, Money T, Bradley D. (2005) A single amino acid converts a repressor to an activator of flowering. Proc Natl Acad Sci USA 102: 7748–7753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y. (2012) Chromatin regulation of flowering. Trends Plant Sci 17: 556–562 [DOI] [PubMed] [Google Scholar]
- Hou CJ, Yang CH. (2009) Functional analysis of FT and TFL1 orthologs from orchid (Oncidium Gower Ramsey) that regulate the vegetative to reproductive transition. Plant Cell Physiol 50: 1544–1557 [DOI] [PubMed] [Google Scholar]
- Hsu CY, Adams JP, Kim HJ, No K, Ma CP, Strauss SH, Drnevich J, Vandervelde L, Ellis JD, Rice BM, et al. (2011) FLOWERING LOCUS T duplication coordinates reproductive and vegetative growth in perennial poplar. Proc Natl Acad Sci USA 108: 10756–10761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingestad T (1979) Mineral requirements of Pinus silverstris and Picea abies seedlings. Physiol Plant 45: 373–380 [Google Scholar]
- Jaeger KE, Wigge PA. (2007) FT protein acts as a long-range signal in Arabidopsis. Curr Biol 17: 1050–1054 [DOI] [PubMed] [Google Scholar]
- Karlberg A, Bako L, Bhalerao RP. (2011) Short day-mediated cessation of growth requires the downregulation of AINTEGUMENTALIKE1 transcription factor in hybrid aspen. PLoS Genet 7: e1002361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlgren A, Carlsson J, Gyllenstrand N, Lagercrantz U, Sundstrom JF. (2009) Non-radioactive in situ hybridization protocol applicable for Norway spruce and a range of plant species. J Vis Exp [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlgren A, Gyllenstrand N, Källman T, Sundström JF, Moore D, Lascoux M, Lagercrantz U. (2011) Evolution of the PEBP gene family in plants: functional diversification in seed plant evolution. Plant Physiol 156: 1967–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Ono N, Hayashi Y, Morimoto S, Nakamura S, Soda M, Kato Y, Ohnishi M, Nakano T, Inoue S, et al (2011) FLOWERING LOCUS T regulates stomatal opening. Curr Biol 21: 1232–1238 [DOI] [PubMed] [Google Scholar]
- Klintenas M, Pin PA, Benlloch R, Ingvarsson PK, Nilsson O. (2012) Analysis of conifer FLOWERING LOCUS T/TERMINAL FLOWER1-like genes provides evidence for dramatic biochemical evolution in the angiosperm FT lineage. New Phytol 196: 1260–1273 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T. (1999) A pair of related genes with antagonistic roles in mediating flowering signals. Science 286: 1960–1962 [DOI] [PubMed] [Google Scholar]
- Krasowski MJ, Owens JN. (1989) Development of the crown (nodal diaphragm) in coastal Douglas-fir seedlings. Can J Bot 67: 2473–2483 [Google Scholar]
- Lifschitz E, Eviatar T, Rozman A, Shalit A, Goldshmidt A, Amsellem Z, Alvarez JP, Eshed Y. (2006) The tomato FT ortholog triggers systemic signals that regulate growth and flowering and substitute for diverse environmental stimuli. Proc Natl Acad Sci USA 103: 6398–6403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed R, Wang CT, Ma C, Shevchenko O, Dye SJ, Puzey JR, Etherington E, Sheng XY, Meilan R, Strauss SH, et al (2010) Populus CEN/TFL1 regulates first onset of flowering, axillary meristem identity and dormancy release in Populus. Plant J 62: 674–688 [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Shimamoto K, Kyozuka J. (2002) Overexpression of RCN1 and RCN2, rice TERMINAL FLOWER 1/CENTRORADIALIS homologs, confers delay of phase transition and altered panicle morphology in rice. Plant J 29: 743–750 [DOI] [PubMed] [Google Scholar]
- Navarro C, Abelenda JA, Cruz-Oró E, Cuéllar CA, Tamaki S, Silva J, Shimamoto K, Prat S. (2011) Control of flowering and storage organ formation in potato by FLOWERING LOCUS T. Nature 478: 119–122 [DOI] [PubMed] [Google Scholar]
- Nystedt B, Street NR, Wetterbom A, Zuccolo A, Lin YC, Scofield DG, Vezzi F, Delhomme N, Giacomello S, Alexeyenko A, et al. (2013) The Norway spruce genome sequence and conifer genome evolution. Nature 497: 579–584 [DOI] [PubMed] [Google Scholar]
- Pin PA, Benlloch R, Bonnet D, Wremerth-Weich E, Kraft T, Gielen JJL, Nilsson O. (2010) An antagonistic pair of FT homologs mediates the control of flowering time in sugar beet. Science 330: 1397–1400 [DOI] [PubMed] [Google Scholar]
- Pin PA, Nilsson O. (2012) The multifaceted roles of FLOWERING LOCUS T in plant development. Plant Cell Environ 35: 1742–1755 [DOI] [PubMed] [Google Scholar]
- Pnueli L, Carmel-Goren L, Hareven D, Gutfinger T, Alvarez J, Ganal M, Zamir D, Lifschitz E. (1998) The SELF-PRUNING gene of tomato regulates vegetative to reproductive switching of sympodial meristems and is the ortholog of CEN and TFL1. Development 125: 1979–1989 [DOI] [PubMed] [Google Scholar]
- Qamaruddin M, Dormling I, Ekberg I, Eriksson G, Tillberg E. (1993) Abscisic-acid content at defined levels of bud dormancy and frost tolerance in 2 contrasting populations of Picea abies grown in a phytotron. Physiol Plant 87: 203–210 [Google Scholar]
- Qamaruddin M, Ekberg I, Dormling I, Norell L, Clapham D, Eriksson G. (1995) Early effects of long nights on budset, bud dormancy and abscisic acid content in two populations of Picea abies. For Genet 2: 207–216 [Google Scholar]
- Ratcliffe OJ, Amaya I, Vincent CA, Rothstein S, Carpenter R, Coen ES, Bradley DJ. (1998) A common mechanism controls the life cycle and architecture of plants. Development 125: 1609–1615 [DOI] [PubMed] [Google Scholar]
- Repinski SL, Kwak M, Gepts P. (2012) The common bean growth habit gene PvTFL1y is a functional homolog of Arabidopsis TFL1. Theor Appl Genet 124: 1539–1547 [DOI] [PubMed] [Google Scholar]
- Rinne PL, Kaikuranta PM, van der Schoot C. (2001) The shoot apical meristem restores its symplasmic organization during chilling-induced release from dormancy. Plant J 26: 249–264 [DOI] [PubMed] [Google Scholar]
- Rohde A, Bhalerao RP. (2007) Plant dormancy in the perennial context. Trends Plant Sci 12: 217–223 [DOI] [PubMed] [Google Scholar]
- Saidi Y, Finka A, Chakhporanian M, Zrÿd JP, Schaefer DG, Goloubinoff P. (2005) Controlled expression of recombinant proteins in Physcomitrella patens by a conditional heat-shock promoter: a tool for plant research and biotechnology. Plant Mol Biol 59: 697–711 [DOI] [PubMed] [Google Scholar]
- Shannon S, Meeks-Wagner DR. (1991) A mutation in the Arabidopsis TFL1 gene affects inflorescence meristem development. Plant Cell 3: 877–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn EJ, Rojas-Pierce M, Pan S, Carter C, Serrano-Mislata A, Madueño F, Rojo E, Surpin M, Raikhel NV. (2007) The shoot meristem identity gene TFL1 is involved in flower development and trafficking to the protein storage vacuole. Proc Natl Acad Sci USA 104: 18801–18806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström JF (2001) Evolution of genetic mechanism regulating reproductive development in plants. PhD thesis. Uppsala University, Acta Universitatis Upsaliensis, Uppsala, Sweden
- Takada S, Goto K. (2003) TERMINAL FLOWER2, an Arabidopsis homolog of HETEROCHROMATIN PROTEIN1, counteracts the activation of FLOWERING LOCUS T by CONSTANS in the vascular tissues of leaves to regulate flowering time. Plant Cell 15: 2856–2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torti S, Fornara F, Vincent C, Andrés F, Nordström K, Göbel U, Knoll D, Schoof H, Coupland G. (2012) Analysis of the Arabidopsis shoot meristem transcriptome during floral transition identifies distinct regulatory patterns and a leucine-rich repeat protein that promotes flowering. Plant Cell 24: 444–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaftaris A, Pasentsis K, Kalivas A, Michailidou S, Madesis P, Argiriou A. (2012) Isolation of a CENTRORADIALIS/TERMINAL FLOWER1 homolog in saffron (Crocus sativus L.): characterization and expression analysis. Mol Biol Rep 39: 7899–7910 [DOI] [PubMed] [Google Scholar]
- Tsuji H, Nakamura H, Taoka K, Shimamoto K. (2013) Functional diversification of FD transcription factors in rice, components of florigen activation complexes. Plant Cell Physiol 54: 385–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Arnold S, Clapham D. (2008) Spruce embryogenesis. Methods Mol Biol 427: 31–47 [DOI] [PubMed] [Google Scholar]
- Wang R, Albani MC, Vincent C, Bergonzi S, Luan M, Bai Y, Kiefer C, Castillo R, Coupland G. (2011) Aa TFL1 confers an age-dependent response to vernalization in perennial Arabis alpina. Plant Cell 23: 1307–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chen B, Hu Y, Li J, Lin Z. (2005) Inducible excision of selectable marker gene from transgenic plants by the cre/lox site-specific recombination system. Transgenic Res 14: 605–614 [DOI] [PubMed] [Google Scholar]
- Wigge PA, Kim MC, Jaeger KE, Busch W, Schmid M, Lohmann JU, Weigel D. (2005) Integration of spatial and temporal information during floral induction in Arabidopsis. Science 309: 1056–1059 [DOI] [PubMed] [Google Scholar]