A red/far-red light photoreceptor controls flowering time in barley by stimulating a flowering promoter gene in the most downstream part of the genetic pathways for flowering.
Abstract
The spring-type near isogenic line (NIL) of the winter-type barley (Hordeum vulgare ssp. vulgare) var. Hayakiso 2 (HK2) was developed by introducing VERNALIZATION-H1 (Vrn-H1) for spring growth habit from the spring-type var. Indo Omugi. Contrary to expectations, the spring-type NIL flowered later than winter-type HK2. This phenotypic difference was controlled by a single gene, which cosegregated only with phytochrome C (HvPhyC) among three candidates around the Vrn-H1 region (Vrn-H1, HvPhyC, and CASEIN KINASE IIα), indicating that HvPhyC was the most likely candidate gene. Compared with the late-flowering allele HvPhyC-l from the NIL, the early-flowering allele HvPhyC-e from HK2 had a single nucleotide polymorphism T1139C in exon 1, which caused a nonsynonymous amino acid substitution of phenylalanine at position 380 by serine in the functionally essential GAF (3′, 5′-cyclic-GMP phosphodiesterase, adenylate cyclase, formate hydrogen lyase activator protein) domain. Functional assay using a rice (Oryza sativa) phyA phyC double mutant line showed that both of the HvPhyC alleles are functional, but HvPhyC-e may have a hyperfunction. Expression analysis using NILs carrying HvPhyC-e and HvPhyC-l (NIL [HvPhyC-e] and NIL [HvPhyC-l], respectively) showed that HvPhyC-e up-regulated only the flowering promoter FLOWERING LOCUS T1 by bypassing the circadian clock genes and flowering integrator CONSTANS1 under a long photoperiod. Consistent with the up-regulation, NIL (HvPhyC-e) flowered earlier than NIL (HvPhyC-l) under long photoperiods. These results implied that HvPhyC is a key factor to control long-day flowering directly.
The flowering time of barley (Hordeum vulgare ssp. vulgare) is a complex character that is composed of three different subcharacters: earliness per se, vernalization requirement, and photoperiod sensitivity (Takahashi and Yasuda, 1970). The first character determines flowering time alone, while the latter two modify it in response to environmental signals including temperature and photoperiod. These are essential for successful seed set and maximizing yield by contributing greatly to adaptation to different climatic regions (Knüpffer et al., 2003; von Bothmer et al., 2003).
In winter-type barley, the vernalization requirement avoids frost injury by delaying flower induction until the extended period of cold temperature during winter satisfies the requirement. On the other hand, spring-type barley does not have such a requirement. The vernalization requirement is controlled by three major genes: VERNALIZATION-H1 (Vrn-H1), Vrn-H2, and Vrn-H3 (former SPRING GROWTH HABIT2 [Sgh2], Sgh1, and Sgh3, respectively). Vrn-H1 encodes a protein highly similar to Arabidopsis (Arabidopsis thaliana) APETALA1 (AP1)/FRUITFULL (FUL) that determines meristem identity, flower organ formation, and flowering time (Yan et al., 2003). Vrn-H2 encodes ZCCT protein with a putative zinc finger and a CCT (CONSTANS, CONSTANS-LIKE, and TIMING OF CHLOROPHYLL A/B BINDING PROTEIN EXPRESSION1) domain, which is expected to be involved in transcriptional regulation and is expressed under long photoperiods (Yan et al., 2004). Vrn-H3, which encodes an ortholog of Arabidopsis flowering promoter FLOWERING LOCUS T (FT) and long photoperiods up-regulate Vrn3 expression (Turner et al., 2005; Yan et al., 2006). It was proposed that they form a feedback loop and interact to regulate their expression (Trevaskis et al., 2007; Distelfeld et al., 2009; Shimada et al., 2009).
Barley is a long-day plant in which photoperiod sensitivity delays flowering time under a short photoperiod compared with that under a long photoperiod. It is well known that photoperiod sensitivity greatly contributes to adaptation (Knüpffer et al., 2003). Two genes that influence photoperiod sensitivity are PHOTOPERIOD-H1 (Ppd-H1) and Ppd-H2 (Laurie et al., 1995). Ppd-H1 controls flowering time under long photoperiods and encodes pseudoresponse regulator (PRR) whose ortholog is involved in circadian clock function in Arabidopsis (Turner et al., 2005). Ppd-H2 controls flowering time under short photoperiods, and it encodes HvFT3, which belongs to the gene family of FT (Kikuchi et al., 2009).
In addition to the above-mentioned genes, novel gene resources for early flowering will be important to elucidate the genetic mechanism of the flowering time and future breeding programs. Recent comparative studies in genetic pathways for flowering revealed that temperate grass species share a similar gene set with dicot species Arabidopsis, especially for photoperiodic pathways, although it has been disclosed gradually that evolutionary distinct genes and pathways are associated with the photoperiodic pathways (Trevaskis et al., 2007; Higgins et al., 2010). These pathways include photoreceptors (phytochromes, cryptochromes, and phototropin) that perceive daily light/dark cycles, the circadian clock (CIRCADIAN CLOCK ASSOCIATED1, TIMING OF CHLOROPHYLL A/B-BINDING PROTEIN EXPRESSION1, PRRs [such as Ppd-H1], and GIGANTEA [GI; HvGI in barley]), which is entrained by the signals from photoreceptors, and downstream genes (CONSTANS [CO; HvCO1 in barley], FT [HvFT1 in barley], and AP1/FUL [Vrn-H1]), which integrate and transmit the signals from photoreceptors and the circadian clock. However, natural variation in many of such genes has not been characterized yet in barley.
Yasuda (1969) developed a series of near isogenic lines (NILs) carrying different spring alleles Sgh2 (Vrn-H1) in a Japanese winter var. Hayakiso 2 (HK2) that had a winter allele sgh2 (vrn-H1) originally. These NILs, compared with HK2, showed unexpectedly later flowering under autumn-sowing field conditions irrespective of their spring growth habit. Such behavior may be ascribable to the pleiotropic effect of Vrn-H1 or an unknown flowering-time gene tightly linked with Vrn-H1. According to previous studies (Szücs et al., 2006; Kato et al., 2008), Vrn-H1 is located closely to other two candidate genes for photoperiod sensitivity, Phytochrome C (HvPhyC) and Casein Kinase II alpha (HvCK2α). HvPhyC encodes the apoprotein of photoreceptor PHYC, which is involved in red/far-red light perception. PhyC orthologs in other species are also associated with flowering time: the rice (Oryza sativa) ortholog controls photoperiod sensitivity (Takano et al., 2005) and Arabidopsis alleles showed latitudinal cline, which suggested the association of photoperiod sensitivity (Balasubramanian et al., 2006). HvCK2α encodes the α subunit of CK2 protein. A rice flowering-time gene, Heading date6, which is considered to be an ortholog of HvCK2α by cross-referencing syntheny among barley, wheat (Triticum aestivum), and rice, controls photoperiod sensitivity (Takahashi et al., 2001; Ogiso et al., 2010; Kato et al., 2002, 2008).
In this study, we first conducted genetic and linkage analyses to identify a causal gene for early flowering in HK2. Secondly, the gene effect was evaluated by growing the NILs under different photoperiods. Third, functional assay using a rice transformation system was conducted to evaluate the functionality of the flowering-time gene. Finally, based on the results of expression analysis, the role of the flowering-time gene in the genetic pathways for flowering was discussed.
RESULTS
Genetic and Linkage Analyses
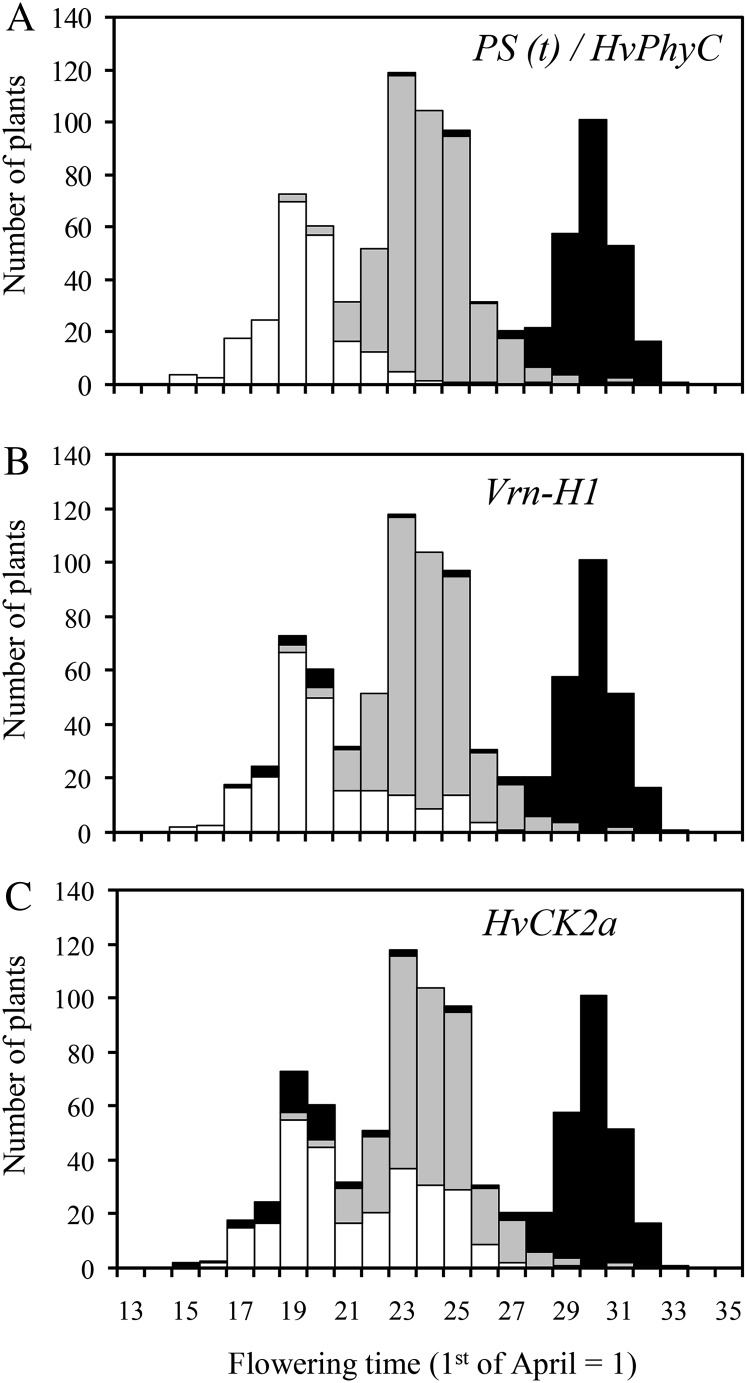
HK2 was crossed with a late-flowering NIL carrying the spring allele Vrn-H1 from var. Indo Omugi (hereafter, NIL [Vrn-H1]). Frequency distribution for flowering time in the F2 population was continuous but trimodal, suggesting the segregation of early, intermediate, and late types caused by a single gene (Fig. 1). For accurate genotyping, a progeny test was conducted using F3 lines derived from F2 plants with flowering time within the overlap regions between different types. As a result, the F2 population was found to segregate 217 early, 446 intermediate, and 229 late types, which fit the ratio of 1:2:1 (χ2 = 0.323, P = 0.851) for single gene segregation. Hereafter, the gene was designated tentatively as PS (t).
Figure 1.
Frequency distribution of flowering time in the F2 population derived from a cross between HK2 and its NIL carrying the spring allele for Vrn-H1 (NIL [Vrn-H1]) under field conditions. The 892 F2 plants could be classified into three types by the genotype of a causal gene for flowering time (PS [t]; A) as well as its candidate genes HvPhyC (A), Vrn-H1 (B), and HvCK2α (C). White, gray, and black rectangles indicate homozygote for HK2 allele, heterozygote, and homozygote for NIL (Vrn-H1) allele.
Linkage analysis using the same population showed that HvPhyC was linked by Vrn-H1 and HvCK2α with genetic distances 1.5 and 3.1 centimorgans, respectively, and the gene order was estimated to be Vrn-H1 – HvPhyC – HvCK2α. Among these three genes, HvPhyC cosegregated with PS (t) (Fig. 1A), strongly suggesting that it is the causal gene for PS (t). By contrast, several critical recombinations were observed between Vrn-H1 and PS (t) and between HvCK2α and PS (t) (Fig. 1, B and C). From this result, Vrn-H1 and HvCK2α could be ruled out as candidates. Hereafter, we designate the early-flowering (HK2) and late-flowering (NIL [Vrn-H1]) alleles for the flowering-time gene (HvPhyC) as HvPhyC-e and HvPhyC-l, respectively.
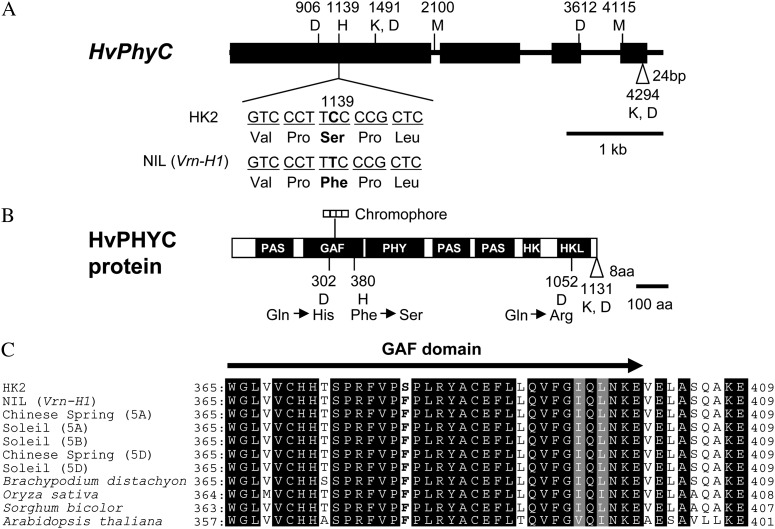
Sequence analysis of HvPhyC alleles revealed a single nucleotide polymorphism (SNP) in exon 1 (at the position 1,139 from the start codon) that causes nonsynonymous substitution at the C-terminal side of the GAF (3′, 5′-cyclic-GMP phosphodiesterase, adenylate cyclase, formate hydrogen lyase activator protein) domain (at position 380) in the deduced amino acid sequence (Fig. 2, A and B). The deduced amino acid residue from HvPhyC-l had Phe at this position, which was well conserved among several plant species (wheat, rice, sorghum [Sorghum bicolor], Brachypodium distachyon, and Arabidopsis), while that from HvPhyC-e had Ser, suggesting it to be a mutant allele (Fig. 2C).
Figure 2.
Structure of HvPhyC gene and its protein. A, HvPhyC gene sequences from HK2 and NIL (Vrn-H1) were compared with those from var. Morex (DQ238106), var. Dicktoo (DQ201145), and var. Kompolti Korai (DQ201146). Black boxes and horizontal lines between them indicate exons and introns, respectively. Vertical line indicates SNP when NIL (Vrn-H1) was compared with other varieties. White triangle indicates a 24-bp insertion. NIL (Vrn-H1), HK2, and Morex have this insertion, which results in an 8-amino acid insertion. Numbers and letters at polymorphic sites indicate the position and polymorphic varieties, respectively. H, M, D, and K indicate HK2, Morex, Dicktoo, and Kompolti Korai, respectively. B, Deduced amino acid sequences were compared based on the HvPhyC gene sequences. Black boxes indicate domains. Polymorphisms are shown in the same way as HvPhyC gene. HK, His kinase domain; HKL, His kinase-like ATPase domain. C, Deduced amino acid sequences around the C-terminal end of GAF domain were compared based on the genomic sequences of barley, wheat (GenBank: AY672995.1, AY673002.1, AY672998.1, AY672999.1, and AY673000.1), B. distachyon (Gbrowse: Bradi1g08400.1), rice (RAP-DB: Os03g0752100-01), and sorghum (GDB: Sb01g007850.1). The same and similar sequences are highlighted in black and gray, respectively.
Effect of HvPhyC on Flowering Time under Different Photoperiods
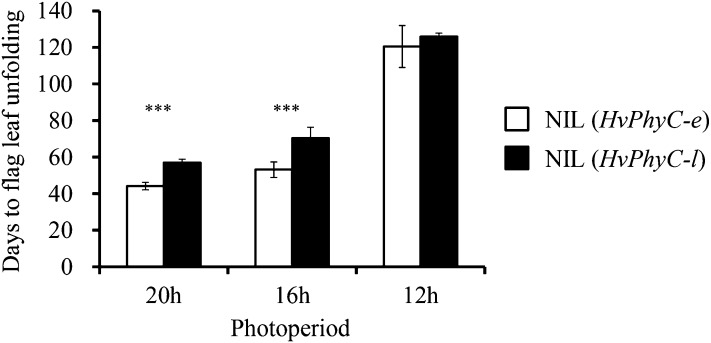
Each two independent NILs carrying HvPhyC-e and HvPhyC-l (four NILs) were selected out of the F4 progenies of the mapping population (Table I). All of these NILs have the same genotype for the other flowering-time genes, because the alleles from the NIL (Vrn-H1) were selected for Vrn-H1 and HvCK2α, which segregated in the mapping population. Each two NILs carrying HvPhyC-e and HvPhyC-l were mixed together and designated as NIL (HvPhyC-e) and NIL (HvPhyC-l), respectively, because the same genotype NILs showed statistically the same flowering time. Under long (16-h) and extremely long (20-h) photoperiods, NIL (HvPhyC-e) flowered much earlier than NIL (HvPhyC-l) (22.5% and 24.4% reduction in days from sowing to flag leaf unfolding, respectively; Fig. 3). By contrast, no significant difference (only 4.3% reduction) was observed under a short (12-h) photoperiod. The result showed that HvPhyC controls photoperiod sensitivity under long photoperiods.
Table I. Genotype for flowering-time genes in HK2 and its NILs determined by diagnostic markers.
| Photoperiod Sensitivity |
Vernalization Requirement |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variety/Line | Flowering Time | HvPhyC | HvCK2αa | Ppd-H1b | Ppd-H2c | Vrn-H1d | Vrn-H2d | Vrn-H3d | Growth Habit |
| HK2 | Early | HvPhyC-e | H | Ppd-H1 | Ppd-H2 | vrn-H1 | Vrn-H2 | vrn-H3 | Winter |
| NIL (Vrn-H1) | Late | HvPhyC-l | N | Ppd-H1 | Ppd-H2 | Vrn-H1 | Vrn-H2 | vrn-H3 | Spring |
| NIL (HvPhyC-l) | Late | HvPhyC-l | N | Ppd-H1 | Ppd-H2 | Vrn-H1 | Vrn-H2 | vrn-H3 | Spring |
| NIL (HvphyC-e) | Early | HvphyC-e | N | Ppd-H1 | Ppd-H2 | Vrn-H1 | Vrn-H2 | vrn-H3 | Spring |
H and N indicate the alleles from HK2 and NIL (Vrn-H1), respectively. bRecessive allele for Ppd-H1 confers late flowering under long photoperiod. cDominant allele for Ppd-H2 confers early flowering under short photoperiod. dDominant allele for Vrn-H1 (identical with HvVRN1-10 in Hemming et al. [2009]), recessive allele for Vrn-H2, and dominant allele for Vrn-H3 confer spring growth habit.
Figure 3.
Photoperiodic response of the NILs carrying different HvPhyC alleles. Days from sowing to flag leaf unfolding (parallel with flowering time) of NIL (HvPhyC-e) and NIL (HvPhyC-l) were compared under three different (20-h, 16-h, and 8-h) photoperiod conditions. Triple asterisks indicate that the difference between the NILs is statistically significant (P < 0.001).
Functional Assay of HvPhyC Using Rice Transformation System
To evaluate the function of HvPhyC-e from HK2 and HvPhyC-l from NIL (Vrn-H1), we introduced these two alleles under the control of the Cauliflower mosaic virus 35S promoter into a rice phyA phyC double mutant line as the recipient with a genetic background of the Japanese var. Nipponbare, because the phyA phyC double mutant line flowers significantly earlier than the original var. Nipponbare under a natural (long) photoperiod (Takano et al., 2005).
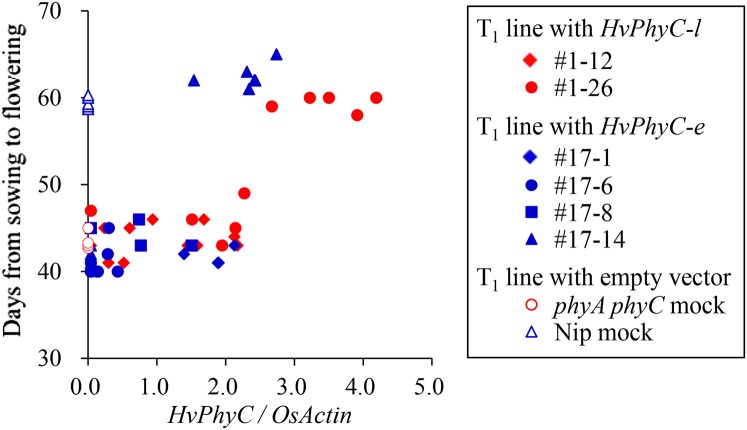
The T1 control lines carrying the empty vector in Nipponbare and phyA phyC double mutant line flowered 59.6 and 45.6 d after sowing under a natural (long) photoperiod, respectively, confirming the effect of PhyC and PhyA genes on flowering time under a long photoperiod (Fig. 4).
Figure 4.
Functional assay of HvPhyC in rice by introducing different HvPhyC alleles (T1 generation). Mutant allele (HvPhyC-e; red-filled marks) and wild-type allele (HvPhyC-l; blue-filled marks) were introduced into rice phyA phyC double mutant lines with a var. Nipponbare genetic background via the A. tumefaciens-mediated transformation method. As the control, empty vector was introduced into phyA phyC double mutant (phyA phyC mock; open red circle) and var. Nipponbare (Nip mock; open blue triangle). All of the T1 lines were grown under natural (long) photoperiod conditions in a greenhouse. Expression level of HvPhyC was analyzed by semiquantitative RT-PCR using OsActin as the internal control.
One (no. 1-26) out of two T1 lines carrying HvPhyC-l (wild-type allele) segregated late-flowering (Nip mock-type) and early-flowering (phyA phyC mock-type) plants. All of the late-flowering plants expressed HvPhyC-l at a high level, while early-flowering plants expressed HvPhyC-l at a low level (Fig. 4). The other T1 line (no. 1-12) was composed of only early-flowering plants that expressed HvPhyC-l at a low level. Therefore, it was confirmed that high enough expression of HvPhyC-l can recover from the phyA phyC double mutant phenotype.
One (no. 17-14) out of four T1 lines carrying HvPhyC-e (mutant allele) segregated late-flowering plants, all of which expressed HvPhyC-e at a high level, while the others (nos. 17-1, 17-6, and 17-8) were composed of only early-flowering plants (Fig. 4). To our surprise, this result strongly suggested that the HvPhyC-l and HvPhyC-e alleles are both functional. However, even a lower expression level of HvPhyC-e, compared with HvPhyC-l, could recover from the phyA phyC double mutant phenotype, suggesting the hyperfunction of HvPhyC-e.
Expression Analysis of the Flowering Pathway Genes
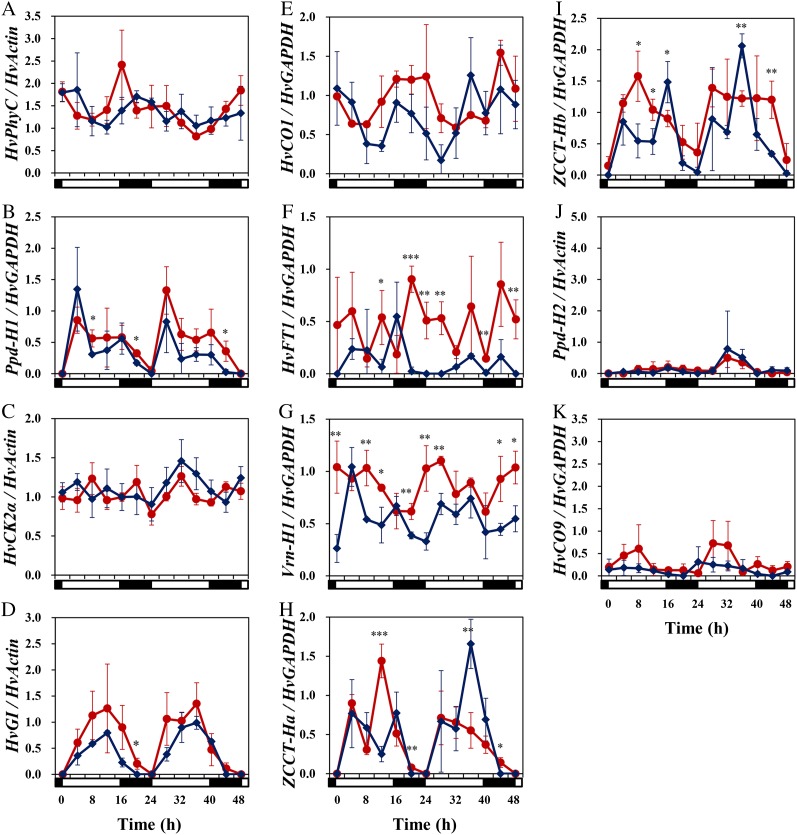
There were no apparent differences in HvPhyC expression between NIL (HvPhyC-e) and NIL (HvPhyC-l), despite their allelic differences (Fig. 5A; Supplemental Fig. S1A). In both of the NILs, HvPhyC was expressed all day and seemed to show diurnal fluctuation under both long (16-h) and short (8-h) photoperiod conditions with the trend that it was up-regulated around dusk and down-regulated during the day. This result led to the hypothesis that the SNP (T1139C) in the GAF domain might affect its protein function rather than its own expression pattern and also the expression pattern of downstream genes interacting with HvPHYC protein.
Figure 5.
Expression pattern of flowering-time genes under a long (16-h) photoperiod. Red circles and blue diamonds indicate NIL (HvPhyC-e) and NIL (HvPhyC-l), respectively. Expression level is the average of band intensity obtained by semiquantitative RT-PCR from three biological replications. White and black horizontal bars below each graph indicate light and dark conditions, respectively. Vertical bar indicates sd. Asterisks above plots indicate that the expression level was significantly different between two lines when they were compared at the same time points. Single, double, and triple asterisks indicate significance at P = 0.05, 0.01, and 0.001, respectively.
Subsequently, we compared the expression of circadian clock-related genes HvCK2α, HvGI, and Ppd-H1, because the circadian clock is considered to interact with photoreceptors. Unexpectedly, two NILs showed similar expression patterns for all three genes (Fig. 5, B–D; Supplemental Fig. S1, B–D). HvCK2α was expressed all day and did not show apparent diurnal fluctuation under both long and short photoperiod conditions. HvGI and Ppd-H1 were up-regulated during the day and down-regulated during the night under both long and short photoperiod conditions, consistent with previous studies (Dunford et al., 2005; Turner et al., 2005). Therefore, it was suggested that HvPhyC does not affect circadian clock-related genes but affects more downstream genes of both photoreceptors and the circadian clock.
HvCO1, which integrates signals from the circadian clock and photoreceptors, showed diurnal expression patterns under long and short photoperiod conditions (Fig. 5E; Supplemental Fig. S1E), consistent with previous studies (Turner et al., 2005; Campoli et al., 2012), although there were no clear differences between the two NILs. On the other hand, clear-cut differences were detected for HvFT1 (Fig. 5F), which is expected to be the direct target of HvCO1 protein and acts as the flowering promoter “florigen” (HvFT1 is also known as the vernalization requirement gene Vrn-H3). In the late-flowering NIL (HvPhyC-l), HvFT1 showed a diurnal expression pattern that had a small peak early in the day and a large peak around dusk under long photoperiod conditions, which was consistent with Turner et al. (2005) and Campoli et al. (2012). In the early-flowering NIL (HvPhyC-e), HvFT1 also showed diurnal expression, but small and large peaks appeared slightly later (by 4 h) than NIL (HvPhyC-l). Furthermore, NIL (HvPhyC-e) expression of HvFT1 was considerably higher than in NIL (HvPhyC-l). This result was expected from several previous studies showing that the early-flowering line expressed HvFT1 at a higher level (Turner et al., 2005; Campoli et al., 2012; Faure et al., 2012). Under short photoperiod conditions, HvFT1 was not expressed in either NIL (Supplemental Fig. S1F), because the photoperiod was noninductive and NILs were still in the vegetative growth stage at the sampling time (15 d after sowing).
Another vernalization requirement gene Vrn-H1 showed a diurnal expression pattern in both NILs under long and short photoperiod conditions (Fig. 5G; Supplemental Fig. S1G), which was consistent with Campoli et al. (2012). Under long photoperiod conditions, the expression level was slightly higher in NIL (HvPhyC-e) than in NIL (HvPhyC-l), while no such difference was observed under short photoperiod conditions. To confirm the difference under long photoperiod conditions, expression analysis with real-time reverse transcription (RT)-PCR was conducted (Supplemental Materials and Methods S1). As a result, a similar trend was observed as semiquantitative RT-PCR, but the differences were not significant at most of the time points (Supplemental Fig. S2). Vrn-H2 locus deploys two family genes, ZCCT-Ha and ZCCT-Hb, both of which were expressed during the day and had two peaks early in the day and around dusk under long photoperiod conditions, while they were not expressed under short photoperiod conditions (Fig. 5, H and I; Supplemental Fig. S1, H and I). Their expression patterns were consistent with Trevaskis et al. (2006). There was no apparent difference in expression pattern between the NILs for both ZCCT-H genes.
We analyzed the expression of other photoperiodic response genes, Ppd-H2 (HvFT3) and HvCO9, which control flowering time under short photoperiods (Kikuchi et al., 2009, 2012). There were no clear differences in either gene between the NILs (Fig. 5, J and K; Supplemental Fig. S1, J and K). Ppd-H2 showed a diurnal expression pattern under short photoperiod conditions and much lower expression under long photoperiod conditions, which were consistent with a previous study (Kikuchi et al., 2009). HvCO9 also showed a diurnal expression pattern under long and short photoperiod conditions and was up-regulated during the day. The expression level was higher under short than long photoperiod conditions. This was consistent with the observation by Kikuchi et al. (2012).
Epistatic Interaction between HvPhyC and Vrn-H3
Flowering times under a field condition were compared among HK2 NILs developed in this study and by Yasuda (1969). NILs carrying a winter allele vrn-H3 flowered on April 27.4 on average, while those carrying a spring allele Vrn-H3 flowered on April 16.3 on average (Table II). Within the group of NILs carrying vrn-H3, the NILs with HvPhyC-e flowered earlier than those with HvPhyC-l by 1 week. Contrarily, flowering times were not different between the NILs with HvPhyC-e and HvPhyC-l, all of which have Vrn-H3. The result strongly suggested that Vrn-H3 is epistatic to HvPhyC.
Table II. Flowering time of HK2 NILs carrying different alleles for HvPhyC, Vrn-H1, and Vrn-H3.
| Genotypea |
|||||
|---|---|---|---|---|---|
| Line | HvPhyC | Vrn-H1b | Vrn-H3 | HvCK2αc | Flowering Time ± sdd |
| HK2 | HvPhyC-e | vrn-H1 | vrn-H3 | H | 24.6 ± 1.1p |
| NIL (HvPhyC-e) | HvPhyC-e | Vrn-H1 | vrn-H3 | N | 21.1 ± 1.3q |
| NIL (HvPhyC-l) | HvPhyC-l | Vrn-H1 | vrn-H3 | N | 30.0 ± 0.9r |
| NIL (Vrn-H1) | HvPhyC-l | Vrn-H1 | vrn-H3 | N | 33.8 ± 1.1s |
| NIL (Vrn-H3) | HvPhyC-e | vrn-H1 | Vrn-H3 | H | 16.4 ± 1.8t |
| NIL (Vrn-H1, Vrn-H3) | HvPhyC-l | Vrn-H1 | Vrn-H3 | N | 16.2 ± 1.3t |
All lines carry Vrn-H2 (winter allele), Ppd-H1(early-flowering allele), and Ppd-H2 (early-flowering allele) in common. bAll the dominant alleles for Vrn-H1 are identical with HvVRN1-10 (Hemming et al., 2009). They were derived from the var. Indo Omugi except for the allele in NIL (Vrn-H1, Vrn-H3) that was derived from the Japanese var. Marumi 16. cH and N indicate the alleles from HK2 and NIL (Vrn-H1), respectively. dApril 1 = 1. Values with different letters (p, q, r, s, or t) indicate significant difference (P < 0.01) by the Tukey test.
Haplotype Analysis for HvPhyC and Vrn-H1
By using diagnostic markers (Supplemental Table S1), haplotype for HvPhyC and Vrn-H1 was determined in Japanese varieties (Table III). Among 12 varieties, four had the same haplotype with HK2 that deploys HvPhyC-e and vrn-H1 (identical with HvVRN1 in Hemming et al. [2009]), while three had the same haplotype with NIL (Vrn-H1) that deploys HvPhyC-l and Vrn-H1 (identical with HvVRN1-10 in Hemming et al. [2009]). Other five varieties carried a recombinant type that was comprised of HvPhyC-l and vrn-H1.
Table III. Haplotypes for HvPhyC and Vrn-H1 in 12 Japanese varieties.
| Haplotype |
|||
|---|---|---|---|
| Variety | HvPhyC | Vrn-H1a | Growth Habit |
| Ishuku Shirazu | HvPhyC-e | vrn-H1 | Spring |
| Ichibanboshi | HvPhyC-e | vrn-H1 | Winter |
| Kawasaigoku | HvPhyC-e | vrn-H1 | Spring |
| Haruna Nijo | HvPhyC-e | vrn-H1 | Spring |
| Silky Snow | HvPhyC-l | vrn-H1 | Winter |
| Kirarimochi | HvPhyC-l | vrn-H1 | Spring |
| Sayakaze | HvPhyC-l | vrn-H1 | Spring |
| Suzukaze | HvPhyC-l | vrn-H1 | Spring |
| Shunrai | HvPhyC-l | vrn-H1 | Spring |
| Hozoroi | HvPhyC-l | Vrn-H1 | Spring |
| Marumi 16 | HvPhyC-l | Vrn-H1 | Spring |
| Kinai 5 | HvPhyC-l | Vrn-H1 | Spring |
vrn-H1 and Vrn-H1 are identical with HvVRN1 and HvVRN1-10 (Hemming et al., 2009), respectively.
DISCUSSION
There have been several studies on phytochromes in model plant species such as rice and Arabidopsis using natural variations and mutants (Takano et al., 2005; Franklin and Quail, 2010). Such studies proved their importance in development and physiological responses, including the control of flowering time. By contrast, in barley, little is known about the effects of phytochromes, although it was suggested that one of the phytochrome genes, HvPhyC, might be involved in photoperiod sensitivity through the detection of a photoperiod sensitivity quantitative trait loci around the HvPhyC region (Szücs et al., 2006). In this study, we successfully identified the natural variation for HvPhyC that controlled flowering time under long photoperiods by affecting the expression of flowering-time genes downstream of HvPhyC.
In linkage analysis, the flowering-time gene PS (t) cosegregated only with HvPhyC among three candidate genes. Therefore, HvPhyC was considered to be the most likely candidate gene for the difference in flowering time between HK2 and NIL (Vrn-H1). According to Szücs et al. (2006), barley has a HvPhyC pseudogene in addition to the intact gene. They mapped the pseudogene at the same position as Vrn-H1, which is distinct from the intact HvPhyC by genetic mapping (Szücs et al., 2006). Furthermore, physical mapping located the pseudogene next to Vrn-H1 (Yan et al., 2005; Szücs et al., 2006). Because of several recombinations between PS (t) and Vrn-H1 in our linkage analysis, PS (t) was considered to be distinct from the pseudogene. Szücs et al. (2006) also showed that the pseudogene was separated into two segments (remnants) by a large (17-kb) insertion, including retro-elements and miniature inverted-repeat transposable elements. Successful PCR amplification of the pseudogene in both HK2 and NIL (Vrn-H1) using the primers specific to the insertion and the genomic regions on opposite sides of the insertion across the pseudogene segments (Szücs et al., 2006) implied that both lines carry the pseudogene, which was nonfunctional (Supplemental Fig. S3). Therefore, it was concluded that the pseudogene is not the cause of the flowering-time difference between HK2 and NIL (Vrn-H1).
Another flowering-related gene AGAMOUS-LIKE GENE1 (AGLG1), encoding a grass-specific SEPALLATA-like MADS-box protein, was expected to exist around Vrn-H1 region because of the syntheny between barley and einkorn wheat (Triticum monococcum) genomes (Yan et al., 2005). Although little is known about AGLG1 function in barley and wheat, a rice ortholog PANICLE PHYTOMER2 (PAP2) has been characterized in detail (Gao et al., 2010; Kobayashi et al., 2010, 2012). PAP2 protein is involved in inflorescence meristem identity by forming protein complex with three AP1/FUL-like MADS-box proteins, OsMADS14, OsMADS15, and OsMADS18 (Kobayashi et al., 2012). Quadruple knockdown plants showed severely affected phenotype, including discordant transition from vegetative phase to reproductive phase that resulted in delayed flowering and impaired inflorescence development. In addition, PAP2 alone functions as a positive regulator of spikelet meristem identity and a suppressor of the extra elongation of glumes (Gao et al., 2010; Kobayashi et al., 2010). Contrary to the quadruple knockdown plants, pap2 single mutant showed milder phenotype, including increase in number of primary branch of rachis and elongation of sterile lemma and rudimentary glume, and did not affect flowering time (Gao et al., 2010; Kobayashi et al., 2010). From these results, AGLG1 single mutation in barley was assumed to affect spike and spikelet morphology rather than flowering time. However, we did not find any differences in spike and spikelet morphology between HK2 and NIL (Vrn-H1). Therefore, AGLG1 was ruled out from the candidate genes for PS (t).
By linkage analysis, the gene order was estimated to be Vrn-H1 – HvPhyC – HvCK2α, which was supported by the barley EST map (CMap for Barley EST; http://map.lab.nig.ac.jp:8085/cmap; Sato et al., 2009) constructed using the double haploid (DH) population of a Japanese var. Haruna Nijo and the Hordeum vulgare ssp. spontaneum line H602. However, order Vrn-H1 – HvPhyC – HvCK2α from proximal to distal on chromosome 5HL was inconsistent with previous reports. Szücs et al. (2006) mapped HvPhyC on the proximal side of Vrn-H1 using the Dicktoo × Morex DH mapping population. HvCK2α was mapped to a more distal region on 5HL using the Steptoe × Morex DH population (Kato et al., 2008). Taken together, their results suggested that the order is HvPhyC – Vrn-H1 – HvCK2α from proximal to distal on 5HL. This order is consistent with that in the corresponding regions of wheat (Kato et al., 2002; Yan et al., 2003), B. distachyon (Gbrowse; http://www.phytozome.net/cgi-bin/gbrowse/brachy/), rice (Rice Annotation Project Database; http://rapdb.dna.affrc.go.jp), and sorghum (Sorghum bicolor Genome Database; http://www.plantgdb.org/XGDB/phplib/resource.php?GDB=Sb), showing that this gene order is prevalent in grass species. Therefore, it was considered that the chromosomal rearrangement in this region might be shared in some barley varieties, including the Japanese var. HK2 and var. Haruna Nijo, the Taiwanese var. Indo Omugi, and H. vulgare ssp. spontaneum line H602.
A “supergene” is formed by a group of cosegregating genes whose allelic combinations (haplotypes) facilitate integrated control of adaptive phenotypes. “Supergenes” have been described in various species, e.g. S locus controlling heterostyly and self-incompatibility in Primula species (Primula sinensis; Mather, 1950) and P locus controlling wing color pattern in mimetic butterflies (Clarke et al., 1968; Brown and Benson, 1974; Nijhout, 2003). In a mimetic butterfly, Heliconius numata, chromosomal rearrangements (inversions) around the P locus prevented recombination within this supergene locus, and inversions that formed specific haplotypes were completely associated with corresponding wing color patterns (Joron et al., 2011). In this study, formation of a “supergene” was expected in chromosome 5HL, where four flowering-related genes (Vrn-H1, HvPhyC, HvCK2α, and HvAGLG1) are clustered and closely linked to each other. Especially for the inversion including the Vrn-H1 – HvPhyC region might played evolutionary and adaptive roles by establishing specific haplotypes (vrn-H1/HvPhyC-e [HK2 type] and Vrn-H1/HvPhyC-l [NIL {Vrn-H1} type]) that could control vernalization requirement and photoperiod sensitivity at the same time. Contrary to expectation, our preliminary analysis using Japanese varieties showed that there was a recombinant type (vrn-H1/HvPhyC-l) in addition to the above two types (Table III). To clarify the evolutionary and adaptive roles of Vrn-H1 and HvPhyC as well as other flowering-related genes, worldwide landrace collection needs to be analyzed comprehensively.
To conduct functional assay of HvPhyC, we adopted the Agrobacterium tumefaciens-mediated transformation system of rice. The result showed that even the mutant allele (HvPhyC-e) is functional and may have a hyperfunction compared with the wild-type allele (HvPhyC-l). If this is the case, functional PhyC has a promoting effect in long-day plant barley under long photoperiod conditions, and conversely, it has a delaying effect in short-day plant rice under the same conditions. There is an another example in which orthologous genes affect flowering time conversely between long-day and short-day plants: in the long-day plant Arabidopsis, the functional CO promotes flowering under long photoperiod conditions, whereas the functional rice ortholog Hd1 delays flowering under the same condition (Putterill et al., 1995; Yano et al., 2000). Rice is a reasonable model plant for functional assay of HvPhyC gene, because phyC null mutant lines are available in a var. Nipponbare genetic background (Takano et al., 2005), which facilitates transformation. On the other hand, transformation of barley is possible only in a ‘Golden Promise’ genetic background, and no HvPhyC null mutant genes are available so far. However, the best way to evaluate precise function of HvPhyC is complementation analysis introducing HvPhyC-e and HvPhyC-l under the control of native promoter into a HvPhyC-deficient mutant of barley (native genetic background), because it is unclear how the ectopic expression from viral promoter and how the differences of photoperiodic pathways between barley and rice affect HvPhyC gene function in rice (heterogeneous) genetic background.
Phytochrome protein deploys several domains: a GAF domain, a PHY (phytochrome-specific, GAF-related) domain, three PAS (Drosophila period protein, vertebrate aryl hydrocarbon receptor nuclear translocator protein, and Drosophila single-minded protein) domains, and two His kinase-related domains (Fig. 1B). The first half of this protein, including N-terminal PAS, GAF, and PHY domains, is considered to be crucial for light perception and signal transduction. And several missense mutations in this region caused a deficiency in signal transduction, chromophore incorporation and spectral integrity, and Pfr stabilization (Nagatani, 2010). In this study, an exon 1 SNP (T1139C) was found near the 3′ end of GAF domain containing a chromophore-binding site. This mutation resulted in nonsynonymous substitution at position 380 from nonpolar and hydrophobic Phe to polar and hydrophilic Ser. Because mutation at this position is unknown in plants including Arabidopsis, it remains unknown how the mutation affects the biochemical function of the protein. However, this amino acid residue is well conserved among many plant species (wheat, B. distachyon, rice, sorghum, and Arabidopsis), suggesting its importance for protein function (Fig. 1C). On the C-terminal side of the protein, both HK2 and NIL (Vrn-H1) had an 8-amino acid insertion, which was previously reported in var. Morex by Szücs et al. (2006; Fig. 1B). No such insertion was found in wheat, B. distachyon, rice, and sorghum PHYC proteins. However, this insertion is located close to the C-terminal end and outside the His kinase-like ATPase domain (Fig. 1B). In addition, the N-terminal side is rather important for light perception and signal transduction compared with the C-terminal side, although it is associated with dimerization (Nagatani, 2010). Therefore, the effect of the insertion on flowering time was considered to be small. Consistent with this idea, constitutive expression of both HvPhyC alleles in rice phyA phyC mutant could recover the wild-type phenotype, although the recovering levels were different (Fig. 4).
Expression analysis showed that SNP (T1139C) in HvPhyC did not affect the expression pattern of HvPhyC itself, circadian clock genes, and most of the downstream genes (Fig. 5, A–E and H–K; Supplemental Fig. S1, A–K). The only exception was HvFT1 (Vrn-H3), which was up-regulated by the mutant allele HvPhyC-e under a long photoperiod (Fig. 5F). This was consistent with physiological analysis in which HvPhyC affected flowering time only under long photoperiods. Therefore, HvPhyC was considered to bypass most of the genes that function in relatively upstream parts of the genetic pathways for flowering (e.g. circadian clock genes and HvCO1) and affect the gene (HvFT1) that functions in the most downstream part of the genetic pathways. Consistent with this, complete loss of three phytochromes in rice did not affect the expression of circadian clock genes and CO, while it affected the expression of FT (Izawa et al., 2002), although the sole effect of PhyC remains unknown. Furthermore, field experiment in this study also supported our idea (Table II). HvPhyC-e, compared with HvPhyC-l, had a promoting effect when coexisting with vrn-H3, while it did not have such effect when coexisting with Vrn-H3, which was derived from the Finnish var. Tammi. The Tammi allele for Vrn-H3 was shown to include four copies of HvFT1, which is associated with earlier transcriptional up-regulation of themselves (Nitcher et al., 2013). Therefore, it was considered that the spring allele Vrn-H3 (high expression of HvFT1) is epistatic to that of HvPhyC-e and HvPhyC functions in the same pathway as Vrn-H3.
Based on the feedback loop models (Trevaskis et al., 2007; Distelfeld et al., 2009; Shimada et al., 2009), up-regulation of HvFT1 was expected to give rise to concordant up-regulation of Vrn-H1 and down-regulation of Vrn-H2. Our result showed that Vrn-H1 expression tended to be slightly higher in NIL (HvPhyC-e) than NIL (HvPhyC-l) (Fig. 5G; Supplemental Fig. S2). However, their differences were confirmed to be nonsignificant (Supplemental Fig. S2). This might be attributable to that both of the NILs carry the spring allele Vrn-H1. Because the spring allele Vrn-H1 expresses at a high level even in young seedlings (Trevaskis et al., 2003; von Zitzewitz et al., 2005), further up-regulation of Vrn-H1 by higher expression of HvFT1 will be marginal even if it occurs. Consistent with this idea, the expression levels of Vrn-H2 were not different between both NILs (Fig. 5, H and I). This might also be attributable to that expression of Vrn-H2 was repressed by the spring allele Vrn-H1 (Hemming et al., 2008) to a considerably low level where the differences were not detectable. Distelfeld and Dubcovsky (2010) suggested that lack of the entire TaPhyC gene down-regulated the ZCCT genes in the maintained vegetative phase mutant of diploid einkorn wheat. To disclose the effect of HvPhyC on Vrn-H2 (perhaps through affecting Vrn-H1), a F2 population segregating for HvPhyC and Vrn-H2 and carrying the winter allele vrn-H1 needs to be developed.
Molecular genetic studies of Arabidopsis have disclosed the essential roles of phytochromes in transcriptional and posttranscriptional control of CO. PHYB is associated with posttranscriptional regulation of CO by degrading CO protein early in the day under a long photoperiod (Valverde et al., 2004). PHYB protein is known to form not only a homodimer, but also heterodimers with PHYC and PHYD proteins, while PHYC and PHYD proteins do not form a homodimer (Clack et al., 2009). If PHYB/PHYC heterodimer participates in CO protein stability, PhyC may affect flowering time through a CO-mediated photoperiodic pathway. Similarly, PHYC protein can participate in transcriptional regulation of CO by forming a heterodimer with PHYB. Clack et al. (2009) showed that PHYC protein, probably by forming a heterodimer with PHYB protein, can interact with Phytochrome Interacting Factor3 (PIF3), which is involved in signal transduction from phytochromes. Oda et al. (2004) showed that suppression of PIF3 resulted in up-regulation of CO and FT but did not affect the expression of circadian clock genes, including LHY (LATE ELONGATED HYPOCOTYL) and CCA1 (CIRCADIAN CLOCK ASSOCIATED1), irrespective of possessing a possible binding sequence in their promoter. Our expression analysis suggests that the mutation (T1139C) in HvPhyC affects the posttranscriptional control of CO rather than the transcriptional control of CO, because HvFT1 was up-regulated but HvCO1 was not in NIL (HvPhyC-e). Taken together, our results indicate that HvPhyC is a key factor to control long-day flowering directly.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
NIL (Vrn-H1) was developed by backcrossing more than 10 times using the Japanese barley (Hordeum vulgare ssp. vulgare) var. HK2 as the recurrent parent and the Taiwanese var. Indo Omugi as the nonrecurrent parent (Vrn-H1 donor; this NIL was originally developed by Shozo Yasuda who conducted backcrossings six times [Yasuda, 1969]. After publication, he conducted further backcrosses more than four times.).
The F2 population (918 individuals) and F3 lines (87 lines, 10–15 plants each) of HK2 × NIL (Vrn-H1) were subjected to genetic and linkage analyses for flowering-time gene. They were grown under natural conditions in the experimental field (the Faculty of Agriculture, Okayama University; 34° 41′N, 133° 55′E, 4 m above sea level). The sowing date of the F2 population was November 25, 2005, and those of F3 lines were December 20, 2006, December 21, 2007, and November 21, 2008. Flowering time was scored for each plant as the date when the first ear appeared from the sheath.
For evaluation of the gene effect on photoperiod sensitivity and the expression pattern of flowering-time genes, the NILs with different alleles for HvPhyC (Table I) were selected from F4 families using allele-specific DNA markers, as summarized in Supplemental Table S1. For the evaluation of photoperiod sensitivity, seeds of NIL (HvPhyC-e) and NIL (HvPhyC-l) were soaked in water at 4°C for 24 h and subsequently kept at 20°C for 24 h for germination. Germinated seeds were sown on the soil (1:1 mixture of field soil and bark compost). Each three plants per line (six plants for each genotype) were grown under short (12-h-light/12-h-dark), long (16-h-light/8-h-dark), and extremely long (20-h-light/4-h-dark) photoperiod conditions in growth chambers (LH-350SP; Nippon Medical and Chemical Instruments), where temperature was kept at 20°C. The light source was fluorescent lamps, and photon flux density was approximately 160 μmol m–2 s–1. For expression analysis, the same lines were grown in the same way under short (8-h-light/16-h-dark) and long (16-h-light/8-h-dark) photoperiods. From 15 d after sowing, second and third leaves (three biological replicates for each genotype at every time point) were collected at 4-h intervals for 2 d.
For validation of the interaction between HvPhyC and Vrn-H3, flowering time of additional two HK2 NILs carrying Vrn-H3 (Yasuda, 1969) as well as the NILs described above were evaluated under a natural condition. Five plants per each line were grown in the same field as described above. Sowing date was December 17, 2010.
Young seedlings of 12 Japanese varieties were grown for the HvPhyC/Vrn-H1 haplotype analysis (Table III). Leaves of these varieties were collected for DNA extraction.
Functional Assay of HvPhyC Using Rice Transformation System
We adopted the rice (Oryza sativa) phyA phyC double mutant line as the recipient, instead of the phyC single mutant line, with a genetic background of the Japanese var. Nipponbare (Takano et al., 2005). This is because the phenotypic effect of the PhyC gene is prominent when PhyA is nonfunctional: the phyA phyC double mutant line flowers much earlier than the original var. Nipponbare and even earlier than the phyC single mutant line under a natural (long) photoperiod, while the phyA single mutant line flowers at the same time as the var. Nipponbare under the same conditions (Takano et al., 2005).
HvPhyC-e and HvPhyC-l cDNAs driven by the Cauliflower mosaic virus 35S promoter were introduced into the phyA phyC double mutant line via Agrobacterium tumefaciens-mediated transformation, as described by Kikuchi et al. (2009). Four T1 lines expressing HvPhyC-e and two T1 lines expressing HvPhyC-l developed from independent T0 plants were subjected to the analysis. T1 lines carrying the empty vector (mock) with the phyA phyC and Nipponbare variety background (phyA phyC mock and Nip mock, respectively) were used as the control. Their seeds were sown on July 26, 2012 in plastic pots, and plants were grown until flowering under a natural photoperiod in a greenhouse, as described by Kikuchi et al. (2009). Flag leaves were collected at 9 am at the end of September for HvPhyC expression analysis.
DNA Extraction and Genotyping
Genomic DNA was extracted from each plant of the F2 population, F3 lines, their parents, NILs, and varieties following the cetyl trimethyl ammonium bromide method (Murray and Thompson, 1980).
For linkage analysis, PCR amplification was conducted using allele-specific DNA markers for Vrn-H1, HvPhyC, and HvCK2α (Supplemental Table S1). PCR products or digested PCR products were separated in agarose gels by electrophoresis. PCR products were visualized with ethidium bromide. A genetic map was constructed using MAPMAKER/EXP3.0 (Lander et al., 1987).
In addition to Vrn-H1, HvPhyC, and HvCK2α, other flowering-time genes, Ppd-H1, Ppd-H2, Vrn-H2, and Vrn-H3, were also genotyped for HK2, its NILs, and varieties using diagnostic markers (Supplemental Table S1).
Sequence Analysis
The HvPhyC gene region was amplified by long PCR using appropriate primers (Supplemental Table S2) and Phusion High-Fidelity DNA polymerase (Thermo Scientific) and cloned using the TOPO TA Cloning Kit (Invitrogen) following the manufacturers’ instructions. Three clones per single PCR product were sequenced using a PRISM 3730 DNA Analyzer (Applied Biosystems).
Gene Expression Analysis
Young leaves (100 mg) of barley NILs and transgenic rice were frozen in liquid nitrogen and ground using a Multibeads shocker (Yasui apparatus). Total RNA was extracted from the ground leaf using Sepasol RNAI Super (Nacalai Tesque) and first-strand complementary DNA was synthesized using ReverTra Ace (TOYOBO) following manufacturers’ instructions. Semiquantitative RT-PCR was conducted using specific primers for flowering-time genes and internal controls (Supplemental Table S3). PCR products were electrophoresed in agarose gels and stained by ethidium bromide. Band intensity was analyzed by Scion Image.
Sequence data from this article can be found in the GenBank database under the following accession numbers: HvPhyC-e (AB827939) and HvPhyC-l (AB827940).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Expression pattern of flowering time genes under a short (8-h) photoperiod.
Supplemental Figure S2. Expression pattern of Vrn-H1 analyzed by real-time RT-PCR under a long (16-h) photoperiod.
Supplemental Figure S3. PCR amplification of HvPhyC pseudogene.
Supplemental Table S1. Primer sets for linkage analysis and diagnostic markers for flowering time genes.
Supplemental Table S2. Primer sets for sequence analysis of HvPhyC.
Supplemental Table S3. Primer sets for functional assay of HvPhyC, expression analysis of flowering-related genes, and amplification of HvPhyC pseudogene.
Supplemental Materials and Methods S1. Materials and methods for Vrn-H1 expression analysis by real-time RT-PCR.
Acknowledgments
We thank Shozo Yasuda (Institute of Plant Science and Resources, Okayama University) and Makoto Takano (National Institute of Agrobiological Sciences) for providing HK2 NILs and the rice phyA phyC double mutant line, respectively, and Koichiro Ushijima (Okayama University) for technical advice on the real-time RT-PCR analysis.
Glossary
- NIL
near isogenic line
- HK2
var. Hayakiso 2
- SNP
single nucleotide polymorphism
- RT
reverse transcription
- QTL
quantitative trait loci
- DH
double haploid
- ZCCT
zinc finger-CONSTANS, CONSTANS-LIKE, or TIMING OF CHLOROPHYLL A/B-BINDING PROTEIN EXPRESSION 1
References
- Balasubramanian S, Sureshkumar S, Agrawal M, Michael TP, Wessinger C, Maloof JN, Clark R, Warthmann N, Chory J, Weigel D. (2006) The PHYTOCHROME C photoreceptor gene mediates natural variation in flowering and growth responses of Arabidopsis thaliana. Nat Genet 38: 711–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KS, Benson WW. (1974) Adaptive polymorphism associated with multiple Müllerian mimicry in Heliconius numata. Biotropica 6: 205–228 [Google Scholar]
- Campoli C, Drosse B, Searle I, Coupland G, von Korff M. (2012) Functional characterisation of HvCO1, the barley (Hordeum vulgare) flowering time ortholog of CONSTANS. Plant J 69: 868–880 [DOI] [PubMed] [Google Scholar]
- Clack T, Shokry A, Moffet M, Liu P, Faul M, Sharrock RA. (2009) Obligate heterodimerization of Arabidopsis phytochromes C and E and interaction with the PIF3 basic helix-loop-helix transcription factor. Plant Cell 21: 786–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke CA, Sheppard PM, Thornton IWB. (1968) The genetics of the mimetic butterfly Papilio memnon. Philos Trans R Soc Lond, B 254: 37–89 [DOI] [PubMed] [Google Scholar]
- Distelfeld A, Dubcovsky J. (2010) Characterization of the maintained vegetative phase deletions from diploid wheat and their effect on VRN2 and FT transcript levels. Mol Genet Genomics 283: 223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distelfeld A, Li C, Dubcovsky J. (2009) Regulation of flowering in temperate cereals. Curr Opin Plant Biol 12: 178–184 [DOI] [PubMed] [Google Scholar]
- Dunford RP, Griffiths S, Christodoulou V, Laurie DA. (2005) Characterisation of a barley (Hordeum vulgare L.) homologue of the Arabidopsis flowering time regulator GIGANTEA. Theor Appl Genet 110: 925–931 [DOI] [PubMed] [Google Scholar]
- Faure S, Turner AS, Gruszka D, Christodoulou V, Davis SJ, von Korff M, Laurie DA. (2012) Mutation at the circadian clock gene EARLY MATURITY 8 adapts domesticated barley (Hordeum vulgare) to short growing seasons. Proc Natl Acad Sci USA 109: 8328–8333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KA, Quail PH. (2010) Phytochrome functions in Arabidopsis development. J Exp Bot 61: 11–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Liang W, Yin C, Ji S, Wang H, Su X, Guo C, Kong H, Xue H, Zhang D. (2010) The SEPALLATA-like gene OsMADS34 is required for rice inflorescence and spikelet development. Plant Physiol 153: 728–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemming MN, Fieg S, Peacock WJ, Dennis ES, Trevaskis B. (2009) Regions associated with repression of the barley (Hordeum vulgare) VERNALIZATION1 gene are not required for cold induction. Mol Genet Genomics 282: 107–117 [DOI] [PubMed] [Google Scholar]
- Hemming MN, Peacock WJ, Dennis ES, Trevaskis B. (2008) Low-temperature and daylength cues are integrated to regulate FLOWERING LOCUS T in barley. Plant Physiol 147: 355–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JA, Bailey PC, Laurie DA. (2010) Comparative genomics of flowering time pathways using Brachypodium distachyon as a model for the temperate grasses. PLoS ONE 5: e10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa T, Oikawa T, Sugiyama N, Tanisaka T, Yano M, Shimamoto K. (2002) Phytochrome mediates the external light signal to repress FT orthologs in photoperiodic flowering of rice. Genes Dev 16: 2006–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joron M, Frezal L, Jones RT, Chamberlain NL, Lee SF, Haag CR, Whibley A, Becuwe M, Baxter SW, Ferguson L, et al. (2011) Chromosomal rearrangements maintain a polymorphic supergene controlling butterfly mimicry. Nature 477: 203–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Kidou S, Miura H. (2008) Molecular cloning and mapping of casein kinase 2 α and β subunit genes in barley. Genome 51: 208–215 [DOI] [PubMed] [Google Scholar]
- Kato K, Kidou S, Miura H, Sawada S. (2002) Molecular cloning of the wheat CK2α gene and detection of its linkage with Vrn-A1 on chromosome 5A. Theor Appl Genet 104: 1071–1077 [DOI] [PubMed] [Google Scholar]
- Kikuchi R, Kawahigashi H, Ando T, Tonooka T, Handa H. (2009) Molecular and functional characterization of PEBP genes in barley reveal the diversification of their roles in flowering. Plant Physiol 149: 1341–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi R, Kawahigashi H, Oshima M, Ando T, Handa H. (2012) The differential expression of HvCO9, a member of the CONSTANS-like gene family, contributes to the control of flowering under short-day conditions in barley. J Exp Bot 63: 773–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knüpffer H, Terentyeva I, Hammer K, Kovaleva O, Sato K (2003) Ecogeographical diversity – a Vavilovian approach. In R von Bothmer, T van Hintum, H Knüpffer, K Sato, eds, Diversity in Barley (Hordeum vulgare). Elsevier, Amsterdam, pp 54–76 [Google Scholar]
- Kobayashi K, Maekawa M, Miyao A, Hirochika H, Kyozuka J. (2010) PANICLE PHYTOMER2 (PAP2), encoding a SEPALLATA subfamily MADS-box protein, positively controls spikelet meristem identity in rice. Plant Cell Physiol 51: 47–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Yasuno N, Sato Y, Yoda M, Yamazaki R, Kimizu M, Yoshida H, Nagamura Y, Kyozuka J. (2012) Inflorescence meristem identity in rice is specified by overlapping functions of three AP1/FUL-like MADS box genes and PAP2, a SEPALLATA MADS box gene. Plant Cell 24: 1848–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Newberg LA. (1987) MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1: 174–181 [DOI] [PubMed] [Google Scholar]
- Laurie DA, Pratchett N, Snape JW, Bezant JH. (1995) RFLP mapping of five major genes and eight quantitative trait loci controlling flowering time in a winter × spring barley (Hordeum vulgare L.) cross. Genome 38: 575–585 [DOI] [PubMed] [Google Scholar]
- Mather K. (1950) The genetical architecture of heterostyly in Primula sinensis. Evolution 4: 340–352 [Google Scholar]
- Murray MG, Thompson WF. (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8: 4321–4325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatani A. (2010) Phytochrome: structural basis for its functions. Curr Opin Plant Biol 13: 565–570 [DOI] [PubMed] [Google Scholar]
- Nijhout HF. (2003) Polymorphic mimicry in Papilio dardanus: mosaic dominance, big effects, and origins. Evol Dev 5: 579–592 [DOI] [PubMed] [Google Scholar]
- Nitcher R, Distelfeld A, Tan C, Yan L, Dubcovsky J. (2013) Increased copy number at the HvFT1 locus is associated with accelerated flowering time in barley. Mol Genet Genomics 288: 261–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda A, Fujiwara S, Kamada H, Coupland G, Mizoguchi T. (2004) Antisense suppression of the Arabidopsis PIF3 gene does not affect circadian rhythms but causes early flowering and increases FT expression. FEBS Lett 557: 259–264 [DOI] [PubMed] [Google Scholar]
- Ogiso E, Takahashi Y, Sasaki T, Yano M, Izawa T. (2010) The role of casein kinase II in flowering time regulation has diversified during evolution. Plant Physiol 152: 808–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putterill J, Robson F, Lee K, Simon R, Coupland G. (1995) The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell 80: 847–857 [DOI] [PubMed] [Google Scholar]
- Sato K, Nankaku N, Takeda K. (2009) A high-density transcript linkage map of barley derived from a single population. Heredity (Edinb) 103: 110–117 [DOI] [PubMed] [Google Scholar]
- Shimada S, Ogawa T, Kitagawa S, Suzuki T, Ikari C, Shitsukawa N, Abe T, Kawahigashi H, Kikuchi R, Handa H, et al. (2009) A genetic network of flowering-time genes in wheat leaves, in which an APETALA1/FRUITFULL-like gene, VRN1, is upstream of FLOWERING LOCUS T. Plant J 58: 668–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szücs P, Karsai I, von Zitzewitz J, Mészáros K, Cooper LLD, Gu YQ, Chen THH, Hayes PM, Skinner JS. (2006) Positional relationships between photoperiod response QTL and photoreceptor and vernalization genes in barley. Theor Appl Genet 112: 1277–1285 [DOI] [PubMed] [Google Scholar]
- Takahashi R, Yasuda S (1970) Genetics of earliness and growth habit in barley. In RA Nilan, ed, Barley Genetics II. Washington State University Press, Pullman, Washington, pp 388–408 [Google Scholar]
- Takahashi Y, Shomura A, Sasaki T, Yano M. (2001) Hd6, a rice quantitative trait locus involved in photoperiod sensitivity, encodes the α subunit of protein kinase CK2. Proc Natl Acad Sci USA 98: 7922–7927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano M, Inagaki N, Xie X, Yuzurihara N, Hihara F, Ishizuka T, Yano M, Nishimura M, Miyao A, Hirochika H, et al. (2005) Distinct and cooperative functions of phytochromes A, B, and C in the control of deetiolation and flowering in rice. Plant Cell 17: 3311–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevaskis B, Bagnall DJ, Ellis MH, Peacock WJ, Dennis ES. (2003) MADS box genes control vernalization-induced flowering in cereals. Proc Natl Acad Sci USA 100: 13099–13104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevaskis B, Hemming MN, Dennis ES, Peacock WJ. (2007) The molecular basis of vernalization-induced flowering in cereals. Trends Plant Sci 12: 352–357 [DOI] [PubMed] [Google Scholar]
- Trevaskis B, Hemming MN, Peacock WJ, Dennis ES. (2006) HvVRN2 responds to daylength, whereas HvVRN1 is regulated by vernalization and developmental status. Plant Physiol 140: 1397–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A, Beales J, Faure S, Dunford RP, Laurie DA. (2005) The pseudo-response regulator Ppd-H1 provides adaptation to photoperiod in barley. Science 310: 1031–1034 [DOI] [PubMed] [Google Scholar]
- Valverde F, Mouradov A, Soppe W, Ravenscroft D, Samach A, Coupland G. (2004) Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science 303: 1003–1006 [DOI] [PubMed] [Google Scholar]
- von Bothmer R, Sato K, Komatsuda T, Yasuda S, Fischbeck G (2003) The domestication of cultivated barley. In R von Bothmer, T van Hintum, H Knüpffer, K Sato, eds, Diversity in Barley (Hordeum vulgare). Elsevier, Amsterdam, pp 9–27 [Google Scholar]
- von Zitzewitz J, Szucs P, Dubcovsky J, Yan L, Francia E, Pecchioni N, Casas A, Chen TH, Hayes PM, Skinner JS. (2005) Molecular and structural characterization of barley vernalization genes. Plant Mol Biol 59: 449–467 [DOI] [PubMed] [Google Scholar]
- Yan L, Fu D, Li C, Blechl A, Tranquilli G, Bonafede M, Sanchez A, Valarik M, Yasuda S, Dubcovsky J. (2006) The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proc Natl Acad Sci USA 103: 19581–19586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Loukoianov A, Blechl A, Tranquilli G, Ramakrishna W, SanMiguel P, Bennetzen JL, Echenique V, Dubcovsky J. (2004) The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 303: 1640–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Loukoianov A, Tranquilli G, Helguera M, Fahima T, Dubcovsky J. (2003) Positional cloning of the wheat vernalization gene VRN1. Proc Natl Acad Sci USA 100: 6263–6268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, von Zitzewitz J, Skinner JS, Hayes PM, Dubcovsky J. (2005) Molecular characterization of the duplicated meristem identity genes HvAP1a and HvAP1b in barley. Genome 48: 905–912 [DOI] [PubMed] [Google Scholar]
- Yano M, Katayose Y, Ashikari M, Yamanouchi U, Monna L, Fuse T, Baba T, Yamamoto K, Umehara Y, Nagamura Y, et al. (2000) Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell 12: 2473–2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda S. (1969) Physiology and genetics of ear emergence in barley and wheat. VIII. Effects of four genes for spring habit on earliness in barley. Nogaku Kenkyu 53: 99–113 [Google Scholar]