Abstract
Purpose:
Corticosteroids are widely used for the treatment of B-cell malignancies, including non-Hodgkin lymphoma, chronic lymphocytic leukemia (CLL), and acute lymphoblastic leukemia; however, this class of drug is associated with undesirable off-target effects. Herein, we developed novel milatuzumab-conjugated liposomes as a targeted dexamethasone carrier for therapeutic delivery in CD74+ B-cell malignancies and explored its effect against the disease.
Experimental Design:
The targeting efficiency of milatuzumab-targeted liposomes to CD74+ cells was evaluated in vitro. The effect of CD74-targeted liposomal dexamethasone was compared with free dexa-methasone in primary CLL cells and cell lines in vitro. The therapeutic efficacy of CD74-targeted liposomal dexamethasone was evaluated in a Raji-severe combined immunodeficient (SCID) xenograft model in vivo.
Results:
Milatuzumab-targeted liposomes promoted selective incorporation of carrier molecules into transformed CD74-positive B cells as compared with CD74-negative T-cells. The CD74-dexamethasone-targeted liposomes (CD74-IL-DEX) promoted and increased killing in CD74-positive tumor cells and primary CLL cells. Furthermore, the targeted drug liposomes showed enhanced therapeutic efficacy against a CD74-positive B-cell model as compared with free, or non-targeted, liposomal dexamethasone in SCID mice engrafted with Raji cells in vivo.
Conclusions:
These studies provide evidence and support for a potential use of CD74-targeted liposomal dexamethasone as a new therapy for B-cell malignancies.
Introduction
Treatment options for patients with B-cell malignancies, including non–Hodgkin lymphoma (NHL), acute lymphoblastic leukemia (ALL), andchronic lymphocytic leukemia (CLL), include chemotherapy, antibody therapy, and corticosteroids (1-4). Potent corticosteroids such as dexamethasone (DEX) can induce apoptosis in malignant B cells by numerous pathways, including caspase activation, interleukin regulation, upregulation of proapoptotic protein BIM, and modulation of prosurvival factors, suchas Bcl-2, Bcl-xL, AP-1, and NF-κB (5-7). In addition, corticosteroids can antagonize microenvironmental stimuli that promote tumor cell survival and can act in a p53-independent manner (2, 8). This is beneficial especially to patients who respond poorly to standard treatment due to p53 chromosomal abnormalities (1,9). While corticosteroids are highly active in virtually every type of B-cell malignancy, they have significant side effects, including immunosuppression, hyperlipidemia, proximal muscle wasting, and osteoporosis (10, 11). Developing a strategy to selectively target corticosteroids to B-cell malignancies represents an attractive treatment approach.
Liposomal delivery can alter drug pharmacokinetics, which can influence both efficacy and toxicity (12, 13). Examples include clinically approved liposomal daunorubicin (Daunoxome; ref. 14) and liposomal doxorubicin (Doxil; ref. 15) that have lower Cmax values and extended terminal half-lives as compared with their parental-free drugs and are associated with reduced cardiac toxicity (13). Liposomal corticosteroids have been evaluated for the treatment of arthritis and neoplasia in preclinical animal models (16-18). Immunoliposomes, which are coated with antibodies, can mediate targeted delivery of the payload to cells expressing the specific antigen recognized by the antibody. Internalizing antibodies have been shown to be superior for therapeutic effects of immunoliposomes (19-22). There has been limited evaluation of immunoliposomes for delivery of corticosteroids (23, 24), and to date, targeted delivery of corticosteroids to B-cell malignancies has not been explored. Development of B-cell directed immunoliposomes for corticosteroids would require a B-cell selective target antigen that mediates internalization.
Translational Relevance.
Corticosteroids are widely used for the treatment of B-cell malignancies, including non–Hodgkin lymphoma, chronic lymphocytic leukemia, and acute lymphoblastic leukemia; however, this class of drug is associated with undesirable off target effects. Herein, we developed novel milatuzumab-conjugated liposomes as a targeted dexamethasone carrier for therapeutic delivery in CD74+ B-cell neoplasia and explored its effect against the disease.
These studies provide preclinical evidence and support for a potential use of CD74-targeted liposomal dexamethasone in overcoming dexamethasone-mediated adverse effects and as a new therapy for B-cell malignancies.
CD74 has the attributes desired for immunoliposome construction for B-cell–specific targeting of corticosteroids. It is a type II transmembrane protein with increased expression on the surface of malignant NHL, ALL, and CLL cells and shows robust internalization upon antibody binding (25-27). Milatuzumab, hLL1, is a humanized monoclonal antibody directed against CD74 that internalizes rapidly and is in clinical trials as a potential therapeutic for NHL, ALL, and CLL. We hypothesized that specificity of steroid treatment could be enhanced using immunoliposomes coated with milatuzumab. We have shown previously that milatuzumab immunoliposomes (CD74-ILs) potentiate the cytotoxic effect of the antibody, offering advantageous alternative to conventional treatments (28). Using this vehicle to transport dexamethasone could represent a strategy for improving efficacy and reducing systemic toxicity. Herein, we present preclinical evaluation of CD74-IL-DEX for the therapeutic efficacy in in vitro and in vivo models of B-cell neoplasia.
Materials and Methods
Cell lines and primary CLL cells
Signed informed consent was obtained to procure cells from patients with previously diagnosed CLL as defined by the modified NCI criteria (29). Raji, Jurkat, and 697 cell lines were obtained from the American Type Culture Collection. Milatuzumab was provided by Immunomedics, Inc. Trastuzumab (Genentech) and goat anti-human IgG antibody (Fc gamma fragment-specific, anti-Fc, Jackson ImmunoResearch Laboratories) were obtained commercially.
Flow cytometry assays
Viability was determined by staining with Annexin VFITC (fluorescein isothiocyanate) and propidium iodide (PI) as described previously (30). Milatuzumab was given at 5 μg/mL and dexamethasone at 10 μmol/L. For surface staining, CLL cells were washed in PBS and stained with antibodies to CD19, CD20, CD74 (BD Biosciences) and/or fluorescence-labeled immunoliposomes. Cells were analyzed using a Beckman-Coulter model EPICS XL cytometer (Beckman-Coulter). For internalization studies, 1 × 106 cells were treated with fluorescently labeled antibodies for 30, 60, and 120 minutes at 37°C, washed with glycine acidic buffer (pH 3) to remove surface-bound antibodies and processed for flow cytometry as previously described (31). Percent internalization at each time point was obtained by the formula: [MFI (exp.) – MFI (0% control)]/[MFI (100% control) – MFI (0% control)] × 100. MFI is the geometric mean fluorescence intensity; 100% control corresponds to CD74 expression on the cell surface of cells treated with PBS alone; and 0% control refers to cells incubated with isotype IgG as control at the designated time points.
MTS assay
MTS assay was conducted as previously described (32). Briefly, 1 × 106 cells were plated in 96-well plates and treated with appropriate drugs for 24 hours. Flavopiridol was used as a positive control. The plates were read 12 hours after addition of MTS solution and at 490 nm, and the absorbance values were normalized to media.
Immunoblot analysis
Immunoblots were conducted as described (30). Antibodies used included GilZ (Abcam) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Santa Cruz Biotechnology).
Preparation of immunoliposomes
CD74-ILs were prepared as previously described (28). Briefly, a post-insertion method was used to incorporate antibody into preformed liposomes, and CD74-ILs were prepared with antibody-to-lipid ratios of 1:1,000.Methoxypolyethylene glycol (MW = 2,000 Da) distearoylphosphatidylethanolamine (PEG-DSPE) and egg phosphatidylcholine (Egg-PC) were obtained from Lipoid. Cholesterol (Chol) and DSPE–PEG–maleimide (DSPE–PEG–Mal) were purchased from Avanti Polar Lipids, Inc. 2-Iminothiolane (Traut’s reagent,) 5,5′-dithiobis-(2-nitrobenzoic acid; Ellman’s reagent), and other chemicals were purchased from Sigma Chemical Co. For liposome preparation, lipids [Chol: Egg-PC: PEG-DSPE (molar ratio ¼ 33.5: 65: 1.5)] were dissolved in ethanol, dried to a thin film, and then rehydrated with 0.2 mol/L calcium acetate. The liposomes were dialyzed against HEPES-buffered saline (HBS; 145 mmol/L NaCl, 20 mmol/L HEPES, pH 7.4) overnight, using a DispoDialyzer (Spectrum Labs) with a molecular weight cutoff value of 10,000 Da. Liposome size distribution was analyzed by dynamic light scattering on a NICOMP Particle Sizer Model 370 (Particle Sizing Systems). Volume-weighted analysis showed an average particle size of 103 nm. Dexamethasone phosphate (Sigma-Aldrich) was incubated with the liposomes at 37°C for 1 hour and then free drug was removed by Sepharose CL-4B column separation to yield liposomal DEX (L-DEX). A post-insertion method was adopted to incorporate antibody ligands into preformed L-DEX. In this method, milatuzumab was reacted with 20× Traut’s reagent (2 hours, room temperature) to yield sulfhydryl-modified antibodies. The anti-CD74-SH was then reacted to micelles of Mal-PEG-DSPE at a molar ratio of 1:10 and then incubated with L-DEX for 1 hour at 37°C. Immunoliposomes with CD74-PEG-DSPE-to-lipid ratios of 1:1,000 were thus prepared.
Fluorescence-labeled liposomes and antibodies
Liposomes were fluorescently labeled with either calcein or octadecyl rhodamine B chloride (R18; Molecular Probes, Inc.). Milatuzumab and the goat anti-human IgG antibody (Fc γ fragment–specific, anti-Fc) were fluorescently conjugated with AlexaFluor 488 5-SDP ester (Invitrogen), as described (33). Localization of CD74-ILs and control IgG-ILs in Raji and Jurkat cells was examined by laser scanning confocal microscopy as described (33).
In vivo studies
C.B-17 SCID (Taconic) female mice were injected with 2 × 106 Raji Burkitt lymphoma cells through the tail vein using a mouse tail illuminator (Braintree Scientific Inc.). Dexamethasone was given intraperitoneally at 5 mg/kg in free or liposomal form 3 times a week for 5 weeks. Antibodies were also given at 5 mg/kg. Mice were monitored daily and sacrificed when they developed hind limb paralysis. Tissue samples obtained from tumor-bearing severe combined immunodeficient (SCID) mice that showed early signs of paralysis were submitted to Ohio State University (OSU; Columbus, OH) Pathology Core Facility for histologic analysis to confirm the presence of human leukemic cells. In addition, bone marrow cells were obtained for flow cytometric analysis by flushing femurs with cold PBS following sacrifice. Cells were counted and stained with anti-human CD20 antibody and isotype controls for flow cytometric analysis. Absolute counts were obtained by multiplying total number of cells with the percentage of CD20-positive cells. Animals were monitored daily for signs of illness and sacrificed immediately if hind limb paralysis, respiratory distress, or more than 20% body weight loss was noted. Survival time as determined by hind limb paralysis was the primary endpoint of the study.
Statistical analysis
All reported statistical evaluations were conducted in the Center for Biostatistics at OSU.One-way ANOVA was used to analyze cell line experiments. Linear mixed-effects models were used for analyses of patient samples and in vivo experiments. Kaplan–Meier estimates of survival for treatments and engraftments were plotted, and the survival for each treatment was calculated with 95% confidence intervals. A significance level of α = 0.05 was used for all tests. SAS software (version 9.2, SAS Institute, Inc.) was used for all statistical analyses.
Results
CD74-ILs are internalized in target CD74+ tumor cells
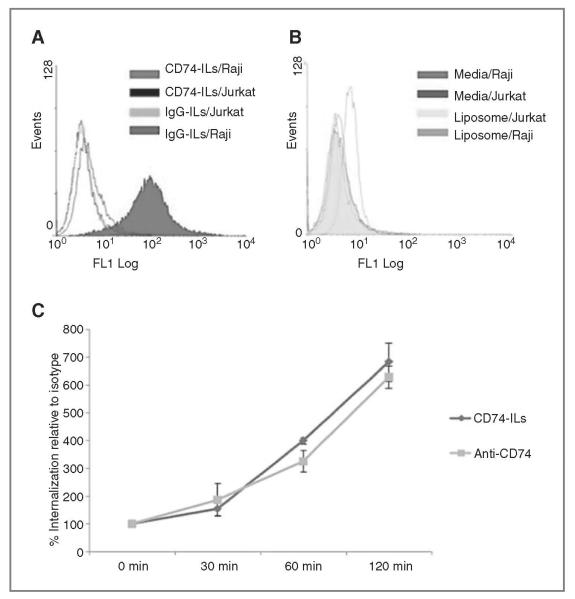
Rapidly internalizing antibodies are preferred for immunoliposomes due to enhanced intracellular drug delivery and therapeutic effects (19-21). Previous work has established that the CD74 antibody, milatuzumab, can be internalized rapidly by target cells as compared with anti-CD19 and anti-CD20 (27, 34, 35). We first evaluated CD74-ILs binding and internalization in vitro. Calcein-labeled CD74-ILs were tested in CD74+ Raji Burkitt lymphoma cells (26, 27, 36) and CD74− Jurkat T lymphoblasts. Nontargeted liposomes were used as a negative control. We observed specific binding of CD74-ILs (representative shown in Fig. 1A) to Raji B cells but not to control Jurkat T cells (Raji MFI 14.7 vs. Jurkat MFI 3.5, P = 0.002). Control IgG-ILs (MFI, 3.9; Fig. 1A) and nonconjugated liposomes (MFI, 2.8; Fig. 1B) did not bind specifically to Raji cells. Collectively, this showed specificity of CD74-ILs for the CD74+ target cells.
Figure 1.
CD74-ILs bind to and are internalized into CD74+ Raji cells. CD74-ILs labeled with calcein are shown by flow cytometry to bind to CD74(+) Raji B cells (A) but not CD74− Jurkat T cells (B). Nonspecific IgG-ILs do not bind to Raji cells (AandB). Internalization of CD74-ILs and anti-CD74 (n = 3) is shown in Raji cells (C), over time portrayed by change in MFI. The results were normalized to IgG isotype and IgG-ILs.
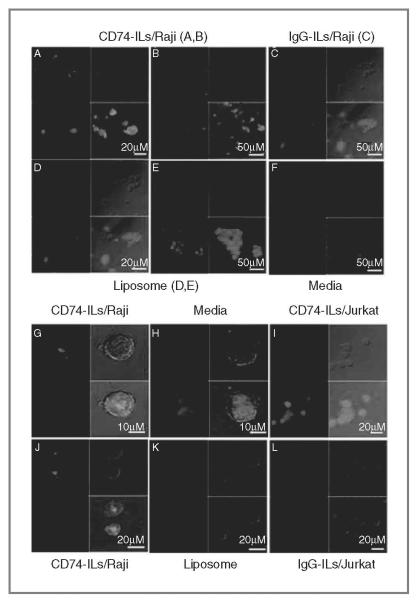
Next, we sought to determine the efficiency of CD74-ILs internalization into Raji cells. In Fig. 1C, we showed that CD74-ILs can be internalized rapidly into target cells similar to that observed with anti-CD74 antibody alone (n = 3, CD74-ILs vs. CD74; P > 0.20 for 30, 60, 120 minutes, respectively). In contrast, IgG-ILs showed no internalization with similar MFI throughout the time points, which was thus used for normalization of results. To confirm these findings and determine the localization of the CD74-ILs in the target cells, we conducted confocal microscopy. Our findings further showed that CD74-ILs were localized to the cell membrane and were internalized in the target Raji cells (Fig. 2A, B, and G), whereas controls did not. CD74-ILs (panel I) did not bind nor internalize in CD7− Jurkat cells. Collectively, these results indicate that CD74-ILs can bind with specificity to target cells and are internalized rapidly, which justifies further development of CD74-ILs.
Figure 2.
Localization of CD74-ILs in target cells. CD74-ILs, after 1-hour incubation with Raji cells (A, B, G, J) visualized by confocal microscopy. CD74-ILs are observed inside the cells and also on the cell membrane. Controls such as IgG-ILs (C and L) and nontargeted liposomes (D, E, K) did not enter/bind the cells. CD74-ILs in CD74− cell line Jurkat (I) did not bind/enter the cells. All lipids are R18-labeled.
Creation of CD74-ILs containing dexamethasone
CD74-ILs loaded with dexamethasone were synthesized. Milatuzumab anti-CD74 antibody was incorporated after drug loading. The immunoliposomes had a mean size of 103 ± 12 nm. The drug was incorporated by remote loading with a pH gradient generated by calcium acetate (37, 38). The efficiency of drug loading of the particle was 92% to 94% (data not shown).
In vitro activity of CD74-IL-DEX
CD74-IL-DEX was tested for in vitro cytotoxicity against lymphoid cell line and primary CLL cells. Previously, we have shown that in vitro CD74-ILs are highly effective in killing B-CLL cells and mimic cross-linked CD74-mediated cytotoxicity (28). Primary B-CLL cells were incubated for 24 hours with CD74-ILs, CD74 with cross-linker, CD74-IL-DEX, or free DEX. The cells were stained with PI and processed for flow cytometry. The results shown in Fig. 3A indicate that CD74-IL-DEX can induce apoptosis to B-CLL cells to a higher degree than empty CD74-ILs (n = 14,% PI positive cells 25.07 vs.15.92 respectively, P < 0.0001) and free DEX (PI-positive cells 16.03, P < 0.0001). Cross-linked milatuzumab had comparable levels of PI-positive staining as CD74-IL-DEX (27.92 vs. 25.07, respectively). Similar results were observed examining viability by assessment of mitochondrial activity, as shown in Fig. 3B. In Fig. 3A, we observed that L-DEX–treated cells had lower PI-positive staining than free DEX (10.41%vs. 16.03%PI+, respectively; n = 14, P = 0.003) and higher MTS assessed mitochondrial activity than free DEX (Fig. 3B, n = 8, 77.62% vs. 46%, P < 0.0001, respectively). This implies that the encapsulation of dexamethasone into liposomes altered the free drug dynamics over time and diminished the efficacy.
Figure 3.
CD74-IL-DEX decreases viability and mitochondrial activity in CLL cells. Primary CLL cells (n = 14) were treated for 24 hours with dexamethasone (10 μmol/L) in free or liposomal forms with cross-linked milatuzumab (CD74+Fc) and all CD74 concentrations at 5 μg/mL (A). Flow cytometric analysis of percentage of PI-positive cells indicated that CD74-IL-DEX kills significantly more B-CLL cells than CD74-ILs (P < 0.0001) and dexamethasone (P < 0.0001). MTS assay analysis (B) shows mitochondrial activity in CLL primary cells (n = 8) after 24 hours of treatments with similar conditions as for flow cytometric assay. CD74-IL-DEX reduces mitochondrial activity at a higher degree than CD74-IL (P- < 0.0001) and L-DEX (P < 0.0001). CD74-ILs are significantly different from CD74+Fc (P < 0.0001) for both assays. C, 24 and 48 hours Annexin V/PI staining in Raji cells (n = 3). D, 24 and 48 hours Annexin V/PI staining in 697 cells (n = 3). Free dexamethasone with CD74 or CD74-ILs does not significantly alter cytotoxicity of cells in comparison with free CD74 antibody or CD74-ILs; however, liposomal dexamethasone kills cell significantly compared with free dexamethasone and CD74.
In our previous study with Raji cells, milatuzumab-ILs showed potent apoptosis due to liposome-mediated crosslinking of the milatuzumab used (28). To appreciate the pharmacologic effect of DEX in CD74-ILs encapsulated dexamethasone formulation, we optimized the concentrations of milatuzumab in CD74-IL-DEX formulation to a suboptimal level so that the CD74-ILs effects were minimized and the effects of the delivered dexamethasone could be evaluated. Our findings indicate that encapsulating dexamethasone into liposomes and targeting it with milatuzumab can induce target cell apoptosis and affect normal cellular functions, resulting in significant apoptosis of target cells compared with either milatuzumab or empty CD74-ILs at the corresponding concentration, as shown with Raji lymphoma (Fig. 3C) and 697 ALL (Fig. 3D) cell lines.
To further confirm the cytotoxicity of CD74-IL-DEX is dependent upon dexamethasone rather than CD74-ILs, immunoblot analysis on the glucocorticoid-induced leucine zipper (GilZ) protein was selected to show cellular glucocorticoid receptor level response (Fig. 4). This shows that after 48-hour treatment with dexamethasone at 10 μmol/L and CD74 accordingly, the levels of GilZ were induced by CD74-IL-DEX and L-DEX but not significantly by other treatment, including free dexamethasone.
Figure 4.
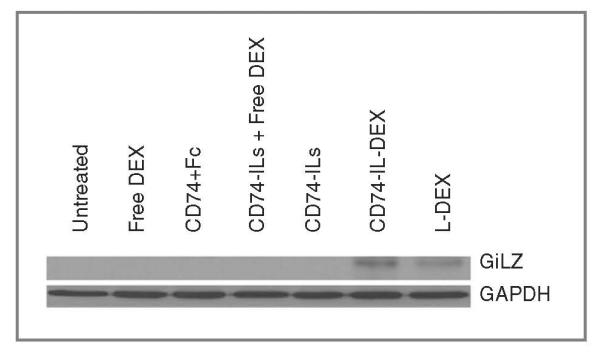
CD74-IL-DEX and L-DEX increase cellular GilZ level. Immunoblot analysis of GilZ induction in whole-cell lysates isolated from Raji cells treated with different formulations of DEX at 10 μmol/L and equivalent CD74.
CD74-IL-DEX increases survival of SCID mice bearing Raji xenografts
The in vitro cytotoxicity of CD74-IL-DEX showed promising results that justify further in vivo evaluation. Therefore, we sought to determine the effectiveness of the CD74-IL-DEX as a therapeutic agent. The disseminated Raji xenograft model described by our group previously (39, 40) was obtained by engrafting SCID mice with Raji cells. The malignant cells infiltrated the central nervous system and bone marrow and, within approximately 2 weeks, mice developed hind limb paralysis requiring sacrifice. This disease model is commonly used to study lymphoproliferative malignancies (27, 40) and is pertinent for our in vivo studies, as CD74-ILs specificity has been shown with Raji cells. Three days postengraftment, mice were treated with CD74-IL-DEX (n = 16), empty CD74-ILs (n = 16), CD74 (n = 16), free dexamethasone (n = 8), or L-DEX (n = 8). Control groups also included Herceptin-IL-DEX (n = 16), Herceptin(HER)-ILs (n = 8), Herceptin (HER, n = 7), empty liposomes (n = 7), and PBS (n = 8). Treatment was administered with intraperitoneal injections 3 times a week for 5 weeks. Antibodies and drug were given at 5 mg/kg in free or liposomal form. All control mice died within 2 weeks postengraftment due to hind limb paralysis. As seen in Fig. 5, milatuzumab anti-CD74 antibody alone increased survival over control groups, but mice treated with CD74-ILs survived approximately 6.35 days longer than CD74 alone (P = 0.04) corroborating our previous in vitro data that CD74-ILs enhanced direct killing of this antibody mentioned earlier and also shown previously (28). Mice receiving CD74-IL-DEX survived longer than all other groups (n = 16, P = 0.0002). The CD74-IL-DEX group survived an average of 13.56 days longer (Table 1) than the CD74-ILs group, and 29.4 days longer than control groups. Consistent with this finding, flow cytometric analysis of bone marrow from CD74-IL-DEX–treated mice showed reduced human CD20+ cells (25%, 38%, and 40% hCD20+ cells in CD74-IL-DEX-, CD74-IL-, and CD74-treated groups, respectively). These results suggest that incorporating dexamethasone into liposomes and delivering it with anti-CD74 to malignant cells can achieve better survival rates than the drug or antibody given separately.
Figure 5.
CD74-IL-DEX extends survival of SCID mice bearing Raji xenografts. A, survival curve of SCID mice treated with various drugs. All control groups died within days 14 to 15 days postengraftment. CD74-ILs increased the mean survival by 6.35 days compared with CD74 treatment alone (P = 0.0405). CD74-IL-DEX liposome group survived an average of 13.56 days longer than CD74-ILs group (P = 0.0002) and increased mean survival by 29.4 days compared with controls (P = 0.0002). B, statistical analysis using ANOVA to compare mean days of survival among groups. A summary of statistical results is presented in Table 1. HER, Herceptin.
Table 1.
Survival statistics from SCID-Raji xenograft study
| Comparisons | Estimate (difference in days of survival) |
P | *95% CI |
|---|---|---|---|
| A: CD74-ILs vs. CD74 | 6.19 | 2.77–9.61 | |
| B: Herceptin-ILs vs. Herceptin | −0.16 | −5.17 to 4.85 | |
| A–B: Interaction between liposome and CD74 | 6.35 | 0.0405 | 0.28–12.41 |
| C: CD74-IL-DEX vs. CD74-ILs | 13.56 | 10.41–16.98 | |
| D: Herceptin-IL-DEX vs. Herceptin-ILs | 0.13 | −4.07 to 4.30 | |
| C–D: Interaction between CD74 and DEX (with liposome) | 13.44 | 0.0002 | 8.03–18.84 |
| CD74-IL-DEX vs. Herceptin-IL-DEX | 29.4 | 0.0002 | 25.95–32.78 |
Discussion
Treatment schemes for B-cell malignancies such as CLL, NHL, and ALL commonly include corticosteroids, such as dexamethasone. This potent drug has great immunosuppressive, anti-inflammatory, and proapoptotic properties desirable for malignancies, as well as other diseases such as asthma and arthritis. Nonetheless, the benefits of corticosteroids sometimes are compromised by their numerous side effects. Here, we describe how encapsulation of dexamethasone into liposomes and delivery with milatuzumab, anti-CD74 antibody can achieve target specificity and improved therapeutic effects. Our findings show that CD74-ILs bind and are internalized in CD74+ Raji cells, offering a robust vehicle for drug encapsulation. CD74-IL-DEX induced cell death in malignant primary CLL cells and disturbed mitochondrial activity to a higher degree than cross-linked antibody or empty CD74-ILs. Free dexamethasone and L-DEX did not mediate such effects, showing the need for drug encapsulation and antibody to improve selective targeted delivery. The promising in vitro results obtained were further evaluated in SCID mice engrafted with CD74+ Raji cells. These studies showed that CD74-IL-DEX treatment significantly prolonged survival of mice by 29.4 days as compared withcontrol groups (P = 0.0002). This was also consistent with the decreased human CD20+ cell sobserved in the bone marrow of the CD74-IL-DEX–treated group. The bone marrow microenvironment provides survival and growth advantage for leukemic B cells (41), and reduction of malignant B cells in the bone marrow using CD74-IL-DEX has clinical relevance to the disease. Consistent with this, treatment with CD74-IL-DEX exhibited superior in vivo efficacy compared with nontargeted and control formulations.
In the current therapy studies, we used 5 mg/kg of antibodies and DEX, based on previous data and our preliminary studies (27, 33). We also adopted the 5-week treatment scheme to mimic clinical dosing of milatuzumab (42, 43) and to address the aggressiveness of the Raji xenograft model. Effects of various dosing schemes of CD74-IL-DEX and alterations in the drug and antibody ratios that can alter the therapeutic in vivo efficacy of the used formulations remain to be evaluated. As preliminary in vitro studies failed to show significant advantage in combining CD74-ILs with free dexamethasone, we focused our in vivo analysis to evaluate the efficacy of targeted delivery using CD74-IL-DEX and compared with isotype control Herceptin-IL-DEX and free dexamethasone.
Numerous studies have established that liposomal drugs behave differently than their free counterpart (15, 44, 45). Here, we also show that L-DEX has different effects than free drug in vivo. Results from a pilot pharmacokinetic study indicated that free and L-DEX had different pharmacokinetics (manuscript in submission). Our findings showed that the Cmax of L-DEX was approximately 2 times lower than free dexamethasone. The area under the curve was about 1.4 times higher with L-DEX than with free dexamethasone. The clearance of free drug was also faster than L-DEX and the bioavailability of the L-DEX was higher than free dexamethasone, likely due to slower drug clearance as than free drug. Furthermore, the study presented in ICR mice showed that L-DEX did not alter lymphocyte numbers from the spleen, but free drug reduced CD19+ splenic lymphocytes, supporting our in vitro cytotoxicity data (data not shown). Interestingly, this effect was not seen with CD3+ lymphocytes, which remained similar in both free and L-DEX groups. There are multiple studies showing that corticosteroids induce lymphocytic apoptosis, but theway that dexamethasone induces apoptosis in lymphocytes is intricate, variable, and has not been fully elucidated (46-48). For mature T cells, studies in peripheral lymphocytes have shown that the effect is mainly attributed to redistribution of cells to other compartments. The effect could be specific to spleen cells and dependent on the dosing and endpoint of the study and could indicate a higher sensitivity of B cells in spleen to dexamethasone apoptosis than in T cells. Regardless, the findings are intriguing and additional studies could possibly explain this observation. In the same study, we also observed that free dexamethasone increased serum cholesterol levels significantly posttreatment (Supplementary Fig. S1), a common corticosteroid side effect (11). Interestingly, in contrast to free dexamethasone, L-DEX failed to alter serum cholesterol levels. The aggressive Raji-SCID model precluded cholesterol evaluation beyond 2-week time. Nevertheless, the CD74-IL-DEX induced less cholesterol levels compared with free dexamethasone. Development of appropriate leukemic mouse models expressing human CD74 will allow better characterization of toxicity associated with targeted formulations in longer duration. The results show that liposomal drug has a different action than free drug and the potential to avoid freedrug-associated side effects.Thus, ourresultsindicate that L-DEX has preferred attributes over the free drug, which are further enhanced by targeting with anti-CD74. The lack of clinical advantage with corticosteroid liposomes is likely to be overcome with targeted delivery formulations. Detailed pharmacokinetic profile of immunoliposomal drug will allow defining the clinical advantage of this formulation. Nevertheless, previous and ongoing studies indicate faster plasma clearance of immunoliposome-formulated drugspresumably through efficient delivery and capture by the targeted cells or tissues. In this context, it is possible to increase the dose of dexamethasone delivered to desired cell type minimizing adverse toxicity.
Limited studies have been conducted with corticosteroids carried in antibody-directed immunoliposomes (23, 24). Most corticosteroid liposomes have been tested in nonhematologic malignancy models and do not offer the advantage of antibody targeting. Here, we present an innovative strategy to package dexamethasone into CD74-ILs and thereby alter the properties of the drug, limit side effects, and enhance its efficacy. Immunoliposomal delivery for hematologic malignancies is likely to have added benefit as opposed to solid tumors, due to the fact that many of the malignant cells are readily accessible in the blood stream. Moreover, keeping liposome size to 100 to 200 nm range can achieve better specificity into tumor homing compartments such as the bone marrow and achieve extended drug circulation time (49, 50).
The findings show therapeutic enhancement of DEX targeted with CD74-ILs. Our studies can have applications to various B-cell malignancies treated with corticosteroids. Furthermore, CD74-IL-DEX could serve as platform for other corticosteroid liposome development with different antibodies to achieve disease-specific therapy. The post-insertion method we have used, however, warrants conjugation of milatuzumab onto the surface of liposome and this may result in random orientation of the conjugated antibodies. We are currently working on combining an anti-CD20 antibody with milatuzumab for dual targeting of immunoliposomes to target cells. We speculate that the combination of rapidly internalizing milatuzumab and clinically established rituximab or other anti-CD20 antibodies may offer an added advantage to L-DEX targeting. Overall, this theme seeks to pursue novel ways to administer drugs that are active but limited by their toxicity. Our work is an example of how we can successfully develop immunoliposomes for hematologic malignancies and combine antibodies with drugs in one system for ease of use, enhanced targeting, andimproved pharmacokinetics with the goal of improving treatment of these diseases.
Supplementary Material
Acknowledgments
Grant Support
This work is supported by The Leukemia and Lymphoma Society, P50-CA140158, PO1-CA95426, PO1 CA81534, R01 CA135332, and The D. Warren Brown Foundation.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Y. Mao and G. Triantafillou contributed equally to this work.
R.J. Lee, J.C. Byrd, and N. Muthusamy are senior authors and contributed equally to this work.
Disclosure of Potential Conflicts of Interest
D.M. Goldenberg has stock and income from Immunomedics, Inc., where he also serves as an officer. No potential conflicts of interest were disclosed by the other authors.
References
- 1.Byrd JC, Stilgenbauer S, Flinn IW. Chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Prog. 2004:163–83. doi: 10.1182/asheducation-2004.1.163. [DOI] [PubMed] [Google Scholar]
- 2.Greenstein S, Ghias K, Krett NL, Rosen ST. Mechanisms of glucocorticoid-mediated apoptosis in hematological malignancies. Clin Cancer Res. 2002;8:1681–94. [PubMed] [Google Scholar]
- 3.Friedenberg WR, Tallman MS, Brodsky I, Paietta E, Rowe JM, Lee SJ, et al. Modified VAD and PSC-833 in the treatment of resistant or relapsing chronic lymphocytic leukemia (E4996): a trial of the Eastern Cooperative Oncology Group. Leuk Res. 2004;28:813–19. doi: 10.1016/j.leukres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Gupta N, Kavuru S, Patel D, Janson D, Driscoll N, Ahmed S, et al. Rituximab-based chemotherapy for steroid-refractory autoimmune hemolytic anemia of chronic lymphocytic leukemia. Leukemia. 2002;16:2092–95. doi: 10.1038/sj.leu.2402676. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt S, Rainer J, Ploner C, Presul E, Riml S, Kofler R. GlucocorticoidInduced apoptosis and glucocorticoid resistance: molecular mechanisms and clinical relevance. Cell Death Differ. 2004;11(Suppl 1):S45–55. doi: 10.1038/sj.cdd.4401456. [DOI] [PubMed] [Google Scholar]
- 6.Iglesias-Serret D, de Frias M, Santidrián AF, Coll-Mulet L, Cosialls AM, Barragan M, et al. Regulation of the proapoptotic BH3-only protein BIM by glucocorticoids, survival signals and proteasome in chronic lymphocytic leukemia cells. Leukemia. 2006;21:281–87. doi: 10.1038/sj.leu.2404483. [DOI] [PubMed] [Google Scholar]
- 7.Almawi WY, Melemedjian OK, Jaoude MM. On the link between Bcl-2 family proteins and glucocorticoid-induced apoptosis. J Leukocyte Biol. 2004;76:7–14. doi: 10.1189/jlb.0903450. [DOI] [PubMed] [Google Scholar]
- 8.Gine E, Crespo M, Muntanola A, Calpe E, Baptista MJ, Villamor N, et al. Induction of histone H1.2 cytosolic release in chronic lymphocytic leukemia cells after genotoxic and non-genotoxic treatment. Haematologica. 2008;93:75–82. doi: 10.3324/haematol.11546. [DOI] [PubMed] [Google Scholar]
- 9.Rosenwald A, Chuang EY, Davis RE, Wiestner A, Alizadeh AA, Arthur DC, et al. Fludarabine treatment of patients with chronic lymphocytic leukemia induces a p53-dependent gene expression response. Blood. 2004;104:1428–34. doi: 10.1182/blood-2003-09-3236. [DOI] [PubMed] [Google Scholar]
- 10.Cooper MS. Sensitivity of bone to glucocorticoids. Clin Sci (Lond) 2004;107:111–23. doi: 10.1042/CS20040070. [DOI] [PubMed] [Google Scholar]
- 11.Kronen berg H, Williams RH. Williams textbook of endocrinology. 11th Saunders/Elsevier; Philadelphia, PA: 2008. [Google Scholar]
- 12.Noble CO, Kirpotin DB, Hayes ME, Mamot C, Hong K, Park JW, et al. Development of ligand-targeted liposomes for cancer therapy. Expert Opin Ther Targets. 2004;8:335–53. doi: 10.1517/14728222.8.4.335. [DOI] [PubMed] [Google Scholar]
- 13.Batist G, Ramakrishnan G, Rao CS, Chandrasekharan A, Gutheil J, Guthrie T, et al. Reduced cardiotoxicity and preserved antitumor efficacy of liposome-encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized, multicenter trial of metastatic breast cancer. J Clin Oncol. 2001;19:1444–54. doi: 10.1200/JCO.2001.19.5.1444. [DOI] [PubMed] [Google Scholar]
- 14.Lowis S, Lewis I, Elsworth A, Weston C, Doz F, Vassal G, et al. A phase I study of intravenous liposomal daunorubicin (DaunoXome) in paediatric patients with relapsed or resistant solid tumours. Br J Cancer. 2006;95:571–80. doi: 10.1038/sj.bjc.6603288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marina NM, Cochrane D, Harney E, Zomorodi K, Blaney S, Winick N, et al. Dose escalation and pharmacokinetics of pegylated liposomal doxorubicin (Doxil) in children with solid tumors: a pediatric oncology group study. Clin Cancer Res. 2002;8:413–18. [PubMed] [Google Scholar]
- 16.Metselaar JM, Wauben MH, Wagenaar-Hilbers JP, Boerman OC, Storm G. Complete remission of experimental arthritis by joint targeting of glucocorticoids with long-circulating liposomes. Arthritis Rheum. 2003;48:2059–66. doi: 10.1002/art.11140. [DOI] [PubMed] [Google Scholar]
- 17.Rauchhaus U, Kinne RW, Pohlers D, Wiegand S, Wolfert A, Gajda M, et al. Targeted delivery of liposomal dexamethasone phosphate to the spleen provides a persistent therapeutic effect in rat antigen-induced arthritis. Ann Rheum Dis. 2009;68:1933–34. doi: 10.1136/ard.2009.108985. [DOI] [PubMed] [Google Scholar]
- 18.Schiffelers RM, Metselaar JM, Fens MH, Janssen AP, Molema G, Storm G. Liposome-encapsulated prednisolone phosphate inhibits growth of established tumors in mice. Neoplasia. 2005;7:118–27. doi: 10.1593/neo.04340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sapra P, Allen TM. Internalizing antibodies are necessary for improved therapeutic efficacy of antibody-targeted liposomal drugs. Cancer Res. 2002;62:7190–94. [PubMed] [Google Scholar]
- 20.Sugano M, Egilmez NK, Yokota SJ, Chen FA, Harding J, Huang SK, et al. Antibody targeting of doxorubicin-loaded liposomes suppresses the growth and metastatic spread of established human lung tumor xenografts in severe combined immunodeficient mice. Cancer Res. 2000;60:6942–49. [PubMed] [Google Scholar]
- 21.Park JW, Hong K, Kirpotin DB, Colbern G, Shalaby R, Baselga J, et al. Anti-HER2 immunoliposomes: enhanced efficacy attributable to targeted delivery. Clin Cancer Res. 2002;8:1172–81. [PubMed] [Google Scholar]
- 22.Lundberg BB, Griffiths G, Hansen HJ. Cellular association and cytotoxicity of doxorubicin-Loaded immunoliposomes targeted via Fab' fragments of an anti-CD74 antibody. Drug Delivery. 2007;14:171–75. doi: 10.1080/10717540601036831. [DOI] [PubMed] [Google Scholar]
- 23.Hegeman M, Cobelens P, Kamps J, Hennus MP, Jansen NJ, Schultz MJ, et al. Liposome-encapsulated dexamethasone attenuates ventilator-induced lung inflammation. Br J Pharmacol. 2011;163:1048–1058. doi: 10.1111/j.1476-5381.2011.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asgeirsdottir SA, Zwiers PJ, Morselt HW, Moorlag HE, Bakker HI, Heeringa P, et al. Inhibition of proinflammatory genes in anti-GBM glomerulonephritis by targeted dexamethasone-loaded AbEsel liposomes. Am J Physiol Renal Physiol. 2008;294:F554–61. doi: 10.1152/ajprenal.00391.2007. [DOI] [PubMed] [Google Scholar]
- 25.Sapra P, Stein R, Pickett J, Qu Z, Govindan SV, Cardillo TM, et al. Anti-CD74 antibody-doxorubicin conjugate, IMMU-110, in a human multiple myeloma xenograft and in monkeys. Clin Cancer Res. 2005;11:5257–64. doi: 10.1158/1078-0432.CCR-05-0204. [DOI] [PubMed] [Google Scholar]
- 26.Griffiths GL, Mattes MJ, Stein R, Govindan SV, Horak ID, Hansen HJ, et al. Cure of SCID mice bearing human B-lymphoma xenografts by an anti-CD74 antibody-anthracycline drug conjugate. Clin Cancer Res. 2003;9:6567–71. [PubMed] [Google Scholar]
- 27.Stein R, Mattes MJ, Cardillo TM, Hansen HJ, Chang CH, Burton J, et al. CD74: anew candidate target for the immunotherapy of B-cell neoplasms. Clin Cancer Res. 2007;13:5556s–63s. doi: 10.1158/1078-0432.CCR-07-1167. [DOI] [PubMed] [Google Scholar]
- 28.Hertlein E, Triantafillou G, Sass EJ, Hessler JD, Zhang X, Jarjoura D, et al. Milatuzumab immunoliposomes induce cell death in CLL by promoting accumulation of CD74 on the surface of B cells. Blood. 2010;116:2554–58. doi: 10.1182/blood-2009-11-253203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheson BD, Bennett JM, Grever M, Kay N, Keating MJ, O'Brien S, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–97. [PubMed] [Google Scholar]
- 30.Lapalombella R, Yu B, Triantafillou G, Liu Q, Butchar JP, Lozanski G, et al. Lenalidomide down-regulates the CD20 antigen and antagonizes direct and antibody-dependent cellular cytotoxicity of rituximab on primary chronic lymphocytic leukemia cells. Blood. 2008;112:5180–89. doi: 10.1182/blood-2008-01-133108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li N, Hill KS, Elferink LA. Analysis of receptor tyrosine kinase internal-ization using flow cytometry. Methods Mol Biol. 2008;457:305–317. doi: 10.1007/978-1-59745-261-8_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konopleva M, Tari AM, Estrov Z, Harris D, Xie Z, Zhao S, et al. Liposomal Bcl-2 antisense oligonucleotides enhance proliferation, sensitize acute myeloid leukemia to cytosine-arabinoside, and induce apoptosis independent of other antiapoptotic proteins. Blood. 2000;95:3929–38. [PubMed] [Google Scholar]
- 33.Alinari L, Yu B, Christian BA, Yan F, Shin J, Lapalombella R, et al. Combination anti-CD74 (milatuzumab) and anti-CD20 (rituximab) monoclonal antibody therapy has in vitro and in vivo activity in mantle cell lymphoma. Blood. 2011;117:4530–41. doi: 10.1182/blood-2010-08-303354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansen HJ, Ong GL, Diril H, Valdez A, Roche PA, Griffiths GL, et al. Internalization and catabolism of radiolabelled antibodies to the MHC class-II invariant chain by B-cell lymphomas. Biochem J. 1996;320:293–300. doi: 10.1042/bj3200293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burton JD, Ely S, Reddy PK, Stein R, Gold DV, Cardillo TM, et al. CD74 is expressed by multiple myeloma and is a promising target for therapy. Clin Cancer Res. 2004;10:6606–11. doi: 10.1158/1078-0432.CCR-04-0182. [DOI] [PubMed] [Google Scholar]
- 36.Lundberg BB, Griffiths G, Hansen HJ. Cellular association and cytotoxicityofanti-CD74-targeted lipid drug-carriers in Blymphoma cells. J Control Release. 2004;94:155–61. doi: 10.1016/j.jconrel.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 37.Avnir Y, Ulmansky R, Wasserman V, Even-Chen S, Broyer M, Barenholz Y, et al. Amphipathic weak acid glucocorticoid prodrugs remote-loaded into sterically stabilized nanoliposomes evaluated in arthritic rats and in a Beagle dog: a novel approach to treating autoimmune arthritis. Arthritis Rheumatism. 2008;58:119–29. doi: 10.1002/art.23230. [DOI] [PubMed] [Google Scholar]
- 38.Waterhouse DN, Tardi PG, Mayer LD, Bally MB. A comparison of liposomal formulations of doxorubicin with drug administered in free form: changing toxicity profiles. Drug Saf. 2001;24:903–20. doi: 10.2165/00002018-200124120-00004. [DOI] [PubMed] [Google Scholar]
- 39.Zhao X, Lapalombella R, Joshi T, Cheney C, Gowda A, Hayden-Ledbetter MS, et al. Targeting CD37-positive lymphoid malignancies with a novel engineered small modular immunopharmaceutical. Blood. 2007;110:2569–77. doi: 10.1182/blood-2006-12-062927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lapalombella R, Zhao X, Triantafillou G, Yu B, Jin Y, Lozanski G, et al. A novel Raji-Burkitt's lymphoma model for preclinical and mechanistic evaluation of CD52-targeted immunotherapeutic agents. Clin Cancer Res. 2008;14:569–78. doi: 10.1158/1078-0432.CCR-07-1006. [DOI] [PubMed] [Google Scholar]
- 41.Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell'Aquila M, Kipps TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96:2655–63. [PubMed] [Google Scholar]
- 42.Christian B, Alinari L, Earl CT, Wilding CQ, Lustberg M, Benson DM, et al. A Phase I study of milatuzumab, a humanized anti-CD74 antibody, and veltuzumab, a humanized anti-CD20 antibody, in patients with relapsed and refractory B-cell non-Hodgkin's lymphoma. Blood (ASH Annual Meeting Abstracts) 2010:116. Abstract nr 2788. [Google Scholar]
- 43.Kaufman J, Niesvizky R, Stadtmauer EA, Chanan-Khan A, Siegel D, Horne H, et al. Dose-escalation trial of milatuzumab (humanizedanti-CD74 monoclonal antibody) in multiple myeloma. J Clin Oncol. 2009;27:15s. doi: 10.1111/bjh.12565. (suppl; abstr 8593). [DOI] [PubMed] [Google Scholar]
- 44.Gabizon A, Papahadjopoulos D. Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors. Proc Natl Acad Sci U S A. 1988;85:6949–53. doi: 10.1073/pnas.85.18.6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson R, Franch A, Castell M, Perez-Cano FJ, Brauer R, Pohlers D, et al. Liposomal encapsulation enhances and prolongs the anti-inflammatory effects of water-soluble dexamethasone phosphate in experimental adjuvant arthritis. Arthritis Res Ther. 2010;12:R147. doi: 10.1186/ar3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith LK, Cidlowski JA. Glucocorticoid-induced apoptosis of healthy and malignant lymphocytes. Prog Brain Res. 2010;182:1–30. doi: 10.1016/S0079-6123(10)82001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Planey SL, Litwack G. Glucocorticoid-induced apoptosis in lymphocytes. Biochem Biophys Res Commun. 2000;279:307–12. doi: 10.1006/bbrc.2000.3922. [DOI] [PubMed] [Google Scholar]
- 48.Tuckermann JP, Kleiman A, McPherson KG, Reichardt HM. Molecular mechanisms of glucocorticoids in the control of inflammation and lymphocyte apoptosis. Crit Rev Clin Lab Sci. 2005;42:71–104. doi: 10.1080/10408360590888983. [DOI] [PubMed] [Google Scholar]
- 49.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5:505–15. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drummond DC, Meyer O, Hong K, Kirpotin DB, Papahadjopoulos D. Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol Rev. 1999;51:691–743. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.