Abstract
Chronic rhinosinusitis (CRS) presents distinct inflammatory and remodeling patterns in different populations and environments. Tibetan ethnic groups live at high altitudes and in cold weather conditions. We sought to examine whether Tibetans exhibit distinct CRS pathology or characteristics. Sinonasal polyps and mucosal tissue were obtained from 14 Tibetan patients with CRS and nasal polyps (CRSwNPs), 13 patients with CRS without nasal polyps (CRSsNPs), and 12 Tibetan controls. Tissue homogenates and serum samples were assayed for several T-helper (TH) cell cytokines and mediators using enzyme linked immunosorbent assay profiles were measured using quantity polymerase chain reaction. Several key inflammatory cells were examined for immunohistochemical markers. CRSwNPs were characterized by increased mediator promoting eosinophilic inflammation (interleukin [IL]-5, eosinophil cationic protein, and total immunoglobulin E) and slight synergism with expression of IL-8, IL-2sRa, IL-1beta, IL-6, and myeloperoxidase, and a predominance of eosinophils, mast cells, and neutrophils. GATA-3 transcription factor was significantly increased and Foxp3 showed a tendency to be impaired in CRSwNPs compared with controls. CRSsNPs were characterized by significantly high levels of transforming growth factor beta1, increased interferon γ, and a significant enhancement of Foxp3 and T-beta compared with CRSwNPs. There were reduced numbers of inflammatory cells but increased levels of macrophages in CRSsNPs. Compared with CRSsNPs, CRSwNPs present a severe inflammatory reaction and show a TH2 milieu with apparently impaired regulatory T cells (Treg) function and increased inflammatory cells infiltration predominated by eosinophilic and mast cells. In contrast, TH1 polarization with enhanced Treg function and increased levels of macrophages appear in CRSsNPs.
Keywords: Chronic rhinosinusitis, eosinophils, high altitude, interleukin-5, nasal polyps, neutrophils, T-cell subsets, Tibetan patients, transcription factor
Tibet is a plateau region in China, northeast of the Himalayas with average altitude of >4000 m above sea level. The climate in Tibet is characterized by long-hour sunshine, low humidity, and thin air.1
The Tibetans live in a comparatively harsh environment with very limited access to medical facilities. Many Tibetans in remote nomadic and agricultural areas seem to be reluctant to use Western medicine.2 Many Tibetans suffer from a higher prevalence of rhinosinusitis.3,4 However, apart from some ancient traditional Tibetan medicine prescriptions for rhinosinusitis,5,6 the records regarding it are extremely deficient and, to our knowledge, there have been no studies focusing on rhinosinusitis reported in the literature.
Rhinosinusitis is a very common disease. It affects almost 20% of the U.S. and European populations7 and has been increasing in prevalence and incidence worldwide. Chronic rhinosinusitis (CRS) is a longer-lasting inflammatory reaction of the mucosa of the nasal sinuses. Pathologically, it can be classified into two subgroups based on the absence (CRS without nasal polyps [CRSsNPs]) or presence (CRS with nasal polyps [CRSwNPs]) of nasal polyps.8 Nevertheless, clinical symptoms and signs mostly overlap in all patients with chronic sinusitis whether or not they have grown polyps, and even after CRSwNP polypectomy there remains a strong tendency to relapse and present as recalcitrant.9,10
So far, specific conditions of CRS can be lightly differentiated in different populations as previously reported.11 Based on immunopathology showing that CRSwNPs and CRSsNPs display distinctive inflammatory cell and mediator profiles12 and specific features of remodeling,13 most studies of white populations indicated a CRS subset of CRSwNPs was characterized by a T-helper (TH) 2–skewed eosinophilic inflammation, whereas CRSsNPs was characterized as a TH1 inflammatory response.14,15 However, a study of Koreans16 found that eosinophilic NPs only comprised one-third of 30 NP samples. Moreover, earlier studies in Thai,17 Japanese,18 and Chinese12,19,20 patients with CRSwNPs had suggested they exist with poorly expressed eosinophilia and a lack of interleukin (IL)-5 and eotaxin expression in the tissue and, conversely, relatively enriched neutrophilic cell cytokines. Thus, they showed a characteristic of CRSwNPs in Asian countries presenting as a primarily neutrophilic pattern of inflammation with a relative lack of eosinophils. Nevertheless, a study comparing histological aspects of nasal polyposis in black African, Chinese, and white populations showed that the number of mast cells was similar for the three groups of patients. However, a significantly greater number of eosinophils were observed in African patients with polyps. Lymphocytes as well as plasmocytes were rare in African patients but abundant in polyps from both Chinese and white patients.21
Obviously, the appearance of clear differences in inflammatory patterns associated with CRS exist in different ethnic groups, although CRSsNPs represented a predominant TH1 milieu and enhanced regulatory T-cell function in both white and Asian patients.11 However, in contrast, a recent large sample study in middle China showed TH2 skewed inflammation with predominant TH17 reactions in eosinophilic CRSwNPs and with impaired regulatory T-cell function in CRSsNPs.20
We hypothesize that high-altitude environmental and genetic factors could possibly cause a disparity in immunopathological mechanisms in CRS. Thus, in this study, we aimed, initially, to describe and to identify differences in the profile of inflammatory mediators and cellular characteristics for CRS in patients living in high altitudes.
MATERIALS AND METHODS
Patients and Study Design
Nasal tissue and serum were obtained from 14 CRSwNP patients, 13 CRSsNP patients, and 12 control subjects, All patients and controls were from the Tibet autonomous region and Tibetan district of Sichuan province in China, and they all had native Tibetan ethnicity and had lived above the 4000-m plateau for a long time. They had consulted the Department of Otorhinolaryngology of the West China Hospital for surgical treatment. Both subsets of CRS samples (polyps or ethmoid tissue) were obtained during routine endonasal sinus surgery. Patients undergoing septoplasty because of anatomic variations causing symptoms such as headache and frequent epistaxis were considered as control subjects and those exclude sinusitis by computed tomography (CT) scan and samples were obtained from the inferior turbinate during septal surgery. The diagnosis of sinus disease followed the EP3OS guidelines.22 CT images were graded as per Lund-Mackay,23and individual rhinosinusitis symptoms were evaluated according to the Davos classification.23 The atopic status was evaluated by skin-prick tests to common inhalant allergens as previously described.24 The diagnosis of asthma was performed by a pneumologist. None of the subjects used treatment such as oral or nasal corticosteroids, antibiotic or Tibetan tradition medicine for at least 4 weeks before surgery. All patients gave their written informed consent and the Ethics Committee of the Sichuan University approved this study. Ba Luo and Liu Feng contributed equally to this work.
Measurement of Cytokines, Mediators, and Immunoglobulin E
Freshly obtained tissue specimens were weighed and snap frozen; 1 mL of 0.9% NaCl solution was added per every 0.1 g of tissue, and all samples were additionally treated with a protease inhibitor cocktail (Complete Roche, Mannheim, Germany). The tissue was then homogenized using a Braun homogenizer at 1500 rmp for 3 minutes on ice. After homogenization, the suspension was centrifuged at 3000 rpm for 15 minutes at 4°C and the supernatants were separated and stored at −80°C until analysis. All samples were assayed for IL-1β, IL-2sRa, IL-5, interferon (IFN) γ, IL-8, IL-6, IL-17, and transforming growth factor (TGF) β1 using commercially available ELISA kits (Quantikine ELISA; R&D Systems, Minneapolis, Portland, MN; myeloperoxidase [MPO]; Oxis International, OR) and using a Luminex system (Luminex 100a System; Luminex Corp., Ausin, TX). MPO, eosinophil cationic protein (ECP) in tissue and total immunoglobulin E (IgE) in both tissue and serum were measured by the Unicap system (Unicap100a; Pharmacia, Uppsala, Sweden).
Quantitative Real-Time Polymerase Chain Reaction
mRNA levels of the transcription factors GATA-3, T-bet, Foxp3, and RORc were determined by means of real-time polymerase chain reaction (PCR). Snap-frozen tissue samples were placed in liquid nitrogen and thoroughly ground with a mortar and pestle and homogenized with Lysis Buffer (Qiagen GmbH, Hilden, Germany). Total RNA was purified using the RNeasy kit (Qiagen GmbH) following manufacturer's instructions. The 0.5 μg of total RNA was then reverse transcribed to generate cDNA with the Prime Script RT Reagent Kit (Takara Biotechnology Co., Ltd., Dalian, China) as instructed by the supplier. cDNA equivalent to 40 ng of total RNA was used to perform quantitative PCR. Amplification reactions were performed on an iCycler iQ Real-Time PCR Detection System (BioRad Laboratories, Hercules, CA) by using their specific primer sequences PCR reactions processed with SYBR Premix Ex Taq II kit (Takara Biotechnology Co.) and PCR protocol consisted of 1 cycle at 95°C for 10 seconds followed by 40 cycles at 95°C for 5 seconds and at 60°C for 45 seconds. The expression of three housekeeping genes—actin β, hydroxymethylbilane synthase, and elongation factor 1—was used to normalize for transcription and amplifcation variations among samples after a validation with geNorm software (Ghent University, Ghent, Belgium). The relative expression units of each gene per 20 ng of cDNA sample were determined by using the qBase program (Version 1.3.5; Ghent University), which consists of a collection of Microsoft Excel sheets that automatically analyze real-time quantitative PCR data, combining the ΔCT relative quantification model with PCR efficiency correction and multiple reference gene normalization.
Assessment of Inflammatory Cells in Nasal Tissue
Paraffin sections were stained with 1% anti-human CD4 antibody (AF-379-NA; R&D) or 1% mouse monoclonal antibodies CD20 (clone L26; Dako; Carpinteria, CA), CD68 (clone EBM11; Dako), CD138 (clone MI15; Dako), MPO (clone 2C7; Serotec, Oxford, U.K.), and mast cell tryptase (clone AA1; Dako), for 45 minutes at 30°C. At the end of this incubation all samples were washed with Tris-buffered saline for 10 minutes and incubated for an additional 45 minutes at 30°C with EnVisiona (Dako), using an Autostainer (Dako). The samples were counterstained with Mayer's hematoxylin stain and mounted as mentioned previously. For evaluation of eosinophils, air-dried sections were stained with hematoxylin–eosin for 2 minutes at room temperature. After staining, the sections were washed in two changes of acidified water, dehydrated in three changes of 100% ethanol, and mounted in Permount TM Mounting Medium (Bioss, Beijing, China) before analysis by light microscopy.
All stained sections were examined by light microscopy at 400× magnification using an Olympus CX-40 microscope (Olympus, Tokyo, Japan) by two independent observers blinded to the experimental and clinical data. Briefly, the observers evaluated 10 random fields in each section and graded the number of positively stained cells for individual cell markers on a scale and evaluated as previously mentioned.19
Statistical Analysis
Statistical analysis was performed using the SPSS Version 12.0 (SPSS, Inc., Chicago, IL). Data were expressed as box-and-whisker plots. When comparisons were made between groups, a Kruskal-Wallis test was used to establish the significant intergroup variability. A Mann-Whitney U test was then used for between-group comparisons. Immunohistochemical staining and baseline variables were analyzed by using a one-way ANOVA or Fisher's exact test. The significance level was set to a value of α = 0.05.
RESULTS
Patient Characteristics
Thirty-nine native Tibetan patients were enrolled in this study. Groups were comparable in terms of baseline clinical characteristics such as age, female/male ratio, and clinical symptom scores. The groups were similar with regard to demographic characteristics, and both subforms of CRS shared a significantly higher total symptom score. However, nasal congestion and anosmia were the most predominant symptoms with a significantly higher bilateral CT score in patients with CRSwNPs; only two patients were diagnosed with comorbid asthma in the CRSwNP group, whereas CRSsNP patients showed a higher symptom score for headache. Of note was a higher incidence of nasal bleeding in the control group and CRSsNP patients than in CRSwNP subjects (Table 1). Our skin-prick tests revealed in total eight subjects sensitized by single or multiallergen, which includes six subjects for house-dust mite, two subjects for hazel tree and birch, and three subjects plurisensitized to more than three allergens. Most of the subjects with a positive skin-prick test were significantly higher in serum total IgE and specific Dermatophagoides pteronyssinus 1 (IgE-d1).
Table 1.
Clinical features and symptom scores
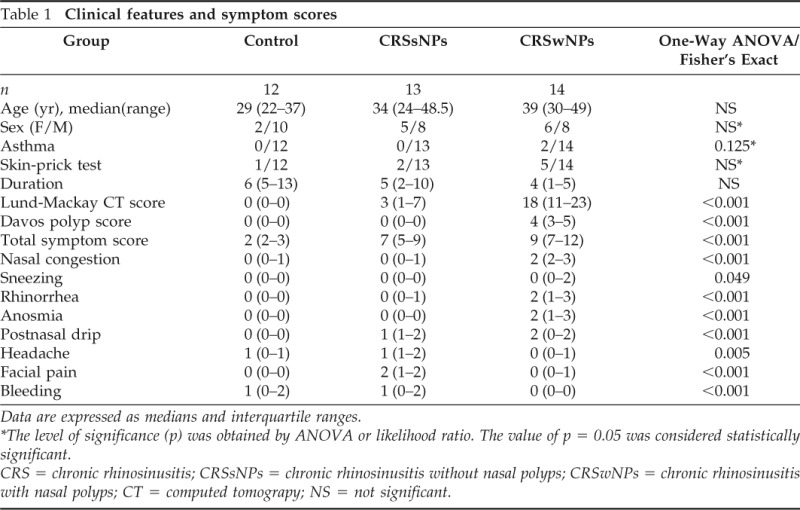
Data are expressed as medians and interquartile ranges.
*The level of significance (p) was obtained by ANOVA or likelihood ratio. The value of p = 0.05 was considered statistically significant.
CRS = chronic rhinosinusitis; CRSsNPs = chronic rhinosinusitis without nasal polyps; CRSwNPs = chronic rhinosinusitis with nasal polyps; CT = computed tomograpy; NS = not significant.
Immunoassay
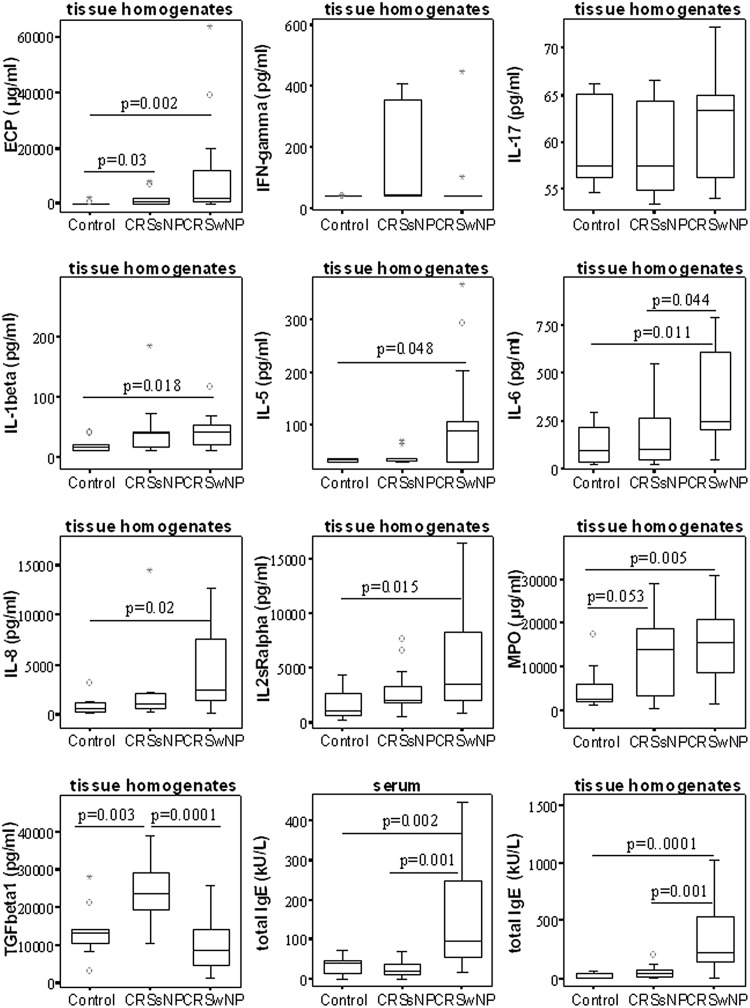
CRSwNPs showed significantly increased amounts of IL-5, ECP, and IL-6 in tissue and a higher level of total IgE in both serum and tissue than in CRSsNP patients and control subjects (p < 0.05); moreover, increased expression of IL-8, IL-2sRa, and IL-1β was found in tissue from CRSwNPs than from tissue in control subjects. In contrast, CRSsNPs is characterized by significantly high levels of TGF-β1 and, apparently, increased levels of IFN-γ but the difference was not significant. Eosinophilic markers of ECP and neutrophilic markers of MPO were significantly increased in both CRS subsets than in control subjects (p < 0.05; Fig. 1).
Figure 1.
Protein concentrations levels of several key cytokines and mediators in tissue homogenates and serum of each group. Values are reported as medians and interquartile ranges. A tow-tailed Mann-Whitney U test was used for between-group comparison. Significance was accepted at p < 0.05.
Gene Expression Quantitative Real-Time PCR
Expression of mRNA for GATA-3 was significantly up-regulated in CRSwNP patients versus CRSsNP patients and control subjects, whereas mRNA expression for T-bet was apparently higher in CRSsNP patients versus control subjects. Foxp3 was significantly overexpressed in CRSsNPs but appeared down-regulated in CRSwNPs, whereas there was no significant difference in RORc expression (Fig. 2).
Figure 2.
mRNA expression levels for T-bet, GATA-3, RORc, and Foxp3 transcription factors normalized to the levels of β-actin, hydroxymethylbilane synthase, and elongation factor 1 as housekeeping genes in each group. Values are reported as medians and interquartile ranges for each group. The significance level was set at a value of p = 0.05.
Inflammatory Cell Types in Both Subsets of CRS
Significantly more CD4+, CD20+, CD138+, and MPO+ inflammatory cell types were found in both subsets of CRS compared with control subjects. Comparison of the inflammatory cell types within the two CRS subsets further showed that CRSwNPs contained significantly more eosinophils and MPO+ cells than CRSsNPs. Additionally, more tryptase-positive cells were found in CRSwNPs and more CD68+ cells were found in CRSsNPs compared with control tissue (Table 2).
Table 2.
IHC staining results: Patient characteristics and symptom scores
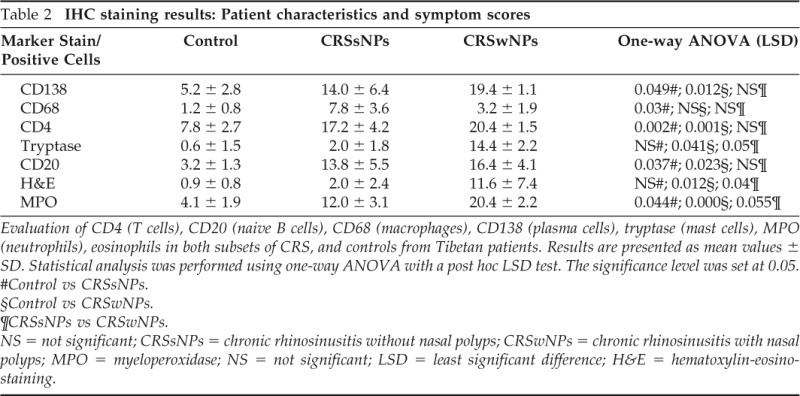
Evaluation of CD4 (T cells), CD20 (naive B cells), CD68 (macrophages), CD138 (plasma cells), tryptase (mast cells), MPO (neutrophils), eosinophils in both subsets of CRS, and controls from Tibetan patients. Results are presented as mean values ± SD. Statistical analysis was performed using one-way ANOVA with a post hoc LSD test. The significance level was set at 0.05.
#Control vs CRSsNPs.
§Control vs CRSwNPs.
¶CRSsNPs vs CRSwNPs.
NS = not significant; CRSsNPs = chronic rhinosinusitis without nasal polyps; CRSwNPs = chronic rhinosinusitis with nasal polyps; MPO = myeloperoxidase; NS = not significant; LSD = least significant difference; H&E = hematoxylin-eosinostaining.
DISCUSSION
Although a majority of white subjects showed a TH2-skewed eosinophilic inflammation in CRSwNPs and a TH1-predominant inflammation in CRSsNPs,25 research on Asian subjects has indicated that they present a TH1-skewed neutrophilic pattern of inflammations in CRSwNPs and have a lesser incidence of asthma comorbidity than in Western patients,26 and CRSsNP patients presented relatively enriched neutrophilic cytokines and a relative lack of eosinophils irrespective of ethnicity.11,20,25
Interestingly, our study initially showed CRSwNPs presenting with a greater immune reaction and higher degree of inflammatory cells infiltration than CRSsNPs, suggesting different potential mechanisms underlying the pathogenic processes. Immunologic assays indicated increased levels of TH2 profile cytokine of IL-5, ECP with a high level of IgE, and concurrent increase of IL-6 in CRSwNPs, but only a significant increase of TGF-β1 and an apparently enhanced TH1 profile of IFN-γ in CRSsNPs. This is consistent with findings of significantly higher levels of GATA-3 signal transduction factor mRNA in CRSwNPs and increased expression of Foxp3 mRNA in CRSsNPs but no significantly higher levels in control subjects. We concluded that there was also regulatory T cells (Treg) enrichment in CRSsNPs but this seems significantly lower in CRSwNPs, consistent with previous studies by Li13 in Chinese subjects and Bruaene et al.14 in a Western population. However, this contrasts with the study by Cao,20 who reported that mucosal samples from Chinese subjects with CRSsNPs were characterized by a decrease in T-regulatory cells and TGF-β1 expression.
However, further combination with inflammatory features showed a high degree of inflammatory cell infiltration in both forms of CRS. It was obvious that eosinophils, MPO+ (neutrophils), CD4+ cells (T lymphocytes), CD138+ cells (plasma cells), and CD20+ cells (B lymphocytes) were enriched in both subsets of CRS. CRSwNPs contained a significant predominance of eosinophils and tryptase-positive cells (mast cells) compared with CRSsNPs, whereas CD68+ cells (macrophages) were predominant in CRSsNPs. Additionally, MPO+ (neutrophils) and several other lymphocytes contribute to the pathology of CRS in this population. Particularly in CRSsNPs, we believe that local mucus bacterial impact increased the numbers of invading macrophages27 and it most likely led to a remodeling effect in rhinosinusitis.28
Therefore, although our study showed that the levels most marks were slightly higher in CRSwNPs than in CRSsNPs, indicating a stronger inflammation reaction in CRSwNPs, neutrophil infiltration was predominant in CRSwNPs. These findings differ slightly from other Asian populations.12,17–20 Perhaps, this may be attributed to a high-altitude environment1 or genetic factor influencing processes of CRS among the Tibetans. Therefore, our findings may provide new insight into the pathogenic mechanism of CRS.
Despite results indicating that the pathogenesis of CRS is based on a common T-cell regulation mechanism and is consistent in different populations, the inflammatory pattern in CRSwNPs presented a predominant TH2-biased mechanism with both eosinophilic and neutrophils contributing to the inflammation reaction in CRSwNPs. This finding is slightly different from those found in previous studies of other Asians groups living at lower altitudes. However, an unexclusion bias may exist because of the limitations of our sample size.
We speculate that the differences may be associated with infections possibly by fungi or other pathogens as result of the poor environmental conditions and through the poor use of antibiotics by this population or antimicrobial resistance as described in the literature.29 The detail of the underlying causes or other implications of this association are not clear.
Our findings regarding asthma comorbidity in the Tibetan CRSwNP patients are in accordance with Bachert26 who showed that increased expression of IL-5 or total IgE marked an increased risk of asthma irrespective of ethnicity. Similarly, our analysis found both serum and tissue total IgE was significantly higher in CRSwNPs and was associated with high levels of IL-5 and ECP, which was also consistent with a results found in a study by Armengot et al.15 The difference in ECP reached statistical significance in CRSsNP patients compared with control subjects, and in the case of ECP, it may also be released by cells other than eosinophils.
Most of our clinical symptoms resembled previous presentations that CRSwNP patients had a stressed nasal obstruction and anosmia, whereas CRSsNP patients commonly complain of headaches rather than postnasal drip.15 However, headaches and nasal obstruction are common symptoms that also appeared in control groups.30 Scoring of bilateral sinus CT images with the Lund-Mackay scale revealed that CRSwNP patients have more extensive opacifications compared with CRSsNP patients.
Our skin-prick tests revealed a total of eight subjects sensitized by single or multiallergen, which included six subjects for house-dust mite and two subjects for hazel tree and birch; one subject positive for rye grass; one subject for olive tree and rye grass; and three subjects polysensitized to more than three allergens. Most of the subjects with positive skin-prick tests had significantly higher serum total IgE and specific IgE-d1. Furthermore, some research literature have reported the major allergens in Tibetan urban areas such as Lhasa are mostly house-dust mite and osier tree as well as some grasses.31,32
In our study epistaxis was a more common symptom among CRSsNP patients than CRSwNP patients. This was considered an effect of the typical high-altitude environment, which usually caused a substantial decrease of inspiratory oxygen pressure, intense solar radiation, and cold weather that may contribute to reduced relative humidity and temperature in the nasal cavity and impaired mucosa of the nose.33 Conversely, in CRSwNP patients, polyp tissue may have a protective effect on the nasal mucosa.
In summary, our findings clearly showed that a high degree of inflammatory cell infiltration presented in Tibetan patients from high altitudes with CRS both with and without NPs. Furthermore, a strong immune reaction showing a TH2 milieu with apparently impaired Treg function and with a predominance of eosinophilic and mast cells presented in CRSwNP patients, whereas TH1 polarization with an enhanced Treg function and increased macrophages was apparently found in CRSsNP patients. This suggests that the pathogenesis of CRS in Tibetan patients is slightly different from that in other Asian populations. This may be the result of specific environmental conditions or genetic variability contributing to pathogenic processes in CRS. Larger sample studies are needed to further investigate the immunopathological mechanisms involved in CRS to determine any ethnicity differences.
Footnotes
Funded by the Program of the National Natural Science Foundation of China (Grant 30973291 and 81260158) and by a collaboration between Ghent University and Sichuan University, by the Flemish Scientific Research Board, FWO, Nr. A12/5-HB-KH3, Project G.0436.04, 3G.0489, G.0642.10N; and by the Global Allergy and Asthma European Network (GA2LEN)
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Gonpo T, Yan L. On the recognition to the ecological safety issues of Qinghai–Tibet Plateau. Tibetan Stud 1:97–103, 2012 [Google Scholar]
- 2. Chen TS, Chen PS. The healing Buddha. J Med Biogr 12:239–241, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Xu L, Zheng K, Jia Y. Census report of the common diseases of otorhinolaryngology from 5229 ordinary person in Bayi region of Tibet. Tibetan Med 1:77–81, 1979 [Google Scholar]
- 4. Droma Y, Kunii O, Yangzom Y, et al. Prevalence and severity of asthma and allergies in schoolchildren in Lhasa, Tibet. Clin Exp Allergy 37:1326–1333, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Zuskin E, Lipozencić J, Pucarin-Cvetković J, et al. Ancient medicine—A review. Acta Dermatovenerol Croat 16:149–157, 2008 [PubMed] [Google Scholar]
- 6. Wang J, Tie J, Zhu Q. Introduction of treatment of rhinosinusitis with topical Tibetan drug—Musks. J Qi Nghaimed Coll 31:216, 2010 [Google Scholar]
- 7. Platts-Mills TA, Rosenwasser LJ. Chronic sinusitis consensus and the way forward. J Allergy Clin Immunol 114:1359–1361, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Huvenne W, van Bruaene N, Zhang N, et al. Chronic rhinosinusitis with and without nasal polyps: What is the difference? Curr Allergy Asthma Rep 9:213–220, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Bachert C, Van Bruaene N, Toskala E, et al. Important research questions in allergy and related diseases: 3-Chronic rhinosinusitis and nasal polyposis—A GALEN study. Allergy 64:520–533, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Reh DD, Wang Y, Ramanathan M, Jr, Lane AP. Treatment-recalcitrant chronic rhinosinusitis with polyps is associated with altered epithelial cell expression of interleukin-33. Am J Rhinol Allergy 24:105–109, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang N, Van Zele T, Perez-Novo C, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol 122:961–968, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Shi J, Fan Y, Xu R, et al. Characterizing T-cell phenotypes in nasal polyposis in Chinese patients. J Investig Allergol Clin Immunol 19:276–282, 2009 [PubMed] [Google Scholar]
- 13. Li X, Meng J, Qiao X, et al. Expression of TGF, matrix metalloproteinases, and tissue inhibitors in Chinese chronic rhinosinusitis. J Allergy Clin Immunol 125:1061–1068, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Van Bruaene N, Perez-Novo C A, Basinski T M, et al. T-cell regulation in chronic paranasal sinus disease. J Allergy Clin Immunol 121:1435–1441, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Schlosser RJ, Mulligan RM, Casey SE, et al. Alterations in gene expression of complement components in chronic rhinosinusitis. Am J Rhinol Allergy 24:21–25, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Kim JW, Hong SL, Kim YK, et al. Histological and immunological features of non-eosinophilic nasal polyps. Otolaryngol Head Neck Surg 137:925–930, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Jareoncharsri P, Bunnag C, Muangsomboon S, et al. Clinical and histopathological classification of nasal polyps in Thais. Siriraj Hosp Gaz 54:689–697, 2002 [Google Scholar]
- 18. Pawankar R, Nonaka M. Inflammatory mechanisms and remodeling in chronic rhinosinusitis and nasal polyps. Curr Allergy Asthma Rep 7:202–208, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Zhang N, Holtappels G, Claeys C, et al. Pattern of inflammation and impact of Staphylococcus aureus enterotoxins in nasal polyps from southern China. Am J Rhinol 20:445–450, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Cao PP, Li HB, Wang BF, et al. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol 124:478–484, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Lacroix JS, Zheng CG, Goytom SH, et al. Histological comparison of nasal polyposis in black African, Chinese and Caucasian patients. Rhinology 40:118–121, 2002 [PubMed] [Google Scholar]
- 22. Fokkens W, Lund V, Bachert C, et al. EAACI position paper on rhinosinusitis and nasal polyps executive summary. Allergy 60:583–601, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Lund VJ, Kennedy DW. Quantification for staging sinusitis. The Staging and Therapy Group. Ann Otol Rhinol Laryngol Suppl 167:17–21, 1995 [PubMed] [Google Scholar]
- 24. Ba L, Zhang N, Meng J, et al. The association between bacterial colonization and inflammatory pattern in Chinese chronic rhinosinusitis patients with nasal polyps. Allergy 66:1296–1303, 2011 [DOI] [PubMed] [Google Scholar]
- 25. Polzehl D, Moeller P, Riechelmann H, Perner S. Distinct features of chronic rhinosinusitis with and without nasal polyps. Allergy 61:1275–1279, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Bachert C, Zhang N, Holtappels G, et al. Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma. J Allergy Clin Immunol 126:961–968, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Patadia M, Dixon J, Conley D, et al. Evaluation of the presence of B-cell attractant chemokines in chronic rhinosinusitis. Am J Rhinol Allergy 24:11–16, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kouzaki H, Seno S, Fukui J, et al. Role of platelet-derived growth factor in airway remodeling in rhinosinusitis. Am J Rhinol Allergy 23:273–280, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Kingdom TT, Swain RE., Jr The microbiology and antimicrobial resistance patterns in chronic rhinosinusitis. Am J Otolaryngol 25:323–328, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Zheng Y, Zhao Y, Lv D, et al. Correlation between computed tomography staging and quality of life instruments in patients with chronic rhinosinusitis. Am J Rhinol Allergy 24:e41–e45, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Ma E, Yang F. Intradermal test analysis of inhaled aeroallergens in patients with seasonal allergic rhinitis in Lhasa region. Tibetan Med J 30:25–26, 2009 [Google Scholar]
- 32. Yang F, Ma E, Zhang G, et al. Investigation of main sensitization pollen in patients with summer hay fever in Lhasa, Tibet. Tibetan Med J 27:3–4, 2006 [Google Scholar]
- 33. Dursun E, Battal B. Clinical outcomes of nasal septal surgery at high altitude. Eur Arch Otorhinolaryngol 266:1579–1581, 2009 [DOI] [PubMed] [Google Scholar]