Two transcription factors link light and abscisic acid networks to regulate plant growth and development.
Abstract
Light and the phytohormone abscisic acid (ABA) regulate overlapping processes in plants, such as seed germination and seedling development. However, the molecular mechanism underlying the interaction between light and ABA signaling is largely unknown. Here, we show that FAR-RED ELONGATED HYPOCOTYL3 (FHY3) and FAR-RED IMPAIRED RESPONSE1 (FAR1), two key positive transcription factors in the phytochrome A pathway, directly bind to the promoter of ABA-Insensitive5 and activate its expression in Arabidopsis (Arabidopsis thaliana). Disruption of FHY3 and/or FAR1 reduces the sensitivity to ABA-mediated inhibition of seed germination, seedling development, and primary root growth. The seed germination of the fhy3 mutant is also less sensitive to salt and osmotic stress than that of the wild type. Constitutive expression of ABA-Insensitive5 restores the seed germination response of fhy3. Furthermore, the expression of several ABA-responsive genes is decreased in the fhy3 and/or far1 mutants during seed imbibition. Consistently, FHY3 and FAR1 transcripts are up-regulated by ABA and abiotic stresses. Moreover, the fhy3 and far1 mutants have wider stomata, lose water faster, and are more sensitive to drought than the wild type. These findings demonstrate that FHY3 and FAR1 are positive regulators of ABA signaling and provide insight into the integration of light and ABA signaling, a process that may allow plants to better adapt to environmental stresses.
Light is an important environmental signal that affects multiple plant processes, such as seed germination and seedling growth. Plants utilize a set of photoreceptors, including phytochromes and cryptochromes, to monitor the light environment and transduce the signals to downstream mediators (Chory, 2010). Numerous intermediate regulators that play important roles in the light signaling network have been identified. Among them, FAR-RED ELONGATED HYPOCOTYL3 (FHY3) and FAR-RED IMPAIRED RESPONSE1 (FAR1) were originally identified as positive regulators of the phytochrome A (phyA) signaling pathway (Hudson et al., 1999; Wang and Deng, 2002). FHY3 and FAR1 encode novel transcription factors derived from ancient mutator-like transposases and belong to the FAR1-Related Sequences gene family, which is specific to plants (Hudson et al., 2003; Lin and Wang, 2004; Lin et al., 2007). These two homologous proteins act redundantly to activate the expression of FHY1 and FHY1-Like, whose proteins promote the nuclear translocation of phyA, resulting in the activation of phyA signaling (Hiltbrunner et al., 2006; Lin et al., 2007). Accumulating studies report that FHY3 and FAR1 are required for regulating various aspects of plant processes, such as far-red-mediated seedling deetiolation, the circadian clock, chloroplast division, and chlorophyll biosynthesis (Hudson et al., 1999; Wang and Deng, 2002; Allen et al., 2006; Li et al., 2011; Ouyang et al., 2011; Tang et al., 2012). Molecular evidence demonstrated that these two transcription factors bind to promoter regions containing the FBS (for FHY3/FAR1-binding site) motif of downstream targets and activate their expression (Lin et al., 2007; Li et al., 2011; Ouyang et al., 2011; Tang et al., 2012). A recent genome-wide analysis suggested that FHY3 has numerous putative direct targets in Arabidopsis (Arabidopsis thaliana; Ouyang et al., 2011), suggesting that FHY3 might have broad functions in plant growth and development, most of which, however, are unknown.
The phytohormone abscisic acid (ABA) regulates many plant processes that are also regulated by light, such as seed germination and seedling development. During seed maturation and under unfavorable conditions, such as drought and salinity, ABA accumulates to high levels and plays important roles, including maintaining seed dormancy, inhibiting seedling growth, and closing stomata (Finkelstein et al., 2002). Genetic studies identified a number of ABA-responsive components, such as transcription factors, protein kinases, phosphatases, and RNA metabolic proteins, that are essential for regulating these processes (Finkelstein et al., 2002, 2008; Cutler et al., 2010). Mutations in a group of ABA-insensitive (ABI) loci resulted in insensitivity to ABA during seed germination, whereas overexpression of these genes led to hypersensitivity to ABA (for review, see Leung and Giraudat, 1998; Finkelstein and Rock, 2002). ABI3, ABI4, and ABI5 encode transcription factors, while ABI1 and ABI2 encode protein phosphatase 2Cs (Leung et al., 1997; Finkelstein et al., 2002). ABI5 was identified by screening for mutants with ABA insensitivity at germination or during seedling growth or with altered ABA-induced transcription (Finkelstein, 1994; Lopez-Molina and Chua, 2000; Carles et al., 2002). ABI5 is a member of a small subfamily of basic leucine zipper transcription factors and is highly expressed in mature seeds and young seedlings exposed to ABA or water deficit stresses (Finkelstein and Lynch, 2000). The loss-of-function abi5 mutant germinates and grows well even in the presence of high concentrations of ABA (Lopez-Molina and Chua, 2000).
Although cross talk between ABA and light signaling pathways has been observed (e.g. ABA metabolism in seeds is regulated by phytochrome; Seo et al., 2006, 2009), the underlying molecular mechanism is largely unknown. In this study, we show that knockout mutants of FHY3 and/or FAR1 have reduced sensitivity to ABA-mediated inhibition of seed germination and seedling growth, lose water faster, and are less tolerant to drought stress than are wild-type plants. We demonstrate that FHY3 directly activates ABI5 expression and that overexpression of ABI5 rescues the seed germination defect of fhy3. FHY3 and FAR1 transcription is induced by ABA and abiotic stresses, and these proteins confer drought tolerance. Our study suggests that FHY3 and FAR1 are positive regulators of ABA signaling.
RESULTS
FHY3 and FAR1 Directly Activate ABI5 Expression
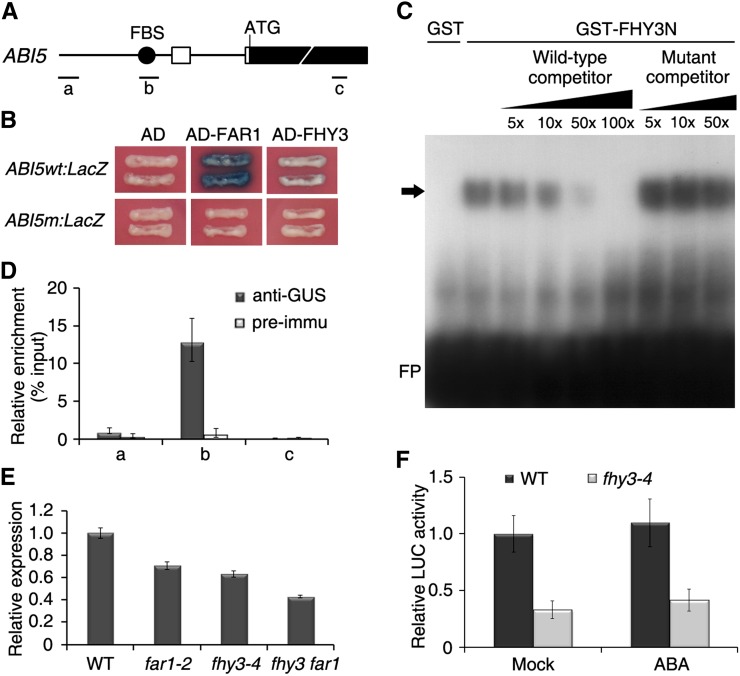
Our previous chromatin immunoprecipitation (ChIP)-based sequencing study revealed that FHY3 binds to numerous downstream targets involved in various hormonal responses and that ABI5 is one of the targets precipitated by FHY3 in dark-grown seedlings (Ouyang et al., 2011). We then focused on ABI5 and performed detailed experiments to further elucidate the relationship between FHY3 (together with its homolog FAR1) and ABI5. By analyzing the promoter sequence of ABI5, we found that a putative FBS (with core sequence CACGCGC) is present 1,009 bp upstream of the ATG start codon of ABI5 (Fig. 1A). A yeast one-hybrid assay showed that AD-FAR1 (fused with the B42 activation domain) was able to bind to wild-type ABI5 oligonucleotides containing the FBS sequence upstream of a LacZ reporter gene (ABI5wt:LacZ) and strongly activated LacZ expression, while AD-FHY3 activated the reporter gene to a lesser extent. Mutations in the FBS motif (ABI5m:LacZ; in which CACGCGC was changed into CACttGC) abolished the activation of the LacZ reporter (Fig. 1B). Next, we performed an electrophoretic mobility shift assay (EMSA) and showed that a FHY3 recombinant protein (N-terminal 250 amino acids of FHY3 fused with glutathione S-transferase [GST], GST-FHY3N; Lin et al., 2007) caused an up-shifted band with ABI5 probes labeled with 32P, and this band was abolished by excess amounts of unlabeled wild-type oligonucleotides but not by unlabeled mutants (Fig. 1C). To further investigate whether FHY3 interacts with the ABI5 promoter in vivo, we carried out a ChIP assay using 35S:GUS-FHY3 (GUS fused with FHY3) transgenic seedlings (Wang and Deng, 2002). The promoter fragment containing the FBS motif (b in Fig. 1A), but not fragments farther upstream in the promoter (a) and coding region (c), was drastically enriched in samples precipitated by the anti-GUS antibody but not by the serum control (Fig. 1D). Together, these results confirm that FHY3 directly binds to the ABI5 promoter through the FBS motif, both in vitro and in vivo.
Figure 1.
FHY3 directly activates ABI5 expression. A, Schematic diagram of ABI5. Black rectangles represent exons, and white rectangles denote untranslated regions. The circle indicates the FBS motif (CACGCGC). a, b, and c indicate fragments used for ChIP-PCR. ATG is the ABI5 translational start codon. B, Yeast one-hybrid assay showing the activity of LacZ reporters driven by either wild-type (ABI5wt:LacZ) or mutant (ABI5m:LacZ) ABI5 and activated by activation domain (AD) fusion effectors. C, EMSA showing the binding activity of GST-FHY3N or GST recombinant proteins with 32P-labeled wild-type ABI5 oligonucleotides in the presence of excess amounts of unlabeled competitors (wild-type and mutant probes). The arrow indicates shifted bands of protein-DNA complexes. FP denotes free probe. D, ChIP assay showing the specific precipitation of the ABI5 fragment by GUS antibody in extracts from 35S:GUS-FHY3 transgenic plants. Precipitation by preimmune serum served as the negative control. ChIP DNA was quantified by real-time PCR with primers targeting fragments as shown in A. Values are means ± sd; n = 3. E, Relative ABI5 expression in the seeds of various mutants and the wild type (WT) after imbibition for 12 h. Values are means ± sd; n = 3. F, Relative activity of the LUC reporter gene in protoplasts isolated from wild-type and fhy3 mutant seedlings transformed with both ABI5p:LUC and 35S:GUS. After transformation, the protoplasts were incubated without (Mock) or with 50 μm ABA in weak light for 12 h. Relative activities are expressed as the ratio of LUC to GUS (internal control). Values are means ± sd; n = 5. [See online article for color version of this figure.].
We next examined how FHY3 and FAR1 regulate ABI5 expression using quantitative reverse transcription (qRT)-PCR. The level of ABI5 transcript was modestly decreased in far1-2 and was even lower in fhy3-4 single and fhy3far1 double mutants (Fig. 1E), suggesting that FHY3 and FAR1 up-regulate ABI5 expression. Consistent with this, nuclear targeting of FHY3 (induced by 1 μm dexamethasone) in the FHY3p:FHY3-GR transgenic plants (Lin et al., 2007) promoted ABI5 expression compared with mock treatment (Supplemental Fig. S1). Next, a LUCIFERASE (LUC) reporter gene under the control of the ABI5 promoter (ABI5p:LUC) was transformed into Arabidopsis protoplasts isolated from wild-type and fhy3 mutant seedlings. This transient expression assay showed that LUC activity was remarkably reduced in fhy3 compared with the wild type regardless of ABA treatment (Fig. 1F), further confirming that FHY3 activates ABI5 expression.
Disruption of FHY3 and FAR1 Reduces ABA Sensitivity in Seed Germination
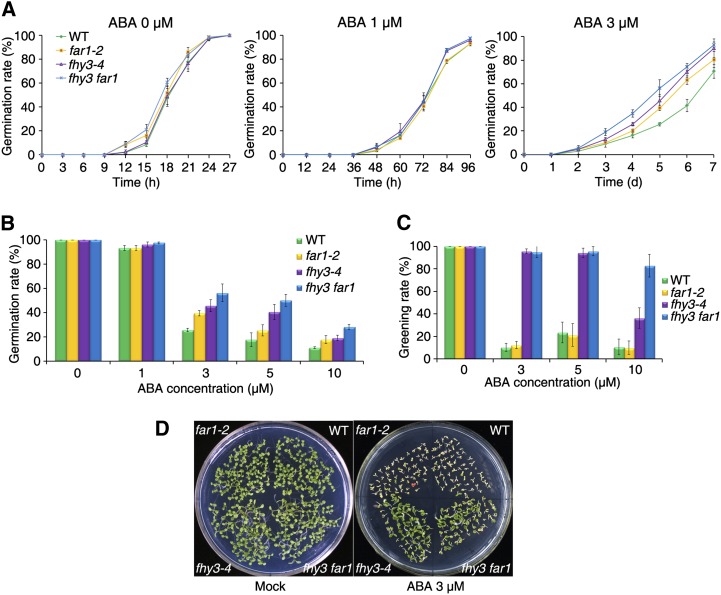
ABI5 is a critical positive regulator of seed germination and seedling establishment in the ABA pathway (Finkelstein and Lynch, 2000; Lopez-Molina and Chua, 2000; Lopez-Molina et al., 2001). Previous public data (http://bar.utoronto.ca/efp/cgi-bin/efpWeb.cgi; Winter et al., 2007) show that FHY3 and FAR1 transcripts accumulate in dry seeds and are down-regulated by imbibition during seed germination in a similar pattern to ABI5 (Supplemental Fig. S2). We thus speculated that FHY3 and FAR1 might be involved in regulating seed germination. To test this possibility, we examined the germination response of fhy3-4, far1-2, and fhy3 far1 mutants and wild-type seeds on Murashige and Skoog (MS) medium in the absence or presence of various concentrations of ABA. As shown in Figure 2, A and B, in the absence of ABA or in the presence of low ABA concentrations (less than 1 μm), the germination rate of the fhy3-4, far1-2, and fhy3 far1 mutants was indistinguishable from that of the wild-type seeds. However, in the presence of high ABA concentrations (3, 5, and 10 μm), the germination rate of fhy3-4 and far1-2 was higher than that of the wild type, and the fhy3 far1 double mutant had the highest germination rate (Fig. 2, A and B; Supplemental Fig. S3). Seedling establishment is also sensitive to ABA. We further found that, 3 weeks after seed germination on plates containing 3 μm ABA, the fhy3-4 and fhy3 far1 mutants were less sensitive to ABA than were the wild type and the far1-2 mutant (Fig. 2, C and D). Therefore, similar to ABI5, FHY3 and FAR1 positively regulate ABA-mediated inhibition of seed germination and seedling greening.
Figure 2.
FHY3 and FAR1 knockout mutants are hyposensitive to ABA-mediated inhibition of seed germination and seedling greening. A, Percentage of seed germination of the NO wild type (WT) and fhy3-4, far1-2, and fhy3 far1 mutants on medium containing various concentrations of ABA. Germination rate was monitored at the indicated time points. Values are means ± sd; n = 3. B, Percentage of seed germination 5 d after imbibition as shown in A and Supplemental Figure S1. Values are means ± sd; n = 3. C, Greening rate of seedlings grown in various concentrations of ABA for 4 weeks. Values are means ± sd; n = 3. D, Representative images of seedlings grown in medium without (Mock; 7 d old) or with 3 μm ABA (21 d old).
The fhy3 Mutant Is Less Sensitive to Salinity and Osmatic Stresses
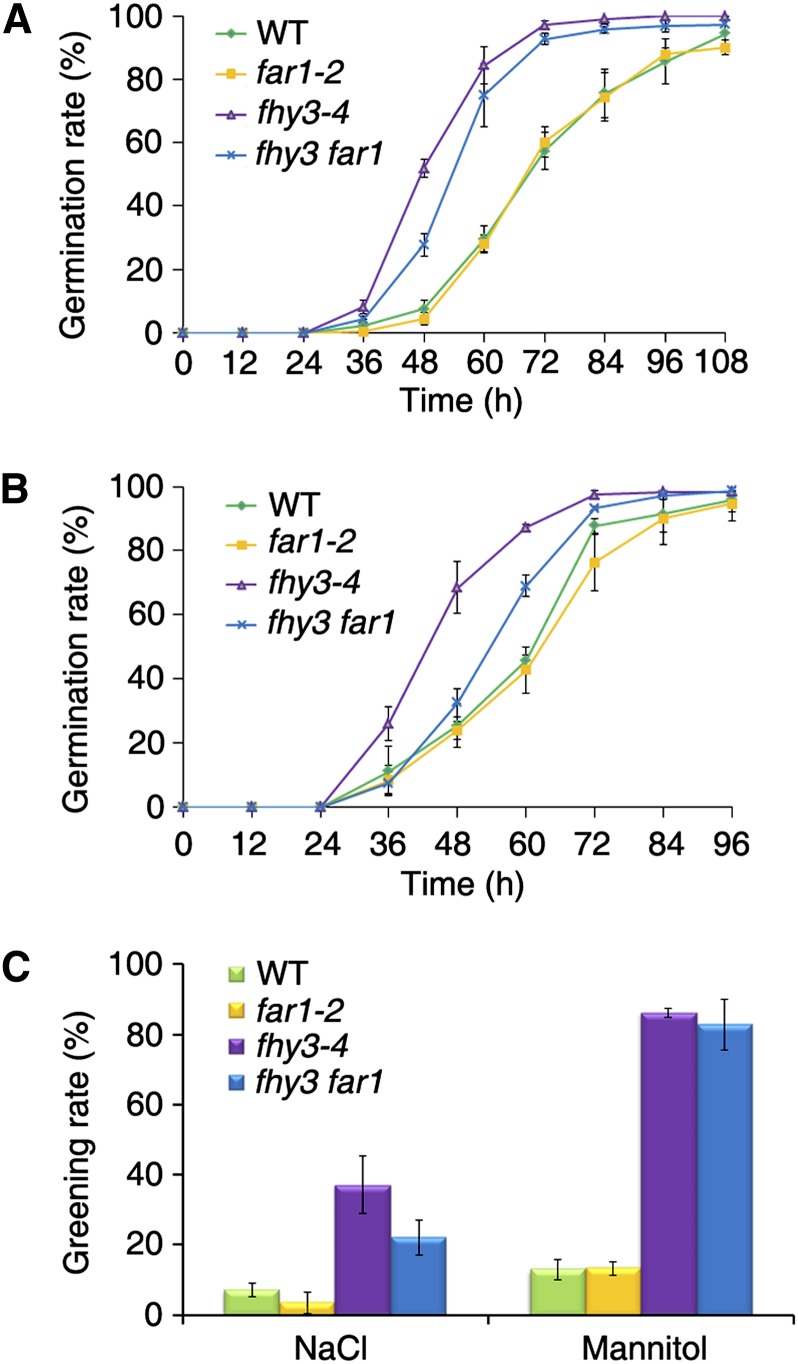
Under abiotic stress, such as salt and osmotic stresses, plants often trigger the accumulation of ABA (Finkelstein et al., 2002). Therefore, we evaluated seed germination of the fhy3 and far1 mutants in response to these stresses. In the presence of 200 mm NaCl, approximately 81% and 75% of fhy3-4 and fhy3 far1 seeds, respectively, germinated within 60 h, but only about 25% of far1-2 and wild-type seeds germinated under these conditions (Fig. 3A). Similarly, when seeds were germinated on medium containing 400 mm mannitol for 60 h, 85% of fhy3 and 75% of fhy3 far1 seeds germinated, whereas only about 40% of far1 and wild-type seeds germinated (Fig. 3B). Consistent with these results, the cotyledon greening rates of the fhy3 and fhy3 far1 mutants were also higher than those of far1-2 and the wild type (Fig. 3C). Thus, disruption of FHY3 causes hyposensitivity of seeds to high salt and osmotic stress, and FAR1 might have a slight opposite effect to FHY3.
Figure 3.
The fhy3 mutant is less sensitive to salinity and osmotic stress. A and B, Kinetics of seed germination on medium containing 200 mm NaCl (A) and 400 mm mannitol (B). Germination rate was monitored at the times indicated. Values are means ± sd; n = 3. C, Quantification of seedlings with green cotyledons shown in A and B. The greening rate was recorded 21 and 14 d after germination for NaCl and mannitol treatment, respectively. Values are means ± sd; n = 3.
FHY3 and FAR1 Are Required for ABA-Inducible Gene Expression during Seed Germination
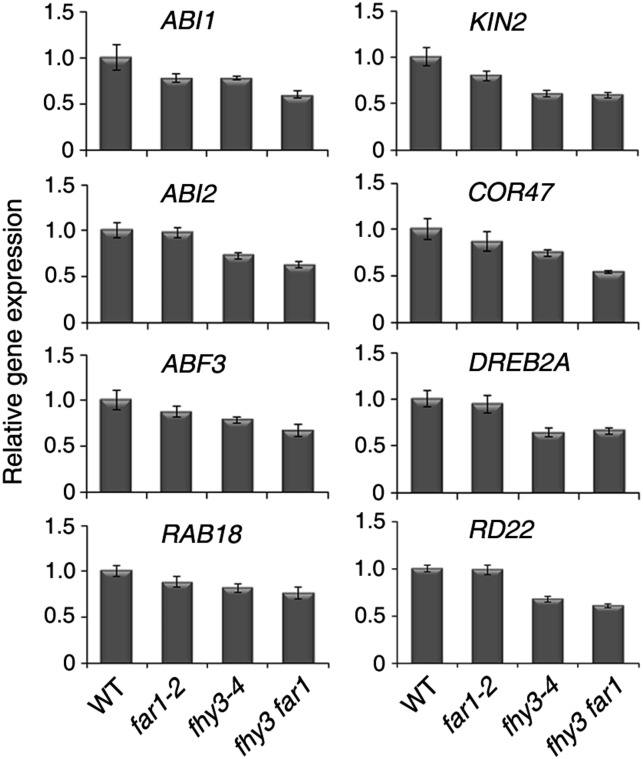
To further verify the involvement of FHY3 and FAR1 in regulating ABA signaling at the molecular level, we examined the expression of a number of ABA- and stress-responsive markers, including ABI1 (Gosti et al., 1999), ABI2 (Leung et al., 1997), ABF3 (Kang et al., 2002), RAB18 (Lång and Palva, 1992), KIN2 (Kurkela and Borg-Franck, 1992), COR47 (Gilmour et al., 1992), DREB2A (Liu et al., 1998), and RD22 (Yamaguchi-Shinozaki and Shinozaki, 1993), in the mutant and wild-type lines. Seeds of the wild type and the fhy3, far, and fhy3 far1 mutants were imbibed for 12 h, and RNA was isolated for qRT-PCR analysis. We found that the transcripts of these genes were moderately down-regulated in the fhy3 and fhy3 far1 mutants compared with the wild type. The expression of ABI1, KIN2, COR47, and ABF3 was also slightly reduced in the far1 mutant seedlings (Fig. 4). These results suggest that FHY3 and FAR1 transcription factors affect the expression of these genes during seed imbibition.
Figure 4.
FHY3 and FAR1 are required for ABA-responsive gene expression. Total RNA was isolated from wild-type (WT), fhy3-4, far1-2, and fhy3 far1 seeds after 12 h of imbibition. qRT-PCR was performed using specific primers as listed in Supplemental Table S1. Values are means ± sd; n = 3.
Overexpression of ABI5 Restores the fhy3 Mutant Phenotypes
To test the genetic relationship between FHY3 and ABI5 in regulating the ABA response, we overexpressed ABI5 (35S:ABI5; Dai et al., 2013) in the fhy3-4 mutant background and used lines homozygous for the transgene in the following experiments. Transgenic plants overexpressing ABI5 are hypersensitive to ABA (Lopez-Molina et al., 2001). In the presence of 3 or 5 μm ABA, the seed germination and seedling greening rates of fhy3 were restored to near wild-type levels or even below those of the wild type by ABI5 overexpression (Fig. 5). These data demonstrate that constitutive expression of ABI5 rescues the ABA responsiveness of the fhy3 mutant and that ABI5 acts downstream of FHY3.
Figure 5.
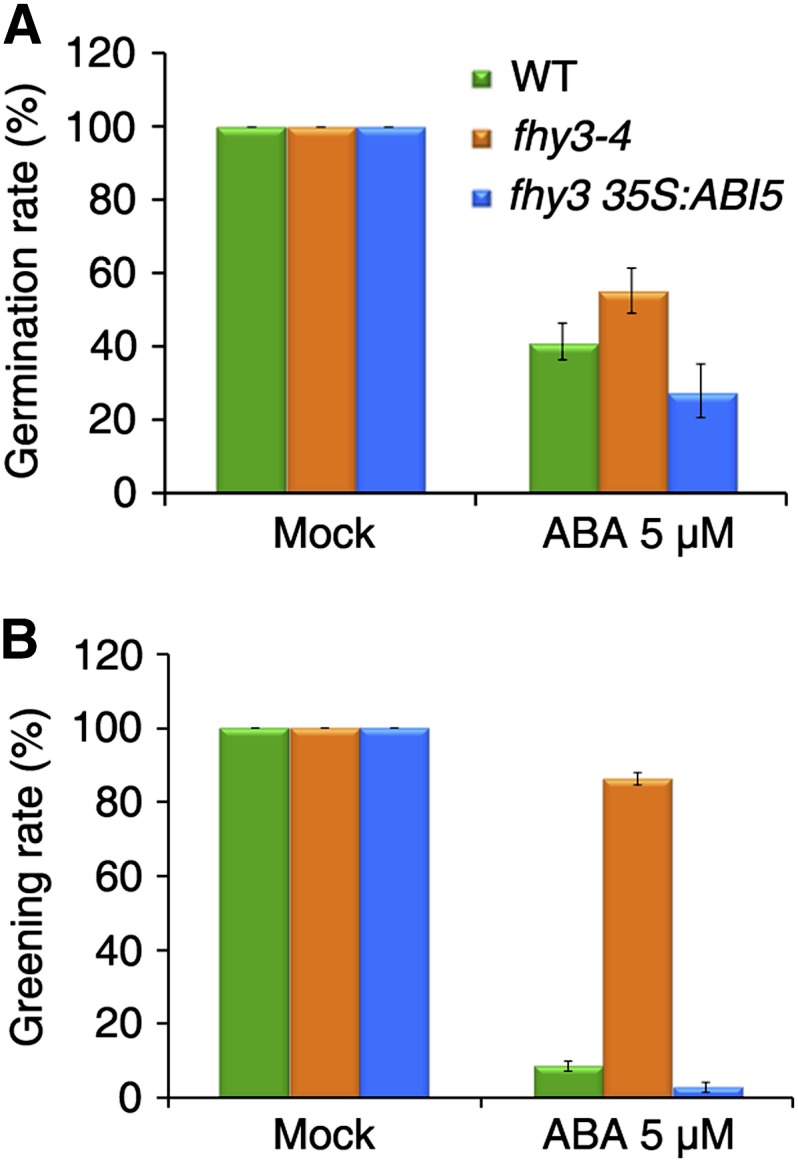
Overexpression of ABI5 rescues fhy3 mutant phenotypes. The percentage of seed germination (A) and of seedlings with green cotyledons (B) on medium in the absence (Mock) or presence of 5 μm ABA is shown. The germination rate was recorded after 5 d, and the greening rate was calculated after 4 weeks. Values are means ± sd; n = 3. WT, Wild type.
Up-Regulation of FHY3 and FAR1 by ABA and Abiotic Stresses
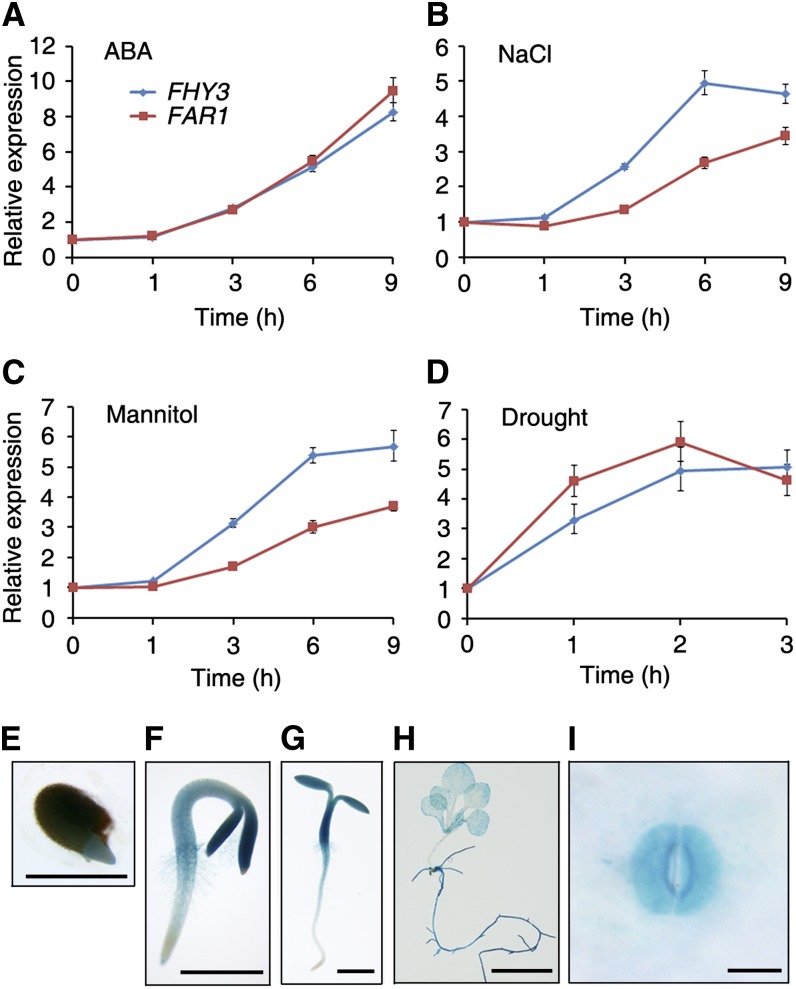
To investigate how the endogenous signal and exogenous stress input into the pathway, we determined the effect of ABA, salt, and osmotic stresses on the expression patterns of FHY3 and FAR1 by qRT-PCR. When 5-d-old seedlings were treated with 100 μm ABA, FHY3 and FAR1 transcript levels gradually increased over time, with an 8-fold induction after 9 h of treatment (Fig. 6A). Furthermore, FHY3 or FAR1 expression was also induced in seedlings treated with 200 mm NaCl or 400 mm mannitol, respectively (Fig. 6, B and C). In addition, when seedlings were subjected to drought treatment for up to 3 h, the mRNA levels of both FHY3 and FAR1 were also remarkably up-regulated (Fig. 6D). These data indicate that ABA and abiotic stresses induce FHY3 and FAR1 transcription, consistent with their roles in regulating the ABA response.
Figure 6.
Expression patterns of FHY3 and FAR1. A to C, Seven-day-old NO wild-type seedlings were transferred to medium containing 100 μm ABA (A), 200 mm NaCl (B), or 400 mm mannitol (C) for various periods of time. D, Seven-day-old NO wild-type seedlings were placed on filter paper under normal growth conditions for up to 3 h. The expression of FHY3 and FAR1 was analyzed by qRT-PCR. Relative expression levels were normalized to that of UBQ. Values are means ± sd; n = 3. E to I, GUS staining of FHY3p:GUS transgenic plants during seed germination (E) and of 2-d-old (F), 3-d-old (G), and 3-week-old (H) FHY3p:GUS plants and the guard cells of 3-week-old FHY3p:GUS plants (I). Bars = 0.5 mm (E), 1 mm (F and G), 5 mm (H), and 10 μm (I).
To test whether FHY3 has tissue-specific expression, we used the FHY3p:GUS transgenic line, in which the GUS reporter gene is driven by the FHY3 promoter (Lin and Wang, 2004). The GUS histochemical staining assay showed that FHY3 was strongly expressed in germinating seeds, the whole seedling during establishment, the roots, and the mature leaves (Fig. 6, E–H). Most strikingly, strong GUS activity was detected in the guard cells (Fig. 6I). These expression patterns suggest that FHY3 has additional roles in roots and leaves.
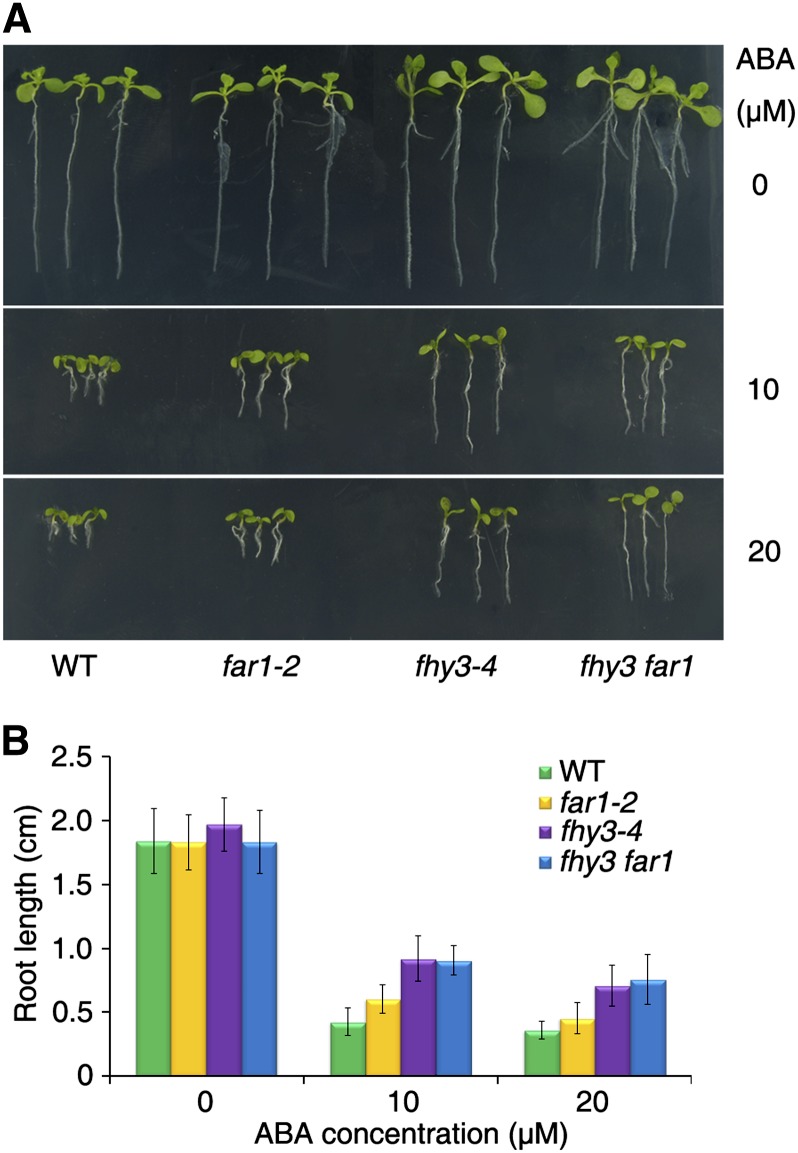
FHY3 Regulates ABA-Mediated Root Elongation
Since high concentrations of ABA inhibit root growth, we grew the seedlings in MS medium for 2 d and then transferred them to medium supplemented with various concentrations of ABA. In the presence of 10 or 20 μm ABA, far1-2 developed longer and fhy3-4 and fhy3 far1 exhibited much longer roots than did the wild type. The root growth of these mutant seedlings was comparable to the wild type in medium lacking ABA (Fig. 7). These observations support the notion that FHY3 also plays a role in ABA-mediated root growth. We also noticed that, without ABA treatment, the single and particularly the double mutants have more lateral roots than the wild type, indicating a reduced sensitivity of these seedlings to endogenous ABA.
Figure 7.
FHY3 and FAR1 regulate ABA-mediated root growth. A, Representative images of root growth on medium with or without ABA. Two-day-old seedlings were transferred to MS medium containing various concentrations of ABA and grown for an additional 7 d. B, Quantification of primary root length of the seedlings shown in A. Values are means ± sd; n = 20. WT, Wild type.
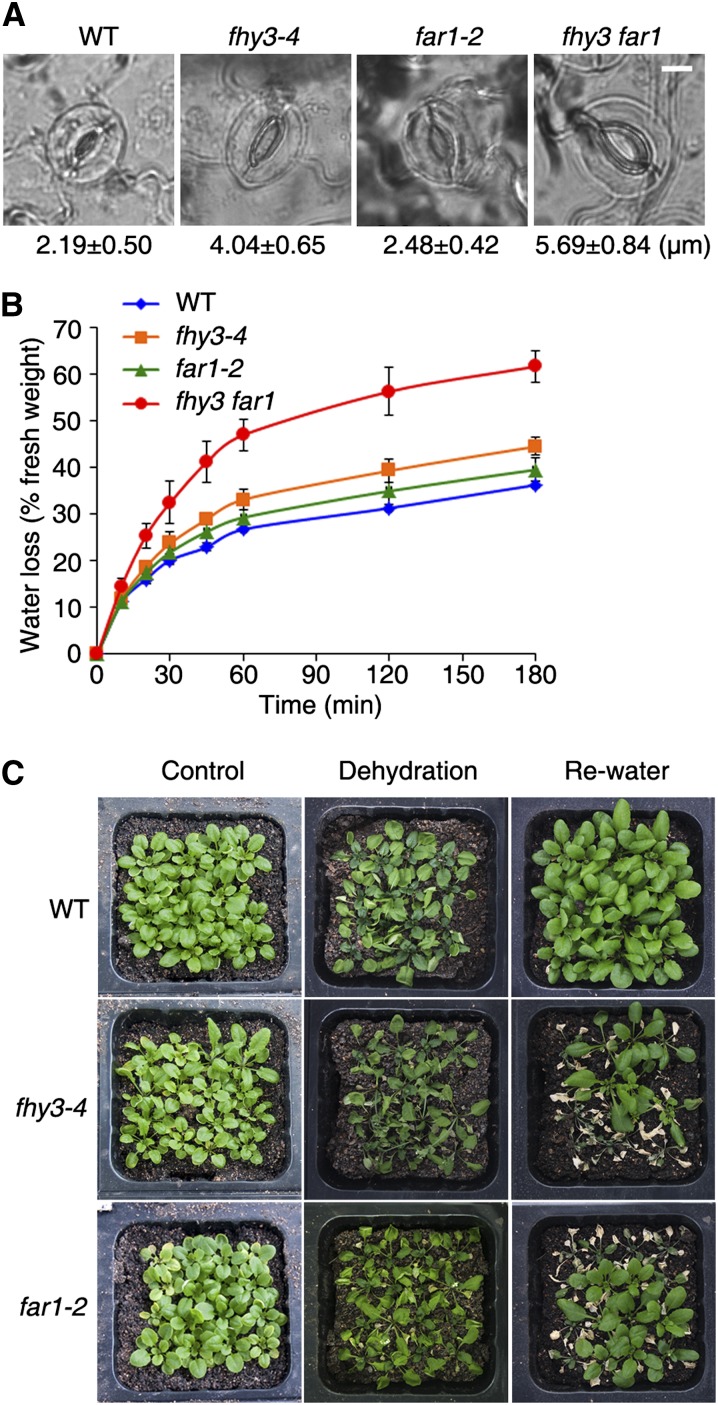
FHY3 and FAR1 Regulate Stomatal Movement and Confer Drought Tolerance
Since FHY3 is strongly expressed in guard cells and stomatal movement is regulated by ABA, we then compared the stomatal apertures of 4-week-old plants of the wild type and fhy3-4, far1-2, and fhy3 far1 mutants. The epidermal peels from rosette leaves at the same developmental stage were observed with a microscope. As shown in Figure 8A, far1-2 displayed slightly wider stomatal apertures than did the wild type. The stomatal apertures of fhy3-4 and especially of fhy3 far1 were much wider than those of far1-2 and the wild type. We also measured the stomatal apertures of fhy3-4 and the wild type in the presence or absence of ABA. Mutants with loss of FHY3 function were less sensitive to both ABA-promoted stomatal closure and ABA-inhibited stomatal opening (Supplemental Fig. S4).
Figure 8.
FHY3 and FAR1 regulate stomatal movement and confer drought tolerance. A, Representative images of stomata. Values below the images are quantification of the stomatal aperture. Values are means ± sd; n = 20. Bar = 10 μm. B, Water loss from detached plants. Three-week-old plants of various genotypes were measured at different periods of time. Values are means ± sd; n = 3. C, Reduced drought tolerance of fhy3 and far1 mutant plants. Three-week-old soil-grown seedlings were subjected to dehydration by withholding water for 2 weeks and then rewatered normally for 3 d. Three independent assays were performed with similar results, and representative images are shown. WT, Wild type.
The impaired stomatal regulation of fhy3 and far1 mutants prompted us to further test whether their water loss was affected. We found that the detached plants of fhy3 and far1 lost water more quickly than those of the wild-type, with the effect being less pronounced for far1 (Fig. 8B). Next, when 3-week-old plants were exposed to dehydration by withholding water for 2 weeks, fhy3 and far1 mutant plants showed a more severe drought stress phenotype than did the wild type. After rewatering for 3 d, the majority of fhy3 and some of the far1 plants died, whereas all of the wild-type plants survived (Fig. 8C), indicating that FHY3 and FAR1 promote drought tolerance.
DISCUSSION
In this study, we collected molecular and genetic evidence that FHY3 and FAR1 are essential regulators of seed germination and ABA signaling that function by activating the expression of the ABI5 transcription factor. We show that fhy3 and/or far1 mutants are hyposensitive to ABA-mediated inhibition of seed germination and seedling greening (Fig. 2). The fhy3 mutant phenotype can be restored to the wild type by the overexpression of ABI5 (Fig. 5). At the molecular level, FHY3 and FAR1 physically bind to the promoter region of ABI5 through the FBS cis-element and directly activate its gene expression (Fig. 1). Moreover, the expression of several ABA- and stress-responsive marker genes was down-regulated by mutations in FHY3 and/or FAR1 during seed imbibition (Fig. 4). Interestingly, fhy3 and far1 mutants possess an altered seed germination response in the presence of relatively high concentrations of exogenous ABA. Since ABI5 is the direct target of FHY3 and FAR1, mutation of ABI5 also promotes seed germination in the presence of high concentrations of ABA (Finkelstein, 1994). In addition, we found that plants deficient in FHY3 or FAR1 are less sensitive to ABA-mediated stomatal movement than are wild-type plants; therefore, FHY3 and FAR1 confer increased resistance to drought (Fig. 8). The drought-sensitive phenotype of fhy3 may be partly caused by the reduced sensitivity of guard cell movement under drought stress conditions, which may induce the production of ABA. In agreement with this, FHY3 is highly expressed in guard cells (Fig. 6I). We propose that other targets of FHY3/FAR1 are involved in mediating these processes at the adult stage, as ABI5 is mainly expressed in seeds. Interestingly, ABI5 expression is induced by drought stress, and plants that overexpress this gene retained water more efficiently than did wild-type plants (Lopez-Molina et al., 2001). Nevertheless, our study reveals that FHY3 and FAR1 are positive regulators of ABA responses.
It should be noted that FHY3 and FAR1 have redundant functions in ABA-mediated seed germination, seedling growth, and drought responses, with FHY3 playing the predominant role. However, FAR1 might have an opposite effect on FHY3 in modulating seed germination in response to salt and osmotic stresses (Fig. 2). A recent study reported that FHY3, but not FAR1, functions in the early photomorphogenic UV-B response (Huang et al., 2012). Thus, these two proteins could have divergent roles likely through protein subfunctionalization (Lin et al., 2008). The functional diversity of homologous proteins was also observed for two other light signaling components, ELONGATED HYPOCOTYL5 (HY5) and HY5 HOMOLOGY (Holm et al., 2002; Sibout et al., 2006). Since plant salt and drought stress responses involve ABA-dependent and ABA-independent pathways (Liu et al., 1998; Kizis et al., 2001), we could not exclude the possibility that the hyposensitivity of fhy3 to salt and drought is due to ABA-independent signaling. It is worth noting that FAR1 interacts more strongly with the ABI5 promoter than does FHY3 (Fig. 1B), whereas the fhy3 mutant has stronger ABA-insensitive phenotypes than far1 (Fig. 2, A–C). This is likely due to a higher transcription activity of FHY3 compared with FAR1. A similar observation was also made regarding FHY3’s other direct targets, including FHY1 and HEMB1 in photomorphogenic response and chlorophyll biosynthesis, respectively (Lin et al., 2007; Tang et al., 2012).
Whereas light is an environmental signal, phytohormones such as ABA are endogenous cues that regulate diverse plant growth and developmental processes. The existence of a regulatory loop between light and ABA signaling pathways has been proposed (Seo et al., 2009). Red light decreases whereas far-red light augments endogenous ABA levels. It has been documented that PIL5, a phytochrome-interacting factor also known as PIF1, represses phyB-mediated seed germination partly by activating the expression of ABA biosynthetic genes and repressing an ABA catabolic gene, consequently increasing ABA levels (Oh et al., 2004, 2007). In addition, PIL5 interacts with ABI3 to activate SOMNUS (SOM) expression in imbibed seeds, suggesting that the SOM promoter integrates ABA and light signaling to regulate seed germination (Park et al., 2011). It has been shown that disruption of HY5 confers tolerance to the inhibitory effect of ABA on lateral root growth, seedling growth, and seed germination (Chen et al., 2008). Although interplay between light and ABA has been observed, the underlying molecular basis was hitherto largely unknown (Lau and Deng, 2010).
FHY3 and FAR1 were identified as key positive components in the phyA-mediated photomorphogenic pathway (Hudson et al., 1999; Wang and Deng, 2002). Later, they were shown to play essential roles in converting the light signal to regulate other plant growth and developmental programs, such as the circadian clock and chlorophyll synthesis (Li et al., 2011; Ouyang et al., 2011; Tang et al., 2012). Here, we demonstrate that FHY3 and FAR1 are also involved in ABA signaling. These two transcription factors thus act as a convergence point that integrates light and ABA signaling during seed germination and early seedling development. Consistent with this notion, FHY3 and FAR1 transcripts are up-regulated by light, ABA, and abiotic stresses (Tang et al., 2012; Fig. 6). The expression of ABI5 is also activated by ABA and light (Lopez-Molina et al., 2001; Chen et al., 2008). We propose that, in the presence of abiotic stresses (e.g. salt, osmotic, and drought), FHY3 and FAR1 transcription is induced; consequently, the expression of ABA-responsive and (or) stress-related genes (e.g. ABI5; Ouyang et al., 2011) is promoted, which up-regulates the ABA signaling network, resulting in adaptation of the plant to various environments by shaping their growth and development. This functionality is significant, since abiotic stresses affect plant biomass and productivity.
Studies demonstrated that the levels of ABI5 protein play important roles in mediating ABA signaling and are tightly regulated (Lopez-Molina et al., 2001, 2003; Stone et al., 2006; Lee et al., 2010; Dai et al., 2013). Our results support the notion that ABI5 is regulated at the transcriptional level and identify two transcription factors that directly bind to the ABI5 promoter. PIL5, ABI3, and HY5 also function as essential regulators upstream of ABI5 (Lopez-Molina et al., 2002; Chen et al., 2008; Oh et al., 2009). HY5 also directly activates ABI5 expression, and overexpressing ABI5 rescues ABA sensitivity in hy5 (Chen et al., 2008). Previous studies demonstrated that FHY3/FAR1 and HY5 physically interact and thereafter either coordinately or antagonistically regulate EARLY FLOWERING4 or FHY1/FHY1 HOMOLOG expression, respectively (Li et al., 2010, 2011). In these cases, the cis-elements of the FBS motif (bound by FHY3/FAR1) and the ACGT-containing element (bound by HY5) in the downstream promoters are close to each other (less than 20 bp away; Li et al., 2010, 2011). However, their respective cis-elements are more than 130 bp away in the ABI5 promoter, suggesting that FHY3/FAR1 and HY5 might not physically interact at the promoter of ABI5. Rather, these transcription factors likely have independent regulatory modes in mediating the ABA response. Consistent with this notion, we observed that, compared with the wild type, fhy3 seeds have a higher germination rate in the presence of relatively high concentrations of ABA (greater than 3 μm). Moreover, fhy3 mutant plants display reduced sensitivity to ABA-induced stomata movement and are less tolerant to drought stress than the control plants (Fig. 8), whereas the hy5 mutation does not have such effects (Chen et al., 2008). Therefore, FHY3 and HY5 play both overlapping and distinct roles regarding the regulation of plant growth and development in response to ABA.
Accumulating studies reveal that FHY3 and FAR1, two transposase-derived transcription factors, function broadly in the life of higher plants (Hudson et al., 1999; Wang and Deng, 2002; Allen et al., 2006; Li et al., 2011; Ouyang et al., 2011; Stirnberg et al., 2012; Tang et al., 2012; this study). FHY3 achieves these physiological responses largely through physically binding to the promoter of the corresponding target genes via the FBS motif (Ouyang et al., 2011). FHY3 might have additional effects on plant growth and development. Nevertheless, our study provides insight into the functional divergence of these transposase-derived proteins in plants during evolution.
MATERIALS AND METHODS
Plant Materials and Conditions
The fhy3-4, far1-2, and fhy3 far1 mutants are of the Arabidopsis (Arabidopsis thaliana) Nossen (NO) ecotype (Lin et al., 2007). 35S:GUS-FHY3 (Wang and Deng, 2002) and FHY3p:FHY3-GR (Lin et al., 2007) are transgenic plants in the fhy3-4 mutant background. FHY3p:GUS (Lin and Wang, 2004) and 35S:ABI5 (Dai et al., 2013) were described previously. fhy3/35S:ABI5 was generated by genetic crossing, and a homozygous line was used. After sterilization, seeds were sown on MS medium containing 1% Suc, 0.8% agar, and various concentrations of ABA, NaCl, or mannitol as described. Seeds were incubated at 4°C in darkness for 3 d, followed by irradiation for 9 h with white light to promote uniform germination.
Seed Germination and Root Growth Assay
Seeds of different genotypes were harvested on the same day from plants grown in identical conditions. Seed germination was observed with a microscope and determined based on the appearance of radicle protrusion. The greening rate was determined by calculating the percentage of seedlings with dark green cotyledons. For the root elongation assay, seedlings were grown on normal MS plates for 2 d and were then transferred to plates containing the indicated concentrations of ABA for an additional 7 d before measurement.
Stomatal Aperture Measurement
Epidermal peels from rosette leaves were floated in KCl-Tris solution (50 mm KCl, 10 mm MES, and using 1 M of pH 8.0 Tris-HCl to adjust the solution pH to 5.7) and exposed to light (100 μmol m−2 s−1) or kept in the dark for 3 h to induce stomatal opening or closure, respectively. Subsequently, the peels were incubated in KCl-Tris solution with or without 50 m ABA under the light condition for an additional 3 h. Stomata were photographed with a dissecting microscope (Olympus), and stomatal aperture was measured using NIH ImageJ software (http://rsbweb.nih.gov/ij/).
Water Loss and Drought Tolerance Assays
Rosette leaves from 4-week-old long-day-grown plants were detached and weighed immediately. The leaves were then placed on a laboratory bench (50% relative humidity) and weighed at various time points. Relative water loss was expressed as the percentage of fresh weight to the initial weight of the leaves.
For drought tolerance experiments, plants were grown at similar density in pots under identical growth conditions for 3 weeks and exposed to dehydration by withholding water for an additional 2 weeks. They were then rewatered to examine the survival status.
Plasmid Construction
To produce LacZ reporters under the control of the ABI5 promoter with a wild-type or mutant FBS motif, 39-bp oligonucleotides were synthesized as two complementary primers (ABI5wF and ABI5wR for the wild type and ABI5mF and ABI5mR for the mutant; for sequences, see Supplemental Table S1) with an EcoRI site overhang at the 5′ end and an XhoI site overhang at the 3′ end. The annealed DNA was ligated into the EcoRI-XhoI sites of pLacZi2µ (Lin et al., 2007), resulting in ABI5wt:LacZ and ABI5m:LacZ, respectively.
To generate the LUC reporter gene driven by the ABI5 promoter, a 2.1-kb fragment upstream of the ABI5 ATG translational start codon was PCR amplified with primers ABI5PF and ABI5PR from ecotype Columbia genomic DNA. The PCR fragment was inserted into the pGEM-T Easy (Promega) vector to produce pGEM-ABI5P and verified by sequencing. The promoter fragment was released from pGEM-ABI5P cut with HindIII and BamHI and then ligated into the HindIII-BamHI site of the LUC vector (Chen et al., 2013) to produce ABI5p:LUC.
The yeast vectors AD-FHY3 and AD-FAR1 and the recombinant protein construct GST-FHY3N were described previously (Lin et al., 2007).
Yeast One-Hybrid Assay
The activation domain fusion constructs (AD-FAR1 and AD-FHY3) were cotransformed with the LacZ reporter plasmids (ABI5wt:LacZ and ABI5m:LacZ) into yeast strain EGY48. Transformants were grown on synthetic dropout plates without tryptophan or uracil containing 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside for color development.
ChIP Assay
Five-day-old 35S:GUS-FHY3 transgenic seedlings were used in the ChIP assay following a previously described procedure (Tang et al., 2012). Chromatin complexes were incubated with anti-GUS (Invitrogen) or the serum control. The precipitated DNA fragments were quantified by real-time PCR using the primers shown in Supplemental Table S1.
EMSA
EMSA was performed as described by Tang et al. (2012).
LUC Activity Assay
The ABI5p:LUC reporter plasmid and the 35S:GUS internal control were cotransformed into Arabidopsis protoplasts isolated from wild-type and fhy3-4 mutant seedlings. After overnight incubation, the activity of LUC and GUS was quantified using a Modulus Luminometer/Fluometer (Promega) as described previously (Tang et al., 2012). Relative ABI5 expression was expressed as the ratio of LUC to GUS.
GUS Histochemical Analysis
Seeds or seedlings of the FHY3p:GUS transgenic line were subjected to GUS staining as described previously (Jing et al., 2013).
RNA Extraction and qRT-PCR
The treatment of seeds or seedlings is described in the text. Plant total RNA was extracted using an RNA extraction kit (Tiangen), and the first-strand complementary DNA was synthesized by reverse transcriptase (Invitrogen). Real-time PCR was performed using the SYBR Premix ExTaq kit (Takara) and a LightCycler 480 thermal cycler (Roche), following the manufacturers’ instructions. Three biological replicates were performed for each sample, and the expression levels were normalized to those of UBQ. Primers are listed in Supplemental Table S1.
The Arabidopsis Genome Initiative locus numbers for the major genes discussed in this article are as follows: FHY3 (At3g22170), FAR1 (At4g15090), ABI5 (At2g36270), ABI1 (At4g26080), ABI2 (At5g57050), ABF3 (At4g34000), KIN1 (At1g14370), COR47 (At1g20440), DREB2A (AT5g05410), RAB18 (At5g66400), RD22 (At5g25610), RD29B (AT5g52300), and UBQ1 (At3g52590).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. ABI5 expression in FHY3p:FHY3-GR transgenic seedlings.
Supplemental Figure S2. Expression of FHY3, FAR1, and ABI5 during seed germination.
Supplemental Figure S3. Kinetics of seed germination on medium containing high concentrations of ABA.
Supplemental Figure S4. Regulation of stomatal aperture in the wild type and the fhy3 mutant by ABA.
Supplemental Table S1. List of primers used in this study.
Glossary
- FBS
FHY3/FAR1-binding site
- ABA
abscisic acid
- ChIP
chromatin immunoprecipitation
- EMSA
electrophoretic mobility shift assay
- GST
glutathione S-transferase
- qRT
quantitative reverse transcription
- MS
Murashige and Skoog
- NO
Nossen
References
- Allen T, Koustenis A, Theodorou G, Somers DE, Kay SA, Whitelam GC, Devlin PF. (2006) Arabidopsis FHY3 specifically gates phytochrome signaling to the circadian clock. Plant Cell 18: 2506–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carles C, Bies-Etheve N, Aspart L, Léon-Kloosterziel KM, Koornneef M, Echeverria M, Delseny M. (2002) Regulation of Arabidopsis thaliana Em genes: role of ABI5. Plant J 30: 373–383 [DOI] [PubMed] [Google Scholar]
- Chen D, Xu G, Tang W, Jing Y, Ji Q, Fei Z, Lin R. (2013) Antagonistic bHLH/bZIP transcription factors form transcriptional modules that integrate light and reactive oxygen species signaling in Arabidopsis. Plant Cell 25: 1657–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zhang J, Neff MM, Hong SW, Zhang H, Deng XW, Xiong L. (2008) Integration of light and abscisic acid signaling during seed germination and early seedling development. Proc Natl Acad Sci USA 105: 4495–4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory J. (2010) Light signal transduction: an infinite spectrum of possibilities. Plant J 61: 982–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. (2010) Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol 61: 651–679 [DOI] [PubMed] [Google Scholar]
- Dai M, Xue Q, McCray T, Margavage K, Chen F, Lee JH, Nezames CD, Guo L, Terzaghi W, Wan J, et al. (2013) The PP6 phosphatase regulates ABI5 phosphorylation and abscisic acid signaling in Arabidopsis. Plant Cell 25: 517–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R, Reeves W, Ariizumi T, Steber C. (2008) Molecular aspects of seed dormancy. Annu Rev Plant Biol 59: 387–415 [DOI] [PubMed] [Google Scholar]
- Finkelstein RR. (1994) Mutation at two new Arabidopsis ABA response loci are similar to the abi3 mutations. Plant J 5: 765–771 [Google Scholar]
- Finkelstein RR, Gampala SSL, Rock CD. (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell (Suppl) 14: S15–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR, Lynch TJ. (2000) The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell 12: 599–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR, Rock CD (2002) Abscisic acid biosynthesis and response. The Arabidopsis Book 1:e0058, /10.1199/tab.0058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour SJ, Artus NN, Thomashow MF. (1992) cDNA sequence analysis and expression of two cold-regulated genes of Arabidopsis thaliana. Plant Mol Biol 18: 13–21 [DOI] [PubMed] [Google Scholar]
- Gosti F, Beaudoin N, Serizet C, Webb AAR, Vartanian N, Giraudat J. (1999) ABI1 protein phosphatase 2C is a negative regulator of abscisic acid signaling. Plant Cell 11: 1897–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltbrunner A, Tscheuschler A, Viczián A, Kunkel T, Kircher S, Schäfer E. (2006) FHY1 and FHL act together to mediate nuclear accumulation of the phytochrome A photoreceptor. Plant Cell Physiol 47: 1023–1034 [DOI] [PubMed] [Google Scholar]
- Holm M, Ma L-G, Qu L-J, Deng XW. (2002) Two interacting bZIP proteins are direct targets of COP1-mediated control of light-dependent gene expression in Arabidopsis. Genes Dev 16: 1247–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Ouyang X, Yang P, Lau OS, Li G, Li J, Chen H, Deng XW. (2012) Arabidopsis FHY3 and HY5 positively mediate induction of COP1 transcription in response to photomorphogenic UV-B light. Plant Cell 24: 4590–4606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson M, Ringli C, Boylan MT, Quail PH. (1999) The FAR1 locus encodes a novel nuclear protein specific to phytochrome A signaling. Genes Dev 13: 2017–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson ME, Lisch DR, Quail PH. (2003) The FHY3 and FAR1 genes encode transposase-related proteins involved in regulation of gene expression by the phytochrome A-signaling pathway. Plant J 34: 453–471 [DOI] [PubMed] [Google Scholar]
- Jing Y, Zhang D, Wang X, Tang W, Wang W, Huai J, Xu G, Chen D, Li Y, Lin R. (2013) Arabidopsis chromatin remodeling factor PICKLE interacts with transcription factor HY5 to regulate hypocotyl cell elongation. Plant Cell 25: 242–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JY, Choi HI, Im MY, Kim SY. (2002) Arabidopsis basic leucine zipper proteins that mediate stress-responsive abscisic acid signaling. Plant Cell 14: 343–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizis D, Lumbreras V, Pagès M. (2001) Role of AP2/EREBP transcription factors in gene regulation during abiotic stress. FEBS Lett 498: 187–189 [DOI] [PubMed] [Google Scholar]
- Kurkela S, Borg-Franck M. (1992) Structure and expression of kin2, one of two cold- and ABA-induced genes of Arabidopsis thaliana. Plant Mol Biol 19: 689–692 [DOI] [PubMed] [Google Scholar]
- Lång V, Palva ET. (1992) The expression of a rab-related gene, rab18, is induced by abscisic acid during the cold acclimation process of Arabidopsis thaliana (L.) Heynh. Plant Mol Biol 20: 951–962 [DOI] [PubMed] [Google Scholar]
- Lau OS, Deng XW. (2010) Plant hormone signaling lightens up: integrators of light and hormones. Curr Opin Plant Biol 13: 571–577 [DOI] [PubMed] [Google Scholar]
- Lee JH, Yoon HJ, Terzaghi W, Martinez C, Dai MQ, Li J, Byun MO, Deng XW. (2010) DWA1 and DWA2, two Arabidopsis DWD protein components of CUL4-based E3 ligases, act together as negative regulators in ABA signal transduction. Plant Cell 22: 1716–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J, Giraudat J. (1998) Abscisic acid signal transduction. Annu Rev Plant Physiol Plant Mol Biol 49: 199–222 [DOI] [PubMed] [Google Scholar]
- Leung J, Merlot S, Giraudat J. (1997) The Arabidopsis ABSCISIC ACID-INSENSITIVE2 (ABI2) and ABI1 genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction. Plant Cell 9: 759–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Siddiqui H, Teng Y, Lin R, Wan XY, Li J, Lau OS, Ouyang X, Dai MQ, Wan J, et al. (2011) Coordinated transcriptional regulation underlying the circadian clock in Arabidopsis. Nat Cell Biol 13: 616–622 [DOI] [PubMed] [Google Scholar]
- Li J, Li G, Gao S, Martinez C, He G, Zhou Z, Huang X, Lee JH, Zhang H, Shen Y, et al. (2010) Arabidopsis transcription factor ELONGATED HYPOCOTYL5 plays a role in the feedback regulation of phytochrome A signaling. Plant Cell 22: 3634–3649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Ding L, Casola C, Ripoll DR, Feschotte C, Wang H. (2007) Transposase-derived transcription factors regulate light signaling in Arabidopsis. Science 318: 1302–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Teng Y, Park HJ, Ding L, Black C, Fang P, Wang H. (2008) Discrete and essential roles of the multiple domains of Arabidopsis FHY3 in mediating phytochrome A signal transduction. Plant Physiol 148: 981–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Wang H. (2004) Arabidopsis FHY3/FAR1 gene family and distinct roles of its members in light control of Arabidopsis development. Plant Physiol 136: 4010–4022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. (1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10: 1391–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Molina L, Chua NH. (2000) A null mutation in a bZIP factor confers ABA-insensitivity in Arabidopsis thaliana. Plant Cell Physiol 41: 541–547 [DOI] [PubMed] [Google Scholar]
- Lopez-Molina L, Mongrand S, Chua NH. (2001) A postgermination developmental arrest checkpoint is mediated by abscisic acid and requires the ABI5 transcription factor in Arabidopsis. Proc Natl Acad Sci USA 98: 4782–4787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Molina L, Mongrand S, Kinoshita N, Chua NH. (2003) AFP is a novel negative regulator of ABA signaling that promotes ABI5 protein degradation. Genes Dev 17: 410–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Molina L, Mongrand S, McLachlin DT, Chait BT, Chua NH. (2002) ABI5 acts downstream of ABI3 to execute an ABA-dependent growth arrest during germination. Plant J 32: 317–328 [DOI] [PubMed] [Google Scholar]
- Oh E, Kang H, Yamaguchi S, Park J, Lee D, Kamiya Y, Choi G. (2009) Genome-wide analysis of genes targeted by PHYTOCHROME INTERACTING FACTOR 3-LIKE5 during seed germination in Arabidopsis. Plant Cell 21: 403–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E, Kim J, Park E, Kim JI, Kang C, Choi G. (2004) PIL5, a phytochrome-interacting basic helix-loop-helix protein, is a key negative regulator of seed germination in Arabidopsis thaliana. Plant Cell 16: 3045–3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E, Yamaguchi S, Hu J, Yusuke J, Jung B, Paik I, Lee H-S, Sun T-P, Kamiya Y, Choi G. (2007) PIL5, a phytochrome-interacting bHLH protein, regulates gibberellin responsiveness by binding directly to the GAI and RGA promoters in Arabidopsis seeds. Plant Cell 19: 1192–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang X, Li J, Li G, Li B, Chen B, Shen H, Huang X, Mo X, Wan X, Lin R, et al. (2011) Genome-wide binding site analysis of FAR-RED ELONGATED HYPOCOTYL3 reveals its novel function in Arabidopsis development. Plant Cell 23: 2514–2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Lee N, Kim W, Lim S, Choi G. (2011) ABI3 and PIL5 collaboratively activate the expression of SOMNUS by directly binding to its promoter in imbibed Arabidopsis seeds. Plant Cell 23: 1404–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo M, Hanada A, Kuwahara A, Endo A, Okamoto M, Yamauchi Y, North H, Marion-Poll A, Sun TP, Koshiba T, et al. (2006) Regulation of hormone metabolism in Arabidopsis seeds: phytochrome regulation of abscisic acid metabolism and abscisic acid regulation of gibberellin metabolism. Plant J 48: 354–366 [DOI] [PubMed] [Google Scholar]
- Seo M, Nambara E, Choi G, Yamaguchi S. (2009) Interaction of light and hormone signals in germinating seeds. Plant Mol Biol 69: 463–472 [DOI] [PubMed] [Google Scholar]
- Sibout R, Sukumar P, Hettiarachchi C, Holm M, Muday GK, Hardtke CS. (2006) Opposite root growth phenotypes of hy5 versus hy5 hyh mutants correlate with increased constitutive auxin signaling. PLoS Genet 2: e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirnberg P, Zhao S, Williamson L, Ward S, Leyser O. (2012) FHY3 promotes shoot branching and stress tolerance in Arabidopsis in an AXR1-dependent manner. Plant J 71: 907–920 [DOI] [PubMed] [Google Scholar]
- Stone SL, Williams LA, Farmer LM, Vierstra RD, Callis J. (2006) KEEP ON GOING, a RING E3 ligase essential for Arabidopsis growth and development, is involved in abscisic acid signaling. Plant Cell 18: 3415–3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Wang W, Chen D, Ji Q, Jing Y, Wang H, Lin R. (2012) Transposase-derived proteins FHY3/FAR1 interact with PHYTOCHROME-INTERACTING FACTOR1 to regulate chlorophyll biosynthesis by modulating HEMB1 during deetiolation in Arabidopsis. Plant Cell 24: 1984–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Deng XW. (2002) Arabidopsis FHY3 defines a key phytochrome A signaling component directly interacting with its homologous partner FAR1. EMBO J 21: 1339–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ. (2007) An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS ONE 2: e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K. (1993) The plant hormone abscisic acid mediates the drought-induced expression but not the seed-specific expression of rd22, a gene responsive to dehydration stress in Arabidopsis thaliana. Mol Gen Genet 238: 17–25 [DOI] [PubMed] [Google Scholar]