Synopsis
Drug induced liver injury (DILI) represents a broad spectrum of liver manifestations. However, the most common manifestation is hepatocyte death following drug intake. DILI can be predictable and dose dependent with notable example of acetaminophen toxicity. Idiosyncratic DILI occurs in an unpredictable fashion at low frequencies implying that environmental and genetic factors alter the susceptibility of individuals to the insult (drugs). An biochemical stress is usually initiated by drugs and their reactive metabolites through covalent binding or direct damage to mitochondria, which leads to oxidative stress, activation of stress signaling pathways, impairment of mitochondrial function, endoplasmic reticulum stress, etc. The ultimate cell death pathways converges at mitochondria through acting on mitochondrial outer-membrane permeability (MOMP) or mitochondrial permeability transition (MPT). The striking HLA associations with idiosyncratic DILI highlight the critical role of the adaptive immune response in pathogenesis, which is now believed to be unmasked in genetically susceptible individuals by the biochemical stress in the liver triggered by drug and/or metabolites. The drug-induced biochemical stress may also contribute to the severity of injury by sensitizing hepatocytes to the lethal effects of the immune response. Adaptive mechanisms including antioxidant signaling (such as Nrf2 signaling) , mitophagy, autophagy, unfolded protein response, anti-inflammatory and immune tolerance dampen and ameliorate injury. All together, the development and severity of injury is determined on the battle between the hazardous stress and adaptive responses within the hepatocytes and the innate and adaptive immune systems.
Keywords: Idiosyncratic drug-induced liver injury, Cell death, Reactive metabolites, Oxidative stress, Stress signaling, Mitochondria, Adaptive immunity, HLA associations
Drug-induced liver injury (DILI) occurs in an incidence of 10 to 15 in 10, 000∼100,000 in USA1, 2, yet it has caused remarkable fatality from acute liver failure yearly. Acetaminophen (APAP) alone accounts for the half of the overall cases of acute liver failure in USA3, 4. DILI can also mimic all forms of acute or chronic liver diseases (hepatitis, cholestasis or a mixed)5, which is often under-recognized due to the complexity of clinical scenarios. Most of DILI cases are idiosyncratic. A threshold dose (50 to 100mg) may be required for DILI to occur6, 7. When the dose threshold is exceeded, injury occurs in very small number of individuals. Although acetaminophen hepatotoxicity is dose dependent, idiosyncratic injury has been observed with a lower dose (less than 4 gram per day) depending on individual susceptibility (i.e. alcohol intake, fasting).
Over the past decades, much effort has been put into research to understand the mechanisms of hepatotoxicity and explore biomarkers for DILI surveillance. The central question of pathogenesis is how a drug or its metabolites initiate and propagate cell death within the liver with the assistance of surrounding immune cells.
Necrosis, Apoptosis and Necroptosis
The fundamental process in DILI is the death of hepatocytes (in some circumstances, cholangiocytes or endothelial cells) in the background or recruitment of inflammation. DILI manifests clinically with hepatocellular injury, cholestasis or a mixture of both.
Necrosis and apoptosis, theoretically, are two distinct modes of cell death. The fundamental differences between necrosis and apoptosis are the integrity of the plasma membrane, and involvement of caspase activation. Apoptosis is a sterile, “clean” programmed cell death characterized by cell shrinkage and chromatin fragmentation. The rapid removal of apoptotic cells by phagocytes or other cells minimizes the surrounding inflammation. Mechanistically, apoptosis is mediated by ATP-dependent intracellular proteolytic cascades involving caspases. This is triggered by the extrinsic pathway related to death ligand/receptors binding at plasma membrane, or intrinsic pathways triggered by oxidative stress, radiation, DNA damage or toxins leading to increasing permeability of mitochondrial outer membrane. Idiosyncratic DILI is mainly mediated by the innate or adaptive immune response involving death ligands such as TNF-α and FasL. TNF-α and FasL bind to death receptors of hepatocytes, triggering their demise of apoptosis.
Necrosis involves cell swelling, membrane bleb formation and eventually the rupture of plasma membrane. The release of cellular components from necrotic cells elicits an inflammatory response. Necrosis is considered an oncotic lysis caused by loss of ion homeostasis as a result of severe mitochondria dysfunction and profound ATP depletion.
Necrosis was conventionally considered as an incidental, “unwanted” cell death in a non-regulated manner. Increasing evidence has shown that necrosis can be tightly regulated. One of the remarkable observations is that when an apoptotic pathway was initiated upon TNF-α/TNFR binding in L929 cells8, inhibition of apoptosis with caspase inhibitors or ATP depletion leads to the shifting of cell demise to necrosis. Many terms have been used to categorize this type of cell death, such as necroptosis, programmed necrosis, regulated necrosis, etc. It involves activation of receptor-interacting protein kinases9, 101 and 3 (RIP1 and RIP3), and participation of mitochondria. This cell death has been implicated in pathophysiology of many diseases such as acute pancreatitis11, brain injury12, and viral infection13, 14.
Acetaminophen-induced cell death of hepatocytes has characteristic morphologic changes of necrosis. Mechanistically, the reactive metabolite, N-acetyl-p-benzo-quinoneimine (NAPQI), leads to profound mitochondrial GSH depletion, covalent binding, severe impairment of mitochondrial function and cessation of ATP production, which lead to disruption of ion homeostasis and consequently oncotic necrosis. Interestingly, many stress kinases such as c-Jun N-terminal kinase (JNK)15, glycogen synthase kinase-3b (GSK-3β)16, apoptosis signal-regulating kinase-1 (ASK1)17, mixed-lineage kinase-3 (MLK3)18, PKC19, RIP1(unpublished from our lab) have been found to actively regulate the process. This suggests that APAP-induced necrosis might be a programmed necrosis.
The Involvement of Reactive Metabolites
The fact that liver is the central organ for drug metabolism places it as a prime target for reactive metabolites of drugs. In most cases, drugs or their reactive metabolites are detoxified via phase II conjugation (glucuronidation, acetylation, sulphation, glutathione conjugation, etc) and excreted out of cells through multi-drug resistance-associated protein (MRP) transporters (phase III). Reactive metabolites are often produced through oxidation and reduction by cytochrome P450 (Phase I). A balance between production and detoxification/transport are critical in determining the most upstream aspects of DILI, namely exposure of hepatocytes to some threshold level of reactive chemicals. Dose and lipophilicity (favoring hepatic distribution) are key additional factors in determining achievement of a threshold exposure to initiate hepatocellular stress or injury. DILI is often primed by reactive metabolites and their covalent binding to cellular proteins. Inhibition of bile salt export pump (BSEP) may aggravate hepatotoxicity20, 21 not only by causing cholestasis in some cases, but more importantly by bile acid retention causing mitochondrial and endoplasmic reticulum (ER) stress which may amplify injury or sensitize hepatocytes to other injury mechanisms.
APAP metabolism has been well characterized. APAP is predominantly metabolized through sulphation and glucuronidation. A small proportion of APAP is oxidized by cytochrome P450 isoform 2E1 (to lesser extent 1A2 and 3A4) to a reactive form, N-acetyl-p-benzo-quinoneimine (NAPQI). NAPQI readily attacks free thiols, leading to rapid and selective glutathione (GSH) depletion in cytosol and mitochondria. When GSH is depleted, NAPQI covalently binds to thiol groups of cellular and mitochondrial proteins causing mitochondria dysfunction, and the production of mitochondrial reactive oxygen species (mROS), MAP kinase activation and downstream events leading to necrosis (see below).
Oxidative stress and covalent binding activate not only toxic signaling, but also protective and adaptive pathways. One such pathway is nuclear factor erythroid 2-related factor 2 (Nrf2)/Keap1 signaling22, 23. Nrf2 is usually maintained at a very low level in the cytosol as newly synthesized Nrf2 is rapidly bound to Kelch-like ECH-associated protein 1 (Keap1), an adaptor of Culin E3 ligase, which shuttles Nrf2 to Culin E3 ligase complex for ubiquitin proteasomal degradation24. Keap 1 has 25 cysteine residues and acts as a redox and electrophilic sensor. The Keap-1 homodimer binds to a single Nrf2 molecule at DLG motif with a low affinity and ETGE motif with a high affinity25, 26. According to “hinge and latch” model25, 26, when thiols of Keap1 are oxidized or covalently bound to electrophiles such as NAPQI, the conformation of Keap1 changes and Nrf2 dissociates from DLG binding site while remaining bound to Keap1 at ETGE motif. Keap1 is thus occupied by Nrf2 that is not further ubiquitinated. This allows de novo newly synthesized Nrf2 to translocate to the nucleus where it binds to the antioxidant response element (ARE) promoters and activate the transcription of many antioxidant genes including glutamate-cysteine ligase (GCL), thioredoxin reductase, peroxiredoxin and glutathione S-transferase27, 28. This increases glutathione synthesis and ROS detoxification. Meanwhile, increased expression of MRPs by Nrf2 activation enhances the export of drugs/metabolites out of cells.
Involvement of Stress Signaling
C-Jun N-terminal kinase (JNK) activation in response to TNF-α is usually rapidly dampened by NF-κB transcription of survival genes. Thus the activation is transient and often nontoxic. Sustained JNK activation, however, leads to lethal consequences. This has been extensively studied in APAP mouse model. Inhibition of JNKs with a small synthetic molecule (SP600125) or silencing of JNK expression with siRNA protects against APAP hepatotoxicity15, 29. JNK is a family of serine/threonine kinases belonging to the MAPK family. Upstream is MAP3K (e.g. ASK1 or MLK3) that phosphorylates and activates MAP2K (e.g. MKK4/7) which in turn phosphorylates JNK. Knockout of ASK1 blunts JNK activation and attenuates APAP toxicity17. Silencing GSK-3β or MLK3 blunts early phase of JNK activation and also exhibits a similar protective effect16, 18.
JNK can be activated by many stressors such as ROS, UV light and cytokines. In APAP models, mitochondrial ROS seems to play a crucial role in prolonged JNK activation. We observed that sustained JNK activation occurred upon profound GSH depletion and covalent binding in mitochondria in APAP model. This might at least be achieved by modifying several redox-sensitive regulators of JNKs such as thioredoxin (Trx), GSH S-transferase Pi (GST-Pi) and JNK phosphatases. ASK1 is held inactive by Trx at physiologic conditions and Trx oxidation allows the dissociation of ASK1 for activation30. GSH S-transferase Pi (GST-Pi) interacts directly with JNK as an inhibitor in non-stressed cells. ROS induces polymerization of GST-Pi via intermolecular disulfides, causing dissociation of GSTr polymer from JNK31.
A crucial downstream target of JNK is mitochondria. Sustained, activated JNK was found to translocate to mitochondria, further impairing mitochondrial function, and amplifying oxidative stress. This self-amplifying process eventually leads to collapse of mitochondrial function and cell death. Our lab has identified an important mitochondrial outer membrane protein Sab (SH3 domain-binding protein that preferentially associates with Btk) which binds JNK and mediates its effect on mitochondria32. Silencing Sab expression abolished sustained JNK activation, blocked JNK translocation and attenuated APAP toxicity. The details of how Sab, once phosphorylated by JNK, mediates inhibition of the electron transport (↑TROS), is currently not known.
The Involvement of Mitochondrial Dysfunction
The mechanisms of hepatocyte apoptosis and necrosis converge on mitochondria. The release of cytochrome C, apoptosis-inducing factor (AIF) and Smac from the mitochondrial intermembrane space are crucial to activate caspases and execute apoptosis in the presence of adequate ATP33. This requires permeabilization of the outer mitochondrial membrane (OMM). Necrosis occurs via mitochondrial permeability transition (MPT). MPT is composed of voltage-dependent anion channel (VDAC) from OMM, adenine nucleotide translocase (ANT) from the inner mitochondrial membrane (IMM), and cyclophilin D (CypD) from the matrix. This putative pore spans the mitochondrial outer and inner membranes. Its opening dissipates the proton gradient, resulting in the collapse of mitochondrial membrane potential and cessation of ATP production. As consequence, mitochondria swell and OMM ruptures releasing pro-apoptotic factors34. However, necrosis is the more likely the outcome in the setting of MPT opening, since MPT causes profound ATP depletion and MPT usually occurs in the context of oxidative stress which inactivates caspases.
Selectively permeabilizing OMM allows the release of the pro-apoptotic factors from the intermembrane space without disrupting IMM. Mitochondrial outer membrane permeability (MOMP) is primarily governed by Bax and Bak, which oligomerize and insert into the outer mitochondrial membrane to create pores for the release of cytochrome c and Smac/Diablo. Bax/Bak is regulated by pro-survival Bcl-2 members (Bcl-2, Bcl-XL, and Mcl1) and pro-apoptotic BH3-only members (Bim, Bid, Puma, Bad and Noxa)35, 36. The pro-survival factors such as Bcl-2 and Bcl-XL, directly inhibit Bax/Bak, whereas, pro-apoptotic factors such as tBid and Bim directly activate Bak/Bax, or de-repress Bak/Bax through binding and inhibiting Bcl-2 and Bcl-XL.
Programmed necrosis is considered as an “aborted” apoptosis. When an innate immune response is activated by lipopolysaccharide (LPS), TNF-α is released and binds to the membrane receptors, promoting cell death cascades, particularly JNK signaling. Necrosis occurs when the cell death signaling propagates upon ligand binding (TNF-α/TNFR) at the cytoplasmic membrane, but fails to execute apoptosis while the executioner caspases are suppressed with inhibitors such as Z-VAD-FMK. The caspases destroy RIP1 and RIP3. Recent studies37, 38 showed that when caspases are inhibited under these conditions, RIP1/RIP3 form a complex which translocates to mitochondria where it activates mitochondrial fission necessary for cell death.
ROS/RNS generated by mitochondria are crucial in the mitochondrial death pathway in APAP model. This has been clearly demonstrated with a regioisomer of APAP, 3′-hydroxyacetanilide (AMAP)39. AMAP has a comparative metabolic profile to APAP, but the attack of its reactive metabolite spares mitochondria. With AMAP treatment, mitochondrial GSH is preserved and no hepatotoxicity occurs40, 41. The unsaturated lipid at mitochondrial membrane, such as cardiolipin, is particularly vulnerable to mROS attack. Cardiolipin collaborates with Bax polymer to promote OMM opening42. Some researchers43 also showed that cardiolipin retains cytochrome c at IMM through electrostatic interaction. Cardiolipin peroxidation abrogates this association and frees cytochrome c, a necessary step for the release of cytochrome c to execute apoptosis. Release of mROS is able to activate JNK signaling in the cytoplasm as noted above. JNK translocates to mitochondria and leads to a self-amplifying cycle of JNK activation. Sustained JNK activation can alter the balance of Bcl-2 family by activating pro-apoptotic members and inactivating anti-apoptotic members or lead to sufficient ROS generation to cause MPT opening (Fig.1).
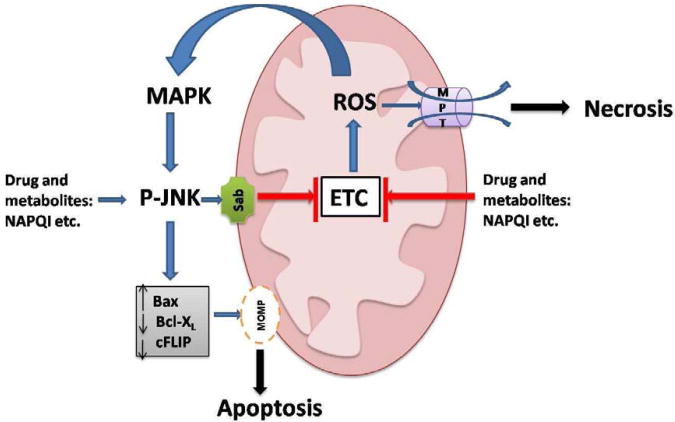
Fig. 1.
c-Jun N-terminal kinase (JNK) signaling and mitochondrial involvement in the death of hepatocytes. Sustained JNK activation and mitochondrial reactive oxygen species (ROS) generation are critical to induce cell death, particularly in the APAP model. In the APAP model, profound glutathione (GSH) depletion and covalent binding of NAPQI, lead to generation of mitochondrial ROS which activates mitogen activated protein kinases (MAPK) including apoptosis signal-regulating kinase-1 (ASK1) and mixed-lineage kinase-3 (MLK3). Both ASK1 and MLK3 activate MKK4/7 which in turn phosphorylates and activates JNK. Phosphorylated JNK translocates to mitochondria where it binds to and phosphorylates Sab, a mitochondrial outer membrane scaffold protein. This somehow leads to further impairment of electron transport chain (ETC) and enhancement of mitochondrial ROS generation. This self-amplifying process leads to sustained JNK activation and overwhelming production of mitochondrial ROS in the APAP model. As a result, mitochondrial permeability transition (MPT) collapses ATP production and necrosis occurs. In the model of tumor necrosis factor-a (TNF-a)-TNF/D-galactosamine, sustained JNK activation leads to mitochondrial outer membrane permeabilization (MOMP) and apoptosis by modulating the Bcl2 family.
Unfit mitochondria may influence the susceptibility to drug-induced liver injury. This is exemplified by heterozygous mouse knockout of superoxide dismutase 2 (Sod2+/-) which exhibits greater vulnerability to liver injury from drugs such as troglitzone44 and flutamide45 as well as APAP46. SOD2(MnSOD) resides solely in mitochondrial matrix and regulates mitochondrial redox by scavenging superoxide. Null knockout (Sod2-/-) is not viable. The heterozygous knockout has preserved GSH/GSSG level, glutathione peroxidase and catalase at birth, but exhibits cumulative mitochondrial oxidative stress over time. With extrinsic insults (such as a drug) to mitochondria, the threshold of mitochondrial damage and/or mROS is lowered to elicit apoptosis or necrosis.
The Involvement of Mitochondrial Adaptation
Mitochondria provide primary energy source for the cell to function and cope with stress. Cyclical fusion and fission coupled with mitophagy are key in maintaining quality control and mitochondrial fitness47. Mitochondrial fission is mediated by Drp1, a large GTPase in the dynamin family. Drp1 is recruited from cytosol by a group of adaptor proteins including Mff, Mid49 and Mid51, and constricts both OMM and IMM at the site where mitochondria make contact with endoplasmic reticulum. Mitochondrial fusion involves OMM fusion and IMM fusion. OMM fusion is mediated by Mfn1 and Mfn2. IMM fusion is mediated by Opa1.
Many environmental insults or drugs (tetracycline, amiodarone, valproate and various antiviral nucleoside analogues) can directly damage mitochondria and deplete mitochondrial DNA. Inhibition of the electron transport chain leads to accumulation of reducing equivalents which generate ROS. Damage from ROS, such as oxidation of mitochondrial proteins, lipids and DNA, builds up within mitochondria. DILI related to mitochondrial toxicity is generally characterized by microvesicular steatosis, focal necrosis and cholestasis. The number of mitochondria in these cases is decreased. This represents a special case when the drug or metabolite directly targets mitochondria and selective mitochondrial dysfunction is the cause of the phenotype. This should be distinguished from examples where the immune system is playing a major role in inducing the injury and the effects of the drug/metabolite on mitochondria influence the susceptibility or severity of immune (innate or adaptive)-mediated killing.
Mitochondrial fusion-fission is a self-repair mechanism by which the damage to mtDNA/proteins/lipids is dissipated by fusion with healthy mitochondria and cumulative damage is contained for elimination. Fusion allows component exchange/sharing of healthy mitochondria with damaged ones, thus rescuing stress and mitigating damage. The cumulative damage would eventually pose harm to the cells. Elimination of the damaged ones is necessary to further maintain quality control. Two major enzymes: PINK1 and PARKIN coordinate and flag the damaged ones for autophagic degradation48. The hypothesis is that the damaged components aggregate at the tip of mitochondria. Upon mitochondrial depolarization, PINK1, a membrane kinase, concentrates on the outer membrane of dysfunctional mitochondria. PINK1 recruits PARKIN, an E3 ligase, which ubiquintinates outer-membrane proteins for proteosomal and autophagic degradation49-51. Mitochondrial fission then allows the segregation of damaged parts which are subsequently engulfed by the autophagosome for elimination.
When the damage is overwhelming and/or repair system is severely impaired, stressed cells commit suicide. There is a close link between mitochondrial fission and cell death(both apoptosis and necrosis). Bax is found to co-localize with Drp1 and Mffs52. Oligermerization of Bax (to promote MOMP) is accompanied by Drp1-dependent fission. Lack of Drp1 delays cell death by decreasing cytochrome C release53. However, inhibition of Drp1 (sequestered in cytoplasm) also protects against necrosis, e.g. APAP and necroptosis models. The link between JNK, ROS, MOMP, MPT with Drp1 is not completely understood. There have even been suggestions that partial membrane remodeling without full fission is implicated in cell death.
The Role of Adaptive Immunity and Innate Immunity in Dili
Some DILI cases (For example: sulindac, phenytoin, and amoxicillin-clavulanic acid ) have classic features of an allergic reaction such as a rash, fever and eosinophilia. The hypothesis is the drug or its metabolites act as haptens and covalently bind to a liver protein such as cytochrome p450. The drug-protein adducts are further processed in the macrophage/dendritic cell and presented as an antigen in complex with major histocompatibility complex (MHC) class II molecules, triggering the adaptive immune response by binding to T cell receptors of CD4 cells. This leads to CD8 cytotoxic T-cell activation. The sensitized CD8 T cells express FasL, TNF-alpha, and perforin that mediate cell death of hepatocytes. Although, most idiosyncratic DILI cases lack features of the systemic allergic reaction, adaptive immunity is believed to play a pivotal role in initiation and propagation of liver injury54. Several genome-wide association studies have revealed striking HLA haplotype associations with DILI. HLA-DRB1*1501 allele has a strong association with Augmentin-induced cholestatic liver injury55-57. The same allele is also associated with an increased risk of lumiracoxib-related hepatitis58. In the case of flucloxacillin, there is a strong link between HLA-B*5701 and DILI59. The carriers of this specific allele have a 80-fold increased risk in developing liver injury. Striking HLA haplotype associations suggest that DILI occurs as a consequence of a genetic predisposition targeted at adaptive immune response and antigen presentation/recognition. However haptenization alone is not sufficient to trigger the injury. Intrahepatic or extrahepatic stress caused by inflammation, infection or oxidative stress is believed to co-stimulate the adaptive immune response as well as to predispose hepatocytes for immune-mediated cell death.
Following some extent of initial cell death, the released cellular content of dead cells may activate innate immune cells including Kupffer cells, infiltrating monocytes and neutrophils in a paracrine fashion. High mobility group box 1 (HMGB1), as well as heat shock proteins and DNAs, are released from necrotic hepatocytes 60, 61. These molecules have been termed damage-associated molecular patterns (DAMPS). DAMPs are able to bind to toll-like receptors of innate immune cells and promote the production of the cytokines such as TNF-alpha, IFNγ and IL-1 which could further modulate the intracellular events. Hepatic inflammation is frequently observed in DILI. It is conceivable these pro-inflammatory cytokines sensitize hepatocytes to biochemical stress, or regulate the adaptive immune-mediated cell injury. Trovafloxacin causes idiosyncratic liver injury in human, but is nontoxic to mice. Co-administration of LPS and trovafloxacin renders mice susceptible to severe hepatic necrosis62, 63. This suggests that an innate immune response could mediate DILI. However, clinical relevance of this model is unclear since the injury caused by this drug in humans appears to involve the adaptive immune system. The role of innate immune response in APAP-induced hepatic necrosis is quite controversial. It is believed that innate immunity is more likely beneficial in the clearance of necrotic cells and promoting tissue repair.
Some drugs, especially biologic immune modulators can activate underlying autoimmune hepatitis. In addition, a few other drugs (e.g. minocycline, nitrofurantoin) appear to cause a rare form of IDILI indistinguishable from autoimmune hepatitis, but responsive to drug withdrawal 64. These cases represent an interesting liver manifestation as a result of interaction between drugs and the host immune system.
Conclusions
Idiosyncratic DILI is the result of the interplay between the environment, drugs and host (genetic, age, sex, immune factors, pre-existing diseases etc.) (Fig.2). Idiosyncratic DILI is often mediated by the adaptive immune response. Meanwhile, some drugs and metabolites can directly damage mitochondria, produce ROS and alter signaling pathway. To defend against the hazards induced by drugs, hepatocytes exhibit adaptive mechanisms including upregulation of Nrf2 signaling, mitophagy and autophagy to cope with stress. Furthermore, the innate and adaptive immune systems can adapt to dampen the response. Ultimately, the battle between hazardous and adaptive responses determines the development of severe injury, restoration of the liver after mild injury (so-called adaptation) or no injury at all.
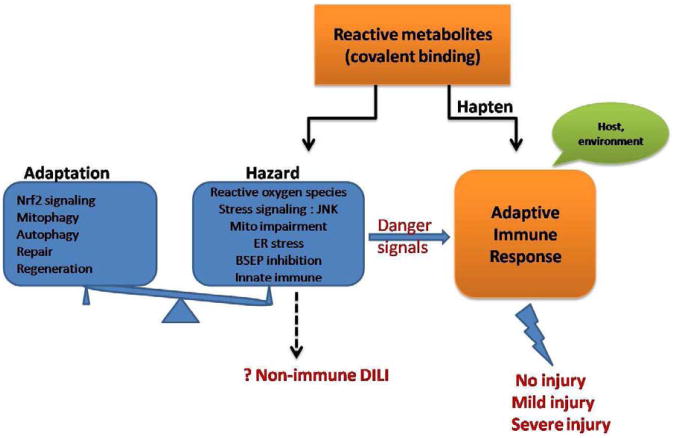
Fig. 2.
Hypothetical mechanisms of idiosyncratic drug-induced liver injury (IDILI). Adaptive immune response plays a major role in IDILI. This may be to some extent regulated by cellular events induced by drug and reactive metabolites. Specifically, drug or reactive metabolites may generate hazards in hepatocytes by inducing endoplasmic reticulum (ER) stress, generating ROS, activating stress signaling, impairing mitochondrial function, etc. To defend against these hazards, hepatocytes respond through adaptive mechanisms including nuclear factor erythroid 2-related factor 2 (Nrf-2) signaling, mitophagy and autophagy to cope with stress. The innate and adaptive immune systems can adapt to dampen the response. The ultimate outcome of liver is no injury, mild injury or severe injury. Although non-immune IDILI remains a theoretical possibility as a consequence of the hazards of reactive metabolites, aside from a small number of drugs which directly damage mitochondria, there are very few, if any, proven examples of this possibility.
Idiosyncratic DILI is the result of the interplay between the environment, drugs and host (genetic, age, sex, immune factors, pre-existing diseases etc.)
Idiosyncratic DILI is often mediated by the adaptive immune response. Meanwhile, some drugs and metabolites can directly damage mitochondria, produce ROS and alter signaling pathway.
To defend against the hazards induced by drugs, hepatocytes exhibit adaptive mechanisms including upregulation of Nrf2 signaling, mitophagy and autophagy to cope with stress.
The innate and adaptive immune systems can adapt to dampen the response.
The battle between hazardous and adaptive responses determines the development of severe injury, restoration of the liver after mild injury (so-called adaptation) or no injury at all.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaplowitz N. Idiosyncratic drug hepatotoxicity. Nat Rev Drug Discov. 2005;4(6):489–499. doi: 10.1038/nrd1750. [DOI] [PubMed] [Google Scholar]
- 2.Holt M, Ju C. Drug-induced liver injury. Handb Exp Pharmacol. 2010;(196):3–27. doi: 10.1007/978-3-642-00663-0_1. [DOI] [PubMed] [Google Scholar]
- 3.Khandelwal N, James LP, Sanders C, et al. Unrecognized acetaminophen toxicity as a cause of indeterminate acute liver failure. Hepatology. 2011;53(2):567–576. doi: 10.1002/hep.24060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42(6):1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 5.Abboud G, Kaplowitz N. Drug-induced liver injury. Drug Saf. 2007;30(4):277–294. doi: 10.2165/00002018-200730040-00001. [DOI] [PubMed] [Google Scholar]
- 6.Lammert C, Einarsson S, Saha C, et al. Relationship between daily dose of oral medications and idiosyncratic drug-induced liver injury: search for signals. Hepatology. 2008;47(6):2003–2009. doi: 10.1002/hep.22272. [DOI] [PubMed] [Google Scholar]
- 7.Lammert C, Bjornsson E, Niklasson A, et al. Oral medications with significant hepatic metabolism at higher risk for hepatic adverse events. Hepatology. 2010;51(2):615–620. doi: 10.1002/hep.23317. [DOI] [PubMed] [Google Scholar]
- 8.Vercammen D, Beyaert R, Denecker G, et al. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med. 1998;187(9):1477–1485. doi: 10.1084/jem.187.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Declercq W, Vanden Berghe T, Vandenabeele P. RIP kinases at the crossroads of cell death and survival. Cell. 2009;138(2):229–232. doi: 10.1016/j.cell.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Vandenabeele P, Declercq W, Van Herreweghe F, et al. The role of the kinases RIP1 and RIP3 in TNF-induced necrosis. Sci Signal. 2010;3(115):re4. doi: 10.1126/scisignal.3115re4. [DOI] [PubMed] [Google Scholar]
- 11.He S, Wang L, Miao L, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137(6):1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Chavez-Valdez R, Martin LJ, Flock DL, et al. Necrostatin-1 attenuates mitochondrial dysfunction in neurons and astrocytes following neonatal hypoxia-ischemia. Neuroscience. 2012;219:192–203. doi: 10.1016/j.neuroscience.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho YS, Challa S, Moquin D, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137(6):1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Upton JW, Kaiser WJ, Mocarski ES. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe. 2010;7(4):302–313. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunawan BK, Liu ZX, Han D, et al. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131(1):165–178. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 16.Shinohara M, Ybanez MD, Win S, et al. Silencing glycogen synthase kinase-3beta inhibits acetaminophen hepatotoxicity and attenuates JNK activation and loss of glutamate cysteine ligase and myeloid cell leukemia sequence 1. J Biol Chem. 2010;285(11):8244–8255. doi: 10.1074/jbc.M109.054999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakagawa H, Maeda S, Hikiba Y, et al. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology. 2008;135(4):1311–1321. doi: 10.1053/j.gastro.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Sharma M, Gadang V, Jaeschke A. Critical role for mixed-lineage kinase 3 in acetaminophen-induced hepatotoxicity. Mol Pharmacol. 2012;82(5):1001–1007. doi: 10.1124/mol.112.079863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saberi B, Shinohara M, Ybanez MD, et al. Regulation of H(2)O(2)-induced necrosis by PKC and AMP-activated kinase signaling in primary cultured hepatocytes. Am J Physiol Cell Physiol. 2008;295(1):C50–63. doi: 10.1152/ajpcell.90654.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dawson S, Stahl S, Paul N, et al. In vitro inhibition of the bile salt export pump correlates with risk of cholestatic drug-induced liver injury in humans. Drug Metab Dispos. 2012;40(1):130–138. doi: 10.1124/dmd.111.040758. [DOI] [PubMed] [Google Scholar]
- 21.Morgan RE, Trauner M, van Staden CJ, et al. Interference with bile salt export pump function is a susceptibility factor for human liver injury in drug development. Toxicol Sci. 2010;118(2):485–500. doi: 10.1093/toxsci/kfq269. [DOI] [PubMed] [Google Scholar]
- 22.Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47(9):1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niture SK, Kaspar JW, Shen J, et al. Nrf2 signaling and cell survival. Toxicol Appl Pharmacol. 2010;244(1):37–42. doi: 10.1016/j.taap.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaspar JW, Niture SK, Jaiswal AK. Antioxidant-induced INrf2 (Keap1) tyrosine 85 phosphorylation controls the nuclear export and degradation of the INrf2-Cul3-Rbx1 complex to allow normal Nrf2 activation and repression. J Cell Sci. 2012;125(Pt 4):1027–1038. doi: 10.1242/jcs.097295. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Tong KI, Kobayashi A, Katsuoka F, et al. Two-site substrate recognition model for the Keap1-Nrf2 system: a hinge and latch mechanism. Biol Chem. 2006;387(10-11):1311–1320. doi: 10.1515/BC.2006.164. [DOI] [PubMed] [Google Scholar]
- 26.Tong KI, Padmanabhan B, Kobayashi A, et al. Different electrostatic potentials define ETGE and DLG motifs as hinge and latch in oxidative stress response. Mol Cell Biol. 2007;27(21):7511–7521. doi: 10.1128/MCB.00753-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Copple IM, Goldring CE, Jenkins RE, et al. The hepatotoxic metabolite of acetaminophen directly activates the Keap1-Nrf2 cell defense system. Hepatology. 2008;48(4):1292–1301. doi: 10.1002/hep.22472. [DOI] [PubMed] [Google Scholar]
- 28.Copple IM, Goldring CE, Kitteringham NR, et al. The Nrf2-Keap1 defence pathway: role in protection against drug-induced toxicity. Toxicology. 2008;246(1):24–33. doi: 10.1016/j.tox.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 29.Kaplowitz N, Shinohara M, Liu ZX, et al. How to protect against acetaminophen: don't ask for JUNK. Gastroenterology. 2008;135(4):1047–1051. doi: 10.1053/j.gastro.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 30.Saitoh M, Nishitoh H, Fujii M, et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17(9):2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adler V, Yin Z, Fuchs SY, et al. Regulation of JNK signaling by GSTp. EMBO J. 1999;18(5):1321–1334. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Win S, Than TA, Han D, et al. c-Jun N-terminal kinase (JNK)-dependent acute liver injury from acetaminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice. J Biol Chem. 2011;286(40):35071–35078. doi: 10.1074/jbc.M111.276089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang C, Youle RJ. The role of mitochondria in apoptosis*. Annu Rev Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Honda HM, Korge P, Weiss JN. Mitochondria and ischemia/reperfusion injury. Ann N Y Acad Sci. 2005;1047:248–258. doi: 10.1196/annals.1341.022. [DOI] [PubMed] [Google Scholar]
- 35.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9(1):47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 36.Youle RJ. Cell biology. Cellular demolition and the rules of engagement. Science. 2007;315(5813):776–777. doi: 10.1126/science.1138870. [DOI] [PubMed] [Google Scholar]
- 37.Zhang DW, Shao J, Lin J, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325(5938):332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z, Jiang H, Chen S, et al. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148(1-2):228–243. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 39.Rashed MS, Streeter AJ, Nelson SD. Investigations of the N-hydroxylation of 3′-hydroxyacetanilide, a non-hepatotoxic positional isomer of acetaminophen. Drug Metab Dispos. 1989;17(4):355–359. [PubMed] [Google Scholar]
- 40.Rashed MS, Nelson SD. Characterization of glutathione conjugates of reactive metabolites of 3′-hydroxyacetanilide, a nonhepatotoxic positional isomer of acetaminophen. Chem Res Toxicol. 1989;2(1):41–45. doi: 10.1021/tx00007a007. [DOI] [PubMed] [Google Scholar]
- 41.Rashed MS, Myers TG, Nelson SD. Hepatic protein arylation, glutathione depletion, and metabolite profiles of acetaminophen and a non-hepatotoxic regioisomer, 3′-hydroxyacetanilide, in the mouse. Drug Metab Dispos. 1990;18(5):765–770. [PubMed] [Google Scholar]
- 42.Kuwana T, Mackey MR, Perkins G, et al. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111(3):331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- 43.Ott M, Robertson JD, Gogvadze V, et al. Cytochrome c release from mitochondria proceeds by a two-step process. Proc Natl Acad Sci U S A. 2002;99(3):1259–1263. doi: 10.1073/pnas.241655498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee YH, Chung MC, Lin Q, et al. Troglitazone-induced hepatic mitochondrial proteome expression dynamics in heterozygous Sod2(+/-) mice: two-stage oxidative injury. Toxicol Appl Pharmacol. 2008;231(1):43–51. doi: 10.1016/j.taap.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 45.Kashimshetty R, Desai VG, Kale VM, et al. Underlying mitochondrial dysfunction triggers flutamide-induced oxidative liver injury in a mouse model of idiosyncratic drug toxicity. Toxicol Appl Pharmacol. 2009;238(2):150–159. doi: 10.1016/j.taap.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 46.Fujimoto K, Kumagai K, Ito K, et al. Sensitivity of liver injury in heterozygous Sod2 knockout mice treated with troglitazone or acetaminophen. Toxicol Pathol. 2009;37(2):193–200. doi: 10.1177/0192623308329282. [DOI] [PubMed] [Google Scholar]
- 47.Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337(6098):1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jin SM, Youle RJ. PINK1- and Parkin-mediated mitophagy at a glance. J Cell Sci. 2012;125(Pt 4):795–799. doi: 10.1242/jcs.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jin SM, Lazarou M, Wang C, et al. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol. 2010;191(5):933–942. doi: 10.1083/jcb.201008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lazarou M, Narendra DP, Jin SM, et al. PINK1 drives Parkin self-association and HECT-like E3 activity upstream of mitochondrial binding. J Cell Biol. 2013;200(2):163–172. doi: 10.1083/jcb.201210111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Narendra DP, Jin SM, Tanaka A, et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8(1):e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22(12):1577–1590. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Montessuit S, Somasekharan SP, Terrones O, et al. Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization. Cell. 2010;142(6):889–901. doi: 10.1016/j.cell.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaplowitz N. Dealing with stress. Hepatology. 2012;55(1):3–13. doi: 10.1002/hep.25515. [DOI] [PubMed] [Google Scholar]
- 55.Hautekeete ML, Horsmans Y, Van Waeyenberge C, et al. HLA association of amoxicillin-clavulanate--induced hepatitis. Gastroenterology. 1999;117(5):1181–1186. doi: 10.1016/s0016-5085(99)70404-x. [DOI] [PubMed] [Google Scholar]
- 56.Lucena MI, Molokhia M, Shen Y, et al. Susceptibility to amoxicillin-clavulanate-induced liver injury is influenced by multiple HLA class I and II alleles. Gastroenterology. 2011;141(1):338–347. doi: 10.1053/j.gastro.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O'Donohue J, Oien KA, Donaldson P, et al. Co-amoxiclav jaundice: clinical and histological features and HLA class II association. Gut. 2000;47(5):717–720. doi: 10.1136/gut.47.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singer JB, Lewitzky S, Leroy E, et al. A genome-wide study identifies HLA alleles associated with lumiracoxib-related liver injury. Nat Genet. 2010;42(8):711–714. doi: 10.1038/ng.632. [DOI] [PubMed] [Google Scholar]
- 59.Daly AK, Donaldson PT, Bhatnagar P, et al. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat Genet. 2009;41(7):816–819. doi: 10.1038/ng.379. [DOI] [PubMed] [Google Scholar]
- 60.Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology. 2012;143(5):1158–1172. doi: 10.1053/j.gastro.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 61.Antoine DJ, Williams DP, Kipar A, et al. High-mobility group box-1 protein and keratin-18, circulating serum proteins informative of acetaminophen-induced necrosis and apoptosis in vivo. Toxicol Sci. 2009;112(2):521–531. doi: 10.1093/toxsci/kfp235. [DOI] [PubMed] [Google Scholar]
- 62.Luyendyk JP, Maddox JF, Cosma GN, et al. Ranitidine treatment during a modest inflammatory response precipitates idiosyncrasy-like liver injury in rats. J Pharmacol Exp Ther. 2003;307(1):9–16. doi: 10.1124/jpet.103.054288. [DOI] [PubMed] [Google Scholar]
- 63.Shaw PJ, Hopfensperger MJ, Ganey PE, et al. Lipopolysaccharide and trovafloxacin coexposure in mice causes idiosyncrasy-like liver injury dependent on tumor necrosis factor-alpha. Toxicol Sci. 2007;100(1):259–266. doi: 10.1093/toxsci/kfm218. [DOI] [PubMed] [Google Scholar]
- 64.Czaja AJ. Drug-induced autoimmune-like hepatitis. Dig Dis Sci. 2011;56(4):958–976. doi: 10.1007/s10620-011-1611-4. [DOI] [PubMed] [Google Scholar]