Abstract
Objective
Hepatic ATP binding cassette transporter A1 (ABCA1) expression is critical for maintaining plasma HDL concentrations, but its role in macrophage reverse cholesterol transport (RCT) and atherosclerosis is not fully understood. We investigated atherosclerosis development and RCT in hepatocyte specific ABCA1 knockout (HSKO) mice in the LDL receptor knockout (LDLrKO) C57BL/6 background.
Approach and Results
Male and female LDLrKO and HSKO/LDLrKO mice were switched from chow at 8 wks of age to an atherogenic diet (10% palm oil, 0.2% cholesterol) for 16 wks. Chow-fed HSKO/LDLrKO mice had HDL concentrations 10–20% of LDLrKO mice, but similar VLDL and LDL concentrations. Surprisingly, HSKO/LDLrKO mice fed the atherogenic diet had significantly lower (40–60%) VLDL, LDL, and HDL concentrations (50%) compared to LDLrKO mice. Aortic surface lesion area and cholesterol content were similar for both genotypes of mice, but aortic root intimal area was significantly lower (20–40%) in HSKO/LDLrKO mice. Although macrophage 3H-cholesterol efflux to apoB lipoprotein-depleted plasma was 24% lower for atherogenic diet-fed HSKO/LDLrKO vs. LDLrKO mice, variation in percentage efflux among individual mice was <2-fold compared to a 10-fold variation in plasma HDL concentrations, suggesting that HDL levels, per se, were not the primary determinant of plasma efflux capacity. In vivo RCT, resident peritoneal macrophage sterol content, biliary lipid composition, and fecal cholesterol mass were similar between both genotypes of mice.
Conclusions
The markedly reduced plasma HDL pool in HSKO/LDLrKO mice is sufficient to maintain macrophage RCT, which, along with reduced plasma VLDL and LDL concentrations, prevented the expected increase in atherosclerosis.
Keywords: cardiovascular disease, atherosclerosis, lipids, lipoproteins, cholesterol
Introduction
Atherosclerosis-associated cardiovascular disease (CVD) is the leading cause of death worldwide1. The inverse relationship between plasma HDL levels and CVD risk has made raising HDL levels a popular potential therapeutic target for CVD prevention. ATP binding cassette transporter A1 (ABCA1) belongs to a large family of the ATP binding cassette transporters2. ABCA1 mediates cellular free cholesterol (FC) and phospholipid (PL) efflux to apolipoprotein A-I (apoA-I), resulting in the formation of nascent HDL that undergo subsequent maturation to become plasma HDL2. The critical role of ABCA1 in HDL formation was established when it was found to be the genetic defect in Tangier disease, a disorder in which HDL levels are <5% of normal3–5. Studies with animal models have also documented the essential function of ABCA1 in maintaining plasma HDL levels6–8.
Despite the well-established role of ABCA1 in HDL formation, its effect on atherogenesis is less clear. Premature atherosclerosis has been reported in some, but not all, people with Tangier disease9. Common genetic variants in ABCA1 have been reported to influence the risk and severity of CVD10–12; however, low HDL caused by loss-of-function mutations in ABCA1 does not contribute to increased risk of CVD in the general population13. Controversial results also exist in studies with mouse models. Overexpression of human ABCA1 (hABCA1) in the liver and macrophages of B6 mice resulted in an anti-atherogenic lipid profile and lower aortic atherosclerosis, whereas in apoE KO mice, overexpressing hABCA1 increased atherosclerosis with minimal effect on plasma lipids14. Physiological overexpression of a full-length hABCA1 containing bacterial artificial chromosome (BAC) in apoE KO and LDLrKO mice both revealed an atheroprotective role of ABCA115, 16. Significantly larger lesions occurred in ApoE or LDLrKO mice transplanted with bone marrow from mice with total body ABCA1 deficiency17–20. In contrast, total body ABCA1 deficiency in apoE KO or LDLrKO mice did not result in increased atherosclerosis compared to control mice18. The complex relationship between global ABCA1 expression and atherosclerosis susceptibility observed in humans and mouse models of atherosclerosis was at least partially attributed to reduction in atherogenic lipoproteins concomitant with ABCA1 deficiency, or to the use of different promoters for transgenic overexpression.
Subsequent studies with hepatocyte-specific ABCA1 knockout (HSKO) mice suggested a major role for the liver in maintaining systemic HDL levels, leading to investigation of the role of hepatic ABCA1 in atherogenesis6. Joyce et al found that liver-specific overexpression of ABCA1 in LDLrKO mice led to increased atherosclerosis, presumably due to increased plasma concentrations of apoB-containing lipoproteins (apoB Lp) concomitant with a significant increase in plasma HDL21. A more recent study of hepatic ABCA1 deletion in chow-fed apoE KO mice showed a significant increase in early-stage atherosclerosis16. However, several issues were not addressed in that study. First, only early atherosclerosis was examined; mice consumed a chow diet for 12 wks, and total plasma cholesterol (TPC) concentrations were relatively low (250–400 mg/dl). Thus, the effect of hepatocyte deletion of ABCA1 on more advanced atherosclerosis is unknown. Second, HDL cholesterol concentrations are quite low in apoE KO mice (~29 mg/dl). Therefore, the difference between apoE KO and HSKO-apoE KO strains may be too small to differentially affect atherosclerosis. Third, apoE has atheroprotective roles at the level of the arterial wall that are absent in the apoE KO background22. Finally, no in vivo RCT studies were performed.
To address these gaps in knowledge, we investigated the influence of hepatic ABCA1 expression on RCT and development of more advanced atherosclerotic lesions in LDLrKO mice. Our results suggest a minimal impact of hepatic ABCA1 deletion on in vivo macrophage RCT and atherogenesis development in atherogenic diet-fed LDLrKO mice, even though plasma HDL concentrations were markedly reduced in HSKO/LDLrKO mice compared to LDLrKO mice. This surprising outcome likely resulted from the significant reduction in atherogenic lipoproteins (i.e., VLDL and LDL) observed in diet-fed HSKO/LDLrKO mice, as well as sufficient HDL to mediate RCT.
Materials and Methods
Materials and Methods are available in the online-only Supplement.
Results
Hepatocyte ABCA1 deletion reduces plasma lipids
To induce atherosclerosis development, mice were switched from chow to an atherogenic diet at 8 wks of age for 16 wks. After 2 wks of diet feeding, TPC and FC increased for both groups of mice, but values were significantly lower in HSKO/LDLrKO mice throughout the 16-wk study (area under curve; P<0.01) (Figure 1A–B). A consistent trend in plasma triglyceride concentrations was not observed (Figure 1A–B). We previously reported that HDL cholesterol (HDL-C) levels in chow-fed HSKO mice were ~80% lower than WT mice6, 23. FPLC fractionation of plasma lipoproteins showed that chow-fed HSKO/LDLrKO had significant reductions in HDL-C (1.74 mmol/L vs. 0.21 mmol/L in males; 67.3 mg/dl vs. 8.1 mg/dl in males, P<0.0001;1.50 mmol/L vs. 0.28 mmol/L in females; 58.0 mg/dl vs. 10.9 mg/dl in females, P<0.0001), contributing to the lower TPC levels in HSKO/LDLrKO vs. LDLrKO mice (4.48 mmol/L vs. 2.68 mmol/L in males; 173.1 mg/dl vs. 103.6 mg/dl in males, P<0.0001; 5.17 mmol/L vs. 3.44 mmol/L in females; 199.7 mg/dl vs. 133 mg/dl in females, P=0.0004). VLDL-C and LDL-C concentrations were similar between genotypes (Figure 1C). The less pronounced hyperlipidemia in atherogenic diet-fed HSKO/LDLrKO mice was mainly attributed to lower VLDL-C (14.43 mmol/L vs. 6.08 mmol/L in males; 557.9 mg/dl vs. 235.0 mg/dl in males, P=0.0050; 16.49 mmol/L vs. 10.05 mmol/L in females; 637.7 mg/dl vs. 388.7 mg/dl in females, P=0.0283) and LDL-C levels (16.19 mmol/L vs. 11.65 mmol/L in males; 626.2 mg/dl vs. 450.5 mg/dl in males, P=0.0221; 11.80 mmol/L vs. 10.32 mmol/L in females; 456.3 mg/dl vs. 399.0 mg/dl in females, P=0.3441), whereas HDL-C concentrations remained significantly different between genotypes (2.0 mmol/L vs. 1.05 mmol/L in males; 77.4 mg/dl vs. 40.5 mg/dl in males, P=0.0007; 0.98 mmol/L vs. 0.36 mmol/L in females; 37.9 mg/dl vs. 14.0 mg/dl in females, P<0.0001) (Figure 1D). These data document the critical role of hepatocyte ABCA1 in maintaining the plasma HDL-C pool in hyperlipidemic mice and demonstrate a potential role for hepatic ABCA1 in regulating plasma apoB-containing lipoprotein concentrations during atherogenesis.
Figure 1.
Plasma lipid and lipoprotein concentrations during atherosclerosis progression. Fasting (4h) plasma total cholesterol (TPC), free cholesterol (FC), and triglyceride (TG) concentrations were measured by enzymatic assays in male (A) and female (B) mice of the indicated genotype during a 16 wk atherosclerosis progression phase (n=9–13). Plasma lipoprotein cholesterol distribution of mice before (C; chow-fed at 7–9 wks of age) or after (D) 16 wks of atherogenic diet consumption were determined after FPLC fractionation of plasma (n=9–19). Data expressed as mean ± SEM. * P<0.05. HSKO, hepatocyte-specific ABCA1 knockout.
VLDL catabolism is increased in HSKO/LDLrKO mice
To determine the explanation for reduced VLDL and LDL concentrations in atherogenic diet-fed HSKO/LDLrKO mice, we investigated VLDL production and catabolism. VLDL TG production was measured after in vivo inhibition of TG lipolysis with intravenous Triton administration to fasted mice. The rate of hepatic VLDL TG mass accumulation during Triton block of lipolysis was similar for both genotypes of mice (Supplemental Figure I A–B). Similarly, in a separate experiment, secretion rates of TG and radiolabeled apoB were not significantly different between groups (data not shown). We next investigated VLDL particle turnover using 125I-VLDL from LDLrKO mice, which was indistinguishable in chemical composition from HSKO/LDLrKO mouse VLDL (Supplemental Table I). VLDL particles were enriched in CE (41–43%) at the expense of TG (5%), typical of β-VLDL24, likely due to the prolonged residence time in plasma in the absence of active LDLr25. VLDL particle turnover, measured as decay of 125I-VLDL apoB clearance from plasma, was more rapid in HSKO/LDLrKO vs. LDLrKO recipient mice (area under curve; P<0.05) (Supplemental Figure II), suggesting that the lower VLDL-C concentrations in HSKO/LDLrKO mice were due to increased VLDL particle catabolism. Expression of hepatic genes involved in VLDL catabolism was similar for HSKO/LDLrKO and LDLrKO mice (Supplemental Figure II C). However, plasma apoE levels were lower in atherogenic diet-fed HSKO/LDLrKO mice in addition to the anticipated reduction in apoA-I levels due to low plasma HDL concentrations (Supplemental Figure II D). Furthermore, most of the plasma apoE as well as apoA-I migrated in the HDL size range (8–10 nm) on non-denaturing gradient gels (Supplemental Figure II E and F). Given these results, we speculate that lower VLDL-C levels in HSKO/LDLrKO mice were likely due to decreased competition by apoE-containing plasma HDL for hepatic VLDL uptake, resulting in increased removal of VLDL particles from plasma in HSKO/LDLrKO vs. LDLrKO mice.
Effect of hepatocyte ABCA1 deletion on hepatic and biliary lipids
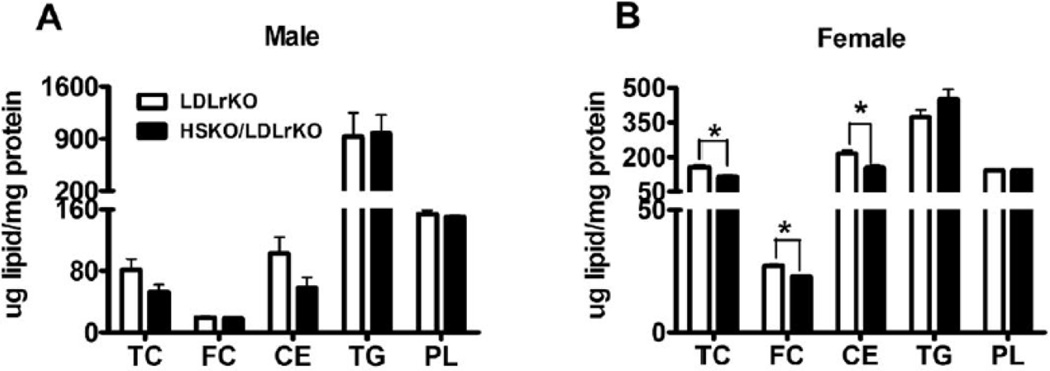
To address whether hepatocyte ABCA1 ablation impacts liver lipid metabolism, we measured hepatic and biliary lipid levels. Unlike our previous study in which similar hepatic lipid content was observed for chow-fed HSKO vs. WT mice23, atherogenic diet-fed HSKO/LDLrKO mice had lower (significant in female mice) hepatic TC, FC, and cholesterol ester (CE) concentrations relative to LDLrKO mice (Figure 2A–B). Hepatic TG and phospholipid (PL) concentrations were similar between the two genotypes (Figure 2A–B). However, there was no significant difference in biliary TC, PL, and bile acid (BA) concentrations or molar percentage composition between the two genotypes (Supplemental Figure III A–D) and fecal cholesterol excretion was similar (Supplemental Figure III E). To investigate whether the decreased liver cholesterol content was associated with transcriptional regulation concomitant with ABCA1 ablation, we measured expression of genes involved in hepatic lipid metabolism. SREBP1c was significantly downregulated, and several other genes (HMGCoA synthase, ACC1) showed downward trends in expression in HSKO/LDLrKO mouse liver, suggesting decreased de novo lipogenesis (Figure 2C). Liver expression of several LXR target genes was similar in HSKO/LDLrKO and LDLrKO mice (Figure 2C).
Figure 2.
Hepatic lipid content and gene expression. Lipid content was determined using detergent-based enzymatic assays of hepatic lipid extracts from 4h-fasted male (A) or female (B) mice after 16 wks of atherogenic diet consumption (n=7–12). (C) Hepatic gene expression in male mice (n=7). Data was expressed in mean ± SEM. * P<0.05.
Impact of hepatic ABCA1 on atherosclerosis development
To investigate the impact of hepatocyte ABCA1 deficiency on atherosclerosis development in LDLrKO mice, three measurements of atherosclerosis were made. En face aortic surface lesion area (Figure 3A, C) and aortic cholesterol content (Figure 3B) were similar for HSKO/LDLrKO and LDLrKO mice, although there was a trend towards reduced aortic cholesterol content in HSKO/LDLrKO mice. Furthermore, cross-sectional analysis of oil red O stained aortic root sections revealed significantly smaller lesions in both female (0.51mm2 vs 0.40 mm2, p=0.0141) and male (0.48 mm2 vs 0.29 mm2, p=0.0341) HSKO/LDLrKO mice vs. their LDLrKO counterparts (Figure 3D–E), suggesting that deletion of hepatocyte ABCA1 expression may actually protect against more advanced atherosclerotic lesion development in the aortic root. Additional support for this concept was obtained with additional analysis of lesion complexity; aortic root sections stained with Masson’s trichrome showed less necrosis, acute inflammation, and adventitial inflammation in lesions of HSKO/LDLrKO vs. LDLrKO mice fed the atherogenic diet for 16 wks (Supplemental Figure IV A). In a separate experiment to evaluate very early stages of aortic atherosclerosis (i.e., 5 wks atherogenic diet feeding), we observed similar aortic cholesterol content and aortic root lesion area between genotypes (Supplemental Figure IV B–C). Taken together, unlike previous findings in apoE KO mice16, the absence of hepatic ABCA1 did not accelerate early-stage atherogenesis in LDLrKO mice and appeared to protect against late-stage, more advanced atherosclerosis.
Figure 3.
Atherosclerosis evaluation. (A) Aorta surface lesion area was expressed as the percentage of total aorta surface area. (B) Aortas were lipid extracted for quantification of total cholesterol (TC) and free cholesterol (FC) content using gas-liquid chromatography. Cholesterol ester (CE) content was calculated as (TC-FC) x1.67. (C) Representative en face aorta from a LDLrKO (left) and HSKO/LDLrKO (right) mouse. (D) Representative LDLrKO (left) and HSKO/LDLrKO (right) mouse aortic root sections stained with Oil Red O. (E) Aortic root lesion area. Each point represents the average lesion area of 3 sections per mouse. Horizontal lines denote the mean ± SEM for each genotype. * P<0.05.
Role of hepatocyte ABCA1 in macrophage RCT in vivo
One atheroprotective mechanism proposed for HDL is the transport of excess macrophage cholesterol to the liver for excretion (i.e., RCT)26. To determine whether the large reduction of plasma HDL in HSKO/LDLrKO mice diminished RCT, we performed in vivo macrophage RCT assays. 3H-cholesterol-radiolabeled J774 macrophages were injected into the peritoneal cavity of HSKO/LDLrKO or LDLrkO mice after 5 wk of atherogenic diet feeding. The plasma 3H-cholesterol closely tracked with lipoprotein cholesterol mass (Figure 4A–B). The amount of 3H tracer in plasma 48h after injection was significantly lower in HSKO/LDLrKO mice, likely reflecting the lower levels of plasma lipoproteins in these mice (Figure 4C). However, the tracer levels in the liver, bile, and feces were similar between the two groups (Figure 4D–F), suggesting that in vivo macrophage RCT was not impaired in HSKO/LDLrkO mice, despite the much lower HDL-C in these mice. A similar outcome was obtained using radiolabeled bone marrow-derived macrophages (BMM) injected into mice fed the atherogenic diet for 16 wk (Supplemental Figure V). We also measured cholesterol content of resident peritoneal macrophages in atherogenic diet-fed mice and observed no significant difference between the two genotypes, although there was a trend toward decreased cholesterol in HSKO/LDLrKO mice (Supplemental Figure VI). Collectively, these data suggest that in vivo macrophage RCT is not impaired in HSKO/LDLrKO mice, despite significantly lower plasma steady-state HDL-C levels.
Figure 4.
Macrophage RCT. 3H-cholesterol radiolabeled, cholesterol-loaded J774 macrophages were injected into the peritoneal cavity of mice fed the atherogenic diet for 5 wks. Plasma cholesterol mass (A) and 3H-cholesterol (B) distribution were determined after FPLC separation of plasma. Plasma 3H-cholesterol radiolabel at different time points (C) and in liver (D), bile (E) and feces (F) 48 hours after injection. Data are expressed as mean ± SEM, n=7–8. * P<0.05.
Plasma cholesterol efflux capacity
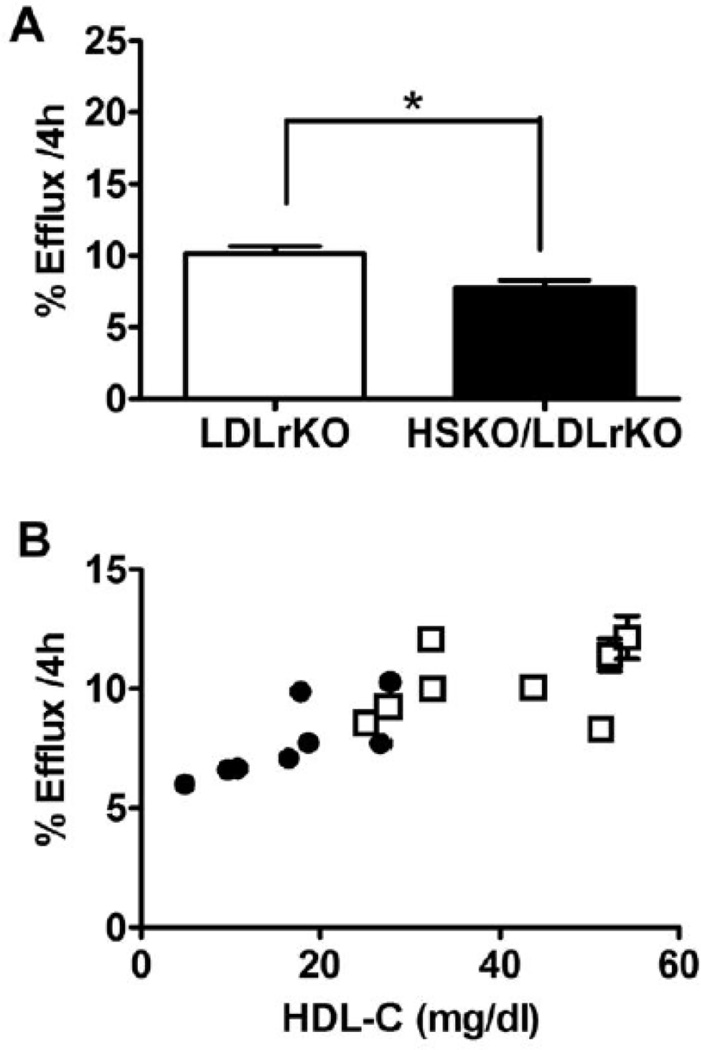
In vivo RCT results suggested that the plasma HDL pool in HSKO/LDLrKO mice was sufficient to maintain normal cholesterol efflux from macrophages for ultimate excretion into feces. One possible explanation for this outcome could be a fraction of mouse plasma HDL that is highly efficient at effluxing macrophage FC, compensating for low plasma HDL levels in RCT in HSKO/LDLrKO mice. For example, pre-β 1 appears to be the preferred acceptor for ABCA1-mediated FC efflux in human plasma, but its concentration is typically <10% of total HDL27. To examine this possibility, we measured the ability of apoB lipoprotein-depleted plasma from atherogenic diet-fed HSKO/LDLrKO and LDLrKO mice to efflux 3H-cholesterol from cholesterol-loaded macrophages and observed a significant reduction (24%) in HSKO/LDLrKO vs. LDLrKO plasma (10.14% vs. 7.75%, p=0.0067; Figure 5A). However, analysis of individual animal HDL-C concentrations vs. percent of efflux values (Figure 5B) demonstrated < 2-fold variation in percentage efflux values compared to a 10-fold variation in plasma HDL-C concentrations, suggesting that HDL-C concentration, per se, is not a primary determinant of plasma efflux capacity.
Figure 5.
Plasma cholesterol efflux capacity. (A) Cholesterol efflux to apoB lipoprotein-depleted plasma. J774 macrophages were radiolabeled with 3H-cholesterol for 24 h and then incubated for 4 h with medium containing 2.8% plasma from LDLrKO or HSKO/LDLrKO mice fed an atherogenic diet (n=8). An aliquot of medium and cellular lipid extract was taken for scintillation counting to determine percentage cholesterol efflux during the 4h incubation. * P<0.05. (B) Percentage cholesterol efflux was plotted against each animal’s plasma HDL-C level. Open symbols=LDLrKO; closed symbols=HSKO/LDLrKO. (C) apo-B depleted plasma medium 4h after cholesterol efflux underwent FPLC separation; radioactivity of each fraction was counted by scintillation counting.
After the cholesterol efflux experiment, we fractionated a subset of individual plasma-containing efflux media using an FPLC column capable of separating plasma HDL particles, pre-β 1 HDL and lipid-free apoA-I from one another (Supplemental Figure VII). The distribution of 3H-cholesterol between the main HDL peak (fractions 41–50) and pre-β 1 HDL elution region (fractions 51–55) did not reveal a disproportionate amount of 3H-FC in the pre-β 1 peak in HSKO/LDLrKO vs. LDLrKO plasma (Figure 5C) and the percentage of 3H-FC in the pre-β 1 peak relative to the entire HDL elution region (i.e. fractions 41–55) was 20.9 ± 3.4% and 20.7 ± 5.2%, respectively (n=6/genotype). In addition, apoA-I Western blot analysis of plasma separated by agarose gel electrophoresis did not show an increase in pre-β 1 HDL for HSKO/LDLrKO compared to LDLrKO mice (Supplemental Figure VII D). Taken together, these results suggest that HSKO/LDLrKO mice do not compensate for reduced plasma HDL levels with an increase in amount or cholesterol efflux efficiency of pre-β 1 HDL to maintain in vivo RCT at levels comparable with LDLrKO mice.
Discussion
Hepatocyte ABCA1 plays a crucial role in HDL biogenesis2, but its role in atherogenesis is less clear. In the current study, we addressed this gap in knowledge by performing atherosclerosis and in vivo macrophage RCT studies in atherogenic diet-fed HSKO mice crossed into the LDLrKO background. Compared to LDLrKO (control) mice, HSKO/LDLrKO mice had reduced TPC, primarily due to a 40–50% reduction in VLDL and LDL, and similar or reduced atherosclerosis. Furthermore, in vivo macrophage RCT to feces was similar for both genotypes of mice, although efflux of macrophage 3H-FC was significantly reduced in apoB lipoprotein-depleted plasma from HSKO/LDLrKO mice. In agreement with the in vivo RCT data, resident peritoneal macrophage cholesterol content, biliary lipid composition, and fecal cholesterol mass were similar for mice with or without hepatocyte ABCA1 expression. These results suggest that the markedly reduced plasma HDL pool in HSKO/LDLrKO mice is sufficient to maintain macrophage RCT, which, together with decreased VLDL and LDL levels, prevented the expected increase in atherosclerosis.
Atherogenic diet-fed HSKO/LDLrKO mice displayed similar en face aortic lesion area and cholesterol content, but reduced aortic root lesion size relative to LDLrKO mice (Figure 3). The fact that development of atherosclerosis in the aortic root precedes that in the whole aorta and is typically more advanced28 may suggest that hepatic ABCA1 deficiency is protective in more advanced stages of atherosclerosis, perhaps by preserving RCT during extended periods of hyperlipidemia (see below). Only one other study has investigated the role of hepatocyte ABCA1 deficiency in atherogenesis16. In that study, aortic lesion size and cholesterol content were significantly increased in HSKO/apoE KO vs. apoE KO mice fed chow for 12 wks, supporting an anti-atherogenic role for hepatic ABCA1. There are several differences between the studies that may explain the divergent outcomes. First, chow-fed apoE KO mice had modest hypercholesterolemia (TPC= 250–400 mg/dl) and early atherosclerotic lesions, with low amounts of aortic cholesterol16, whereas atherogenic-diet fed LDLrKO mice in the present study had much higher TPC concentrations (~1000 mg/dl) and more aortic cholesterol accumulation (~60-fold more TC). In a follow-up study of very early atherosclerosis (5 wks atherogenic diet feeding), we again observed no difference in aortic cholesterol content between HSKO/LDLrKO and LDLrKO mice (Supplemental Figure IV B), although aortic TC levels were 4–6 fold less than mice fed the atherogenic diet for 16 wks (compare Figure 3B to Supplemental figure IV B), but 10-fold higher than aortic TC levels of HSKO/apoEKO mice fed chow for 12 wks16. VLDL and LDL concentrations were significantly lower in the HSKO/apoEKO vs. apoEKO mice, but the magnitude of the reduction (45 mg/dl for VLDL; 60 mg/dl for LDL) was much less than HSKO/LDLrKO mice (250–300 mg/dl for VLDL-C; 60–170 mg/dl for LDL-C) compared to LDLrKO mice. Although the percentage reduction in HDL-C compared to their respective controls was similar for both studies (~50%), the absolute HDL-C values were much lower in HSKO/apoEKO mice compared with HSKO/LDLrKO mice, which may have been low enough to limit RCT; however, macrophage RCT was not measured in the Brunham study. Finally, apoE expression is atheroprotective independent of plasma lipoprotein concentrations29–31. One or more of these differences may explain why hepatocyte ABCA1 deletion was neutral or atheroprotective in LDLrKO mice and atherogenic in apoE KO mice.
Epidemiological studies have documented an inverse association between plasma HDL-C concentrations and coronary heart disease (CHD), supporting HDL’s role as an anti-atherogenic lipoprotein32. The protective role of HDL in CHD is primarily attributed to RCT, but HDL also inhibits lipoprotein oxidation, inflammation, and hematopoiesis and maintains endothelial function, all of which are atheroprotective33, 34. However, recent studies have challenged the assumption that raising HDL-C levels will uniformly translate into reductions in CHD35, 36. Other studies have suggested the HDL particle number and size (i.e., subfraction distribution) are better predictors of CHD risk than HDL-C37–39 and that HDL function may be more important in preventing CHD than HDL-C40, 41. Animal studies have shown a more consistent association between increased atherosclerosis and decreased macrophage RCT than with reduced HDL-C42. In support of the concept that HDL function may be more important than HDL-C in determining CHD risk, we show that a substantial reduction of plasma HDL-C in atherogenic diet-fed HSKO/LDLrKO mice did not significantly affect aortic atherogenesis, in vivo macrophage RCT, or fecal cholesterol excretion. These results are compatible with the idea of a small, dynamic HDL pool that efficiently removes cholesterol from arterial macrophage foam cells and rapidly transports it to the liver for excretion without a detectable increase in plasma HDL-C. Pre-β 1 HDL is a preferential acceptor for macrophage cholesterol efflux via ABCA1 and may function in this regard, although it is generally <10% of total HDL mass27. However, a large HDL pool would not be necessary for this mechanism to be operational, since aortic CE mass in atherogenic-diet fed HSKO/LDLrKO mice is <1% of the steady-state plasma cholesterol pool. Overall, these observations support the concept that HDL quality or function may be a better predictor of atheroprotection than HDL-C, per se.
A recent study by the Rader lab supports our results that a marked decrease in plasma HDL did not affect macrophage RCT43. They found that pharmacologic inhibition of ABCA1 by probucol resulted in an 80% reduction of plasma HDL-C in chow-fed WT mice, but macrophage RCT was unaffected, although the flux of 3H-cholesterol from plasma HDL into the liver and feces was increased. In addition, probucol treatment of SR-BI knockout mice reduced plasma HDL-C by 63% and stimulated macrophage RCT. Based on these combined results, Yamamoto et al suggested that hepatocyte ABCA1 may normally function to counterbalance RCT by mobilizing FC from hepatocytes back into plasma to help maintain plasma HDL-C. However, probucol, an anti-oxidant drug with a long history of clinical use, has broad pharmacological properties (including effects relevant to whole body lipid metabolism) and is not specific for ABCA144. Here, we show that specific genetic deletion of hepatocyte ABCA1 supports the conclusions of Yamamoto and coworkers. Results from both studies suggest that the plasma HDL pool remaining after whole body pharmacological inhibition of ABCA1 or genetic deletion of hepatocyte ABCA1, though small, is quantitatively sufficient or functionally efficient to mediate macrophage cholesterol transport back to the liver for excretion. As discussed above, maintaining macrophage RCT in the face of considerable reductions in the plasma HDL pool appear to result in atheroprotection over long periods of hyperlipidemia when advanced atherosclerosis development is ongoing (Figure 3E).
The similar atherosclerosis outcome in aortas of HSKO/LDLrKO and LDLrKO mice and the aortic root atheroprotection in HSKO/LDLrKO mice may be partially ascribed to the significant reduction in plasma VLDL and LDL levels in atherogenic diet-fed HSKO/LDLrKO mice (Figure 1D). Whole body ABCA1 KO mice in LDLrKO or apoEKO backgrounds also had similar extent of atherosclerosis that was attributed to reduced plasma apoB Lp concentrations18. Altered apoB Lp metabolism is also a feature of Tangier disease, in which plasma LDL levels are ~ 50% of normal due to a two-fold increase in the fractional catabolic rate (FCR) of LDL45. Chow-fed HSKO mice demonstrated a 50% lower plasma LDL concentration, a two-fold increase in 125I-LDL tracer removal rate from plasma, and a two-fold increase in hepatic LDLr expression relative to WT mice, suggesting that increased hepatic LDLr expression may be responsible for the decreased plasma LDL concentrations23. Crossing chow-fed HSKO mice into the LDLrKO background normalized (i.e., increased) plasma LDL concentrations to those of chow-fed LDLrKO mice, lending additional support that increased hepatic LDLr expression is the key mediator of reduced plasma LDL in chow-fed HSKO mice (Figure 1C). However, atherogenic diet-fed HSKO/LDLrKO mice exhibited diminished VLDL-C and LDL-C relative to LDLrKO mice, which was due to increased VLDL catabolism (Supplemental Figure II) with no difference in VLDL production (Supplemental Figure I). Since these mice lack functional LDLr, increased catabolism of VLDL had to be mediated through an LDLr-independent pathway. Hepatic LDLr related protein (LRP) mRNA (Supplemental Figure II C) and protein levels (data not shown) were similar between groups, suggesting the involvement of other potential pathways, such as heparan sulfate proteoglycans46. However, expression of hepatic lipoprotein catabolic genes such as SR-BI, syndecan 1, hepatic lipase, lipoprotein lipase, and apoC3 were similar between genotypes (Figure 2B). Van Eck et al have shown that HDL can effectively compete for hepatic uptake of βVLDL particles via SR-BI dependent and independent-mediated mechanisms24. In the absence of functional LDLr, VLDL residence time in plasma is significantly prolonged25, allowing alternate hepatic VLDL particle uptake pathways to predominate. We show that the pool of apoE-enriched HDL, a likely competitor for alternate hepatic VLDL particle uptake pathways, is diminished in atherogenic diet-fed HSKO/LDLrKO mice (Supplemental figure II F). Based on our combined results and the previous studies by Van Eck et al24, we propose that the increased VLDL particle catabolism in HSKO/LDLrKO mice is mediated through decreased competition for hepatic uptake of VLDL by apoE-containing HDL. Regardless of the exact mechanism, hepatic ABCA1 expression appears to be an important regulator of plasma apoB Lp as well as HDL levels, both of which modulate atherosclerosis progression.
In conclusion, we investigated the impact of hepatocyte ABCA1 deletion on relatively advanced atherosclerosis and macrophage RCT during disease progression. Unexpectedly, we found that hepatocyte ABCA1 deletion did not exacerbate lesion development in atherogenic diet-fed LDLrKO mice, and was atheroprotective in the aortic root, likely due to reduced apoB Lp levels and maintenance of macrophage RCT, despite a large reduction in plasma HDL-C. Our results are also compatible with the idea proposed by Rader and colleagues43 that hepatic ABCA1 may normally recycle a significant amount of plasma HDL-C removed by the liver back into plasma to maintain the plasma HDL-C pool. If true, these findings would result in a paradigm shift, since decreased hepatic ABCA1 expression, resulting in lower plasma HDL-C, may actually increase RCT and reduce CHD risk by reducing the recycling of hepatic cholesterol back into plasma through nHDL formation by ABCA1.
MATERIALS AND METHODS
Animals and Diet
HSKO mice in the C57BL/6 background (>99%) were generated as described previously1, 2 and crossed with LDLrKO mice in the C57Bl/6 background (Jackson Laboratories) to generate heterozygous HSKO/LDLrKO. Mice used for atherosclerosis studies were generated by crosses of heterozygous HSKO/LDLrKO mice; genotypes of offspring were determined by PCR1. Female and male HSKO/LDLrKO and LDLrKO littermate control mice were fed a chow diet until 7–9 wks of age, and then switched to an atherogenic diet containing 10% palm oil and 0.2% cholesterol for 16 wks in most experiments, unless otherwise indicated. Diet composition has been published previously3. Mice were maintained on a 12h light/dark cycle. All protocols and procedures were approved by the Institutional Animal Care and Use Committee.
Lipid and lipoprotein analysis
Plasma was collected by tail bleeding or cardiac puncture from mice fasted for 4 hr. TPC, free cholesterol (FC), and triglycerides (TG) concentrations were determined by enzymatic assays using commercial kits4. Cholesterol distribution among lipoproteins was determined after fractionation of plasma by gel filtration chromatography using a Superose 6 10/300 GL column (GE Healthcare). An aliquot of plasma containing approximately 15 µg total cholesterol was injected onto the column and eluted with 0.9% saline containing 0.01% EDTA and 0.01% sodium azide at a flow rate of 0.4 ml/min. The column effluent was mixed with a commercially available enzymatic total cholesterol reagent (Pointe Scientific, Inc., Canton, MI) delivered at 0.125 ml/min. After passing through a knitted reaction coil maintained at 37°C, the absorbance of the reaction mixture was read at 500 nm using a UV-VIS detector. The area under the VLDL, LDL, and HDL peaks was calculated using Chromperfect Spirit (Justice Laboratory Software) chromatography software. To calculate the cholesterol concentration in each lipoprotein fraction, the ratio of the respective peak area to total peak area was multiplied by the total plasma cholesterol. Liver lipid analysis was performed by enzymatic assay of detergent-extracted liver5.
Analysis of atherosclerotic lesions
Mice were sacrificed after 16 wks of atherogenic diet consumption. First they were anesthetized with ketamine/xylazine, and the vasculature then was perfused with cold PBS. Aortas were isolated and fixed in 10% buffered formalin. After fixation, aortas were cleaned of adventitial fat and pinned open for measurement of surface lesion areas. Images of en face aortas were analyzed using WCIF Image J software. Aortas were then lipid extracted for quantification of total and FC content using gas-liquid chromatography, as described previously3. Aortic roots were embedded in Optimal Cutting Temperature (Tissuetek) media in a plastic mold, frozen, and cut at 8 µm intervals. Sections were collected from the aortic region moving toward the apex of the heart and sequentially placed on 8 slides, such that each slide had sections 64 µm apart. The sections were fixed in 10% buffered formalin, stained in 0.5% Oil Red O for 25 minutes and counterstained with hematoxylin. Stained sections were photographed and Image-Pro software (Media Cybernetics Inc., Rockville, Md.) was used to quantify lesion area. The lesion areas of three sections representing different regions were averaged for each mouse.
In vivo Macrophage RCT
Macrophage RCT assays were conducted as described by Rader and colleagues6 with minor modifications7. J774 mouse macrophages or bone marrow-derived macrophages (BMM) from LDLrKO mice were radiolabeled and cholesterol-loaded with 3H-cholesterol and acetylated LDL, respectively. Cells were then injected into the peritoneal cavity of recipient mice fed the atherogenic diet. Plasma samples were collected at 6h, 24h, and 48h after injection. Feces were collected throughout the 48h study. At necropsy, tissues were harvested and 3H-tracer levels in plasma, liver, bile, and feces were then quantified after lipid extraction and liquid scintillation counting. Aliquots of plasma were also fractionated by FPLC and cholesterol mass, and radiolabel distribution among lipoprotein fractions was quantified.
Real-time PCR Analysis
At sacrifice, tissues were harvested and snap frozen in liquid nitrogen. Total RNA was isolated from livers of male mice using TRIzol (Invitrogen), and real-time RCR was performed as reported previously 4. Primer sequences were the same as described previously2. GAPDH was used as the endogenous control.
Cellular cholesterol efflux
Cholesterol efflux from J774 macrophages to plasma of mice fed the atherogenic diet was measured as previously reported, with minor modifications8. Briefly, 350,000 J774 cells were plated into each well of a 24-well plate and incubated in labeling medium (RPMI 1640 medium containing 1% FBS and 2 µCi 3H-cholesterol) for 24 hours. Cells were then washed once and incubated with efflux medium (MEM-HEPES media containing 2.8% apoB lipoprotein-depleted plasma). Four hours later, medium was harvested and cellular lipid was extracted with isopropanol. A 100 µl aliquot of medium and cellular lipid extract was taken for scintillation counting to determine percentage cholesterol efflux during incubation. Aliquots of efflux medium were fractionated on a Superdex 200HR column (1×30 cm), and radioactivity of each fraction was determined by scintillation counting.
Biliary lipids
A measured volume of gallbladder bile collected from mice (n=5–10) was subjected to neutral lipid extraction. 5-α cholestane (5 µg) was added to each extraction tube as an internal standard. Aliquots of the bottom organic phase were used to determine total cholesterol (TC) content by gas-liquid chromatography and phospholipid (PL) content by enzymatic assay (4). Bile acid (BA) was measured as reported previously using the top phase of the lipid extract9.
Fecal cholesterol excretion
Two-day fecal collections were subjected to lipid extraction, and cholesterol content was measured as described previously10.
Macrophage cholesterol content
Peritoneal cells were harvested by lavage from 4h-fasted mice. Cells were suspended in RPMI-1640 medium containing 1% Nutridoma-SP (Roche Applied Science), 100 units/ml penicillin, 100 µg/ml streptomycin, and 2 mM L-glutamine and cultured at 37° C for 2 hours. Then, nonadherent cells were removed by washing with PBS and adherent macrophages were extracted with isopropanol at room temperature overnight. TC and FC content was determined by gasliquid chromatography and cellular protein was measured by Lowry protein assay after NaOH digestion, as previously reported4.
In vivo VLDL TG secretion rate determination
Tyloxapol (500 mg/kg body weight) was injected intravenously into 4h-fasted, anesthetized, atherogenic diet-fed mice (n=4–6). Blood was collected before (0 min), 30, 60, and 90 min after injection for measurement of plasma triglyceride (TG) concentration by enzymatic assay. VLDL TG secretion rate was determined by calculating the slope of the time vs. plasma TG concentration plot for each animal using linear regression analysis.
VLDL/IDL composition and size analysis
Plasma was collected from LDLrKO mice (n=4/group) fed an atherogenic diet for 16 wk after they were fasted for 4h. VLDL/IDL were isolated by ultracentrifugation at d=1.019 g/ml (100,000 rpm for 4h, Beckman Coulter TLA100.2 rotor) and chemical composition was determined by enzymatic assay and chemical assays {Rong, 2012 #48}. Lipid and protein content were expressed as percentage of total mass. Aliquots of VLDL/IDL were used to measure particle size using a Zetasizer Nano S dynamic light scattering instrument (Malvern Instruments Ltd., Worcestershire, UK). Particle sizes are reported as the major peak mean by volume analysis.
In vivo VLDL turnover
VLDL were separated from plasma of fasted LDLrKO mice fed an atherogenic diet for 16 wk by ultracentrifugation at d=1.006 g/ml. The VLDL preparation was refloated at d=1.006 gm/ml and radiolabeled with 125I using the iodine monochloride method11. Ninety-seven percent of the radioactivity was trichloroacetic acid-precipitable. VLDL tracer (0.25 × 106 cpm) was diluted to 200 µl with saline for retro-orbital injection into 4h-fasted, anesthetized recipient mice (n=5/genotype) fed an atherogenic diet for 16 wk. Blood samples were collected at 2 and 30 min and 1, 3, 5, 8 and 24 h after injection and 125I radiolabel in plasma was determined by gamma radiation counting. ApoB was precipitated from plasma using isopropyl alcohol and 125I radiolabel in apoB was quantified by gamma radiation counting12. Turnover curves were plotted as the percentage of injected tracer remaining in plasma or as the percentage of 125I-apoB remaining in plasma relative to the injected dose vs. time.
Gel electrophoresis
Plasma (1 µl) from LDLrKO or HSKO/LDLrKO mice fed an atherogenic diet for 19 wk was fractionated by 0.7% agarose gel electrophoresis. Following a 2h capillary transfer of the fractionated plasma from the agarose gel to a 0.2 µm nitrocellulose membrane, Western blot analysis was performed using anti-mouse apoA-I (Meridian Life Science, Inc. K23600G). SDSPAGE and 4–30% non-denaturing gradient gel electrophoresis and Western blot analysis was performed as previously described1.
Statistical analysis
Results are reported as mean ± standard error of the mean. Data were analyzed using twotailed Student’s t test (with Welch’s correction in case of unequal variance) using Graphpad Prism software. P <0.05 was considered statistically significant.
Supplementary Material
Significance.
Hepatocyte ABCA1 plays a pivotal role in maintaining plasma HDL levels, but its impact on macrophage RCT and atherogenesis is less clear. In this study, we show the importance of hepatic ABCA1 in regulating both plasma HDL and apoB lipoprotein metabolism under hyperlipidemic conditions. Despite a 50% reduction in plasma HDL cholesterol (HDL-C) in the absence of hepatic ABCA1, atherosclerosis was not worsened, likely due to the maintenance of in vivo macrophage RCT and the concomitant paradoxical 40–50% reduction in plasma VLDL and LDL levels. In addition, macrophage cholesterol efflux to apoB lipoprotein-depleted plasma varied <2-fold compared to a 10-fold variation in plasma HDL-C concentrations, supporting the concept that steady-state HDL-C concentration is not the primary determinant of plasma cholesterol efflux capacity. Therapeutic interventions targeting hepatic ABCA1 expression to alleviate cardiovascular burden should take into consideration that paradoxical effects on apoB lipoprotein metabolism may oppose atheroprotection.
Acknowledgments
The authors gratefully acknowledge Karen Klein (Office of Research, Wake Forest School of Medicine) for editing the manuscript and Dr. Mark Cline (Professor of Pathology, Wake Forest School of Medicine) for examining atherosclerotic lesion complexity in aortic root sections.
Sources of funding- This study was supported by National Institutes of Health Grants HL49373 and HL94525.
Non-standard Abbreviations
- ABC
ATP-binding cassette transporter
- TPC
Total plasma cholesterol
- FC
Free cholesterol
- CE
Cholesteryl ester
- PL
Phospholipid
- ApoB Lp
ApoB containing lipoproteins
- HSKO
Hepatocyte-specific ABCA1 knockout
- PM
Peritoneal macrophages
- BMM
Bone marrow-derived macrophages
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures- None
References
- 1.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 2.Oram JF, Heinecke JW. ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiol Rev. 2005;85:1343–1372. doi: 10.1152/physrev.00005.2005. [DOI] [PubMed] [Google Scholar]
- 3.Bodzioch M, Orso E, Klucken J, et al. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet. 1999;22:347–351. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- 4.Brooks-Wilson A, Marcil M, Clee SM, et al. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet. 1999;22:336–345. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- 5.Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette JC, Deleuze JF, Brewer HB, Duverger N, Denefle P, Assmann G. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet. 1999;22:352–355. doi: 10.1038/11921. [DOI] [PubMed] [Google Scholar]
- 6.Timmins JM, Lee JY, Boudyguina E, Kluckman KD, Brunham LR, Mulya A, Gebre AK, Coutinho JM, Colvin PL, Smith TL, Hayden MR, Maeda N, Parks JS. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J Clin Invest. 2005;115:1333–1342. doi: 10.1172/JCI23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poernama F, Subramanian R, Cook ME, Attie AD. High density lipoprotein deficiency syndrome in chickens is not associated with an increased susceptibility to atherosclerosis. Arterioscler Thromb. 1992;12:601–607. doi: 10.1161/01.atv.12.5.601. [DOI] [PubMed] [Google Scholar]
- 8.McNeish J, Aiello RJ, Guyot D, Turi T, Gabel C, Aldinger C, Hoppe KL, Roach ML, Royer LJ, de Wet J, Broccardo C, Chimini G, Francone OL. High density lipoprotein deficiency and foam cell accumulation in mice with targeted disruption of ATP-binding cassette transporter-1. Proc Natl Acad Sci U S A. 2000;97:4245–4250. doi: 10.1073/pnas.97.8.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaefer EJ, Zech LA, Schwartz DE, Brewer HB., Jr Coronary heart disease prevalence and other clinical features in familial high-density lipoprotein deficiency (Tangier disease) Ann Intern Med. 1980;93:261–266. doi: 10.7326/0003-4819-93-2-261. [DOI] [PubMed] [Google Scholar]
- 10.Brousseau ME, Bodzioch M, Schaefer EJ, Goldkamp AL, Kielar D, Probst M, Ordovas JM, Aslanidis C, Lackner KJ, Bloomfield Rubins H, Collins D, Robins SJ, Wilson PW, Schmitz G. Common variants in the gene encoding ATP-binding cassette transporter 1 in men with low HDL cholesterol levels and coronary heart disease. Atherosclerosis. 2001;154:607–611. doi: 10.1016/s0021-9150(00)00722-x. [DOI] [PubMed] [Google Scholar]
- 11.Clee SM, Zwinderman AH, Engert JC, Zwarts KY, Molhuizen HO, Roomp K, Jukema JW, van Wijland M, van Dam M, Hudson TJ, Brooks-Wilson A, Genest J, Jr, Kastelein JJ, Hayden MR. Common genetic variation in ABCA1 is associated with altered lipoprotein levels and a modified risk for coronary artery disease. Circulation. 2001;103:1198–1205. doi: 10.1161/01.cir.103.9.1198. [DOI] [PubMed] [Google Scholar]
- 12.Frikke-Schmidt R, Nordestgaard BG, Jensen GB, Steffensen R, Tybjaerg-Hansen A. Genetic variation in ABCA1 predicts ischemic heart disease in the general population. Arterioscler Thromb Vasc Biol. 2008;28:180–186. doi: 10.1161/ATVBAHA.107.153858. [DOI] [PubMed] [Google Scholar]
- 13.Frikke-Schmidt R, Nordestgaard BG, Stene MC, Sethi AA, Remaley AT, Schnohr P, Grande P, Tybjaerg-Hansen A. Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. JAMA. 2008;299:2524–2532. doi: 10.1001/jama.299.21.2524. [DOI] [PubMed] [Google Scholar]
- 14.Joyce CW, Amar MJ, Lambert G, Vaisman BL, Paigen B, Najib-Fruchart J, Hoyt RF, Jr, Neufeld ED, Remaley AT, Fredrickson DS, Brewer HB, Jr, Santamarina-Fojo S. The ATP binding cassette transporter A1 (ABCA1) modulates the development of aortic atherosclerosis in C57BL/6 and apoE-knockout mice. Proc Natl Acad Sci U S A. 2002;99:407–412. doi: 10.1073/pnas.012587699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singaraja RR, Fievet C, Castro G, James ER, Hennuyer N, Clee SM, Bissada N, Choy JC, Fruchart JC, McManus BM, Staels B, Hayden MR. Increased ABCA1 activity protects against atherosclerosis. J Clin Invest. 2002;110:35–42. doi: 10.1172/JCI15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunham LR, Singaraja RR, Duong M, Timmins JM, Fievet C, Bissada N, Kang MH, Samra A, Fruchart JC, McManus B, Staels B, Parks JS, Hayden MR. Tissue-specific roles of ABCA1 influence susceptibility to atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:548–554. doi: 10.1161/ATVBAHA.108.182303. [DOI] [PubMed] [Google Scholar]
- 17.Van Eck M, Bos IST, Kaminski WE, Orsó E, Rothe G, Twisk J, Böttcher A, Van Amersfoort ES, Christiansen-Weber TA, Fung-Leung W-P, Van Berkel TJC, Schmitz G. Leukocyte ABCA1 controls susceptibility to atherosclerosis and macrophage recruitment into tissues. Proceedings of the National Academy of Sciences. 2002;99:6298–6303. doi: 10.1073/pnas.092327399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aiello RJ, Brees D, Bourassa PA, Royer L, Lindsey S, Coskran T, Haghpassand M, Francone OL. Increased atherosclerosis in hyperlipidemic mice with inactivation of ABCA1 in macrophages. Arterioscler Thromb Vasc Biol. 2002;22:630–637. doi: 10.1161/01.atv.0000014804.35824.da. [DOI] [PubMed] [Google Scholar]
- 19.Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, Li R, Welch C, Tall AR. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. The Journal of Clinical Investigation. 2007;117:3900–3908. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Y, Pennings M, Hildebrand RB, Ye D, Calpe-Berdiel L, Out R, Kjerrulf M, Hurt-Camejo E, Groen AK, Hoekstra M, Jessup W, Chimini G, Van Berkel TJC, Van Eck M. Enhanced Foam Cell Formation, Atherosclerotic Lesion Development, and Inflammation by Combined Deletion of ABCA1 and SR-BI in Bone Marrow–Derived Cells in LDL Receptor Knockout Mice on Western-Type Diet. Circulation Research. 2010;107:e20–e31. doi: 10.1161/CIRCRESAHA.110.226282. [DOI] [PubMed] [Google Scholar]
- 21.Joyce CW, Wagner EM, Basso F, et al. ABCA1 overexpression in the liver of LDLr-KO mice leads to accumulation of pro-atherogenic lipoproteins and enhanced atherosclerosis. J Biol Chem. 2006;281:33053–33065. doi: 10.1074/jbc.M604526200. [DOI] [PubMed] [Google Scholar]
- 22.Curtiss LK, Boisvert WA. Apolipoprotein E and atherosclerosis. Curr Opin Lipidol. 2000;11:243–251. doi: 10.1097/00041433-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Chung S, Timmins JM, Duong M, Degirolamo C, Rong S, Sawyer JK, Singaraja RR, Hayden MR, Maeda N, Rudel LL, Shelness GS, Parks JS. Targeted deletion of hepatocyte ABCA1 leads to very low density lipoprotein triglyceride overproduction and low density lipoprotein hypercatabolism. J Biol Chem. 2010;285:12197–12209. doi: 10.1074/jbc.M109.096933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Eck M, Hoekstra M, Out R, Bos IST, Kruijt JK, Hildebrand RB, Van Berkel TJC. Scavenger receptor BI facilitates the metabolism of VLDL lipoproteins in vivo. Journal of Lipid Research. 2008;49:136–146. doi: 10.1194/jlr.M700355-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. The Journal of Clinical Investigation. 1993;92:883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab. 2008;7:365–375. doi: 10.1016/j.cmet.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 27.de la Llera-Moya M, Drazul-Schrader D, Asztalos BF, Cuchel M, Rader DJ, Rothblat GH. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol. 2010;30:796–801. doi: 10.1161/ATVBAHA.109.199158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tangirala RK, Rubin EM, Palinski W. Quantitation of atherosclerosis in murine models: correlation between lesions in the aortic origin and in the entire aorta, and differences in the extent of lesions between sexes in LDL receptor-deficient and apolipoprotein Edeficient mice. Journal of Lipid Research. 1995;36:2320–2328. [PubMed] [Google Scholar]
- 29.Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo CL, Wang M, Sanson M, Abramowicz S, Welch C, Bochem AE, Kuivenhoven JA, Yvan-Charvet L, Tall AR. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest. 2011;121:4138–4149. doi: 10.1172/JCI57559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linton MF, Atkinson JB, Fazio S. Prevention of atherosclerosis in apolipoprotein Edeficient mice by bone marrow transplantation. Science. 1995;267:1034–1037. doi: 10.1126/science.7863332. [DOI] [PubMed] [Google Scholar]
- 31.Bellosta S, Mahley RW, Sanan DA, Murata J, Newland DL, Taylor JM, Pitas RE. Macrophage-specific expression of human apolipoprotein E reduces atherosclerosis in hypercholesterolemic apolipoprotein E-null mice. The Journal of Clinical Investigation. 1995;96:2170–2179. doi: 10.1172/JCI118271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emerging Risk Factors C. Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG, Danesh J. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis GF, Rader DJ. New Insights Into the Regulation of HDL Metabolism and Reverse Cholesterol Transport. Circulation Research. 2005;96:1221–1232. doi: 10.1161/01.RES.0000170946.56981.5c. [DOI] [PubMed] [Google Scholar]
- 34.Zhu X, Parks JS. New Roles of HDL in Inflammation and Hematopoiesis. Annual Review of Nutrition. 2012;32:161–182. doi: 10.1146/annurev-nutr-071811-150709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. The Lancet. 2012;380:572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rader DJ, Tall AR. The not-so-simple HDL story: Is it time to revise the HDL cholesterol hypothesis? Nat Med. 2012;18:1344–1346. doi: 10.1038/nm.2937. [DOI] [PubMed] [Google Scholar]
- 37.El Harchaoui K, Arsenault BJ, Franssen R, Després J-P, Hovingh GK, Stroes ESG, Otvos JD, Wareham NJ, Kastelein JJP, Khaw K-T, Boekholdt SM. High-Density Lipoprotein Particle Size and Concentration and Coronary Risk. Annals of Internal Medicine. 2009;150:84–93. doi: 10.7326/0003-4819-150-2-200901200-00006. [DOI] [PubMed] [Google Scholar]
- 38.Otvos JD. The surprising AIM-HIGH results are not surprising when viewed through a particle lens. J Clin Lipidol. 2011;5:368–370. doi: 10.1016/j.jacl.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 39.Mackey RH, Greenland P, Goff DC, Jr, Lloyd-Jones D, Sibley CT, Mora S. High-Density Lipoprotein Cholesterol and Particle Concentrations, Carotid Atherosclerosis, and Coronary Events: MESA (Multi-Ethnic Study of Atherosclerosis) Journal of the American College of Cardiology. 2012;60:508–516. doi: 10.1016/j.jacc.2012.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heinecke JW. The not-so-simple HDL story: A new era for quantifying HDL and cardiovascular risk? Nat Med. 2012;18:1346–1347. doi: 10.1038/nm.2930. [DOI] [PubMed] [Google Scholar]
- 42.Rader DJ, Alexander ET, Weibel GL, Billheimer J, Rothblat GH. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res. 2009;50(Suppl):S189–S194. doi: 10.1194/jlr.R800088-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamamoto S, Tanigawa H, Li X, Komaru Y, Billheimer JT, Rader DJ. Pharmacologic suppression of hepatic ATP-binding cassette transporter 1 activity in mice reduces highdensity lipoprotein cholesterol levels but promotes reverse cholesterol transport. Circulation. 2011;124:1382–1390. doi: 10.1161/CIRCULATIONAHA.110.009704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamashita S, Matsuzawa Y. Where are we with probucol: a new life for an old drug? Atherosclerosis. 2009;207:16–23. doi: 10.1016/j.atherosclerosis.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Schaefer EJ, Brousseau ME, Diffenderfer MR, Cohn JS, Welty FK, O'Connor J, Jr, Dolnikowski GG, Wang J, Hegele RA, Jones PJ. Cholesterol and apolipoprotein B metabolism in Tangier disease. Atherosclerosis. 2001;159:231–236. doi: 10.1016/s0021-9150(01)00688-8. [DOI] [PubMed] [Google Scholar]
- 46.Stanford KI, Bishop JR, Foley EM, Gonzales JC, Niesman IR, Witztum JL, Esko JD. Syndecan-1 is the primary heparan sulfate proteoglycan mediating hepatic clearance of triglyceride-rich lipoproteins in mice. The Journal of Clinical Investigation. 2009;119:3236–3245. doi: 10.1172/JCI38251. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 1.Timmins JM, Lee JY, Boudyguina E, Kluckman KD, Brunham LR, Mulya A, Gebre AK, Coutinho JM, Colvin PL, Smith TL, Hayden MR, Maeda N, Parks JS. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J Clin Invest. 2005;115:1333–1342. doi: 10.1172/JCI23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung S, Timmins JM, Duong M, Degirolamo C, Rong S, Sawyer JK, Singaraja RR, Hayden MR, Maeda N, Rudel LL, Shelness GS, Parks JS. Targeted deletion of hepatocyte ABCA1 leads to very low density lipoprotein triglyceride overproduction and low density lipoprotein hypercatabolism. J Biol Chem. 2010;285:12197–12209. doi: 10.1074/jbc.M109.096933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudel LL, Kelley K, Sawyer JK, Shah R, Wilson MD. Dietary monounsaturated fatty acids promote aortic atherosclerosis in LDL receptor-null, human ApoB100-overexpressing transgenic mice. Arterioscler Thromb Vasc Biol. 1998;18:1818–1827. doi: 10.1161/01.atv.18.11.1818. [DOI] [PubMed] [Google Scholar]
- 4.Zhu X, Lee JY, Timmins JM, Brown JM, Boudyguina E, Mulya A, Gebre AK, Willingham MC, Hiltbold EM, Mishra N, Maeda N, Parks JS. Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J Biol Chem. 2008;283:22930–22941. doi: 10.1074/jbc.M801408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr TP, Andresen CJ, Rudel LL. Enzymatic determination of triglyceride, free cholesterol, and total cholesterol in tissue lipid extracts. Clin Biochem. 1993;26:39–42. doi: 10.1016/0009-9120(93)90015-x. [DOI] [PubMed] [Google Scholar]
- 6.Naik SU, Wang X, Da Silva JS, Jaye M, Macphee CH, Reilly MP, Billheimer JT, Rothblat GH, Rader DJ. Pharmacological activation of liver X receptors promotes reverse cholesterol transport in vivo. Circulation. 2006;113:90–97. doi: 10.1161/CIRCULATIONAHA.105.560177. [DOI] [PubMed] [Google Scholar]
- 7.Temel RE, Sawyer JK, Yu L, Lord C, Degirolamo C, McDaniel A, Marshall S, Wang N, Shah R, Rudel LL, Brown JM. Biliary sterol secretion is not required for macrophage reverse cholesterol transport. Cell Metab. 2010;12:96–102. doi: 10.1016/j.cmet.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de la Llera-Moya M, Drazul-Schrader D, Asztalos BF, Cuchel M, Rader DJ, Rothblat GH. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol. 2010;30:796–801. doi: 10.1161/ATVBAHA.109.199158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turley SD, Dietschy JM. Re-evaluation of the 3 alpha-hydroxysteroid dehydrogenase assay for total bile acids in bile. J Lipid Res. 1978;19:924–928. [PubMed] [Google Scholar]
- 10.Temel RE, Lee RG, Kelley KL, Davis MA, Shah R, Sawyer JK, Wilson MD, Rudel LL. Intestinal cholesterol absorption is substantially reduced in mice deficient in both ABCA1 and ACAT2. J Lipid Res. 2005;46:2423–2431. doi: 10.1194/jlr.M500232-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Parks JS, Rudel LL. Different kinetic fates of apolipoproteins A-I and A-II from lymph chylomicra of nonhuman primates. Effect of saturated versus polyunsaturated dietary fat. J Lipid Res. 1982;23:410–421. [PubMed] [Google Scholar]
- 12.Rong S, Cao Q, Liu M, Seo J, Jia L, Boudyguina E, Gebre AK, Colvin PL, Smith TL, Murphy RC, Mishra N, Parks JS. Macrophage 12/15 lipoxygenase expression increases plasma and hepatic lipid levels and exacerbates atherosclerosis. J Lipid Res. 2012;53:686–695. doi: 10.1194/jlr.M022723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.