Abstract
Fasciolosis is a zoonosis caused by the trematode Fasciola hepatica, prevalent in cattle, that is actually emerging as a cause of disease in humans. The goal of this work was to describe the characteristics of fasciolosis in arroyo El Juncal region, La Toma, San Luis province, Argentina. In order to get this objective, a transversal, quantitative study was carried out by a fieldwork that allowed the collection of data, human, animal, and environmental samples. The materials were processed by direct, immunological and/or molecular diagnostic techniques. According to the geographical characteristics and in presence of all the definitive and intermediate hosts, reservoirs, and sources of infection, it was possible to describe the persistence of fasciolosis in the area. The prevalence was 11.90 % in humans (by serology), 5.26 % in cattle (by coprological analysis) and 61.76 % in snails (by PCR). The situation that was found for this area indicates that any measure of intervention for the control of this zoonosis should be adopted by multidisciplinary teams.
Keywords: Fasciolosis, Fasciola hepatica, Epidemiology
Introduction
Fasciolosis is a zoonotic parasitic disease caused by the trematode Fasciola hepatica which is common in animals and is actually emerging as a cause of disease in humans.
Fasciolosis hepatica is distributed worldwide (Chen and Mott 1990; Rim et al. 1994; Organización Mundial de la Salud 1995). Human fasciolosis occurs in endemic forms or as outbreaks and it has been registered in numerous countries of America, Europe, Africa, and Asia (Organización Mundial de la Salud 1995; Esteban et al. 1998; Mas-Coma et al. 1999b). In Argentina, the situation of human fascioliasis has been analysed in a recent exhaustive and critical review (Mera y Sierra et al. 2011) of 619 reported cases, their majority (97.7 %) from high altitudes, with diagnosis mainly relied on egg finding, followed by serology, intradermal reaction, surgery, and erratic fluke observation.
The effect of this parasitic disease over human health depends on parasite charge and duration of the infection. The migration of juveniles thought the parenquima of the liver produces traumatic and necrotic lesions. The chronic phase starts when the worms reach the bile ducts: progressive inflammation leads to fibrosis and thickening of the walls of the biliary system and of the surrounding hepatic tissue. Biliary colic pain due to blockage of the bile ducts and jaundice are possible complications (Villegas et al. 2012). In severe infections, with a high number of parasites, biliary stasis, liver atrophy and periportal cirrhosis have been reported. In chronic cases frequently occurs cholecystitis and cholelithiasis (Acha and Szyfres 1986; Chen and Mott 1990; Arjona et al. 1995; Bryan and Michelson 1995; Binkley and Sinniah 1997; Mas-Coma et al. 1999a, Villegas et al. 2012).
Diagnosis is usually carried out by serial coprological analysis, with the disadvantages of low sensitivity and no detection in the acute phase (Knobloch et al. 1985; Apt et al. 1992; Mas-Coma et al. 1999a). Numerous immunodiagnostic techniques have been described and applied in different countries (Knobloch 1985; Espino et al. 1987; Espino Hernández et al. 1991; Hillyer et al. 1992; Sampaio Silva et al. 1996; O′Neill et al. 1998; O′Neill et al. 1999; Carnevale et al. 2001a, 2001b; Figueroa-Santiago et al. 2011).
In animals infection occurs in cattle, sheep, horses, donkeys, camelids, pigs, cervids and goats, with severe economic losses due to mortality, weight loss, reproductive efficiency diminution, and liver seizure (Ngategize et al. 1993; Behm and Sangster 1999). The morbidity and mortality rates vary between regions. In endemic areas it is common to find infection rates over 50 %. The more susceptible domestic species is sheep, followed by cattle (Acha and Szyfres 1986; Olaechea 1994). The diagnosis in animals is based on necropsy, by parasite observation. The coprological analysis is also employed and immunological test have been developed for antibody detection against the parasite.
The main strategies for liver fluke control have been grazing management to avoid intermediate host snails habitats and the use of flukicide treatments (Mas-Coma et al. 1999a). In Argentina, triclabendazole resistance of F. hepatica in calves has been described (Olaechea et al. 2011).
The delimitation of this work was performed according to the previous identification of human cases of F. hepatica infection in the study area and the high prevalence reported for bovine fasciolosis (Rossanigo et al. 1983). The main goal was to describe the characteristics of fasciolosis in the region of Arroyo El Juncal, La Toma, Province of San Luis. The specific objectives included to determine the prevalence of human fasciolosis in the inhabitants of the area, to estimate fasciolosis in animals, to identify factors involved in F. hepatica life cycle installation, to apply different diagnostic methods to demonstrate the presence of F. hepatica in definitive and intermediate hosts including direct, immunological and molecular techniques and to identify the circulating haplotype of the parasite.
Materials and methods
Study area
The study was carried out in the area of Arroyo El Juncal, La Toma, Province of San Luis, Argentina, at an altitude between 500 and 600 m above sea level (32º57′24″LS; 65º41′39″LO), occupying about 600 km2. This is a rocky region in the south while a zone with vegetation is located in the North. The hydrography belongs to the Quinto river basin, with the rivers de la Carpa and Rosario as permanent and a high number of intermittent brooks that depend on seasonal rainfall.
According to the physiognomy of natural vegetation in the area, represented mainly by grasslands and shrublands, the main use of soils is the breeding of cattle, sheep, goats and horses.
Human activities in the area are characteristic of a rural population, with no towns but detached isolated settlements in the area where their labour sources are located.
The roads of the area are related to human activity that takes place, with unpaved roads, and without railways.
During the month of March in which the fieldwork was conducted, for the study area there was an average temperature of 17.4 °C and monthly rainfall of 48.5 mm. The area is between the isohyets of 500–600 mm.
Description of the study
The study was transversal, quantitative, employing essentially primary data.
We included in the study all the human population living in the bank of the stream El Juncal (n = 42), cattle, sheep and horses grazing in the area (n = 19), aquatic vegetables of human consumption, snails compatible with the genus Lymnaea (n = 34).
Primary data
Geographic information
For detailed location of the study area, we consulted the writings and drawings of some of the properties visited provided at owner’s discretion. With these data and information provided verbally locating schemes were made for each of the visited families.
Households were classified into the following types: house, with direct access to the outside, with ceramic, tile, mosaic, wood or cement floor, water supply piped into the home and toilet flushing; hut, housing with direct access to the outside, made of inferior materials or waste; “rancho”, with direct access to the outside, with adobe walls, dirt floors and thatched or tin roof, typical of rural areas.
Humans
We employed a questionnaire designed for data collection about personal characteristics, habits and houses. Each individual was evaluated with a clinical history in order to describe symptoms and signs such as dyspepsia, diarrhoea, jaundice, abdominal pain, vomiting, hepatomegaly, fever. We employed an informed consent for the study.
Faecal samples were collected daily for 3 days. Stool specimens were fixed in 5 % formalin saline solution. Faeces were processed by formalin-ether concentration (Telemann 1901), flotation in salt (Willis 1921) with superficial and bottom microscopic observation, eggs quantification (Stoll and Hausheer 1926), concentration in Sheather’s solution, Kinyoun and modified Trichrome stain (Weber et al. 1992).
We also obtained serum samples and thin blood smears. Serum samples were assayed by ELISA for the detection of anti-recombinant procathepsin L1 of F. hepatica (Fh-rproCL1) antibodies (Carnevale et al. 2001a). The expression and purification of the antigen was performed according to our previous development (Carnevale et al. 2001a). Thin blood smears were fixed and stained for eosinophilia level determination.
Livestock animals
Samples were collected from the soil after defecation of each animal and stored at 4 °C. These fresh faeces were weighed and 1 g of sample was dissolved in 5 % formalin saline solution. After homogenisation, samples were filtered with double gauze and the following steps were carried out essentially as described for human samples. We also employed 5 g of samples for simple sedimentation.
Snails
Snails morphologically compatible with the genus Lymnaea were collected from the water bodies by direct observation of the surface and margins. Sampling was carried out by time counting during 30 min by the same observer in an extension of 5 m on both margins.
Collected snails were taxonomically determined by shell and internal organs characteristics (Paraense 1976, 1982) by professionals from the Laboratorio de Parasitología General, Unidad de Ecología de Reservorios y Vectores de Parásitos, Departamento de Ecología, Genética y Evolución, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires. Specimens were analysed by dissection in order to verify infection due to F. hepatica or other trematodes.
The soft parts of the snails were employed for DNA extraction according to Cucher et al. (2006) with inhibitors removal.
The PCR assay was used to determine F. hepatica infection accordingly to our previous published protocols (Cucher et al. 2006), based on the amplification of a 405 bp fragment of the cytochrome c oxidase subunit 1 gene (CO1). Twenty microlitres of the amplification products were electrophoretically resolved in 1.2 % agarose gels and stained with ethidium bromide.
Aquatic vegetables
Watercress plants were collected in different locations from the water bodies, especially near the houses. These watercress plants (Rorippa nasturtium-aquaticum, (L) Hayek), were washed and the washing solutions were concentrated by centrifugation. Pellets were observed by light microscopy at low magnification (100×) for metacercariae examination. Molecular diagnosis was essentially performed as described above for snail samples.
Phylogenetic analysis
In order to determine the F. hepatica haplotype present in the area, a 447 bp portion of the CO1 gene of F. hepatica was also amplified from two infected snails with primers JB3 (5′-TTTTTTGGGCATCCTGAGGTTTAT-3′) and JB4.5 (5′-TAAAGAAAGAACATAATGAAAATG-3′) (Bowles et al. 1992). The procedure was carried out according to a previous report (Ai et al. 2011). Briefly, reactions were carried out in a Biorad thermocycler, in a total volume of 25 μl containing 2.5 μM each primer, 200 μM each dNTP, 1.25 U Taq DNA polymerase (Fermentas International Inc.), 50 mM KCl, 10 mM Tris–HCl pH 8.8, 0.08 % Nonidet P40, 2 mM MgCl2, 1 μl template DNA. Cycling conditions consisted of an initial denaturation step at 94 °C for 5 min; then 35 cycles of 94 °C for 30 s (denaturation); 55 °C for 30 s (annealing); 72 °C for 30 s (extension); followed by a final extension at 72 °C for 5 min. Eight microlitres of amplification products were analysed by 2 % agarose gel electrophoresis, stained with ethidium bromide and UV visualised. Amplicons were purified from 1 % agarose gels with the centrifugal filter device Ultrafree®-DA (Millipore) and sequenced in both directions using a Hitachi 3130XL Genetic Analyzer (Applied Biosystems) with the BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) employing the same primers used in the PCR.
Sequences from F. hepatica, F. gigantica and Paragonimius westermani mitochondrial CO1 gene were retrieved from NCBI (http://www.ncbi.nlm.nih.gov/). CO1 sequences were analysed by MaGat software and redundant sequences were removed. The remained non redundant sequences were aligned with ClustalX (v2.0.12) and manually edited with BioEdit (v7.1.3). They were 313 bp in length from 17 isolates (including two from our study). Using Maximum likelihood and maximum parsimony analysis, implemented in PHYLIP package phylogenetic trees were constructed with P. westermani as outgroup and 1,000 bootstrap replicates. The same tree topology was obtained with both methods. Also, we used F. gigantica CO1 sequences (Accession no. GU112464) as outgroup and found similar group of sequences.
Secondary data collection
With the aim to complement the identification of factors that influence F. hepatica life cycle, weather (average temperature and rainfall), geographic (hydrography, topography, fauna and flora), agricultural and farming activities and demographic data were collected.
Data analysis
All statistical analyses were carried out employing the Epi Info, STATS and EPIDAT softwares. The prevalence of infection calculated by different methods was compared by the χ2 test. Concordance between methods was calculated using the kappa statistic. Association of disease with personal habits and housing characteristics was studied by Odds Ratio (OR) y χ2 test.
Complementary activities
A diary record of the activities was taken, including population behaviour related to the work. Population and health personnel’s training was also carried out.
Results
Housing
We studied 23 families who lived predominantly in “ranchos” (56.52 %; 13/23); with a lower number in houses (39.13 %; 9/23) or huts (4.35 %; 1/23).
Most of the homes in the area lacked drinking water and electricity. The main drinking water supply was surface water without filtration. With regard to sewage disposal, the main type corresponded to the latrine (56.52 %; 13/23).
The scarcity of resources was related to the fact that only in 30.43 % (7/23) of the visited households the family income was fixed. The variable income was a reflection of the work activity of the household head who worked in rural tasks.
Animal breeding was recorded in 91.30 % (21/23) of the households, predominating companion animals (100.00 %), followed by cattle (95.23 %) and poultry (61.90 %).
Study population
The study population included 42 individuals, 54.76 % (23/42) males and 45.24 % (19/42) females. They represented all people living in the area. Among males, 47.83 % (11/23) were younger than 15 years while among females, they were 31.58 % (6/19).
All people more than 15 years (25 inhabitants) were active workers. The main occupational category among men was represented entirely by farm workers, while among women corresponded to housewives.
Data on education of the study population indicated that all children between 6 and 15 years (17 persons) were attending the primary level.
The information collected about the habits of people (assuming a total of 40 since two were younger than 3 years and could not answer the questions in the questionnaire) indicated that 97.50 % (39/40) recognised wild edible plants in the stream identifying them as watercress plants. Among those who recognised the watercress, 89.74 % (35/39) reported that ate it, with this intake throughout the year at 91.43 % (32/35) and only during the summer at 8.57 % (3/35).
Animal slaughter was done by 77.50 % (31/40) of the population. Among those surveyed, 80.00 % (32/40) knew F. hepatica, and 77.50 % (31/40) reported having seen these worms in viscera.
When collecting information about the use of boiled water, only 7.50 % (3/40) indicated that he did for consumption, while no one used it for vegetable washing or personal hygiene.
Contact with animals was reported by the 95.00 % (38/40) of surveyed persons, among them being the 71.05 % (27/38) only near the houses and the 28.95 % (11/38) near the houses and during work. Contact with birds was indicated by the 100.00 % (40/40), with cattle in 95.00 % (38/40), with sheep in 80.00 % (32/40), with goats in 55.00 % (22/40), with horses in 22.50 % (9/40), with pigs in 20.00 % (8/40), with mules in 10.00 % (4/40).
Humans
Laboratory diagnosis
The microscopic observations showed that only one patient had parasite eggs in faeces. The ELISA for detection of antibodies against Fh-rproCL1 revealed five positive patients including the one detected by stool examination. Therefore, by stool examination, the prevalence of infection with F. hepatica in the inhabitants of the study area was 2.38 % (95 % CI = 1.16–3.60), whereas this value rose to 11.90 % (95 % CI = 10.65–13.15) when antibodies were detected by ELISA. The difference between the two techniques was statistically significant (p < 0.05). The analysis of concordance between both methods resulted in a kappa value of 0.31 (95 % CI = −0.16 to 0.77), with an observed agreement of 90.48 %, kappa minimum of −0.05 and maximum of 0.81.
Association with personal variables, habits and housing
Among the data collected by questionnaire, we selected those fields that could be associated with infection by F. hepatica in humans.
No statistically significant associations were found with the variables of sex (OR = 1.82, 95 % CI = 0.32–10.45, p = 0.50), age (OR = 9.39, 95 % CI = 0.48–182.03, p = 0.08), watercress consumption (OR = 0.43, 95 % CI = 0.05–3.48, p = 0.42), water source (OR = 1.48, 95 % CI = 0.07–31.44, p = 0.80), consumption of unboiled water (OR = 0.24, 95 % CI = 0.02–3.31, p = 0.26).
Association with symptoms
There were not significant positive associations with the signs and symptoms of fever (OR = 0.53, 95 % CI = 0.05–5.55, p = 0.38), jaundice (OR = 0.87, 95 % CI = 0.12–6.32, p = 0.89), urticaria (OR = 3.46, 95 % CI = 0.56–21.57, p = 0.17), vomiting (OR = 0.68, 95 % CI = 0.09–4.87, p = 0.70), hepatomegaly (OR = 1.00, 95 % CI = 0.14–7.30, p = 1.00), splenomegaly (OR = 1.27, 95 % CI = 0.11–14.95, p = 0.59), and eosinophilia (OR = 2.14, 95 % CI = 0.36–12.72, p = 0.39).
There were statistically significant negative associations with abdominal pain (OR = 0.05, 95 % CI = 0.01–0.40, p = 0.01) and diarrhoea (OR = 0.07, 95 % CI = 0.01–0.51, p = 0.01).
There was also no association with previous cholecystectomy (OR = 1.62, 95 % CI = 0.21–12.32, p = 0.64).
Description of patients infected with F. hepatica
The characteristics of the five patients with fasciolosis detected by ELISA are described in Table 1.
Table 1.
Description of five cases of fasciolosis diagnosed by ELISA in the study area
| Case number | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Sex/age (years) | Female (52) | Female (49) | Male (58) | Female (39) | Male (22) |
| Educational level | Incomplete tertiary | Primary | Primary | Complete tertiary | Primary |
| Working activity | Housewife | Housewife | Ambulance driver | Nurse | Farm worker |
| Time of residence (years) | 33 | 49 | 58 | 39 | 22 |
| Water supply for consumption | Boiled surface water without filtration | Unboiled surface water without filtration | Unboiled surface water without filtration | Stream, without filtration or boiling | Well water bucket without filtration or boiling |
| Raised animals | Cattle, horses, birds, dogs, cats | Cattle, sheep, poultry, dogs | Cattle, sheep, goats, pigs | Cattle, sheep, goats, pigs, poultry, companion animals | Cattle, sheep, goats, mules, poultry, dogs, cats |
| Contact with animals | In the peridomestic area | In the peridomestic area | In the dwellings | In the peridomestic area | In the peridomestic area and at work |
| Watercress eating | During all the year | During all the year | During all the year | During all the year | No |
| Know F. hepatica? | No | Yes | Yes | Yes | Yes |
| Clinical symptoms and signs | RUQ abdominal pain, vomiting, diarrhoea, and hepatomegaly | Asymptomatic | Abdominal pain, diarrhoea, vomiting and painful hepatomegaly | Jaundice, RUQ abdominal pain, diarrhoea | Asymptomatic |
| Stool examination | F. hepatica eggs (epg = 100) | Endolimax nana cysts | Microsporidia spores* | Negative | Negative |
| ELISA for F. hepatica (A 410) | Positive (0.705) | Positive (0.458) | Positive (0.498) | Positive (0.475) | Positive (0.581) |
| Eosinophilia (%) | 34 | 2 | 7 | 2 | 2 |
| Additional information | Previous abdominal US without diagnosis. Repeated US showed the presence of F. hepatica adult worms, thickening and dilation of bile ducts, gallbladder enlarged and thickened wall | ND | Previous cholecystectomy | Previous abdominal US without diagnosis |
EPG eggs per gram; RUQ right upper quadrant; US ultrasonography; ND no data; A 410 absorbance value at 410 nm (cut-off = 0.410)
* Enterocytotoon bieneusi confirmed by PCR
Farm animals
During the fieldwork faecal samples were collected from grazing animals corresponding to nine cows, three calves, six sheep and one horse.
Only one sample was positive for F. hepatica eggs, belonging to a bovine (cow). Therefore, by stool examination, the prevalence of infection with F. hepatica in farm animals was estimated at 5.26 % (95 % CI = 2.57–7.96), and referred exclusively to cattle was 8.33 % (95 % CI = 4.07–12.59).
Intermediate hosts (snails)
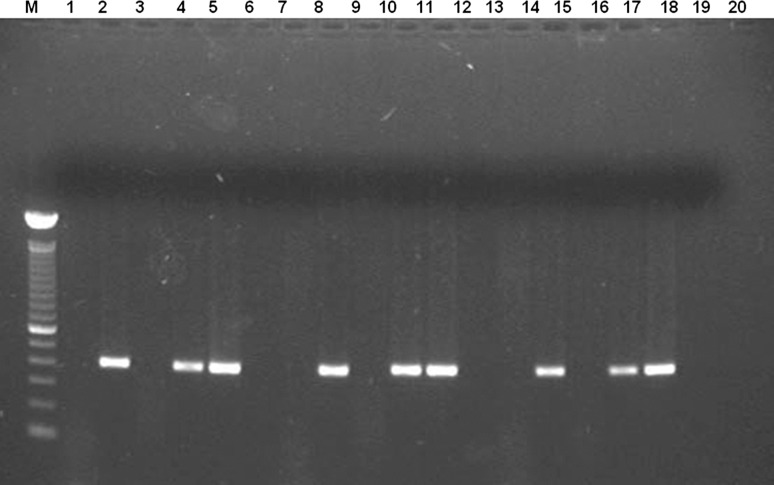
The 34 collected snails were determined as Lymnaea viatrix. The microscopic observations showed one out of the 34 snails with immature redia, but parasite species could not be identified. For subsequent analysis, we considered as positive that snail infected with trematodes by microscopy. The PCR revealed 21 positive snails (Fig. 1), including that detected by direct observation. The prevalence of infection by F. hepatica in snails was 2.94 (95 % CI = 1.44–4.45) when microscopic examination was used, but this value reached 61.76 % (95 % CI = 60.19–63.33) when using molecular amplification method. The difference between the two techniques was statistically significant (p < 0.005). The agreement between methods was not acceptable with a kappa value of 0.04 (95 % CI = −0.04–0.11), with an observed agreement of 41.18 %, kappa minimum of −0.42 and maximum of 0.13.
Fig. 1.
Agarose gel electrophoresis of amplification products, showing the feasibility of the PCR technique to detect F. hepatica in snails collected in the field. Lane M, molecular size marker (100 bp ladder), lane 1, reaction mixture, lane 2, 2.5 ng of F. hepatica DNA, lanes 3–20 template DNA from different sample specimens
Aquatic plants
Washing samples of watercress plants concentrated by centrifugation were observed by light microscopy but it was not possible to detect the presence of metacercariae. However, the PCR technique for F. hepatica was positive for one of the samples analysed.
Phylogenetic analysis
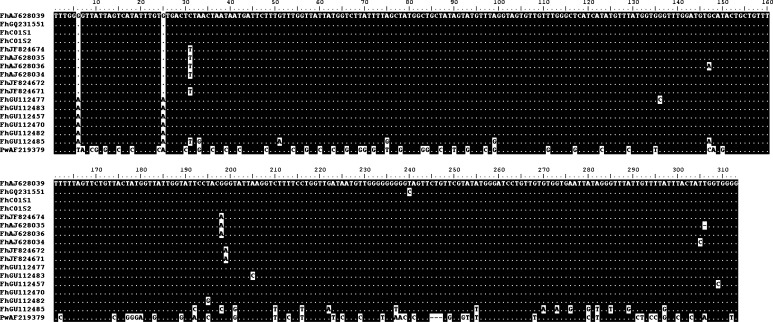
The alignment of the 17 sequences, including two from our studied snails identified as FhCO1S1 and FhCO1S2 is shown in Fig. 2.
Fig. 2.
Alignment of CO1 sequences employed for the phylogenetic tree construction. FhCO1S1 and FhCO1S2 correspond to the analysed sequences of this study. Letters and numbers following Fh indicate the GenBank accession no. of the corresponding sequences. PwAF219379 corresponds to Paragonimus westermani CO1
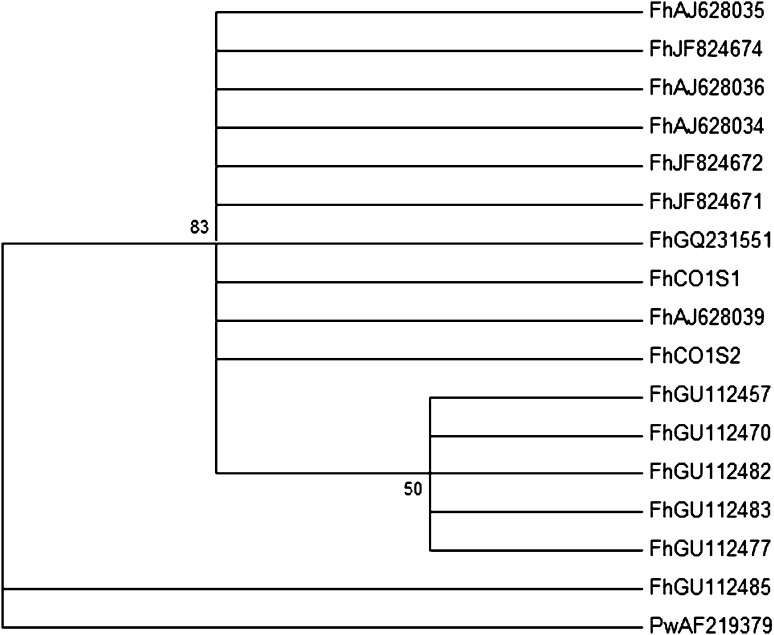
The topology of the tree, using Maximum likelihood and maximum parsimony analysis, showed that the two sequences, FhCO1S1 and FhCO1S2, belong to the FhAJ628034/5/6/9, FhJF824671/2/4, FhGQ231551 group (Fig. 3). This is in concordance with the high conservation between them, ranging from 99.4 to 100.0 % of sequence identity.
Fig. 3.
Phylogenetic relationships among of F. hepatica based on partial sequences of the mitochondrial cytochrome oxidase subunit I (COI) gene. P. westermani (AF219379) was used as outgroup. The phylogenetic tree was constructed using maximum likelihood analysis, conducted in PHYLIP package. Numbers at nodes represent percentage occurrence of clades in 1,000 bootstrap replications of the data. Maximum parsimony analysis resulted in the same tree topology (data not shown)
Discussion
The definitive diagnosis of human fasciolosis is performed by the finding of eggs in stool, but the method has low sensitivity, detecting the infection when the adult parasite is already installed in the bile ducts and requiring the collection of serial samples, resulting unreliable (Levine et al. 1980; Chen and Mott 1990).
In this work it was possible to confirm one case by coproparasitological analysis, corresponding to a symptomatic patient possibly in latent or chronic phase. However, due to the limitations of stool examination, we used ELISA for detection of antibodies using Fhr-proCL1 as antigen. In the study area, the serology by ELISA using Fhr-proCL1 allowed the detection of five positive cases, including that one detected by stool examination. According to the validity parameters of the method previously reported (Carnevale et al. 2001a) cases that were only serologically positive could also considered as infected with F. hepatica. Among these four cases, two of them were asymptomatic, indicating that might be in incubation, acute or latent phase. The other two cases had some symptoms and signs of acute phase and a low to moderate eosinophilia. As we could not demonstrate the presence of eggs in faeces for these four cases and the presence of antibodies only indicates exposure to the parasite, we could not confirm if these cases presented an active or pass infection.
The estimated prevalence for cattle in the study area, which reached 8.33 %, is in concordance with the few existing data for the region that indicate values of 9.9 % in slaughterhouses (Rossanigo et al. 1983). It is important to note that in the case of livestock, the estimated prevalence represents only animals found in the chronic stage of the disease, i.e. those in whom the diagnosis in faeces by centrifugation were positive, leaving out of consideration those found in the acute phase of illness or in which the eggs are accumulated with little release into the intestine. The stool diagnostic method presented data according to local veterinary inspection.
Given the economic importance of fasciolosis due to losses that may result from mortality, seizure of livers, reduced production and reproductive efficiency, the estimated prevalence value should be considered in the context of the study area where in the 86.96 % of the visited households the inhabitants raised cattle, resulting the main subsistence of the families for their own consumption and for sale of their own livestock or their derivatives.
The diagnosis of F. hepatica infections in the intermediate host is conventionally performed by observation of cercariae emission or by microscopic examination of developing stages in the mollusc with or without crushing (Khallaayoune et al. 1991). These procedures are tedious and time-consuming, and also required trained personnel for parasite identification because infections in the prepatent period (without emission of cercariae) are difficult to identify due to the fact that sporocysts and redia of different trematodes are very similar, making it nearly impossible to differentiate. In addition, the miracidium which recently entered can only be observed by histological sections. Therefore, these methodologies offer low sensitivity for detecting the early stages of the parasite (Kaplan et al. 1995). In our analysed sample the prevalence of infection detected by microscopy was within normal values reported in the literature (2.94 %). However, there was a significant difference (p < 0.005) with the result obtained by PCR (61.76 %). The PCR confirmed the taxonomic identity of immature stages (sporocysts and redia) that could not be identified by direct analysis, established a better estimate of the number of intermediate hosts that were invaded, allowing a better appreciation of the parasite offer, or infection in pastures.
The F. hepatica haplotype identified in the present study is the first one described in Argentina. As the molecular variation may reflect differences in virulence, host specificity and drug susceptibility (Criscione et al. 2005), different geographical isolates from Argentina need to be studied with the use of additional molecular markers.
This paper shows that fasciolosis is a disease that should be included in the list of public health problems of the area. Although fasciolosis is a common parasitic disease of cattle in the study region according to the local slaughter (Rossanigo et al. 1983), it is not considered relevant for humans. For the prevention of human infection is important that plants, especially watercress, are consumed from fenced and irrigated orchards with safe water, and wild watercress that grows spontaneously in the areas of access to the animals never be used. This prevention strategy, although it seems very simple, requires an intensive health education and the need to change a habit deeply rooted in the population, which also implies a change in family finances by removing feeding a product with no cost that get directly from nature.
The situation that was found for this area indicates that any measure of intervention for the control of this zoonosis should be adopted by multidisciplinary teams.
References
- Acha PN, Szyfres B. Fascioliasis. In: Acha PN, Szyfres B, editors. Zoonosis y enfermedades transmisibles comunes al hombre y los animales. 2. Washington DC: Organización Panamericana de la Salud; 1986. pp. 689–698. [Google Scholar]
- Ai L, Weng YB, Elsheikha HM, Zhao GH, Alasaad S, Chen JX, Li J, Li HL, Wang CR, Chen MX, Lin RQ, Zhu XQ. Genetic diversity and relatedness of Fasciola spp. isolates from different hosts and geographic regions revealed by analysis of mitochondrial DNA sequences. Vet Parasitol. 2011;181:329–334. doi: 10.1016/j.vetpar.2011.03.057. [DOI] [PubMed] [Google Scholar]
- Apt W, Aguilera X, Vega F, Zulantay I, Retamal C, Apt P, Sandoval J. Human fascioliasis in rural areas of Central Chile. Rev Med Chile. 1992;120:621–626. [PubMed] [Google Scholar]
- Arjona R, Riancho J, Aguado J, Salesa R, Gonzalez-Macias J. Fascioliasis in developed countries: a review of classic and aberrant forms of the disease. Medicine (Baltimore) 1995;74:13–23. doi: 10.1097/00005792-199501000-00002. [DOI] [PubMed] [Google Scholar]
- Behm CA, Sangster NC. Pathology, pathophysiology and clinical aspects. In: Dalton JP, editor. Fasciolosis. New York: CABI Publishing; 1999. pp. 185–224. [Google Scholar]
- Binkley CE, Sinniah B. Fascioliasis. In: Connor DH, Chandler FW, Schwartz DA, Manz HJ, Lack EE, editors. Pathology of infectious diseases. Stamford: Appleton & Lange; 1997. pp. 1419–1425. [Google Scholar]
- Bowles J, Blair D, McManus DP. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol Biochem Parasitol. 1992;54:165–173. doi: 10.1016/0166-6851(92)90109-W. [DOI] [PubMed] [Google Scholar]
- Bryan RT, Michelson MK. Parasitic infections of the liver and biliary tree. In: Surawicz C, Owen RW, editors. Gastrointestinal and hepatic infections. Philadelphia: W.B. Saunders; 1995. pp. 405–454. [Google Scholar]
- Carnevale S, Rodríguez MI, Guarnera EA, Carmona C, Tanos T, Angel S. Immunodiagnosis of fasciolosis using recombinant procathepsin L cystein proteinase. Diagn Microbiol Infect Dis. 2001;41:43–49. doi: 10.1016/S0732-8893(01)00288-7. [DOI] [PubMed] [Google Scholar]
- Carnevale S, Rodríguez MI, Santillan G, Labbé JH, Cabrera MG, Bellegarde EJ, Velásquez JN, Trgovcic JE, Guarnera EA. Immunodiagnosis of human fascioliasis by enzyme-linked immunosorbent assay (ELISA) and Micro-ELISA. Clin Diagn Lab Immunol. 2001;8:174–177. doi: 10.1128/CDLI.8.1.174-177.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MG, Mott KE. Progress in assessment of morbidity due to Fasciola hepatica infection: a review of recent literature. Trop Dis Bull. 1990;87:R1–R38. [Google Scholar]
- Criscione CD, Poulin R, Blouin MS. Molecular ecology of parasites: elucidating ecological and micro-evolutionary processes. Mol Ecol. 2005;14:2247–2257. doi: 10.1111/j.1365-294X.2005.02587.x. [DOI] [PubMed] [Google Scholar]
- Cucher MA, Carnevale S, Prepelitchi L, Labbé JH, Wisnivesky-Colli C. PCR diagnosis of Fasciola hepatica in field-collected Lymnaea columella and Lymnaea viatrix snails. Vet Parasitol. 2006;137:74–82. doi: 10.1016/j.vetpar.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Espino Hernández AM, Seuret N, Morlote C, Dumenigo Ripoll B. Antígenos de Fasciola hepatica. Su utilidad en el diagnóstico de la fascioliasis humana. Rev Cubana Med Trop. 1991;43:151–155. [PubMed] [Google Scholar]
- Espino AM, Dumenigo BE, Fernandez R, Finlay CM. Immunodiagnosis of human fascioliasis by enzyme-linked immunosorbent assay using excretory-secretory products. Am J Trop Med Hyg. 1987;37:605–608. doi: 10.4269/ajtmh.1987.37.605. [DOI] [PubMed] [Google Scholar]
- Esteban JG, Bargues MD, Mas-Coma S. Geographical distribution, diagnosis and treatment of human fasciolosis: a review. Res Rev Parasitol. 1998;58:13–48. [Google Scholar]
- Figueroa-Santiago O, Delgado B, Espino AM. Fasciola hepatica saposin-like-2 protein based ELISA for the serodiagnosis of chronic human fascioliasis. Diagn Microbiol Infect Dis. 2011;70:355–361. doi: 10.1016/j.diagmicrobio.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyer GV, Soler de Galanes M, Rodriguez-Perez J, Bjorland J, De Lagrava MS, Ramirez Guzman S, Bryan RT. Use of the Falcon assay™ screening test-enzyme linked immunosorbent assay (FAST-ELISA) and the enzyme-linked immunoelectrotransfer blot (EITB) to determine the prevalence of human fascioliasis in the Bolivian Altiplano. Am J Trop Med Hyg. 1992;46:603–609. doi: 10.4269/ajtmh.1992.46.603. [DOI] [PubMed] [Google Scholar]
- Kaplan R, Dame JB, Reddy GR, Courtney CH. A repetitive DNA probe for the sensitive detection of Fasciola hepatica infected snails. Int J Parasitol. 1995;25:601–610. doi: 10.1016/0020-7519(94)00159-L. [DOI] [PubMed] [Google Scholar]
- Khallaayoune KH, Stromberg BE, Dakkak A, Malone JB. Seasonal dynamics of Fasciola hepatica burdens in grazing timahdit sheep in Morocco. Int J Parasitol. 1991;21:307–314. doi: 10.1016/0020-7519(91)90032-3. [DOI] [PubMed] [Google Scholar]
- Knobloch J. Human fascioliasis in Cajamarca/Peru: humoral antibody response and antigenaemia. Trop Med Parasitol. 1985;36:91–93. [PubMed] [Google Scholar]
- Knobloch J, Delgado AE, Alvarez GA, Reymann U, Bialek R. Human fascioliasis in Cajamarca/Perú. I. Diagnostic methods and treatment with praziquantel. Trop Med Parasitol. 1985;36:88–90. [PubMed] [Google Scholar]
- Levine DM, Hillyer GV, Flores SI. Comparison of counterelectrophoresis, the enzyme-linked immunosorbent assay and Kato faecal examination for the diagnosis of fascioliasis in infected mice and rabbits. Am J Trop Med Hyg. 1980;29:602–608. doi: 10.4269/ajtmh.1980.29.602. [DOI] [PubMed] [Google Scholar]
- Mas-Coma S, Bargues MD, Esteban JG. Human Fasciolosis. In: Dalton JP, editor. Fasciolosis. New York: CABI Publishing; 1999. pp. 411–434. [Google Scholar]
- Mas-Coma S, Esteban JG, Bargues MD. Epidemiology of human fascioliasis: a review and proposed new classification. Bull WHO. 1999;77:340–346. [PMC free article] [PubMed] [Google Scholar]
- Mera y Sierra R, Agramunt VH, Cuervo P, Mas-Coma S (2011) Human fascioliasis in Argentina: retrospective overview, critical analysis and baseline for future research. Parasit Vectors 4:104. doi:10.1186/1756-3305-4-104 [DOI] [PMC free article] [PubMed]
- Ngategize PK, Bekele T, Tilahun G. Financial losses caused by ovine fasciolosis in the Ethiopian highlands. Trop Anim Health Prod. 1993;25:155–161. doi: 10.1007/BF02236234. [DOI] [PubMed] [Google Scholar]
- Olaechea FV. Epidemiología y control de Fasciola hepatica en Argentina. In: Nari A, Fiel C, editors. Enfermedades parasitarias de importancia económica en bovinos. Montevideo: Editorial Agropecuaria Hemisferio Sur; 1994. pp. 325–362. [Google Scholar]
- Olaechea F, Lovera V, Larroza M, Raffo F, Cabrera R. Resistance of Fasciola hepatica against triclabendazole in cattle in Patagonia (Argentina) Vet Parasitol. 2011;178:364–366. doi: 10.1016/j.vetpar.2010.12.047. [DOI] [PubMed] [Google Scholar]
- ONeill SM, Parkinson M, Strauss W, Angles R, Dalton JP. Immunodiagnosis of Fasciola hepatica infection (fascioliasis) in a human population in the Bolivian Altiplano using purified cathepsin L cysteine proteinase. Am J Trop Med Hyg. 1998;58:417–423. doi: 10.4269/ajtmh.1998.58.417. [DOI] [PubMed] [Google Scholar]
- ONeill SM, Parkinson M, Dowd AJ, Strauss W, Angles R, Dalton JP. Short report: immunodiagnosis of human fascioliasis using recombinant Fasciola hepatica cathepsin L1 cysteine proteinase. Am J Trop Med Hyg. 1999;60:749–751. doi: 10.4269/ajtmh.1999.60.749. [DOI] [PubMed] [Google Scholar]
- Organización Mundial de la Salud (1995) Lucha contra las Trematodiasis de Transmisión Alimentaria. OMS, Serie de Informes Técnicos No. 849. Organización Mundial de la Salud, Ginebra
- Paraense WL. Lymnaea viatrix: a study of topotypic specimens (Mollusca: Lymnaeidae) Rev Bras Biol. 1976;36:419–428. [Google Scholar]
- Paraense WL. Lymnaea viatrix and Lymnaea collumella in the neotropical region: a distributional outline. Mem Inst Oswaldo Cruz. 1982;77:181–188. [Google Scholar]
- Rim HJ, Farag HF, Sornmani S, Cross JH. Food-borne trematodes: ignored or emerging? Parasitol Today. 1994;10:207–209. doi: 10.1016/0169-4758(94)90111-2. [DOI] [Google Scholar]
- Rossanigo CE, Avila JD, Vásquez R, Sager L. Incidencia, distribución e identificación del huésped intermediario de la distomatosis bovina en la provincia de San Luis. Gaceta Veterinaria XLV. 1983;382:739–746. [Google Scholar]
- Sampaio Silva ML, Correia da Costa JM, Viana da Costa AM, Pires MA, Lopes SA, Castro AM, Monjour L. Antigenic components of excretory-secretory products of adult Fasciola hepatica recognized in human infections. Am J Trop Med Hyg. 1996;54:146–148. doi: 10.4269/ajtmh.1996.54.146. [DOI] [PubMed] [Google Scholar]
- Stoll NR, Hausheer WC. Concerning two options in dilution egg counting: small drop and displacement. Am J Hyg. 1926;6:134–145. [Google Scholar]
- Telemann W. Eine methode zur erleichterung der auffindung von parasiteneiern in den faeces. Dtsch Med Wochenschr. 1901;35:1510–1511. [Google Scholar]
- Villegas F, Angles R, Barrientos R, Barrios G, Valero MA, Hamed K, Grueninger H, Ault SK, Montresor A, Engels D, Mas-Coma S, Gabrielli AF. Administration of triclabendazole is safe and effective in controlling fascioliasis in an endemic community of the Bolivian Altiplano. PLoS Negl Trop Dis. 2012;6:e1720. doi: 10.1371/journal.pntd.0001720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber R, Bryan RT, Owen RL, Wilcox CM, Gorelkin L, Visvesvara GS, The Enteric Opportunistic Infections Working Group (1992) Improved light-microscopical detection of microsporidia spores in stool and duodenal aspirates. N Engl J Med 326:161–166 [DOI] [PubMed]
- Willis HH. A simple levitation method for the detection of hookworm ova. Med J Aust. 1921;29:375–376. [Google Scholar]