Abstract
Present study was conducted to investigate the pathological effects of Dioctophymatid nematode (Eustrongylides sp.) in the freshwater fish, Glossogobius giuris. It is bright red colored nematode, measuring 30–55.26 mm in length and 0.525–0.630 mm in width, was found encapsulated or free in the body muscles, swim bladder, liver, intestine and ovaries of host fish. The overall prevalence of parasite was 38.47 ± 18.234 % and high prevalence of 68.75 and 50.0 % was observed in month of November, 2008 and March, 2008 respectively i.e. maximum infection of parasite was during pre-spawning and post-spawning period. Only the ovaries of host fish were found infected with encapsulated larvae. Ovaries showed 11.2 ± 0.088 % of prevalence and 0.259 ± 1.176 of relative intensity. The gonado-somatic index and fecundity of infected fishes were observed to decrease as compared to the uninfected ones. The histo-pathological damage caused by parasites in ovaries is characterized by damaged and disrupted germinal epithelium, damaged oocytes showing the sign of necrosis, liquification of yolk globules and reduction in the formation of yolk. The present observation attributes towards the reduction in fecundity and further decline in the population of the host fish.
Keywords: Glossogobius giuris, Eustrongylides, Gonado-somatic index, Fecundity, Pathological
Introduction
Parasites act as one of the factors regulating the host population by affecting the host survival and reproduction. Pathological damage caused by parasites may directly or indirectly influence the host size, growth and development of gonads. According to Rohde (1993) parasites of the gonads and coelomic cavity may lead to complete castration and reduction in egg numbers in the host. Experimental and observational studies on philometrid nematode infections have soon that a heavy worm burden may reduce the host’s reproductive potential and also delay sexual maturity in the fish.
Knowledge of the effect of parasites on fish reproduction is scanty. The impact of some digeneans on the reproductive success of declining frog population is has been reviewed by Johnson et al. (2004). Although, many workers viz. Ramachandran (1975), Oliva et al. (1992), Moravec et al. (1997, 2002), Hesp et al. (2002), Clarke et al. (2006) and Perez et al. (2009) was explored the effect of Dracunculoid nematode on the ovaries and reproductive potential in number of fish hosts. But a very little work has been done on the effect of Eustrongylides sp. in relation to gonadal development. Nematodes belonging to the genus Eustrongylides are heteroxenous in their life cycle, involving aquatic annelids (oligochaetes) as first intermediate hosts and fish as second intermediate hosts (Karmanova 1968; Anderson 2000). Eustrongylides sp. is pathogenic to fish in its final larval stage of development (Sloboda et al. 2010) and its larvae have been reported from 17 orders of fish worldwide (Spalding and Forrester 1993). Studies on the pathological effect of Eustrongylides sp. larvae on the ovaries and egg number of fish remain scarce. Therefore, an attempt was made to document the effects of this nematode in naturally infected ovaries of freshwater teleost, Glossogobius giuris.
Materials and methods
Collection of fish specimens and parasites
Live specimens of host fish were collected from the Lower Lake and from the local fish markets of Bhopal (MP), India. They were brought to the laboratory and examined morphologically. The host fish, G. giuris was collected continuously for one year at regular intervals. Fish specimens were dissected out in physiological saline (0.75 % NaCl solution) for collecting nematode parasites from different organs. Nematodes collected were washed thoroughly in normal saline. Then they are killed and fixed in hot 70 % alcohol, stored in glycerine alcohol (1:3) and studied as wet mounts or temporary mounts in glycerine (Meyer and Olsen 1975). Taxonomical identification of nematode parasites was done by adopting the works of Anderson et al. (1974–1983) and Soota (1983).
Histo-pathological study
The infected ovaries of G. giuris were taken out and fixed in alcoholic Bouin’s fluid for 24 h. After complete removal of picric acid, the tissue was dehydrated, cleared in xylene and processed for preparation of paraffin wax blocks. The tissue was then cut at 4–5 μm thickness by rotatary microtome and stained routinely with haematoxylin and eosin (H–E) for histo-pathological examination (Luna 1968). Stained histo-pathological sections were examined under Olympus research microscope. Histo-pathological changes observed were photographed and interpreted in comparison to the work of others.
Gonado-somatic index (GSI) and fecundity estimation
Gonado-somatic index: Gonad weight was measured for infected and non-infected females. The ovaries were first removed and weighed. The GSI was calculated according to the formula given by Dahlgren (1979), as stated below
GSI (%) = (weight of gonad/total body weight) × 100.
Fecundity: it was estimation of ova content in the ovary. It was calculated from the counts of mature ova, by adopting the method of Sinha (1975). It is estimated by using following formula
Fecundity (F) = W × (n1 + n2 + n3)/(w1 + w2 + w3)
where W = total weight of ovary and (w1 + w2 + w3) and (n1 + n2 + n3) are the respective weight and ova counts of each subsample.
Ecological and statistical analysis
Ecological analysis of parasites was done according to the method of Margolis et al. (1982). All the data were analyzed statistically. One-way analysis of variance was done by SPSS program (11.5 version).
Results
Description and location of worm
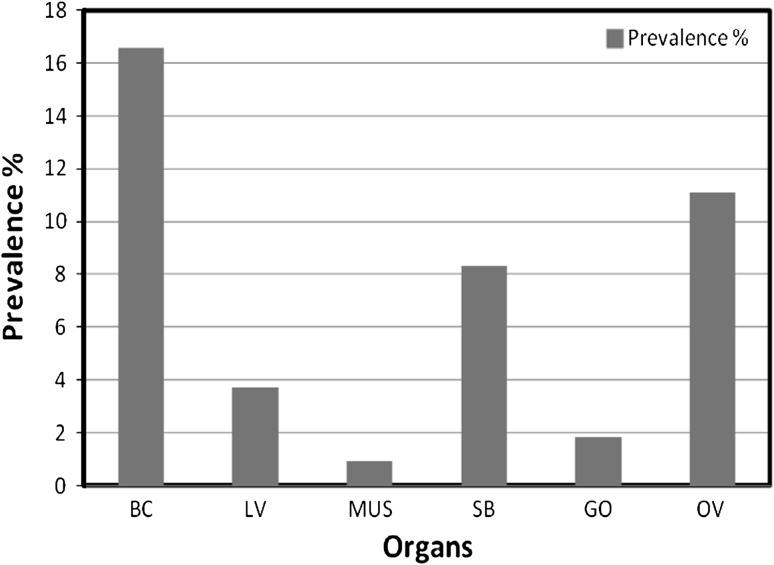
Out of 195 specimens of Glossogobius giuris examined, 56 were found infected with Eustrongylides sp. larvae. Bright red colored larvae (Fig. 1), measuring 30–55.36 mm in length and 0.525–0.630 mm in width. It is found encapsulated or free in the body cavity, muscles, liver, swim-bladder, gill opening and ovaries of host fish. Organ-wise prevalence of nematode infection was given in Fig. 2. According to its occurrence, the body cavity and ovaries is most favorable site of infection.
Fig. 1.
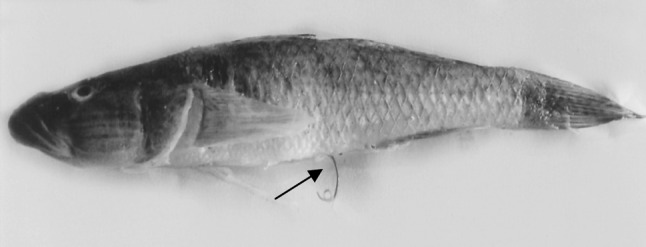
Photograph of G. giuris infected by the larvae of Eustrongylides sp. showing the larva extruding from anus (arrow)
Fig. 2.
Organ-wise prevalence of Eustrongylides sp. larvae in Glossogobius giuris. BC body cavity, LV liver, MUS muscles, SB swim bladder, GO gill opening, OV ovary
Prevalence and intensity
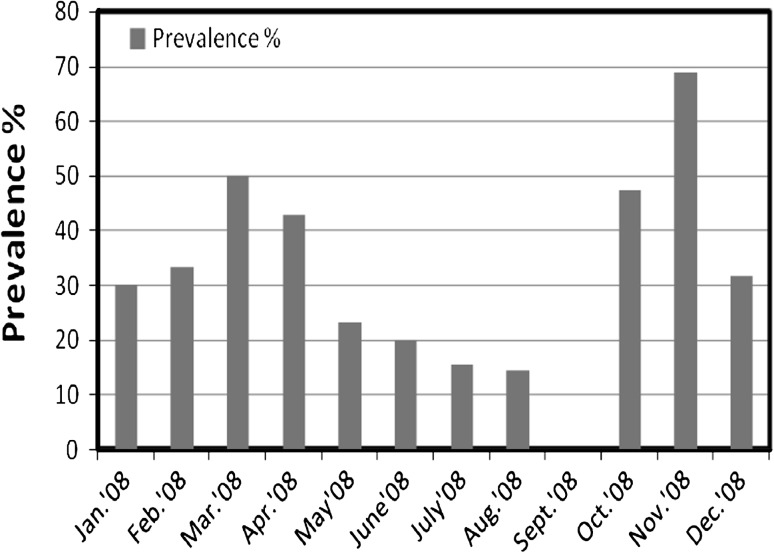
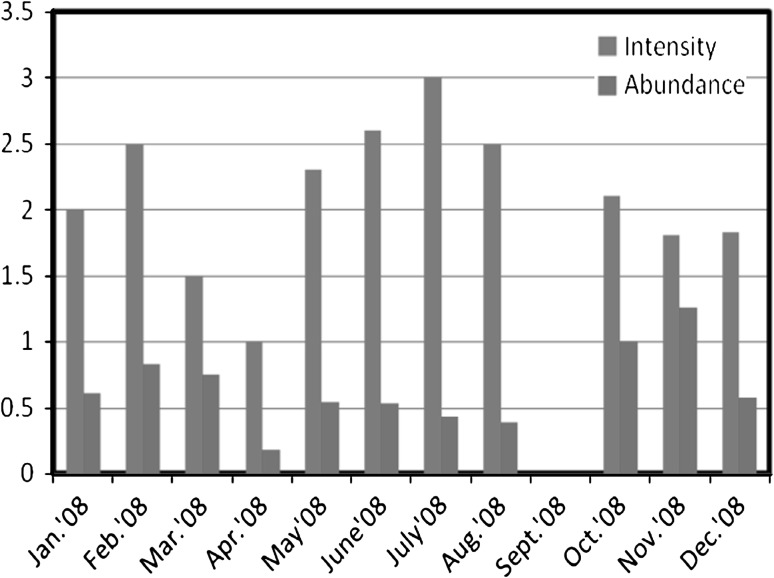
The overall prevalence of 38.47 ± 18.234 % was recorded during the year of survey i.e. January, 2008–December, 2008. The high prevalence of 68.75 and 50.0 % was recorded in months of November, 2008 and March, 2008 respectively i.e. during pre-spawning and post-spawning period. A sharp decrease in prevalence was observed during spawning period, with abrupt decline in infection in the month of September, 2008. In G. giuris, the maximum intensity (3.0) of the parasite was recorded in the month of July, 2008 i.e. during spawning season and the minimum intensity (1.0) was recorded in April, 2008. The recorded value of abundance ranged from 0.176 to 1.25. The highest value of abundance was recorded in November, 2008 and lowest value (0.176) in April, 2008. The monthly variations in prevalence, intensity and abundance of parasite in G. giuris were given in Figs. 3 and 4.
Fig. 3.
Monthly variations in the percentage of prevalence of nematode infection in Glossogobius giuris
Fig. 4.
Monthly variations in the intensity and abundance of nematode infection in Glossogobius giuris
Effect on ovaries
Quantitative analysis showed significant change in the GSI (0.305 ± 0.11) and fecundity (2632.49 ± 2.09) values of the ovaries of infected G. giuris as compared to GSI (6.194 ± 2.26) and fecundity (4055.19 ± 3.9) of non-infected ovaries. There is a significant positive correlation between the GSI of non-infected and infected ovaries (R = 0.999**, P = 0.000). However, negative correlation was found between the fecundity of non-infected and infected ovaries (R = −0.165, P = 0.395) (Table 1).
Table 1.
Mean values (±SD) and significance of the GSI and fecundity of G. giuris in infected and non-infected specimens
| S No. | Parameters | Non-infected (mean ± SD) | Infected (mean ± SD) | R | P |
|---|---|---|---|---|---|
| 1. | Gonado-somatic index | 6.194 ± 2.26 | 0.305 ± 0.11 | 0.999** | 0.000 |
| 2. | Fecundity | 4055.19 ± 3.9 | 2632.49 ± 2.09 | −0.165 | 0.395 |
** Correlation is significant at the 0.01 level (2-tailed)
Histo-pathological effects
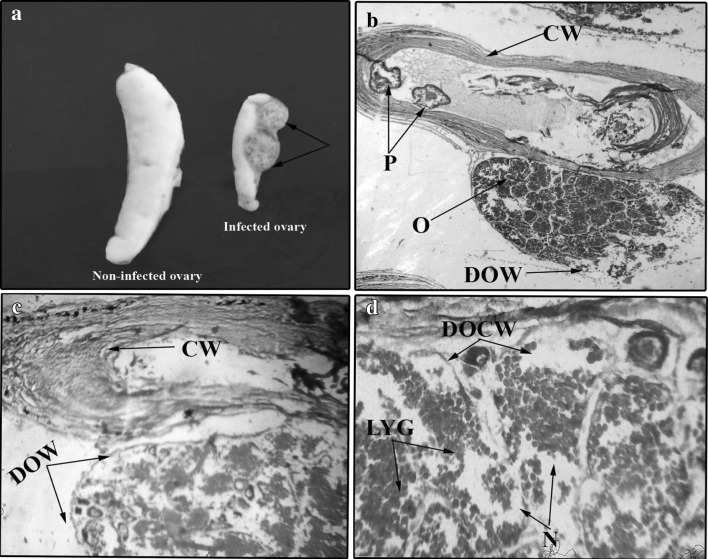
Morphologically, the infected ovary was significantly reduced in size as compared to uninfected ovary. Infected ovaries were found surrounded by 1–2 encapsulated parasites, attached to the wall of the ovary (Fig. 5a). Each cyst consisted of only one coiled compactly packed nematode. Histologically, the cyst is characterized by several concentric whorls of fibrotic tissue. Outer whorl of cyst was found in close association with ovarian wall which may exert pressure on ovarian wall and cause its disruption, damage and thinning (Fig. 5b). Lumen of the cyst was filled with several transverse sections of parasite. The pathological effects were characterized by disrupted and damaged germinal epithelium which might be due to the parasitic activity. Most of the oocytes were seen in vitellogenic stage with indistinguishable margins and showed a sign of necrosis. Liquification of yolk globules was the dominant pathological response which shows the reduction in the yolk formation in oocytes (Fig. 5c, d).
Fig. 5.
a Photograph showing the encysted Eustrongylides sp. larva (arrows) on the ovary of Glossogobius giuris in comparison to the non-infected ovary on left side. b Microphotograph of a cross section of infected ovary of G. giuris showing the ovary (O), cyst wall (CW), parasites (P) and damaged ovarian wall (DOW) ×25. c Microphotograph of a cross section of infected ovary of G. giuris showing cyst wall (CW) and damaged ovarian wall (DOW) ×40. d Microphotograph of a cross section of infected ovary of G. giuris showing necrosis (N), damaged oocyte wall (DOCW) and liquification of yolk globules (LYG) ×150
Discussion
Larvae of this genus were reported by Fernando and Furtado (1963) from three species of fishes, viz. Heteropneustes fossilis, Ompok bimaculatus and Wallago attu from Sri Lanka. Kalyankar (1974) reported Eustrongylides from Mystus seenghala from Maharashtra.
Chen (1973) and Wang et al. (1997) reported the larvae of Eustrongylides from the fish species of the families Engraulidae, Cyprinidae, Siluridae, Bagridae, Channidae and Percichthyidae. Ibiwoye et al. (2004) studied the density of infection caused by the larvae of Eustrongylides africanus in Clarias from Bida floodplain. They emphasized that the female fishes are more frequently infected with the parasite than the males. The same is observed under present investigation. Ibiwoye et al. (2008) reported the occurrence of Eustrongylidesafricanus larvae in Clarias species in the months of January, March and June. Moravec et al. (1995) further explains the prevalence of Philometra margolisi (varies from 14 to 28 %) in the gonads of Epinephelus morio, which depends on locality. The same was not observed during present investigation. The percentage of prevalence was relatively high (38.47 %) and showed seasonal variation. Frederick et al. (1996) and Brugni and Viozzi (1999) reported comparatively low percentage of prevalence in fish other than gobiids like Gambusia holbrooki (2.45 %) and G. maculates (0.78–8.0 %), respectively. The infection was heavy in winter months, which may be due to decrease in water temperature during winter season, which reduces the immune response of fish and increases its vulnerability to parasitic infection.
In the present investigation, the ovaries of infected Glossogobius giuris exhibited necrosis and decrease in the yolk formation due to disappearance of vitellogenic oocytes. It was also been observed that the GSI and fecundity of infected G. giuris decreased as compared to non-infected specimens. The present observations are consistent with the work of Heins and Baker (2003) who found that mean egg size in three spined stickleback (Gasterosteus aculeatus) was smaller in fish infected by the cestode, Schistocephalus solidus than in non-infected fish. Oliva et al. (1992) further suggested that philometrid infection resulted in reduced fecundity in Chilean sea bass (Paralabraxhumeralis) because the effective volume of the ovaries was reduced. Necrosis was described in the ovaries of striped mullet (Mugil cephalus) due to infection by Philometracephalus (Ramachandran 1975). Atrophy in the ovaries of New Zealand snapper (Chrysophrys auratus) due to infection by Philometra sp. was described by Hine and Anderson (1981). Similarly Moravec et al. (1997), Hesp et al. (2002) and Moravec et al. (2002) reported Philometra spp. causes extensive damage in the ovaries of various reef- fishes. Recent investigation of Deaton (2011) on the effects of larval nematode Eustrongylides ignotus on the reproduction of Mosquitofish, Gambusia affinis revealed a significant reduction in fecundity. Thus, infestation of nematode in the ovaries of female host fish may possessed a potential effect on reproductive biology. The present observation attributes towards the reduction in GSI and fecundity which may further decline the population of host fish.
Acknowledgments
Authors are thankful to Department of Zoology and Applied Aquaculture, Bhopal (MP), for providing infrastructure facilities.
References
- Anderson RC (2000) Nematode parasites of vertebrates. Their development and transmission, 2nd edn. CAB International Publishing. Wallingford
- Anderson RC, Chabaud AG and Willmott S (eds) (1974–1983) CIH keys to the nematode parasites of vertebrates, nos 1–10. Commonwealth Agricultural Bureaux, Farnham Royal, Bucks
- Brugni NL, Viozzi GP. Presence of Eustrongylides sp. (Jagerskiold, 1909) (Nematoda: Dioctophymatoidea) in Galaxias maculates (Jenyns, 1842) (Pisces: Galaxiidae) from Patagonia Argentina. J Helminthol Soc Wash. 1999;66:62–94. [Google Scholar]
- Chen C. An illustrated guide to the fish diseases and causative pathogenic fauna and flora in the Hubei province. Beijing: Science Press; 1973. pp. 1–456. [Google Scholar]
- Clarke LM, Dove ADM, Conover DO. Prevalence, intensity and effect of a nematode (Philometra saltatrix) in the ovaries of bluefish (Pomatomus saltatrix) Fish Bull. 2006;104:118–124. [Google Scholar]
- Dahlgren BT. Effects of population density on fecundity and fertility in the guppy, Poecilia reticulate (Peters) J Fish Biol. 1979;15:71–91. doi: 10.1111/j.1095-8649.1979.tb03573.x. [DOI] [Google Scholar]
- Deaton R. Parasitic nematodes decrease fecundity in the Western Mosquitofish. Gambusia affinis. Southwest Nat. 2011;56(2):262–265. doi: 10.1894/N05-RJE-03.1. [DOI] [Google Scholar]
- Fernando CH, Furtado JI. A study of some helminths parasites of freshwater fishes in Ceylon. Z ParasitKde. 1963;23:141–162. [Google Scholar]
- Frederick PC, McGehee SM, Spalding MG. Prevalence of Eustrongylides ignotus in mosquitofish (Gambusia holbrooki) in Florida: historical and regional comparisons. J Wildl Dis. 1996;32:552–555. doi: 10.7589/0090-3558-32.3.552. [DOI] [PubMed] [Google Scholar]
- Heins DC, Baker JA. Reduction of egg size in natural populations of threespines stickleback infected with a cestode macroparasite. J Parasitol. 2003;89:1–6. doi: 10.1645/0022-3395(2003)089[0001:ROESIN]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Hesp SA, Hobbs RP, Potter IC. Infection of the gonads of Glaucosoma hebraicum by the nematode Philometra lateolabracis: occurrence and host response. J Fish Biol. 2002;60:663–673. [Google Scholar]
- Hine PM, Anderson CD (1981) Diseases of the gonads and kidneys of New Zealand snapper, Chrysophrys auratus Forster (F. Sparidae). In: Fowler ME (ed) Wildlife diseases of the Pacific Basin and other countries. Academic Press, London, pp 166–170
- Ibiwoye TII, Balogun AM, Ogunsusi RA, Agbontale JJ. Determination of the infection densities of mudfish Eustrongylides in Clarias gariepinus and C. anguillaris from Bida floodplain of Nigeria. J Appl Sci Environ Manag. 2004;8(2):39–44. [Google Scholar]
- Ibiwoye TII, Okarme AN, Ogunsusi RA, Balogun AM. First report and record of nematode Eustrongylides africanus larvae in a vertebrate host mudfish Clarias species from Bida floodplain of Nigeria. Afr Sci. 2005;6(2):47–55. [Google Scholar]
- Ibiwoye TII, Ogunsusi RA, Balogun AM and Agbontale JJ (2008) The Contributions of environmental and haematological factors to the distributions and estimations of Eustrongylides africanus larvae densities in Clarias gariepinus and Clarias anguillaris from Bida floodplain of Nigeria. J Fish Aquat Sci 3:195−205
- Johnson PTJ, Sutherland DR, Kinsella JM, Lunde KB. Review of the trematode genus Ribeiroia (Psilostomidae): ecology, life history and pathogenesis with special emphasis on the amphibian malformation problem. Adv Parasitol. 2004;57:191–253. doi: 10.1016/S0065-308X(04)57003-3. [DOI] [PubMed] [Google Scholar]
- Kalyankar SD. First report on Eustrongylides larva (Nematoda: Dioctophymidae) from fish in India. Acta Parasit Pol. 1974;22(30):331–333. [Google Scholar]
- Karmanova EM. Dioctophymidae of animals and man and diseases caused by them. Fundam Nematol. 1968;20:123–206. [Google Scholar]
- Luna LC (1968) Manual of histological staining methods. Armed forces institute of pathology, 3rd edn. McGraw Hill Book Company, New York
- Margolis L, Esch GW, Holmes JC, Kuris AM, Shad GA. The use of ecological terms in parasitology Report of an Ad Hoc Committee of American Society of Parasitology. J Parasitol. 1982;68:131–133. doi: 10.2307/3281335. [DOI] [Google Scholar]
- Meyer MC, Olsen OW (1975) Essentials of parasitology, 2nd edn. Wm. C. Brown Co, Iowa
- Moravec F, Vidal-Martinez VM, Aquirremacedo L. Philometra margolisi n. sp. (Nematoda, Philometridae) from the gonads of the red grouper, Epinephelus morio (Pisces, Serranidae), in Mexico. Can J Fish Aquat Sci. 1995;52(suppl. 1):161–165. doi: 10.1139/f95-522. [DOI] [Google Scholar]
- Moravec F, Vidal-Martinez VM, Vargas-Vazquez J, Vivas-Rodriguez C, Gonzalez-Solis D, Mendoza-Franco E, Sima-Alvarez R, Guemez-Ricalde J. Helminths parasites of Epinephelus morio (Pisces: Serranidae) of the Yucatan peninsula, southeastern Mexico. Folia Parasitol. 1997;44:255–266. [PubMed] [Google Scholar]
- Moravec F, Ogawa K, Suzuki M, Miyazaki K, Donai H. On two species of Philometra (Nematoda: Philometridae) from the serranid fish Epinephelus septemfascistus in Japan. Acta Parasitol. 2002;47:34–40. [Google Scholar]
- Oliva ME, Srquez ASB, Olivares AN. Sexual status of Paralabrax humeralis (Serranidae) and infection by Philometra sp. (Nematoda: Dracunculoidea) J Fish Biol. 1992;40:979–980. doi: 10.1111/j.1095-8649.1992.tb02645.x. [DOI] [Google Scholar]
- Perez GR, Roumillat WA, Levesque EM, Connors VA, Buron I. Synchronization of occurrence of the ovarian philometrid Philometra carolinensis, with the spawning season of its fish host, the spotted seatrout, Cynoscion nebuloscus. Parasitol Res. 2009;104:1079–1085. doi: 10.1007/s00436-008-1291-y. [DOI] [PubMed] [Google Scholar]
- Ramachandran P. Philometra cephalus sp. n. infecting the gonads of the striped mullet, Mugil cephalus L. from the Arabian coast of Kerala, India, with a note on its pathology. Zool Anz. 1975;19:140–144. [Google Scholar]
- Rohde K (1993) Ecology of marine parasites; an introduction to marine parasitology, 2nd edn. CAB International, Wallingford
- Sinha M (1975) Observation on the biology of Puntius sarana (Ham.) of Loni reservoir (MP). J Inland Fish Soc 7:49–57
- Sloboda M, Mihalca AD, Falka I, Petrzelkova K, Carlsson M, Ghira I, Modry D. Are gobiid fish more susceptible to predation if parasitized by Eustrongylides excisus? An answer from robbed snakes. Ecol Res. 2010;25:469–473. doi: 10.1007/s11284-009-0676-4. [DOI] [Google Scholar]
- Soota TD. Studies on nematode parasites of Indian Vertebrates. 1 fishes. Rec Zool Surv India. Misc Publ Occ Paper. 1983;54:1–352. [Google Scholar]
- Spalding MG, Forrester DJ. Pathogenesis of Eustrongylides ignotus (Nematoda: Dioctophymatoidea) in Ciconiiformes. J Wildl Dis. 1993;29(2):250–260. doi: 10.7589/0090-3558-29.2.250. [DOI] [PubMed] [Google Scholar]
- Wang W, Li L, Yu Y, Feng W, Xiao C, Wang G, Liu J, Yao W, Feng S. Parasite fauna of fishes in Wuling Mountains area. In: Song D, editor. Invertebrates of Wuling Mountains area. Beijing: Science Press; 1997. pp. 73–146. [Google Scholar]