Abstract
Objectives
The relative influences of hypoxia and other environmental stressors on growth at altitude remain unclear. Previous work demonstrated an association between peripheral arterial oxygen saturation (SpO2) and anthropometry (especially tibia length) among Tibetan and Han children at altitude. We investigated whether similar associations exist among Andeans, and the patterning of associations between SpO2 and anthropometry.
Methods
Stature, head-trunk height, total upper and lower limb lengths, zeugopod (ulna and tibia) and autopod (hand and foot) lengths were measured in Peruvian children (0.5–14 years) living at >3000 m altitude. SpO2 was measured by pulse oximetry. Anthropometry was converted to internal z scores. Correlation and multiple regression were used to examine associations between anthropometry z scores and SpO2, altitude, or SpO2 adjusted for altitude since altitude is a major determinant of variation in SpO2.
Results
SpO2 and altitude show weak, significant correlations with zeugopod length z scores and still weaker significant correlations with total upper and lower limb length z scores. Correlations with z scores for stature, head-trunk height, or autopod lengths are not significant. Adjusted for altitude, there is no significant association between anthropometry and SpO2.
Conclusions
Associations between SpO2 or altitude and total limb and zeugopod length z scores exist among Andean children. However, the relationships are relatively weak, and while the relationship between anthropometry and altitude may be partly mediated by SpO2, other factors that covary with altitude (e.g., socioeconomic status, health) are likely to influence anthropometry. The results support suggestions that zeugopod lengths are particularly sensitive to environmental stressors. Am. J. Hum. Biol., 25:629–636, 2013. © 2013 Wiley Periodicals, Inc.
As an environmental stressor that cannot be culturally mitigated (Baker, 1976), high altitude hypoxia has been the subject of considerable research to identify the genetic, physiological, and morphological means by which humans adapt to these conditions. Whether hypoxia directly affects human growth has been extensively investigated, and the negative impact of high altitude pregnancy on birth weight is well documented (Beall, 1981; Giussani et al., 2001; Haas et al., 1977; McClung, 1969; Moore et al., 1998; Unger et al., 1988; Zamudio and Moore, 2000). While a number of studies report reduced child and adult stature at altitude, the growing consensus is that socioeconomic differences account for the greatest part of the deficit, while a reduction of just 1–2 cm in adult stature is likely attributable to hypoxia (Greksa, 2006). This is important because growth deficits due to the direct effects of hypoxia may be difficult to resolve, while deficits due to other factors can be effectively addressed by interventions to improve growth.
The delivery of oxygen to the tissues is not only critical for maintaining immediate function, but also for growth. This is demonstrated by the fact that populations who have lived at altitude for many generations (e.g., Andeans, Tibetans) are partially protected from hypoxia-related fetal growth reduction by genetic adaptations that increase oxygen delivery to the fetus (Bennett et al., 2008; Giussani et al., 2001; Julian et al., 2007,2009,2011; Moore, 2003). The body employs various mechanisms to counteract ambient hypoxia at high altitude and thus maintain cellular and tissue oxygen homeostasis (reviewed in Beall, 2001; Moore et al., 1998; West et al., 2007). These include erythrocytosis and increased ventilation and heart rates on acute exposure to hypoxia, and in the case of Tibetan high altitude natives, increased tissue blood flow (perfusion: Andeans remain untested in this respect) (Beall et al., 2001; Erzurum et al., 2007).
There is evidence that exposure to hypoxia in utero or post-natally affects body size and proportions, particularly the relative size of the limbs and trunk (Bailey et al., 2007; Lampl et al., 2003; Stinson 2009), although the extent to which hypoxia exerts a direct influence and the mechanisms by which it does so remain unclear. Recently the specific impact of oxygen delivery on human postnatal growth at altitude has been investigated using peripheral arterial oxygen saturation (SpO2). SpO2 measures the percentage of hem groups in hemoglobin which are bound to oxygen (Moore et al., 1998), and influences tissue oxygen delivery. SpO2 decreases with increasing altitude, even among populations adapted to high altitude hypoxia (Beall, 2007). SpO2 represents only one component of blood oxygen content (the total amount of oxygen the blood carries) which is also a function of erythrocyte and hemoglobin concentrations (Beall, 2001,2007). The advantage of SpO2 is that it can be measured simply, inexpensively and non-invasively by pulse oximetry (Schult and Canelo-Aybar, 2011), and evidence for a major gene among Tibetans that increases oxygen saturation (Beall et al., 1994,1997) demonstrates that SpO2 is sufficiently important to have been under natural selection in this native high altitude population. However, Andeans do not seem to share this adaptation (Beall et al., 2004).
Bailey and co-workers (Bailey and Hu, 2002; Bailey et al., 2007) demonstrated that higher SpO2 and better lung function are associated with greater stature and with relatively longer tibiae among 8- to 11-year-old Tibetan and Han children at high altitude. A similar relationship between anthropometry and SpO2 has not yet been observed in highland Andeans. The purpose of this study is to investigate the relationship between SpO2 and measures of body size, limb, and trunk lengths; specifically stature, head-trunk (sitting) height, total limb lengths and limb segment lengths (zeugopod: ulna or tibia; and autopod: hand or foot) among Peruvian infants and children at high altitude (>3000 m). While relative total lower limb length, and sometimes tibia length, have been investigated in studies of body proportions in relation to environmental stress, relationships between total upper limb length, ulna length, or autopod (hand or foot) length and SpO2 have not. The investigation of total upper and lower limb lengths, as well as limb segment lengths, may help to elucidate the mechanisms underlying altered body proportions under stress conditions (Pomeroy et al., 2012). These mechanisms remain unclear, but are relevant to understanding reported associations between early life conditions, body proportions, and chronic disease risk (reviewed in Bogin and Varela-Silva, 2010; Samaras, 2007) and how humans adapt to poor environmental conditions during growth. We hypothesize that, as among Tibetan and Han children, SpO2 in Andeans will be more strongly positively associated with zeugopod lengths than trunk length or stature. Furthermore, in light of evidence that the effects of environmental stress on anthropometry rank as follows: zeugopod > total limb > autopod > trunk (Pomeroy et al., 2012), we hypothesize that a similar ranking will be seen with SpO2.
Methods
The study received ethical approval from the Institutional Ethics Committee at the Universidad Peruana Cayetano Heredia, Lima, and from the Health Directorate for Ayacucho Region (Dirección Régional de Salud Ayacucho, DIRESA). Participation was voluntary and the study was conducted according to international ethical standards (World Medical Association, 2008). Children aged 6 months to 14 years who were born and raised in highland communities in Ayacucho Region, Peru (Fig. 1), were included in the study. First language was predominantly Quechua, and participants came from small rural communities living from subsistence agriculture and herding at altitudes from 3100–4400 m. Written informed consent was obtained from a parent or legal guardian by signature or fingerprint (where not literate) once the study had been explained in full to them and to the participant in age-appropriate terms. Participants aged 6 years or over also gave their assent, either in writing or verbally where not literate.
Figure 1.
Map showing location of study sites in the central Peruvian highlands. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Anthropometry was measured by a single trained observer (EP) using standard methods as previously described (Pomeroy et al., 2012). Measurements were converted to age–sex-specific z scores based on a combined sample of highland and lowland children who participated in a larger study (Pomeroy et al., 2012), though only highland children are considered here. Z scores adjust a measurement for age and sex and express it in standard deviation units. Therefore their use permits analyses combining data varying by age and sex so as to maximize statistical power. Z scores were derived by the LMS method (Cole, 1990; Cole and Green, 1992) using LMS Chartmaker Light version 2.43 (Pan and Cole, 2010). Subsequently, references to any anthropometric measurements are to their z scores.
SpO2 was measured by pulse oximetry, which uses differences in the wavelength of light absorbed by oxygenated and deoxygenated blood to estimate arterial oxygen saturation (SaO2). As deoxyhemoglobin absorbs more red light (600–750 nm wavelength) than oxyhemoglobin, which has higher infrared absorption (850–1000 nm), the ratio of light absorption in the red and infrared spectra indicates SaO2 (Fouzas et al., 2011). SpO2 is typically measured using a fingertip clip that passes red and infrared light through the finger and measures light transmission (Fouzas et al., 2011).
A Nonin 8500 pulse oximeter (Nonin Medical, Plymouth, MN) was used to measure SpO2. Probes were selected based on the participant’s weight according to the manufacturer’s recommendations, and attached to the index finger of the left hand, or to the big toe of infants whose fingertip thickness was less than 5 mm. Individuals were measured in a calm, resting state (if they were visibly distressed the measurement was not done). As movement can reduce measurement accuracy (Fouzas et al., 2011), the child’s hand or foot was held still during the measurement where necessary. Insufficient tissue perfusion can also lead to inaccurate readings (Fouzas et al., 2011), but results were only recorded when the oximeter’s heart rate indicator showed that there was sufficient blood flow for a reliable measurement. The manufacturer reports accuracy of ±2%, consistent with that for pulse oximetry in general (Jensen et al., 1998; Ross and Helms, 1990), and the 8500 model is approved for use up to 12,000 m altitude and is certified for aeromedical use by the US Air Force.
The normality of distributions for SpO2 and the anthropometric data were assessed visually using histograms. Anthropometry z score distributions were normal after removing a single strong outlier, and similarly for SpO2 after excluding two individuals with unusually low readings. Despite previous studies reporting an association between SpO2 and age (Schult and Canelo-Aybar, 2011), no such relationship was found in our data (Pearson’s correlation, P = 0.1). Sex differences in SpO2 were also absent (t-test, P = 0.3). Thus age and sex were not included in the analyses, as the anthropometry z scores were already age–sex-adjusted.
The altitude at which the children were studied varied, and SpO2 decreases with increasing altitude. The sample fell into three natural groups in terms of altitude (Fig. 2), so the data were analyzed in three altitude groups and the correlations between altitude and SpO2 assessed using Spearman’s rank correlation coefficient. Pearson’s correlation was derived between SpO2 and anthropometry. As other factors varying with altitude, such as socioeconomic status (SES) and healthcare (see “Introduction” section), might also impact on body size and proportions, correlations between altitude of measurement and anthropometry z scores were also conducted to investigate whether the relationships were similar to those between anthropometry and SpO2, and thus whether a direct effect of SpO2, rather than other factors associated with altitude, might be inferred. Therefore, Spearman’s correlations were derived between altitude group and anthropometry z scores.
Figure 2.
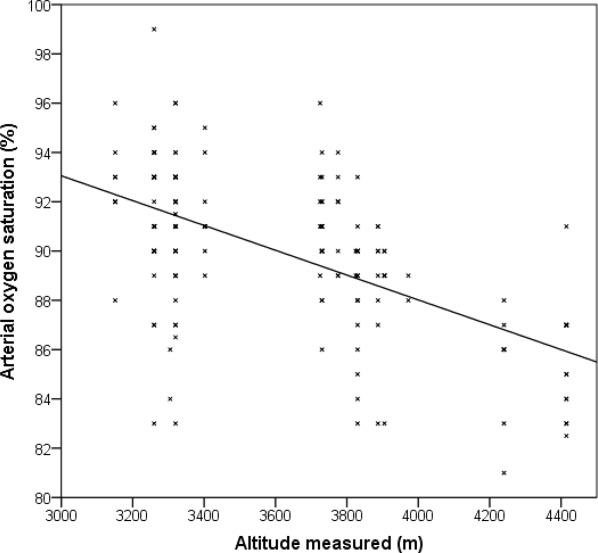
Scatter plot of peripheral arterial oxygen saturation (SpO2) against altitude where measurements were taken, demonstrating the expected decrease in SpO2 with increasing altitude.
Multiple regression analyses of anthropometry z scores on SpO2 were used to assess their associations including an adjustment for altitude group. To confirm that the use of z scores did not bias the results, the analyses were repeated using raw anthropometry as outcomes, adjusting for sex, age and age2 (to cater for nonlinear relationships). Analyses were performed using SPSS 21.0 for Windows, with statistical significance defined at P < 0.05.
Results
Table 1 summarizes the characteristics of the study sample. Summary statistics on stature, sitting height, and weight by age group and sex are given in Supporting Information Table S1, and further summary anthropometry can be found in Pomeroy et al. (2012).
Table 1.
Summary statistics of the study sample
| Variable | Statistic | Value |
|---|---|---|
| Sample size | n (males, females) | 165 (82, 83) |
| Altitude of measurement (m) | Median | 3564 |
| Interquartile range | 3306–3823 | |
| Range | 3150–4415 | |
| SpO2 (%) | Median | 90 |
| Interquartile range | 88–92 | |
| Range | 81–99 | |
| Age (years) | Mean | 5.25 |
| Standard deviation | 3.59 | |
| Range | 0.5–14.4 |
The correlation between altitude of measurement and SpO2 (Fig. 2) is negative, as expected (r = −0.55, P < 0.001). Correlations between anthropometry and SpO2 (Table 2) are significant for ulna, tibia, total upper limb and total lower limb length z scores, but not for stature, head-trunk height, foot, or hand length z scores. Overall the correlations are low (r ≤0.23), indicating weak relationships even where significant.
Table 2.
Correlations between SpO2 and anthropometry z scores
| Outcome z score | Pearson r | P | n |
|---|---|---|---|
| Ulna length | 0.23 | 0.003 | 163 |
| Tibia length | 0.22 | 0.004 | 164 |
| Total upper limb length | 0.19 | 0.02 | 159 |
| Total lower limb length | 0.17 | 0.03 | 165 |
| Stature | 0.15 | 0.06 | 165 |
| Head-trunk height | 0.08 | 0.3 | 165 |
| Foot length | 0.07 | 0.4 | 164 |
| Hand length | 0.05 | 0.5 | 156 |
Bold indicates significant P values.
Correlations between altitude of measurement and anthropometry z scores rank similarly to those for SpO2, with those for zeugopod length highest, and those for autopod lengths and head-trunk height low and insignificant (Table 3). Furthermore, these correlations with altitude are generally similar in magnitude to those with SpO2 (Table 2). However, none of the anthropometry z scores are significantly related to SpO2 when adjusted for altitude group (Table 4). The results are confirmed by repeating the analyses using raw anthropometry, which leads to the same conclusions (Supporting Information Tables S2–S4).
Table 3.
Correlations between altitude and anthropometry z scores
| Outcomez score | Spearmanr | P | n |
|---|---|---|---|
| Tibia length | −0.28 | <0.001 | 164 |
| Ulna length | −0.26 | 0.001 | 163 |
| Total lower limb length | −0.3 | 0.001 | 165 |
| Total upper limb length | −0.18 | 0.03 | 159 |
| Stature | −0.1 | 0.07 | 165 |
| Hand length | −0.08 | 0.3 | 156 |
| Foot length | −0.04 | 0.6 | 164 |
| Head-trunk height | 0.006 | 0.9 | 165 |
Bold indicates significant P values.
Table 4.
Results of multiple regression models of anthropometry z scores on SpO2 and altitude
| 3700–4000 m altitudea | 4200–4500 m altitude | SpO2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome z score | B | SE | P | B | SE | P | B | SE | P |
| Tibia length | −0.32 | 0.11 | 0.004 | −0.67 | 0.19 | 0.001 | 0.007 | 0.018 | 0.7 |
| Total lower limb length | −0.37 | 0.12 | 0.004 | −0.71 | 0.22 | 0.002 | −0.002 | 0.021 | 0.9 |
| Ulna length | −0.24 | 0.10 | 0.02 | −0.43 | 0.18 | 0.02 | 0.018 | 0.017 | 0.3 |
| Total upper limb length | −0.22 | 0.11 | 0.05 | −0.12 | 0.19 | 0.5 | 0.027 | 0.018 | 0.1 |
| Stature | −0.13 | 0.12 | 0.3 | −0.42 | 0.22 | 0.06 | 0.009 | 0.021 | 0.6 |
| Foot length | −0.00 | 0.13 | 0.9 | −0.20 | 0.22 | 0.4 | 0.004 | 0.021 | 0.8 |
| Hand length | −0.02 | 0.13 | 0.9 | −0.04 | 0.22 | 0.8 | 0.009 | 0.021 | 0.7 |
| Head-trunk height | 0.13 | 0.13 | 0.3 | 0.04 | 0.23 | 0.8 | 0.021 | 0.022 | 0.3 |
Bold indicates significant P values.
Two dummy variables for altitude group with lowest altitude group (3150–3400 m) as the reference group.
Discussion
Oxygen saturation and anthropometry z scores are significantly associated in this population of high altitude Andean children. However, anthropometry z scores correlate similarly with altitude and SpO2, and adjusting for altitude the SpO2—anthropometry associations vanish. This suggests that the relationship between anthropometry and altitude may be partly mediated through SpO2, which itself is negatively correlated with altitude, but that other factors covarying with altitude also independently influence anthropometry. The low correlations between SpO2 and anthropometry indicate that, even if the association is causal, factors other than SpO2 substantially influence anthropometry, in line with evidence that nutritional and other factors, rather than hypoxia, most likely explain the height deficit in highland populations (Greksa, 2006).
The results are consistent with previous evidence that total lower limb length is more sensitive to the effects of environmental stress than trunk length (Bogin and Varela-Silva, 2010; Frisancho, 2007; Gunnell et al., 1998; Whitley et al., 2008), and that zeugopod lengths are more sensitive to environmental stress than total limb lengths (Meadows Jantz and Jantz, 1999; Pomeroy et al., 2012). With specific reference to hypoxia, the results agree with those of Bailey and colleagues on Tibetan and Han children, which also showed a stronger association between SpO2 and absolute or relative tibia length than with total lower limb length or stature (Bailey et al., 2007). They are also consistent with a study into the effect of prenatal hypoxia on rats demonstrating reduced total fore- and hind-limb lengths, and that zeugopod lengths were more strongly affected than stylopod (humerus or femur) lengths (Hunter and Clegg, 1973). However that study also reported significant reductions in paw length under hypoxic conditions which contrast with our results. Work examining relative tibia, femur, and total upper limb lengths in hypoxia-exposed fetuses also suggests that the tibia was shortened relative to the femur, but total upper limb length was unaffected (Lampl et al., 2003), again in contrast with our results.
The results suggest that factors other than SpO2 that covary with altitude may explain much of the altitude-related anthropometric variation. As already outlined, SpO2 is only one aspect of tissue oxygen delivery, and variation in altitude may have additional hypoxia-related effects on growth that are not captured by SpO2. SpO2 also varies diurnally in individuals, for example tending to be lower during sleep (Fouzas et al., 2011) and exercise (Brutsaert et al., 2000), so the single measurement of SpO2 recorded in this study may not have captured between-individual variation in SpO2 that may have had significant impacts on growth. Furthermore, environmental characteristics like SES, temperature and healthcare access decrease with increasing altitude (Leonard et al., 1990; Niermeyer et al., 2009; Rivera-Ch et al., 2008; West et al., 2007) while respiratory infection rates increase (Niermeyer et al., 2009; Subhi et al., 2009), and these may also impact on growth, body size, and body proportions.
Distinguishing between the influences of these different environmental stressors is challenging, since factors such as SES, ambient temperature, and tissue oxygenation (the end product of various mechanisms of oxygen delivery at altitude) are hard to characterize. In this study, participants came from small rural communities where SES is very low and varies little, while diet and access to healthcare are similarly poor. Bailey et al. (2007) reported little variation in SES in their sample of Tibetan and Han children, so argued this could not have accounted for their results, but it remains untested whether even such limited variation could still influence morphology at altitude. Variation in other factors known to influence growth and body size, including maternal phenotype, health and nutrition, and intergenerational effects on maternal and offspring phenotype (Wells, 2010) are important considerations for future research. Temperature is unlikely to explain the results, which are inconsistent with the mechanisms thought to link limb growth and temperature (Pomeroy et al., 2012). Given the difficulty of separating different influences on morphology at altitude where exposure to multiple stressors is correlated, experimental animal models where other factors can be controlled are likely key to understanding the effects of hypoxia on body size and proportions.
In terms of underlying mechanisms, our results are inconsistent with a proximo-distal decrease in available resources along the limb, as suggested in Lampl’s distal blood flow model (Lampl et al., 2003). This hypothesis states that fetuses exposed to hypoxia due to maternal diabetes or smoking show reduced tibia length, but not reduced total upper limb or femur lengths, due to the nature of the fetal circulation and the diminution of blood oxygen availability with distance from the placenta, reaching its lowest levels in the distal lower limb (tibia). The model implies that hands and feet are most affected by hypoxia, though this was not investigated by Lampl et al. (2003). However, this was not the pattern observed here.
The results are more consistent with the thrifty phenotype hypothesis as applied to limb lengths (Pomeroy et al., 2012). The thrifty phenotype hypothesis (Hales and Barker, 1992) states that when resources are limited, growth is prioritized in organs or parts of the body where function would be most compromised by inadequate growth. In comparing children from the Peruvian highlands (including the sample from this study) with those from the Peruvian lowlands who experience markedly lower levels of environmental stress in terms of socioeconomic factors, healthcare access, hypoxia and cold exposure, we documented a similar pattern (Pomeroy et al., 2012). Zeugopod lengths showed the greatest differences between populations, followed by total limb lengths, while differences were smaller in hand and foot lengths and smallest in head-trunk height. The trunk may be relatively protected as it houses the major organs, while autopod lengths may also be protected due to their critical roles in manipulation and substrate interaction during locomotion. While this suggestion has not been demonstrated empirically, it has been proposed that greater canalization of autopod size compared with the stylopod and zeugopod may be explained in this way (Rolian, 2008,2009; Young and Hallgrímsson 2005). Our analyses suggest that SpO2 represents one altitude-associated signal of supply, to which the limb components respond in a hierarchical manner.
In terms of proximate mechanisms, trunk size may be maintained at the expense of limb lengths through peripheral vasoconstriction which may reduce nutrient delivery to the limbs, a response observed in humans and animal models exposed to hypoxia or nutritional stress (Burrage et al., 2009; Celander, 1960; Edelstone and Rudolph, 1979; Gardner et al., 2002; Giussani et al., 1994,2005; Hawkins et al., 2000; Kidd et al., 1966; Krampl et al., 2001; Llanos et al., 2007; Morrison, 2008; Mulder et al., 1998; Powers and Swyer, 1977; Rudolph, 1984; Williams et al., 2005). Existing studies of regional blood flow generally measure only lower limb blood supply, and data on upper limb circulation are lacking, as are direct studies of blood flow in relation to limb segment lengths in animals or humans. Studies of limb segment blood flow demonstrate proportionally greater flow (corrected for element size) to the stylopod than the zeugopod, although there is evidence for variation with age (proximo-distal gradients decrease with age) and among species (Morris and Kelly, 1980; Nakano et al., 1986; Tothill et al., 1985; Tothill and MacPherson, 1986). In addition, it is well accepted that blood supply affects limb growth (Brashear, 1963; Brodin, 1955; Serrat, 2007; Tomita et al., 1986).
A strength of this study is the predominance of infants and young children in the sample, since plasticity is thought to be greatest at younger ages (Lucas, 1991; Mei et al., 2004; Smith et al., 1976) and thus patterns of body size and proportion in relation to SpO2 are potentially strongest in such individuals. While fetal growth may be the most plastic of all, prenatal environmental influences are mediated through maternal phenotype (Wells, 2003). Limitations of this study include its short timescale, so it was not possible to test whether the patterns maintain into adulthood, or to investigate the interaction between hypoxia and nutritional status on growth suggested by Bailey et al. (2007). It also remains to be demonstrated how other aspects of oxygen delivery relate to body proportions and growth at altitude. Nonetheless the study has important implications for public health policy. As correlations between SpO2 and anthropometry were low, even where significant, this implies that other factors exert much greater influences on growth and variation in body size and proportions, likely nutrition and healthcare as others have argued previously (e.g., Greksa, 2006). Therefore, interventions that serve to improve nutrition and health in highland communities are likely to be effective in improving growth.
In conclusion, this study demonstrates significant correlations between SpO2 or altitude and limb and limb segment length z scores among Andean children. The results indicate that associations are strongest with zeugopod length and then total limb length z scores, but weaker and insignificant with head-trunk height and autopod length z scores. Part of the association between altitude and limb measurement z scores seems to be mediated by SpO2, although correlations are relatively weak, and other factors that covary with altitude are likely to play a major role in influencing body size and proportions. Future work should aim to explore further the effects of hypoxia on growth and body proportions to elucidate the details of the underlying mechanisms, and to distinguish the effects of hypoxia and other environmental stress exposures at altitude.
Acknowledgments
Thank you to Lilia Cabrera and Angela Huamán Gómez of PRISMA, and all the field staff for their assistance in participant recruitment and data collection. Thanks to Dr. Antonio Bernabe Ortiz for assistance with establishing the project, and to Rie Goto for advice. We are also grateful to the Ayacucho Department Health Directorate (DIRESA) for their support, and especially to all the participants and their families who generously gave their time to take part in the study. Thanks also to the editor and two anonymous reviewers whose comments helped to improve the manuscript.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Literature Cited
- Bailey SM, Hu XM. High-altitude growth differences among Chinese and Tibetan children. In: Gilli G, Schell LM, Benso L, editors. Human growth from conception to maturity. London: Smith-Gordon; 2002. pp. 237–247. [Google Scholar]
- Bailey SM, Xu J, Feng JH, Hu X, Zhang C, Qui S. Tradeoffs between oxygen and energy in tibial growth at high altitude. Am J Hum Biol. 2007;19:662–668. doi: 10.1002/ajhb.20667. [DOI] [PubMed] [Google Scholar]
- Baker PT. Evolution of a project: theory, method and sampling. In: Baker PT, Little MA, editors. Man in the Andes: a multidisciplinary study of high altitude Quechua. Stroudsburg, Pennsylvania: Dowden, Hutchinson and Ross, Inc; 1976. pp. 1–20. [Google Scholar]
- Beall CM. Optimal birthweights in Peruvian populations at high and low altitudes. Am J Phys Anthropol. 1981;56:209–216. doi: 10.1002/ajpa.1330560302. [DOI] [PubMed] [Google Scholar]
- Beall CM. Adaptations to altitude: a current assessment. Annu Rev Anthropol. 2001;30:423–456. [Google Scholar]
- Beall CM. Two routes to functional adaptation: Tibetan and Andean high-altitude natives. Proc Natl Acad Sci USA. 2007;104(Suppl 1):8655–8660. doi: 10.1073/pnas.0701985104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall CM, Blangero J, Williams-Blangero S, Goldstein MC. Major gene for percent of oxygen saturation of arterial hemoglobin in Tibetan highlanders. Am J Phys Anthropol. 1994;95:271–276. doi: 10.1002/ajpa.1330950303. [DOI] [PubMed] [Google Scholar]
- Beall CM, Laskowski D, Strohl KP, Soria R, Villena M, Vargas E, Alarcon AM, Gonzales C, Erzurum SC. Pulmonary nitric oxide in mountain dwellers. Nature. 2001;414:411–412. doi: 10.1038/35106641. [DOI] [PubMed] [Google Scholar]
- Beall CM, Song K, Elston RC, Goldstein MC. Higher offspring survival among Tibetan women with high oxygen saturation genotypes residing at 4,000 m. Proc Natl Acad Sci USA. 2004;101:14300–14304. doi: 10.1073/pnas.0405949101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall CM, Strohl KP, Blangero J, Williams-Blangero S, Decker MJ, Brittenham GM, Goldstein MC. Quantitative genetic analysis of arterial oxygen saturation in Tibetan highlanders. Hum Biol. 1997;69:597–604. [PubMed] [Google Scholar]
- Bennett A, Sain SR, Vargas E, Moore LG. Evidence that parent-of-origin affects birth-weight reductions at high altitude. Am J Hum Biol. 2008;20:592–597. doi: 10.1002/ajhb.20784. [DOI] [PubMed] [Google Scholar]
- Bogin B, Smith P, Orden AB, Varela Silva MI, Loucky J. Rapid change in height and body proportions of Maya American children. Am J Hum Biol. 2002;14:753–761. doi: 10.1002/ajhb.10092. [DOI] [PubMed] [Google Scholar]
- Bogin B, Varela-Silva MI. Leg length, body proportion, and health: a review with a note on beauty. Int J Environ Res Public Health. 2010;7:1047–1075. doi: 10.3390/ijerph7031047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brashear HR., Jr Epiphyseal avascular necrosis and its relation to longitudinal bone growth. J Bone Joint Surg Am. 1963;45:14231438. [PubMed] [Google Scholar]
- Brodin H. Longitudinal bone growth, the nutrition of the epiphyseal cartilages and the local blood supply; an experimental study in the rabbit. Acta Orthop Scand Suppl. 1955;20:1–92. [PubMed] [Google Scholar]
- Brutsaert TD, Araoz M, Soria R, Spielvogel H, Haas JD. Higher arterial oxygen saturation during submaximal exercise in Bolivian Aymara compared to European sojourners and Europeans born and raised at high altitude. Am J Phys Anthropol. 2000;113:169–181. doi: 10.1002/1096-8644(200010)113:2<169::AID-AJPA3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Burrage DM, Braddick L, Cleal JK, Costello P, Noakes DE, Hanson MA, Green LR. The late gestation fetal cardiovascular response to hypoglycaemia is modified by prior peri-implantation undernutrition in sheep. J Physiol. 2009;587:611–624. doi: 10.1113/jphysiol.2008.165944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celander O. Blood flow in the foot and calf of the newborn. A plethysmographic study. Acta Pediatr. 1960;49:488–496. doi: 10.1111/j.1651-2227.1960.tb07763.x. [DOI] [PubMed] [Google Scholar]
- Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr. 1990;44:45–60. [PubMed] [Google Scholar]
- Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med. 1992;11:1305–1319. doi: 10.1002/sim.4780111005. [DOI] [PubMed] [Google Scholar]
- Edelstone DI, Rudolph AM. Preferential streaming of ductus venosus blood to the brain and heart in fetal lambs. Am J Physiol Heart Circ Physiol. 1979;237:H724–H729. doi: 10.1152/ajpheart.1979.237.6.H724. [DOI] [PubMed] [Google Scholar]
- Erzurum SC, Ghosh S, Janocha AJ, Xu W, Bauer S, Bryan NS, Tejero J, Hemann C, Hille R, Stuehr DJ, Feelisch M, Beall CM. Higher blood flow and circulating NO products offset high-altitude hypoxia among Tibetans. Proc Natl Acad Sci USA. 2007;104:17593–17598. doi: 10.1073/pnas.0707462104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouzas S, Priftis KN, Anthracopoulos MB. Pulse oximetry in pediatric practice. Pediatrics. 2011;128:740–752. doi: 10.1542/peds.2011-0271. [DOI] [PubMed] [Google Scholar]
- Frisancho AR. Relative leg length as a biological marker to trace the developmental history of individuals and populations: growth delay and increased body fat. Am J Hum Biol. 2007;19:703–710. doi: 10.1002/ajhb.20676. [DOI] [PubMed] [Google Scholar]
- Gardner DS, Fletcher AJW, Bloomfield MR, Fowden AL, Giussani DA. Effects of prevailing hypoxaemia, acidaemia or hypoglycaemia upon the cardiovascular, endocrine and metabolic responses to acute hypoxaemia in the ovine fetus. J Physiol. 2002;540:351–366. doi: 10.1113/jphysiol.2001.013434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA, Phillips PS, Anstee S, Barker DJ. Effects of altitude versus economic status on birth weight and body shape at birth. Pediatr Res. 2001;49:490–494. doi: 10.1203/00006450-200104000-00009. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Spencer JAD, Hanson MA. Fetal cardiovascular reflex responses to hypoxaemia. Fetal Matern Med Rev. 1994;6:17–37. [Google Scholar]
- Giussani DA, Thakor AS, Frulio R, Gazzolo D. Acute hypoxia increases S100β protein in association with blood flow redistribution away from peripheral circulations in fetal sheep. Pediatr Res. 2005;58:179–184. doi: 10.1203/01.PDR.0000169999.66157.C0. [DOI] [PubMed] [Google Scholar]
- Greksa LP. Growth and development of Andean high altitude residents. High Alt Med Biol. 2006;7:116–124. doi: 10.1089/ham.2006.7.116. [DOI] [PubMed] [Google Scholar]
- Gunnell DJ, Smith GD, Frankel SJ, Kemp M, Peters TJ. Socio-economic and dietary influences on leg length and trunk length in childhood: a reanalysis of the Carnegie (Boyd Orr) survey of diet and health in prewar Britain (1937–39) Paediatr Perinat Epidemiol. 1998;12(Suppl 1):96–113. doi: 10.1046/j.1365-3016.1998.0120s1096.x. [DOI] [PubMed] [Google Scholar]
- Haas JD, Baker PT, Hunt EE., Jr The effects of high altitude on body size and composition of the newborn infant in southern Peru. Hum Biol. 1977;49:611–628. [PubMed] [Google Scholar]
- Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- Hawkins P, Steyn C, McGarrigle HHG, Calder NA, Saito T, Stratford LL, Noakes DE, Hanson MA. Cardiovascular and hypothalamic-pituitary-adrenal axis development in late gestation fetal sheep and young lambs following modest maternal nutrient restriction in early gestation. Reprod Fertil Dev. 2000;12:443–464. doi: 10.1071/rd99071. [DOI] [PubMed] [Google Scholar]
- Hunter C, Clegg EJ. Changes in skeletal proportions of the rat in response to hypoxic stress. J Anat. 1973;114:201–219. [PMC free article] [PubMed] [Google Scholar]
- Jensen LA, Onyskiw JE, Prasad NGN. Meta-analysis of arterial oxygen saturation monitoring by pulse oximetry in adults. Heart Lung J Acute Crit Care. 1998;27:387–408. doi: 10.1016/s0147-9563(98)90086-3. [DOI] [PubMed] [Google Scholar]
- Julian CG, Hageman JL, Wilson MJ, Vargas E, Moore LG. Lowland origin women raised at high altitude are not protected against lower uteroplacental O2 delivery during pregnancy or reduced birth weight. Am J Hum Biol. 2011;23:509–516. doi: 10.1002/ajhb.21167. [DOI] [PubMed] [Google Scholar]
- Julian CG, Vargas E, Armaza JF, Wilson MJ, Niermeyer S, Moore LG. High-altitude ancestry protects against hypoxia-associated reductions in fetal growth. Arch Dis Child Fetal Neonatal Ed. 2007;92:F372–F377. doi: 10.1136/adc.2006.109579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian CG, Wilson MJ, Lopez M, Yamashiro H, Tellez W, Rodriguez A, Bigham AW, Shriver MD, Rodriguez C, Vargas E, Moore LG. Augmented uterine artery blood flow and oxygen delivery protect Andeans from altitude-associated reductions in fetal growth. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1564–R1575. doi: 10.1152/ajpregu.90945.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd L, Levison H, Gemmel P, Aharon A, Swyer PR. Limb blood flow in the normal and sick newborn: a plethysmographic study. Am J Dis Child. 1966;112:402–407. doi: 10.1001/archpedi.1966.02090140074004. [DOI] [PubMed] [Google Scholar]
- Krampl E, Chalubinski K, Schatten C, Husslein P. Does acute hypoxia cause fetal arterial blood flow redistribution? Ultrasound Obstet Gynecol. 2001;18:175–177. doi: 10.1046/j.1469-0705.2001.00501.x. [DOI] [PubMed] [Google Scholar]
- Lampl M, Kuzawa CW, Jeanty P. Prenatal smoke exposure alters growth in limb proportions and head shape in the midgestation human fetus. Am J Hum Biol. 2003;15:533–546. doi: 10.1002/ajhb.10140. [DOI] [PubMed] [Google Scholar]
- Leonard WR, Leatherman TL, Carey JW, Thomas RB. Contributions of nutrition vs. hypoxia to growth in rural Andean populations. Am J Hum Biol. 1990;2:613–626. doi: 10.1002/ajhb.1310020605. [DOI] [PubMed] [Google Scholar]
- Llanos AJ, Riquelme RA, Herrera EA, Ebensperger G, Krause B, Reyes RV, Sanhueza EM, Pulgar VM, Behn C, Cabello G, Parer JT, Giussani DA, Blanco CE, Hanson MA. Evolving in thin air: lessons from the llama fetus in the altiplano. Respir Physiol Neurobiol. 2007;158:298–306. doi: 10.1016/j.resp.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Lucas A. Programming by early nutrition in man. In: Bock GR, Whelan J, editors. The childhood environment and adult disease. Chichester: Wiley; 1991. pp. 38–55. [Google Scholar]
- McClung J. Effects of high altitude on human birth: observations on mothers, placentas, and the newborn in two Peruvian populations. Cambridge, MA: Harvard University Press; 1969. [Google Scholar]
- Meadows Jantz L, Jantz RL. Secular change in long bone length and proportion in the United States, 1800–1970. Am J Phys Anthropol. 1999;110:57–67. doi: 10.1002/(SICI)1096-8644(199909)110:1<57::AID-AJPA5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Mei Z, Grummer-Strawn LM, Thompson D, Dietz WH. Shifts in percentiles of growth during early childhood: analysis of longitudinal data from the California child health and development study. Pediatrics. 2004;113:e617–e627. doi: 10.1542/peds.113.6.e617. [DOI] [PubMed] [Google Scholar]
- Moore LG. Fetal growth restriction and maternal oxygen transport during high altitude pregnancy. High Alt Med Biol. 2003;4:141–156. doi: 10.1089/152702903322022767. [DOI] [PubMed] [Google Scholar]
- Moore LG, Niermeyer S, Zamudio S. Human adaptation to high altitude: regional and life-cycle perspectives. Yrbk Phys Anthropol. 1998;27:25–64. doi: 10.1002/(sici)1096-8644(1998)107:27+<25::aid-ajpa3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Morris M, Kelly P. Use of tracer microspheres to measure bone blood flow in conscious dogs. Calcif Tissue Int. 1980;32:69–76. doi: 10.1007/BF02408523. [DOI] [PubMed] [Google Scholar]
- Morrison JL. Sheep models of intauterine growth restriction: fetal adaptations and consequences. Clin Exp Pharmacol Physiol. 2008;35:730–743. doi: 10.1111/j.1440-1681.2008.04975.x. [DOI] [PubMed] [Google Scholar]
- Mulder ALM, van Golde JC, Prinzen FW, Blanco CE. Cardiac output distribution in response to hypoxia in the chick embryo in the second half of the incubation time. J Physiol. 1998;508:281–287. doi: 10.1111/j.1469-7793.1998.281br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Thompson JR, Christopherson RJ, Aherne FX. Blood flow distribution in hind limb bones and joint cartilage from young growing pigs. Can J Vet Res. 1986;50:96–100. [PMC free article] [PubMed] [Google Scholar]
- Niermeyer S, Andrade Mollinedo P, Huicho L. Child health and living at high altitude. Arch Dis Child. 2009;94:806–811. doi: 10.1136/adc.2008.141838. [DOI] [PubMed] [Google Scholar]
- Pan H, Cole TJ. 2010. LMSchartmaker, a program to construct growth references using the LMS method. Version 2.43. Available online at http://www.healthforallchildren.co.uk/
- Pomeroy E, Stock JT, Stanojevic S, Miranda JJ, Cole TJ, Wells JCK. Trade-offs in relative limb length among Peruvian children: extending the thrifty phenotype hypothesis to limb proportions. PLoS One. 2012;7:e51795. doi: 10.1371/journal.pone.0051795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers WF, Swyer PR. Diminished limb blood flow in infants with transposition of the great vessels: an adaptation to chronic hypoxia? Acta Paediatr. 1977;66:205–209. doi: 10.1111/j.1651-2227.1977.tb07834.x. [DOI] [PubMed] [Google Scholar]
- Rivera-Ch M, Castillo A, Huicho L. Hypoxia and other environmental factors at high altitude. Int J Environ Health. 2008;2:92–106. [Google Scholar]
- Rolian C. Developmental basis of limb length in rodents: evidence for multiple divisions of labor in mechanisms of endochondral bone growth. Evol Dev. 2008;10:15–28. doi: 10.1111/j.1525-142X.2008.00211.x. [DOI] [PubMed] [Google Scholar]
- Rolian C. Integration and evolvability in primate hands and feet. Evol Biol. 2009;36:100–117. [Google Scholar]
- Ross RI, Helms PJ. Comparative accuracy of pulse oximetry and transcutaneous oxygen in assessing arterial saturation in pediatric intensive care. Crit Care Med. 1990;18:725–727. doi: 10.1097/00003246-199007000-00009. [DOI] [PubMed] [Google Scholar]
- Rudolph AM. The fetal circulation and its response to stress. J Dev Physiol. 1984;6:11–19. [PubMed] [Google Scholar]
- Samaras TT, editor. Human Body Size and the Laws of Scaling: Physiological, Performance, Growth, Longevity and Ecological Ramifications. New York: Nova Science Publishers Inc; 2007. [Google Scholar]
- Schult S, Canelo-Aybar C. Oxygen saturation in healthy children aged 5 to 16 years residing in Huayllay, Peru at 4340 m. High Alt Med Biol. 2011;12:89–92. doi: 10.1089/ham.2009.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrat MA. Kent State University; 2007. Experimentally determined tissue temperature modulates extremity growth in mammals: a potential comprehensive explanation of Allen’s rule. Unpublished PhD Thesis: [Google Scholar]
- Smith DW, Truog W, Rogers JE, Greitzer LJ, Skinner AL, McCann JJ, Sedgwick Harvey MA. Shifting linear growth during infancy: illustration of genetic factors in growth from fetal life through infancy. J Pediatr. 1976;89:225–230. doi: 10.1016/s0022-3476(76)80453-2. [DOI] [PubMed] [Google Scholar]
- Stinson S. Nutritional, developmental, and genetic influences on relative sitting height at high altitude. Am J Hum Biol. 2009;21:606–613. doi: 10.1002/ajhb.20918. [DOI] [PubMed] [Google Scholar]
- Subhi R, Smith K, Duke T. When should oxygen be given to children at high altitude? A systematic review to define altitude-specific hypoxaemia. Arch Dis Child. 2009;94:6–10. doi: 10.1136/adc.2008.138362. [DOI] [PubMed] [Google Scholar]
- Tomita Y, Tsai TM, Steyers C, Ogden L, Jupiter JB, Kutz JE. The role of the epiphyseal and metaphyseal circulations on longitudinal growth in the dog: an experimental study. J Hand Surg Am. 1986;11:375–382. doi: 10.1016/s0363-5023(86)80145-9. [DOI] [PubMed] [Google Scholar]
- Tothill P, Hooper G, McCarthy I, Hughes S. The variation with flow-rate of the extraction of bone-seeking tracers in recirculation experiments. Calcif Tissue Int. 1985;37:312–317. doi: 10.1007/BF02554880. [DOI] [PubMed] [Google Scholar]
- Tothill P, MacPherson JN. The distribution of blood flow to the whole skeleton in dogs, rabbits and rats measured with microspheres. Clin Phys Physiol Meas. 1986;7:117–123. doi: 10.1088/0143-0815/7/2/002. [DOI] [PubMed] [Google Scholar]
- Unger C, Weiser JK, McCullough RE, Keefer S, Moore LG. Altitude, low birth weight, and infant mortality in Colorado. JAMA. 1988;259:3427–3432. [PubMed] [Google Scholar]
- Wells JC. The thrifty phenotype hypothesis: thrifty offspring or thrifty mother? J Theor Biol. 2003;221:143–161. doi: 10.1006/jtbi.2003.3183. [DOI] [PubMed] [Google Scholar]
- Wells JCK. Maternal capital and the metabolic ghetto: an evolutionary perspective on the transgenerational basis of health inequalities. Am J Hum Biol. 2010;22:1–17. doi: 10.1002/ajhb.20994. [DOI] [PubMed] [Google Scholar]
- West JB, Schoene RB, Milledge JS, Ward MP. High altitude medicine and physiology. London. 2007:Hodder Arnold. [Google Scholar]
- Whitley E, Gunnell D, Davey Smith G, Holly JMP, Martin RM. Childhood circumstances and anthropometry: The Boyd Orr cohort. Ann Hum Biol. 2008;35:518–534. doi: 10.1080/03014460802294250. [DOI] [PubMed] [Google Scholar]
- Williams SJ, Campbell ME, McMillen IC, Davidge ST. Differential effects of maternal hypoxia or nutrient restriction on carotid and femoral vascular function in neonatal rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R360–R367. doi: 10.1152/ajpregu.00178.2004. [DOI] [PubMed] [Google Scholar]
- World Medical Association. 2008. WMA Declaration of Helsinki—ethical principles for medical research involving human subjects. World Medical Association: http://www.wma.net/en/30publications/10policies/b3/. Accessed 03/05/2011.
- Young NM, Hallgrímsson B. Serial homology and the evolution of mammalian limb covariation structure. Evolution. 2005;59:2691–2704. [PubMed] [Google Scholar]
- Zamudio S, Moore LG. Altitude and fetal growth: current knowledge and future directions. Ultrasound Obstet Gynecol. 2000;16:6–8. doi: 10.1046/j.1469-0705.2000.00155.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


