Abstract
A new Beauveria bassiana isolate, showing high activity against Musca domestica L. (Diptera: Muscidae) adults (mortality—100.0 %), larvae (mortality—72.3 %) and pupae (Infection in emerged flies—96.7 %) was used. The isolate was subjected to a combinational approach towards selection of process parameters for its growth optimization. Initial screening of several carbon and nitrogen sources revealed glucose and NaNO3 as the most suitable source for optimal biomass and spore production. Further, optimization through Placket–Burman and a 25 full factorial central composite design revealed highly significant effect of glucose and pH. The optimum composition for maximum biomass yield was (g/l): glucose 28; NaNO3 2.43; KH2PO4 1.32; MgSO4 0.60; and pH 7.00. Glucose concentration showed almost linear relationship with biomass yield, indicating its significant contribution in medium composition for fungal growth. Highly significant interactions were observed between glucose and pH, followed by glucose and NaNO3 concentration.
Keywords: Beauveria bassiana, Pathogenecity, Nutrition, RSM, Optimization
Introduction
The entomopathogenic fungi, Beauveria bassiana has a broad host range among Lepidoptera, Coleoptera, Hemiptera, Orthoptera and Diptera (Fuguet et al. 2004). Its activity against Musca domestica L. (housefly), a vector of significance for several medical and veterinary pathogens, has widely been reported (Watson et al. 1995; Geden et al. 1995; Carswell et al. 1998; Lecuona et al. 2005; Siri et al. 2005; Mishra et al. 2011). The broad host range and cosmopolitan nature of B. bassiana guarantee its prominent role in bio pest management. However, pathogenicity of B. bassiana varies with strain type, which in turn is influenced by geographical and nutritional condition (Jackson and Schisler 1992). Moreover, nutritional conditions also affect spore yield as well as their virulence potential (Jackson and Schisler 1992; Hallsworth and Magan 1996; Safavi et al. 2007; Gao et al. 2007). Hence, the studies elucidating the effect of various media components on growth of the organism are critical before taking up the requisite organism in mass cultivation for biopesticide production.
For entomopathogenic fungi, most of the studies have been done using one variable-at-a-time (OVAT) approach, however, some recent studies have utilized statistical optimization in enzyme production and fermentation (Rao et al. 2006; Bhanu Prakash et al. 2008). Statistical approach, such as response surface methodology (RSM) is a powerful tool of statistics which simplifies the optimization by studying the mutual interactions among variables over a range of values in a statistically valid manner (Bhanu Prakash et al. 2008). This multivariate approach also improves statistical interpretation possibilities, and evaluates the relative significance of several affecting factors even in the presence of complex interactions (Rao et al. 2006). The present study aimed at evaluating the pathogenicity potential and optimizing various nutritional factors for the growth of newly isolated entomopathogenic fungi, B. bassiana (HQ917687). The OVAT methodology was used for initial screening of carbon and nitrogen sources, followed by identification of the critical nutrients through Plackett–Burman (P–B). Finally, the selected parameters were optimized through central composite design (CCD) of RSM.
Materials and methods
Microorganism
Beauveria bassiana HQ917687 used in the present study was isolated from soil samples collected from Northern Uttar Pradesh, India (Data unpublished). The fungal isolate was maintained on Potato Dextrose Agar slants at 4 °C. For preparation of spore suspensions, spores were harvested from 5 days cultured slants by adding 10 ml of 0.1 % sterile Tween 80, vortexed for 5 min, and the resulting spore suspension filtered through a sterilized 8 μm membrane filter disk. The filtered spore suspension was enumerated and checked for viability of spores using an Automatic Cell Counter (Cellometer® Vision HSL, Nexcelom Bioscience). For each experiment, freshly revived culture was used.
Bioassay against Musca domestica
Insecticidal activity of B. bassiana HQ917687 was evaluated against M. domestica, to establish its efficacy. Rearing of the M. domestica and the bioassay against its adults and larvae were done by the method described earlier (Mishra et al. 2011). In a petri plate (150 × 25 mm, BOROSIL®) assay, 10 adult flies (2–3 days old) or larvae (2nd instar) were placed on a filter paper (Whatmann No. 1) containing a diet of wheat bran and groundnut oil cake (ratio, 1:3), and sprayed with 1 ml of spore suspension (108 conidia/ml). Mortality of M. domestica adults and larvae was recorded after 5–6 days of observation. Activity against M. domestica pupae was determined by standard dipping method, proposed for pupicidal assay by Mochi et al. (2010). In the assay, M. domestica pupae (10 in no., 2–3 days old) were dipped in fungal spore suspension (108 conidia/ml) for 1 min and then air dried and placed on filter paper in a petri plates. Treated pupae were observed for the suppression in adult emergence as well as the infection percentage in the emerged adult flies. In control treatments, assay was performed with 1 ml of sterile distilled water containing 0.1 % Tween 80. Petri plates were incubated at 28 ± 5 °C, 65 % RH and 14:10 photoperiod. All the bioassays were replicated thrice and the values presented as mean.
Nutritional optimization
All the studies concerning nutritional optimization i.e. selection of carbon and nitrogen source by OVAT, initial screening of nutritional components by P–B, and final media optimization by RSM were performed using basal media. The composition of the basal media was (g/l): glucose 20, NH4NO3 2, KH2PO4 1, MgSO4 0.5, NaCl 0.5, FeSO4 0.007, ZnSO4 0.007 and CuSO4 0.007.
Evaluation of nitrogen and carbon sources
Five inorganic nitrogen sources; NaNO3, KNO3, NH4NO3 (NH4)2SO4 and NH4Cl at a fixed concentration of 2 g/l, and five carbon sources; glucose, galactose, sucrose, fructose and mannose at a fixed concentration of 20 g/l, were tested separately as the alternative nitrogen (to NH4NO3) or carbon sources (to glucose) in the basal medium. The test flasks were then inoculated with 1 ml of seed culture (108 conidia/ml) and incubated at 28 ± 2 °C and 180 rpm for 5 days. After 5 days, the spore yield and biomass production from each flask was evaluated and the data obtained was compared by one-way ANOVA.
Statistical experimental design
After identifying the carbon and nitrogen variables affecting beauveria growth by ‘OVAT’ approach, a screening test was constructed by P–B design to examine the effects of medium components (Glucose, NaNO3, KH2PO4, MgSO4, NaCl, ZnSO4, FeSO4, CuSO4) and pH on biomass production. P–B design allows screening of n variables with n + 1 runs, each variable set at two levels, high (H) and low (L) (Table 1). Critical ranges of selected parameters were determined by preliminary experiments based on the literature review (Deacon 2006). Twelve runs were designed to identify the significant factors affecting biomass production.
Table 1.
Levels and values of variables in Plackett–Burman design (concentrations in g/L)
| Level | Glucose | NaNO3 | KH2PO4 | MgSO4 | NaCl | ZnSO4 | FeSO4 | CuSO4 | pH |
|---|---|---|---|---|---|---|---|---|---|
| High | 28.00 | 2.80 | 1.40 | 0.70 | 0.70 | 0.05 | 0.07 | 0.05 | 7.00 |
| Low | 12.00 | 1.20 | 0.60 | 0.30 | 0.30 | 0.03 | 0.05 | 0.03 | 4.00 |
Screened out factors which formed critical subset of media components (glucose, NaNO3, KH2PO4, MgSO4 and pH) were further optimized through CCD of RSM. A 25 factorial CCD with five factors was described to depict the nature of the response surface in the optimum region. Each independent variable was coded at three levels between −1 and +1 (Table 2). A central composite design has three groups of design points: (a) axial points, (b) center points, and (c) factorial points. The design includes 8 axial or star points which has one factor at an axial distance to the centre of ±a, and the other factors at level ‘0’. The axial points make the design rotatable. For the center points all factors are set at their mid values (0, 0). Centre points helps in understanding the curvature while replication of centre points (seven replicates) helps in estimation of experimental error and for determination of confidence intervals. The design points decided by factorial points be 2k, where k is the number of variables while the total design points (experiments) would be N = 2k (factorial points) + 2k (axial points) + g0 (replicates of centre point) = 50.
Table 2.
Independent variables and their levels in CCD
| Variables | Symbol | Code level | ||||
|---|---|---|---|---|---|---|
| −α | −1 | 0 | +1 | +α | ||
| Glucose | A | 0.97 | 12.00 | 20.00 | 28.00 | 39.03 |
| NaNO3 | B | 0.10 | 1.20 | 2.00 | 2.80 | 3.90 |
| KH2PO4 | C | 0.05 | 0.60 | 1.00 | 1.40 | 1.95 |
| MgSO4 | D | 0.02 | 0.30 | 0.50 | 0.70 | 0.98 |
| pH | E | 1.93 | 4.00 | 5.50 | 7.00 | 9.07 |
The biomass production from the fungus was taken as the dependent variable or response. The response was fitted to a second order polynomial equation for analyzing the interaction between dependent and independent variables of the experiment. The fitted polynomial equation was expressed as three-dimensional response surface and contour presentations to find the concentration of each factor for maximum response. Response was subjected to multiple nonlinear regressions using the statistical software package Design-Expert, Stat-Ease Inc., Minneapolis, USA. The data was also subjected to the analysis of variance (ANOVA) and the F test was used to evaluate the significance of the models.
Results
Bioassay against M. domestica
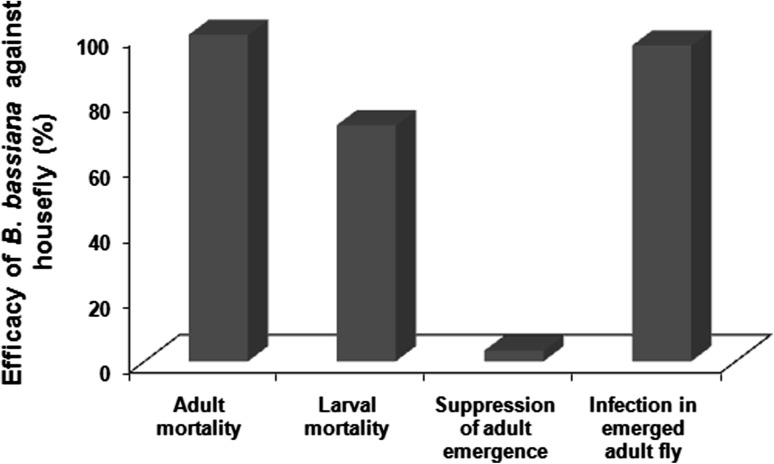
The fungus isolate used in the study was highly effective for M. domestica control with 100 and 72.3 % mortality of adults and larvae, respectively, on 5th day of observation (Fig. 1). The B. bassiana isolate showed only 3.3 % suppression in adult emergence during assay with M. domestica pupae. However, the emerged flies showed fungal infection at 96.7 %, revealing propagation of infection to the next generation.
Fig. 1.
Insecticidal activity of Beauveria bassiana against different life stage of Musca domestica (HF)
Evaluation of nitrogen and carbon sources
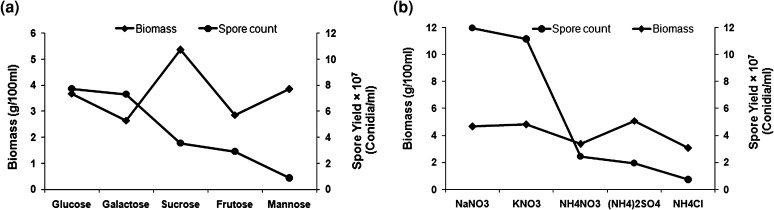
Screening of various carbon and nitrogen sources showed maximum biomass production in presence of sucrose (5.36 g/l) followed by mannose (3.86 g/l), glucose (3.68 g/l), fructose (2.86 g/l) and galactose (2.65 g/l). However, for spore production, glucose (7.7 × 107 conidia/ml) was the best substrate while mannose and sucrose performed poorly (Fig. 2a). Therefore, glucose with moderate biomass production and maximum spore yield was chosen as carbon source for media.
Fig. 2.
Biomass and spore yield by Beauveria bassiana using different a carbon, and b nitrogen sources, through one-variable-at-a-time approach
Amongst various nitrogen sources, (NH4)2SO4 (5.10 g/l) produced maximum biomass followed by KNO3 (4.82 g/l), NaNO3 (4.66 g/l), NH4NO3 (3.37 g/l) and NH4Cl (3.06 g/l) (Fig. 2b). For the spore production, NaNO3 (1.2 × 108 conidia/ml) was found to be best performer followed by KNO3. Amongst nitrogen sources, NaNO3 with good yield of biomass and spore was chosen for further optimization study over (NH4)2SO4 which had maximum biomass production but very poor spore yield.
Screening of significant factors using Placket–Burman design
The ANOVA data for the experimental results from P–B screening test had R2 of 0.989 and Adj R2 of 0.944. Of the nine factors tested, 6 factors, glucose, NaNO3, KH2PO4, MgSO4, NaCl and pH showed positive while others (ZnSO4, FeSO4, and CuSO4) had negative effect on biomass production. Five factors namely, glucose, NaNO3, KH2PO4, MgSO4, and pH had significant effect (P < 0.05) and hence are expected to play important role in growth and biomass production. Hence, five factors (glucose, NaNO3, KH2PO4, MgSO4, and pH) were taken as the variables in the 25 factorial CCD optimization experiments.
Optimization of biomass production
Optimization studies help in understanding interactions among the nutrients at varying concentrations and in calculating the optimal concentration of each nutrient for a given target. Biomass production efficiency was predicted through quadratic model equation generated by CCD in terms of coded factors using second order polynomial equation:
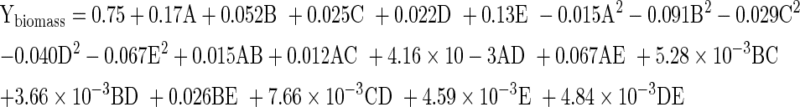 |
1 |
The model reveals that glucose concentration (x1) had a significant effect (P < 0.01) on Ybiomass as it had the largest coefficient followed by MgSO4 (x5). Positive coefficient of x1, x2, x3, x4, and x5 indicated a linear effect to increase Ybiomass. However, quadratic terms x11, x22, x33, x44 and x55 had negative effects. The coefficients of the response surface model given by Eq. (1) were evaluated by their regression analysis. Student t test indicated that the linear coefficients (A, B, C, D and E), quadratic term (A2, B2, C2, D2 and E2) and four cross product term (AB, AC, AE and BE) were highly significant (all P < 0.05). However, to minimize the errors, all the coefficients were included in the model.
The statistical significance of the model equation was evaluated by the F test for analysis of variance (ANOVA). The ANOVA result for the response is shown in Table 3, which indicated that the quadratic models could be used to navigate the design space. The Model F value of 109.37 implied significance of model with only a 0.01 % chance of its occurrence due to noise. The value of R2 (0.9869) implied explanation of 98.69 % variance in model. The Pred R2 of 0.9541 was in reasonable agreement with the Adj R2 of 0.9779, indicating good agreement between experimental and predicted values. The adequate precision value of 42.08 indicated adequate signal and confirmed utility of model to navigate the design space. The “lack of fit tests” compares the residual error to the “Pure error” from replicated experimental design points. The lack of fit F value of 2.61 implied that it was not significant relative to pure error.
Table 3.
Analysis of variance for fitted quadratic model for optimization of biomass production from B. bassiana
| Source | Sum of square | DF | Mean square | F value | Prob > F |
|---|---|---|---|---|---|
| Model | 2.98 | 20 | 0.15 | 109.37 | <0.0001 |
| Residual | 0.039 | 29 | 1.361 × 10−3 | ||
| Lack of fit | 0.035 | 22 | 1.599 × 10−3 | 2.61 | 0.0978 |
| Pure error | 4.292 × 10−3 | 7 | 6.131 × 10−4 | ||
| R 2 = 0.9869 | |||||
| Adeq precision = 42.08 | |||||
| Adj R 2 = 0.9779 | |||||
Perturbation plot
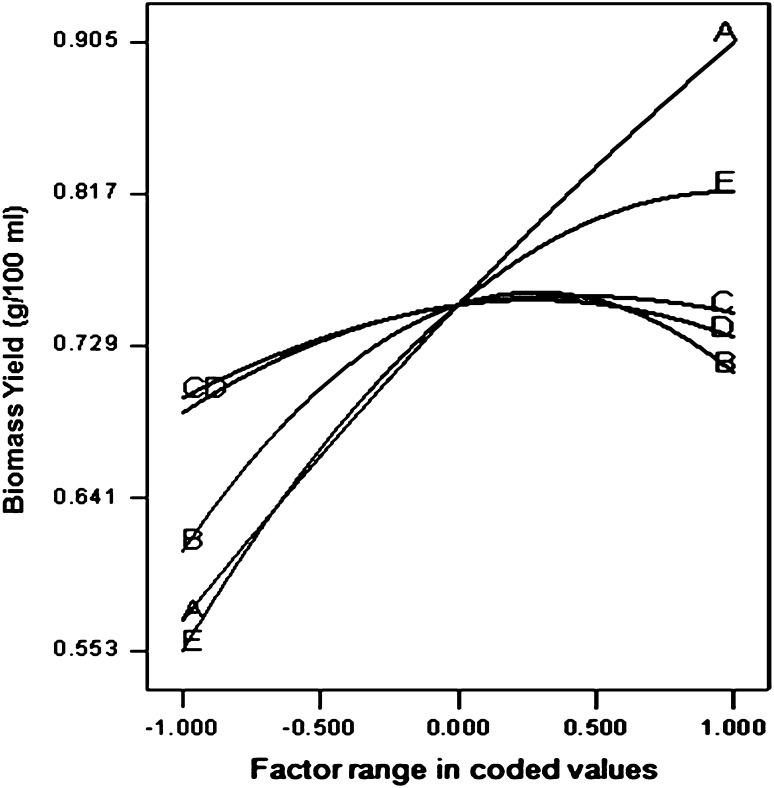
Perturbation plot for biomass production (Fig. 3) shows a linear curvature for glucose while NaNO3 and pH showed highly steep curvature. At the same time, curvature for KH2PO4 and MgSO4 were relatively less steep. The pattern of deviation from the reference point showed that in the range studied, biomass production increased with increase in concentrations of all the factors except NaNO3, which showed a sharp decrease in biomass production with increase in its concentration after midpoint. The glucose concentration emerged to be the most dominant factor affecting biomass production.
Fig. 3.
Perturbation plots for Biomass yield by Beauveria bassiana A glucose, B NaNO3, C KH2PO4, D MgSO4, and E pH
3D surface plots
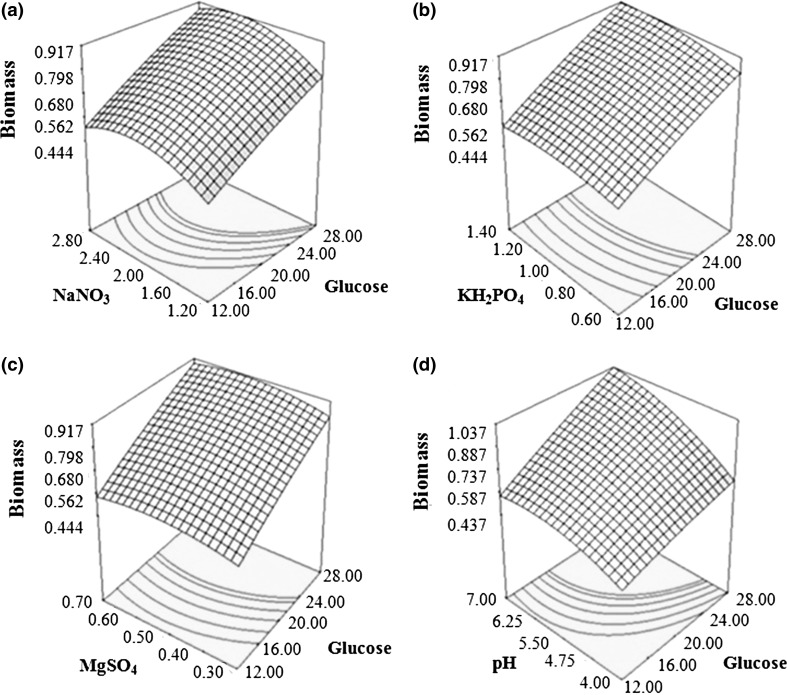
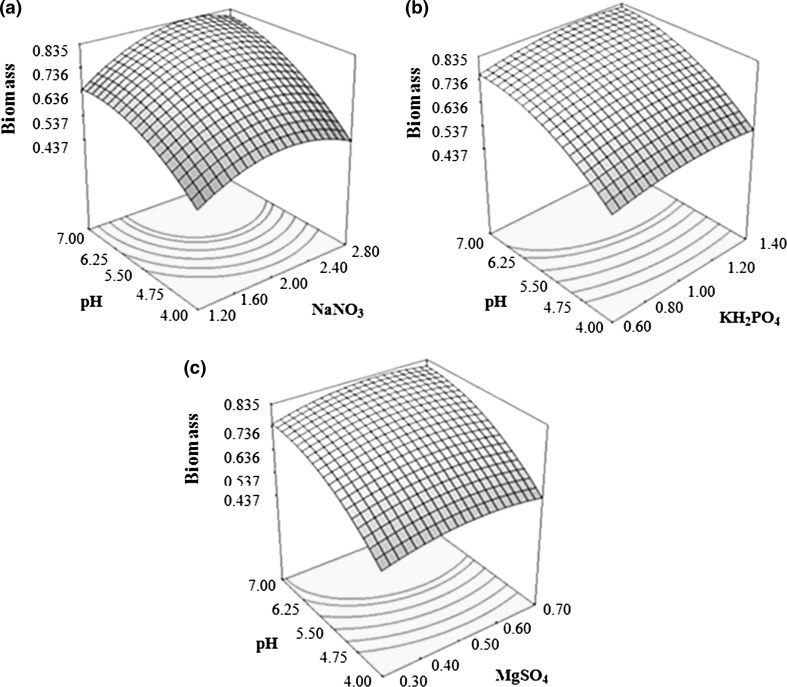
3D surface plots presented in Fig. 4 showed the relative interaction between glucose and other factors on biomass production from B. bassiana. Figure 4a represented an increase in biomass production contemporaneous to glucose and NaNO3 concentration. However, with NaNO3, trend only followed till midpoint, where after still higher concentration of NaNO3 caused significant depression in biomass production. Hence, proper choice of the levels for both glucose and nitrate source is requisite for maximizing the biomass production.
Fig. 4.
Response surface curves for biomass yield (g/100 ml) by Beauveria bassiana showing interaction between a glucose and NaNO3, b glucose and KH2PO4, c glucose and MgSO4 and d glucose and pH
Figure 4b, c revealed change in biomass production wrt glucose and KH2PO4 and glucose and MgSO4 concentrations, respectively. Increase in concentration of all the factors showed increase in biomass production; albeit with slightly different slopes. Glucose showed synergistic effect on biomass production with both the variables; however, their interaction was rather weak. Figure 4d depicted increase in biomass production relative to glucose concentration and initial pH change; with slope of increase being almost same for both variables.
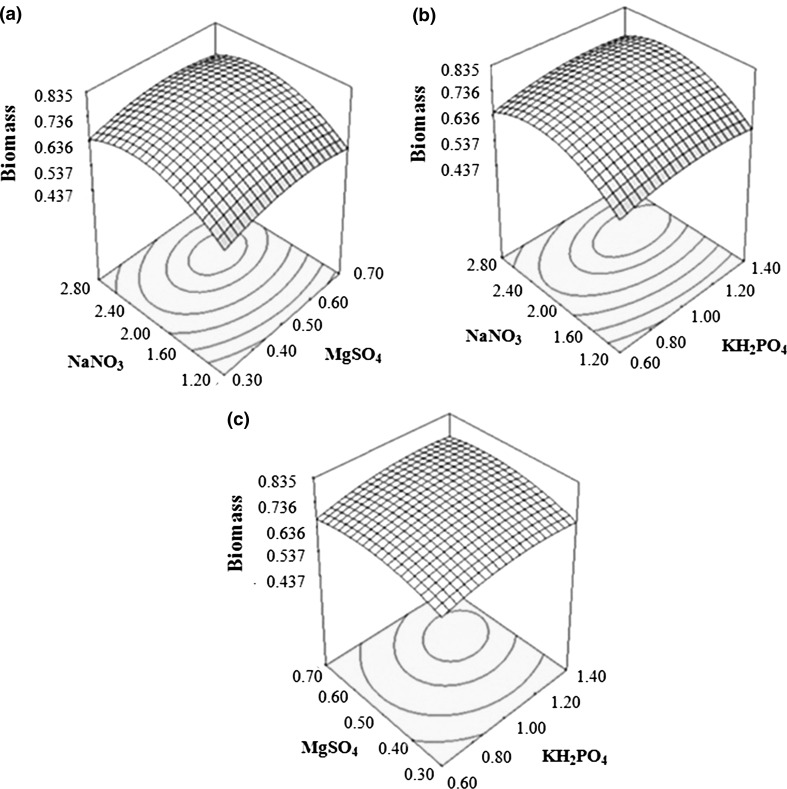
Figure 5 represented interaction between pH and nutritional factors for biomass production from B. bassiana. Increase in biomass production was observed with increase in pH and NaNO3 concentration (Fig. 5a). With NaNO3 concentration, increase in biomass production was observed only to a certain point, after which slight decrease was apparent. Moreover, increase in biomass production was higher for interaction term, than either of the individual factors. Figure 5b, c representing the interaction between pH and KH2PO4 and pH and MgSO4, respectively, showed non significant interaction. Figure 6a, b depicted non-significant interaction between nutritional factors (NaNO3 and MgSO4 and NaNO3 and KH2PO4) while Fig. 6c represented a weak interaction between KH2PO4 and MgSO4 concentration for biomass production.
Fig. 5.
Response surface curves for biomass yield (g/100 ml) by Beauveria bassiana showing interaction between a pH and NaNO3, b pH and KH2PO4, and c pH and MgSO4
Fig. 6.
Response surface curves for biomass yield (g/100 ml) by Beauveria bassiana showing interaction between a NaNO3 and MgSO4, b NaNO3 and KH2PO4, and c MgSO4 and KH2PO4
Desirability function
Desirability function of the software optimized the parameters at (g/l): glucose 28; NaNO3 2.43; KH2PO4 1.32; MgSO4 0.60 and pH at 7.00. The predicted response for biomass production was 10.86 g/l with a desirability level of 0.983.
Discussion
There are varying reports on the activity of B. bassiana (100 % mortality in 5–15 days) against M. domestica adults (Watson et al. 1995; Geden et al. 1995; Carswell et al. 1998; Siri et al. 2005; Mishra et al. 2011). Against M. domestica larvae, B. bassiana is reported to have significantly lower activity, at 40 % (Barson et al. 1994) and 97 % (Mishra et al. 2011) larval mortalities. Few studies describing B. bassiana control activity against M. domestica pupae, reported no significant activity on treated pupae with indirect effect on emerged flies (Lecuona et al. 2005, 2007). Further, Barson et al. (1994) inferred that the infected flies have reduced fecundity. The present study confirmed the earlier findings and established the new B. bassiana isolate as a pathogen of significance against M. domestica. The B. bassiana isolate showed holistic control activity against different life stages of fly. With the significantly reduced time of kill for adults and larvae, the isolate was better than most of the B. bassiana isolates used in the earlier studies. Moreover, B. bassiana infection in flies emerged from treated pupae, hold significance in terms of decreased oviposition and ultimate reduction in overall flies’ population density. Therefore, further growth optimization for the isolate was conducted to facilitate mass production.
Nutrients are substances used in biosynthesis and energy release and therefore serve as cardinal impetus towards the viability, survival and sustenance of any organism (Safavi et al. 2007; Mustafa and Kaur 2009). Fungi respond differently to different carbohydrates. The present study reported glucose as best carbon source for spore production, while for biomass production, it followed close behind mannose. The results were supported by findings of Campbell et al. (1983), who reported d-glucose along with d-sucrose and d-trehalose as best carbon source for sporulation of B. bassiana conidia. Li and Holdom (1995) found mannose as good source for mycelial growth in Metarhzium anisopliae but contradictory to the result of the present study where mannose was poor substrate for spore yield, in their study, mannose also supported good sporulation. Glucose being most utilizable carbon source for fungi, gets readily incorporated into the cell, and occupies the central position of the glycolytic pathway while other carbon sources being less preferred, reduces fungal growth (Rangel et al. 2006).
In the past, various studies had dedicated to the cause of investigation of carbon and nitrogen sources for fungal growth (Campbell et al. 1983; Li and Holdom 1995; Rangel et al. 2006; Safavi et al. 2007). In the present study along with different essential nutrient factors, effect of initial pH of the medium has also been attempted. Previous studies have suggested that for commendable growth, the microorganism must be able to incorporate appreciable quantities of carbon, nitrogen, phosphorus and sulphur, which are the essential ingredients of growth factors viz., amino acids, purines, pyrimidines and vitamins (Mustafa and Kaur 2009). Similarly, initial pH value is the critical factor in biomass accumulation and metabolite formation by affecting morphology of fungal mycelia (Kim et al. 2003).
Gillespie (1984) correlated increase in filamentous growth and blastospore production in M.anisopliae with increase in glucose concentration. However, a very high glucose concentration led to reduction in blastospore production, which was attributed to decrease in water activity of the medium due to increased glucose (Humphreys et al. 1989). Sabu et al. (2000) reported maximal yield of l-glutaminase enzyme production from marine Beauveria sp. in presence of 1 % d-glucose, whereas (St. Leger 1995) observed de-repression of Pr1 and Pr2 proteases in M. anisopliae and B. bassiana, by addition of exogenous carbon and nitrogen in basal media. Ratio of carbon and nitrogen in medium might also influence conidial yield and fungal pathogenicity (Safavi et al. 2007). Medium containing low C/N (10:1) ratio had higher yield of conidia with higher Pr1 activity and virulence than the medium with higher C/N ratio (Safavi et al. 2007). Vega et al. (2003) reported highest spore yield in media containing carbon at 36 g/l and C:N ratio of 10:1. Optimized value of carbon and nitrogen for maximal fungal growth obtained in this study corresponds with the above studies with C:N ratio (28:2.43) coming near 10:1. The optimum pH for maximal fungal growth obtained in the present study was 7.0, supported by the study of Hallsworth and Magan (1996), who determined pH optima of 5–8 for fungal growth and conidial physiology of the entomopathogenic fungi, B. bassiana, M. anisopliae, and Paecilomyces farinosus.
Nutritional parameters play a key role in growth, spore production and virulence of fungi. The studies by Gao et al. (2007) and Mustafa and Kaur (2009) established strain specificity in nutritional requirements. Therefore, it is imperative to know nutritional aspects for any entomopathogenic strain before being developed for field application (Kant et al. 1996). Rather than using the OVAT approach as reported in earlier studies, it is desirable to study the influence of various nutritional inputs in totality using statistical optimization. In case of entomopathogens, Bhanu Prakash et al. (2008) had optimized M. anisopliae conidiospores production in solid-state fermentation while Rao et al. (2006) optimized cultivation processes for extracellular protease production from B. bassiana. The present study has optimized the crucial nutrients and pH requirements for the growth of new B. bassiana isolate through series of OVAT and statistical methodology, which could eventually help in various fermentation process and other large scale productional set-ups.
Overall, the present study attempted biological control of M. domestica using a new isolate of B. bassiana. Significant mortality of adults (100 %) and larvae (72.3 %) obtained after 5 days of monitoring, established the potential of the tested strain for large scale applications. In order to obtain efficient alternates to high cost complex media, several low cost carbon and nitrogen sources were screened. The results revealed glucose and NaNO3 as the most suitable source for optimal biomass and spore production. Further, optimization through P–B and RSM revealed highly significant effect of glucose and pH. Also, interaction factor between glucose and pH and NaNO3 and pH showed significant effect on response. Thus, the present study suggests careful selection and monitoring of these crucial factors while going for large scale productional set-ups with entomopathogenic fungi.
Acknowledgments
This work was supported by Indian Council of Medical Research (IRIS_ID No. 2010-07860), India. CSIR fellowship to one of the authors (SM) is gratefully acknowledged. The authors acknowledge Mr. Peeyush Kumar (IIT Delhi, India) for his help in experimental work.
References
- Barson G, Renn N, Bywater AF. Laboratory evaluation of six species of entomopathogenic fungi for the control of house fly (Musca domestica L.), a pest of intensive animal units. J Invertebr Pathol. 1994;64:107–113. doi: 10.1006/jipa.1994.1078. [DOI] [PubMed] [Google Scholar]
- Bhanu Prakash GVS, Padmaja V, Siva Kiran RR. Statistical optimization of process variables for the large-scale production of Metarhizium anisopliae conidiospores in solid-state fermentation. Bioresour Technol. 2008;99:1530–1537. doi: 10.1016/j.biortech.2007.04.031. [DOI] [PubMed] [Google Scholar]
- Campbell RK, Barnes GL, Cartwright BO, Eikenbary RD. Growth and sporulation of Beauveria bassiana and Metarhizium anisopliae in a basal medium containing various carbohydrate sources. J Invertebr Pathol. 1983;41:117–121. doi: 10.1016/0022-2011(83)90242-2. [DOI] [Google Scholar]
- Carswell I, Spooner-Hart R, Milner RJ. Laboratory susceptibility of Musca domestica L. (Diptera: Muscidae) and Bactrocera tryoni (Frogatt) (Diptera: Tephritidae) to an isolate of Metarhizium anisopliae (Metsch.) Sorokin. Aust J Entomol. 1998;37:281–284. doi: 10.1111/j.1440-6055.1998.tb01584.x. [DOI] [Google Scholar]
- Deacon JW. A chemically defined liquid culture media for fungus. Fungal biology. 4. Oxford: Blackwell Publishing limited; 2006. [Google Scholar]
- Fuguet R, The’raud M, Vey A. Production in vitro of toxic macromolecules by strains of Beauveria bassiana, and purification of a chitosanase-like protein secreted by a melanizing isolate. Comp Biochem Physiol Part C. 2004;138:149–161. doi: 10.1016/j.cca.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Gao Li, Sun MH, Liu XZ, Yong CS. Effects of carbon concentration and carbon to nitrogen ratio on the growth and sporulation of several biocontrol fungi. Mycol Res. 2007;111:87–92. doi: 10.1016/j.mycres.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Geden CJ, Rutz DA, Steinkraus DC. Virulence of different isolates and formulation of Beauveria bessiana for house flies and the parasitoid Muscidifurax raptor. Biol Control. 1995;5:615–621. doi: 10.1006/bcon.1995.1073. [DOI] [Google Scholar]
- Gillespie AT (1984) The potential of entomogenous fungi to control glasshouse pests and brown plant hopper of rice. Ph.D. Thesis, Southampton University
- Hallsworth JE, Magan N. Culture, age, temperature and pH effect the polyol and trehalose contents of fungal propagules. Appl Environ Microbiol. 1996;62:2435–2447. doi: 10.1128/aem.62.7.2435-2442.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys AM, Matewele P, Trinci APJ, Gillespie AT. Effects of water activity on morphology, growth and blastospore production of Metarhizium anisopliae, Beauveria bassiana and Paecilomyces farinosus in batch and fed-batch culture. Mycol Res. 1989;92:257–264. doi: 10.1016/S0953-7562(89)80063-2. [DOI] [Google Scholar]
- Jackson MA, Schisler DA. The composition and attributes of Colletotrichum truncatum spores are altered by the nutritional environment. Appl Environ Microbiol. 1992;58:2260–2265. doi: 10.1128/aem.58.7.2260-2265.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant R, Pandey SD, Sharma SK. Role of biological agents for the control of mosquito breeding in rice fields. Indian J Malariol. 1996;33:209–215. [PubMed] [Google Scholar]
- Kim S-W, Hwang H-J, Xu C-P, Sung J-M, Choi J-W, Yun J-W. Optimization of submerged culture process for the production of mycelial biomass and exo-polysaccharides by Cordyceps militaris C738. J Appl Microbiol. 2003;94:120–126. doi: 10.1046/j.1365-2672.2003.01754.x. [DOI] [PubMed] [Google Scholar]
- Lecuona R, Turica M, Tarocco F, Crespo D. Microbial control of Musca domestica (Diptera: Muscidae) with entomopathogenic fungi. J Med Entomol. 2005;42:332–336. doi: 10.1603/0022-2585(2005)042[0332:MCOMDD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Lecuona R, Crespo D, Rossa FL. Populational parameters of Spalangia endius walker (Hymenoptera: Pteromalidae) on Pupae of Musca domestica L. (Diptera: Muscidae) treated with two strains of Beauveria bassiana (Bals.) Vuil. (Deuteromycetes) Neotrop Entomol. 2007;36(4):537–541. doi: 10.1590/S1519-566X2007000400010. [DOI] [PubMed] [Google Scholar]
- Li DP, Holdom DG. Effects of nutrients on colony formation, growth, and sporulation of Metarhizium anisopliae (Deuteromycotina: Hyphomycetes) J Invertebr Pathol. 1995;65:253–260. doi: 10.1006/jipa.1995.1039. [DOI] [Google Scholar]
- Mishra S, Kumar P, Malik A, Satya S. Adulticidal and larvicidal activity of Beauveria bassiana and Metarhizium anisopliae against housefly, Musca domestica (Diptera: Muscidae), in laboratory and simulated field bioassays. Parasitol Res. 2011;108:1483–1492. doi: 10.1007/s00436-010-2203-5. [DOI] [PubMed] [Google Scholar]
- Mochi DA, Monteiro AC, Machado ACR, Yoshida L. Efficiency of entomopathogenic fungi in the control of eggs and larvae of the horn fly Haematobia irritans (Diptera: Muscidae) Vet Parasitol. 2010;167:62–66. doi: 10.1016/j.vetpar.2009.09.046. [DOI] [PubMed] [Google Scholar]
- Mustafa U, Kaur G. Effects of carbon and nitrogen sources and ratio on the germination, growth and sporulation characteristics of Metarhizium anisopliae and Beauveria bassiana isolates. Afr J Agric Res. 2009;3:922–930. [Google Scholar]
- Rangel DEN, Anderson AJ, Roberts DW. Growth of Metarhizium anisopliae on non-preferred carbon sources yields conidia with increased UV-B tolerance. J Invertebr Pathol. 2006;93:127–134. doi: 10.1016/j.jip.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Rao YK, Lu S-C, Liu B-L, Tzeng Y-M. Enhanced production of an extracellular protease from Beauveria bassiana by optimization of cultivation processes. Biochem Eng J. 2006;28:57–66. doi: 10.1016/j.bej.2005.09.005. [DOI] [Google Scholar]
- Sabu A, Keerthi TR, Kumar SR, Chandrasekaran M. L-Glutaminase production by marine Beauveria sp. under solid state fermentation. Process Biochem. 2000;35:705–710. doi: 10.1016/S0032-9592(99)00127-2. [DOI] [Google Scholar]
- Safavi SA, Farooq AS, Aziz KP, Reza RG, Ali RB, Tariq MB. Effect of nutrition on growth and virulence of the entomopathogenic fungus Beauveria bassiana. FEMS Microbiol Lett. 2007;270:116–123. doi: 10.1111/j.1574-6968.2007.00666.x. [DOI] [PubMed] [Google Scholar]
- Siri A, Scorsetti AC, Dikgolz VE, Lopez Lastra CC. Natural infection caused by the fungus Beauveria bassiana as a pathogen of Musca domestica in the neotropic. Biol Control. 2005;50:937–940. [Google Scholar]
- St. Leger RJ. The role of cuticle-degrading proteases in fungal pathogens of insects. Can J Bot. 1995;73:1119–1125. doi: 10.1139/b95-367. [DOI] [Google Scholar]
- Vega FE, Jackson MA, Mercadier G, Poprawski TJ. The impact of nutrition on spore yields for various fungal entomopathogens in liquid culture. World J Microbiol Biotechnol. 2003;19:363–368. doi: 10.1023/A:1023924304456. [DOI] [Google Scholar]
- Watson DW, Geden CJ, Long SJ, Rutz DA. Efficacy of Beaauveria bassiana for the controlling of the House fly and Stable fly (Diptera: Muscadiae) Biol Control. 1995;5:405–411. doi: 10.1006/bcon.1995.1048. [DOI] [Google Scholar]